Abstract
Perilla frutescens (L.) Britton, an important pharmaceutical and nutraceutical crop, is widely cultivated in East Asian countries. In this review, we present the latest research findings on the phytochemistry and pharmacological activities of P. frutescens. Different databases, including PubMed, Scopus, CNKI, Agricola, Scifinder, Embase, ScienceDirect, DOAJ, and Web of Science, were searched to present the best review. In this review, we clearly represent the active constituents responsible for each and every pharmacological activity, plausible mechanism of action, and maximum inhibitory concentrations, as well as IC50 values. Approximately 400 different bioactive compounds, including alkaloids, terpenoids, quinines, phenylpropanoids, polyphenolic compounds, flavonoids, coumarins, anthocyanins, carotenoids, neolignans, fatty acids, polycosanols, tocopherols, and sitosterols, have been reported in the leaves, seeds, roots, and aerial parts of P. frutescens. The bioactive constituents of P. frutescens exhibited different enzyme-inhibition properties, including antihyaluronidase effects and aldose reductase inhibitory, α-glucosidase inhibitory, xanthine oxidase inhibitory, and tyrosinase inhibitory properties. P. frutescens showed strong anti-inflammatory, antidepressant, anti-spasmodic, anticancer, antioxidant, antimicrobial, insecticidal, neuroprotective, and hepatoprotective effects. Hence, the active constituents of P. frutescens used in the treatment of diabetes and diabetic complications (retinopathy, neuropathy, and nephropathy), prevention of hyperuricemia in gout patients, hyper pigmentation, allergic conditions, skin inflammation, skin allergy, atopic dermatitis, periodontosis, androgenic alopecia, gastric inflammation, oesophagitis, carcinogenesis, cardiovascular, Alzheimer’s, Parkinson’s, and cerebral ischemic disorders. Furthermore, we revealed the most active constituents and possible mechanisms of the pharmacological properties of P. frutescens.
Keywords: Perilla frutescens, secondary metabolites, bioactive compounds, pharmacological activities
1. Introduction
Perilla frutescens (L.) Britton, belonging to the family Lamiaceae, is widely distributed in East Asian countries, such as Japan, China, Korea, and Vietnam. Since ancient times, it has been used in traditional Chinese medicine and is cultivated as an edible crop in mainland China, Japan, and Korea. The leaves of Perilla were used in preparing vegetable curries, pickles, and chutneys. Several studies have reported the presence of rich polyphenolic compounds, which exhibit high antioxidant capacity, in Perilla leaves. Green tea prepared with Perilla leaves is highly popular in East Asian countries, due to its high antioxidant capacity, and has been used to treat fish and crab poisoning in these countries. Perilla essential oil (PEO), extracted from P. frutescens leaves, is a complex mixture of volatile components, constituting approximately 150 to 200 different compounds, exhibiting high antioxidant, anticancer, anti-inflammatory, insecticidal, and antimicrobial activities; hence, it is mainly used in processed meat, bakery products, frozen foods, puddings, soups, and preservatives. Perilla oil, extracted from seeds, was regularly used as an edible oil in Mainland China. Modern medical research has revealed that different plant parts of P. frutescens possess enormous amounts of bioactive secondary metabolites, including terpenoids, flavonoids, alkaloids, steroids, quinines, and phenolic compounds, which exhibit a wide range of biological activities and have immense potential applications as pharmaceuticals, nutraceuticals, agrochemicals, biopesticides, flavours, fragrances, colours, and food additives. Different biological activities, including anti-allergic, anti-depressant, hypolipidemic, hepatoprotective, neuroprotective, anti-inflammatory, anticancer, antioxidant, and antimicrobial activities, reported in P. frutescens were attributed to the presence of bioactive secondary metabolites in the different plant parts. In this review, we aim to present up-to-date research on P. frutescens. The present review reveals practically all compounds/metabolites present in different plant parts of P. frutescens, and discusses different biological activities of P. frutescens, mechanisms of biological activities, and compounds involved in the biological activities of P. frutescens.
2. Phytoconstituents of P. frutescens
Different types of phytoconstituents, including alkaloids, phenylpropanoids, terpenoids (monoterpenes, diterpenes and triterpenoids), phenolic acids, flavonoids (flavones, flavonols, flavanones, isoflavanones, aurones and chalcones), anthocyanins, coumarins, carotenoids, neolignans, fatty acids, policosanols, tocopherols, sitosterols, glycosides, glucosides, peptides and benzoxipen derivatives, have been reported from the seeds, leaves, and aerial parts of P. frutescens (Table 1).
Table 1.
Different types of phytoconstituens reported from P. frutescens.
| S. No | Phytochemical Name |
Molecular Formula |
Plant Parts | Reference |
|---|---|---|---|---|
| Monoterpenes | ||||
| Acyclic type | ||||
| 1. | β-Myrcene | C10H16 | Leaves | [1,2] |
| 2. | Geraniol | C10H18O | Leaves | [3,4] |
| 3. | β-Citronellene | C10H18 | Leaves | [3] |
| 4. | Nerol | C10H18O | Leaves | [2] |
| 5. | Linalool | C10H18O | Leaves, Aerial parts | [4,5] |
| 6. | Ocimene | C10H16 | Leaves | [3] |
| Monocyclic type | ||||
| 7. | α-Terpineol | C10H18O | Leaves, Aerial parts | [3,5] |
| 8. | β-Terpineol | C10H18O | Aerial parts | [6] |
| 9. | Thymol | C10H14O | Leaves, Aerial parts | [1,5] |
| 10. | α-Phellandrene | C10H16 | Leaves | [1] |
| 11. | β-Phellandrene | C10H16 | Leaves, Aerial parts | [3,6] |
| 12. | 1,8-Cineole | C10H18O | Leaves | [1,2] |
| 13. | Damascenone | C13H18O | Leaves | [4] |
| 14. | Terpinen-4-ol | C10H18O | Leaves | [3] |
| 15. | Terpinene | C10H16 | Leaves | [3] |
| 16. | Carvacrol | C10H14O | Leaves, Aerial parts | [1,5] |
| 17. | Carvone | C10H14O | Leaves, Aerial parts | [4,7] |
| 18. | Piperitone | C10H16O | Leaves | [4] |
| 19. | Piperitenone | C10H14O | Leaves | [3,4] |
| 20. | Limonene | C10H16 | Leaves, Aerial parts | [1,2] |
| 21. | Menthone | C10H18O | Leaves | [3] |
| 22. | Carveole | C10H16O | Leaves | [4] |
| 23. | Pulegone | C10H16O | Leaves | [2] |
| Bicyclic type | ||||
| 24. | δ -2-Carene | C10H16 | Leaves | [4] |
| 25. | Camphane | C10H18 | Leaves | [3] |
| 26. | Verbenol | C10H16O | Leaves | [3] |
| 27. | Sabinene | C10H16 | Leaves | [2,3] |
| Furanoid type | ||||
| 28. | Frutescenone A | C10H10O4 | Aerial parts | [8] |
| 29. | Frutescenone B | C10H14O3 | Aerial parts | [8] |
| 30. | Frutescenone C | C15H19N5O2 | Aerial parts | [8] |
| 31. | Isoegomaketone | C10H12O2 | Aerial parts | [8,9] |
| 32. | 9-hydroxyisoegoma ketone | C10H12O3 | Aerial parts | [8,9] |
| 33. | (3S,4R)-3-hydroxy perillaldehyde | C10H14O | Aerial parts | [8] |
| 34. | Perillic acid | C10H14O2 | Aerial parts | [8] |
| Sesquiterpenes | ||||
| Acyclic type | ||||
| 35. | α-Farnesene | C15H24 | Leaves, Aerial parts | [2,3,4,5] |
| 36. | β -Farnesene | C15H24 | Leaves, Aerial parts | [3,10] |
| 37. | Farnesol | C15H26O | Leaves | [3] |
| 38. | Nerolidol | C15H26O | Leaves, Aerial parts | [2,5] |
| Monocyclic type | ||||
| 39. | α-Humulene | C15H24O | Leaves, Aerial parts | [2,5] |
| 40. | Bisabolene | C15H24 | Leaves | [3] |
| 41. | Germacrene | C15H24 | Leaves | [1] |
| 42. | Elemene | C15H24 | Leaves | [1] |
| 43. | β-Ionone | C13H20O | Leaves, Aerial parts | [1,5] |
| Bicyclic type | ||||
| 44. | α-pinene | C10H16 | Leaves, Aerial parts | [1,5] |
| 45. | β-pinene | C10H16 | Leaves, Aerial parts | [1] |
| 46. | β-Caryophyllene | C15H24 | Leaves, Aerial parts | [5,11] |
| 47. | ε-Muurolene | C15H24 | Leaves | [3] |
| 48. | α-Cadinene | C15H24 | Leaves | [2] |
| 49. | β-Cadinene | C15H24 | Leaves | [3] |
| 50. | α-Santalol | C15H24O | Leaves | [3] |
| 51. | α-Bulnesene | C15H24 | Leaves | [3] |
| 52. | β-Gurjunene | C15H24 | Leaves | [3] |
| 53. | β-Selinene | C15H24 | Leaves | [3] |
| 54. | α-Fenchene | C10H16 | Leaves | [3] |
| 55. | α-Cadinol | C15H26O | Leaves | [12] |
| 56. | Eremophilene | C15H24 | Leaves | [3] |
| 57. | Calarene | C15H24 | Leaves | [3] |
| 58. | Valencene | C15H24 | Aerial parts | [10] |
| Tricyclic type | ||||
| 59. | Spathulenol | C15H24O | Leaves, Aerial parts | [1,13] |
| 60. | Viridiflorene | C15H24 | Leaves | [3] |
| 61. | Cubebene | C15H24 | Leaves | [2] |
| 62. | Alloaromadendrene | C15H24 | Leaves | [3] |
| 63. | Patchoulane | C15H26 | Leaves | [3] |
| 64. | α-Copaene | C15H24 | Leaves, Aerial parts | [3,10] |
| 65. | Longifolen | C15H24 | Leaves | [3] |
| 66. | Ylangene | C15H24 | Leaves, Aerial parts | [4] |
| Diterpenoids | ||||
| 67. | Phytol | C20H40O | Leaves, Aerial parts |
[1,5,11] |
| Triterpenoids | ||||
| 68. | Ursolic acid | C30H48O3 | Leaves | [14,15] |
| 69. | Corosolic acid | C30H48O4 | Leaves | [15,16] |
| 70. | 3-epicorosolic acid | C30H48O4 | Leaves | [15] |
| 71. | Pomolic acid | C30H48O4 | Leaves | [15] |
| 72. | Tormentic acid | C30H48O5 | Leaves | [15,16] |
| 73. | Hyptadienic acid | C30H46O4 | Leaves | [15] |
| 74. | Oleanolic acid | C30H48O3 | Leaves | [15] |
| 75. | Augustic acid | C30H48O4 | Leaves | [15] |
| 76. | 3-epimaslinic acid | C30H48O4 | Leaves | [15] |
| 77. | Sericoside | C36H58O11 | Leaves | [17] |
| Phenyl propanoids | ||||
| 78. | Elemicin | C12H16O3 | Aerial parts Leaves |
[8,18] |
| 79. | Isoelemicin | C12H16O3 | Aerial parts | [8] |
| 80. | Myristicin | C11H12O3 | Aerial parts Leaves |
[8,18] |
| 81. | Eugenol | C10H12O2 | Leaves Aerial parts |
[1,2] |
| 82. | Isoeugenol | C10H12O2 | Leaves | [3,4] |
| 83. | Perilloside E | C17H22O9 | Leaves | [19] |
| 84. | Dillapiole | C12H14O4 | Leaves | [18] |
| 85. | Nothoapiole | C13H16O5 | Leaves | [18] |
| 86. | Allyltetramethoxy benzene | Leaves | [18] | |
| 87. | α-Asarone | C12H16O3 | Leaves, Aerial Parts | [3,10] |
| 88. | Estragole | C10H12O | Leaves | [1] |
| Alkaloids | ||||
| 89. | Neoechinulin A | C19H21N3O2 | Aerial parts | [8] |
| 90. | 1H-indole-3-carboxylic acid |
C9H7NO2 | Aerial parts | [8] |
| 91. | Indole-3-carboxaldehyde | C9H7NO | Aerial parts | [8] |
| Phenolic compounds | ||||
| 92. | Rosmarinic acid | C18H16O8 | Leaves, Fruits Seeds |
[20,21,22,23] |
| 93. | Methyl rosmarinic acid | C19H18O8 | Leaves Seeds |
[24,25] |
| 94. | Rosmarinic acid-3-O-glucoside | C24H26O13 | Seeds | [22,23,24] |
| 95. | 3′-dehydroxyl-rosmar inicacid-3-O-glucoside | C24H25O12 | Seeds | [24] |
| 96. | Caffeic acid | C9H8O4 | Leaves, Seeds |
[22,24,26] |
| 97. | Ethyl caffeate | C11H12O4 | Aerial parts (leaves and stems) | [8] |
| 98. | Methyl caffeate | C10H10O4 | Aerial parts (leaves and stems) | [27] |
| 99. | Vinyl caffeate | C11H10O4 | Aerial parts (leaves and stems) | [27] |
| 100. | Trans-p-menthenyl caffeate | leaves | [27] | |
| 101. | Caffeic acid-3-O-glucoside | C15H18O9 | Seeds | [23,24] |
| 102. | Protocatechuic acid | C7H6O4 | Leaves | [25,28,29] |
| 103. | Protocatechuic aldehyde | C7H6O3 | Aerial parts (leaves and stems) | [27] |
| 104. | Chlorogenic acid | C16H18O9 | Leaves | [25,29] |
| 105. | Vanillic acid | C8H8O4 | Seeds | [24] |
| 106. | Isovanillic acid | C8H8O4 | Leaves | [29] |
| 107. | Sinapic acid | C11H12O5 | Leaves | [29] |
| 108. | Gallic acid | C7H6O5 | Leaves | [29,30] |
| 109. | Ferulic acid | C10H10O4 | Leaves Seeds |
[29,31] |
| 110. | 4-coumaric acid | C9H8O3 | Leaves | [32] |
| 111. | Coumaroyl tartaric acid | C13H12O8 | Leaves | [30] |
| 112. | 4-hydroxyphenyl lactic acid | C9H10O4 | Leaves | [33] |
| 113 | Sagerinic acid | C36H32O16 | Leaves | [16] |
| 114. | Cimidahurinine | C14H20O8 | Seeds | [24] |
| 115. | p-Hydroxybenzoic acid | C7H6O3 | Leaves | [29] |
| 116. | 3,4-DHPEA (Hydroxy tyrosol) | C8H10O6S | Leaves | [30] |
| Flavonoids | ||||
| Flavones | ||||
| 117. | Luteolin | C15H10O6 | Leaves seeds, fruits |
[21,22,23] |
| 118. | Luteolin-7-O-glucuronide | C21H18O12 | Leaves | [34] |
| 119. | Luteolin-7-O-diglucuronide | C27H26O18 | Leaves | [32,34] |
| 120. | Luteolin 7-O- glucuronide -6”-methyl ester |
C22H20O12 | Leaves | [35] |
| 121. | Luteolin-5-O-glucoside | C21H20O11 | Seeds | [24] |
| 122. | Luteolin-7-O-glucoside | C21H20O11 | Leaves, Seeds | [36,37] |
| 123. | Apigenin | C15H10O5 | Leaves, seeds, fruits | [21,22,23,28] |
| 124. | Apigenin-7-O-glucuronide | C21H18O11 | Leaves | [32] |
| 125. | Apigenin-7-O-diglucuronide | C27H26O17 | Leaves | [32,34] |
| 126. | Apigenin-7-O-glucoside | C21H20O10 | Seeds | [37] |
| 127. | Apigenin 7-O-caffeoylglucoside | C30H26O13 | Leaves | [34] |
| 128. | Diosmetin | C16H12O6 | Seeds | [38] |
| 129. | Chrysoeriol | C16H12O6 | Fruits, seeds | [21,22] |
| 130. | Scutellarin | C21H18O12 | Leaves | [17] |
| 131. | Scutellarein | C15H10O6 | Leaves | [17] |
| 132. | Scutellarein -7-O-glucuronide | C21H18O12 | Leaves | [32,34,39] |
| 133. | Scutellarein 7-O-diglucuronide | C27H26O18 | Leaves | [32,34,39] |
| 134. | Negletein | C16H12O5 | Leaves | [17,28] |
| 135. | Vicenin-2 | C27H30O15 | Leaves | [40] |
| 136. | Catechin | C15H14O6 | Leaves Seeds |
[31] |
| Flavanones | ||||
| 137. | Shisoflavanone A | C17H16O5 | Leaves | [28] |
| 138. | Liquiritigenin | Leaves | [16] | |
| 139. | 5,8-dihydroxy-7-methoxyflavanone | C16H14O5 | Leaves | [28] |
| 140. | (2S)-5,7-dimethoxy-8,4′-dihydroxy flavanone | C17H16O6 | Leaves | [41] |
| 141. | 8-hydroxy-5,7-dimethoxyflavanone | C17H16O5 | Leaves | [42] |
| Chalcones | ||||
| 142. | 2′,4′-dimethoxy-4,5′,6′-trihydroxychalcone | C17H16O6 | Leaves | [41] |
| 143. | 2′,3′-dihydroxy-4′,6′-dimethoxychalcone | C17H16O5 | Leaves | [43] |
| Aurones | ||||
| 144. | (Z)-4,6-dimethoxy-7,4′-dihydroxyaurone | C17H14O6 | Leaves | [41] |
| Anthocyanins | ||||
| 145. | Shisonin (Perillanin) (cyanidin 3-coumaroyl-glucoside- 5-glucoside) |
