Abstract
We reported a new method dealing with the synthesis of novel pharmacologically relevant α-aminophosphonate derivatives via a lipase-catalyzed Kabachnik−Fields reaction with yields of up to 93%. The advantages of this protocol are excellent yields, mild reaction conditions, low costs, and sustainability. The developed protocol is applicable to a range of H-phosphites and organic amines, providing a wide substrate scope. A new class of α-aminophosphonate analogues possessing P-chiral centers was also synthesized. The synthesized compounds were characterized on the basis of their antimicrobial activities against E. coli. The impact of the various alkoxy groups on antimicrobial activity was demonstrated. The crucial role of the substituents, located at the aromatic rings in the phenylethyloxy and benzyloxy groups, on the inhibitory action against selected pathogenic E. coli strains was revealed. The observed results are especially important because of increasing resistance of bacteria to various drugs and antibiotics.
Keywords: α-aminophosphonates, Kabachnik−Fields reaction, antimicrobial activity
1. Introduction
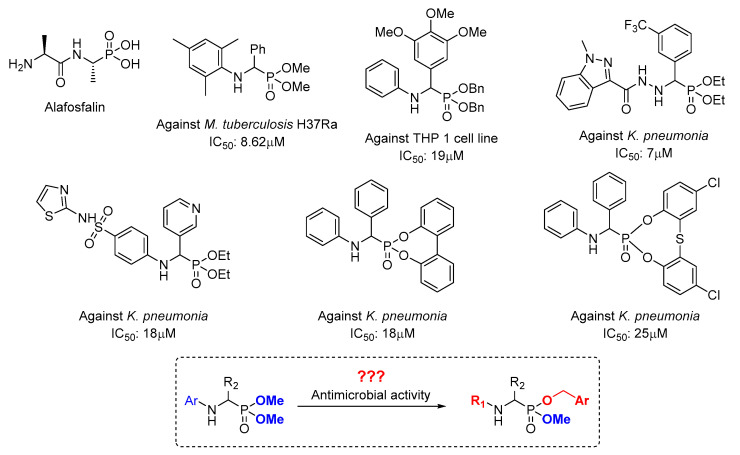
α-Aminophosphonates appear to be a very important class of organic compounds because of their potential biological activities [1,2,3,4]. The distinctive character of bioactive organophosphorus compounds has established their wide applicability in agricultural and medicinal chemistry [5,6,7,8,9,10,11,12]. α-Aminophosphonates (Figure 1) play a crucial role as a platform to design new drugs [13,14,15,16,17,18,19,20]. Among other advantages, there are several reports regarding their antimicrobial activity; alafosfalin, for example, a simple dialkyl α-aminophosphonate, exhibits activity against pathogenic E. coli, S. aureus, Bacillus, and K. pneumonia strains (Figure 1) [21,22,23,24,25,26,27,28,29,30]. However, the application of alafosfalin in medicine is limited due to its instability. It is shown that the correct design of the alkoxyl groups in the H-phosphite used for these compounds may significantly increase their antimicrobial properties (Figure 1) [1,2,3,31,32,33,34,35,36].
Figure 1.
Biologically active antimicrobial α-aminophosphonate derivatives.
The aim of the work is to develop a metal-free protocol for the preparation of α-aminophosphonate derivatives with P-chiral centers on bacterial strains K12 and R2−R4.
2. Materials and Methods
2.1. Microorganisms and Media
All microorganisms and media were accurately described in detail in the previous work [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] and analyzed by a Tukey test.
2.2. General Methods of Synthesis α-Aminophosphonate Derivatives
All the chemicals were described in detail in the previous work [74]. All specific strains, such as Pseudomonas cepacia (PcL) and wheat germ, were provided by Sigma-Aldrich (Merck). The bovine acetone powder was prepared in our laboratory according to the literature procedure [38]. Symmetrical and unsymmetrical H-phosphites were obtained via alcoholysis of the dimethyl phosphite with the appropriate alcohol according to the literature procedure [40,41,42,43,44,45,46] (see Supplementary Materials).
3. Results
3.1. Chemistry
Organophosphorus compounds show a variety of relevant biological activities [47,48,49]. The Kabachnik–Fields reaction is the most efficient method for the formation of carbon−phosphorus bonds using an aldehyde, amine, and H-phosphite. A number of other synthetic approaches have also been reported for the preparation of α-aminophosphonates [7,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95]. These methods are generally conducted in the presence of various organic and inorganic bases [56,57,58] as well as Lewis and Bronsted acids, such as zirconium tetrachloride (ZrCl4), aluminum chloride (AlCl3), tantalum pentachloride (TaCl5), or lanthanide triflates [59,60,61,62,63]. Therefore, it is necessary to further develop an efficient one-pot, multicomponent synthesis of α-aminophosphonates that is devoid of these problems and fulfils requirements of the pharmaceutical industry. Enzymes, which are natural catalysts with high catalytic activity, seem to be the best alternative leading to the development of new synthesis methods that meet the requirements related to safety and environmental protection. In addition, enzymes enable the synthesis of compounds without metal contamination, which is especially appreciated by the pharmaceutical industries. Among other uses, hydrolases are most often used as biocatalysts in organic synthesis. Our work is more focused on discovering new unnatural catalytic activities of hydrolases. This phenomenon was defined as enzymatic promiscuity. Recently, a number of unnatural reactions catalyzed by hydrolases have been reported, such as the aza-Henry reaction [64], Michael additions [65,66], 1,2-addition of thiols to imines [67], and Morita–Baylis–Hillman reaction [68,69,70]. Although some chemical strategies work towards the synthesis of α-aminophosphonates, the biocatalytic preparation of target α-aminophosphonates remains unexploited. It was shown that some selected α-aminophosphonates could be obtained from aniline derivatives by the Kabachnik–Fields reaction using Candida antarctica lipase B (CAL-B) as a catalyst [71,72,73].
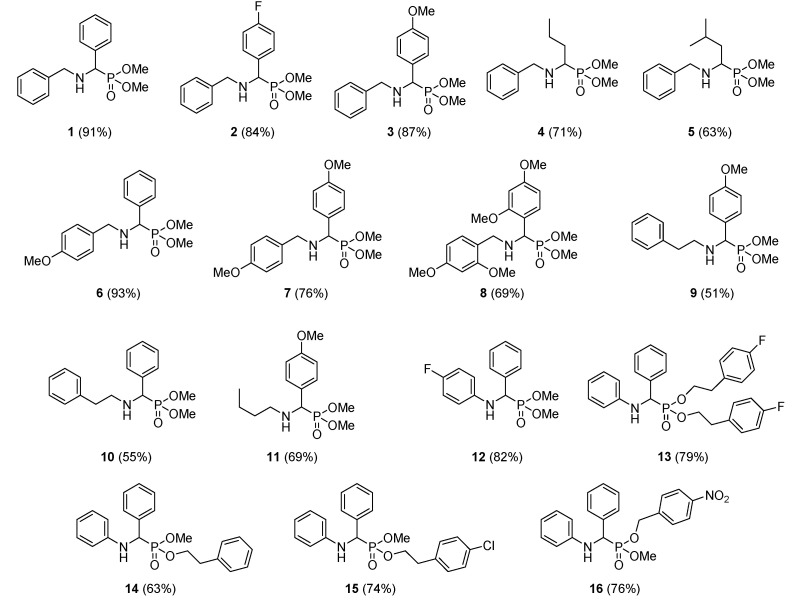
As a continuation of our research on seeking new catalytic activities of hydrolases [74,75,76,77,78,79,80,94,95], we focused our efforts on elaborating a sustainable metal-free method towards desired α-aminophosphonates 1–16 (Figure 2).
