Abstract
Centronuclear myopathy (CNM) is a congenital myopathy characterised by centralised nuclei in skeletal myofibers. T-tubules, sarcolemmal invaginations required for excitation-contraction coupling, are disorganised in the skeletal muscles of CNM patients. Previous studies showed that various endocytic proteins are involved in T-tubule biogenesis and their dysfunction is tightly associated with CNM pathogenesis. DNM2 and BIN1 are two causative genes for CNM that encode essential membrane remodelling proteins in endocytosis, dynamin 2 and BIN1, respectively. In this review, we overview the functions of dynamin 2 and BIN1 in T-tubule biogenesis and discuss how their dysfunction in membrane remodelling leads to CNM pathogenesis.
Keywords: centronuclear myopathy, T-tubules, dynamin, BIN1, membrane remodelling
1. Introduction
Centronuclear myopathy (CNM) is a hereditary muscular disorder that is diagnosed by the clinical features of congenital myopathy and pathological characteristic of centralised nuclei in the skeletal muscle biopsy [1]. Clinical features of CNM patients are broad spectra of onset age and symptoms, and the disease course of an individual patient is often unpredictable. Seven causative genes for CNM, MTM1, SPEG, BIN1, DNM2, RYR1, TTN and CCDC78, have been identified [2,3,4,5]. Among these CNM causative genes, MTM1, BIN1 or DNM2 variants cause disorganisation of T-tubules (transverse tubules) and triads in the skeletal muscle, suggesting their function in a common pathway during T-tubule biogenesis (Figure 1) [6]. DNM2 and BIN1 encode essential membrane remodelling proteins, dynamin 2 and BIN1 (also called amphiphysin II), respectively, and they are required for T-tubule biogenesis in skeletal muscle development [6,7]. BIN1- and DNM2-associated CNM patients show normal or slightly elevated levels of serum creatine kinase and slowly progressive muscle weakness [8,9,10,11]. This review will overview the functions of dynamin 2 and BIN1 in T-tubule biogenesis and discuss possible pathogenic mechanisms of CNM caused by their membrane remodelling defects, aiming for compensating other excellent reviews [2,12,13,14,15,16].
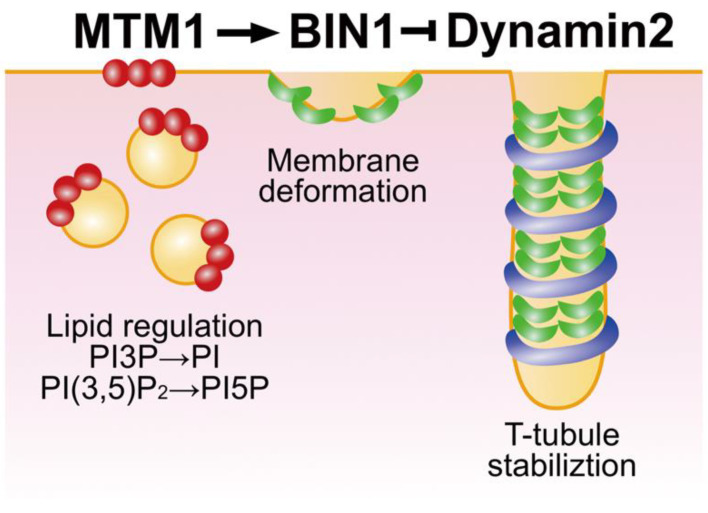
Figure 1.
Functions of MTM1, BIN1 and DNM2 in T-tubule biogenesis. CNM causative genes MTM1, BIN1 and DNM2 contribute to T-tubule biogenesis in a common pathway by respectively regulating lipid homeostasis, membrane deformation and T-tubule stabilisation.
2. T-Tubules: Sarcolemmal Invaginations Essential for E-C Coupling
Rapid and coordinated contraction of striated muscles is achieved by coupled voltage- and calcium-dependent processes called excitation-contraction (E-C) coupling [17]. T-tubules are sarcolemmal invaginations required for the E-C coupling in both skeletal- and cardiac muscles [6]. In skeletal muscle cells, T-tubules are associated with sarcoplasmic reticulum (SR) on either side to form closely apposed membrane contacts termed “triad”, whereas, in cardiac muscle cells, their contact occurs only on one side to form “diad”. T-tubules are enriched with specific lipids such as PI(4,5)P2 (phosphatidylinositol 4,5-bisphosphate) and cholesterol and they serve as a platform for localizing T-tubule specific ion channels or lipid-binding proteins [18,19,20,21]. In the E-C coupling, excitation (depolarisation) of the T-tubule membrane induces conformational changes of a voltage-gated L-type calcium channel DHPR (dihydropyridine receptors), which in turn opens RyR1 (ryanodine receptor 1), a Ca2+ channel on SR, to allow Ca2+ release from SR to induce muscle contraction [22]. In the skeletal muscle cells, DHPR directly interacts with RyR1 to enable rapid signal transmission (within 2 ms) [23,24,25]. Thus, the structural and functional integrity of T-tubules is crucial for proper E-C coupling of the skeletal muscles. Not surprisingly, abnormalities in T-tubule structures cause various muscle diseases including congenital myopathies [26].
3. BIN1: A BAR Domain Protein-Inducing Membrane Curvature
3.1. BIN1 Functions in T-Tubule Biogenesis
BIN1 (Bridging Integrator 1) belongs to the conserved BAR domain superfamily that senses and induces membrane curvature [27,28,29]. BIN1 contains an N-terminal amphipathic helix Bin/Amphiphysin/Rvs-homology (N-BAR) domain that forms a “crescent-shaped” dimer, and its positively charged concave surface binds to negatively charged phospholipids to induce membrane curvature [30]. Human and mouse BIN1 are alternatively spliced to express tissue-specific isoforms [27,31,32,33]. The skeletal muscle-specific BIN1 isoform, isoform 8, has been shown to localise on T-tubules [33]. Conditional Bin1 knockout mice in skeletal muscle exhibit neonatal lethality [34] and acute knock-down of BIN1 in adult mice caused structural and functional defects of T-tubules [35], indicating that BIN1 plays essential roles in the development and maintenance of the skeletal muscle. The BIN1 ortholog in Drosophila, Amph, is also required for muscle contraction, but not for synaptic vesicle trafficking, suggesting that it has a similar function as human BIN1 [36].
BIN1 isoform 8 consists of four functional domains: H0, N-BAR, PI and Src homology 3 (SH3) domains from N- to C-terminus [27] (Figure 2). H0 is an amphipathic helix that is folded and inserted into one leaflet of the membrane to initiate oligomerisation of N-BAR domains and membrane tubulation [37,38]. N-BAR domain of BIN1 induces clustering of PI(4,5)P2 and in turn, recruits a downstream partner dynamin 2 to enhance membrane tubulation in T-tubule biogenesis [39,40,41]. Thus, BIN1 contributes to T-tubule biogenesis by regulating lipid composition and protein interaction in a positive feedback manner. N-BAR domain of BIN1 also interacts with F-actin to regulate its organisation via stabilisation or bundle formation of actin filaments [42]. Actin regulatory function of BIN1 is required for proper T-tubule biogenesis in cardiac muscle cells [43]. In contrast, the formation of BIN1-mediated T-tubule like structures (TLS) in mouse myoblast C2C12 cells is antagonised by actin polymerisation [44]. The PI domain that exists only in BIN1 isoform 8 interacts with PI(4,5)P2 [44]. Neuronal BIN1 isoform 1 that lacks the PI domain diffusely localises in the cytoplasm of CHO cells, suggesting essential roles of the PI domain in membrane invaginations required for T-tubule biogenesis [19]. Indeed, skipping of the PI domain in BIN1 by dysregulated alternative splicing causes aberrant T-tubule formation in CNM and myotonic dystrophy [45,46]. Lack of the PI domain does not affect muscle development per se, but it causes defects in the formation of T-tubule network and muscle regeneration due to a reduced pool of satellite cells [33]. The C-terminal SH3 domain of BIN1 interacts with PR domain-containing proteins such as dynamin 2 [9,47]. The SH3 domain of BIN1 also binds to its PI domain intramolecularly to form a closed auto-inhibitory conformation [41]. The autoinhibition of BIN1 is released upon PI(4,5)P2 binding to the PI domain that in turn recruits its partner proteins dynamin 2 and myotubularin to the PI(4,5)P2-rich membrane domains [41,48,49]. Interestingly, deletion of exon 20 that encodes the SH3 domain of BIN1 causes defects in T-tubule formation at E18.5 embryonic muscle fibres, but the triad structures in adult skeletal muscle are not affected [33]. This result suggests that the BIN1 SH3 domain is required for T-tubule formation, but not for its maintenance, at the early stages of skeletal muscle development.
