Abstract
Over the last years, prepectoral implant-based breast reconstruction has undergone a renaissance due to several technical advancements regarding mastectomy techniques and surgical approaches for the placement and soft tissue coverage of silicone implants. Initially abandoned due to the high incidence of complications, such as capsular contraction, implant extrusion, and poor aesthetic outcome, the effective prevention of these types of complications led to the prepectoral technique coming back in style for the ease of implant placement and the conservation of the pectoralis muscle function. Additional advantages such as a decrease of postoperative pain, animation deformity, and operative time contribute to the steady gain in popularity. This review aims to summarize the factors influencing the trend towards prepectoral implant-based breast reconstruction and to discuss the challenges and prospects related to this operative approach.
Keywords: prepectoral breast reconstruction, synthetic mesh, biologic mesh, implant-based breast reconstruction, mastectomy, hybrid breast reconstruction
1. Introduction
Attempts to surgically remove breast cancer lesions date back thousands of years. Even the oldest known surgical document, the Edwin Smith Egyptian papyrus, contains reasonings on how to treat breast tumors [1]. Even though early attempts were mostly contained to rapid excisions and/or cauterization due to the lack of anesthesia, usually brutal experimental procedures continued to be performed despite the excessive disfigurement, morbidity, and mortality [2]. In 1804, Japanese surgeon Seishu Hanaoka used a self-concocted anesthetic mixture to perform what some believe to be the world’s first procedure under general anesthesia, a mastectomy [3]. Only 90 years later, Halsted published his landmark paper “The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889, to January 1894” [4]. In what is today known as Halsted’s radical mastectomy, all suspected tissues, including the pectoralis major muscle, were resected en bloc to prevent the spread of the breast cancer and to cure the disease. Later in his career, Halsted recommended an even more extensive dissection, including, for instance, the supraclavicular lymph nodes [5]. His procedure continued to be the standard of care up until the 1940s, when the additional use of radiation therapy became more widespread. Techniques such as the modified radical mastectomy by Madden [6], which spared the pectoralis muscle, or the simple mastectomy showed similar oncological outcomes to Halsted’s mastectomy when combined with radiation therapy [2]. Advances in cancer biology, a better understanding of the pathophysiology, and consistent screening methods resulted in the development of the concept of breast conservation surgery, as well as substantially changed approaches to mastectomy. Freeman described the subcutaneous or skin preserving mastectomy for benign lesions of the breast as early as 1962 [7], though the technique was not used for the treatment of breast cancer until decades later [8]. In addition, nipple sparing mastectomy techniques were popularized over time, as they allowed for an excellent aesthetic outcome close to the original aspect of the breast [9].
Parallel to this development, reconstructive procedures continued to evolve, including the search for suitable implants. Starting with the transplantation of a lipoma to fill the defect left by a partial mastectomy [10], ivory, glass, rubber, cartilage, wool, polyethylene chips, and even sponges have been used as breast implants [11]. In the 1960s, Cronin and Gerow reported the use of a silicone gel breast implant for breast augmentation, and this was soon after followed by the initial use of silicone implants for breast reconstruction [12,13]. The initial pre-pectoral placement was soon abandoned, due to massive rates of capsular contraction, implant extrusion, infection, and poor aesthetic outcome (Figure 1) [14]. The subsequent shift to the subpectoral plane offered an increased coverage of the implant and the effective prevention of some of these complications [14]. Newer operative techniques led to initially pleasing results [15]. However, subpectoral implant placement in turn is frequently associated with chronic muscle related pain, muscle spasms or contractions, animation deformity of the reconstructed breast, reduced physical mobility of the upper extremity, and eventually a reduction in physical strength of the patient (Figure 2) [16,17]. Therefore, prepectoral implant-based breast reconstruction (IBBR) has undergone a renaissance, utilizing various technical advancements to control and prevent the initially encountered challenges of the technique (Supplementary Video S1). Among these advancements are the continued optimization of mastectomy techniques, advances in radiation therapy, the use of alloplastic adjuncts and autologous fat grafts, as well as new implant designs (Figure 3).
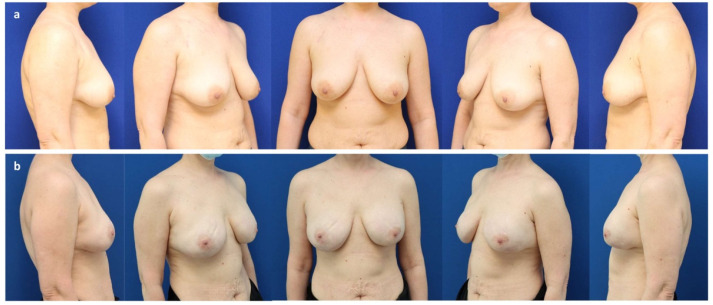
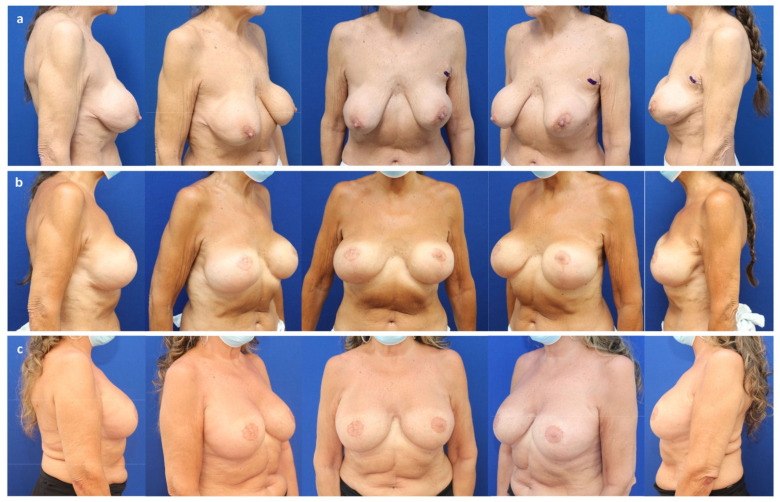
Figure 1.
(a) A 41-year-old patient with invasive-ductal carcinoma (pT2 pN1a (3/3) M0) of the left breast and a BRCA-1 mutation after neo-adjuvant chemotherapy with mild volume asymmetry and breast ptosis grade II (Regnault classification). (b) Six years after the bilateral nipple sparing mastectomy and immediate subpectoral implant-based breast reconstruction (Motiva Ergonomix® ERSD 475 cc, Establishment Labs Motiva, Alajuela, Costa Rica) and ADM (Strattice™ tissue matrix, LifeCell Corporation; Branchburg, NJ, USA) through a periareolar access with lateral extension to correct the ptosis and adjuvant radiotherapy of the left breast. Note the rippling of the right breast (upper inner quadrant), skin retraction, and capsular contraction grade II (Baker classification) of the left breast.
Figure 2.
(a) A 45-year-old patient after a bilateral nipple-sparing mastectomy through a lateral access for invasive-ductal carcinoma (pT2 pN0 M0) of the right breast and a prophylactic mastectomy of the left breast after immediate subpectoral implant-based reconstruction elsewhere. Note the capsular contraction grade IV (Baker classification) and implant displacement on the right. (b) Six years after bilateral partial capsulectomy, reconstruction of the subpectoral implant pocket using a resorbable synthetic mesh (Vicryl®, Ethicon, Cincinnati, OH, USA), and implant exchange (Motiva Ergonomix® ERSD 300 cc).
