Abstract
In late 2019, the emergence of a new viral strain, later referred to as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) took the shape of a global pandemic, affecting millions of lives and deteriorating economies around the globe. Vaccines were developed at an exceptional rate to combat the viral desolation, all of them being rolled out once they displayed sufficient safety and efficacy. However, assorted adverse events came into attention, one of them being Transverse Myelitis (TM), an infrequent, immune-mediated, focal disease of the spinal cord. This disorder can lead to severe neurological complications including autonomic, sensory, and motor deficits. The literature aims to shed light on TM and its various etiologies, specifically in line with the vaccine, and a comprehensive treatment plan. Discussing and reducing the number of vaccines related adverse events can help succor in bringing down the vaccine hesitancy and ultimately combatting the pandemic.
Keywords: Coronavirus, Neurological manifestation, Vaccine, Transverse myelitis
Highlights
-
•
The COVID-19 global vaccination program achieved significant results in reducing mortality.
-
•
Transverse myelitis (TM) is an immune-mediated, focal disease of the spinal cord that may involve one or more levels.
-
•
According to Vaccine Adverse Event Reporting System, 593 TM cases following COVID-19 vaccination has been reported.
-
•
This review highlights the potential mechanism, diagnosis, and management of COVID-19 vaccine-associated TM.
-
•
Despite rare occurrences, it is imperative to overcome this vaccine hesitancy, occurring secondary to adverse events.
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) which originated in Wuhan, China towards the end of 2019, was declared a pandemic by the World Health Organization (WHO) in March 2020 following its drastic spread [1]. To date, December 26, 2021, 276.4 million confirmed cases have been reported and 5.4 million deaths have occurred worldwide due to the virus [2]. Other than healthcare consequences, the detrimental economic implications of the pandemic have rendered numerous unemployed and financial markets and global economies unstable [3]. Furthermore, according to the United Nations Educational, Scientific and Cultural Organization (UNESCO), lockdowns and closures disrupted over half of the student population worldwide [4].
To overcome the debilitating effects of the virus, worldwide efforts led to the development of several COVID-19 vaccines within the first year of the pandemic. The currently authorized vaccines include can (i) The viral vector vaccines comprising AstraZeneca, Sputnik, and Janssen incorporating spike protein gene into the Adenovirus DNA, which then induces the synthesis of anti-spike protein antibodies. (ii)modern technology-based RNA vaccines including Pfizer and Moderna directly delivering the messenger RNA code for spike protein [5]. The DNA and RNA vaccines use immunologic liposomes as delivery vehicles to obtain maximum antigen levels within the target cells [6,7]. (iii) Sinopharm and Sinovac which makes use of live attenuated virus to stimulate a protective immune response [8]. These vaccines employ adjuvants like Aluminum hydroxide to enhance their effects [6,9].
Despite having different modes of action, these vaccines were approved after rigorous trials and demonstrated an adequate safety profile [10]. As of December 26, 2022, 8.6 billion doses have been administered [2]. Injection site pain, headache, fever, chills, myalgias, and fatigue constitute the commonly reported adverse effects following vaccine administration. All these effects are mild, short-lived, and self-limited [11]. However, a few serious adverse events including tinnitus [12], vaccine-induced thrombotic thrombocytopenia (VITT) [13], myocarditis [14], uveitis [15], Guillain Barre Syndrome (GBS) [16] have also been reported. Although the current literature evidence the beneficial effects of COVID-19 vaccines in reducing hospital admissions and severe outcomes [17,18], rare side effects and spread of misinformation significantly continue to contribute toward vaccine hesitancy [19].
More recently, several cases of transverse myelitis have been reported following COVID-19 vaccination. According to Vaccine Adverse Event Reporting System (VAERS), 593 cases of TM following COVID-19 vaccination have been reported [20]. Although the incidence of these cases is rare, that is compared to the total vaccine doses administered (11.7 billion) [21]. Although the incidence of these cases is rare, understanding the precise pathophysiology, predispositions and management are integral towards countering vaccine hesitancy. In this review, we evaluate the currently available literature to highlight the potentially involved mechanisms and management of such cases.
2. Transverse myelitis: what we know about it?
Transverse myelitis (TM) is an infrequent, immune-mediated, focal disease of the spinal cord that may involve one or more levels, in the absence of a compressive lesion [22]. This form of acute inflammation leads to morbid changes in the spinal cord segments [23]. Around 1.8 million people are affected by this disease every year around the globe with maximal incidence reported between 20 and 40 years of age [24]. It is estimated that 3 in every 100,000 people suffer from this illness and about 66% of these have some degree of residual disability [25].
While the disease can result from a broad spectrum of etiologies, the most prevalent causes include demyelinating illnesses such as multiple sclerosis and neuromyelitis optica. It is also associated with infections like Herpes Simplex Virus (HSV), Varicella Zoster Virus (VZV), Epstein Barr Virus (EBV), and Cytomegalovirus (CMV), immunizations, neoplastic lesions, and connective tissue diseases [24]. Furthermore, numerous cases have been reported in individuals with systemic autoimmune conditions like systemic lupus erythematosus (SLE), Sjogren's syndrome, etc [24,26]. Lastly, the cause of TM is unknown in up to 30% of cases [27]. It is clinically defined by the onset of acute or sub-acute motor, sensory, and autonomic dysfunction [24,28].
This disorder is known to cause neurological symptoms including autonomic, sensory, and motor deficits [23]. Paresthesia and numbness are initial symptoms among adults. Patients also complain of pain in the spinal region, extremities, and abdomen. Weakness is characterized by expeditious development of ascending paraparesis, starts in the lower limbs and then rapidly involves the upper extremities. The genitourinary system is greatly affected with diseased experiencing urinary incontinence, difficulty in voiding, increased urgency, erectile dysfunction, ejaculatory disorders, and markedly decreased lubrication in females. Pudendal nerve lesion diminishes the sensations in both men and women which makes orgasm arduous and causes sexual impairment. Involuntary bowel movements and constipation are the usual manifestations of the gastrointestinal system [29].
This disorder is classified based on the area and extent of the lesion, and the diagnosis is made using spinal cord imaging particularly magnetic resonance imaging (MRI) [30] and lumbar puncture with microscopic analysis of the cerebrospinal fluid (CSF) which identifies inflammatory markers, oligoclonal bands, specific proteins, and enzymes [31]. Longitudinally extensive transverse myelitis (LETM) is a type of TM that involves three or more vertebral segments and is associated with serious morbidity and increased risk for recurrence [32].
