Abstract
This pilot study compares the compositions of bacterial biofilms in pipe networks supplied with water containing either high levels of biodegradable organic matter (BOM) or low levels of BOM (conventionally or biologically treated, respectively). The Microbial Identification System for fatty acid analysis was utilized in this study to identify a large number of organisms (>1,400) to determine population changes in both conventionally and biologically treated water and biofilms. Data generated during this study indicated that suspended bacteria have little impact on biofilms, and despite treatment (conventional or biological), suspended microbial populations were similar following disinfection. Prechlorination with free chlorine resulted not only in reduced plate count values but also in a dramatic shift in the composition of the bacterial population to predominately gram-positive bacteria. Chlorination of biologically treated water produced the same shifts toward gram-positive bacteria. Removal of assimilable organic carbon by the biologically active filters slowed the rate of biofilm accumulation, but biofilm levels were similar to those found in conventionally treated water within several weeks. Iron pipes stimulated the rate of biofilm development, and bacterial levels on disinfected iron pipes exceeded those for chlorinated polyvinyl chloride pipes. The study showed that the iron pipe surface dramatically influenced the composition, activity, and disinfection resistance of biofilm bacteria.
Biological treatment provides numerous benefits for drinking water utilities, including removal of micropollutants, improved treatment processes (i.e., coagulation, color removal, and oxidation of iron and manganese), taste and odor control, and reduced chlorine demand of treated water (16, 32). One of the primary reasons that utilities implement biological treatment is to reduce nutrient levels in the treated water, which in turn can limit the potential for bacterial regrowth in the distribution system. Although concerns regarding the higher levels of heterotrophic plate count (HPC) bacteria in the effluents of biological filters have been raised, the changes in the composition of distribution system microbial populations resulting from a shift in the nutrient levels following biological treatment may have a much more dramatic impact on water quality. While the numbers of organisms in conventionally and biologically treated systems may not differ significantly, the population composition may reveal the largest impact of biologically activated filtration.
The goals of our work were to use pilot-scale conventional and biological treatment to study (i) microbial population changes throughout the distribution system, including biofilm samples and water column samples; (ii) the impact of postdisinfection with free chlorine on microbial populations; and (iii) the impact of corrosion control and pipe materials on microbial populations. Identification and characterization of these populations were made possible through the use of fatty acid analysis.
MATERIALS AND METHODS
Construction and operation of pilot-scale system.
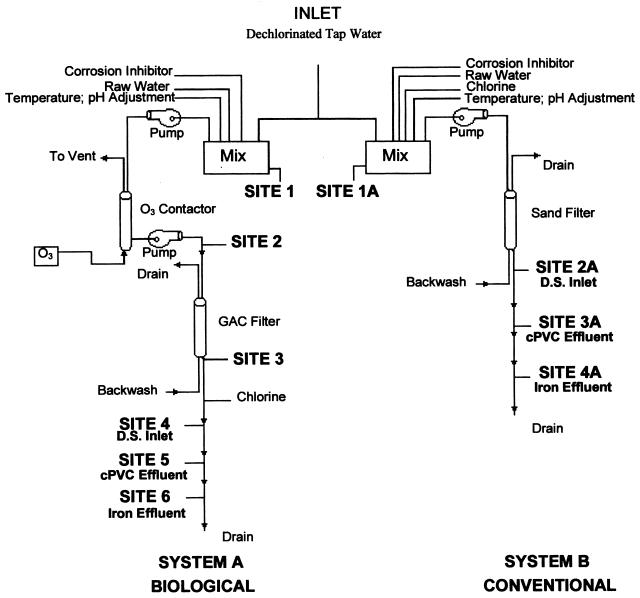
The pilot distribution system at the American Water Works Service Company, Inc., Belleville Laboratory was designed to examine the impact of conventional and biological treatment, postdisinfection, and corrosion control on the microbial quality of the water (Fig. 1). Two small distribution systems (A and B) were constructed from 17 1-ft (30.5-cm) sections of 3/4-in. (1.91-cm)-diameter chlorinated polyvinyl chloride (cPVC) pipe and 14 1-ft (30.5-cm) sections of 1/2-in. (1.27-cm)-diameter black iron pipe connected with Lasc-O-Tite plastic quick-disconnect couplings (United States Plastics Corp., Lima, Ohio). The quick-disconnect couplings provided easy removal of the pipe sections for biofilm analysis. Free chlorine was used to determine the effect of postdisinfection on bacterial levels and populations. Both cPVC and iron pipe surfaces were used in each system to determine the impact of corrosion on disinfection efficiencies and bacterial populations.
FIG. 1.
Schematic diagram of pilot treatment system. D.S., distribution system.
The source water for both the conventionally treated system and the biologically active system was tap water which was dechlorinated by using a column of granular activated carbon (GAC). The source water was supplemented with bacteria and natural organic material from Mississippi River water (diluted 1/50) and a nutrient spike consisting of the supernatant from centrifuged raw sewage (diluted 1/5,000) to obtain assimilable organic carbon (AOC) levels of >500 μg/liter. Both systems included a 12- by 12-in. (30.5- by 30.5-cm) reservoir tank in which source water was mixed with river water, nutrient spike, and AquaMag polyphosphate corrosion inhibitor (10 mg of phosphate per liter; Kjell Corporation, Beloit, Wis.). A circulator-temperature controller (model D1; Haake, Berlin, Germany) was used to maintain proper mixing and temperature control. pH adjustment was achieved in the reservoir tank by using sodium hydroxide or hydrochloric acid and a pH-metering pump (model DP; Cole-Parmer Instrument Co., Chicago, Ill.). A neutral pH was chosen for the study to be representative of water utility operations (albeit on the lower range of common practice) (22) and to minimize the complex interactions between chlorine chemistry, corrosion, and optimal microbial growth rates, etc.