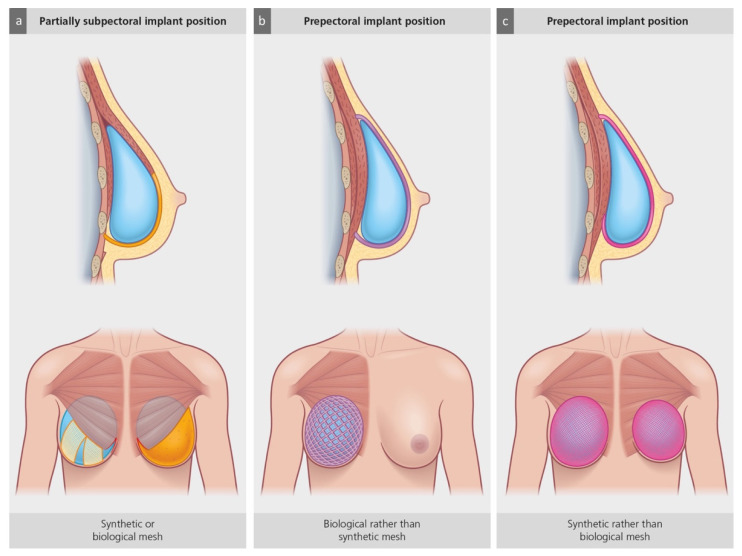
Figure 3.
Schematic drawing of three different clinical situations during implant-based breast reconstruction (BR). (a) Subpectoral placement of an expander or implant requires the partial detachment of the greater pectoral muscle with partial medial disinsertion. Absence of fixation using synthetic mesh strips (right hemithorax) or biologic meshes (acellular dermal matrix; ADM; left hemithorax) will result in cranial muscle retraction. (b) Prepectoral expander or implant placement in patients with thin mastectomy flaps may benefit from thickened adipocutaneous implant coverage, due to ADM or synthetic meshes with tissue-integrative potential. (c) Prepectoral implant placement in patients with a mismatch between the large footprint of the breast after a mastectomy (e.g., breast hypertrophy) or a wide implant pocket in revisional breast surgery (e.g., down-sizing of breast implant volume) and implant size. When using a smaller implant, these patients may benefit from implant positioning and fixation using synthetic, pocket-shaped meshes rather than ADMs.
With the present narrative review, we want to give an overview of the factors that have contributed to the current trend towards prepectoral breast reconstruction and discuss the related chances and challenges, as well as illustrate them with appropriate clinical cases.
2. Current Mastectomy Techniques and Their Implications for Implant-Based Breast Reconstruction
The continuously growing share of women desiring breast reconstruction after mastectomy has markedly contributed to the rise in IBBR, as autologous procedures, including microvascular flaps, are generally less available [18]. The trend towards bilateral procedures has also added to this effect [19]. Such bilateral procedures are commonly performed as either a contralateral prophylactic mastectomy in patients with unilateral cancer, or as a bilateral prophylactic mastectomy in women with an increased breast cancer risk. While a clear risk reduction for the development of breast cancer could be demonstrated in high-risk populations, such as carriers of genetic mutations [20,21], an increasing number of patients that do not carry gene mutations still decide to undergo a contralateral prophylactic mastectomy anyways [22], without clear evidence for a substantial survival benefit [23,24,25,26,27]. In these cases, however, the use of prepectoral IBBR seems particularly attractive, as the minimally invasive muscle sparing approach appeals to the generally young and both functionally, as well as aesthetically demanding patient cohort [28,29]. Moreover, bilateral implant placement improves the perception of breast symmetry [27], and unlike autologous flap-based reconstruction, it does not result in a collateral damage due to tissue harvesting.
Besides a clear shift in the quantity, it is above all the technique and quality of performed mastectomies that has changed over time. Surgical mastectomy has gradually undergone a paradigm shift from the “maximum tolerable” to the “minimum effective” treatment [30]. Conservative surgical approaches, such as skin-sparing mastectomies (SSM) and nipple-sparing mastectomies (NSM), have become the standard of care, whenever the resection of the entire mammary gland is oncologically warranted or desired by the patient, both in the curative and prophylactic setting. By preserving as much as possible of the soft tissue envelope of the breast, these techniques attribute a much greater value to a women’s body image and well-being after treatment and ultimately represent a new attitude towards breast cancer therapy. While SSM has been universally recognized to be an oncologically safe and effective treatment option [31], NSM was initially scrutinized due to the small amount of residual ductal tissue behind the nipple–areola complex (NAC), considered to be at risk of an occult NAC involvement and subsequent local recurrence [32,33]. However, recent studies report few or no nipple recurrences and overall survival rates of up to 98–99%, thus supporting the safety of this procedure [34]. Nonetheless, histopathological analyses of retroareolar biopsies have become part of the standard procedure [35]. Even though patients experience a significant loss of sensation [36], the preservation of the breast envelope is associated with an improved postoperative body image and sexual function and has ultimately led to the clinical implementation of SSM and NSM techniques whenever possible [37,38]. Moreover, future technical advancements will continue to change the standards of care in breast cancer surgery. Robotic NSM, for instance, has emerged as a novel approach that promises to push the efforts to conserve the breast envelope even further [39,40,41]. Even though its safety and feasibility are still being evaluated, the initial meta-analysis did not find significant differences in complication rates when comparing robotic NSM to traditional NSM [42].
If conservative mastectomy is indicated, several patient-related factors, such as body-mass-index, breast volume and grade of ptosis, active smoking habits and co-morbidities such as diabetes, as well as disease-related factors, including the need for adjuvant therapy, influence the surgical course of action and the possibility of prepectoral IBBR. Patients with hypertrophic and ptotic breasts remain a challenging subgroup for prepectoral IBBR, as they have a higher risk of developing complications such as NAC-necrosis or delayed wound healing [43,44]. Skin reducing mastectomy (SRM) has emerged as a way to utilize the skin excess for additional soft tissue coverage [43,45]. Skin reduction is achieved by using common mastopexy incision patterns, with the Wise pattern being used most frequently. This results in a de-epithelialized inferior adipocutaneous flap that is used for additional soft tissue coverage of the inferior implant pole [46]. In selected cases, these flaps can also be used to stabilize the implant in its subcutaneous pocket. In addition, new incision patterns continue to be described with the intention to perfect this technique [47,48]. Another interesting approach is the technique of “preshaping” [49,50,51]. For this approach, mastopexy or reduction mastoplasty is performed as a first step, followed by risk-reducing NSM and prepectoral IBBR a few months later.
Once the mammary gland excision has been performed, it is part of the competence of the breast surgeon to adequately evaluate the intraoperative situation regarding thickness, dimensions, and perfusion of the mastectomy flap, as adequate soft tissue coverage is essential for successful prepectoral IBBR. Should the intraoperative perfusion of the mastectomy flap remain dubious, conversion to a subpectoral approach, at least temporarily, may decrease the likelihood of mastectomy skin flap necrosis and reconstructive failure [52]. If prepectoral placement of a tissue expander or implant was carried out, the used surgical approach should also influence subsequent treatment decisions. For instance, if minor skin flap necrosis occurs, aggressive management with early debridement may be necessary to avoid exposure of the device or any implanted materials, as the pectoralis muscle cannot function as a barrier between the mastectomy flap and the surgical pocket [52].
3. The Use of Biologic and Synthetic Meshes in Prepectoral IBBR
Another important factor that has influenced the trend towards prepectoral breast reconstruction is the increasing use of biologic meshes (acellular dermal matrices = ADMs) and synthetic meshes. Initially popularized for direct-to-implant (DTI) reconstructions [53], they have become increasingly popular in both single- and two-stage IBBR. Over time, different types of alloplastic adjuncts have become available, including ADMs derived from human, bovine, and porcine dermis, as well as absorbable, partially absorbable, and non-absorbable synthetic meshes [54]. Even though they bear a certain endogenous potential for complications, such as infection, seroma formation, and red breast syndrome [55,56], ADMs and meshes became an important player in the prevention of implant-associated complications in prepectoral IBBR [57]. Their use ensures an improved implant placement and soft tissue coverage by wrapping them around the entire implant or using them to cover its front side (Figure 4). The implant can thus be fixed to the pectoralis major muscle or its fascia without dissecting the muscle, reducing postoperative pain and facilitating a fast recovery [58]. The use of ADMs and meshes also decreases the pressure on the caudal mastectomy flap, preventing ischemic wound complications during the acute postoperative phase or a “bottoming out” of the implant over time. This improved fixation is particularly beneficial when un-textured implants are used, as they do not adhere to the surrounding soft tissue over time and therefore progressively expand the skin. Furthermore, the use of alloplastic adjuncts can compensate for an unfavorable mismatch between the size of the tissue pocket and the implant’s width after mastectomy or in revisional surgery after IBBR. In this case, the internal fixation of the implant further helps avoid complications, such as the lateral displacement of the implant in the supine position.
