Abstract
Respiratory diseases are the cause of millions of deaths annually around the world. Despite the recent growth of our understanding of underlying mechanisms contributing to the pathogenesis of lung diseases, most therapeutic approaches are still limited to symptomatic treatments and therapies that only delay disease progression. Several clinical and preclinical studies have suggested stem cell (SC) therapy as a promising approach for treating various lung diseases. However, challenges such as the potential tumorigenicity, the low survival rate of the SCs in the recipient body, and difficulties in cell culturing and storage have limited the applicability of SC therapy. SC-derived extracellular vesicles (SC-EVs), particularly SC-derived exosomes (SC-Exos), exhibit most therapeutic properties of stem cells without their potential drawbacks. Similar to SCs, SC-Exos exhibit immunomodulatory, anti-inflammatory, and antifibrotic properties with the potential to be employed in the treatment of various inflammatory and chronic respiratory diseases. Furthermore, recent studies have demonstrated that the microRNA (miRNA) content of SC-Exos may play a crucial role in the therapeutic potential of these exosomes. Several studies have investigated the administration of SC-Exos via the pulmonary route, and techniques for SCs and SC-Exos delivery to the lungs by intratracheal instillation or inhalation have been developed. Here, we review the literature discussing the therapeutic effects of SC-Exos against respiratory diseases and advances in the pulmonary route of delivery of these exosomes to the damaged tissues.
Keywords: extracellular vesicles, nanosized vesicles, lung diseases, stem cell-derived exosomes
1. Introduction
Respiratory diseases are among the top causes of death globally. It was estimated that cumulatively, respiratory diseases were the third largest cause of death and were responsible for approximately 4 million deaths worldwide in 2019 [1]. Novel approaches, such as nanomedicine and regenerative medicine, could be beneficial in the diagnosis or treatment of various lung disorders [2,3,4,5]. Despite advances in modern medicine and drug design techniques, no drug has been developed to cure major respiratory diseases. Most available drugs mainly help control and restrict the progress of the disease or prevent complications. Cell-based therapies hold great promise for treating various lung diseases. In cell therapy, viable cells are introduced into the body via different routes to exert their particular therapeutic effects. Stem cells (SCs) have been found to have protective and regenerative properties on different tissues, and numerous studies have investigated the advantages of SCs in the treatment of disorders. SCs can be obtained from four different sources: embryonic tissues, fetal tissues, adult tissues (e.g., mesenchymal stem cells (MSCs) derived from different tissues), and differentiated somatic cells resulting from genetic manipulation, known as induced pluripotent SCs (iPSCs) [6]. The administration of SCs has been shown to be safe and effective in various respiratory diseases, such as chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), and pulmonary fibrosis (PF) [7]. However, the therapeutic use of SCs has its downsides: (I) SCs secrete varied growth factors that may have the potential to induce tumors; (II) the survival rate of SCs after administration is low; (III) SCs may block some small-diameter pulmonary blood vessels due to their size, and (IV) specific requirements make it challenging to culture and store SCs [8].
SC-derived exosomes (SC-Exos) as a cell-free therapy have been shown to be a promising strategy in addressing challenges associated with the administration of SCs, according to their negligible tumorigenicity, easier manipulation, standard storage requirements, and availability [9]. Exosomes are the smallest type of lipid-bilayer EVs with a 30–120 nm diameter size, similar to other EVs (microvesicles and apoptotic bodies), take part in intracellular communications as a paracrine factor, and can be secreted from many cell types including SCs [10,11]. Although exosomes secreted from different sources share a conserved general composition (Figure 1), they can be wildly diverse depending on the origin of the cell type and its microenvironmental stresses and conditions [12]. Accordingly, by inheriting their origin cell molecular pattern in their cargo composition, SC-Exos can affect their recipient cells similarly to their parental cells [13].
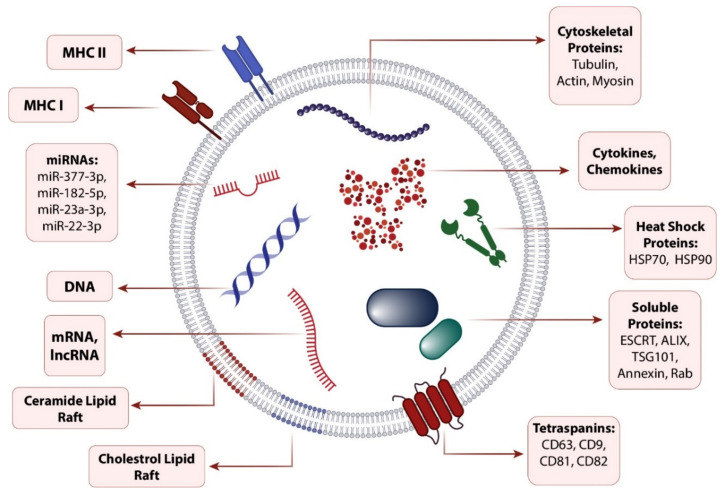
Figure 1.
Exosome composition. Exosomes share a conserved cargo composition consisting of proteins, DNA, mRNAs, and miRNAs, which can be regulated according to the secreting cell type and its microenvironment. Abbreviations: MHC, Major histocompatibility complex; miRNA, microRNAs; lncRNA, long noncoding RNA.
The administration route is one of the determining factors in the success and effectiveness of therapeutic agents. Introducing drugs directly to the lungs is suitable for treating respiratory diseases, mainly because the therapeutic agent could reach the region affected by the disease without entering circulation [14]. Therapeutic ingredients can be injected directly into the trachea (i.e., intratracheal instillation) or introduced to the lungs in aerosolized form using devices such as nebulizers and inhalers. Instillation via an endotracheal tube temporarily placed in the trachea is more invasive than inhalation; however, both approaches are considered noninvasive compared to invasive methods such as parenteral administration [15,16,17]. Several clinical and preclinical studies have investigated the potential of pulmonary delivery of SC-EVs and SC-Exos via intratracheal instillation and inhalation. Further in this review, we discuss the biogenesis of SC-Exos, their role and mechanism of action in treating lung diseases, and their potential to be administered via the direct pulmonary route.
2. Exosome Biogenesis
Exosome biogenesis starts with the formation of an early endosome that matures into a late endosome. Exosomal cargos are then segregated into microdomains on the late endosome membrane. Exosome biogenesis starts with the formation of an early endosome that forms by several endocytic vesicle fusion. Exosomal cargos are then segregated into microdomains on the endosome membrane. Further, these microdomains bud into the lumen of the endosome and form a multivesicular body (MVB), also referred to as a late endosome, containing numerous intraluminal vesicles (ILVs) that can be secreted upon fusion of this MVB with the plasma membrane or become degraded by MVB fusion with the lysosome [18]. Although this pathway is generally accepted, it does not seem to be the only mechanism of exosome biogenesis. Multiple studies suggested that exosomes can be formed and secreted by direct budding from the plasma membrane in some cell types and circumstances [19,20,21].
The protein sorting process into ILVs can be regulated by both endosomal sorting complexes required for transport (ESCRT)-dependent and independent pathways. ESCRT machinery consists of four main ESCRT complexes, including ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, and sort proteins into ILVs in ubiquitin-dependent and independent pathways. In the ubiquitin-dependent pathway, ubiquitylated proteins are recognized and encountered by ESCRT-0. By joining ESCRT-I and ESCRT-II to the complex, a strong recognition domain with a high affinity for ubiquitinated substrates forms on the endosomal membrane and buds into the inside. With ESCRT-III convergence with the complex, these buds can further be released into the intraluminal space, and the cargo proteins can be deubiquitinated by the deubiquitylating enzyme to rescue the ILVs from lysosomal degradation. In addition, nonubiquitinated proteins can be sorted into ILVs via the ESCRT pathway intersection by syntenin and ALIX (ALG-2-interacting protein X; an ESCRT accessory protein) proteins, which clusters nonubiquitinated proteins into microdomains and bridges them and the Vps32 (Vacuolar sorting-associated protein 32) ESCRT-III subunit, respectively [22]. Additionally, ESCRT-independent pathways mediated by ceramide (ESCRT-independent ILV budding), tetraspanins such as CD63, CD81, CD82, and CD9 (ESCRT-independent endosomal sorting regulation), and HSP70 and HSC70 (selective cytosolic protein cosorting into ILVs) can result in ILV formation and regulation separately from the ESCRT machinery. However, the role of both the ESCRT-dependent and independent pathway in determining ILV composition is necessary and routinely regulates ILV formation simultaneously [23].
3. Stem Cell-Derived Exosome Therapeutic Effect on Lung Diseases
3.1. Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is a clinical condition resulting from various risk factors, including pneumonia, sepsis, aspiration of gastric contents, trauma, and other less common precipitants [24,25]. ARDS is considered a more severe form of acute lung injury as it shares similar pathophysiological conditions. ARDS is associated with increased alveolar–capillary permeability to fluid, proteins, neutrophils, and inflammatory cells resulting in enhanced inflammation, accelerated autophagy, epithelial-mesenchymal transition (EMT), and decreased alveolar fluid clearance, leading to pulmonary edema, impaired gas exchange, hypoxemia, and PF [26,27,28]. Various stimuli such as hypoxia, cytokines, chemokines, thrombins, primed leukocytes, lipopolysaccharides (LPS), and damage-associated molecular patterns can shift the alveolar-capillary membrane toward dysfunction [24]. With the relatively high rate of ARDS-associated mortality and morbidity [28], and, on the other hand, the promising SC and SC-Exo regenerative and immunomodulatory properties, several studies evaluated SC-Exo effects on ARDS models and investigated the underlying mechanisms (Table 1) [29,30,31,32,33,34].
