Abstract
The growth of Lactobacillus delbrueckii subsp. bulgaricus (L. delbrueckii subsp. bulgaricus) on lactose was altered upon aerating the cultures by agitation. Aeration caused the bacteria to enter early into stationary phase, thus reducing markedly the biomass production but without modifying the maximum growth rate. The early entry into stationary phase of aerated cultures was probably related to the accumulation of hydrogen peroxide in the medium. Indeed, the concentration of hydrogen peroxide in aerated cultures was two to three times higher than in unaerated ones. Also, a similar shift from exponential to stationary phase could be induced in unaerated cultures by adding increasing concentrations of hydrogen peroxide. A significant fraction of the hydrogen peroxide produced by L. delbrueckii subsp. bulgaricus originated from the reduction of molecular oxygen by NADH catalyzed by an NADH:H2O2 oxidase. The specific activity of this NADH oxidase was the same in aerated and unaerated cultures, suggesting that the amount of this enzyme was not directly regulated by oxygen. Aeration did not change the homolactic character of lactose fermentation by L. delbrueckii subsp. bulgaricus and most of the NADH was reoxidized by lactate dehydrogenase with pyruvate. This indicated that NADH oxidase had no (or a very small) energetic role and could be involved in eliminating oxygen.
Lactobacillus delbrueckii subsp. bulgaricus (L. delbrueckii subsp. bulgaricus) is an important species of lactic acid bacteria currently used in the industrial production of fermented milk products. L. delbrueckii subsp. bulgaricus is an aerotolerant anaerobe that obtains most of its energy from homolactic fermentation (12). It does not require strict anaerobic growth conditions and tolerates the concentration of O2 in air. Even though L. delbrueckii subsp. bulgaricus does not use O2 in its energetic metabolism, it is likely that the presence of oxygen in its environment can influence its physiology. Indeed, some lactic acid bacteria possess oxidases that utilize molecular oxygen to oxidize substrates such as pyruvate (22) or NADH (2, 5, 8, 16, 23–25). As these oxidation reactions cannot occur under anaerobic conditions, metabolism in the presence of oxygen cannot be identical to that in the absence of oxygen. Also, the activities of these oxidases can produce partially reduced oxygen species such as the superoxide radical (O2.−), hydrogen peroxide (H2O2), the hydroxyl radical (HO.), and other peroxyl radicals or peroxides that will cause an oxidative stress in the cell. It is therefore expected that the presence of oxygen will induce a specific cellular response to such oxidative stress. In this work, a difference in the growth of L. delbrueckii subsp. bulgaricus in the presence and absence of oxygen was observed. It was also found that L. delbrueckii subsp. bulgaricus could reduce oxygen into hydrogen peroxide with an NADH oxidase, probably to eliminate the oxygen present. However, this detoxification of oxygen led to an overproduction of hydrogen peroxide that caused oxidative stress and triggered an early entry of the cells into stationary phase.
MATERIALS AND METHODS
Chemicals.
NADH and NAD+ were purchased from Boehringer Mannheim, sodium dithionite was purchased from Merck, and thiamine pyrophosphate, flavin adenine dinucleotide (FAD), and hydrogen peroxide were purchased from Sigma. Peroxidase (EC 1.11.1.7), pyruvate oxidase (EC 1.2.3.3), catalase (EC 1.11.1.6), and superoxide dismutase (EC 1.15.1.1) were obtained from Boehringer Mannheim.
Bacterial strains and growth conditions.
L. delbrueckii subsp. bulgaricus B107 was obtained from the Centre International de Recherche Daniel Carasso, Danone group. Bacteria were grown at 42°C in modified MRS medium (6) without manganese, supplemented with 2% lactose, initial pH 6.5. Unaerated and aerated cultures were grown under the same conditions of medium and temperature, except that permanent aeration was provided to aerated cultures by vigorous shaking, whereas no shaking was used for unaerated cultures.
Lactose metabolism by growing cells of L. delbrueckii subsp. bulgaricus.
The concentrations of residual lactose and accumulated d-lactate in the medium were determined at different times with commercially available kits (Boehringer Mannheim), after eliminating the cells by centrifugation for 4 min at 18,000 × g.
Determination of H2O2 production.
