Abstract
An efficient method of producing quinine derivatives via reaction of acylation with 4,5-dichloroisothiazole-3-, 5-arylisoxazole-3-, adamantane- and hydrochlorides of pyridine-3- and pyridine-4-carbonyl chlorides was developed. All synthesized compounds were tested for antiviral, antimicrobial and analgesic activity. The most pronounced antibacterial activity was shown by the compounds 2e, 3b, 3c and 3e with isoxazole and pyridine fragments. It was found that most of the tested compounds showed significant analgesic activity reducing the pain response of animals to the irritating effect of acetic acid.
Keywords: quinine, esters, isoxazole, isothiazole, pyridine, adamantane, quaternary pyridinium salts, antiviral activity, antimicrobial activity, analgesic activity
1. Introduction
Quinine is the main alkaloid of the bark of various cinchona (Cinchona) species, which has a pronounced activity against malarial plasmodia and made it possible to use quinine as an effective treatment for malaria for a long time [1]. However, quinine at the same time has a toxic side effect [2,3], causing tinnitus, hearing and vision impairment, dizziness, heart palpitations, vomiting, hypotension, hypoglycemia and kidney failure. This was the reason for replacing quinine with more effective and safer antimalarial drugs, such as artemisinin [4].
Along with its antimalarial activity, quinine has been reported to possess antipyretic, antibacterial and antifungal properties [5,6,7]. Currently, quinine and its derivatives are considered as potential treatments for COVID-19 [8,9,10,11]. Taking into account the high potential activity, numerous studies are underway in the world aimed at searching for new drugs among quinine derivatives, including the esterification reaction of the C-9 hydroxyl group [12,13,14,15].
Nowadays, antimicrobial resistance is considered one of the greatest problems facing humans, because many bacterial strains have become resistant to available antibiotics. Thus, the discovery of new effective antimicrobial agents, especially from traditional medicinal plants and their derivatives, is urgently needed. Moreover, a screening study of some quinine esters suggested that they have moderate antimicrobial activity against human pathogenic bacteria strains Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus substillis; thus, the compounds potentially work as antimicrobial agents for both Gram-negative and Gram-positive bacterial strains [12]. In this present study, we synthesized several derivative compounds of quinine with specific functional groups in order to understand how different functional groups serve different antimicrobial actions.
Pyridine, isoxazole and isothiazole heterocycles are widely used structural units in the design and synthesis of new biologically active compounds and are included in the molecular structures of a large number of bioactive substances used in medical practice [16,17,18,19,20,21]. The combination of alkaloid, pyridine and 1,2-azole fragments in one molecule can improve the activity and impart new useful properties to target compounds, and the high lipophilicity with the bulk structure of the adamantane radical can significantly promote and modify the pharmacological action of various biologically active compounds by creating favorable conditions for their transport through biological membranes [22].
Promising data on the antiviral activity of quinine from numerous literary sources [8,9,10,11,23,24] prompted us to study the antiviral properties of obtained derivatives. We also examined the analgesic and fungicidal activity of the target compounds.
The use of an ester bond for the covalent bonding of various pharmacophore groups and structural fragments of natural as well as synthetic origin can serve as a prime example of molecular design. Drugs with an ester group often perform prodrug functions. In the body, “prodrugs” undergo biotransformation and turn into the true drug. Prodrugs are common tools for overcoming drawbacks typically associated with drug formulation and delivery, with ester prodrugs providing a classic strategy for masking polar alcohol and carboxylic acid functionalities and improving cell permeability [25,26]. The ester group in a drug molecule can also perform the following functions: (a) be part of the pharmacophore—a grouping of atoms that determines the pharmacological effect of the substance; (b) protect reactive groups from exposure to adverse environmental factors (air oxygen, light) and the internal environment of the body (enzymes); (c) reduce the toxicity and irritant properties of the substance; (d) improve the pharmacokinetic properties of the substance. An additional factor that contributes to the popularity of esters is that the synthesis of an ester is often straightforward.
2. Results and Discussion
2.1. Chemistry
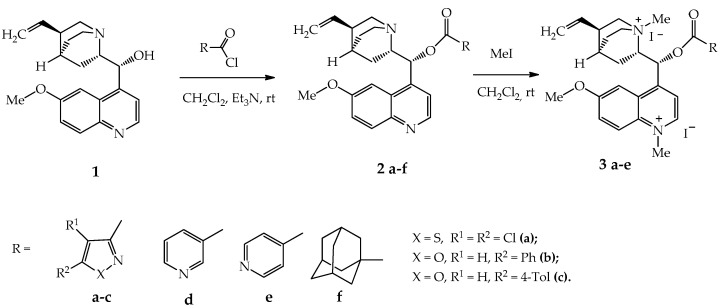
In the framework of this work, quinine esters with heterocyclic 1,2-azole, pyridine and adamantane fragments were obtained. It should be noted that quinine esters with isoxazole and isothiazole fragments have not been previously obtained; however, there are methods for the synthesis of quinine derivatives with a pyridine heterocycle [14,15], and some of them have shown high insecticidal activity against Mythimna separata. The article [14] presents a one-step synthesis method using DCC and DMAP as a catalyst, and the yield of esters of nicotinic and isonicotinic acids and quinine was 44 and 24%, respectively.
The method proposed by us is based on the reaction of quinine acylation with hydrochlorides of pyridine-3- and pyridine-4-carbonyl chlorides. It can compete with that stated in [14] due to higher yields (>80%) and the absence of the need for further purification of the products isolated from the reaction mixture. Esters 2a–f were obtained by acylation of quinine 1 with various acid chlorides in dichloromethane in the presence of triethylamine at room temperature in 86–91% yield (Scheme 1).
Scheme 1.
Synthesis of quinine esters with 1,2-azole and pyridine fragments 2a–f and their iodomethylates 3a–e.
Based on the synthesized derivatives 2a–f, quaternary pyridinium salts (iodomethylates) were obtained. Quaternization proceeded with the participation of quinoline, quinuclidine and pyridine nitrogen atoms and led to the formation of diiodomethylates 3a–c in 94–98% yield and triiodomethylates 3d,e in 83–85% yield. The quaternization reaction of the obtained esters proceeds completely with a 4-fold excess of the alkylating agent, and the resulting salts precipitate out of the solution.
Quaternization of quinine esters makes it possible to increase the water solubility of compounds, which is important for choosing the most rational ways of introducing drugs into the body. Pyridinium salts are also known to inhibit the growth of various microorganisms such as bacteria, viruses, and fungi. Thus, quinine alkylation displayed an increase in antibacterial activity of obtained amphiphiles against a number of Gram-positive and Gram-negative strains [27].
The obtained compounds were identified on the basis of IR, UV, mass and NMR spectra (1H and 13C), as well as elemental analysis (please see the Supplementary Materials).
All synthesized compounds were tested for antiviral, antimicrobial and analgesic activity.
2.2. Evaluation of the Biological Activity
2.2.1. Antiviral Activity
Cytotoxicity and chicken embryo lethality of test compounds were studied.
The acute toxicity of the samples was assessed at various doses in various in vitro models and in a 10-day-old chick embryo model. The study of acute toxicity of quinine derivatives “in vitro” was carried out on macrophage models of outbred mice. The interval of the dose range was determined, first of all, by the interval of acceptable values for the number of compounds used in further studies for antiviral activity. The analysis of acute cytotoxicity of compounds “in vitro” was carried out in the dose range of 0.003–0.4 mg, corresponding to effective doses of compounds with antiviral properties. Cytotoxicity of substances was determined by studying the effect of various doses of compounds on cell viability, by the method of dehydrogenase activity detection (MTT test).
It was found that in the tested dose range, all the studied compounds did not reach the LD50. At the maximum dose of 0.4 mg/chick embryo, the toxicity (LD50) of the test compounds was not manifested; therefore, further presence study of the antiviral activity was carried out in the dose range from 0.4 mg/chick embryo or less. Thus, in the determination of acute toxicity “in vitro” and on the model of 10-day-old chicken embryos, the studied compounds did not reveal toxic properties at the maximum of the tested doses.
The virus-inhibiting activity of the samples was studied.
The virus-inhibiting activity of the preparations was studied at concentrations from 0.0016% to 0.2%, which corresponded to doses of 0.003–0.4 mg per chick embryo (0.06–8 mg/kg). It was found that in the given dose range, the studied compounds suppress the reproduction of the human influenza A/Almaty/8/98 (H3N2) virus by no more than 12%. At the same time, compounds 2e, 3b, 3c, 3d and 3e showed more pronounced antiviral properties in relation to the human influenza virus strain A/Almaty/8/98 (Table 1) than other compounds.
Table 1.
