Abstract
Crowd gatherings are an important cause of COVID-19 outbreaks. However, how the scale, scene and other factors of gatherings affect the spread of the epidemic remains unclear. A total of 184 gathering events worldwide were collected to construct a database, and 99 of them with a clear gathering scale were used for statistical analysis of the impact of these factors on the disease incidence among the crowd in the study. The results showed that the impact of small-scale (less than 100 people) gathering events on the spread of COVID-19 in the city is also not to be underestimated due to their characteristics of more frequent occurrence and less detection and control. In our dataset, 22.22% of small-scale events have an incidence of more than 0.8. In contrast, the incidence of most large-scale events is less than 0.4. Gathering scenes such as “Meal” and “Family” occur in densely populated private or small public places have the highest incidence. We further designed a model of epidemic transmission triggered by crowd gathering events and simulated the impact of crowd gathering events on the overall epidemic situation in the city. The simulation results showed that the number of patients will be drastically reduced if the scale and the density of crowds gathering are halved. It indicated that crowd gatherings should be strictly controlled on a small scale. In addition, it showed that the model well reproduce the epidemic spread after crowd gathering events better than does the original SIER model and could be applied to epidemic prediction after sudden gathering events.
Keywords: Gathering events, Simulation, Epidemic model, COVID-19 pandemic, Epidemic prediction
1. Introduction
Since 2019, a novel coronavirus (SARS-CoV-2) has resulted in a worldwide pandemic and developed into coronavirus disease 2019 (COVID-19). In addition to the strong transmission, COVID-19 also has a certain mortality rate (Yuan et al., 2020). As variants of SARS-CoV-2 are found in an increasing number of countries (Wise, 2020; Islam et al., 2021), the prevention and control of the COVID-19 epidemic have faced more increasing challenges. The factors that influence the spread of COVID-19 are complex. Studies have shown that human-to-human interactions are an important cause of the spread of COVID-19. For example, studies on European countries have shown that business activities are closely related to the incidence of COVID-19 (Bontempi and Coccia, 2021a) and that highly international trade activities are likely to increase the risk of virus importation (Bontempi et al., 2021). Moreover, city lockdown policies affect morbidity (Askitas et al., 2021). Countries with longer lockdown periods have a higher proportion of patients than countries with short lockdowns (Coccia, 2021a). In addition, meteorological and environmental factors also affect the outbreak and spread of COVID-19 (Diao et al., 2021; Coccia, 2021b; Coccia, 2021c; Domingo et al., 2020; Coccai, 2020). A study in China showed a positive linear relationship between average temperature and the number of COVID-19 cases with a threshold of 3 °C (Xie and Zhu, 2020). A study in California showed that air pollutant concentrations were significantly associated with the COVID-19 epidemic (Bashir et al., 2020). Air pollutants can act as carriers of SARS-CoV-2 in the air, maintaining viral concentrations (Coccia, 2021d). The incidence of COVID-19 may also vary across income groups (Chang et al., 2021) and ethnic groups (Pan et al., 2020). Social distancing and self-quarantine are still effective means of epidemic prevention until the epidemic is fully controlled. Therefore, understanding the requirements of social distancing and self-quarantining in different scenarios and predicting epidemic development after gathering events are particularly crucial tasks for epidemic prevention.
Substantial evidence indicates that crowd gatherings may be an important cause of the rapid increase in the number of infections. Historically, large-scale crowd gatherings for religion, sports, music and other purposes are substantial causes of widespread epidemics (Memish et al., 2019). For example, studies have found that the 1817–24 Asia cholera pandemic was associated with the Hindu religious pilgrimage festival, the Kumbh Mela (Hays, 2005), which is one of the largest mass gathering events in the world. Large-scale crowd gatherings create hotbeds for the spread of diseases (Memish et al., 2019). The simulation results also show that during the H1N1 influenza epidemic, the crowd gatherings and people travelling increased the number of cases and even lead to a second outbreak (Shi et al., 2010). The Islamic pilgrimage (Rashid et al., 2008) and the Winter Olympics (Gundlapalli et al., 2007) also significantly exacerbate influenza. The COVID-19 pandemic is no exception. For example, one study shows that more than 35% of COVID-19 cases in Malaysia are related to the Sri Petaling mass gathering that occurred between February 27, 2020 and March 1, 2020. More than 19,000 people from different countries participated in this event (Che et al., 2020).
