Abstract
Background: Clotting is a major drawback of continuous renal replacement therapy (CRRT) performed on critically ill pediatric patients. Although anticoagulation is recommended to prevent clotting, limited results are available on the effect of each pharmacological strategy in reducing filter clotting in pediatric CRRT. This study defines which anticoagulation strategy, between regional citrate anticoagulation (RCA) and systemic anticoagulation with heparin, is safer and more efficient in reducing clotting, patient mortality, and treatment complications during pediatric CRRT. Methods: A systematic literature review was run considering papers published in English until December 2021 and describing patients’ and treatments’ complications in CRRT performed with heparin and RCA on patients aged less than 18 years. Results: Eleven studies were considered, cumulatively comprising 1.706 CRRT sessions (62% with systemic anticoagulation and 38% with RCA). Studies have consistently identified RCA’s superiority over systemic anticoagulation with heparin in prolonging circuit life. The pooled estimate (95% CI) of filter clotting risk showed that RCA is a protective factor for clotting risk (RR = 0.204). Conclusions: RCA has a potential role in prolonging circuit life and seems superior to systemic anticoagulation with heparin in decreasing the risk of circuit clotting during CRRT performed in critically ill pediatric patients.
Keywords: continuous renal replacement therapy, pediatric intensive care unit, anticoagulation methods, systemic anticoagulation, regional anticoagulation
1. Introduction
Acute kidney injury (AKI) is a severe condition in critically ill children, affecting short and long-term patients’ outcomes [1]. Diagnosis and staging are identified by the Kidney Disease Improving Global Outcomes (KDIGO) criteria [2]. Like adult patients, the current management of AKI is based on early diagnosis, primary and secondary prevention, etiology identification with the aim of establishing pathophysiologically driven treatments, and supporting organ function [3]. In the case of severe AKI, children may need renal replacement therapy. Critically ill children with AKI can benefit from continuous renal replacement therapy (CRRT), which supports kidney function, and potentially improves short-term outcomes [4]. The Prospective Pediatric CRRT Registry showed that CRRT is applied in critically ill children in very different clinical settings and with extreme variability in prescription and management among different centers [5].
Indeed, the definition of best practices for CRRT specifically performed in pediatric patients is needed. Similar to other extracorporeal blood purification therapies, preventing filter clotting is significantly challenging during CRRT. It decreases treatment efficiency, increases treatment downtime, and leads to unexpected filter substitution with increased patient blood loss and health care costs [6]. Thus, efficient anticoagulation protocols are required to prevent clotting and prolong circuit life (CL). Unfractionated systemic heparin administration and regional anticoagulation with sodium citrate are applied with this aim. In adult patients, regional citrate anticoagulation (RCA) has been demonstrated to be more effective than systemic anticoagulation with heparin in reducing clotting, prolonging filter efficiency, and reducing the risk of bleeding. Indeed, the 2012 KDIGO guidelines recommend using RCA for adult patients at increased risk of bleeding and in the absence of contraindications for citrate infusion [7]. Nonetheless, few studies have investigated factors affecting CL in critically ill pediatric patients treated with CRRT [6], and limited results are available nowadays on the effect of each pharmacological strategy in reducing filter clotting in this population.
This systematic review aims to define which anticoagulation strategy, between RCA and systemic anticoagulation with heparin, is safer and more efficient in reducing clotting, patient mortality, and treatment complications during pediatric CRRT.
2. Materials and Methods
We performed a systematic literature review (SLR) on critically ill pediatric patients treated with CRRT. Outcomes referring to both the patient (e.g., mortality rate) and the treatment (e.g., circuit life or clotting rate), as well as treatment-related complications (e.g., electrolyte and acid-base disturbances), were compared between groups of patients undergoing systemic anticoagulation with heparin or RCA. Table 1 shows the PICO criteria used for this SLR.
Table 1.
PICO. PICU: pediatric intensive care unit.
| Population | Intervention | Comparison | Outcome | Study Types |
|---|---|---|---|---|
| Patients < 18 years old admitted in PICU undergoing continuous renal replacement therapy | Use of anticoagulation during CRRT (systemic with heparin or regional citrate anticoagulation) | Heparin vs. RCA | Circuit life (CL) OR clotting rate; Complications (bleeding, blood transfusion rate, electrolyte OR metabolic disturbances); Survival | Prospective and retrospective observational studies; randomized clinical trials |
This SLR was developed following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) methodology [8]. Databases such as PubMed, EMBASE, CINAHL, and Cochrane Library were considered for the literature review and data extraction. Strings were developed and used for each database, using different keywords: “citrate”, “heparin”, “dialysis”, “hemodialysis”, “hemofiltration”, “kidney replacement therapy”, “continuous renal replacement therapy”, “CRRT”, “pediatric”, and “children”. No time restrictions and no filters were used, and the last search was performed on 28 December, 2021. Strings are reported as supplemental material.
