Abstract
Soluble suppressor of tumorigenicity (sST)-2 plasma concentration is related to atherosclerosis. The aim of this study was to assess the prognostic impact of sST2 and its membrane-associated form (ST2L) in patients with carotid atherosclerotic plaque who underwent endarterectomy (CEA). Eighty-two consecutive patients (age range: 48–86 years) who underwent CEA were enrolled. Anthropometric, clinical, instrumental, and laboratory evaluations were gathered. Thirty-seven (45%) patients were symptomatic of cerebrovascular diseases. Patients underwent a five-year follow-up. Phone calls and the analysis of national and regional databases were performed in order to evaluate the occurrence of the primary outcome (all-cause mortality). The population was divided according to survival status. Statins were administered in 81% and 87.5% of survivors and non-survivors, respectively. sST2 levels were higher in non-survivors than in survivors (117.0 ± 103.9 vs. 38.0 ± 30.0 ng/mL, p < 0.001) and in symptomatic individuals, compared with asymptomatic (80.3 ± 92.1 ng/mL vs. 45.4 ± 41.4 ng/mL, p = 0.02). ROC curve analysis identified sST2 cut-off: >98.44 ng/mL as the best predictor for mortality. At the one-year follow-up, the survival rate decreased up to 20% in patients with sST2 higher than the cut-off value. A multivariate regression analysis revealed that only sST2 (HR: 1.012, 95% CI: 1.008–1.016, p < 0.0001) and triglycerides plasma levels (HR: 1.008, 95% CI: 1.002–1.015, p = 0.0135) remained significantly associated with all-cause mortality. ST2L was not associated with all-cause mortality risk. sST2 may act as an independent prognostic determinant of all-cause mortality and symptomatic cerebrovascular diseases in patients with carotid atherosclerotic plaque who underwent CEA.
Keywords: sST2, ST2L, carotid plaque, atherosclerosis, biomarkers, prognosis
1. Introduction
The suppressor of tumorigenicity (ST2) protein has been described as the receptor for interleukin (IL)-33, which is a member of the IL-1 cytokine family [1]. The st2 gene is located on chromosome 2q12 and transcripts for two proteins—IL1RL1-b or ST2L, i.e., the membrane receptor member of the IL-1 receptor family, and IL1RL1-a or sST2, the truncated soluble receptor [1].
sST2 has recently been considered a reliable prognostic biomarker in patients with heart failure (HF) [2,3,4]. Specifically, it seemed to act as an independent predictor of all-cause mortality in patients with chronic HF [5,6]. Nevertheless, the role of sST2 and ST2L in atherosclerosis is less known and still controversial. Miller et al. [7] demonstrated that the IL-33–ST2L pathway might inhibit the development of atherosclerosis in apoE−/− mice fed on a high-fat diet while promoting plaque development when sST2 was administered. Plasma concentrations in sST2 were related to soluble P-selectin and tissue factor (TF) expressions [8], while the concentration of ST2L in carotid plaque significantly correlated with the degree of inflammatory cells infiltration, thus promoting the vulnerability of the atherosclerotic plaques [9].
The impact of sST2 plasma concentrations and ST2L expression on the membranes of the cells of the atheroma on the overall prognosis of patients with overt atherosclerotic cardiovascular disease (ASCVD) is still a matter of debate. Willems et al. [10] observed no association between serum levels in sST2 and major adverse cardiovascular events (MACE) patients who underwent carotid endarterectomy. The KAROLA study demonstrated the association of sST2 concentration to short- and long-term all-cause mortality in patients with stable coronary heart disease [11], while a recent meta-analysis [12] reported an association between sST2 concentration and all-cause mortality in patients with the acute coronary syndrome (ACS).
The aims of this study were to (1) assess the association between sST2 serum levels concentrations and the immunohistochemical expression of ST2L in atherosclerotic plaques from internal carotid arteries of patients who underwent endarterectomy and (2) evaluate the prognostic impact of sST2 and ST2L expression on the overall survival rate of patients with ASCVD.
2. Materials and Methods
2.1. Study Population
We collected the atherosclerotic carotid plaques from 82 consecutive patients (age range: 48–86 years) who were admitted to the Vascular Surgery Section (Department of Emergency and Organ Transplantation) of the University of Bari to undergo internal carotid endarterectomy (CEA).
All patients underwent anthropometric, clinical, instrumental, and laboratory evaluations, the data of which were collected and gathered in the final dataset. Patients were enrolled between March 2016 and September 2017.
Thirty-seven (45%) patients were defined “symptomatic” in agreement with the guidelines of the European Society of Vascular Surgery (ESVS)—namely, those showing signs or symptoms of transient ischemic attack (TIA) or stroke within the last 6 months, homolateral hemispheric ischemia and/or homolateral retinal ischemia, in the absence of other embolic foci (atrial fibrillation, intracranial stenosis, etc); the presence of homolateral hemispheric lesions shown with imaging techniques (computer tomography (CT) or magnetic resonance imaging (MRI)) [13,14].
