Graphical abstract
Keywords: Inhalation, Lung drug delivery, Pulmonary drug delivery, Aerosol(s), Antiinfective(s)
Abstract
Coronavirus Disease 2019 (COVID-19) pandemic has been on the agenda of humanity for more than 2 years. In the meantime, the pandemic has caused economic shutdowns, halt of daily lives and global mobility, overcrowding of the healthcare systems, panic, and worse, more than 6 million deaths. Today, there is still no specific therapy for COVID-19. Research focuses on repurposing of antiviral drugs that are licensed or currently in the research phase, with a known systemic safety profile. However, local safety profile should also be evaluated depending on the new indication, administration route and dosage form. Additionally, various vaccines have been developed. But the causative virus, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has undergone multiple variations, too. The premise that vaccines may suffice to eradicate new and all variants is unreliable, as they are based on earlier versions of the virus. Therefore, a specific medication therapy for COVID-19 is crucial and needed in order to prevent severe complications of the disease. Even though there is no specific drug that inhibits the replication of the disease-causing virus, among the current treatment options, systemic antivirals are the most medically appropriate. As SARS-CoV-2 directly targets the lungs and initiates lung damage, treating COVID-19 with inhalants can offer many advantages over the enteral/parenteral administration. Inhaled drug delivery provides higher drug concentration, specifically in the pulmonary system. This enables the reduction of systemic side effects and produces a rapid clinical response. In this article, the most frequently (systemically) used antiviral compounds are reviewed including Remdesivir, Favipiravir, Molnupiravir, Lopinavir-Ritonavir, Umifenovir, Chloroquine, Hydroxychloroquine and Heparin. A comprehensive literature search was conducted to provide insight into the potential inhaled use of these antiviral drugs and the current studies on inhalation therapy for COVID-19 was presented. A brief evaluation was also made on the use of inhaler devices in the treatment of COVID-19. Inhaled antivirals paired with suitable inhaler devices should be considered for COVID-19 treatment options.
Introduction
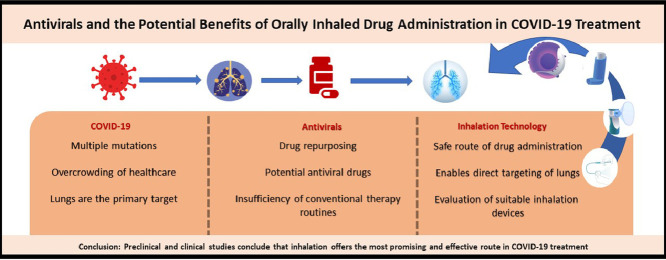
COVID-19 is the third severe respiratory outbreak caused by the coronaviruses in this century, after Severe Acute Respiratory Syndrome (SARS) in 2002 and Middle East Respiratory Syndrome (MERS) in 2006.1 Distinctly COVID-19 was declared as a pandemic.2 SARS-CoV-2, which causes COVID-19 targets lungs and initiates a lung damage leading to death due to respiratory failure. Today, more than 2 years after the beginning of the pandemic, approximately 529 million COVID-19 cases were confirmed, the death toll has exceeded 6 million globally.3 Disease spreading can be reduced using social distancing, face masks, adequate ventilation, general hygiene and travel restrictions but these measures are not generally popular and many countries have lifted them entirely or partly. Therefore, readily accessible self-administered products with good safety and efficacy profiles for early intervention and treatment of SARS-COV-2 infections would be desirable.
Various vaccines have been developed, but resource limitations and economic disparity hinder an even distribution across the world. Moreover, the effectiveness of the vaccines on forthcoming mutations remains unclear and booster doses are likely to be required frequently and their effectiveness and duration of action in the future is unknown. The elderly population, pregnant women, immunocompromised patients and those with chronic disease are especially at a higher risk for severe complications of COVID-19, despite being vaccinated. A medication therapy for COVID-19 is of great import to prevent complications of COVID-19 for those subgroups as well as those with no access to vaccines. Plus, there are still patient groups for whom vaccines are yet to be approved.
Although the lungs are the primary target of the virus, no inhaled drug has been approved. Principally, pulmonary administration are highly preferred in lung diseases such as asthma, chronic obstructive pulmonary disease and cystic fibrosis.4 , 5 Inhaled delivery of drugs provides two advantages: an effective treatment with lower doses and minimum side effects.6 As COVID-19 can cause acute respiratory distress syndrome (ARDS) and lung damage, targeting the lungs directly promises greater efficacy and various drugs are in clinical trials using inhalers.5 This review aims to summarize ongoing antiviral treatments in COVID-19, to discuss possible inhalation approaches, and briefly evaluate inhalation devices.
Antiviral Treatment Approaches in COVID-19
In terms of antiviral therapy, current research focuses on repurposing of antiviral drugs that are licensed or currently in the research phase, with a known systemic safety profile. Despite WHO's statement in an interim guidance7 that “Investigational anti-COVID-19 therapeutics should be used only in approved, randomized, controlled trials”, several compounds are being tried around the world based on animal models, cell lines or even virtual screening models. This article reviews the most frequently used antivirals, which include Remdesivir, Favipiravir, Molnupiravir, Lopinavir-Ritonavir, Umifenovir, Chloroquine, Hydroxychloroquine and Heparin. These were classified based on their antiviral groupings, along with mechanisms of action and dosage forms (Table 1 ).7, 8, 9
Table 1.
Some antiviral drugs for treatment of COVID-19
| Group | Drugs | Mechanism of Action | Clinical Dosing |
|---|---|---|---|
|
Inhibitors of viral RNA polymerase/RNA synthesis |
Remdesivir | Adenosine nucleotide analogue, prodrug, RdRpa inhibitor |
Day 1: 200 mg, IV Day 2–10: 100 mg/day, IV |
| Favipiravir | Guanosine nucleotide analogue, prodrug, RdRpa inhibitor |
Day 1: 2 × 1600 mg Day 2–7 (or 10): 2 × 600 mg/day, orally |
|
| Molnupiravir | N-hydroxycytidine ribonucleoside analogue, prodrug, RdRpa inhibitor | Day 1-5: 2 × 800 mg/day, orally | |
|
Inhibitors of viral protein Synthesis |
Lopinavir-Ritonavir | Protease inhibitor |
Day 1–10 (or 14): 2 × 400mg/100mg /day, orally |
| Viral entry inhibitors | Umifenovir | Fusion inhibitor | Day 1-7 (or 10): 3 × 200 mg, orally |
| Chloroquine | Increasing endosomal pH which disrupts virus-cell fusion, as well as interfering with the glycosylation of cellular receptors (ACE-2)b of SARS-CoV |
Day 1–5 (or 10): 2 × 500 mg/ day, orally |
|
| Hydroxychloroquine |
Day 1–5: 2 × 200 mg/day, orally |
||
| Heparin | Competition with host cell surface glycoproteins or proteoglycans | 5000 IU x 2 /day, subcutaneously |
RdRp: RNA-dependent RNA polymerase
ACE-2: Angiotensin Converting Enzyme-2
Remdesivir
Remdesivir, an antiviral drug that is an RNA polymerase inhibitor adenosine nucleotide analogue, was developed for treatment of Ebola virus, and was used intravenously to treat the first COVID-19 case in the USA. It is concluded randomized controlled studies are needed to evaluate effectiveness, confidence and robustness of collected data.10 In a study on compassionate-use of Remdesivir, 53 COVID-19 patients were treated with the drug for 10 days. Although data on viral load were not collected to confirm antiviral effects of Remdesivir, authors observed an improvement in the oxygen-support status of 68% of patients.11 A randomized, double-blind placebo-controlled, multicenter study observed that Remdesivir provide no significant clinical or antiviral effect in severe COVID-19 patients (158 to Remdesivir and 79 to placebo), meanwhile it provided an improvement in some clinical parameters.12 A study from 1062 patients (541 assigned to Remdesivir and 521 to placebo) indicated that those who received Remdesivir had a median recovery time of 10 days, compared with 15 days in the placebo group. All-cause mortality was 11.4% in the Remdesivir group and 15.2% in the placebo. The study supports use of Remdesivir for COVID-19 patients as it may have prevented the progression to more severe respiratory status, and provided a lower incidence of new oxygen use among patients. However, it was stated that treatment with an antiviral drug alone is not likely to suffice due to high likelihood of mortality despite the use of Remdesivir.13
The Japanese health authority approved Remdesivir for patients with severe COVID-19 on 7 May 2020 as the first officially authorized drug in the country.14 On 22 October 2020, FDA approved “New Drug Application (NDA)” for Remdesivir, for adults and pediatric patients (≥40 kg and 12 years), and issued an Emergency Use Authorization (EUA) to treat suspected or laboratory-confirmed COVID-19 hospitalized pediatric patients weighing between 3.5 kg and 40 kg, or hospitalized pediatric patients less than 12 years of age and ≥3.5 kg.15 , 16 On 19 November 2020, the FDA issued an EUA for the drug Baricitinib, in combination with Remdesivir, for the treatment of suspected or laboratory-confirmed COVID-19 in hospitalized adults and pediatric patients ≥2 years of age requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).17 In a double-blind, randomized, placebo-controlled trial based on this approval, the combination of Baricitinib plus Remdesivir was found to shorten the recovery time, and accelerate the improvement of the clinical situation, and decrease death rate and adverse reactions compared with the Remdesivir treatment only.18