Table 1.
Effects of stem cell-derived exosomes (SC-Exos) on acute respiratory distress syndrome (ARDS) models.
| Factor | Study Model | Cell Source | Target | Effect | Reference |
|---|---|---|---|---|---|
| miR-182-5p, miR-23a-3p | Lipopolysaccharide (LPS)-induced ARDS | Mesenchymal stem cells (MSCs) | Ikbkb, USP5 | Immunomodulation and tissue recovery via IKKβ/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/hedgehog suppression | [35] |
| miR-377-3p | LPS-induced ARDS | Umbilical cord-derived MSCs (UCMSCs) | Regulatory associated protein of mammalian target of mTOR (RPTOR) | Injury recovery by RPTOR suppression and inducing autophagy | [36] |
| miR-22-3p | LPS-induced ARDS | Umbilical cord blood-derived MSCs (UCBMSCs) | Frizzled class receptor 6 (FZD6)/Wnt | Immunomodulation via decreasing FZD6 expression, leading to NF-κB suppression | [37] |
| miR-150 | LPS-induced ARDS | Bone marrow-derived MSCs (BMSCs) | MAPK | Immunomodulation via suppression of MAPK-associated proteins and cleaved caspase-3 and B-cell lymphoma 2 (Bcl-2) promotion | [38] |
| miR-30b-3p | LPS-induced ARDS | MSCs | SAA3 | Immunomodulation via suppression of serum amyloid A-3 (SAA3) | [39] |
| miR-425 | HYRX-induced ARDS | BMSCs | PTEN | PI3K/AKT upregulation via suppressing PTEN | [40] |
| miR-126 | Histone-induced ARDS | Adipose-derived MSCs (AMSCs) | PI3K/AKT | PI3K/AKT upregulation | [41] |
| Unknown | IIR-induced ARDS | BMSCs | TLR4/NF-κB | Immunomodulation via downregulation of toll-like receptor 4 (TLR4)/NF-κB pathway | [42] |
| miR-384-5p | LPS-induced ARDS | BMSCs | Beclin-1 | Immunomodulation via inducing autophagy in disordered macrophages | [43] |
| miR-451 | Burn-induced ARDS | UCMSCs | TLR4/NF-κB | Immunomodulation via TLR4 and NF-κB downregulation | [31] |
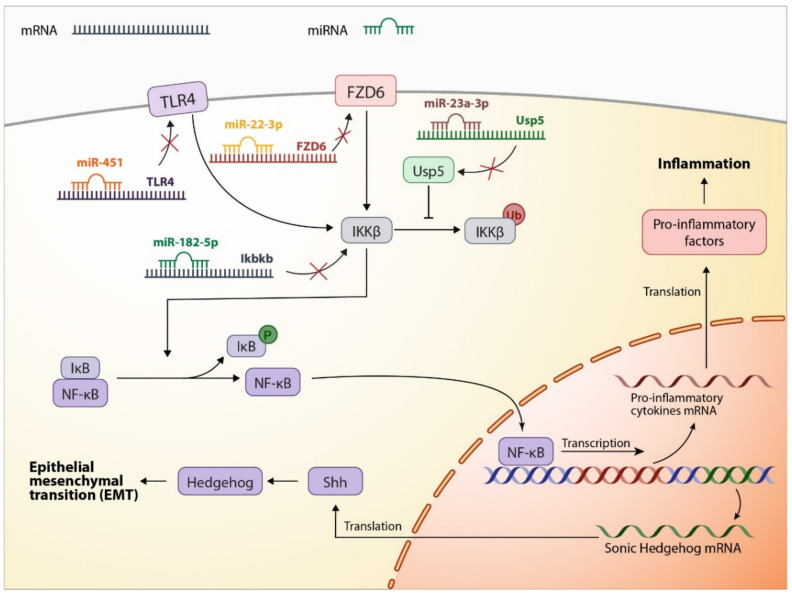
Various in vitro and in vivo studies on LPS-induced ARDS models reported a significant increase in inflammatory factors, leading to enhanced autophagy and EMT progress. Investigations on an LPS-induced murine lung epithelial-12 (MLE-12) model revealed that upregulation in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway is the main factor in LPS-induced inflammation, which further initiates EMT by the hedgehog signaling pathway activation via sonic hedgehog protein transcription. Additional treatment with MSC-Exos suggests NF-κB regulation, leading to inflammation suppression and EMT reverse. Further investigations into the underlying MSC-Exo mechanism of action demonstrate that MSC-Exo contains miR-182-5p and miR-23a-3p, targeting Ikbkb and Usp5 genes, respectively. Ikbkb and Usp5 encode IKKβ (inhibitor of nuclear factor kappa-B kinase subunit beta) and Usp5 (ubiquitin-specific peptidase 5) proteins, and their silencing causes a decrease in IKKβ expression and an increase in its ubiquitination, leading to a coinhibition of the NF-κB pathway [35]. A study on LPS-induced NR8383 cell treatment with UCMSC-Exos demonstrated that UCMSC-Exos can reduce the inflammatory reaction, suppress oxidative stress response, and decrease NF-κB expression via frizzled class receptor 6 (FZD6) downregulation by transferring miR-22-3p into LPS-induced cells and targeting FZD6 directly [37]. Although FZD6 mainly serves as an initiator in the Wnt signaling pathway, it can lead to NF-κB activation via Wnt/β-Catenin signaling pathway mediation [44]. Serum amyloid A-3 (SAA3) is a downstream gene in the NF-κB signaling pathway whose activation enhances proinflammatory cytokine activation [45], and its upregulation has been shown to play a role in inflammation occurrence in MLE-12 cells after LPS treatment. It is shown that MSC-derived exosomal miR-30b-3p is another anti-inflammatory factor in MSC-Exos that can suppress inflammation and enhance cell proliferation by decreasing SAA3 expression [39]. In addition, it is reported that MSC-Exos inhibit inflammation in an intestinal ischemia reperfusion-induced (IIR-induced) ARDS model by NF-κB suppression via downregulating toll-like receptor-4 (TLR4); however, the underlying mechanism has not yet been identified [42].
In addition to NF-κB, MSC-Exos are reported to ameliorate ARDS by manipulating other signaling pathways in different ARDS models. A recent study on an LPS-induced mice ARDS model suggested that hUCMSC-Exos treatment can effectively reverse LPS induction complications, including inflammatory infiltration, increased bronchoalveolar lavage fluid protein concentration, and increased interleukin (IL)-6 and IL-1β concentrations. Moreover, it was revealed that the administration of hUCMSC-Exos significantly enhances autophagy. Further investigations on LPS-treated human pulmonary alveolar epithelial cells and the underlying mechanism of hUCMSC-Exos in autophagy induction stand for the exosomal miR-377-3p pivotal role, which targets the regulatory associated protein of mTOR (RPTOR). Although the exact mechanism by which RPTOR silencing induces autophagy is still unclear, it is shown that exosomal miR-377-3p-mediated RPTOR downregulation can effectively improve LPS-induced ARDS in vitro and in vivo [36]. Figure 2 demonstrates the underlying mechanism of actions revealed by the mentioned studies in which SC-Exos improve alveolar cells in ARDS.
Figure 2.
SC-Exo miRNA cargo regulates dysregulated pathways in ARDS. NF-κB is one of the main pathways in which out of balance activity in ARDS leads to lung damage. NF-κB upregulation during ARDS leads to an increased level of proinflammatory factors and sonic hedgehog (Shh) protein, which triggers the hedgehog signaling pathway and begins epithelial–mesenchymal transition in alveolar-epithelial cells. SC-Exos can restore balance to the NF-κB pathway activity and prevent inflammation and EMT, mediated by several miRNAs targeting different proteins upstream of the NF-κB, including TLR4 and IKKβ. FZD6 is another target of SC-Exo miRNA, and its suppression can lead to NF-κB downregulation; however, it is mainly associated with the Wnt/β-Catenin pathway, and the underlying relation between FZD6 and NF-κB is not fully determined.
As one of the pivotal players in ARDS occurrence and progression, alveolar macrophages can also be affected by MSC-Exos, resulting in ARDS improvement. It is demonstrated that bone marrow-derived MSC (BMSC)-derived exosomes (BMSC-Exos) uptake by alveolar macrophages in LPS-induced ARDS mice can induce their differentiation from a proinflammatory type into an anti-inflammatory type via inhibiting M1 polarization and promoting M2 polarization [46]. Further, it is shown that by targeting and suppressing Beclin-1 mediated by exosomal miR-384-5p, BMSC-Exo treatment can inhibit the viability loss, prevent apoptosis, and attenuate autolysosome and autophagosome punctum formation in LPS-induced alveolar macrophages, which leads to controlling the alveolar macrophage autophagy induced by LPS [43].