3. Literature search and data extraction
Three authors (FNN, SFSH, MDR) independently conducted a thorough literature search over PubMed and google scholar from inception till November 25, 2021. The following key terms separated by BOOLEAN Operators ‘OR’ and ‘AND’ were employed: “SARS-CoV-2 vaccine”, “Coronavirus vaccine”, “COVID-19 vaccine”, “transverse myelitis”, “spinal cord inflammation”, “longitudinally extensive transverse myelitis”, “LETM”. Grey literature and bibliographies of the relevant articles were also screened without any restriction of location and language. Any discrepancies were resolved by discussion with a fourth author (SW). The results of the literature search are shown in Fig. 1. Following a comprehensive literature search, the retrieved full-length articles were screened and recruited for inclusion in this review. After study selection, two authors (SHA, TGS) independently tabulated all the relevant data, as depicted in Table 1.
Fig. 1.
Prisma Flowchart
PRISMA: Preferred reporting items for systemic review and meta-analyses.
Table 1.
A tabulation of the outcomes of literature view.
| Author Country | Age Sex | Past Medical History | Vaccine Administered Time from vaccination to onset of symptoms | Presenting Complaint | Clinical Findings | Investigations and Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Tahir et al.24 USA | 44 y/o Female | Non-significant | Ad26.COV2. S vaccine (Johnson & Johnson/Janssen) 10 days |
Back pain along with nausea and urinary retention for three days. Numbness and weakness in lower extremities along with fever, chills and body aches was also present. | Exaggerated (+3) deep tendon reflex in both extremities and positive Babinski sign bilaterally. Decreased vibration in bilateral toes, and mild paresthesia in neck and abdomen. | MRI showed increased signal throughout the spinal cord extending from the C2-C3 segment. Lumbar puncture showing WBC count of 227 μ/L and RBC count of 25 μ/L. A total cell count of 100 with 96% of lymphocytes, 3% of monocytes, and 1% of eosinophils.CSF chemistry revealed glucose of 71 mg/dL, protein of 43 mg/dL, albumin 0.6 g/dL and lactase dehydrogenase 8 units/L. The myelin basic protein was 2.8 mcg/L and IgG index was 0.67 | Plasma exchange for five treatments over ten days was started after the completion of three-day course of methylprednisolone | Discharged |
| Alshararni et al.33 Saudi Arabia | 38 y/o Male | History of lower extremities pain and numbness | BNT162b2 mRNA-vaccine Pfizer 1 day after first dose. |
Pain and weakness in lower extremities along with severe headache | N/A | The findings of the MRI diagnosis of the dorsal spinal cord with contrast indicate expanded edematous faint enhancing spinal cord at the level of D11 and D12 with anterior cortical and subcortical abnormal signal hyperintense in T1 hypointense in T2 and STIR surrounding by the sclerotic margin. The findings of the lumbosacral spine observed on the MRI are similar to the dorsal spine findings. CSF protein was 621 mq/L (NR: 150–450 mq/L). WBC, RBC, and albumin were within normal range. | N/A | N/A |
| McLean et al.34 USA | 69 y/o Female | Surgically treated cervical cancer, hypothyroidism, hyperlipidemia, restless leg syndrome, and right leg sciatica | BNT162b2 mRNA-vaccine Pfizer 3 days after first dose. |
Weakness and paresthesia bilaterally in hands | Patient was afebrile on admission. There was bilateral weakened grip strength and finger extension. Reflexes | MRI of cervical spine revealed extensive T2 signal abnormalities mostly in anterior aspect and in mid-cord extending from C3-4 down to T2-3. Serum was positive for Coxsackie B5 with titers of 1:8, and Coxsackie B6 with titers of 1:16 (clinically insignificant) | Patient was treated with 1g per day of methylprednisolone for five days along with aggressive physical and occupational training. | Discharged |
| Khan et al.23 NA | 67 y/o Female | Known case of chronic kidney disease, coronary artery disease, neuropathy and previous colon rupture with colostomy | mRNA Vaccine Moderna 1 day after first dose |
Tingling in right lower extremity and difficulty in ambulating requiring assistance for walking | Motor strength was low in right lower (3/5) and right upper (4/5). Upper motor neuron sign was bilaterally present in both lower extremities with +3 reflexes. Babinski sign was also positive bilaterally along with marked loss of vibration in ankle. | Hemoglobin was 8.5 g/dL (NR: 12.0–15.5 g/dL), hematocrit 27% (NR: 36–48%), platelet count 1,30,000 platelets/uL (150,000–450,000 platelets/uL). Calcium 8.4 mg/dL (8.6–10.3 mg/dL, total protein 5.8 g/dL (6–8.3 g/dL), albumin 3.2 g/dL (3.4–5.4 g/dL). Creatinine was elevated to 1.32 mg/dL (0.7–1.2 mg/dL) and D-dimer elevated to 1.28 (range<0.5). Brain MRI revealed scattered patchy foci nonspecific for white matter signal change suggestive of chronic microvascular changes. MRI of the cervical spine revealed hyperintense lesions in the upper cervical spine and cord edema extending from C1-C3 with patchy post-contrast enhancement. CSF study revealed cell count 2, glucose 77 mg/dl, serum glucose 125 mg/dl, CSF protein 56 mg/dl, oligoclonal bands 2 in CSF and 2 in serum, with 0 isolated bands, IgG index 0.48 | IV solumedrol (IVMP) 1 g daily for 3 days but there was no improvement, so PLEX therapy were initiated for 5 days. | Discharged |
| Pagenkopf et al.35 Germany | 45 y/o Male | Actopic dermatitis | ChAdOx1 nCoV-19 (AstraZeneca) 11 days after first dose |
Fever, headache, weakness, thoracic back pain, and urinary retention. | Within one day after admission the patient developed an acute flaccid tetra paresis, emphasizing lower limbs, and a sensory level at Th9. | MRI revealed a LETM lesion showing T2 hyperintense signal of the spinal cord with wide axial and longitudinal extent reaching from C3 to Th2 without gadolinium enhancement. The brain MRI was normal CSF analysis showed a predominantly polymorphonuclear pleocytosis of 481 cells/μl (67% granulocytes), increased protein (1.4 g/L), increased lactate (3.98 mmol/L) and decreased glucose (CSF/serum ratio 0.43). There was no evidence of intrathecal Ig-synthesis or unique oligoclonal bands in CSF. | The patient was given anti-infective combination therapy with acyclovir, ceftriaxone and ampicillin and additionally an anti-oedematous medication with 100 mg prednisolone IV. As soon as a specific infection of the spinal cord was excluded, a pulse treatment with high dose corticosteroids was initiated applying 1 g methylprednisolone per day for five consecutive days followed by oral tapering. | Discharged |