Figure 2.
α-Aminophosphonates 1–16 obtained via an enzyme-catalyzed Kabachnik–Fields reaction. Yields in brackets provided for isolated products 1–16.
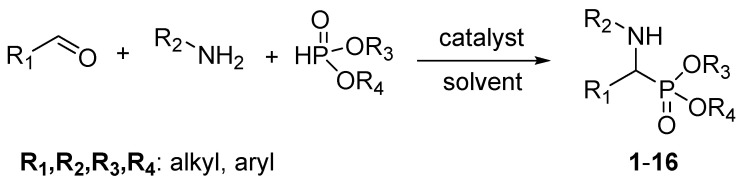
Regarding the promiscuous activity of lipases, [71] the model Kabachnik–Fields reaction of benzyl amine (1 mmol), benzaldehyde (1 mmol), and dimethyl phosphite (1 mmol) was conducted in neat at 25 °C (Scheme 1 and Table 1, entry 1).
Scheme 1.
Enzyme-catalyzed synthesis of α-aminophosphonates 1–16.
Table 1.
Model Kabachnik–Fields reaction catalyzed by enzymes. [a] Optimization studies.
| Entry | Catalyst | T (°C) | Solvent | Yield [%] f |
|---|---|---|---|---|
| 1 | None | 25 | neat | <5 |
| 2 | Porcine pancreas lipase (PpL) | 25 | neat | 73 |
| 3 | Porcine pancreas lipase (PpL) | 25 | Toluene | 64 |
| 4 | Porcine pancreas lipase (PpL) | 25 | EtOAc | 18 |
| 5 | Porcine pancreas lipase (PpL) | 25 | THF | 52 |
| 6 | Porcine pancreas lipase (PpL) | 25 | 2-Me THF | 55 |
| 7 | Porcine pancreas lipase (PpL) | 25 | TBME | 88 |
| 8 | Porcine pancreas lipase (PpL) | 30 | TBME | 91 |
| 9 | Porcine pancreas lipase (PpL) | 40 | TBME | 87 |
| 10 | Porcine pancreas lipase (PpL) b | 30 | TBME | 93 |
| 11 | Wheat germ lipase | 20 | neat | 29 |
| 12 | Pseudomonas cepacia lipase (PfL) | 20 | neat | 44 |
| 13 | Candida cylindracea lipase (CcL) | 20 | neat | 57 |
| 14 | Candida rugosa lipase (CrL) | 20 | neat | 35 |
| 15 | Novozym 435 | 20 | neat | 67 |
| 16 | Bovine serum albumin (BSA) | 30 | TBME | 9 |
| 17 | Bovine liver acetone powder (BLAP) c | 20 | neat | 43 |
| 18 | Denatured PpL d | 30 | TBME | <1 |
| 19 | CuI e | 25 | neat | 39 |
| 20 | Cu2O e | 25 | neat | 24 |
| 21 | Cu(OAc)2 e | 25 | neat | 33 |
| 22 | PhB(OH)2 e | 25 | neat | 14 |
a Reaction conditions: benzaldehyde (1 mmol), benzylamine (1 mmol), dimethyl phosphite (1 mmol), and enzyme (50 mg) in a solvent (2 mL) for 24 h, 200 rpm; b PpL (80 mg); c domestically prepared; d thermally deactivated at 100 °C for 24 h; e 10 mol%; f yield of the isolated product 1 after chromatography on silica gel.
As shown in Table 1, lipase from a porcine pancreas (PpL) was found as the best catalyst among the tested lipases for this addition reaction (Table 1, entry 2). The α-aminophosphonate 1 obtained agood yield (73%) after 24 h in neat at 25 °C. The yield did not increase substantially after 24 h. In the absence of enzyme only traces of the target product 1 was formed (Table 1, entry 1). In addition, four different nonenzymatic catalysts were reported in the literature as sustainable promoters of the Kabachnik–Fields reaction [81,82,83,94,95]; copper(I) iodide, copper(I) oxide, copper(II) acetate, and phenylboronic acid were tested under similar reaction conditions, leading to the target product 1 with up to a 39% yield (Table 1, entries 19–22). It is well recognized that the type of solvent used has a great impact on enzyme stability and activity [84]. Product 1 was provided with the highest yield of 88% in TBME (Table 1, entry 7); therefore, this solvent was applied in the following optimization. Furthermore, the model reaction was carried out at elevated temperatures; however, the yield of product 1 was reduced at temperatures above 30 °C (Table 1, entries 8 and 9). Next, we studied if the amount of enzyme used had any impact on the reaction yield, and we found out that the yield of target compound 1 increased slightly by raising the amount of PpL from 50 mg to 80 mg. Thus, the 80 mg of PpL was the optimal amount for the further investigations [71,94,95].
Finally, we used the elaborated enzymatic protocol with various aromatic and aliphatic amines, aldehydes, and symmetrical as well as unsymmetrical H-phosphites [41] (Figure 2). The enzymatic Kabachnik–Fields reaction with aliphatic aldehydes and amines as well as 2-phenylethylamine provided products 4, 5, and 9–11 with lower yield ranges from 51% to 71% (Figure 2). A similar reduction in the reaction yield was observed for sterically bulky electron-rich aldehyde and amine with methoxy groups located at the phenyl ring, which resulted in product 8 with a 69% yield. Finally, the application of unsymmetrical H-phosphonates provided P-chiral products 14–16 as a mixture of diasteroisomers (1:1) with yields up to 76%. The structures of all obtained compounds 1–16 are presented in the experimental section (Supplementary Materials Figures S4–S65).
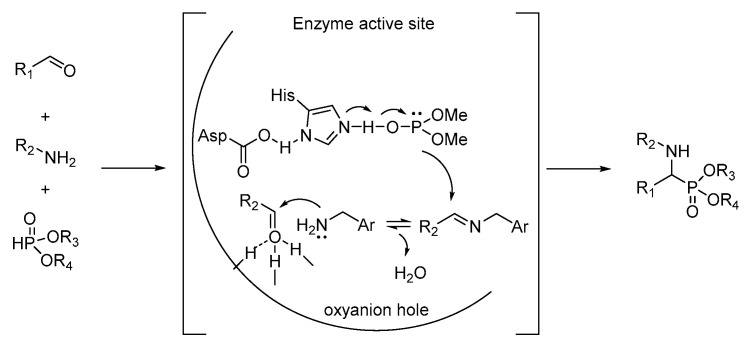
Additional experiments were performed to gather insights on the reaction pathway. Under developed conditions, N-(4-methoxylbenzylidene)benzylamine was used together with dimethyl H-phosphite in the presence of PpL as a catalyst, which resulted in an excellent yield of 95% of the target α-aminophosphonate 1. This observation constitutes the initial formation of an imine in the presence of lipase (Scheme 2).
Scheme 2.
Plausible mechanism of the porcine pancreas lipase-catalyzed Kabachnik−Fields reaction.
