Abstract
Porcine deltacoronavirus (PDCoV) is a novel coronavirus that causes diarrhea in pigs of various ages, especially in suckling piglets, and there are no effective measures to prevent and control PDCoV currently. In this study, two adjuvants Al(OH)3 and ODN2395 working through different mechanisms were used to prepare inactivated PDCoV vaccines, and the immune effects of PDCoV inactivated vaccines were assessed in mice. From the results, we found that both PDCoV/Al(OH)3 vaccine and PDCoV/2395 vaccine could induce IgG and neutralizing antibodies with high levels in mice. At the same time, cytokines of IFN-γ, IL-4 and chemokine ligand of CXCL13 in serum were significantly increased after immunization, and reached the highest levels in PDCoV/2395 vaccine group, which suggested that PDCoV/2395 could promote the production of both Th1 and Th2 polarized cytokines. In addition, histopathological observations showed that vaccination helped mice resist PDCoV infection. These results indicated that both the two inactivated vaccines have good immune effects. Moreover, the PDCoV/2395 vaccine worked better than the PDCoV/Al(OH)3 vaccine for PDCoV/2395 having the good ability to induce both humoral and cellular immunogenicity. The PDCoV/2395 inactivated vaccine developed in this study might be an effective tool for the prevention of PDCoV infection.
Keywords: PDCoV, Inactivated vaccine, Al(OH)3, ODN2395, Immunogenicity
Abbreviations: ODN, Oligodeoxynucleotides; IFN-γ, Interferon-γ; IL-4, Interleukin-4; CXCL13, chemokine CXC ligand 13
1. Introduction
Porcine deltacoronavirus (PDCoV) is a kind of porcine coronavirus belonging to the genus of Deltacoronavirus, and family of Coronaviridae with a worldwide distribution [1]. PDCoV was first reported in Hong Kong, China in 2012 [2]. In 2015, the first strain of PDCoV named OH-FD22 was isolated and identified from the intestinal contents of diarrheic pigs in the United States [3]. Then, PDCoV was subsequently reported in many countries around the world, such as Canada, South Korea, Thailand, Laos, Vietnam and Japan [4], [5], [6], [7], [8]. Clinical symptoms caused by PDCoV are similar to those of porcine epidemic diarrhea virus (PEDV) and porcine transmissible gastroenteritis virus (TGEV) [9], including vomiting, dehydration, diarrhea, and which are more severe in piglets. PDCoV is one of the hazards to the global swine industry with its widespread tissue tropism and significant viremia [10].
In addition, PDCoV could spread across species. Studies have shown that it can infect cells of swine, humans, calves, and chickens in vitro [11], [12], and diarrhea can be observed in PDCoV-inoculated chicks and turkey poults [13], [14]. Recently the first case of human infection has been reported [15], and reports that PDCoV-related viruses have also been found in wild animals highlight the importance of wildlife in the cross-species spread of the coronavirus [16]. While the lack of medicines and vaccines for PDCoV poses a considerable risk to public health safety all over the world. Therefore, rapid and efficient preventive methods are the key to prevent and control PDCoV, and vaccination remains the most effective tool. As a classic vaccine development route, inactivated vaccines have been widely used in the biomedical industry. However, adjuvants are usually needed to achieve high immunogenicity and provide excellent stimulation to the immune system. Adjuvants are vital in animal and human vaccine development, and many compounds of organic, inorganic, synthetic and natural origin have been demonstrated to increase the immune response with effective adjuvant ingredients [17]. Adjuvants could be roughly divided into two categories: immune enhancers including cytokines, saponins and toll-like receptor (TLR) agonists, and delivery agents which contain emulsions, particulates and mineral salts. Antigen-presenting cells (APCs) are stimulated by immune enhancers and enhance the secretion of a variety of cytokines, while delivery agents can maintain the conformation of an antigen and deliver it to the APCs and provide continuous immune stimulation for the slow release of the antigen. Immune stimulants such as TLR agonists enhance the recruitment of immune cells and the secretion of cytokines, while emulsions and mineral salts mainly play a storage role at the injection site, prolonging antigen release time and continuously stimulating immune cells [18]. Aluminum salts were one of the earliest adjuvants used in human and animal vaccines. CpG ODN (Cytosine-phosphate-guanine Oligodeoxynucleotides) is an oligonucleotide chain that contains cytosine-phosphate-guanine dinucleotides, single-stranded DNA containing one or more unmethylated CpG motifs. CpG ODN is a novel immune enhancer with advantages in promoting immune responses, which has become a hot spot in the field of disease prevention and control in recent years. CpG ODN 2395 is a type of CpG ODN, which contains a full phosphorothioate backbone and a palindromic CpG-containing motif at the 3′-end [19].
In our study, two different kinds of adjuvants (Al(OH)3 and ODN2395) were used to prepare inactivated PDCoV vaccines, and the immune effects of PDCoV inactivated vaccines were evaluated in mice. These data may help us choose a low-cost and effective adjuvant for the preparation of the inactivated vaccine.
2. Materials and methods
2.1. Cells and virus
The LLC-porcine kidney (LLC-PK1) cells were cultured in minimum essential medium (MEM) (Gibco, Carlsbad, CA, USA) with 5% fetal bovine serum (Gibco), 1% MEM-nonessential amino acids (NEAA, Gibco), 1% Antibiotic-Antimycotic (Gibco), and 1% HEPES (Gibco) at 37 °C with 5% CO2. The virulent PDCoV strain of HNZK-02 (GenBank accession number MH708123) was isolated and identified in our laboratory. PDCoV was propagated in LLC-PK1 cells and the virus titer was determined as 108 TCID50/0.1 mL.