Two independent reviewers (E.B. and G.V.) assessed the identified papers. Titles and abstracts were evaluated for eligibility criteria; conflicts and disagreements were discussed with all other authors, and a unified list of eligible papers was defined. Only English-written manuscripts were considered. Studies on patients affected by liver failure were excluded and those exploring intermittent or prolonged renal replacement therapy. Commentaries, letters, editorials, case reports, case series, reviews, and meta-analyses were not included in the analysis.
The quality of eligible papers was analyzed, and studies noncompliant to standard methodologies were excluded from the final analysis. In particular, observational studies (Table S5) were evaluated using the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statements [9], while randomized clinical trials (Table S6) were evaluated using the Consolidated Standards of Reporting Trials (CONSORT) statements [10]. For those papers included in the analysis, experimental design, sample size, numbers of CRRT circuits, patients’ features, severity, circuit life span, and total CRRT duration were reported.
In order to evaluate the difference in clotting rate, the proportion of clotting and its 95% confidence interval was used for each article and pooled weighting for sample size. The I2 statistic was used to quantify the heterogeneity. I2 was calculated using the formula proposed by Higgins and Thompson [11]. Statistical analysis was performed using SAS® software version 9.2.
3. Results
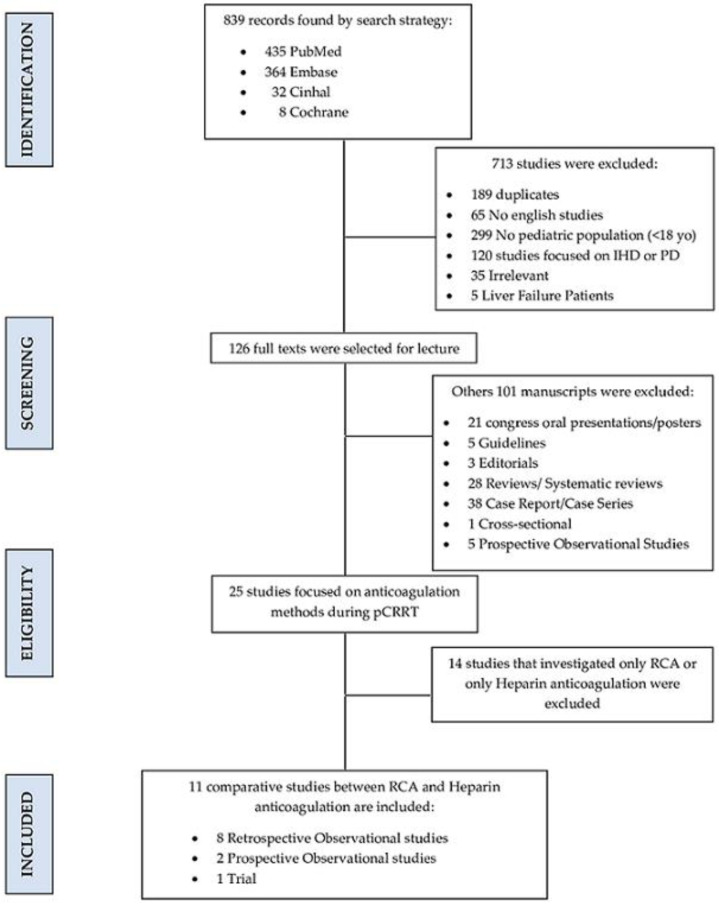
Eight hundred and thirty-nine records were extracted from the literature. Figure 1 shows the selection process. Eleven studies comparing RCA with systemic anticoagulation using heparin during pediatric CRRT were ultimately considered (Table 2).
Figure 1.
PRISMA flow chart. yo: years old; IHD: intermittent hemodialysis; PD: peritoneal dialysis; pCRRT: pediatric continuous renal replacement therapy; RCA: regional citrate anticoagulation.
Table 2.
Characteristics of studies included. Abbreviations: RCA, regional citrate anticoagulation; CL, circuit life.
