Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder that may lead to gas exchange abnormalities, including hypercapnia. Chronic hypercapnia is an independent risk factor of mortality in COPD, leading to epithelial dysfunction and impaired lung immunity. Moreover, chronic hypercapnia affects the cardiovascular physiology, increases the risk of cardiovascular morbidity and mortality, and promotes muscle wasting and musculoskeletal abnormalities. Noninvasive ventilation is a widely used technique to remove carbon dioxide, and several studies have investigated its role in COPD. In the present review, we aim to summarize the causes and effects of chronic hypercapnia in COPD. Furthermore, we discuss the use of domiciliary noninvasive ventilation as a treatment option for hypercapnia while highlighting the controversies within the evidence. Finally, we provide some insightful clinical recommendations and draw attention to possible future research areas.
Keywords: airway immunity, chronic obstructive pulmonary disease, hypercapnia, noninvasive ventilation
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease that is caused by aberrant airway inflammation due to chronic exposure to noxious gases or particles, such as tobacco or biomass fuel smoke [1]. COPD is responsible for 250,000 deaths yearly in the European Union and is the third leading cause of mortality worldwide [2]. It is usually considered to be a progressive disease, and the ultimate worsening of airflow limitation and impaired gas exchange may result in the development of hypercapnia [3]. The presence of alveolar hypoventilation, and therefore the development of consequential hypercapnia, is more common in severe forms of COPD [4], although hypercapnia is present in even moderate disease in a proportion of patients [5]. The prevalence of hypercapnia is around 30–50% in patients with very severe COPD (predicted forced expiratory volume in the first second (FEV1) <30%) [5]. Although hypercapnia may be beneficial to mitigate pulmonary inflammation [6], there is an increasing amount of evidence suggesting that the deleterious effects outweigh the protective ones [7,8,9,10]. Hypercapnia causes alveolar epithelial dysfunction, which results in alveolar oedema formation and further deterioration in gas exchange [7,11]. Moreover, the repair mechanisms of the airway epithelial cells are also damaged as hypercapnia causes mitochondrial dysfunction and impaired cell proliferation [12]. Overall, chronic hypercapnia is an independent risk factor for hospitalizations and mortality in COPD and has thus attracted attention as a possible treatable trait [10,13,14,15,16].
Several mechanisms, which are detailed in this review article, may lead to alveolar hypoventilation and result in hypercapnia in COPD. Correction of hypoventilation and acute respiratory acidosis using noninvasive ventilation (NIV) is a widely used and evidence-based treatment for acute hypercapnic respiratory failure (AHRF) [17]. It is also available at domiciliary settings for patients with chronic type II respiratory failure (i.e., hypoxemia with hypercapnia), including those with COPD [18]. However, the effects of long-term NIV (LT-NIV) on hard endpoints (mortality and exacerbation frequency) are unclear due to the contradictory results of previous studies in COPD [18,19].
The mechanisms leading to hypercapnia in COPD are well documented, and we aim to summarize them only briefly. Instead, our review will focus on the pulmonary and systemic effects of hypercapnia and evidence on the effects of long-term NIV treatment in COPD. We highlight controversies, provide clinical recommendations, and outline an agenda for further research.
2. The Mechanisms of Hypercapnia in Stable COPD
As COPD is a heterogenous disorder, it is not surprising that mechanisms leading to hypercapnia are also numerous and not necessarily interrelated. Identification of these processes as treatable traits is important at the individual level. The following risk factors are described to be associated with the development of chronic hypercapnia in patients with COPD: breathing pattern, inspiratory muscle weakness, smoking habit, low FEV1, high body mass index, reduced forced vital capacity, and high arterial bicarbonate level [3,20,21,22].
Arterial carbon dioxide pressure (PaCO2) is determined by the ratio of CO2 production and alveolar ventilation. Although CO2 production may be accelerated due to hypoxemia and sarcopenia, the predominant process leading to hypercapnia is alveolar hypoventilation [23]. Alveolar ventilation is determined by two main processes: minute ventilation and dead space ventilation [24]. Processes leading to decreased respiratory drive, including reduced central nervous system control (“the patient will not breathe”) and impaired respiratory muscle strength (“the patient cannot breathe”), decrease minute ventilation, whereas increased dead space area due to airway, parenchymal, and vascular changes in COPD promotes dead space ventilation. Most importantly, hypercapnia occurs when a patient is unable to sustain satisfactory ventilation due to overload of the respiratory system.
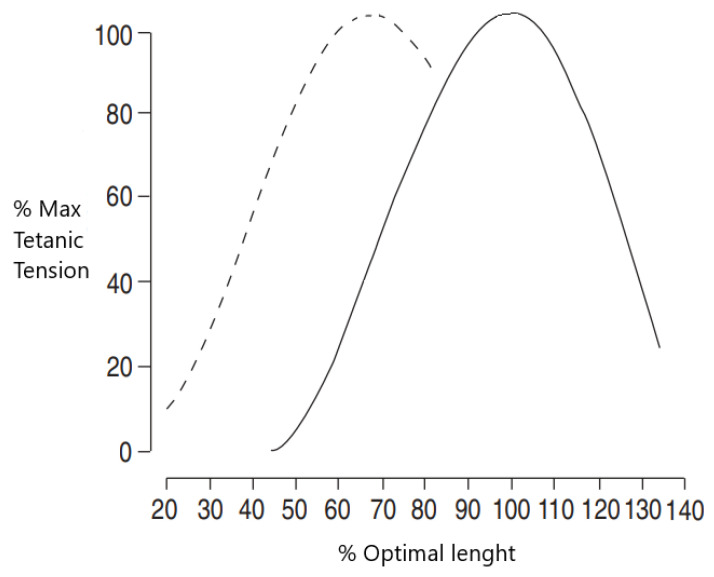
The “overload” that challenges the thoracopulmonary system is due to increased resistance of the airways, pulmonary hyperinflation, and increased ventilatory demand [25,26]. Pulmonary hyperinflation plays a central role in the pathogenesis of hypercapnia in COPD [27], especially in severe disease [28]. Static pulmonary hyperinflation, i.e., the increase in functional residual capacity and the ratio of residual volume/total lung capacity (RV/TLC) at rest, causes the thoracopulmonary system to breathe at rest at higher volumes during full expiration on a portion of the most unfavorable length–tension curve [29] (Figure 1). This “disadvantage” causes muscle fibers to adapt by shortening diaphragmatic sarcomeres and increasing the proportion of type I fibers. Despite the presence of these compensatory mechanisms, pulmonary hyperinflation impairs ventilatory capacity in patients with COPD, with the system unable to increase both tidal volume and expiratory volume when ventilatory demand increases as it occurs during exercise or exacerbation. Furthermore, hyperinflation decreases the resting length of the diaphragm and the rib cage muscles [30,31], causing diaphragmatic dysfunction with a significant increase in respiratory work at rest.
Figure 1.
Diaphragmatic length–tension curve in subjects without (solid line) and with (dashed line) emphysema. In emphysema, the curve is shifted towards the left, thus shifting to shorter length.
This “overloaded system” is superimposed on an unfavorable muscular condition that is unable to support this increased workload [32,33,34]. The unfavorable alterations in the muscles are also due to compromised oxygen delivery, the nutritional deficit that typically occurs in patients with severe COPD and systemic inflammation [34,35]. However, not all authors agree on defining the muscular system of hypercapnic COPD patients as “weaker”. Topeli et al. showed that there is evidence of greater activation of the diaphragm following increased ventilatory demand in hypercapnic COPD patients compared to normocapnic COPD patients [36].
Due to progressive alveolar tissue loss, the physiological ventilation/perfusion (V/Q) imbalance can be accelerated in patients with COPD. The alveolar tissue loss is usually heterogenous and leads to poorly ventilated but well perfused (lower lobes) or well ventilated but poorly perfused (upper lobes) areas, thereby increasing the physiological dead space areas [37]. An increase in dead space ventilation naturally leads to decreased alveolar ventilation. The V/Q can further be worsened by the presence of comorbidities (e.g., coagulopathy, heart disease, and pulmonary hypertension). The central respiratory drive can also be reduced in a number of patients with COPD [38]. The control of ventilation depends on the interaction of signals from central chemoreceptors and pressure receptors on the rib cage and respiratory muscles. Moreover, downregulation of the breathing centers, with the consequent alveolar hypoventilation, seems to be a protective factor put in place to “save energy” and avoid additional burden on the respiratory system [39,40].
Usually, hypoxemia precedes hypercapnia in patients with COPD. Long-term O2 treatment (LTOT) is beneficial in these patients; however, it may induce hypercapnia in otherwise normocapnic individuals [41], and routine blood gas analyses are therefore recommended in these patients.
In addition, COPD may be accompanied by other disorders associated with hypercapnia, such as obesity [42], chest wall diseases [43], and neuromuscular disease [44]. Of note, obesity may also occur due to immobility commonly seen in patients with COPD. Recognizing them during the diagnostic workup is essential when tailoring individualized treatment.
3. The Mechanisms of Hypercapnia during Exacerbation of COPD
Many COPD exacerbations are characterized by hypercapnic hypoxemic respiratory failure, which is a major risk factor for mortality [45,46]. Acute hypercapnia can be found both in patients who are already hypercapnic at rest and in patients with normocapnia, in whom exacerbation imposes an excessive load on the thoracopulmonary system that leads to difficulty in sustaining the ventilatory flow. A history of severe exacerbations in previous years is the most important risk factor for the occurrence of future flare-ups [47,48,49]. Furthermore, developing hypercapnia during an exacerbation correlates with the risk of mortality in the following 12 months [50], thus representing a negative prognostic factor.
Alterations in the V/Q ratio represent one of the first and main mechanisms that occurs during COPD exacerbation and leads to the development of hypercapnic respiratory failure [51,52]. Many regions of the parenchyma are perfused but not ventilated due to the presence of bronchospasm, edema, and secretions [53], which increase dead space ventilation. In addition, there is an increase in respiratory and muscular work, with a consequent rise in oxygen demand [54]. Barbera et al. showed that hypercapnic respiratory failure during COPD exacerbation is mainly linked to alterations in V/Q as a result of remodeling of the airways, bronchospasm, and hypersecretions [55].