2.2. Vaccine preparation
PDCoV was inactivated by β-propiolactone (BPL, Acros Organics, Belgium) for the final concentration of 0.01% (w/v) with 12 h. The infectivity and sterility were confirmed as described previously [20]. The Al(OH)3 adjuvant was prepared as described previously [21]. CPG-ODN-2395 (5′-TCGTCGTTTTCGGCGCGCGCCG-3′, 1 μg/μL) was synthesized by Sangon Biotech (Shanghai, China) and mixed with ISA 201 VG (SEPPIC, Paris, France) at a ratio of 1:20 (v/v) to prepare the 2395 adjuvant. The inactivated PDCoV was emulsified with Al(OH)3 adjuvant and 2395 adjuvant at a ratio of 1:1 (v/v) separately to produce the inactivated PDCoV vaccines, and the vaccines were stayed at 4 °C for 72 h to test their stabilities.
2.3. Immunization and samples collection
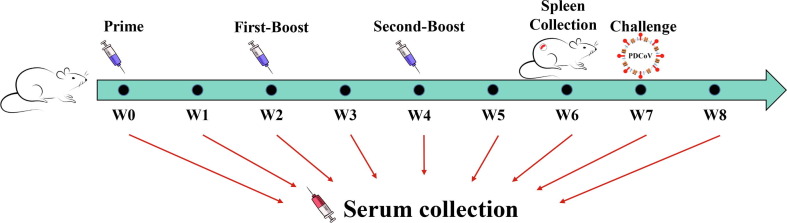
Thirty-nine six-week-old female BALB/c mice were provided by Henan Province Laboratory Animal Management Committee in China. Before immunization with PDCoV vaccines, all mice were confirmed negative for the PDCoV antibody by enzyme linked immunosorbent assay (ELISA) [22]. The mice were then subdivided into four groups. Group 1 was vaccinated with 200 μL of PDCoV/2395 vaccine, group 2 was vaccinated with 200 μL of PDCoV/Al(OH)3 vaccine, group 3 was vaccinated with 200 μL of inactivated PDCoV vaccine, and group 4 was vaccinated with 200 μL PBS as the negative control. There were 9 mice in group 1, 2 and 3 that immunized with the vaccines, but 12 mice in group 4. All the mice were subcutaneously vaccinated into their backs. The mice were injected in a prime-boost manner at week 0, with a first-boost after 2 weeks and second-boost at week 4. Serum was collected every week, and spleens were collected at week 6 for the isolation of lymphocytes. Mice were challenged orally with PDCoV at week 7 and euthanized after being challenged 3 days. Animal welfare and experiments were conducted in accordance with the regulations of the Animal Research Ethics Board of Henan Agricultural University in this study.
2.4. PDCoV specific IgG detection by ELISA
As previously described, PDCoV-N-specific IgG from immunized mice were detected by ELISA [22]. Briefly, the 96-well plates were coated with PDCoV-N protein of 1 μg/mL and incubated at 4 °C for 12 h. After blocked with 10 μg/mL of bovine serum albumin (BSA), the serum was diluted with PBS and incubated on the ELISA plate at 37 °C for 1 h. Then, a peroxidase-conjugated goat anti-mouse IgG secondary antibody (Sigma, Germany) was added to the plate and incubated at 37 °C for 1 h. After extensive washing, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Solarbio, China) was added for 15 min to develop a colorimetric reaction. Finally, read the optical density (OD) at 450 nm.
2.5. Cytokines and chemokine ligand detection
Cytokines of interferon-gamma (IFN-γ), interleukin 4 (IL-4) and chemokine CXC ligand 13 (CXCL13) in mice serum at week 6 were assayed using corresponding ELISA kits (mlbio, China) according to the manufacturer’s instruction.
2.6. Neutralizing antibodies measurement
Virus neutralization test (VNT) was performed on the HNZK-02 strain of PDCoV to determine the levels of neutralizing antibodies in the serum of mice at week 7. Briefly, the serum of mice was heated at 56 °C for 30 min. Then, 100 μL two-fold serial dilutions serum was co-incubated with 100 μL of 100 TCID50 PDCoV at 37 °C for 1 h. Next, 200 μL of the above mixture was added to the LLC-PK1 cells to determine the VNT according to the highest dilution of serum showing at least 50% cytopathic effect (CPE), and the neutralizing antibodies levels were confirmed by the Reed-Muench method.
2.7. Lymphocyte proliferation assay
Spleens of mice in different groups were collected at week 6 aseptically, and lymphocytes were isolated as described previously [20]. The isolated lymphocytes were cultured in 96-well flat-bottom plates, and stimulated with concanavalin A (ConA, Sigma) at a final concentration of 50 μg/mL or 20 μL of inactivated PDCoV (1 × 108 TCID50/0.1 mL), respectively. Lymphocytes added with DMEM were used as the negative control. All processing is in triplicate. After the plates were incubated at 37 °C for 24 h, 10 μL/well of CCK-8 was added and further incubated at 37 °C for 4 h. The OD 450 value was determined, and the stimulation index (SI) was calculated as the value of (OD sample well − OD blank well)/(OD negative well − OD blank well).
2.8. Cytotoxic T Lymphocytes (CTL) killing assay
Cytotoxic T Lymphocytes (CTL) could kill target cells which were infected with PDCoV. To prepare the target cells, LLC-PK1 cells cultured in 96-well plates reaching 80–90% confluent monolayers were infected with 100 TCID50 PDCoV per well. After absorption for 1 h, the cells were washed by maintenance medium for 3 times, and then another 100 μL of maintenance medium with 10 μg/mL of trypsin was added to the cells. The cells were incubated at 37 °C with 5% CO2 for 24 h. To analyze CTL effect in vitro, splenocytes were collected as the effector cells. Splenocytes were isolated to co-incubate with target cells in the ratio of 25:1 at 37 °C with 5% CO2 for 6 h. The natural release group (without effector cells) and the maximum release group (cracking target cells with Triton-X 100) were set as controls. Supernatant was collected and the cytotoxicity was analyzed by LDH Cytotoxicity Assay Kit (Nanjing Jancheng Bioengineering Institute, China). And the CTL was calculated as the value of (OD sample well − OD natural release group well)/(OD maximum release group well − OD natural release group well).