An average free chlorine residual of 1.2 mg/liter was maintained in the reservoir tank of the conventionally treated system, which fed a 24-in. (61-cm)-deep 4-in. (10.2-cm)-diameter sand filter. The biologically active system consisted of a nonchlorinated reservoir tank which fed an 8-ft (2.4-m)-high, 4-in. (10.2-cm)-diameter ozone contactor column. Ozone was generated by using a model GTC-0.5C generator (Griffin Technics Inc., Lodi, N.J.) at a dose sufficient to maintain concentrations in the column averaging 0.16 mg/liter (concentration · time = 3.2 mg · min/liter). The water was then filtered through a 24-in. (61-cm)-deep, 4-in. (10.2-cm)-diameter biologically active GAC filter. The carbon for this filter was obtained from a full-scale GAC filter at the East St. Louis, Ill., treatment plant. For both systems, the filtered water entered the distribution system at a flow rate of 250 ml/min. Free chlorine was used as a postdisinfectant (Tables 1 and 2). Masterflex digital peristaltic pumps (Cole-Parmer Instrument Co.) were used to add all chemicals and disinfectants and to maintain flow through the distribution systems. Corrosion rates were measured with a corrator (model RCS 9000; Rohrback-Cosasco Systems, Santa Fe, Calif.). The three-electrode carbon steel corrator probes were incorporated into each system by using compression tees located at the end of each distribution system.
TABLE 1.
Summary of water quality parameters for the conventionally treated distribution systems
| Parameter measured | Valuea at the following site:
|
||
|---|---|---|---|
| Free-chlorinated raw water reservoir tank | Conventional filter effluent | Free-chlorinated distribution system | |
| Free chlorine residual (mg/liter) | 1.19 (0.65) | 1.18 (0.84) | 1.12 (0.73) |
| Total chlorine residual (mg/liter) | 1.31 (0.71) | 1.32 (0.86) | 1.21 (0.73) |
| pHb | 6.78 (0.16) | 7.00 (0.19) | 6.97 (0.15) |
| Temp (°C) | 14.4 (1.5) | 15.9 (1.5) | 17.6 (1.5) |
| AOC (μg of C/liter)c | 602 (190–1,045) | NDd | ND |
| TOCe (mg/liter) | 1.62 (0.26) | 1.66 (0.25) | 1.69 (0.24) |
| Turbidity (NTUf) | 0.87 (0.62) | 0.31 (0.18) | 1.42 (1.91)g |
| Ammonia (mg/liter) | 0.03 (0.03) | 0.03 (0.04) | 0.04 (0.04) |
| Corrosion rate (mils/yr) | ND | ND | 0.28 (0.10) |
| Dissolved oxygen (mg/liter) | 9.42 (0.83) | 8.89 (1.47) | 8.33 (1.23) |
Chemical parameter measurements are averages from each site, with standard deviations given in parentheses, unless otherwise indicated.
The system pH was targeted at levels found in previously studied distribution systems (22).
AOC levels are reported as geometric means, with ranges given in parentheses.
ND, not determined.
TOC, total organic carbon.
NTU, nephelometric turbidity units.
Turbidity values for free-chlorinated distribution system effluent exceeded 3.0 NTU on three of six occasions, resulting in a high average value.
TABLE 2.
Summary of water quality parameters for the biologically treated distribution systems
| Parameter measured | Valuea at the following site:
|
|||
|---|---|---|---|---|
| Raw water reservoir tank | Ozone contactor | Biologically active carbon filter | Free-chlorinated distribution system | |
| Free chlorine residual (mg/liter) | NAb | NA | NA | 1.68 (0.29) |
| Total chlorine residual (mg/liter) | NA | NA | NA | 1.79 (0.28) |
| Ozone residual (mg/liter) | NA | 0.16 (0.04) | NA | NA |
| pHc | 5.76 (0.14) | 6.93 (0.13) | 6.74 (0.22) | 6.82 (0.23) |
| Temp (°C) | 24.0 (1.0) | 20.5 (1.9) | 20.1 (1.8) | 20.2 (1.4) |
| AOC (μg of C/liter)d | 720 (180–1,700) | 384 (114–630) | 39 (12–63) | NA |
| TOCe (mg/liter) | 1.68 (0.32) | 1.73 (0.33) | 1.26 (0.15) | 1.26 (0.12) |
| Turbidity (NTUf | 1.80 (1.03) | 1.97 (0.99) | 0.14 (.03) | 0.88 (2.57) |
| Ammonia (mg/liter) | 0.08 (0.04) | 0.14 (0.07) | 0.05 (0.06) | 0.03 (0.03) |
| Corrosion rate (mils/yr) | NA | NA | NA | 0.73 (0.68) |
| Dissolved oxygen (mg/liter) | 8.17 (1.23) | 8.05 (1.22) | 6.96 (1.02) | 6.41 (1.08) |
Chemical parameter measurements are averages from each site, with standard deviations given in parentheses, unless otherwise indicated.
NA, not applicable.
The system pH was targeted at levels found in previously studied distribution systems (22).
AOC levels are reported as geometric means, with ranges given in parentheses.
TOC, total organic carbon.
NTU, nephelometric turbidity units.
The empty-bed contact time for both filters was 20 min, and both columns utilized false-floor underdrain systems, using nylon screen to prevent loss of the filter media. The filters were backwashed once each week during normal operations. During backwash, the systems were taken offline and the filters were backwashed for 20 min at a rate sufficient to expand the filter media to twice the normal volume by using dechlorinated tap water. In an effort to discourage biological activity in the conventional filter, hyperchlorination (>100 mg of chlorine per liter) and air scouring of the filter were performed during backwash every other week. To prevent passage of high levels of chlorine to the distribution system following hyperchlorination, the filter was flushed for 20 min with dechlorinated tap water, and chlorine residuals were measured before the filter was put back into service. Sample collection occurred at least 24 h after backwashing of filters.
Sampling.