H2O2 concentrations were measured after eliminating the cells by centrifugation for 4 min at 18,000 × g, as described by Green and Hill (9), from the amount of quinoneimine formed by the oxidation of 4-aminoantipyrine and phenol by hydrogen peroxide. The assay mixture contained 0.4 mM phosphate buffer (pH 6.9), 2% H2O-saturated phenol, 0.4 mg of 4-aminoantipyrine (Sigma) per ml, and 0.04 U of peroxidase per ml, and the change in the absorbance was measured at 505 nm (A505) with an extinction coefficient of ɛ = 6,400 M−1 cm−1 for the quinoneimine.
Preparation of crude extracts.
Cells in their early stationary phase were harvested by centrifugation, washed with 100 mM phosphate buffer (pH 6.9), and broken with alumina (ca. 2 g of alumina per g of wet cells) in the same buffer. The crude extract was obtained by centrifugation for 30 min at 18,000 × g at 4°C, and its protein concentration was determined according to Bradford (4), with immunoglobulin G as a standard.
Enzyme assays.
NADH oxidase activity was determined spectrophotometrically by measuring the initial rate of NADH oxidation at 25°C with ɛ = 6,220 M−1 cm−1 as the extinction coefficient of NADH at 340 nm. The assay mixture contained 100 to 250 μM NADH and 20 μM FAD in 50 mM phosphate buffer at pH 6. Pyruvate oxidase was assayed by the method of Risse et al. (18) in a reaction mixture composed of 5 mM pyruvate, 2 mM 4-aminoantipyrine, 7 mM 2-hydroxy-3,5 dichlorobenzene sulfonate, and 0.1 U of peroxidase per ml in 50 mM phosphate buffer (pH 6.5) and 10% glycerol. d-Lactate dehydrogenase (LDH) was assayed according to Le Bras and Garel (14) in 400 μM NADH and 7.5 mM pyruvate in 0.4 M potassium acetate (pH 5.5) by the changes at A340 with an optical path of 0.5 cm. For all enzymes assayed, 1 U of activity was defined as the amount that converted 1 μmol of substrate per min at 25°C.
RESULTS AND DISCUSSION
Influence of aeration on the growth of L. delbrueckii subsp. bulgaricus.
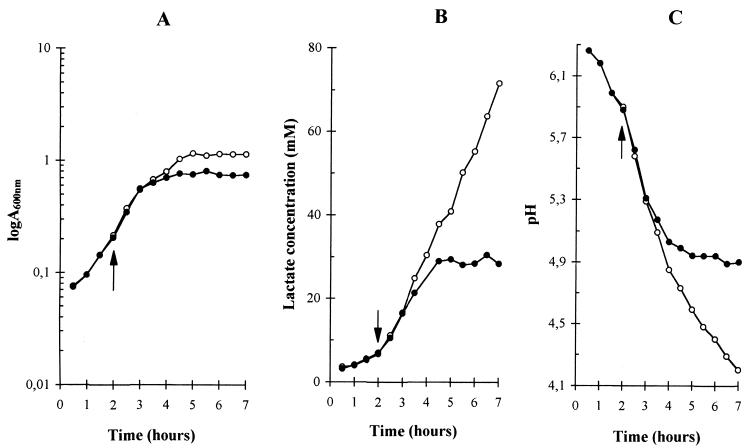
In the following discussion, the unaerated cultures of L. delbrueckii subsp. bulgaricus grown without shaking will be called “still cultures,” as opposed to those grown with vigorous shaking, which will be called “aerated cultures.” In order to eliminate any influence of aeration on the lag phase and to monitor only the influence of aeration on growth, a common inoculum in the same medium was split into two halves that were both grown without shaking until they reached A600 = 0.2. At this point, one half was shifted to aerated conditions and grown with strong shaking, while the other half was left still and further grown as an unaerated culture without shaking. The still and aerated cultures showed the same maximal growth rate of 0.8 ± 0.1 h−1, but the aerated culture entered into its stationary phase earlier than the still culture (Fig. 1). The result of this earlier entry into stationary phase upon aeration was a reduction of about 40% in the biomass produced (Fig. 1A). The absorbance of the aerated cultures at A600 remained constant for about 3 h, suggesting that no bacterial lysis occurred during stationary phase. The early entry into stationary phase was associated with a stop in lactose consumption. The interruption in the growth of the aerated cultures was not, however, due to a lack of energy or carbon source, since more than half of the initial lactose was still present in the medium. The still cultures did not stop growing because of exhaustion of their lactose supply, as they utilized less than two-thirds of the initial lactose before settling into stationary phase.