2.9. Challenge and samples collection
At week 7, three mice were randomly chosen from each group and challenged with 107 TCID50 of PDCoV strain of HNZK-02 orally. And three mice in control group were left untreated as controls. The clinical signs were observed daily. Three days after the challenge, the mice were euthanized to observe the pathological changes. The fresh and formalin-fixed tissue samples were collected separately. The fresh samples including lung, jejunum, ileum and duodenum were used for viral distribution detection, and the formalin-fixed tissues (jejunum, ileum and duodenum) were served as the pathological examination.
2.10. Viral extraction and RT-qPCR
The fresh tissues of the lung and small intestines including duodenum, jejunum and ileum were collected, and total RNA was extracted by Trizol Reagent. Reverse transcription (RT) was followed by using the reverse transcription kit (Vazyme, China). As previously reported, the PDCoV distributions in mice were determined by qRT-PCR assay [13].
2.11. Gross pathology and histopathology
The tissues of the duodenum, jejunum and ileum of mice were fixed in 10% (v/v) phosphate-buffered formalin for 48 h and embedded in paraffin. Then they were sectioned and stained with hematoxylin and eosin (H&E). Slides were examined for light microscopy examination by conventional light microscopy. The values of villus height (VH) and crypt depth (CD) of duodenum, jejunum and ileum were measured according to the computerized image system previously described [23], and the ratio of VH/CD was calculated.
2.12. Statistical analysis
Graph Pad Prism software was used for statistical analysis (version 7 for Windows, California USA). The data was represented as the mean ± standard deviation (SD). P values were calculated using one way ANOVA (followed by Tukey’s multiple comparisons test). P value with *p < 0.05 is considered to be statistically high, **p < 0.01 is considered to be significantly high and ***p < 0.001 is considered to be extremely high, respectively.
3. Results
3.1. Detection of PDCoV-specific IgG by ELISA
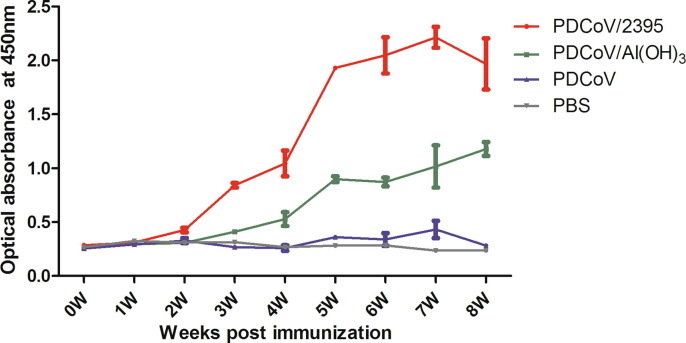
To confirm the immune effects of the two PDCoV inactivated vaccines, mice were subcutaneously immunized for three times with them (Fig. 1 ). PDCoV-specific IgG antibody in serum of mice was evaluated by ELISA assay every week, and the results were shown in Fig. 2 . The titers of PDCoV N-specific IgG elicited by PDCoV/2395 and PDCoV/Al(OH)3 inactivated vaccines were higher than that of the inactivated PDCoV and PBS control groups. In PDCoV/2395 group, increased IgG antibody levels were observed in the sera of boosted mice and remained high for longer, which peaked at week 7. In PDCoV/Al(OH)3 group, the increase rate of IgG antibody titer was relatively slow, and the antibody increased significantly at week 4 and was always lower than that of PDCoV/2395 group. For the PDCoV group without adjuvant, the titer was not significantly higher than that of the PBS control group.
Fig. 1.
Schematic of BALB/c mice vaccination. Mice from each group were prime/boost-vaccinated with different vaccines at week 0, week 2 and week 4. Serum was collected every week. The spleens were collected at week 6. Three mice from each group were challenged orally with PDCoV at week 7.
Fig. 2.
Detection of PDCoV-specific IgG in mice sera. PDCoV-specific IgG titers of immunized BALB/c mice at each time point were detected by ELISA, and the IgG titers of serum in each week were calculated and plotted as time-course curve. Bars represent the mean (standard deviation) of three replicates per treatment in one experiment.
3.2. Expression levels of IFN-γ, IL-4 and CXCL13
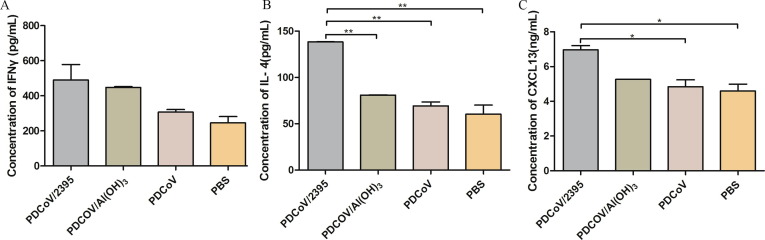
In order to further investigate the immune effects of the vaccines, the cytokines in the serum of immunized mice were analyzed. Sera were collected from three mice in each group at week 6 after the first immunization, and IFN-γ, IL-4 and CXCL13 levels were confirmed by commercial ELISA kits. As shown in Fig. 3 A, the concentration of IFN-γ in PDCoV/2395 and PDCoV/Al(OH)3 groups was slightly higher than that of the PBS control group (P > 0.05). The level of IL-4 expression in the PDCoV/2395 group was observed significantly higher than that of the PDCoV/Al(OH)3, PDCoV and PBS control groups (P < 0.01) (Fig. 3B). As shown in Fig. 3C, CXCL13 was obviously increased in the mice immunized with PDCoV/2395 compared with those injected with PDCoV and PBS (P < 0.05). Although the concentration of CXCL13 in PDCoV/2395 group was higher than that of the PDCoV/Al(OH)3 group, there was no statistical difference (P > 0.05).