C36H37O18+ | Leaves | [39,44] |
| 146. | Cis-Shisonin | [Cl-]C36H37O17[O+] | Leaves | [44] |
| 147. | Cis-malonyl shisonin | C39H39O21+ | Leaves | [44] |
| 148. | Cyanidin 3-O-feruloyl glucoside-5-O-glucoside | C43H49O24 | Leaves | [44] |
| 149. | Cyanidin 3-O-caffeoyl glucoside-5-Oglucoside | C36H37O19+ | Leaves | [44] |
| 150. | Cyanidin 3-O-caffeoyl glucoside-5-O-malonyl glucoside | C30H27O14+ | Leaves | [44] |
| 151. | Peonidin 3-O-malonyl glucoside-5-O-p-coumarylglucoside | C38H41O17+ | Leaves | [45] |
| Coumarins | ||||
| 152. | Esculetin | C9H6O4 | Leaves | [17,28] |
| 153. | 6,7-dihydroxycoumarin | C9H6O4 | Leaves and stems | [27] |
| Carotenoids | ||||
| 154. | Loliolide | C11H16O3 | Leaves | [17] |
| 155. | Isololiolide | C11H16O3 | Leaves | [17] |
| Neolignans | ||||
| 156. | Magnosalin | C24H32O6 | Leaves | [46] |
| 157. | Andamanicin | C24H36O6 | Leaves | [46] |
| Glucosides | ||||
| 158. | Perillanolide A | C16H26O7 | Leaves | [17] |
| 159. | Perillanolide B | C16H26O7 | Leaves | [17] |
| 160. | Perilloside A | C16H26O6 | Leaves | [19] |
| 161. | Perilloside B | C16H24O7 | Leaves | [19] |
| 162. | Perilloside C | C16H28O6 | Leaves | [19] |
| 163. | Perilloside E | C17H22O9 | Leaves | [19] |
| 164. | Loganin(Iridoid glucoside) | C17H26O10 | Leaves | [16] |
| 165. | 5’-β-d-glucopyranosyl oxyjasrnonic acid; | C18H28O9 | Leaves | [16,19] |
| 166. | 3-β-d-glucopyrano syl-3-epi-2-isocucur bic acid | C18H30O8 | Leaves | [19] |
| 167. | 3-β-d-glucopyranosyl oxy-5-phenylvaleric acid | C17H24O8 | Leaves | [19] |
| 168. | N-octanoyl-β-d-fructofuranosyl-α-d-glucopyranoside | C20H36O12 | Leaves | [16] |
| 169. | Eugenyl-β-d-glucoside | C16H22O7 | Leaves | [19] |
| 170. | Benzyl-β-d-glucoside | C13H18O6 | Leaves | [19] |
| 171. | β-sitosteryl β-d-glucoside | C35H60O6 | Leaves | [19] |
| 172. | Prunasin | C14H17NO6 | Leaves | [19] |
| 173. | Sambunigrin | C14H17NO6 | Leaves | [19] |
| Benzoxepin derivatives | ||||
| 174. | Perilloxin | C16H18O4 | Stems | [47] |
| 175. | Dehydroperilloxin | C16H16O4 | Stems | [47] |
| Policosanols | ||||
| 176. | Eicosnaol | C20H42O | Seeds | [48,49,50] |
| 177. | Heneicosanol | C21H44O | Seeds | |
| 178. | Docosanol | C22H46O | Seeds | |
| 179. | Tricosanol | C23H48O | Seeds | |
| 180. | Tetracosanol | C24H50O | Seeds | |
| 181. | Pentacosanol | C25H52O | Seeds | |
| 182. | Hexacosanol | C26H54O | Seeds | |
| 183. | Heptacosanol | C27H56O | Seeds | |
| 184. | Octacosanol | C28H58O | Seeds | |
| 185. | Nonacosanol | C29H60O | Seeds | |
| 186. | Triacontanol | C30H62O | Seeds | |
| Phytosterols | ||||
| 187. | Stigmasterol | C29H48O | Seeds | [50,51] |
| 188. | β-sitosterol | C31H52O2 | Seeds | [50,51] |
| 189. | Campesterol | C28H48O | Seeds | [50] |
| 190. | β-amyrin | C30H50O | Seeds | [50] |
| 191. | β-cholestanol | C27H48O | Seeds | [50] |
| 192. | 5α-cholestane | C27H48 | Seeds | [50,52] |
| Tocopherols | ||||
| 193. | δ-tocopherol | C27H46O2 | Seeds | [50,51] |
| 194. | γ-tocopherol | C28H48O2 | Seeds | |
| 195. | β-tocopherol | C28H48O2 | Seeds | |
| 196. | α-tocopherol | C29H50O2 | Seeds | |
| Fatty acids | ||||
| 197. | Lauric acid | C12H24O2 | Seeds | [53] |
| 198. | Myristic acid | C14H28O2 | Seeds | [53] |
| 199. | Pentadecanoic acid | C15H30O2 | Seeds | [50] |
| 200. | Palmitic acid | C16H32O2 | Seeds | [50] |
| 201. | Palmitoleic acid | C16H30O2 | Seeds | [53] |
| 202. | Heptadecanoic acid | C17H34O2 | Seeds | [53] |
| 203. | Stearic acid | C18H36O2 | Seeds | [50] |
| 204. | Oleic acid | C18H34O2 | Seeds | [50] |
| 205. | Linoleic acid | C18H32O2 | Seeds | [50] |
| 206. | Linolenic acid | C18H30O2 | Seeds | [50] |
| 207. | Arachidic acid | C20H40O2 | Seeds | [50] |
| 208. | Eicosenoic acid | C20H38O2 | Seeds | [50] |
| 209. | Eicosadienoic acid | C20H36O2 | Seeds | [53] |
| 210. | Eicosatrienoic acid | C20H34O2 | Seeds | [53] |
| 211. | Behenic acid | C22H44O2 | Seeds | [53] |
| Other important compounds | ||||
| 212. | p-Hydroxybenzaldehyde | C7H6O2 | Leaves | [17] |
| 213. | p-Hydroxyacetophenone | C8H8O2 | Leaves | [17] |
| 214. | trans-p-Hydroxycinnamic acid | C9H8O3 | Leaves | [17] |
| 215. | 3′,4′,5′-trimethoxycinnamyl alcohol | C12H16O4 | Aerial parts | [8] |
2.1. Alkaloids, Phenylpropanoids, and Terpenoids
Wang et al. [8] reported that the aerial parts of P. frutescens contain an important alkaloid, neoechinulin A, which inhibits mitogen-activated protein kinase (MAPK) phosphorylation. Other alkaloids present in the aerial parts include 1H-indole-3-carboxylic acid and indole-3-carboxaldehyde. Phenylpropanoids, organic compounds mainly present in plants, are biosynthesised from phenylalanine or tyrosine, via the shikimate pathway. Many phenylpropanoids, also, act as precursors or intermediate materials for the biosynthesis of flavonoids, coumarins, and lignins. The leaves and other aerial parts of P. frutescens contain various important phenylpropanoids, such as elemicin, isoelemicin, myristicin, and methylisoeugenol. Elemicin and myristicin are widely accepted psychoactive compounds. Isoelemicin and methylisoeugenol exhibit antiplasmodial effects against Plasmodium falciparum strains. The leaves, also, contain antidepressant and antioxidant phenylpropanoids, including dillapiole, nothoapiole, perilloside E (phenylpropanoid glucoside), and allyltetramethoxybenzene. Phenylpropanoids, such as elemicin, isoelemicin, dillapiole, nothoapiole, and allyltetramethoxybenzene, have also been found to effectively inhibit proinflammatory cytokines during lung inflammation. Various terpenoids in the leaves and other aerial parts of P. frutescens include monoterpenoids, sesquiterpenoids, and triterpenoids. Monoterpenoids of P. frutescens are of the perilla-ketone and perillaldehyde type. Perilla-ketone-type monoterpenoids include frutescenone A, frutescenone B, frutescenone C, isoegomaketone, and 9-hydroxyisoegomaketone [54,55] which exhibit anti-inflammatory activity, by inhibiting the expression of proinflammatory cytokines. Perillaldehyde-type monoterpenoids include 3-hydroxyperillaldehyde and perillic acid [56]. Furthermore, leaves and other aerial parts of P. frutescens, also, contain acyclic, monocyclic, and bicyclic monoterpenoids. Acyclic monoterpenoids include β-myrcene, geraniol, β-citronellene, nerol, linalool, and ocimene. Monocyclic monoterpenoids include terpineol, thymol, phellandrene, 1,8-Cineole, damascenone, terpinene, carvacrol, carvone, piperitone, piperitenone, limonene, menthone, carveole, and pulegone [1,5,7]. Bicyclic monoterpenoids present in the leaves include δ -2-carene, camphene, verbenol, and sabinene. P. frutescens leaves are rich in sesquiterpenoids, which include acyclic, monocyclic, bicyclic, and tricyclic types. Acyclic sesquiterpenoids include farnesene, farnesol, and nerolidol. Monocyclic sesquiterpenoids include α-humulene, bisabolene, germacrene, elemene, and β-ionone [22]. The leaves are, particularly, rich in bicyclic and tricyclic sesquiterpenoids. Bicyclic sesquiterpenoids include α-pinene, β-pinene, β-caryophyllene, ε-muurolene, α-cadinene, β-cadinene, α-santalol, α-bulnesene, β-gurjunene, β-selinene, α-fenchene, α-cadinol, eremophilene, calarene, and valencene. Tricyclic sesquiterpenoids include spathulenol, viridiflorene, cubebene, alloaromadendrene, patchoulane, α-copaene, longifolen, and ylangene. To date, only one diterpenoid phytol has been reported from the leaves and aerial parts of P. frutescens. There are about 200 different types of volatile components that have been reported from the leaves and other aerial parts of P. frutescens (Table 2). The leaves of P. frutescens, also, constitute pentacyclic triterpenoids, including ursolic acid, corosolic acid, 3-epicorosolic acid, pomolic acid, tormentic acid, hyptadienic acid, oleanolic acid, augustic acid, and 3-epimaslinic acid [14], which exhibit cytotoxicity against various cancers, including leukaemia, breast, and hepatic carcinomas.
Table 2.
Different volatile components present in the leaves and aerial parts of P. frutescens.
| S. No. | Component | Mol. Formula | Parts | Reference |
|---|---|---|---|---|
| 1. | α-Farnesene | C15H24 | Leaves, Aerial parts | [3,5,11] |
| 2. | β -Farnesene | C15H24 | Leaves, Aerial parts | [3,10] |
| 3. | α-Caryophyllene | C15H24 | Leaves, Aerial parts | [3,7] |
| 4. | β-Caryophyllene | C15H24 | Leaves, Aerial parts | [5,11] |
| 5. | Isocaryophyllene | C15H24 | Leaves, Aerial parts | [3,10] |
| 6. | Caryophyllene oxide | C15H24O | Leaves, Aerial parts | [1,3,5] |
| 7. | Phytol | C20H40O | Leaves, Aerial parts | [1,5,11] |
| 8. | Alloaromadendrene | C15H24 | Leaves | [3] |
| 9. | Thymoquinone | C10H12O2 | Leaves | [11,57] |
| 10. | Bergamotene | C16H24 | Leaves, Aerial parts | [4,11] |
| 11. | Diisooctyl adipate | C22H42O4 | Leaves | [11] |
| 12. | α-pinene | C10H16 | Leaves, Aerial parts | [1,5] |
| 13. | β-pinene | C10H16 | Leaves, Aerial parts | [1,5] |
| 14. | Eugenol | C10H12O2 | Leaves, Aerial parts | [1,2,7] |
| 15. | Isoeugenol | C10H12O2 | Leaves | [3,4] |
| 16. | Methyl eugenol | C11H14O2 | Leaves | [3] |
| 17. | Methyl isoeugenol | C11H14O2 | Leaves, Aerial parts | [3,8] |
| 18. | Spathulenol | C15H24O | Leaves, Aerial parts | [1,5,13] |
| 19. | Viridiflorene | C15H24 | Leaves | [3] |
| 20. | Viridiflorol | C15H26O | Leaves | [3] |
| 21. | ε-Muurolene | C15H24 | Leaves | [3] |
| 22. | γ-Terpinene | C10H16 | Leaves | [2] |
| 23. | β-Terpinene | C10H16 | Leaves | [3] |
| 24. | Terpinen-4-ol | C10H18O | Leaves | [3] |
| 25. | Nerolidol | C15H26O | Leaves, Aerial parts | [2,5] |
| 26. | α-Cadinene | C15H24 | Leaves | [2] |
| 27. | β-Cadinene | C15H24 | Leaves | [3] |
| 28. | δ-Cadinene | C15H24 | Leaves, Aerial parts | [3,5] |
| 29. | α-Asarone | C12H16O3 | Leaves, Aerial parts | [3,10] |
| 30. | Linalool | C10H18O | Leaves, Aerial parts | [4,5] |
| 31. | Linalool propanoate | C14H24O2 | Leaves | [1] |
| 32. | Linalool formate | C11H18O2 | Leaves | [1] |
| 33. | Linalool oxide | C10H18O2 | Leaves | [58] |
| 34. | Carvacrol | C10H14O | Leaves, Aerial parts | [1,5] |
| 35. | α-Santalol | C15H24O | Leaves | [3] |
| 36. | α-Bulnesene | C15H24 | Leaves | [3] |
| 37. | β-Gurjunene | C15H24 | Leaves | [3] |
| 38. | β-Selinene | C15H24 | Leaves | [3] |
| 39. | Germacrene A | C15H24 | Leaves | [1] |
| 40. | Germacrene B | C15H24 | Leaves | [1] |
| 41. | Germacrene D | C15H24 | Leaves, Aerial parts | [7,12] |
| 42. | Bicyclogermacrene | C15H24 | Leaves | [2] |
| 43. | Estragole | C10H12O | Leaves | [1] |
| 44. | α-Cubebene | C15H24 | Leaves | [2] |
| 45. | β-Cubebene | C15H24 | Leaves | [2] |
| 46. | Carvone | C10H14O | Leaves, Aerial parts | [4,7] |
| 47. | Shisofuran | C10H12O | Leaves | [1,4] |
| 48. | Piperitone | C10H16O | Leaves | [4] |
| 49. | Piperitenone | C10H14O | Leaves | [3,4] |
| 50. | Farnesol | C15H26O | Leaves | [3] |
| 51. | Phytone | C18H36O | Leaves | [3] |
| 52. | α-citral | C10H16O | Leaves | [3] |
| 53. | β-citral | C10H16O | Leaves | [1] |
| 54. | Ocimene | C10H16 | Leaves | [3] |
| 55. | Cosmene | C10H14 | Leaves | [3] |
| 56. | γ-Pyronene | C10H16 | Leaves | [3] |
| 57. | Perillene | C10H14O | Leaves, Aerial parts | [3,13] |
| 58. | Perillaldehyde | C10H14O | Leaves, Aerial parts | [1,3,5] |
| 59. | Perilla ketone | C10H14O2 | Leaves, Aerial parts | [1,13] |
| 60. | Egoma ketone | C10H12O2 | Leaves, Aerial parts | [5,12] |
| 61. | Isoegomaketone | C10H12O2 | Leaves, Aerial parts | [12,13] |
| 62. | Perilla alcohol | C10H16O | Leaves, Aerial parts | [1,2,5] |
| 63. | Perillic acid | C10H14O2 | Aerial parts | [5] |
| 64. | Methy perillate | C11H16O2 | Aerial parts | [5] |
| 65. | Elscholtzia ketone | C10H14O2 | Leaves, Aerial parts | [1,13] |
| 66. | dehydro-elsholtzia ketone | C10H12O2 | Leaves, Aerial parts | [1] |
| 67. | Naginata ketone | Leaves | [3] | |
| 68. | α-Terpineol | C10H18O | Leaves, Aerial parts | [3,5] |
| 69. | Elemicin | C12H16O3 | Leaves, Aerial parts | [3,10] |
| 70. | Isoelemicin | C12H16O3 | Leaves, Aerial parts | [3,8] |
| 71. | Myristicin | C11H12O3 | Leaves, Aerial parts | [10] |
| 72. | Dillapiol | C12H14O4 | Leaves | [18] |
| 73. | Nothoapiol | C13H16O5 | Leaves | [18] |
| 74. | Patchoulane | C15H26 | Leaves | [3] |
| 75. | α-Patchoulene | C15H24 | Leaves | [3] |
| 76. | o-Cymene | C10H14 | Aerial parts | [7] |
| 77. | p-Cymene | C10H14 | Aerial parts | [5] |
| 78. | Pulegone | C10H16O | Leaves | [2] |
| 79. | Isopulegone | C10H16O | Leaves | [3] |
| 80. | β-Bourbonene | C15H24 | Leaves | [3] |
| 81. | α-Humulene | C15H24 | Leaves, Aerial parts | [2,5] |
| 82. | Humulene epoxide II | C15H24O | Leaves, Aerial parts | [3,5] |
| 83. | α-Bisabolene epoxide | C15H24O | Leaves | [3] |
| 84. | Isoaromadendrene epoxide | C15H24O | Aerial parts | [6] |
| 85. | Sabinene | C10H16 | Leaves | [2,3] |
| 86. | Styrene | C8H8 | Leaves | [3] |
| 87. | Limonene | C10H16 | Leaves, Aerial parts | [2,10] |
| 88. | Limonene oxide | C10H16O | Leaves | [2] |
| 89. | Limonene aldehyde | C11H18O | Leaves | [1] |
| 90. | Isolimonene | C10H16O | Leaves | [3] |
| 91. | Pseudolimonene | C10H16 | Leaves, Aerial parts | [3,10] |
| 92. | α-Copaene | C16H26 | Leaves, Aerial parts | [3,10] |
| 93. | α-Fenchene | C10H16 | Leaves | [3] |
| 94. | Anisole | C7H8O | stems | [59] |
| 95. | Eucalyptol | C10H18O | Leaves, Aerial parts | [3,6] |
| 96. | β-Myrcene | C10H16 | Leaves | [1,2] |
| 97. | Geranyl acetone | C13H22O | Leaves | [1,2] |
| 98. | Hexahydrofarnesyl acetone | C18H36O | Leaves, Aerial parts | [5,13] |
| 99. | Methyl geranate | C11H18O2 | Leaves | [1,2] |
| 100. | Camphene | C10H16 | Leaves | [1,2] |
| 101. | Longifolen | C15H24 | Leaves | [3] |
| 102. | 1,8-Cineole | C10H18O | Leaves | [1,2] |
| 103. | Damascenone | C13H18O | Leaves | [4] |
| 104. | α-Cadinol | C15H26O | Leaves | [12] |
| 105. | Tau-Cadinol | C15H26O | Leaves | [1] |