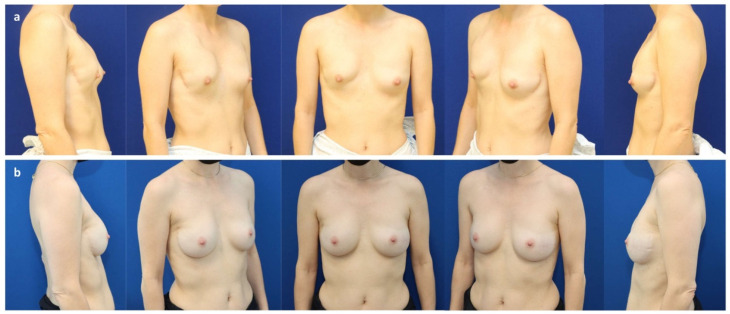
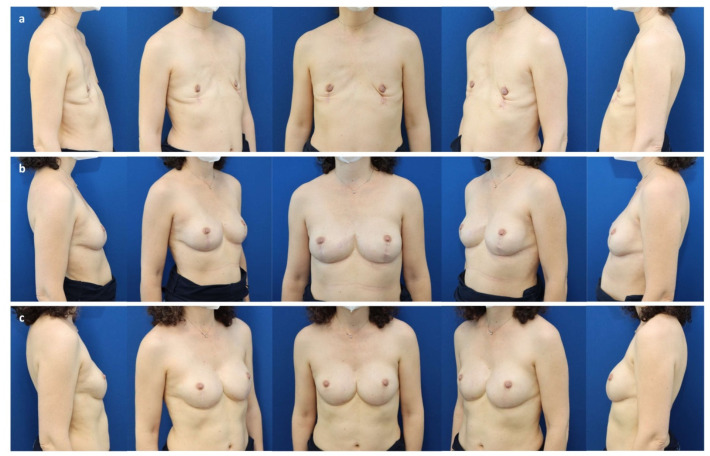
Figure 4.
(a) A 43-year-old patient with multifocal invasive-ductal carcinoma (pT1c pN1mi M0) of the right breast. (b) Six months after a nipple sparing mastectomy through a lateral hemi-areolar access with vertical extension and prepectoral expander placement (Motiva Flora® XMF-58 440 cc) and ADM (SurgiMend® PRS meshed, Integra LifeSciences, Princeton NJ, USA) use. (c) One year after expander exchange for a definitive implant (Motiva Ergonomix® ERSD 300 cc) and autologous fat grafting to the mastectomy flap (140 cc) and mastopexy of the left breast (“auto-augmentation”).
Furthermore, the use of ADMs and meshes makes IBBR in irradiated patients a feasible option [59]. Despite the added costs and potentially higher complication rates, surgeons advocate the use of ADMs in irradiated or otherwise complex patients, who might particularly benefit from their use [60,61]. Several studies have demonstrated a significantly lower rate of capsular contraction due to reduced chronic inflammation and subsequent tissue elastosis in this therapeutic setting [60,62,63,64,65,66].
Alloplastic adjuncts should, however, not be used without careful consideration. Instead, the surgeon should implant as little foreign material as possible without compromising the desired stabilization and coverage of the implant. In this context, a retrospective analysis showed that thicker ADMs are associated with an increased incidence of seroma, infection, or skin necrosis [67]. This is probably due to a slower neovascularization and thus, a later integration of the ADM into the surrounding tissues of the mastectomy flap [68]. It does, therefore, remain dubitable whether the prepectoral position should still be used in borderline cases such as particularly slim patients or under very thin mastectomy flaps, creating an unfavorable ratio between patient tissue and matrix to be integrated with a disproportionately high risk of complications.
4. Autologous Fat Grafting and Hybrid Prepectoral Reconstruction
An indirect contribution of ADM-use to the trend towards prepectoral IBBR is the fact that biological meshes do not only increase the thickness of the thin mastectomy flaps, but they can also serve as a well vascularized recipient tissue for subsequent autologous fat grafting (AFG) after their successful integration (Figure 5). The oncological safety of AFG after breast cancer has been demonstrated in several clinical trials, including long term follow up studies [69,70]. AFG thickens the soft tissue coverage of the inserted implant and, thus, improves the aesthetic outcome of IBBR by reducing complications such as implant rippling or contour irregularities. In fact, several studies have shown that this procedure significantly improves patient satisfaction after breast cancer surgery and IBBR [71,72,73].
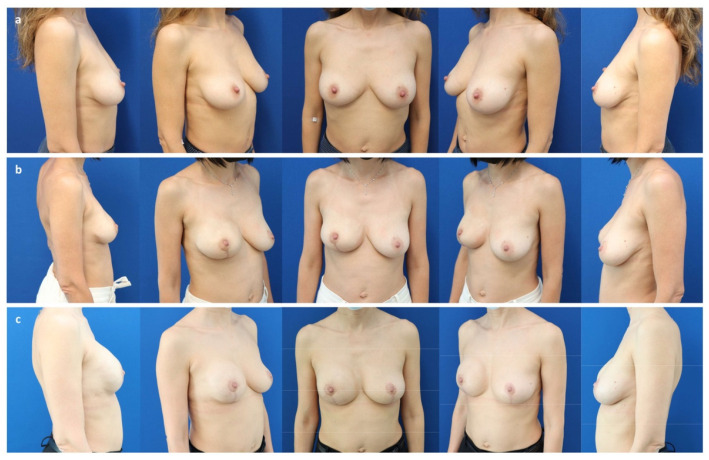
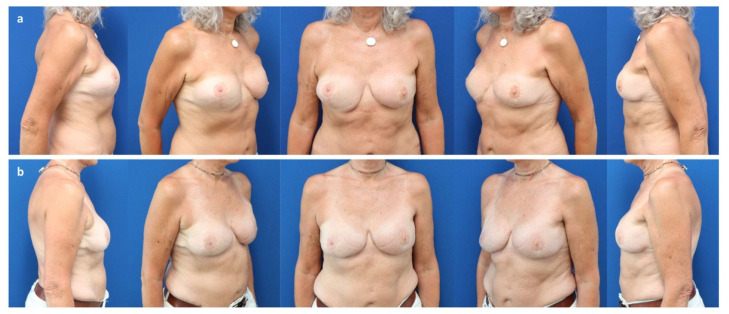
Figure 5.
(a) A 62-year-old patient with persistent in situ ductal carcinoma (pTis pN0 M0) of the left breast after tumorectomy, as well as bilateral ptosis grade II (Regnault classification) and capsular contracture grade IV (Baker) 30 years after prepectoral augmentation mastoplasty. (b) Six months after a bilateral skin reducing mastectomy, free nipple graft, and prepectoral expander placement (Mentor CPX4 550 cc, Mentor Worldwide LLC, Irvine, CA, USA). (c) Two years after a bilateral expander exchange for a definitive implant (Motiva Ergonomix® ERSD 575 cc), synthetic pocket-like mesh (TiLOOP® Bra Pocket, pfm medical AG, Cologne, Germany), and autologous fat grafting to the mastectomy flaps (210 cc/breast).
Due to the beneficial effects of AFG, some surgeons have begun to postulate hybrid or composite concepts that combine prepectoral DTI reconstruction with AFG during the same operative procedure [74]. A more gradual surgical approach is the so called “reverse expansion”, where the placed tissue expander is drained in a stepwise fashion once the desired skin expansion is reached, and the removed volume is replaced with several AFG sessions (Figure 6) [75]. By markedly augmenting the subcutaneous fat compartment, a more desirable ratio between smaller implant volume and thicker soft-tissue coverage can be achieved. In addition to the increased quantity of tissue, AFGs have also been shown to improve its quality [76]. This aspect of AFG is particularly beneficial for patients that have previously been irradiated [73]. For these patients, the risk of early complications, reconstruction failure, and poor aesthetic results can reach up to 50% [77]. Carrying out AFG, either before the tissue expander is substituted with the definitive implant or even before the prophylactic mastectomy is performed, significantly improved surgical outcomes [78,79,80].