3.2. Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a multifunctional chronic lung disease associated with prematurity and is one of the most common complications in preterm infants with low birth weight [47]. BPD can be associated with airway distortion, bronchial muscle hypertrophy, pulmonary hypertension leading to capillary remodeling, and severe inflammation with extensive fibrotic changes, which altogether can cause impaired pulmonary function and reduced exercise tolerance that continues into adulthood [48,49]. The improvement in infant viability in past decades has resulted in more premature infants with diagnosed BPD surviving into adulthood, and, as a consequence, more adult cases with BPD are diagnosed [50]. Therefore, new and effective treatments are required for treating and reversing BPD complications, and MSC-Exos are shown to be promising to address this issue. It is shown that exposing newborn mice to hyperoxia (HYRX; 75% O2) from postnatal day (PN) 1 to PN7 and returning them to room air (NRMX) for the next seven days demonstrates similar histological patterns to the human BPD, characterized by alveolar growth impairment, large airspaces, and incomplete alveolar septation. A more severe model can be achieved by exposing newborn mice for an extra seven days (from PN1 to PN14), resulting in additional collagen deposition. These animal models and MSC-Exos from human bone marrow and the umbilical cord Wharton’s Jelly effects on them were investigated in a study in the short- and long-term. It has been shown that treatment of the HYRX-exposed group with a single dose of intravenous injection of BMSC-Exos or Wharton’s Jelly—a mucoid connective tissue—MSC (WJMSC)-derived exosomes (WJMSC-Exos) at PN4 can dramatically improve the alveolarization and restore lung architecture almost completely in the short-term (PN14) compared with the untreated HYRX-exposed group. This effect was compared with human dermal fibroblast-derived exosomes as a control, which demonstrated no significant protective effect as assessed at PN14. Over a long-term assessment, these effects were studied at PN42, suggesting robust improvement in pulmonary development, significantly decreased collagen deposition, peripheral pulmonary arterial remodeling, HYRX-induced PH improvement, and overall lung function improvement after HYRX-induced lung injury. Additional whole-organ RNA sequencing and a gene ontology analysis on the NRMX- and HYRX-exposed groups at PN7 indicate an upregulation in genes associated with adaptive immune response, inflammatory response, and leukocyte-mediated immunity in the HYRX-exposed group. This HYRX-mediated immune response induction is demonstrated to be modulated in the WJMSC-Exo-treated group by blunting genes involved in inflammation, adaptive immune response, IFN-γ-mediated signaling, and production of cytokines and granulocytes leading to suppressing the M1 (proinflammatory phenotype) macrophage and augmenting the M2-like (anti-inflammatory phenotype) macrophage and shifting the overall transcriptome profile of the HYRX group toward the NRMX group [51]. Another study compared the effect of “early” and “late” intervention of WJMSC-Exos on prolonged neonatal HYRX-induced lung injury. In this study, newborn mice were exposed to HYRX (75% O2) for 14 days and divided the animals into two groups, treated with a single dose of MSC-Exos at PN4 (early intervention) or a serial dose of MSC-Exos at PN18, 25, 32, and 35 (late intervention). It is demonstrated that the late intervention of MSC-Exos can dramatically improve alveolarization, reverse elevations in pulmonary vascular muscularization, ameliorate alterations in the right ventricular hypertrophy, and restore functional exercise capacity in HYRX-exposed mice at PN60, with no substantial difference with the early intervention [52]. Studies on the MSC-Exos mechanism of action suggest tumor necrosis factor-inducible gene 6 protein (TSG-6), an immunomodulatory protein, as a crucial factor in MSC-Exo effects on BPD models. After validating the human WJMSC-Exo immunomodulatory and regenerative effect on BPD models, BPD models and human tracheal aspirates in patients with BPD development were checked for TSG-6 RNA level change, demonstrating a significant elevation in the expression of TSG-6 in comparison with the non-BPD group. However, in the WJMSC-Exo-treated group, the TSG-6 level decreased to that of the NRMX group. By Western blotting, TSG-6 was detected in WJMSC-Exos. Human recombinant TSG-6 was injected intraperitoneally into PBD mouse models at PN2 and 4 to investigate whether TSG-6 in WJMSC-Exos acts as one of the mediators in improving the BPD models, which results in significant immunomodulation and an overall lung architecture improvement. Additionally, loss of function of TSG-6 was performed by the administration of the TSG-6 neutralizing antibody or the introduction of a TSG-6 short interfering RNA (siRNA) into exosome donor cells (25 weeks gestational age human WJMSC). A further analysis suggests no difference between BPD and the exosome-treated group, demonstrating TSG-6 plays a crucial role in the WJMSC-Exo effect on attenuating BPD in mouse models [53].
3.3. Pulmonary Hypertension
Pulmonary hypertension (PH) is a chronic disorder defined as an increase in mean pulmonary arterial pressure (greater than 25 mm Hg at rest). PH has been divided into five subtypes: (I) pulmonary arterial hypertension (PAH); (II) PH caused by left-sided heart disease; (III) PH due to lung disease and/or hypoxia; (IV) chronic thromboembolic PH; and (V) PH with unclear or multifactorial etiologies [54]. Several factors can induce PH, and currently, most therapies focus on improving the symptoms of PH. In recent years, advances in the understanding of underlying molecular mechanisms of PH, such as vascular remodeling and the role of leukocytes, led to the development of new therapies that potentially can reverse the underlying mechanisms of the disease. SCs and their secretome have been proven to reverse the PH process by simultaneously affecting several pathways [55].
Recent studies indicated that SC-Exos are effective in PH treatment. A 2012 study demonstrated that MSC-Exos and human umbilical cord-derived MSC (UCMSC)-derived exosomes (hUCMSC-Exos) inhibit PH through several mechanisms. The injection of MSC-conditioned media into the hypoxia-induced PH mouse model suppressed the increase in the hypoxia-induced mitogenic factor and monocyte chemoattractant protein-1. Further experiments showed that MSC-conditioned, which is depleted from exosomes, had no considerable inhibitory effect, but isolated MSE-derived exosomes suppressed hypoxic inflammation in the animal model. MSC-Exos exert their function by inhibiting signal transducer and the activator of transcription 3 (STAT3); its activation has a crucial role in respiratory epithelial inflammatory responses. In addition, MSC-Exos affect levels of several types of miRNA by suppressing STAT3; the transcription of the miR-17 superfamily is upregulated in pulmonary artery endothelial cells in response to hypoxic conditions. Additionally, the miR-204 level in pulmonary artery smooth muscle cells (PASMCs) is inversely related to the severity of PH. The study suggests that MSC-Exos might attenuate PH by preventing the hypoxic induction of the miR17 superfamily and enhancing the levels of miR-204 [55,56,57].
PH, particularly PAH, is mainly associated with a progressive remodeling of small pulmonary arteries, causing right heart failure and death [58]. Studies indicated that dysregulation of some signaling pathways, such as the Wnt pathway, could involve PAH pathogenesis [59,60]. Wnt5a is a signaling protein that activates the Wnt pathway and regulates cell fate and embryo development. The reduction of Wnt5a has been found as a role player in regulating PAH through vascular remodeling [61]. Zhang et al., demonstrated that MSC-Exos are effective against vascular remodeling and right ventricular hypertrophy caused by monocrotaline-induced PH by regulating Wnt5a. The study also showed that the mechanism of action of MSC-Exos might be related to controlling the bone morphogenetic protein type II receptor (BMPRII). Mutations in the BMPRII gene, as a transmembrane receptor associated with the bone morphogenetic protein pathway, are related to PAH’s pathogenesis. Furthermore, MSC-Exos prevented the endothelial to mesenchymal transition process by regulating the Wnt5a/BMPRII signaling pathway [62,63,64].
Mitochondrial dysfunction is another factor involved in the pathogenesis of PH [65,66]. Sirtuin 4 (SIRT4), a mitochondrial protein, is a member of the mammalian sirtuin family with ADP-ribosyltransferase activity. In response to hypoxic conditions, the expression of the SIRT4 gene increases to block the tricarboxylic acid cycle and protect the cell against oxygen deficiency. However, chronic activation of SIRT4 might lead to mitochondrial dysfunction [67,68]. Hogan et al., indicated that MSC-Exos could decrease SIRT4 gene expression in hypoxia-exposed PASMCs and the semaxinib/hypoxia rat model of PAH. It is proposed that reducing SIRT4 increases tricarboxylic acid cycle metabolites and, consequently, mitochondrial metabolism [69].
3.4. Pulmonary Fibrosis
Pulmonary fibrosis (PF) is a progressive and destructive lung condition driven by the fibrotic response due to various factors. Idiopathic PF (IPF), the most common form of PF, is identified by replacing healthy lung tissue with extracellular matrix and transforming alveolar structure [70,71]. Currently, there are two to drugs approved by the Food and Drug Administration (FDA) for PF, but neither of them can reverse the disease process, and the only available cure is lung transplantation, which has its own risks. According to studies, dysregulation of hedgehog, transforming growth factor beta (TGF-β), notch and fibroblast growth factor signaling pathways associate lung development and repair. However, dysregulation of these pathways can lead to different diseases such as PF [72]. BMSC-Exos have been shown to attenuate PF in the rat model of silica-induced PF. BMSC-Exos can reduce levels of the TGF-β1 and Wnt/β-catenin signaling pathways and inhibit the progress of the EMT process [73].
In recent years, the understanding of the function of miRNA in IPF and related pathways has increased. An alteration of about 10 percent of miRNA in an IPF lung indicates that miRNAs might play essential roles in treating PF. Previous studies demonstrated the role of some types of miRNA, such as let-7, miR-155, miR-21, and miR-29, in the pathogenesis of IPF [74]. Xu et al., showed that human UCMSC-Exos could transfer let-7i-5p into fibroblast cells and interfere with TGFBR1/Smad3 signaling pathways. The effect of UCMSC-Exos against the silica-induced mice model was more beneficial than MRC-5 (human embryonic lung fibroblasts)-derived exosomes (Figure 3a) [75]. Sun et al., demonstrated that menstrual blood-derived SC-derived exosomes contain Let-7 miRNA and relieved bleomycin-induced PF and alveolar epithelial cell damage (Figure 3b). Exosomal Let-7 suppresses the expression of lectin-like oxidized low-density lipoprotein scavenger receptor-1 (LOX1) and has a protective effect on alveolar epithelial cells [76]. Moreover, the Let-7 family and two other miRNAs, miR-30a and miR-99, have been identified in lung spheroid cell-derived exosomes (LSC-Exos). LSCs are derived from adult lungs and contain lung progenitor cells and supporting stromal cells and can be used for therapeutic applications. It was indicated that LSC-Exos, compared with MSC-Exos, have a better performance against the rat model of bleomycin- and silica-induced PF [77,78].