| 106. | α-Terpinolene | C10H16 | Leaves | [1,2] |
| 107 | Menthol | C10H20O | Leaves | [3] |
| 108. | Menthone | C10H18O | Leaves | [3] |
| 109. | Isomenthone | C10H18O | Leaves | [3] |
| 110. | Eremophilene | C15H24 | Leaves | [3] |
| 111. | Carveole | C10H16O | Leaves | [4] |
| 112. | Dihydrocarveol | C10H18O | Leaves, Aerial parts | [3,5] |
| 113. | Dihydrocarveol acetate | C12H20O2 | Leaves | [1,3] |
| 114. | Isodihydrocarveol acetate | C12H20O2 | Leaves | [58] |
| 115. | Geraniol | C10H18O | Leaves | [3,4] |
| 116. | α-Terpineol | C10H18O | Leaves, Aerial parts | [3,5] |
| 117. | β-Terpineol | C10H18O | Aerial parts | [6] |
| 118. | β-Elemene | C15H24 | Leaves | [1,2] |
| 119. | δ -Elemene | C15H24 | Leaves | [2,3] |
| 120. | β-Citronellene | C10H18 | Leaves | [3] |
| 121. | δ -2-Carene | C10H16 | Leaves | [4] |
| 122. | Calarene | C15H24 | Leaves | [3] |
| 123. | Camphane | C10H18 | Leaves | [3] |
| 124. | Ylangene | C15H24 | Leaves, Aerial parts | [4] |
| 125. | Nerol | C10H18O | Leaves | [2] |
| 126. | Cadina 3,9-diene | C15H24 | Aerial parts | [7] |
| 127. | Neophytadiene | C20H38 | Leaves | [12] |
| 128. | β-Ionone | C13H20O | Leaves, Aerial parts | [1,5] |
| 129. | α -Fenchene | C10H16 | Leaves | [3] |
| 130. | Thymol | C10H14O | Leaves, Aerial parts | [1,5] |
| 131. | α-Phellandrene | C10H16 | Leaves | [1] |
| 132. | β-Phellandrene | C10H16 | Leaves, Aerial parts | [3,6] |
| 133. | Santolina triene | C10H16 | Leaves | [3] |
| 134. | Verbenol | C10H16O | Leaves | [3] |
| 135. | trans-Shisool | C10H18O | Leaves, Aerial parts | [1,6] |
| 136. | Thujyl alcohol | C10H18O | Leaves | [3] |
| 137. | Furfuryl alcohol | C5H6O2 | Leaves | [3] |
| 138. | 2-Hexanoylfuran | C10H14O2 | Leaves, Aerial parts | [10,11] |
| 139. | 2-acetylfuran | C6H6O2 | Leaves | [3] |
| 140. | β-terpinyl acetate | C12H20O2 | Aerial parts | [7] |
| 141. | trans-Valerenyl acetate | C17H26O2 | Leaves | [11] |
| 142. | Isomenthyl acetate | C12H22O2 | Leaves | [1] |
| 143. | Isobornyl acetate | C12H20O2 | Aerial parts | [6] |
| 144. | Bornyl acetate | C12H20O2 | Leaves | [58] |
| 145. | Nerol acetate | C12H20O2 | Leaves | [3] |
| 146. | 2-furyl methyl ketone | C6H6O2 | Aerial parts | [7] |
| 147. | Valencene | C15H24 | Aerial parts | [10] |
| 148. | laurolene | C8H14 | Leaves | [3] |
| 149. | α-curcumene | C15H22 | Stems | [59] |
| 150. | Elixene | C15H24 | Leaves | [59] |
| 151. | Curlone | C15H22O | Stems | [59] |
| 152. | Isopiperitenol | C10H16O | Leaves | [60] |
| 153. | Isopiperitenone | C10H14O | Leaves | [60] |
| 154. | Neral | C10H16O | Leaves | [60] |
| 155. | Geranial | C10H16O | Leaves | [60] |
| 156. | Geraniol | C10H18O | Leaves | [60] |
| 157. | α-naginatene | C10H14O | Leaves | [60] |
| 158. | β -cyclocitral | C10H16O | Leaves | [58] |
| 159. | Pthalic acid | C8H6O4 | Stems | [59] |
| 160. | 2-Butylamine | C4H11N | Leaves | [3] |
| 161. | 2-Pyrimidinamine | C4H5N3 | Aerial parts | [10] |
| 162. | 2-Hydroxypyridine | C5H5NO | Leaves | [3] |
| 163. | Phenylacetaldehyde | C8H8O | Leaves | [12] |
| 164. | p-Mentha-3,8-diene | C10H16 | Leaves | [3] |
| 165. | Methyl thymyl ether | C11H16O | Seeds | [59] |
| 166. | p-mentha-2,4(8)-diene | C10H16 | Leaves, Aerial parts | [4,7] |
| 167. | p-Menth-2-en-1-ol | C10H18O | Leaves | [2] |
| 168. | p-Menth-1-en-8-ol | C10H18O | Leaves, Aerial parts | [2,4,7] |
| 169. | p-Mentha-1,8-dien-7-ol | C10H16O | Leaves, Aerial parts | [2,7] |
| 170. | p-Menth-4(8)-en-3-one | C10H16O | Leaves | [2] |
| 171. | 2-Butanone | C4H8O | Leaves | [2] |
| 172. | 1-Pentene-3-one | C5H8O | Leaves | [12] |
| 173. | 3-Pentanone | C5H10O | Leaves | [12] |
| 174. | 2-Cyclopentenone | C5H6O | Leaves | [3] |
| 175. | 4,4-Dimethyl-2-cyclopenten-1-one | C7H10O | Leaves | [3] |
| 176. | 2-Methylcyclopentanone | C6H10O | Leaves | [3] |
| 177. | 2-Methyl-2-cyclopentenone | Leaves | [3] | |
| 178. | Cyclohexanone | C6H8O | Leaves | [3] |
| 179. | Methyl heptenone | C8H14O | Leaves | [3] |
| 180. | 1-octen-3-one | C8H14O | Leaves | [12] |
| 181. | 1-Pentene-3-ol | C5H10O | Leaves | [12] |
| 182. | 2-Pentenol | C5H10O | Leaves | [12] |
| 183. | 2-Hexenol | C6H12O | Leaves | [12] |
| 184. | 3-Hexenol | C6H12O | Leaves | [12] |
| 185. | 1-Hexanol | C6H14O | Leaves | [12] |
| 186. | 1-Octen-3-ol | C8H16O | Leaves, Aerial parts | [3,4,13] |
| 187. | 3-Octanol | C8H18O | Leaves, Aerial parts | [4,10] |
| 188. | Octadienol | C8H14O | Leaves | [2] |
| 189. | Benzaldehyde | C7H6O | Leaves, Aerial parts | [2,5,13] |
| 190. | 3-Pentenal | C5H8O | Leaves | [2,12] |
| 191. | Hexanal | C6H12O | Leaves | [2,12] |
| 192. | 2-Hexenal | C6H10O | Leaves | [2,12] |
| 193. | 3-Hexenal | C6H10O | Leaves | [2,12] |
| 194. | 2,4-Hexadienal | C6H8O | Leaves | [2,12] |
| 195. | 2,4-Heptadienal | C7H10O | Leaves | [2,12] |
| 196. | Octanal | C8H16O | Leaves | [2,12] |
2.2. Polyphenolic Compounds
The leaves, stems, and seeds of P. frutescens are rich in different types of phenolic compounds. The leaves constitute rosmarinic acid, methylrosmarinic acid, caffeic acid and its derivatives including ethyl caffeate, methylcaffeate, vinyl caffeate, trans-p-menthenyl caffeate, caffeic acid-3-O-glucoside, (Z, E)-2-(3,4-dihydroxyphenyl)ethenyl ester of caffeic acid, and (Z,E)-2-(3,5-dihydroxyphenyl)ethenyl ester of caffeic acid [25]. The leaves, also, contain protocatechuic acid, protocatechuic aldehyde, chlorogenic acid, isovanillic acid, sinapic acid, gallic acid, ferulic acid, 4-coumaric acid, coumaroyl tartaric acid, 4-hydroxyphenyl lactic acid, sagerinic acid, p-hydroxybenzoic acid, and hydroxytyrosol. The phenolic compounds, present in P. frutescens seeds, include rosmarinic acid, methyl rosmarinic acid, rosmarinic acid-3-O-glucoside, 3′-dihydroxyl-rosmarinicacid-3-O-glucoside, caffeic acid, caffeic acid-3-O-glucoside, vanillic acid, ferulic acid, and cimidahurinine [23]. P. frutescens stems contains caffeic acid and their derivatives, mainly ethyl caffeate, methyl caffeate, vinyl caffeate, and protocatechuic aldehyde.
2.3. Flavonoids
The leaves, stems, fruits, and seeds of P. frutescens constitute different types of flavonoids, including flavones, flavanones, chalcones, and aurones. P. frutescens leaves contain flavones, such as luteolin, apigenin, scutellarein, negletein, vicenin-2, and catechin [17,28,40] along with several derivatives of luteolin, apigenin, and scutellarein [38] which include luteolin-7-O-glucuronide, luteolin-7-O-diglucuronide, luteolin 7-0-glucuronide -6″-methyl ester, luteolin-7-O-glucoside, apigenin-7-O-glucuronide, apigenin-7-O-diglucuronide, apigenin-7-O-glucoside, apigenin 7-O-caffeoylglucoside, scutellarein-7-O-glucuronide, and scutellarein 7-O-diglucuronide. The flavones in P. frutescens fruits include luteolin, apigenin, and chrysoeriol, while those in seeds include luteolin, luteolin-5-O-glucoside, luteolin-7-O-glucoside, apigenin, apigenin-7-O-glucoside, diosmetin, chrysoeriol, and catechin [22]. The flavanones in P. frutescens leaves include shisoflavanone A, liquiritigenin, 5,8-dihydroxy-7-methoxyflavanone, (2S)-5,7-dimethoxy-8,4′-dihydroxy flavanone, and 8-hydroxy-5,7-dimethoxyflavanone. The chalcones in P. frutescens leaves include 2′,4′-dimethoxy-4,5′,6′-trihydroxychalcone, and 2′,3′-dihydroxy-4′,6′-dimethoxychalcone, along with an aurone, (Z)-4,6-dimethoxy-7,4′-dihydroxyaurone [41].
2.4. Anthocyanins, Coumarins, Carotenoids, and Neolignans
P. frutescens leaves contain anthocyanins and their glucosides, which include shisonin, cis-shosinin, malonyl shisonin, cis-malonyl shisonin, cyanidin 3-O-feruloyl glucoside-5-O-glucoside, cyanidin 3-O-caffeoyl glucoside-5-O-glucoside, cyanidin 3-O-caffeoyl glucoside-5-O-malonyl glucoside, and peonidin 3-O-malonyl glucoside-5-O-p-coumarylglucoside [44]. P. frutescens leaves constitute esculetin and 6,7-dihydroxycoumarin, which possess xanthine oxidase (XO)-inhibitory and anti-inflammatory activities. The leaves constitute carotenoids, such as loliolide and isololiolide, which, also, possess XO-inhibitory activity [20] Two neolignans in P. frutescens leaves, magnosalin, and amanicin, have the potential to treat endotoxemia and inflammation [46].
2.5. Fatty Acids, Policosanols, Tocopherols, and Sitosterols
Perilla seed oil constitutes 40% of the total seed weight. P. frutescens seed oil contains saturated fatty acids, such as lauric acid, myristic acid, pentadecanoic acid, palmitic acid, heptadecanoic acid, stearic acid, arachidic acid, and behenic acid. The unsaturated fatty acids in the seeds include monounsaturated fatty acids (MUFAs), such as palmitoleic acid (16:1 cis-7), oleic acid (18:1 cis-9), eicosenoic acid (20:1 cis-11), and polyunsaturated fatty acids (PUFAs) such as linoleic acid (18:2 cis-9,12), α-linolenic acid (18:3 cis-9,12,15), eicosadienoic acid (20:2 cis-11,14), and eicosatrienoic acid (20:3 cis-11,14,17). The perilla seed oil is, mainly, rich in unsaturated fatty acids and is constituted by 54–64% of α-linolenic acid, 14–23% of oleic acid, 11–16% of linoleic acid, and 7–8% of saturated fatty acids. Furthermore, P. frutescens seed oil contains long-chain alcohols, known as policosanols. P. frutescens seeds are highly rich in policosanols and contain 427.83 milligrammes of policosanols per kilogramme of perilla seed oil. Policosanols present in P. frutescens seed include eicosanol, heneicosanol, docosanol, tricosanol, tetracosanol, pentacosanol, hexacosanol, heptacosanol, octacosanol, nonacosanol, and triacontanol. Tetracosanol, hexacosanol, and octacosanol account for 88% of all policosanols. P. frutescens seeds, also, contain important vitamin-E-related antioxidant compounds, known as tocopherols, which include δ-tocopherol, γ-tocopherol, β-tocopherol, and α-tocopherols. The γ-tocopherol content in perilla seeds was, approximately, equal to that of α-linolenic acid and constitutes 70–80% of the total tocopherol content. The seeds, also, contain phytosterols, such as campesterol, stigmasterol, β-sitosterol, β-amyrin, β-cholestanol, and 5α-cholestane.
2.6. Glucosides, Peptides, Benzoxepin Derivatives, and Other Constituents
P. frutescens leaves, reportedly, contain different types of glucosides, including dehydrovomifoliol, perillanolide A, perillanolide B, perilloside A, perilloside B, perilloside C, perilloside D (monoterpene glucosides) [20] loganin (iridoid glucoside), 5′-β-d-glucopyranosyl oxyjasrnonic acid, 3-β-d-glucopyranosyl-3-epi-2-isocucurbic acid, 3-β-d-glucopyranosyloxy-5-phenylvalericacid, n-octanoyl-β-d-fructofuranosyl-α-d-glu- copyranoside, and 4-(3,4-Dihydroxybenzoyloxymethyl)phenyl-O-β-d-glucopyranoside (polyphenolic glucoside). The leaves, also, contain five β-d-glucosides (eugenyl-β-d-glucoside, benzyl-β-d-glucoside, β-sitosteryl-β-d-glucoside, prunasin, and sambunigrin) and methyl-α-d-galactoside [19]. The seeds contain oligopeptides (PSO), and dipeptides, such as Tyr-Leu and Phe-Tyr. The glycoprotein Pf-gp6 has, also, been reported in P. frutescens leaves. P. frutescens stems contain benzoxepin derivatives, such as perilloxin and dehydroperilloxin. Other constituents, such as p-hydroxybenzaldehyde, p-hydroxyacetophenone, trans-p-hydroxycinnamic acid, and 3,4,5-trimethoxycinnamyl alcohol, are reportedly present in P. frutescens leaves [20].
3. Biological Functions of P. frutescens
3.1. Aldose Reductase Inhibitory Activity
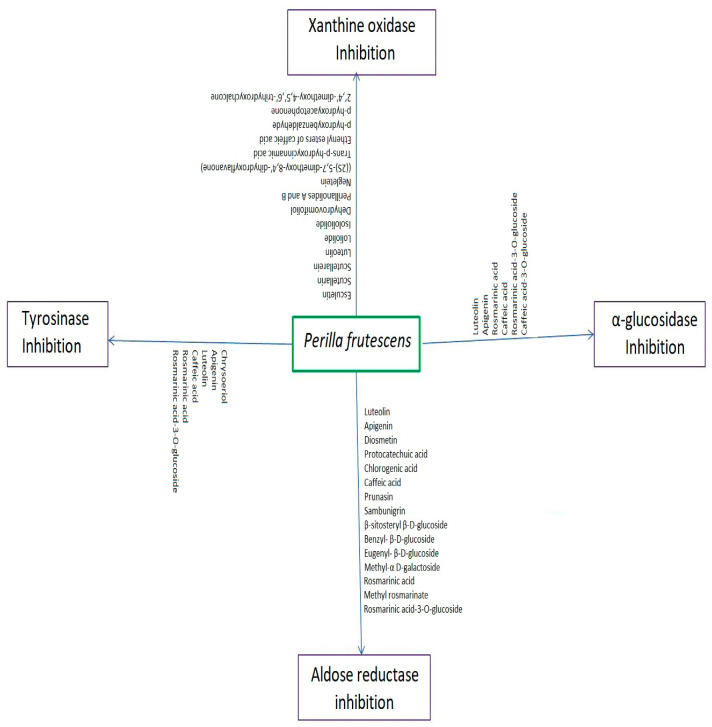
Aldose reductase, or aldehyde reductase (AR), an important NADPH-dependent enzyme, is, ubiquitously, present at higher concentrations in the heart, eyes, neurons, and kidneys. This enzyme catalyses the reduction in monosaccharides, into their respective sugar alcohols. AR converts glucose into sorbitol, via the polyol pathway of glucose metabolism [61]. Excessive sorbitol is severely damaging, and its accumulation in the eyes, neurons, and kidneys of diabetic patients causes retinopathy, neuropathy, and nephropathy, respectively [62]. AR inhibitors are a class of drugs that averts sorbitol accumulation, by preventing or delaying the action of AR in tissues such as eyes, kidneys, and neurons, and are, consecutively, used to prevent the corresponding diabetic complications. Different bioactive compounds from plants possess AR inhibitory activity [63]. The rosmarinic acid, chlorogenic acid, caffeic acid, protocatechuic acid, and methyl rosmarinic acid contained in P. frutescens leaves possess significant AR-inhibitory activity. Figure 1 represents the different phytoconstituents of P. frutescens involved in aldosereductase-inhibitory activity. Rosmarinic acid exhibited strong inhibition against AR, with an IC50 of 2.77 µM, followed by chlorogenic acid (IC50 = 3.16 µM) and methyl rosmarinic acid (IC50 = 4.03 µM). However, caffeic acid and protocatechuic acid exhibited weak AR-inhibitory activity (Paek et al., 2013). Luteolin, apigenin, and diosmetin, isolated from P. frutescens seeds, also, showed AR inhibitory activity. Luteolin was found to be a strong AR inhibitor, with an IC50 of 1.89 µM, followed by apigenin (IC50 = 4.18 µM) and diosmetin (Lee et al., 2016). Different glycosides isolated from P. frutescens leaves, including four monoterpene glycosides (perilloside A-D), five β-d-glucosides (eugenyl-β-d-glucoside, benzyl-β-d-glucoside, β-sitosteryl β-d-glucoside, prunasin, and sambunigrin), and methyl-α-d-galactoside, significantly inhibited AR. In particular, perilloside A and perilloside C have been proven to be potent AR inhibitors that show competitive inhibition, as demonstrated by L–B plots [19]. Caffeic acid-3-O-glucoside and rosmarinic acid-3-O-glucoside isolated from P. frutescens seeds also exhibited AR inhibitory activity. Thus, these bioactive constituents in P. frutescens may be useful as a remedy to treat diabetic complications.
Figure 1.
Different bioactive compounds of P. frutescens involved in enzyme inhibitory activities.