3.2. Cytotoxic Studies of the Library of α-Hydroxy Phosphonate Derivatives
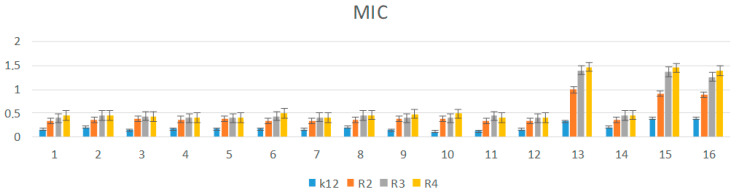
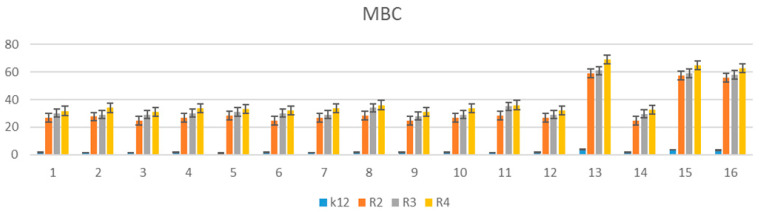
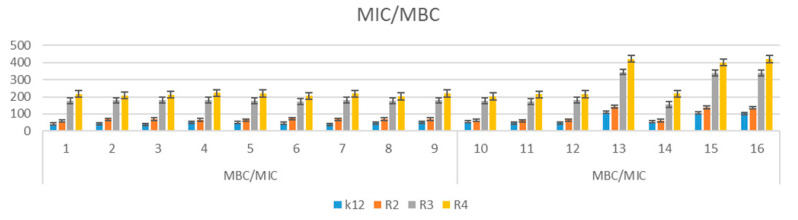
It is worth noting that the introduction of a fluorine atom into the structure of all 16 tested compounds did not have a significant effect on the activity of 2 and 12, which is often observed for various types of compounds exhibiting antibacterial activity [12] (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
Figure 3.
Minimum inhibitory concentration (MIC) of the phosphonate derivatives in model bacterial strains. The x-axis features compounds 1–16 used sequentially. The y-axis shows the MIC value in µg/mL−1. Investigated strains of E. coli K12 as the control (blue), R2 strains (orange), R3 strains (grey), and R4 strains (yellow). The order in which the compounds were applied to the plate is shown in Supplementary Materials Figure S1.
Figure 4.
Minimum bactericidal concentration (MBC) of the phosphonate derivatives. The x-axis features compounds 1–16 used sequentially. The y-axis shows the MIC value in µg/mL−1. Investigated strains of E. coli K12 as control (blue), R2 strains (orange), R3 strains (grey), and R4 strains (yellow). The order in which the compounds were applied to the plate is shown in Supplementary Materials Figure S1.
Figure 5.
The ratio of MBC/MIC of the phosphonate derivatives. The x-axis features compounds 1–16 used sequentially. The y-axis shows the MIC value in µg/mL−1. Investigated strains of E. coli K12 as control (blue), R2 strains (orange), R3 strains (grey), and R4 strains (yellow). The order in which the compounds were applied to the plate is shown in Supplementary Materials Figure S1.
Figure 6.
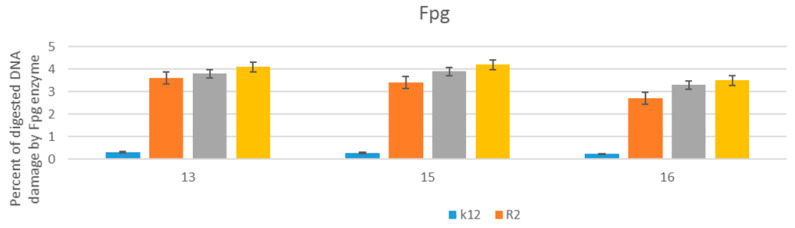
Percentage of plasmid DNA recognized by the Fpg enzymes (y-axis) with model bacterial, K12, and R2–R4 strains (x-axis). All analyzed compounds numbered were statistically significant at <0.05 (see Table 2).
Figure 7.
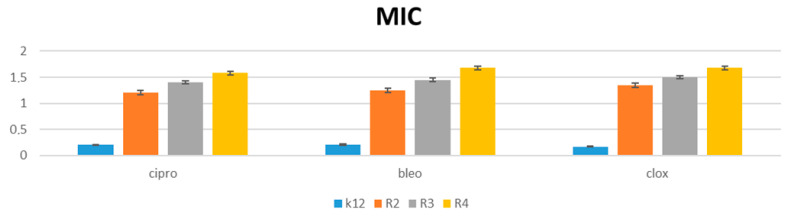
Examples of MIC with model bacterial strains K12, R2, R3, and R4 for studying the antibiotics ciprofloxacin (cipro), bleomycin (bleo), and cloxacillin (clox). The x-axis features antibiotics used sequentially. The y-axis features the MIC value in µg/mL−1.
The analyzed bacterial strains used in the experiments were used in 48-well plates. (Figure 3, Figure 4 and Figure 5 and Table 2).
Table 2.
Statistical analysis of all analyzed compounds by MIC, MBC, and MBC/MIC; <0.05 *, <0.01 **, <0.001 ***.
| No. of Samples | 13 | 15 | 16 | Type of Test |
|---|---|---|---|---|
| K12 | *** | *** | *** | MIC |
| R2 | *** | *** | *** | MIC |
| R3 | *** | *** | *** | MIC |
| R4 | *** | *** | *** | MIC |
| K12 | ** | * | ** | MBC |
| R2 | ** | * | ** | MBC |
| R3 | ** | * | ** | MBC |
| R4 | ** | * | ** | MBC |
| K12 | *** | ** | ** | MBC/MIC |
| R2 | *** | ** | ** | MBC/MIC |
| R3 | *** | ** | ** | MBC/MIC |
| R4 | *** | ** | ** | MBC/MIC |
3.3. Analysis of R2–R4 E. coli Strains Modified with α-Aminophosphonate Derivatives
The obtained MIC values as well as our previous studies with various types of the analyzed compounds [85,86,87,88,89,90,91,92,93,94,95] indicate that α-aminophosphonate derivatives also show a strong toxic effect on the analyzed bacterial model strains. The three analyzed compounds were selected for further analysis by modifying their DNA. Modified bacterial DNA was digested with Fpg as described earlier [85,86,87,88,89,90,91,92,93]. All selected analyzed α-aminophosphonate derivatives (Figure 6), including different types of alkoxy groups, substituents located at the phenyl ring, and the length of the alkyl chain, can strongly change the topology of bacterial DNA. After digestion with Fpg, approximately 3.5% of oxidative damage was identified, which very strongly indicates oxidative damage in bacterial DNA, similar to the previous observations [85,86,87,88,89,90,91,92,93]. The different types of alkoxy groups, substituents located at the phenyl ring, and the length of the alkyl affected this outcome (Figure 6).
3.4. R2-R4 E. coli Strains with Tested α-Aminophosphonate Derivatives
The performed studies prove that the analyzed and newly synthesized compounds can potentially be used as “substitutes” for the currently used antibiotics in hospital and clinical infections, (Figure 7 and Figure 8 and Supplementary Materials Figure S3).
Figure 8.
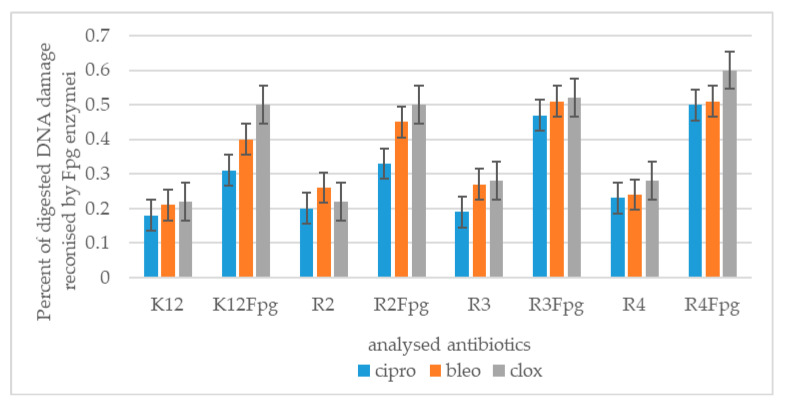
Percentage of bacterial DNA recognized by the Fpg enzymes in model bacterial strains after ciprofloxacin, bleomycin, and cloxacillin treatment. The compounds were statistically significant at p < 0.05.