The establishment of dynamic pulmonary hyperinflation, i.e., the increase in end-expiratory lung volume (EELV) above relaxation volume [56], can contribute to an increase in PaCO2 during exacerbations, which also develops in patients with flow limitation during exercise. During exacerbations, the resistance of the airways, and therefore the flow restriction, increases due to bronchoconstriction, edema of the walls, and accumulation of bronchial secretions [57]. This is superimposed on hyperactivation of the respiratory neural drive with rapid shallow breathing pattern and consequent lengthening of the time constant and air entrapment [57]. These elements cause a progressive vicious circle underlying dyspnea, a key symptom of COPD exacerbations. As a “mechanical” consequence, patients find themselves breathing in an even more unfavorable portion of the compliance curve, in which large changes in pressure cause small changes in volume with a consequent increase in respiratory work.
The abovementioned factors may lead to the presence of positive static end-expiratory elastic recoil pressure called intrinsic positive end expiratory pressure (PEEP), which represents an additional mechanical load that the respiratory system must overcome in order to guarantee respiratory gas exchanges [58]. This implies that the respiratory muscles must first overcome the intrinsic PEEP threshold in order to induce an adequate inspiratory flow. Furthermore, regional differences in intrinsic PEEP contribute to poor pulmonary ventilation distribution and thus to impaired gas exchange.
All these elements contribute to create an additional load on the already compromised thoracopulmonary system, with consequent muscle weakness and overload, particularly at the diaphragmatic level [59]. In this way, the work necessary to ensure respiratory exchanges is significantly increased, with hyperactivation of the central respiratory tracts that does not correspond to a proportional increase in muscle activation due to fatigue [57].
An option to balance the load and capacity of the ventilatory muscles is the use of mechanical ventilation. Positive-pressure support ventilation unloads fatigued respiratory muscles, thus enabling recovery of the respiratory system and leading to improvement in lung function parameters, correction of hypercapnia, and reversal of acidosis [60]. The main types of ventilatory support are invasive and noninvasive ventilation. According to current guidelines, due to its several advantages over invasive ventilation (no need for sedation; maintained ability to eat, drink, cough, and expectorate; and lower risk of ventilator-associated pneumonia) in the rightly selected population, NIV is the recommended initial therapy for acute hypercapnic respiratory failure during an acute exacerbation of COPD [61].
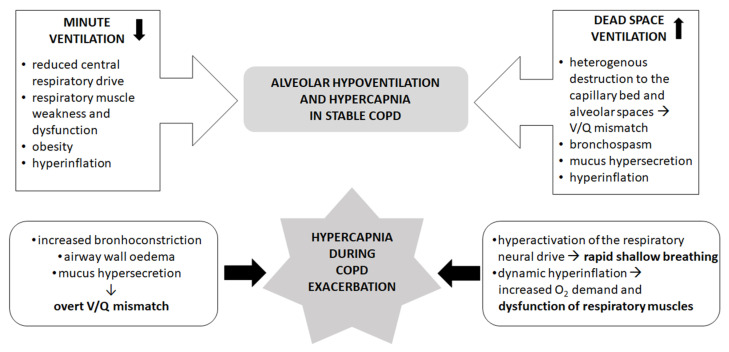
The mechanisms leading to hypercapnia in stable and exacerbated conditions are summarized in Figure 2.
Figure 2.
Mechanisms leading to hypercapnia in stable (upper panel) and exacerbated (lower panel) COPD. V/Q: ventilation/perfusion of the alveoli. References are listed in the text.
4. Effects of Hypercapnia on the Lung
Carbon dioxide is not only a by-product of physiological processes but also a signaling molecule, and it can affect many different cell and tissue types [62]. The pathways through which CO2 impacts cell functions include pH-dependent and pH-independent routes via soluble and transmembrane receptor proteins [63,64,65]. In the respiratory system, hypercapnia affects the alveolar epithelial function and cell repair mechanisms, the inflammatory response and immunity of the airways, and the airway mechanics [66].
4.1. Effects on Alveolar Epithelium
The most thoroughly studied effect of hypercapnia on the respiratory system is its relationship with alveolar epithelial dysfunction. The deleterious effects of hypercapnia are multifactorial. Firstly, in vitro and rodent models have been used to show that resorption of the alveolar fluid is impaired due to endocytosis of Na+/K+-ATPase enzyme, leading to reduced clearance of lung oedema. The reduction of Na+/K+-ATPase activity is mediated through protein kinase C and A pathways [7,64,67]. In addition, it has been reported that hypercapnia attenuates epithelial cell repair by disruption of cell migration via reduced NF-κB activation [68]. The repair mechanism is further impaired through disturbed plasma membrane wound resealing [69,70] and decreased rate of proliferation of alveolar epithelial cells due to mitochondrial dysfunction [12].
4.2. Effects on Immunity and Inflammatory Response of the Respiratory System
Hypercapnia also affects the immunity of the airways. Although the elevation of CO2 levels alters the expression of several hundred genes [71], the key regulator of the response to hypercapnia may be the NF-κB pathway [72]. Several studies have reported that hypercapnia hinders the activation of NF-κB, which regulates genes involved in innate immunity and inflammation [73,74,75]. The consequence of NF-κB suppression is a complex disturbance in the innate immunity, which involves impaired phagocytic capacity of alveolar macrophages and decreased production of proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α [9,76]. Moreover, the antiviral activity of macrophages is also impaired in hypercapnia. In a combined in vivo and in vitro model, Casalino-Matsuda et al. showed that hypercapnia increased the replication of influenza A virus in mice while inhibiting the antiviral gene and protein expression in macrophages through activation of the Akt1 pathway [77]. Furthermore, in a recent study, gene clusters that are associated with innate immunity and nucleosome assembly were found to be downregulated by hypercapnia, whereas lipid metabolism genes were upregulated [71].
Besides in vitro models, the effects of hypercapnia on host defense can also be observed in preclinical rodent models of acute lung injury, although the results are controversial. Chonghaile et al. reported that hypercapnic acidosis may attenuate the lung injury induced by E. coli pneumonia but only when CO2 is administered with antibiotic therapy [78]. Masterson and her colleagues also found that hypercapnic acidosis improved survival and reduced lung inflammation in rats and that the mechanism behind this effect was the inhibition of NF-κB [75]. On the contrary, O’Croinin observed that hypercapnia did not have a protective effect against pneumonia-induced lung injury [79] or even worsened the damage [80].
4.3. Effects on Airway Mechanics
Hypercapnia regulates the bronchial muscle tone through vagal nerve stimulation, CO2-signalling pathways, and pH-dependent mechanisms. However, the results are controversial as several studies have reported increased airway contractility [81,82,83] while others found that hypercapnia augments airway relaxation [84,85,86,87,88,89,90].
Historically, the dominant explanation of the bronchoconstrictor effect of hypercapnia had been that CO2 stimulates vagal efferent nerves, which play an important role in regulating bronchial tone. Later, however, it was proven that the constrictor effect cannot be entirely antagonized by the blockage of the vagal nerve, and other factors also contribute to the observed effect of CO2 [65]. Subsequently, in a mouse and human airway smooth muscle cell culture model, Shigemura et al. found that hypercapnia increased acetylcholine-mediated cell contraction, and the effect was independent of oxygen saturation and extracellular pH [81]. Importantly, they validated the in vitro results in a small cohort of human subjects with COPD. It was found that hypercapnic COPD patients had higher airway resistance, which decreased after the normalization of CO2 levels [81]. In line with this, using a forced oscillation technique, Uno and his colleagues found that respiratory resistance was higher in hypercapnia patients with COPD [83]. These findings indicate that hypercapnia may contribute to airflow limitation, which severely impacts the quality of life and mortality, particularly in COPD [91,92]. Indeed, randomized controlled trials that aimed to correct chronic hypercapnia in COPD have reported an improvement in lung function parameters, health-related quality of life, and mortality [93,94].
Hypercapnia can also be associated with bronchodilation [84,85,86,87,88,89,90]. In vivo animal models have shown that the inhalation of CO2 can reverse the bronchoconstrictor effect of drugs or pulmonary artery occlusion [85,87,90]. A possible explanation for this effect is that carbon dioxide rapidly dissolves into H+ and HCO3− in a watery solution, thus lowering the intracellular pH in smooth muscle cells. The intracellular acidosis then reduces the influx of ionized calcium (Ca2+) through voltage-dependent Ca2+ channels, which would be necessary for the active contraction of the muscle [86,88,89]. Moreover, El Mays et al. described a pH-independent, epithelium-mediated mechanism of bronchial relaxation induced by CO2. In a rodent in vitro model, they found that substance P provoked relaxation in bronchial rings pretreated with methacholine independently of extracellular H+ concentration [84]. Overall, the relaxing effect of hypercapnia seems to be significant only on an already pathologically constricted smooth muscle.
4.4. Effects on Pulmonary Circulation
Hypercapnia has also been linked to increased pulmonary vascular resistance and the development of pulmonary hypertension (PH) in COPD. It is estimated that 1–4% of patients with COPD present with severe pulmonary hypertension as defined by mean pulmonary arterial pressure (PAP) ≥ 35 mmHg [95,96]. Of clinical relevance, it has recently been shown that severe PH is an independent risk factor of mortality in COPD [97].
Several lines of evidence confirm the role of hypercapnia in the development of PH in COPD. In an animal model, it was shown that chronic intermittent hypoxia with hypercapnia resulted in elevated pulmonary and systemic arterial pressures and an increase in hematocrit [98]. Importantly, PH in end-stage COPD is not only predicted by hypoxemia but also by hypercapnia [96]. Furthermore, in experimental settings, the development of hypercapnia is associated with an increase in pulmonary vascular resistance [99,100], impaired right ventricular function [100,101], and reduced right ventricular ejection fraction [100]. Hypercapnia can lead to an increase in PAP via acidosis [102], and chronic hypercapnic acidosis is suspected to contribute to heart failure in PH [103]. Importantly, in a small cohort of patients with PH related to hypoventilation due to COPD alone or in combination with obesity hyperventilation syndrome, NIV reduced PAP and improved exercise capacity and cardiac function as assessed by serum N-terminal probrain natriuretic peptide levels [104]. The positive relationship between PAP and body mass index and night-time CO2 tension, but not oxygen tension, lung volumes, or left ventricular function, suggests that PH in this population is driven by body mass index and hypercapnic pulmonary vasoconstriction [104].