3.2. α-Glucosidase Inhibitory Activity
α-glucosidase is a hydrolytic enzyme that breaks down starch, oligosaccharides, and disaccharides into simple sugars, such as glucose molecules, by acting on α (1–4) bonds to facilitate intestinal absorption of carbohydrates. Their action drastically increases post-meal blood sugar levels in diabetes patients. α-Glucosidase inhibitors prevent or delay the breakdown of carbohydrates into simple sugars (glucose molecules), which delays the intestinal absorption of glucose, and consequently the increase in blood sugar levels after meals [64]. Hence, α-glucosidase inhibitors have been successfully used in treating type II diabetes mellitus. Figure 1 show the bioactive compounds involved in α-glucosidase inhibitory activity of P. frutescens. Ha et al. [23] reported that the five phenolic compounds isolated from P. frutescens seeds exhibited α-glucosidase inhibitory activity in a dose-dependent manner. Luteolin was found to be a potent α-glucosidase inhibitor with an IC50 value of 45.4 µM, and inhibited α-glucosidase in a non-competitive manner with an inhibition constant (KI) of 45.0 µM and Michaelis-Menton’s constant (Km) of 259.3 µM. Caffeic acid-3-O-glucoside, rosmarinic acid, rosmarinic acid-3-O-glucoside, and apigenin exhibited α-glucosidase inhibitory activity at concentrations higher than 100 µM. Owing to their α-glucosidase inhibitory activity, bioactive compounds of P. frutescens can be formulated into oral antidiabetic medications.
3.3. Xanthine Oxidase (XO) Inhibitory Activity
Xanthine oxidase or xanthine oxidoreductase (XO) enzyme plays an important role in purine catabolism, wherein, it catalyses the oxidative hydroxylation step; hypoxanthine is first converted to xanthine which is further converted to uric acid. Hence, the XOinhibitors significantly reduce uric acid production when treating gout and hyperuricemia [65] Oxidative hydroxylation step catalysed by XO produces reactive oxygen species (ROS) such as superoxide (O2−.) and hydrogen peroxide (H2O2) radicals. ROS cause oxidative stress, which is associated with several diseases such as cardiovascular, neurological, and aging. Hence, XO inhibitors play a role in ROS inhibition [66,67]. Synthetic XO inhibitors cause adverse effects such as anaphylactic shock, Stevens-Johnsonsyndrome, hepatotoxicity, and epidermal necrolytic effects [68,69]; hence, this necessitates isolating natural XO inhibitors from different plant sources. Different bioactive compounds isolated from P. frutescens leaves showed potent XO-inhibitory activity (Figure 1). Two caffeic acid esters, (Z-E)-2-(3,4-dihydroxyphenyl) ethenyl ester and (Z-E)-2-(3,5-dihydroxyphenyl) ethenyl esters exhibit potent XO inhibitory activity with IC50 values of 0.021 and 0.121 µg/mL, respectively. In particular, (Z-E)-2-(3,4-dihydroxyphenyl) ethenyl ester exhibited XO inhibition equal to that of the standard drug, allopurinol. The L–B plots demonstrated that these caffeic acids exhibited non-competitive inhibition [70]. A chalcone,2′,4′-dimethoxy-4,5′,6′-trihydroxychalcone and a flavone, luteolin (3′,4′,5,7-Tetrahydroxyflavone) possess XO inhibitory activity with IC50 values of 0.21 and 2.18 µM, respectively. The L–B plots demonstrated that chalcone exhibited mixed-type inhibition, whereas luteolin exhibited competitive inhibition [41]. Furthermore, flavonone, ((2S)-5,7-dimethoxy-8,4′-dihydroxyflavanone); coumarin, esculetin (6,7-dihydroxycoumarin); and scutellarein, (4′,5,6,7-Tetrahydroxyflavone) also exhibited good XO inhibitory activities with IC50 values of 18.44, 32.56, and 48.66 µM, respectively. The Lineweaver–Burk plots indicated that flavonone and scutellarin exhibited mixed-type inhibition, while esculetin displayed competitive inhibition. An aurone, ((Z)-4,6-dimethoxy-7,4′-dihydroxyaurone), also, presented good XO inhibition. Negletein (5,6-dihydroxy-7-methoxyflavone), scutellarein-7-glucuronide (breviscapin), sericoside (triterpene), loliolide, isololiolide (carotenoids), dehydrovomifoliol, perillanolide A, perillanolide B (monoterpeneglycosides), 4-(3,4-dihydroxybenzoyloxymethyl) phenyl-O-β-d-glucopyranoside, trans-p-hydroxy cinnamic acid, p-hydroxybenzaldehyde, and p-hydroxyacetophenone, also, exerted XO-inhibitory activities, with IC50 values greater than 200 µM [17]. Thus, the bioactive compounds of P. frutescens could be used in treating hyperuricemia in patients with gout.
3.4. Tyrosinase Inhibitory Activity
Tyrosinases are oxidases involved in the initial stages of melanin biosynthesis. Overproduction of melanin causes hyper pigmentation and other skin disorders, such as melasma, solar melanosis, senile lentigos, and ephelides [71]. The modern lifestyle, junk foods, insomnia, industrial smoke, pollution, and synthetic drugs cause oxidative stress, which leads to aging and skin disorders. Hence, development of natural tyrosinase inhibitors with antioxidant capacity, to reduce hyper pigmentation, aging, and skin disorders, is essential. Several flavonoids and polyphenolic compounds possess both antioxidant and anti-tyrosinase activities (Figure 1). Kim et al. [22] reported that the methanolic extracts of P. frutescens seeds exhibit dose-dependent inhibition of tyrosinase and radical scavenging against DPPH and ABTS free radicals. The potential anti-tyrosinase activity of P. frutescens seeds is attributed to phenolic compounds, such as rosmarinic acid, rosmarinic acid-3-O-glucoside, luteolin, apigenin, chrysoeriol, and caffeic acid. Rosmarinic acid exhibited potential anti-tyrosinase activity, with an IC50 value of 20.8 µM, followed by luteolin (24.6 µM), chrysoeriol (35.8 µM), apigenin (49.3 µM), rosmarinic acid-3-O-glucoside (57.9 µM), and caffeic acid (>300 µM). Furthermore, all of these compounds showed significant antioxidant activity, as evidenced by their DPPH and ABTS radical-scavenging capacities. Based on these activities, P. frutescens seeds can be used as health additives in food preparations.
3.5. Antispasmodic Effect
Vicenin-II, a bis C-glycosylflavonoid, displayed no direct spasmolytic effect, but significantly reduced Ba2+-or acetylcholine-induced smooth muscle contractions in the rat ileum (a part of the small intestine of the gastrointestinal tract), demonstrating an antispasmodic effect. The antispasmodic effect on smooth muscles relieves gastrointestinal discomfort and bowel disease symptoms, thus maintaining gut health. Hence, vicenin-II can be, prophylactically, used in maintaining and improving gut health [40].
3.6. Insecticidal Activity
Different active constituents reported in the essential oil, such as, R-(+)-carvone, perilla aldehyde, limonene, perillic acid, caryophyllene oxide, methyl perillate, perilla alcohol, and 2-furyl methyl ketone, obtained from P. frutescens aerial-dried parts, exhibited stronger insecticide and-repellent activities, against different insects. For example, 2-Furyl methyl ketone (LD50 = 0.86 mg/L air) exhibited stronger fumigant toxicity against Lasioderma serricorne, followed by R-(+)-carvone(LD50 = 1.83 mg/L air), perilla aldehyde (LD50 = 3.03 mg/L air), and the crude essential oil (LD50 = 4.16 mg/L air) of P. frutescens. The 2-Furyl methyl ketone, also, exhibited strong insecticidal activity against Tribolium castaneum, with an LD50 of 1.32 mg/L air (fumigant toxicity). Limonene, also, exhibited strong insecticidal activity against both Lasioderma serricorne and Tribolium castaneum, with LD50 values of 14.07 mg/L air and 6.21 mg/L air, respectively [7]. Limonene, perilla aldehyde, perillic acid, caryophyllene oxide, methyl perillate, and perilla alcohol exhibit stronger larvicidal activity against dengue-fever-causing mosquitoes, Aedes aegypti [5] Methyl perillate was the most toxic against Aedes aegypti, with an LC50 value of 16.0 ppm, followed by limonene (LC50 of 29.1 ppm), caryophyllene oxide (LC50 of 29.8 ppm), perilla aldehyde (LC50 of 35.3 ppm), perilla alcohol (LC50 of 39.1 ppm), and perillic acid (LC50 of 56.5 ppm). A sesquiterpenoid, α-farnesene, isolated from the whole plant extract of P. frutescens, exhibited insecticidal activity against third instar larvae of Plutella xylostella, with an LD50 of 53.7 ppm [72]. The results indicate that the essential oil of P. frutescens and isolated compounds have the potential to be developed into natural repellents or insecticides, for controlling insects in stored products. The development of natural pesticides would help to decrease the negative effects, such as pesticide residues, resistance, and environmental pollution, caused by synthetic insecticides and repellents.
3.7. Anti-Allergic Activity
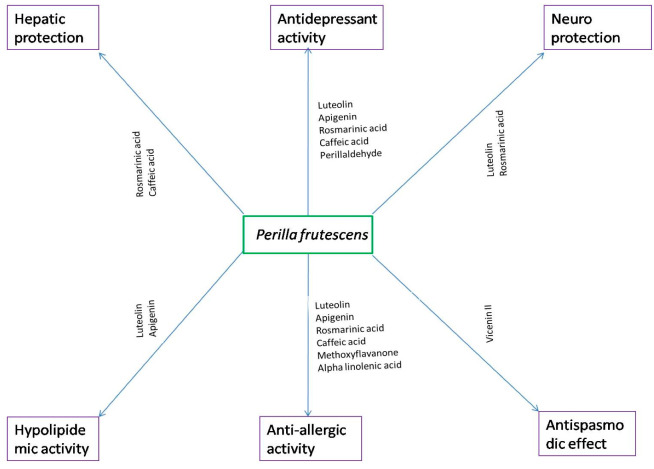
Different bioactive components of P. frutescens, including rosmarinic acid, caffeic acid, luteolin, apigenin, methoxyflavanone and α-linolenic acid, were found to possess antiallegic activity (Figure 2) [73]. Kamei et al. [42] reported the presence of 8-hydroxy-5,7-dimethoxyflavanone in P. frutescens leaves, and named it Perilla-derived methoxyflavanone (PDMF). PDMF may be used to prevent IgE-driven type I hypersensitivity reactions. Akt phosphorylation and intracellular Ca2+ influx, the two critical molecular events involved in mast cell degranulation in allergic reactions, are suppressed by PDMF, which acts as a potent anti-allergic compound. The other polyphenols of P. frutescens, such as rosmarinic acid, luteolin, apigenin, and caffeic acid, also, suppress IgE-mediated type I hypersensitivity reactions. However, PDMF exhibits a more potent histamine-release inhibitory activity than known derived anti-inflammatory polyphenols. Further PDMF stimulation suppresses histamine release from RBL-2H3 cells in a dose-dependent manner, and the IC50 value (68.5 mM) was found to be considerably lower than that of apigenin (96.8 mM), luteolin (174.1 mM), caffeic acid (620.4 mM), and rosmarinic acid (>1000 mM).
Figure 2.
Different bioactive compounds of P. frutescens, exhibiting different biological functions, including antiallergic activity, antidepressant activity, antispasmodic effect, hypolipidemic, hepatoprotection, and neuroprotection activities.
Specific matrix proteins, such as matrix metalloproteinase (MMP), periostin, interleukin (IL-31), thymus, and activation-regulated chemokine (TARC), are sensitive markers for the clinical diagnosis of skin health [74]. These protein levels are drastically elevated during conditions of skin damage, such as skin injuries, skin inflammation, skin allergy, and atopic dermatitis; atopic dermatitis or atopic eczema is a type of severe skin inflammation, with symptoms such as swelling, redness, cracking, and itchy skin. Heo et al. [75] reported that an aqueous extract of P. frutescens mitigates DNFB (2,4-dinitrofluorobenzene)-induced atopic dermatitis, in a mouse model. The anti-dermatitis activity of the aqueous extract of P. frutescens is, possibly, due to the downregulation of matrix metalloproteinase-9 (MMP-9) and IL-31 expression levels, and upregulation of T-bet activity. Komatsu et al. [76] demonstrated that P. frutescens leaf extract prevents atopic dermatitis induced by house dust mite allergens (Dermatophagoides farina), in an NC/Nga AD mouse model. Moreover, P. frutescens leaf extract significantly decreased the serum levels of inflammatory markers, such as IgE, periostin, and TARC. Furthermore, P. frutescens leaf extract significantly decreased the allergen-stimulated CD4+/CD8+ T cell ratio, in spleen lymphocytes.
Shin et al. [77] demonstrated that an aqueous extract of P. frutescens effectively inhibited immediate mast-cell-triggered allergy and inhibited the fatal systemic allergy, using compound 48/80 (a potent inducer of systemic allergy, by stimulating mast cell degranulation and promoting histamine release), in a dose-dependent manner. Furthermore, they reported that an aqueous extract of P. frutescens significantly inhibited the local allergic reaction, induced by anti-DNP IgE. The anti-allergic effects of the aqueous extract of P. frutescens may be due to the inhibition of histamine released from mast cells, TNF-α production, and passive cutaneous anaphylaxis (PCA). Jeon et al. [78] reported that luteolin obtained from the methanolic extracts of P. frutescens exhibited potential antiallergic and antipruritic effects. The antiallergic effect of luteolin is due to the inhibition of histamine release from mast cells, induced by compound 48/80. Luteolin significantly inhibited the scratching behaviour and vascular permeability induced by serotonin and compound 48/80, respectively, in an ICR mouse model. Hyaluronidase is one of the major enzymes regulating mast cell degranulation, and histamine release is, primarily, controlled by protein kinase C and Ca+2. Asada et al. [79] reported that a glycoprotein from P. frutescens hot-water extracts prevented the degranulation of mast cells, possibly due to inhibition of protein kinase C and hyaluronidase activity. Chen et al. [80] reported that the ethanolic extract of P. frutescens successfully exhibited an antiallergic effect in an ovalbumin (allergen)-sensitised asthma mouse model. The ethanolic extract suppressed serum IgE levels, downregulated allergen-stimulated Th2 cytokines (IL-5 and IL-13), and decreased the secretion of allergic mediators (histamine and eotaxin). Furthermore, the ethanolic extract of P. frutescens inhibits airway hyper-responsiveness, by suppressing cell infiltration and reducing lung and bronchiole inflammation. Sanbongi et al. [81] reported that oral administration of rosmarinic acid extract of P. frutescens inhibits allergic inflammation, caused by the mite allergen D. farina. D. farina-sensitised C3H/He mice exhibited severe eosinophilic inflammation and elevated expression levels of IL-4, IL-5, and eotaxin in the lungs; rosmarinic acid extract significantly decreased the influx of eosinophils in the lungs and inhibited the lung tissue expression of IL-4, IL-5, and eotaxin proteins. In another study, by Takano et al. [82] rosmarinic acid-rich P. frutescens extract inhibited allergic rhinoconjunctivitis in humans, at least partly by inhibiting the infiltration of polymorphonuclear leukocytes (PMNL) into the nostrils. In this study, rosmarinic acid-rich extract significantly decreased the number of PMNL and inhibited the levels of inflammatory mediators, such as histamine, eotaxin, IL1-β, IL-8, and IgE levels, in nasal lavage fluid. Makino et al. [83] demonstrated that hot-water extracts of P. frutescens significantly suppressed the mice ear–PCA reaction, thereby concluding that rosmarinic acid is the active constituent, mainly, responsible for anti-allergic reactions. Dietary perilla oil, rich in α-linolenic acid, significantly suppressed serum lipid levels as well as IgG1 and IgA levels, in ovalbumin-sensitised mice. Based on these results, Chang et al. [84] reported that dietary perilla oil might, moderately, suppress asthmatic allergy. P. frutescens, PDMF, and rosmarinic acid could be, medially, useful in treating allergic diseases.
3.8. Anti-Depressant Activity
Figure 2 represents the different bioactive components of P. frutescens exhibiting antidepressant activity. Rosmarinic acid and caffeic acid showed significant antidepressant activity; this was demonstrated by Takeda et al. [85] in a forced swimming test in mice, which is a well-accepted stress model of depression. The antidepressant activity was revealed via mechanisms other than the inhibition of monoamine transporters and monoamine oxidase. In neuropharmacological studies, neither rosmarinic acid nor caffeic acid affected either the uptake of monoamines to synaptosomes or mitochondrial monoamine oxidase activity in the mouse brain. The detailed mechanisms involved in the antidepressive-like properties of rosmarinic acid and caffeic acids are unclear. However, previous pharmacological studies have revealed that rosmarinic acid inhibits histamine release from mast cells [86], as well as that caffeic acid can activate the a1-adrenoreceptor system [87] and inhibit the production and release of nitric oxide (NO) [88,89]. Furthermore, previous studies using the forced swimming test have suggested that either the activation of α1-adrenoreceptors or the inhibition of NO production may be involved in the expression of antidepressive-like effects. Their effects may, reportedly, involve direct modulation of a second messenger system. Caffeic acid, reportedly, inhibits both protein kinase A and protein kinase C activity in vitro. Increased evidence suggests that the therapeutic effects of existing antidepressants are associated with adaptive changes in post-receptor signalling, rather than with their primary action. Furthermore, EOPF exhibited significant antidepressant activity in a chronic, unpredictable, mild stress (CUMS)-induced mouse model, a widely used rodent model of depression. Brain-derived neurotrophic factor (BDNF) is a nerve growth factor essential for neuronal survival, and promotes the growth, differentiation, and maintenance of neurons, as well as synaptic plasticity, cognitive function, and long-term memory. BDNF is present throughout the brain and spinal cord, and its levels are decreased in conditions of depression, aging, and neurological disorders, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and Lou Gehrig’s disease. However, the hippocampal region of the brain shows high densities of BDNF. CUMS significantly decreased hippocampal BDNF protein levels, by downregulating the mRNA expression of BDNF. Administration of EOPF significantly elevates BDNF protein levels, by upregulating BDNF mRNA expression levels [90]. Thus, EOPF showed antidepressant activity, by enhancing BDNF protein levels. Chronic stress significantly decreases serotonin concentrations (5-hydroxytryptamine) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), in the hippocampus of the brain. Furthermore, chronic mild stress could induce a proinflammatory response, by increasing the plasma levels of pro-inflammatory cytokines, including IL-1, IL-6, and TNF-α. CUMS also reduced open-field activity and sucrose consumption as well as increased the duration of immobility in the forced swimming and tail suspension tests. Administering EOPF effectively increased the concentrations of serotonin and 5-HIAA and reduced the levels of IL-6, IL-1β, and TNF-α. Furthermore, EOPF significantly enhanced open-field activity and sucrose consumption as well as reduced the duration of immobility. Ji et al. [91] demonstrated that perillaldehyde, a major constituent of EOPF, exhibited an antidepressant effect in an LPS-induced depression mouse model. In this experiment, perillaldehyde elevated the reduced levels of monoamines, such as norepinephrine and 5-hydroxytryptamine, in the prefrontal cortex of LPS-depressed mice. Furthermore, perillaldehyde decreased proinflammatory cytokine levels (IL-6 and TNF-α) in the prefrontal cortex of mice, and significantly reduced the LPS-enhanced immobility duration in the forced swimming and tail suspension tests.