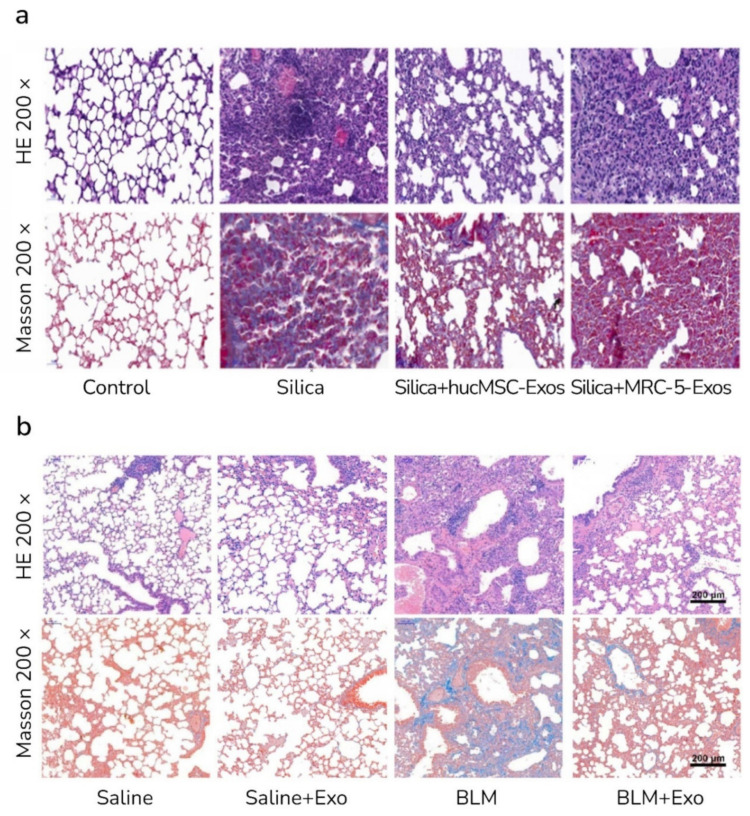
Figure 3.
Effects of SC-Exos on PF in mice. (a) Human UCMSC-derived exosomes (hucMSC-Exos) but not MRC-5-Exos alleviated silica-induced mice model of pulmonary fibrosis (PF) (H&E and Masson staining; magnifications of 200× via light micrograph [75]. (b) Menstrual blood-derived SC-derived exos showed protective effects in bleomycin (BLM)-induced PF in mice (H&E and Masson staining; the scale bar represents 200 μm) [76].
The immune system is another factor associated with the pathogenesis of IPF [79]. It is well known that SC-Exos have immunomodulatory properties, and recent studies showed that at least part of the protective effects of SC-Exos against PF is due to the immune system’s regulation [8]. SC-Exos are reported to regulate the function and behavior of different immune system cell types, such as neutrophil, macrophage, and T cells, and their immunomodulatory properties are effective in IPF treatment [80]. Mansouri et al., showed that MSC-Exos could inhibit and revert the process of bleomycin-induced PF and suggested that this effect may be due to the systemic modulation of monocyte phenotypes [81]. Furthermore, iPSC-derived exosomes (iPSC-Exos) can alleviate PF by suppressing M2-type macrophages. A recent study showed that iPSC-Exos contain high levels of MiR-302a-3p miRNA and detected the binding between MiR-302a-3p and ten-eleven translocation 1 (TET1). TET1 functions as a DNA methylation and gene transcription modulator and may have roles in fibrosis by regulating M2-type macrophages. The study suggests that MiR-302a-3p is a critical mediator in iPSC-Exos that ameliorates PF by TET1 and, consequently, M2-type macrophages [82].
3.5. Asthma
Asthma is one of the most common chronic and nontransmissible lung diseases affecting children and adults. Asthma has been regarded as a heterogeneous condition caused by a complex interplay of genetic and environmental factors. Inhaled corticosteroids remain the mainstay intervention to control asthma, but their effects vary based on the subtype of disease [83,84]. SCs and SC-Exos may be a potential therapy that may use as a complement or alternative medication [85]. Immune system malfunction and disruption in signaling pathways have been associated with the pathophysiology of asthma [86,87], and SC-Exos may be beneficial by regulating the involved pathways [88]. Dong et al., demonstrated that MSC-Exos could reduce lung inflammation and pulmonary hyper-responsiveness in the mice model of severe steroid-resistant asthma. Experiments on LPS-stimulated RAW 264.7 cells showed that MSC-Exos regulate macrophage polarization activation of the NF-κB and PI3K/AKT signaling pathways through tumor necrosis factor receptor-associated factor 1 protein [89]. Moreover, another study found that MSC-Exos could moderately increase lung interstitial macrophages and promote protective effects in ovalbumin-sensitized mice. Interstitial macrophages are shown to produce IL-10 and improve allergic asthma in mice [90]. The miRNA content also plays some roles in the function of exosomes against asthma. A study identified miR-301a-3p in adipose-derived MSC (ASC)-derived exosomes (ASC-Exos) and tested its function against platelet-derived growth factor-treated airway smooth muscle cells (ASMCs). The results imply that the exosomal miR-301a-3p reduces remodeling and inflammation of PDGF-BB-treated ASMCs by targeting the 3ʹUTR region of STAT3 [90].
3.6. Other Pulmonary Disorders
Sepsis is a life-threatening condition caused by an improper host response to infection. The lung is the most common site of the infection and may fail during sepsis [91]. Recent studies proposed some roles for SC-Exos in improving sepsis-induced lung failure. SC-Exos could modulate various pathways involved in sepsis-induced lung injury pathogenesis and regulate the immune system. IL-27 is a cytokine that increases during sepsis, and its suppression could be a potential target in treating sepsis [92]. ASC-Exos have been shown to attenuate lung injury in septic mice by decreasing pulmonary macrophages and reducing their IL-27 expression [93]. The molecular mechanism of action of SC-Exos could be related to their miRNA content. A study identified exosomal miR-16-5p derived from ASC as a regulator of TLR4 in septic mice. By this mechanism, ASC-Exos promoted macrophage polarization and alleviated sepsis-induced lung injury [93].
SC-Exos have been shown to be effective against cigarette smoke (CS)-induced lung inflammation. Xu et al., demonstrated that BMSC-Exos could regulate inflammatory and apoptosis-related factors by repressing the HMGB1/NF-κB pathways. Regulatory effects of exosomes reduced CS-induced lung injury in vivo and in vitro [94]. In another study, immunomodulatory effects of ASC-Exos against CS-induced lung injury were determined. The results suggest that alveolar macrophage pyroptosis is associated with CS-induced lung inflammation, and ASC-Exos ameliorate the inflammation and mucus hypersecretion in CS-exposed mice by preventing the pyroptosis process in alveolar macrophages [95]. Some recent studies reported the benefits of SC-Exos to treat other pulmonary conditions, such as ventilator-induced lung injury [95], diffuse alveolar hemorrhage [96], ischemia/reperfusion injury (IRI) [97], pulmonary embolism [98], and basement membrane-induced fibrosis [99].
4. Pulmonary Delivery of Stem Cell-Derived Exosomes
Several routes may be used to deliver drugs into the body, and preferring one depends on multiple factors such as the disease, the affected organ, and the drug’s properties [100]. The pulmonary route has been used for thousands of years and has several advantages over other delivery routes: The lungs have a large surface area that is suitable for the rapid absorption of drugs; there are no extreme PH and distractive enzymes that exist in other routes; drugs do not need to pass first-pass liver; drugs rapidly transfer from the alveolar area to the lungs, and, finally, delivering drugs directly to the lungs when the lungs are the disease site minimizes the side effects on other organs [101,102,103]. However, the lung has its defense mechanism; for instance, macrophages and mucus capture and remove foreign particles in the airway, and to deliver therapeutic agents, this removal by macrophages should be taken into account [104]. Nevertheless, the pulmonary route is appropriate for drug delivery and is used for various respiratory and lung diseases such as asthma [105]. Intratracheal instillation and inhalation are two noninvasive pulmonary drug delivery approaches recently used to introduce SC-Exos to the lungs.