| Jian-Gao et al.36 Taiwan | 76 y/o Female | Hypertension and right sided hearing impairment | mRNA Vaccine Moderna 2 days after first dose |
Low grade fever, right upper limb paresthesia that extended from the distal to the proximal limb areas, and to the right lower limb, progressive gait disturbance and sacral paresthesia | Exhibited good muscle strength, decreased proprioceptive sensation below the right T4 dermatome, impaired joint position sense and thermal analgesia in the right limbs. The deep tendon reflex of the right limbs was relatively brisk and Babinski sign showed a right extensor plantar response |
C-spine MRI revealed extensive intramedullary hyperintensity at C2–C5 levels on T2-weighted images, and at the C3 level with T1 ring enhancement of the cervical cord. Brain MRI and magnetic resonance angiography were unremarkable. Cerebrospinal fluid (CSF) analysis showed mild pleocytosis (15/μL) with neutrophil predominance (73%) and increased protein levels (57.2 mg/dL). CSF RPR, TPPA, HIV, cytology, serum AQP4 antibodies were all negative. It also revealed a vitamin B12 deficiency at 131 pg/mL. The patient was diagnosed with LETM |
Pulse therapy with intravenous methylprednisolone (1 g/day for five days). Following which, oral prednisolone (60 mg/day) was administered and then was gradually tapered off. Hydroxocobalamin (1 mg/day) was included in the regimen |
Discharged |
| Hsiao et al.37 Taiwan | 41 y/o male | Well controlled Diabetes | ChAdOx1 nCoV-19 (AstraZeneca) 2 weeks after first dose |
left peripheral facial palsy, a tingling sensation over T4 dermatome,progressive paresthesia below T4, lower-limb weakness | bilateral pinprick sensation loss below T4, decreased lower-limb muscle power, severe over left side, loss of joint position, and vibration over bilateral lower limbs, increased bilateral knee reflex | Contrast-enhanced MRI of the spine revealed intramedullary-enhancing lesion over the spinal cord at the T1 to T6vertebral levels. CSF analysis demonstrated mild pleocytosis (WBC:11/μL, lymphocyte predominant: 100%) and mild elevated protein levels (44.3 mg/dL). | Pulse therapy with 1000 mg of methylprednisolone daily for 5 days, and tapered off as symptoms improved | Discharged |
| Albokhari et al.38 Saudia Arabia | 16 y/0 Female | Non-significant | BNT162b2 mRNA-vaccine Pfizer 13 days after second dose |
lower extremity weakness and difficulty in walking, progressed upper extremity with numbness of both lower limbs | Moderate decline in the power of all extremities, decrease fine sensation and pain stimuli in the lower extremity, increased tone with spasticity pattern, hyperreflexia with positive Babinski sign. | MRI represented an acute inflammation on the spine. CBC was unremarkable. Brucellosis titer was negative | N/A | Discharged |
| Notghi et al.39 England | 58 y/o Male | Type 2 diabetes mellitus and pulmonary sarcoidosis diagnosed at the age of 32 years | ChAdOx1 nCoV-19 (AstraZeneca) 7 days after first dose |
Progressive numbness in lower limbs, allodynia up to chest level, genital dysesthesia, an episode of urinary incontinence | Hyperesthesia below T7, hyperreflexia in all four limbs, post-void urinary retention and normal cranial nerves. | Contrast MRI of the head and whole spine revealed an extensive T2-weighted hyperintense signal abnormality up to C1 level. Repeat images of the thoracic cord suggested flow voids. Cerebrospinal fluid (CSF) analysis revealed a raised protein of 1.68 g/L, lymphocytic pleocytosis and oligoclonal bands of an identical band pattern to that found in the serum. CT of the thorax showed calcified mediastinal lymph nodes, nodules distributed peri-lymphatically and within the pulmonary fissures. Subsequent CT –positron emission tomography (CT-PET) showed no evidence of fluorodeoxyglucose uptake within these nodules nor elsewhere to suggest active sarcoidosis | intravenous methylprednisolone 1 g/day for 5 days followed by oral prednisolone at 60 mg/day. 5 days of plasma exchange (PLEX) after 10 days of steroid | Recovering |
| Wee Yong Tan et al.40 Malaysia | 25 y/o Female | Non-significant | ChAdOx1 nCoV-19 (AstraZeneca) 16 days after first dose |
fever, myalgia of lower limbs with progressive bilateral weakness, urinary retention | Afebrile with normal vital signs, numbness, allodynia below the T8 spinal level, bilateral hypertonia of lower limbs with reduced power (3/5 proximally and distally), exaggerated deep tendon reflexes at the knees and ankles with upgoing plantar. | Gadolinium-enhanced MRI of the whole spine revealed multi-segment T2-hyperintensities (T3-T5, T7-T8 and T11-L1), which showed variable cord enhancement post-contrast at T7-T8 lesions. CSF examination showed clear-appearing CSF with an elevated protein count of 546 mg/L (normal range: 150–400) and CSF glucose of 3.1 mmol/L (serum glucose of 5.6 mmol/L). Blood investigations revealed haemoglobin of 15.0 g/dL with total white cells of 8.12 x 103 μL (81% neutrophils and 15% lymphocytes) and platelets of 285 x 103 μL. ESR was 21 mm/h. Urine microscopy revealed the presence of leucocytes and bacteria | Intravenous (IV) methylprednisolone 1000 mg daily for 5 days. IV ceftriaxone covering for urinary tract infection for 5 days and subcutaneous enoxaparin for deep venous thrombosis prophylaxis. | Discharged |
| Fitzsimmons et al.41 USA | 63 y/o M | Non-significant | mRNA Vaccine Moderna 1 day after second dose | sharp shooting pain from the buttocks down through the legs into bottoms of the feet with greater severity in the left leg, pain in the lower legs and ankles, numbness of left calf, both ankles and both feet, unable to urinate, constipation. | Patient had left foot drop and brisk patellar and Achilles reflexes | Cervical and lumbar spines appear within normal limits. Increased T2 cord signal seen in the distal spinal cord and conus with questionable associated enhancement. MRI was repeated two days later of brain and few punctate T2/FLAIR signal hyperintensities in bilateral corona radiata, nonspecific were seen. CSF findings included glucose 74 mg/dL (40–75); total protein 37 mg/dL (15–45); cell count and differential normal; total nucleated cell count 3 | IVIG 0.5 g/kg on 10 Apr and 11 Apr (2 doses); Methylprednisolone IV 1 G/day 11–15 Apr (5 doses) followed by oral prednisone | Discharged |
N/A: Data not available, CSF: Cerebrospinal fluid, WBC: White blood cell, RBC: Red blood count, NR: Normal range, PLEX: Plasmapheresis, LETM: Longitudinal extensive transverse myelitis, MRI: Magnetic Resonance Imaging, RPR: Rapid Plasma Reagin, TPPA: treponema pallidum hemagglutination, HIV: Human Immunodeficiency Virus, AQP4: Anti-aquaporin 4, ESR: Electrocyte Sedimentation Rat, USA: United States of America.