Patients who did not satisfy such characteristics were considered “asymptomatic”. Specifically, they were considered “asymptomatic” if they had no sign or symptom related to transient ischemic attack (TIA)/stroke within 6 months before study entry and showed unstable carotid plaques at imaging analysis (stenosis progression >20%, large and/or echolucent plaques, hypoechoic areas, intraplaque hemorrhage and/or necrotic core, surface irregularity, silent infarction on CT/MRI).
Cardiovascular risk factors were defined according to international guidelines [15]. Briefly, diabetes was defined as fasting plasma glucose levels of 126 mg/dL in at least two determinations, or use of antidiabetic drugs; dyslipidemia was defined as total cholesterol plasma levels >200 mg/dL, high-density lipoprotein levels <45 mg/dL, triglycerides >150 mg/dL, and/or use of lipid-lowering agents; hypertension was defined as systolic/diastolic blood pressure >140/90 mmHg [15]. Smoking habit was defined when patients assumed >10 cigarettes/die within the last 3 months. All patients underwent standard biochemical examination.
Patients underwent a five-year follow-up. Phone calls and analysis of national and regional databases were adopted in order to evaluate the occurrence of the primary outcome (all-cause mortality). Phone evaluations and rechecks of the national and regional databases were performed every six months till the end of the follow-up. All of the patients were followed up until the primary endpoint occurred.
Informed consent was obtained from each patient before enrollment. This study was performed in agreement with the ethical guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Bari.
2.2. Tissue Samples
All carotid plaques removed during CEA surgery were subjected to histological analysis. After surgical dissection, the plaques were fixed in 10% buffered neutral formalin for 24–48 h and cut into 5 mm length segments. All of them were sequentially numbered to reconstruct the entire plaque length from the proximal common carotid artery to the distal segment of the internal carotid artery and included in paraffin blocks.
Paraffin blocks were cut into 4 μm thick sections and stained with hematoxylin–eosin, Masson’s tri-chrome, and elastic fiber stains.
2.3. Immunohistochemistry
All of the plaque specimens were examined by immunohistochemistry for the expression of ST2L receptor in the whole section, using a rabbit polyclonal anti-ST2 antibody (IL1RL1 interleukin receptor 1, 1:500 dilution; Sigma Aldrich, St. Louis, MO, USA). The specimens were evaluated in the next days after the surgical removal. The pathologist was unaware of the clinical characteristics of the patients, and no information was according to the occurrence of the primary endpoint. All of the immunostainings were performed via an automated immunostainer (Dako Autostainer, Ft. Collins, CO, USA). Slices were pretreated with DAKO-PT-link in a pH 9 EDTA retrieval solution. Substitution of the primary antibody with PBS served as a negative control. After the preparation, the sections were simultaneously evaluated by two observers to ensure the reproducibility of the semiquantitative assessment of the plaques.
The histological examination considered both the common morphologic features of the atherosclerotic plaques—namely, fibrosis, calcification, the amplitude of the necrotic core, and the presence of complications (erosion, thrombosis, hemorrhage)—and the evaluation of the ST2L expression on the cellular component of the carotid plaques. In particular, we examined the expression of ST2L on the endothelium of the newly formed vessels. A semiquantitative method was adopted for grading the expression of ST2L on macrophages and endothelial cells. The plaques were considered negative in the absence of ST2L expression in any kind of cell (score 0–1: LOW = 0/1+). A focal positivity in cells/capillaries was graded as a moderately expressing receptor (score 2: MODERATE = 2+). A diffuse and strong expression in a large number of macrophages and capillaries was graded as high (score 3: HIGH = 3+).
2.4. ELISA Test
sST2 serum levels were defined using the ELISA test, and a solid-phase enzyme immunoassay was carried out to detect the presence of the ligand in a liquid sample by means of antibodies.
The ST2 ELISA kit measures soluble human ST2 protein via sandwich ELISA, with a recombinant human ST2 antibody. The assay uses two monoclonal antibodies against two different epitopes of human ST2. The ready-to-use kit is composed of the microtiter plate coated with a monoclonal antibody, peroxidase-conjugated streptavidin, and a biotinylated ST2-specific antibody. At the end of the process, the intensity of the generated color is read at 450 nm.
2.5. Statistics and Data Analysis
The comparisons between groups were performed with Student’s t-test or Mann–Whitney U test where appropriate. Receiver-operating characteristic (ROC) curve analysis was performed to calculate the area under the curve (AUC) values, and the optimal cut-off values for mortality were calculated as the point of maximum sensitivity and specificity. Survival was calculated using Kaplan–Meier analysis.
Univariate and multivariate Cox regression analyses were performed to evaluate independent predictors of mortality. p-values below 0.05 were defined as statistically significant. The analyses were performed using STATA software, version 12 (StataCorp, College Station, TX, USA).
3. Results
Table 1 gathered the main characteristics of the two groups.
Table 1.