4.1. Intratracheal Instillation
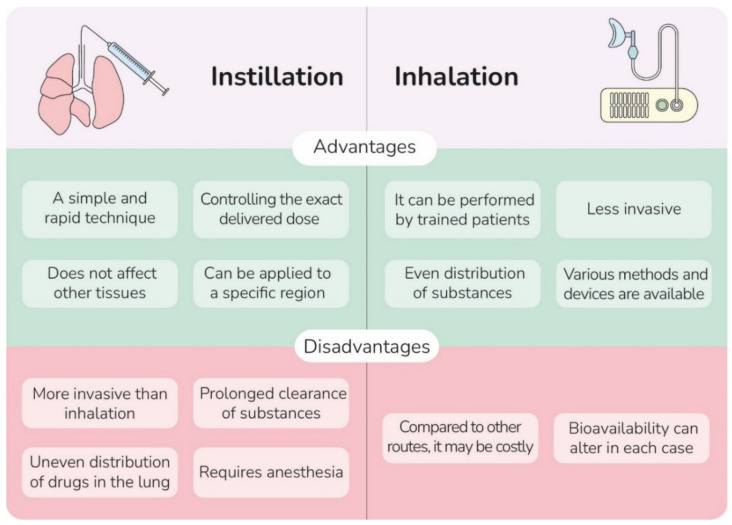
The intratracheal instillation process can expose chemical substances directly to the trachea [15]. Instillation has some advantages over inhalation; compared to inhalation, installation is a simple technique, allows precise determination of delivered dose to the lungs, prevents the exposure of administered substances to the skin, and permits introducing substances to a specific region in the lungs. However, the use of this method is limited mainly because of its invasive nature, uneven distribution, and prolonged clearance of substances in the lungs (Figure 4) [106]. Instillation conventionally has been used to evaluate the respiratory toxicity of substances and the induction of pulmonary inflammation in animal models, but many studies employed this method to administer therapeutic agents [107]. A few studies investigated the effects of pulmonary instillation of SC-Exos on animal models of some lung disorders. Table 2. summarizes some of these studies.
Figure 4.
A comparison of advantages and disadvantages of intratracheal instillation and inhalation related to human use.
Table 2.
Preclinical studies that used intratracheal instillation for delivering SC-Exos.
| Disease | Source of Exosomes |
Instilled Dose | Animal Model | Refs |
|---|---|---|---|---|
| Severe steroid-resistant asthma (SSRA) | Human UCMSCs |
100 μg Exos in 50 μL Phosphate-buffered saline (PBS) | Ovalbumin (OVA)/: (complete Freud’s adjuvant) CFA-induced SSRA mice | [89] |
| Ischemia/reperfusion (I/R) injury | Murine BMSC | Exos derived from 2 × 106 in 30 μL PBS | I/R model mice | [97] |
| Cigarette smoke (CS)-induced lung injury |
Human ASC | 30 μL of purified Exos solution (as explained in the original paper) | CS-exposed C57BL/6 mice | [95] |
| ARDS | BMSC | 50 mg Exos in 10 mL PBS and 100 mg Exos in 10 mL PBS | LPS-induced ARDS C57BL/6 mice | [46] |
| Phosgene-induced acute lung injury (ALI) | Rat BMSC | Exos isolated from 3 × 106 MSC (50 mL) | Phosgene-induced ALI Sprague–Dawley rat | [108] |
4.2. Inhalation
Inhalation therapy has become more prevalent in recent years as it offers a noninvasive and efficient drug delivery route to the lungs. For a therapeutic agent to be inhaled, in combination with the aerosol generator of choice, it should form an aerosol droplet within a highly respirable distribution, usually considered to be between 1 and 5 μm (Andrersen Cascade Impactor, operated at 28.3 liters per minute). Additionally, aerosol generator devices are medical devices that aerosolize drugs to deliver them via inhalation [109]. Currently, there are three prevalent aerosol generator technologies in commercial use, with many new technologies under development or as of yet not commercially available: (I) pressurized metered-dose inhalers (pMDIs); (II) dry powder inhalers; and (III) medical nebulizers [110]. The choice of device should consider the required target lung dose and the likely patient intervention at the time of administration, as both have been shown to have significant influence [111,112]. The pMDIs are the most prescribed device for lung diseases such as COPD and asthma, but regarding clinical research, nebulizers are the most used instrument [113]. Nebulizers are preferred for early experiments because formulating a liquid is more accessible than making dry powder, and also nebulizers are appropriate for use on animal models [114]. Among the three common types of nebulizers—jet, ultrasonic, and vibrating mesh nebulizers (VMNs)—the jet nebulizers have been the most commonly used [115]. Some studies investigated jet and VMNs to deliver SCs into the lungs. Ultrasonic nebulizers are not considered appropriate for SC delivery, primarily because of the excessive heat produced and its effect on SC viability [116]. A 2013 study showed that, among three nebulizers, MSCs had the highest survival rate when jet nebulizers were used as the delivery device; however, aerosol generator technologies continue to advance, and so they warrant further investigations [117,118,119].
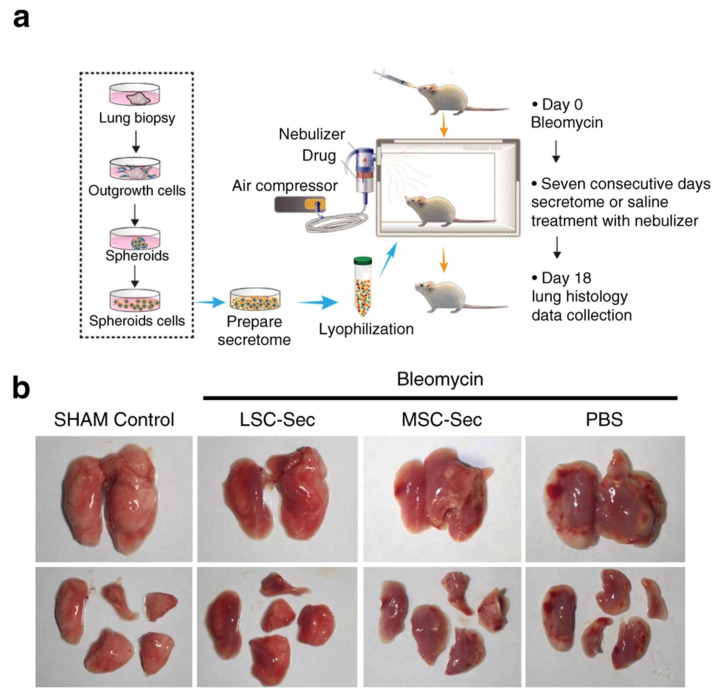
A few studies have investigated the effects of nebulization on SC-secretome or exosomes and its application in pulmonary diseases. The VMN was shown to be a suitable device to deliver BMSC and UCMSC secretome to the lungs [116]. Moreover, Dinh et al., used a jet nebulizer to introduce MSC- and LSC-Exos to the silica model of induced PF. Approximately 10 × 109 exosome particles per kg of body weight were given through the nebulizer, and any adverse effects were observed on the heart, kidneys, liver, and spleen after treatment. Interestingly, nebulized secretomes and exosomes alleviated PF in silica-induced models and improved lung health (Figure 5) [77]. Inhalation delivery of exosomes is being investigated in several recent clinical trials. The following section will mainly focus on the role of exosomes and their delivery route in these trials.
Figure 5.
Administration of SC-Exos via the inhalation route. (a) The schematic representation of lung spheroid cell (LSC)-secretome (LSC-sec) preparation and animal model treatment with aerosolized secretome. The mice model of PF was obtained by intratracheal injection of bleomycin, and after ten days, 10 mg of secretome protein per kg of body weight was introduced to mice by a nebulizer for seven consecutive days. On day 18, animals were euthanized, and lungs were harvested for histological analysis. (b) Macroscopic view of harvested lungs from bleomycin-induced mice. LSC-sec and MSC-sec improved the lung condition in PF mice [77].
5. Clinical Trials
Some clinical trials have been conducted to evaluate the benefits of SC-Exos against lung disorders. Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that was first isolated from the respiratory epithelium of patients suffering from unexplained pneumonia in 2019 [120,121]. COVID-19 affects different organs and causes serious complications, such as pneumonia, ARDS, acute liver injury, and multiorgan failure [122,123]. SCs and SC-Exos, with their immunomodulatory and protective properties, can help control and prevent destructive COVID-19 complications [8]. Currently, a number of clinical and preclinical trials are underway in order to evaluate the safety and efficacy of SCs and SC-Exos in severe COVID-19 patients. Table 3 indicates some of the clinical trials that assessed the safety and effectiveness of SC-Exos against respiratory diseases.
Table 3.
Clinical trials conducted with SC-Exos on patients with respiratory diseases (www.clinicaltrials.gov; accessed on 20 April 2022.).
| Trail Identification | Official Title | Conditions | Source of Exosomes |
Administration Route | Status |
|---|---|---|---|---|---|
| NCT04602104 | A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS | ARDS | Human MSCs | Inhalation | Recruiting |
| NCT04544215 | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for the Treatment of Pulmonary Infection | Drug-resistant | Human ASCs | Inhalation | Recruiting |
| NCT04313647 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes In Healthy Volunteers | Healthy | MSCs | Inhalation | Completed |
| NCT04602442 | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia | COVID-19 Pneumonia |
MSCs | Inhalation | Enrolling by invitation |
| NCT04491240 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. | COVID-19 Pneumonia |
MSCs | Inhalation | Completed |
| NCT04276987 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | COVID-19 Pneumonia |
Allogenic ASCs | Inhalation | Completed |
| NCT05216562 | Efficacy and Safety of EXOSOME-MSC Therapy to Reduce Hyper-inflammation In Moderate COVID-19 Patients | SARS-CoV2 Infection | MSCs | Intravenous | Recruiting |
| NCT04798716 | The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19 | COVID-19 ARDS |
MSCs | Intravenous | Not yet recruiting |
According to the ongoing and completed trials, inhalation is the preferred route in clinical studies (rows 1–6 in Table 3). In one study, preclinical and clinical efficacy and safety of MSC-derived EVs were determined. Nebulizing human ASC-derived EVs (haMSC-EVs) using VMNs showed an 80 percent increase in survival rate in P. aeruginosa-induced murine lung injury model. Considerable side effects were not observed after administering haMSC-EVs nebulization to twenty-four volunteers. Based on these results, a randomized, double-blind, placebo-controlled clinical study is in progress to determine the efficacy of haMSC-EV nebulizers for treating ARDS (NCT04313647) [124]. In another nonrandomized open-label clinical trial, 24 COVID-19 patients were treated with exosomes (ExoFloTM) derived from allogeneic BMSC. At the end of the study, the intravenous administration of exosomes led to a survival rate of 83 percent. In addition, ExoFloTM improved oxygenation, reduced cytokine storm, and regulated the immune system [125]. Future clinical trials are needed to determine all aspects of SC-Exos in the treatment of respiratory diseases.