Large modifications of plasmid DNA were observed for the three analyzed compounds numbered 13, 15, and 16, showing high superselectivity.
4. Conclusions
Our developed protocol provides an efficient mild and metal-free synthesis of the target products with a high yield (51–93%). Among the studied derivatives, the compounds possessing alkoxy groups with halogen atoms or nitro groups in phosphate moieties 13, 15, and 16 turned out to be the most active compared to derivatives with the dimethyl groups (Figure 2). Finally, the reported α-aminophosphonate derivatives are more cytotoxic in the model bacterial cells than the following commonly used antibiotics: ciprofloxacin, bleomycin, and cloxacillin.
Acknowledgments
The authors thank Jolanta Łukasiewicz from the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy (Polish Academy of Sciences) for providing the strains of E. coli.
Abbreviations
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| Oc | open circle |
| Ccc | covalently closed circle |
| BER | base excision repair |
| Fpg | DNA-formamidopyrimidine glycosylase |
Supplementary Materials
The supporting information can be downloaded at the following: https://www.mdpi.com/article/10.3390/ma15113846/s1. Figure S1: examples of MIC and MBC on microplates with different concentrations of studied compounds (mg L−1). Figure S2: an example of an agarose gel electrophoresis separation of isolated plasmids DNA from R4 strains modified with selected compounds. Figure S3: an example of an agarose gel electrophoresis separation of isolated plasmids DNA from R4 strains modified with the following antibiotics: cloxacilline, ciprofloxaclinie, and bleomycine digested (or not) with repair Fpg enzymes. NMR spectra of α-aminophosphonate derivatives 1–16. Figure S4: 1HNMR (400 MHz, CDCl3) spectra of compound 1. Figure S5: 13CNMR (100 MHz, CDCl3) spectra of compound 1. Figure S6: 31PNMR (162 MHz, CDCl3) spectra of compound 1. Figure S7: 1HNMR (400 MHz, CDCl3) spectra of compound 2. Figure S8: 13CNMR (100 MHz, CDCl3) spectra of compound 2. Figure S9: 31PNMR (162 MHz, CDCl3) spectra of compound 2. Figure S10: 1HNMR (400 MHz, CDCl3) spectra of compound 3. Figure S11: 13CNMR (100 MHz, CDCl3) spectra of compound 3. Figure S12: 31PNMR (162 MHz, CDCl3) spectra of compound 3. Figure S13: 1HNMR (400 MHz, CDCl3) spectra of compound 4. Figure S14: 13CNMR (100 MHz, CDCl3) spectra of compound 4. Figure S15: 31PNMR (162 MHz, CDCl3) spectra of compound 4. Figure S16: 1HNMR (400 MHz, CDCl3) spectra of compound 5. Figure S17: 13CNMR (100 MHz, CDCl3) spectra of compound 5. Figure S18: 31PNMR (162 MHz, CDCl3) spectra of compound 5. Figure S19: 1HNMR (400 MHz, CDCl3) spectra of compound 6. Figure S20: 13CNMR (100 MHz, CDCl3) spectra of compound 6. Figure S21: 31PNMR (162 MHz, CDCl3) spectra of compound 6. Figure S22: 1HNMR (400 MHz, CDCl3) spectra of compound 7. Figure S23: 13CNMR (100 MHz, CDCl3) spectra of compound 7. Figure S24: 31PNMR (162 MHz, CDCl3) spectra of compound 7. Figure S25: 1HNMR (400 MHz, CDCl3) spectra of compound 8. Figure S26: 13CNMR (100 MHz, CDCl3) spectra of compound 8. Figure S27: 31PNMR (162 MHz, CDCl3) spectra of compound 8. Figure S28: 1HNMR (400 MHz, CDCl3) spectra of compound 9. Figure S29: 13CNMR (100 MHz, CDCl3) spectra of compound 9. Figure S30: 31PNMR (162 MHz, CDCl3) spectra of compound 9. Figure S31: 1HNMR (400 MHz, CDCl3) spectra of compound 10. Figure S32: 13CNMR (100 MHz, CDCl3) spectra of compound 10. Figure S33: 31PNMR (162 MHz, CDCl3) spectra of compound 10. Figure S34: 1HNMR (400 MHz, CDCl3) spectra of compound 11. Figure S35: 13CNMR (100 MHz, CDCl3) spectra of compound 11. Figure S36: 31PNMR (162 MHz, CDCl3) spectra of compound 11. Figure S37: 1HNMR (400 MHz, CDCl3) spectra of compound 12. Figure S38: 13CNMR (100 MHz, CDCl3) spectra of compound 12. Figure S39: 31PNMR (162 MHz, CDCl3) spectra of compound 12. Figure S40: 1HNMR (400 MHz, CDCl3) spectra of compound 13. Figure S41: 13CNMR (100 MHz, CDCl3) spectra of compound 13. Figure S42: 31PNMR (162 MHz, CDCl3) spectra of compound 13. Figure S43: 1HNMR (400 MHz, CDCl3) spectra of compound 14. Figure S44: 13CNMR (100 MHz, CDCl3) spectra of compound 14. Figure S45: 31PNMR (162 MHz, CDCl3) spectra of compound 14. Figure S46: 1HNMR (400 MHz, CDCl3) spectra of compound 15. Figure S47: 13CNMR (100 MHz, CDCl3) spectra of compound 15. Figure S48: 31PNMR (162 MHz, CDCl3) spectra of compound 15. Figure S49: 1HNMR (400 MHz, CDCl3) spectra of compound 16. Figure S50: 13CNMR (100 MHz, CDCl3) spectra of compound 16. Figure S51: 31PNMR (162 MHz, CDCl3) spectra of compound 16. Figure S52: 1HNMR (400 MHz, CDCl3) spectra of bis(4-fluorophenylethyl)phosphite. Figure S53: 13CNMR (100 MHz, CDCl3) spectra of bis(4-fluorophenylethyl)phosphite. Figure S54: 31PNMR (162 MHz, CDCl3) spectra of bis(4-fluorophenylethyl)phosphite. Figure S55: 1HNMR (400 MHz, CDCl3) spectra of methyl (phenylethyl) phosphite. Figure S56: 13CNMR (100 MHz, CDCl3) spectra of methyl (phenylethyl) phosphite. Figure S57: 31PNMR (162 MHz, CDCl3) spectra of methyl (phenylethyl) phosphite. Figure S58: 1HNMR (400 MHz, CDCl3) spectra of methyl (4-chlorophenylethyl) phosphite. Figure S59: 13CNMR (100 MHz, CDCl3) spectra of methyl (4-chlorophenylethyl) phosphite. Figure S60: 31PNMR (162 MHz, CDCl3) spectra of methyl (4-chlorophenylethyl) phosphite. Figure S61: 1HNMR (400 MHz, CDCl3) spectra of methyl (4-nitrobenzyl) phosphite. Figure S62: 13CNMR (100 MHz, CDCl3) spectra of methyl (4-nitrobenzyl) phosphite. Figure S63: 31PNMR (162 MHz, CDCl3) spectra of methyl (4-nitrobenzyl) phosphite. Figure S64: 1HNMR (400 MHz, CDCl3) spectra of N-(4-methoxylbenzylidene)benzylamine. Figure S65: 13CNMR (100 MHz, CDCl3) spectra of N-(4-methoxylbenzylidene)benzylamine.