Virus-inhibiting activity of the test compounds when exposed to a strain of human influenza virus A/Almaty/8/98 (H3N2).
| Compound | Virus-Inhibiting Activity (%) at a Dose of mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 0 | 0 |
| 2b | 0 | 0 | 0 | 0 |
| 2d | 0 | 2.1 | 3.5 | 5.6 |
| 2e | 3.6 | 5.5 | 7.4 | 11.3 |
| 2f | 1.8 | 2.4 | 3.0 | 3.6 |
| 3a | 0 | 0 | 2.1 | 4.5 |
| 3b | 0 | 0 | 5.2 | 10.1 |
| 3c | 0 | 1.7 | 4.9 | 10.8 |
| 3d | 0 | 0 | 4.5 | 10.6 |
| 3e | 1.6 | 2.9 | 6.7 | 11.1 |
| Amizon | 1.9 | 3.4 | 5.2 | 9.8 |
The antiviral activity of the compounds was also studied on the model of avian influenza virus strain A/FPV/34/1 (H7N1). It was shown that 2b and 2f did not exhibit virus-inhibiting properties at all doses studied. Compounds 2e, 3b, 3d and 3e at a dose of 0.4 mg/chick embryo suppressed the reproduction of the influenza virus strain A/FPV/34/1 (H7N1) up to 16.5% (Table 2).
Table 2.
Virus-inhibiting activity of the test compounds when exposed to a strain of avian influenza virus A/FPV/34/1 (H7N1).
| Compound | Virus-Inhibiting Activity (%) at a Dose of Mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 0 | 0 |
| 2b | 0 | 1.7 | 3.5 | 4.7 |
| 2d | 0 | 2.1 | 3.9 | 5.6 |
| 2e | 0 | 3.5 | 7.3 | 16.5 |
| 2f | 0 | 0 | 0 | 0 |
| 3a | 0 | 0 | 0 | 0 |
| 3b | 0 | 5.4 | 7.1 | 11.2 |
| 3c | 0 | 2.6 | 4.2 | 5.3 |
| 3d | 0 | 4.6 | 8.5 | 10.4 |
| 3e | 0 | 5.8 | 9.3 | 14.7 |
| Amizon | 0 | 4.3 | 7.2 | 8.6 |
Further study of the antiviral activity of preparations 2b, 2e, 2f and 3b was carried out on the model of swine influenza virus strain A/swine/Iowa/30 (H1N1). It was found that at a dose of more than 0.08 mg/embryo, all the studied compounds showed mild antiviral properties. With the increase in the dose to 0.4 mg/chick embryo, the antiviral activity of the compounds 2e, 3b, 3c and 3e increased to 30% (Table 3).
Table 3.
Virus-inhibiting activity of the test compounds when exposed to the swine influenza A/swine/Iowa/30 (H1N1) strain.
| Compound | Virus-Inhibiting Activity (%) at a Dose of Mg/Chick Embryo | |||
|---|---|---|---|---|
| 0.003 | 0.02 | 0.08 | 0.4 | |
| 2a | 0 | 0 | 6.8 | 8.5 |
| 2b | 3.2 | 5.0 | 7.0 | 9.2 |
| 2d | 2.5 | 4.9 | 8.2 | 10.5 |
| 2e | 3.4 | 5.5 | 20.6 | 35.2 |
| 2f | 0 | 5.2 | 7.2 | 8.1 |
| 3a | 0 | 5.3 | 7.5 | 9.6 |
| 3b | 3.4 | 5.4 | 16.3 | 21.6 |
| 3c | 3.4 | 5.5 | 19.2 | 28.5 |
| 3d | 2.9 | 4.1 | 8.5 | 11.7 |
| 3e | 3.5 | 5.6 | 23.3 | 36.4 |
| Amizon | 3.6 | 5.8 | 7.5 | 10.9 |
The virucidal activity of the samples was studied in the dose range 0.003–0.4 mg/chick embryo against influenza virus strains A/Almaty/8/98 (H3N2), A/FPV/34/1 (H7N1) and A/swine/Iowa/30 (H1N1).
On three strains of the influenza virus, it was found that the compounds 2b, 2e, 2f and 3b do not have virucidal properties.
Thus, it was shown that the majority of the tested compounds did not show pronounced antiviral properties in the range of the studied doses. However, it was found that in a number of compounds under study, samples 2e, 3b, 3c and 3e with isoxazole and pyridine-4- fragments have more pronounced antiviral properties and exceed the activity of the reference drug Amizon in their action. Derivatives with an isonicotinic fragment were found to have a higher antiviral activity against all studied strains (Table 1, Table 2 and Table 3).
2.2.2. Antimicrobial Activity
According to the literature data, it has been established that quinine derivatives have a pharmacological effect against Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa and Escherichia coli [28]. It has been proven that quinoline derivatives inhibit DNA synthesis, promoting the cleavage of bacterial DNA gyrase, resulting in the death of bacterial cells [29].
The ability of compounds of the quinoline series, such as β-lactam antibiotics used to prevent pathogenic processes in the body, caused in particular by Staphylococcus aureus, to participate in the irreversible inhibition of the activity of transpeptidase, a penicillin-binding protein that catalyzes the formation of peptidoglycan, an essential component of the bacterial cell wall, is described and leads to the death of the pathogen.
The above data indicate the need to study the antimicrobial properties of the new derivatives of quinoline compounds synthesized by us in order to identify potential antimicrobial agents.
As a result of the antimicrobial study, it was found that the test compounds exhibit antibacterial activity in varying degrees of severity against the presented opportunistic test strains (Table 4).
Table 4.
Minimum inhibitory concentration (MIC) of test compounds in relation to reference test strains.
| Compound | MIC (μM) | ||||
|---|---|---|---|---|---|
|
Staphylococcus
aureus ATCC 6538 |
Bacillus
subtilis ATCC 6633 |
Escherichia
coli ATCC 25922 |
Pseudomonas
aeruginosa ATCC 27853 |
Candida
albicans ATCC 10231 |
|
| 2a | - | - | 99 | - | - |
| 2b | 50 | - | 101 | - | - |
| 2c | - | - | - | - | - |
| 2d | 29 | 116 | 116 | - | - |
| 2e | 15 | 58 | 58 | - | - |
| 2f | 51 | - | - | - | 103 |
| 3a | 63 | - | - | - | - |
| 3b | 8 | 64 | - | - | - |
| 3c | 8 | 63 | 32 | - | - |
| 3d | 29 | - | 59 | - | 59 |
| 3e | 7 | - | 15 | - | - |
| Ceftriaxone | 11 | 22 | 11 | 22 | - |
| Nystatin | - | - | - | - | 14 |
Analysis of the antimicrobial activity of the test substances showed that its manifestation depends on the type of pathogenic microorganism.
Test strain Staphylococcus aureus is the most sensitive to all the presented compounds (except 2a, 2c), for which their minimum inhibitory concentration is between 7 and 63 µM.
The most pronounced antibacterial activity was shown by the compounds 2e, 3b, 3c and 3e against the Gram-positive test strain of Staphylococcus aureus ATCC 6538. The antibacterial effect of these compounds against this test strain reached 7 μM, even better than that of ceftriaxone.
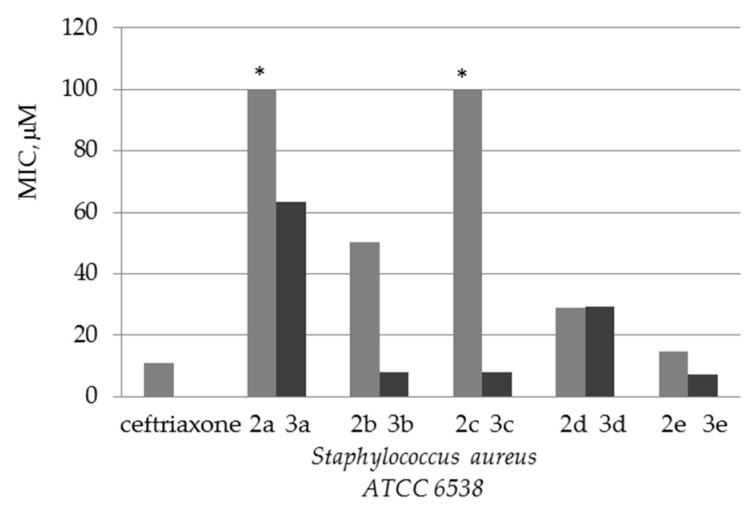
The obtained data on antimicrobial activity against Staphylococcus aureus allow us to conclude that quaternization of quinine esters increases antibacterial activity compared to the initial substrates (Figure 1).
Figure 1.
Diagram comparing the activity of esters 2a–e and their quaternary salts 3a–e against the Gram-positive test strain of Staphylococcus aureus ATCC 6538. * Test strain is non-sensitive to compounds 2a and 2c (MIC >> 100).
For test strains of Bacillus subtilis and Escherichia coli, the minimum inhibitory concentration of a number of test compounds is in the range of 14.6–116.4 µM. At the same time, the antibacterial activity of these compounds against Bacillus subtilis and Escherichia coli is lower than that of the reference drug.
The Gram-negative test strain of Pseudomonas aeruginosa turned out to be the most resistant to the action of these compounds. None of the test compounds showed antibacterial activity against this microorganism.
Antifungal activity was revealed in the compounds 2f and 3d. Antifungal activity against the yeast-like fungus Candida albicans, which causes human opportunistic fungal infections, was observed in these substances at concentrations of 58.5 and 102.7 μM.