Characteristics of the study population: survivors vs. non-survivors.
| Characteristics | Survivors (n = 58) |
Non-Survivors (n = 24) |
p-Values |
|---|---|---|---|
| Age (years) | 71.3 ± 7.9 | 73.1 ± 8.6 | 0.36 |
| Female (n/%) | 38 (65.5) | 20 (83.3) | 0.12 |
| Weight (kg) | 78.4 ± 11.9 | 76.6 ± 12.9 | 0.55 |
| Height (cm) | 167.4 ± 7.9 | 166.2 ± 9.0 | 0.53 |
| BMI (kg/m2) | 28.0 ± 3.6 | 27.8 ± 4.4 | 0.84 |
| Systolic arterial pressure (mmHg) | 130.3 ± 14.4 | 130.6 ± 10.5 | 0.91 |
| Diastolic arterial pressure (mmHg) | 76.3 ± 8.0 | 75.8 ± 7.5 | 0.81 |
| Heart rate (bpm) | 63.6 ± 9.2 | 67.8 ± 12.6 | 0.09 |
| Symptomatic (n/%) | 23 (39.6) | 14 (58.3) | 0.12 |
| Diabetes (n/%) | 18 (31.0) | 8 (33.3) | 0.84 |
| Hypertension (n/%) | 53 (91.4) | 22 (91.7) | 0.97 |
| Smokers (n/%) | 8 (13.8) | 6 (25) | 0.22 |
| Ex-smokers (n/%) | 22 (37.9) | 7 (29.2) | 0.46 |
| C-reactive protein (mg/L) | 4.2 ± 3.9 | 5.2 ± 3.5 | 0.90 |
| Leucocytes (admission) (×103/mm3) | 7.3 ± 2.0 | 8.4 ± 2.4 | 0.049 |
| Leucocytes (peak) (×103/mm3) | 11.9 ± 4.3 | 11.5 ± 3.3 | 0.74 |
| Total Cholesterol (mg/dL) | 158.6 ± 35.3 | 159.4 ± 51.7 | 0.93 |
| LDL-C (mg/dL) | 85.9 ± 33.1 | 85.8 ± 42.5 | 0.99 |
| HDL-C (mg/dL) | 51.9 ± 19.7 | 45.5 ± 11.3 | 0.14 |
| Triglycerides (mg/dL) | 104.0 ± 45.6 | 140.5 ± 66.4 | 0.005 |
| Troponin I (ng/mL) | 0.023 ± 0.035 | 0.027 ± 0.028 | 0.61 |
| Plaque instability features in asymptomatic pts | |||
| - Stenosis progression > 30% (n/%) | 9 (25.7) | 3 (30) | 0.79 |
| - Large plaque (n/%) | 10 (28.6) | 6 (25) | 0.07 |
| - Necrotic core (n/%) | 7 (20) | 4 (16.7) | 0.20 |
| - Echolucent plaque (n/%) | 7 (20) | 4 (16.7) | 0.20 |
| - Hypoechoic areas (n/%) | 6 (17.1) | 4 (16.7) | 0.13 |
| - Intraplaque hemorrhages (n/%) | 7 (20) | 5 (20.8) | 0.06 |
| - Surface irregularity (n/%) | 17 (48.6) | 8 (33.3) | 0.08 |
| - Silent infarction on CT/MRI (n/%) | 10 (28.6) | 6 (25) | 0.07 |
| sST2 (ng/mL) | 38.0 ± 30.0 | 117.0 ± 103.9 | <0.001 |
| Pharmacological treatments | |||
| ACEi/sartans (n/%) | 51 (87.9) | 20 (83.3) | 0.58 |
| Beta-blockers (n/%) | 42 (72.4) | 17 (70.8) | 0.88 |
| Diuretics (n/%) | 20 (34.5) | 9 (37.5) | 0.80 |
| MRA (n/%) | 11 (19.0) | 5 (20.8) | 0.84 |
| Statins (n/%) | 47 (81.0) | 21 (87.5) | 0.48 |
| Ezetimibe (n/%) | 10 (17.2) | 5 (20.8) | 0.71 |
| PCSK9i (n/%) | 4 (6.9) | 2 (8.3) | 0.82 |
Data are expressed as number ± standard deviation and/or number and percentages. Abbreviations: ACEi: angiotensin-converting enzyme inhibitors; BMI: body mass index; CT: computer tomography; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MRA: mineralocorticoid receptor antagonist; MRI: magnetic resonance imaging; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitors; sST2: soluble suppressor of tumorigenicity. BMI: body mass index.
All patients were on optimal medical therapy. Statins were administered in 81% and 87.5% of survivors and non-survivors, respectively, with implementation of therapies with ezetimibe in order to achieve lipid targets. Some patients (6.9% and 8.3%, respectively) were on proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), but no statistically significant differences were found between the two groups. Non-survivors demonstrated higher levels in leucocytes (8.4 ± 2.4 × 103/mm3 vs. 7.3 ± 2.0 × 103/mm3, p = 0.049), triglycerides (140.5 ± 66.4 mg/dL vs. 104.0 ± 45.6 mg/dL, p = 0.005) and sST2 (117.0 ± 103.9 ng/mL vs. 38.0 ± 30.0 ng/mL, p < 0.001) at admission.