Many studies have conducted quantitative research on the impact of crowds gathering events on disease transmission. Rainey et al. (2014) used video to track, analyze and estimate the number of contacts and contact time between participants in mass gathering activities. Hu et al. (2013) established a spatial contact model based on population thresholds and population mobility models to describe the relationship between contact rate and population density and explore the contact rate in different locations. Devices such as the internet and infinite sensors are also used to monitor mass gathering events to assess and control the risk of disease transmission during gatherings (Nsoesie et al., 2015). Overall, the previous research on the involvement of crowd gathering events in the spread of infectious diseases is gradually being refined and quantified. However, most of the existing studys only focus on typical events and lack the collection and sorting of relevant data, as well as a summary of such events and an exploration of the overall rules. Additionally, a concise model that can be used to study and predict the epidemic spread among crowds in gathering events is also needed.
Therefore, we collected data during the COVID-19 pandemic to establish a crowd gathering event dataset, and designed a model of epidemic transmission caused by crowd gathering events to quantitatively simulate how crowd gathering events affect disease transmission in the overall population. We collected 184 crowd gathering events of different scales that occurred worldwide from January 2020 to February 2021. Several outbreaks were triggered by these events, 99 of which had clear gathering scale and specific numbers of patients. We categorized these cases and explored the relationship between the incidence and the scale of crowd gathering events. Then, we use the model to simulate typical crowds gathering events of different scales and to simulate the changes in the number of patients after their scale is reduced proportionally to explore the hazards and influencing factors of crowd gathering events on the spread of the epidemic.
2. Materials and methods
2.1. Sample and data
We collected 184 gathering events in more than 23 countries from articles and news. The information collected includes the scene, the number and the time of gathering events. We define the scale of a gathering event with fewer than 100 persons as “small”, an event with between 100 persons (including 100 persons) and 500 persons (not including 500 persons) as “middle”, and an event with more than 500 persons as “large”. In addition, we classify events into 25 categories such as “Party” and “Meeting”. Among them, 99 events had a clear gathering scale, and we used these events to explore the impact of the gathering scale and aggregation scene on the incidence.
The true case data of the two events we used for the simulation came from the Health Commission of Liaoning Province (http://wsjk.ln.gov.cn/wst_zdzt/xxgzbd/yqtb/index_6.html) and the Changsha Municipal Health Commission of (http://wsjkw.changsha.gov.cn/ztzl_1/fkxxgzbd/fkdt/index_26.html).
2.2. Measures of variables
2.2.1. The gathering model
We use the extended SEIR model (Godio et al., 2020; Peng et al., 2020; Cheynet, 2020) to simulate the change in the number of cases after the gathering size changes. In this model, the total population is divided into seven groups: the susceptible (S), the protected (P), the exposed (E, infected cases in a latent period), the infective (I, infected cases that have not been quarantined), the quarantined (Q, confirmed and quarantined cases), the recovered (R), and dead (D). The sum of these seven populations is always equal to the total population (N).
| (1) |
The model consists of the following formulas:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
is the rate at which the susceptible (S) individuals become protected (P). Moreover, the susceptible may also become exposed (E) at the rate of (infection rate). When a person is infected, he or she will first become exposed, and after a certain period of time (incubation period, ), the person will become infected (I). is the rate at which people enter in quarantine. Quarantined (Q) individuals will eventually become recovered (R) or dead (D). and are the cure rate and death rate respectively (Cheynet, 2020; Liu et al., 2021). These parameters () are fitted from actual cases. Where and are determined by functions and their values vary over time (Cheynet, 2020). See Text S1 for more details. This study considers the impact of crowd gatherings on the epidemic spread and improves upon the model. During a gathering, potentially infected persons are present in the crowd. Infection also occurs inside the gathering, and the process also follows the abovementioned rules of transmission of infectious diseases. In the gathering, infectious individuals have more opportunities to come in connect more people, so the infection rate at gatherings is higher than the overall infection rate in society. After a gathering event, the event participants return to society and continue to participate in the spread of the epidemic throughout society. The overall process is shown in Fig. 1 .
Fig. 1.