Thus, in the series of new synthesized derivatives of quinine, compounds with antibacterial activity comparable to the activity of the drug ceftriaxone were identified. This allows us to consider this series of compounds as very promising for the search for new potential antibacterial drugs, which requires further in-depth studies.
2.2.3. Analgesic Activity In Vivo
In the course of studying the analgesic activity induced by novel compounds, the animals were observed from the moment of modeling the vinegar writhing. It was found that most of the test compounds, when administered once at a dose of 25 mg/kg 1 h before the stimulus, significantly reduce the pain response of animals to the irritating effect of acetic acid (Table 5).
Table 5.
Analgesic activity of test compounds.
| Compound | Dose, mg/kg | Writhing Number | Decrease in the Vinegar Writhing Number (%) |
|---|---|---|---|
| control | - | 106.2 ± 11.2 | 100 |
| sodium diclofenac | 8 | 49.5 ± 10.7 | 53.3 |
| 2a | 25 | 95.4 ± 10.9 | 10.2 |
| 2b | 25 | 55.6 ± 11.3 * | 47.6 |
| 2d | 25 | 62.2 ± 11.2 * | 41.4 |
| 2e | 25 | 53.7 ± 11.6 * | 49.4 |
| 3a | 25 | 96.8 ± 11.2 | 8.8 |
| 3b | 25 | 83.8 ± 10.4 | 21.1 |
| 3c | 25 | 57.2 ± 7.5 * | 46.1 |
| 3d | 25 | 75.4 ± 10.5 | 29.0 |
| 3e | 25 | 50.2 ± 10.3 * | 52.7 |
| 2f | 25 | 54.4 ± 12.3 | 48.8 |
Note: *—p < 0.05 compared to control.
The greatest analgesic effect among the studied contaminated forms and potential pharmaceutical substances of 2b, 2d, 2e, 2f, 3c and 3e caused a significant decrease in the amount of vinegar writhing in mice by 47.6, 41.4, 49.4, 48.8, 46.1 and 52.7% respectively. The analgesic activity level of these compounds is comparable to sodium diclofenac.
Compounds 2b, 2d, 2e, 2f, 3c and 3e at doses of 25 mg/kg showed analgesic activity in the model of chemical irritation of the peritoneum (test “vinegar cramps”), showing a significant decrease in the pain response of visceral nociceptors to the irritating effect of acetic acid in comparison with the control.
3. Materials and Methods
3.1. General Chemistry Section
UV spectra were recorded on a Varian Cary 300 spectrophotometer using quartz cuvettes with l = 1 cm. The concentration of the studied compounds in methanol was 4–6 × 10−5 mol/L. IR spectra were registered on a Thermo Nicolet Protege 460 Fourier transform spectrometer in KBr pellets.
1H and 13C NMR spectra were acquired on a Bruker Avance 500 spectrometer (500 and 125 MHz, respectively) in DMSO-d6 and CDCl3. The residual solvent signals (DMSO-d6, δH 2.5, δC 40.1 ppm; CDCl3, δH 7.26, δC 77.2 ppm) were used as internal standard. The assignment of signals in the 13C NMR spectra was performed using the DEPT technique.
Liquid chromatography–mass spectrometry spectra were recorded on an Agilent 1200 LC-MS system with an Agilent 6410 Triple Quad Mass Selective Detector with electrospray ionization in the positive ion registration mode (MS2 scanning mode). An Agilent ZORBAX Eclipse XDB-C18 (4.6 × 50 mm, 1.8 μm) column was used. The mobile phase was MeCN–H2O + 0.05% HCO2H with gradient elution from 40 to 90% MeCN in 10 min. A flow rate of 0.5 mL/min was used.
Elemental analysis was performed on a Vario MICRO cube CHNS-analyzer. The halogen content was determined by classical microanalysis using a modified Pregl’s method. Melting points were determined on a Kofler bench.
Reagents and solvents used were of analytical grade with the content of the main component being more than 99.5%. Dichloromethane was preliminarily kept for 1 day over CaCl2 to remove 0.5% of ethanol used for stabilizing dichloromethane. 5-Arylisoxazole-3-carboxylic and 4,5-dichloroisothiazole-3-carboxylic acids and acid chlorides were synthesized according to previously described procedures [30,31].
3.2. In Vitro Biological Assays
3.2.1. Antiviral Activity
The virus-inhibiting properties of the compounds were studied in experiments with orthomyxoviruses on chick embryos. Determination of antiviral properties was performed by the “screening test” method, designed to neutralize the virus in the amount of 100 EID50 given concentrations of the tested compounds. The presence of a difference in virus titer in comparison with the control was considered as a criterion for antiviral activity. In this case, as a rule, only complete suppression of the virus titer was taken into account.
The virucidal activity of the studied samples was determined by treating the virus-containing material with chemical compounds at 37 °C for 30 min, followed by titration of the infectivity of the treated material. The real virucidal effect was taken as the difference between the virus titer in the sample without exposure and its titer after. If the difference in titers was 1.0–2.0 lg, then the substance was considered to have moderate or pronounced antiviral activity [32]. The infectious titer of viruses was determined by the method of Reed and Muench [33].
The antiviral drug Amizon was used as a reference drug.
The following viruses were used in the work:
Orthomyxoviruses: avian influenza virus, strain A/FPV/34/1 (H7N1); human influenza virus, strain A/Almaty/8/98 (H3N2); and swine influenza virus A/swine/Iowa/30 (H1N1) were obtained from the collection of microorganisms at the Research Institute for Biological Safety Problems (Kazakhstan). Viruses were grown in the allantoic cavity of 10-day-old chicken embryos for 36 h at 37 °C.
The hemagglutinating activity of viruses was determined according to the standard method [34] using a 0.75% suspension of chicken erythrocytes.
Phosphate buffered saline (PBS, pH 7.4) was purchased from Amresco (Solon, OH, USA). Ten-day-old chicken eggs and 50% chicken red blood cell (cRBC) suspensions were obtained from the Almaty chicken factory farm (Almaty, Kazakhstan).
To assess the toxicity of the obtained test compounds, the MTT cytotoxicity test using macrophages of outbred mice was used. The technique is used to evaluate the cytotoxicity of solutions of compounds in the experiment and is based on the ability of living cell dehydrogenases to reduce unstained forms of 3–4,5-dimethylthiazol-2-yl-2,5-diphenylterarazole (MTT reagent) to blue crystalline formazan, soluble in dimethyl sulfoxide.
Acute toxicity was determined in a 10-day-old chick embryo model. The toxicity of the test materials was determined by inoculating 0.2 mL of the compound into the chorioallantoic cavity of chicken embryos (embryotoxicity). The toxicity of the preparations was determined by the death of chicken embryos within 4 days after the inoculation of materials.
Standard methods for finding the mean values and their mean errors were used for the statistical processing of the results.
3.2.2. Antimicrobial Activity
The antimicrobial activity of the samples was studied on the reference test micro-organisms recommended by the State Pharmacopoeia of the Republic of Kazakhstan: facultative anaerobic Gram-positive cocci Staphylococcus aureus ATCC 6538, aerobic Gram-positive spore-forming rods Bacillus subtilis ATCC 6633, Gram-negative facultative anaerobe rods Escherichia coli ATCC 25922, aerobic Pseudomonas aeruginosa ATCC 27853 and yeast fungus Candida albicans ATCC 10231 by the method of random dilutions with the determination of the minimum inhibitory concentration (MIC) [35,36]. The test strains of microorganisms used in the study were obtained from the American Type Culture Collection [29].
The antibacterial drug ceftriaxone and the antifungal drug nystatin were used as reference drugs.
The minimum inhibitory concentration (MIC) of the samples was determined by the method of serial dilution of ethanol solutions of the test samples in nutrient broth. For serial dilutions, suspensions of test strains at the concentration of 106 CFU/mL were used. The suspension of test strains of microorganisms was prepared from daily cultures grown on slant agar at the temperature of 37 °C for 24 h; for the yeast fungus Candida albicans, cultures were grown at 30 °C for 48 h.
Then, the test tube containing a clear suspension and the lowest concentration of the antimicrobial agent was selected by visual determination of turbidity in each test tube. This concentration was in line with the MIC. The results were averaged over the data of three experiments.
3.3. Analgesic Activity In Vivo
The experimental part was carried out in accordance with the “Rules of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes” and in accordance with the requirements for the study of new pharmacological substances [37]. The analgesic effect of the synthesized compounds was carried out using chemical stimulus on outbred white mice with weight in the range of 23 to 35 g. The experimental animals were kept in standard vivarium conditions on a normal diet. Five groups containing six animals each were formed (control, reference drug “diclofenac sodium”, three novel substances).
The analgesic effect of the samples was evaluated in the chemical irritation test of the peritoneum (test “vinegar cramps”). The abdominal constriction test is a visceral inflammatory pain model (acute peritonitis model). When visceral receptors are irritated with acetic acid, abdominal muscle contraction, hind limb extension and body elongation are observed [38]. A 0.75% solution of acetic acid was injected intraperitoneally in an amount of 0.1 mL per 10 g of animal weight. The potential pharmaceutically active substances were injected intragastrically at a dose of 25 mg/kg 30 min before the administration of the acetic acid. Immediately after the introduction of the stimulus, the latent time of the onset of the pain reaction “writhing” was recorded, and the writhings were counted for 30 min. The analgesic effect of compounds was determined by the ability to reduce the number of “writhings” counted for 10, 15, 20 and 30 min, compared with the corresponding indicators in the control animal group. The model drug was the non-steroidal anti-inflammatory drug diclofenac sodium, which was tested at an effective dose of 8 mg/kg (ED50 = 8 mg/kg). Control animals received the equivalent volume of starchy mucus.
