Abstract
Severe cases of Coronavirus Disease 2019 (COVID-19) can present with multiple neurological symptoms. The available neuropathological studies have described different lesions; the most frequent was the presence of neuroinflammation and vascular-related lesions. The objective of this study was to report the neuropathological studies performed in a medical institution, with abundant long intensive care unit stays, and their associated clinical manifestations. This is a retrospective monocentric case series study based on the neuropathological reports of 13 autopsies with a wide range of illness duration (13–108 days). A neuroinflammatory score was calculated based on the quantification of CD8- and CD68-positive cells in representative areas of the central nervous system. This score was correlated afterwards with illness duration and parameters related to systemic inflammation. Widespread microglial and cytotoxic T-cell activation was found in all patients. There was no correlation between the neuroinflammatory score and the duration of the illness; nor with parameters of systemic inflammation such as the peak of IL-6 or the HScore (a parameter of systemic macrophage activation syndrome). Two patients had global hypoxic ischaemic damage and five patients had subacute infarcts. One patient had many more brain vascular microthrombi compared to the others and multiple subacute pituitary infarcts. SARS-CoV-2 RNA was not detected with qRT-PCR. The proportion of brain lesions in severe COVID-19 patients could be related to illness duration. In our series, with abundant long hospitalisation stays, neuroinflammation was present in all patients and was more prominent between day 34 and day 45 after onset of symptoms. Clinical correlation showed that two patients with the highest neuroinflammatory scores had severe encephalopathies that were not attributable to any other cause. The second most frequent lesions were related to vascular pathology.
Key words: COVID-19, autopsy, neuropathology, neuroinflammation, encephalopathy
Introduction
Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is mainly a respiratory illness that may lead to a respiratory distress syndrome. However, signs and symptoms are not exclusive of the respiratory system and a variety of neurological symptoms have been described, including anosmia, ageusia, mood disorders, headache, dizziness, altered consciousness, and paresthesia, among others.1 Several different mechanisms of disease were initially hypothesised: a neurotropic potential of SARS-CoV-2, a secondary toxic encephalopathy, and a secondary acute cerebrovascular disease.2 The presence of SARS-CoV-2 in brain tissue has been demonstrated with variable success and its direct role is currently under debate.3, 4, 5
The evaluation of the brain in the context of autopsies case series and reports has shown a variety of neuropathological findings.6 , 7 Some early series proved an extensive hypoxic-ischaemic damage8 and the presence of acute/subacute infarcts6; while other authors have emphasised the presence of neuroinflammation.5 , 9 , 10
Neuroinflammation in COVID-19 is mainly described as a widespread activated microgliosis with few infiltrating lymphocytes.5 , 9 The pathophysiology and clinical relevance of this finding is currently poorly understood, mainly due to the limited data available: microgliosis was described in 74 of 197 brains compiled in a recent review.7 In addition, clinical information is scarce in many reports.
It is known that COVID-19 patients have high levels of proinflammatory cytokines, and this cytokine storm is associated with disease severity.11 Moreover, some authors have noticed that the cytokine profile described in these patients is like that described in macrophage activation syndrome and in secondary haemophagocytic lymphohistiocytosis (sHLH).12 In addition, the presence of haemophagocytosis pointing towards a systemic macrophage activation has been repeatedly described in COVID-19 patients.13 , 14 Among all proinflammatory cytokines, IL-6 is increased in COVID-19 and a meta-analysis has shown that high IL-6 levels are associated with worse outcomes.15 However, there is little evidence correlating systemic inflammation and brain neuropathology.
The objective of this article is to report the neuropathological studies performed in a medical institution, with abundant long intensive care unit (ICU) stays and their associated clinical manifestations. Moreover, neuroinflammatory findings are correlated with illness length, clinical parameters related to systemic inflammation and the HScore, which is used as a clinical parameter of systemic macrophage activation.
Materials and methods
Patient selection and clinical data
Patients with a pre-mortem confirmed diagnosis of SARS-CoV-2 infection using nasopharyngeal SARS-CoV-2 RNA quantitative RT-PCR, were autopsied at the Hospital Universitario Ramón y Cajal (Madrid, Spain) between April 2020 and May 2021, representing approximately 1.5% of patients who died from COVID-19 during this period. Autopsies were requested by the medical staff according to clinical interest; most autopsies corresponded to patients with severe respiratory diseases (n=12, 92%) and were requested by the ICU (n=10, 77%). Patients were numbered according to the duration of their illness, measured from the onset of symptoms to death.