Luteolin and apigenin, isolated from the fruit of Perilla frutescens (L.) Britton enhance monoamine uptake, either on monoamine-transporter transgenic Chinese hamster ovary (CHO) cells or on wild dopaminergic cell lines, with higher specificity for dopamine (DA) uptake than for norepinephrine (NE) and serotonin (5HT) uptake, with a greater potency and efficacy for luteolin than for apigenin. Furthermore, in the transgenic cells, the principal NE/DA uptake activated by luteolin was significantly prevented by the respective transporter inhibitor, and the transmitter-uptake-enhancing action was independent of its ligands, which supports the compounds as monoamine transporter activators. Furthermore, luteolin, markedly, inhibited the cocaine-targeted effect in CHO cells overexpressing the dopamine transporter. Thus, luteolin and apigenin function as monoamine transporter activators, which would improve several hypermonoaminergicneuropsychological disorders, especially cocaine dependence, by upregulating monoamine transporter activity [92].
Luteolin and apigenin, both isolated from FP, are novel monoamine transporter activators, and luteolin is the most potent DAT activator, which, strikingly, prevents in vitro cocaine-targeted action. Luteolin, therefore, could be effective for hyperfunctional monoamine-associated neuropsychological disorders (e.g., mania, schizophrenia, and drug problems, especially cocaine dependence). The mechanism of the enhancement of DAT/NET function is unknown and needs to be determined in future studies [93].
3.9. Hepatoprotective Activity
Rosmarinic acid, a major polyphenolic component of P. frutescens, reduces lipopolysaccharide-induced liver injury in d-galactosamine-sensitised mice. The mechanism of action of perilla-derived rosmarinic acid, in reducing liver injury induced by DGalN and LPS, is, mainly, attributed to the scavenging of superoxide or peroxynitirite. Furthermore, rosmarinic acid decreases the mRNA expression levels of the proinflammatory cytokine TNF-α, a prime cause of liver injury. Additionally, it decreases the mRNA expression levels of inducible nitric oxide synthase (iNOS), an important enzyme that produces nitric oxide (NO), which plays an important role in the apoptosis of injured hepatocytes [93].
Caffeic acid and rosmarinic acid from P. frutescens leaves exhibit significant hepatoprotection against tert-butylhydroperoxide (t-BHP)-induced oxidative stress damage in the liver and suppress oxidative stress damage in different ways. They increase the levels of intracellular GSH, by enhancing the activity of γ-GCS, and enhance the activity of various antioxidant enzymes, such as SOD, GPx, and CAT. They, further, reduce the levels of oxidative stress biomarker enzymes, such as aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), and lipid peroxidation. Their combination showed synergistic hepatoprotective activity, compared to their individual action [94].
3.10. Hair Growth Promotion Activity
The butanolic fraction of P. frutescens extract (BFPE) promoted hair regrowth in C57BL/6 mice; BFPE successfully induced the anagen phase of hair growth in mice seven days after shaving. Furthermore, BFPE stimulated hair elongation through the proliferation and differentiation of hair follicles and prevented androgenic alopecia. The hair growth-promoting activity of BFPE is, mainly, due to its active constituent, rosmarinic acid, which increased the viability of PHFC (primary hair follicle fibroblast) cells. Furthermore, rosmarinic acid reduced testosterone- and dihydrotestosterone-induced androgenic alopecia. The antimicrobial, anti-inflammatory, anti-leukotriene B4, and anti-androgenic alopecia activities of rosmarinic acid in P. frutescens leaves, synergistically, promote hair growth [95].
3.11. Hypolipidemic Activity
The total flavonoid extract of P. frutescens (TFP), mainly, contains apigenin and luteolin. TFP inhibited hyperlipidemia in rats fed with a high-fat diet. TFP decreases lipid accumulation in adipose tissues and serum levels of triacylglycerols, total cholesterol, and low-density lipoprotein cholesterol (bad cholesterol), accompanied by increased levels of high-density lipoprotein cholesterol (good cholesterol). Furthermore, TFP suppressed oxidative stress in hyperlipidemic rats, by increasing the levels of antioxidant enzymes, such as SOD and GPx, following the inhibition of lipid peroxidation, by decreasing serum malondialdehyde (MDA) levels in the serum of high-fat-diet-fed rats. Based on this evidence, P. frutescens can be used as a food additive to prevent atherosclerosis [96].
3.12. Inotropic and Lusitropic Effects
Korotkich et al. [97] reported that P. frutescens extract (PFE) exhibited positive inotropic and lusitropic effects on the myocardium of rabbits. PFE increased myocardial contraction (inotropic) and relaxation (lusitropic) effects in a dose-dependent manner and demonstrated that the inotropic and lusitropic effects were attributed to the metabolism of calcium in cardiac muscle cells. An increase in the influx of calcium ions, through L-type membrane channels, increases the velocity of contraction (inotropic), and the acceleration of the sarcoplasmic reticulum uptake of calcium ions increases the relaxation velocity (lusitropic).
3.13. Neuroprotective Activity
Luteolin from the ripe seed of P. frutescens exhibits neuroprotective effects against ROS-induced cytotoxicity in primary cortical neurons. Luteolin enhanced the viability of H2O2 intoxicated primary neurons [98] inhibited ROS production in primary neurons treated with H2O2, and enhanced mitochondrial membrane potential in a concentration-dependent manner. Furthermore, luteolin decreased oxidative stress in primary cortical neurons, by enhancing the levels of antioxidant enzymes, such as CAT and GSH. Hence, luteolin can be used as a dietary supplement to prevent neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and cerebral ischemia disorders. Kim et al. [99] reported that luteolin, from the alcoholic extract of P. frutescens, inhibited NO production in microglial cells, by suppressing the mRNA expression levels of iNOS. Rosmarinic acid from the methanolic extract of P. frutescens exhibited neuroprotective effects, against oxidative stress induced by H2O2 in C6 glial cells [100]. H2O2-intoxicated glial cells showed elevated levels of ROS, NO production, and lipid peroxidation, which are associated with apoptosis of glial cells. H2O2 intoxication upregulated the mRNA expression levels of iNOS and COX-2. Rosmarinic acid treatment protects glial cells, by reducing ROS, NO, and lipid peroxidation. The neuroprotective effect of rosmarinic acid was due to the downregulation of protein and mRNA expression levels of iNOS and COX-2. Lee et al. [101] demonstrated that the methanolic extract of P. frutescens and its active constituent rosmarinic acid effectively improved cognitive function and exhibited objective discrimination in an amyloid β (Aβ25–35)-induced mouse model of Alzheimer’s disease (AD). The methanolic extract of P. frutescens and rosmarinic acid inhibited the production of NO (neurotoxicant) and MDA (causing cell membrane damage by lipid peroxidation) in Aβ25–35-injected mouse brain. Perilla seed oil and its active constituent α-linolenic acid (ALA) protect SH-SY5Y (human neuroblastoma) cells from H2O2-induced oxidative stress and apoptosis; the cell death of SH-SY5Y cells is attenuated, by downregulating the Bax/Bcl-2 ratio, cleaved PARP, cleaved caspase-3, and caspase-9 (Lee et al., 2018). β-site amyloid precursor protein cleaving enzyme 1 (BACE 1) is one of the three enzymes required for the production of β-amyloid, which is a neurotoxic peptide that plays a major role in Alzheimer’s disease. Inhibition of BACE 1 limits or stops the production of β-amyloid, which, consequently, slows the pathology of Alzheimer’s disease. Choi et al. [102] reported that luteolin and rosmarinic acid from methanolic extracts of P. frutescens inhibited the BACE 1 enzyme activity, in a dose-dependent manner, with IC50 values of 0.5 and 21 µM, respectively, and inhibited the BACE 1 enzyme in a non-competitive manner. Perilla seed oil and leaf extract showed neuroprotective effects against β-amyloid-peptide-induced toxicity in pheochromocytoma (PC12) cells, reducing oxidative stress and inhibiting hyperphosphorylation of tau protein. Tau protein is a microtubule-associated protein that maintains the normal shape and structure of neurons, which is hyperphosphorylated in neurodegenerative diseases and is incapable of maintaining the normal neuron morphology. Perilla seed oil and leaf extract successfully inhibited tau protein phosphorylation and enhanced neurite-outgrowth-bearing cells [103].
3.14. Anti-Inflammatory Activity
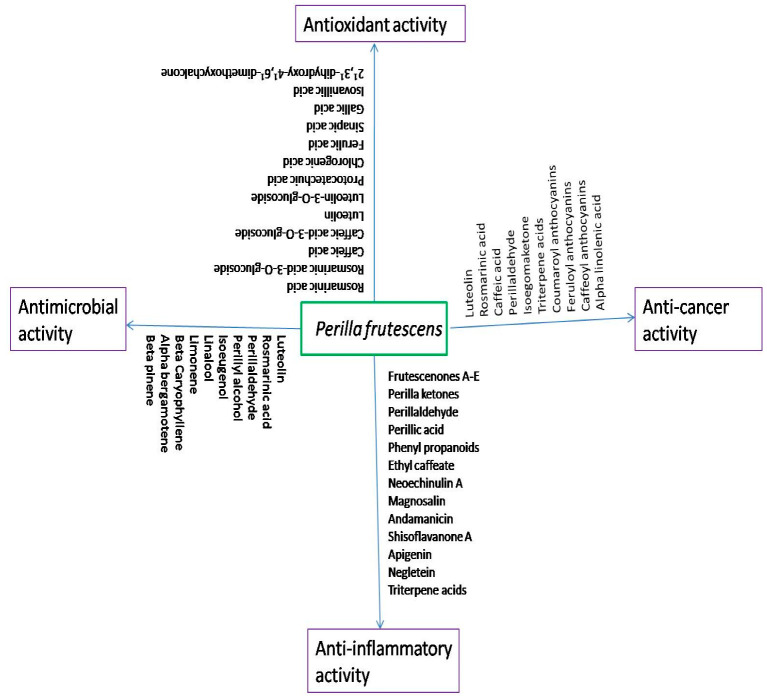
More than ten different bioactive compounds of P. frutescens showed significant anti-inflammatory activity (Figure 3). Wang et al. [8] reported that the aerial parts of P. frutescens contain seven monoterpenoids, which include three new furanoid monoterpenoids, frutescenone A (furanoid with 2,3-bifuran skeleton), frutescenone B (8R-hydroxyperillaketone), frutescenone C (perillaketone-adenine hybrid heterodimer), isoegomaketone, 9-hydroxyisoegomaketone (perillaketone monoterpenoids), (3S,4R)-3-hydroxyperillaldehyde, and (S)-(−)-perillic acid; six phenylpropanoids, such as methylisoeugenol, elemicin, isoelemicin, 3,4,5-trimethoxycinnamyl alcohol, myristicin, and ethyl caffeic acid; and three alkaloids, indole-3-carboxaldehyde, 1H-indole-3-carboxylicacid, and neoechinulin A. These monoterpenoids, phenylpropanoids, and alkaloids exhibit anti-inflammatory effects, by inhibiting the production of proinflammatory cytokines (TNF-α and IL-6) and proinflammatory mediators (NO) in LPS-stimulated RAW264.7 cells. Two neolignans, magnosalin and amanicin, have the potential to treat endotoxemia and inflammation, accompanied by the overproduction of NO and TNF-α.
Figure 3.
Various bioactive compounds of P. frutescens, exhibiting different biological functions, including antioxidant, anti-inflammatory, anticancer, and antimicrobial activities.
The inflammatory mediator NO is a marker that evaluates the anti-inflammatory effect of a drug or compound and is synthesised by the inducible NO synthase (iNOS) enzyme. The iNOS gene expression is induced by the proinflammatory cytokine IL-1β. Different compounds, such as luteolin, apigenin, negletein, shisoflavanone A (8-hydroxy-6,7-dimethoxyflavanone), 5,8-dihydroxy-7-methoxyflavanone, esculetin, and protocatechuic acid, exhibit significant anti-inflammatory activity, by decreasing IL-1β-induced NO production [28]. These compounds, further, decrease the mRNA expression levels of iNOS and TNF-α and suppress NO production in a dose-dependent manner. Shisoflavanone A, apigenin, and negletein exhibited strong NO suppression activity, with IC50 values of 10 µM, 12 µM, and 15 µM, respectively. Esculetin, luteolin, and 5,8-dihydroxy-7-methoxyflavanone, also, suppressed NO production, with IC50 values of 34 µM, 39 µM, and 55 µM, respectively.
Huang et al. [104] reported that the methanolic leaf extract of P. frutescens exhibited anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated macrophage (RAW264.7) cells, which ameliorates LPS-induced inflammation, by downregulating the mRNA expression levels of proinflammatory markers (IL-2, IL-6, and TNF-α) and inflammatory mediators (iNOS and COX-2). Their study, also, elucidated the underlying mechanism of inhibition of proinflammatory markers and inflammatory mediators. Phosphorylation of MAPKs (ERK1/2, JNK, and p38) and nuclear translocation of NF-κB are the two main factors associated with the production and expression of proinflammatory cytokines and inflammatory mediators. P. frutescens leaf extract inhibits MAPK phosphorylation, nuclear translocation of NF-κB, and cytosolic IkBα degradation, in LPS-stimulated macrophages. Chang et al. [84] reported that P. frutescens oil, rich in α-linolenic acid, suppresses bronchoalveolar inflammation in an ovalbumin-sensitised-asthmatic-mouse model. P. frutescens oil suppresses the levels of proinflammatory markers, such as IL-1β, IL-2, IL-4, IL-5, IL-6, and IL-10.
Triterpene acids from P. frutescens leaves inhibit ear oedema inflammation, induced by tetradecanoyl phorbol acetate (TPA), in ICR female mice. Tormentic acid exhibits strong anti-inflammatory effect, with an IC50 value of 0.03 mg/ear, followed by corosolic acid and augustic acid (IC50 of 0.09 mg), ursolic acid and 3-epimaslinic acid (IC50 of 0.10 mg), pomolic acid (IC50 of 0.12 mg), hyptadienic acid (IC50 of 0.13 mg), and oleanolic acid (IC50 of 0.3 mg) [14]. The ethanolic extract of P. frutescens leaves (PFLE) showed anti-inflammatory effects on human neutrophils, stimulated by N-formyl-Met-Leu-Phe (fMLF), a potent macrophage activator and PMNL chemotactic factor (Chen et al., 2015). The underlying mechanisms revealed that PFLE exhibited dose-dependent inhibition of elastase release, ROS formation, superoxide anion production, cell migration, and CD11b expression. Furthermore, PFLE inhibits the activation of Src family kinases, including Src (Tyr416) and Lyn (Tyr396), and decreased intracellular Ca+2 mobilization. Urushima et al. [105] demonstrated that P. frutescens extract suppressed inflammatory bowel disease, induced by dextran sulfate sodium in a C57/BL6 mouse model, and reported that P. frutescens extract inhibits excessive secretion of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-17A) in the colon and upregulates anti-inflammatory cytokines (IL-10 and TGF-β). Immunocompetent cells, such as CD4+Foxp3+Tregs, regulate the pathogenesis of ulcerative colitis, and, mainly, induce the secretion of anti-inflammatory cytokines. Furthermore, Urushima et al. [105] elucidated that the prepared extract, mainly, comprises three compounds, luteolin, apigenin, and rosmarinic acid, wherein luteolin suppressed the production of TNF-α, IL-1β, IL-6, and IL-17A; apigenin suppressed IL-1β and IL-17A levels; and rosmarinic acid significantly reduced the levels of IL-1β and enhanced the levels of IL-10 and TGF-β, by upregulating the mRNA expression levels of Tregs. Apigenin enhanced the levels of IL-10 levels through other mechanisms; however, luteolin could not induce IL-10 production. Jeon et al. [78] reported that luteolin from P. frutescens methanolic extracts exhibited potential anti-inflammatory effects. Luteolin inhibited the production of TNF-α and IL-1β in human mast cells, induced by PMA and Ca+2 ionophore.
Isoegomaketone, isolated from P. frutescens essential oil, exhibited anti-inflammatory effects in LPS-induced mouse macrophages (RAW 264.7 cells) and suppressed the production of NO (inflammatory mediators), IL-6 (inflammatory cytokine), and monocyte chemoattractant type-1 (MCP-1, a protein that attracts monocytes and macrophages to the areas of inflammation). Park et al. [54] prepared different synthetic derivatives of isoegomaketone. One of the isoegomaketone derivatives inhibited NO, MCP-1, and IL-6 more effectively than its precursor. Park et al. [54] also, demonstrated that isoegomaketone and its derivatives suppressed the NF-kB pathway and activator protein-1 (AP-1), which are two inflammation-associated genes involved in the production of inflammatory mediators and inflammatory cytokines. Moreover, 9-hydroxy-isoegomaketone from P. frutescens leaves inhibited NO production in the LPS-stimulated mouse macrophage cell line RAW 264.7, as 9-hydroxy-isoegomaketone inhibits NO production, in a dose-dependent manner, with an IC50 value of 14.4 µM [55].
Yang et al. [20] reported that rosmarinic acid from P. frutescens leaves showed anti-inflammatory effects in cecal ligation and puncture (CLP)-induced septic mice and in human endothelial cells. Rosmarinic acid suppresses phorbol myristateacetate (PMA), TNF-α, IL-1β, and CLP-mediated endothelial protein C receptor (EPCR) shedding, by inhibiting the expression of TNF-α and converting enzyme (TACE). Furthermore, rosmarinic acid suppressed the phosphorylation of p38, ERK1/2, and JNK stimulated by PMA [106]. High-mobility group box-1 (HMGB-1) is a DNA-binding nuclear protein that acts as a proinflammatory cytokine, which plays an important role in infections, injury, and sepsis-related inflammation. Rosmarinic acid suppressed CLP-or LPS-stimulated HMGB1 release, and inhibited HMGB1 mediated inflammation in endothelial cells and sepsis-related mortality. Based on the above studies, rosmarinic acid can be used as a potential therapeutic agent for treating vascular inflammatory diseases, by inhibiting the HMGB-1 signalling pathway.