4. Demographics
11 studies, comprising data from 11 vaccinated patients (6 females, 5 males) with a mean age 49.27 ± 5.47 were short-listed for the review. Fig. 2 illustrates the geographical distribution of reported cases included in this review [23,24,[33], [34], [35], [36], [37], [38], [39], [40], [41]]. Literature suggests that TM affects women and men, equally in general [42], and minor to no indifference is discerned in the occurrence pattern between Euro/American-born and Afro/Asian-born populations [43], which also appears to be valid for the included cases in this review. However, despite a diverse geographical location, any potential connection among ethnicities, races, or environmental factors that led to TM in a group of people post-vaccination, while sparing others, who got their shots requires exploration. To reach a submissive conclusion and establish missing links, it is pivotal that while conducting trials, geographical links must be investigated critically.
Fig. 2.
Geographical distributions of the reported cases.
5. Transverse myelitis following previous vaccines
Vaccines are probably one of the greatest achievements in the public health sector reducing the number of deaths and illnesses associated with it [44]. Despite being able to significantly reduce mortality and morbidity, vaccines share wavering public confidence due to safety concerns [45]. One such complication of post-vaccination, although very rare, is the manifestation of neurological conditions, including the TM, which is mostly immune mediated [46].
There are several vaccines, along with SARS-CoV-2 vaccines, that are associated with TM. One such example is the recombinant hepatitis B vaccine which was approved in 1986 [47]. An article published in Lupus 2009 has reported 13 cases of TM following HBV vaccination since 1982 making it the most common vaccine associated with TM [46] during the above-mentioned period.
Another example is the live attenuated oral polio vaccine (OPV) which is widely used globally to battle poliovirus but very rarely it can cause poliovirus which itself is associated with TM. The Institute of Medicine of the National Academies of the United States in 1993 mentioned a casual relation between TM and OPV vaccine [46]. Primarily because of this along with several other disadvantages, many countries have shifted to inactivated poliovirus vaccine (IPV) [48].
Other vaccines, including DPT, Influenza, and rabies vaccination may also result in neurological complications including the TM [46]. Prolonged periods between vaccination and the onset of symptoms are another debatable topic and might point towards other underlying complications, genetic predisposition, and environmental triggers. The exact cause and the mechanism behind vaccine-induced TM are not clear but it is proposed that since the host's response to the vaccine is similar to the response by an infective agent and can cause auto-immunity, it could be assumed that the recombinant or live attenuated vaccines may trigger TM through the same mechanism [49].
6. Transverse myelitis following SARS-CoV-2 vaccines
As summarized in the table below (Table 1), most of the cases presented with varied neurological manifestations of sensory, motor, and autonomic dysfunction. Clinical presentations of the majority cases included pain and weakness of lower limbs [33,40,41] along with paresthesia [23,34] and numbness [24,38,39,41]of lower limbs. Some cases also presented with autonomic symptoms such as urinary retention [24,35,40,41], urinary incontinence [39], and fecal retention [41] accompanying sensory and motor deficits of limbs. Cerebrospinal fluid (CSF) analyses of most of the cases revealed elevated proteins and white blood cells (WBC), and relatively normal glucose. MRI spine of these patients revealed abnormal hyperintense signals from the level of the cervical spine down to the thoracic spine indicating acute inflammation of the spinal cord. The combination of the high abnormal CSF protein test and acute inflammation of the spine observed from the MRI findings were confirmed evidence of acute transverse myelitis [33] after the administration of the SARS-CoV-2 vaccine in the cases under review.
7. Pathophysiology
Usually, TM's pathogenesis involves an atypical immune response causing injury to the spinal cord. At times, infections result in the transcription of a protein that mimics the self-antigen which stimulates T-lymphocytes against the body's own tissue, resulting in immune-mediated destruction. Similarly, the administration of vaccines can also induce the same response. When the body's immune system cannot distinguish between foreign antigens and host antigens, it triggers autoimmunity which leads to the destruction of host cells [23,46,49]. The following mechanisms may induce autoimmunity in a person:
-
A.
Molecular Mimicry
The concept of molecular mimicry implied to post-infection neurological disorder could be possible pathogenesis eliciting TM after SARS-CoV-2 vaccination. The proposed mechanism of the post-infection “Molecular Mimicry” suggests that microorganism epitope shares a similar structure to the host's antigen. The cross-reaction between the epitope and self-antigen activates B lymphocyte and the bystander activation of T cells, which induces an immune response. This mechanism appears to be the explanation for vaccines with viral antigen adjuvants, which may mediate immune responses targeting spinal cords [23,24,29,37,50]. AZD1222 and Johnson & Johnson COVID-19 vaccines, both contain adenovirus antigens, thus may induce acute TM by the same pathogenesis [24,51].
The mRNA vaccine consists of spike glycoprotein sequencing as their main target [52], hence the proposed mechanism of molecular mimicry in such vaccines can be cross-reactivity with a structurally similar host protein causing an acute autoimmune reaction [34,46]. A study reported massive commonality between the SARS-CoV-2 glycoprotein and human proteomes, thereby further supporting molecular mimicry as a possible pathogenesis mechanism [53].
-
B.