The effect of crowd gatherings on the development of the epidemic. a, propagation process. Blue indicates uninfected, and red indicates infected and potentially infected. The circle represents the area in which the infection occurs (the large circle represents the entire city, regardless of external transportation; the small circle on the left represents gatherings that occur at the same time, and the infection occurs inside). The large circle on the left is the situation at the time of the gatherings, and the large circle on the right is the situation after the mass gatherings. b, model setting. When the gathering event has not occurred or has ended, the model is shown in the block diagram above. During a gathering event, the inside of the gathering place shows the same propagation pattern as the outside world (shown in the box below), and there are susceptible cases (SG), protected cases (PG), exposed cases (EG), infective cases (IG), quarantined cases (QG), recovered cases (RG) and dead cases (DG) in the gathering events. The infection rate is . When the gathering activity starts, the parameters (α, γ, δ, λ, κ) describing the spread of the epidemic in the city are used in the prediction process of the gathering event, but during the gathering process, there is another infection rate (βG). After the gathering, the variation of people in various categories generated by the gathering process are added to the population of the city, and changed the number of various groups of people in the city, and the spread of the epidemic in the overall population of the city continues. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We use the data of real cases from the first week or so days (depending on the quality of the data) of an outbreak in a city to fit the parameters. In this study, the model is also used to simulate the spread of the epidemic within the gathering event. There are individuals who are exposed before the gathering event (EG). These people carry the virus before participating in the gathering activity but do not know that they are sick. During the event, they show contagiousness. The results of the gathering event simulation are returned to the model for the overall crowd simulation, the numbers of various groups of people in the crowd are adjusted, and the next step of the simulation is performed.
The infection rate () consists of two parts: virus transmission rate (, determined by the nature of the virus) and contact rate ():
| (9) |
Thus, the infection rate is related to the contagiousness of the virus itself and human-to-human contact. In our model, only the virus transmission process from person to person during the crowd gatherings is considered. So we only change during the crowd gathering process.
Based on the contact model, Rhodes and Anderson (2008) deduced the expression of the infection rate in the gathering population. When the activity capacity and body resistance of human are constant, the infection rate of crowd gathering is proportional to the population density of the gathering place (Rhodes and Anderson, 2008). Therefore, for the gathering events in cities, based on the change in the population density of the gathering place compared with the overall population density, the infection rate of crowd gathering () is expressed as follows:
| (10) |
where is the contact rate within the gathering place. and are the population density of the gathering and urban infection density, respectively. and are the contact rate and prevalent infection rate in the whole city, respectively.
is expressed as follows:
| (11) |
where and are the number of people in the gathering and the area of gathering place, respectively.
is expressed as follows:
| (12) |
where is the resident population of the city, is the area of administration and public services of the city, is the area of commercial and business facilities of the city, is the area of municipal utilities of the city, is the area of green space and squares of the city. Few people live in some areas in the city, such as industrial areas, farmland, wetlands, lakes, and swamps; there is almost no human-to-human transmission of diseases in these areas. Therefore, only the areas where citizens are concentrated in the city are counted, that is, the above four parts.
2.2.2. Calculation of relative error
The relative error is calculated as:
| (13) |
where is the number of cases simulated by the model, and is the number of true cases generated by the gathering event.
2.3. Data analysis and case simulation
We classified and counted 99 events with detailed information on gathering scale and scenarios to explore the impact of different factors on the incidence of gathering activities. In addition, we use the gathering model in Section 2.2.1 to simulate two gathering events of different scales (gathering events in a canteen in Zhuanghe University town in Dalian, China and in two buses in Changsha, China) to further explore the impact of gathering events on the spread of COVID-19 in the overall population. All calculating and charting work were performed using MATLAB R2020a, OriginPro 2018C and Edraw Mind Map 7.9.
3. Results and discussion
3.1. The impact of the scale and scene of gathering events on the incidence
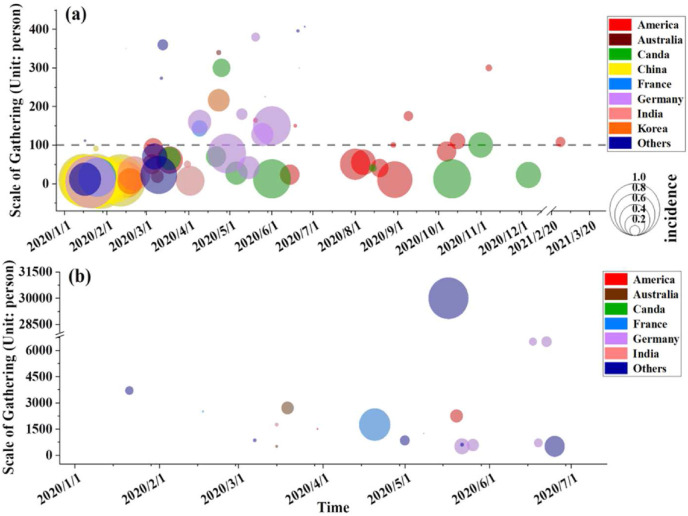
We collected 99 gathering events at a specific scale (Fig. 2 ). These events involved 20 countries and 31,874 cases (only direct transmission in a gathering was considered). Among these incidents, small-scale gathering events accounted for the majority. A total of 79.80% of the gathering events had fewer than 500 persons, and events with fewer than 100 people accounted for 54.55%. In the 20 countries involved in the case, gatherings of less than 500 people occurred. It shows that small-scale incidents are more frequent. The overall incidence of gathering events with fewer than 500 people was 23.35%, among which the incidence of gathering events with fewer than 100 people was 45.16%. The incidence of events between 100 persons (including 100 persons) and 500 persons (not including 500 persons) was 17.78%. The overall incidence of gatherings of more than 500 people was only 8.77%, with the exception of the two gatherings on the French ship Charles de Gaulle and the Mexican market. The time distribution of small-scale gatherings is more even. Except for January 2020, which was affected by the Chinese New Year, when there are more gathering events, in other months, gatherings of less than 500 people have almost the same frequency.