Clinical and laboratory data were taken from the medical records. IL-6 levels were requested based on clinical criteria of poor clinical progress. Pre-mortem neurological diagnoses were determined by consensus of two neurologists (IC and JM) after reviewing the medical records, blinded to the neuropathological findings. A neurological diagnosis was considered not available for patients who were neurologically asymptomatic before the need for orotracheal intubation and who received sedative-analgesic medications until death. Encephalopathy was diagnosed in the presence of decreased level of consciousness in patients without sedative drugs and without focal neurological signs and in the absence of meningitis or structural lesions in neuroimaging. Acute confusional state (ACS) was diagnosed in the presence of attention deficit, with disorientation and agitation, without focal neurological signs.
HScore is used as a diagnostic tool for sHLH. It was calculated for all the patients based on clinical, laboratory and bone marrow evaluation at the time of autopsy (Supplementary Table 1, Appendix A), using a tool freely available online (http://saintantoine.aphp.fr/score/).16
Standard protocol approvals, registrations and patient consents
Consented autopsies were performed following the recommended security measures.17 This study was approved by the Hospital Universitario Ramón y Cajal Research Ethics Committee (reference: Necropsias_Covid19; approval code: 355/20) and tissue samples were managed through the Hospital Universitario Ramón y Cajal-IRYCIS Biobank.
Autopsy and histological procedures
Brains were removed at the time of autopsy (no longer than 24 hours after death) and fixed in buffered 4% formaldehyde for 21 days. After fixation, brains were weighed, examined macroscopically, and underwent routine neuropathological workup. This included histological examination stained with haematoxylin and eosin (H&E) of a minimum of 20 standardised blocks from both olfactory bulbs, superior frontal gyrus, parietal cortex, superior temporal cortex, insular cortex, calcarine cortex basal ganglia, hippocampus, cerebellar cortex and cerebellar dentate nucleus, whole brainstem, and superior cervical medulla. In addition, sections from macroscopically identified lesions were included. In selected sections, Luxol fast blue was used to evaluate myelin, and Congo red to evaluate amyloid deposits. The pituitary gland histopathological study was also considered in the present work. Bone marrow was evaluated from decalcified rib tissue as previously described.13 No peripheral nerves were taken at the time of autopsy.
Immunohistochemical methods
The following primary antibodies were employed: CD8 to identify cytotoxic T cells (clone C8/144B; Agilent, United States); CD68 to identify activated microglia in the brain and haemophagocytic cells in the bone marrow (clone PG-M1; Agilent); CD61, a platelet marker, to identify microthrombi (clone Y2/51, Agilent); and cytomegalovirus (clones CCH2+DDG9, Agilent). Immunohistochemistry visualisation was performed using EnVision FLEX system (Agilent). Dual immunostainings were performed using EnVision FLEX DAB+Chromogen (Agilent) to obtain a brown colour and EnVision FLEX HRP Magenta (Agilent) to obtain a magenta colour.
Quantifications were performed in four selected areas: frontal cortex, basal ganglia, hippocampus, and midbrain. Cytotoxic T lymphocytes assessed by CD8 immunostaining and activated microgliosis by CD68 immunostaining were evaluated following the semi-quantitative approach described by Matschke et al. 5 in the selected areas. Positive cells were counted per high power field (HPF) of 0.332 mm2 in both perivascular and parenchymal areas independently, and categorised in the following grades: none, mild (1–9 cells per HPF), moderate (10–49 cells per HPF), and severe (≥50 cells per HPF). For perivascular evaluation, only transversally sectioned vessels were considered. A neuroinflammatory score was obtained from the sum of all values obtained after CD68/CD8 immunohistochemistry evaluation for each patient. The presence of microthrombi, defined as compact intravascular CD61 positive casts, was evaluated using a semi-quantitative score in a 20× field (1.131 mm2): none, mild (1 or 2 vessels occluded by platelet deposit per field), moderate (3–5 vessels occluded by platelet deposit per field) and severe (≥6 vessels occluded by platelet deposit per field).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of SARS-CoV-2.
Hippocampus formalin-fixed, paraffin-embedded (FFPE) blocks from all patients and other FFPE blocks with microglial nodules were selected for SARS-CoV-2 identification. RNA was extracted from 10 sections of 5μm obtained from FFPE blocks using RecoverAll Total Nucleic Acid Isolation Kit (Invitrogen, USA), following the manufacturer's instructions. RNA quantity was measured fluorometrically with Qubit RNA high sensitivity assay kit (Invitrogen) and gRNA SARS-CoV-2 was detected using TaqmanTM 2019 nCoV assay (ThermoFisher, USA).
Study design and statistics
STROBE guidelines have been followed for the design of the study and the writing of the manuscript. Numerical and categorical variables were summarised as median (range) and as frequencies and percentages, respectively. Spearman correlation was calculated using the statistical software IBM SPSS v19 (IBM, USA). Differences were considered significant with p values <0.05.