Interaction between Spike Protein Antibody and Host Protein
The Moderna COVID-19 (mRNA-1273) vaccine is composed of an mRNA encoding the pre-fusion spike protein encapsulated in lipid nanoparticles, with no adjuvants [54]. Therefore, other mechanisms might be involved in the development of autoimmunity in such cases. A study suggested that the immunological reaction between the SARS-CoV-2 spike protein antibody and tissue proteins, such as myelin basic protein, maybe a plausible cause for the occurrence of demyelinating autoimmune diseases [36,55].
-
C.
Role of Angiotensin-converting Enzyme 2 Receptors
It can be speculated that post-vaccination spike protein elicits a similar response in the body, as that of post-SARS-CoV-2 infection resulting in the development of TM via interaction of spike protein and angiotensin-converting enzyme 2 (ACE-2) receptors.
The mechanism by which COVID-19 causes ATM is not well comprehended but has been presumed to be that of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1). SARS-CoV-1 was thought to cause extra-pulmonary manifestations through its functional receptor; ACE-2, which is abundantly expressed on the endothelial layer of blood vessels of all organs. Entrance to the nervous system can take place through two pathways: directly or indirectly. The direct pathway is via trans-synaptic transmission from the peripheral nervous system or by hematogenous spread into the blood-brain barrier (BBB) through ACE-2 [56]. Whilst the indirect pathway is through a systemic immune response that prompts the release of a cytokine storm, especially interleukin-6 (IL-6) [57,58]. Therefore, the inflammatory response triggered by the interaction between spike proteins and angiotensin-converting enzyme 2 (ACE-2) receptors present in endothelial cells of the blood-brain barrier or spinal neurons may be another possible mechanism of demyelination [36,51,59].
-
D.
Factors predisposing to the development of Autoimmunity
The fact that not all individuals receiving the SARS-CoV-2 vaccine developed TM suggests a plausible role of predisposing factors in the development of autoimmunity. Following are some of the reported and suggested factors.
-
i.
Genetic Predisposition
Individuals developing the SARS-CoV-2 vaccine-associated TM may have certain genetic mutations thereby making them susceptible to autoimmunity development [60,61]. A mutation in VPS37A has been reported in individuals with idiopathic transverse myelitis [62]. Similarly, post-vaccination TM may be associated with certain genetic mutations. However, further research is required to identify the genetic role.
-
ii.
Environmental Triggers
Autoimmune diseases arise in genetically predisposed individuals but require an environmental trigger. Of the many potential environmental factors, infections are the most likely cause [60]. In a few discrete illnesses such as reactive arthritis, rheumatic fever, or hepatitis B virus (HBV) associated vasculitis, the inciting microbial agent is relatively well defined. Similarly, viral vaccines can be implicated as an environmental trigger or factor associated with the development of autoimmunity [63,64].
-
ii.
Comorbidities
An underlying chronic disease or chronic inflammatory process can be correlated to the autoimmunity development in some individuals. Some of the cases reviewed in this study had chronic diseases including hypothyroidism, chronic kidney disease, coronary artery disease, pulmonary sarcoidosis, neuropathy, and atopic dermatitis. Moreover, two cases had a history of diabetes mellitus (DM) should not be ignored as a coincidence. However, the establishment of a potential link between DM and the development of the SARS-CoV-2 vaccine-associated TM needs more evidence and can be elucidated in future research.
However, further investigations are crucial to identify complex interactions between specific predisposing factors and underlying chronic diseases for TM development. Moreover, strong exploration is vital to establish links between the interaction of spike protein with ACE-2 receptors. Lastly, the development of transverse myelitis regardless of viral vector or mRNA vaccine supports the idea of spike protein as the key pathogenesis of SARS-CoV-2 vaccine-associated TM which can be elucidated further in future studies.
8. Diagnostic criteria
Owing to a broad differential for TM, reaching a terminating conclusion may be tricky. Hence, physicians are advised to search and devise a cost-effective strategy. This can be done via an extensive patient's clinical history, thorough examination, CSF pleocytosis and magnetic resonance imaging (MRI) findings [65].
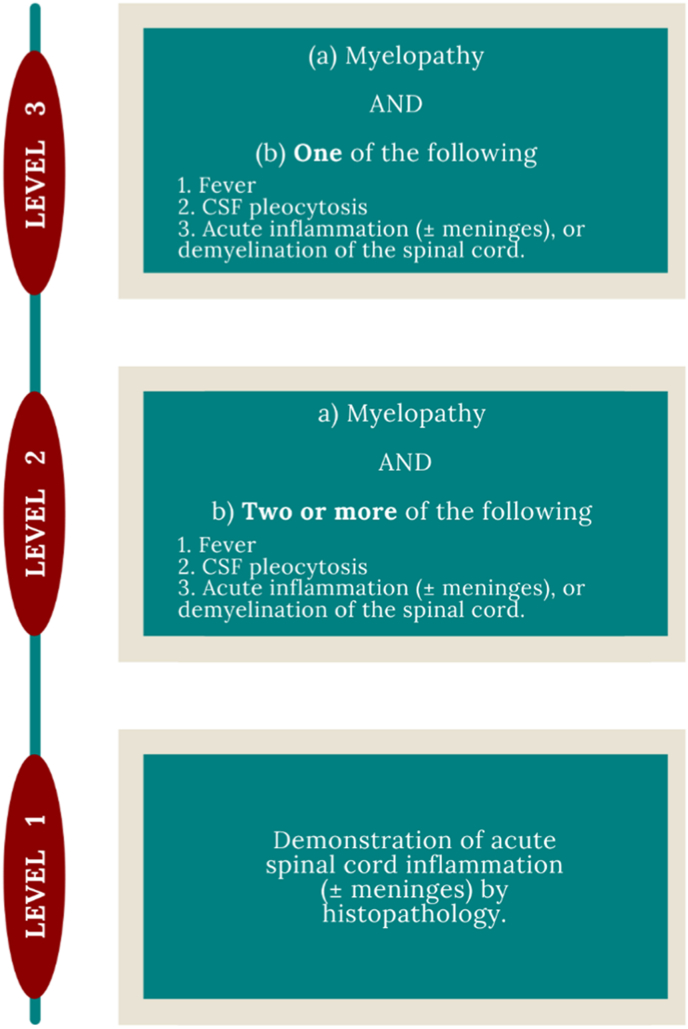
The Brighton Collaboration Encephalomyelitis guideline can be utilized to confirm whether the inflammation is of post-vaccine etiology. The Collaboration group designed a set case definition and diagnostic criteria for encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM), exemplified in Fig. 3, which can be applied in diverse health care settings and different geographical areas [66].