Weekly water samples were collected from both systems to ensure proper functioning of the pilot system. Various water quality parameters (Tables 1 and 2) were measured as previously described (20). In addition, ozone concentrations were determined by using HACH (Loveland, Colo.) indigo colorimetric reagents and a colorimeter (model DR100; HACH). Dissolved oxygen levels were monitored with a dissolved oxygen meter (model 59; YSI Corporation, Yellow Springs, Ohio), and AOC levels were measured by the rapid AOC method published by LeChevallier et al. (17). Average values for water quality parameters are listed in Tables 1 and 2. The conventionally treated system was sampled at seven sites and the biologically treated system was sampled at nine sites to ensure that sampling would adequately represent all aspects of the treatment process (Fig. 1).
Biofilm levels were also monitored on a weekly basis via plate counts. Pipe sections were removed from the system, and biofilms were scraped and washed from the pipe interior, homogenized, and enumerated on R2A agar at 22°C for 7 days (20). Ten-milliliter portions of the biofilm samples were shipped for Mycobacterium analysis (done by Joseph Falkinham, Virginia Technical Institute, Blacksburg). The samples were plated without a decontamination step on a Tween 80-containing, low-pH selective medium and analyzed for both rapid- and slow-growing acid-fast organisms exhibiting colonial and microscopic morphologies consistent with Mycobacterium (13). The isolates were identified by comparing large restriction fragments separated by pulsed-field gel electrophoresis (2, 25).
Microbial identification.
On two separate sampling days, 50 bacterial cultures were isolated for identification from each water column sampling site, as well as from biofilm samples from both cPVC and iron pipe surfaces in each system. In an attempt to adequately represent the populations throughout these systems, >1,400 isolates were identified during the course of this project. To reduce the bias associated with colony selection, all isolates were chosen from a selected quadrant of a plate, as opposed to selection based on colony morphology. The plates were visually inspected, and an attempt was made to chose the most representative section possible. If the plate counts had fewer than 50 colonies, we took all of the isolates from one plate and from a portion of another plate. When there were more than 50 colonies on a plate, isolates were selected from one point on the plate and we worked our way across the plate, selecting all colonies, quadrant by quadrant, until 50 colonies had been removed. When sections of plates were crowded with isolates growing together, these sections were avoided if possible to minimize the selection of multiple colonies. The selected isolates were streaked on R2A agar to confirm purity and incubated at 22°C for 7 days. Cells were then washed from the plates and stored at −80°C in a 2% peptone–20% glycerol solution for future identification.
The Microbial Identification System (Microbial ID, Inc., Newark, Del.) was utilized in this study to identify a large number of organisms to determine population changes in both conventionally and biologically treated water and biofilms.
All previously frozen isolates were removed from storage, streaked onto four quadrants on R2A agar plates, and incubated at 28°C for 48 h in preparation for identification. Based on the manufacturer's recommendation, cells were harvested in a late log growth phase to obtain the most stable fatty acid compositions. Fatty acids were extracted and methylated according to the manufacturer's instructions. The samples were then analyzed by using the Microbial Identification System (Microbial ID, Inc.). This system was composed of a Hewlett-Packard (Palo Alto, Calif.) 5890 series II gas chromatograph with a 7673 autosampler, a 3396 series II integrator, and a 7673 controller. The system was controlled by a Hewlett-Packard Vectra QS/20 computer with a VGA monitor. The resulting profiles were compared to two libraries, one based on cultures grown on R2A agar (American Water Works Service Company generated; described below), and the other based on cultures grown on Trypticase soy broth agar (version 3.9; Microbial ID, Inc.).
To monitor the extraction process, a positive control (Stenotrophomonas maltophilia ATCC 13637) and a reagent blank (containing no cells) were processed at the beginning and end of each sample set. At no time during the project was the positive control or the reagent blank found to be unacceptable. In addition, a standard calibration mix purchased from Microbial ID, Inc., was analyzed prior to each run and after every 10 samples during the run.
Gram reaction determinations.
The occurrence of specific peaks in the fatty acid profile of an organism has been found to correlate with the Gram reaction, allowing Gram reaction determinations to be made for many organisms based upon the fatty acid composition of that organism (6). In cases where fatty acid profiles did not clearly indicate Gram reactions, determinations were confirmed by using the nonstaining KOH technique (30).
Statistical analyses.
All statistical analyses were performed with Instat-Instant Biostatistics (version 2.0; Graphpad Software, San Diego, Calif.). Analyses included paired t test, unpaired t test, and one-way analysis of variance. All statistical calculations were based on a 95% confidence interval.
RESULTS
Performance of the pilot system.
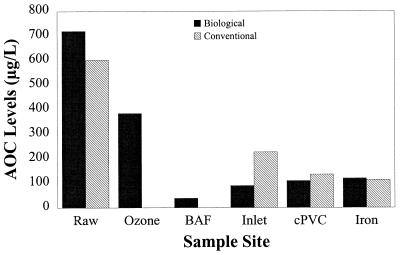
Pilot system raw water was spiked with nutrients from a sewage extract to simulate a polluted water source with a high regrowth potential. Source water AOC levels ranged between 200 and 2,000 μg/liter and averaged between 600 and 720 μg/liter (Tables 1 and 2). In the biologically active treatment chain, AOC levels were decreased by ozonation and biologically active filtration, but they increased following postdisinfection to an average level of 90 μg/liter at the inlet of the pipe system (Fig. 2). AOC levels averaged between 110 and 120 μg/liter at the sampling points at the ends of the cPVC and iron pipe sections. In addition to removing AOC, the biologically active filters also reduced total organic carbon levels on average from 1.7 to 1.3 mg/liter through the treatment process (Table 2).
FIG. 2.
Changes in AOC levels in the biologically active and conventional treatment trains. Geometric means are based on six samples for the raw water and five samples for all other sites. BAF, biologically active filter; inlet, distribution system inlet; cPVC, effluent from the cPVC section of the system; iron, effluent from the iron section of the system.