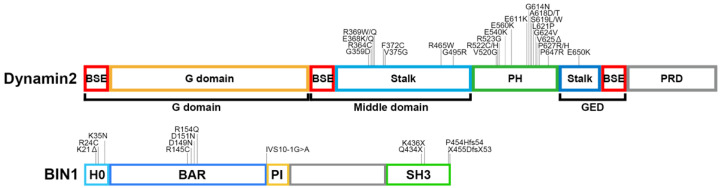
Figure 2.
Domain structures of dynamin 2 and BIN1. Schematic illustrations of domain structures and CNM-associated mutations in dynamin 2 and BIN1.
3.2. CNM Pathogenesis Caused by Defective Membrane Remodelling of BIN1 Variants
Multiple pathogenic BIN1 variants have been identified in CNM patients (Figure 2). CNM-associated variants in the H0 helix, K21del, R24C and K35N, have been reported to cause abnormalities in T-tubule structures due to decreased abilities to generate membrane curvature [44,48]. CNM-associated variants in the N-BAR domain, D151N and R154Q, are defective both in membrane binding and in curvature sensing possibly due to oligomerisation defects [33,44]. D151N is also defective in the clustering of PI(4,5)P2 both in cellulo and in vitro systems using a flat membrane sheet [39]. Another variant in the N-BAR domain, D149N, exhibits decreased membrane deformation abilities in cellulo [40]. Since membrane tubulation defects of K35N and D149N can be restored by supplementing with PI(4,5)P2, these variants are deficient in recruiting PI(4,5)P2 probably due to reduced membrane binding affinity [40]. CNM-associated variant IVS10-1G>A in exon 11 causes deletion of the PI domain, resulting in defective triad formation both in humans and dogs [45]. Thus, CNM-associated variants in H0, N-BAR and PI domains are likely to induce abnormal T-tubule structures due to their membrane deformation disabilities.
Two recessive CNM variants of BIN1, Q434X and K436X, that partially truncate the SH3 domain shows suppressed interaction with dynamin 2 [9,41,47]. In the skeletal muscle biopsies from CNM patients with these variants, abnormal T-tubule morphology with aggregated caveolae-positive membranous structures is observed [49]. Partial truncation of the SH3 domain by Q434X and K436X variants also keeps BIN1 in a constitutively open conformation with altered membrane deformation abilities [41]. The loss of autoinhibition by the CNM mutant BIN1 also causes enhanced interaction with myotubularin, which is a phosphatidylinositol-3-phosphatase for PI(3)P or PI(3,5)P2 encoded by a CNM causative gene MTM1 [50]. The SH3 domain of BIN1 also interacts with N-WASP, an activator of Arp2/3 dependent actin polymerisation [51]. BIN1 mutants with truncated SH3 show suppressed N-WASP interaction and induce collapsed T-tubule structures [51]. Thus, the structural abnormalities of T-tubules caused by CNM-associated BIN1 variants are caused by abnormal protein–protein and/or protein–lipid interactions.
4. Dynamin: A Membrane Fission Catalyser in Endocytosis
4.1. Structure and Function of Dynamin
Dynamin is a large GTPase essential for membrane fission in clathrin-dependent and independent endocytic pathways [52,53,54]. There are three dynamin isoforms in mammals: dynamin 1 and 3, two tissue-specific isoforms highly expressed in neurons, and dynamin 2, a ubiquitously expressed isoform [55,56,57]. These isoforms are similar in amino acid sequences and share the same functional domains: G, middle, pleckstrin homology (PH), GTPase effector (GE) and PR domains from N- to C-terminus (Figure 2). The G domain is responsible for GTP binding and hydrolysis [58]. The middle and GED form a “stalk” structure that serves as interacting platforms in the formation of dimer or tetramer [59]. PH domain binds to negatively charged phospholipids such as PI(4,5)P2 and plays a role in clustering the phosphoinositides [60,61]. PH domain also senses membrane curvature by being hydrophobically inserted into the lipid bilayer [62]. Furthermore, the PH domain can bind to stalk structure intramolecularly to form autoinhibitory “closed” conformation that prevents untimely self-assembly [63]. The C-terminal PR domain binds to other SH3 domain-containing proteins such as BIN1, amphiphysin 1, and endophilin [9,64,65]. PR domain is also involved in actin organisation at invadosomes, membranous protrusions required for myoblast fusion [64,66].
Structural studies using cryo-EM, X-ray crystallography and high-speed atomic force microscopy (HS-AFM) gave mechanistic insights into dynamin-mediated membrane fission. Dynamin exists as a tetramer in a physiological condition in the absence of lipids [63], while it assembles into a helical polymer at the neck of endocytic pits [65] or on membrane tubules reconstituted in vitro from liposomes [67,68]. Conformational changes of dynamin helical polymer coupled with binding and hydrolysis of GTP promote membrane constriction and fission [69,70]. Although precise mechanisms of the dynamin-mediated membrane fission are still under debate, a few decades of studies in the past strongly support the following consensus views: (1) Dynamin polymerises into a helical polymer in the absence of GTP; (2) the dynamin polymer constricts in the presence of GTP and (3) dynamin sever membrane upon GTP hydrolysis [52]. Various models for dynamin-mediated membrane fission have been proposed such as the “constrictase model” in which the dynamin helical polymer constricts and mechanically severs the membrane and the “two-stage model” in which constriction and dissociation of dynamin helical polymer are required for membrane cleavage [52]. By using HS-AFM, we and other groups observed cluster formation by dynamin helices upon GTP hydrolysis [71,72]. We also observed that membrane fission occurs between the clustered dynamin helices proposing a novel “clusterase model” [72]. GTP hydrolysis also causes the twisting motion of the dynamin helical polymer that provides torsion at the neck of the endocytic pits to promote membrane fission [73,74]. Thus, dynamin severs membrane by a combination of various mechanical stresses caused by structural changes and depolymerisation upon GTP hydrolysis.
4.2. Dynamin 2 Functions in T-Tubule Biogenesis
Dynamin 2 is ubiquitously expressed in various tissues, but its expression level is relatively high in skeletal muscles [75]. In skeletal muscles, dynamin 2 localises to T-tubules at the early stages of development and regulates T-tubule orientation [34,76]. In cellulo reconstitution assay for T-tubule-like structures (TLS) revealed that dynamin 2 is required for stabilisation of the TLS [47]. GTPase activity of dynamin 2 is inhibited by BIN1 in a stoichiometry-dependent manner to allow dynamin 2 to stabilise TLS (Figure 1) [34,47]. CNM-associated BIN1 mutants with partially truncated SH3 domain fail to bind to dynamin 2 and induce TLS formation [47]. The expression level of BIN1 is increased as skeletal muscle development progresses, while that of dynamin 2 remains unchanged [19]. Thus, it is interesting to speculate that BIN1 contributes not only to membrane tubulation per se but also supports dynamin 2-mediated membrane stabilisation by suppressing GTPase activity to organise the T-tubule system during the normal development of skeletal muscles.