5. Systemic Effects of Hypercapnia
As hypercapnia is a systemic condition, it affects all parts of the human body. Evidence suggests a significant role of carbon dioxide in regulating coronary perfusion as well as muscle function and contractility [105,106,107].
5.1. Cardiovascular Effects
Cardiovascular diseases often accompany COPD and impact COPD-related morbidity and mortality [108]. Therefore, cardiovascular consequences of hypercapnia are worth acknowledging.
Elevated PaCO2 and the consequent change in pH have regulatory effects on coronary blood flow and oxygenation [105,109]. Hypercapnia significantly increased myocardial blood flow in healthy subjects assessed with positron emission tomography [110,111]. This effect is driven by the direct vasodilating effect of CO2 and decreased pH as well as changes in the activity of the autonomic nervous system mediated by central and peripheral chemoreceptor stimulation [105]. Furthermore, hypercapnia promotes improvement in tissue oxygenation through the rightward shift of the oxyhemoglobin dissociation curve, which decreases the affinity of hemoglobin to oxygen, thereby enhancing cellular O2 diffusion and uptake (“Bohr effect”) [112]. In addition, elevated CO2 tension potentiates hypoxia-induced pulmonary vasoconstriction, thus reducing intrapulmonary shunt and improving the V/Q ratio [113,114]. Besides enhanced oxygenation and coronary perfusion, hypercapnia decreases the total peripheral resistance, consequently lowering the afterload of the left heart and increasing the cardiac output and cardiac index [115,116].
5.2. Musculoskeletal Effects
Muscle wasting and skeletal muscle dysfunction are common features of COPD [117]. Muscle loss is independently associated with unfavorable outcomes, such as higher mortality and hospital readmission rates and lower quality of life [118,119,120]. However, the exact link between hypercapnia and muscle dysfunction has been demonstrated only in recent years [117]. Jaitovich and his colleagues studied mice and cultured myotubes that were exposed to high CO2 [121]. They observed that the muscle atrophy and reduced anabolic capacity was mediated through AMP-activated kinase (AMPK) subunit α2 activation, which led to an upregulation of muscle-specific ring finger protein-1 (MuRF-1) expression. MuRF-1 is a specific type of ubiquitin ligase responsible for protein degradation [122]. The same research group also showed that AMPKα2 downregulated ribosomal biogenesis and thus decreased muscle protein synthesis, further negatively influencing the skeletal muscle turnover [106]. Furthermore, the regeneration of myoblasts is also impaired due to increased fatty acid oxidation caused by an enhanced rate of oxidative phosphorylation [8].
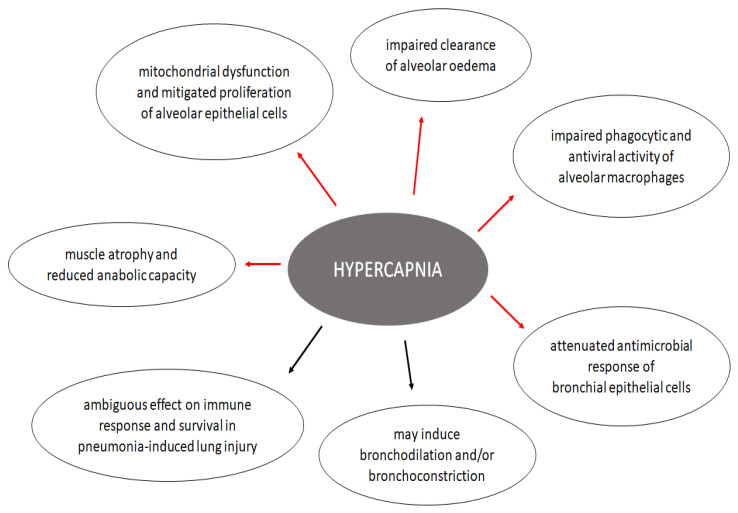
The possible cellular effects of hypercapnia in patients with COPD are shown in Figure 3.
Figure 3.
Possible cellular effects of hypercapnia in COPD. Red arrows indicate a harmful effect, while black arrows show that current evidence is inconclusive. References are listed in the text.
6. Noninvasive Ventilation in COPD
Noninvasive ventilation refers to techniques that provide ventilatory support without endotracheal intubation [123]. Nowadays, the most frequently used form of NIV is positive-pressure ventilation delivered through a tight-fitting mask or helm, while negative-pressure support has a historical relevance and a narrow range of indications [124].
NIV is a widely used standard of care in an acute care setting as treatment for acute or acute-on-chronic hypercapnic respiratory failure [61]. Acute exacerbations (AE) of COPD are the most common indication of NIV as COPD patients hospitalized due to AE frequently develop acute respiratory failure (ARF) and acidosis [125]. The beneficial effects of NIV in ARF are robust and well proven; it reduces the need for endotracheal intubation, decreases the rate of complications, and lowers the cost of care [61,126]. Furthermore, NIV can also be used as long-term treatment for chronic hypercapnic respiratory failure in COPD [18,19].
6.1. NIV in Stable Hypercapnic COPD
Several studies have investigated the effects of long-term NIV (LT-NIV) on various outcomes in hypercapnic stable COPD, including mortality [93,127,128,129,130]; hospitalization rate [93,128,130,131]; and patient-centered outcomes, such as exercise tolerance [93,128,131,132,133,134,135], symptom severity [127,128,132,133,134,135,136], or health-related quality of life (HRQL) [93,128,129,132,134]. However, the results of these studies are controversial. The reasons for contradictions include differences in study design (observational or controlled trials), selection criteria (i.e., hypercapnic vs. normocapnic patients), patient population (frequent vs. infrequent exacerbator and inclusion vs. exclusion of obese patients or those with obstructive sleep apnea), or target PaCO2 (aiming to normalize hypercapnia or without a set PaCO2 target). Most importantly, the ventilator setups were different between studies. Increasing evidence suggests that appropriate intensity NIV that is able to normalize hypercapnia is the only method associated with any mortality benefit [93]. It has better efficacy in improving dyspnea, lung function, and quality of life compared to low-intensity NIV [137].
In 2014, Köhnlein et al. found that lowering carbon dioxide by at least 20% or to PaCO2 < 6.4 kPa significantly improved the one-year survival rate compared to standard therapy [93]. Furthermore, patients in the intervention group experienced a higher improvement in HRQL and several physiological parameters, such as pH, PaCO2, SaO2, HCO3−, and FEV1. Clini et al. found no difference in mortality between NIV and the control group with 2 years of follow-up, but the quality of life improved at a higher rate in the group receiving ventilatory support [128]. Additionally, when comparing the number of hospitalizations over the 2-year study period to the previous 3 years, they found that the hospitalization rate in the LT-NIV group decreased by 45%, while it increased by 27% in the control group. Casanova et al. did not find any difference in mortality, readmission rate, hospitalization, or endotracheal intubation rate between the NIV and the LTOT control group, but patients in the intervention arm were observed as having decreased dyspnea [127]. McEvoy et al. found a slight survival benefit in patients treated with NIV and LTOT compared to LTOT alone, but contrary to the previous studies, they reported reduced HRQL [129]. Márquez-Martín et al. assessed the effect of exercise training, NIV, and the combined use of these interventions on exercise capacity, quality of life, systemic inflammatory markers, gas exchange, peripheral muscle strength. and BODE index [135]. They reported a significant improvement in dyspnea, HRQL. and BODE index in all three treatment arms, with no statistical difference between the groups. Although there were differences in the other individual outcomes, the combination of NIV and exercise training were found to improve all parameters to a higher extent than each of the interventions separately. Duiverman et al. and Garrod et al. both evaluated whether the addition of NIV to a pulmonary rehabilitation program would produce greater benefits to HRQL and functional status than rehabilitation alone [134,138]. They observed greater improvements in HRQL scores (Chronic Respiratory Disease Questionnaire and Maugeri Respiratory Failure Questionnaire) and gas exchange parameters (PaCO2 and PaO2) in the group receiving NIV and rehabilitation than in the rehabilitation group alone.
Even though the results regarding primary outcomes of the studies are inconclusive, improved exercise tolerance, improved quality of life, and decreased dyspnea seem to be universal, which is also supported by observational studies [139,140]. This effect of NIV is paramount because, according to a recent large-scale international survey conducted by a European Respiratory Society (ERS) taskforce committee, these outcomes are the most important for patients living with severe COPD [141]. Based on these improvements in patient-centered outcomes and the fact that only minor adverse events occurred during the use of NIV, both the ERS and the American Thoracic Society (ATS) guidelines recommend the use of domiciliary NIV in chronic stable hypercapnic COPD [18,19].
6.2. NIV during Acute Exacerbations of COPD
Acute exacerbations are important events during COPD as they are a key cause of mortality, accelerate lung function decline, contribute to worsening quality of life, and enhance the risk of a future relapse [142,143]. A large proportion of patients with AECOPD admitted to hospital develop acute respiratory failure necessitating mechanical ventilation.
The ultimate treatment for life-threatening ARF is invasive mechanical ventilation. However, this is associated with a number of short- and long-term complications, such as ventilator-associated lung injury, laryngeal and tracheal injuries, nosocomial pneumonia, difficultly weaning, and prolonged ICU stay [144,145]. The large costs [146] and high risk of complications raises the need for the development of alternative therapies.
Noninvasive ventilation has been effectively used for decades for the acute treatment of ARF in AECOPD [147]. The review of the available literature would extend the scope of our article, and as it has been carried out on multiple instances by international review boards [17,61], we aim to summarize its benefits only briefly.
Several randomized controlled trials (RCT) have assessed the effectiveness of NIV vs. usual care in AECOPD patients experiencing an AHRF episode who do not initially require endotracheal intubation and mechanical ventilation. A meta-analysis of 17 RCTs involving more than 1200 patients found that NIV reduces the risk of mortality by 46% and the need for intubation by 65%. The quality of this evidence was graded “moderate” according to the GRADE criteria. The authors also concluded that NIV decreased the length of hospital stay and improved pH and blood gas parameters [17]. Similarly, in their joint guideline, the taskforce committees of the ERS and ATS also reported that, besides benefits in mortality and prevention of intubation, NIV shortens the length of intensive care unit (ICU) stay, decreases the rate of infectious complications, and improves dyspnea [61]. Based on the high certainty of the evidence, the ERS/ATS guideline strongly recommends the use of bilevel NIV for AECOPD patients with AHRF.
