Abstract
Background:
The primary objective of this analysis is to assess if greater exposure to any major antihypertensive drug class was associated with reduced primary composite outcome events in SPRINT.
Methods:
This is a secondary analysis of the SPRINT trial evaluating whether longitudinal, time varying exposure to any major antihypertensive drug class had any impact on primary outcome events, after adjusting for effects of randomization arm, time varying achieved systolic blood pressure, other drug class exposure, and baseline characteristics.
Results:
9,252 participants were included. After adjustments, exposure of one year or greater to thiazide-type diuretics or RAS blockers was associated with significantly fewer primary events than exposure of less than one year (hazard ratio (HR), 0.78; 95% confidence interval [CI], 0.64 to 0.94). There was no significant difference with longer versus shorter exposure to calcium channel blockers. Greater exposure to beta-blockers was associated with an increase in primary events compared to exposure of less than one year (HR, 1.35; 95% CI, 1.13 to 1.62). Furthermore, thiazide-type diuretics were associated with a reduction in heart failure events and RAS blockers with reduced myocardial infarction. Both were associated with less cardiovascular deaths.
Conclusions:
The SPRINT trial demonstrated a lower target blood pressure led to reductions in adverse cardiovascular events. This analysis suggests greater exposure to thiazide-type diuretics and RAS blockers also contributed to reduced adverse cardiovascular events. Greater exposure to beta-blockers was associated with increased cardiovascular events.
Keywords: Hypertension, Antihypertensive Agents, Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Antagonists, Adrenergic beta-Antagonists, Thiazide Diuretics, Calcium Channel Blockers, Treatment Outcome
Graphical Abstract
Introduction
The Systolic Blood Pressure Intervention (SPRINT) Trial was a landmark study demonstrating a lower systolic blood-pressure target of less than 120 mm Hg (intensive-treatment) was superior to a target of less than 140 mm Hg (standard-treatment) in reducing fatal and nonfatal cardiovascular events in hypertensive patients at elevated risk without diabetes, prior stroke, or heart failure.1 Subjects randomized to intensive-treatment experienced a 25% relative risk reduction of the primary composite outcome (first occurrence of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or cardiovascular death) compared to the standard-treatment arm. Of the individual components of the primary outcome, those randomized to intensive treatment experienced a 38% decrease in the rate of heart failure events and a 43% decrease in cardiovascular death rate.
Aside from randomization to a blood pressure target strategy arm, another inherent variable introduced after randomization was the eventual selection of different antihypertensive drug classes and regimens. Due to the need for greater blood pressure reduction, subjects randomized to the intensive-treatment arm received a greater number of antihypertensive agents and thus were more likely to receive those drug classes shown to reduce clinical events in randomized controlled trials (RCT). It is unclear if greater use of these drug classes in the intensive arm may have contributed to these improved outcomes. Others have also questioned whether differences in drug class exposure between the two strategy arms may have impacted outcomes.2–4
The main objective of this analysis is to assess if longer exposure to any of the major antihypertensive drug classes was associated with a reduced primary composite outcome in SPRINT after adjustment for randomization arm, time varying systolic blood pressure achieved, exposure to respective drug classes under study, and specified baseline characteristics.
Methods
Anonymized SPRINT data have been made publicly available through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository (BioLINCC) repository and can be accessed at https://biolincc.nhlbi.nih.gov/home/. The methods for conducting the SPRINT trial have been previously reported.1,5 Briefly, SPRINT was a randomized, controlled, open-label trial designed to compare a blood pressure lowering strategy of targeting treatment to a systolic blood pressure less than 120 mm Hg (intensive-treatment) versus a control group target of 135–139 mm Hg (standard-treatment). At baseline 9,361 enrolled subjects were 50 years of age or older with systolic blood pressure of 130 to 180 mm Hg, most receiving antihypertensive therapy prior to study entry. Subjects were considered at high risk for a future cardiovascular event based on the presence of preexisting cardiovascular disease or any of the following: subclinical cardiovascular disease, Framingham risk score of 15% or greater, age of 75 years and older, or an estimated glomerular filtration rate of 20 to 59 ml per minute per 1.73 m2 of body surface area (by four-variable Modification of Diet in Renal disease equation). Subjects with diabetes mellitus, prior stroke, advanced chronic kidney disease, dementia, or living in a nursing home were excluded. Those with symptomatic heart failure or left ventricular ejection fraction less than 35% (measured within 6 months prior to baseline) were also excluded.
Study visits to measure blood pressure and adjust antihypertensive medications were scheduled monthly for the first 3 months, then every 3 months thereafter. Additional “as needed” visits could be arranged to resolve clinical issues, manage adverse effects, or for extra monitoring. Blood pressure was measured after a 5-minute rest period using the mean of three different measurements taken 1 minute apart.5,6 The antihypertensive regimens before and after each visit were documented in the SPRINT database. Regimens were left to the discretion of individual investigators at each of 102 study sites, however a drug formulary and treatment algorithm advised use the of preferred antihypertensive drug classes at the time of study initiation.7 The focus of this investigation are the antihypertensive drug classes associated with reduced cardiovascular outcomes including 1) thiazide-type diuretics, 2) renin-angiotensin system (RAS) blockers, 3) calcium channel blockers and 4) beta-blockers. For this analysis, RAS blockers consist of either an angiotensin converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) due to their similar pharmacological effects and typical practice of using one or the other, but not both to treat hypertension. In SPRINT, other drug classes could be added to these first-line drug classes if needed to attain blood pressure goals but were not a focus of this investigation.