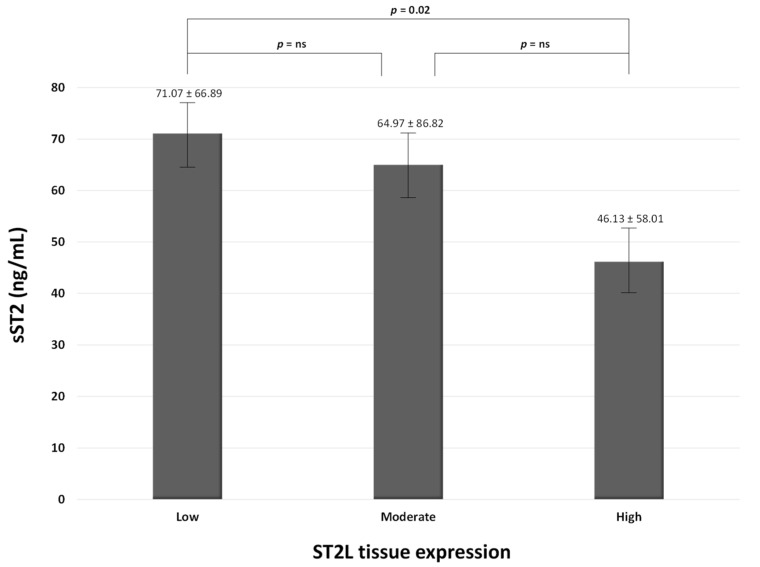
Figure 1 compares plasma levels in sST2 and the expression of ST2L on the surface of the cells on the carotid plaques.
Figure 1.
Association between the degree of ST2L expression on carotid plaques and serum levels of sST2. ns: not significant.
In particular, the higher the expression of ST2L in carotid plaque, the lower the plasma circulating levels of sST2.
Supplementary Table S1 includes the main characteristics of asymptomatic vs. symptomatic patients. In particular, symptomatic individuals are more likely to show statistically significant increased levels in total and LDL-C, as well as in sST2 (80.3 ± 92.1 ng/mL vs. 45.4 ± 41.4 ng/mL, p = 0.02).
ROC curve analysis for predicting mortality identified the following cut-off: >98.44 ng/mL for sST2 and >105 mg/dL for triglycerides (Table 2).
Table 2.
Performance of anthropometric, clinical, and laboratory characteristics in predicting all-cause mortality and symptomatic cerebrovascular symptoms.
| Characteristic | Survivors (n = 58) | Non-Survivors (n = 24) | Cut-Off | Sensibility | Specificity | AUC | p |
| sST2, ng/mL | 38.0 ± 30.0 | 117.0 ± 103.9 | >98.44 | 54.2 | 98.3 | 0.791 | <0.0001 |
| Tryglicerides, mg/dL | 104.0 ± 45.6 | 140.5 ± 66.4 | >105 | 70.8 | 67.2 | 0.685 | =0.0043 |
| Characteristic | Asymptomatic (n = 37) | Symptomatic (n = 45) | Cut-Off | Sensibility | Specificity | AUC | p |
| Total Cholesterol, mg/dL | 146.6 ± 31.6 | 173.7 ± 45.3 | >175.6 | 56.8 | 88.9 | 0.722 | =0.0002 |
| HDL-C, mg/dL | 54.2 ± 21.3 | 44.9 ± 10.5 | ≤55 | 89.2 | 37.8 | 0.628 | =0.0363 |
| LDL-C, mg/dL | 70.9 ± 26.1 | 104.1 ± 37.9 | >95 | 70.3 | 86.7 | 0.775 | <0.0001 |
| Tryglicerides, mg/dL | 107.5 ± 54.2 | 123.5 ± 54.8 | >94 | 75.7 | 51.1 | 0.623 | =0.0466 |
| sST2, ng/mL | 45.4 ± 41.4 | 80.3 ± 92.1 | >95.43 | 32.4 | 91.1 | 0.609 | 0.0855 |
Abbreviations: AUC: area under the curve; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; sST2: soluble suppressor of tumorigenicity.
Furthermore, ROC curve analysis for predicting symptomatic conditions revealed the following cut-offs: total cholesterol: >175.6 mg/dL, HDL-C: <55 mg/dL, LDL-C: >95 mg/dL, triglycerides: >94 mg/dL, and sT2: >95.43 ng/mL.