FIG. 1.
Effect of aeration on the growth of L. delbrueckii subsp. bulgaricus (A), the lactate concentration (B), and the pH of the culture medium (C). Open circles, still cultures; solid circles, aerated cultures. The arrow shows the time at which aeration was started. MRS medium with 2% lactose was used. Growth was measured through changes in A600. The lactate concentration and pH of the medium of the supernatant were determined after rapidly centrifuging the cells.
The entry into stationary phase of aerated cultures was accompanied by a stop in the production of lactate. The final lactate concentration in the medium of aerated cultures was around 30 mM, significantly lower than that for still cultures, of close to 72 mM (Fig. 1B). The aerated and still cultures showed the same ratio of 1.9 ± 0.1 millimoles of lactate produced per millimoles of lactose consumed, indicating that aeration did not significantly alter the homolactic character of lactose fermentation by L. delbrueckii subsp. bulgaricus. Because aeration lowered the production of lactate, the final pH reached by aerated cultures was about 0.7 pH units higher than that of still cultures (Fig. 1C), suggesting that the early entry into stationary phase of aerated cultures was not caused by excessive acid stress.
Another difference between aerated and still cultures was that aerated cultures showed little, if any, production of lactate and decrease in pH during their stationary phase, whereas still cultures maintained a significant consumption of lactose (not shown), production of lactate (Fig. 1B), and lowering of pH (Fig. 1C) after they stopped growing. This indicated that the physiological states of stationary cells were different in aerated and still cultures, and that the residual maintenance metabolism was almost quiet in aerated cells but still active in unaerated cells. This difference suggested that what caused the early interruption of growth with aeration was absent in still cultures and could possibly be related to oxidative stress.
Production of H2O2 by L. delbrueckii subsp. bulgaricus culture and its effects on bacterial growth.
Oxidative stress results from an overexposure to reactive oxygen derivatives, such as superoxide anion (O2−) and hydrogen peroxide (H2O2). Although superoxide is a charged species, it could diffuse sufficiently across the cell membrane that a significant fraction of intracellular superoxide would be eliminated in the presence of external superoxide dismutase. The addition of superoxide dismutase to the medium had no effect on the growth curves of aerated cultures, indicating that early entrance into stationary phase was not directly related to an excessive concentration of external superoxide.
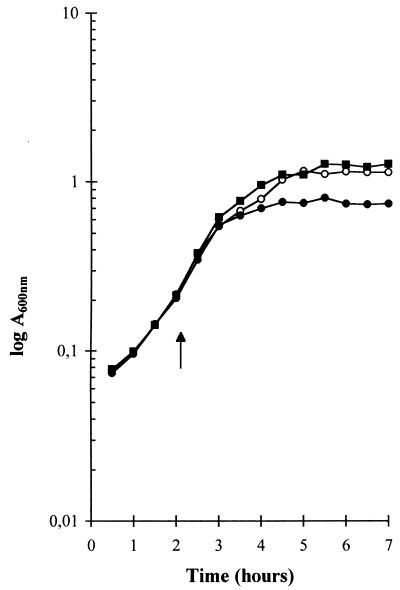
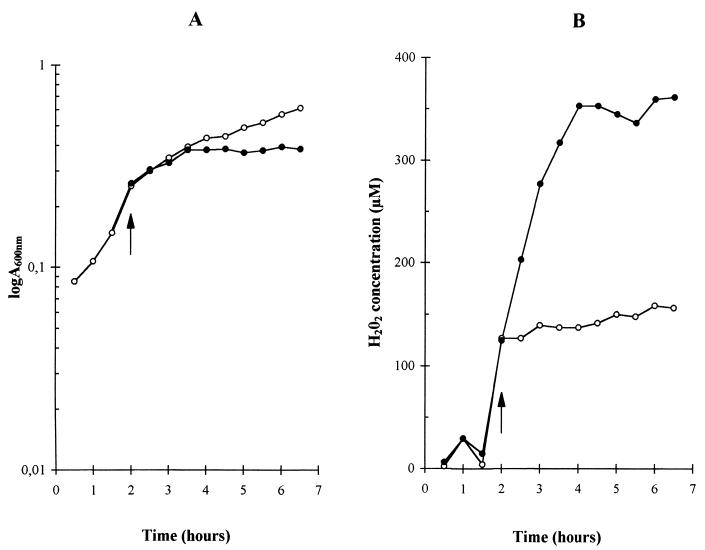
The presence of catalase in the medium of aerated cultures completely prevented the early entry of the bacteria into stationary phase. Indeed, aerated cultures in the presence of catalase showed the same growth curves (Fig. 2), lactate production, final pH, and lactose/lactate ratio (data not shown) as those of still cultures (Fig. 2), strongly suggesting that hydrogen peroxide was somehow involved in the early interruption of growth. The simultaneous presence of catalase and superoxide dismutase had the same influence