4.3. Dysregulation of T-Tubule Function by CNM-Associated Dynamin 2 Variants
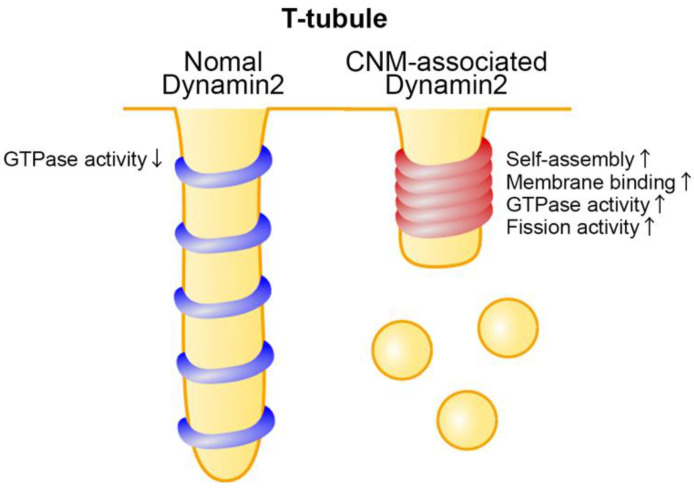
DNM2 is a causal gene for autosomal-dominant CNM and at least 29 pathogenic variants have been identified in the middle, PH, and GE domains [47,77,78,79,80] (Figure 2). Based on the crystal structure of dynamin 1, most of these mutations appear to locate at the interface between the PH domain and the stalk region [63]. As already mentioned in this review, the self-assembly and lipid-binding ability of dynamin are required for efficient membrane fission [81,82,83]. CNM-associated dynamin 2 variants causing mutations in the middle or PH domains formed abnormally stable polymer with elevated lipid binding affinity [47,84,85]. These mutants are gain-of-function because they are featured by elevated GTPase and membrane fission activities [47,86,87,88] (Figure 3). Furthermore, the CNM-associated dynamin 2 mutants induce fragmented T-tubule-like structures in cultured cells because they are resistant to the BIN1-mediated inhibition of GTPase activity [47,88]. Consistently, CNM-model animals (mouse, zebrafish, and fruit fly) expressing mutant dynamin 2 in their skeletal muscles exhibit fragmented or collapsed T-tubules [76,84,85,88,89]. These model animals show reduced calcium release and motor dysfunction that mimic CNM symptoms [84,89]. The molecular dynamics simulation predicts that CNM-associated dynamin 2 mutants form tighter helical structures compared to those with wild type dynamin 2 [90], which may underlie elevated membrane fission activities of CNM-associated dynamin 2 mutants. Further analyses on alterations in structures and dynamics of CNM-associated dynamin 2 mutants will reveal the molecular pathogenesis of CNM.
Figure 3.
Possible mechanisms of defective T-tubule formation caused by CNM-associated dynamin 2 mutant. CNM-associated dynamin 2 exhibits gain-of-function features with elevated GTPase and membrane fission activities compared to normal dynamin 2.
4.4. Correlation between Membrane Fission Activity and Symptom Severities by CNM-Associated Dynamin 2 Variants
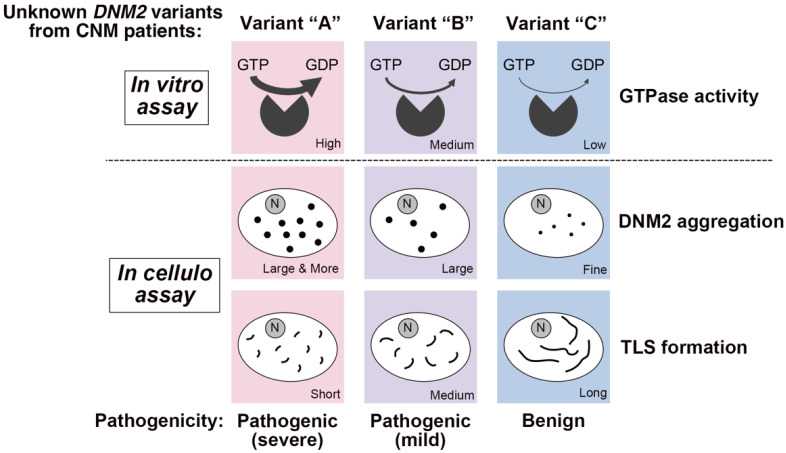
DNM2-associated CNM represents a wide spectrum of clinical features ranging from severe neonatal forms to moderate adult-onset ones with various histopathological phenotypes [78]. CNM-associated DNM2 variants are clustered in exons 8, 11, 14 and 16 and the genotype of these variants are potentially correlated with clinical severities [78]. Most reported CNM-associated DNM2 variants are linked to either early onset and severe phenotype (e.g., p.E368K, p.R369Q and p.S619L) or early onset but milder phenotype (e.g., p.R465W) [78]. In contrast, only a few patients have been reported to develop the late-onset disease. The fission activities of dynamin have been mainly measured based on its GTPase activity and most of the CNM-associated dynamin 2 mutants have been identified as gain-of-function mutants. Interestingly, our quantitative analyses on T-tubule like structures reconstituted in cellulo showed a good correlation between membrane fission activities of CNM-associated variants and pathogenicity [91]. Thus, our approach using simple in vitro and in cellulo assays together with genetic and clinicopathological analyses should contribute to a more precise diagnosis of pathogenicity, especially when muscle biopsy samples are unavailable (Figure 4). Furthermore, from the therapeutic point of view, early diagnosis by our simple assay may also improve the management and care of these patients.
Figure 4.
Determining pathogenicity of novel CNM variants by various analyses. Possible phenotypic summary of unknown variants identified from CNM patients analysed by various assays either in vitro (GTPase activity) or in cellulo (DNM2 aggregation and TLS formation) to determine their pathogenicity. N: nuclei.
4.5. Other Functions of Dynamin 2 in Skeletal Muscle
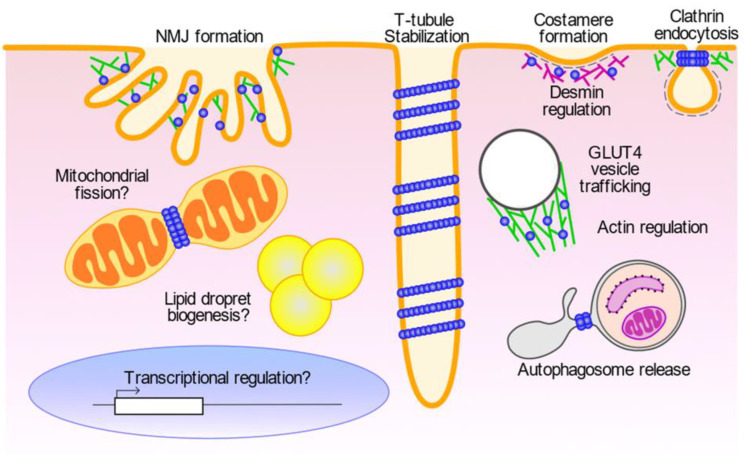
In skeletal muscle cells, dynamin 2 functions not only in T-tubule stabilisation but also regulates multiple processes such as vesicle trafficking, cytoskeletal organisation and satellite cell regeneration (Figure 5).
Figure 5.
Multiple functions of dynamin 2 in skeletal muscle cells. Dynamin 2 (blue) is involved in multiple processes in muscle cells such as T-tubule biogenesis, NMJ formation, costamere formation, endocytosis and vesicle trafficking, autophagy and lipid homeostasis.
Dynamin 2 regulates clathrin-dependent and -independent endocytosis of glucose transporter-4 (GLUT4) [92], which is required for glucose homeostasis via insulin signalling [93]. In the clathrin-dependent endocytosis, GLUT4 binds to adaptor protein AP2 that recruits clathrin at the plasma membrane, and the clathrin-coated bulk is pinched-off by dynamin 2 [94]. A study using L6 myoblasts demonstrated that dynamin 2 is required for cholesterol-dependent GLUT4 endocytosis [92].
Dynamin 2 is also required for the release of autophagosomes from recycling endosomes and autolysosomes [95,96]. Endocytosed vesicles are normally cleaved by dynamin 2 from early endosomes and transported to the plasma membrane via recycling endosomes [97]. In a starvation condition, recycling endosomes serve as a platform for the assembly of core autophagy-related proteins to induce autophagosome formation [98]. Dynamin 2 directly interacts with LC3, a mammalian ortholog of yeast Atg8, that specifically binds to the autophagosomal membrane via its PH domain [95]. Autophagosomes formed on recycling endosomes are released by dynamin 2 and processed for maturation [95]. In homozygous knock-in mice with a CNM-associated mutant dynamin 2 (R465W), the autophagosome maturation process is defected [99]. Dynamin 2 R465W can still interact with LC3, but its function on autophagosome is impaired, because of enhanced interaction with ITSN1, a binding partner of dynamin 2 on the plasma membrane [95].