Fig. 2.
Bubble chart of gathering scale, time of occurrence and incidence (different colors of bubbles indicate different countries, the size of the bubble indicates the incidence, and the horizontal and vertical coordinates are the gathering time and scale, respectively) a, The scale less than 500 people; b, The scale more than 500 people. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In these cases, 22.22% of events with a scale of less than 100 people have an incidence greater than 0.8 (Fig. 3 ). Thus, in small-scale gathering events, the extreme phenomenon of all (or the vast majority) of the attendees in the gathering being infected is likely. In contrast, in events with a scale of more than 500 people, the proportion of events with an incidence rate greater than 0.4 is only about 10 percent. This phenomenon is closely related to the scene. We divided the gathering events into more than 15 categories such as “Work” and “Wedding” according to the scene. The most common scenes are “Meal” and “Family”, both accounting for 14.14% of all events. In these scenarios, the highest incidence rate is “Family”, where 64.29% of events have an incidence rate of more than 0.8. Small-scale incidents occur mostly in private or small public places (bistros, small restaurants, etc.). These locations tend to be indoors, and these small and densely populated places are more likely to lead to infection.
Fig. 3.
The impact of scale and scenario on incidence. a, The proportion of the incidence of gathering events with different scales; b, Incidence and number for each scene of gathering event. (Yellow, pink, red, brown, and brown-black represent incidence of 0–0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8 and 0.8–1.0, respectively). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Using the dataset for statistical analysis, it can be clearly found that gathering events that occur in confined, more closed indoor spaces are generally smaller in scale and tend to have higher incidence. The statistics of the gathering events that occurred in China at the beginning of the COVID-19 outbreak (Qian et al., 2021), as well as the model simulation of the gathering events (Saidan et al., 2020), also illustrate this point. Poor ventilation in small spaces is an important reason for the high incidence. In outdoor environments, supplemental fresh air can dilute the airborne droplet concentration and reduce the risk of virus transmission. Therefore, in a gathering place with a small indoor space, in addition to opening windows for ventilation, it is also necessary to have a gathering density as small as possible to ensure that everyone has sufficient fresh air supply (Guo et al., 2021). Temperature and humidity also affect the spread of COVID-19: studies have shown that the spread is significantly reduced in high-temperature environments (Haque and Rahman, 2020; Notari, 2021; Sarkodie and Owusu, 2020; Rosario et al., 2020). Moreover, in low-temperature environments, the defense function of the human oral mucosa decreases (Guo et al., 2021). The trajectory of droplets is also affected by temperature. In terms of humidity, a dry indoor environment (RH<40%) prolongs the suspension time of droplets in the air, which greatly increases the chance of virus transmission through the air (Ahlawat et al., 2020). Therefore, suitable temperature and humidity in gathering places are important to control the spread of COVID-19 in gathering events. In addition, small-scale gatherings are more frequent, and their occurrence is more hidden and random, and it is difficult to detect and monitor. Therefore, on the whole, the harm of such events to the prevention and control of the spread of COVID-19 in cities cannot be ignored. There are still some limitations in the dataset. The data come from articles and media reports, and there is no guarantee that media reports have no preference for different types of gatherings. Moreover, there is no information about the self-protection steps taken by event participants (such as wearing a mask and consciously reducing physical contact and conversation) when the gathering event occurs. Studies have shown that wearing masks and other personal protective measures impact the spread of the epidemic (Zhai, 2020; Wang et al., 2020). In addition, the events in the dataset were concentrated before virus mutations were discovered. Gathering events after the COVID-19 vaccine was widely available and after the emergence of the variants of SARS-CoV-2 still need to be added to the dataset.