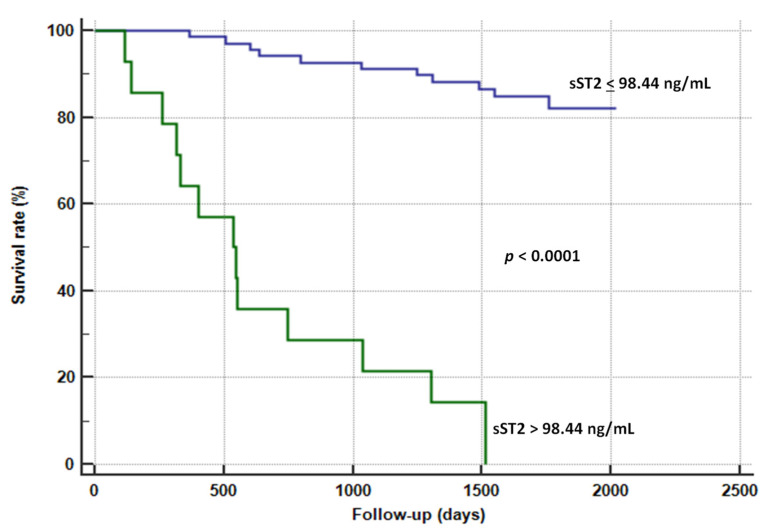
Figure 2 outlines the Kaplan–Meier survival curves for sST2.
Figure 2.
Kaplan–Meier survival curves for sST2 for the prediction of all-cause mortality.
sST2 > 98.44 ng/mL significantly impacted survival rate: at the one-year follow-up, survival rate decreased up to 20% in patients with sST2 higher than the cut-off value.
Therefore, we evaluated the main determinants of all-cause mortality in our population in Table 3.
Table 3.
Univariate and multivariate Cox proportional hazards survival analyses and hazards for cerebrovascular symptoms related to carotid plaques.
| Univariate Cox Regression Analysis | Adjusted Cox Regression Analysis | ||||
|---|---|---|---|---|---|
| Survival Analysis | |||||
| Parameter | HR (95% CI) | p- Value | HR (95% CI) | Wald | p- Value |
| sST2, ng/mL | 1.012 (1.008–1.016) | <0.0001 | 1.012 (1.008–1.016) | 34.6856 | <0.0001 |
| Triglycerides, mg/dL | 1.008 (1.002–1.015) | 0.0058 | 1.008 (1.002–1.015) | 6.0960 | 0.0135 |
| Symptomatic Cerebrovascular Diseases | |||||
| Parameter | HR (95% CI) | p- Value | HR (95% CI) | Wald | p- Value |
| Smoking habit | 2.327 (1.083–4.998) | 0.0304 | |||
| Hemoglobin, g/dL | 0.841 (0.716–0.988) | 0.0347 | 0.744 (0.613–0.903) | 8.9739 | 0.0027 |
| HDL-C, mg/dL | 0.972 (0.946–0–999) | 0.0440 | |||
| LDL-C, mg/dL | 1.010 (1.001–1.020) | 0.0282 | |||
| Triglycerides, mg/dL | 1.007 (1.001–1.013) | 0.0162 | |||
| Ezetimibe | 0.151 (0.021–1.104) | 0.0102 | |||
| sST2, ng/mL | 1.013 (1.008–1.017) | <0.0001 | 1.013 (1.008–1.018) | 21.998 | <0.0001 |
Abbreviations: CI: confidential index; HDL-C: high-density lipoprotein cholesterol; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; sST2: soluble suppressor of tumorigenicity.
The multivariate regression analysis revealed that sST2 (HR: 1.012, 95% CI: 1.008–1.016, p < 0.0001) and triglycerides plasma levels (HR: 1.008, 95% CI: 1.002–1.015, p = 0.0135) significantly impacted all-cause mortality rates in our population. When splitting the population in relation to symptoms before hospital admission, we observed that sST2 plasma levels still predicted the occurrence of cerebrovascular symptoms in patients with carotid atherosclerotic plaques (HR: 1.013, 95% CI: 1.008–1.018, p < 0.0001) when performing multivariate regression analysis.
4. Discussion
The value of sST2 and its membrane-associated isoform ST2L in predicting adverse events in carotid atherosclerotic plaques is still a matter of debate. Our study demonstrated that (1) increased levels of sST2 were associated with long-term all-cause mortality and symptomatic cerebrovascular events in patients with carotid atherosclerotic plaques; (2) the presence of ST2L on the surface of atherosclerotic plaques was not able to predict adverse outcomes; (3) the higher the sST2 plasma levels, the lower the expression of ST2L on the membrane of the cells composing the carotid atherosclerotic plaques.
There is still uncertainty about the exact role of the st2 gene and its transcripts in atherosclerosis. During the last few years, researchers have focused their attention on the description of the IL-33–ST2 axis, the significance of sST2 elevation in plasma, and the role of ST2L in atherosclerotic plaques [2,3,16,17]. Nevertheless, the reciprocal correlation between these two receptors and their prognostic role in atherosclerosis and cerebrovascular diseases are still a matter of debate.
Patients at higher cardiovascular risk and with increased plasma levels in sST2 showed lower survival rates as compared with those with reduced sST2 [18,19].