on the growth curves as that of catalase alone (data not shown). In order to confirm that the early interruption of growth in L. delbrueckii subsp. bulgaricus with aeration was due to the presence of hydrogen peroxide, we measured H2O2 concentrations in the media of still and aerated cultures. One component of MRS medium, beef extract, interfered with the spectrophotometric measurement of H2O2 concentration, and we had to grow L. delbrueckii subsp. bulgaricus in an MRS medium without beef extract. The use of this incomplete MRS medium led to a lower biomass production, but a comparison of still and aerated cultures gave the same results as those observed for cultures grown in complete MRS medium: aeration did not change the maximal growth rate but markedly reduced both biomass (Fig. 1A and 3A) and lactate production. The final lactate concentration in the medium was indeed lowered from 24 mM for still cultures to 14 mM for aerated cultures. The concentration of hydrogen peroxide in the medium was largely increased by aeration (Fig. 3B). In still cultures, hydrogen peroxide concentration rapidly increased to 130 μM at the beginning of the exponential phase and then increased slowly to 160 μM during the rest of the exponential and the stationary phases (Fig. 3). In aerated cultures, hydrogen peroxide concentration increased throughout the exponential phase to reach 360 μM and remained at this level during the stationary phase.
FIG. 2.
Growth of L. delbrueckii subsp. bulgaricus under aerated (●) and nonaerated (○) conditions and in aerated conditions in the presence of 4 U of catalase per ml in the growth medium (■). The arrow shows the time at which aeration was started.
FIG. 3.
Effect of aeration on the growth of L. delbrueckii subsp. bulgaricus (A) and the concentration of hydrogen peroxide concentration in the culture medium (B). Open circles, unaerated still cultures; closed circles, aerated cultures. The arrow shows the time at which aeration was started. MRS medium without beef extract and with 2% lactose was used. Growth was measured through changes at A600. The hydrogen peroxide concentration of the supernatant was determined after rapidly centrifuging the cells.
The levelling off of hydrogen peroxide concentrations in the medium of aerated and still cultures was probably due to different causes. It is shown below that a large amount of the hydrogen peroxide was produced by an NADH:H2O2 oxidase that catalyzed the reduction of molecular oxygen by NADH with a 1/1 stoichiometry. In aerated cultures, the cellular energetic metabolism was very quiet during stationary phase, as judged from the low lactose consumption (data not shown) and low lactate production (Fig. 1B). Therefore, although oxygen was present, the production of H2O2 could have been limited by the availability of NADH. In still cultures, however, cells in late exponential or stationary phase had a metabolism actively producing lactate (Fig. 1B), showing that NADH was not limiting. Rather, the factor limiting H2O2 production could have been the availability of oxygen. Indeed, the final H2O2 concentration of 160 μM in still cultures corresponded roughly to the solubility of molecular oxygen at the growth temperature of 42°C, suggesting that hydrogen peroxide was a waste product of oxygen elimination by the cells.