Results
The clinical and neuropathological findings of the 13 patients are listed in Table 1 . The mean age was 66.7 years (range 52–83), two (15.4%) patients were women and 11 (84.6%) men. They had an average duration of illness of 39.5 days (range 13–108) and an average length of hospitalisation of 33.9 days (range 3–102). Eleven patients (84.6%) were treated in the ICU: the average length of the ICU stay was 34 days (range 12–95).
Table 1.
Summary of clinical and neuropathological data
| Patient | Sex | Age | ID | LH | ICU | Relevant comorbidities | Neurological diagnosis | Sepsis | BW (g) | AHID | Subacute lacunar infarct | Microglial nodules | Ath | Other relevant histological findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT1 | Male | 80 | 13 | 8 | 0 | HTN, AF, COPD | ACS | No | 1245 | Yes (mild) | No | No | Yes | |
| PT2 | Female | 83 | 14 | 3 | 0 | Degenerative dementia | ACS | No | 1055 | No | No | No | Yes | Pathological features of Alzheimer's disease NIA-AA (A2, B2, C2) |
| PT3 | Male | 58 | 16 | 12 | 12 | DM2 | Severe encephalopathy of unknown cause | No | 1490 | Yes (global) | No | No | Yes | |
| PT4 | Male | 69 | 19 | 18 | 17 | HTN, DM2, ischaemic cardiopathy, CKD | Not available | Yes | 1280 | No | No | No | Yes | |
| PT5 | Male | 69 | 25 | 22 | 22 | Ischaemic cardiopathy | Encephalopathy attributed to sepsis and uraemia; probable CIP | Yes | 1325 | No | Yes | No | Yes | |
| PT6 | Female | 60 | 32 | 29 | 19 | No | Not available | Yes | 1075 | Yes (global) | No | No | Yes | Frontal subarachnoid haemorrhage |
| PT7 | Male | 73 | 34 | 26 | 25 | No | Severe encephalopathy, probably anoxic | No | 1195 | No | Yes | Yes | Yes | |
| PT8 | Male | 70 | 36 | 32 | 30 | No | Not available | Yes | 1450 | No | No | Yes | Yes | |
| PT9 | Male | 55 | 40 | 33 | 19 | DM2, DL | Probable CIP | No | 1290 | No | No | Yes | No | Subacute pituitary infarcts |
| PT10 | Male | 63 | 45 | 37 | 36 | Mitral prosthetic valve Staphylocccus aureus endocarditis | Severe encephalopathy of unknown cause | Yes | 1270 | No | Yes | Yes | No | |
| PT11 | Male | 52 | 57 | 50 | 50 | HTN | Confirmed CIM | Yes | 1150 | No | Yes | No | Yes | |
| PT12 | Male | 73 | 74 | 69 | 49 | DL | Not available | Yes | 1345 | No | No | No | Yes | |
| PT13 | Male | 62 | 108 | 102 | 95 | DL | Mild encephalopathy; probable CIP | Yes | 1400 | Yes (mild) | Yes | No | Yes |
ACS, acute confusional state; AF, atril fibrillation; AHID, acute hypoxic-ischaemic damage; Ath, atherosclerosis; BW, brain weight; CIM, critical illness myopathy; CIP, critical illness polyneuropathy; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM2, Type 2 diabetes mellitus; DL, dyslipidaemia; HTN, hypertension; ICU, length of ICU stay; ID, illness duration; LH, length of hospitalisation; NIA-AA, National Institute on Aging and Alzheimer's Association.
Pre-mortem clinical diagnosis was considered not available in four patients (Table 1). ACS was diagnosed in two patients older than 80 years showing disorientation and agitation (PT1, PT2); one of them had been previously diagnosed with degenerative dementia. A diagnosis of encephalopathy of variable degree was established in five patients (PT3, PT5, PT7, PT10, PT13). Encephalopathy was attributed to sepsis and uremia in one patient (PT5), and to cerebral anoxia after resuscitated cardiac arrest in another (PT7). In the other three patients no other cause of encephalopathy was suspected, and episodes of severe hypoxemia or low cardiac output were not recorded in their files. Additionally, three patients (PT5, PT9, PT13) were diagnosed with probable critical illness polyneuropathy (CIP) (no electrophysiological studies were performed), and one patient (PT11) was diagnosed with critical illness myopathy, confirmed using electromyography.
We found CD68-positive activated microglia in the brain parenchyma in all patients, associated with perivascular and occasionally infiltrating CD8-positive T lymphocytes. In addition to the microglial activation, four patients had microglial nodules (PT7, PT8, PT9, PT10) (Fig. 1 A). Interestingly, these patients had a similar clinical course with a duration of illness between 34 and 45 days and an ICU stay of 19–36 days. This finding was most striking in patient PT10, with a diagnosis of Staphylococcus aureus endocarditis, in which there were scattered microglial nodules in a background of glial activation in the hippocampus, thalamus, midbrain, and cervical spinal cord (Fig. 1B). The other three patients had isolated microglial nodules in the dorsal medulla oblongata and cervical spinal cord. Only two of these four patients had sepsis, defined as at least one positive blood culture, during the disease. No parasitic or cytopathic viral changes were observed in these cases. Hippocampus FFPE blocks from all patients and FFPE blocks containing microglial nodules from patients PT7, PT8, PT9 and PT10 were negative for SARS-CoV-2 RNA by qRT-PCR.