Analgesic activity was expressed as a percentage reduction in the number of acetic writhings in experimental rats compared to controls.
Statistical processing was carried out by parametric statistical methods with the calculation of the arithmetic mean and standard error. Differences were considered significant at the achieved significance level p < 0.05.
The analgesic activity level of this compound is comparable to sodium diclofenac.
3.4. Experimental Section
3.4.1. General Procedure for the Synthesis of Compounds 2a–f
Quinine (2.6 g, 0.008 mol) was dissolved in 100 mL of dry dichloromethane. Then, 1.0 g (0.01 mol) of triethylamine and 0.009 mol of 1,2-azole-3- (2a–c) or adamantane- (2f) carbonyl chlorides were successively added to the resulting solution under stirring. The mixture was stirred for 1 h and left for 15 h at 20–23 °C. The mixture was washed with water (2 × 200 mL, 1 h stirring) and 5% sodium bicarbonate solution (2 × 200 mL, 1 h stirring). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed, and the residue was crystallized from a mixture of ether and hexane (1:1).
The procedure for the synthesis of quinine esters with a pyridine fragment (2d,e) is similar to the previous one, except for the amount of the used triethylamine. Here, 2.6 g (0.008 mol) of quinine, 2.4 g (0.024 mol) of Et3N and 1.6 g (0.009) mol of hydrochloride of nicotinic or isonicotinic carbonyl chlorides were taken into the reaction.
(R)-(6-Methoxyquinolin-4-yl)[(1S,2R,4S,5R)-5-vinylquinuclidin-2-yl]methyl 4,5-dichloroisothiazole-3-carboxylate (2a): white solid; yield 88%; mp 131–132 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 232 (4.57), 274 (3.90), 317 (3.70), 333 (3.70) nm; IR (KBr) ν 3075, 3010 (C=C–H); 2996, 2964, 2946, 2906, 2889, 2863 (C–Haliph); 1732 (C=O); 1623, 1589, 1560 1512 (C–Carom); 1464, 1455, 1403, 1354; 1263, 1213 (C–O); 1174, 1082, 1036, 975, 954, 906, 859, 843, 822 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.40–1.49 (1H, m, CH2), 1.55 (1H, dd, J = 12.4, 7.6 Hz, CH2), 1.70–1.77 (1H, m, CH2), 1.77–1.82 (1H, m, CH), 1.93–2.03 (1H, m, CH2), 2.17–2.26 (1H, m, CH–CH=CH2), 2.39–2.49 (2H, m, CH2 + CH2), 2.83 (1H, dd, J = 12.8, 10.5 Hz, CH2), 3.13–3.22 (1H, m, CH2), 3.50 (1H, q, J = 8.3 Hz, CH–N), 3.95 (3H, s, OMe), 4.95–5.07 (2H, m, CH=CH2), 5.89–6.00 (1H, m, CH=CH2), 6.59 (1H, d, J = 8.6 Hz, CH–O), 7.44 (1Hquin, dd, J = 9.2, 2.6 Hz), 7.56 (1Hquin, d, J = 4.5 Hz), 7.63 (1Hquin, d, J = 2.6 Hz), 7.96 (1Hquin, d, J = 9.2 Hz), 8.71 (1Hquin, d, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 25.76 (CH2), 27.62 (CH), 27.74 (CH2), 40.36 (CH–CH=CH2), 42.21 (CH2), 56.10 (OMe), 56.24 (CH2), 60.05 (CH–N), 76.34 (CH–O), 102.75 (1CHquin), 114.91 (=CH2), 119.89 (1CHquin), 122.13 (1CHquin), 131.91 (1CHquin), 142.88 (CH=CH2), 148.13 (1CHquin), 125.16, 127.40, 144.19, 144.55, 151.26, 153.49, 157.90, 158.48 (C=O) (8Cquater); MS m/z (Irel, %) 504.10 [M+H]+ (42.3); Anal. calcd. for C24H23Cl2N3O3S (504.43): C, 57.15; H, 4.60; Cl, 14.06; N, 8.33; S, 6.36%; Found: C, 57.44; H, 4.71; Cl, 13.88; N, 8.13; S, 6.22%.
(R)-(6-methoxyquinolin-4-yl)[(1S,2R,4S,5R)-5-vinylquinuclidin-2-yl]methyl 5-phenylisoxazole-3-carboxylate (2b): white solid; yield 89%; mp 158–159 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 232 (4.56), 272 (4.22), 319 (3.58), 333 (3.67); IR (KBr) ν 3130 (CHisox), 3075, 3000 (C=C–H); 2945, 2924, 2882, 2860 (C–Haliph); 1736 (C=O), 1622, 1591, 1571, 1512 (C–Carom); 1461, 1446, 1320, 1297; 1264, 1242 (C–O); 1172, 1138, 1100, 1084, 1037, 1017, 957, 947, 931, 851, 821, 803, 781, 765, 688, 677 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.42–1.51 (1H, m, CH2), 1.60 (1H, dd, J = 12.7, 7.6 Hz, CH2), 1.70–1.78 (1H, m, CH2), 1.78–1.83 (1H, m, CH), 1.98–2.08 (1H, m, CH2), 2.19–2.27 (1H, m, CH–CH=CH2), 2.41–2.56 (2H, m, CH2 + CH2), 2.88 (1H, dd, J = 13.3, 10.2 Hz, CH2), 3.32–3.39 (1H, m, CH2), 3.50 (1H, q, J = 8.3 Hz, CH–N), 3.96 (3H, s, OMe), 4.96–5.07 (2H, m, =CH2), 5.93–6.02 (1H, m, CH=CH2), 6.62 (1H, d, J = 8.5 Hz, CH–O), 7.45 (1Hquin, J = 9.2, 2.6 Hz), 7.54–7.57 (3HAr, m), 7.57 (CHisox, s), 7.59 (1Hquin, d, J = 4.5 Hz), 7.62 (1Hquin, d, J = 2.6 Hz), 7.94–8.00 (3H, m, 1Hquin+2HAr), 8.72 (1Hquin, d, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 25.44 (CH2), 27.69 (CH), 27.77 (CH2), 39.87 (CH–CH=CH2), 42.23 (CH2), 56.21 (OMe), 56.26 (CH2), 60.06 (CH–N), 76.36 (CH–O), 101.47 (CHisox), 102.70 (1CHquin), 114.94 (=CH2), 119.82 (1CHquin), 122.13 (1CHquin), 126.47 (2CHAr), 129.92 (2CHAr), 131.71 (1CHquin), 131.95 (1CHAr), 142.94 (CH=CH2), 148.14 (1CHquin), 126.53, 127.28, 144.15, 144.58, 157.02, 157.92, 159.31, 172.06 (8Cquater); MS m/z (Irel, %) 496.30 [M+H]+ (44.0); Anal. calcd. for C30H29N3O4 (495.58): C, 72.71; H, 5.90; N, 8.48%; Found: C, 73.09; H, 6.05; N, 8.35%.
(R)-(6-methoxyquinolin-4-yl)[(1S,2R,4S,5R)-5-vinylquinuclidin-2-yl]methyl 5-(p-tolyl)isoxazole-3-carboxylate (2c): white solid; yield 86%; mp 148–149 °C; UV (MeOH c = 6 × 10−5 M) λmax (log ε) 233 (4.58), 278 (4.30), 317 (3.70), 332 (3.70); IR (KBr) ν 3137 (CHisox), 3072, 3029 (C=C–H); 2945, 2923, 2882, 2866 (C–Haliph); 1737 (C=O); 1622, 1592, 1513(C–Carom); 1460, 1446, 1316, 1295; 1265, 1240 (C–O); 1172, 1136, 1112, 1084, 1037, 1020, 999, 947, 928, 851, 823, 812, 800, 781, 715, 687, 677, 567, 501 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.41–1.50 (1H, m, CH2), 1.58 (1H, dd, J = 13.2, 7.6 Hz, CH2), 1.70–1.77 (1H, m, CH2), 1.78–1.84 (1H, m, CH), 1.98–2.07 (1H, m, CH2), 2.18–2.27 (1H, m, CH–CH=CH2), 2.37 (3H, s, Me), 2.40–2.49 (2H, m, CH2 + CH2), 2.85 (1H, dd, J = 13.5, 10.0 Hz, CH2), 3.15–3.24 (1H, m, CH2), 3.50 (1H, q, J = 8.3 Hz, CH–N), 3.96 (3H, s, OMe), 4.96–5.07 (2H, m, =CH2), 5.92–6.02 (1H, m, CH=CH2), 6.59 (1H, d, J = 8.4 Hz, CH–O), 7.37 (2HAr, d, J = 8.1 Hz), 7.45 (1Hquin, dd, J = 9.2, 2.6 Hz), 7.50 (CHisox, s), 7.58 (1Hquin, d, J = 4.5 Hz), 7.62 (1Hquin, d, J = 2.6 Hz), 7.85 (2HAr, d, J = 8.1 Hz), 7.97 (1Hquin, d, J = 9.2 Hz), 8.72 (1Hquin, d, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 21.59 (Me), 25.45 (CH2), 27.68 (CH), 27.76 (CH2), 39.88 (CH–CH=CH2), 42.22 (CH2), 56.19 (OMe), 56.26 (CH2), 60.07 (CH–N), 76.30 (CH–O), 100.79 (CHisox), 102.69 (1CHquin), 114.92 (=CH2), 119.82 (1CHquin), 122.12 (1CHquin), 126.40 (2CHAr), 130.44 (2CHAr), 131.94 (1CHquin), 142.94 (CH=CH2), 148.14 (1CHquin), 123.88, 127.88, 141.75, 144.17, 144.58, 156.94, 157.90, 159.35, 172.21 (9Cquater); MS m/z (Irel, %) 510.20 [M + H]+ (52.7); Anal. calcd. for C31H31N3O4 (509.61): C, 73.06; H, 6.13; N, 8.25%; Found: C, 73.44; H, 6.36; N, 8.11%.