The SPRINT trial was terminated early based on the recommendation of the data and safety monitoring board when preset criteria for superiority was exceeded on two consecutive occasions in the intensive-treatment arm. Data and outcomes were censored as of August 20, 2015 for the initial publication of results, however patients continued to receive medication and study contact until final close-out visits through July 29, 2016 at which time a final adjudication of outcomes was collected.8 This analysis includes this observational post intervention period data up to each subject’s last visit. As a study site, we received permission to download the final de-identified Expanded Internal Data Release database from the SPRINT trial website.
Statistical Analysis
We extracted and adjusted for the following static baseline characteristics: age (categorized as less than 75 or equal or greater than 75 years), sex, race (black vs. nonblack), baseline systolic blood pressure, presence or absence of baseline clinical cardiovascular disease and chronic kidney disease, and randomization arm. Time varying cumulative systolic blood pressure was calculated as the area under the blood pressure curve, utilizing the trapezoid rule9,10 To create this variable as well as the 4 drug class exposure variables, data at all visits were utilized. All drug class variable exposures were treated as cumulative, time varying. For this analysis, exposure to each antihypertensive drug was calculated by counting the number of days from the clinic visit date first prescribed until the visit date of discontinuance. If discontinued but added back (or another drug from the same drug class) to their regimen at a later clinic visit, the number of days of exposure was added to the previous count. Thus, the time varying medication variables reflect the total days on each antihypertensive drug classes over the study duration. For study comparisons this cumulative time was categorized as an exposure time of less than 1 year versus exposure time of one year or greater. The effect of exposure time of each antihypertensive agent class was measured after controlling for baseline variables of randomization arm, sex, race, age, chronic kidney disease, cardiovascular disease, and the cumulative time varying variables of systolic blood pressure and exposure to each drug class.
The primary composite outcome for this analysis was the same as used in SPRINT, namely the first adjudicated occurrence of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from a cardiovascular cause.5 Secondary outcomes included each of the individual components of the primary outcome.
Subjects without any blood pressure or antihypertensive drug data beyond the randomization visit were excluded. Analysis was conducted in SAS 9.4®. This project proposal was reviewed and approved by the Minneapolis Veterans Affairs Health Care System’s Research and Development Committee.
Results
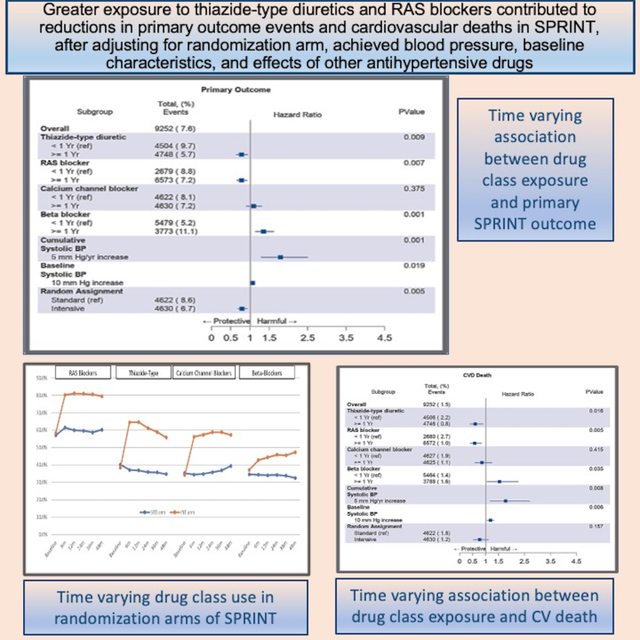
Of the 9,361 subjects enrolled in SPRINT, 109 were excluded from this analysis due to a lack of documented blood pressure measurements or antihypertensive medication after randomization, leaving a total of 9,252. Baseline characteristics based on category of exposure to each antihypertensive drug class are shown in Table 1. Prior to randomization, 90.7% of all subjects were taking an antihypertensive medication. After randomization to the respective strategy arms, most major medication adjustments in the intensive arm were complete by twelve months, then remained relatively stable throughout the study (Figure 1). From baseline, intensive-treatment arm subjects increased their use of thiazide-type diuretics (by 68%), RAS blockers (by 39%), calcium channel blockers (by 68%), and beta-blockers (by 20%). In contrast, for those randomized to the standard-treatment arm, the frequency of drug class use remained consistent from baseline to study end. The individual drugs most frequently used in from each antihypertensive drug class in SPRINT are shown in Table 2.
Table 1.