The conventional treatment train employed free chlorine to limit microbial activity within the pilot system. Despite the presence of a free-chlorine residual, AOC levels declined to 227 μg/liter in the effluent of the sand filter (Fig. 2). AOC levels continued to decline within the pipe system to a geometric mean of 114 μg/liter at the effluent of the iron pipe segment.
The higher level of chlorine demand resulting from high organic carbon and ammonia content in the conventional system made maintenance of a free chlorine residual difficult. Free chlorine residuals in the prechlorination tank averaged 1.2 mg/liter but ranged from 0.6 to 2.2 mg/liter (Table 1). In contrast, the use of preozone and GAC filtration allowed maintenance of consistent chlorine residuals (average of 1.68 ± 0.29 mg/liter) in the biologically treated system (Table 2).
Water column studies. (i) Bacterial levels.
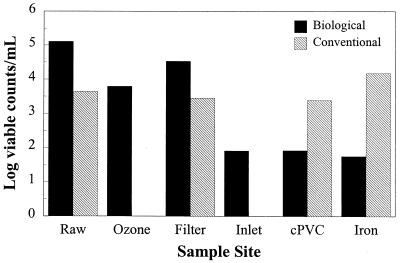
The raw, untreated water used for the pilot studies contained approximately 105 CFU of HPC bacteria per ml. Following free chlorine predisinfection in the conventional treatment chain, bacterial levels were reduced to a geometric mean of 4.4 × 103 CFU/ml (range, 1.7 × 103 to 9.0 × 103 CFU/ml) (Fig. 3). Bacterial levels remained at this approximate level through the sand filter and in the part of the model distribution system that contained cPVC pipe. However, bacterial levels increased substantially (geometric mean, 1.51 × 104 CFU/ml; range, 4.2 × 102 to 5.3 × 105 CFU/ml) at the effluent of the iron pipe section of the pilot system (Fig. 3).
FIG. 3.
HPC bacterial levels throughout biological and conventional treatment and distribution with free chlorine. Filter, filter effluent; inlet, distribution system inlet; cPVC, effluent from the cPVC section of the system; iron, effluent from the iron section of the system.
Ozone reduced HPC bacterial levels by 1.5 log units, but bacterial levels increased 0.76 log unit in the effluent of the biologically active GAC filters (Fig. 4). Statistical analysis (analysis of variance) revealed that the changes in bacterial levels were significantly different (P < 0.0001). Treatment of the biologically active filter effluent water with free chlorine (1.7 mg/liter) (Table 2) reduced bacterial levels in the water column to a geometric mean of 8.1 × 101 CFU/ml (range, 6.7 × 100 to 2.1 × 103 CFU/ml), and plate counts gradually declined to a geometric mean of 5.6 × 101 CFU/ml (range, 1.5 × 101 to 1.7 × 103 CFU/ml) at the end of the model distribution system (Fig. 3).
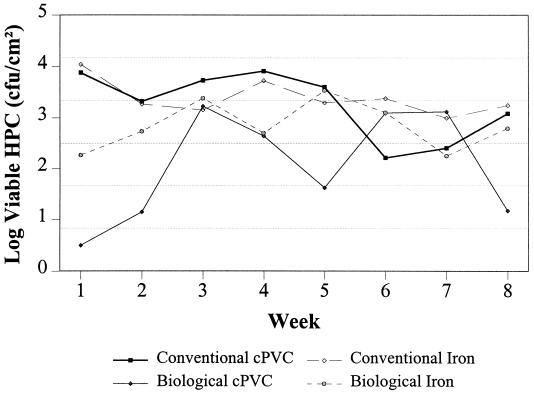
FIG. 4.
Biofilm levels on cPVC pipe surfaces with conventional or biological treatment and free chlorine or monochloramine.
(ii) Microbial populations.
To characterize microbial populations suspended in the water column throughout the pilot system, 100 isolates were collected from each sampling site following system stabilization. Analysis of the bacterial populations based on the Gram reaction showed that prechlorination resulted in a rapid shift from predominately gram-negative bacteria (97%) in the raw water to mostly gram positive-organisms (98%) in the chlorinated water. This predominately gram-positive population remained stable throughout the remainder of the treatment process.
Preozonation, however, did not dramatically alter the composition of culturable raw water bacteria. Nearly 80% of the bacteria isolated from water treated with ozone at 3.2 mg · min/liter were gram negative, and the population of bacteria in the effluent of the biologically active GAC filter remained predominately (86%) gram negative. Exposure of the biologically treated water to free chlorine resulted in the same shift to predominately gram-positive organisms as seen with conventional treatment (100% gram positive).
Grouping of bacterial genera identified by using the Microbial Identification System showed a diverse population of gram-negative bacteria in the raw-water sample (Table 3). Acinetobacter spp., Pseudomonas spp., and Klebsiella spp. predominated among the 20 genera identified in the raw water. Ozonation reduced the microbial diversity to 13 genera, dominated by Pseudomonas spp., and Rhodococcus spp. (Table 3).
TABLE 3.
Microbial populations isolated from the water column during biological treatmenta
| Bacterial identification | % Population in:
|
|||||
|---|---|---|---|---|---|---|
| Raw water | Ozone contactor | Filter effluent | Distribution system influent | cPVC pipe effluent | Iron pipe effluent | |
| Gram negative | ||||||
| Acidovorax spp. | 2 | 4 | 7 | |||
| Acinetobacter spp. | 29 | 6 | ||||
| Agrobacterium spp. | 18 | |||||
| Alcaligenes spp. | 12 | 2 | 1 | 12 | ||
| Alteromonas spp. | 2 | |||||
| Comamonas spp. | 1 | 3 | ||||
| Enterobacter spp. | 2 | 5 | ||||
| Flavobacterium spp. | 2 | 5 | ||||
| Hydrogenophaga spp. | 8 | 3 | 1 | |||
| Klebsiella spp. | 10 | 1 | 3 | |||
| Methylobacterium spp. | 1 | 2 | ||||
| Pseudomonas spp. | 14 | 53 | 22 | 12 | ||
| Rhodobacter spp. | 2 | 1 | ||||
| Sphingomonas spp. | 2 | 2 | 19 | |||
| Stenotrophomonas spp. | 2 | 1 | 2 | 6 | ||
| Xanthobacter spp. | 3 | |||||
| Otherb | 2 | 1 | 5 | |||
| Gram positive | ||||||
| Bacillus spp. | 7 | 6 | 6 | |||
| Micrococcus spp. | 6 | |||||
| Nocardia spp. | 1 | 3 | 7 | 53 | 46 | 82 |
| Rhodococcus spp. | 16 | 4 | ||||
| Staphylococcus spp. | 1 | 1 | ||||
| Other | 1 | 1 | 1 | |||
| Unidentified | 3 | 9 | 16 | 33 | 0 | 6 |
One hundred isolates from each site were identified.