Lopinavir–Ritonavir
Lopinavir–Ritonavir was proposed as a treatment option for COVID-19 on the basis of in vitro activity, preclinical studies and observational studies. Lopinavir is a HIV type 1 aspartate protease inhibitor. Ritonavir is combined with Lopinavir as it increases Lopinavir's plasma half-life via cytochrome P450 inhibition. The efficacy of the oral Lopinavir-Ritonavir combination in patients infected with SARS-CoV-2 was evaluated in a randomized, controlled study. No superiority was found for Lopinavir-Ritonavir combined therapy (99 patients) compared to standard therapy (100 patients).19 Similarly, no significant time difference in discharge from hospital was observed between both Lopinavir–Ritonavir (374 patients) and usual care (767 patients) groups. Lopinavir–Ritonavir was not associated with reduction in 28-day mortality, hospital stay, or risk of progression to invasive mechanical ventilation or death. The use of Lopinavir–Ritonavir in patients admitted to hospital with COVID-19 was not supported.20 Many other observational studies also show that use of Lopinovir-Ritonovir provide no significant improvement for COVID-19 patients.21, 22, 23
Umifenovir
Umifenovir (Arbidol) is a small indole derivative molecule licensed for the prophylaxis and treatment of influenza and other respiratory viral infections in Russia and China.24 , 25 It is a broad-spectrum antiviral agent against a number of enveloped and non-enveloped viruses, such as Ebola, Hepatitis B and C. For the influenza virus, Umifenovir was shown to increase the stability of the hemagglutinin glycoprotein (HA) and prevent HA from transitioning to a low pH-induced fusogenic state, which inhibits the virus from entering the cell.25
In a clinical pilot study of COVID-19 patients in Wuhan, Umifenovir treatment was shown to reduce viral load and mortality compared with the control group.26 The antiviral effects and safety of Umifenovir and Lopinavir–Ritonavir in COVID-19 patients were compared in a study, where 50 patients were divided into a Lopinavir–Ritonavir group (34 cases) and a Umifenovir group (16 cases). On the 14th day, no viral load was detected in the Umifenovir group, but the viral load was found in 15 patients (44.1%) treated with Lopinavir–Ritonavir. Patients in the Umifenovir group had a shorter duration of positive RNA test compared with those in the Lopinavir–Ritonavir group. No apparent side effects were found in both groups. The authors concluded that Umifenovir therapy may be superior to Lopinavir–Ritonavir in treating COVID-19.27 In a retrospective cohort study, some of the COVID-19 patients were treated with Lopinavir–Ritonavir combined with oral Umifenovir, while others were treated with oral Lopinavir–Ritonavir only. In the combination group, the rate of conversion of the coronavirus test from positive to negative was “significantly higher” compared to the Lopinavir–Ritonavir only group. A significant improvement in chest tomography has been associated with combined therapy.28 In contrast, a retrospective clinical research study conducted with 134 COVID-19 patients in Shanghai observed that Lopinavir–Ritonavir and Umifenovir did neither relieve symptoms nor accelerate virus clearance after treatment for 5 days.29 Similarly, Li et al.30 reported that Lopinavir–Ritonavir or Umifenovir monotherapy presents “little benefit” for improving the clinical outcome of the patients hospitalized with mild/moderate COVID-19 over supportive care. It is stated that a higher dose is needed to successfully suppress SARS-CoV-2 to achieve an effect comparable with the in vitro cytotoxicity tests. But this may not be possible in practice due to the side effects caused by both drugs. In a single-blind randomized controlled trial carried out on a total of 100 patients with COVID-19, patients were randomly assigned to Hydroxychloroquine alone and Hydroxychloroquine plus Umifenovir groups. The administration of both Umifenovir and Hydroxychloroquine has led to an improvement of the hematological parameters. The study introduced Umifenovir as an effective treatment for moderate to severe patients, showing not only a reduced time of CRP (C-reactive protein) normalization, but also a decreased hospitalization stay and mortality compared with those reported for Hydroxychloroquine.31 Conversely, another study has shown that additional Umifenovir was not effective in decreasing the duration of SARS-CoV-2 in severe patients and improving the prognosis in non-intensive care unit (non-ICU) patients and mortality.32 A recent retrospective study concluded that Umifenovir did not provide a significant improvement in the treatment of COVID-19 for non-ICU patients and that randomized clinical trials should be performed.33 In a single-center, randomized, open-label clinical study, the intervention group was given Lopinavir-Ritonavir + Hydroxychloroquine + Interferon-β1a + Umifenovir. The control group was given the same dose of Lopinavir-Ritonavir + Hydroxychloroquine + Interferon-β1a. The results revealed that the addition of Umifenovir did not improve prognosis and mortality in severe and non-ICU COVID-19 patients, and Umifenovir was once again found to be ineffective for COVID-19.32
Chloroquine and Hydroxychloroquine
Chloroquine (CQ), which belongs to the 4-aminoquinolines group of compounds, has long been used to treat malaria and amebiasis. However, Plasmodium falciparum developed widespread resistance to the drug, and with the development of new antimalarials, it has become a choice for the prophylaxis of malaria.34 , 35 Besides its antimalarial activity, by accumulating in the acidic organelles, CQ exerts both direct antiviral effects on enveloped viruses and decreases activation of several cell types involved in the immune response.36 The antiviral mechanism is achieved by increasing endosomal pH, which disrupts virus-cell fusion. The glycosylation of cellular receptors of SARS-CoV is also disturbed.37, 38, 39
A study demonstrated that CQ functioned at both entry, and at post entry stages of SARS-CoV-2 in Vero E6 cells.40 However, it was shown that engineered expression of TMPRSS2, a cellular protease that activates SARS-CoV-2 for entry into lung cells, renders SARS-CoV-2 infection of Vero cells insensitive to CQ. Moreover, CQ does not block infection with SARS-CoV-2 in the TMPRSS2-expressing human lung cell line Calu-3. It was concluded that CQ targets a pathway that is not active in lung cells, and thus is unlikely to protect against the spread of SARS-CoV-2 among patients.41
After a number of subsequent clinical trials in China, it was observed that CQ phosphate were superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, and promoting a virus negative conversion, hence shortening the disease course. An Expert Consensus in China recommended CQ phosphate tablets, 500 mg twice per day for 10 days for patients diagnosed with mild, moderate and severe cases of novel coronavirus pneumonia, if without contraindications to CQ.42 , 43 In a randomized clinical trial of high and low dose CQ in COVID-19 patients, the high-dosage group (i.e., 600 mg CQ twice daily for 10 days) presented greater QTc interval and higher mortality rates (39.0% versus 15.0%) than the low-dosage group (i.e., 450 mg twice daily on day 1 and once daily for 4 days). The higher CQ dosage is not recommended for critically ill patients with COVID-19, particularly when taken concurrently with Azithromycin or/and Oseltamivir due to potentially synergistic cardiac toxic effects.44
Hydroxychloroquine (HCQ), a derivative of CQ, was first synthesized in 1946 by introducing a hydroxyl group into CQ and was demonstrated to be 2 to 3 times less toxic than CQ in animals.45 HCQ is still widely available to treat autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis.34 Theoretically both agents are similar in their antiviral activity, but there is more clinical data available on the anti-coronaviral activity of CQ than that of HCQ. Of note, CQ is not as widely available as HCQ in some countries.46
HCQ (EC50=0.72 μM) was more potent than CQ (EC50=5.47 μM) in vitro and it was recommended orally in a loading dose of 400 mg twice daily of HCQ sulphate, followed by a maintenance dose of 200 mg given twice daily for 4 days as a recommendation for SARS-CoV-2 infection.47 In a randomized clinical trial, after 5 days of HCQ treatment, the symptoms of COVID-19 patients were significantly relieved, the recovery time from cough and fever have shortened.48On the contrary, there are other clinical studies that show HCQ did not improve the clinical status of COVID-19 patients compared with standard care.49, 50, 51, 52 There are retrospective and randomized clinical studies showing that HCQ was not effective in decreasing viral shedding in subjects with mild to moderate COVID-19 symptoms.53 Similarly, results of another study suggest no important antiviral effect of HCQ in humans infected with SARS-CoV-2. HCQ did not significantly decrease SARS-CoV-2 oropharyngeal viral load compared to standard care alone.54 In addition, it has been reported that the calculated free lung concentrations that would result from proposed dosing regimens are well below the in vitro EC50/EC90 values, making the antiviral effect against SARS-CoV-2 unlikely to be achieved with a safe oral dosing.55 Moreover, there are studies showing that CQ caused enhanced virus replication in various animal models56, 57, 58 concluding that, CQ/HCQ would be ineffective, even be harmful in patients.59