| Source | Study Design | Country | Mean Age (Months) |
Sample Size (N) (Citrate) |
Sample Size (N) (Heparin) |
Outcomes |
|---|---|---|---|---|---|---|
| Chen et al., 2021 [12] | Retrospective Observational | China | 48 | 107 | 49 | Reduced mortality rate with RCA at logistic regression analysis |
| Buccione et al., 2021 [13] | Retrospective Observational | Italy | 48 | 23 | 23 | RCA as a protective factor for clotting at multivariate Cox regression analysis |
| Cortina et al., 2020 [14] | Retrospective Observational | Australia | 61.2 | 61 | 161 | No statistical difference in CL between heparin and RCA at multivariate logistic regression analysis |
| Sik et al., 2019 [15] | Retrospective Observational | Turkey | 72 | 19 | 26 | Median CL was significantly longer for RCA at univariate regression analysis. |
| Kakajiwala et al., 2017 [16] | Retrospective Observational | United States of America | 141.6 | 26 | 26 | Lower risk of clotting with Heparin anticoagulation at univariate Cox regression analysis. |
| Miklaszewska et al., 2017 [17] | Retrospective Observational | Poland | 116.7 | 8 | 32 | No differences in the survival rate between the groups |
| Rico et al., 2017 [18] | Retrospective Observational | Colombia | 1 to 216 | 17 | 15 | Median CL prolonged with RCA at univariate and bivariate regression analysis. |
| Raymakers-Janssen et al., 2017 [19] | Prospective Observational | Netherlands | 15 | 14 | 6 | Median CL was higher with RCA at log-rank |
| Zaoral et al., 2016 [20] | Crossover Trial | Czech Republic | 84 | 63 | 63 | RCA prolongs CL at the Wilcoxon paired test |
| Fernandez et al., 2014 [21] | Prospective Observational | Spain | 34.5 | 12 | 24 | Prolonged CL with RCA at Kaplan–Meier survival analysis |
| Soltysiak et al., 2014 [22] | Retrospective Observational | Poland | 19.7 | 16 | 14 | Higher CL was observed with RCA at Kaplan–Meier survival analysis. |
The studies included in this SLR comprise 1.706 CRRT sessions; 62% (n = 1.058) of these were managed using systemic anticoagulation with heparin, while 38% (n = 648) used RCA.
3.1. Circuit Life and Clotting Rate
Ten of the eleven selected studies report CL or clotting rate as treatment outcomes [13,14,15,16,17,18,19,20,21,22] (Table 3). Eight studies reporting CL as treatment outcomes, and cumulatively counting for 1550 procedures, consistently identify the superiority of RCA over systemic anticoagulation with heparin in prolonging CL. Mean and standard deviation or median and interquartile range of the resulting CL are reported in Table 3.
Table 3.
Circuit lifetime and clotting rate reported by studies included. RCA: regional citrate anticoagulation.
| Source | N Sessions | Circuit Life (h) | Clotting Rate (%) | |||
|---|---|---|---|---|---|---|
| RCA | Heparin | RCA | Heparin | RCA | Heparin | |
| Buccione et al., 2021 [13] | 11 | 72 | N/A | N/A | 18.2 | 60.6 |
| Cortina et al., 2020 [14] | 132 | 355 | 29.3 [25.8–33.1] |
23.8 [19.5–29.2] |
N/A | N/A |
| Sik et al., 2019 [15] | 44 | 57 | 53 [40–70] |
40.25 [22.75–53.5] |
11.36 | 26.31 |
| Kakajiwala et al., 2017 [16] | 22 | 51 | N/A | N/A | 39.2 | 51 |
| Miklaszewska et al., 2017 [17] (HF20/ST60/ST100) |
36 | 15 | 41 ± 25.9 | 33.3 ± 23.8 | 43.9 | 29.8 |
| 15 | 46 | 57 ± 23.5 | 53.1 ± 23.8 | |||
| 15 | 23 | 69.7 ± 8.2 | 57.2 ± 23.3 | |||
| Rico et al., 2017 [18] | 80 | 70 | 72 [48–96] |
18 [12–24] |
70 | 90 |
| Raymakers-Janssen et al., 2017 [19] | 105 | 121 | 45.2 [37.5–52.8] |
21 [14.5–27.5] |
17.1 | 42 |
| Zaoral et al., 2016 [20] | 111 | 111 | 41 [35–51.75] |
36 [31–40] |
N/A | N/A |
| Fernandez et al., 2014 [21] | 34 | 96 | 48 [31.0–93.7] |
31.0 [15.5–71.0] |
18.8 | 76.4 |
| Soltysiak et al., 2014 [22] | 43 | 41 | 58.04 ± 51.18 | 37.64 ± 32.51 | 11.63 | 34.15 |
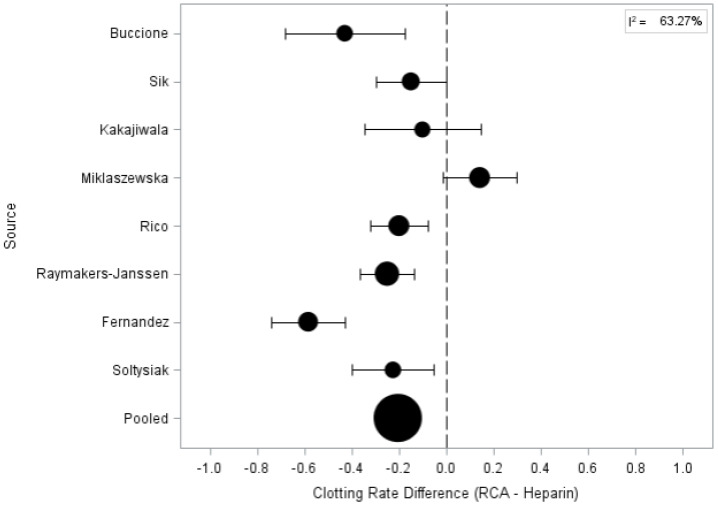
Among the eight studies reporting clotting rate as treatment outcome, only one study (performed on 150 procedures) reports a higher clotting rate for RCA sessions [17]. The remaining seven studies (cumulatively considering 847 procedures) identify systemic anticoagulation with heparin as associated with a higher clotting rate [13,14,15,16,18,19,20,21,22]. Furthermore, the pooled estimate (95% CI) of filter clotting risk showed that RCA is a protective factor for clotting risk (RR = 0.204) with a high study heterogeneity (I2 = 63.27%) (Table 4 and Figure 2).