Includes organisms isolated from only one site at a frequency of 1%.
Following biologically active GAC filtration, 19 genera were identified in the filter effluent, the majority of which (63%) matched isolates observed in the raw water (Table 3). The predominant genera were Pseudomonas spp. and Sphingomonas spp. These organisms are widely distributed in the environment and can grow on a variety of carbon substrates. Chlorination of biologically treated water resulted in a shift to Nocardia spp. following distribution through the pipe system (Table 3). Identification of isolates in the conventional treatment chain revealed 72% fewer genera than in the biologically treated water, with Nocardia spp. dominating in the free-chlorinated water.
Biofilm studies. (i) Rate of biofilm development.
Despite the application of continuous disinfection, biofilms rapidly developed within the model distribution system. For conventionally treated water, biofilm levels on cPVC pipe surfaces averaged 104 CFU/cm2 within the first week (Fig. 4). Biofilms attached to cPVC pipe surfaces in the biologically treated system developed at a lower rate, requiring 3 to 4 weeks to reach the same levels as in the conventionally treated system. Biofilm levels stabilized in both systems following the initial 3- to 4-week growth period.
Figure 4 shows the rate of biofilm development on iron pipes for conventionally and biologically treated water. Although biofilm development for biologically treated water was slower than that for conventionally treated water, the bacterial densities were similar within 3 weeks. The data shown in Fig. 4 reveal that the type of pipe material also influenced the rate of biofilm development. After 1 week, bacterial densities on iron pipe surfaces were substantially (>100-fold) higher than levels on cPVC surfaces for biologically treated water. Average viable counts for biofilms attached to iron pipe surfaces were significantly higher (1.1 × 104 CFU/cm2) following conventional treatment than following biological treatment (3.2 × 103 CFU/cm2) (P < 0.0001). The choice of pipe material, however, had a lesser impact for conventionally treated water, with biofilm levels on cPVC pipe surfaces (3.2 × 103 CFU/cm2) not being statistically different (P = 0.1341) from biofilm levels on cPVC pipe surfaces following biologically active filtration (1.7 × 103 CFU/cm2).
(ii) Impact of pipe surface and disinfectant.
The data shown in Fig. 4 also illustrate that biofilms can rapidly form even in the presence of a 1- to 2-mg/liter free chlorine residual. Corrosion products have been previously shown to provide increased protection from free chlorine disinfection (19). cPVC pipe surfaces were incorporated into the pilot system, in part, to study disinfection efficiencies in the absence of corrosion. While corrosion products were not eliminated from the iron section of the pilot system, the addition of a polyphosphate corrosion inhibitor limited corrosion rates (0.3 to 0.7 mils/yr) and the amount of interference experienced during free chlorine disinfection of iron pipe surfaces. Samples collected at between 3 and 8 weeks of colonization showed that the average viable counts for free-chlorinated iron and cPVC pipe surfaces (5.1 × 103 CFU/cm2 and 1.1 × 103 CFU/cm2, respectively), differed significantly (P = 0.0002), indicating that the disinfection efficiency of free chlorine was impacted by iron pipe surface.
(iii) Microbial populations. (a) Generic diversity.
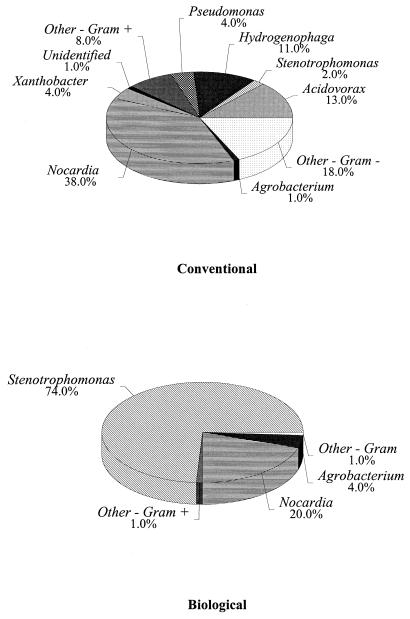
The effects of various nutrient levels, different pipe surfaces, and postdisinfection with free chlorine not only may change the growth rate and density of biofilm bacteria but also may alter the composition of the biofilm populations. To examine the impact of these parameters on the types of bacteria present in the biofilms, 100 isolates were collected from each site following an 8-week growth period. Figure 5 summarizes populations isolated from cPVC pipe surfaces exposed to chlorination and conventionally and biologically treated water. The data indicate that biofilms on cPVC pipe surfaces from the conventionally treated system were more diverse than biofilms isolated under the same conditions from the biologically treated system. Conventionally treated cPVC pipe surfaces treated with free chlorine were colonized with 11 different genera of bacteria, primarily Acidovorax spp., Acinetobacter spp., Pseudomonas spp., Sphingomonas spp., Stenotrophomonas spp., and Nocardia spp. Following biological treatment, only five genera were found on free-chlorinated cPVC pipes and populations were primarily composed of Stenotrophomonas spp., and Nocardia spp. The dramatic shift in the composition of the cPVC populations is shown in Fig. 5. The combination of lower nutrient levels and free chlorination resulted in selective pressures that permitted only a few genera of bacteria to survive. This shift in populations is significant, considering that the bacterial densities were not significantly different between conventionally and biologically treated cPVC surfaces (Fig. 4). Examination of microbial populations grown on iron pipe surfaces showed that the biofilms were less diverse following biological treatment than with conventional treatment (Fig. 6), although the shift was not as pronounced as was seen with cPVC biofilms. Free-chlorinated biofilm populations following conventional treatment contained 12 genera, consisting primarily of Acidovorax spp., Methylobacterium spp., Pseudomonas spp., Stenotrophomonas spp., and Nocardia spp. (Fig. 6). Following biological treatment, seven genera were isolated and included Acidovorax spp., Pseudomonas spp., Xanthobacter spp., and Nocardia spp. as the predominant organisms.