(R)-(6-methoxyquinolin-4-yl)[(1S,2R,4S,5R)-5-vinylquinuclidin-2-yl]methyl nicotinate (2d): white solid; yield 86%; mp 149–150 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 208 (4.57), 222 (4.59), 264 (3.78), 322 (3.70), 334 (3.70); IR (KBr) ν 3049 (C=C–H); 2975, 2940, 2872, 2854 (C–Haliph); 1716 (C=O); 1621, 1587, 1564, 1510 (C–Carom); 1462, 1443, 1420, 1334, 1288; 1266, 1246 (C–O); 1194, 1175, 1112, 1073, 1041, 1021, 990, 921, 855, 847, 815, 767, 739, 712, 701, 677, 643, 623, 562 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.38–1.47 (1H, m, CH2), 1.54 (1H, dd, J = 13.0, 7.7 Hz, CH2), 1.63–1.72 (1H, m, CH2), 1.73–1.78 (1H, m, CH), 2.03–2.13 (1H, m, CH2), 2.15–2.24 (1H, m, CH–CH=CH2), 2.38–2.45 (1H, m, CH2), 2.45–2.53 (1H, m, CH2), 2.83 (1H, dd, J = 13.4, 10.0 Hz, CH2), 3.11–3.23 (1H, m, CH2), 3.54 (1H, q, J = 8.5 Hz, CH–N), 3.94 (3H, s, OMe), 4.94–5.04 (2H, m, =CH2), 5.93–6.05 (1H, m, CH=CH2), 6.57 (1H, d, J = 8.8 Hz, CH–O), 7.43 (1Hquin, dd, J = 9.2, 2.7 Hz), 7.56–7.61 (1Hquin, m), 7.61–7.65 (1Hquin+1HPy, m), 7.96 (1Hquin, d, J = 9.2 Hz), 8.38 (1HPy, dt, J = 8.1, 2.0 Hz), 8.70 (1Hquin, d, J = 4.6 Hz), 8.84 (1HPy, dd, J = 4.8, 1.7 Hz), 9.23 (1HPy, dd, J = 2.2, 0.6 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 25.71 (CH2), 27.72 (CH), 27.89 (CH2), 40.10 (CH–CH=CH2), 42.18 (CH2), 56.19 (OMe), 56.41 (CH2), 60.40 (CH–N), 75.33 (CH–O), 102.66 (1CHquin), 114.82 (=CH2), 119.74 (1CHquin), 121.99 (1CHquin), 124.62 (1CHPy), 131.90 (1CHquin), 137.6 (1CHPy), 143.02 (CH=CH2), 148.14 (1CHquin), 150.76 (1CHPy), 154.63 (1CHPy), 125.77, 127.46, 144.54, 144.98, 157.85, 164.65 (6Cquater); MS m/z (Irel, %) 430.30 [M+H]+ (100); Anal. calcd. for C26H27N3O3 (429.52): C, 72.71; H, 6.34; N, 9.78%; Found: C, 73.01; H, 6.50; N, 9.61%.
(R)-(6-methoxyquinolin-4-yl)[(1S,2R,4S,5R)-5-vinylquinuclidin-2-yl]methyl isonicotinate (2e): white solid; yield 88%; mp 153–154 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 209 (4.59), 231 (4.51), 278 (3.78), 320 (3.60), 333 (3.70); IR (KBr) ν 3071, 3033 (C=C–H); 2927, 2862 (C–Haliph), 1732 (C=O), 1621, 1593, 1560, 1508 (C–Carom); 1474, 1432, 1407, 1363, 1324; 1273, 1226 (C–O); 1116, 1063, 1029, 991, 913, 849, 830, 754, 706, 673 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.40–1.50 (1H, m, CH2), 1.54 (1H, dd, J = 13.1, 7.9 Hz, CH2), 1.65–1.72 (1H, m, CH2), 1.76–1.81 (1H, m, CH), 2.03–2.13 (1H, m, CH2), 2.18–2.26 (1H, m, CH–CH=CH2), 2.39–2.42 (1H, m, CH2), 2.42–2.52 (1H, m, CH2), 2.84 (1H, dd, J = 13.6, 10.1 Hz, CH2), 3.11–3.21 (1H, m, CH2), 3.53 (1H, q, J = 8.5 Hz, CH–N), 3.94 (3H, s, OMe), 4.97–5.07 (2H, m, =CH2), 5.96–6.06 (1H, m, CH=CH2), 6.55 (1H, d, J = 8.8 Hz, CH–O), 7.44 (1Hquin, dd, J = 9.2, 2.7 Hz), 7.58–7.63 (2Hquin, m), 7.92 (2HPy, m, J = 6.0, 1.7 Hz), 7.96 (1Hquin, d, J = 9.2 Hz), 8.70 (1Hquin, d, J = 4.6 Hz), 8.84 (2HPy, dd, J = 6.0, 1.6 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 25.75 (CH2), 27.70 (CH), 27.88 (CH2), 40.06 (CH–CH=CH2), 42.18 (CH2), 56.24 (OMe), 56.35 (CH2), 60.45 (CH–N), 75.69 (CH–O), 102.65 (1CHquin), 114.91 (=CH2), 119.68 (1CHquin), 122.06 (1CHquin), 123.15 (2CHPy), 131.91 (1CHquin), 143.06 (CH=CH2), 148.16 (1CHquin), 151.54 (2CHPy), 127.48, 136.90, 144.51, 144.88, 157.87, 164.61 (6Cquater); MS m/z (Irel, %) 430.30 [M + H]+ (100); Anal. calcd. for C26H27N3O3 (429.52): C, 72.71; H, 6.34; N, 9.78%; Found: C, 73.01; H, 6.50; N, 9.61%.
(R)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl (3R,5R,7R)-adamantane-1-carboxylate (2f): white solid; yield 91%; mp 162–163 °C; UV (MeOH c = 5 × 10−5 M) λmax (log ε) 232 (4.46), 279 (3.54), 320 (3.60), 333 (3.70); IR (KBr) ν 3075 (C=C–H), 2933, 2906, 2851 (C–Haliph); 1803, 1736 (C=O); 1621, 1591, 1508 (C–Carom), 1470, 1453, 1431, 1344; 1240, 1227 (C–O); 1156, 1103, 1079, 1032, 997, 973, 935, 855, 830, 717, 643 cm−1; 1H NMR (CDCl3, 500 MHz) δ 1.44–1.57 (2H, m, CH2), 1.64–1.78 (6H, m), 1.78–1.84 (1H, m), 1.84–1.89 (1H, m), 1.90–1.95 (6H, m), 1.95–2.02 (1H, m), 2.02–2.08 (3H, m), 2.23–2.31 (1H, m), 2.60–2.70 (1H, m), 3.07 (1H, dd, J = 13.7, 10.3 Hz), 3.10–3.17 (2H, m), 3.40–3.51 (1H, m), 3.88 (3H, s, OMe), 4.87–4.99 (2H, m, =CH2), 5.53 (1H, d, J = 4.1 Hz, CH–O), 5.68–5.77 (1H, m, CH=CH2), 7.21 (1Hquin, d, J = 2.5 Hz), 7.29 (1Hquin, dd, J = 9.2, 2.6 Hz), 7.47 (1Hquin, d, J = 4.5 Hz), 7.94 (1Hquin, d, J = 9.2 Hz), 8.60 (1Hquin, d, J = 4.5 Hz); 13C NMR (CDCl3, 125 MHz) δ 21.99 (CH2), 27.77 (CH2), 27.84 (CH), 28.02 (CH), 36.48 (3CH2), 38.45 (3CH2), 40.08 (CH–CH=CH2), 43.37 (CH2), 55.86 (OMe), 57.12 (CH2), 60.13 (CH–N), 72.10 (CH–O), 101.50 (1CHquin), 114.59 (=CH2), 118.64 (1CHquin), 121.65 (1CHquin), 131.71 (1CHquin), 141.95 (CH–CH=CH2), 147.71 (1CHquin), 36.57, 126.80, 144.39, 147.76, 157.90, 173.56 (6Cquater); Anal. calcd. for C31H38N2O3 (486.66): C, 76.51; H, 7.87; N, 5.76%; Found: C, 76.81; H, 7.98; N, 5.52%.