Luteolin reported from the P. frutescens alcoholic extracts inhibits neuro-inflammatory response in LPS-activated microglial (BV-2) cells, through the suppression of NO production [99]. Luteolin suppressed NO production in a dose-dependent manner, with an IC50 value of 6.9 µM, and inhibited NO production by suppressing the mRNA expression levels of iNOS. Deep-mechanism studies demonstrated that luteolin suppressed the degradation of Ik-Bα, an inhibitor of NF-kB, which is a protein family that mediates inflammatory responses, by producing inflammatory mediators and proinflammatory cytokines. Thus, luteolin inhibits the neurotoxic effector (NO) in the central nervous system, by suppressing the mRNA expression levels of iNOS [99].
The essential oil of P. frutescens (EOPF) suppressed chronic stress-induced neuro-inflammatory responses, by inhibiting the plasma levels of proinflammatory cytokines, including IL-1, IL-6, and TNF-α. Ji et al. [91] reported that perillaldehyde, a major constituent of EOPF, could reduce neuroinflammation by inhibiting IL-6 and TNF-α levels in the prefrontal cortex of an LPS-induced mouse model. Reflux of gastric contents from the stomach into the oesophagus causes inflammation of mucosal tissue and oxidative stress, a condition called reflux oesophagitis. Arya et al. [107] demonstrated that P. frutescens fixed oil successfully inhibited experimental reflux oesophagitis in Wistar albino rats. In this experiment, P. frutescens fixed oil exhibited different activities, such as antioxidant, lipoxygenase-inhibitory, anticholinergic, and antihistamine activities. These activities of P. frutescens fixed oil are, mainly, due to the presence of α-linolenic acid. Together, these activities provide protection against reflux oesophagitis in experimental rats.
3.15. Antioxidant Activity
Oxidative stress is, mainly, referred to as an imbalance between reactive oxygen species (free radicals) and antioxidant capacity, occurring when ROS production exceeds antioxidant capacity. It causes severe cellular damage, by disrupting cellular defence systems by interfering with cellular survival mechanisms, and is associated with several human diseases, including aging, neurodegenerative, cardiovascular, carcinogenesis, hepatic, and kidney diseases [108]. Oxidative stress-induced cell death can be weakened, by suppressing intracellular ROS production and by inducing the expression of antioxidant enzymes and cytoprotective proteins, such as superoxide dismutase (SOD), glutathione peroxidase (GPX), thioredoxin, catalase, γ-glutamylcysteine synthetase (g-GCS), glutathione S-transferase (GST), hemeoxygenase-1 (HO-1), and NAD(P)H: quinone oxidoreductase-1 (NQO1). Moreover, the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element (ARE) pathway controls the expression of these proteins and enzymes, and its activation protects cells against oxidative stress, thus functioning as a cellular defence system [109].
In addition, 21,31-dihydroxy-41,61-dimethoxychalcone (DDC), a flavonoid isolated from the leaves of green perilla, suppresses intracellular ROS production. Furthermore, it stimulates the expression of antioxidant enzymes, such as γ-GCS, NQO1, and HO-1. DDC increases glutathione content and antioxidant response element (ARE) activity in a concentration-dependent manner. Nrf2 induces the expression of antioxidant genes, by binding to AREs, which is crucial in the antioxidant genes expression, to overcome the cellular damage caused by oxidative stress. DDC significantly induced Nrf2-dependent ARE activation. Furthermore, phosphorylation of Nrf2 plays an important role in nuclear translocation and transcriptional activation, through AREs. The Nrf2-ARE pathway is regulated by different signalling kinases, including p38 MAPK (p38 mitogen-activated protein kinase), PI3K, and protein kinase C. DDC, effectively, increased the p38 MAPK and PI3K/Akt pathways. Thus, DDC from green Perilla acts as an effective antioxidant [43]. Figure 4 depicts a schematic representation of Nrf2-dependent ARE pathway activation, by the DDC of P. frutescens.
Figure 4.
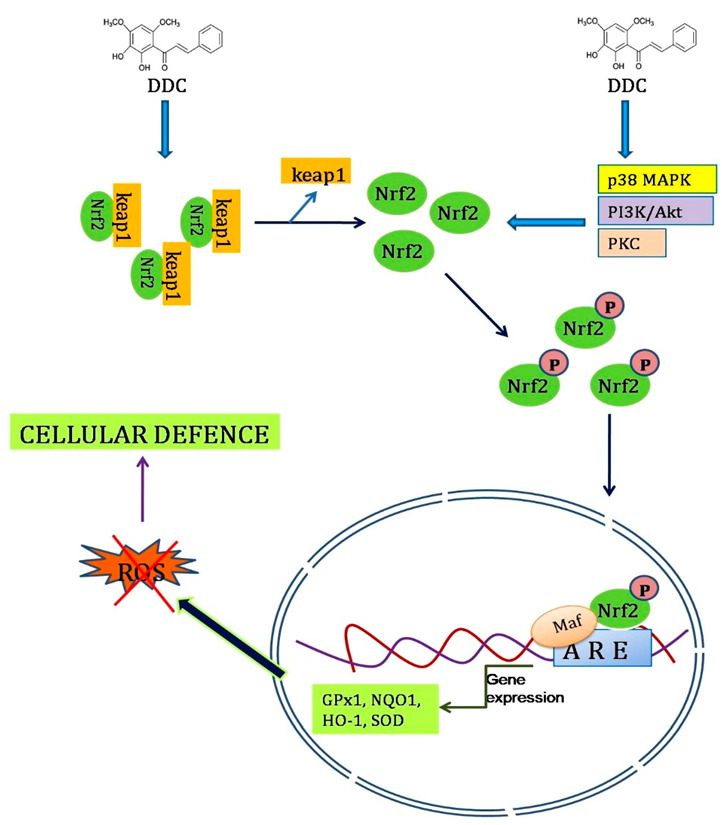
Schematic representation of Nrf2-ARE activation, by 21,31-dihydroxy-41,61-dimethoxychalcone from P. frutescens.
γ-Glutamylcysteine synthetase (γ-GCS) catalyses the major regulatory (rate-limiting) step in the de novo synthesis of glutathione (GSH), a major antioxidant tripeptide that protects cells from cellular damage caused by ROS. Park et al. [26] reported that caffeic acid from the leaves of green perilla increased γ-GCS activity in a dose-dependent manner. Similarly, GSH content increased, according to γ-GCS activity. This result revealed that the elevation of γ-GCS activity by caffeic acid is responsible for the de novo increase in GSH synthesis.
The ethanol extract of purple perilla leaves contains different phenolic acids, such as hydroxybenzoic acids, which include isovanillic acid, protocatechuic acid, gallic acid; and hydroxycinnamic acids, which include rosmarinic acid, caffeic acid, sinapic acid, ferulic acid, chlorogenic acid, rosmarinic acid-3-O-glucoside, caffeic acid-3-O-glucoside, and luteolin-5-O-glucoside, which exhibits effective in vitro antioxidant activity against ABTS, DPPH, BHT, and H2O2 radicals. Among these compounds, rosmarinic acid is a strong natural antioxidant. Rosmarinic acid and luteolin exhibit strong scavenging activities against DPPH free radicals, with IC50 values of 8.61 mM and 7.50 mM, respectively [24].
3.16. Anticancer Activity
The ethanolic leaf extract of P. frutescens (PFLE) inhibited the viability of HL-60 human leukemia cells, in a concentration-dependent manner. PFLE caused DNA defragmentation and arrested the cell cycle in the G1-phase. Its treatment increased the activities of apoptotic proteins, including caspase-3, caspase-8, and caspase-9, in a dose-dependent manner, and induced the cleavage of poly (ADP-ribose) protein (PARP), a DNA-repair enzyme. Furthermore, PFLE upregulated the levels of the proapoptotic protein Bax and downregulated the levels of the anti-apoptotic protein Bcl-2. PFLE treatment increased the levels of glucose-regulated protein-78 (GRP-78), phosphorylated eIF-2α, and JNK and p21 levels, in a concentration-dependent manner. Kwak et al. (2009) demonstrated that PFLE strongly induced the apoptosis of HL-60 through different mechanisms, including mitochondria-mediated (upregulation of caspase-9 by releasing cytochrome C from mitochondria), death-receptor-mediated (upregulation of key initiator caspase-8), and endoplasmic-reticulum-stress-mediated (upregulation of GRP78 and activation of eIF-2α and JNK by phosphorylation) pathways. The activity of PFLE towards apoptosis of HL-60 might be attributed to its active constituent luteolin.
P. frutescens leaf extract (PLE) inhibited hepatic carcinoma (Hep-G2) in a dose-dependent manner, with an IC50 value of 105 µg/mL, and exhibited antiproliferative activity in Hep-G2 cells, by inducing apoptosis in both caspase-dependent and caspase-independent pathways. PLE upregulated the mRNA expression levels of pro-apoptotic proteins caspase-8 and Bax, whereas the mRNA expression levels of the anti-apoptotic protein Bcl-2 were downregulated by PLE treatment. PLE upregulated apoptotic inducers, including c-Jun, Jun-B, and Fos-B, in the FOS/JUN-pathway, and NFkBIA and TNFSF9, through the autocrine pathway. The apoptotic effect of PLE on Hep-G2 cells was attributed to its active constituents, such as rosmarinic acid, caffeic acid, luteolin, and triterpene acids [110].
Kwak et al. [111] demonstrated that the ethanol extract of perilla leaf (PLE) inhibited colorectal cancer (HCT116) and non-small cell lung cancer (H1299), in a dose-dependent manner, with IC50 values of 50 µg/mL and 67 µg/mL, respectively. The PLE-treated cells exhibited nuclear fragmentation and chromatin condensation. PLE treatment significantly enhanced the sub-G1 cell population and inhibited the migration of non-small cell lung cancer cells and their adhesion.
Akihisa et al. [15] reported nine pentacyclic triterpene acids from ethanolic extracts of P. frutescens leaves, including six ursane types (ursolic acid, corosolic acid, epicorosolic acid, pomolic acid, tormentic acid, and hyptadienic acid) and three oleanane types (oleanolic acid, augustic acid, and epimaslinic acid), and evaluated their anticancer activities against human cancer cell lines, including leukaemia (HL-60), breast cancer (MCF-7), and hepatic carcinoma (Hep-G2). Ursolic acid, corosolic acid, tormentic acid, oleanolic acid, and 3-epimaslinic acid showed dose-dependent cytotoxicity against all cancer cell lines. Ursolic acid, corosolic acid, and oleanolic acid exhibited significant cytotoxicity against leukaemia, breast, and hepatic carcinomas, with IC50 values between 12–48 µM. Tormentic acid and 3-epimaslinic acid exhibited moderate cytotoxicity, with IC50 values greater than 50 µM. Among the nine pentacyclic triterpene acids, ursolic acid, reportedly, exhibited strong anticancer activity against leukaemia, breast, and hepatic carcinomas, with IC50 values of 11.8 µM, 14.9 µM, and 27.6 µM, respectively.
Banno et al. [14] reported that lipophilic triterpene acids, such as tormentic acid and oleanolic acid from the ethanolic extracts of Perilla leaves, showed inhibitory activity against skin carcinoma, through the protein kinase C signalling pathway. Lipophilic pentacyclic triterpene acids, such as ursolic acid, oleanolic acid, maslinic acid, 3-epimaslinic acid, corosolic acid, and 3-epicorosolic acid, inhibit skin carcinoma promoted by 12-O-tetradecanoylphorbol-13-acetate (TPA) in an ICR mouse model. All pentacyclic triterpene acids inhibited the TPA-induced epidermal proliferation. Furthermore, they inhibited skin inflammation by suppressing the expression of inflammatory genes. However, maslinic acid and 3-epicorsolic acid were more effective against TPA-mediated skin tumour promotion. They inhibited epidermal proliferation and skin inflammation compared to other pentacyclic triterpene acids. Maslinic acid and 3-epicorsolic acid, effectively, inhibited skin carcinoma, by suppressing the expression of Cox-2 and Twist-1 proteins, and inhibited the phosphorylation (activation) of epidermal signalling pathway proteins, such as IGF-1βR, Src, STAT3, JNK1/2, and c-Jun. Furthermore, they enhanced the levels of the tumour-suppressor protein, Pdcd4 [80].
Seven anthocyanins reported from P. frutescens leaves, such as shisonin, cis-shisonin, malonyl-shisonin, malonyl-cis-shisonin (coumaroyl anthocyanins), two caffeoyl anthocyanins, and one feruloyl anthocyanin, exhibited significant apoptotic effects on human cervical adenocarcinoma (HeLa) cell lines, in a dose-dependent manner [44].
Isoegomaketone, from the aerial parts of green perilla, inhibited the viability of mouse melanoma (B16) cells, in a concentration-dependent manner. It inhibits melanoma growth in tumour-transplanted mice. The possible effects of the antitumor/anticancer activity of isoegomaketone are, also, explained. Isoegomaketone induces apoptosis of melanoma cells, via both caspase-dependent and caspase-independent pathways. It induces ROS generation, nuclear fragmentation, cell shrinkage, and cell membrane blebbing, and increases caspase-3 and caspase-9 activities, in a concentration-dependent manner. Isoegomaketone upregulates Bax (pro-apoptotic protein) and downregulates Bcl-2 (anti-apoptotic protein). Furthermore, isoegomaketone significantly increases Bax/Bcl-2 [9].
Dietary perilla oil inhibits methyl nitrosourea (MNU)-induced colon cancer in F344/NSlc rats. Narisawa et al. [112] demonstrated that perilla oil inhibits colonic tumour development by MNU, mainly because of the presence of α-linolenic acid, a ω-3 PUFA. α-Linolenic acid modified the sensitization of colonic cell membranes to carcinogens, such as MNU.
Perillaldehyde, a major volatile component of the essential oil of P. frutescens, inhibited the viability of mouse gastric cancer cell lines (MFCs). Perillaldehyde inhibits MFCs by inducing autophagy in MFCs, which is dependent on adenosine monophosphate-activated protein kinase (AMPK), and AMPK activation is, LKB1 dependent, a primary upstream kinase of AMPK. Zhang et al. [113] reported that PAH activates AMPK, via phosphorylation of the Thr172 residue. Phosphorylated AMPK induces autophagy in gastric cell lines. Furthermore, perillaldehyde upregulated the levels of apoptotic proteins, such as caspase-3 and p53, and promoted apoptosis of gastric cancer cells in vivo.
3.17. Antimicrobial Activity
The leaves of P. frutescens contain different volatile compounds such as terpenoids, which include perillaldehyde, β-caryophyllene, limonene, α-bergamotene, perillyl alcohol, isoeugenol, and linalool. Perillaldehyde showed broad-spectrum activity against different microbes, including Gram-negative bacteria (Enterobacter aerogenes, Escherichia coli, Salmonella choleraesuis, and Pseudomonas aeruginosa), Gram-positive bacteria (Bacillus subtilis, Propionibacterium acnes, Staphylococcus aureus, and Streptococcus mutans), and fungi and yeast (Aspergillus niger, Candida albicans, Candida utilis, Penicillium chrysogenum, Mucor mucedo, and Saccharomyces cerevisiae). Limonene and β-caryophyllene exhibited excellent antimicrobial activity against Gram-positive bacteria, especially A. naeslundii and P. acnes, respectively. β-Pinene, perillyl alcohol, isoeugenol, and linalool exhibited weak-butbroad-spectrum antimicrobial activity, against both Gram-positive bacteria and fungi (Kang et al., 1992) [114]. Luteolin, one of the phenolic compounds of Perilla seed, exhibited the strongest effect on the oral pathogenic bacterium Porphyromonas gingivalis (Yamamoto et al., 2002) [115].
Perilla essential oil showed antifungal activity against phytopathogenic fungi, including A. niger, A. flavus, A. oryzae, Rhizopus oryzae, and Alternaria alternata [3]. Rosmarinic acid, and its extracts from P. frutescens leaves, exhibited strong antimicrobial activity against different bacterial and fungal pathogens, including E. coli, S. aureus, B. subtilis, A. niger and C. albicans. Rosmarinic acid exhibits antibacterial activity, by targeting deformylase, which is a bacterial enzyme peptide that is essential in synthesising bacterial functional polypeptides. Rosmarinic acid shows fungal inhibitory activity, by targeting the fungal co/post-translational enzyme N-myristoyltransferase [116].
Among all compounds reported from P. frutescens, rosmarinic acid and luteolin were found to show a wide variety of biological applications. Figure 5 shows the different biological functions of the rosmarinic acid and luteolin of P. frutescens.
Figure 5.
Different biological functions of rosmarinic acid and luteolin, reported from P. frutescens.
Perillafrutescens shows no toxicity and no side effects to human. However, it shows few side effects for cattle and livestock. However, the side effects are negligible, when the cattle eat limited P. frutescens. Perilla frutescens contains a pneumotoxin in the leaves and seeds that when metabolised in the rumen produces toxic intermediaries. Perilla ketone, from the essential oil of Perilla frutescens, is a pulmonary edemagenic agent for laboratory animals and livestock, when they take a high amount of P. frutescens.