Figure 6.
(a) A 44-year-old patient with mild structural deformity of the anterior chest wall and state after bilateral implant-removal for infection following a nipple-sparing mastectomy and immediate subpectoral implant-based reconstruction elsewhere for multifocal invasive-ductal carcinoma (pT2 pN 1a (3/3) M0) associated with an extended in situ component of the right breast and BRCA-2 mutation. (b) Three months after completed expansion (Motiva Flora® XMF-58 440 cc) and before the first session of autologous fat grafting. (c) One year after the expander-to-implant based prepectoral breast reconstruction and two sessions of autologous fat grafting (70–90 cc per breast: reversed expansion, “hybrid breast reconstruction”; Motiva Ergonomix® ERSM 275 cc).
Ultimately, no amount of soft tissue coverage can completely eradicate complications associated with the use of silicone implants, but breast surgeons may be able to increase and prolong the durability of prepectoral IBBR, especially in irradiated patients prone to implant failure, and therefore reduce the need for implant-related revisional surgery. However, general implant-related complications such as breast asymmetry remain a concern regarding the long-term outcome of prepectoral IBBR [81]. While it is an appropriate surgical approach for patients that lack sufficient tissue or refuse flap-based breast reconstruction [82], it may be prudent to consider that the cost of repeated AFG sessions, implant placement, and substitutions rivals the cost of flap-based autologous breast reconstruction without the benefit of being a lifetime solution.
5. Radiation Therapy and Prepectoral IBBR
Even though radiation therapy is an integral part of breast cancer treatment, the use of postmastectomy radiotherapy considerably complicates breast reconstruction, in particular when implants are used (Figure 7) [83]. In addition, a considerable part of patients undergoing a mastectomy has previously undergone breast conserving treatment consisting of tumorectomy and adjuvant radiotherapy. However, IBBR in irradiated patients has become more feasible with the introduction of ADMs and the use of AFG to counteract radiation damage and complications such as capsular contracture or implant extrusion [59,79,80,84]. Interestingly, prepectoral implant placement has been shown to be beneficial in this context. Subpectorally placed implants exhibited a significantly higher rate of capsular contractures when compared to prepectoral implants in irradiated patients [85]. In addition, the observed cases of capsular contractures were markedly more pronounced in subpectorally placed implants when graded according to the Baker classification [85].
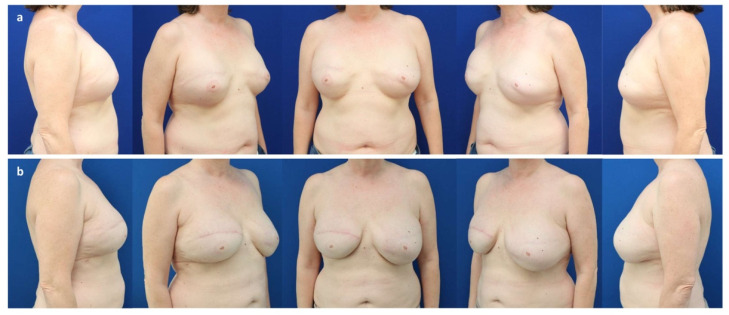
Figure 7.
(a) A 59-year-old patient after a bilateral skin reducing mastectomy for invasive lobular carcinoma (pT3 pN2 (10/10) M0) of the left breast and immediate subpectoral implant-based reconstruction elsewhere, followed by adjuvant radiotherapy. Exchange of implants with microvascular flaps from the abdomen (DIEP). Salvage of the breast pocket with an implant on the right for flap failure. Note the caudal implant displacement and asymmetry of breast shape. (b) Three years after a pocket change from subpectoral to prepectoral, exchange of the implant (Motiva Ergonomix® ERSM 400 cc), reconstruction of the inframammary fold, and autologous fat grafting to the mastectomy flap (220 cc).
Another development that may further ease the combination of radiation therapy and IBBR is the emerging concept of preoperative “neo-adjuvant” radiation protocols [86,87,88,89]. Combining preoperative radiation therapy with immediate reconstruction could bear several advantages, as it avoids the direct irradiation of the implant and prevents the need for secondary reconstruction after tissue expander placement. Ideally, the initial consolidation and wound healing can thus take place before any chronic radiation damage, such as fibrosis or microangiopathy, reaches its full extend [90]. Initial studies do not show relevant differences in overall survival when comparing preoperative and postoperative radiation [91]. Even though such protocols rely on a close interdisciplinary collaboration and on adhering to a strict perioperative treatment sequence, this optimization may further decrease the incidence of surgical complications in breast reconstruction.
6. Further Technical Developments
Other technical advances have contributed to the trend towards prepectoral IBBR in smaller, yet interesting ways. Several techniques have been described to increase the survival rate and tissue quality of mastectomy flaps; for instance, there is the use of local heat preconditioning [92,93] or hyperbaric oxygen therapy [94]. Furthermore, the development and clinical implication of intraoperative indocyanine green angiography (ICG) for the real time visualization of tissue perfusion has been another crucial step toward perfecting prepectoral IBBR [95]. The immediate intraoperative assessment of the perfusion of the mastectomy skin flap to guide excision of inadequately perfused areas has since translated to improved clinical outcomes. By preventing necrosis or wound dehiscence, ICG has proven to be a helpful tool in reconstructive breast surgery, though large randomized clinical trials are still warranted to confirm its effectiveness [96]. Other issues that need to be addressed in the future are the currently missing standardization and the possible overprediction of necrosis [97].
Nowadays, surgeons can also choose from a much larger range of implants to best suit the patient’s needs. Textured implants, which fuse better with the surrounding tissue, prevent a “bottoming out” of the implant. Although the risk is very low, the risk of implant-associated anaplastic large cell lymphoma (BIA-ALCL) must be considered and discussed with the patient, but they may still be indicated in specific situations [98]. Besides textured implants, newer lightweight implants are an additional option to reduce pressure on the caudal mastectomy flap [99]. Especially when large implants are necessary, as often is the case in reconstructive breast surgery, this may be beneficial for the initial wound healing process after surgery and long-term prevention of bottoming out (Figure 8).
Figure 8.
(a) A 54-year-old patient with state after a bilateral mastectomy for multifocal invasive ductal carcinoma (pT3 pN2 (10/10) M0) of the right breast and prophylactic mastectomy of the left breast, as well as immediate subpectoral expander-to-implant-based reconstruction elsewhere, followed by adjuvant immuno-chemotherapy. Note the bilateral capsular contracture (Baker classification grade IV). (b) Two years after a pocket change from subpectoral to prepectoral, exchange of the implant (Motiva Ergonomix® ERSD 575 cc), and autologous fat grafting to the mastectomy flaps (220 cc/side) without mesh-support. Note bottoming out of the left.
7. Conclusions
Taken together, prepectoral implant placement after mastectomy has become a valid surgical alternative for breast reconstruction. By maintaining the breast envelope and increasing soft tissue coverage, complications such as implant extrusion and capsular contracture can effectively be decreased, even in irradiated patients. Short-term benefits such as a rapid recovery and maintained pectoralis muscle function contribute to the patient’s psychosocial well-being and high satisfaction with the reconstructive result. Though many long-term complications related to subpectoral implant placement, including breast animation and impaired muscle function, are effectively avoided, general implant-related complications, such as breast asymmetry, remain a concern for prepectoral IBBR. It does, however, provide good to excellent aesthetic and functional results for patients that cannot or do not wish to undergo autologous breast reconstruction, for instance because they lack sufficient tissue.