In the course of autophagy, dynamin 2 localises not only to recycling endosomes but also localises to autolysosomes [96]. At autolysosomes, the fission activities of dynamin 2 contribute to lipophagy, which is the autophagic degradation of lipid droplet (LD) required for lipid homeostasis [100]. Dynamin 2 depletion or loss of its GTPase activities in hepatocytes results in defective lipophagy [96]. Similarly, loss of dynamin 2 in skeletal muscles also causes defects in lipid homeostasis by altering LD biogenesis and mitochondrial morphology [101]. Dynamin 2 has been implicated in mitochondrial fission cooperatively with Drp1 (dynamin-related protein 1) in COS-7, Sk-Mel2 and HeLa cells [102]. However, dysfunction of CNM-associated dynamin 2 variants in LD biogenesis, lipophagy or mitochondrial fission and their implications in CNM pathogenesis remains to be elucidated.
Dynamin 2 is also implicated in cytoskeletal regulation, especially in the organisation of actin. Dynamin 2 regulates intracellular trafficking of the GLUT4-containing vesicles by controlling actin polymerisation [93]. The actin regulation by dynamin 2 is also required for insulin-dependent exocytosis of GLUT4 to supply intracellular membrane components to T-tubules [103,104,105]. Expression of CNM-associated mutant dynamin 2 disrupts de novo actin filament formation in muscle cells [93]. Consistently, in the CNM model mouse expressing CNM mutant dynamin 2 (R465W), translocation of GLUT4 to the plasma membrane is impaired due to disorganised actin filaments, and abnormal perinuclear accumulation of GLUT4 is observed in CNM patient’s muscle biopsy [93].
Actin regulation by dynamin 2 is also required for skeletal muscle development in myoblast fusion [64,66] and the formation of neuromuscular junctions (NMJ) [106]. Invadosomes are actin-rich membrane protrusions required for degradation of the extracellular matrix (ECM), and they play essential roles in myoblast fusion and NMJ formation [107]. In invadosomes, dynamin 2 is involved in actin organisation either by itself via the PR domain [64] or with its interacting proteins such as Tks5 (tyrosine kinase substrate with 5 SH3 domain) [66,106]. Dynamin 2 is also required for the formation and function of invadosomes cooperatively with various BAR domain proteins such as BIN1 [108], endophilin [109] and pacsin 2 [110]. Expression of CNM-associated dynamin 2 mutant (A618T) in C2C12 cells enhances formation of invadosomes with abnormal matrix degradation by inducing F-actin bundles [106].
Costameres, sub-sarcolemmal adhesion sites associated with Z-lines in skeletal muscle, play mechanical and signalling roles during muscle contraction [111]. Costameres consist of multiple components such as integrin [112], actin [113], clathrin [114] and dynamin 2 [115] and they are required for the stabilisation of skeletal muscle fibres by attaching sarcolemma to myofibrils [111]. Dynamin 2 regulates clathrin plaque formation in costameres by interacting with desmin and N-WASP [114,115]. In the CNM-model mouse expressing dynamin 2 mutant and the CNM patient’s biopsy, costameres are defected because of disorganised desmin filaments and clathrin plaques [114,116].
The nuclear positioning to the periphery of skeletal muscle cells requires crosslinking of myofibrils by desmin which is regulated by the arp2/3 complex [117]. Dynamin 2 is required for peripheral nuclear positioning by interacting with N-WASP, an activator of the Arp2/3 complex [51,118,119,120]. CNM mutant dynamin 2 localises around centralised nuclei and their size and numbers are impaired in the adult skeletal muscles in Dnm2-KI mice [121,122]. These abnormal nuclei are possibly produced by defective regeneration of satellite cells due to decreased transcription [123]. However, it is still unclear how the function of dynamin 2 around the nuclei is impaired. Further analyses are required for unveiling yet unknown transcriptional regulation by dynamin 2.
4.6. Therapeutic Approaches for CNM
CNM-associated dynamin 2 variants cause gain-of-function features in membrane fission activities because of elevated GTPase activity [47,86,87,88]. Likewise, overexpression of wild-type dynamin 2 also induces CNM phenotypes such as muscle weakness, abnormal histology and altered T-tubule structures in mice and Drosophila [79,89,119]. Based on these findings, gene silencing approaches are developed to reduce or normalise the expression level of dynamin 2 using AAV-mediated expression of shRNA targeting Dnm2 mRNA or antisense oligonucleotides against Dnm2 pre-mRNA and mRNA [124,125,126]. These gene silencing approaches improve CNM phenotypes of moderate Dnm2R465W/+ and severe Dnm2S619L/+ mouse models [124,125,126]. The expression level of dynamin 2 protein is increased in muscle lysates from Mtm1-KO mouse and XLMTM1 patients [127]. Therefore, gene silencing approaches targeting Dnm2 also improved the CNM symptoms in Mtm1-KO mice [127,128]. As already mentioned in this review, BIN1 negatively regulates GTPase activities of dynamin 2 in a stoichiometry dependent manner [34,47]. Skeletal muscle-specific Bin1-KO mouse shows CNM phenotypes including reduced muscle mass and force, and T-tubule abnormalities with a slight increase of dynamin 2 protein level [34,129]. Thus, downregulation of dynamin 2 by gene silencing tunes its relative amount for BIN1 protein resulting in normal survival, muscular force and triad structures [34,129]. In zebrafish, knockout of a CNM causal gene SPEG (striated preferentially expressed protein kinase) that encodes a myosin light chain kinase family protein show T-tubule abnormalities with the increased expression level of dynamin 2 protein [130]. Since SPEG has been shown to interact with MTM1 [5], SPEG may regulate dynamin 2 function together with MTM1 and BIN1 in skeletal muscle. Although it is still unclear if SPEG is also a negative regulator of dynamin 2, gene silencing of DNM2 may be a potential therapeutic approach for CNM caused by variants in DNM2 gene as well as for CNM associated with variants in other genes such as MTM1, BIN1, SPEG. Indeed, a clinical trial using investigational antisense medicine DYN101 is ongoing for DNM2-associated CNM (NCT04033159).
5. Perspectives
In this review, we overviewed the function of BIN1 and dynamin 2 in T-tubule biogenesis and discussed possible molecular mechanisms of CNM pathogenesis caused by their membrane remodelling defects. Abnormal membrane remodelling by CNM-associated variants of BIN1 and dynamin 2 has been greatly elucidated using multidisciplinary approaches. However, the impact of CNM-associated variants on multifunctional features of dynamin 2 at various cellular organelles is still largely unknown. A comprehensive understanding of dysregulated functions of dynamin 2 in the multiple cellular processes may contribute to a better elucidation of pathomechanisms of CNM and the development of more precise diagnosis, management and care of CNM patients. Although we focused on the T-tubule biogenesis by BIN1 and dynamin 2, there are a variety of other proteins involved in T-tubule formation, and many of them are associated with muscle diseases [6,131]. A more comprehensive understanding of protein functions that affect T-tubule formation is required for a better understanding of the CNM pathogenesis caused by abnormal membrane remodelling.
Acknowledgments
The authors would like to thank Ichizo Nishino (NCNP) and Kohji Takei (Okayama University) for their scientific insights during our study.