Baseline Characteristics*
| Characteristic | Thiazide-type diuretic exposure | RAS blocker exposure | Calcium channel blocker exposure | Beta-blocker exposure | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| <1 year 4504 |
≥1 year 4748 |
<1 year 2676 |
≥1 year 6573 |
<1 year 4622 |
≥1 year 4630 |
<1 year 2503 |
≥1 year 2127 |
|
|
| ||||||||
| Intensive arm (%) | 38.3% | 61.2% | 35% | 56.2% | 39.7% | 60.3% | 45.7% | 56.4% |
| Age ≥75 yr (%) | 31.4% | 25.0% | 31.0% | 26.9% | 28.4% | 27.8% | 26.9% | 29.8% |
| Female sex (%) | 33.8% | 37.1% | 36.0% | 35.3% | 36.1% | 34.9% | 35.3% | 35.8% |
| Black race (%) | 26.1% | 36.3% | 35.2% | 29.7% | 25.4% | 37.2% | 32.6% | 29.5% |
| Cardiovascular disease (%) | 22.8% | 17.4% | 19.1% | 20.4% | 20.6% | 19.4% | 12.8% | 30.6% |
| Chronic kidney disease (%)† | 31.4% | 25.1% | 28.7% | 28.0% | 26.9% | 29.4% | 24.4% | 33.7% |
| Baseline systolic BP | 138.2±15.3 | 141.1±15.8 | 137.4±15.1 | 140.6±15.7 | 137.9±15.4 | 141.5±15.6 | 139.1±15.0 | 140.5±16.3 |
| Baseline diastolic BP | 76.9±11.6 | 79.2±12.2 | 77.5±11.7 | 78.4±12.0 | 77.6±11.5 | 78.6±12.4 | 78.4±11.6 | 77.6±12.4 |
| Smoking status (%) | ||||||||
| Never smoked | 44.6% | 43.6% | 42.6% | 44.7% | 44.8% | 43.5% | 45.6% | 47.9% |
| Former smoker | 43.0% | 42.3% | 42,7% | 42.7% | 43.2% | 42.1% | 41.6% | 44.3% |
| Current smoker | 12.3% | 14.1% | 14.7% | 12.6% | 12.0% | 14.4% | 12.8% | 13.8% |
| Aspirin use (%) | 52.5% | 49.8% | 48.0% | 51.8% | 51.2% | 51.0% | 46.0% | 58.5% |
| Serum creatinine (mg/dl) | 1.12±1.54 | 1.12±3.51 | 1.11±2.0 | 1.12±3.0 | 1.11±2.9 | 1.13±2.6 | 1.11±3.15 | 1.13±1.97 |
| Estimated GFR‡ (ml/min/1.73m2) | 69.3±21.4 | 72.8±20.8 | 71.2±21.8 | 71.1±20.9 | 71.6±20.6 | 70.6±21.8 | 72.6±20.9 | 68.9±21.4 |
| Body-mass index§ | 29.9±5.8 | 30.0±5.8 | 29.9±5.8 | 29.9±5.8 | 29.9±5.8 | 30.0±5.8 | 30.0±5.9 | 29.7±5.6 |
| Fasting plasma Glucose | 98.1±13.0 | 99.5±13.9 | 98.2±12.7 | 99.1±13.8 | 98.7±13.5 | 98.9±13.5 | 98.2±13.4 | 99.7±13.6 |
| Fasting total cholesterol (mg/dl) | 188.1±41.4 | 192.1±40.9 | 191.3±41.7 | 189.7±41.0 | 190.4±41.5 | 190.0±40.9 | 193.7±40.4 | 185.0±41.8 |
| Fasting HDL|| cholesterol (mg/dl) | 52.9±14.8 | 52.8±14.2 | 53.5±15.0 | 52.6±14.2 | 52.7±14.3 | 53.1±14.6 | 53.4±14.5 | 51.3±14.2 |
| Fasting total Triglycerides (mg/dl) | 124.7±84.6 | 127.2±95.0 | 123.4±103.5 | 127.1±84.0 | 127.2±81.8 | 124.8±97.7 | 120.6±82.8 | 133.8±99.2 |
| Ratio of urinary albumin (mg) to creatinine (g) | 46.6±187.6 | 34.7±135.1 | 41.7±176.1 | 40±157.2 | 34.5±162.8 | 46.5±162.8 | 32.1±132.9 | 52.8±198.0 |
| Using antihypertensive drugs at baseline | 89.1% | 92.3% | 87.1% | 92.2% | 87.9 | 93.5 | 87.3% | 95.8% |
| # of antihypertensive drugs at baseline | 1.7±1.0 | 1.9±1.0 | 1.6±1.0 | 1.9±1.0 | 1.6±1.0 | 2.0±1.0 | 1.6±1.0 | 2.2±1.0 |
± values are standard deviation units.
Chronic kidney disease: estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 of body surface area.
GFR: glomerular filtration rate
BMI: Body-mass index is the weight (in kilograms) divided by height (m2)
HDL: High-density lipoprotein
To convert serum creatinine values to micromoles per liter, multiply by 88.4. To convert plasma glucose values to millimoles per liter, multiply by 0.05551. To convert cholesterol values to millimoles per liter, multiply by 0.02586. To convert triglyceride values to millimoles per liter, multiply by 0.01129
Figure 1.

Antihypertensive drug class use at baseline, and at 6, 12, 24, 36, and 48 month study visits
Table 2.
Individual drugs within each antihypertensive agent class as used in SPRINT
| Antihypertensive agent class and individual drugs | Frequency of use at 12-month visit within drug class |
|---|---|
|
| |
| Thiazide-type diuretic | |
| chlorthalidone | 89% |
| hydrochlorothiazide | 11% |
|
| |
| RAS blocker | |
| lisinopril | 55% |
| losartan | 25% |
| valsartan | 17% |
|
| |
| Calcium channel blocker | |
| amlodipine | 90% |
| diltiazem or verapamil | 10% |
|
| |
| Beta-blocker | |
| metoprolol | 57% |
| atenolol | 35% |
| carvedilol | 5% |
RAS: renin-angiotensin system; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker
Primary Outcome Results
A total of 706 primary composite outcome events were recorded in the SPRINT Expanded Data Release including 268 myocardial infarctions, 91 acute coronary syndrome events, 168 strokes, 208 decompensated heart failure events and 136 cardiovascular deaths. After adjustment for covariates, exposure of one year or greater to thiazide-type diuretics (hazard ratio (HR), 0.777; 95% confidence interval [CI], 0.646 to 0.933) or RAS blockers (HR, 0.766; 95% confidence interval [CI], 0.646 to 0.933) was associated with significantly fewer primary outcome events than exposure of less than one year (Figure 2). There was no significant difference with longer versus shorter exposure to calcium channel blockers. Longer exposure to beta-blockers was associated with an increased occurrence of the primary outcome compared to exposure of less than one year (HR, 1.35; 95% CI, 1.13 to 1.61). All results were similar whether antihypertensive drug class exposure was analyzed as a continuous measure (cumulative time of exposure) or as a categorical time of exposure (less than one year versus one year or greater) (Table S1). Separating out individual drugs within each antihypertensive drug class did not reveal differences in effect on the primary outcome (Table S2).