In conclusion, the collective learning curve of prepectoral IBBR has not reached a plateau yet, though massive strides have been made in the last years. Besides the shared experiences of breast surgeons worldwide, large scale clinical trials will continue to provide crucial information and opportunities to further improve the technique. Surgeons should therefore strive to incorporate new developments into their clinical routines as they emerge to provide patients with the best possible care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11113079/s1, Supplementary Video S1: Prepectoral breast reconstruction.
Author Contributions
Conceptualization, A.W., D.S. and Y.H.; writing—original draft preparation, A.W. and D.B.; writing—review and editing, D.S. and Y.H.; visualization, A.W., D.S., D.B. and Y.H.; supervision, Y.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breasted J.H. The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes. JAMA J. Am. Med. Assoc. 1931;96:1534. doi: 10.1001/jama.1931.02720440082042. [DOI] [Google Scholar]
- 2.Freeman M.D., Gopman J.M., Salzberg C.A. The Evolution of Mastectomy Surgical Technique: From Mutilation to Medicine. Gland. Surg. 2018;7:308–315. doi: 10.21037/gs.2017.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izuo M. Medical History: Seishu Hanaoka and His Success in Breast Cancer Surgery under General Anesthesia Two Hundred Years Ago. Breast Cancer. 2004;11:319–324. doi: 10.1007/BF02968037. [DOI] [PubMed] [Google Scholar]
- 4.Halsted W.S. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann. Surg. 1894;20:497–555. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halsted W.S. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann. Surg. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madden J.L. Modified Radical Mastectomy. Surg. Gynecol. Obstet. 1965;121:1221–1230. doi: 10.1097/00000658-197205000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Freeman B.S. Subcutaneous Mastectomy for Benign Breast Lesions with Immediate or Delayed Prosthetic Replacement. Plast. Reconstr. Surg. 1962;30:676–682. doi: 10.1097/00006534-196212000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Toth B.A., Lappert P. Modified Skin Incisions for Mastectomy: The Need for Plastic Surgical Input in Preoperative Planning. Plast. Reconstr. Surg. 1991;87:1048–1053. doi: 10.1097/00006534-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Murthy V., Chamberlain R.S. Defining a Place for Nipple Sparing Mastectomy in Modern Breast Care: An Evidence Based Review. Breast J. 2013;19:571–581. doi: 10.1111/j.1524-4741.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 10.Czerny V. Drei Plastische Operationen. III. Plastischer Ersatz Der Brustdruse Durch Ein Lipom. Arch. F Klin. Chir. 1895;50:216–217. [Google Scholar]
- 11.Young V.L., Watson M.E. Breast Implant Research: Where We Have Been, Where We Are, Where We Need to Go. Clin. Plast. Surg. 2001;28:451–483. doi: 10.1016/S0094-1298(20)32389-0. [DOI] [PubMed] [Google Scholar]
- 12.Snyderman R.K., Guthrie R.H. Reconstruction of the Female Breast Following Radical Mastectomy. Plast. Reconstr. Surg. 1971;47:565–567. doi: 10.1097/00006534-197106000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Cronin T.D., Brauer R.O. Augmentation Mammaplasty. Surg. Clin. N. Am. 1971;51:441–452. doi: 10.1016/S0039-6109(16)39388-4. [DOI] [PubMed] [Google Scholar]
- 14.Sbitany H. Pre-Pectoral Breast Reconstruction: A Less Invasive Option. Gland. Surg. 2019;8:1–2. doi: 10.21037/gs.2018.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigo M.H., Piccinini P.S., Sartori L.D.P., de Carvalho L.A.R., Uebel C.O. SMS—Split Muscle Support: A Reproducible Approach for Breast Implant Stabilization. Aesthetic Plast. Surg. 2020;44:698–705. doi: 10.1007/s00266-019-01565-5. [DOI] [PubMed] [Google Scholar]
- 16.Kraenzlin F., Chopra K., Kokosis G., Venturi M.L., Mesbahi A., Nahabedian M.Y. Revision Breast Reconstruction with Prepectoral Pocket Conversion of Submuscular Breast Implants. Plast. Reconstr. Surg. 2021;147:743e–748e. doi: 10.1097/PRS.0000000000007885. [DOI] [PubMed] [Google Scholar]
- 17.Leonardis J.M., Lyons D.A., Giladi A.M., Momoh A.O., Lipps D.B. Functional Integrity of the Shoulder Joint and Pectoralis Major Following Subpectoral Implant Breast Reconstruction. J. Orthop. Res. 2019;37:1610–1619. doi: 10.1002/jor.24257. [DOI] [PubMed] [Google Scholar]
- 18.Mennie J.C., Mohanna P.N., O’Donoghue J.M., Rainsbury R., Cromwell D.A. National Trends in Immediate and Delayed Post-Mastectomy Reconstruction Procedures in England: A Seven-Year Population-Based Cohort Study. Eur. J. Surg. Oncol. 2017;43:52–61. doi: 10.1016/j.ejso.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Cemal Y., Albornoz C.R., Disa J.J., Mccarthy C.M., Mehrara B.J., Pusic A.L., Cordeiro P.G., Matros E. A Paradigm Shift in U.S. Breast Reconstruction: Part 2. the Influence of Changing Mastectomy Patterns on Reconstructive Rate and Method. Plast. Reconstr. Surg. 2013;131:320e–326e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 20.de Felice F., Marchetti C., Musella A., Palaia I., Perniola G., Musio D., Muzii L., Tombolini V., Benedetti Panici P. Bilateral Risk-Reduction Mastectomy in BRCA1 and BRCA2 Mutation Carriers: A Meta-Analysis. Ann. Surg. Oncol. 2015;22:2876–2880. doi: 10.1245/s10434-015-4532-1. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig K.K., Neuner J., Butler A., Geurts J.L., Kong A.L. Risk Reduction and Survival Benefit of Prophylactic Surgery in BRCA Mutation Carriers, a Systematic Review. Am. J. Surg. 2016;212:660–669. doi: 10.1016/j.amjsurg.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 22.You Q., Chen K., Li Y., Lai J., Fang Y., Shen S., Liu Y., Su F., Yu F. Factors Associated with the Increasing Trend of Contralateral Prophylactic Mastectomy among Patients with Ductal Carcinoma in Situ: Analysis of Surveillance, Epidemiology, and End Results Data. Breast. 2018;40:147–155. doi: 10.1016/j.breast.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Nash R., Goodman M., Lin C.C., Freedman R.A., Dominici L.S., Ward K., Jemal A. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004–2012. JAMA Surg. 2017;152:648–657. doi: 10.1001/jamasurg.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Zhang P., Zhang M., Wang M., Bai F., Wu K. Growing Trends of Contralateral Prophylactic Mastectomy and Reconstruction in Young Breast Cancer. J. Surg. Res. 2019;239:224–232. doi: 10.1016/j.jss.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B., Coopey S.B., Gadd M.A., Hughes K.S., Chang D.C., Oseni T.O. Trends in Unilateral and Contralateral Prophylactic Mastectomy Use in Ductal Carcinoma In Situ of the Breast: Patterns and Predictors. Ann. Surg. Oncol. 2019;26:3863–3873. doi: 10.1245/s10434-019-07628-w. [DOI] [PubMed] [Google Scholar]