6.3. NIV after an Acute Exacerbation
In a proportion of patients surviving an AHRF episode due to AECOPD, hypercapnia persists after hospital discharge and even in stable state [125]. It raises the question whether these patients would benefit from long-term NIV therapy initiated right after a life-threatening episode of AE.
Cheung et al. carried out a pilot RCT involving patients surviving a life-threatening AE in which they compared the continuation of NIV after hospital discharge to a control sham continuous positive airway pressure (CPAP) therapy in hypercapnic patients [148]. They reported a significant difference in recurring ARF during the 1-year follow-up favoring NIV (38.5% in NIV group vs. 60.2% in control group, p = 0.039). However, the definition of AE was unclear, and the drop-out rate was high from an already limited sample size (25% of 49 patients). Notably, the LT-NIV or control therapy was initiated after >48 h of successful weaning of the original ventilation but before hospital discharge. De Backer et al. also conducted a pilot RCT comparing maximal pharmacological treatment to maximal pharmacological treatment combined with NIV therapy for 6 months after an episode of AE [149]. Although the sample size was very small (n = 15), they did not report any deaths or relapses during the study period. However, the primary outcomes of the study were not mortality or relapse rate but rather arterial blood gas values and functional imaging of the lungs. Importantly, they found that NIV provided better ventilation/perfusion match and thus improved the arterial blood gas and lung function parameters. Furthermore, two large-scale international RCTs also investigated the efficacy of LT-NIV + LTOT in reducing mortality and readmission rate after an AE compared to LTOT alone [150,151]. The RESCUE trial involving 201 hypercapnic COPD patients failed to detect any differences in the main outcomes between the two groups during the 1-year follow-up [151]. In contrast, the HOT-HMV trial found a remarkable difference in the 12-month risk of readmission or death (63.4% in the NIV group vs. 80.4% in the LTOT alone group) and in the median time to readmission or death (4.3 months vs. 1.4 months, respectively). This huge discrepancy may be explained by the different criteria of hypercapnia (>45 mmHg in RESCUE vs. >53 mmHg in the HOT-HMV trial) and by the different timepoints of initial assessment and enrolment of patients. In the RESCUE trial, the randomization was carried out after >48 h of independence from the ventilator, which may have led to the inclusion of patients who had a spontaneously reversible hypercapnia [152], thus masking the beneficial effects of NIV in the right population. In the HOT-HMV trial, however, the assessment of hypercapnia and the inclusion was carried out 2–4 weeks after the exacerbation.
Lastly, Funk and his colleagues carried out a study from a different approach [153]. They provided LT-NIV therapy to 26 consecutive COPD patients who remained hypercapnic after an episode of ARF, and after 6 months of therapy, they randomized the participants to either continue or withdraw from NIV. They observed that the risk of clinical worsening was higher in the withdrawal group, and the six-minute walk distance was reduced compared to the ventilation group. Table 1 describes these trials and summarizes their main findings.
Table 1.
Clinical trials investigating the effect of LT-NIV in chronic hypercapnic COPD on different outcomes.
| Study Name | Population | Primary Outcome | Favors NIV | Baseline PaCO2, kPa | AE Frequency at Baseline |
BMI | OSA | Normalizing Hypercapnia, Yes/No | NIV Mode |
|---|---|---|---|---|---|---|---|---|---|
| Casanova 2000 [127] | Stable | Number of AEs | No | 6.8 ± 1.1 | No data | 25 ± 4 | Excluded | No | Nasal BiPAP, S mode, EPAP: 4 cmH2O; IPAP: 12 cmH2O |
| Clini 2002 [128] | Stable | Arterial blood gas values, hospital and ICU admissions, total hospital and ICU length of stay, HRQL | Partly | 7.2 ± 0.6 | No data | 26 ± 5 | Excluded | Yes (5% decrease) | Nasal BiPAP, S/T mode, backup frequency: 8/min; EPAP: 2–5 cmH2O; IPAP: maximal tolerated pressure |
| Duiverman 2008 [134] | Stable | HRQL, functional status and gas exchange parameters | Yes | 6.89 ± 0.68 | No data | 27.1 ± 6.4 | Excluded | Yes (PaCO2 < 6.0 kPa) | BiPAP, S/T mode; IPAP: maximal tolerated pressure titrated towards an optimal correction of nocturnal arterial blood gases (PaCO2 6.0 kPa and PaO2 8.0 kPa) |
| Garrod 2000 [138] | Stable | Exercise capacity and health status | Yes | 5.9 ± 0.9 | No data | No data | Not excluded | No | Nasal BiPAP, S mode overnight or minimum 8 h/day, settings adjusted individually to obtain the maximal pressure tolerated; EPAP: 4 (4–6) cmH2O; IPAP: 16 (13–24) cmH2O |
| Köhnlein 2014 [93] | Stable | 1-year all-cause mortality | Yes | 7.8 ± 0.8 | No data | 24.8 ± 5.8 | Not excluded | Yes (>20% decrease or PaCO2 < 6.5 kPa) | Pressure support ventilation with high backup rates minimum 6 h/day, preferably during sleep (face or nasal mask). Aim: to reduce ≥20% baseline PaCO2 or PaCO2 < 6.5 kPa |
| Marquez-Martin 2014 [135] | Stable | Exercise capacity | Favors ventilation/training combined group over ventilation alone | NIV group: median 51, NIV-ET group: median 50 | No data | No data | Excluded | No | Nocturnal nasal BiPAP, S/T mode, backup frequency 12/min, 6–8 h/night; EPAP: 4 cmH2O; IPAP: initially 10 cmH2O and increased progressively to a maximum of 20 cmH2O, depending on patient tolerance, clinical response and SpO2 |
| McEvoy 2009 [129] | Stable | Survival | Yes | 7.01 [6.80–7.23] | No data | 25.5 [24.3–26.7] | Excluded | No | BiPAP, VPAP mode, EPAP: lowest possible level (~3 cm H2O); IPAP: gradually increased during daytime and night-time trials to the maximum tolerated with a target PS of ≥10 cm H2O |
| Cheung 2010 [148] | Post AE (>48 h after successful weaning of acute NIV) | Recurrent severe AE with AHRF requiring acute NIV, intubation or resulting in death in the first year | Yes | 7.7 ± 1.0 | Previous acute NIV: 1 [0–3], previous intubation: 0 (0–1), no other data | 19.2 ± 3.6 | Excluded | No | BiPAP, S/T mode, backup frequency: 14/min; EPAP: 5 cmH2O; IPAP: 10–20 cmH2O |
| De Backer 2011 [149] | Post AE (5–12 days after admission) | Arterial blood gas values and functional imaging of the lungs | Yes | 7.39 ± 1.03 | No data | No data | Excluded | Yes (5% decrease) | BiPAP for >5 h a day with a full face mask; modes were adapted until O2 saturation was >90% during 90% of the time, and PaCO2 was decreased 5% in 1 h |
| Funk 2011 [153] | Post AE (before discharge from the ICU or immediately after transfer to regular wards) | Time to clinical worsening Defined as an escalation of mechanical ventilation | Yes | 7.6 ± 1.7 | No data | 24.2 ± 4.3 | Excluded | No | BiPAP EPAP: ~5 cmH2O; IPAP: increasingly raised from 10 to ~20 cmH2O. The inspiratory time was limited to a maximum of 1.3 s |
| Murphy 2017 [150] | Post AE (2–4 weeks after resolution of respiratory acidemia) | Time to readmission or death within 12 months adjusted for the number of previous COPD admissions, previous use of long-term oxygen, age, and BMI | Yes | 7.87 ± 0.93 | ≥3 COPD-related readmissions within past year: NIV-LTOT group: N = 30 (53%) vs. LTOT group: N = 31 (53%) | 21.5 (18.8–24.5) | Excluded | Yes (reduce tcCO2 by at least 4 mmHg) | BiPAP, PS mode, recommended initial titration settings: IPAP 18 cmH2O, EPAP 4 cmH2O, backup rate 14–16/min; target IPAP ≥25 cmH2O. NIV settings and O2 flow rate were titrated to maintain SpO2 >88% and to reduce tcCO2 by ≥4 mmHg |
| Struik 2014 [151] | Post AE (>48 h after termination of ventilatory support) | Time to readmission for respiratory cause or death | No | 7.9 ± 1.2 | Median: 2, min–max: 1–9 | 24.6 ± 5.4 | Excluded | Yes (to achieve normocapnia) | BiPAP, S/T mode starting with a backup frequency of 12/min; IPAP: initial 14 cmH2O and gradually increased to a maximal tolerated level; EPAP: initial 4 cmH2O and increased if auto-PEEP was present or when patients used respiratory muscles to trigger the ventilator. Respiratory rate was set as close as possible to the that of the patient. I:E ratio was 1:3, with a short rise time and then titrated on comfort and effectiveness |
Abbreviations: AE, acute exacerbation; AHRF, acute hypercapnic respiratory failure; BiPAP, bilevel positive airway pressure; BMI, body mass index; COPD, chronic obstructive pulmonary disease; EPAP, expiratory positive airway pressure; I:E, ratio of inhalation to exhalation; IPAP, inspiratory positive airway pressure; LT, long-term; NIV, noninvasive ventilation; OSA, obstructive sleep apnea syndrome; PaCO2, partial arterial carbon dioxide pressure; PEEP, positive end-expiratory pressure; PS, pressure support; SpO2, arterial oxygen saturation; S/T mode, spontaneous/timed mode.
As a consequence of the abovementioned evidence, the ATS and ERS recommend a careful selection of patients benefiting from LT-NIV therapy following an ARF, with the reassessment of hypercapnia 2–4 weeks after hospital discharge [18,19].
7. Discussion
7.1. Clinical Recommendations
Hypercapnia is common in patients with severe and very severe COPD, and it is a risk factor for hospitalization and mortality. Correction of PaCO2 values might therefore be beneficial in selected patients. Based on the available evidence, the authors make the following clinical recommendations (Table 2).
Table 2.