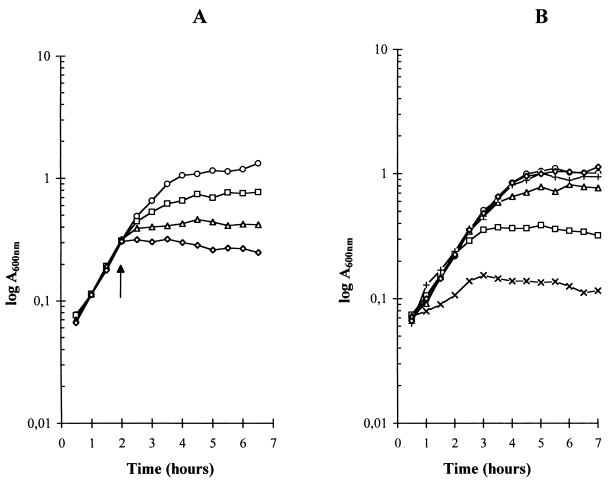
The influence of hydrogen peroxide on the growth of L. delbrueckii subsp. bulgaricus was studied directly by adding either different H2O2 concentrations, from 0.5 to 5 mM, during the exponential phase of still cultures or the same H2O2 concentration of 0.75 mM at different times during the exponential phase. It was found that such additions of hydrogen peroxide caused still cultures to enter into stationary phase (Fig. 4A) independently of the time of addition during exponential phase (Fig. 4B). Absorbance at 600 nm remained constant for several hours after the addition of up to 5 mM H2O2, indicating that no bacterial lysis was occurring. That millimolar concentrations of hydrogen peroxide were sufficient to stop the growth of L. delbrueckii subsp. bulgaricus suggested that the earlier entry into stationary phase and subsequent lower biomass production of aerated cultures were due to the higher accumulation of H2O2 in the medium.
FIG. 4.
Influence of hydrogen peroxide on the growth of L. delbrueckii subsp. bulgaricus under nonaerated conditions. MRS medium with 2% lactose was used. Growth was measured through changes in A600. (A) Influence of hydrogen peroxide concentration. Hydrogen peroxide was added at the time indicated by the arrow to reach final concentrations of 0 (○), 0.5 (□), 1 (▵), and 5 (◊) mM. (B) Influence of the time of addition. Hydrogen peroxide was added at different times to give the same final concentration of 0.75 mM in parallel unaerated cultures. H2O2 was added immediately after inoculation (X), when A600 = 0.150 (□), when A600 = 0.350 (▵), when A600 = 0.600 (+), and when A600 = 1 (◊). ○, growth without addition of hydrogen peroxide.
Some strains of Streptococcus mutans also accumulated high levels (up to 2 mM) of hydrogen peroxide during growth, because they produced H2O2 faster than they eliminated it (24). Since little was known of the metabolism (production and/or elimination) of hydrogen peroxide in L. delbrueckii subsp. bulgaricus, a characterization of the enzyme(s) that could be involved in H2O2 formation was undertaken.
Evidence for the presence of an NADH:H2O2 oxidase in L. delbrueckii subsp. bulgaricus.
L. delbrueckii subsp. bulgaricus has been reported to lack a superoxide dismutase that could have produced hydrogen peroxide by the dismutation of O2− (3). Therefore, the reactions that were the best candidates for the formation of H2O2 in L. delbrueckii subsp. bulgaricus were those catalyzed by oxidases. Three H2O2-producing oxidases (lactate, pyruvate, and NADH oxidases) were assayed for activity in crude extracts from L. delbrueckii subsp. bulgaricus. The lactate and pyruvate oxidases were assayed by their H2O2 production, and NADH oxidase was assayed by its consumption of NADH. These assays showed that no lactate oxidase could be detected, and that some pyruvate oxidase was present. Pyruvate oxidase activity was nondialyzable and heat labile, suggesting that it was associated with a protein. It also had properties similar to those of the pyruvate oxidase from Lactobacillus plantarum (18), in that it required FAD and was largely enhanced by adding 50 μM thiamine pyrophosphate and 1 mM Mn2+ ion to the assay. It is thus likely that a part of the H2O2 produced by L. delbrueckii subsp. bulgaricus was due to pyruvate oxidase.