6. Conclusions and Future Perspective
Cell-free therapy has gained much momentum and focus in recent years, and numerous studies have investigated the therapeutic potential of exosomes. In this review, we discussed the clinical and preclinical studies around SC-Exos for improving the condition of patients suffering from lung diseases. Further, we talked about the potential of pulmonary drug delivery as a promising route for administering SC-Exos. Although our knowledge of exosomes has increased steadily over recent years, the exact mechanism of action of exosomes remains to be elucidated. Recent studies attributed the function of exosomes to their RNA cargos, but the precise role of other components is not clearly understood. In addition, limitations such as high costs and technical challenges make it difficult to isolate and purify considerable amounts of particular exosomes; however, recent progress in detecting exosomal bioactive molecules responsible for their therapeutic effects can lead to designing exosome-mimic particles with similar therapeutic effects. Another challenge is to immortalize SCs in order to obtain excess quantities of exosomes. However, the cell should be manipulated with its risk and limitations to achieve this goal. Further studies should be carried out to determine the potential of aerosolized SC-Exos and identify the proper device to administer them.
Author Contributions
M.H.A. and N.G. writing—original draft preparation and editing, M.D. project administration— review and editing, R.M. review and editing. M.D. and R.M. supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ritchie H., Roser M. Causes of Death. [(accessed on 20 April 2022)]. Available online: https://ourworldindata.org/causes-of-death.
- 2.Doroudian M., Azhdari M.H., Goodarzi N., O’Sullivan D., Donnelly S.C. Smart Nanotherapeutics and Lung Cancer. Pharmaceutics. 2021;13:1972. doi: 10.3390/pharmaceutics13111972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doroudian M., MacLoughlin R., Poynton F., Prina-Mello A., Donnelly S.C. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74:965–976. doi: 10.1136/thoraxjnl-2019-213037. [DOI] [PubMed] [Google Scholar]
- 4.Popowski K., Lutz H., Hu S., George A., Dinh P.U., Cheng K. Exosome therapeutics for lung regenerative medicine. J. Extracell. Vesicles. 2020;9:1785161. doi: 10.1080/20013078.2020.1785161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doroudian M., O’Neill A., O’Reilly C., Tynan A., Mawhinney L., McElroy A., Webster S.S., MacLoughlin R., Volkov Y., Armstrong M.E., et al. Aerosolized drug-loaded nanoparticles targeting migration inhibitory factors inhibit Pseudomonas aeruginosa-induced inflammation and biofilm formation. Nanomedicine. 2020;15:2933–2953. doi: 10.2217/nnm-2020-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacakova L., Zarubova J., Travnickova M., Musilkova J., Pajorova J., Slepicka P., Kasalkova N.S., Svorcik V., Kolska Z., Motarjemi H., et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Geiger S., Hirsch D., Hermann F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017;26:170044. doi: 10.1183/16000617.0044-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi Dehbidi M., Goodarzi N., Azhdari M.H., Doroudian M. Mesenchymal stem cells and their derived exosomes to combat COVID-19. Rev. Med. Virol. 2022;32:e2281. doi: 10.1002/rmv.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashemian S.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S.E., Hossieni H., Keshel S.H., Naderpour Z., Hajizadeh-Saffar E., et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Res. Ther. 2021;12:91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanjani N.A., Esmaelizad N., Zanganeh S., Gharavi A.T., Heidarizadeh P., Radfar M., Omidi F., MacLoughlin R., Doroudian M. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit. Rev. Oncol. Hematol. 2022;169:103565. doi: 10.1016/j.critrevonc.2021.103565. [DOI] [PubMed] [Google Scholar]
- 11.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamzah R.N., Alghazali K.M., Biris A.S., Griffin R.J. Exosome Traceability and Cell Source Dependence on Composition and Cell-Cell Cross Talk. Int. J. Mol. Sci. 2021;22:5346. doi: 10.3390/ijms22105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C., Zhao L., Zhang L., Bao Q., Li L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020;11:377. doi: 10.1186/s13287-020-01895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruge C.A., Kirch J., Lehr C.M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013;1:402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto Y., Izumi H., Yoshiura Y., Fujishima K., Yatera K., Yamamoto K. Usefulness of Intratracheal Instillation Studies for Estimating Nanoparticle-Induced Pulmonary Toxicity. Int. J. Mol. Sci. 2016;17:165. doi: 10.3390/ijms17020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin B.K., Williams R.W. Emerging aerosol drug delivery strategies: From bench to clinic. Adv. Drug Deliv. Rev. 2014;75:141–148. doi: 10.1016/j.addr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.MacLoughlin R., Telfer C., Clark A., Fink J. Aerosol: A Novel Vehicle for Pharmacotherapy in Neonates. Curr. Pharm. Des. 2017;23:5928–5934. doi: 10.2174/1381612823666170918122136. [DOI] [PubMed] [Google Scholar]
- 18.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 20.Booth A.M., Fang Y., Fallon J.K., Yang J.M., Hildreth J.E., Gould S.J. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y., Wu N., Gan X., Yan W., Morrell J.C., Gould S.J. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 24.Meyer N.J., Gattinoni L., Calfee C.S. Acute respiratory distress syndrome. Lancet. 2021;398:622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson B.T., Chambers R.C., Liu K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 26.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoulikha M., Xiao Q., Boafo G.F., Sallam M.A., Chen Z., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan E., Brodie D., Slutsky A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 29.Loy H., Kuok D.I.T., Hui K.P.Y., Choi M.H.L., Yuen W., Nicholls J.M., Peiris J.S.M., Chan M.C.W. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. J. Infect. Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao G.C., Gong C.C., Wang Z., Sun M.X., Pei Z.P., Meng W.Q., Cen J.F., He X.W., Lu Y., Xu Q.Q., et al. BMSC-derived exosomes ameliorate sulfur mustard-induced acute lung injury by regulating the GPRC5A-YAP axis. Acta Pharmacol. Sin. 2021;42:2082–2093. doi: 10.1038/s41401-021-00625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J.S., Du J., Cheng X., Zhang X.Z., Li Y., Chen X.L. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J. Chin. Med. Assoc. 2019;82:895–901. doi: 10.1097/JCMA.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 32.Kaspi H., Semo J., Abramov N., Dekel C., Lindborg S., Kern R., Lebovits C., Aricha R. MSC-NTF (NurOwn(R)) exosomes: A novel therapeutic modality in the mouse LPS-induced ARDS model. Stem Cell Res. Ther. 2021;12:72. doi: 10.1186/s13287-021-02143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Deng X., Ji X., Shi X., Ying Z., Shen K., Xu D., Cheng Z. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf-2/ARE and NF-kappaB signaling pathways. PeerJ. 2020;8:e9928. doi: 10.7717/peerj.9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui X., Liu W., Liu Z. Exosomal lncRNA-p21 derived from mesenchymal stem cells protects epithelial cells during LPS-induced acute lung injury by sponging miR-181. Acta Biochim. Biophys. Sin. 2021;53:748–757. doi: 10.1093/abbs/gmab043. [DOI] [PubMed] [Google Scholar]
- 35.Xiao K., He W., Guan W., Hou F., Yan P., Xu J., Zhou T., Liu Y., Xie L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-kappaB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. doi: 10.1038/s41419-020-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X., Yi X., Lv H., Sui X., Lu P., Li L., An Y., Yang Y., Yi H., Chen G. MicroRNA-377-3p released by mesenchymal stem cell exosomes ameliorates lipopolysaccharide-induced acute lung injury by targeting RPTOR to induce autophagy. Cell Death Dis. 2020;11:657. doi: 10.1038/s41419-020-02857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y., Liu J., Chen P., Lin L., Luo Y., Ma X., Lin J., Shen Y., Zhang L. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021;269:119004. doi: 10.1016/j.lfs.2020.119004. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Xu D., Yu Z., Fu Z., Lv Z., Meng L., Zhao X. Exosomal miR-150 partially attenuated acute lung injury by mediating microvascular endothelial cells and MAPK pathway. Biosci. Rep. 2022;42:BSR20203363. doi: 10.1042/BSR20203363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi X., Wei X., Lv H., An Y., Li L., Lu P., Yang Y., Zhang Q., Yi H., Chen G. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp. Cell Res. 2019;383:111454. doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y., Li J., Yuan R., Deng Z., Wu X. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425. Arch. Biochem. Biophys. 2021;697:108712. doi: 10.1016/j.abb.2020.108712. [DOI] [PubMed] [Google Scholar]