- 26.Tuttle T.M., Habermann E.B., Grund E.H., Morris T.J., Virnig B.A. Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend toward More Aggressive Surgical Treatment. J. Clin. Oncol. 2007;25:5203. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 27.Gutnik L., Fayanju O.M. Controversies in Breast Cancer Surgery. Surg. Clin. N. Am. 2021;101:1033–1044. doi: 10.1016/j.suc.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruccia M., Elia R., Tedeschi P., Gurrado A., Moschetta M., Testini M., Giudice G. Prepectoral Breast Reconstruction: An Ideal Approach to Bilateral Risk-Reducing Mastectomy. Gland Surg. 2021;10:2997–3006. doi: 10.21037/gs-21-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezai M., Strauß S., Kimmig R., Kern P. Risk-Reducing, Conservative Mastectomy-Analysis of Surgical Outcome and Quality of Life in 272 Implant-Based Reconstructions Using TiLoop® Bra versus Autologous Corial Flaps. Gland Surg. 2016;5:1–8. doi: 10.3978/j.issn.2227-684X.2015.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veronesi U., Stafyla V., Luini A., Veronesi P. Breast Cancer: From “Maximum Tolerable” to “Minimum Effective” Treatment. Front. Oncol. 2012;2:125. doi: 10.3389/fonc.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galimberti V., Vicini E., Corso G., Morigi C., Fontana S., Sacchini V., Veronesi P. Nipple-Sparing and Skin-Sparing Mastectomy: Review of Aims, Oncological Safety and Contraindications. Breast. 2017;34:S82–S84. doi: 10.1016/j.breast.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de La Cruz L., Moody A.M., Tappy E.E., Blankenship S.A., Hecht E.M. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple–Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann. Surg. Oncol. 2015;22:3241–3249. doi: 10.1245/s10434-015-4739-1. [DOI] [PubMed] [Google Scholar]
- 33.Valero M.G., Muhsen S., Moo T.A., Zabor E.C., Stempel M., Pusic A., Gemignani M.L., Morrow M., Sacchini V.S. Increase in Utilization of Nipple-Sparing Mastectomy for Breast Cancer: Indications, Complications, and Oncologic Outcomes. Ann. Surg. Oncol. 2020;27:344–351. doi: 10.1245/s10434-019-07948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vicini E., de Lorenzi F., Invento A., Corso G., Radice D., Bozzo S., Fontana S.K.R., Caldarella P., Veronesi P., Galimberti V. Is Nipple-Sparing Mastectomy Indicated after Previous Breast Surgery? A Series of 387 Institutional Cases. Plast. Reconstr. Surg. 2021;148:21–30. doi: 10.1097/PRS.0000000000008097. [DOI] [PubMed] [Google Scholar]
- 35.Balci F.L., Kara H., Dulgeroglu O., Uras C. Oncologic Safety of Nipple-Sparing Mastectomy in Patients with Short Tumor-Nipple Distance. Breast J. 2019;25:612–618. doi: 10.1111/tbj.13289. [DOI] [PubMed] [Google Scholar]
- 36.Akdeniz Dogan Z., Farhadi J., Farhadi J. Evaluation of Sensation on Mastectomy Skin Flaps Following Immediate Breast Reconstruction. J. Reconstr. Microsurg. 2020;36:420–425. doi: 10.1055/s-0040-1702157. [DOI] [PubMed] [Google Scholar]
- 37.Didier F., Arnaboldi P., Gandini S., Maldifassi A., Goldhirsch A., Radice D., Minotti I., Ballardini B., Luini A., Santillo B., et al. Why Do Women Accept to Undergo a Nipple Sparing Mastectomy or to Reconstruct the Nipple Areola Complex When Nipple Sparing Mastectomy Is Not Possible? Breast Cancer Res. Treat. 2012;132:1177–1184. doi: 10.1007/s10549-012-1983-y. [DOI] [PubMed] [Google Scholar]
- 38.Metcalfe K.A., Cil T.D., Semple J.L., Li L.D.X., Bagher S., Zhong T., Virani S., Narod S., Pal T. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann. Surg. Oncol. 2015;22:3324–3330. doi: 10.1245/s10434-015-4761-3. [DOI] [PubMed] [Google Scholar]
- 39.Houvenaeghel G., Bannier M., Rua S., Barrou J., Heinemann M., Knight S., Lambaudie E., Cohen M. Robotic Breast and Reconstructive Surgery: 100 Procedures in 2-Years for 80 Patients. Surg. Oncol. 2019;31:38–45. doi: 10.1016/j.suronc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Lai H.W., Wang C.C., Lai Y.C., Chen C.J., Lin S.L., Chen S.T., Lin Y.J., Chen D.R., Kuo S.J. The Learning Curve of Robotic Nipple Sparing Mastectomy for Breast Cancer: An Analysis of Consecutive 39 Procedures with Cumulative Sum Plot. Eur. J. Surg. Oncol. 2019;45:125–133. doi: 10.1016/j.ejso.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Sarfati B., Struk S., Leymarie N., Honart J.F., Alkhashnam H., Tran de Fremicourt K., Conversano A., Rimareix F., Simon M., Michiels S., et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2018;25:2579–2586. doi: 10.1245/s10434-018-6555-x. [DOI] [PubMed] [Google Scholar]
- 42.Filipe M.D., de Bock E., Postma E.L., Bastian O.W., Schellekens P.P.A., Vriens M.R., Witkamp A.J., Richir M.C. Robotic Nipple-Sparing Mastectomy Complication Rate Compared to Traditional Nipple-Sparing Mastectomy: A Systematic Review and Meta-Analysis. J. Robot. Surg. 2021;16:265–272. doi: 10.1007/s11701-021-01265-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman M.K. Reconstruction of the Ptotic Breast Using Wise Pattern Skin Deepithelialization. Plast. Reconstr. Surg.-Glob. Open. 2016;4:e1077. doi: 10.1097/GOX.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rammos C.K., Mammolito D., King V.A., Yoo A. Two-Stage Reconstruction of the Large and Ptotic Breasts: Skin Reduction Mastectomy with Prepectoral Device Placement. Plast. Reconstr. Surg.-Glob. Open. 2018;6:e1853. doi: 10.1097/GOX.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruccia M., Elia R., Gurrado A., Moschetta M., Nacchiero E., Bolletta A., Testini M., Giudice G. Skin-Reducing Mastectomy and Pre-Pectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast. Surg. 2020;44:664–672. doi: 10.1007/s00266-020-01616-2. [DOI] [PubMed] [Google Scholar]
- 46.Safran T., Al-Halabi B., Viezel-Mathieu A., Boileau J.F., Dionisopoulos T. Skin-Reducing Mastectomy with Immediate Prepectoral Reconstruction: Surgical, Aesthetic, and Patient-Reported Outcomes with and without Dermal Matrices. Plast. Reconstr. Surg. 2021;147:1046–1057. doi: 10.1097/PRS.0000000000007899. [DOI] [PubMed] [Google Scholar]
- 47.Movassaghi K., Stewart C.N. The “Smile Mastopexy”: A Novel Technique to Aesthetically Address the Excess Skin Envelope in Large, Ptotic Breasts While Preserving Nipple Areolar Complex during Prosthetic Breast Reconstruction. Aesthetic Surg. J. 2022;42:NP393–NP403. doi: 10.1093/asj/sjac021. [DOI] [PubMed] [Google Scholar]
- 48.Albright W.B., Hawkes P.J. The Bell Pattern: A Novel Breast Incision Approach to Skin-Reducing Mastectomies. Aesthetic Surg. J. Open Forum. 2020;2:ojz031. doi: 10.1093/asjof/ojz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Momeni A., Kanchwala S., Sbitany H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast. Reconstr. Surg. 2020;145:914–920. doi: 10.1097/PRS.0000000000006657. [DOI] [PubMed] [Google Scholar]
- 50.Gunnarsson G.L., Bille C., Reitsma L.C., Wamberg P., Thomsen J.B. Prophylactic Nipple-Sparing Mastectomy and Direct-to-Implant Reconstruction of the Large and Ptotic Breast: Is Preshaping of the Challenging Breast a Key to Success? Plast. Reconstr. Surg. 2017;140:449–454. doi: 10.1097/PRS.0000000000003621. [DOI] [PubMed] [Google Scholar]