FIG. 5.
Microbial populations isolated from free-chlorinated cPVC pipe surfaces following conventional or biological treatment.
FIG. 6.
Microbial populations isolated from free-chlorinated iron pipe surfaces following conventional or biological treatment.
(b) Gram reactions.
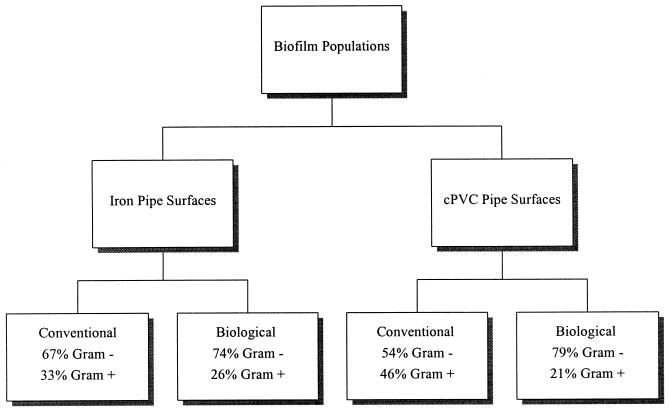
Biofilm isolates were also grouped according to their Gram reaction, which was determined by fatty acid profiles or by using the KOH string test (30). Gram reactions for biofilm populations attached to iron and cPVC pipe surfaces from all four distribution systems showed similar ratios of gram-positive organisms (average, 30%) to gram-negative organisms (average, 70%) (Fig. 7).
FIG. 7.
Summary of biofilm populations isolated from iron and cPVC pipe surfaces, grouped according to Gram reaction determinations.
(c) Isolation of Mycobacterium spp.
The genus Mycobacterium includes opportunistic pathogens that may pose special risks to immunocompromised individuals (14, 28). These acid-fast microbes are known to grow in drinking water systems and are very resistant to chlorine disinfection (6, 10, 26). It was of interest, therefore, to determine if Mycobacterium spp. were present in any of the pilot system biofilms. Mycobacterium spp. were found in 75% (three of four samples) of the biofilm samples from conventionally treated water, but no Mycobacterium spp. were isolated from the biologically treated system with free chlorine used as a postdisinfectant. Three species of Mycobacterium (M. intracellulare, M. fortuitum, and M. phlei) were isolated from chlorinated biofilms.
DISCUSSION
The definition of biological filtration implies that microbial activity is encouraged within the treatment filter so that the maximum amount of biodegradable organic material is removed. The result of this microbial activity is elevated bacterial levels in the filter effluent. The effective disinfection of bacteria released from biological filters has been widely reported by researchers and operating utilities as well (5, 11, 15, 31). The fact that bacterial counts in the effluent of biologically active filters may not vary substantially from levels seen in some conventional GAC filter-adsorbers (3, 15) demonstrates that conventional disinfection practices that already produce acceptable microbial quality for conventional GAC filters can reliably control bacterial levels from biologically active filters as well.
The pilot system and model pipe network was designed to compare the impact of biologically active treatment to results for conventionally treated water with a variety of distribution system configurations. AOC levels for biologically treated pilot water (90 μg/liter) were similar to the median AOC level of 92 μg/liter reported by LeChevallier et al. (21) for 24 filtered water systems in North America, indicating that nutrient levels in the test system were comparable to those in full-scale distribution systems.
Lower levels of suspended HPC bacteria were isolated from the biologically treated system, which could result from a combination of the reduced AOC level and higher chlorine residuals (Tables 1 and 2). Due to fluctuating ammonia levels in the feed water, maintenance of identical free chlorine residuals in the conventional and biological systems was difficult. Ozonation and biological filtration remove the ammonia in the treated water prior to chlorination, making residual maintenance less difficult. Therefore, lower bacterial counts in chlorinated biologically treated water must contribute to the combined impact of biologically activated filtration and higher chlorine residuals.
The pilot work showed that chlorine disinfection not only reduced the bacterial viable count but also selected for gram-positive bacteria. LeChevallier et al. (18) previously reported that gram-positive bacteria survived free chlorination better than gram-negative HPC bacteria. It was hypothesized that the thicker cell wall of gram-positive bacteria aided in survival with chlorination.
Population shifts following chlorination indicate that chlorine disinfection plays a substantial role in determining the composition of suspended microbial populations. Ozone, however, may not have as large an impact on bacteria as it has for waterborne protozoa. The results of this study are supported by other research that has shown that preozonation and GAC treatment did not change the composition of bacterial populations (7).
The observed shift to gram-positive organisms resulted in domination of free-chlorinated waters with Nocardia spp. These organisms are widely distributed in the environment (soil, freshwater, and marine environments, etc.) and possess characteristic fatty acids that are closely related to those of Rhodococcus, Mycobacterium, and Corynebacterium (33). The partially acid-fast cell wall and the possession of catalase enzyme, which is able to break down hydrogen peroxide, are important factors that enable the organism to survive ozonation. Nocardia spp. are nutritionally versatile but typically grow slowly (33). Although some strains may be pathogenic, the majority of isolates have little or no known health importance.