- 41.Mizuta Y., Akahoshi T., Guo J., Zhang S., Narahara S., Kawano T., Murata M., Tokuda K., Eto M., Hashizume M., et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res. Ther. 2020;11:508. doi: 10.1186/s13287-020-02015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J., Chen T., Lei P., Tang X., Huang P. Exosomes Released by Bone Marrow Mesenchymal Stem Cells Attenuate Lung Injury Induced by Intestinal Ischemia Reperfusion via the TLR4/NF-kappaB Pathway. Int. J. Med. Sci. 2019;16:1238–1244. doi: 10.7150/ijms.35369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X., Gao C., Wang Y., Niu L., Jiang S., Pan S. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxid. Med. Cell. Longev. 2021;2021:9973457. doi: 10.1155/2021/9973457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma B., Hottiger M.O. Crosstalk between Wnt/beta-Catenin and NF-kappaB Signaling Pathway during Inflammation. Front. Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djurec M., Grana O., Lee A., Troule K., Espinet E., Cabras L., Navas C., Blasco M.T., Martin-Diaz L., Burdiel M., et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc. Natl. Acad. Sci. USA. 2018;115:E1147–E1156. doi: 10.1073/pnas.1717802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng H., Wu L., Liu M., Zhu L., Chen Y., Zhou H., Shi X., Wei J., Zheng L., Hu X., et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate LPS-Induced ARDS by Modulating Macrophage Polarization Through Inhibiting Glycolysis in Macrophages. Shock. 2020;54:828–843. doi: 10.1097/SHK.0000000000001549. [DOI] [PubMed] [Google Scholar]
- 47.Thebaud B., Goss K.N., Laughon M., Whitsett J.A., Abman S.H., Steinhorn R.H., Aschner J.L., Davis P.G., McGrath-Morrow S.A., Soll R.F., et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers. 2019;5:78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilfillan M., Bhandari A., Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974. doi: 10.1136/bmj.n1974. [DOI] [PubMed] [Google Scholar]
- 49.Cassady S.J., Lasso-Pirot A., Deepak J. Phenotypes of Bronchopulmonary Dysplasia in Adults. Chest. 2020;158:2074–2081. doi: 10.1016/j.chest.2020.05.553. [DOI] [PubMed] [Google Scholar]
- 50.Bancalari E., Jain D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology. 2019;115:384–391. doi: 10.1159/000497422. [DOI] [PubMed] [Google Scholar]
- 51.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., Kwong A., Mitsialis S.A., Kourembanas S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willis G.R., Fernandez-Gonzalez A., Reis M., Yeung V., Liu X., Ericsson M., Andrews N.A., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J. Extracell. Vesicles. 2020;9:1790874. doi: 10.1080/20013078.2020.1790874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Cao J., Lv W., Mou H. CircRNA_100395 Carried by Exosomes From Adipose-Derived Mesenchymal Stem Cells Inhibits the Malignant Transformation of Non-Small Cell Lung Carcinoma Through the miR-141-3p-LATS2 Axis. Front. Cell Dev. Biol. 2021;9:663147. doi: 10.3389/fcell.2021.663147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humbert M., Montani D., Evgenov O.V., Simonneau G. Definition and classification of pulmonary hypertension. Handb. Exp. Pharmacol. 2013;218:3–29. doi: 10.1007/978-3-642-38664-0_1. [DOI] [PubMed] [Google Scholar]
- 55.Klinger J.R., Pereira M., Del Tatto M., Brodsky A.S., Wu K.Q., Dooner M.S., Borgovan T., Wen S., Goldberg L.R., Aliotta J.M., et al. Mesenchymal Stem Cell Extracellular Vesicles Reverse Sugen/Hypoxia Pulmonary Hypertension in Rats. Am. J. Respir. Cell Mol. Biol. 2020;62:577–587. doi: 10.1165/rcmb.2019-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Courboulin A., Paulin R., Giguere N.J., Saksouk N., Perreault T., Meloche J., Paquet E.R., Biardel S., Provencher S., Cote J., et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pullamsetti S.S., Doebele C., Fischer A., Savai R., Kojonazarov B., Dahal B.K., Ghofrani H.A., Weissmann N., Grimminger F., Bonauer A., et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 58.Thompson A.A.R., Lawrie A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol. Med. 2017;23:31–45. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Ueland T., Abraityte A., Norum H., Varathalingam S., Gullestad L., Aukrust P., Andreassen A.K. Circulating regulators of the wingless pathway in precapillary pulmonary hypertension. Respirology. 2021;26:574–581. doi: 10.1111/resp.14048. [DOI] [PubMed] [Google Scholar]
- 60.Laumanns I.P., Fink L., Wilhelm J., Wolff J.C., Mitnacht-Kraus R., Graef-Hoechst S., Stein M.M., Bohle R.M., Klepetko W., Hoda M.A., et al. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2009;40:683–691. doi: 10.1165/rcmb.2008-0153OC. [DOI] [PubMed] [Google Scholar]
- 61.Yuan K., Shamskhou E.A., Orcholski M.E., Nathan A., Reddy S., Honda H., Mani V., Zeng Y., Ozen M.O., Wang L., et al. Loss of Endothelium-Derived Wnt5a Is Associated with Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation. 2019;139:1710–1724. doi: 10.1161/CIRCULATIONAHA.118.037642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z., Ge L., Zhang S., Wang J., Jiang W., Xin Q., Luan Y. The protective effects of MSC-EXO against pulmonary hypertension through regulating Wnt5a/BMP signalling pathway. J. Cell. Mol. Med. 2020;24:13938–13948. doi: 10.1111/jcmm.16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hautefort A., Mendes-Ferreira P., Sabourin J., Manaud G., Bertero T., Rucker-Martin C., Riou M., Adao R., Manoury B., Lambert M., et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation. 2019;139:932–948. doi: 10.1161/CIRCULATIONAHA.118.033744. [DOI] [PubMed] [Google Scholar]
- 64.Dunmore B.J., Jones R.J., Toshner M.R., Upton P.D., Morrell N.W. Approaches to treat pulmonary arterial hypertension by targeting BMPR2: From cell membrane to nucleus. Cardiovasc. Res. 2021;117:2309–2325. doi: 10.1093/cvr/cvaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Culley M.K., Chan S.Y. Mitochondrial metabolism in pulmonary hypertension: Beyond mountains there are mountains. J. Clin. Investig. 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marshall J.D., Bazan I., Zhang Y., Fares W.H., Lee P.J. Mitochondrial dysfunction and pulmonary hypertension: Cause, effect, or both. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314:L782–L796. doi: 10.1152/ajplung.00331.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood J.G., Schwer B., Wickremesinghe P.C., Hartnett D.A., Burhenn L., Garcia M., Li M., Verdin E., Helfand S.L. Sirt4 is a mitochondrial regulator of metabolism and lifespan in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2018;115:1564–1569. doi: 10.1073/pnas.1720673115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B., Che W., Xue J., Zheng C., Tang K., Zhang J., Wen J., Xu Y. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell. Physiol. Biochem. 2013;32:655–662. doi: 10.1159/000354469. [DOI] [PubMed] [Google Scholar]
- 69.Hogan S.E., Rodriguez Salazar M.P., Cheadle J., Glenn R., Medrano C., Petersen T.H., Ilagan R.M. Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L723–L737. doi: 10.1152/ajplung.00058.2018. [DOI] [PubMed] [Google Scholar]
- 70.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 71.Kishore A., Petrek M. Roles of Macrophage Polarization and Macrophage-Derived miRNAs in Pulmonary Fibrosis. Front. Immunol. 2021;12:678457. doi: 10.3389/fimmu.2021.678457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chanda D., Otoupalova E., Smith S.R., Volckaert T., De Langhe S.P., Thannickal V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang E., Geng X., Shan S., Li P., Li S., Li W., Yu M., Peng C., Wang S., Shao H., et al. Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/beta-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol. Mech. Methods. 2021;31:655–666. doi: 10.1080/15376516.2021.1950250. [DOI] [PubMed] [Google Scholar]
- 74.Pandit K.V., Milosevic J., Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Xu C., Hou L., Zhao J., Wang Y., Jiang F., Jiang Q., Zhu Z., Tian L. Exosomal let-7i-5p from three-dimensional cultured human umbilical cord mesenchymal stem cells inhibits fibroblast activation in silicosis through targeting TGFBR1. Ecotoxicol. Environ. Saf. 2022;233:113302. doi: 10.1016/j.ecoenv.2022.113302. [DOI] [PubMed] [Google Scholar]
- 76.Sun L., Zhu M., Feng W., Lin Y., Yin J., Jin J., Wang Y. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid. Med. Cell. Longev. 2019;2019:4506303. doi: 10.1155/2019/4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinh P.C., Paudel D., Brochu H., Popowski K.D., Gracieux M.C., Cores J., Huang K., Hensley M.T., Harrell E., Vandergriff A.C., et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020;11:1064. doi: 10.1038/s41467-020-14344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry E., Cores J., Hensley M.T., Anthony S., Vandergriff A., de Andrade J.B., Allen T., Caranasos T.G., Lobo L.J., Cheng K. Adult Lung Spheroid Cells Contain Progenitor Cells and Mediate Regeneration in Rodents with Bleomycin-Induced Pulmonary Fibrosis. Stem Cells Transl. Med. 2015;4:1265–1274. doi: 10.5966/sctm.2015-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shenderov K., Collins S.L., Powell J.D., Horton M.R. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J. Clin. Investig. 2021;131:e143226. doi: 10.1172/JCI143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan J.L., Lau S.N., Leaw B., Nguyen H.P.T., Salamonsen L.A., Saad M.I., Chan S.T., Zhu D., Krause M., Kim C., et al. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl. Med. 2018;7:180–196. doi: 10.1002/sctm.17-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mansouri N., Willis G.R., Fernandez-Gonzalez A., Reis M., Nassiri S., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4:e128060. doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y., Gao Y., Zhang W., Chen Y., Jin M., Yang Z. Exosomes derived from induced pluripotent stem cells suppresses M2-type macrophages during pulmonary fibrosis via miR-302a-3p/TET1 axis. Int. Immunopharmacol. 2021;99:108075. doi: 10.1016/j.intimp.2021.108075. [DOI] [PubMed] [Google Scholar]