Author Contributions
K.F., S.N. and T.T. wrote and edited the manuscript. K.F. designed the figures. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by JSPS KAKENHI, Grant numbers 18K07198, 19KK0180, The Takeda Science Foundation, Wesco Scientific Promotion Foundation and Ryobi Teien Memory Foundation for T.T. This research was also funded by Intramural Research Grant for Neuronal and Psychiatric Disorders of NCNP (29-4, 2-5 for T.T. and Ichizo Nishino, 2-6, 3-9 for S.N.), and AMED under Grant Numbers JP19ek0109285h0003 for Ichizo Nishino and S.N.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jungbluth H., Wallgren-Pettersson C., Laporte J. Centronuclear (myotubular) myopathy. Orphanet. J. Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jungbluth H., Gautel M. Pathogenic mechanisms in centronuclear myopathies. Front. Aging Neurosci. 2014;6:339. doi: 10.3389/fnagi.2014.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero N.B. Centronuclear myopathies: A widening concept. Neuromuscul. Disord. 2010;20:223–228. doi: 10.1016/j.nmd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Majczenko K., Davidson A.E., Camelo-Piragua S., Agrawal P.B., Manfready R.A., Li X., Joshi S., Xu J., Peng W., Beggs A.H., et al. Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am. J. Hum. Genet. 2012;91:365–371. doi: 10.1016/j.ajhg.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal P.B., Pierson C.R., Joshi M., Liu X., Ravenscroft G., Moghadaszadeh B., Talabere T., Viola M., Swanson L.C., Haliloglu G., et al. SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am. J. Hum. Genet. 2014;95:218–226. doi: 10.1016/j.ajhg.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Qusairi L., Laporte J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet. Muscle. 2011;1:26. doi: 10.1186/2044-5040-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling J.J., Gibbs E.M., Feldman E.L. Membrane traffic and muscle: Lessons from human disease. Traffic. 2008;9:1035–1043. doi: 10.1111/j.1600-0854.2008.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitoun M., Maugenre S., Jeannet P.Y., Lacene E., Ferrer X., Laforet P., Martin J.J., Laporte J., Lochmuller H., Beggs A.H., et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 2005;37:1207–1209. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]
- 9.Nicot A.S., Toussaint A., Tosch V., Kretz C., Wallgren-Pettersson C., Iwarsson E., Kingston H., Garnier J.M., Biancalana V., Oldfors A., et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat. Genet. 2007;39:1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 10.Fischer D., Herasse M., Bitoun M., Barragan-Campos H.M., Chiras J., Laforet P., Fardeau M., Eymard B., Guicheney P., Romero N.B. Characterization of the muscle involvement in dynamin 2-related centronuclear myopathy. Brain. 2006;129:1463–1469. doi: 10.1093/brain/awl071. [DOI] [PubMed] [Google Scholar]
- 11.Bohm J., Yis U., Ortac R., Cakmakci H., Kurul S.H., Dirik E., Laporte J. Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation. Orphanet. J. Rare Dis. 2010;5:35. doi: 10.1186/1750-1172-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Oca R., Cowling B.S., Laporte J. Common Pathogenic Mechanisms in Centronuclear and Myotubular Myopathies and Latest Treatment Advances. Int. J. Mol. Sci. 2021;22:11377. doi: 10.3390/ijms222111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling J.J., Weihl C.C., Spencer M.J. Molecular and cellular basis of genetically inherited skeletal muscle disorders. Nat. Rev. Mol. Cell Biol. 2021;22:713–732. doi: 10.1038/s41580-021-00389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M., Maani N., Dowling J.J. Dynamin 2 (DNM2) as Cause of, and Modifier for, Human Neuromuscular Disease. Neurotherapeutics. 2018;15:966–975. doi: 10.1007/s13311-018-00686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowling B.S., Toussaint A., Muller J., Laporte J. Defective membrane remodeling in neuromuscular diseases: Insights from animal models. PLoS Genet. 2012;8:e1002595. doi: 10.1371/journal.pgen.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durieux A.C., Prudhon B., Guicheney P., Bitoun M. Dynamin 2 and human diseases. J. Mol. Med. 2010;88:339–350. doi: 10.1007/s00109-009-0587-4. [DOI] [PubMed] [Google Scholar]
- 17.Franzini-Armstrong C. The relationship between form and function throughout the history of excitation-contraction coupling. J. Gen. Physiol. 2018;150:189–210. doi: 10.1085/jgp.201711889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosemblatt M., Hidalgo C., Vergara C., Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J. Biol. Chem. 1981;256:8140–8148. doi: 10.1016/S0021-9258(18)43399-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee E., Marcucci M., Daniell L., Pypaert M., Weisz O.A., Ochoa G.C., Farsad K., Wenk M.R., De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 20.Flucher B.E. How is SR calcium release in muscle modulated by PIP(4,5)2? J. Gen. Physiol. 2015;145:361–364. doi: 10.1085/jgp.201511395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrientos G., Llanos P., Hidalgo J., Bolanos P., Caputo C., Riquelme A., Sanchez G., Quest A.F., Hidalgo C. Cholesterol removal from adult skeletal muscle impairs excitation-contraction coupling and aging reduces caveolin-3 and alters the expression of other triadic proteins. Front. Physiol. 2015;6:105. doi: 10.3389/fphys.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calderon J.C., Bolanos P., Caputo C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014;6:133–160. doi: 10.1007/s12551-013-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fill M., Copello J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 24.Cui Y., Tae H.S., Norris N.C., Karunasekara Y., Pouliquin P., Board P.G., Dulhunty A.F., Casarotto M.G. A dihydropyridine receptor alpha1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int. J. Biochem. Cell Biol. 2009;41:677–686. doi: 10.1016/j.biocel.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Hu H., Wang Z., Wei R., Fan G., Wang Q., Zhang K., Yin C.C. The molecular architecture of dihydropyrindine receptor/L-type Ca2+ channel complex. Sci. Rep. 2015;5:8370. doi: 10.1038/srep08370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowling J.J., Lawlor M.W., Dirksen R.T. Triadopathies: An emerging class of skeletal muscle diseases. Neurotherapeutics. 2014;11:773–785. doi: 10.1007/s13311-014-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prokic I., Cowling B.S., Laporte J. Amphiphysin 2 (BIN1) in physiology and diseases. J. Mol. Med. 2014;92:453–463. doi: 10.1007/s00109-014-1138-1. [DOI] [PubMed] [Google Scholar]
- 28.Frost A., Unger V.M., De Camilli P. The BAR domain superfamily: Membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suetsugu S., Toyooka K., Senju Y. Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Semin Cell Dev. Biol. 2010;21:340–349. doi: 10.1016/j.semcdb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Casal E., Federici L., Zhang W., Fernandez-Recio J., Priego E.M., Miguel R.N., DuHadaway J.B., Prendergast G.C., Luisi B.F., Laue E.D. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry. 2006;45:12917–12928. doi: 10.1021/bi060717k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamuro D., Elliott K.J., Wechsler-Reya R., Prendergast G.C. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat. Genet. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- 32.Butler M.H., David C., Ochoa G.C., Freyberg Z., Daniell L., Grabs D., Cremona O., De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J. Cell Biol. 1997;137:1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prokic I., Cowling B.S., Kutchukian C., Kretz C., Tasfaout H., Gache V., Hergueux J., Wendling O., Ferry A., Toussaint A., et al. Differential physiological role of BIN1 isoforms in skeletal muscle development, function and regeneration. Dis. Model. Mech. 2020;13:dmm044354. doi: 10.1242/dmm.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowling B.S., Prokic I., Tasfaout H., Rabai A., Humbert F., Rinaldi B., Nicot A.S., Kretz C., Friant S., Roux A., et al. Amphiphysin (BIN1) negatively regulates dynamin 2 for normal muscle maturation. J. Clin. Investig. 