The results suggest that iron pipes play an important role in stimulating the development of biofilms and enhancing biofilm densities, especially in low-nutrient waters. Previous investigations have shown that iron tubercles can concentrate organic nutrients (1, 34). However, in the studies presented here, effective corrosion control limited the amount of iron tuberculation. Camper (8) also observed faster biofilm development and high biofilm densities on iron pipes. This effect may be due to a combination of nutrient accumulation, surface roughness, and disinfectant demand that favors iron surfaces as sites for bacterial growth.
Integrating the biofilm density data (Fig. 4) with the microbial identifications (Fig. 6) shows that for iron pipes, the density of cells in the biofilms in the biologically treated, free-chlorinated system was significantly higher, although the compositions of the populations were somewhat similar to those in the free-chlorinated conventional system. These data indicate that the impacts of nutrient level, disinfectant type, and pipe material interact in a complex manner to influence both the rate and amount of biofilm development and the composition of the microbial populations.
Research has shown that shear stress can cause thicker biofilms to slough off, resulting in higher bacterial counts in the water column (9, 12, 23). This study verified those results in that increases in bacterial levels in iron pipes following conventional treatment were noted. Elevated biofilm levels on these pipe surfaces resulted in sloughing of bacterial clumps into the water column.
While bacterial levels in the water column may be affected by biofilm levels, comparison of the microbial population attached to pipe surfaces with the bacteria present in the water column shows clear differences between the microbes in the different environments. For example, more than 90% of the isolates from the chlorinated water column were gram positive, compared to the predominately gram-negative biofilms. One explanation for these differences may be the varying attachment capabilities of gram-positive and gram-negative bacteria. Murgel et al. (27) found that Arthrobacter spp. (gram-positive organisms) were capable of superior growth in the fluid phase but had limited attachment and biofilm growth capabilities.
The population variations between suspended and biofilm isolates may also be due to the selective pressures on suspended bacteria posed by disinfection. Attachment to pipe surfaces provides adequate protection for the survival of gram-negative cells which are not capable of surviving as suspended cells. Once the pipe surfaces are colonized, it appears that protection levels and growth rates are sufficient to maintain a substantial level of gram-negative cells in the biofilm, despite exposure to chlorinated waters containing predominately gram-positive organisms. Because many of the gram-positive bacteria grow slower than the gram-negative cells, following an initial colonization period the microbial composition of the water column may have little effect on biofilm composition. Pedersen (29) found that initial surface attachment was dominated by other biofilm processes, such as growth, product formation, and debris entrapment, indicating that the microbial composition initially colonizing the surface has only a limited impact on the mature biofilm. Banks and Bryers (4) found that biofilms were quickly dominated by faster-growing organisms. Slow-growing organisms were not eliminated from the biofilm but increased in numbers over time.
Bacteria released from biologically active filters would first be exposed to chlorine disinfection, which would shift microbial populations to predominately gram-positive bacteria. Even if low levels of these organisms were released from the filters, they would be unlikely to colonize the distribution system due to high levels of competition imposed by previously established organisms. This scenario is supported by studies in which complex natural environments were colonized only when inoculated with high numbers of organisms (<103 cells/ml) (35). Based on the information above, the organic carbon levels in the water, the amount of residual disinfectant, and the composition of the pipe surfaces are much more important in determining the density and composition of biofilm bacteria than are the types of organisms released from biologically active filters.
The public health significance of many of the bacteria isolated from the water column or biofilm is unknown. Because these bacteria are adapted to grow at low nutrient levels and at low temperatures, they probably have little public health significance (24). Preliminary research presented in this study suggested that Mycobacterium spp., some of which are opportunistic pathogens resistant to disinfection, could be controlled by limiting the level of biodegradable organic carbon. If these data are verified, lowering the levels or changing composition of AOC and biodegradable dissolved organic carbon by biological treatment could be an important mechanism for reducing the public health risk from opportunistic pathogens. It is possible, therefore, that the biostability of water may be an expectation for potable water in the future.
ACKNOWLEDGMENTS
We thank the American Water System for funding and support. This project was also funded by the American Water Works Association Research Foundation.
We thank Stephanie Maralis, project manager for the American Water Works Association Research Foundation, and project advisory committee members Edward J. Bouwer (Johns Hopkins University), Donald J. Reasoner (U.S. Environmental Protection Agency), and Charlotte D. Smith (Charlotte Smith & Associates, Inc.) for their guidance and suggestions. The comments of Morteza Abbaszadegan and Richard H. Moser were also appreciated. We gratefully acknowledge Joseph Falkinham for the analyses of Mycobacterium spp. conducted for this project and Darla Hsiao for graphical assistance.