- 83.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 84.Agache I., Eguiluz-Gracia I., Cojanu C., Laculiceanu A., Del Giacco S., Zemelka-Wiacek M., Kosowska A., Akdis C.A., Jutel M. Advances and highlights in asthma in 2021. Allergy. 2021;76:3390–3407. doi: 10.1111/all.15054. [DOI] [PubMed] [Google Scholar]
- 85.Tynecka M., Moniuszko M., Eljaszewicz A. Old Friends with Unexploited Perspectives: Current Advances in Mesenchymal Stem Cell-Based Therapies in Asthma. Stem Cell Rev. Rep. 2021;17:1323–1342. doi: 10.1007/s12015-021-10137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammad H., Lambrecht B.N. The basic immunology of asthma. Cell. 2021;184:1469–1485. doi: 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 87.Koopmans T., Gosens R. Revisiting asthma therapeutics: Focus on WNT signal transduction. Drug Discov. Today. 2018;23:49–62. doi: 10.1016/j.drudis.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Song J., Zhu X.M., Wei Q.Y. MSCs reduce airway remodeling in the lungs of asthmatic rats through the Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:11199–11211. doi: 10.26355/eurrev_202011_23608. [DOI] [PubMed] [Google Scholar]
- 89.Dong B., Wang C., Zhang J., Zhang J., Gu Y., Guo X., Zuo X., Pan H., Hsu A.C., Wang G., et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021;12:204. doi: 10.1186/s13287-021-02244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren J., Liu Y., Yao Y., Feng L., Zhao X., Li Z., Yang L. Intranasal delivery of MSC-derived exosomes attenuates allergic asthma via expanding IL-10 producing lung interstitial macrophages in mice. Int. Immunopharmacol. 2021;91:107288. doi: 10.1016/j.intimp.2020.107288. [DOI] [PubMed] [Google Scholar]
- 91.Gotts J.E., Matthay M.A. Sepsis: Pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 92.Morrow K.N., Coopersmith C.M., Ford M.L. IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Front. Immunol. 2019;10:1982. doi: 10.3389/fimmu.2019.01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X., Liu D., Zhang X., Yang L., Xia Z., Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022;8:18. doi: 10.1038/s41420-021-00785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu B., Gan C.X., Chen S.S., Li J.Q., Liu M.Z., Guo G.H. BMSC-derived exosomes alleviate smoke inhalation lung injury through blockade of the HMGB1/NF-kappaB pathway. Life Sci. 2020;257:118042. doi: 10.1016/j.lfs.2020.118042. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Z., Lian X., Su X., Wu W., Zeng Y., Chen X. Exosomes derived from adipose-derived stem cells alleviate cigarette smoke-induced lung inflammation and injury by inhibiting alveolar macrophages pyroptosis. Respir. Res. 2022;23:5. doi: 10.1186/s12931-022-01926-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X., Wei Q., Sun H., Zhang X., Yang C., Tao Y., Nong G. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization to Attenuate Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage in Mice. Int. J. Stem Cells. 2021;14:331–340. doi: 10.15283/ijsc20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J.W., Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 98.Mao H.Y., Liu L.N., Hu Y.M. Mesenchymal stem cells-derived exosomal miRNA-28-3p promotes apoptosis of pulmonary endothelial cells in pulmonary embolism. Eur. Rev. Med. Pharmacol. Sci. 2020;24:10619–10631. doi: 10.26355/eurrev_202010_23420. [DOI] [PubMed] [Google Scholar]
- 99.Royce S.G., Patel K.P., Mao W., Zhu D., Lim R., Samuel C.S. Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease. Br. J. Pharmacol. 2019;176:2195–2208. doi: 10.1111/bph.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jain K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020;2059:1–54. doi: 10.1007/978-1-4939-9798-5_1. [DOI] [PubMed] [Google Scholar]
- 101.Ali M. CHAPTER 9—Pulmonary Drug Delivery. In: Kulkarni V.S., editor. Handbook of Non-Invasive Drug Delivery Systems. William Andrew Publishing; Boston, MA, USA: 2010. pp. 209–246. [Google Scholar]
- 102.Brave H., MacLoughlin R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery. Int. J. Mol. Sci. 2020;21:6435. doi: 10.3390/ijms21176435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gulati N., Chellappan D.K., MacLoughlin R., Dua K., Dureja H. Inhaled nano-based therapeutics for inflammatory lung diseases: Recent advances and future prospects. Life Sci. 2021;285:119969. doi: 10.1016/j.lfs.2021.119969. [DOI] [PubMed] [Google Scholar]
- 104.Masterson C.H., McCarthy S.D., O’Toole D., Laffey J.G. The role of cells and their products in respiratory drug delivery: The past, present, and future. Expert Opin. Drug Deliv. 2020;17:1689–1702. doi: 10.1080/17425247.2020.1814732. [DOI] [PubMed] [Google Scholar]
- 105.O’Byrne P., Fabbri L.M., Pavord I.D., Papi A., Petruzzelli S., Lange P. Asthma progression and mortality: The role of inhaled corticosteroids. Eur. Respir. J. 2019;54:1900491. doi: 10.1183/13993003.00491-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Driscoll K.E., Costa D.L., Hatch G., Henderson R., Oberdorster G., Salem H., Schlesinger R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- 107.Bassler D. Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia. Neonatology. 2015;107:358–359. doi: 10.1159/000381132. [DOI] [PubMed] [Google Scholar]
- 108.Xu N., Shao Y., Ye K., Qu Y., Memet O., He D., Shen J. Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhal. Toxicol. 2019;31:52–60. doi: 10.1080/08958378.2019.1597220. [DOI] [PubMed] [Google Scholar]
- 109.Dolovich M.B., Dhand R. Aerosol drug delivery: Developments in device design and clinical use. Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 110.Lavorini F., Buttini F., Usmani O.S. 100 Years of Drug Delivery to the Lungs. Handb. Exp. Pharmacol. 2019;260:143–159. doi: 10.1007/164_2019_335. [DOI] [PubMed] [Google Scholar]
- 111.Naughton P.J., Joyce M., Mac Giolla Eain M., O’Sullivan A., MacLoughlin R. Evaluation of Aerosol Drug Delivery Options during Adult Mechanical Ventilation in the COVID-19 Era. Pharmaceutics. 2021;13:1574. doi: 10.3390/pharmaceutics13101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandez Fernandez E., Joyce M., O’Sullivan A., MacLoughlin R. Evaluation of Aerosol Therapy during the Escalation of Care in a Model of Adult Cystic Fibrosis. Antibiotics. 2021;10:472. doi: 10.3390/antibiotics10050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lexmond A., Forbes B. Drug Delivery Devices for Inhaled Medicines. Handb. Exp. Pharmacol. 2017;237:265–280. doi: 10.1007/164_2016_67. [DOI] [PubMed] [Google Scholar]
- 114.Chow M.Y.T., Chang R.Y.K., Chan H.K. Inhalation delivery technology for genome-editing of respiratory diseases. Adv. Drug Deliv. Rev. 2021;168:217–228. doi: 10.1016/j.addr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elphick M., von Hollen D., Pritchard J.N., Nikander K., Hardaker L.E., Hatley R.H. Factors to consider when selecting a nebulizer for a new inhaled drug product development program. Expert Opin. Drug Deliv. 2015;12:1375–1387. doi: 10.1517/17425247.2015.1014339. [DOI] [PubMed] [Google Scholar]
- 116.McCarthy S.D., Horgan E., Ali A., Masterson C., Laffey J.G., MacLoughlin R., O’Toole D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Antibacterial Properties against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2020;33:140–152. doi: 10.1089/jamp.2019.1542. [DOI] [PubMed] [Google Scholar]
- 117.Averyanov A., Konoplyannikov A., Zabozlaev F., Danilevskaya O., Konoplyannikov M., Kuzovlev O., Kulagina N., Koroleva I. Comparative effects of inhaled and intravenous mesenchymal stem cells in bleomycin-induced pulmonary fibrosis in rabbits. Eur. Respir. J. 2013;42:226. [Google Scholar]
- 118.Clark A.R. Half a Century of Technological Advances in Pulmonary Drug Delivery: A Personal Perspective. Front. Drug Deliv. 2022;2:871147. doi: 10.3389/fddev.2022.871147. [DOI] [Google Scholar]
- 119.Sweeney L., McCloskey A.P., Higgins G., Ramsey J.M., Cryan S.-A., MacLoughlin R. Effective nebulization of interferon-γ using a novel vibrating mesh. Respir. Res. 2019;20:66. doi: 10.1186/s12931-019-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahboubi Mehrabani M., Karvandi M.S., Maafi P., Doroudian M. Neurological complications associated with COVID-19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022:e2334. doi: 10.1002/rmv.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zanganeh S., Goodarzi N., Doroudian M., Movahed E. Potential COVID-19 therapeutic approaches targeting angiotensin-converting enzyme 2; An updated review. Rev. Med. Virol. 2021:e2321. doi: 10.1002/rmv.2321. [DOI] [PubMed] [Google Scholar]
- 124.Shi M.M., Yang Q.Y., Monsel A., Yan J.Y., Dai C.X., Zhao J.Y., Shi G.C., Zhou M., Zhu X.M., Li S.K., et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12134. doi: 10.1002/jev2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.