3.4.2. General Procedure for the Synthesis of Compounds 3a–e
A mixture of 0.02 mol of ester 2a–e in 30 mL of dry dichloromethane and 0.08 mol (5 mL) of dry methyl iodide was kept for 14 days in the dark. Then, the precipitated product was filtered off, washed with 2 × 5 mL of methylene chloride and dried in a vacuum.
(1S,2R,4S,5R)-2-(R)-[(4,5-dichloroisothiazole-3-carbonyl)oxy)(6-methoxy-1-methylquinolin-1-ium-4-yl]methyl-1-methyl-5-vinylquinuclidin-1-ium diiodide (3a): orange solid; yield 98%; mp 174–175 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 254 (4.48), 278 (3.70), 318 (3.60), 356 (3.70); IR (KBr) ν 3072 (C=C–H); 2997, 2925, 2855 (C–Haliph); 1742 (C=O), 1615, 1591, 1532 (C–Carom); 1475, 1460, 1440, 1430, 1415, 1378, 1350; 1274, 1243 (C–O); 1185, 1160, 1118, 1077, 1034, 1020, 1002, 970, 835, 910, 829, 795, 726, 715, 690, 514 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.70–1.81 (1H, m, CH2), 2.01–2.11 (1H, m, CH2), 2.13–2.20 (1H, m, CH), 2.22–2.30 (1H, m, CH2), 2.50–2.59 (1H, m, CH2), 2.86–2.95 (1H, m, CH–CH=CH2), 3.51 (3H, s, MeN), 3.42–3.62 (1H, m, CH2), 3.72–3.81 (1H, m, CH2), 3.83–3.90 (1H, m, CH–N), 4.06–4.15 (2H, m, CH2), 4.18 (3H, s, OMe), 4.64 (3H, s, MeN), 5.04–5.09 (1H, m, CH=CH2), 5.13–5,19 (1H, m, CH=CH2), 5.72–5.83 (1H, m, CH=CH2), 7.31 (1H, s, CH–O), 7.60 (1Hquin, d, J = 9.2, 2.6 Hz), 8.03 (1Hquin, dd, J = 9.7, 2.5 Hz), 8.24 (1Hquin, d, J = 6.1 Hz), 8.56 (1Hquin, d, J = 9.7 Hz), 9.31 (1Hquin, d, J = 6.2 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 21.33 (CH2), 24.76 (CH2), 26.42 (CH), 37.98 (CH–CH=CH2), 46.40 (MeN), 49.65 (MeN), 54.91 (CH2), 57.06 (OMe), 65.10 (CH–N), 65.25 (CH2), 70.23 (CH–O), 104.30 (1CHquin), 117.58 (=CH2), 120.88 (1CHquin), 122.71 (1CHquin), 127.84 (1CHquin), 138.19 (CH=CH2), 146.92 (1CHquin), 125.86, 127.46, 134.66, 149.15, 151.15, 152.74, 156.94, 160.20 (8Cquater); MS m/z (Irel, %) 534.10 [M + H - 2I]+ (6.6); Anal. calcd. for C26H29Cl2I2N3O3S (788.30): C, 39.61; H, 3.71; Cl, 8.99; I, 32.20; N, 5.33; S, 6.00%; Found: C, 39.89; H, 3.79; Cl, 8.72; I, 31.80; N, 5.05; S, 5.68%.
(1S,2R,4S,5R)-2-[(R)-(6-methoxy-1-methylquinolin-1-ium-4-yl)(5-phenylisoxazole-3-carbonyl)oxymethyl]-1-methyl-5-vinylquinuclidin-1-ium diiodide (3b): orange solid; yield 94%; mp 190–192 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 213 (4.74), 254 (4.59), 272 (4.15), 319 (3.70), 356 (3.70); IR (KBr) ν 3073 (C=C–H); 3000, 2923, 2853 (C–Haliph); 1750 (C=O); 1615, 1590, 1570, 1533 (C–Carom); 1476, 1442, 1379; 1275, 1221(C–O); 1163, 1124, 1071, 1036, 1020, 993, 947, 918, 829, 794, 762, 690 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.75–1.85 (1H, m, CH2), 2.07–2.16 (1H, m, CH2), 2.17–2.23 (1H, m, CH), 2.33–2.41 (2H, m, CH2), 2.51–2.60 (1H, m, CH2), 2.87–2.97 (1H, m, CH–CH=CH2), 3.45–3.53 (3H, m, NMe), 3.56 (3H, s, NMe), 3.57–3.67 (1H, m, CH2), 3.74–3.81 (1H, m, CH2), 3.86–3.94 (1H, m, CH2), 4.06–4.15 (2H, m, CH2 +CH–N), 4.19 (3H, s, OMe), 4.66 (3H, s, NMe), 5.05–5.10 (1H, m, CH=CH2), 5.15–5.21 (1H, m, CH=CH2), 5.75 (1H, s, CH–O), 5.75–5.85 (1H, m, CH=CH2), 7.34–7.37 (1Hquin, m), 7.57–7.63 (4H, m, 1Hquin+3HAr), 7.70 (CHisox, s), 8.01–8.07 (3H, m, 1Hquin+2HAr), 8.33 (1Hquin, d, J = 6.2 Hz), 8.58 (1Hquin, d, J = 9.8 Hz), 9.33 (1Hquin, d, J = 6.3 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 21.19 (CH2), 24.80 (CH2), 26.50 (CH), 37.98 (CH–CH=CH2), 46.45 (NMe), 49.71 (NMe), 54.79 (CH2), 57.11 (OMe), 65.13 (CH–N), 65.26 (CH2), 70.28 (CH–O), 101.80 (CHisox), 104.28 (1CHquin), 117.62 (=CH2), 120.92 (1CHquin), 122.76 (1CHquin), 126.49 (2CHAr), 127.85 (1CHquin), 130.04 (2CHAr), 131.88 (1CHAr), 138.16 (CH=CH2), 147.02 (1CHquin), 126.40, 127.46, 134.66, 149.09, 156.97, 158.26, 160.23, 172.15 (8Cquater); MS m/z (Irel, %) 524.40 [M + H - 2I]+ (12.4); Anal. calcd. for C32H35I2N3O4 (779.45): C, 49.31; H, 4.53; I, 32.56; N, 5.39%; Found: C, 49.71; H, 4.79; I, 31.88; N, 5.16%.
(1S,2R,4S,5R)-2-[(R)-(6-methoxy-1-methylquinolin-1-ium-4-yl)(5-(p-tolyl)isoxazole-3-carbonyl)oxymethyl]-1-methyl-5-vinylquinuclidin-1-ium diiodide (3c): orange solid; yield 95%; mp 193–194 °C; UV (MeOH c = 4 × 10−5 M) λmax (log ε) 212 (4.73), 254 (4.58), 277 (4.23), 302 (3.70), 357 (3.70); IR (KBr) ν 3072 (C=C–H); 3000, 2946 (C–Haliph); 1749 (C=O); 1614, 1592, 1533, 1510 (C–Carom); 1477, 1443, 1378; 1275, 1223 (C–O); 1209, 1163, 1129, 1111, 1035, 1020, 993, 947, 917, 825, 794, 759, 715, 677, 500 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.73–1.84 (1H, m, CH2), 2.07–2.16 (1H, m, CH2), 2.17–2.23 (1H, m, CH), 2.32–2.39 (2H, m, CH2), 2.40 (3H, s, Me), 2.52–2.60 (1H, m, CH2), 2.87–2.97 (1H, m, CH–CH=CH2), 3.54 (3H, s, NMe), 3.56–3.64 (1H, m, CH2), 3.72–3.80 (1H, m, CH2), 3.84–3.93 (1H, m, CH2), 4.03–4.15 (2H, m, CH2 +CH–N), 4.18 (3H, s, OMe), 4.65 (3H, s, NMe), 5.06–5.10 (1H, m, CH=CH2), 5.14–5.21 (1H, m, CH=CH2), 5.75 (1H, s, CH–O), 5.78–5.84 (1H, m, CH=CH2), 7.41 (2HAr, d, J = 8.1 Hz), 7.57–7.64 (1Hquin), 7.61 c (CHisox, m), 7.92 (2HAr, d, J = 8.1 Hz), 8.05 (1Hquin, dd, J = 9.8, 2.5 Hz), 8.30 (1Hquin, d, J = 6.1 Hz), 8.58 (1Hquin, d, J = 9.7 Hz), 9.32 (1Hquin, d, J = 6.2 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 21.18 (CH2), 21.65 (Me), 24.81 (CH2), 26.49 (CH), 37.97 (CH–CH=CH2), 46.43 (NMe), 49.71 (NMe), 54.81 (CH2), 57.09 (OMe), 65.13 (CH–N), 65.31 (CH2), 70.24 (CH–O), 101.12 (CHisox), 104.29 (1CHquin), 117.62 (=CH2), 120.90 (1CHquin), 122.75 (1CHquin), 126.45 (2CHAr), 127.84 (1CHquin), 130.57 (2CHAr), 138.17 (CH=CH2), 147.04 (1CHquin), 123.76, 127.46, 134.67, 141.99, 149.13, 156.90, 158.31, 160.24, 172.34 (9Cquater); Anal. calcd. for C33H37I2N3O4 (793.48): C, 49.95; H, 4.70; I, 31.99; N, 5.30%; Found: C, 50.21; H, 4.81; I, 31.64; N, 5.11%.