4. Conclusions
P. frutescens is an annual herb, belonging to the family Lamiaceae, and is mainly cultivated in China, Japan, Korea, and India. P. frutescens has gained attention from researchers worldwide, owing to its emerging economic importance. The enormous phytochemical constituents, their medicinal properties, and their nutraceutical properties make P. frutescens a unique crop. The leaves, aerial parts, and seeds of P. frutescens are used for culinary and non-culinary purposes. Approximately 400 compounds, including alkaloids, terpenoids, quinines, phenylpropanoids, glycosides, benzoxipen derivatives, polyphenolic compounds, flavonoids, chalcones, coumarins, carotenoids, anthocyanins, neolignans, fatty acids, policosanols, tocopherols, and phytosterols, were reported from P. frutescens. Rosmarinic acid, luteolin, apigenin, caffeic acid, and their derivatives, which are present in leaves, seeds, and aerial parts, were the most active constituents of P. frutescens. Rosmarinic acid, luteolin, apigenin, and caffeic acid are the key bioactive constituents responsible for many biological activities, including antimicrobial, antioxidant, anticancer, antidepressant, anti-allergic, anti-inflammatory, antidiabetic, and hypolipidemic activities. Rosmarinic acid, luteolin, apigenin, and caffeic acid exhibited neuroprotective, hepatoprotective, and cardioprotective effects. Further Rosmarinic acid and luteolin exhibited xanthine oxidase, tyrosinase, alpha-glucosidase, hyaluronidase, and aldose reductase inhibitory activities. The antimicrobial, anti-inflammatory, anti-leukotriene B4, and anti-androgenic alopecia activities of rosmarinic acid and luteolin were, synergistically, involved in the hair growth promotion of P. frutescens leaf. Rosmarinic acid was found at very high concentrations in P. frutescens, compared to caffeic acid, luteolin, and apigenin. The essential oil of P. frutescens (EOPF) exhibited stronger insecticidal, antiplasmodial, antifungal, antibacterial, and antiviral activities, and demonstrated anticarcinogenic effects, in different cancer cell lines. EOPF suppresses the neuroinflammatory response, inhibits inflammation of the mucosal layer of the oesophagus, and exhibits anti-inflammatory effects. Furthermore, EOPF shows radical scavenging, lipoxygenase inhibitory, anticholinergic, and antihistamine activities. All of these EOPF activities were due to the presence of 200 different volatile substances, including monoterpenes, sesquiterpenes, and phenylpropanoids. Isoegomaketone and perillal were found to be the most active constituents of EOPF, with anti-inflammatory, insecticidal, antimicrobial, and anticarcinogenic effects. P. frutescens leaves constitute triterpene acids, anthocyanins, coumarins, carotenoids, and neolignans. Nine different types of triterpene acids present in P. frutescens exhibited anticarcinogenic effects against different types of human cancer cell lines. Seven different types of anthocyanins present in P. frutescens showed anti-inflammatory and anticancer activities. Coumarins and carotenoids present in P. Frutescens demonstrated xanthine-oxidase-inhibitory, anti-inflammatory, antioxidant, and anticancer activities. Two neolignans present in P. frutescens can, potentially, treat endotoxemia and inflammation. Perilla leaves are of industrial importance because of their medicinal and nutraceutical properties. Perilla leaves are edible as well as nutritious and are used as leafy vegetables and spicy herbs in Asian cuisine. The leaves are used in pickles, soups, salads, condiments, garnishes, food flavours, and wraps. Perilla seed oil, extracted from toasted seeds, is used for cooking in China, Japan, and Korea. Perilla seed oil is an important edible oil used for flavour enhancement, cooking, roasting, and dipping sauce. The seed oil is rich in polyunsaturated fatty acids, policosanols, phytosterols, and tocopherols. α-linolenic acid constitutes about 55–65% of the polyunsaturated fatty acids of P. frutescens. Perilla seed oil contains the highest proportion of omega-3-fatty acids, compared to any other plant oil worldwide. Perilla seed oil and α-linolenic acid exhibit strong neuroprotective effects, and tocopherols show strong antioxidant effects. Perilla seeds are, also, rich in flavonoids and polyphenolic compounds. Perilla seed oil, extracted from untoasted seeds, has been used for fueling lamps, printing ink, varnishes, paints, linoleum, lacquers, and waterproof coatings. The seed cake obtained after oil extraction has been used as an animal feed and a biofertiliser. Thus, in this review, we discuss the phytochemical constituents, biological properties, medicinal uses, and pharmaceutical and nutraceutical importance of P. frutescens. This review can serve as a ground work for subsequent studies on P. frutescens.
Author Contributions
Conceptualization, T.H.; V.R.N. and Z.Z.; methodology, Y.X.; H.L. and H.Z.; Formatting, Y.X.; H.L. and H.Z.; validation, T.H.; V.R.N. and Z.Z.; investigation, T.H.; V.R.N. and Z.Z.; resources, Y.X.; H.L. and H.Z.; data curation, H.Z. and Y.X.; writing—original draft preparation, T.H.; V.R.N. and H.L. writing—review and editing, T.H.; V.R.N.; H.L. and Z.Z. visualization, H.Z. and Y.X.; supervision, T.H.; V.R.N. and Z.Z.; project administration, T.H. and Z.Z. funding acquisition, T.H. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We are very grateful to the funding agencies to execute this investigation at great levels. Shanxi Scholarship Council of China: HGKY2019075; Jinzhong Science and Technology Key R&D Plan of Shanxi Province: 202003D03111123.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed H.W., Szilvia T.-S. Identification and quantification of essential oil content and composition total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019;275:730–738. doi: 10.1016/j.foodchem.2018.09.155. [DOI] [PubMed] [Google Scholar]
- 2.Lin L.Y., Peng C.C., Wang H.E., Liu Y.W., Shen K.H., Chen K.C., Peng R.Y. Active volatile constituents in Perilla frutescens essential oils and improvement of antimicrobial and anti-inflammatory bioactivity by fractionation. J. Essen. Oil Bear. Plants. 2016;19:1957–1983. doi: 10.1080/0972060X.2016.1226962. [DOI] [Google Scholar]
- 3.Tian J., Zeng X., Zhang S., Wang Y., Zhang P., Lü A., Peng X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 2014;59:69–79. doi: 10.1016/j.indcrop.2014.04.048. [DOI] [Google Scholar]
- 4.Ito M., Toyoda M., Honda G. Chemical composition of the essential oil of Perilla frutescens. Nat. Med. 1999;53:32–36. [Google Scholar]
- 5.Tabanca N., Demirci B., Ali A., Ali Z., Blythe E.K., Khan I.A. Essential oils of green and red Perilla frutescens as potential sources of compounds for mosquito management. Ind. Crops Prod. 2015;1:36–44. doi: 10.1016/j.indcrop.2014.11.043. [DOI] [Google Scholar]
- 6.Ye Q., Zheng D. Rapid analysis of the essential oil components of dried Perilla frutescens (L) by magnetic nanoparticle-assisted microwave distillation and simultaneous headspace solid-phase microextraction followed by gas chromatography-mass spectrometry. Anal. Methods. 2009;1:39–44. doi: 10.1039/b9ay00035f. [DOI] [PubMed] [Google Scholar]
- 7.You C.X., Yang K., Wu Y., Zhang W.J., Wang Y., Geng Z.F., Chen H.P., Jiang H.Y., Du S.S., Deng Z.W., et al. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L) Britt aerial parts against two stored product insects. Eur. Food Res. Technol. 2014;239:481–490. doi: 10.1007/s00217-014-2242-8. [DOI] [Google Scholar]
- 8.Wang X.-F., Li H., Jiang K., Wang Q.-Q., Zheng Y.-H., Tang W., Tan C.-H. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW2.647 cells. Fitoterapia. 2018;130:61–65. doi: 10.1016/j.fitote.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Kwon S.J., Lee J.H., Moon K.D., Jeong I.Y., Ahn D.U., Lee M.K., Seo K.I. Induction of apoptosis by isoegomaketone from Perilla frutescens L in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent-independent pathway. Food Chem. Toxicol. 2014;65:97–104. doi: 10.1016/j.fct.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Luo W., Du Z., Zheng Y., Liang X., Huang G., Zhang Q., Liu Z., Zhang K., Zheng X., Lin L., et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crops Prod. 2019;133:357–364. doi: 10.1016/j.indcrop.2019.03.025. [DOI] [Google Scholar]
- 11.Li N., Zhang Z.J., Li X.J., Li H.Z., Cui L.X., He D.L. Microcapsules biologically prepared using Perilla frutescens (L) Britt essential oil and their use for extension of fruit shelf life. J. Sci. Food Agric. 2018;98:1033–1041. doi: 10.1002/jsfa.8552. [DOI] [PubMed] [Google Scholar]
- 12.Seo W.H., Baek H.H. Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. J. Agric. Food Chem. 2009;57:11537–11542. doi: 10.1021/jf902669d. [DOI] [PubMed] [Google Scholar]
- 13.Baser K.H.C., Demirci B., Donmez A.A. Composition of the essential oil of Perilla frutescens (L) Britton from Turkey. Flavour Fragr. J. 2003;18:122–123. doi: 10.1002/ffj.1174. [DOI] [Google Scholar]
- 14.Banno N., Akihisa T., Tokuda H., Yasukawa K., Higashihara H., Ukiya M., Watanabe K., Kimura Y., Hasegawa J.I., Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 15.Akihisa T., Satoshi K., Taketo U., Hiroyuki A., Norihiro B., Yosuke T., Ken Y. Cytotoxic activity of Perilla frutescens var japonica leaf extract is due to high concentrations of oleanolic and ursolic acids. J. Nat. Med. 2006;60:331–333. doi: 10.1007/s11418-006-0015-9. [DOI] [Google Scholar]
- 16.Lee Y.H., Kim B., Kim S., Kim M.S., Kim H., Hwang S.R., Kim K., Lee J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017;25:776–788. doi: 10.1016/j.jfda.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Liu X.-H., Zhou S., Hua G., Li G.-L., Guo W.-J., Fang X.-Y., Wang W. Perillanolides A and B new monoterpene glycosides from the leaves of Perilla frutescens. Rev. Bras. Farmacogn. 2017;27:564–568. doi: 10.1016/j.bjp.2017.06.003. [DOI] [Google Scholar]
- 18.Lim H.J., Woo K.W., Lee K.R., Lee S.K., Kim H.P. Inhibition of proinflammatory cytokine generation in lung inflammation by the leaves of Perilla frutescens and its constituents. Biomol. Ther. 2014;22:62–67. doi: 10.4062/biomolther.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita T., Ohira K., Miyatake K., Nakano Y., Nakayama M. Inhibitory effect of perilloside A and C and related monoterpene glucosides on aldose reductase and their structure-activity relationships. Chem. Pharm. Bull. 1995;43:920–926. doi: 10.1248/cpb.43.920. [DOI] [PubMed] [Google Scholar]
- 20.Yang E.J., Ku S.K., Lee W., Lee S., Lee T., Song K.S., Bae J.S. Barrier protective effects of rosmarinic acid on HMGB1-induced inflammatory responses in vitro and in vivo. J. Cell. Physiol. 2013;228:975–982. doi: 10.1002/jcp.24243. [DOI] [PubMed] [Google Scholar]
- 21.Gu L., Wu T., Wang Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var acuta. LWT Food Sci. Technol. 2009;42:131–136. doi: 10.1016/j.lwt.2008.04.006. [DOI] [Google Scholar]
- 22.Kim D.H., Lee J.H. Comparative evaluation of phenolic phytochemicals from perilla seeds of diverse species and screening for their tyrosinase inhibitory and antioxidant properties. S. Afr. J. Bot. 2019;123:341–350. doi: 10.1016/j.sajb.2019.03.015. [DOI] [Google Scholar]
- 23.Ha T.J., Lee J.H., Lee M.-H., Lee B.W., Kwon H.S., Park C.-H., Shim K.B., Kim H.T., Baek I.Y., Jang D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012;135:1397–1403. doi: 10.1016/j.foodchem.2012.05.104. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X.J., Yan L.L., Yin P.P., Shi L.L., Zhang J.H., Liu Y.J., Ma C. Structural characterization and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var arguta seed flour. Food Chem. 2014;164:150–157. doi: 10.1016/j.foodchem.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 25.Paek J.H., Shin K.H., Kang Y.H., Lee J.Y., Lim S.S. Rapid identification of aldose reductase inhibitory compounds from Perilla frutescens. BioMed Res. Int. 2013;2013:679463. doi: 10.1155/2013/679463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H.Y., Nam M.H., Lee H.S., Jun W., Hendrich S., Lee K.W. Isolation of caffeic acid from Perilla frutescens and its role in enhancing γ-glutamylcysteine synthetase activity and glutathione level. Food Chem. 2010;119:724–730. doi: 10.1016/j.foodchem.2009.07.020. [DOI] [Google Scholar]
- 27.Tada M., Matsumoto R., Yamaguchi H., Chiba K. Novel antioxidants isolated from Perilla frutescens Britton var crispa (Thunb) Biosci. Biotechnol. Biochem. 1996;60:1093–1095. doi: 10.1271/bbb.60.1093. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima A., Yamamoto Y., Yoshinaka N., Namba M., Matsuo H., Okuyama T., Yoshigai E., Okumura T., Nishizawa M., Ikeya Y. A new flavanone and other flavonoids from green perilla leaf extract inhibit nitric oxide production in interleukin 1β-treated hepatocytes. Biosci. Biotechnol. Biochem. 2015;79:138–146. doi: 10.1080/09168451.2014.962474. [DOI] [PubMed] [Google Scholar]
- 29.Jun H.I., Kim B.T., Song G.S., Kim Y.S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var acuta) leaves. Food Chem. 2014;148:367–372. doi: 10.1016/j.foodchem.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Asif M. Health effects of omega-369 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011;11:51–59. doi: 10.1007/s13596-011-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Y., Ye J., Kong J. Determination of phenolic compounds in Perilla frutescens L by capillary electrophoresis with electrochemical detection. J. Agric. Food Chem. 2005;53:8141–8147. doi: 10.1021/jf051360e. [DOI] [PubMed] [Google Scholar]
- 32.Assefa A.D., Jeong Y.J., Kim D.J., Jeon Y.A., Ok H.C., Baek H.J., Sung J.S. Characterization identification and quantification of phenolic compounds using UPLC-Q-TOF-MS and evaluation of antioxidant activity of 73 Perilla frutescens accessions. Food Res. Int. 2018;111:153–167. doi: 10.1016/j.foodres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Kagawa N., Iguchi H., Henzan M., Hanaoka M. Drying the leaves of Perilla frutescens increases their content of anticancer nutraceuticals. Food Sci. Nutr. 2019;7:1494–1501. doi: 10.1002/fsn3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng L., Lozano Y., Bombarda I., Gaydou E.M., Li B. Polyphenol extraction from eight Perilla frutescens cultivars. Comptes Rendus Chim. 2009;12:602–611. doi: 10.1016/j.crci.2008.04.011. [DOI] [Google Scholar]
- 35.Makino T., Ito M., Kiuchiu F., Ono T., Muso E., Honda G. Inhibitory effect of decoction of Perilla frutescens on cultured murine mesangial cell proliferation and quantitative analysis of its active constituents. Planta Med. 2001;67:24–28. doi: 10.1055/s-2001-10878. [DOI] [PubMed] [Google Scholar]
- 36.Iwai M., Ohta M., Tsuchiya H., Suzuki T. Enhanced accumulation of caffeic acid rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J. Funct. Foods. 2010;2:66–70. doi: 10.1016/j.jff.2009.11.002. [DOI] [Google Scholar]
- 37.Lee J.H., Park K.H., Lee M.H., Kim H.T., Seo W.D., Kim J.Y., Baek I.Y., Jang D.S., Ha T.J. Identification characterization and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013;136:843–852. doi: 10.1016/j.foodchem.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 38.Lee J., Rodriguez J.P., Quilantang N.G., Lee M.H., Cho E.J., Jacinto S.D., Lee S. Determination of flavonoids from Perilla frutescens var japonica seeds and their inhibitory effect on aldose reductase. Appl. Biol. Chem. 2017;60:155–162. doi: 10.1007/s13765-017-0260-5. [DOI] [Google Scholar]
- 39.Yoshida K., Kameda K., Kondo T. Diglucuronoflavones from purple leaves of Perilla ocimoides. Phytochemistry. 1993;33:917–919. doi: 10.1016/0031-9422(93)85304-A. [DOI] [PubMed] [Google Scholar]
- 40.Verspohl E.J., Fujii H., Homma K., Sybille B.-W. Testing of Perilla frutescens extract and vicenin 2 for their antispasmodic effect. Phytomedicine. 2013;20:427–431. doi: 10.1016/j.phymed.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Hou Y., Si Y., Wang W., Zhang S., Sun S., Liu X., Wang R., Wang W. Isolation, characterization, and xanthine oxidase inhibitory activities of flavonoids from the leaves of Perilla frutescens. Nat. Prod. Res. 2020;34:2566–2572. doi: 10.1080/14786419.2018.1544981. [DOI] [PubMed] [Google Scholar]
- 42.Kamei R., Fujimura T., Matsuda M., Kakihara K., Hirakawa N., Baba K., Ono K., Arakawa K., Kawamoto S. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochem. Biophys. Res. Commun. 2017;483:674–679. doi: 10.1016/j.bbrc.2016.12.083. [DOI] [PubMed] [Google Scholar]
- 43.Izumi Y., Matsumura A., Wakita S., Akagi K.I., Fukuda H., Kume T., Irie K., Yuki T.-T., Sugimoto H., Hashimoto T., et al. Isolation identification and biological evaluation of Nrf2-ARE activator from the leaves of green perilla (Perilla frutescens var crispa f viridis) Free Rad. Biol. Med. 2012;53:669–679. doi: 10.1016/j.freeradbiomed.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 44.He Y., Yao Y.-Y., Chang Y.-N. Characterization of anthocyanins in Perilla frutescens var acuta extract by advanced UPLC-ESI-IT-TOF-MS method and their anticancer bioactivity. Molecules. 2015;20:9155–9169. doi: 10.3390/molecules20059155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki M., Nakajima J.I., Yamanashi M., Sugiyama M., Makita Y., Springob K., Awazuhara M., Saito K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry. 2003;62:987–995. doi: 10.1016/S0031-9422(02)00721-5. [DOI] [PubMed] [Google Scholar]