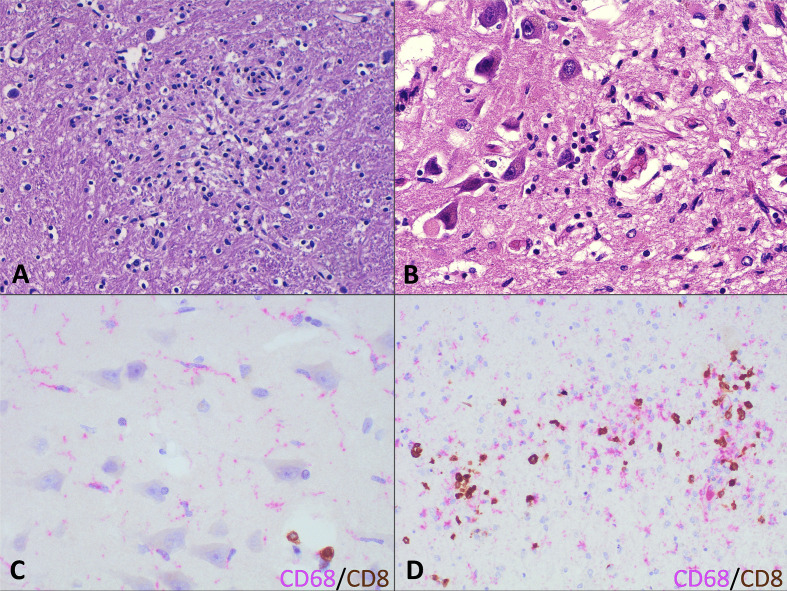
Fig. 1.
Neuroinflammation in fatal COVID-19 patients. (A) Microglial nodule in PT10 (H&E). (B) Area of hippocampal cortical destruction by a microglial reaction in PT10 (H&E). (C) Double staining highlighting microglial activated cells (CD68-positive in pink) and scattered T cytotoxic lymphocytes (CD8-positive in brown). (D) Double staining (CD68-positive in pink and CD8-positive in brown) of a microglial nodule highlighting the presence of numerous macrophages with interspersed lymphocytes.
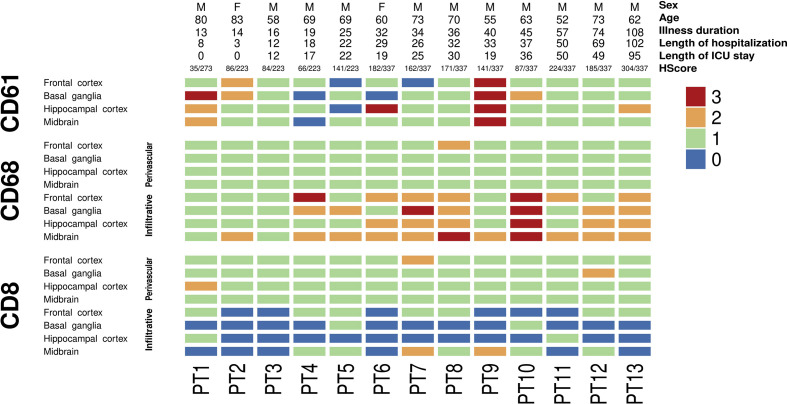
Immune infiltrates studied by double immunohistochemistry against CD68 as an active microglia marker and CD8 as a cytotoxic T-cell marker were evaluated in four selected regions (frontal cortex, basal ganglia, hippocampus, and midbrain) following the same score employed by Matschke et al. 5 in their COVID-19 neuropathology series (Fig. 2 A). Perivascular and infiltrating cells were evaluated independently. Neuroinflammation, and more specifically microgliosis, was more prominent, with an increased duration of symptoms and more prominent in the midbrain compared with the other areas examined (Fig. 1C, Fig. 2). Microglial nodules were mainly composed of CD68-positive cells, with scattered CD8 cells (Fig. 1D).
Fig. 2.
Heatmap showing the distribution of inflammation and microthrombi in the cohort according to the length of illness.
Neuroinflammation was the only prominent pathological finding in the brain of four patients with a pre-mortem diagnosis of different degrees of encephalopathy (PT5, PT7, PT10, PT13). The two patients with more severe microgliosis (PT7, PT10) had severe encephalopathy. Other patients with prominent neuroinflammation did not have pre-mortem neurological evaluation (PT6, PT8, PT12).