Clinical recommendations on the screening, assessment, and treatment of stable hypercapnic COPD.
| Category | Recommendation |
|---|---|
| Screening | Patients with severe and very severe COPD and those on long-term oxygen therapy should have regular blood gas assessment. |
| Patients with acute hypercapnic respiratory failure should have a blood gas assessment at 2–4 weeks following discharge. | |
| Assessment | Pharmacological and nonpharmacological COPD treatment and other disorders causing hypercapnia (i.e., obesity, neuromuscular, and chest wall diseases) should be evaluated during assessment. |
| Routine sleep study should be offered to explore the presence of obstructive sleep apnoea and to identify variable (i.e., sleep-phase or positional) episodes of hypoventilation. | |
| Treatment | Pharmacological therapy should be optimised to improve symptoms and reduce the number of exacerbations. |
| Treatable traits contributing to hypercapnia (i.e., obesity and sarcopenia) should be addressed in parallel with NIV. | |
| Long-term NIV should be offered to those with persistent hypercapnic respiratory failure (PaCO2 ≥ 52 mmHg (>6.8 kPa)). | |
| The effect of long-term NIV therapy should be assessed with routine blood gas tests, sleep studies, and COPD-related outcomes (i.e., symptoms, quality of life, and the number of exacerbations). | |
| NIV treatment should be titrated to normalise PaCO2 (PaCO2 < 52 mmHg (<6.8 kPa)). |
7.2. Future Research Directions
Previous studies have clearly highlighted that not all patients with hypercapnia benefit from long-term NIV treatment, and the effect is also variable. This could be due to the poor understanding about the role of hypercapnia on the course of COPD and also due to the fact that NIV trials did not systematically assess physiological and biological variables. Based on this, we make the following recommendations for future research directions:
Observational studies are needed to understand the impact and stability of the hypercapnia exacerbator phenotype.
Phase III long-term NIV trials with physiological and biological outcomes are needed in patients with chronic hypercapnic respiratory failure.
Studies designed to understand the impact of long-term NIV on cardiovascular outcomes are needed.
Studies designed to understand factors predicting treatment response to long-term NIV are needed.
Studies designed to establish minimal hours of usage necessary with long-term NIV are needed.
Author Contributions
Conceptualization, A.B. (Andras Bikov); writing—original draft preparation, B.C., M.R.V., S.D. and Z.L.; writing—review and editing, A.B. (Andras Bikov), Z.L., A.B. (Andrew Bentley) and T.F.; visualization, Z.L.; supervision, A.B. (Andras Bikov) and Z.L.; A.B. (Andras Bikov) and Z.L. have equally contributed to the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Timothy Felton and Andras Bikov are supported by the NIHR Manchester Biomedical Research Centre.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report) [(accessed on 9 September 2021)]. Available online: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
- 2.Gibson G.J., Loddenkemper R., Lundbäck B., Sibille Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013;42:559–563. doi: 10.1183/09031936.00105513. [DOI] [PubMed] [Google Scholar]
- 3.Dreher M., Neuzeret P.C., Windisch W., Martens D., Hoheisel G., Groschel A., Woehrle H., Fetsch T., Graml A., Kohnlein T. Prevalence of Chronic Hypercapnia in Severe Chronic Obstructive Pulmonary Disease: Data from the HOmeVent Registry. Int. J. Chronic Obstr. Pulm. Dis. 2019;14:2377–2384. doi: 10.2147/COPD.S222803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saure E.W., Eagan T.M., Jensen R.L., Voll-Aanerud M., Aukrust P., Bakke P.S., Hardie J.A. Explained variance for blood gases in a population with COPD. Clin. Respir. J. 2012;6:72–80. doi: 10.1111/j.1752-699X.2011.00248.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Roisin R., Drakulovic M., Rodriguez D.A., Roca J., Barbera J.A., Wagner P.D. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. (1985) 2009;106:1902–1908. doi: 10.1152/japplphysiol.00085.2009. [DOI] [PubMed] [Google Scholar]
- 6.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Briva A., Vadasz I., Lecuona E., Welch L.C., Chen J., Dada L.A., Trejo H.E., Dumasius V., Azzam Z.S., Myrianthefs P.M., et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceco E., Celli D., Weinberg S., Shigemura M., Welch L.C., Volpe L., Chandel N.S., Bharat A., Lecuona E., Sznajder J.I. Elevated CO2 Levels Delay Skeletal Muscle Repair by Increasing Fatty Acid Oxidation. Front. Physiol. 2020;11:630910. doi: 10.3389/fphys.2020.630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N., Gates K.L., Trejo H., Favoreto S., Jr., Schleimer R.P., Sznajder J.I., Beitel G.J., Sporn P.H. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24:2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadi Z., Bornefalk-Hermansson A., Franklin K.A., Midgren B., Ekstrom M.P. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: A population-based prospective study. Respir. Res. 2014;15:30. doi: 10.1186/1465-9921-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecuona E., Trejo H.E., Sznajder J.I. Regulation of Na,K-ATPase during acute lung injury. J. Bioenerg. Biomembr. 2007;39:391–395. doi: 10.1007/s10863-007-9102-1. [DOI] [PubMed] [Google Scholar]
- 12.Vohwinkel C.U., Lecuona E., Sun H., Sommer N., Vadasz I., Chandel N.S., Sznajder J.I. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011;286:37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agusti A., Bel E., Thomas M., Vogelmeier C., Brusselle G., Holgate S., Humbert M., Jones P., Gibson P.G., Vestbo J., et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 14.Burrows B., Earle R.H. Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patients. N. Engl. J. Med. 1969;280:397–404. doi: 10.1056/NEJM196902202800801. [DOI] [PubMed] [Google Scholar]
- 15.Neff T.A., Petty T.L. Tolerance and survival in severe chronic hypercapnia. Arch. Intern. Med. 1972;129:591–596. doi: 10.1001/archinte.1972.00320040067008. [DOI] [PubMed] [Google Scholar]
- 16.Nizet T.A., van den Elshout F.J., Heijdra Y.F., van de Ven M.J., Mulder P.G., Folgering H.T. Survival of chronic hypercapnic COPD patients is predicted by smoking habits, comorbidity, and hypoxemia. Chest. 2005;127:1904–1910. doi: 10.1378/chest.127.6.1904. [DOI] [PubMed] [Google Scholar]
- 17.Osadnik C.R., Tee V.S., Carson-Chahhoud K.V., Picot J., Wedzicha J.A., Smith B.J. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017;7:Cd004104. doi: 10.1002/14651858.CD004104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ergan B., Oczkowski S., Rochwerg B., Carlucci A., Chatwin M., Clini E., Elliott M., Gonzalez-Bermejo J., Hart N., Lujan M., et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur. Respir. J. 2019;54:1901003. doi: 10.1183/13993003.01003-2019. [DOI] [PubMed] [Google Scholar]
- 19.Macrea M., Oczkowski S., Rochwerg B., Branson R.D., Celli B., Coleman J.M., Hess D.R., Knight S.L., Ohar J.A., Orr J.E., et al. Long-Term Noninvasive Ventilation in Chronic Stable Hypercapnic Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020;202:e74–e87. doi: 10.1164/rccm.202006-2382ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner P.D., Dantzker D.R., Dueck R., Clausen J.L., West J.B. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J. Clin. Investig. 1977;59:203–216. doi: 10.1172/JCI108630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorini M., Misuri G., Corrado A., Duranti R., Iandelli I., De Paola E., Scano G. Breathing pattern and carbon dioxide retention in severe chronic obstructive pulmonary disease. Thorax. 1996;51:677–683. doi: 10.1136/thx.51.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez D.A., Jover L., Drakulovic M.B., Gómez F.P., Roca J., Barberà J.A., Wagner P.D., Rodríguez-Roisin R. Below what FEV1 should arterial blood be routinely taken to detect chronic respiratory failure in COPD? Arch. Bronconeumol. 2011;47:325–329. doi: 10.1016/j.arbres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Mathews A.M., Wysham N.G., Xie J., Qin X., Giovacchini C.X., Ekström M., MacIntyre N.R. Hypercapnia in Advanced Chronic Obstructive Pulmonary Disease: A Secondary Analysis of the National Emphysema Treatment Trial. Chronic Obstr. Pulm. Dis. 2020;7:336–345. doi: 10.15326/jcopdf.7.4.2020.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon C.S., Tin C., Song G. Submissive hypercapnia: Why COPD patients are more prone to CO2 retention than heart failure patients. Respir. Physiol. Neurobiol. 2015;216:86–93. doi: 10.1016/j.resp.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roussos C., Fixley M., Gross D., Macklem P.T. Fatigue of inspiratory muscles and their synergic behavior. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979;46:897–904. doi: 10.1152/jappl.1979.46.5.897. [DOI] [PubMed] [Google Scholar]
- 26.Calverley P.M., Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–1061. doi: 10.1016/S0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell D.E., D’Arsigny C., Fitzpatrick M., Webb K.A. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am. J. Respir. Crit. Care Med. 2002;166:663–668. doi: 10.1164/rccm.2201003. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell D.E., Webb K.A., Neder J.A. Lung hyperinflation in COPD: Applying physiology to clinical practice. COPD Res. Pract. 2015;1:4. doi: 10.1186/s40749-015-0008-8. [DOI] [Google Scholar]
- 29.Decramer M. Hyperinflation and respiratory muscle interaction. Eur. Respir. J. 1997;10:934–941. [PubMed] [Google Scholar]
- 30.Dodd D.S., Yarom J., Loring S.H., Engel L.A. O2 cost of inspiratory and expiratory resistive breathing in humans. J. Appl. Physiol. (1985) 1988;65:2518–2523. doi: 10.1152/jappl.1988.65.6.2518. [DOI] [PubMed] [Google Scholar]