Author Contributions
Conceptualization, P.K. and D.K.; methodology, P.K., D.K., P.Ś., J.S.-G. and A.W.; software P.Ś., J.S.-G., K.K., investigation, P.K., A.W. and M.S.; validation, P.Ś., J.S.-G., K.K., formal analysis P.K., D.K., R.O., A.W., P.Ś., J.S.-G., K.K. resources, P.K., D.K., K.K.; data curation, K.K., M.S. and A.W.; writing—original draft preparation, P.K., R.O. and D.K.; writing—review and editing, D.K., R.O. and P.K.; visualization P.K., D.K., R.O.; project administration P.K., D.K., R.O.; supervision, P.K., D.K.; funding acquisition K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
On request of those interested.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a grant from the Medical University of Białystok SUB/2/DN/22/001/2201 and by National Science Center, Poland project OPUS No. 2019/33/B/ST4/01118.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albrecht L., Albrecht A., Krawczyk H., Jorgensen K.A. Organocatalytic Asymmetric Synthesis of Organophosphorus Compounds. Chem. Eur. J. 2010;16:28–48. doi: 10.1002/chem.200902634. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D., Wang R. Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles. Chem. Soc. Rev. 2012;41:2095–2108. doi: 10.1039/C1CS15247E. [DOI] [PubMed] [Google Scholar]
- 3.Hiratake J., Oda J. Aminophosphonic and Aminoboronic Acids as Key Elements of a Transition State Analogue Inhibitor of Enzymes. Biosci. Biotechnol. Biochem. 1997;61:211–218. doi: 10.1271/bbb.61.211. [DOI] [Google Scholar]
- 4.Kafarski P., Lejczak B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991;63:193–215. doi: 10.1080/10426509108029443. [DOI] [Google Scholar]
- 5.Nassan M.A., Aldhahrani A., Amer H.H., Elhenawy A., Swelum A.A., Ali O.M., Zaki Y.H. Investigation of the Anticancer Effect of α-Aminophosphonates and Arylidine Derivatives of 3-Acetyl-1-aminoquinolin-2(1H)-one on the DMBA Model of Breast Cancer in Albino Rats with In Silico Prediction of Their Thymidylate Synthase Inhibitory Effect. Molecules. 2022;27:756. doi: 10.3390/molecules27030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezaei Z., Khabnadideh S., Zomorodian K., Pakshir K., Nadali S., Mohtashami N., Mirzaei E.F. Design, Synthesis, and Antifungal Activity of New α-Aminophosphonates, Design, Synthesis, and Antifungal Activity of New α-Aminophosphonates. Int. J. Med. Chem. 2011;2011:678101. doi: 10.1155/2011/678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga P.R., Keglevich G. Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules. 2021;26:2511. doi: 10.3390/molecules26092511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwanejko J., Samadaei M., Pinter M., Senfter D., Madlener S., Kochel A., Rohr-Udilova N., Wojaczyńska E. Cytotoxic Activity of Piperazin-2-One-Based Structures: Cyclic Imines, Lactams, Aminophosphonates, and Their Derivatives. Materials. 2021;14:2138. doi: 10.3390/ma14092138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packialakshmi P., Gobinath P., Ali D., Alarifi S., Alsaiari N.S., Idhayadhulla A., Surendrakumar R. Synthesis and Characterization of Aminophosphonate Containing Chitosan Polymer Derivatives: Investigations of Cytotoxic Activity and in Silico Study of SARS-CoV-19. Polymers. 2021;13:1046. doi: 10.3390/polym13071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Francés A., del Corte X., de Marigorta E.M., Palacios F., Vicario J. Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents. Molecules. 2021;26:1654. doi: 10.3390/molecules26061654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Yan K., Song B., Xu G., Yang S., Xue W., Hu D., Lu P., Ouyang G., Jin L., et al. Synthesis and Antiviral Bioactivities of α-Aminophosphonates Containing Alkoxyethyl Moieties. Molecules. 2006;11:666–676. doi: 10.3390/11090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song B.-A., Wu Y.-L., Yang S., Hu D.-Y., He X.-Q., Jin L.-H. Synthesis and Bioactivity of a-Aminophosphonates Containing Fluorine. Molecules. 2003;8:186–192. doi: 10.3390/80100186. [DOI] [Google Scholar]
- 13.Onita N., Şisu I., Penescu M., Purcarea V.L., Kurunczi L. Synthesis Characterization and Biological activity of some α-aminophoshonates. Farmcia. 2010;58:531–545. [Google Scholar]
- 14.Bhagat S., Shah P., Garg S.K., Mishra S., Kaur P.K., Singh S., Chakraborti A.K. α-Aminophosphonates as novel anti-leishmanialchemotypes: Synthesis, biological evaluation, and CoMFA studies. Med. Chem. Commun. 2014;5:665–669. doi: 10.1039/C3MD00388D. [DOI] [Google Scholar]
- 15.Lewkowski J., Malinowski Z., Matusiak A., Morawska M., Rogacz D., Rychter P. The Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants: A Seedling Emergence and Growth Test. Molecules. 2016;21:694. doi: 10.3390/molecules21060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I.-H., Park Y.-K., Nishiwaki H., Hammock B.D., Nishi K. Structure–activity relationships of amide–phosphonate derivatives as inhibitors of the human soluble epoxide hydrolase. Bioorg. Med. Chem. 2015;23:7199–7210. doi: 10.1016/j.bmc.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Klimczak A.A., Matusiak A., Lewkowski J., Bitner J., Szemraj J., Kontek R. Dimethyl (2-Furyl)-N-(2-Methoxyphenyl)Aminomethylphosphonate Induces Apoptosis in Esophageal Squamous Cancer Cells. Structure Versus Activity of its Selected Analogs. Phosphorus Sulfur Silicon Relat. Elem. 2015;190:1088–1099. doi: 10.1080/10426507.2014.965821. [DOI] [Google Scholar]
- 18.Klimczak A.A., Kuropatwa A., Lewkowski J., Szemraj J. Synthesis of new N-arylamino(2-furyl)methylphosphonic acid diesters, and in vitro evaluation of their cytotoxicity against esophageal cancer cells. Med. Chem. Res. 2012;22:852–860. doi: 10.1007/s00044-012-0065-3. [DOI] [Google Scholar]
- 19.Kafarski P., Lejczak B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anti-Cancer Agents. 2001;1:301–312. doi: 10.2174/1568011013354543. [DOI] [PubMed] [Google Scholar]
- 20.Li C., Song B., Yan K., Xu G., Hu D., Yang S., Jin L., Xue W., Lu P. One Pot Synthesis of α-Aminophosphonates Containing Bromo and 3,4,5-Trimethoxybenzyl Groups under Solvent-free Conditions. Molecules. 2007;12:163–172. doi: 10.3390/12020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonar S.S., Sadaphal S.A., Labade V.B., Shingate B.B., Shingare M.S. An Efficient Synthesis and Antibacterial Screening of Novel Oxazepine α-Aminophosphonates by Ultrasound Approach. Phosphorus Sulfur Silicon Relat. Elem. 2009;185:65–73. doi: 10.1080/10426500802713259. [DOI] [Google Scholar]