Table 4.
Pooled estimate of filter clotting risk for regional citrate anticoagulation vs. heparin anticoagulation.
| Source | Clotting Rate Difference | 95% CI |
|---|---|---|
| Buccione | −0.429 | 0.684–0.175 |
| Sik | −0.150 | 0.297–0.002 |
| Kakajiwala | −0.101 | 0.348–0.146 |
| Miklaszewska | 0.142 | −0.013–0.296 |
| Rico | −0.200 | 0.323–0.077 |
| Raymakers-Janssen | −0.250 | 0.364–0.136 |
| Fernandez | −0.584 | 0.738–0.430 |
| Soltysiak | −0.225 | 0.399–0.051 |
| POOLED | −0.204 | 0.265–0.144 |
Figure 2.
Forest plot for clotting rate difference between regional citrate anticoagulation vs. heparin anticoagulation.
3.2. Complications
Six studies describe the complications associated with the CRRT [15,18,19,20,21,22]. Complications described within these studies are detailed in Table 5.
Table 5.
Complications according to anticoagulant methods. RCA: regional citrate anticoagulation; RBC, red blood cells.
| Complications | p-Value | Complication | ||
|---|---|---|---|---|
| Source | RCA | Heparin | ||
| Sik et al., 2019 [15] | 7.01% | 6.41% | 0.956 | Metabolic alkalosis |
| 12.28% | 2.56% | <0.05 | Hypocalcemia | |
| 14.03% | 10.25% | <0.05 | Hypernatremia | |
| 0.8 [0.3–2.0] | 1.65 [0.5–2.38] | 0.32 | Units of RBC transfused | |
| Rico et al., 2017 [18] | 30% | 32.6% | 0.605 | Severe bleeding events |
| Raymakers-Janssen et al., 2017 [19] | 3 [2.0–5.0] | 6.5 [1.5–23.8] | 0.12 | Units of RBC transfused |
| Zaoral et al., 2016 [20] | 0.17 [0.0–1.0] | 0.36 [0.0–2.0] | 0.003 | Units of RBC transfused |
| Fernandez et al., 2014 [21] | 45.5% | 0% | <0.01 | Hypochloremia |
| 27.3% | 0% | 0.045 | Hypomagnesemia | |
| 0% | 27.8 | 0.06 | Hypophosphatemia | |
| Soltysiak et al., 2014 [22] | 18.75% | 0% | N/A | Hyponatremia |
| Soltysiak et al., 2014 [22] | 18.75% | 14.3% | N/A | Hypernatremia |
| 12.5% | 21.4% | N/A | Hyperkalemia | |
| 62.5% | 28.6% | N/A | Hypokalemia | |
| 43.75% | 64.3% | N/A | Hypercalcemia | |
| 43.75% | 0% | N/A | Hypocalcemia | |
| 43.75% | 42.9% | N/A | Metabolic acidosis | |
| 25% | 14.3% | N/A | Metabolic alkalosis | |
Three studies [15,21,22] (cumulatively counting 315 procedures) describe a significantly higher rate of electrolyte disturbances for patients treated with RCA. In particular, hypokalemia (p < 0.05), hypernatremia (p < 0.05), hypochloremia (p < 0.01), hypomagnesemia (p = 0.045), and mild metabolic alkalosis (p = 0.036) have been reported.