REFERENCES
- 1.Allen M J, Geldreich E E. Proceedings of the American Water Works Association Water Quality Technology Conference. Denver, Colo: American Water Works Association; 1977. Distribution line sediments and bacterial regrowth; pp. 1–6. [Google Scholar]
- 2.Arbalt R D, Slutsky A, Barber T W, Maslow J N, Niemczyk S, Falkinham III J O, O'Connor G T, Fordham von Reyn C. Genetic diversity among strains of Mycobacterium avium causing monoclonal and polyclonal bacteremia in patients with AIDS. J Infect Dis. 1993;167:1384–90. doi: 10.1093/infdis/167.6.1384. [DOI] [PubMed] [Google Scholar]
- 3.Bablon G, Ventresque C, Aim R B. Developing a sand-GAC filter to achieve high-rate biological filtration. J Am Water Works Assoc. 1988;80(12):47–53. [Google Scholar]
- 4.Banks M K, Bryers J D. Bacterial species dominance within a binary culture biofilm. Appl Environ Microbiol. 1991;57:1974–1979. doi: 10.1128/aem.57.7.1974-1979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbigot M M, Dodin A, Lheritier R. Proceedings of the American Water Works Association Annual Conference. Denver, Colo: American Water Works Association; 1982. Limiting bacterial aftergrowth in distribution systems by removing biodegradable organics; pp. 871–886. [Google Scholar]
- 6.Briganti L A, Wacker S C. Fatty acid profiling and the identification of environmental bacteria for drinking water utilities. Denver, Colo: American Water Works Association Research Foundation and American Water Works Association; 1995. [Google Scholar]
- 7.Burlingame G A, Suffet I H, Pipes W O. Predominant bacterial genera in granular activated carbon water treatment systems. Can J Microbiol. 1986;32:226–230. doi: 10.1139/m86-045. [DOI] [PubMed] [Google Scholar]
- 8.Camper A K. Factors limiting microbial growth in distribution systems: laboratory and pilot-scale experiments. Denver, Colo: American Water Works Association Research Foundation and American Water Works Association; 1996. [Google Scholar]
- 9.Characklis W G. Fouling biofilm development: a process analysis. Biotechnol Bioeng. 1981;23:1923–1960. doi: 10.1002/bit.22227. [DOI] [PubMed] [Google Scholar]
- 10.Collins C H, Grange J M, Yates M D. Mycobacteria in water. J Appl Bacteriol. 1984;57:193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 11.Faust S D, Aly O M. Adsorption process for water treatment. Stoneham, Mass: Butterworth Publishers; 1987. pp. 443–470. [Google Scholar]
- 12.Fletcher M, Marshall K C. Are solid surfaces of ecological significance to aquatic bacteria? In: Marshall K C, editor. Advances in microbial ecology. Vol. 6. New York, N.Y: Plenum Press; 1982. pp. 199–236. [Google Scholar]
- 13.George K L, Falkinham J O., III Selective medium for the isolation and enumeration of Mycobacterium avium-intracellulare and M. scrofulaceum. Can J Microbiol. 1986;32:10–14. doi: 10.1139/m86-003. [DOI] [PubMed] [Google Scholar]
- 14.Horsburgh C R. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 15.LeChevallier, M. W., W. C. Becker, P. Schorr, and R. G. Lee. 1992. Evaluating the performance of biologically active rapid filters. J. Am. Water Works Assoc. 84:(4):136–146.
- 16.LeChevallier M W. Coliform regrowth in drinking water: a review. J Am Water Works Assoc. 1990;82(11):74–86. [Google Scholar]
- 17.LeChevallier M W, Shaw N J, Kaplan L A, Bott T L. Development of a rapid assimilable organic carbon method for water. Appl Environ Microbiol. 1993;59:1526–1531. doi: 10.1128/aem.59.5.1526-1531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeChevallier M W, Seidler R J, Evans T M. Enumeration and characterization of standard plate count bacteria in chlorinated and raw water supplies. Appl Environ Microbiol. 1980;40:922–930. doi: 10.1128/aem.40.5.922-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeChevallier M W, Lowry C D, Lee R G, Gibbon D L. Examining the relationship between iron corrosion and the disinfection of biofilm bacteria. J Am Water Works Assoc. 1993;85(7):111–123. [Google Scholar]
- 20.LeChevallier M W, Lowry C D, Lee R G. Disinfecting biofilms in a model distribution system. J Am Water Works Assoc. 1990;82(7):87–99. [Google Scholar]
- 21.LeChevallier M W, Welch N J, Smith D B. Full-scale studies of factors related to coliform regrowth in drinking water. Appl Environ Microbiol. 1996;62:2201–2211. doi: 10.1128/aem.62.7.2201-2211.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeChevallier M W, Welch N J, Smith D B. Factors limiting microbial growth in distribution systems: full-scale experiments. Denver, Colo: American Water Works Association Research Foundation and American Water Works Association; 1996. [Google Scholar]
- 23.Lu C, Biswas P, Clark R M. Simultaneous transport of substrates, disinfectants and microorganisms in water pipes. Water Res. 1995;29:881–894. [Google Scholar]
- 24.Lye D J, Dufour A P. Virulence characteristics of heterotrophic bacteria commonly isolated from potable water. Environ Toxicol Water Quality. 1993;8:13–23. [Google Scholar]
- 25.Mazurek G H, Hartman S, Zhang Y, Brown B A, Hector J S, Murphy D, Wallace R J., Jr Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993;31:390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Means E G, Scott K N, Lee M L, Wolfe R L. Proceedings of the American Water Works Association Annual Conference. Denver, Colo: American Water Works Association; 1986. Bacteriological impact of a changeover from chlorine to chloramine disinfection in a water distribution system. [Google Scholar]
- 27.Murgel G A, Lion L W, Acheson C, Shuler M L, Emerson D, Ghiorse W C. Experimental apparatus for selection of adherent microorganisms under stringent growth conditions. Appl Environ Microbiol. 1991;57:1987–1996. doi: 10.1128/aem.57.7.1987-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Wynne B A. Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen K. Biofilm development on stainless steel and PVC surfaces in drinking water. Water Res. 1990;24:239–243. [Google Scholar]
- 30.Powers E M. Efficacy of the Ryu nonstaining KOH technique for rapidly determining Gram reactions of food-borne and waterborne bacteria and yeasts. Appl Environ Microbiol. 1995;61:3756–3758. doi: 10.1128/aem.61.10.3756-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittmann B E, Huck P M. Biological treatment of public water supplies. Crit Rev Environ Control. 1989;19:119–184. [Google Scholar]
- 32.Snoeyink V L. Adsorption of organic compounds. In: Pontius F W, editor. Water quality and treatment. New York, N.Y: McGraw-Hill, Inc.; 1990. pp. 781–875. [Google Scholar]
- 33.Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 3. Baltimore, Md: The Williams & Wilkins Co.; 1989. [Google Scholar]
- 34.Victoreen H T. Proceedings of the American Water Works Association Water Quality Technology Conference. Denver, Colo: American Water Works Association; 1984. The role of rust in coliform regrowth; pp. 253–264. [Google Scholar]
- 35.Warren T M, Williams V, Fletcher M. Influence of solid surface, adhesive ability, and inoculum size on bacterial colonization in microcosm studies. Appl Environ Microbiol. 1992;58:2954–2959. doi: 10.1128/aem.58.9.2954-2959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]