- 22.Sayed I.E.T.E., Fathy G., Ahmed A.A.S. Synthesis and Antibacterial Activity of Novel Cyclic α-Aminophsophonates. Biomed. J. Sci. Tech. Res. 2019;23:17609–17614. doi: 10.26717/BJSTR.2019.23.003936. [DOI] [Google Scholar]
- 23.Abdel-Megeed M.F., Badr B.E., Azaam M.M., El–Hiti G.A. Antimicrobial Activities of a Series of Diphenyl (4′-(Aryldiazenyl)Biphenyl-4-Ylamino)(Pyridin-3-YL)Methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2012;187:1202–1207. doi: 10.1080/10426507.2012.680084. [DOI] [Google Scholar]
- 24.Kudzin Z.H., Kudzin M.H., Drabowicz J., Stevens C.V. Aminophosphonic Acids—Phosphorus Analogues of Natural Amino Acids. Part 1: Syntheses of α-Aminophosphonic Acids. Curr. Org. Synth. 2011;15:2015–2071. doi: 10.2174/138527211795703612. [DOI] [Google Scholar]
- 25.Allen J.G., Atherton F.R., Hall M.J., Hassall C.H., Holmes S.W., Lambert R.W., Nisbet L.J.P., Ringrose S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978;272:56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- 26.Shaik M.S., Nadiveedhi M.R., Gundluru M., Katike U., Obulam V.S.R., Cirandur S.R. Efficient catalyst free green synthesis and in vitro antimicrobial, antioxidant and molecular docking studies of α-substituted aromatic/heteroaromatic aminomethylene bisphosphonates. Synth. Commun. 2021;51:747–764. doi: 10.1080/00397911.2020.1853778. [DOI] [Google Scholar]
- 27.Mulla S.A.R., Pathan M.Y., Chavan S.S., Gample S.P., Sarkar D. Highly efficient one-pot multi-component synthesis of α-aminophosphonates and bis-α-aminophosphonates catalyzed by heterogeneous reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) at ambient temperature and their antitubercular evaluation against Mycobactrium Tuberculosis. RSC Adv. 2014;4:7666–7672. doi: 10.1039/c3ra45853a. [DOI] [Google Scholar]
- 28.Ali N.S., Zakir S., Patel M., Farooqui M. Synthesis of new α aminophosphonate system bearing Indazole moiety and their biological activity. Eur. J. Med. Chem. 2012;50:39–43. doi: 10.1016/j.ejmech.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Sampath C., Vani K.V., Kotaiah Y., Krishna N.H., Raju C.N., Rao C.V. A facile and efficient One-pot Three Component Reaction (Kabachinik-Fields Reaction) for the Synthesis of Novel α-Aminophosphonates by 1, 4-Dimethylpiperazine as a new catalyst. J. Chem. Pharm. Res. 2012;4:1375–1382. [Google Scholar]
- 30.Maruyama H.B., Arisawa M., Sawada T. Alafosfalin, a new inhibitor of cell wall biosynthesis: In vitro activity against urinary isolates in Japan and potentiation with beta-lactams. Antimicrob. Agents Chemother. 1979;16:444–451. doi: 10.1128/AAC.16.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasthuraiah M., Kumar K.A., Reddy C.S., Reddy C.D. Syntheses, spectral property, and antimicrobial activities of 6-α-amino dibenzo [d,f][1,3,2]dioxaphosphepin 6-oxides. Heteroat. Chem. 2007;18:2–8. doi: 10.1002/hc.20226. [DOI] [Google Scholar]
- 32.Kasthuraiah M., Balakrishna A., Reddy K.R.K.K., Kumar B.S., Reddy C.S., Nagaraju C. Synthesis and Antimicrobial Activity of New 2,10-Dichloro-6-phenylaminobenzyl-dibenzo[d,g] [1,3,6,2]dioxathiaphosphocin 6-Oxides. Heterocycl. Chem. 2008;45:103–107. doi: 10.1002/jhet.2008.45.1.103. [DOI] [Google Scholar]
- 33.Tajtihttps Á., Bálinthttps E., Keglevich G. Microwave-assisted synthesis of α-aminophosphonates and related derivatives by the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2019;194:379–381. doi: 10.1080/10426507.2018.1547729. [DOI] [Google Scholar]
- 34.Maestro A., del Corte X., López-Francés A., de Marigorta E.M., Palacios F., Vicario J. Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives. Molecules. 2021;26:3202. doi: 10.3390/molecules26113202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viveros-Ceballos J.L., Ordóñez M., Sayago F.J., Cativiela C. Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives. Molecules. 2016;21:1141. doi: 10.3390/molecules21091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bálint E., Tajti Á., Kalocsai D., Mátravölgyi B., Karaghiosoff K., Czugler M., Keglevich G. Synthesis and utilization of optically active α-aminophosphonate derivatives by Kabachnik-Fields reaction. Tetrahedron. 2017;73:5659–5667. doi: 10.1016/j.tet.2017.07.060. [DOI] [Google Scholar]
- 37.Tajti Á., Bálint E., Keglevich G. Synthesis of Ethyl Octyl-α-Aminophosphonate Derivatives. Curr. Org. Synth. 2016;13:638–645. doi: 10.2174/1570179413666151218202757. [DOI] [Google Scholar]
- 38.Philippe N., Denivet F., Vasse J., Santos J., Levacher V., Dupas G. Highly stereoselective Friedel-Crafts type cyclization. Facile access to enantiopure 1,4-dihydro-4-phenyl isoquinolinones. Tetrahedron. 2003;59:8049–8056. doi: 10.1016/S0040-4020(03)01236-5. [DOI] [Google Scholar]
- 39.Bálint E., Tajti Á., Drahos L., Ilia G., Keglevich G. Alcoholysis of Dialkyl Phosphites Under Microwave Conditions. Curr. Org. Chem. 2013;17:555–562. doi: 10.2174/1385272811317050010. [DOI] [Google Scholar]
- 40.Gao Y., Huang Z., Zhuang R., Xu J., Zhang P., Tang G., Zhao Y. Direct Transformation of Amides into α-Amino Phosphonates via a Reductive Phosphination Process. Org. Lett. 2013;15:4214–4217. doi: 10.1021/ol4019419. [DOI] [PubMed] [Google Scholar]
- 41.Maier L., Diel P.J. Organic Phosphorus compounds 941 preparation, physical and biological properties of amino-arylmethylphosphonic and phoshonous acids. Phosphorus Sulfur Silicon Relat. Elem. 1991;57:57–64. doi: 10.1080/10426509108038831. [DOI] [Google Scholar]
- 42.Bhagat S., Chakraborti A.K. Zirconium(IV) Compounds As Efficient Catalysts for Synthesis of α-Aminophosphonates. J. Org. Chem. 2008;73:6029–6032. doi: 10.1021/jo8009006. [DOI] [PubMed] [Google Scholar]
- 43.Bedolla-Medrano M., Hernández-Fernández E., Ordóñez M. Phenylphosphonic Acid as Efficient and Recyclable Catalyst in the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett. 2014;25:1145–1149. doi: 10.1002/chin.201444196. [DOI] [Google Scholar]
- 44.Tibhe G.D., Bedolla-Medrano M., Cativiela C., Ordóñez M. Phenylboronic Acid as Efficient and Eco-Friendly Catalyst for the One-Pot, Three-Component Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett. 2012;23:1931–1936. doi: 10.1002/chin.201248190. [DOI] [Google Scholar]