(1S,2R,4S,5R)-2-[(R)-(6-methoxy-1-methylquinolin-1-ium-4-yl)((1-methylpyridin-1-ium-3-carbonyl)oxy)methyl]-1-methyl-5-vinylquinuclidin-1-ium triiodide (3d): reddish orange solid; yield 85%; mp 178 °C (with decomposition); UV (MeOH c = 4 × 10−5 M) λmax (log ε) 217 (4.81), 254 (4.49), 272 (3.70), 318 (3.70), 355 (3.70); IR (KBr) ν 3075 (C=C-H), 3025, 2994, 2923, 2854 (C–Caliph); 1740 (C=O); 1636, 1617, 1591, 1532 (C–Carom); 1475, 1437, 1371, 1293; 1276, 1243 (C–O); 1214, 1165, 1098, 1034, 1020, 1000, 947, 927, 797, 727, 659 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.73–1.87 (1H, m, CH2), 2.02–2.13 (1H, m, CH2), 2.13–2.21 (1H, m, CH2), 2.26–2.38 (1H, m, CH), 2.67–2.78 (1H, m, CH2), 2.85–2.97 (1H, m, CH–CH=CH2), 3.32 (3H, s, NMe), 3.52–3.64 (1H, m, CH2), 3.75–3.84 (1H, m, CH2), 3.85–3.92 (1H, m, CH2), 3.92–4.01 (1H, m, CH2), 4.02–4.11 (1H, m, CH–N), 4.20 (3H, s, OMe), 4.54 (3H, s, NMe), 4.65 (3H, s, NMe), 5.05 (1H, d, =CH2), 5.17 (1H, d, =CH2), 5.76–5.85 (1H, m, CH=CH2), 7.37–7.44 (1H, m, CH–O), 7.65 (1Hquin, d, J = 1.5 Hz), 8.05 (1Hquin, dd, J = 9.7, 2.6 Hz), 8.40 (1Hquin, dd, J = 8.0, 6.2 Hz), 8.47 (1HPy, d, J = 6.1 Hz), 8.57 (1Hquin, d, J = 9.7 Hz), 9.16 (1Hquin, d, J = 8.2 Hz), 9.30 (1HPy, d, J = 6.2 Hz), 9.37 (1HPy, d, J = 6.3 Hz), 9.67–9.71 (1HPy, m); 13C NMR (DMSO-d6, 125 MHz) δ 21.07 (CH2), 25.21 (CH2), 26.58 (CH), 37.97 (CH–CH=CH2), 46.37 (NMe), 49.25 (NMe), 49.69 (NMe), 54.74 (CH2), 57.17 (OMe), 64.85 (CH–N), 65.36 (CH2), 70.51 (CH–O), 104.48 (1CHquin), 117.56 (=CH2), 121.09 (1CHquin), 122.72 (1CHquin), 127.70 (1CHquin), 128.50 (1CHPy), 138.48 (CH=CH2), 146.07 (1CHPy), 146.82 (1CHquin), 147.27 (1CHPy), 149.87 (1CHPy), 127.55, 129.03, 134.60, 149.01, 160.31, 161.07 (6Cquater); Anal. calcd. for C29H36I3N3O3 (855.33): C, 40.72; H, 4.24; I, 44.51; N, 4.91%; Found: C, 40.96; H, 4.51; I, 44.11; N, 4.75%.
(1S,2R,4S,5R)-2-[(R)-(6-methoxy-1-methylquinolin-1-ium-4-yl)((1-methylpyridin-1-ium-4-carbonyl)oxy)methyl]-1-methyl-5-vinylquinuclidin-1-ium triiodide (3e): reddish orange solid; yield 83%; mp 200 °C (with decomposition); UV (MeOH c = 4 × 10−5 M) λmax (log ε) 219 (4.81), 254 (4.46), 279 (3.70), 318 (3.70), 355 (3.70); IR (KBr) ν 3075 (C=C–H); 3001, 2924, 2854 (C–Haliph); 1745 (C=O); 1639, 1616, 1592, 1531 (C–Carom); 1450, 1432, 1376; 1270, 1244 (C–O); 1218, 1162, 1001, 1035, 1019, 1000, 943, 917, 900, 824, 723, 671 cm−1; 1H NMR (DMSO–d6, 500 MHz) δ 1.77–1.87 (1H, m, CH2), 2.02–2.14 (1H, m, CH2), 2.15–2.22 (1H, m, CH2), 2.24–2.34 (1H, m, CH), 2.64–2.72 (1H, m, CH2), 2.87–2.96 (1H, m, CH–CH=CH2), 3.33 (3H, s, NMe), 3.52–3.64 (1H, m, CH2), 3.76–3.84 (1H, m, CH2), 3.85–3.94 (1H, m, CH2), 3.95–4.03 (1H, m, CH2), 4.04–4.14 (1H, m, CH–N), 4.20 (3H, s, OMe), 4.54 (3H, s, NMe), 4.65 (3H, s, NMe), 5.03–5.08 (1H, m, =CH2), 5.14–5.21 (1H, m, =CH2), 5.77–5.86 (1H, m, CH=CH2), 7.29–7.36 (1H, m, CH–O), 7.62 (1Hquin, d, J = 1.5 Hz), 8.05 (1Hquin, dd, J = 9.7, 2.6 Hz), 8.43 (1Hquin, d, J = 6.2 Hz), 8.57 (1Hquin, d, J = 9.8 Hz), 8.71–8.76 (2HPy, m), 9.30 (2HPy, d, J = 6.7 Hz), 9.38 (1HPy, d, J = 6.4 Hz); 13C NMR (DMSO-d6, 125 MHz) δ 20.98 (CH2), 25.09 (CH2), 26.54 (CH), 37.92 (CH–CH=CH2), 46.38 (NMe), 49.31 (NMe), 49.63 (NMe), 54.39 (CH2), 57.16 (OMe), 64.91 (CH–N), 65.29 (CH2), 70.77 (CH–O), 104.37 (1CHquin), 117.60 (=CH2), 120.96 (1CHquin), 122.73 (1CHquin), 127.72 (1CHquin), 127.84 (2CHPy), 138.36 (CH=CH2), 146.91 (1CHquin), 147.55 (2CHPy), 127.53, 134.58, 143.11, 148.91, 160.27, 161.61 (6Cquater); Anal. calcd. for C29H36I3N3O3 (855.33): C, 40.72; H, 4.24; I, 44.51; N, 4.91%; Found: C, 40.96; H, 4.51; I, 44.11; N, 4.75%.
4. Conclusions
A convenient method for the preparation of natural alkaloid quinine derivatives via an acylation reaction with 4,5-dichloroisothiazole-3-, 5-arylisoxazole-3-, adamantane- and hydrochlorides of pyridine-3- and pyridine-4-carbonyl chlorides has been developed. According to bioassay data, all tested substances did not show pronounced antiviral properties in the range of doses studied, except for compounds 2e, 3b, 3c and 3e, which showed more pronounced antiviral properties. However, the presence of antimicrobial and analgesic activity in newly synthesized compounds, particularly compounds 2b, 2e, 2f, 3b, 3c and 3e with isoxazole, pyridine and adamantane fragments, makes it possible to consider them promising for further study of their pharmacological properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27113476/s1, NMR, UV, IR and mass spectra of synthesized compounds 2a–f, 3a–e.