In an internationally collaborative meta-analysis of randomized trials, treatment with HCQ was associated with increased mortality in COVID-19 patients, and there was no benefit of CQ.60 The interim results published by the Solidarity Therapeutics Trial on 15 October 2020, coordinated by the WHO, indicate that Remdesivir, HCQ, Lopinavir-Ritonavir and Interferon regimens appeared to have little or no effect on 28-day mortality or on the hospital course of COVID-19 patients.61
Favipiravir
Favipiravir is a nucleoside analogue antiviral drug that targets RNA-dependent viral RNA polymerase. It is a selective and potent inhibitor of viral RNA polymerase of influenza, and is effective against all subtypes and strains of influenza viruses. Since it shows antiviral activity against many RNA viruses, Favipiravir is a promising drug for the treatment of many RNA-related infections, and is approved for influenza treatment in Japan.62 , 63
In a COVID-19 treatment study, Favipiravir was compared with the combination of Lopinavir-Ritonavir. The mean viral clearance time was 4 days in the Favipiravir group (35 patients) and 11 days in the Lopinavir-Ritonavir group (45 patients). Consequently, the recovery rate in chest tomography was reported to be higher in the Favipiravir group.64 In another study of 240 COVID-19 patients, either Favipiravir or Umifenovir was given and clinical recovery rates were compared over 7 days, but no significant difference was found between the two.65 A randomized controlled open-label trial stated that there was no difference in clinical outcomes between Favipiravir plus inhaled Interferon-β1b and standard HCQ treatment in adults hospitalized with moderate to severe COVID-19 pneumonia.66
A systematic review and meta‑analysis of clinical trials on the efficacy of Favipiravir in COVID-19 states that the mortality rate in the Favipiravir group is approximately 30% less than in the control group, but this difference is deemed to be not statistically significant, thus Favipiravir demonstrating no significant beneficial effect in terms of mortality. It is stated that use of antiviral after once the patient has developed symptoms may be too late to be effective, which explains its low efficacy in the clinic.67 According to another systematic review and meta-analysis, Favipiravir can promote viral clearance within 7 days and clinical improvement within 14 days, especially in patients with mild-to-moderate COVID-19. The early initiation of treatment with Favipiravir can contribute to positive outcomes for COVID-19.68 Another systematic review on the effectiveness of Favipiravir stated that there were no significant difference in fatality rate and the mechanical ventilation requirement between Favipiravir treatment and the standard care in moderate and severe COVID-19 patients.69 In a Phase 3 trial entitled as PRESECO (PREventing SEvere COVID-19) evaluating oral antiviral Favipiravir's at home use for mild-to-moderate COVID-19 patients, the preliminary results were reported as not to be yet statistically significant on the primary endpoint of time to sustained clinical recovery.70
Molnupiravir
Molnupiravir, is a prodrug that is metabolized into active antiviral ribonucleoside analogue β-D-N4-hydroxycytidine (NHC). It has activity against a number of RNA viruses, including SARS-CoV-2, SARS-CoV, MERS-CoV, and seasonal and pandemic influenza viruses. NHC shows its effect on RNA-dependent RNA-polymerase enzyme, which plays a role in viral genome transcription and replication.71
In a ferret model, Molnupiravir treatment completely blocked transmission to untreated animals. If ferret-based inhibition data of SARS-CoV-2 transmission are predictive for humans, patients with COVID-19 could become non-infectious within 24–36 h after the onset of an oral treatment.72 Molnupiravir's antiviral activity was also evaluated in a SARS-CoV-2 hamster infection model combined with Favipiravir. The results indicate that the combined treatment is effective on virus, and reduces the transmission to the uninfected contacts. These findings may form the basis for clinical trial design for combined therapies.73 In an analysis of the results from Phase 1, Phase 2 and Phase 3 clinical trials, Molnupiravir was safe, and caused no major adverse reactions.74 According to an interim analysis of a randomized, double-blind, placebo controlled Phase 2/3 study, 7.3% of patients, who received Molnupiravir were hospitalized through Day 29, compared with 14.1% of placebo-treated patients, who were hospitalized or died.75 , 76
Heparin
Heparin, a well-tolerated anticoagulant pharmaceutical, has been used safely for over 80 years. Alongside anticoagulant and anti-inflammatory properties, it has been known to prevent viral infection, such as SARS-CoV,77 , 78 Zika Virus,79 Herpes Simplex Virus,80 Influenza81 and HIV.82 , 83 Furthermore, its anticoagulant potential mainly with low molecular weight heparin (LMWH) is associated with better prognosis in severe COVID-19 patients with sepsis-induced coagulopathy score ≥ 4 or with D-dimer > 6-fold of upper limit of normal.84 The WHO interim guidance recommends LMWHs daily in prophylaxis or subcutaneous unfractionated Heparin twice daily.7 Mycroft-West et al. 85 showed that addition of Heparin (100 μg/ml) to Vero cells inhibits invasion by SARS-CoV-2 by 70%. Heparin binds to the Spike (S1) protein receptor-binding domain (RBD), inducing a conformational change. Mycroft-West et al. also reported that Enoxaparin, a low-molecular-weight clinical anticoagulant, binds to the S1 RBD protein inducing a conformational change. Heparin is 30-fold-more potent than Enoxaparin.85 These findings suggest Heparin as a potent antiviral against SARS-CoV-2.
Potential of Inhaled Antiviral Therapy in COVID-19
SARS-CoV-2 directly targets the lungs and initiates lung damage. Patients usually lose their lives due to respiratory failure.86 Although the virus is a respiratory one, the predominant focus has been on those dosage forms that provide systemic effects in the treatment. Unlike systemic dosage forms, inhaled ones can maximize direct delivery to the site of disease without first-pass metabolism, in order to boost the local antiviral activity in the lungs, while minimizing the side effects of systemic drug delivery.87 Just at this point, the inhalation route can be considered as the most promising route for the treatment of COVID-19. However, critical drug-attributes should be considered as they are different for inhaled and systemically administered formulations.88 There is currently no specific treatment for COVID-19 and some previously licensed antiviral drugs are intended to be repurposed for the treatment. The drug product of Remdesivir is administered by intravenous injection and Heparin is administered by subcutaneous or intravenous injection. They both are having a systemic effect and can only be administered in the hospital. But most patients are not hospitalized either due to pandemic conditions, or they have no access to an injectable administration of Remdesivir and Heparin. Sun89 hypothesized that for Remdesivir to be effective, the drug must be distributed to the lungs and reach sufficient concentration level. However, based on available data in humans and animals, it is unlikely that so called ‘sufficient concentrations’ to kill SARS-CoV-2 in the human lung can be achieved with an intravenous dose of Remdesivir (100–200 mg). The author stated that direct pulmonary administration of Remdesivir can provide a higher drug concentration in the lung, and also reduce systemic toxicity in patients. Since COVID-19 patients often have lymphopenia in addition to lung damage, inhibition of the virus in lymphocytes, lymph nodes, spleen, blood vessels, and other organs is indeed necessary, as well as inhibition of SARS-CoV-2 in the lung. Sun states that an intravenous Remdesivir is also necessary in combination with the pulmonary route with an adjustment of the intravenous dose.89 Favipiravir, Molnupiravir, Lopinavir-Ritonavir, Umifenovir, CQ and HCQ are administered in oral tablet or capsule forms. However, some patient subgroups (i.e. intubated or pediatric ones) are unable to take antivirals in oral forms. Therefore, Lopinavir-Ritonavir tablets are crushed in hospitals and given to those patients as “reformulated on-site” in liquid form, a common pandemic practice.90 , 91 But, the alteration of this formulation causes the formation of unstable crystal structure leading to approximately 50% loss in bioavailability.92 The alteration process of formulation puts an extra burden on healthcare professionals in the hospital. Wang and Chen93 reported that the reason why Lopinavir could not be beneficial in COVID-19 patients may be due to the insufficient concentration level of oral Lopinavir in the lungs to inhibit SARS-CoV-2 replication. They base their hypothesis on a tissue distribution study of orally administered Lopinavir-Ritonavir in rats by Kumar et al.94 This study revealed that the concentration of oral Lopinavir in lung tissue remains relatively low. In a biodistribution study of [18F] Favipiravir in mice, lungs were the ‘lowest accumulation’ site of the antiviral agent, whereas the highest accumulation was seen in kidney, liver and stomach.95 Theoretically however, an antiviral used in the treatment of COVID-19 should accumulate primarily in the lungs. Data on COVID-19 treatment so far suggest that antiviral agents may not provide an effective concentration in the lungs, demolishing the promise for a definitive antiviral accumulation in the lungs when administered systemically.