We did not find any correlation between the neuroinflammatory score and the duration of illness (r=0.450, p=0.123), nor between the neuroinflammatory score and the length of ICU stay (r=0.346, p=0.297) exclusively considering those patients that were admitted to ICU (n=11). To evaluate the influence of systemic inflammation on neuroinflammation, correlation between the highest of IL-6 and neuroinflammatory score was calculated showing a non-significant negative association (r = –0.505, p=0.113). However, the range of IL-6 was very wide (1.27–3900.00) (Supplementary Table 1, Appendix A). No correlation was observed between the HScore and the neuroinflammatory score (r=0.204, p=0.503) (Supplementary Fig. 1, Appendix A).
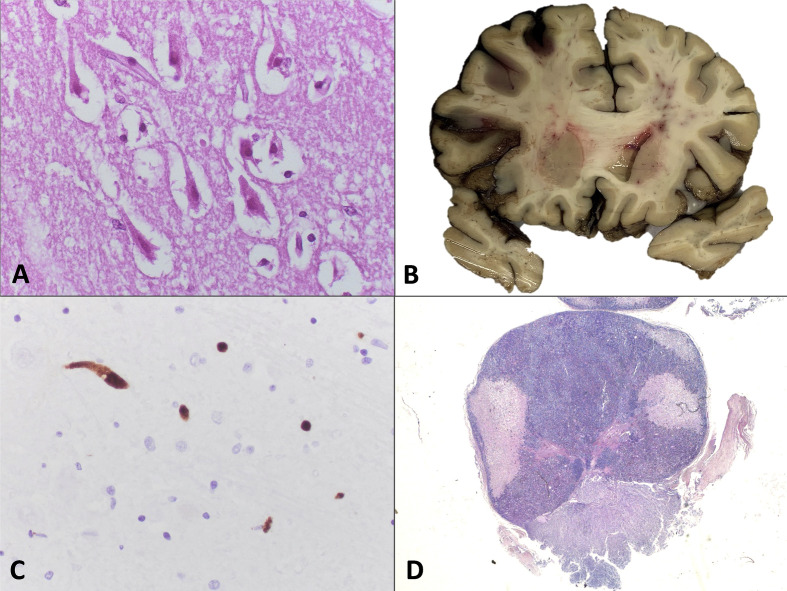
The second most frequent findings were related to vascular pathology. Two patients (PT3, PT6) (15.4%) presented global acute hypoxic ischaemic damage (Fig. 3 A). Moreover, patient PT6 had a frontal predominant subarachnoid haemorrhage without a ruptured aneurysm or other vascular lesions (Fig. 3B). This patient had a cytomegalovirus pneumonitis over diffuse alveolar damage in the lung; however, no cytomegalovirus cytopathic changes were found and the immunohistochemistry for cytomegalovirus we performed in selected regions was negative. This patient was receiving unfractionated heparin due to extracorporeal membrane oxygenation and suffered a sudden drop in the bispectral index scale (BIS), but neurological evaluation could not be performed. Patient PT3 was transferred to our hospital due to acute deterioration of consciousness, under a suspected diagnosis of brainstem stroke, which was not confirmed. No episodes of severe hypoxaemia were detected during his hospital stay. Two additional patients had mild focal signs of acute hypoxic ischaemic damage.
Fig. 3.
Vascular neuropathological findings in fatal COVID-19 patients. (A) Eosinophilic neurons with pyknotic nuclei from the hippocampal cortex characteristic of hypoxic-ischaemic damage (H&E) in PT6. (B) Macroscopy of a coronal section showing a subarachnoid haemorrhage in PT6. (C) A high density of microthrombi is highlighted by CD61 immunohistochemistry in PT9 (CD61). (D) Pituitary gland from PT9 showing several areas of parenchymal disruption that correspond with ischaemic infarcts (H&E).
Five patients (38.5%) presented small subacute infarcts that were less than 1 cm in size located in the basal ganglia, pons, or cerebellum. In addition, PT10 had an acute infarct in the right cerebellar cortex. No episode consistent with acute stroke was recorded in any of these patients. Amyloid angiopathy was excluded in all patients. Atherosclerosis, mainly located in the arteries of the circle of Willis, was a common finding present in 11 patients (84.6%).
Scattered thrombi were found in some of the brains examined with H&E. CD61 immunohistochemistry highlighted scattered microthrombi in most patients (Fig. 3C), and they were very prominent in patient PT9. However, no symptoms of stroke or significant encephalopathy were detected. The pituitary gland was studied in 12 patients (92.3%). The only relevant finding was the presence of several foci of subacute infarcts in patient PT9, the one who also presented microthrombi (Fig. 3D).