Figure 2.

Association of antihypertensive drug class exposure with primary composite outcome*
Footnotes:
* First occurrence of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or cardiovascular death.
Medication exposure and cumulative systolic blood pressure were treated as time varying in the models.
Other baseline variables controlled for but not shown in the forest plot include gender, age, gender, race (black vs. nonblack), and presence or absence of clinical cardiovascular disease or chronic kidney disease.
Secondary Outcomes (individual components of primary outcome)
For the outcome of cardiovascular death, longer exposure to thiazide-type diuretics or RAS blockers was associated with a 40% (HR, 0.60; 95% CI, 0.39 to 0.91) and 42% (HR, 0.58; 95% CI, 0.40 to 0.85) reduction in events, respectively (Figure 3A). Longer exposure to a thiazide-type diuretic was associated with a significant reduction of heart failure events (HR, 0.53; 95% CI, 0.37–0.75) (Figure 3B) while longer exposure to RAS blockers reduced myocardial infarction (HR, 0.64; 95% CI, 0.47–0.87) (Figure 3C). In contrast, calcium channel blockers did not show protective effects with longer exposure for any outcome and trended towards harm for heart failure events. Greater exposure to the beta-blockers, was associated with an increased hazard of myocardial infarction (HR, 1.38; 95% CI, 1.03 to 1.84), heart failure (HR, 1.67; 95% CI, 1.21 to 2.30) and cardiovascular death (HR, 1.53; 95% CI, 1.03 to 2.24). No drug class exposure had significant effect on the individual outcomes of stroke or non-myocardial infarction acute coronary syndrome (Figure 3D and 3E).
Figure 3.

Antihypertensive drug class exposure with individual components of the primary composite outcome
A. CV Death
B. Heart Failure
C. Myocardial Infarction
D. Stroke
E. Non-Myocardial Infarction ACS
Additional analyses explored the interaction between race (black versus non-black) and each antihypertensive drug class. This did not reveal any significant interactions between each of the four drug classes and race. In a separate model, the interaction between sex (male versus female) with each drug class on primary outcome events was also explored. This did not reveal any significant interactions between sex and any of the drug classes on primary outcome. (Tables S3, S4)
Discussion
The question put forward in this analysis of the SPRINT study database is “did greater exposure to thiazide-type diuretics, RAS blockers, calcium channel blockers or beta-blockers, contribute to reduced outcomes above or beyond the effect of randomization arm, baseline covariates, achieved systolic blood pressure, and exposures to any of the other antihypertensive drug class studied?” Results suggest that, in SPRINT, longer exposure to a thiazide-type diuretic or RAS blocker was associated with a reduced rate of the primary composite outcome, cardiovascular deaths, heart failure (thiazide-type diuretics only) and myocardial infarction (RAS blockers only). Notably, longer exposure to the beta-blocker class was associated with harm as expressed by its association with a greater number of primary outcome events, myocardial infarction, heart failure events, and cardiovascular deaths. Exposure to the calcium channel blocker drug class was equivocal after all adjustments.
Meta-analytic analyses of the many RCT’s comparing antihypertensive drugs consistently suggest no significant differences between drug classes in reducing a broad, composite of cardiovascular outcomes.11–18 This has led most to conclude that the blood pressure reduction achieved, not the use of a specific drug class, is most important in reducing overall cardiovascular outcomes. However, these same meta-analytic studies do reveal notably consistent differences between drug classes in reducing individual outcomes that comprise these broad outcomes.