2017;127:4477–4487. doi: 10.1172/JCI90542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tjondrokoesoemo A., Park K.H., Ferrante C., Komazaki S., Lesniak S., Brotto M., Ko J.K., Zhou J., Weisleder N., Ma J. Disrupted membrane structure and intracellular Ca2+ signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS ONE. 2011;6:e25740. doi: 10.1371/journal.pone.0025740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razzaq A., Robinson I.M., McMahon H.T., Skepper J.N., Su Y., Zelhof A.C., Jackson A.P., Gay N.J., O’Kane C.J. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isas J.M., Ambroso M.R., Hegde P.B., Langen J., Langen R. Tubulation by amphiphysin requires concentration-dependent switching from wedging to scaffolding. Structure. 2015;23:873–881. doi: 10.1016/j.str.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam J., Basnet N., Mizuno N. Structural insights into the cooperative remodeling of membranes by amphiphysin/BIN1. Sci. Rep. 2015;5:15452. doi: 10.1038/srep15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picas L., Viaud J., Schauer K., Vanni S., Hnia K., Fraisier V., Roux A., Bassereau P., Gaits-Iacovoni F., Payrastre B., et al. BIN1/M-Amphiphysin2 induces clustering of phosphoinositides to recruit its downstream partner dynamin. Nat. Commun. 2014;5:5647. doi: 10.1038/ncomms6647. [DOI] [PubMed] [Google Scholar]
- 40.Gowrisankaran S., Wang Z., Morgan D.G., Milosevic I., Mim C. Cells Control BIN1-Mediated Membrane Tubulation by Altering the Membrane Charge. J. Mol. Biol. 2020;432:1235–1250. doi: 10.1016/j.jmb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Wu T., Baumgart T. BIN1 membrane curvature sensing and generation show autoinhibition regulated by downstream ligands and PI(4,5)P2. Biochemistry. 2014;53:7297–7309. doi: 10.1021/bi501082r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drager N.M., Nachman E., Winterhoff M., Bruhmann S., Shah P., Katsinelos T., Boulant S., Teleman A.A., Faix J., Jahn T.R. Bin1 directly remodels actin dynamics through its BAR domain. EMBO Rep. 2017;18:2051–2066. doi: 10.15252/embr.201744137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong T., Yang H., Zhang S.S., Cho H.C., Kalashnikova M., Sun B., Zhang H., Bhargava A., Grabe M., Olgin J., et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat. Med. 2014;20:624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu T., Shi Z., Baumgart T. Mutations in BIN1 associated with centronuclear myopathy disrupt membrane remodeling by affecting protein density and oligomerization. PLoS ONE. 2014;9:e93060. doi: 10.1371/journal.pone.0093060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohm J., Vasli N., Maurer M., Cowling B.S., Shelton G.D., Kress W., Toussaint A., Prokic I., Schara U., Anderson T.J., et al. Altered splicing of the BIN1 muscle-specific exon in humans and dogs with highly progressive centronuclear myopathy. PLoS Genet. 2013;9:e1003430. doi: 10.1371/journal.pgen.1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fugier C., Klein A.F., Hammer C., Vassilopoulos S., Ivarsson Y., Toussaint A., Tosch V., Vignaud A., Ferry A., Messaddeq N., et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat. Med. 2011;17:720–725. doi: 10.1038/nm.2374. [DOI] [PubMed] [Google Scholar]
- 47.Fujise K., Okubo M., Abe T., Yamada H., Nishino I., Noguchi S., Takei K., Takeda T. Mutant BIN1-Dynamin 2 complexes dysregulate membrane remodeling in the pathogenesis of centronuclear myopathy. J. Biol. Chem. 2021;296:100077. doi: 10.1074/jbc.RA120.015184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohm J., Biancalana V., Malfatti E., Dondaine N., Koch C., Vasli N., Kress W., Strittmatter M., Taratuto A.L., Gonorazky H., et al. Adult-onset autosomal dominant centronuclear myopathy due to BIN1 mutations. Brain. 2014;137:3160–3170. doi: 10.1093/brain/awu272. [DOI] [PubMed] [Google Scholar]
- 49.Toussaint A., Cowling B.S., Hnia K., Mohr M., Oldfors A., Schwab Y., Yis U., Maisonobe T., Stojkovic T., Wallgren-Pettersson C., et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 2011;121:253–266. doi: 10.1007/s00401-010-0754-2. [DOI] [PubMed] [Google Scholar]
- 50.Royer B., Hnia K., Gavriilidis C., Tronchere H., Tosch V., Laporte J. The myotubularin-amphiphysin 2 complex in membrane tubulation and centronuclear myopathies. EMBO Rep. 2013;14:907–915. doi: 10.1038/embor.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falcone S., Roman W., Hnia K., Gache V., Didier N., Laine J., Aurade F., Marty I., Nishino I., Charlet-Berguerand N., et al. N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol. Med. 2014;6:1455–1475. doi: 10.15252/emmm.201404436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonny B., Burd C., De Camilli P., Chen E., Daumke O., Faelber K., Ford M., Frolov V.A., Frost A., Hinshaw J.E., et al. Membrane fission by dynamin: What we know and what we need to know. EMBO J. 2016;35:2270–2284. doi: 10.15252/embj.201694613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferguson S.M., De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmid S.L., Frolov V.A. Dynamin: Functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- 55.Cao H., Garcia F., McNiven M.A. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook T., Mesa K., Urrutia R. Three dynamin-encoding genes are differentially expressed in developing rat brain. J. Neurochem. 1996;67:927–931. doi: 10.1046/j.1471-4159.1996.67030927.x. [DOI] [PubMed] [Google Scholar]
- 57.Cook T.A., Urrutia R., McNiven M.A. Identification of dynamin 2, an isoform ubiquitously expressed in rat tissues. Proc. Natl. Acad. Sci. USA. 1994;91:644–648. doi: 10.1073/pnas.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reubold T.F., Eschenburg S., Becker A., Leonard M., Schmid S.L., Vallee R.B., Kull F.J., Manstein D.J. Crystal structure of the GTPase domain of rat dynamin 1. Proc. Natl. Acad. Sci. USA. 2005;102:13093–13098. doi: 10.1073/pnas.0506491102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faelber K., Posor Y., Gao S., Held M., Roske Y., Schulze D., Haucke V., Noe F., Daumke O. Crystal structure of nucleotide-free dynamin. Nature. 2011;477:556–560. doi: 10.1038/nature10369. [DOI] [PubMed] [Google Scholar]
- 60.Zhenga J., Cahill S.M., Lemmon M.A., Fushmana D., Schlessinger J., Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: Implications for stimulation of GTPase activity. J. Mol. Biol. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- 61.Bethoney K.A., King M.C., Hinshaw J.E., Ostap E.M., Lemmon M.A. A possible effector role for the pleckstrin homology (PH) domain of dynamin. Proc. Natl. Acad. Sci. USA. 2009;106:13359–13364. doi: 10.1073/pnas.0906945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehrotra N., Nichols J., Ramachandran R. Alternate pleckstrin homology domain orientations regulate dynamin-catalyzed membrane fission. Mol. Biol. Cell. 2014;25:879–890. doi: 10.1091/mbc.e13-09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reubold T.F., Faelber K., Plattner N., Posor Y., Ketel K., Curth U., Schlegel J., Anand R., Manstein D.J., Noe F., et al. Crystal structure of the dynamin tetramer. Nature. 2015;525:404–408. doi: 10.1038/nature14880. [DOI] [PubMed] [Google Scholar]