Three studies [15,19,20] (cumulatively counting for 549 procedures) show a higher transfusion requirement in patients treated with systemic anticoagulation with heparin; nevertheless, only one [20] shows a significant difference (p = 0.003) between the two groups. Beyond the results reporting the number of units of red blood cells transfused to the patients, preliminary data are also available for bleeding events and platelet abnormalities. A single study [18] performed on 150 procedures highlights a percentage of severe bleeding events of 32 vs. 30% between patients treated with systemic anticoagulation with heparin and RCA, respectively. Finally, a paper [21] reporting data from 130 procedures describes a significant drop in platelet levels at 72 h from CRRT initiation during systemic anticoagulation with heparin.
3.3. Survival
Seven studies (cumulatively counting for 632 patients) report the patients’ survival rate at PICO or hospital discharge, and most of them do not show any differences between both groups. Only one study [12] reporting data from 156 patients shows a statistically higher survival rate in the RCA group (Table 6). Only one study reports long-term patients outcomes about 12 of 28 patients studied: 42% of these did not develop any form of kidney dysfunction; 8% developed low-grade proteinuria; 25% developed CKD; and 25% developed ESKD at least at one-year follow-up, with a mean follow-up length of 3.5 ± 2.0 years [13].
Table 6.
Survival rate according to anticoagulant methods. RCA: regional citrate anticoagulation; *: statistically significant.
| Source | Time-Point | Survival Rate (%) | p-Value | |
|---|---|---|---|---|
| RCA | Heparin | |||
| Chen et al., 2021 [12] | PICU discharge | 53.2 | 34.7 | 0.031 * |
| Sik et al., 2019 [15] | PICU discharge | 68.42 | 69.23 | 0.954 |
| Miklaszewska et al., 2017 [17] | PICU discharge | 62.5 | 34.4 | N/A |
| Rico et al., 2017 [18] | PICU discharge | 83.3 | 81.2 | 0.859 |
| Raymakers-Janssen et al., 2017 [19] | PICU discharge | 50 | 50 | N/A |
| Fernandez et al., 2014 [21] | PICU discharge | 25 | 25 | N/A |
| Soltysiak et al., 2014 [22] | Hospital discharge | 37.5 | 14.3 | N/A |
3.4. Dialysis Targets
Unfortunately, few studies systematically report dialysis prescriptions. Dialysis targets are detailed in Table 7. Four studies [17,18,21,22] report blood pump flow (Qb) according to mL/min/kg. Qb ranges from 2 to 5 mL/kg/min. Another four studies [13,14,15,20] report blood pump flows in mL/min, and values range from 60 to 96 mL/min. No difference seems to be between RCA and heparin treatments. Finally, all the four studies [13,20,21,22] that describe the heparin dose report a lower dose of 20 UI/kg/h.
Table 7.
Dialysis targets. RCA: regional citrate anticoagulation; Qb: blood pump flow.
| Qb (mL/min) | Dialysate (mL/h) | Heparin Dose (IU/kg/h) | Net Ultrafiltration (mL/h) | Replacement (mL/min) | Citrate (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | RCA | Heparin | RCA | Heparin | RCA | Heparin | RCA | Heparin | RCA | Heparin | RCA | Heparin |
| Buccione et al., 2021 [13] | 60 (40–80) | 60 (40–80) | 400 (200–600) | 400 (200–600) | N/A | 13.9 | 40 (25–70) | 40 (25–70) | 200 (50–400) | 200 (50–400) | N/A | N/A |
| Cortina et al., 2020 [14] | 96 (16–400) | 96 (16–400) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sik et al., 2019 [15] | 60 (50–80) | 60 (50–80) | 700 (500–900) | 500 (350–800) | N/A | N/A | N/A | N/A | N/A | N/A | 4 (4–5) | N/A |
| Miklaszewska et al., 2017 [17](HF20/ST60/ST100) | 3.5/kg (.5) | 3.5/kg (.5) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 2.1/kg (1.5) | 2.1/kg (1.5) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| 2/kg (.9) | 2/kg (.9) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Rico et al., 2017 [18] | 3.4/kg | 3.5/kg | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Zaoral et al., 2016 [20] | 90 (70–100) | 90 (70–100) | 60.34/kg (48.5–118.5) |
53.57/kg (38–85) |
N/A | 15 (13.2–17.9) | N/A | N/A | N/A | N/A | N/A | N/A |
| Fernandez et al., 2014 [21] | 3.2/kg (2–3.8) | 5/kg (3.8–5.6) | 325 (50–600) | 300 (140–500) | N/A | 15 (12–25) | 75 (50–97.5) | 60 (50–90) | 50 (0–50) | 300 (140–500) | 2.6 (2.3–2.9) | N/A |
| Soltysiak et al., 2014 [22] | 3.49/kg ± 1.56 | 2.88/kg ± 0.80 | 52.32/kg ± 35.63 | 71.71/kg ± 39.39 | N/A | 17 ± 10 | N/A | N/A | N/A | N/A | 4.05 ± 2.30 | N/A |
4. Discussion
Results described in this systematic review show the potential role of RCA in prolonging CL and its superiority with respect to systemic anticoagulation with heparin in decreasing the risk of circuit clotting during CRRT performed in critically ill pediatric patients.