Fig. 3.
Cytokines expression post immunization. Levels of secreted IFN-γ (A), IL-4 (B) and CXCL13 (C) were measured by ELISA. Data shown represent the mean SD of fold change of three independent experiments, with each determination performed in duplicate. Statistical significance was indicated by *P < 0.05(significant) and **P < 0.01(extremely significant) compared with other group.
3.3. Neutralization antibodies titer detection
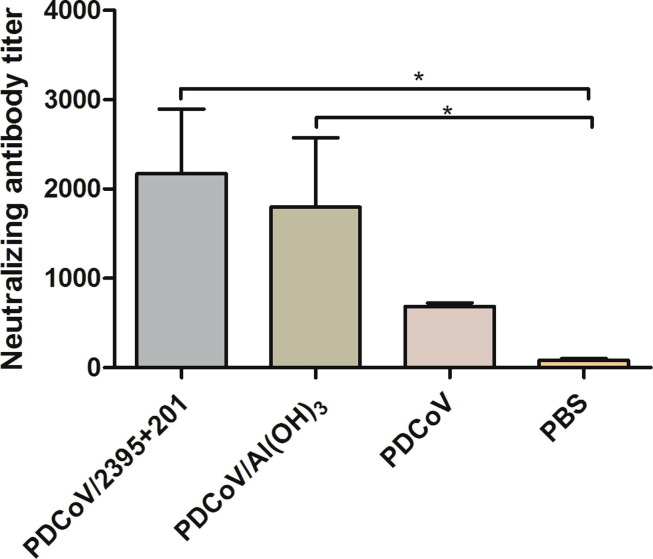
To investigate whether vaccine-induced antibodies inhibit virus entry into cells, serum samples were collected at week 7, and VNT was used to examine the level of neutralizing antibody (nAbs) against PDCoV which was induced by inactivated vaccines. As shown in Fig. 4 , both the PDCoV/2395 and PDCoV/Al(OH)3 vaccines had induced high levels of nAbs in mice. In contrast, the inactivated PDCoV group and PBS control group were only able to induce low levels of nAbs against PDCoV on LLC-PK1 cells.
Fig. 4.
The levels of neutralizing antibodies. The levels of neutralizing antibodies in mice serum at week 7 were determined using PDCoV strain HNZK-02 with a virus neutralization test. Bars represent the mean (standard deviation) of three replicates per treatment in one experiment. Statistical significance was indicated by *P < 0.05 (significant) compared with control group.
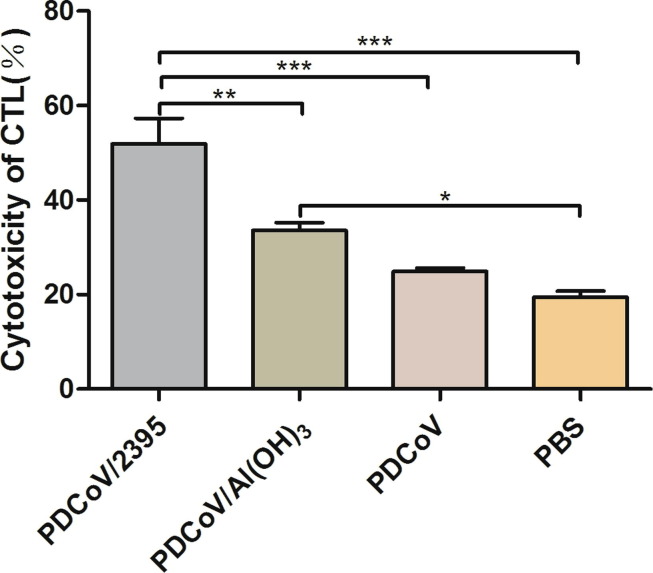
3.4. Result of CTL killing assay ex vivo
In order to assess the induction of decent cytotoxic T lymphocytes in response to the vaccination, the cytotoxic capacity of splenocytes isolated from vaccinated mice were tested ex vivo. As shown in Fig. 5 , PDCoV/2395 group elicited more powerful cytotoxicity compared with PDCoV/Al(OH)3 group (P < 0.01), and the cytotoxicity was significantly enhanced compared with the PDCoV group and PBS control group (P < 0.001). The cytotoxicity induced by PDCoV/Al(OH)3 vaccine group was slightly higher than that of the PDCoV vaccine group (P > 0.05), and significantly higher than that of the PBS control group (P < 0.05).
Fig. 5.
The cytotoxic capacity of splenocytes. To analyze CTL effect in vitro, splenocytes were collected at week 7, and the cytotoxicity was analyzed by LDH Cytotoxicity Assay Kit. Data were presented as mean SD of three duplicate samples. Statistical significance was indicated by *P < 0.05(significant), **P < 0.01(extremely significant) and ***P < 0.001(extremely significant) compared with other group.