Fig. 3.
The Brighton Collaboration Diagnostic Criteria for Myelitis
Myelopathy: development of sensory, motor, or autonomic dysfunction attributable to the spinal cord, including upper- and/or lower-motor neuron weakness, sensory level, bowel and/or bladder dysfunction, erectile dysfunction; Fever: Temp ≥38 °C; CSF pleocytosis: >5 WBC/mm3 in children >2 months of age; >15 WBC/mm3 in children <2 months of age).
9. Treatment
-
A.
Glucocorticoids
Transverse myelitis (TM) is an inflammatory disorder for which the first-line therapy is the administration of intravenous glucocorticoid [67]. They act by altering the gene expression of inflammatory mediators, thereby reducing their levels in the body, and suppressing the inflammation [68]. Nine of the included studies reported the administration of glucocorticoids, such as methylprednisolone, followed by oral prednisone in three of the studies [36,39,41]. The remaining two studies [33,38] did not report the treatment. Administration through intramuscular and intravenous routes is of great advantage as it surpasses the liver biotransformation and gastrointestinal symptoms that occur when given orally [69]. Five of the included studies reported the administration of intravenous methylprednisolone [23,36,[39], [40], [41]]. This route might also be preferred due to rapid action (onset of action is 1 h) in emergency situations [69]. However, the use of glucocorticoids needs to be strictly regulated as the high dosage and long-term use of these drugs leads to several adverse effects such as resistance of insulin, hypertension, Cushing-like symptoms, and hyperglycemia [70]. In addition, the use of these drugs is contraindicated in the situations such as uncontrolled medical disorders, administration of live vaccines, and systemic infections [68].
-
B.
Plasma Exchange
The plasma exchange can also be initiated if the management by corticosteroids is not optimum [67]. It is the procedure to remove the abnormally located substances from the body such as the removal of cytokines or viral load from the body in COVID-19 [71]. This can be seen in 3 of the included studies [23,24,39], where this line of treatment was started after methylprednisolone. However, the plasma exchange is associated with anaphylactic shock and disruption of electrolytes such as calcium and magnesium [71]. Most of the symptoms occurring are minor with urticaria and pruritus being the most common [72]. Hence, plasma exchange can be used safely with appropriate monitoring.
-
C.
Other medications
The patients should be started with antibiotics if the cerebrospinal fluid (CSF) study shows elevated proteins and an abundance of neutrophils [67]. Consequently, in two of the studies [35,40], antibiotics were administered due to the elevated protein levels in CSF examination in both of the studies and increased granulocytes in Pagenkopf et al. [35]. The common antibiotic given in both studies was ceftriaxone, which is a third-generation drug of cephalosporins and inhibits the synthesis of bacteria's cell walls [73].
Patients with TM can be given rituximab or immunomodulatory therapy such as cyclophosphamide to prevent the resistant TM [22,74]. Rituximab or immunomodulatory therapy helps by decreasing the attacks of diseases such as transverse myelitis or preventing the occurrence of neuromyelitis optica, which is an inflammatory central nervous disease [75,76]. None of the studies, included in this review, reports the usage of these medications. This might be because of different presentations in each case and different genetic factors such as age and co-morbidities.
10. Other neurological manifestations
A wide spectrum of neurological manifestations, ranging from mild to moderate, following COVID-19 vaccines has been reported in the literature. Severe adverse events following immunization included facial nerve palsy [77,78], thrombotic complications [79], ischemic stroke [80], Guillain-Barre Syndrome [81], and cerebral venous thrombosis [82], among many others. Some of the numerous manifestations witnessed post-COVID-19 vaccination are illustrated in Fig. 4. Despite several case reports, the fact that COVID-19 vaccines are the causative agents of all the manifestations is yet to be proven, suggesting the potential need for large-scale collaborative trials and studies to determine the exact relationship between the vaccines and neurological manifestation.
Fig. 4.
Other neurological manifestations following COVID-19 Vaccination.
NMOSD: Neuromyelitis optica spectrum disorders; LETM: Longitudinally extensive transverse myelitis; CVST: Cerebral venous sinus thrombosis.
11. Conclusion
This review highlights the potential pathophysiology and management of SARS-CoV-2 vaccine-induced transverse myelitis in light of the currently available literature. Despite a rare incidence, it is integral to elucidate its precise pathogenesis that may help redefine vaccine administration criteria to eliminate incidence. While corticosteroids remained the mainstay of treatment, there is an overwhelming need to evaluate other treatment options in terms of both short and long-term effects. Further research must emphasize on investigating the genetic and environmental predispositions, risk populations, economic diagnostic measures, and potential treatments options in order to combat vaccine hesitancy and ensure the success of the global vaccination program.
Sources of funding
N/A.
Ethical approval
Not required.
Consent
N/A.
Author contributions
F·N·N and S·F·S·H conceived the idea, F·N·N, S·F·S·H, M.D.R and S·W, retrieved the data, did write up of letter, and finally, S·H.A and T.G.S reviewed and provided inputs. All authors approved the final version of the manuscript.
Registration of research studies
-
1.
Name of the registry:
-
2.
Unique Identifying number or registration ID:
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Summaiyya Waseem, MBBS, Corresponding author.
Declaration of competing interest
N/A.
Acknowledgments
None.
Contributor Information
Fatima Naz Naeem, Email: fatimanaeem173@gmail.com.
Syeda Fatima Saba Hasan, Email: fatimasabadps@gmail.com.
Muskaan Doulat Ram, Email: muskaanlohana@gmail.com.
Summaiyya Waseem, Email: summaiyyawaseem@gmail.com.
Syed Hassan Ahmed, Email: syedhassanahmed99@gmail.com.
Taha Gul Shaikh, Email: tahagul946@gmail.com.