- 46.Ryu J.-H., Haeng J.S., Lee S.H., Sohn D.H. Two neolignans from Perilla frutescens and their inhibition of nitric oxide synthase and tumor necrosis factor-α expression in murine macrophage cell line Raw 2.647. Bioorg. Med. Chem. Lett. 2002;12:649–651. doi: 10.1016/S0960-894X(01)00812-5. [DOI] [PubMed] [Google Scholar]
- 47.Liu J., Steigel A., Reininger E., Bauer R. Two new prenylated 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta. J. Nat. Prod. 2000;63:403–405. doi: 10.1021/np990362o. [DOI] [PubMed] [Google Scholar]
- 48.Jung S., Lee W.Y., Yong S.J., Shin K.C., Kim C.W., Lee J.H., Kim S.H. Occupational asthma caused by inhaling smoke from roasting perilla seeds. Allergy Asthma Respir. Dis. 2013;1:90–93. doi: 10.4168/aard.2013.1.1.90. [DOI] [Google Scholar]
- 49.Adhikari P., Hwang K.T., Park J.N., Kim C.K. Policosanol content and composition in perilla seeds. J. Agric. Food Chem. 2006;54:5359–5362. doi: 10.1021/jf060688k. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.K., Park S.Y., Na J.K., Seong E.S., Yu C.Y. Metabolite profiling based on lipophilic compounds for quality assessment of perilla (Perilla frutescens) cultivars. J. Agric. Food Chem. 2012;60:2257–2263. doi: 10.1021/jf204977x. [DOI] [PubMed] [Google Scholar]
- 51.Da Silva M.V., Da Silva A.V., Bonafe E.G., De Souza N.E., Visentainer J.V. Perilla frutescens: A potential ingredient for the enhancement of white bread as a source of Omega-3. Acta Sci. Technol. 2016;38:399–405. doi: 10.4025/actascitechnol.v38i4.27436. [DOI] [Google Scholar]
- 52.Longvah T., Deosthale Y.G., Kumar P.U. Nutritional and short tem toxicological evaluation of Perilla seed oil. Food Chem. 2000;70:13–16. doi: 10.1016/S0308-8146(99)00263-0. [DOI] [Google Scholar]
- 53.Sirilun S., Sivamaruthi B.S., Pengkumsri N., Saelee M., Chaiyasut K., Tuntisuwanno N., Suttajit M., Peerajan S., Chaiyasut C. Impact of different pre-treatment strategies on the quality of fatty acid composition, tocols content and metabolic syndrome related activities of Perilla frutescens seed oil. J. Appl. Pharm. Sci. 2016;6:1–8. doi: 10.7324/JAPS.2016.60201. [DOI] [Google Scholar]
- 54.Park Y.D., Jin C.H., Choi D.S., Byun M.W., Jeong I.Y. Biological evaluation of isoegomaketone isolated from Perilla frutescens and its synthetic derivatives as anti-inflammatory agents. Arch. Pharm. Res. 2011;34:1277–1282. doi: 10.1007/s12272-011-0806-8. [DOI] [PubMed] [Google Scholar]
- 55.Nam B., Yangkang S., Kim H.Y., Kim J.B., Jin C.H., Han A.R. A New Monoterpene from the leaves of a radiation mutant cultivar of Perilla frutescens var crispa with inhibitory activity on LPS-induced NO production. Molecules. 2017;22:1471. doi: 10.3390/molecules22091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji W.W., Wang S.Y., Ma Z.Q., Li R.P., Li S.S., Xue J.S., Li W., Niu X.X., Yan L., Zhang X., et al. Effects of perillaldehyde on alternations in serum cytokines and depressive-like behaviour in mice after lipopolysaccharide administration. Pharm. Biochem. Behav. 2014;116:1–8. doi: 10.1016/j.pbb.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 57.Lee A.Y., Choi J.M., Lee M.H., Lee J., Lee S., Cho E.J. Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide. Nutr. Res. Pract. 2018;12:93. doi: 10.4162/nrp.2018.12.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bumblauskiene L., Jakstas V., Janulis V., Mazdzieriene R., Ragazinskiene O. Preliminary analysis on essential oil composition of Perilla L cultivated in Lithuania. Acta Pol. Pharm. Drug Res. 2009;66:409–413. [PubMed] [Google Scholar]
- 59.Liu J., Wan Y., Zhao Z., Chen H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J. 2013;7:61. doi: 10.1186/1752-153X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura M., Ito M. Bioconversion of essential oil components of Perilla frutescens by Saccharomyces cerevisiae. J. Nat. Med. 2020;74:189–199. doi: 10.1007/s11418-019-01363-y. [DOI] [PubMed] [Google Scholar]
- 61.Kubo E., Miyoshi N., Fukuda M., Akagi Y. Cataract formation through the polyol pathway is associated with free radical production. Exp. Eye Res. 1999;68:457–464. doi: 10.1006/exer.1998.0624. [DOI] [PubMed] [Google Scholar]
- 62.Carper D.A., Hohman T.C., Old S.E. Residues affecting the catalysis and inhibition of rat lens aldose reductase. Biochim. Biophys. Acta. 1995;1246:67–73. doi: 10.1016/0167-4838(94)00182-G. [DOI] [PubMed] [Google Scholar]
- 63.Oates P.J., Mylari B.L. Aldose reductase inhibitors: Therapeutic implications for diabetic complications. Expert Opin. Investig. Drugs. 1999;8:2095–2119. doi: 10.1517/13543784.8.12.2095. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S., Collier A. Churchill’s Pocketbook of Diabetes. Churchill Livingstone; London, UK: 2012. Section 3—Management of Diabetes; pp. 83–125. [Google Scholar]
- 65.Smelcerovic A., Tomovic K., Smelcerovic Z., Petronijevic Z., Kocic G., Tomasic T., Jakopin Z., Anderluh M. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties predicted pharmacokinetics and toxicity. Eur. J. Med. Chem. 2017;135:491–516. doi: 10.1016/j.ejmech.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 66.Yuan H.Y., Zhang X.H., Zhang X.L., Wei J.F., Meng L. Analysis of patents on anti-gout therapies issued in China. Expert Opin. Ther. Pat. 2014;24:555–572. doi: 10.1517/13543776.2014.895325. [DOI] [PubMed] [Google Scholar]
- 67.Zalawadiya S.K., Veeranna V., Mallikethi-Reddy S., Bavishi C., Lunagaria A., Kottam A., Afonso L. Uric acid and cardiovascular disease risk reclassification: Findings from NHANES III. Eur. J. Prev. Cardiol. 2015;22:513–518. doi: 10.1177/2047487313519346. [DOI] [PubMed] [Google Scholar]
- 68.Lin M.S., Dai Y.S., Pwu R.F., Chen Y.H., Chang N.C. Risk estimates for drugs suspected of being associated with Stevens-Johnson syndrome and toxic epidermal necrolysis: A case-control study. Intern. Med. J. 2005;35:188–190. doi: 10.1111/j.1445-5994.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 69.Mockenhaupt M., Viboud C., Dunant A., Naldi L., Halevy S., Bavinck J.N., Sidoroff A., Schneck J., Roujeau J.C., Flahault A. Stevens–Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J. Investig. Derm. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi T., Nishi M., Inada A., Obata H., Tanabe M., Abe S., Wakashiro M. Two new potent inhibitors of xanthine oxidase from leaves of Perilla frutescens britton var Acuta Kudo. Chem. Pharm. Bull. 1990;38:1772–1774. doi: 10.1248/cpb.38.1772. [DOI] [PubMed] [Google Scholar]
- 71.Chang T.-M. Tyrosinase and tyrosinase inhibitors. J. Biocatal. Biotransform. 2012;1:2. doi: 10.4172/2324-9099.1000e106. [DOI] [Google Scholar]
- 72.Kim H.K., Cho S.R., Kim G.H. Insecticidal and antifeeding activity of Perilla frutescens-derived material against the diamondback moth Plutella xylostella L. Entomol. Res. 2019;49:55–62. doi: 10.1111/1748-5967.12336. [DOI] [Google Scholar]
- 73.Oh H.A., Park C.S., Ahn H.J., Park Y.S., Kim H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011;236:99–106. doi: 10.1258/ebm.2010.010252. [DOI] [PubMed] [Google Scholar]
- 74.Sbardella D., Fasciglione G.F., Gioia M., Ciaccio C., Tundo G.R., Marini S., Coletta M. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Asp. Med. 2012;33:119–208. doi: 10.1016/j.mam.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Heo J.C., Nam D.-Y., Myung S.S., Le S.-H. Alleviation of atopic dermatitis-related symptoms by Perilla frutescens Britton. Int. J. Mol. Med. 2011;28:733–737. doi: 10.3892/ijmm.2011.763. [DOI] [PubMed] [Google Scholar]
- 76.Komatsu K.I., Takanari J., Maeda T., Kitadate K., Sato T., Mihara Y., Uehara K., Wakame K. Perilla leaf extract prevents atopic dermatitis induced by an extract of Dermatophagoides farinae in NC/Nga mice. Asian Pac. J. Allergy Immunol. 2016;34:272–277. doi: 10.12932/AP0717. [DOI] [PubMed] [Google Scholar]
- 77.Shin T.Y., Kim S.H., Kim S.H., Kim Y.K., Park H.J., Chae B.S., Jung H.J., Kim H.M. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Perilla frutescens. Immunopharmacol. Immunotoxicol. 2000;22:489–500. doi: 10.3109/08923970009026007. [DOI] [PubMed] [Google Scholar]
- 78.Jeon I.H., Kim H.S., Kang H.J., Lee H.S., Jeong S.I., Kim S.J., Jang S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P frutescens L.) leaves. Molecules. 2014;19:6941–6951. doi: 10.3390/molecules19066941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asada M., Fukumori Y., Inoue M., Nakagomi K., Sugie M., Fujita Y. Glycoprotein derived from the hot water extract of mint plant Perilla frutescens Britton. J. Agric. Food Chem. 1999;47:468–472. doi: 10.1021/jf9802777. [DOI] [PubMed] [Google Scholar]
- 80.Chen C.Y., Leu Y.L., Fang Y., Lin C.F., Kuo L.M., Sung W.C. Anti-inflammatory effects of Perilla frutescens in activated human neutrophils through two independent pathways: Src family kinases and calcium. Sci. Rep. 2015;5:18204. doi: 10.1038/srep18204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanbongi C., Takano H., Osakabe N., Sasa N., Natsume M., Yanagisawa R., Inoue K.I., Sadakane K., Ichinose T., Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen in a mouse model. Clin. Exp. Allergy. 2004;34:971–977. doi: 10.1111/j.1365-2222.2004.01979.x. [DOI] [PubMed] [Google Scholar]
- 82.Takano H., Osakabe N., Sanbongi C., Yanagisawa R., Inoue K.I., Yasuda A., Natsume M., Baba S., Ichiishi E.I., Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid a polyphenolic phytochemical inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004;229:247–254. doi: 10.1177/153537020422900305. [DOI] [PubMed] [Google Scholar]
- 83.Makino T., Furuta Y., Wakushima H., Fujii H., Saito K.I., Kano Y. Antiallergic effect of Perilla frutescens and its active consti3tuents. Phytother. Res. 2003;17:240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 84.Chang H.H., Chen C.S., Lin J.Y. Dietary perilla oil lowers serum lipids and ovalbumin-specific IgG1 but increases total IgE levels in ovalbumin-challenged mice. Food Chem. Toxicol. 2009;47:848–854. doi: 10.1016/j.fct.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Takeda H., Tsuji M., Masato I., Egashira T., Matsumiya T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002;449:261–267. doi: 10.1016/S0014-2999(02)02037-X. [DOI] [PubMed] [Google Scholar]
- 86.Rimando A.M., Inoshiri S., Otsuka H. Screening for mast cell histamine release inhibitory activity of Philippine medicinal plants Active constituent of Ehretia. Microphylla. 1987;41:242–247. [Google Scholar]
- 87.Cheng J.T., Liu I.M. Stimulatory effect of caffeic acid on α 1A-adrenoceptors to increase glucose uptake into cultured C 2 C 12 cells. Naunyn-Schmiedebergs Arch. Pharm. 2000;362:122–127. doi: 10.1007/s002100000274. [DOI] [PubMed] [Google Scholar]
- 88.Soliman K.F., Mazzio E.A. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc. Soc. Exp. Biol. Med. 1998;218:390–397. doi: 10.3181/00379727-218-44309. [DOI] [PubMed] [Google Scholar]
- 89.Yokozawa T., Chen C.P. Role of Salviae Miltiorrhizae Radix extract and its compounds in enhancing nitric oxide expression. Phytomedicine. 2000;7:55–61. doi: 10.1016/S0944-7113(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 90.Yi L.T., Li J., Geng D., Liu B.B., Fu Y., Tu J.Q., Liu Y., Weng L.-J. Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in mice. J. Ethnopharmacol. 2013;147:245–253. doi: 10.1016/j.jep.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 91.Ji W.-W., Li R.P., Li M., Wang S.Y., Zhang X., Ni X.X., Li W., Ya L., Yang W.A., Qiang F.U. Antidepressant-like effect of essential oil of Perilla frutescens in a chronic unpredictable mild stress-induced depression model mice. Chin. J. Nat. Med. 2014;12:0753–0759. doi: 10.1016/S1875-5364(14)60115-1. [DOI] [PubMed] [Google Scholar]
- 92.Nakazawa T., Yasuda T., Ueda J., Ohsawa K. Antidepressant-like effects of apigenin and 2 4 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- 93.Osakabe N., Yasuda A., Natsume M., Sanbongi C., Kato Y., Osawa T., Yoshikawa T. Rosmarinic acid a major polyphenolic component of Perilla frutescens reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Rad. Biol. Med. 2002;33:798–806. doi: 10.1016/S0891-5849(02)00970-X. [DOI] [PubMed] [Google Scholar]
- 94.Yang S.Y., Hong C.O., Lee G.P., Kim C.T., Lee K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013;55:92–99. doi: 10.1016/j.fct.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 95.Li J.J., Li Z., Gu L.J., Choi K.J., Kim D.S., Kim H.K., Sung C.K. The promotion of hair regrowth by topical application of a Perilla frutescens extract through increased cell viability and antagonism of testosterone and dihydrotestosterone. J. Nat. Med. 2018;72:96–105. doi: 10.1007/s11418-017-1116-3. [DOI] [PubMed] [Google Scholar]
- 96.Feng L.J., Yu C.H., Ying K.J., Hua J., Dai X.Y. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011;44:404–409. doi: 10.1016/j.foodres.2010.09.035. [DOI] [Google Scholar]
- 97.Korotkich I., Senikiene Z., Simoniene G., Lazauskas R., Laukeviciene A., Kevelaitis E. Inotropic and lusitropic effects of Perilla frutescens (L) Britton extract on the rabbit myocardium. Medicina. 2006;42:406–412. [PubMed] [Google Scholar]
- 98.Zhao G., Yao-Yue C., Qin G.W., Guo L.H. Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiol. Aging. 2012;33:176–186. doi: 10.1016/j.neurobiolaging.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 99.Kim J.S., Lee H.J., Lee M.H., Kim J., Jin C., Ryu J.H. Luteolin inhibits LPS-stimulated inducible nitric oxide synthase expression in BV-2 microglial cells. Planta Med. 2006;72:65–68. doi: 10.1055/s-2005-873145. [DOI] [PubMed] [Google Scholar]
- 100.Lee A.Y., Hwang B.R., Lee M.H., Lee S., Cho E.J. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25–35 induced impairment of cognition and memory function. Nutr. Res. Pract. 2016;10:274. doi: 10.4162/nrp.2016.10.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee A.Y., Wu T.T., Hwang B.R., Lee J., Lee M.H., Lee S., Cho E.J. The neuro-protective effect of the methanolic extract of Perilla frutescens var. japonica and rosmarinic acid against H2O2-induced oxidative stress in C6 glial cells. Biomol. Ther. 2016;24:338. doi: 10.4062/biomolther.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi S.H., Hur J.M., Yang E.J., Jun M., Park H.J., Lee K.B., Moon E., Song K.S. β-Secretase (BACE1) inhibitors from Perilla frutescens var acuta. Arch. Pharmacal Res. 2008;31:183–187. doi: 10.1007/s12272-001-1139-9. [DOI] [PubMed] [Google Scholar]
- 103.Senavong P., Kongkham S., Saelim S., Suangkavathin V. Neuroprotective effect of perilla extracts on PC12 cells. J. Med. Assoc. Thail. 2016;99:S256–S264. doi: 10.1055/s-0036-1596545. [DOI] [PubMed] [Google Scholar]
- 104.Huang B.P., Lin C.H., Chen Y.C., Kao S.H. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW2647 cells. Mol. Med. Rep. 2014;10:1077–1083. doi: 10.3892/mmr.2014.2298. [DOI] [PubMed] [Google Scholar]
- 105.Urushima H., Nishimura J., Mizushima T., Hayashi N., Maeda K., Ito T. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G32–G41. doi: 10.1152/ajpgi.00294.2014. [DOI] [PubMed] [Google Scholar]
- 106.Ku S.K., Yang E.J., Song K.S., Bae J.S. Rosmarinic acid down-regulates endothelial protein C receptor shedding in vitro and in vivo. Food Chem. Toxicol. 2013;59:311–315. doi: 10.1016/j.fct.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Arya E., Saha S., Saraf S.A., Kaithwas G. Effect of Perilla frutescens fixed oil on experimental esophagitis in albino wistar rats. BioMed Res. Int. 2013;2013:981372. doi: 10.1155/2013/981372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan B.L., Norhaizan M.E., Liew W.P., Rahman S.H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018;16:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeong W.S., Jun M., Kong A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 110.Lin C.S., Kuo C.L., Wang J.P., Cheng J.S., Huang Z.W., Chen C.F. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J. Ethnopharmacol. 2007;112:557–567. doi: 10.1016/j.jep.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 111.Kwak Y., Ju J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth migration and adhesion of human cancer cells. Nutr. Res. Pract. 2015;9:11–16. doi: 10.4162/nrp.2015.9.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Narisawa T., Fukaura Y., Yazawa K., Ishikawa C., Isoda Y., Nishizawa Y. Colon cancer prevention with a small amount of dietary perilla oil high in alpha-linolenic acid in an animal model. Cancer. 1994;73:2069–2075. doi: 10.1002/1097-0142(19940415)73:8<2069::AID-CNCR2820730810>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y., Liu S., Feng Q., Huang X., Wang X., Peng Y., Zhao Z., Liu Z. Perilaldehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. J. Cell. Biochem. 2019;120:1716–1725. doi: 10.1002/jcb.27491. [DOI] [PubMed] [Google Scholar]
- 114.Kang R., Helms R., Stout M.J., Jaber Z., Chen Z., Nakatsu T. Antimicrobial activity of the volatile constituents of Perilla frutescens and its synergistic effects with polygodial. J. Agric. Food Chem. 1992;40:2328–2330. doi: 10.1021/jf00023a054. [DOI] [Google Scholar]
- 115.Yamamoto H., Ogawa T. Antimicrobial activity of Perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002;66:921–924. doi: 10.1271/bbb.66.921. [DOI] [PubMed] [Google Scholar]
- 116.Li H.Z., Zhiqing R., Reddy N.V., Hou T., Zhang Z. In silico evaluation of antimicrobial antihyaluronidase and bioavailability parameters of rosmarinic acid in Perilla frutescens leaf extracts. SN Appl. Sci. 2020;2:1547. doi: 10.1007/s42452-020-03323-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.