Only one patient, PT2, had a pre-existing neurological disease, which consisted of a clinical diagnosis of degenerative dementia. The neuropathological study showed amyloid plaques and neurofibrillary tangles that were Tau-positive, in the absence of Lewy bodies. The final neuropathological diagnosis was Alzheimer disease (NIA-AA A2, B2, C2).
Discussion
We report 13 cases of fatal COVID-19 in whom neuropathological examination revealed a variety of abnormalities. The most common finding was widespread neuroinflammation in all patients, with the presence of microglial nodules in four patients. In addition, there were six patients with vascular related lesions: four of them showed subacute infarcts and two presented global acute hypoxic ischaemic damage.
A variety of neuropathological findings have been described in COVID-19; therefore, it is difficult that one isolated alteration could explain all the neurological symptoms in these patients. In one of the early series published, Solomon et al. 8 found acute hypoxic ischaemic damage as a common feature in all patients. We found this lesion as the main finding in only two patients, in addition to a subarachnoid haemorrhage in one of them. Hypoxic lesions are mainly a consequence of diffuse alveolar damage in the lung parenchyma found in fatal COVID-19 patients.18 , 19 However, some larger studies, like Matschke et al. 5 including 43 neuropathological studies in COVID-19 patients, did not find acute necrotising lesions or small-vessel thrombosis.
Widespread microgliosis was present in all fatal COVID-19 patients referred for autopsy. Similar findings were described by Matschke et al. 5 in a larger series. They described astrogliosis in 86% of patients, with occasional microglial nodules and concluded that neuroinflammation in brainstem is the most common finding, an affirmation supported by our data. In addition, Meinhardt et al. 20 found microglial nodules in 13 of 25 brains. Similar findings were described in isolated cases by other authors,21 , 22 and there are clinical cases reporting brainstem encephalitis in COVID-19 patients.23 Not all studies describe in detail the evolution of the disease in the patients, and for this reason comparison is difficult. However, it seems that a prolonged evolution is necessary to see an increase in neuroinflammation. That could explain the scarcity of neuroinflammation reported by Solomon et al. 8 in patients with a mean of 10.4 days from onset of symptoms to death, and by Bryce et al. 24 with a median hospitalisation stay of 9 days. In contrast, studies reporting more widespread neuroinflammation had a longer survival period. Meinhardt et al. 20 patients had a median survival period from the onset of symptoms to death of 31 days, Thakur et al. 25 patients had an average length of hospitalisation of 19 days, and our patients had a mean survival period of 39.5 days from onset of symptoms to death.
Neuroinflammation was more accentuated with the increase in the duration of the disease, although long-term patients (with more than 50 days since onset of symptoms), had reduced scores compared with those with intermediate illness durations. Interestingly, in our series, patients presenting microglial nodules had very similar duration of illness (between 34 and 45 days) and length of ICU stay (between 19 and 36 days). This timeline may reflect that described for plasma neurofilament light chain protein, a marker of neuronal injury in severe COVID-19 patients that peaks between 30 and 70 days after the onset of symptoms and decreases after 100 days.26 , 27
Microglial nodules are not exclusive of viral encephalitis, but they are also present in autoimmune encephalitis as well as in multiple sclerosis.28 Once the presence of the virus by qRT-PCR in the brain parenchyma is excluded, we cannot rule out post-infectious autoimmune mechanisms. Other aetiologies should also be considered. Microglial nodules have been described in a patient with sepsis due to staphylococcal endocarditis in probable relation with pre-existent emboli29 and patient PT10 in our series had an endocarditis caused by Staphylococcus aureus during the course of the disease. Considering the whole series, 62% of the patients had sepsis defined as the presence of any positive blood culture, during their disease course; but only two of four patients also presented microglial nodules. More detailed investigations have described that the microglial state in these patients is different from the microglial cell states in human degenerative10 and neuroinflammatory diseases.30
To understand the relationship of neuroinflammation and systemic inflammation using retrospective clinical data, we analysed the correlation between the neuroinflammatory score and the peak of the proinflammatory cytokine IL-6. We found a negative non-significant correlation between both parameters. However, this result should be treated with caution due to the wide range of IL-6 values (1.27–3900.00) and the fact that PT9, which showed microglial nodules, had the highest IL-6 value. Knowing that COVID-19 neuroinflammation is mainly characterised by microglial activation,5 , 10 , 30 and based on prior reports describing a cytokine storm syndrome like sHLH and macrophage activation syndrome,31 we wondered if the HScore used to diagnose sHLH16 may help to identify patients with the highest scores of neuroinflammation. However, our analysis did not show any significant correlation. Despite the presence of haemophagocytic figures in the bone marrow in almost all COVID-19 patients, we did not find evidence of haemophagocytic syndrome in the studied brains such as leptomeningeal histiocytosis or haemophagocytic figures related to lymphohistiocytic lesions.32