- 64.Zhang R., Lee D.M., Jimah J.R., Gerassimov N., Yang C., Kim S., Luvsanjav D., Winkelman J., Mettlen M., Abrams M.E., et al. Dynamin regulates the dynamics and mechanical strength of the actin cytoskeleton as a multifilament actin-bundling protein. Nat. Cell Biol. 2020;22:674–688. doi: 10.1038/s41556-020-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takei K., McPherson P.S., Schmid S.L., De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 66.Chuang M.C., Lin S.S., Ohniwa R.L., Lee G.H., Su Y.A., Chang Y.C., Tang M.J., Liu Y.W. Tks5 and Dynamin-2 enhance actin bundle rigidity in invadosomes to promote myoblast fusion. J. Cell Biol. 2019;218:1670–1685. doi: 10.1083/jcb.201809161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/S0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 68.Sweitzer S.M., Hinshaw J.E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/S0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 69.Chappie J.S., Acharya S., Leonard M., Schmid S.L., Dyda F. G domain dimerization controls dynamin’s assembly-stimulated GTPase activity. Nature. 2010;465:435–440. doi: 10.1038/nature09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganichkin O.M., Vancraenenbroeck R., Rosenblum G., Hofmann H., Mikhailov A.S., Daumke O., Noel J.K. Quantification and demonstration of the collective constriction-by-ratchet mechanism in the dynamin molecular motor. Proc. Natl. Acad. Sci. USA. 2021;118:e2101144118. doi: 10.1073/pnas.2101144118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colom A., Redondo-Morata L., Chiaruttini N., Roux A., Scheuring S. Dynamic remodeling of the dynamin helix during membrane constriction. Proc. Natl. Acad. Sci. USA. 2017;114:5449–5454. doi: 10.1073/pnas.1619578114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda T., Kozai T., Yang H., Ishikuro D., Seyama K., Kumagai Y., Abe T., Yamada H., Uchihashi T., Ando T., et al. Dynamic clustering of dynamin-amphiphysin helices regulates membrane constriction and fission coupled with GTP hydrolysis. eLife. 2018;7:e30246. doi: 10.7554/eLife.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roux A., Uyhazi K., Frost A., De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 74.Cheng X., Chen K., Dong B., Yang M., Filbrun S.L., Myoung Y., Huang T.X., Gu Y., Wang G., Fang N. Dynamin-dependent vesicle twist at the final stage of clathrin-mediated endocytosis. Nat. Cell Biol. 2021;23:859–869. doi: 10.1038/s41556-021-00713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diatloff-Zito C., Gordon A.J., Duchaud E., Merlin G. Isolation of an ubiquitously expressed cDNA encoding human dynamin II, a member of the large GTP-binding protein family. Gene. 1995;163:301–306. doi: 10.1016/0378-1119(95)00275-B. [DOI] [PubMed] [Google Scholar]
- 76.Cowling B.S., Toussaint A., Amoasii L., Koebel P., Ferry A., Davignon L., Nishino I., Mandel J.L., Laporte J. Increased expression of wild-type or a centronuclear myopathy mutant of dynamin 2 in skeletal muscle of adult mice leads to structural defects and muscle weakness. Am. J. Pathol. 2011;178:2224–2235. doi: 10.1016/j.ajpath.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biancalana V., Romero N.B., Thuestad I.J., Ignatius J., Kataja J., Gardberg M., Heron D., Malfatti E., Oldfors A., Laporte J. Some DNM2 mutations cause extremely severe congenital myopathy and phenocopy myotubular myopathy. Acta Neuropathol. Commun. 2018;6:93. doi: 10.1186/s40478-018-0593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bohm J., Biancalana V., Dechene E.T., Bitoun M., Pierson C.R., Schaefer E., Karasoy H., Dempsey M.A., Klein F., Dondaine N., et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum. Mutat. 2012;33:949–959. doi: 10.1002/humu.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casar-Borota O., Jacobsson J., Libelius R., Oldfors C.H., Malfatti E., Romero N.B., Oldfors A. A novel dynamin-2 gene mutation associated with a late-onset centronuclear myopathy with necklace fibres. Neuromuscul. Disord. 2015;25:345–348. doi: 10.1016/j.nmd.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Hohendahl A., Roux A., Galli V. Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2. J. Struct. Biol. 2016;196:37–47. doi: 10.1016/j.jsb.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marks B., Stowell M.H., Vallis Y., Mills I.G., Gibson A., Hopkins C.R., McMahon H.T. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 82.Warnock D.E., Hinshaw J.E., Schmid S.L. Dynamin self-assembly stimulates its GTPase activity. J. Biol. Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- 83.Vallis Y., Wigge P., Marks B., Evans P.R., McMahon H.T. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr. Biol. 1999;9:257–260. doi: 10.1016/S0960-9822(99)80114-6. [DOI] [PubMed] [Google Scholar]
- 84.Gibbs E.M., Davidson A.E., Telfer W.R., Feldman E.L., Dowling J.J. The myopathy-causing mutation DNM2-S619L leads to defective tubulation in vitro and in developing zebrafish. Dis. Model. Mech. 2014;7:157–161. doi: 10.1242/dmm.012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao M., Smith L., Volpatti J., Fabian L., Dowling J.J. Insights into wild-type dynamin 2 and the consequences of DNM2 mutations from transgenic zebrafish. Hum. Mol. Genet. 2019;28:4186–4196. doi: 10.1093/hmg/ddz260. [DOI] [PubMed] [Google Scholar]
- 86.Wang L., Barylko B., Byers C., Ross J.A., Jameson D.M., Albanesi J.P. Dynamin 2 mutants linked to centronuclear myopathies form abnormally stable polymers. J. Biol. Chem. 2010;285:22753–22757. doi: 10.1074/jbc.C110.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kenniston J.A., Lemmon M.A. Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients. EMBO J. 2010;29:3054–3067. doi: 10.1038/emboj.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chin Y.H., Lee A., Kan H.W., Laiman J., Chuang M.C., Hsieh S.T., Liu Y.W. Dynamin-2 mutations associated with centronuclear myopathy are hypermorphic and lead to T-tubule fragmentation. Hum. Mol. Genet. 2015;24:5542–5554. doi: 10.1093/hmg/ddv285. [DOI] [PubMed] [Google Scholar]
- 89.Kutchukian C., Szentesi P., Allard B., Trochet D., Beuvin M., Berthier C., Tourneur Y., Guicheney P., Csernoch L., Bitoun M., et al. Impaired excitation-contraction coupling in muscle fibres from the dynamin2(R465W) mouse model of centronuclear myopathy. J. Physiol. 2017;595:7369–7382. doi: 10.1113/JP274990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hinostroza F., Neely A., Araya-Duran I., Maraboli V., Canan J., Rojas M., Aguayo D., Latorre R., Gonzalez-Nilo F.D., Cardenas A.M. Dynamin-2 R465W mutation induces long range perturbation in highly ordered oligomeric structures. Sci. Rep. 2020;10:18151. doi: 10.1038/s41598-020-75216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujise K., Okubo M., Abe T., Yamada H., Takei K., Nishino I., Takeda T., Noguchi S. Imaging-based evaluation of pathogenicity by novel DNM2 variants associated with centronuclear myopathy. Hum. Mutat. 2021;43:169–179. doi: 10.1002/humu.24307. [DOI] [PubMed] [Google Scholar]
- 92.Antonescu C.N., Diaz M., Femia G., Planas J.V., Klip A. Clathrin-dependent and independent endocytosis of glucose transporter 4 (GLUT4) in myoblasts: Regulation by mitochondrial uncoupling. Traffic. 2008;9:1173–1190. doi: 10.1111/j.1600-0854.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Jamett A.M., Baez-Matus X., Olivares M.J., Hinostroza F., Guerra-Fernandez M.J., Vasquez-Navarrete J., Bui M.T., Guicheney P., Romero N.B., Bevilacqua J.A., et al. Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells. Sci. Rep. 2017;7:4580. doi: 10.1038/s41598-017-04418-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Hasani H., Kunamneni R.K., Dawson K., Hinck C.S., Muller-Wieland D., Cushman S.W. Roles of the N- and C-termini of GLUT4 in endocytosis. J. Cell Sci. 2002;115:131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- 95.Puri C., Manni M.M., Vicinanza M., Hilcenko C., Zhu Y., Runwal G., Stamatakou E., Menzies F.M., Mamchaoui K., Bitoun M., et al. A DNM2 Centronuclear Myopathy Mutation Reveals a Link between Recycling Endosome Scission and Autophagy. Dev. Cell. 2020;53:154–168.e6. doi: 10.1016/j.devcel.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 96.Schulze R.J., Weller S.G., Schroeder B., Krueger E.W., Chi S., Casey C.A., McNiven M.A. Lipid droplet breakdown requires dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. J. Cell Biol. 2013;203:315–326. doi: 10.1083/jcb.201306140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mesaki K., Tanabe K., Obayashi M., Oe N., Takei K. Fission of tubular endosomes triggers endosomal acidification and movement. PLoS ONE. 2011;6:e19764. doi: 10.1371/journal.pone.0019764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Birgisdottir A.B., Johansen T. Autophagy and endocytosis—Interconnections and interdependencies. J. Cell Sci. 2020;133:jcs228114. doi: 10.1242/jcs.228114. [DOI] [PubMed] [Google Scholar]