- 31.Bégin P., Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1991;143:905–912. doi: 10.1164/ajrccm/143.5_Pt_1.905. [DOI] [PubMed] [Google Scholar]
- 32.Bellemare F., Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983;55:8–15. doi: 10.1152/jappl.1983.55.1.8. [DOI] [PubMed] [Google Scholar]
- 33.Cohen C.A., Zagelbaum G., Gross D., Roussos C., Macklem P.T. Clinical manifestations of inspiratory muscle fatigue. Am. J. Med. 1982;73:308–316. doi: 10.1016/0002-9343(82)90711-2. [DOI] [PubMed] [Google Scholar]
- 34.Gea J., Sancho-Muñoz A., Chalela R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 2018;10:S1332–S1354. doi: 10.21037/jtd.2018.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byun M.K., Cho E.N., Chang J., Ahn C.M., Kim H.J. Sarcopenia correlates with systemic inflammation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:669–675. doi: 10.2147/COPD.S130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topeli A., Laghi F., Tobin M.J. The voluntary drive to breathe is not decreased in hypercapnic patients with severe COPD. Eur. Respir. J. 2001;18:53–60. doi: 10.1183/09031936.01.00014101. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert R., Keighley J., Auchincloss J.H. Mechanisms of Chronic Carbon Dioxide Retention in Patients with Obstructive Pulmonary Disease. Am. J. Med. 1965;38:217–225. doi: 10.1016/0002-9343(65)90175-0. [DOI] [PubMed] [Google Scholar]
- 38.Lourenço R.V., Miranda J.M. Drive and performance of the ventilatory apparatus in chronic obstructive lung disease. N. Engl. J. Med. 1968;279:53–59. doi: 10.1056/NEJM196807112790201. [DOI] [PubMed] [Google Scholar]
- 39.Richter D.W., Ballanyi K., Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr. Opin. Neurobiol. 1992;2:788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 40.Neubauer J.A., Melton J.E., Edelman N.H. Modulation of respiration during brain hypoxia. J. Appl. Physiol. (1985) 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- 41.Abdo W.F., Heunks L.M. Oxygen-induced hypercapnia in COPD: Myths and facts. Crit. Care. 2012;16:323. doi: 10.1186/cc11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaw R., Hernandez A.V., Walker E., Aboussouan L., Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: A systematic review and metaanalysis of cohort studies. Chest. 2009;136:787–796. doi: 10.1378/chest.09-0615. [DOI] [PubMed] [Google Scholar]
- 43.Kjensli A., Falch J.A., Ryg M., Blenk T., Armbrecht G., Diep L.M., Ellingsen I. High prevalence of vertebral deformities in COPD patients: Relationship to disease severity. Eur. Respir. J. 2009;33:1018–1024. doi: 10.1183/09031936.00073908. [DOI] [PubMed] [Google Scholar]
- 44.Gea J., Agusti A., Roca J. Pathophysiology of muscle dysfunction in COPD. J. Appl. Physiol. (1985) 2013;114:1222–1234. doi: 10.1152/japplphysiol.00981.2012. [DOI] [PubMed] [Google Scholar]
- 45.Brown H., Dodic S., Goh S.S., Green C., Wang W.C., Kaul S., Tiruvoipati R. Factors associated with hospital mortality in critically ill patients with exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:2361–2366. doi: 10.2147/COPD.S168983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Q.F., Sheng Y., Zhu N., Tan Y., Xie X.H., Wang S.Y., Cai J.F. The v-DECAF score can predict 90-day all-cause mortality in patients with COPD exacerbation requiring invasive mechanical ventilation. Clin. Respir. J. 2019;13:438–445. doi: 10.1111/crj.13028. [DOI] [PubMed] [Google Scholar]
- 47.Chu C.M., Chan V.L., Lin A.W., Wong I.W., Leung W.S., Lai C.K. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax. 2004;59:1020–1025. doi: 10.1136/thx.2004.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartl S., Lopez-Campos J.L., Pozo-Rodriguez F., Castro-Acosta A., Studnicka M., Kaiser B., Roberts C.M. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur. Respir. J. 2016;47:113–121. doi: 10.1183/13993003.01391-2014. [DOI] [PubMed] [Google Scholar]
- 49.Csoma B., Bikov A., Tóth F., Losonczy G., Müller V., Lázár Z. Blood eosinophils on hospital admission for COPD exacerbation do not predict the recurrence of moderate and severe relapses. ERJ Open Res. 2021;7:00543-2020. doi: 10.1183/23120541.00543-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seneff M.G., Wagner D.P., Wagner R.P., Zimmerman J.E., Knaus W.A. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–1857. doi: 10.1001/jama.1995.03530230038027. [DOI] [PubMed] [Google Scholar]
- 51.Marthan R., Castaing Y., Manier G., Guenard H. Gas exchange alterations in patients with chronic obstructive lung disease. Chest. 1985;87:470–475. doi: 10.1378/chest.87.4.470. [DOI] [PubMed] [Google Scholar]
- 52.Torres A., Reyes A., Roca J., Wagner P.D., Rodriguez-Roisin R. Ventilation-perfusion mismatching in chronic obstructive pulmonary disease during ventilator weaning. Am. Rev. Respir. Dis. 1989;140:1246–1250. doi: 10.1164/ajrccm/140.5.1246. [DOI] [PubMed] [Google Scholar]
- 53.Kuwano K., Bosken C.H., Paré P.D., Bai T.R., Wiggs B.R., Hogg J.C. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1993;148:1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 54.Fleury B., Murciano D., Talamo C., Aubier M., Pariente R., Milic-Emili J. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am. Rev. Respir. Dis. 1985;131:822–827. doi: 10.1164/arrd.1985.131.6.822. [DOI] [PubMed] [Google Scholar]
- 55.Barberà J.A., Roca J., Ferrer A., Félez M.A., Díaz O., Roger N., Rodriguez-Roisin R. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 1997;10:1285–1291. doi: 10.1183/09031936.97.10061285. [DOI] [PubMed] [Google Scholar]
- 56.Calverley P.M., Koulouris N.G. Flow limitation and dynamic hyperinflation: Key concepts in modern respiratory physiology. Eur. Respir. J. 2005;25:186–199. doi: 10.1183/09031936.04.00113204. [DOI] [PubMed] [Google Scholar]
- 57.O’Donnell D.E., Parker C.M. COPD exacerbations·3: Pathophysiology. Thorax. 2006;61:354–361. doi: 10.1136/thx.2005.041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haluszka J., Chartrand D.A., Grassino A.E., Milic-Emili J. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1990;141:1194–1197. doi: 10.1164/ajrccm/141.5_Pt_1.1194. [DOI] [PubMed] [Google Scholar]
- 59.Orozco-Levi M., Lloreta J., Minguella J., Serrano S., Broquetas J.M., Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- 60.Ram F.S.F., Picot J., Lightowler J., Wedzicha J.A. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2004;3:CD004104. doi: 10.1002/14651858.CD004104.pub3. [DOI] [PubMed] [Google Scholar]
- 61.Rochwerg B., Brochard L., Elliott M.W., Hess D., Hill N.S., Nava S., Navalesi P., Antonelli M., Brozek J., Conti G., et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 62.Cummins E.P., Strowitzki M.J., Taylor C.T. Mechanisms and Consequences of Oxygen and Carbon Dioxide Sensing in Mammals. Physiol. Rev. 2020;100:463–488. doi: 10.1152/physrev.00003.2019. [DOI] [PubMed] [Google Scholar]
- 63.Turner M.J., Saint-Criq V., Patel W., Ibrahim S.H., Verdon B., Ward C., Garnett J.P., Tarran R., Cann M.J., Gray M.A. Hypercapnia modulates cAMP signalling and cystic fibrosis transmembrane conductance regulator-dependent anion and fluid secretion in airway epithelia. J. Physiol. 2016;594:1643–1661. doi: 10.1113/JP271309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vadasz I., Dada L.A., Briva A., Trejo H.E., Welch L.C., Chen J., Toth P.T., Lecuona E., Witters L.A., Schumacker P.T., et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Investig. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shigemura M., Sznajder J.I. Elevated CO2 modulates airway contractility. Interface Focus. 2021;11:20200021. doi: 10.1098/rsfs.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigemura M., Lecuona E., Sznajder J.I. Effects of hypercapnia on the lung. J. Physiol. 2017;595:2431–2437. doi: 10.1113/JP273781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lecuona E., Sun H., Chen J., Trejo H.E., Baker M.A., Sznajder J.I. Protein kinase A-Ialpha regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO(2) concentrations. Am. J. Respir. Cell Mol. Biol. 2013;48:626–634. doi: 10.1165/rcmb.2012-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Toole D., Hassett P., Contreras M., Higgins B.D., McKeown S.T., McAuley D.F., O’Brien T., Laffey J.G. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- 69.Doerr C.H., Gajic O., Berrios J.C., Caples S., Abdel M., Lymp J.F., Hubmayr R.D. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am. J. Respir. Crit. Care Med. 2005;171:1371–1377. doi: 10.1164/rccm.200309-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortes-Puentes G.A., Westerly B., Schiavo D., Wang S., Stroetz R., Walters B., Hubmayr R.D., Oeckler R.A. Hypercapnia Alters Alveolar Epithelial Repair by a pH-Dependent and Adenylate Cyclase-Mediated Mechanism. Sci. Rep. 2019;9:349. doi: 10.1038/s41598-018-36951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casalino-Matsuda S.M., Wang N., Ruhoff P.T., Matsuda H., Nlend M.C., Nair A., Szleifer I., Beitel G.J., Sznajder J.I., Sporn P.H.S. Hypercapnia Alters Expression of Immune Response, Nucleosome Assembly and Lipid Metabolism Genes in Differentiated Human Bronchial Epithelial Cells. Sci. Rep. 2018;8:13508. doi: 10.1038/s41598-018-32008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masterson C., Horie S., McCarthy S.D., Gonzalez H., Byrnes D., Brady J., Fandino J., Laffey J.G., O’Toole D. Hypercapnia in the critically ill: Insights from the bench to the bedside. Interface Focus. 2021;11:20200032. doi: 10.1098/rsfs.2020.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cummins E.P., Oliver K.M., Lenihan C.R., Fitzpatrick S.F., Bruning U., Scholz C.C., Slattery C., Leonard M.O., McLoughlin P., Taylor C.T. NF-kappaB links CO2 sensing to innate immunity and inflammation in mammalian cells. J. Immunol. 2010;185:4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- 74.Keogh C.E., Scholz C.C., Rodriguez J., Selfridge A.C., von Kriegsheim A., Cummins E.P. Carbon dioxide-dependent regulation of NF-kappaB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J. Biol. Chem. 2017;292:11561–11571. doi: 10.1074/jbc.M116.755090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masterson C., O’Toole D., Leo A., McHale P., Horie S., Devaney J., Laffey J.G. Effects and Mechanisms by Which Hypercapnic Acidosis Inhibits Sepsis-Induced Canonical Nuclear Factor-kappaB Signaling in the Lung. Crit. Care Med. 2016;44:e207–e217. doi: 10.1097/CCM.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 76.Lang C.J., Dong P., Hosszu E.K., Doyle I.R. Effect of CO2 on LPS-induced cytokine responses in rat alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L96–L103. doi: 10.1152/ajplung.00394.2004. [DOI] [PubMed] [Google Scholar]