Kim et al. [20] observed that patients with stable coronary artery diseases (CAD) and higher plasma levels in sST2 showed a 13.7-fold increase in major cardiovascular events (MACE)—namely, cardiac death, non-fatal myocardial infarction, coronary revascularization (90 days after ICA), and ischemic stroke. The risk for death in CAD patients with increased plasma concentration of sST2 was twofold higher as compared with those in the lowest percentiles [21,22]. Indeed, a meta-analysis from Liu et al. [12] indicated that elevated baseline sST2 levels promote a 74% increase in the long-term risk of MACE in patients with the acute coronary syndrome (ACS), thus considering a possible implication of sST2 in vulnerable plaques.
Nevertheless, the literature is scant according to the evaluation of the association among sST2/ST2L, carotid atherosclerotic plaques, and prognosis. Marzullo et al. [17] previously demonstrated that ST2L was more expressed on the membrane of macrophages and neo-vessels of carotid plaques in symptomatic patients. We observed that plaques with higher ST2L expression were related to lower plasma levels in sST2 (Figure 1). Nevertheless, ST2L expression did not provide any prognostic information in our population. We rather observed that sST2 was an independent predictor of all-cause mortality in patients with carotid plaques, as it demonstrated an AUC = 0.791, a sensibility level of 54.2%, and a specificity rate of 98.3% for a cut-off value >98.44 ng/mL (Table 2 and Figure 2). This relationship remained stable using the multivariate Cox regression analysis (Table 3). Definitely, we demonstrated that patients with higher sST2 plasma levels were more prone to die and to be symptomatic of their carotid disease. Although we observed a trend toward an inverse relationship between sST2 plasma levels and ST2L expression on the carotid atherosclerotic specimens, this correlation was lost when we performed the multivariate analysis, as well as the predictive value of the ST2L expression.
ST2L effectively failed to exert a prognostic impact in our cohort of patients, as statistical analysis did not demonstrate a predictive value for ST2L as a determinant of all-cause mortality. Similar negative results were for the discrimination between symptomatic and asymptomatic patients. We indeed believe that further analysis should be considered and performed in ad hoc clinical trials in order to better validate this relationship.
Tian et al. [23] reported an increased risk of new stroke event (ischemic or hemorrhagic) and all-cause mortality at 90-day and 1-year follow-up in patients with a transient ischemic attack (TIA)/ischemic stroke and concomitant increased plasma levels in sST2. sST2 seemed to improve the net reclassification improvement (NRI) in this specific category of patients [23]. It has been observed that increased levels in sST2 soon after stroke were related to poor outcomes (defined as modified Rankin Scale 3–6) and a 3.36-fold increase in all-cause mortality [24].
Andersson et al. [25] observed that higher sST2 plasma levels predicted subclinical brain injury, cognitive impairment, and incident stroke, thus suggesting sST2 as a potential biomarker in such a context [25,26]. Our study demonstrated that sST2 plasma level >95.43 ng/mL remained a significant predictor of symptomatic cerebrovascular diseases (Table 2).
Nevertheless, Willems et al. [10] found no correlation between sST2 plasma levels and cerebrovascular events, as well as with histopathological features of a rupture-prone carotid plaque.
A possible explanation might be found in studies that tried to demonstrate the role of interleukin-33 (IL-33), i.e., the main ligand for ST2, on cerebrovascular diseases [27,28,29,30]. Nevertheless, further research should be performed in order to better define the relationship between the carotid plaque, ST2, and the occurrence of cerebrovascular diseases.
Limitations
The small sample size is a limitation of this study. Nevertheless, the number of formalin-fixed, paraffin-embedded carotid plaque samples is the largest seen in the literature on this specific topic. Indeed, dedicated trials are needed in order to definitely confirm the results. Furthermore, this is a single-center study; however, a multicenter approach will more likely produce more intriguing results.
5. Conclusions
Plasma levels in sST2 may act as independent prognostic determinants of all-cause mortality in patients with carotid atherosclerotic plaque who underwent CEA. Increased sST2 concentrations may also discriminate symptomatic versus asymptomatic cerebrovascular diseases in patients with carotid atherosclerotic disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11113142/s1, Table S1: Characteristics of the study population: Symptomatic vs. asymptomatic.