Due to these limitations of the conventional drug therapy, it can be considered the inhalation therapy may provide a superior alternative with greater benefits in COVID-19. In support of this hypothesis, various inhalation studies have already been carried out. Sahakijpijarn et al. produced high-potency Remdesivir dry powder formulations for inhalation, using the thin film freezing technique, to maximize delivery to the lungs. It has been stated that the formulations were suitable to treat patients with COVID-19 on an outpatient basis and earlier in the disease course.96 Vartak et al. developed a nebulized and scalable nanoliposomes of Remdesivir against COVID-19. The nanoliposomes have an optimal particle size, effective aerosol characteristic, high drug entrapment efficiency, minimal mammalian cell toxicities and prolonged drug release.97 Vermillion et al. investigated the pharmacokinetics and efficacy of Remdesivir administered by inhalation in African green monkeys to make Remdesivir more convenient for non-hospitalized patients. Relative to an intravenous administration at 10 mg/kg, an about 20-fold lower dose administered by inhalation produced comparable concentrations of the pharmacologically active form in lower respiratory tract tissues. Inhaled Remdesivir resulted in lower systemic exposures as compared with intravenous route. The efficacy study with repeated dosing of inhaled Remdesivir demonstrated reductions in viral replication in bronchoalveolar lavage fluid and respiratory tract tissues compared with placebo.98 Albariqi et al. successfully prepared an inhalable HCQ crystalline powder for potential treatment of COVID-19.99 Tai et al. showed that an inhalable form of liposomal HCQ had desirable pharmacokinetics in a rat model. It was shown that inhalable liposomal HCQ can be delivered directly to the lungs for COVID-19 pulmonary treatment with less frequent dosing and at a relatively lower dose. For delivery, a disposable closed-loop system, which minimizes the spreading of aerosols and reduces contamination risk, was developed and connected to a nebulizer.100
Currently, there are several clinical trials on the inhalation treatment of COVID-19. One of them is a Phase 1 study of inhaled aerosolized HCQ that has been administered via the Aerogen nebulizer. It has been reported that administering aerosolized HCQ at a lower dose than the oral dose may rapidly achieve a respiratory tract concentration that would suffice to inhibit SARS-CoV-2.101 , 102 Another Phase 1 study has been performed with inhaled dry powder HCQ that has been administrated via Cyclops dry powder inhaler. The rationale of the study was that HCQ administered as dry powder via inhalation in SARS-CoV-2 could be safer than oral HCQ, hence allowing for higher and more effective pulmonary concentrations without dose-induced toxic effects.103 , 104 Further phase studies are required for inhaled aerosolized or dry powder HCQ. An ongoing study aims at developing new "Ready-to-Use" inhalable forms of HCQ that can be used directly through nebulization or using dry powder inhalers for the COVID-19 viral infection.105 Another clinical trial characterized the impact of inhaled Remdesivir on viral load in participants with early stage COVID-19, but the study was halted by the company conducting it, due to “unsatisfactory lung deposition”.106 Another ongoing study weighs inhaled nanoparticle formulation of Remdesivir in terms of its safety, tolerability and pharmacokinetics on healthy subjects.107
Many clinical studies on the inhalation of Heparin and LMWH were conducted before the pandemic.108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120 According to van Haren et al., there is a strong scientific and biological basis to test the nebulized Heparin as a therapy for COVID-19 pneumonia and ARDS. Besides, delivering Heparin via inhalation to the upper airways, the main entry point of the virus, may provide a prophylaxis.121 And it should not be a surprise that there are some completed and ongoing clinical studies on the use of nebulized Heparin and LMWH in COVID-19,122, 123, 124, 125, 126, 127, 128, 129 which investigate therapeutic effects of Heparin and LMWH on symptom management of COVID-19-induced inflammation, and on vascular pulmonary thrombosis. In one of the ongoing meta-trial, it was aimed to investigate that whether the administration of nebulized Heparin to the hospitalized COVID-19 patients would reduce intubation rates and the risk of death.130 At the end of the study, it was concluded that inhaled nebulized Heparin was safe in hospitalized COVID-19 patients and urgent randomized studies are needed to evaluate the efficacy.131 Our study group has recently completed a clinical Phase 2b trial that proposes inhaled LMWH given via soft-mist inhaler significantly improves hypoxemia in COVID-19 patients. Soft-mist inhaled LMWH was well-tolerated and markedly decreased the need for the oxygen treatment (vs. reservoir masks, high-flow oxygen therapy) at the end of the 10-day treatment.132 However, randomized studies with larger patient groups are needed to support this study.
Evaluation of Inhaler Devices for Antiviral Therapy in COVID-19
Pressurized metered-dose inhalers (pMDIs) are the most popular delivery systems in the pulmonary field.133 The main action of pMDIs is the activation of the device and the synchronization of the patient's breathing, where aerosol formation occurs with the help of a propellant gas.134 The key components here include a container, propellants, actuator, formulation and metering valve. Initially developed using chlorofluorocarbon propellants, this system has been modified over the years due to environmental concerns regarding the irreversible damage to the atmospheric ozone layer. Current devices include hydrofluoroalkanes as an environmentally safe alternative.135 pMDIs are highly unlikely for a target group with a decreased breathing capacity, like COVID-19 patients, to perform adequately; an action even normal patients find challenging. Moreover pMDIs tend to leave a “cold feeling” during administration and induce bronchospasm in some patients.135 Although these disadvantages may leave pMDIs an undesirable option, especially for the severe COVID-19 patients, there are newly developed pMDIs which has spacers and valved holding chambers suitable especially for children.88 Under the right supervision in hospital settings, the pMDI plus spacer may be similarly effective as nebulizers.136 In addition, pMDIs reduces the risk of aerosol generating particles compared with nebulizers. But this advantage resulted in high demand for pMDIs and caused a shortage.137 For ambulatory patients with mild COVID-19 symptoms, pMDIs may be advantageous over nebulizers as they are portable and transmission risk is lower comparing to nebulizers due to the closed system in the pMDIs. However, it is not possible to use for mechanically ventilated patients. Modern dry powder inhalers (DPIs) were developed as an alternative to pMDIs.138 Regarding the mechanism of DPIs; the micronised drug is weakly bound to a carrier (e.g.,α-lactose monohydrate) which prevents aggregation and provides sufficient flowability in powder form.139 Patients are typically encouraged to breathe forcefully and deeply when using a DPI 140; but severe COVID-19 patients may find this action to be difficult to perform and inadequate dose uptake could be seen. DPIs can be suitable for mild-to-moderate COVID-19 patients who does not have breathing difficulties. Although DPIs are eco-friendly devices, one of the reasons pMDIs are still frequently prescribed is their low cost compared to DPIs.141 Albeit, they are easier to carry and thought to cause less risk of transmission compared to nebulizers, as with pMDIs, it is not possible to use DPIs in mechanically ventilated patients with severe symptoms. In addition, in the early days of the pandemic, developing dry powder formulations for COVID-19 could not be considered as an urgent therapy in hospitals as it would take more time than formulating nebules containing liquid dosage forms.