References
- 1.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. https://click.endnote.com/viewer?doi=10.1136%2Fbmj.m1036&token=WzM2MjI5MzEsIjEwLjExMzYvYm1qLm0xMDM2Il0.BLT2elVlT65SNev0eE7eZm2SD6o [DOI] [PubMed]
- 2.WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/
- 3.Nicola M., Alsafi Z., Sohrabi C., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg Lond Engl. 2020;78:185. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Educational Disruption and Response. UNESCO; 2020. https://en.unesco.org/news/covid-19-educational-disruption-and-response https://plus.google.com/+UNESCO. [Google Scholar]
- 5.Wat zijn de verschillen tussen diverse vaccins tegen COVID-19? NTVT; 2021. http://www.ntvt.nl/tijdschrift/editie/artikel/t/wat-zijn-de-verschillen-tussen-diverse-vaccins-tegen-covid-19 [DOI] [PubMed] [Google Scholar]
- 6.Hofman K., Shenoy G.N., Chak V., Balu-Iyer S.V. Pharmaceutical aspects and clinical evaluation of COVID-19 vaccines. Immunol. Invest. 2021;50:743–779. doi: 10.1080/08820139.2021.1904977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowalczyk A., Doener F., Zanzinger K., et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34:3882–3893. doi: 10.1016/j.vaccine.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Delrue I., Verzele D., Madder A., Nauwynck H.J. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges. Expert Rev. Vaccines. 2012;11:695–719. doi: 10.1586/erv.12.38. [DOI] [PubMed] [Google Scholar]
- 9.Heinz F.X., Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. Npj Vaccines. 2021;6:1–13. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Z.-P., Yang M., Lai C.-L. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharm Basel Switz. 2021;14:406. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernández A.F., Calina D., Poulas K., et al. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol Rep. 2021;8:871. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S.H., Waseem S., Shaikh T.G., et al. SARS-CoV-2 vaccine-associated-tinnitus: a review. Ann Med Surg. 2022;75 doi: 10.1016/j.amsu.2022.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed S.H., Shaikh T.G., Waseem S., et al. Vaccine-induced thrombotic thrombocytopenia following coronavirus vaccine: a narrative review. Ann Med Surg. 2022;73 doi: 10.1016/j.amsu.2021.102988. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B., Kaur P., Cedeno L., et al. COVID-19 mRNA vaccine and myocarditis. Eur J Case Rep Intern Med. 2021;8 doi: 10.12890/2021_002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ElSheikh R.H., Haseeb A., Eleiwa T.K., Elhusseiny A.M. Acute uveitis following COVID-19 vaccination. Ocul. Immunol. Inflamm. 2021;29:1207–1209. doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 16.Hasan T., Khan M., Khan F., Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghadas SM, Vilches TN, Zhang K, et al The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv. 10.1101/2020.11.27.20240051. [DOI]
- 18.Moline H.L. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥65 Years — COVID-NET, 13 States, february–april 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70 doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri N., Coomes E.A., Haghbayan H., Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum. Vaccines Immunother. 2020;16:2586–2593. doi: 10.1080/21645515.2020.1780846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The vaccine adverse event reporting system (VAERS) request. https://wonder.cdc.gov/vaers.html [DOI] [PubMed]
- 21.Ritchie H., Mathieu E., Rodés-Guirao L., et al. Our World Data; 2020. Coronavirus Pandemic (COVID-19) [Google Scholar]
- 22.Lim P.A.C. Transverse myelitis. Essent Phys Med Rehabil. 2020;952 doi: 10.1016/B978-0-323-54947-9.00162-0. [DOI] [Google Scholar]
- 23.Khan E., Shrestha A.K., Colantonio M.A., et al. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J. Neurol. 2021 doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahir N., Koorapati G., Prasad S., et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021;13 doi: 10.7759/cureus.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West T.W., Hess C., Cree B.A.C. Acute transverse myelitis: demyelinating, inflammatory, and infectious myelopathies. Semin. Neurol. 2012;32:97–113. doi: 10.1055/s-0032-1322586. [DOI] [PubMed] [Google Scholar]
- 26.Barnes G., Benjamin S., Bowen J.D., et al. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/WNL.59.4.499. [DOI] [PubMed] [Google Scholar]
- 27.Bhat A., Naguwa S., Cheema G., Gershwin M.E. The epidemiology of transverse myelitis. Autoimmun. Rev. 2010;9:A395–399. doi: 10.1016/j.autrev.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Sakakibara R., Hattori T., Yasuda K., Yamanishi T. Micturition disturbance in acute transverse myelitis. Spinal Cord. 1996;34:481–485. doi: 10.1038/sc.1996.82. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan C. Transverse myelitis: pathogenesis, diagnosis and treatment. Front. Biosci. 2004;9:1483. doi: 10.2741/1351. [DOI] [PubMed] [Google Scholar]
- 30.Bachhuber A. [Inflammatory spinal cord diseases and transverse myelitis] Radiol. 2021;61:251–257. doi: 10.1007/s00117-021-00816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drozdowski W. [Transverse myelitis] Przegl. Epidemiol. 2008;62(Suppl 1):30–38. [PubMed] [Google Scholar]
- 32.Kitley J.L., Leite M.I., George J.S., Palace J.A. The differential diagnosis of longitudinally extensive transverse myelitis. Mult Scler Houndmills Basingstoke Engl. 2012;18:271–285. doi: 10.1177/1352458511406165. [DOI] [PubMed] [Google Scholar]
- 33.Alshahrani A. Acute transverse myelitis associated with COVID-19 vaccine: a case report. Int. J. Res. Pharm. Sci. 2021;12:2083–2087. doi: 10.26452/ijrps.v12i3.4818. [DOI] [Google Scholar]
- 34.McLean P., Trefts L. Transverse myelitis 48 hours after the administration of an mRNA COVID 19 vaccine. Neuroimmunol Rep. 2021;1 doi: 10.1016/j.nerep.2021.100019. [DOI] [Google Scholar]
- 35.Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J.-J., Tseng H.-P., Lin C.-L., et al. Acute transverse myelitis following COVID-19 vaccination. Vaccines. 2021;9:1008. doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao Y.-T., Tsai M.-J., Chen Y.-H., Hsu C.-F. Acute transverse myelitis after COVID-19 vaccination. Med Kaunas Lith. 2021;57:1010. doi: 10.3390/medicina57101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albokhari A., Alsawas A., Adnan M.A., et al. Acute inflammatory transverse myelitis post pfizer-BioNTech-COVID-19 vaccine in 16-year-old. Sci Prepr. 2021 doi: 10.14293/S2199-1006.1.SOR-.PPVXII5.v1. [DOI] [Google Scholar]
- 39.Notghi A.A., Atley J., Silva M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin. Med. 2021;21:e535–e538. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan W.Y., Yusof Khan A.H.K., Mohd Yaakob M.N., et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol. 2021;21:395. doi: 10.1186/s12883-021-02427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzsimmons W., Nance C.S. Social Science Research Network; Rochester, NY: 2021. Sudden Onset of Myelitis after COVID-19 Vaccination: an Under-recognized Severe Rare Adverse Event. [Google Scholar]