- 77.Casalino-Matsuda S.M., Chen F., Gonzalez-Gonzalez F.J., Nair A., Dib S., Yemelyanov A., Gates K.L., Budinger G.R.S., Beitel G.J., Sporn P.H.S. Hypercapnia Suppresses Macrophage Antiviral Activity and Increases Mortality of Influenza A Infection via Akt1. J. Immunol. 2020;205:489–501. doi: 10.4049/jimmunol.2000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chonghaile M.N., Higgins B.D., Costello J., Laffey J.G. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology. 2008;109:837–848. doi: 10.1097/ALN.0b013e3181895fb7. [DOI] [PubMed] [Google Scholar]
- 79.O’Croinin D.F., Hopkins N.O., Moore M.M., Boylan J.F., McLoughlin P., Laffey J.G. Hypercapnic acidosis does not modulate the severity of bacterial pneumonia-induced lung injury. Crit. Care Med. 2005;33:2606–2612. doi: 10.1097/01.CCM.0000186761.41090.C6. [DOI] [PubMed] [Google Scholar]
- 80.O’Croinin D.F., Nichol A.D., Hopkins N., Boylan J., O’Brien S., O’Connor C., Laffey J.G., McLoughlin P. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Crit. Care Med. 2008;36:2128–2135. doi: 10.1097/CCM.0b013e31817d1b59. [DOI] [PubMed] [Google Scholar]
- 81.Shigemura M., Lecuona E., Angulo M., Homma T., Rodriguez D.A., Gonzalez-Gonzalez F.J., Welch L.C., Amarelle L., Kim S.J., Kaminski N., et al. Hypercapnia increases airway smooth muscle contractility via caspase-7-mediated miR-133a-RhoA signaling. Sci. Transl. Med. 2018;10:aat1662. doi: 10.1126/scitranslmed.aat1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodarte J.R., Hyatt R.E. Effect of acute exposure to CO2 on lung mechanics in normal man. Respir. Physiol. 1973;17:135–145. doi: 10.1016/0034-5687(73)90057-1. [DOI] [PubMed] [Google Scholar]
- 83.Uno T., Homma T., Shigemura M., Fukuda Y., Kimura T., Onitsuka C., Kawahara T., Sato H., Akimoto K., Suganuma H., et al. Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD. J. Clin. Med. 2020;9:2819. doi: 10.3390/jcm9092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Mays T.Y., Saifeddine M., Choudhury P., Hollenberg M.D., Green F.H. Carbon dioxide enhances substance P-induced epithelium-dependent bronchial smooth muscle relaxation in Sprague-Dawley rats. Can. J. Physiol. Pharmacol. 2011;89:513–520. doi: 10.1139/y11-052. [DOI] [PubMed] [Google Scholar]
- 85.Astin T.W., Barer G.R., Shaw J.W., Warren P.M. The action of carbon dioxide on constricted airways. J. Physiol. 1973;235:607–623. doi: 10.1113/jphysiol.1973.sp010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Croxton T.L., Lande B., Hirshman C.A. Role of intracellular pH in relaxation of porcine tracheal smooth muscle by respiratory gases. Am. J. Physiol. 1995;268:L207–L213. doi: 10.1152/ajplung.1995.268.2.L207. [DOI] [PubMed] [Google Scholar]
- 87.Tisi G.M., Wolfe W.G., Fallat R.J., Nadel J.A. Effects of O2 and CO2 on airway smooth muscle following pulmonary vascular occlusion. J. Appl. Physiol. 1970;28:570–573. doi: 10.1152/jappl.1970.28.5.570. [DOI] [PubMed] [Google Scholar]
- 88.Twort C.H., Cameron I.R. Effects of PCO2, pH and extracellular calcium on contraction of airway smooth muscle from rats. Respir. Physiol. 1986;66:259–267. doi: 10.1016/0034-5687(86)90078-2. [DOI] [PubMed] [Google Scholar]
- 89.Yamakage M., Lindeman K.S., Hirshman C.A., Croxton T.L. Intracellular pH regulates voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Am. J. Physiol. 1995;268:L642–L646. doi: 10.1152/ajplung.1995.268.4.L642. [DOI] [PubMed] [Google Scholar]
- 90.Sterling G.M., Holst P.E., Nadel J.A. Effect of CO2 and pH on bronchoconstriction caused by serotonin vs. acetylcholine. J. Appl. Physiol. 1972;32:39–43. doi: 10.1152/jappl.1972.32.1.39. [DOI] [PubMed] [Google Scholar]
- 91.Lutter J.I., Jorres R.A., Kahnert K., Schwarzkopf L., Studnicka M., Karrasch S., Schulz H., Vogelmeier C.F., Holle R., for the COSYCONET Study Group Health-related quality of life associates with change in FEV1 in COPD: Results from the COSYCONET cohort. BMC Pulm. Med. 2020;20:148. doi: 10.1186/s12890-020-1147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budweiser S., Hitzl A.P., Jorres R.A., Schmidbauer K., Heinemann F., Pfeifer M. Health-related quality of life and long-term prognosis in chronic hypercapnic respiratory failure: A prospective survival analysis. Respir. Res. 2007;8:92. doi: 10.1186/1465-9921-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohnlein T., Windisch W., Kohler D., Drabik A., Geiseler J., Hartl S., Karg O., Laier-Groeneveld G., Nava S., Schonhofer B., et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2014;2:698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 94.Windisch W., on behalf of the Quality of Life in Home Mechanical Ventilation Study Group Impact of home mechanical ventilation on health-related quality of life. Eur. Respir. J. 2008;32:1328–1336. doi: 10.1183/09031936.00066407. [DOI] [PubMed] [Google Scholar]
- 95.Chaouat A., Bugnet A.S., Kadaoui N., Schott R., Enache I., Ducolone A., Ehrhart M., Kessler R., Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 96.Andersen K.H., Iversen M., Kjaergaard J., Mortensen J., Nielsen-Kudsk J.E., Bendstrup E., Videbaek R., Carlsen J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J. Heart Lung Transplant. 2012;31:373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Kovacs G., Avian A., Bachmaier G., Troester N., Tornyos A., Douschan P., Foris V., Sassmann T., Zeder K., Lindenmann J., et al. Severe Pulmonary Hypertension in COPD: Impact on Survival and Diagnostic Approach. Chest. 2022 doi: 10.1016/j.chest.2022.01.031. [DOI] [PubMed] [Google Scholar]
- 98.McGuire M., Bradford A. Chronic intermittent hypercapnic hypoxia increases pulmonary arterial pressure and haematocrit in rats. Eur. Respir. J. 2001;18:279–285. doi: 10.1183/09031936.01.00078801. [DOI] [PubMed] [Google Scholar]
- 99.Foex P., Prys-Roberts C. Effects of changes in PaCO2 on pulmonary input impedance. J. Appl. Physiol. 1975;38:52–57. doi: 10.1152/jappl.1975.38.1.52. [DOI] [PubMed] [Google Scholar]
- 100.Viitanen A., Salmenpera M., Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology. 1990;73:393–400. doi: 10.1097/00000542-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Dessap A.M., Charron C., Devaquet J., Aboab J., Jardin F., Brochard L., Vieillard-Baron A. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brimioulle S., Lejeune P., Vachiery J.L., Leeman M., Melot C., Naeije R. Effects of acidosis and alkalosis on hypoxic pulmonary vasoconstriction in dogs. Am. J. Physiol. 1990;258:H347–H353. doi: 10.1152/ajpheart.1990.258.2.H347. [DOI] [PubMed] [Google Scholar]
- 103.Rose C.E., Jr., Van Benthuysen K., Jackson J.T., Tucker C.E., Kaiser D.L., Grover R.F., Weil J.V. Right ventricular performance during increased afterload impaired by hypercapnic acidosis in conscious dogs. Circ. Res. 1983;52:76–84. doi: 10.1161/01.RES.52.1.76. [DOI] [PubMed] [Google Scholar]
- 104.Held M., Walthelm J., Baron S., Roth C., Jany B. Functional impact of pulmonary hypertension due to hypoventilation and changes under noninvasive ventilation. Eur. Respir. J. 2014;43:156–165. doi: 10.1183/09031936.00147712. [DOI] [PubMed] [Google Scholar]
- 105.Crystal G.J. Carbon Dioxide and the Heart: Physiology and Clinical Implications. Anesth. Analg. 2015;121:610–623. doi: 10.1213/ANE.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 106.Korponay T.C., Balnis J., Vincent C.E., Singer D.V., Chopra A., Adam A.P., Ginnan R., Singer H.A., Jaitovich A. High CO2 Downregulates Skeletal Muscle Protein Anabolism via AMP-activated Protein Kinase alpha2-mediated Depressed Ribosomal Biogenesis. Am. J. Respir. Cell Mol. Biol. 2020;62:74–86. doi: 10.1165/rcmb.2019-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corfield D.R., McKay L.C. Regional Cerebrovascular Responses to Hypercapnia and Hypoxia. Adv. Exp. Med. Biol. 2016;903:157–167. doi: 10.1007/978-1-4899-7678-9_11. [DOI] [PubMed] [Google Scholar]
- 108.Chen W., Thomas J., Sadatsafavi M., FitzGerald J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 109.Curley G., Laffey J.G., Kavanagh B.P. Bench-to-bedside review: Carbon dioxide. Crit. Care. 2010;14:220. doi: 10.1186/cc8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pelletier-Galarneau M., deKemp R.A., Hunter C., Klein R., Klein M., Ironstone J., Fisher J.A., Ruddy T.D. Effects of Hypercapnia on Myocardial Blood Flow in Healthy Human Subjects. J. Nucl. Med. 2018;59:100–106. doi: 10.2967/jnumed.117.194308. [DOI] [PubMed] [Google Scholar]
- 111.Yang H.J., Dey D., Sykes J., Klein M., Butler J., Kovacs M.S., Sobczyk O., Sharif B., Bi X., Kali A., et al. Arterial CO2 as a Potent Coronary Vasodilator: A Preclinical PET/MR Validation Study with Implications for Cardiac Stress Testing. J. Nucl. Med. 2017;58:953–960. doi: 10.2967/jnumed.116.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turek Z., Kreuzer F. Effect of shifts of the O2 dissociation curve upon alveolar-arterial O2 gradients in computer models of the lung with ventilation-perfusion mismatching. Respir. Physiol. 1981;45:133–139. doi: 10.1016/0034-5687(81)90055-4. [DOI] [PubMed] [Google Scholar]