Nebulizers are one of the common devices for delivery of drug formulations to the lungs. They produce aerosol droplets from solutions or suspensions of liquid or solid particles. Although administration of higher doses is possible, longer administration times disfavors nebulizers in comparison to pMDIs and DPIs.142 Generally, nebulizers can be classified into three groups: jet nebulizers, ultrasonic nebulizers and vibrating mesh nebulizers.143
Jet nebulizers are the earliest nebulizer technology. The mechanism of jet nebulizers is the flowing of a gas stream from a compressor through a capillary tube with a high speed in which the fluid is drawn up to be atomized through the capillary tube. Contact of large aerosol droplets with the baffle inside the nebulizer prevents production of large droplets. Atomization force may cause unwanted changes in formulation. In the case of ultrasonic nebulizers, instead of compressed air gas, high frequency vibrating piezoelectric crystals produce sound waves which in return form liquid droplets. Ultrasonic nebulizers cause heat formation and result in degradation of heat sensitive substances. Also, ultrasonic nebulizers are less efficient in nebulization of suspension and viscous liquid due to the decreased aerosolization force and are far more expensive compared to jet nebulizers. Lastly, vibrating mesh is a relatively new nebulizer technology. It also uses piezoelectric crystals to force liquids extrusion through vibrating mesh. They can reach deep areas of the lung with shortened time compared to jet nebulizers.144 , 145
During nebulization, the leakage of formulation aerosols into the air by the patient's breathing pattern causes undesired drug loss. It is noteworthy to underline that in the case of COVID-19, the risk of contamination increases via nebulization.146, 147, 148, 149 Current guidelines warn against the use of nebulizers.150 As nebulized drug may induce cough in patient, the caregiver thus may face an increased risk of virus contamination.151 However, there is no conclusive evidence regarding transmission of SARS-CoV-2 via nebulizers and these statements remain as a debate. Despite the disadvantages, nebulization is the easiest and fastest way to administer inhaled treatment to unconscious patients with severe symptoms.
In soft-mist inhalers, the drug is dissolved in a solution, so it is relatively less affected by moisture than dry powders, hence more suitable for use in humid ambient conditions. Its relatively low speed and long spray time facilitates a reproducible inhalation of the aerosol.152, 153, 154 Most distinctively, soft-mist inhalers are active systems that do not require propellant gas. The energy required for aerosol generation comes from a mechanical force, and is independent of the patient's respiratory capacity. The versatile design of soft-mist inhalers may reduce airborne particulate contamination by allowing the patient to breathe through the mouth and exhale through the nose, which is a critical factor in COVID-19.
Soft mist inhalers are more optimal in terms of increased drug accumulation in the lungs. Clinical studies conducted in volunteers using radiolabeled drug particles and gamma scintigraphy, indicate that drug aerosols administered with Respimat®, a soft mist inhaler, had a higher accumulation in lung with a mean (range) of 51%, particularly in peripheral lung tissues.155 , 156 Also, a recent study by our research team has shown that fine particle fraction was 44.4% with Pulmospray®, which is a specially designed soft mist inhaler.132 A key advantage of soft-mist inhalers is its easy administration. The drug is administered in soluble form with a specific volume produced from a single-use dosage form or multidose, and provide the opportunity of repeated administration with various ranges of doses 157 , 158 . The single-use dosage form can be advantageous in COVID-19 patients in terms of reducing the risk of contamination. In addition, being liquid dosage forms that do not contain propellant and can be easily carried makes it possible to use them in all patients with mild or severe COVID-19.
Formulation and device suitability can be evaluated according to the characteristics of the devices and the above-mentioned antiviral agents. When evaluated in terms of solubility, all active substances except Molnupiravir and Heparin show low solubility in water. Molnupiravir and Heparin formulations can be prepared for use in nebulizers, pMDIs and soft mist inhalers as their solubility in water is 39.7 mg/mL and 50 mg/mL respectively. For other antiviral agents, various solubility enhancing formulation strategies can be developed and solution forms of these active substances can be prepared. Cyclodextrins can be used as solubilizing agent for the formulation of poorly soluble active ingredients. The formulation of Remdesivir, which is currently available in the market as intravenous, was prepared using cyclodextrins due to the solubility problem of the active substance.159 Although their use in the lungs has not been approved by the FDA yet, EMA's report states that cyclodextrins are safe for nasal and pulmonary use.160, 161 Therefore, as solubility enhancers, cyclodextrins can be used in the preparation of above-mentioned antivirals in solution formulation. In addition, the preparation of dry powder formulations and pMDI formulations with co-solvent is a suitable option for these agents that have solubility problems.
Several reports for inhaled HCQ stated that doses ranging from 2 to 4 mg twice daily to 20 mg once daily can be effective in treating COVID-19 symptoms.162, 163, 164 Oral dose of HCQ for COVID-19 ranging from 200 mg to 800 mg.165 Considering that the dose of the drug is so low when administered as pulmonary, treatment can be achieved at very low doses with similar calculations for other antiviral agents.
Conclusion
The primary target of SARS-CoV-2 is the lungs. However, the medical approaches form a contradiction in terms of systemic administration of drugs for the treatment of COVID-19. Here, inhalation is a superior administration route in terms of inhibition of airborne viruses in the lungs. Hence, it is able to achieve higher lung accumulation of the drug and elimination of the side effects. Preclinical and clinical studies conclude that inhalation technology offers a promising and effective route in COVID-19 treatment that should be taken into consideration. Development of inhaler forms of antiviral drugs, some of which are currently used for COVID-19 treatment, will be more effective in the lungs and safer than conventional dosage forms. As for inhaler devices, apart from their features the potential environmental effect should also be considered while prescribing. It should be kept in mind that the effects of such a widespread disease are bound to leave behind serious ecological waste. Although it is true that a disposable product would cause ecological concerns, there is a serious issue of sanitizing reusable devices such as nebulizers. Overall, for the treatment of COVID-19 the health professions should consider the suitability of the specific device for the patient and associated risks. Although each device has a separate practical advantage, soft mist inhalers might be a suitable choice for pandemic conditions. Further clinical studies are necessary to determine the future of soft mist inhaler technology in the treatment protocols.
Author Contributions
GS wrote the original draft and contributed to editing of the review and the literature research. NB, AE, MC contributed equally to the literature research and writing of the review. OAD and SS contributed to writing and editing of the review and the literature research. AYP conceptualized, designed and supervised the review. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this study.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Sally Louise McDonald for editing the manuscript and Cahit Erdem Pekoz for his valuable contributions. This publication is dedicated to Ayse Sahin, the mother of GS, who unfortunately passed away due to COVID-19.