It was also found that extracts of L. delbrueckii subsp. bulgaricus contained a nondialyzable and heat-labile activity that could rapidly reoxidize NADH into NAD+. NADH reoxidation was not dependent on the addition of any electron acceptor and apparently needed only dissolved molecular oxygen, as indicated by the marked reduction of the rate of reoxidation upon degassing the assay buffer and flushing it with nitrogen. The involvement of oxygen in this reoxidation of NADH was confirmed by the complete disappearance of this activity in the presence of the oxygen scavenger sodium dithionite in the assay. The rate of NADH reoxidation was strongly increased by the addition of FAD to the assay mixture, suggesting that the NADH oxidase from L. delbrueckii subsp. bulgaricus was a flavoprotein, as are NADH oxidases from other gram-positive bacteria (1, 13, 20, 21). Other evidence for NADH oxidase being a flavoprotein was the inhibition caused by diphenyleneiodonium chloride, a reagent that binds covalently to reduced flavins (S. Arnould and J.-M. Camadro, personal communication).
The reoxidation of NADH by NADH oxidase could produce either H2O or H2O2, depending on the number of electrons used to reduce molecular oxygen. Two types of NADH oxidases have indeed been described, the first one catalyzing the four-electron reduction of O2 into 2 H2O (13, 17, 19, 21) and the second the two-electron reduction of O2 to H2O2 (2, 10, 11, 24). These enzymes are present in several lactic acid bacteria that possess either a NADH-H2O2 or a NADH-H2O oxidase and sometimes both (5, 7). The NADH oxidase from L. delbrueckii subsp. bulgaricus showed a ratio of 1 ± 0.05 between the amounts of NADH reoxidized and that of H2O2 liberated, indicating a stoichiometric reaction. This indicated not only the involvement of a NADH:H2O2 oxidase in the production of H2O2, but also the absence of any NADH:H2O oxidase. The NADH:H2O2 activity in extracts of L. delbrueckii subsp. bulgaricus decreased very slowly over time during storage at 4°C, indicating that the NADH oxidase was not a fragile enzyme. The optimum pH for its activity was around pH 5.5.
This NADH oxidase contributed much more strongly to the production of hydrogen peroxide than did pyruvate oxidase. Indeed, under the same conditions, the specific activity of NADH oxidase was 25-fold higher than that of pyruvate oxidase, 0.65 and 0.025 μmol/min/mg of protein, respectively. This NADH oxidase thus appeared as an important enzyme in H2O2 production by L. delbrueckii subsp. bulgaricus.
The specific activity of NADH oxidase was determined throughout the growth of L. delbrueckii subsp. bulgaricus with crude extracts taken from still and aerated cultures to check whether or not the presence of oxygen and/or the growth phase had any influence on the cellular amount of this enzyme. The same specific activity of 0.65 ± 0.2 μmol/min/mg of protein was measured for NADH oxidase with and without aeration, and it remained constant during the exponential and stationary phases (Table 1). Therefore, the expression of the NADH oxidase gene was apparently not regulated by its substrate, oxygen, or its product, hydrogen peroxide. Also, no regulation of the amount of NADH oxidase was associated with a shift from exponential to stationary phase. The higher production of H2O2 by aerated cultures of L. delbrueckii subsp. bulgaricus was thus not due to a higher amount of NADH oxidase but probably to a higher concentration of oxygen. This was consistent with the above hypothesis that H2O2 production by still cultures was limited by oxygen availability, and that dissolved oxygen could be eliminated efficiently from the medium by this NADH oxidase.
TABLE 1.
Specific activity of NADH:H2O2 oxidase and LDHa
| t (h) | Sp act and absorbance in:
|
|||||
|---|---|---|---|---|---|---|
| Nonaerated culture
|
Aerated culture
|
|||||
| A600 | NADH oxidase | LDH | A600 | NADH oxidase | LDH | |
| 2 | 0.2 | 0.56 | 9.16 | 0.2 | 0.56 | 9.16 |
| 2.5 | 0.42 | 0.69 | 8.31 | 0.40 | 0.57 | 9.94 |
| 3.5 | 0.86 | 0.52 | 9.28 | 0.62 | 0.55 | 8.09 |
| 4.5 | 1.04 | 0.67 | 12.2 | 0.67 | 0.51 | 7.17 |
| 6 | 1.2 | 0.61 | 12 | 0.68 | 0.75 | 9.28 |
Values represent specific activity in micromoles of NADH reoxidized per minute per milligram of protein during the growth of L. delbrueckii subsp. bulgaricus in MRS medium with 2% lactose at 42°C in aerated and nonaerated cultures.