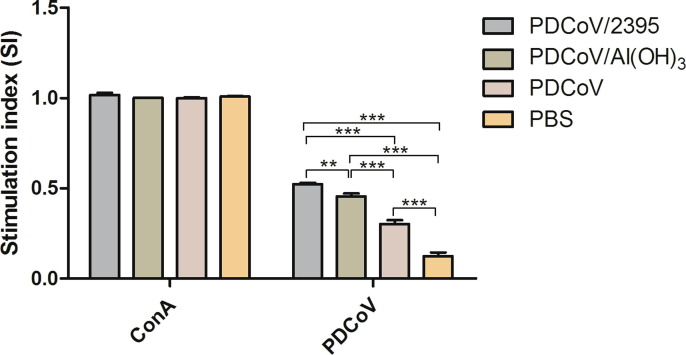
3.5. Result of spleen lymphocyte proliferation
On the 42 day after the first immunization, spleen lymphocytes were isolated for proliferation test. As shown in Fig. 6 , we found that there was no significant difference among ConA non-specific stimulation groups (P > 0.05). For specific PDCoV antigen stimulation groups, the SI values of the PDCoV/2395 group and the PDCoV/Al(OH)3 group were much higher than those of the PDCoV group and PBS group. Under specific stimulation of PDCoV antigen, the SI value of the PDCoV/2395 group was significantly higher than that of the PDCoV/Al(OH)3 group (P < 0.01) and the PBS control group (P < 0.001). SI value of the PDCoV/Al(OH)3 group was significantly higher than that of the PDCoV group and PBS group (P < 0.001), and the SI value of PDCoV group was significantly higher than of the PBS group (P < 0.001). These results indicated that the PDCoV/2395 group was more potent in inducing the proliferation of spleen lymphocytes.
Fig. 6.
The proliferation result of spleen lymphocyte by CCK-8 assay. Spleens of three mice in each group were collected at week 7, respectively (n = 3). Bars represent the mean (standard deviation) of three replicates per treatment in one experiment. Statistical significance was indicated by**P < 0.01(extremely significant) and ***P < 0.001(extremely significant) compared with other group.
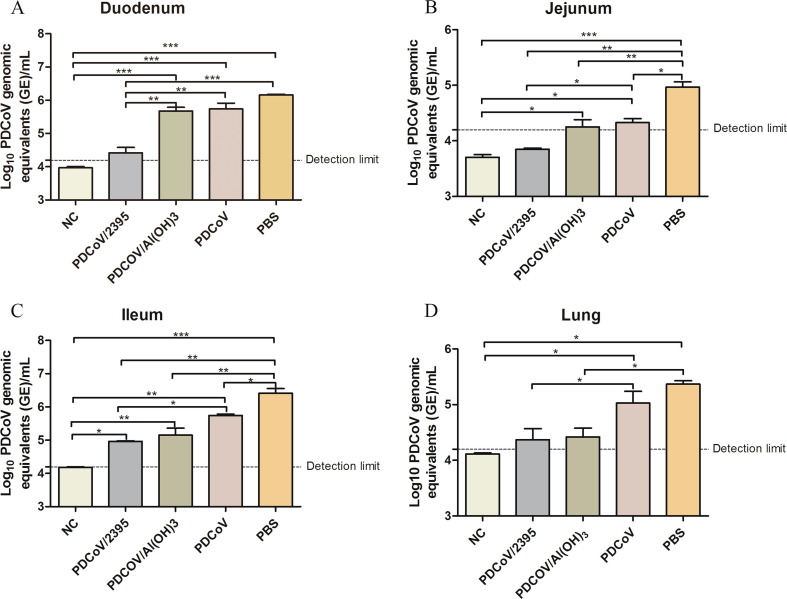
3.6. Viral RNA detection in mice tissues
The viral RNA of PDCoV from mice tissue samples including lung, duodenum, jejunum and ileum was extracted after 3 days of challenge with PDCoV, and then quantified by qRT-PCR assay. As shown in Fig. 7 , the PDCoV viral RNA levels of duodenum, jejunum, ileum and lung in PDCoV/2395 group were significantly lower than those in other immunized groups, and no PDCoV was detected in jejunum. The PDCoV viral RNA levels in mice tissues of PDCoV/Al(OH)3 group were lower than those of the PDCoV group and PBS control group. And the PDCoV loads in the PBS control group were higher than that in PDCoV group.
Fig. 7.
Viral loading in tissues of mice. (A) Viral RNA shedding titers of PDCoV in the duodenum (A), jejunum (B), ileum (C) and lung (D) of mice at 3 dpi. Error bars indicate the standard deviations from each group (n = 3). Statistical significance was indicated by**P < 0.01(extremely significant) and ***P < 0.001(extremely significant) compared with other group.
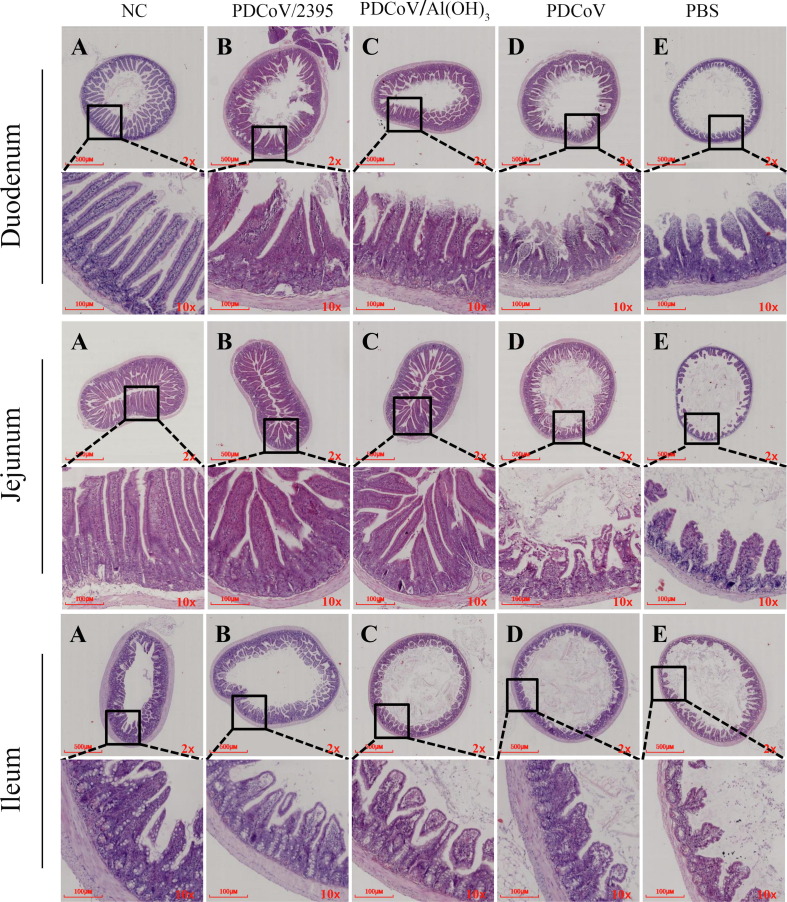
3.7. Histological lesions in mice intestinal tissues
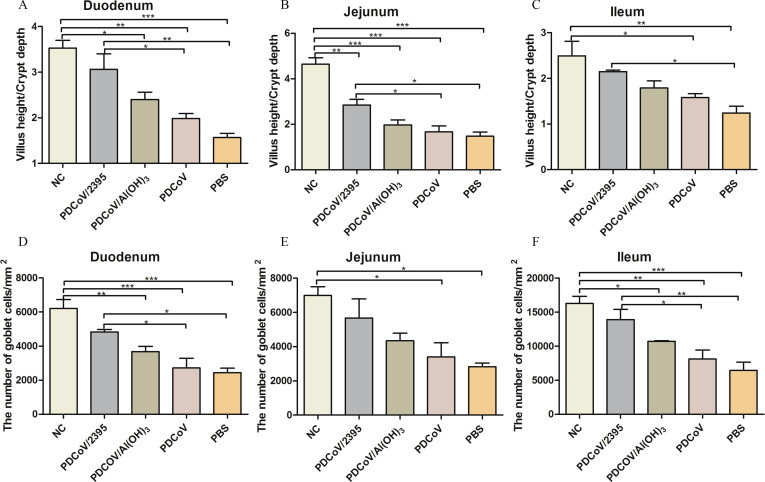
The tissues of duodenum, jejunum and ileum were collected at 3 days after PDCoV challenge for histopathological analysis. As shown in Fig. 8 , the pathological changes of these tissues were more extensive and severe in PDCoV and PBS groups of mice when compared with the mice of PDCoV/2395 and PDCoV/Al(OH)3 groups. Histopathological changes were characterized by severe necrosis and extensive distribution of intestinal villi in mice, including sloughing and necrosis of intestinal villi and lymphocytosis in the duodenum, jejunum, and ileum. However, mice inoculated with PDCoV/2395 or PDCoV/Al(OH)3 had relatively mild pathological damage. Villous height to crypt depth ratios were reported in Fig. 9 . As shown in Fig. 9A, B and C, the ratios of all challenged mice decreased, and the ratios of the mice inoculated with PDCoV/2395 were higher than the other three groups in the duodenum, jejunum and ileum. The density of goblet cells in each segment of small intestine was decreased in the challenged groups compared with the control group. However, the goblet cell density of mice inoculated with PDCoV/2395 was higher than that of the other three groups.