Author Contributions
Conceptualization, P.S., M.M.C., A.M. and R.P.; methodology, P.S., M.M.C., A.M., P.A.G., E.P.N., F.M., D.A. and R.P.; validation, P.S., M.M.C., A.M., P.A.G., E.P.N., F.M., D.A., F.C., A.Z., A.S., C.G., A.S.C. and R.P.; formal analysis, P.S., M.M.C., A.M., F.M., A.S.C. and R.P.; investigation, P.S., M.M.C., A.M., A.S.C., F.C., A.Z., C.G., A.S. and D.A.; resources, M.M.C., A.M., R.P., A.S.C., E.P.N. and P.A.G.; data curation, P.S., M.M.C., A.M., P.A.G., E.P.N., F.M., D.A., F.C., A.Z., A.S., C.G., A.S.C. and R.P.; writing—original draft preparation, P.S., M.M.C., A.M., P.A.G., E.P.N., F.M., D.A., F.C., A.Z., A.S., C.G., A.S.C. and R.P.; writing—review and editing, P.S., M.M.C., A.M., P.A.G., E.P.N., F.M., D.A., F.C., A.Z., A.S., C.G., A.S.C. and R.P.; visualization, P.S., M.M.C., A.M., F.M., R.P. and D.A.; supervision, P.S., M.M.C., F.M., A.M., R.P., D.A., P.A.G. and E.P.N.; project administration, P.S., M.M.C., A.M. and R.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board Policlinic of Bari, Italy (964/2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on request by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Aimo A., Vergaro G., Passino C., Ripoli A., Ky B., Miller W.L., Bayes-Genis A., Anand I., Januzzi J.L., Emdin M. Prognostic Value of Soluble Suppression of Tumorigenicity-2 in Chronic Heart Failure: A Meta-Analysis. JACC Heart Fail. 2017;5:280–286. doi: 10.1016/j.jchf.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Aimo A., Vergaro G., Ripoli A., Bayes-Genis A., Pascual Figal D.A., de Boer R.A., Lassus J., Mebazaa A., Gayat E., Breidthardt T., et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. 2017;5:287–296. doi: 10.1016/j.jchf.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Aimo A., Januzzi J.L., Jr., Vergaro G., Richards A.M., Lam C.S.P., Latini R., Anand I.S., Cohn J.N., Ueland T., Gullestad L., et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur. J. Heart Fail. 2020;22:2078–2088. doi: 10.1002/ejhf.1701. [DOI] [PubMed] [Google Scholar]
- 5.Hou Z.W., Yu H.B., Liang Y.C., Gao Y., Xu G.Q., Wu M., Mei Z., Wang Z.L., Li Z.G., Li Y.Y., et al. Circulating Soluble ST2 Predicts All-Cause Mortality in Severe Heart Failure Patients with an Implantable Cardioverter Defibrillator. Cardiol. Res. Pract. 2020;2020:4375651. doi: 10.1155/2020/4375651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurora L., Peterson E., Gui H., Zeld N., McCord J., Pinto Y., Cook B., Sabbah H.N., Keoki Williams L., Snider J., et al. Suppression tumorigenicity 2 (ST2) turbidimetric immunoassay compared to enzyme-linked immunosorbent assay in predicting survival in heart failure patients with reduced ejection fraction. Clin. Chim. Acta. 2020;510:767–771. doi: 10.1016/j.cca.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller A.M., Xu D., Asquith D.L., Denby L., Li Y., Sattar N., Baker A.H., McInnes I.B., Liew F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojkovic S., Demyanets S., Kopp C.W., Hengstenberg C., Wojta J., Eichelberger B., Panzer S., Gremmel T. Association of Soluble Suppression of Tumorigenesis 2 (sST2) With Platelet Activation, Monocyte Tissue Factor and Ischemic Outcomes Following Angioplasty and Stenting. Front. Cardiovasc. Med. 2020;7:605669. doi: 10.3389/fcvm.2020.605669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stankovic M., Ljujic B., Babic S., Maravic-Stojkovic V., Mitrovic S., Arsenijevic N., Radak D., Pejnovic N., Lukic M.L. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine. 2019;120:242–250. doi: 10.1016/j.cyto.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Willems S., Quax P.H., de Borst G.J., de Vries J.P., Moll F.L., de Kleijn D.P., Hoefer I.E., Pasterkamp G. Soluble ST2 levels are not associated with secondary cardiovascular events and vulnerable plaque phenotype in patients with carotid artery stenosis. Atherosclerosis. 2013;231:48–53. doi: 10.1016/j.atherosclerosis.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Pfetsch V., Sanin V., Jaensch A., Dallmeier D., Mons U., Brenner H., Koenig W., Rothenbacher D. Increased Plasma Concentrations of Soluble ST2 Independently Predict Mortality but not Cardiovascular Events in Stable Coronary Heart Disease Patients: 13-Year Follow-up of the KAROLA Study. Cardiovasc. Drugs Ther. 2017;31:167–177. doi: 10.1007/s10557-017-6718-1. [DOI] [PubMed] [Google Scholar]