Author Contributions
Conceptualization, G.K.M. and E.A.D.; methodology, E.A.D. and R.B.S.; validation, E.A.D., E.A.A. and S.K.P.; investigation, R.B.S., E.A.D., E.A.A. and S.K.P.; resources, G.K.M., A.R.Z., O.A.N.; writing—original draft preparation, E.A.A. and R.B.S.; writing—review and editing, A.R.Z. and V.I.P.; visualization, E.A.A.; supervision, G.K.M. and V.I.P.; project administration, G.K.M. and V.I.P.; funding acquisition, G.K.M., A.R.Z., O.A.N. and R.B.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
The work was carried out within the framework of project No. АР08855433 with grant financing from the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D’Alessandro U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011;10:144–155. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liles N.W., Page E.E., Liles A.L., Vesely S.K., Raskob G.E., George J.N. Diversity and severity of adverse reactions to quinine: A systematic review. Am. J. Hematol. 2016;91:461–466. doi: 10.1002/ajh.24314. [DOI] [PubMed] [Google Scholar]
- 3.Taylor W.R.J., White N.J. Antimalarial Drug Toxicity. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Heller L.E., Roepe P.D. Artemisinin-Based Antimalarial Drug Therapy: Molecular Pharmacology and Evolving Resistance. Trop. Med. Infect. Dis. 2019;4:89. doi: 10.3390/tropicalmed4020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf R., Baroni A., Greco R., Donnarumma G., Ruocco E., Tufano M.A., Ruocco V. Quinine sulfate and bacterial invasion. Ann. Clin. Microbiol. Antimicrob. 2002;1:5. doi: 10.1186/1476-0711-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharal S.A., Hussain Q., Ali S., Fakhuruddin Quinine is bactericidal. J. Pak. Med. Assoc. 2009;59:208–212. [PubMed] [Google Scholar]
- 7.Leanse L.G., Goh X.S., Dai T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2019;52:569–575. doi: 10.1002/lsm.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majnooni M.B., Fakhri S., Bahrami G., Naseri M., Farzaei M.H., Echeverría J. Alkaloids as Potential Phytochemicals against SARS-CoV-2: Approaches to the Associated Pivotal Mechanisms. Evid.-Based Complementary Altern. Med. 2021;2021:6632623. doi: 10.1155/2021/6632623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latarissa I.R., Barliana M.I., Meiliana A., Lestari K. Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review. Clin. Pharmacol. Adv. Appl. 2021;13:225–234. doi: 10.2147/CPAA.S331660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younis N.K., Zareef R.O., Al Hassan S.N., Bitar F., Eid A.H., Arabi M. Hydroxychloroquine in COVID-19 Patients: Pros and Cons. Front. Pharmacol. 2020;11:597985. doi: 10.3389/fphar.2020.597985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antika L.D., Triana D., Ernawati T. Antimicrobial activity of quinine derivatives against human pathogenic bacteria. IOP Conf. Ser. Earth Environ. Sci. 2020;462:012006. doi: 10.1088/1755-1315/462/1/012006. [DOI] [Google Scholar]
- 13.Ernawati T., Minarti M., Lotulung P.D.N. Structure Modification of Quinine on C-9 Hydroxyl Group via Esterification Reaction. J. Pure Appl. Chem. Res. 2020;9:32–39. doi: 10.21776/ub.jpacr.2020.009.01.505. [DOI] [Google Scholar]
- 14.Che Z., Yang J., Sun D., Tian Y., Liu S., Lin X., Jiang J., Chen G. Combinatorial Synthesis of Novel 9R-Acyloxyquinine Derivatives as Insecticidal Agents. Comb. Chem. High Throughput Screen. 2020;23:111–118. doi: 10.2174/1386207323666200120112714. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Yang D., Han F., Li D., Zhao D., Wang R. Catalytic Asymmetric Construction of Pyrroloindolines via an in Situ Generated Magnesium Catalyst. Org. Lett. 2014;17:176–179. doi: 10.1021/ol503455r. [DOI] [PubMed] [Google Scholar]
- 16.Kletskov A.V., Bumagin N.A., Zubkov F.I., Grudinin D.G., Potkin V.I. Isothiazoles in the design and synthesis of biologically active substances and ligands for metal complexes. Synthesis. 2020;52:159–188. doi: 10.1055/s-0039-1690688. [DOI] [Google Scholar]
- 17.Zhu J., Mo J., Lin H., Chen Y., Sun H. The recent progress of isoxazole in medicinal chemistry. Bioorganic Med. Chem. 2018;26:3065–3075. doi: 10.1016/j.bmc.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Altaf A.A., Shahzad A., Gul Z., Rasool N., Badshah A., Lal B., Khan E. Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015;1:1–11. [Google Scholar]
- 19.Kletskov A.V., Potkin V.I., Dikusar E.A., Zolotar R.M. New Data on Vanillin-Based Isothiazolic Insecticides Synergists. Nat. Prod. Commun. 2017;12:105–106. doi: 10.1177/1934578X1701200130. [DOI] [PubMed] [Google Scholar]
- 20.Kletskov A.V., Potkin V.I., Kolesnik I.A., Petkevich S.K., Kvachonak A.V., Dosina M.O., Loiko D.O., Larchenko M.V., Pashkevich S.G., Kulchitsky V.A. Synthesis and biological activity of novel comenic acid derivatives containing isoxazole and isothiazole moieties. Nat. Prod. Commun. 2018;13:1507–1510. doi: 10.1177/1934578X1801301124. [DOI] [Google Scholar]
- 21.Potkin V.I., Kletskov A.V., Petkevich S.K., Pashkevich S.G., Kazbanov V.V., Denisov A.A., Kulchitsky V.A. Synthesis of water soluble isoxazol-3-yl(isothiazol-3-yl) carboxamides and ureas containing amino acid residues—Potential anticancer agents. Heterocycl. Lett. 2015;1:11–19. [Google Scholar]
- 22.Klimochkin Y.N., Shiryaev V.A., Leonova M.V. Antiviral properties of cage compounds. New prospects. Russ. Chem. Bull. 2015;64:1473–1496. doi: 10.1007/s11172-015-1035-y. [DOI] [Google Scholar]
- 23.Malakar S., Sreelatha L., Dechtawewat T., Noisakran S., Yenchitsomanus P.T., Chu J.J.H., Limjindaporn T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018;255:171–178. doi: 10.1016/j.virusres.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Große M., Ruetalo N., Layer M., Hu D., Businger R., Rheber S., Setz C., Rauch P., Auth J., Fröba M., et al. Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2. Viruses. 2021;13:647. doi: 10.3390/v13040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaumont K., Webster R., Gardner I., Dack K. Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Curr. Drug Metab. 2003;4:461–485. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
- 26.Larsen E.M., Johnson R.J. Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Dev. Res. 2019;80:33–47. doi: 10.1002/ddr.21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce M.D., Jennings M.C., Santiago C.N., Fletcher M.H., Wuest W.M., Minbiole K.P. Natural product-derived quaternary ammonium compounds with potent antimicrobial activity. J. Antibiot. 2015;69:344–347. doi: 10.1038/ja.2015.107. [DOI] [PubMed] [Google Scholar]
- 28.Patel P.R., Joshi H., Shah U., Bapna M., Patel B. New generation of quinazolinone derivatives as potent antimicrobial agents. Asian Pac. J. Health Sci. 2021;8:61–66. doi: 10.21276/apjhs.2021.8.2.12. [DOI] [Google Scholar]
- 29.Badshah S.L., Ullah A. New developments in non quinolone-based antibiotics for the inhibition of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018;152:393–400. doi: 10.1016/j.ejmech.2018.04.059. [DOI] [PubMed] [Google Scholar]
- 30.Potkin V.I., Gadzhily R.A., Dikusar E.A., Petkevich S.K., Zhukovskaya N.A., Aliev A.G., Nagieva S.F. Synthesis of hydroxybenzaldehyde derivatives containing an isoxazole heteroring. Russ. J. Org. Chem. 2012;48:127–136. doi: 10.1134/S1070428012010216. [DOI] [Google Scholar]
- 31.Nechai N.I., Dikusar E.A., Potkin V.I., Kaberdin R.V. Synthesis of 4,5-Dichloroisothiazole-3-carboxylic Acid Amides and Esters. Russ. J. Org. Chem. 2004;40:1009–1014. doi: 10.1023/B:RUJO.0000045195.47004.a9. [DOI] [Google Scholar]
- 32.Makarova N.V., Boreko E.I., Moiseev I.K., Pavlova N.I., Nikolaeva S.N., Zemtsova M.N., Vladyko G.V. Antiviral activity of adamantane-containing heterocycles. Pharm. Chem. J. 2002;36:5–7. doi: 10.1023/A:1015732304631. [DOI] [Google Scholar]
- 33.Rajtar B., Szacoń E., Świątek Ł., Rządkowska M., Matosiuk D., Polz-Dacewicz M. Antiviral activity of 1-(1-arylimidazolidine-2-ylidene)-3-(4-chlorobenzyl)urea derivatives. J. Pre-Clin. Clin. Res. 2013;7:104–106. doi: 10.26444/jpccr/71447. [DOI] [Google Scholar]
- 34.Filippova E.I., Kukushkina T.A., Lobanova I.E., Vysochina G.I., Mazurkova N.A. Antiviral properties of the preparation based on the amount of flavonoids of the cuff (Alchemilla vulgaris L.) in relation to the influenza virus. Fundam. Res. 2015;2:5139–5144. [Google Scholar]
- 35.State Pharmacopoeia of the Republic of Kazakhstan. I. Publishing House “Zhibek Zholy”; Almaty, Kazakhstan: 2015. 720p [Google Scholar]
- 36.Guidelines for Conducting Preclinical Studies of Drugs . In: Mironov A.N., editor. GRIF-K; Moscow, Russia: 2012. p. 206. Part 1. [Google Scholar]
- 37.Council N.R. Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press; Washington, DC, USA: 2011. 220p [Google Scholar]
- 38.Réus G.Z., Stringari R.B., de Souza B., Petronilho F., Dal-Pizzol F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Harmine and Imipramine Promote Antioxidant Activities in Prefrontal Cortex and Hippocampus. Oxidative Med. Cell. Longev. 2010;3:325–331. doi: 10.4161/oxim.3.5.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article or Supplementary Materials.