Fig. 8.
Lesions of small intestinal and lung tissue sections from mice challenged with PDCoV. The intestinal tissues (duodenum, jejunum, ileum) and lung from the mice challenged with PDCoV were collected at 3 days after challenge and then observed by microscopes. Scale bars were shown in each image.
Fig. 9.
The ratio of villous height to crypt depth and the number of goblet cells in tissue sections from mice. Error bars indicate the standard deviations from each group (n = 3). Statistical significance was indicated by *P < 0.05(significant), **P < 0.01(extremely significant) and ***P < 0.001(extremely significant) compared with other group.
4. Discussion
PDCoV is prevalent in many countries, and the mortality rate is from 40% to 80% in suckling piglets [24], [25]. More and more evidences have suggested that PDCoV could spread across species. To prevent the spread of PDCoV, vaccines are direly needed. However, there are no effective vaccines securable for PDCoV. Multiple vaccine research approaches against PDCoV and other enteric coronavirus are currently being evaluated [26]. Zhang et al. [27] reported that the protective efficiency of passive immunization was studied by inoculating pregnant sows with PDCoV inactivated vaccine in Houhai cave, and the protective efficacy of the vaccine on piglets was up to 87.1%. The results suggested that the inactivated PDCoV vaccine could provide protection against virulent PDCoV in piglets, providing data support for the development of PDCoV inactivated vaccine. Huang et al.[28] constructed recombinant pseudorabies virus (PRV) expressing PDCoV spike (S) protein, and confirmed the safety, efficacy and immunogenicity of the recombinant vaccine in mice.
Theoretically, as a classical vaccine, the research and development of inactivated vaccine is mature and easy to produce. However, inactivated vaccines usually require adjuvants and multiple immunizations are needed. The development of an excellent PDCoV inactivated vaccine is urgent and important to the pig industry and human public health. It is generally believed that aluminum salts are effective inducers of Th2 type immune response in mice. Studies have shown that aluminum salts cause some degree of inflammation in animals, which is largely determined by the injection site and the type of aluminum salt [29]. CpG ODNs is a pathogen-associated molecular pattern which can be quickly recognized and internalized by immune cells, and can be used as a ligand to bind to TLR9 in cells to form a complex [30]. CPG-ODN activates TLR9 to produce a variety of immune effects and induces humoral and cellular immunity, including the activation of dendritic cells, monocytes, macrophages and NK cells, leading to antigen presentation and cytokine production. Furthermore, B cells can be activated and increased proliferation upon induction of TLR9. Activation of TLR9 upregulates the production of Th1 polarized cytokines. Cytokines of TNF-α, IL-6, IL-12, interferon and some chemokines promote T cell activation [31], [32]. Due to its good effect of promoting immune response, CpG ODN is often used as an immune enhancer in clinical studies with great application prospect, which has high safety and excellent efficacy in a certain dose range.