- 12.Liu N., Hang T., Gao X., Yang W., Kong W., Lou Q., Yang J. The association between soluble suppression of tumorigenicity-2 and long-term prognosis in patients with coronary artery disease: A meta-analysis. PLoS ONE. 2020;15:e0238775. doi: 10.1371/journal.pone.0238775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor A.R., Ricco J.B., de Borst G.J., Debus S., de Haro J., Halliday A., Hamilton G., Kakisis J., Kakkos S., Lepidi S., et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2018;55:3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Aboyans V., Ricco J.B., Bartelink M.E.L., Björck M., Brodmann M., Cohnert T., Collet J.P., Czerny M., De Carlo M., Debus S., et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2018;55:305–368. doi: 10.1016/j.ejvs.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Visseren F.L.J., Mach F., Smulders Y.M., Carballo D., Koskinas K.C., Bäck M., Benetos A., Biffi A., Boavida J.M., Capodanno D., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 16.Ciccone M.M., Cortese F., Gesualdo M., Riccardi R., Di Nunzio D., Moncelli M., Iacoviello M., Scicchitano P. A Novel Cardiac Bio- Marker: ST2: A Review. Molecules. 2013;18:15314–15328. doi: 10.3390/molecules181215314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzullo A., Ambrosi F., Inchingolo M., Manca F., Devito F., Angiletta D., Zito A., Scicchitano P., Ciccone M.M. ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS ONE. 2016;11:e0156315. doi: 10.1371/journal.pone.0156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardellini M., Rizza S., Casagrande V., Cardolini I., Ballanti M., Davato F., Porzio O., Canale M.P., Legramante J.M., Mavilio M., et al. Soluble ST2 is a biomarker for cardiovascular mortality related to abnormal glucose metabolism in high-risk subjects. Acta Diabetol. 2019;56:273–280. doi: 10.1007/s00592-018-1230-z. [DOI] [PubMed] [Google Scholar]
- 19.Filali Y., Kesäniemi Y.A., Ukkola O. Soluble ST2, a biomarker of fibrosis, is associated with multiple risk factors, chronic diseases and total mortality in the OPERA study. Scand. J. Clin. Lab. Investig. 2021;81:324–331. doi: 10.1080/00365513.2021.1904518. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.L., Lee J.P., Wong N., Lim W.H., Seo J.B., Zo J.H., Kim M.A., Kim S.H. Prognostic value of serum soluble ST2 in stable coronary artery disease: A prospective observational study. Sci. Rep. 2021;11:15203. doi: 10.1038/s41598-021-94714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M., Duan L., Cai Y., Hao B., Chen J., Li H., Liu H. Prognostic value of soluble suppression of tumorigenesis-2 (sST2) for cardiovascular events in coronary artery disease patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2021;20:49. doi: 10.1186/s12933-021-01244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieplinger B., Egger M., Haltmayer M., Kleber M.E., Scharnagl H., Silbernagel G., de Boer R.A., Maerz W., Mueller T. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: Results from the Ludwigshafen risk and cardiovascular health study. Clin. Chem. 2014;60:530–540. doi: 10.1373/clinchem.2013.209858. [DOI] [PubMed] [Google Scholar]
- 23.Tian X., Guo Y., Wang X., Pei L., Wang X., Wu J., Sun S., Li Y., Ning M., Buonanno F.S., et al. Serum soluble ST2 is a potential long-term prognostic biomarker for transient ischaemic attack and ischaemic stroke. Eur. J. Neurol. 2020;27:2202–2208. doi: 10.1111/ene.14419. [DOI] [PubMed] [Google Scholar]
- 24.Hansen C., Sastre C., Wolcott Z., Bevers M.B., Kimberly W.T. Time-dependent, dynamic prediction of fatty acid-binding protein 4, Galectin-3, and soluble ST2 measurement with poor outcome after acute stroke. Int. J. Stroke. 2021;16:660–668. doi: 10.1177/1747493020971166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson C., Preis S.R., Beiser A., DeCarli C., Wollert K.C., Wang T.J., Januzzi J.L., Jr., Vasan R.S., Seshadri S. Associations of Circulating Growth Differentiation Factor-15 and ST2 Concentrations With Subclinical Vascular Brain Injury and Incident Stroke. Stroke. 2015;46:2568–2575. doi: 10.1161/STROKEAHA.115.009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Lin A., Yu Y., Zhang L., Yang G., Hu H., Luo Y. Serum Soluble ST2 as a Novel Inflammatory Marker in Acute Ischemic Stroke. Clin. Lab. 2018;64:1349–1356. doi: 10.7754/Clin.Lab.2018.180105. [DOI] [PubMed] [Google Scholar]
- 27.Sastre C., Bevers M.B., Kimberly W.T. Role of Interleukin-1 Receptor-Like 1 (ST2) in Cerebrovascular Disease. Neurocrit. Care. 2021;35:887–893. doi: 10.1007/s12028-021-01284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y., Zhou Y., Xiao W., Liang Z., Dai J., Weng X., Wu X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen P., Kanninen K.M., Lehtonen Š., Lemarchant S., Puttonen K.A., Oksanen M., Dhungana H., Loppi S., Pollari E., Wojciechowski S., et al. Immunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav. Immun. 2015;49:322–336. doi: 10.1016/j.bbi.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y., Liu H., Zhang H., Ye Q., Wang J., Yang B., Mao L., Zhu W., Leak R.K., Xiao B., et al. ST2/IL-33-Dependent Microglial Response Limits Acute Ischemic Brain Injury. J. Neurosci. 2017;37:4692–4704. doi: 10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request by contacting the corresponding author.