- 113.Brogan T.V., Robertson H.T., Lamm W.J., Souders J.E., Swenson E.R. Carbon dioxide added late in inspiration reduces ventilation-perfusion heterogeneity without causing respiratory acidosis. J. Appl. Physiol. (1985) 2004;96:1894–1898. doi: 10.1152/japplphysiol.00160.2003. [DOI] [PubMed] [Google Scholar]
- 114.Swenson E.R., Robertson H.T., Hlastala M.P. Effects of inspired carbon dioxide on ventilation-perfusion matching in normoxia, hypoxia, and hyperoxia. Am. J. Respir. Crit. Care Med. 1994;149:1563–1569. doi: 10.1164/ajrccm.149.6.8004314. [DOI] [PubMed] [Google Scholar]
- 115.Cullen D.J., Eger E.I. Cardiovascular effects of carbon dioxide in man. Anesthesiology. 1974;41:345–349. doi: 10.1097/00000542-197410000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Akca O., Doufas A.G., Morioka N., Iscoe S., Fisher J., Sessler D.I. Hypercapnia improves tissue oxygenation. Anesthesiology. 2002;97:801–806. doi: 10.1097/00000542-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 117.Balnis J., Korponay T.C., Jaitovich A. AMP-Activated Protein Kinase (AMPK) at the Crossroads between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD) Int. J. Mol. Sci. 2020;21:955. doi: 10.3390/ijms21030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marquis K., Debigare R., Lacasse Y., LeBlanc P., Jobin J., Carrier G., Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 119.Schols A.M., Broekhuizen R., Weling-Scheepers C.A., Wouters E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005;82:53–59. doi: 10.1093/ajcn/82.1.53. [DOI] [PubMed] [Google Scholar]
- 120.Swallow E.B., Reyes D., Hopkinson N.S., Man W.D., Porcher R., Cetti E.J., Moore A.J., Moxham J., Polkey M.I. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaitovich A., Angulo M., Lecuona E., Dada L.A., Welch L.C., Cheng Y., Gusarova G., Ceco E., Liu C., Shigemura M., et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1) J. Biol. Chem. 2015;290:9183–9194. doi: 10.1074/jbc.M114.625715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 123.British Thoracic Society Standards of Care Committee Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Corrado A., Gorini M. Negative-pressure ventilation: Is there still a role? Eur. Respir. J. 2002;20:187–197. doi: 10.1183/09031936.02.00302602. [DOI] [PubMed] [Google Scholar]
- 125.Roberts C.M., Stone R.A., Buckingham R.J., Pursey N.A., Lowe D., On behalf of the National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project (NCROP) Implementation Group Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48. doi: 10.1136/thx.2010.153114. [DOI] [PubMed] [Google Scholar]
- 126.Elliott M.W. Noninvasive ventilation in acute exacerbations of COPD. Eur. Respir. Rev. 2005;14:39–42. doi: 10.1183/09058180.05.00009405. [DOI] [Google Scholar]
- 127.Casanova C., Celli B.R., Tost L., Soriano E., Abreu J., Velasco V., Santolaria F. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118:1582–1590. doi: 10.1378/chest.118.6.1582. [DOI] [PubMed] [Google Scholar]
- 128.Clini E., Sturani C., Rossi A., Viaggi S., Corrado A., Donner C.F., Ambrosino N. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur. Respir. J. 2002;20:529–538. doi: 10.1183/09031936.02.02162001. [DOI] [PubMed] [Google Scholar]
- 129.McEvoy R.D., Pierce R.J., Hillman D., Esterman A., Ellis E.E., Catcheside P.G., O’Donoghue F.J., Barnes D.J., Grunstein R.R., on behalf of the Australian Trial of Non-Invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax. 2009;64:561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 130.Windisch W., Haenel M., Storre J.H., Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int. J. Med. Sci. 2009;6:72–76. doi: 10.7150/ijms.6.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiang L.L., Liu C.Y., Ho S.C., Sheng T.F., Yu C.T., Lin H.C., Kuo H.P. Efficacy of nocturnal nasal positive pressure ventilation in hypercapnic patients with severe obstructive lung diseases. Chang. Gung Med. J. 2004;27:98–106. [PubMed] [Google Scholar]
- 132.Zhou L., Li X., Guan L., Chen J., Guo B., Wu W., Huo Y., Zhou Z., Liang Z., Zhou Y., et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: A short-term prospective, multicenter, randomized, controlled trial. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1279–1286. doi: 10.2147/COPD.S127540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhatt S.P., Peterson M.W., Wilson J.S., Durairaj L. Noninvasive positive pressure ventilation in subjects with stable COPD: A randomized trial. Int. J. Chronic Obstr. Pulm. Dis. 2013;8:581–589. doi: 10.2147/COPD.S53619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duiverman M.L., Wempe J.B., Bladder G., Jansen D.F., Kerstjens H.A., Zijlstra J.G., Wijkstra P.J. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax. 2008;63:1052–1057. doi: 10.1136/thx.2008.099044. [DOI] [PubMed] [Google Scholar]
- 135.Marquez-Martin E., Ruiz F.O., Ramos P.C., Lopez-Campos J.L., Azcona B.V., Cortes E.B. Randomized trial of non-invasive ventilation combined with exercise training in patients with chronic hypercapnic failure due to chronic obstructive pulmonary disease. Respir. Med. 2014;108:1741–1751. doi: 10.1016/j.rmed.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 136.Eman Shebl R., Abderaboh M.M. Bi-level positive airway pressure ventilation for patients with stable hypercapnic chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2015;64:395–398. doi: 10.1016/j.ejcdt.2015.02.004. [DOI] [Google Scholar]
- 137.Dreher M., Storre J.H., Schmoor C., Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: A randomised crossover trial. Thorax. 2010;65:303–308. doi: 10.1136/thx.2009.124263. [DOI] [PubMed] [Google Scholar]
- 138.Garrod R., Mikelsons C., Paul E.A., Wedzicha J.A. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000;162:1335–1341. doi: 10.1164/ajrccm.162.4.9912029. [DOI] [PubMed] [Google Scholar]
- 139.Tsolaki V., Pastaka C., Karetsi E., Zygoulis P., Koutsokera A., Gourgoulianis K.I., Kostikas K. One-year non-invasive ventilation in chronic hypercapnic COPD: Effect on quality of life. Respir. Med. 2008;102:904–911. doi: 10.1016/j.rmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Wilson M.E., Dobler C.C., Morrow A.S., Beuschel B., Alsawas M., Benkhadra R., Seisa M., Mittal A., Sanchez M., Daraz L., et al. Association of Home Noninvasive Positive Pressure Ventilation With Clinical Outcomes in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis. JAMA. 2020;323:455–465. doi: 10.1001/jama.2019.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mathioudakis A.G., Abroug F., Agusti A., Ananth S., Bakke P., Bartziokas K., Beghe B., Bikov A., Bradbury T., Brusselle G., et al. ERS Statement: A core outcome set for clinical trials evaluating the management of chronic obstructive pulmonary disease (COPD) exacerbations. Eur. Respir. J. 2021;59:2102006. doi: 10.1183/13993003.02006-2021. [DOI] [PubMed] [Google Scholar]
- 142.Donaldson G.C., Seemungal T.A., Bhowmik A., Wedzicha J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hurst J.R., Vestbo J., Anzueto A., Locantore N., Mullerova H., Tal-Singer R., Miller B., Lomas D.A., Agusti A., Macnee W., et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 144.Pierson D.J. Complications associated with mechanical ventilation. Crit. Care Clin. 1990;6:711–724. doi: 10.1016/S0749-0704(18)30362-2. [DOI] [PubMed] [Google Scholar]
- 145.Scheinhorn D.J., Hassenpflug M.S., Votto J.J., Chao D.C., Epstein S.K., Doig G.S., Knight E.B., Petrak R.A., Ventilation Outcomes Study G. Post-ICU mechanical ventilation at 23 long-term care hospitals: A multicenter outcomes study. Chest. 2007;131:85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 146.Dasta J.F., McLaughlin T.P., Mody S.H., Piech C.T. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit. Care Med. 2005;33:1266–1271. doi: 10.1097/01.CCM.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 147.Meduri G.U., Conoscenti C.C., Menashe P., Nair S. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest. 1989;95:865–870. doi: 10.1378/chest.95.4.865. [DOI] [PubMed] [Google Scholar]
- 148.Cheung A.P., Chan V.L., Liong J.T., Lam J.Y., Leung W.S., Lin A., Chu C.M. A pilot trial of non-invasive home ventilation after acidotic respiratory failure in chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2010;14:642–649. [PubMed] [Google Scholar]
- 149.De Backer L., Vos W., Dieriks B., Daems D., Verhulst S., Vinchurkar S., Ides K., De Backer J., Germonpre P., De Backer W. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: A randomized controlled pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2011;6:615–624. doi: 10.2147/COPD.S22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murphy P.B., Rehal S., Arbane G., Bourke S., Calverley P.M.A., Crook A.M., Dowson L., Duffy N., Gibson G.J., Hughes P.D., et al. Effect of Home Noninvasive Ventilation With Oxygen Therapy vs. Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial. JAMA. 2017;317:2177–2186. doi: 10.1001/jama.2017.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Struik F.M., Sprooten R.T., Kerstjens H.A., Bladder G., Zijnen M., Asin J., Cobben N.A., Vonk J.M., Wijkstra P.J. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: A randomised, controlled, parallel-group study. Thorax. 2014;69:826–834. doi: 10.1136/thoraxjnl-2014-205126. [DOI] [PubMed] [Google Scholar]
- 152.Costello R., Deegan P., Fitzpatrick M., McNicholas W.T. Reversible hypercapnia in chronic obstructive pulmonary disease: A distinct pattern of respiratory failure with a favorable prognosis. Am. J. Med. 1997;102:239–244. doi: 10.1016/S0002-9343(97)00017-X. [DOI] [PubMed] [Google Scholar]
- 153.Funk G.C., Breyer M.K., Burghuber O.C., Kink E., Kirchheiner K., Kohansal R., Schmidt I., Hartl S. Long-term non-invasive ventilation in COPD after acute-on-chronic respiratory failure. Respir. Med. 2011;105:427–434. doi: 10.1016/j.rmed.2010.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.