In our study, two different adjuvants (ODN 2395 and Al(OH)3) were chosen to prepare inactivated PDCoV vaccines. And the mice were immunized three times to evaluate the effects of different vaccines. The PDCoV/2395 vaccine had induced humoral and cellular immunity obviously. Our results showed that mice developed strong antibody responses after a second immunization with PDCoV/2395 vaccine and peaked at week 7. Compared with other groups, the mice immunized with PDCoV/2395 vaccine were obviously produced high levels of nAbs in serum. Moreover, the mice of PDCoV/2395 vaccine group induced stronger cytotoxicity than that of other groups. PDCoV/2395 vaccine enhanced the production of antibodies and induced CTL effect, which had excellent immune effect.
Helper T cells (Th cells) are a kind of CD4+ T cells. It is involved in a variety of diseases and is an important mediator of adaptive immunity. Th cells differentiate into two lymphocyte subsets, Th1 and Th2. IFN-γ induces the differentiation of Th0 cells into Th1 cells, which could regulate cellular immune responses [33], [34]. PDCoV/2395 induced the highest level of IFN-γ production in serum of mice. It might be that CpG 2395 could promote the production of Th1 polarized cytokines and induce the cellular immune responses. While Th0 cells are induced to differentiate into Th2 cells by IL-4, and Th2 cells mainly control humoral and mucosal immunity [35]. CXCL13 level in serum serves as a surrogate marker for germinal center activation [36]. The levels of IL-4 and CXCL13 in sera of mice which induced by PDCoV/2395 vaccine were higher than those of other groups. It proved that PDCoV/2395 vaccine has a good ability to induce both humoral and cellular immunity.
Research have shown that small intestines were the primary target sites of PDCoV infection [9], [37], and our previous research confirmed that mice infected with PDCoV had higher viral loads in the small intestine and lungs. Therefore, we focused on testing viral content in the tissues of duodenum, jejunum, ileum and lung. Our study also showed that the mice immunized with the PDCoV/2395 vaccine could resist PDCoV infection to some extent, for the loads of viral RNA were lower than other groups. Correspondingly, through pathological examination, we found that the pathological damage of intestinal tissues in the PDCoV and PBS groups were more serious after the challenge, while the pathological changes of intestinal tissues in PDCoV/2395 group and PDCoV/Al(OH)3 group were not obvious.
5. Conclusion
Adjuvants are essential for inactivated vaccines. In our study, two different adjuvants of Al(OH)3 and ODN2395 were mixed with inactivated PDCoV respectively, and two kinds of inactivated PDCoV vaccines were developed. The immunogenicity of the two vaccines were evaluated by detecting IgG, nAbs, CKs, the ability of lymphocyte proliferation, and the ability of challenge protection. PDCoV/2395 group showed the best immunogenicity in both humoral and cellular immunity.
Funding
This work was supported by the National Key Research and Development Program of China [Grant Number 2021YFD1801105]; the Science and Technology Innovation Talents Support Plan of Henan Province Colleges and Universities [Grant Number 21HASTIT039].
Ethics statement
The animal experiments in this study were approved and performed in accordance with the regulations of the Animal Research Ethics Board of Henan Agricultural University (Zhengzhou, China).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Zheng Lanlan reports financial support was provided by The Education Department of Henan Province. Zheng Lanlan reports financial support was provided by Ministry of Science and Technology of the People’s Republic of China.
Acknowledgments
Acknowledgements
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Author contributions
Z.F. performed the experiments and drafted the manuscript; L.L. performed the experiments and collected the data; Z. W., N.W., M. M., J.X., S. L. and Q.X. performed animal work. L.Z. and H.H. provided funding support on this study and made great contribution to manuscript revision. All authors have read and agreed to the published version of the manuscript.
References
- 1.He W., Ji X., He W., Dellicour S., Wang S., Li G., et al. Genomic Epidemiology, Evolution, and Transmission Dynamics of Porcine Deltacoronavirus. Mol Biol Evol. 2020;37:2641–2654. doi: 10.1093/molbev/msaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo P., Lau S., Lam C., Lau C., Tsang A., Lau J., et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H., Jung K., Vlasova A., Chepngeno J., Lu Z., Wang Q., et al. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J Clin Microbiol. 2015;53:1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg Infect Dis. 2014;20:1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S., Lee C. Complete Genome Characterization of Korean Porcine Deltacoronavirus Strain KOR/KNU14-04/2014. Genome Announcements. 2014;2:e01191–14. doi: 10.1128/genomeA.01191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeng-Chuto K., Lorsirigool A., Temeeyasen G., Vui D.T., Stott C.J., Madapong A., et al. Different Lineage of Porcine Deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transboundary & Emerging Diseases. 2017;64:3–10. doi: 10.1111/tbed.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janetanakit T., Lumyai M., Bunpapong N., Boonyapisitsopa S., Chaiyawong S., Nonthabenjawan N., et al. Porcine Deltacoronavirus, Thailand, 2015. Emerg Infect Dis. 2016;22:757–759. doi: 10.3201/eid2204.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T., Shibahara T., Imai N., Yamamoto T., Ohashi S. Genetic characterization and pathogenicity of Japanese porcine deltacoronavirus. Infect Genet Evol. 2018;61:176–182. doi: 10.1016/j.meegid.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg Infect Dis. 2015;21:650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Zhang Y, Liang X, Lou F, Oglesbee M, Krakowka S, et al. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio. 2015;6:e00064. [DOI] [PMC free article] [PubMed]