Comparison between the fluxes of NADH reoxidized by NADH oxidase and lactate dehydrogenase.
In the classical paradigm of lactic fermentation, NADH is reoxidized by LDH. The amount of LDH, like that of NADH oxidase, did not seem to be regulated by aeration or the growth phase (Table 1). The two enzymes, LDH and NADH oxidase, compete for NADH, and their relative contributions to NADH reoxidation could be estimated from the ratio between the concentrations of their respective products, lactate and H2O2, liberated in the medium, assuming that L. delbrueckii subsp. bulgaricus did not rapidly degrade H2O2 (see above). The ratio between lactate and H2O2 was the lowest at the end of aerated cultures, when the concentration of H2O2 reached 360 μM (Fig. 3B) and that of lactate was only 14 mM. The lowest value of the lactate/H2O2 ratio was thus close to 40-fold, indicating that about 97 to 98% of NADH had been reoxidized by LDH and only 2 to 3% by NADH oxidase. Comparing the specific activities of LDH and NADH oxidase in extracts of L. delbrueckii subsp. bulgaricus confirmed that a much higher flux of NADH could be reoxidized with pyruvate than with oxygen. Indeed, during the exponential and stationary phases of still and aerated cultures, the specific activity of LDH was about 15 times higher than that of NADH oxidase (Table 1). Therefore, only a small percentage (at most) of NADH could be reoxidized by oxygen, which suggested that NADH oxidase had little (if any) significant role in energy production in L. delbrueckii subsp. bulgaricus, in agreement with the fermentation remaining homolactic, with the same lactate/lactose ratio of 1.9 ± 0.1 (see above), with and without aeration. Aeration of wild-type L. delbrueckii subsp. bulgaricus was thus not sufficient to induce a visible shift from homolactic to mixed-acid fermentation. However, a very large increase in NADH oxidase activity could result in a significant fraction of NADH being reoxidized with oxygen, so that the pyruvate that would not be reduced into lactate could be metabolized further into acetyl coenzyme A, acetate, acetoin, diacetyl, and other fermentation end products. Indeed, it has been recently shown that a 100-fold overexpression of a foreign NADH oxidase gene in Lactococcus lactis caused a shift from homolactic to mixed-acid fermentation during growth on glucose in the presence of oxygen (15). A similar metabolic shift should occur in L. delbrueckii subsp. bulgaricus if the level of its NADH oxidase activity could be raised by 10- to 100-fold or if the activity of its LDH could be lowered by 10- to 100-fold.
Conclusion.
The present results have shown that aeration provoked an early entry into stationary phase by L. delbrueckii subsp. bulgaricus growing on lactose, probably because of the oxidative stress caused by an excessive liberation of hydrogen peroxide in the medium. This H2O2 production was largely due to NADH oxidase, an enzyme that seemed to be involved in the elimination of dissolved oxygen. This NADH:H2O2 oxidase from L. delbrueckii subsp. bulgaricus could be a flavoprotein, similar to that isolated from S. mutans (11). This NADH oxidase had apparently no crucial energetic role in wild-type L. delbrueckii subsp. bulgaricus, since its activity was much lower than that of LDH. This enzyme could, however, be a valuable target for enzyme engineering, either for increasing the biomass production in presence of oxygen or for shifting lactose fermentation from homolactic to mixed acid.
ACKNOWLEDGMENTS
We are grateful to S. Arnould and J.-M. Camadro for communication of their results prior to publication and to M.-C. de Givry for her help in obtaining the L. bulgaricus stocks.
This work was supported by grants from CNRS (UPR9063), Université Pierre-et-Marie Curie (9270300), and the Danone group.