These results are in line with those reported for adult populations. As an example, in a systematic review including 6 randomized controlled trials published in 2012 and cumulatively considering 658 CRRT sessions, Zhang et al. reported a significantly longer circuit life span for treatments performed with RCA [23]. Unfortunately, few studies are currently available aimed at exploring the role of pharmacological strategies in reducing the risk of clotting, specifically in CRRT performed in pediatric patients. It is well known that clotting is a major drawback of extracorporeal treatments. This is true particularly for pediatric patients due to the younger age, the smaller vascular access available for CRRT, and the more limited blood flow achievable for extracorporeal treatment. Furthermore, considering that monitors specifically designed for pediatric treatment and characterized by miniaturized peristaltic pumps are not commonly available, periodic oscillations in the inflow line pressure may lead to excessively negative pressure and frequent treatment interruptions, ultimately causing circuit clotting [24]. For this reason, pharmacologic strategies for anticoagulation seem to be crucial in pediatric patients treated with CRRT to prevent this complication.
Raina et al. have evaluated the safety and efficacy of the extracorporeal anticoagulants in the pediatric CRRT in a systematic review, including any pediatric study reporting data on anticoagulation (heparin, citrate, or prostacyclin) [25]. In this systematic review, including 24 studies, the authors have demonstrated the association between RCA and an average prolonged circuit life with a relatively higher risk of electrolytes imbalance. Even if similar results were obtained in our systematic review, several differences must be remarked. In particular, our analysis was consistently confined to those studies reporting an explicit comparison between RCA and systemic anticoagulation with heparin for the selected outcomes. Hence, we included three more papers [12,13,14] evaluating direct comparison between RCA and heparin, for a total of three hundred and forty patients. Finally, we excluded a paper [25] included in the analysis by Raina et al., because it considered patients up to twenty years old, who have to be considered as adults, especially from the anticoagulation standpoint.
Interestingly, despite the substantial differences in the methodology used, our study reports consistent results with those reported by Raina et al. [25] for bleeding events. In particular, no difference was reported between RCA and systemic anticoagulation with heparin in terms of severe bleeding events. Nonetheless, heparin was associated with a significant risk of a drop in platelet levels and an increased need for transfusions of red blood cell units, probably due to more frequent circuit changes. Indeed, CRRT prescription for younger and smaller children is affected by problems concerning the extracorporeal blood volume, the need for circuit blood priming, and the adaptation of machines designed for adult-sized patients [26]. Blood priming could be necessary when extracorporeal circuit volume exceeds 10–15% of the patient’s blood volume [27]. The more frequent the circuit clotting is and the circuit substitution, the higher the need for blood transfusions and blood units for circuit priming. Furthermore, papers studied reported lower values of heparin dosage than previously published studies, which reported a dosage of 20 UI/kg/h [28,29].
RCA, reducing circuit clotting and prolonging filter life, might thus have a role in attenuating the needs of hemoderivates. Citrate administration can cause metabolic alkalosis and calcium perturbations [28]. Since 2003, Bunchman et al. worked on a simplified protocol to administer citrate anticoagulation, avoiding potential error risks and complications for the patients [30]. Unfortunately, according to our findings, RCA seems to be more commonly associated with electrolyte imbalance and metabolic alkalosis. These results are in line with those reported by Raina et al. [25], where metabolic alkalosis and electrolyte imbalance were reported in 71.4% and 40% of patients treated with RCA (vs. 16.7% and 0% of those treated with systemic anticoagulation with heparin). Similar results are not confirmed for adult patients, where RCA is usually considered safe and effective in maintaining electrolytes and acid-base homeostasis. Indeed, in a meta-analysis of 6 RCTs for a total of 488 adult patients Wu et al. [31], show no statistically significant difference in terms of metabolic alkalosis and electrolyte imbalance between patients treated with different anticoagulation strategies.
Cumulatively considering the effects on circuit outcomes and treatment complications, an economic advantage might be expected in using RCA for CRRT in pediatric patients. Studies on cost analysis including adult patients are available in this field; unfortunately, the same studies are currently lacking for pediatric settings.
Several drawbacks may be recognized in this systematic review. First, most of the considered studies were retrospective in nature, and only one crossover trial was available. Second, the wide heterogeneity in reporting results across different studies (mean and standard deviation or median and interquartile range) did not allow a meta-analysis for outcomes as the circuit life. Third, limited results are reported for patients’ long-term outcomes as renal functional recovery and dialysis dependence between the two groups of anticoagulation strategies.