- 11.Li W., Hulswit R., Kenney S., Widjaja I., Jung K., Alhamo M., et al. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. PNAS. 2018;115:E5135–E5143. doi: 10.1073/pnas.1802879115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung K., Vasquez-Lee M., Saif L. Replicative capacity of porcine deltacoronavirus and porcine epidemic diarrhea virus in primary bovine mesenchymal cells. Vet Microbiol. 2020;244 doi: 10.1016/j.vetmic.2020.108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Q., Zhang H., Li B., Ding Q., Wang Y., Gao W., et al. Susceptibility of Chickens to Porcine Deltacoronavirus Infection. Viruses. 2019;11:573. doi: 10.3390/v11060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boley P., Alhamo M., Lossie G., Yadav K., Vasquez-Lee M., Saif L., et al. Porcine Deltacoronavirus Infection and Transmission in Poultry, United States. Emerging Infectious Diseases. 2020;26:255–265. doi: 10.3201/eid2602.190346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lednicky JA, Tagliamonte MS, White SK, Elbadry MA, Alam MM, Stephenson CJ, et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature.600:133-1377. [DOI] [PMC free article] [PubMed]
- 16.He W., Hou X., Zhao J., Sun J., He H., Si W., et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell. 2022;185:1117–1129.e8. doi: 10.1016/j.cell.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Giudice G., Rappuoli R., Didierlaurent A. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Li X., Xing R., Xu C., Liu S., Qin Y., Li K., et al. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr Polym. 2021;264 doi: 10.1016/j.carbpol.2021.118050. [DOI] [PubMed] [Google Scholar]
- 19.Vollmer J., Weeratna R., Payette P., Jurk M., Schetter C., Laucht M., et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 20.Zhao F., Liu L., Xu M., Shu X., Zheng L., Wei Z. Assessments of different inactivating reagents in formulating transmissible gastroenteritis virus vaccine. Virology J. 2020;17:163. doi: 10.1186/s12985-020-01433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin X., Zheng L., Song M., Xu W., Kou Y., Zhou Y., et al. A nano silicon adjuvant enhances inactivated transmissible gastroenteritis vaccine through activation the Toll-like receptors and promotes humoral and cellular immune responses. Nanomed Nanotechnol Biol Med. 2018;14:1201–1212. doi: 10.1016/j.nano.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Yan RJ, Zhang YF, Liu LL, Zhang HL, Wang YB, Hu hui. Prokaryotic expression of porcine deltacoronavirus N protein and establishment of indirect ELISA assay. Journal of Northwest A&F(Nat.SCI.Ed.) 2019;47:9.
- 23.Madson D., Magstadt D., Arruda P., Hoang H., Sun D., Bower L., et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet Microbiol. 2014;174:60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 24.McCluskey B., Haley C., Rovira A., Main R., Zhang Y., Barder S. Retrospective testing and case series study of porcine delta coronavirus in U.S. swine herds. Preventive Veterinary Med. 2016;123:185–191. doi: 10.1016/j.prevetmed.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Liang Q., Li B., Cui X., Wei X., Ding Q., et al. Prevalence, phylogenetic and evolutionary analysis of porcine deltacoronavirus in Henan province, China. Preventive Veterinary Med. 2019;166:8–15. doi: 10.1016/j.prevetmed.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang P., Cui E., Song Y., Yan R., Wang J. Porcine deltacoronavirus and its prevalence in China: a review of epidemiology, evolution, and vaccine development. Arch Virol. 2021;166:2975–2988. doi: 10.1007/s00705-021-05226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Chen J., Liu Y., Da S., Shi H., Zhang X., et al. Pathogenicity of porcine deltacoronavirus (PDCoV) strain NH and immunization of pregnant sows with an inactivated PDCoV vaccine protects 5-day-old neonatal piglets from virulent challenge. Wiley-Blackwell. 2020;67:572–583. doi: 10.1111/tbed.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y., Xu Z., Gu S., Nie M., Wang Y., Zhao J., et al. The recombinant pseudorabies virus expressing porcine deltacoronavirus spike protein is safe and effective for mice. BMC veterinary research. 2022;18:16. doi: 10.1186/s12917-021-03115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cain D., Sanders S., Cunningham M., Kelsoe G. Disparate adjuvant properties among three formulations of “alum”. Vaccine. 2013;31:653–660. doi: 10.1016/j.vaccine.2012.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai C., Yu G., Luo Y., Xiang R., Chuang T. Immunostimulatory Activities of CpG-Oligodeoxynucleotides in Teleosts: Toll-Like Receptors 9 and 21. Front Immunol. 2019;10:179. doi: 10.3389/fimmu.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollmer J., Krieg A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H., Gao X.D. Nanodelivery systems for enhancing the immunostimulatory effect of CpG oligodeoxynucleotides. Mater Sci Eng, C. 2017;70:935–946. doi: 10.1016/j.msec.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K.M., Ouyang W., Farrar J.D., Yang J., Ranganath S., Asnagli H., et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 34.Pinto R.A., Arredondo S.M., Bono M.R., Gaggero A.A., Díaz P.V. T Helper 1/T Helper 2 Cytokine Imbalance in Respiratory Syncytial Virus Infection Is Associated With Increased Endogenous Plasma Cortisol. Pediatrics. 2006;117:878–886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q., Li M., Li C.X., Ge W., Gao M. Genetic analysis of differentiation of T-helper lymphocytes. Genetics and Molecular Research: GMR. 2013;12:972–987. doi: 10.4238/2013.April.2.13. [DOI] [PubMed] [Google Scholar]
- 36.Havenar-Daughton C., Lindqvist M., Heit A., Wu J.E., Reiss S.M., Kendric K., et al. CXCL13 is a plasma biomarker of germinal center activity. PNAS. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., Han F., Shu X., Li Q., Ding Q., Hao C., et al. Co-infection of porcine epidemic diarrhoea virus and porcine deltacoronavirus enhances the disease severity in piglets. Transboundary and Emerging Diseases. 2021;00:1–12. doi: 10.1111/tbed.14144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.