Thiazide-type diuretics have consistently been demonstrated to be superior in reducing cardiovascular events compared to other antihypertensive drug classes, particularly heart failure,.12,14–18 Another recent post-hoc analysis of SPRINT using a different methodology than ours evaluating only intensive arm patients, also showed thiazide-type diuretic exposure to be associated with significantly less heart failure.19 This finding is consistent with our study although our analysis includes both randomization arms and revealed improved outcomes beyond heart failure reductions. Of importance, heart failure prevention is an increasingly important outcome due to the aging population, its increasing prevalence, and a well-known association with high morbidity and mortality.20,21 This is exemplified in SPRINT where heart failure was the second most frequent cardiovascular adverse event and contributed to a 26-fold higher risk for future cardiovascular events compared to other components of the primary outcome.22
Longer exposure to an RAS blocker was also associated with reduced primary outcome events and notably, fewer myocardial infarctions. This finding is also supported by previous evidence. Numerous experimental studies reveal protective cardiovascular pharmacological effects of RAS blockers considered independent or in addition to their blood-pressure lowering effects.23–40 Randomized clinical trials also support blood-pressure independent effects of ACE inhibitors including the Heart Outcomes Prevention Evaluation Study (HOPE) and EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA), both demonstrating substantial improvements in cardiovascular outcomes (including myocardial infarction) with their addition to therapeutic regimens, many already receiving other antihypertensive agents.41,42 The inference of RAS blocker pharmacological effects extending beyond blood pressure reduction has also been supported by their wide success in improving outcomes in patients after myocardial infarction or with heart failure or chronic kidney disease.43–51 A Blood Pressure Lowering Treatment Trialists’ Collaboration review of the early evidence concluded ACE inhibitors exhibit protective effects independent of a lower blood pressure with a particular effect on reducing risks of future coronary heart disease events, similar to the outcomes measured in SPRINT.52 There has been less data for the ARB class due to their later discovery and absence from large RCT’s included in previous meta-analyses. However, like the ACE inhibitors, blood-pressure independent benefits of the ARB drug class are supported by randomized clinical trial evidence with positive findings of the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ON-TARGET) and Losartan Intervention For Endpoint reduction in hypertension study (LIFE) trials.53,54 A Cochrane meta-analysis and two recent new-user comparative cohort studies conclude no comparable differences in efficacy between the two drug class.55–58 Overall, our results are consistent with and contribute further to evidence supporting blood-pressure independent protective vascular effects of RAS blockers showing that longer exposure decreased cardiovascular events (including death) and myocardial infarction after accounting for the effects of achieved blood pressure and randomization arm.
Our subgroup analyses in black versus non-black patients did not reveal significant differences, however there was a slightly reduced risk of primary outcome events with longer exposure to thiazide-type diuretics and a slight attenuation of benefit with longer RAS blocker exposure.
It is known that RAS blockers as monotherapy in hypertension are less effective than thiazide-type diuretics and calcium channel blockers in black patients. However, when RAS blockers are used in combination with thiazide-type diuretics or calcium channel blockers, blood pressure lowering efficacy of these combinations do not differ in blacks versus non-blacks and differences in outcome effects are minimized.59,60 Our data (and results) are more reflective of combination drug therapy as this strategy was most prevalent in SPRINT, but caution is advised with any interpretation as it is limited by low power and other problems associated with subgroup analyses.
Longer exposure to the calcium channel blocker class was not associated with differences in the primary composite endpoint or any of the individual components including stroke compared to shorter exposure. This differs from previous evidence of possible calcium channel blocker superiority in stroke reduction compared to RAS blockers, beta-blockers, and primary antihypertensive drug classes as a group.11,13–18 This could be due to a lower incidence of stroke in SPRINT (patients with a baseline history of stroke were excluded) compared to other studies, thus insufficient power to detect true differences. Although not statistically significant, a trend towards increased heart failure events with longer calcium channel blocker use is consistent with meta-analyses showing a higher risk when compared to other antihypertensive drug classes.11–18
Longer exposure to beta-blockers was associated with greater harm as illustrated by an increased occurrence of the primary composite outcome as well as the secondary outcomes of myocardial infarction, heart failure, and cardiovascular death. This is consistent with meta-analyses indicating an overall inferiority of beta-blockers versus other major antihypertensive drug classes in reducing cardiovascular outcomes.11–13,15,16 Their comparative efficacy and historical inclusion as a first-line antihypertensive has been called into question, particularly the use of atenolol.60–63 Accordingly, the most recent American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines exclude beta-blockers as a first-line antihypertensive drug class.60 The European guidelines continue their inclusion as a preferred drug class.64 Our results support their exclusion.
A strength of this study is that SPRINT used a high-quality design providing outcome data with use of primary antihypertensive agents often used in combination that are more reflective of real-world clinical practice. Most individual RCT’s and thus forthcoming meta-analyses often preclude the use of common combinations of first-line agents in order to maintain internal validity for their comparison. This results in earlier use of second line or supplemental antihypertensive drug classes less commonly used today creating what some have described as RCTs comparing “artificial regimens”.65,66 In contrast, SPRINT reflects current state of the art, evidence-based antihypertensive treatment. Limitations to this study include those inherent in a post-hoc analysis of a previously randomized study. We did not use intent to treat methodology and preserve randomization; however, we did adjust for randomization group and results demonstrate a similar association compared to the main SPRINT results. SPRINT was not designed to measure differences between antihypertensive agents. Also, like SPRINT, our results reflect the population being studied that most notably consisted of patients without diabetes mellitus, stroke, or heart failure at baseline. Although we did adjust for time varying blood pressure, drug exposure and randomization status and other major baseline characteristics, residual confounding may remain.
Perspectives
These results imply that in addition to the targeting of a lower blood pressure as shown in SPRINT, the selection of certain antihypertensive drug classes (thiazide-type diuretics and RAS blockers) and avoidance of others (beta-blockers) may further reduce the risk of major cardiovascular events in a population similar to those studied in SPRINT.
Conclusion
This analysis of the trial database shows that longer use of thiazide-type diuretics and RAS blockers contributed to reductions in primary outcome events in SPRINT after adjusting for randomization group, achieved blood pressure, baseline characteristics and the effect of other antihypertensive drugs. Furthermore, thiazide-type diuretics were associated with a reduction in heart failure events and RAS blockers with reduced myocardial infarction. Both were associated with less cardiovascular deaths. A greater use of these drug classes in the intensive-treatment arm may have contributed to the improved outcomes found in that arm. In contrast, beta-blockers were associated with an increase in cardiovascular events and there was no alteration in outcomes associated with calcium channel blockers after adjustments for blood pressure achieved and other variables. These results support the use of thiazide-type diuretics and RAS blockers early in treatment of hypertension for patient populations with characteristics like those included in the SPRINT trial.