References
- 1.De Wit E, Van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Timeline - COVID-19. January 4, 2022. Published 2020. Accessed https://www.who.int/news-room/detail/27-04-2020-who-timeline—covid-19
- 3.WHO . June 5, 2022. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Published 2020. Accessed. [Google Scholar]
- 4.Hickey AJ. Back to the future: inhaled drug products. J Pharm Sci. 2013;102(4):1165–1172. doi: 10.1002/jps.23465. [DOI] [PubMed] [Google Scholar]
- 5.Eedara BB, Alabsi W, Encinas-Basurto D, Polt R, Ledford JG, Mansour HM. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13(1077):1–24. doi: 10.3390/pharmaceutics13071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rau JL. The inhalation of drugs: Advantages and problems. Respir Care. 2005;50(3):367–382. [PubMed] [Google Scholar]
- 7.WHO . January 4, 2022. Clinical Management of Severe Acute Respiratory Infection When COVID-19 is Suspected.https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf?sequence=1&isAllowed=y Published 2020. Accessed. [Google Scholar]
- 8.Kamps BS, Hoffman C. COVID Reference. 3. 2020, 2020. https://covidreference.com/timeline_it
- 9.Şimşek Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turkish J Med Sci. 2020;50:611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. Published online. 2020:1–10. doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YY, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(0):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilead . Accessed January 4, 2022. Gilead Announces Approval of Veklury® (remdesivir) in Japan for Patients With Severe COVID-19.https://www.gilead.com/news-and-press/press-room/press-releases/2020/5/gilead-announces-approval-of-veklury-remdesivir-in-japan-for-patients-with-severe-covid19 [Google Scholar]
- 15.FDA . Accessed January 4, 2022. Veklury (remdesivir) EUA Letter of Approval.https://www.fda.gov/media/137564/download Published 2020. [Google Scholar]
- 16.FDA . Accessed January 4, 2022. Frequently Asked Questions for Veklury (remdesivir)https://www.fda.gov/media/137574/download Published 2020. [Google Scholar]
- 17.FDA . Accessed January 4, 2022. Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19 [Google Scholar]
- 18.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/nejmoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recovery Collaborative Group Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Castelnuovo A, Costanzo S, Antinori A, et al. Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: findings from the multicenter Italian CORIST study. Front Med. 2021;8(June) doi: 10.3389/fmed.2021.639970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph BA, Dibas M, Evanson KW, et al. Efficacy and safety of lopinavir/ritonavir in the treatment of COVID-19: a systematic review. Expert Rev Anti Infect Ther. 2021;19(6):679–687. doi: 10.1080/14787210.2021.1848545. [DOI] [PubMed] [Google Scholar]
- 23.Patel TK, Patel PB, Barvaliya M, Saurabh MK, Bhalla HL, Khosla PP. Efficacy and safety of lopinavir-ritonavir in COVID-19: a systematic review of randomized controlled trials. J Infect Public Health. 2021;14(6):740–748. doi: 10.1016/j.jiph.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaising J, Polyak SJ, Pécheur E-I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107(1):84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci U S A. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Ling Y, Xi X, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chinese J Infect Dis. 2020;12:1–10. doi: 10.3760/cma.j.cn311365-20200210-00050. [DOI] [Google Scholar]
- 30.Li Y, Xie Z, Lin W, et al. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv (Preprint) 2020:1–33. doi: 10.1101/2020.03.19.20038984. Published online. [DOI] [Google Scholar]
- 31.Khodashahi R, Naderi H, Bojdy A, et al. Comparison the effect of arbidol plus hydroxychloroquine vs hydroxychloroquine alone in treatment of COVID-19 disease: a randomized clinical trial. Curr Respir Med Rev. 2021;16(4):252–262. doi: 10.2174/1573398X17666210129125703. [DOI] [Google Scholar]
- 32.Alavi Darazam I, Shokouhi S, Mardani M, et al. Umifenovir in hospitalized moderate to severe COVID-19 patients: A randomized clinical trial. Int Immunopharmacol. 2021;99(July) doi: 10.1016/j.intimp.2021.107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):6–9. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weniger H. Review of side effects and toxicity of chloroqine. World Heal Organ. Published online. 1979:1–26. [Google Scholar]
- 36.Savarino A, Shytaj IL. Chloroquine and beyond: Exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12(1):1–10. doi: 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(69):1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M, Mösbauer K, Hofmann-winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585(September) doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 42.Multicenter Collaboration Group of Department Province of Science and Technology of Guangdong, Province and Health Commission of, Guangdong Novel for Chloroquine in the Treatment of Pneumonia Coronavirus. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- 44.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(1 PART 1):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 46.Sahraei Z, Shabani M, Shokouhi S, Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020;7 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 49.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/nejmoa2019014. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369(April):1–11. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.RECOVERY . Accessed January 4, 2022. No Clinical Benefit From Use of Hydroxychloroquine in Hospitalised Patients with COVID-19.https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-co Published 2020. [Google Scholar]
- 52.Ulrich RJ, Troxel AB, Carmody E, et al. Treating COVID-19 with hydroxychloroquine (TEACH): a multicenter, double-blind randomized controlled trial in hospitalized patients. Open Forum Infect Dis. 2020;7(10) doi: 10.1093/ofid/ofaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C-P, Lin Y-C, Chen T-C, et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19) Atkin SL, ed. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyngbakken MN, Berdal JE, Eskesen A, et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020;11(1):6–11. doi: 10.1038/s41467-020-19056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan J, Zhang X, Liu J, Yang Y, Zheng N, Liu Q. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect : a critical step in treating patients with coronavirus disease 2019. Clin Infect Dis. 2020;Xx Xxxx:1–5. doi: 10.1093/cid/ciaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maheshwari RK, Srikantan V, Bhartiya D. Chloroquine enhances replication of semliki forest virus and encephalomyocarditis virus in mice. J Virol. 1991;65(2):992–995. doi: 10.1128/jvi.65.2.992-995.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roques P, Thiberville SD, Dupuis-Maguiraga L, et al. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses. 2018;10(5):1–18. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seth P, Mani H, Singh AK, et al. Acceleration of viral replication and up-regulation of cytokine levels by antimalarials: implications in malaria-endemic areas. Am J Trop Med Hyg. 1999;61(2):180–186. doi: 10.4269/ajtmh.1999.61.180. [DOI] [PubMed] [Google Scholar]
- 59.Guastalegname M, Vallone A. Could chloroquine /hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12(1):2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO Solidarity trial consortium Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Japan Acad Ser B. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldhill DH, Te Velthuis AJW, Fletcher RA, et al. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci U S A. 2018;115(45):11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19 : an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19 : a randomized clinical trial. medRxiv preprint. 2022 [Google Scholar]
- 66.Khamis F, Al Naabi H, Al Lawati A, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. February 2020;102:538–543. doi: 10.1016/j.ijid.2020.11.008. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. doi: 10.1038/s41598-021-90551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):1–13. doi: 10.1186/s12879-021-06164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Özlüşen B, Kozan Ş, Akcan RE, et al. Effectiveness of favipiravir in COVID-19: a live systematic review. Eur J Clin Microbiol Infect Dis. 2021;40(12):2575–2583. doi: 10.1007/s10096-021-04307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Appili Therapeutics. Appili Therapeutics Provides Update on Phase 3 PRESECO Clinical Trial Evaluating Avigan®/ReeqonusTM. Accessed January 4, 2022. Published 2021; https://www.appilitherapeutics.com/newsfeed/Appili-Therapeutics-Provides-Update-on-Phase-3-PRESECO-Clinical-Trial-Evaluating-Avigan®%2FReeqonusTM
- 71.Painter WP, Holman W, Bush JA, et al. 2019. Molnupiravir, A Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6(1):11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdelnabi R, Foo CS, Kaptein SJF, et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr Clin Res Rev. 2021;15(6) doi: 10.1016/j.dsx.2021.102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ClinicalTrials.gov . Accessed January 4, 2022. Efficacy and Safety of Molnupiravir (MK-4482) in Non-Hospitalized Adult Participants With COVID-19 (MK-4482-002)https://clinicaltrials.gov/ct2/show/NCT04575597 Published 2021. [Google Scholar]