- 99.Durieux A.C., Vassilopoulos S., Laine J., Fraysse B., Brinas L., Prudhon B., Castells J., Freyssenet D., Bonne G., Guicheney P., et al. A centronuclear myopathy—Dynamin 2 mutation impairs autophagy in mice. Traffic. 2012;13:869–879. doi: 10.1111/j.1600-0854.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 100.Kounakis K., Chaniotakis M., Markaki M., Tavernarakis N. Emerging Roles of Lipophagy in Health and Disease. Front. Cell Dev. Biol. 2019;7:185. doi: 10.3389/fcell.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tinelli E., Pereira J.A., Suter U. Muscle-specific function of the centronuclear myopathy and Charcot-Marie-Tooth neuropathy-associated dynamin 2 is required for proper lipid metabolism, mitochondria, muscle fibers, neuromuscular junctions and peripheral nerves. Hum. Mol. Genet. 2013;22:4417–4429. doi: 10.1093/hmg/ddt292. [DOI] [PubMed] [Google Scholar]
- 102.Lee J.E., Westrate L.M., Wu H., Page C., Voeltz G.K. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marette A., Burdett E., Douen A., Vranic M., Klip A. Insulin induces the translocation of GLUT4 from a unique intracellular organelle to transverse tubules in rat skeletal muscle. Diabetes. 1992;41:1562–1569. doi: 10.2337/diab.41.12.1562. [DOI] [PubMed] [Google Scholar]
- 104.Wang W., Hansen P.A., Marshall B.A., Holloszy J.O., Mueckler M. Insulin unmasks a COOH-terminal Glut4 epitope and increases glucose transport across T-tubules in skeletal muscle. J. Cell Biol. 1996;135:415–430. doi: 10.1083/jcb.135.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ploug T., van Deurs B., Ai H., Cushman S.W., Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: Identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin S.S., Hsieh T.L., Liou G.G., Li T.N., Lin H.C., Chang C.W., Wu H.Y., Yao C.K., Liu Y.W. Dynamin-2 Regulates Postsynaptic Cytoskeleton Organization and Neuromuscular Junction Development. Cell Rep. 2020;33:108310. doi: 10.1016/j.celrep.2020.108310. [DOI] [PubMed] [Google Scholar]
- 107.Paterson E.K., Courtneidge S.A. Invadosomes are coming: New insights into function and disease relevance. FEBS J. 2018;285:8–27. doi: 10.1111/febs.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao F., Zhou Y., Liu X., Yu C.H. Podosome formation promotes plasma membrane invagination and integrin-beta3 endocytosis on a viscous RGD-membrane. Commun. Biol. 2020;3:117. doi: 10.1038/s42003-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ochoa G.C., Slepnev V.I., Neff L., Ringstad N., Takei K., Daniell L., Kim W., Cao H., McNiven M., Baron R., et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 2000;150:377–390. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J., Fujise K., Wint H., Senju Y., Suetsugu S., Yamada H., Takei K., Takeda T. Dynamin 2 and BAR domain protein pacsin 2 cooperatively regulate formation and maturation of podosomes. Biochem. Biophys. Res. Commun. 2021;571:145–151. doi: 10.1016/j.bbrc.2021.07.041. [DOI] [PubMed] [Google Scholar]
- 111.Ervasti J.M. Costameres: The Achilles’ heel of Herculean muscle. J. Biol. Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 112.Peter A.K., Cheng H., Ross R.S., Knowlton K.U., Chen J. The costamere bridges sarcomeres to the sarcolemma in striated muscle. Prog. Pediatr. Cardiol. 2011;31:83–88. doi: 10.1016/j.ppedcard.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rybakova I.N., Patel J.R., Ervasti J.M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 2000;150:1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franck A., Laine J., Moulay G., Lemerle E., Trichet M., Gentil C., Benkhelifa-Ziyyat S., Lacene E., Bui M.T., Brochier G., et al. Clathrin plaques and associated actin anchor intermediate filaments in skeletal muscle. Mol. Biol. Cell. 2019;30:579–590. doi: 10.1091/mbc.E18-11-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vassilopoulos S., Gentil C., Laine J., Buclez P.O., Franck A., Ferry A., Precigout G., Roth R., Heuser J.E., Brodsky F.M., et al. Actin scaffolding by clathrin heavy chain is required for skeletal muscle sarcomere organization. J. Cell Biol. 2014;205:377–393. doi: 10.1083/jcb.201309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Massana Munoz X., Buono S., Koebel P., Laporte J., Cowling B.S. Different in vivo impacts of dynamin 2 mutations implicated in Charcot-Marie-Tooth neuropathy or centronuclear myopathy. Hum. Mol. Genet. 2019;28:4067–4077. doi: 10.1093/hmg/ddz249. [DOI] [PubMed] [Google Scholar]
- 117.Roman W., Martins J.P., Carvalho F.A., Voituriez R., Abella J.V.G., Santos N.C., Cadot B., Way M., Gomes E.R. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017;19:1189–1201. doi: 10.1038/ncb3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yarar D., To W., Abo A., Welch M.D. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 1999;9:555–558. doi: 10.1016/S0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- 119.Machesky L.M., Mullins R.D., Higgs H.N., Kaiser D.A., Blanchoin L., May R.C., Hall M.E., Pollard T.D. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Egile C., Loisel T.P., Laurent V., Li R., Pantaloni D., Sansonetti P.J., Carlier M.F. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kierdaszuk B., Berdynski M., Karolczak J., Redowicz M.J., Zekanowski C., Kaminska A.M. A novel mutation in the DNM2 gene impairs dynamin 2 localization in skeletal muscle of a patient with late onset centronuclear myopathy. Neuromuscul. Disord. 2013;23:219–228. doi: 10.1016/j.nmd.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 122.Fongy A., Falcone S., Laine J., Prudhon B., Martins-Bach A., Bitoun M. Nuclear defects in skeletal muscle from a Dynamin 2-linked centronuclear myopathy mouse model. Sci. Rep. 2019;9:1580. doi: 10.1038/s41598-018-38184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Almeida C.F., Bitoun M., Vainzof M. Satellite cells deficiency and defective regeneration in dynamin 2-related centronuclear myopathy. FASEB J. 2021;35:e21346. doi: 10.1096/fj.202001313RRR. [DOI] [PubMed] [Google Scholar]
- 124.Buono S., Ross J.A., Tasfaout H., Levy Y., Kretz C., Tayefeh L., Matson J., Guo S., Kessler P., Monia B.P., et al. Reducing dynamin 2 (DNM2) rescues DNM2-related dominant centronuclear myopathy. Proc. Natl. Acad. Sci. USA. 2018;115:11066–11071. doi: 10.1073/pnas.1808170115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trochet D., Prudhon B., Beuvin M., Peccate C., Lorain S., Julien L., Benkhelifa-Ziyyat S., Rabai A., Mamchaoui K., Ferry A., et al. Allele-specific silencing therapy for Dynamin 2-related dominant centronuclear myopathy. EMBO Mol. Med. 2018;10:239–253. doi: 10.15252/emmm.201707988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Munoz X.M., Kretz C., Silva-Rojas R., Ochala J., Menuet A., Romero N.B., Cowling B.S., Laporte J. Physiological impact and disease reversion for the severe form of centronuclear myopathy linked to dynamin. JCI Insight. 2020;5:e137899. doi: 10.1172/jci.insight.137899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cowling B.S., Chevremont T., Prokic I., Kretz C., Ferry A., Coirault C., Koutsopoulos O., Laugel V., Romero N.B., Laporte J. Reducing dynamin 2 expression rescues X-linked centronuclear myopathy. J. Clin. Investig. 2014;124:1350–1363. doi: 10.1172/JCI71206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tasfaout H., Lionello V.M., Kretz C., Koebel P., Messaddeq N., Bitz D., Laporte J., Cowling B.S. Single Intramuscular Injection of AAV-shRNA Reduces DNM2 and Prevents Myotubular Myopathy in Mice. Mol. Ther. 2018;26:1082–1092. doi: 10.1016/j.ymthe.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silva-Rojas R., Nattarayan V., Jaque-Fernandez F., Gomez-Oca R., Menuet A., Reiss D., Goret M., Messaddeq N., Lionello V.M., Kretz C., et al. Mice with muscle-specific deletion of Bin1 recapitulate centronuclear myopathy and acute downregulation of dynamin 2 improves their phenotypes. Mol. Ther. 2022;30:868–880. doi: 10.1016/j.ymthe.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Espinosa K.G., Geissah S., Groom L., Volpatti J., Scott I.C., Dirksen R.T., Zhao M., Dowling J.J. Characterization of a novel zebrafish model of SPEG-related centronuclear myopathy. Dis. Model. Mech. 2022;15:dmm049437. doi: 10.1242/dmm.049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hall T.E., Martel N., Ariotti N., Xiong Z., Lo H.P., Ferguson C., Rae J., Lim Y.W., Parton R.G. In vivo cell biological screening identifies an endocytic capture mechanism for T-tubule formation. Nat. Commun. 2020;11:3711. doi: 10.1038/s41467-020-17486-w. [DOI] [PMC free article] [PubMed] [Google Scholar]