Supplementary Material
Pathophysiological Novelty and Relevance.
What is new:
Previous guidelines and expert opinions have suggested the degree of blood pressure lowering is most important in reducing cardiovascular outcomes when treating hypertension. This analysis of SPRINT trial outcomes suggests the choice of antihypertensive drug classes may also play a prominent role. After accounting for blood pressure reduction and randomization arm, longer exposure to thiazide-type diuretics and RAS inhibitors reduced major adverse cardiovascular outcomes while longer beta blocker exposure increased these outcome events.
What is Relevant:
Approximately 116 million people in the USA (nearly half the adult population) and an estimated 1.28 billion adults aged 30–79 years worldwide have hypertension. Untreated or poorly treated hypertension places individuals at risk for heart disease and strokes, both major causes of premature death worldwide. This analysis of SPRINT trial data provides findings that may contribute to a greater efficiency in selecting antihypertensive drug classes that provide greater benefit and less harm thereby improving effective treatment rates and reducing the adverse cardiovascular outcomes associated with hypertension.
Clinical/Pathophysiological Implications:
Clinical implications include a strategy for clinicians to select antihypertensive drug therapy more effective in preventing the major cardiovascular adverse outcomes, including cardiovascular death, associated with hypertension. Pathophysiological implications are that the pharmacological mechanisms of action of antihypertensive drugs are relevant to identifying current treatment and importantly, are also relevant for future research to identify more effective pharmacological agents or strategies.
Sources of Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
List of nonstandard abbreviations and nonstandard acronyms used in manuscript text:
- SPRINT
Systolic Blood Pressure Intervention
- RCT
randomized controlled trials
- BioLINCC
National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository
- RAS
renin-angiotensin system
- ACE
angiotensin converting enzyme
- ARB
angiotensin receptor blocker
- HR
hazard ratio
- CI
confidence interval
- HOPE
Heart Outcomes Prevention Evaluation study
- EUROPA
EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease
- ON-TARGET
Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial
- LIFE
Losartan Intervention For Endpoint reduction in hypertension study
- ACC
American College of Cardiology
- AHA
American Heart Association
Footnotes
Disclosures:
None
Contributor Information
Douglas D DeCarolis, Minneapolis VA Health Care System, One Veterans Drive, Pharmacy and Research Services (119), Minneapolis MN 55417 USA.
Amy Gravely, Research Service, Minneapolis VA Health Care System, Minneapolis MN 55417 USA.
Christine M. Olney, Research Service, Minneapolis VA Health Care System, Minneapolis, MN 55417 USA; School of Nursing, University of Minnesota, Minneapolis, MN 55455 USA.
Areef Ishani, Minneapolis VA Health Care System and University of Minnesota, Minneapolis MN 55417 USA.
References
- 1.SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control [published correction appears in N Engl J Med. 2017 Dec 21;377(25):2506]. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjeldsen SE, Narkiewicz K, Hedner T, Mancia G. The SPRINT study: Outcome may be driven by difference in diuretic treatment demasking heart failure and study design may support systolic blood pressure target below 140 mmHg rather than below 120 mmHg. Blood Press. 2016;25(2):63–66. doi: 10.3109/08037051.2015.1130775 [DOI] [PubMed] [Google Scholar]
- 3.Pierard LA. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2016;374(23):2293. doi: 10.1056/NEJMc1602668 [DOI] [PubMed] [Google Scholar]
- 4.Andersson C, Vasan RS. Intensive vs Standard Blood Pressure Control for Older Adults. JAMA. 2016;316(18):1922–1923. doi: 10.1001/jama.2016.14927 [DOI] [PubMed] [Google Scholar]
- 5.Systolic Blood Pressure Intervention Trial (SPRINT); Protocol Version 5.0 October 15, 2015. https://biolincc.nhlbi.nih.gov/media/studies/sprint/SPRINT_Protocol.pdf?link_time=2020-07-14_11:17:59.151117. Accessed December 26, 2020 [Google Scholar]
- 6.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, et al. ; SPRINT Research Group. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018. May;71(5):848–857. doi: 10.1161/HYPERTENSIONAHA.117.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. Epub 2003 May 14. Erratum in: JAMA. 2003 Jul 9;290(2):197. [DOI] [PubMed] [Google Scholar]
- 8.SPRINT Research Group, Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, Cutler JA, Evans GW, Johnson KC, Kitzman DW, et al. Final Report of a Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2021. May 20;384(20):1921–1930. doi: 10.1056/NEJMoa1901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YX, Song L, Xing AJ, Gao M, Zhao HY, Li CH, Zhao HL, Chen SH, Lu CZ, Wu SL. Predictive Value of Cumulative Blood Pressure for All-Cause Mortality and Cardiovascular Events. Sci Rep. 2017. Feb 7;7:41969. doi: 10.1038/srep41969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiang K The SAS® Calculations of Areas Under the Curve (AUC) for Multiple Metabolic Readings, WUSS (Western Users of SAS® software) October 13–15, 2004.