REFERENCES
- 1.Ahmed S A, Claiborne A. The streptococcal flavoprotein NADH oxidase. 1. Evidence linking NADH oxidase and NADH peroxidase cysteinyl redox centers. J Biol Chem. 1989;264:19856–19863. [PubMed] [Google Scholar]
- 2.Anders R F, Hogg D M, Jago G R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970;19:608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald F S, Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280. [Google Scholar]
- 6.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 7.de Vos W M. Metabolic engineering of sugar catabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:223–242. doi: 10.1007/BF00395934. [DOI] [PubMed] [Google Scholar]
- 8.Götz F, Sedewitz B, Elstner E F. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch Microbiol. 1980;125:209–214. doi: 10.1007/BF00446878. [DOI] [PubMed] [Google Scholar]
- 9.Green M J, Hill H A O. Chemistry of dioxygen. Methods Enzymol. 1984;105:3–22. doi: 10.1016/s0076-6879(84)05004-7. [DOI] [PubMed] [Google Scholar]
- 10.Grufferty R C, Condon S. Effect of fermentation sugar on hydrogen peroxide accumulation by Streptococcus lactis C10. J Dairy Res. 1983;50:481–489. [Google Scholar]
- 11.Higuchi M, Shimada M, Matsumoto J, Yamamoto Y, Rhaman A, Kamio Y. Molecular cloning and sequence analysis of the gene encoding the H2O2-forming NADH oxidase from Streptococcus mutans. Biosci Biotech Biochem. 1994;58:1603–1607. doi: 10.1271/bbb.58.1603. [DOI] [PubMed] [Google Scholar]
- 12.Kandler O, Weiss N. Regular, nonsporing Gram-positive rods. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. London, England: Williams and Wilkins; 1986. pp. 1208–1234. [Google Scholar]
- 13.Koike K, Kobayashi T, Ito S. Purification and characterization of NADH oxidase from a strain of Leuconostoc mesenteroides. J Biochem. 1985;97:1279–1288. doi: 10.1093/oxfordjournals.jbchem.a135179. [DOI] [PubMed] [Google Scholar]
- 14.Le Bras G, Garel J-R. Properties of d-lactate dehydrogenase from Lactobacillus bulgaricus: a possible different evolutionary origin for the d- and l-lactate dehydrogenases. FEMS Microbiol Lett. 1991;15:89–94. doi: 10.1016/0378-1097(91)90533-g. [DOI] [PubMed] [Google Scholar]
- 15.Lopez de Felipe F, Kleerebezem M, de Vos W M, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucey C A, Condon S. Active role of oxygen and NADH oxidase in growth and energy metabolism of Leuconostoc. J Gen Microbiol. 1986;132:1789–1796. [Google Scholar]
- 17.Matsumoto J, Higuchi M, Shimada M, Yamamoto Y, Kamio Y. Molecular cloning and sequence analysis of the gene encoding the H2O-forming NADH oxidase from Streptococcus mutans. Biosci Biotech Biochem. 1996;60:39–43. doi: 10.1271/bbb.60.39. [DOI] [PubMed] [Google Scholar]
- 18.Risse B, Stempfer G, Rudolph R, Mollering H, Jaenicke R. Stability and reconstitution of pyruvate oxidase from Lactobacillus plantarum: dissection of the stabilizing effects of coenzyme binding and subunit interaction. Protein Sci. 1992;1:1699–1709. doi: 10.1002/pro.5560011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross R P, Claiborne A. Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J Mol Biol. 1992;227:658–671. doi: 10.1016/0022-2836(92)90215-6. [DOI] [PubMed] [Google Scholar]
- 20.Saeki Y, Nozaki M, Matsumoto K. Purification and properties of NADH oxidase from Bacillus megaterium. J Biochem. 1985;98:1433–1440. doi: 10.1093/oxfordjournals.jbchem.a135411. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt H-L, Stocklein W, Danzer J, Kirch P, Limbach B. Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Eur J Biochem. 1986;156:149–155. doi: 10.1111/j.1432-1033.1986.tb09560.x. [DOI] [PubMed] [Google Scholar]
- 22.Sedewitz B, Schleifer K H, Götz F. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J Bacteriol. 1984;160:273–278. doi: 10.1128/jb.160.1.273-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smart J B, Thomas T D. Effect of oxygen on lactose metabolism in lactic streptococci. Appl Environ Microbiol. 1987;53:533–541. doi: 10.1128/aem.53.3.533-541.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas E L, Pera K A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983;154:1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitzelsberger W, Götz F, Schleifer K H. Distribution of superoxide dismutases, oxidases, and NADH peroxidases in various streptococci. FEMS Microbiol Lett. 1984;21:243–246. [Google Scholar]