3.2. Simulation of gathering events
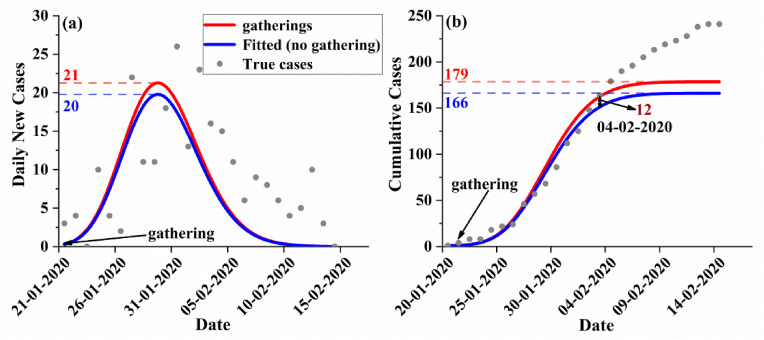
Furthermore, we consider the situation in which individuals in a gathering event continue to infect the entire population and simulate the incidence in the overall population after the event. We select a large-scale event and a small-scale event to simulate separately. For the large-scale event, we chose the event that occurred in the canteen of university town in Dalian, China (Table S1, S2). A COVID-19 outbreak occurred in Dalian in November 2021. Since the first case appeared on November 4 and until the new cases were cleared on the 28th, there were 308 cases in Dalian. Contaminated cold chain food was the source of this epidemic. The rapid spread of the epidemic in universities is an important transmission chain of this event. The university town located in northeast Dalian has two universities. The infected employees of the canteen in the university were the source of the transmission chain of the epidemic in the university. On November 4, teachers and students ate in the canteen. On November 5, dining in the canteen was banned. As of November 27, including canteen staff and students, a total of 81 people in the universities were infected (Table S3). According to our simulation results, compared with what would have occurred with no gathering events occurred in the canteen, the largest daily new cases in Dalian increased by 9 (Fig. 4 a), and as of November 27, the cumulative number of cases in Dalian increased by 62 (Fig. 4 b). The relative error of the simulation is 23.46%.
Fig. 4.
The simulation of COVID-19 transmission in Dalian with gathering events in the canteen in Zhuanghe University town. (The red line and blue line represent the number of cases with and without the gathering event, respectively. Grey dots indicate true cases); a, daily new cases; b, cumulative cases. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
For small gathering events, we selected events that occurred in two buses in Changsha, China (Tables S4, S5). On January 22, 2020, a passenger in Changsha took two buses, bus A with 47 seats and bus B with 17 seats, and the travel times of the two buses were approximately 3 h and 1 h, respectively (Ou et al., 2022). The passenger developed symptoms that day and was later diagnosed (Luo et al., 2020). The passenger was later identified as the initial spreader on the two buses. As of February 24, 13 cases in Changsha were related to the two buses (Table S6). According to the simulation results, compared with no gathering event, the largest daily new cases in Changsha increased by 1 people (Fig. 5 a). As of February 4, the cumulative number of cases in Changsha increased by 12 (Fig. 5 b), with a relative error of 7.69%. In fact, in late January 2020, the first COVID-19 cases appeared in Changsha. In the days before lockdown measures were taken, there were many similar small gatherings, resulting in a relatively rapid increase in cases. Here we have only simulated gathering events on two buses, so the simulated cumulative number of cases is lower than the actual cumulative number of cases.
Fig. 5.
Same as Fig. 4, but for Changsha with gathering events in two buses.
The common feature of the above two cases is that the gathering place is relatively closed and the gathering density is high. For the gathering event of Changsha buses, bus A was completely closed, and bus B only had individual windows opened occasionally. Moreover, the air-conditioning systems of both buses were not turned on (Ou et al., 2022). Furthermore, the concentration of gathering on the bus was very high, so the ventilation conditions were poor, and the concentration of droplets exhaled by individuals could not be quickly diluted. In fact, air circulation alone within a closed environment is not sufficient to prevent the spread of COVID-19; it can even facilitate the spread. Opening doors and windows, as well as adding extra air, can reduce the risk of disease transmission by accelerating the removal of aerosolized virus particles and changing particle trajectories in the room (Farthing and Lanzas, 2021; Ahmadzadeh et al., 2021). For the COVID-19 transmission event in Zhuanghe University town in Dalian, since the gathering occurred during meal time, there is no option protect oneself by wearing a mask. For the gathering event in Changsha, only some passengers wore masks since the COVID-19 pandemic was not declared nationwide at the time (Ou et al., 2022). According to the results, none of these passengers wearing masks were infected. In fact, many experiments and facts have proven that for confined spaces with high population density and less ventilation, masks are a lifesavers (Zhai, 2020; Esposito and Principi, 2020; Asadi et al., 2020).