- 76.Merck . Accessed January 4, 2022. Merck and Ridgeback's Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study.https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ Published 2021. [Google Scholar]
- 77.Vicenzi E, Canducci F, Pinna D, et al. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg Infect Dis. 2004;10(3):413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lang J, Yang N, Deng J, et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6(8):e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghezzi S, Cooper L, Rubio A, et al. Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res. 2017;140(January):13–17. doi: 10.1016/j.antiviral.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skidmore MA, Kajaste-Rudnitski A, Wells NM, et al. Inhibition of influenza H5N1 invasion by modified heparin derivatives. Med Chem Commun. 2015;6(4):640–646. doi: 10.1039/c4md00516c. [DOI] [Google Scholar]
- 82.Harrop HA, Rider CC. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor. CD4. Glycobiology. 1998;8(2):131–137. doi: 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- 83.Rusnati M, Coltrini D, Oreste P, et al. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J Biol Chem. 1997;272(17):11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 84.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mycroft-West CJ, Su D, Pagani I, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv. 2020 doi: 10.1101/2020.04.28.066761. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Nat Sci Reports. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. J Clin Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson S, Atkins P, Bäckman P, et al. Inhaled medicines: past, present, and future. Pharmacol Rev. 2022;74:48–118. doi: 10.1124/pharmrev.120.000108. [DOI] [PubMed] [Google Scholar]
- 89.Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. AAPS J. 2020;22(4) doi: 10.1208/s12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Republic of Turkey Ministry of Health . 2022. COVID-19 Tedavisinde Kullanılacak İlaçlara İlişkin Bilgilendirme Favipiravir 200mg Tablet. [Google Scholar]
- 91.Republic of Turkey Ministry of Health . 2022. COVID-19 Tedavisinde Kullanılacak İlaçlara İlişkin Bilgilendirme-Lopinavir 200mg/Ritonavir 50mg Film Tablet. [Google Scholar]
- 92.Best BM, Capparelli EV, Diep H, et al. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. JAIDS. 2011;58(4):339–385. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Chen L. Lung tissue distribution of drugs as a key factor for COVID-19 treatment. Br J Pharmacol. 2020;177(21):4995–4996. doi: 10.1111/bph.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar GN, Jayanti VK, Johnson MK, et al. Metabolism and disposition of the HIV-1 protease inhibitor lopinavir (ABT-378) given in combination with ritonavir in rats, dogs, and humans. Pharm Res. 2004;21(9):1622–1630. doi: 10.1023/B:PHAM.0000041457.64638.8d. [DOI] [PubMed] [Google Scholar]
- 95.Bocan TM, Basuli F, Stafford RG, et al. Synthesis of [18 F]Favipiravir and biodistribution in C3H/HeN mice as assessed by positron emission tomography. Sci Rep. 2019;9(1785):1–10. doi: 10.1038/s41598-018-37866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahakijpijarn S, Moon C, Koleng JJ, Williams RO. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12(11):1–28. doi: 10.1101/2020.07.26.222109. 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vartak R, Patil SM, Saraswat A, Patki M, Kunda NK, Patel K. Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro. Nanomedicine. 2021;16(14):1187–1202. doi: 10.2217/nnm-2020-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vermillion MS, Murakami E, Ma B, et al. Inhaled remdesivir reduces viral burden in a nonhuman primate model of SARS-CoV-2 infection. Sci Transl Med. 2022;14(633):1–14. doi: 10.1126/scitranslmed.abl8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Albariqi AH, Chang RYK, Tai W, et al. Inhalable hydroxychloroquine powders for potential treatment of COVID-19. J Aerosol Med Pulm Drug Deliv. 2020;34(1):20–31. doi: 10.1089/jamp.2020.1648. [DOI] [PubMed] [Google Scholar]
- 100.Tai TT, Wu TJ, Wu HD, et al. A strategy to treat COVID-19 disease with targeted delivery of inhalable liposomal hydroxychloroquine: a preclinical pharmacokinetic study. Clin Transl Sci. 2021;14(1):132–136. doi: 10.1111/cts.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.ClinicalTrials.gov . Accessed January 4, 2022. A Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Orally Inhaled Aerosolized Hydroxychloroquine Sulfate in Healthy Adult Volunteers.https://clinicaltrials.gov/ct2/show/NCT04461353?term=rockefeller&cond=hydroxychloroquine&draw=2&rank=1 Published 2020. [Google Scholar]
- 102.Bentur O, Hutt R, Brassil D, et al. Phase 1 randomized placebo-controlled study in healthy adult volunteers to evaluate the safety, tolerability, and pharmacokinetics of orally inhaled aerosolized hydroxychloroquine sulfate – a potential treatment for COVID-19. J Allergy Clin Immunol. 2021;147(2):AB237. doi: 10.1016/j.jaci.2020.12.011. [DOI] [Google Scholar]
- 103.ClinicalTrials.gov . Accessed January 4, 2022. Local Tolerability and Pharmacokinetic Evaluation of Cyclops Dry Powder Hydroxychloroquine Inhalation in Healthy Volunteers.https://clinicaltrials.gov/ct2/show/NCT04497519 Published 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Reus YA, Hagedoorn P., Sturkenboom M.G.G., Grasmeijer F, Bolhuis MS, et al. Tolerability and pharmacokinetic evaluation of inhaled dry powder hydroxychloroquine in healthy volunteers. medRxiv. 2020:1–22. doi: 10.1101/2020.12.03.20243162. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.ClinicalTrials.gov . Accessed January 4, 2022. Development and Validation of “Ready-to-Use” Inhalable Forms of Hydroxychloroquine for Treatment of COVID-19.https://clinicaltrials.gov/ct2/show/NCT04477083 Published 2020. [Google Scholar]
- 106.ClinicalTrials.gov . Accessed January 4, 2022. Study in Participants With Early Stage Coronavirus Disease 2019 (COVID-19) to Evaluate the Safety, Efficacy, and Pharmacokinetics of Remdesivir Administered by Inhalation.https://clinicaltrials.gov/ct2/show/NCT04539262 Published 2020. [Google Scholar]
- 107.ClinicalTrials.gov . Accessed January 4, 2022. Safety, Tolerability and Pharmacokinetics of Inhaled Nanoparticle Formulation of Remdesivir (GS-5734) and NA-831 (NEUROSIVIR)https://clinicaltrials.gov/ct2/show/NCT04480333 Published 2020. [Google Scholar]
- 108.Yip LY, Lim YF, Chan HN. Safety and potential anticoagulant effects of nebulised heparin in burns patients with inhalational injury at Singapore General Hospital Burns Centre. Burns. 2011;37(7):1154–1160. doi: 10.1016/j.burns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 109.Markart P, Nass R, Ruppert C, et al. Safety and tolerability of inhaled heparin in idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv. 2010;23(3):161–172. doi: 10.1089/jamp.2009.0780. [DOI] [PubMed] [Google Scholar]
- 110.Yildiz A, John E, Özsoy Y, et al. Inhaled extended-release microparticles of heparin elicit improved pulmonary pharmacodynamics against antigen-mediated airway hyper-reactivity and inflammation. J Control Release. 2012;162(2):456–463. doi: 10.1016/j.jconrel.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 111.Xie N, Huan M, Tian F, Gu Z, Li X. Low molecular weight heparin nebulization attenuates acute lung injury. Biomed Res Int. 2017;2017:1–5. doi: 10.1155/2017/3169179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheuch G, Brand P, Meyer T, et al. Anticoagulative effects of the inhaled low molecular weight heparin certoparin in healthy subjects. J Physiol Pharmacol. 2007;58(Pt 2):603–614. Suppl 5. [PubMed] [Google Scholar]
- 113.Bendstrup KE, Chambers CB, Jensen JI, Newhouse MT. Lung deposition and clearance of inhaled 99mTc-heparin in healthy volunteers. Am J Respir Crit Care Med. 1999;160(5 I):1653–1658. doi: 10.1164/ajrccm.160.5.9809123. [DOI] [PubMed] [Google Scholar]
- 114.Chimenti L, Camprubí-Rimblas M, Guillamat-Prats R, et al. Nebulized heparin attenuates pulmonary coagulopathy and inflammation through alveolar macrophages in a rat model of acute lung injury. Thromb Haemost. 2017;117(11):2125–2134. doi: 10.1160/TH17-05-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation–associated acute lung injury. Crit Care Med. 2014;42(2):413–419. doi: 10.1097/CCM.0b013e3182a645e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Desai MH, M R., Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/acetylcystine therapy. J Burn Care Rehabilition. 1998;19(3):210–212. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and n-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30(2):249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 118.McIntire AM, Harris SA, Whitten JA, et al. Outcomes following the use of nebulized heparin for inhalation injury (HIHI Study) J Burn Care Res. 2017;38(1):45–52. doi: 10.1097/BCR.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 119.Dixon B, Smith RJ, Campbell DJ, et al. Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(4):360–372. doi: 10.1016/S2213-2600(20)30470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suchankova J, Mata M, Cortijo J, Morcillo EJ. Effects of bemiparin on airway responses to antigen in sensitized Brown–Norway rats. Eur J Pharmacol. 2005;507(1-3):261–271. doi: 10.1016/j.ejphar.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 121.van Haren FMP, Page C, Laffey JG, et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit Care. 2020;24(454):1–11. doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.ClinicalTrials.gov . Accessed January 4, 2022. Nebulized Heparin in Severe Acute Respiratory Syndrome COVID-19 (NEBUHEPA)https://clinicaltrials.gov/ct2/show/NCT04530578?cond=heparin+covid&draw=2&rank=2 Published 2020. [Google Scholar]