Neurological clinical and pathological correlations have been difficult to carry out in autopsy studies of COVID-19 patients.25 Information on neurological symptoms or diagnosis have been absent5 or obtained through retrospective revision of electronic medical records,5 , 8 as in the present study. Neurological examination is limited in patients with orotracheal intubation and under sedative-analgesic medications or neuromuscular blockers. There were also difficulties with the performance of complementary neurological studies during the peak of incidence of the pandemic. For these reasons, the clinical significance of the neuroinflammation found in some autopsy series is unknown.5 , 25 Our results suggest that neuroinflammation may explain some cases of encephalopathy in COVID-19 patients. The presence of neuroinflammation was the only significant pathological finding in four patients with a pre-mortem diagnosis of encephalopathy, although it was attributed to sepsis and uraemia in one of them. The severity of the encephalopathy could be correlated with the intensity of microgliosis in one patient. Encephalopathy was the most common pre-mortem neurological diagnosis in the present series (38.5%). Encephalopathy is frequent in hospitalised COVID-19 patients, particularly in ICU patients.33 It represents a clinical challenge since it may be caused through different mechanisms: cerebral anoxia, metabolic derangements, diffuse microvascular lesions, cytokine storm, meningitis, and encephalitis. The different mechanisms may be clinically indistinguishable, which emphasises the need for an exhaustive neurological evaluation in these patients. In the present autopsy study, anoxic encephalopathy was unexpectedly diagnosed in one patient without a clear antecedent of cerebral anoxia, while it was not confirmed in a patient who had suffered cardiac arrest. This shows the challenges existing in the aetiological diagnoses of encephalopathy in these patients, so we suggest that encephalopathy due to neuroinflammation associated with SARS-CoV-2 should be included among the possible differential diagnoses.
Regarding vascular lesions, few series have described the presence of microthrombi in brain vessels. Fabbri et al. 34 described the presence of microthrombi in 10 patients with variable duration of illness (6–35 days). Bryce et al. 24 noticed widespread microthrombi in 17 of the 58 brains studied (29.3%), while other authors have found microthrombi in 18% of patients.20 Because we have found some very scattered thrombi in some brains, we have systematically assessed the presence of microthrombi using CD61 immunohistochemistry as a platelet marker and found one patient with significant microvascular thrombosis. It is important to note that some threshold should be established when assessing thrombi by CD61 immunohistochemistry because small post-mortem platelet precipitates can be misleading.35 The patient with the highest density of microthrombi had several pituitary infarcts. The association between both findings made us wonder about the possible role of microthrombi in pituitary infarcts, which were also present in the pituitary tissue at the time of autopsy. The presence of pituitary infarcts has already been described as infrequent, but it has also been repeatedly reported in autopsy studies from COVID-19 patients.6 , 24
Small subacute lacunar infarcts were found in three patients and one additional patient had a subarachnoid haemorrhage. No lacunar infarcts were found in the patient with increased microthrombi. Acute or subacute brain infarcts have been described in around 20% of COVID-19 patients with neuropathological studies, and spontaneous haemorrhages in around 15%.6 This correlates well with clinical series describing an increased risk of stroke in COVID-19 patients.36 In our series, there were no neurological signs or symptoms directly attributable to these lesions.
We were not able to prove the presence of SARS-CoV-2 by qRT-PCR in these patients. There is currently some debate concerning the presence of neuroinvasion by SARS-CoV-2. Some autopsy series have reported the presence of positive SARS-CoV-2 qRT-PCR in brain tissue, but mostly at low titres.5 , 8 , 20 The rate of positive samples is increased when using more sensitive methods. Gagliardi et al. 4 reported an increase in positivity from 1/9 to 8/9 when using droplet digital PCR instead of qRT-PCR in frozen brain tissue. However, some authors have rejected that, considering those low titres as neuroinvasion, as they do not allow differentiation between neuroinvasion and the presence of circulating virus in brain blood vessels.4 , 10
The limitations of this study are derived from the number of cases and the retrospective nature of the study. Nevertheless, long-term COVID-19 patients have been barely described in the literature. Because our findings are based on autopsy examination of fatal COVID-19 patients, there is an unavoidable selection bias, and our conclusions should be extrapolated to all COVID-19 patients with caution.
Conclusion
We describe a variety of neuropathological findings that could be found in fatal COVID-19 patients. Widespread microgliosis and neuroinflammation were constant findings in the long term in these patients. Moreover, severe neuroinflammation could explain severe encephalopathies that were not attributable to any other cause. The second most frequent neuropathological lesions were related to vascular injury.