In the model, only direct contact and transmission between people are considered. Therefore, the relationship between the infection rate in the gathering place () and the infection rate in the overall population () is only determined by the relationship between the population density of the gathering place and the national population density. That is, is related only to density. Secondary surface transmission of the virus (such as via tables and chairs) and the impact of environmental factors, such as the ventilation of the gathering place, on are not considered here. In addition, the type of gathering activity also impacts on . For example, at funerals in South Africa, guests singing and washing their hands in a basin increase the likelihood of infection (Jaja et al., 2020). Activities of different types and intensities have different effects on (), which still needs to be further refined in the model.
3.3. Simulation of the occurrence of multiple small-scale gathering events
To explore the impact of multiple small-scale gathering events on the spread of the epidemic in the city, we take the event occurring on bus A in Changsha as an example, assuming that there are 50 gathering events on the same day in Changsha, and simulate the number of patients. According to the simulation results, the maximum number of daily new cases will increase by a factor of 3.35 compared with that with no gathering event, with 50 such events occurring at the same time (Fig. 6 a). As of February 4, the confirmed number of cases will increase by a factor of 3.36 (Fig. 6 b). However, if the number of passengers in the car is reduced by half, the number of patients will drop sharply due to the reduction in the density of crowds. Compared with the situation where the number of passengers is not halved, the maximum number of daily new cases will drop by 36.78%, and the confirmed cases as of February 4 will drop by 36.17%.
Fig. 6.
Simulation of cases when multiple small-scale crowd gatherings (such as the COVID-19 transmission event on bus A in Changsha) occur at the same time. (The blue line, the red line, and the black line represent the number of cases without a gathering event, the number of patients after 50 gatherings of the same scale and the number of patients after the scale was halved, respectively.) (a, daily new cases; b, confirmed cases). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Therefore, the density of crowds in gathering places has a considerable impact on the development of the epidemic. When the scale of the gathering decreases, the density of the crowd in the venue also decreases, and the probability of people in the venue being infected decreases. For small-scale gathering events, because they are more frequent and difficult to detect and control, the harm is not less than that of large-scale gathering events. Thus, it is particularly important to strictly control the scale of such gathering activities.
4. Conclusion
In our study, we first constructed a gathering event dataset. The dataset contains 184 cases from January 2020 to February 2021. These cases involved more than 20 countries, including 99 cases with clear information such as the scale of the gathering, the scene, the number of patients, and the time of onset. Based on these cases, we studied the impact of factors such as the size of the gathering, the gathering scene, and the time of the gathering event on the incidence. The results show that small-scale gathering events are more frequent and their occurrence time is more evenly distributed. In these cases, the incidence of gathering events with fewer than 100 people was 45.16%, while the overall incidence of gathering events with more than 500 people, except for individual cases, was only 8.77%. The incidence rate is closely related to the scene, and the most common scenes are “Meal” and “Family”. Gathering events that occur in confined, closed indoor spaces are generally smaller in size and tend to have higher incidence.
Furthermore, we designed a gathering event outbreak-spreading model based on the improved SEIR model and used this model to simulate the impact of gathering events on the development of the epidemic. For large-scale gathering events, we selected the canteen gathering events in Zhuanghe University town in Dalian, China. The number of cases was simulated by considering gathering factors, and the simulation error was 23.46%. The small-scale gathering events selected for simulation were the bus gathering event in Changsha, China. The simulation error was 7.69%. This shows that the model can accurately reproduce the epidemic development following the events. Fig. S1 shows the correlation between the simulated and reported cases in the two cities with Pearson correlation coefficients at the upper-left corner of the sub-figures. There was a high correlation between the simulated cumulative and reported cases in both events (0.986 and 0.972). The model for the spread of infectious diseases can be applied to the prediction of epidemic situations after sudden gathering events. We increased the number of small gatherings and halved the scale for another simulation. After the scale is halved, the population density in the gathering place decreases, and the number of patients eventually decreases substantially. The results show that the population density in the gathering place is crucial to the spread of the epidemic. For small-scale gathering events, the number of gatherings should be strictly controlled due to the more enclosed space. The determination method of model parameters has been verified in the simulation of the case, but it still needs to be further verified and improved with more cases in the future.