- 51.Gunnarsson G.L., Thomsen J.B. Reply: How to Preshape the Breast for a Successful Nipple-Sparing Mastectomy and Direct-to-Implant Breast Reconstruction in the Challenging Breast. Plast. Reconstr. Surg. 2018;141:610e–611e. doi: 10.1097/PRS.0000000000004227. [DOI] [PubMed] [Google Scholar]
- 52.Nahabedian M.Y. Current Approaches to Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. 2018;142:871–880. doi: 10.1097/PRS.0000000000004802. [DOI] [PubMed] [Google Scholar]
- 53.Breuing K.H., Warren S.M. Immediate Bilateral Breast Reconstruction with Implants and Inferolateral AlloDerm Slings. Ann. Plast. Surg. 2005;55:232–239. doi: 10.1097/01.sap.0000168527.52472.3c. [DOI] [PubMed] [Google Scholar]
- 54.Rolph R., Farhadi J. The Use of Meshes and Matrices in Breast Reconstruction. Br. J. Hosp. Med. 2018;79:454–459. doi: 10.12968/hmed.2018.79.8.454. [DOI] [PubMed] [Google Scholar]
- 55.Nahabedian M.Y. Prosthetic Breast Reconstruction and Red Breast Syndrome: Demystification and a Review of the Literature. Plast. Reconstr. Surg.-Glob. Open. 2019;7:e2108. doi: 10.1097/GOX.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer H.F., Perez Colman M.C., Stoppani I. Red Breast Syndrome (RBS) Associated to the Use of Polyglycolic Mesh in Breast Reconstruction: A Case Report. Acta Chir. Plast. 2020;62:50–52. [PubMed] [Google Scholar]
- 57.Cabalag M.S., Rostek M., Miller G.S., Chae M.P., Quinn T., Rozen W.M., Hunter-Smith D.J. Alloplastic Adjuncts in Breast Reconstruction. Gland. Surg. 2016;5:158–173. doi: 10.3978/j.issn.2227-684X.2015.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sigalove S., Maxwell G.P., Sigalove N.M., Storm-Dickerson T.L., Pope N., Rice J., Gabriel A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017;139:287–294. doi: 10.1097/PRS.0000000000002950. [DOI] [PubMed] [Google Scholar]
- 59.Haynes D.F., Kreithen J.C. Vicryl Mesh in Expander/Implant Breast Reconstruction: Long-Term Follow-Up in 38 Patients. Plast. Reconstr. Surg. 2014;134:892–899. doi: 10.1097/PRS.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 60.Moyer H.R., Pinell-White X., Losken A. The Effect of Radiation on Acellular Dermal Matrix and Capsule Formation in Breast Reconstruction: Clinical Outcomes and Histologic Analysis. Plast. Reconstr. Surg. 2014;133:214–221. doi: 10.1097/01.prs.0000437255.01199.42. [DOI] [PubMed] [Google Scholar]
- 61.Seth A.K., Hirsch E.M., Fine N.A., Kim J.Y.S. Utility of Acellular Dermis-Assisted Breast Reconstruction in the Setting of Radiation: A Comparative Analysis. Plast. Reconstr. Surg. 2012;130:750–758. doi: 10.1097/PRS.0b013e318262f009. [DOI] [PubMed] [Google Scholar]
- 62.Chopra K., Buckingham B., Matthews J., Sabino J., Tadisina K.K., Silverman R.P., Goldberg N.H., Slezak S., Singh D.P. Acellular Dermal Matrix Reduces Capsule Formation in Two-Stage Breast Reconstruction. Int. Wound J. 2017;14:414–419. doi: 10.1111/iwj.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J., Hou J., Li Z., Wang B., Sun J. Efficacy of Acellular Dermal Matrix in Capsular Contracture of Implant-Based Breast Reconstruction: A Single-Arm Meta-Analysis. Aesthetic Plast. Surg. 2020;44:735–742. doi: 10.1007/s00266-019-01603-2. [DOI] [PubMed] [Google Scholar]
- 64.Lardi A.M., Ho-Asjoe M., Junge K., Farhadi J. Capsular Contracture in Implant Based Breast Reconstruction-the Effect of Porcine Acellular Dermal Matrix. Gland Surg. 2017;6:49–56. doi: 10.21037/gs.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tessler O., Reish R.G., Maman D.Y., Smith B.L., Austen W.G. Beyond Biologics: Absorbable Mesh as a Low-Cost, Low-Complication Sling for Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2014;133:90e–99e. doi: 10.1097/01.prs.0000437253.55457.63. [DOI] [PubMed] [Google Scholar]
- 66.Dieterich M., Paepke S., Zwiefel K., Dieterich H., Blohmer J., Faridi A., Klein E., Gerber B., Nestle-Kraemling C. Implant-Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLOOP Bra): A Multicenter Study of 231 Cases. Plast. Reconstr. Surg. 2013;132:8e–19e. doi: 10.1097/PRS.0b013e318290f8a0. [DOI] [PubMed] [Google Scholar]
- 67.Rose J.F., Zafar S.N., Ellsworth W.A., IV Does Acellular Dermal Matrix Thickness Affect Complication Rate in Tissue Expander Based Breast Reconstruction? Plast. Surg. Int. 2016;2016:2867097. doi: 10.1155/2016/2867097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia O., Scott J.R. Analysis of Acellular Dermal Matrix Integration and Revascularization Following Tissue Expander Breast Reconstruction in a Clinically Relevant Large-Animal Model. Plast. Reconstr. Surg. 2013;131:741e–751e. doi: 10.1097/PRS.0b013e3182865c6d. [DOI] [PubMed] [Google Scholar]
- 69.Sorrentino L., Regolo L., Scoccia E., Petrolo G., Bossi D., Albasini S., Caruso A., Vanna R., Morasso C., Mazzucchelli S., et al. Autologous Fat Transfer after Breast Cancer Surgery: An Exact-Matching Study on the Long-Term Oncological Safety. Eur. J. Surg. Oncol. 2019;45:1827–1834. doi: 10.1016/j.ejso.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Gale K.L., Rakha E.A., Ball G., Tan V.K., McCulley S.J., Macmillan R.D. A Case-Controlled Study of the Oncologic Safety of Fat Grafting. Plast. Reconstr. Surg. 2015;135:1263–1275. doi: 10.1097/PRS.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 71.Cogliandro A., Barone M., Tenna S., Morelli Coppola M., Persichetti P. The Role of Lipofilling After Breast Reconstruction: Evaluation of Outcomes and Patient Satisfaction with BREAST-Q. Aesthetic Plast. Surg. 2017;41:1325–1331. doi: 10.1007/s00266-017-0912-1. [DOI] [PubMed] [Google Scholar]
- 72.Brown A.W.W., Kabir M., Sherman K.A., Meybodi F., French J.R., Elder E.B. Patient Reported Outcomes of Autologous Fat Grafting after Breast Cancer Surgery. Breast. 2017;35:14–20. doi: 10.1016/j.breast.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Panettiere P., Marchetti L., Accorsi D. The Serial Free Fat Transfer in Irradiated Prosthetic Breast Reconstructions. Aesthetic Plast. Surg. 2009;33:695–700. doi: 10.1007/s00266-009-9366-4. [DOI] [PubMed] [Google Scholar]
- 74.Gronovich Y., Winder G., Maisel-Lotan A., Lysy I., Sela E., Spiegel G., Carmon M., Hadar T., Elami A., Eizenman N., et al. Hybrid Prepectoral Direct-to-Implant and Autologous Fat Graft Simultaneously in Immediate Breast Reconstruction: A Single Surgeon’s Experience with 25 Breasts in 15 Consecutive Cases. Plast. Reconstr. Surg. 2022;149:386e–391e. doi: 10.1097/PRS.0000000000008879. [DOI] [PubMed] [Google Scholar]
- 75.Stillaert F.B.J.L., Lannau B., Van Landuyt K., Blondeel P.N. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plast. Reconstr. Surg.-Glob. Open. 2020;8:e2966. doi: 10.1097/GOX.0000000000002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rigotti G., Marchi A., Galiè M., Baroni G., Benati D., Krampera M., Pasini A., Sbarbati A. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 77.Manyam B.V., Shah C., Woody N.M., Reddy C.A., Weller M.A., Juloori A., Naik M., Valente S., Grobmyer S., Durand P., et al. Long-Term Complications and Reconstruction Failures in Previously Radiated Breast Cancer Patients Receiving Salvage Mastectomy with Autologous Reconstruction or Tissue Expander/Implant-Based Reconstruction. Breast J. 2019;25:1071–1078. doi: 10.1111/tbj.13428. [DOI] [PubMed] [Google Scholar]