- 11.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3 [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289(19):2534–2544. doi: 10.1001/jama.289.19.2534 [DOI] [PubMed] [Google Scholar]
- 13.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. May 19. doi: 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Chen N, Zhou M, Guo J, Zhu C, Zhou J, Ma M, He L. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database Syst Rev. 2021. Oct 17;10(10):CD003654. doi: 10.1002/14651858.CD003654.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fretheim A, Odgaard-Jensen J, Brørs O, Madsen S, Njølstad I, Norheim OF, Svilaas A, Kristiansen IS, Thürmer H, Flottorp S. Comparative effectiveness of antihypertensive medication for primary prevention of cardiovascular disease: systematic review and multiple treatments meta-analysis. BMC Med. 2012;10:33. doi: 10.1186/1741-7015-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015;33(7):1321–1341. doi: 10.1097/HJH.0000000000000614 [DOI] [PubMed] [Google Scholar]
- 17.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahim K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 18.Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR 3rd, Polonsky T, Thompson-Paul AM, Vupputuri S. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018. May 15;71(19):2176–2198. doi: 10.1016/j.jacc.2017.11.004. Erratum in: J Am Coll Cardiol. 2018 May 15;71(19):2272–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujimoto T, Kajio H. Thiazide Use and Decreased Risk of Heart Failure in Nondiabetic Patients Receiving Intensive Blood Pressure Treatment. Hypertension. 2020. Aug;76(2):432–441. doi: 10.1161/HYPERTENSIONAHA.120.15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 21.Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev. 2017. Apr;3(1):7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, et al. ; SPRINT Research Group. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circ Heart Fail. 2017. Apr;10(4):e003613. doi: 10.1161/CIRCHEARTFAILURE.116.003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Morishita R, Moriguchi A, Tomita N, Aoki M, Sakonjo H, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Inhibition of neointima by angiotensin-converting enzyme inhibitor in porcine coronary artery balloon-injury model. Hypertension. 2001. Feb;37(2):270–4. doi: 10.1161/01.hyp.37.2.270. [DOI] [PubMed] [Google Scholar]
- 24.Hornig B, Kohler C, Drexler H. Role of bradykinin in mediating vascular effects of angiotensin-converting enzyme inhibitors in humans. Circulation. 1997. Mar 4;95(5):1115–8. doi: 10.1161/01.cir.95.5.1115. [DOI] [PubMed] [Google Scholar]
- 25.Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Salvetti A. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003. Jun;41(6):1281–6. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 26.Lonn EM, Yusuf S, Jha P, Montague TJ, Teo KK, Benedict CR, Pitt B. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994. Oct;90(4):2056–69. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- 27.Taddei S, Virdis A, Ghiadoni L, Mattei P, Salvetti A. Effects of angiotensin converting enzyme inhibition on endothelium-dependent vasodilatation in essential hypertensive patients. J Hypertens. 1998. Apr;16(4):447–56. doi: 10.1097/00004872-199816040-00006. [DOI] [PubMed] [Google Scholar]
- 28.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001. Feb 13;103(6):799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 29.Hornig B, Arakawa N, Drexler H. Effect of ACE inhibition on endothelial dysfunction in patients with chronic heart failure. Eur Heart J. 1998. Jul;19 Suppl G:G48–53. [PubMed] [Google Scholar]
- 30.Antony I, Lerebours G, Nitenberg A. Angiotensin-converting enzyme inhibition restores flow-dependent and cold pressor test-induced dilations in coronary arteries of hypertensive patients. Circulation. 1996. Dec 15;94(12):3115–22. doi: 10.1161/01.cir.94.12.3115. [DOI] [PubMed] [Google Scholar]
- 31.Prasad A, Husain S, Quyyumi AA. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: a potential role of bradykinin. J Am Coll Cardiol. 1999. Mar;33(3):796–804. doi: 10.1016/s0735-1097(98)00611-1. [DOI] [PubMed] [Google Scholar]
- 32.Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999. Sep 14;100(11):1223–9. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 33.Paul M, Ganten D. The molecular basis of cardiovascular hypertrophy: the role of the renin-angiotensin system. J Cardiovasc Pharmacol. 1992;19 Suppl 5:S51–8. [PubMed] [Google Scholar]
- 34.Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995. Apr;25(4 Pt 2):699–703. doi: 10.1161/01.hyp.25.4.699. [DOI] [PubMed] [Google Scholar]
- 35.Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996. May 15;275(19):1507–13. [PubMed] [Google Scholar]
- 36.Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, Dilley RJ, Cooper ME, Allen TJ. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 2002. Jul 9;106(2):246–53. doi: 10.1161/01.cir.0000021122.63813.32. [DOI] [PubMed] [Google Scholar]
- 37.Fennessy PA, Campbell JH, Mendelsohn FA, Campbell GR. Angiotensin-converting enzyme inhibitors and atherosclerosis: relevance of animal models to human disease. Clin Exp Pharmacol Physiol. 1996. Aug;23(8):S30–2. doi: 10.1111/j.1440-1681.1996.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 38.Pitt B Potential role of angiotensin converting enzyme inhibitors in treatment of atherosclerosis. Eur Heart J. 1995. Nov;16 Suppl K:49–54. doi: 10.1093/eurheartj/16.suppl_k.49. [DOI] [PubMed] [Google Scholar]
- 39.Schölkens BA, Landgraf W. ACE inhibition and atherogenesis. Can J Physiol Pharmacol. 2002. Apr;80(4):354–9. doi: 10.1139/y02-038. [DOI] [PubMed] [Google Scholar]