- 123.ClinicalTrials.gov . Accessed January 4, 2022. Nebulized Heparin for the Treatment of COVID-19 (INHALE-HEP)https://clinicaltrials.gov/ct2/show/NCT04723563?cond=heparin+covid&draw=2&rank=3 Published 2021. [Google Scholar]
- 124.ClinicalTrials.gov . Accessed January 4, 2022. Inhaled Heparin for Hospitalised COVID-19 Patients (INHALE-HEP)https://clinicaltrials.gov/ct2/show/NCT04635241?cond=heparin+covid&draw=2&rank=7 Published 2021. [Google Scholar]
- 125.ClinicalTrials.gov . Accessed January 4, 2022. Nebulized Heparin for COVID19-associated Acute Respiratory Failure.https://clinicaltrials.gov/ct2/show/NCT04842292?cond=heparin+covid&draw=2&rank=9 Published 2021. [Google Scholar]
- 126.ClinicalTrials.gov . Accessed January 4, 2022. Nebulised Heparin in Patients With Severe COVID-19 (CHARTER-MT)https://clinicaltrials.gov/ct2/show/NCT04545541?cond=heparin+covid&draw=2&rank=10 Published 2021. [Google Scholar]
- 127.ClinicalTrials.gov . Accessed January 4, 2022. Nebulized Heparin for the Treatment of COVID-19 Induced Lung Injury.https://clinicaltrials.gov/ct2/show/NCT04397510?cond=heparin+covid&draw=2&rank=13 Published 2021. [Google Scholar]
- 128.ClinicalTrials.gov . Accessed January 4, 2022. Effects of Low Molecular Weight Heparin Therapy With Soft-Mist Inhaler for COVID-19 Induced Hypoxemia.https://clinicaltrials.gov/ct2/show/NCT04990830?cond=heparin+covid &draw=2&rank=14 Published 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.ClinicalTrials.gov . Accessed January 4, 2022. Nebulised Heparin to Reduce COVID-19 Induced Acute Lung Injury (CHARTER-Irl)https://clinicaltrials.gov/ct2/show/NCT04511923?cond=heparin+covid&draw=2&rank=19 Published 2021. [Google Scholar]
- 130.van Haren FMP, Richardson A, Yoon H, et al. INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. Br J Clin Pharmacol. January 19, 2021:1–17. doi: 10.1111/bcp.14714. Published online. [DOI] [PubMed] [Google Scholar]
- 131.van Haren FMP, van Loon LM, Steins A, et al. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br J Clin Pharmacol. December 2021;2022:1–12. doi: 10.1111/bcp.15212. [DOI] [PubMed] [Google Scholar]
- 132.Erelel M, Kaskal M, Akbal-Dagistan O, et al. Early effects of low molecular weight heparin therapy with soft-mist inhaler for covid-19-induced hypoxemia : a phase iib trial. Pharmaceutics. 2021;13(1768):1–13. doi: 10.3390/pharmaceutics13111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lavorini F, Buttini F, Usmani OS. In: Concepts and Principles of Pharmacology-100 Years of the Handbook of Experimental Pharmacology. Barrett JE, Page CP, Michel MC, editors. 2019. 100 Years of drug delivery to the lungs- [DOI] [PubMed] [Google Scholar]
- 134.Gonda I, Schuster J. In: Modified-Release Drug Delivery Technology. 1st Editio. Rathbone MJ, Hadgraft J, Roberts MS, editors. CRC Press; 2002. Pulmonary delivery of drugs by inhalation; pp. 807–815. [DOI] [Google Scholar]
- 135.Cazzola M, Cavalli F, Usmani OS, Rogliani P. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Deliv. 2020;17(5):635–646. doi: 10.1080/17425247.2020.1739021. [DOI] [PubMed] [Google Scholar]
- 136.Roncada C, Andrade J, carolina Bischoff L, márcio Pitrez P. Comparison of two inhalational techniques for bronchodilator administration in children and adolescents with acute asthma crisis: a meta‑analysis. Rev Paul Pediatr. 2018;36(3):364–371. doi: 10.1590/1984-0462/;2018;36;3;00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Elbeddini A, Yeats A. Amid COVID-19 drug shortages: proposed plan for reprocessing and reusing salbutamol pressurized metered dose inhalers (pMDIs) for shared use. Drugs Ther Perspect. 2020;36(7):300–302. doi: 10.1007/s40267-020-00740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Boer AH, Hagedoorn P, Hoppentocht M, Buttini F, Grasmeijer F, Frijlink HW. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv. 2017;14(4):499–512. doi: 10.1080/17425247.2016.1224846. [DOI] [PubMed] [Google Scholar]
- 139.Levy ML, Carroll W, Izquierdo Alonso JL, Keller C, Lavorini F, Lehtimäki L. Understanding dry powder inhalers: key technical and patient preference attributes. Adv Ther. 2019;36(10):2547–2557. doi: 10.1007/s12325-019-01066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tweedie A, Lewis D. Enhancing the performance of dry powder inhalers: breath actuated mechanisms. ONdrugDelivery Mag. Apr 2016;66:34–38. 2016. [Google Scholar]
- 141.Starup-Hansen J, Dunne H, Sadler J, Jones A, Okorie M. Climate change in healthcare: Exploring the potential role of inhaler prescribing. Pharmacol Res Perspect. 2020;8(6):1–6. doi: 10.1002/prp2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hess DR. Nebulizers : principles and performance nebulizers : principles and performance. Respir Care. 2000;45(6):609–622. [PubMed] [Google Scholar]
- 143.Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16(1):1–7. doi: 10.5152/ejp.2014.00087. [DOI] [Google Scholar]
- 144.Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55(7):845–851. [PubMed] [Google Scholar]
- 145.Vecellio L, De Gersem R, Le Guellec S, et al. Deposition of aerosols delivered by nasal route with jet and mesh nebulizers. Int J Pharm. 2011;407(1-2):87–94. doi: 10.1016/j.ijpharm.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 146.Rau JL, Ari A, Restrepo RD. Performance comparison of nebulizer designs: constant-output, breath-enhanced, and dosimetric. Respir Care. 2004;49(2):174–179. [PubMed] [Google Scholar]
- 147.Ari A, De Andrade AD, Sheard M, Alhamad B, Fink JB. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J Aerosol Med Pulm Drug Deliv. 2015;28(4):281–289. doi: 10.1089/jamp.2014.1149. [DOI] [PubMed] [Google Scholar]
- 148.Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med. 2020;167(April) doi: 10.1016/j.rmed.2020.105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benge CD, Barwise JA. Aerosolization of COVID-19 and contamination risks during respiratory treatments. Fed Pract. 2020;37(4):160–163. http://www.ncbi.nlm.nih.gov/pubmed/32322146 [PMC free article] [PubMed] [Google Scholar]
- 150.Kim AY, Gandhi RT. April 8, 2022. COVID-19: Management in Hospitalized Adults.https://www.uptodate.com/contents/covid-19-management-in-hospitalized-adults UpToDate. Published 2022. Accessed. [Google Scholar]
- 151.Public Health Agency of Canada Prevention and control of influenza during a pandemic for all healthcare settings. Can Pandemic Influ Plan Heal Sect. 2011 http://www.phac-aspc.gc.ca/cpip-pclcpi/assets/pdf/ann-f-eng.pdf May. [Google Scholar]
- 152.Wachtel H, Kattenbeck S, Dunne S, Disse B. The Respimat® Development story: patient-centered innovation. Pulm Ther. 2017;3:19–30. doi: 10.1007/s41030-017-0040-8. [DOI] [Google Scholar]
- 153.Iwanaga T, Tohda Y, Nakamura S, Suga Y. The Respimat® Soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39(11):1021–1030. doi: 10.1007/s40261-019-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dalby R, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices Evid Res. 2011;4:145. doi: 10.2147/MDER.S7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Newman SP, Brown J, Steed KP, Reader SJ, Kladde H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines * Comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113(4):957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 156.Pitcairn G, Reader S, Pavia D, Newman S. Deposition of Corticosteroid aerosol in the human lung by Respimat® Soft MistTM inhaler compared to deposition by metered dose inhaler or by Turbuhaler® Dry powder inhaler. J Aerosol Med. 2005;18(3):264–272. doi: 10.1089/jam.2005.18.264. [DOI] [PubMed] [Google Scholar]
- 157.Cipolla DC, Gonda I. Formulation technology to repurpose drugs for inhalation delivery. Drug Discov Today Ther Strateg. 2011;8(3-4):123–130. doi: 10.1016/j.ddstr.2011.07.001. [DOI] [Google Scholar]
- 158.Cipolla D, Chan H-K, Schuster J, Farina D. Personalizing aerosol medicine: development of delivery systems tailored to the individual. Ther Deliv. 2010;1(5):667–682. doi: 10.4155/tde.10.54. [DOI] [PubMed] [Google Scholar]
- 159.Larson N. Published online 2019. Compositions Comprising an RNA Polymerase Inhibitor and Cyclodextrin for Treating Viral Infections.https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019014247 [Google Scholar]
- 160.European Medicines Agency . Accessed May 31, 2022. Cyclodextrins Used as Excipients.; 2017.https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_ en.pdf [Google Scholar]
- 161.US Food and Drug Administration . Accessed May 31, 2022. Inactive Ingredient Search for Approved Drug Products.https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=BasicSearch.page [Google Scholar]
- 162.Kavanagh O, Marie Healy A, Dayton F, et al. Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med Hypotheses. 2020:143. doi: 10.1016/j.mehy.2020.110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fassihi SC, Nabar NR, Fassihi R. Novel approach for low-dose pulmonary delivery of hydroxychloroquine in COVID-19. Br J Pharmacol. 2020;177(21):4997–4998. doi: 10.1111/bph.15167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klimke A, Hefner G, Will B, Voss U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med Hypotheses. 2020:142. doi: 10.1016/j.mehy.2020.109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karatza E, Ismailos G, Marangos M, Karalis V. Optimization of hydroxychloroquine dosing scheme based on COVID-19 patients’ characteristics: a review of the literature and simulations. Xenobiotica. 2021;51(2):127–138. doi: 10.1080/00498254.2020.1824301. [DOI] [PMC free article] [PubMed] [Google Scholar]