- 45.Liu Q., Yu S., Hu L., Hussain M.I., Zhang X., Xiong Y. Cross-dehydrogenative coupling strategy for phosphonation and cyanation of secondary N-alkyl anilines by employing 2,3-dichloro-5,6-dicyanobenzoquinone. Tetrahedron. 2018;74:7209–7217. doi: 10.1016/j.tet.2018.10.058. [DOI] [Google Scholar]
- 46.Lee O.-Y., Law K.-L., Yang D. Secondary Amine Formation from Reductive Amination of Carbonyl Compounds Promoted by Lewis Acid Using the InCl3/Et3SiH System. Org. Lett. 2009;11:3302–3305. doi: 10.1021/ol901111g. [DOI] [PubMed] [Google Scholar]
- 47.Hall R.G. The design and synthesis of biologically active organophosphorus compounds--the role of a central research laboratory. Chimia. 2010;64:34–36. doi: 10.2533/chimia.2010.34. [DOI] [PubMed] [Google Scholar]
- 48.Orsini F., Sello G., Sisti M. Aminophosphonic Acids and Derivatives. Synthesis and Biological Applications. Curr. Med. Chem. 2010;17:264–289. doi: 10.2174/092986710790149729. [DOI] [PubMed] [Google Scholar]
- 49.Ahmadi F., Assadi Y., Hosseini S.M.R.M., Rezaee M. Determination of organophosphorus pesticides in water samples by single drop microextraction and gas chromatography-flame photometric detector. J. Chromatogr. A. 2006;1101:307–312. doi: 10.1016/j.chroma.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Amira A., Aouf Z., K’tir H., Chemam Y., Ghodbane R., Zerrouki R., Aouf N.-E. Recent Advances in the Synthesis of α-Aminophosphonates: A Review. ChemistrySelect. 2021;6:6137–6149. doi: 10.1002/slct.202101360. [DOI] [Google Scholar]
- 51.Shastri R.A. Review on the Synthesis of α-Aminophosphonate Derivatives. Chem. Sci. Trans. 2019;8:359–367. doi: 10.7598/cst2019.1585. [DOI] [Google Scholar]
- 52.Basha M.H., Subramanyam C., Rao K.P. Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants. Main Group Met. Chem. 2020;43:147–153. doi: 10.1515/mgmc-2020-0018. [DOI] [Google Scholar]
- 53.Eyckensa D.J., Henderson L.C. Synthesis of α-aminophosphonates using solvate ionic liquids. RSC Adv. 2017;7:27900–27904. doi: 10.1039/C7RA04407K. [DOI] [Google Scholar]
- 54.Babak K. Surface-Mediated Solid Phase Reactions: A Simple and New Method for the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Chem. Lett. 2001;30:880–881. doi: 10.1246/cl.2001.880. [DOI] [Google Scholar]
- 55.Basha S.K.T., Kalla R.M.N., Varalakshmi M., Sudhamani H., Appa R.M., Hong S.C., Raju C.N. Heterogeneous catalyst SiO2–LaCl3·7H2O: Characterization and microwave-assisted green synthesis of α-aminophosphonates and their antimicrobial activity. Mol. Divers. 2022 doi: 10.1007/s11030-021-10360-x. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Kowalczyk D., Albrecht Ł. An organocatalytic biomimetic approach to α-aminophosphonates. Chem. Commun. 2015;51:3981–3984. doi: 10.1039/C4CC09477H. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y., Li Z., Liu Y., Lin T., Sun H., Yang D., Jiang C. Identification of novel and selective non-peptide inhibitors targeting the polo-box domain of polo-like kinase 1. Bioorg. Chem. 2018;81:278–288. doi: 10.1016/j.bioorg.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Sravya G., Suresh G., Zyryanov G.V., Balakrishna A., Reddy N.B. K2CO3/Al2O3: An Efficient and Recyclable Catalyst for One-Pot, Three Components Synthesis of α-Aminophosphonates and Bioactivity Evaluation. Asian J. Org. Chem. 2019;31:2383–2388. doi: 10.14233/ajchem.2019.22194. [DOI] [Google Scholar]
- 59.Chandrasekhar S., Prakash S.J., Jagadeshwar V., Narsihmula C. Three component coupling catalyzed by TaCl5–SiO2: Synthesis of α-amino phosphonates. Tetrahedron Lett. 2001;42:5561–5563. doi: 10.1016/S0040-4039(01)01053-X. [DOI] [Google Scholar]
- 60.Yadav J.S., Reddy B.V.S., Raj K.S., Reddy K.B., Prasad A.R. Zr4+-Catalyzed Efficient Synthesis of α-Aminophosphonates. Synthesis. 2001;15:2277–2280. doi: 10.1055/s-2001-18444. [DOI] [Google Scholar]
- 61.Manjula A., Rao B.V., Neelakantan P. One-Pot Synthesis of α-Aminophosphonates: An Inexpensive Approach. Synth. Comun. 2003;33:2963–2969. doi: 10.1081/SCC-120022468. [DOI] [Google Scholar]
- 62.Milen M., Ábrányi-Balogh P., Dancsó A., Frigyes D., Pongó L., Keglevich G. T3P®-promoted Kabachnik–Fields reaction: An efficient synthesis of α-aminophosphonates. Tetrahedron Lett. 2013;54:5430–5433. doi: 10.1016/j.tetlet.2013.07.145. [DOI] [Google Scholar]
- 63.Lee S.G., Lee J.K., Song C.-E., Kim D.C. Microwave-assisted Kabachnik-Fields Reaction in Ionic Liquid. Bull. Korean Chem. Soc. 2002;23:667–668. doi: 10.5012/bkcs.2002.23.5.667. [DOI] [Google Scholar]
- 64.Janicki I., Łyżwa P., Kiełbasiński P. The first enzyme-promoted addition of nitromethane to imines (aza-Henry reaction) Bioorg. Chem. 2020;94:103377. doi: 10.1016/j.bioorg.2019.103377. [DOI] [PubMed] [Google Scholar]
- 65.Cai J.-F., Guan Z., He Y.-Y. The lipase-catalyzed asymmetric C-C Michael addition. J. Mol. Catal. B Enzym. 2011;68:240–244. doi: 10.1016/j.molcatb.2010.11.011. [DOI] [Google Scholar]
- 66.Li C., Feng X.-W., Wang N., Zhou Y.-J., Yu X.-Q. Biocatalytic promiscuity: The first lipase-catalysed asymmetric aldol reaction. Green Chem. 2008;10:616–618. doi: 10.1039/b803406k. [DOI] [Google Scholar]
- 67.Albuquerque T.B., da Silva C.D.G., de Oliveira A.R., Santos B.F.d., da Silva B.A.L., Katla R., Rochaa M.P.D., Domingues N.L.C. Lipase catalyzed 1,2-addition of thiols to imines under mild conditions. New J. Chem. 2018;42:1642–1645. doi: 10.1039/C7NJ03387G. [DOI] [Google Scholar]
- 68.Reetz M.T., Mondière R., Carballeira J.D. Enzyme promiscuity: First protein-catalyzed Morita–Baylis–Hillman reaction. Tetrahedron Lett. 2007;48:1679–1681. doi: 10.1016/j.tetlet.2007.01.063. [DOI] [Google Scholar]
- 69.Kapoor M., Gupta M.N. Lipase promiscuity and its biochemical applications. Proc. Biochem. 2012;47:555–569. doi: 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- 70.Dwivedee B.P., Soni S., Sharma M., Bhaumik J., Laha J.K., Banerjee U.C. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect. 2018;3:2441–2466. doi: 10.1002/slct.201702954. [DOI] [Google Scholar]
- 71.Guezane-Lakoud S., Toffano M., Aribi-Zouioueche L. Promiscuous lipase catalyzed a new P–C bond formation: Green and efficient protocol for one-pot synthesis of α-aminophosphonates. Heteroat. Chem. 2017;28:e21408. doi: 10.1002/hc.21408. [DOI] [Google Scholar]