- 78.Lesniak D.M., Sarfati I., Meredith I., Millochau J., Wang K.C., Nos C., Clough K.B. Fat Grafting before Delayed Prophylactic Mastectomy and Immediate Implant Reconstruction for Patients at High Risk of Complications. Plast. Reconstr. Surg. 2022;149:52–56. doi: 10.1097/PRS.0000000000008672. [DOI] [PubMed] [Google Scholar]
- 79.Ribuffo D., Atzeni M., Guerra M., Bucher S., Politi C., Deidda M., Atzori F., Dessi M., Madeddu C., Lay G. Treatment of Irradiated Expanders: Protective Lipofilling Allows Immediate Prosthetic Breast Reconstruction in the Setting of Postoperative Radiotherapy. Aesthetic Plast. Surg. 2013;37:1146–1152. doi: 10.1007/s00266-013-0221-2. [DOI] [PubMed] [Google Scholar]
- 80.Gentilucci M., Mazzocchi M., Alfano C. Effects of Prophylactic Lipofilling after Radiotherapy Compared to Non-Fat Injected Breasts: A Randomized, Objective Study. Aesthetic Surg. J. 2020;40:NP597–NP607. doi: 10.1093/asj/sjaa182. [DOI] [PubMed] [Google Scholar]
- 81.Nahabedian M.Y. What Are the Long-Term Aesthetic Issues in Prepectoral Breast Reconstruction? Aesthetic Surg. J. 2020;40:S29–S37. doi: 10.1093/asj/sjaa164. [DOI] [PubMed] [Google Scholar]
- 82.Sarfati I., Ihrai T., Duvernay A., Nos C., Clough K. Autologous Fat Grafting to the Postmastectomy Irradiated Chest Wall Prior to Breast Implant Reconstruction: A Series of 68 Patients. Ann. Chir. Plast. Esthet. 2013;58:35–40. doi: 10.1016/j.anplas.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Lee B.T., Adesiyun T.A., Colakoglu S., Curtis M.S., Yueh J.H., Anderson K.E., Tobias A.M., Recht A. Postmastectomy Radiation Therapy and Breast Reconstruction: An Analysis of Complications and Patient Satisfaction. Ann. Plast. Surg. 2010;64:679–683. doi: 10.1097/SAP.0b013e3181db7585. [DOI] [PubMed] [Google Scholar]
- 84.Lee J.S., Lee J.H., Ryu J.Y., Park S.H., Park J.Y., Han M.H., Lee J., Park H.Y., Yang J.D. Influence of Irradiation on Capsules of Silicone Implants Covered with Acellular Dermal Matrix in Mice. Aesthetic Plast. Surg. 2021;46:937–946. doi: 10.1007/s00266-021-02618-4. [DOI] [PubMed] [Google Scholar]
- 85.Sinnott C.J., Persing S.M., Pronovost M., Hodyl C., McConnell D., Ott Young A. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann. Surg. Oncol. 2018;25:2899–2908. doi: 10.1245/s10434-018-6602-7. [DOI] [PubMed] [Google Scholar]
- 86.Hughes K., Neoh D. Neoadjuvant Radiotherapy: Changing the Treatment Sequence to Allow Immediate Free Autologous Breast Reconstruction. J. Reconstr. Microsurg. 2018;34:624–631. doi: 10.1055/s-0038-1660871. [DOI] [PubMed] [Google Scholar]
- 87.Tansley P., Ramsey K., Wong S., Guerrieri M., Pitcher M., Grinsell D. New Treatment Sequence Protocol to Reconstruct Locally Advanced Breast Cancer. ANZ J. Surg. 2013;83:630–635. doi: 10.1111/ans.12110. [DOI] [PubMed] [Google Scholar]
- 88.Monrigal E., Dauplat J., Gimbergues P., le Bouedec G., Peyronie M., Achard J.L., Chollet P., Mouret-Reynier M.A., Nabholtz J.M., Pomel C. Mastectomy with Immediate Breast Reconstruction after Neoadjuvant Chemotherapy and Radiation Therapy. A New Option for Patients with Operable Invasive Breast Cancer. Results of a 20 Years Single Institution Study. Eur. J. Surg. Oncol. 2011;37:864–870. doi: 10.1016/j.ejso.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Paillocher N., Florczak A.S., Richard M., Classe J.M., Oger A.S., Raro P., Wernert R., Lorimier G. Evaluation of Mastectomy with Immediate Autologous Latissimus Dorsi Breast Reconstruction Following Neoadjuvant Chemotherapy and Radiation Therapy: A Single Institution Study of 111 Cases of Invasive Breast Carcinoma. Eur. J. Surg. Oncol. 2016;42:949–955. doi: 10.1016/j.ejso.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Kung T.A., Kidwell K.M., Speth K.A., Pang J.C., Jagsi R., Newman L.A., Wilkins E.G., Momoh A.O. Radiation-Induced Skin Changes after Postmastectomy Radiation Therapy: A Pilot Study on Indicators for Timing of Delayed Breast Reconstruction. J. Reconstr. Microsurg. 2019;35:209–215. doi: 10.1055/s-0038-1670650. [DOI] [PubMed] [Google Scholar]
- 91.Deng Y., Li H., Zheng Y., Zhai Z., Wang M., Lin S., Li Y., Wei B., Xu P., Wu Y., et al. Impact of Preoperative vs Postoperative Radiotherapy on Overall Survival of Locally Advanced Breast Cancer Patients. Front. Oncol. 2021;11:779185. doi: 10.3389/fonc.2021.779185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehta S., Cro S.C., Coomber B., Rolph R., Cornelius V., Farhadi J. A Randomised Controlled Feasibility Trial to Evaluate Local Heat Preconditioning on Wound Healing after Reconstructive Breast Surgery: The PreHEAT Trial. Pilot Feasibility Stud. 2018;4:34. doi: 10.1186/s40814-019-0392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mehta S., Rolph R., Cornelius V., Harder Y., Farhadi J. Local Heat Preconditioning in Skin Sparing Mastectomy: A Pilot Study. J. Plast. Reconstr. Aesthetic Surg. 2013;66:1676–1682. doi: 10.1016/j.bjps.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 94.Fredman R., Wiser I., Friedman T., Heller L., Karni T. Skin-Sparing Mastectomy Flap Ischemia Salvage Using Urgent Hyperbaric Chamber Oxygen Therapy: A Case Report. Undersea Hyperb. Med. 2014;41:145–147. [PubMed] [Google Scholar]
- 95.Lauritzen E., Damsgaard T.E. Use of Indocyanine Green Angiography Decreases the Risk of Complications in Autologous- and Implant-Based Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthetic Surg. 2021;74:1703–1717. doi: 10.1016/j.bjps.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 96.Pruimboom T., Schols R.M., van Kuijk S.M.J., van der Hulst R.R.W.J., Qiu S.S. Indocyanine Green Angiography for Preventing Postoperative Mastectomy Skin Flap Necrosis in Immediate Breast Reconstruction. Cochrane Database Syst. Rev. 2020;4:CD013280. doi: 10.1002/14651858.CD013280.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson A.C., Colakoglu S., Chong T.W., Mathes D.W. Indocyanine Green Angiography in Breast Reconstruction: Utility, Limitations, and Search for Standardization. Plast. Reconstr. Surg.-Glob. Open. 2020;8:e2694. doi: 10.1097/GOX.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groth A.K., Graf R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthetic Plast. Surg. 2020;44:1–12. doi: 10.1007/s00266-019-01521-3. [DOI] [PubMed] [Google Scholar]
- 99.Govrin-Yehudain J., Dvir H., Preise D., Govrin-Yehudain O., Govreen-Segal D. Lightweight Breast Implants: A Novel Solution for Breast Augmentation and Reconstruction Mammaplasty. Aesthetic Surg. J. 2015;35:965–971. doi: 10.1093/asj/sjv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.