- 40.Brown NJ, Agirbasli MA, Williams GH, Litchfield WR, Vaughan DE. Effect of activation and inhibition of the renin-angiotensin system on plasma PAI-1. Hypertension. 1998. Dec;32(6):965–71. doi: 10.1161/01.hyp.32.6.965. [DOI] [PubMed] [Google Scholar]
- 41.Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000. Jan 20;342(3):145–53. doi: 10.1056/NEJM200001203420301. Erratum in: 2000 May 4;342(18):1376. Erratum in: N Engl J Med 2000 Mar 9;342(10):748. [DOI] [PubMed] [Google Scholar]
- 42.Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 43.Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. ACE Inhibitor Myocardial Infarction Collaborative Group. Circulation. 1998. Jun 9;97(22):2202–12. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, et al. ; Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003. Nov 13;349(20):1893–906. doi: 10.1056/NEJMoa032292. Epub 2003 Nov 10. Erratum in: N Engl J Med. 2004 Jan 8;350(2):203. [DOI] [PubMed] [Google Scholar]
- 45.SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991. Aug 1;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 46.Yusuf S, Pepine CJ, Garces C, Pouleur H, Salem D, Kostis J, Benedict C, Rousseau M, Bourassa M, Pitt B. Effect of enalapril on myocardial infarction and unstable angina in patients with low ejection fractions. Lancet. 1992. Nov 14;340(8829):1173–8. doi: 10.1016/0140-6736(92)92889-n. [DOI] [PubMed] [Google Scholar]
- 47.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017. Aug;23(8):628–651. doi: 10.1016/j.cardfail.2017.04.014. Epub 2017 Apr 28. [DOI] [PubMed] [Google Scholar]
- 48.Brunner HR, Gavras H. Angiotensin blockade for hypertension: a promise fulfilled. Lancet. 2002. Mar 23;359(9311):990–2. doi: 10.1016/S0140-6736(02)08062-5. [DOI] [PubMed] [Google Scholar]
- 49.López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, et al. ; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004. Aug;25(16):1454–70. doi: 10.1016/j.ehj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. ; American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012. Dec 18;126(25):e354–471. doi: 10.1161/CIR.0b013e318277d6a0. Erratum in: Circulation. 2014 Apr 22;129(16):e463. [DOI] [PubMed] [Google Scholar]
- 51.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 52.Blood Pressure Lowering Treatment Trialists’ Collaboration, Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, Chalmers J, Zanchetti A, MacMahon S. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007. May;25(5):951–8. doi: 10.1097/HJH.0b013e3280bad9b4. Erratum in: J Hypertens. 2007 Jul;25(7):1524. [DOI] [PubMed] [Google Scholar]
- 53.ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008. Apr 10;358(15):1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 54.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, et al. ; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002. Mar 23;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 55.Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014. Aug 22;2014(8):CD009096. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potier L, Roussel R, Elbez Y, Marre M, Zeymer U, Reid CM, Ohman M, Eagle KA, Bhatt DL, Steg PG; REACH Registry Investigators*. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart. 2017. Sep;103(17):1339–1346. doi: 10.1136/heartjnl-2016-310705. [DOI] [PubMed] [Google Scholar]
- 57.Chen R, Suchard MA, Krumholz HM, Schuemie MJ, Shea S, Duke J, Pratt N, Reich CG, Madigan D, You SC, Ryan PB, Hripcsak G. Comparative First-Line Effectiveness and Safety of ACE (Angiotensin-Converting Enzyme) Inhibitors and Angiotensin Receptor Blockers: A Multinational Cohort Study. Hypertension. 2021. Sep;78(3):591–603. doi: 10.1161/HYPERTENSIONAHA.120.16667. Epub 2021 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messerli FH, Bavishi C, Bangalore S. Why Are We Still Prescribing Angiotensin-Converting Enzyme Inhibitors? Circulation. 2022. Feb 8;145(6):413–415. doi: 10.1161/CIRCULATIONAHA.121.057835. Epub 2022 Feb 7. [DOI] [PubMed] [Google Scholar]
- 59.Helmer A, Slater N, Smithgall S. A Review of ACE Inhibitors and ARBs in Black Patients With Hypertension. Ann Pharmacother. 2018. Nov;52(11):1143–1151. doi: 10.1177/1060028018779082. [DOI] [PubMed] [Google Scholar]
- 60.Whelton PK, Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018. May 15;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006Erratum in: J Am Coll Cardiol. 2018 May 15;71(19):2275–2279. [DOI] [PubMed] [Google Scholar]
- 61.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary HTN? A meta-analysis. Lancet. 2005. Oct 29-Nov 4;366(9496):1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 62.Messerli FH, Beevers DG, Franklin SS, Pickering TG. b-Blockers in hypertension-the emperor has no clothes: an open letter to present and prospective drafters of new guidelines for the treatment of hypertension. Am J Hypertens. 2003. Oct;16(10):870–3. doi: 10.1016/s0895-7061(03)01017-3. [DOI] [PubMed] [Google Scholar]
- 63.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004. Nov 6–12;364(9446):1684–9. doi: 10.1016/S0140-6736(04)17355-8. Erratum in: Lancet. 2005 Feb 19;365(9460):656. [DOI] [PubMed] [Google Scholar]
- 64.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018. Sep 1;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. Erratum in: Eur Heart J. 2019 Feb 1;40(5):475. [DOI] [PubMed] [Google Scholar]
- 65.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002. Dec 18;288(23):2981–97. doi: 10.1001/jama.288.23.2981. Erratum in: JAMA 2003 Jan 8;289(2):178. Erratum in: JAMA. 2004 May 12;291(18):2196. [DOI] [PubMed] [Google Scholar]
- 66.Meltzer JI. A specialist in clinical hypertension critiques ALLHAT. Am J Hypertens. 2003. May;16(5 Pt 1):416–20. doi: 10.1016/s0895-7061(03)00570-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