According to our research results, small gatherings that are common and easily overlooked, such as dinner parties, should not be underestimated in terms of the risk of the spread of COVID-19. For such gathering events, the scale should be strictly controlled to reduce the density of gatherings, and attention should be given to the ventilation of gathering places and personal protection. Infected persons should be quarantined immediately to prevent further spread of COVID-19. For this study, more detailed cases with self-protection measures and vaccination data need to be added in the future. The indirect transmission process in the gathering events, the meteorological and environmental factors that affect the transmission, etc., still need to be further considered to improve the model.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded in part through the program National Natural Science Foundation of China 41521004. This work was also supported by the Gansu Provincial Special Fund Project for Guiding Scientific and Technological Innovation and Development (Grant No. 2019ZX-06) and the Fundamental Research Funds for the Central Universities (lzujbky-2021-kb12).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.113604.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ahlawat A., Wiedensohler A., Mishra S.K. An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol Air Qual. Res. 2020;20:1856–1861. [Google Scholar]
- Ahmadzadeh M., Farokhi E., Shams M. Investigating the effect of air conditioning on the distribution and transmission of COVID-19 virus particles. J. Clean. Prod. 2021;316 doi: 10.1016/j.jclepro.2021.128147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Cappa C.D., Barreda S., et al. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askitas N., Tatsiramos K., Verheyden B. Estimating worldwide effects of non-pharmaceutical interventions on COVID-19 incidence and population mobility patterns using a multiple-event study. Sci. Rep. 2021;11:1972. doi: 10.1038/s41598-021-81442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., Ma B.J., Bilal Correlation between environmental pollution indicators and COVID-19 pandemic: a brief study in Californian context. Environ. Res. 2020;187 doi: 10.1016/j.envres.2020.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M. International trade as critical parameter of COVID-19 spread that outclasses demographic, economic, environmental, and pollution factors. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M., Vergalli S., Zanoletti A. Can commercial trade represent the main indicator of the COVID-19 diffusion due to human-to-human interactions?. A comparative analysis between Italy, France, and Spain. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccai M. How (un) sustainable environments are related to the diffusion of COVID-19: the relation between coronavirus disease 2019, air pollution, wind resource and energy. Sustainability. 2020;12:9709. [Google Scholar]
- Coccia M. The relation between length of lockdown, numbers of infected people and deaths of Covid-19, and economic growth of countries: lessons learned to cope with future pandemics similar to Covid-19 and to constrain the deterioration of economic system. Sci. Total Environ. 2021;775 [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ. Sci. Pollut. Control Ser. 2021;28:19147–19154. doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Pandemic prevention: lessons from COVID-19. Encyclopedia. 2021;1:433–444. [Google Scholar]
- Coccia M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2021;78:1–27. [Google Scholar]
- Chang S., Pierson E., Koh P.W. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2021;589:82–87. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- Che N.F., Edinur H.A., Abdul M.K.A., Safuan S. A single mass gathering resulted in massive transmission of COVID-19 in\fections in Malaysia with further international spread. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa059. taaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheynet E. Github; 2020. Generalized SEIR Epidemic Model (Fitting and Computation)https://github.com/ECheynet/SEIR/releases/tag/v4.8.4 [WWW Document] [Google Scholar]
- Diao Y.L., Kodera S., Anzai D., et al. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID-19: a comparative study of scenarios in China, England, Germany, and Japan. One Health. 2021;12 doi: 10.1016/j.onehlt.2020.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Principi N. To mask or not to mask children to overcome COVID-19. Eur. J. Pediatr. 2020;179:1267–1270. doi: 10.1007/s00431-020-03674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing T.S., Lanzas C. Assessing the efficacy of interventions to control indoor SARS-Cov-2 transmission: an agent-based modeling approach. Epidemics. 2021;37 doi: 10.1016/j.epidem.2021.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godio A., Pace F., Vergnano A. SEIR modeling of the Italian epidemic of SARS-CoV-2 using computational swarm intelligence. Int. J. Environ. Res. Publ. Health. 2020;17:3535. doi: 10.3390/ijerph17103535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlapalli A.V., Olson J., Smith S.P., Baza M., Hausam R.R., Eutropius L.J., Pestotnik S.L., Duncan K., Staggers N., Pincetl P., Samore M.H. Hospital electronic medical record–based public health surveillance system deployed during the 2002 Winter Olympic Games. Am. J. Infect. Control. 2007;35:613. doi: 10.1016/j.ajic.2006.08.003. 171. [DOI] [PubMed] [Google Scholar]