Conflicts of interest and sources of funding
This work was supported by the Instituto de Salud Carlos III (grant PI 19/01331), CIBERONC (grant CB16/12/00316), Instituto Ramón y Cajal de Investigación Sanitaria Intramural COVID19 (project grants 2020/0290 and 2020/154), and Merck, Sharp & Dohme (MSD). The authors state that there are no other conflicts of interest to disclose.
Acknowledgements
The authors would like to thank all members of the MACROCOVID and Pathology Departments from Hospital Universitario Ramón y Cajal (Madrid, Spain) for their constant support to the COVID-19 Autopsy Project; to Marta Rosas and María Luisa Zamorano, from the Pathology department of Hospital Universitario Ramón y Cajal (Madrid, Spain), for their technical assistance in the histopathological and immunohistochemistry studies; and Hospital Universitario Ramón y Cajal-IRYCIS Biobank (Madrid, Spain), for the management of tissue samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pathol.2022.03.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guerrero J.I., Barragan L.A., Martinez J.D., et al. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis. 2021;21:515. doi: 10.1186/s12879-021-06185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y., Xu X., Chen Z., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosentino G., Todisco M., Hota N., et al. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: a critical systematic review. Eur J Neurol. 2021;28:3856–3865. doi: 10.1111/ene.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagliardi S., Poloni E.T., Pandini C., et al. Detection of SARS-CoV-2 genome and whole transcriptome sequencing in frontal cortex of COVID-19 patients. Brain Behav Immun. 2021;97:13–21. doi: 10.1016/j.bbi.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matschke J., Lutgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou J.J., Movassaghi M., Gordy D., et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021;2:2. doi: 10.17879/freeneuropathology-2021-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiese A., Manetti A.C., Bosetti C., et al. SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;31 doi: 10.1111/bpa.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon I.H., Normandin E., Bhattacharyya S., et al. Neuropathological features of covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schurink B., Roos E., Radonic T., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang A.C., Kern F., Losada P.M., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGonagle D., Sharif K., O'Regan A., et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunez-Torron C., Ferrer-Gomez A., Moreno Moreno E., et al. Secondary haemophagocytic lymphohistiocytosis in COVID-19: correlation of the autopsy findings of bone marrow haemophagocytosis with HScore. J Clin Pathol. 2021 doi: 10.1136/jclinpath-2020-207337. Mar 15; jclinpath-2020-207337. [DOI] [PubMed] [Google Scholar]
- 14.Prieto-Perez L., Fortes J., Soto C., et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33:2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fardet L., Galicier L., Lambotte O., et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 17.The COVID-19 Autopsy Project The first COVID-19 autopsy in Spain performed during the early stages of the pandemic. Rev Esp Patol. 2020;53:182–187. doi: 10.1016/j.patol.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Mies B., Gomez-Rojo M., Carretero-Barrio I., et al. Pulmonary vascular proliferation in patients with severe COVID-19: an autopsy study. Thorax. 2021;76:1044–1046. doi: 10.1136/thoraxjnl-2020-216714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polak S.B., Van Gool I.C., Cohen D., et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinhardt J., Radke J., Dittmayer C., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 21.Al-Dalahmah O., Thakur K.T., Nordvig A.S., et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol Commun. 2020;8:147. doi: 10.1186/s40478-020-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen M.P., Le Quesne J., Officer-Jones L., et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2021;47:17–25. doi: 10.1111/nan.12662. [DOI] [PubMed] [Google Scholar]
- 23.Khoo A., McLoughlin B., Cheema S., et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 2020;91:1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 24.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: the mount sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur K.T., Miller E.H., Glendinning M.D., et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanberg N., Ashton N.J., Andersson L.M., et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 27.Kanberg N., Simren J., Eden A., et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70:103512. doi: 10.1016/j.ebiom.2021.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troscher A.R., Wimmer I., Quemada-Garrido L., et al. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019;137:619–635. doi: 10.1007/s00401-019-01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weeks S.G., Silva C., Auer R.N., et al. Encephalopathy with staphylococcal endocarditis: multiple neuropathological findings. Can J Neurol Sci. 2001;28:260–264. doi: 10.1017/s0317167100001438. [DOI] [PubMed] [Google Scholar]
- 30.Schwabenland M., Salie H., Tanevski J., et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54:1594–1610. doi: 10.1016/j.immuni.2021.06.002. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein C., Kleinschmidt-DeMasters B.K., Liang X., et al. A review of neuropathological features of familial and adult hemophagocytic lymphohistiocytosis. J Neuropathol Exp Neurol. 2019;78:197–208. doi: 10.1093/jnen/nlz001. [DOI] [PubMed] [Google Scholar]
- 33.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabbri V.P., Foschini M.P., Lazzarotto T., et al. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 2021;31:205–210. doi: 10.1111/bpa.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78:760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fridman S., Bres Bullrich M., Jimenez-Ruiz A., et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.