- 42.Scott T.F., Frohman E.M., De Seze J., et al. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77:2128–2134. doi: 10.1212/WNL.0b013e31823dc535. [DOI] [PubMed] [Google Scholar]
- 43.Berman M., Feldman S., Alter M., et al. Acute transverse myelitis: incidence and etiologic considerations. Neurology. 1981;31:966–971. doi: 10.1212/wnl.31.8.966. [DOI] [PubMed] [Google Scholar]
- 44.Miller E.R., Moro P.L., Cano M., Shimabukuro T.T. Deaths following vaccination: what does the evidence show? Vaccine. 2015;33:3288–3292. doi: 10.1016/j.vaccine.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson H.J., Cooper L.Z., Eskola J., et al. Addressing the vaccine confidence gap. Lancet Lond Engl. 2011;378:526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 46.Agmon-Levin N., Kivity S., Szyper-Kravitz M., Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009;18:1198–1204. doi: 10.1177/0961203309345730. [DOI] [PubMed] [Google Scholar]
- 47.Ho J.K.-T., Jeevan-Raj B., Netter H.-J. Hepatitis B virus (HBV) subviral particles as protective vaccines and vaccine platforms. Viruses. 2020;12 doi: 10.3390/v12020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandyopadhyay A.S., Garon J., Seib K., Orenstein W.A. Polio vaccination: past, present and future. Future Microbiol. 2015;10:791–808. doi: 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- 49.Tishler M., Shoenfeld Y. Vaccination may be associated with autoimmune diseases. Isr Med Assoc J IMAJ. 2004;6:430–432. [PubMed] [Google Scholar]
- 50.Román G.C., Gracia F., Torres A., et al. Acute transverse myelitis (ATM):Clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marino S., Taibi R., Pavone P., et al. Neurotropism of SARS-CoV 2 and others coronavirus in children: mechanisms and clinical manifestations. Eurasian J Med Oncol. 2021;5:91–93. doi: 10.14744/ejmo.2021.78845. [DOI] [Google Scholar]
- 52.Martínez-Flores D., Zepeda-Cervantes J., Cruz-Reséndiz A., et al. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68:310. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald I., Murray S.M., Reynolds C.J., et al. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Npj Vaccines. 2021;6:1–14. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol Orlando Fla. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamming I., Timens W., Bulthuis M.L.C., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakraborty U., Chandra A., Ray A.K., Biswas P. COVID-19–associated acute transverse myelitis: a rare entity. BMJ Case Rep CP. 2020;13 doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali L., Mohammed I., Zada Y., et al. COVID-19-Associated acute transverse myelitis: a case series of a rare neurologic condition. Cureus. 2021;13 doi: 10.7759/cureus.18551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemoto W., Yamagata R., Nakagawasai O., et al. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur. J. Pharmacol. 2020;872 doi: 10.1016/j.ejphar.2020.172950. [DOI] [PubMed] [Google Scholar]
- 60.Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet Lond Engl. 2003;362:1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 61.Todd J.A., Wicker L.S. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15:387–395. doi: 10.1016/s1074-7613(01)00202-3. [DOI] [PubMed] [Google Scholar]
- 62.Genetic mutation found in familial transverse myelitis | SRNA blog. https://wearesrna.org/genetic-mutation-found-familial-transverse-myelitis/
- 63.Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–3886. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Maldonado Y.A. Current controversies in vaccination: vaccine safety. JAMA. 2002;288:3155–3158. doi: 10.1001/jama.288.24.3155. [DOI] [PubMed] [Google Scholar]
- 65.Jacob A., Weinshenker B.G. An approach to the diagnosis of acute transverse myelitis. Semin. Neurol. 2008;28:105–120. doi: 10.1055/s-2007-1019132. [DOI] [PubMed] [Google Scholar]
- 66.Sejvar J.J., Kohl K.S., Bilynsky R., et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 67.West T.W. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov. Med. 2013;16:167–177. [PubMed] [Google Scholar]
- 68.Hodgens A., Sharman T. StatPearls. StatPearls Publishing. 2022. Corticosteroids. Treasure Island (FL) [Google Scholar]
- 69.Ocejo A., Correa R. StatPearls. StatPearls Publishing. 2022. Methylprednisolone. Treasure Island (FL) [Google Scholar]
- 70.Yang R., Yu Y. Glucocorticoids are double-edged sword in the treatment of COVID-19 and cancers. Int. J. Biol. Sci. 2021;17:1530. doi: 10.7150/ijbs.58695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balagholi S., Dabbaghi R., Eshghi P., et al. Potential of therapeutic plasmapheresis in treatment of COVID-19 patients: immunopathogenesis and coagulopathy. Transfus. Apher. Sci. 2020;59 doi: 10.1016/j.transci.2020.102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D S., D B., M G. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J. Clin. Apher. 2007;22 doi: 10.1002/jca.20143. [DOI] [PubMed] [Google Scholar]
- 73.Bui T., Preuss C.V. StatPearls. StatPearls Publishing. 2022. Cephalosporins. Treasure Island (FL) [Google Scholar]
- 74.Simone C.G., Emmady P.D. StatPearls. StatPearls Publishing. 2022. Transverse myelitis. Treasure Island (FL) [PubMed] [Google Scholar]
- 75.Collongues N., Seze J de. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord. 2011;4:111. doi: 10.1177/1756285611398939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jade J.D., Bansi S., Singhal B. Rituximab in neuromyelitis optica spectrum disorders: our experience. Ann. Indian Acad. Neurol. 2017;20:229. doi: 10.4103/aian.AIAN_499_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colella G., Orlandi M., Cirillo N. Bell's palsy following COVID-19 vaccination. J. Neurol. 2021;268:3589–3591. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan E.Y.F., Chui C.S.L., Lai F.T.T., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect. Dis. 2022;22:64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smadja D.M., Yue Q.-Y., Chocron R., et al. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corrêa D.G., Cañete L.A.Q., dos Santos G.A.C., et al. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: cause or coincidence? Clin. Imag. 2021;80:348–352. doi: 10.1016/j.clinimag.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waheed S., Bayas A., Hindi F., et al. Neurological complications of COVID-19: guillain-barre syndrome following pfizer COVID-19 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krzywicka K., Heldner M.R., Sánchez van Kammen M., et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur. J. Neurol. 2021;28:3656–3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]