- Guo M.Y., Xu P., Xiao T., et al. Review and comparison of HVAC operation guidelines in different countries during the COVID-19 pandemic. Build. Environ. 2021;187 doi: 10.1016/j.buildenv.2020.107368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.E., Rahman M. Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ. Sci. Pol. 2020;114:253–255. doi: 10.1016/j.envsci.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J.N. In: Epidemics and Pandemics: Their Impacts on Human History. Hays J.N., editor. ABC-CLIO; Santa Barbara, CA: 2005. First cholera pandemic 1817–1824; pp. 193–200. [Google Scholar]
- Hu H., Nigmatulina K., Eckhoff P. The scaling of contact rates with population density for the infectious disease models. Math. Biosci. 2013;244:125–134. doi: 10.1016/j.mbs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Islam O.K., Ai-Emran H.M., Hasan M., Anwar A., Jahid M.K., Hossain M. Emergence of European and north American mutant variants of SARS‐CoV‐2 in South‐east Asia. Transbound Emerg Dis. 2021:824–832. doi: 10.1111/tbed.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja I.F., Anyanwu M.U., Lwu Jaja C.-J. Social distancing: how religion, culture and burial ceremony undermine the effort to curb COVID-19 in South Africa. Emerg. Microb. Infect. 2020;9:1077–1079. doi: 10.1080/22221751.2020.1769501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.Y., Huang J.P., Li C.Y., et al. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K.W., Lei Z., Hai Z. Transmission of SARS-CoV-2 in public transportation vehicles: a case study in hunan Province, China. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa430. ofaa430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Steffen R., White P., Dar O., Azhar E.I., Sharma A., Zumla A. Mass gatherings medicine: public health issues arising from mass gathering religious and sporting events. Lance. 2019;393:2073–2084. doi: 10.1016/S0140-6736(19)30501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari A. Temperature dependence of COVID-19 transmission. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsoesie E.O., Kluberg S.A., Mekaru S.R., Khan K., Hay S.I., et al. New digital technologies for the surveillance of infectious diseases at mass gathering events. Clin. Microbiol. Infect. 2015;21:134–140. doi: 10.1016/j.cmi.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C.Y., Hu S.X., Luo K.W., et al. Insufficient ventilation led to a probable long-range airborne transmission of SARS-CoV-2 on two buses. Build. Environ. 2022;207 doi: 10.1016/j.buildenv.2021.108414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Sze S., Minhas J.S. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.R., Yang W.Y., Zhang D.Y., et al. Epidemic analysis of COVID-19 in China by dynamical modeling. medRxiv. 2020:1–18. [Google Scholar]
- Qian H., Miao T., Liu L., et al. Indoor transmission of SARS-CoV-2. Indoor Air. 2021;31:639–645. doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Rainey J.J., Cheriyadat A., Radke R.J., Crumly J.S., Koch D.B. Estimating contact rates at a mass gathering by using video analysis: a proof-of-concept project. BMC Publ. Health. 2014;14:1101. doi: 10.1186/1471-2458-14-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid H., Haworth E., Shafi S., Memish Z.A., Booy R. Pandemic influenza: mass gatherings and mass infection. Lancet Infect. Dis. 2008;8:526. doi: 10.1016/S1473-3099(08)70186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C.J., Anderson R.M. Contact rate calculation for a basic epidemic model. Math. Biosci. 2008;216:56–62. doi: 10.1016/j.mbs.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Rosario D.K.A., Mutz Y.S., Bernardes P.C., Conte-Junior C.A. Relationship between COVID-19 and weather: case study in a tropical country. Int. J. Hyg Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2020.113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidan M.N., Shbool M.A., Arabeyyat Q.S. Estimation of the probable outbreak size of novel coronavirus (COVID-19) in social gathering events and industrial activities. Int. J. Infect. Dis. 2020;98:321–327. doi: 10.1016/j.ijid.2020.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkodie S.A., Owusu P.A. Impact of meteorological factors on COVID-19 pandemic: evidence from top 20 countries with confirmed cases. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P.Y., Keskinocak P., Swann J.L., Lee B.Y. The impact of mass gatherings and holiday traveling on the course of an influenza pandemic: a computational model. BMC Publ. Health. 2010;10:778. doi: 10.1186/1471-2458-10-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Pan L.J., Tang S., J J.S., S X.M. Mask use during COVID-19: a risk adjusted strategy. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. Covid-19: new coronavirus variant is identified in UK. BMJ. 2020:m4857. doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- Xie J.G., Zhu Y.J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:2020. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Li M.H., Lv G., Lu Z.K. Monitoring transmissibility and mortality of COVID-19 in Europe. Int. J. Infect. Dis. 2020;95:311–315. doi: 10.1016/j.ijid.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z.Z. Facial mask: a necessity to beat COVID-19. Build. Environ. 2020;175 doi: 10.1016/j.buildenv.2020.106827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.