5. Conclusions
Regional citrate anticoagulation could prolong circuit life and decrease the risk of clotting in CRRT performed in critically ill pediatric patients. Although no difference is observed in severe bleeding events, systemic anticoagulation with heparin is associated with a greater reduction in platelet levels during the treatment and with an increased need for transfusions of red blood cells, with respect to RCA. More frequent circuit substitution and the use of blood for circuit priming may explain this phenomenon.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11113121/s1, Table S1: Search strategy for PubMed database; Table S2: Search strategy for CINAHL database; Table S3: Search strategy for EMBASE database; Table S4: Search strategy for Cochrane database; Table S5: Quality evaluation of observational studies according to STROBE reporting guidelines; Table S6: Quality evaluation of Crossover Trial study according to CONSORT reporting guidelines.
Author Contributions
Conceptualization, E.B., G.V. and T.P.; methodology, G.V.; investigation, E.B.; resources, E.B.; data curation, L.T. and G.V.; writing—original draft preparation, E.B.; writing—review and editing, G.V., S.B., K.E.A. and C.D.P.; visualization, L.R.; supervision, S.R. and Z.R.; funding acquisition, G.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
“Philip” and “Irene Toll Gage Foundation” has supported this study through research grants. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kellum J.A. Diagnostic Criteria for Acute Kidney Injury. Crit. Care Clin. 2015;31:621–632. doi: 10.1016/j.ccc.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Fry A.C. Management of Acute Renal Failure. Postgrad. Med. J. 2006;82:106–116. doi: 10.1136/pgmj.2005.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco F.C., Ortega G., Qureshi F.G. Renal Replacement Therapy in Children. Semin. Pediatr. Surg. 2015;24:25–31. doi: 10.1053/j.sempedsurg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Symons J.M., Chua A.N., Somers M.J., Baum M.A., Bunchman T.E., Benfield M.R., Brophy P.D., Blowey D., Fortenberry J.D., Chand D., et al. Demographic Characteristics of Pediatric Continuous Renal Replacement Therapy: A Report of the Prospective Pediatric Continuous Renal Replacement Therapy Registry. Clin. J. Am. Soc. Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 6.del Castillo J., López-Herce J., Cidoncha E., Urbano J., Mencía S., Santiago M.J., Bellón J.M. Circuit Life Span in Critically Ill Children on Continuous Renal Replacement Treatment: A Prospective Observational Evaluation Study. Crit. Care. 2008;12:R93. doi: 10.1186/cc6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis T.K., Neumayr T., Geile K., Doctor A., Hmeil P. Citrate Anticoagulation during Continuous Renal Replacement Therapy in Pediatric Critical Care. Pediatr. Crit. Care Med. 2014;15:471–485. doi: 10.1097/PCC.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 8.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Schulz K.F., Altman D.G., Moher D. For the CONSORT Group CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z., Wang H., Wu Z., Jin M., Chen Y., Li J., Wei Q., Tao S., Zeng Q. Continuous Renal-Replacement Therapy in Critically Ill Children: Practice Changes and Association with Outcome. Pediatr. Crit. Care Med. 2021;22:E605–E612. doi: 10.1097/PCC.0000000000002751. [DOI] [PubMed] [Google Scholar]
- 13.Buccione E., Guzzi F., Colosimo D., Tedesco B., Romagnoli S., Ricci Z., L’Erario M., Villa G. Continuous Renal Replacement Therapy in Critically Ill Children in the Pediatric Intensive Care Unit: A Retrospective Analysis of Real-Life Prescriptions, Complications, and Outcomes. Front. Pediatr. 2021;9:696798. doi: 10.3389/fped.2021.696798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortina G., McRae R., Chiletti R., Butt W. The Effect of Patient- and Treatment-Related Factors on Circuit Lifespan During Continuous Renal Replacement Therapy in Critically Ill Children. Pediatr. Crit. Care Med. 2020;21:578–585. doi: 10.1097/PCC.0000000000002305. [DOI] [PubMed] [Google Scholar]
- 15.Sık G., Demirbuga A., Annayev A., Citak A. Regional Citrate versus Systemic Heparin Anticoagulation for Continuous Renal Replacement Therapy in Critically Ill Children. Int. J. Artif. Organs. 2020;43:234–241. doi: 10.1177/0391398819893382. [DOI] [PubMed] [Google Scholar]