- 72.Aissa R., Guezane-Lakoud S., Kolodziej E., Toffano M., Aribi-Zouioueche L. Diastereoselective synthesis of bis(α-aminophosphonates) by lipase catalytic promiscuity. New J. Chem. 2019;43:8153–8159. doi: 10.1039/C8NJ06235H. [DOI] [Google Scholar]
- 73.Chavan A.S., Kharat A.S., Bhosle R., Dhumal S.T., Mane R.A. CAL-B accelerated novel synthetic protocols for 3,3’-arylidenebis-4-hydroxycoumarins and dimethyl ((substituted phenyl) (phenylamino)methyl) phosphonates. Res. Chem. Intermed. 2021;47:4497–4512. doi: 10.1007/s11164-021-04535-2. [DOI] [Google Scholar]
- 74.Kowalczyk P., Koszelewski D., Gawdzik B., Samsonowicz-Górski J., Kramkowski K., Wypych A., Lizut R., Ostaszewski R. Promiscuous Lipase-Catalyzed Markovnikov Addition of H-Phosphites to Vinyl Esters for the Synthesis of Cytotoxic α-Acyloxy Phosphonate Derivatives. Materials. 2022;15:1975. doi: 10.3390/ma15051975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koszelewski D., Brodzka A., Madej A., Trzepizur D., Ostaszewski R. Evaluation of gem-Diacetates as Alternative Reagents for Enzymatic Regio- and Stereoselective Acylation of Alcohols. J. Org. Chem. 2021;86:6331–6342. doi: 10.1021/acs.joc.1c00154. [DOI] [PubMed] [Google Scholar]
- 76.Koszelewski D., Ostaszewski R., Śmigielski P., Hrunyk A., Kramkowski K., Laskowski Ł., Laskowska M., Lizut R., Szymczak M., Michalski J., et al. Pyridine Derivatives—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials. 2021;14:5401. doi: 10.3390/ma14185401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koszelewski D., Ostaszewski R. The studies on chemoselective promiscuous activity of hydrolases on acylals transformations. Bioorg. Chem. 2019;93:102825. doi: 10.1016/j.bioorg.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 78.Koszelewski D., Ostaszewski R. Enzyme promiscuity as a remedy for the common problems with Knoevenagel condensation. Chem. Eur. J. 2019;25:10156–10164. doi: 10.1002/chem.201901491. [DOI] [PubMed] [Google Scholar]
- 79.Koszelewski D., Ostaszewski R. Biocatalytic Promiscuity of Lipases in Carbon-Phosphorus Bond Formation. ChemCatChem. 2019;11:2554–2558. doi: 10.1002/cctc.201900397. [DOI] [Google Scholar]
- 80.Albanese D.C.M., Gaggero N. Albumin as a promiscuous biocatalyst in organic synthesis. RSC Adv. 2015;5:10588–10598. doi: 10.1039/C4RA11206G. [DOI] [Google Scholar]
- 81.Fang H., Xie X., Hong B., Zhao Y., Fang M. Copper (I) Iodide-Catalyzed Solvent-Free Synthesis of α-Aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2011;186:2145–2155. doi: 10.1080/10426507.2011.590561. [DOI] [Google Scholar]
- 82.Kandula M.K.R., Gundluru M., Nemallapudi B.R., Gundala S., Kotha P., Zyryanov G.V., Chadive S., Cirandur S.R. Synthesis, antioxidant activity, and α-glucosidase enzyme inhibition of α-aminophosphonate derivatives bearing piperazine-1,2,3-triazole moiety. J. Heterocycl. Chem. 2021;58:172–181. doi: 10.1002/jhet.4157. [DOI] [Google Scholar]
- 83.Azaam M.M., Kenawy E.R., El-din A.S.B., Khamis A.A., El-Magd M.A. Antioxidant and anticancer activities of α-aminophosphonates containing thiadiazole moiety. J. Saudi. Chem. Soc. 2018;22:34–41. doi: 10.1016/j.jscs.2017.06.002. [DOI] [Google Scholar]
- 84.Bollinger A., Molitor R., Thies S., Koch R., Coscolín C., Ferrer M., Jaeger K.-E. Organic-Solvent-Tolerant Carboxylic Ester Hydrolases for Organic Synthesis. Appl. Environ. Microbiol. 2020;86:e00106-20. doi: 10.1128/AEM.00106-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kowalczyk P., Wilk M., Parul P., Szymczak M., Kramkowski K., Raj S., Skiba G., Sulejczak D., Kleczkowska P., Ostaszewski R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials. 2021;14:5725. doi: 10.3390/ma14195725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samsonowicz-Górski J., Kowalczyk P., Koszelewski D., Brodzka A., Szymczak M., Kramkowski K., Ostaszewski R. The Synthesis and Evaluation of Amidoximes as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials. 2021;14:7577. doi: 10.3390/ma14247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kowalczyk P., Trzepizur D., Szymczak M., Skiba G., Kramkowski K., Ostaszewski R. 1,2-Diarylethanols—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials. 2021;14:1025. doi: 10.3390/ma14041025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kowalczyk P., Madej A., Szymczak M., Ostaszewski R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials. 2020;13:5169. doi: 10.3390/ma13225169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kowalczyk P., Borkowski A., Czerwonka G., Cłapa T., Cieśla J., Misiewicz A., Borowiec M., Szala M. The microbial toxicity of quaternary ammonium ionic liquids is dependent on the type of lipopolysaccharide. J. Mol. Liq. 2018;266:540–547. doi: 10.1016/j.molliq.2018.06.102. [DOI] [Google Scholar]
- 90.Borkowski A., Kowalczyk P., Czerwonka G., Cieśla J., Cłapa T., Misiewicz A., Szala M., Drabik M. Interaction of quaternary ammonium ionic liquids with bacterial membranes—Studies with Escherichia coli R1–R4-type lipopolysaccharides. J. Mol. Liq. 2017;246:282–289. doi: 10.1016/j.molliq.2017.09.074. [DOI] [Google Scholar]
- 91.Kowalczyk P., Gawdzik B., Trzepizur D., Szymczak M., Skiba G., Raj S., Kramkowski K., Lizut R., Ostaszewski R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials. 2021;14:2956. doi: 10.3390/ma14112956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maciejewska A., Kaszowska M., Jachymek W., Lugowski C., Lukasiewicz J. Lipopolysaccharide-linked Enterobacterial Common Antigen (ECALPS) Occurs in Rough Strains of Escherichia coli R1, R2, and R4. Int. J. Mol. Sci. 2020;21:6038. doi: 10.3390/ijms21176038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prost M.E., Prost R. Basic parameters of evaluation of the effectiveness of antibiotic therapy. OphthaTherapy. 2017;4:233–236. doi: 10.24292/01.OT.291217.06. [DOI] [Google Scholar]
- 94.Kabachnik M.I., Medved T.Y. Нoвый метoд синтеза сс-аминoфoсфинoвых кислoт [A new method for the synthesis of α-amino phosphoric acids] Doklady Akademii Nauk SSSR. 1952;83:689ff. [Google Scholar]
- 95.Fields E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952;74:1528–1531. doi: 10.1021/ja01126a054. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On request of those interested.