- 16.Kakajiwala A., Jemielita T., Hughes J.Z., Windt K., Denburg M., Goldstein S.L., Laskin B. Membrane Pressures Predict Clotting of Pediatric Continuous Renal Replacement Therapy Circuits. Pediatr. Nephrol. 2017;32:1251–1261. doi: 10.1007/s00467-017-3601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklaszewska M., Korohoda P., Zachwieja K., Kobylarz K., Stefanidis C.J., Sobczak A., Drozdz D. Filter Size Not the Anticoagulation Method Is the Decisive Factor in Continuous Renal Replacement Therapy Circuit Survival. Kidney Blood Press. Res. 2017;42:327–337. doi: 10.1159/000477609. [DOI] [PubMed] [Google Scholar]
- 18.Rico M.P., Fernández Sarmiento J., Rojas Velasquez A.M., González Chaparro L.S., Gastelbondo Amaya R., Mulett Hoyos H., Tibaduiza D., Quintero Gómez A.M. Regional Citrate Anticoagulation for Continuous Renal Replacement Therapy in Children. Pediatr. Nephrol. 2017;32:703–711. doi: 10.1007/s00467-016-3544-9. [DOI] [PubMed] [Google Scholar]
- 19.Raymakers-Janssen P.A.M.A., Lilien M., van Kessel I.A., Veldhoen E.S., Wösten-van Asperen R.M., van Gestel J.P.J. Citrate versus Heparin Anticoagulation in Continuous Renal Replacement Therapy in Small Children. Pediatr. Nephrol. 2017;32:1971–1978. doi: 10.1007/s00467-017-3694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaoral T., Hladík M., Zapletalová J., Trávníček B., Gelnarová E. Circuit Lifetime with Citrate Versus Heparin in Pediatric Continuous Venovenous Hemodialysis. Pediatr. Crit. Care Med. 2016;17:e399–e405. doi: 10.1097/PCC.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 21.Fernández S.N., Santiago M.J., López-Herce J., García M., Del Castillo J., Alcaraz A.J., Bellón J.M. Citrate Anticoagulation for CRRT in Children: Comparison with Heparin. Biomed Res. Int. 2014;2014:786301. doi: 10.1155/2014/786301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soltysiak J., Warzywoda A., Kociński B., Ostalska-Nowicka D., Benedyk A., Silska-Dittmar M., Zachwieja J. Citrate Anticoagulation for Continuous Renal Replacement Therapy in Small Children. Pediatr. Nephrol. 2014;29:469–475. doi: 10.1007/s00467-013-2690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z., Hongying N. Efficacy and Safety of Regional Citrate Anticoagulation in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Intensive Care Med. 2012;38:20–28. doi: 10.1007/s00134-011-2438-3. [DOI] [PubMed] [Google Scholar]
- 24.Garzotto F., Zaccaria M., Vidal E., Ricci Z., Lorenzin A., Neri M., Murer L., Nalesso F., Ruggeri A., Ronco C. Choice of Catheter Size for Infants in Continuous Renal Replacement Therapy: Bigger Is Not Always Better. Pediatr. Crit. Care Med. 2019;20:e170–e179. doi: 10.1097/PCC.0000000000001825. [DOI] [PubMed] [Google Scholar]
- 25.Raina R., Agrawal N., Kusumi K., Pandey A., Tibrewal A., Botsch A. A Meta-Analysis of Extracorporeal Anticoagulants in Pediatric Continuous Kidney Replacement Therapy. J. Intensive Care Med. 2022;37:0885066621992751. doi: 10.1177/0885066621992751. [DOI] [PubMed] [Google Scholar]
- 26.Garzotto F., Zanella M., Ronco C. The Evolution of Pediatric Continuous Renal Replacement Therapy. Nephron Clin. Pract. 2014;127:172–175. doi: 10.1159/000363204. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland S.M., Alexander S.R. Continuous Renal Replacement Therapy in Children. Pediatr. Nephrol. 2012;27:2007–2016. doi: 10.1007/s00467-011-2080-x. [DOI] [PubMed] [Google Scholar]
- 28.John J.C., Taha S., Bunchman T.E. Basics of Continuous Renal Replacement Therapy in Pediatrics. Kidney Res. Clin. Pract. 2019;38:455–461. doi: 10.23876/j.krcp.19.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunchman T.E., Donckerwolcke R.A. Continuous Arterial-Venous Diahemofiltration and Continuous Veno-Venous Diahemofiltration in Infants and Children. Pediatr. Nephrol. 1994;8:96–102. doi: 10.1007/BF00868282. [DOI] [PubMed] [Google Scholar]
- 30.Bunchman T.E., Maxvold N.J., Brophy P.D. Pediatric Convective Hemofiltration: Normocarb Replacement Fluid and Citrate Anticoagulation. Am. J. Kidney Dis. 2003;42:1248–1252. doi: 10.1053/j.ajkd.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Wu M.-Y., Hsu Y.-H., Bai C.-H., Lin Y.-F., Wu C.-H., Tam K.-W. Regional Citrate Versus Heparin Anticoagulation for Continuous Renal Replacement Therapy: A Meta-Analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2012;59:810–818. doi: 10.1053/j.ajkd.2011.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.