Abstract
Artificially designed gelatins comprising tandemly repeated 30-amino-acid peptide units derived from human αI collagen were successfully produced with a Bacillus brevis system. The DNA encoding the peptide unit was synthesized by taking into consideration the codon usage of the host cells, but no clones having a tandemly repeated gene were obtained through the above-mentioned strategy. Minirepeat genes could be selected in vivo from a mixture of every possible sequence encoding an artificial gelatin by randomly ligating the mixed sequence unit and transforming it into Escherichia coli. Larger repeat genes constructed by connecting minirepeat genes obtained by in vivo selection were also stable in the expression host cells. Gelatins derived from the eight-unit and six-unit repeat genes were extracellularly produced at the level of 0.5 g/liter and easily purified by ammonium sulfate fractionation and anion-exchange chromatography. The purified artificial gelatins had the predicted N-terminal sequences and amino acid compositions and a solgel property similar to that of the native gelatin. These results suggest that the selection of a repeat unit sequence stable in an expression host is a shortcut for the efficient production of repetitive proteins and that it can conveniently be achieved by the in vivo selection method. This study revealed the possible industrial application of artificially designed repetitive proteins.
The relationship between function and structure for proteins has been extensively studied, and the consensus sequences for certain functions have become clear for some proteins (6). Structural proteins, e.g., collagen and silk proteins, generally have a repeat consensus sequence which is related to the protein's unique properties. Gelatin can be obtained by the partial hydrolysis of collagen as a mixture of tripeptide repeats with various molecular masses and compositions. The physicochemical properties of collagen and synthetic collagen analogs are well understood and have been extensively reviewed (1). To create new materials having novel properties, artificial repetitive proteins have been designed on the basis of the consensus sequences of structural proteins and expressed in Escherichia coli, which acts as a host (4, 10, 11). However, little effort for the efficient production of repetitive proteins has been made, so the production levels of repetitive proteins remain low and thus are not high enough for industrial applications.
A host-vector system involving Bacillus brevis as a host has been used for the efficient extracellular production of many heterologous proteins, including prokaryotic and eukaryotic proteins (17). This system has two prominent features: heterologous proteins are secreted directly into the culture medium in soluble and biologically active forms, and the secreted proteins are often stable because of the very low extracellular protease activity. However, no application of this system to repetitive proteins has been reported, except for the production of the casoxin D repeat by fusion to the epidermal growth factor (9). Therefore, we explored the possibility of efficient secretion of tandem repetitive proteins. Here, we report the direct secretion and some properties of artificial uniform gelatin analogs designed as tandem repeats of tripeptide units.
MATERIALS AND METHODS
Strains, plasmids, media, and reagents.
Plasmid pAN21 was derived from pAN3 (17) by elimination of the restriction sites PpuMI, AvaI, and BanII. Gene cloning experiments were performed on E. coli XL1-Blue. B. brevis 31-OK was used throughout this work for expression studies (7). Plasmids pNU212 (17), pNU211L4 (14), and pNU211R2L4 (14) containing the signal peptide of middle wall protein (MWP), the signal peptide L4, and the signal peptide R2L4, respectively, were described previously. pNH326 was constructed from pNH300 (12) by changing the MWP signal peptide to an R2L6 (14) signal peptide. YC and YC-P2 media (8) were used for the production of artificial gelatins.
Stability of insert DNA in the host cells.
The DNAs encoding the repeat units of NEU were synthesized exactly according to the sequences selected by the in vivo selection method. To compare the stability in the host cells, a repeat unit DNA designed on the basis of the codon usage of E. coli was also synthesized. These DNAs were ligated into the BanII site in pAN21Neu under the same conditions. E. coli HB101 was transformed with a ligation reaction mixture containing 10 μg of insert DNA. The stability of an insert DNA in the host cells was evaluated as the transformation efficiency of each insert DNA.
Immunoblot analysis.
Immunoblot analysis was performed with the use of anti-NEU antiserum and alkaline phosphatase-coupled goat anti-rabbit immunoglobulin G (Bio-Rad Laboratories, Hercules, Calif.). 5-Bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium were used for visualization. Anti-NEU antiserum was prepared as follows. A 3-month-old New Zealand White rabbit was immunized with the synthesized NEU peptide in Freund's complete adjuvant. After three subsequent immunizations with the NEU peptide in Freund's incomplete adjuvant, the rabbit was bled from the auricular artery, the blood was allowed to clot, and then the antiserum was collected by centrifugation.
Purification of artificial gelatins.
Culture supernatants, after 6 days of culture, were fractionated by the ammonium sulfate precipitation method. Large portions of NEU8 and PHI6 were included in the fractions obtained by 30 to 45% and 70 to 90% saturation with ammonium sulfate, respectively. Each precipitate was dissolved in a small volume of 20 mM Tris-HCl, pH 7.5 (buffer A) and then dialyzed against the same buffer. After removal of the insoluble materials by centrifugation, the dialyzed sample was applied to a Mono Q HR 5/5 column (Pharmacia) equilibrated with buffer A. Elution was performed with an NaCl gradient (0 to 1 M) in buffer A. The eluate was dialyzed against distilled water and then freeze-dried.
Amino acid sequencing and composition analysis and molecular mass determination.
Amino acid sequencing was performed with an automated amino acid sequencer (model 473A; Applied Biosystems). The amino acid composition was determined by the method of Dreyer and Bynum (3) with a Piko-Tag system (Millipore; Waters). Molecular masses were determined by gel permeation chromatography. The purified NEU8 and PHI6 were each put on a column of TSK gel G3000 SWXL (0.78 by 30 cm; Tosoh, Tokyo, Japan). Equilibration and elution were performed with 100 mM sodium phosphate buffer (pH 7.0) containing 0.15 M sodium chloride with or without 5 M guanidine hydrochloride (GnHCl) at the flow rate of 1 ml/min. Ribonuclease A, ovalbumin, bovine serum albumin, chymotrypsinogen, and catalase were used as molecular markers.
Viscosity of gelatin solutions.
The purified Neu8 and Phi6 were dissolved in phosphate buffer (100 mM NaH2PO4, pH 7.0), and their viscosities were measured with a corn-plate viscometer (EL; Tokisangyo, Tokyo, Japan) after equilibrium was obtained at various temperatures. The viscosities of NEU8 and PHI6 solutions were compared with that of gelatin from bovine skin (approximately 75 bloom, G6650; Sigma, St. Louis, Mo.). Protein concentrations were determined by the dry weight method.
RESULTS
Design and gene synthesis of artificial gelatins.
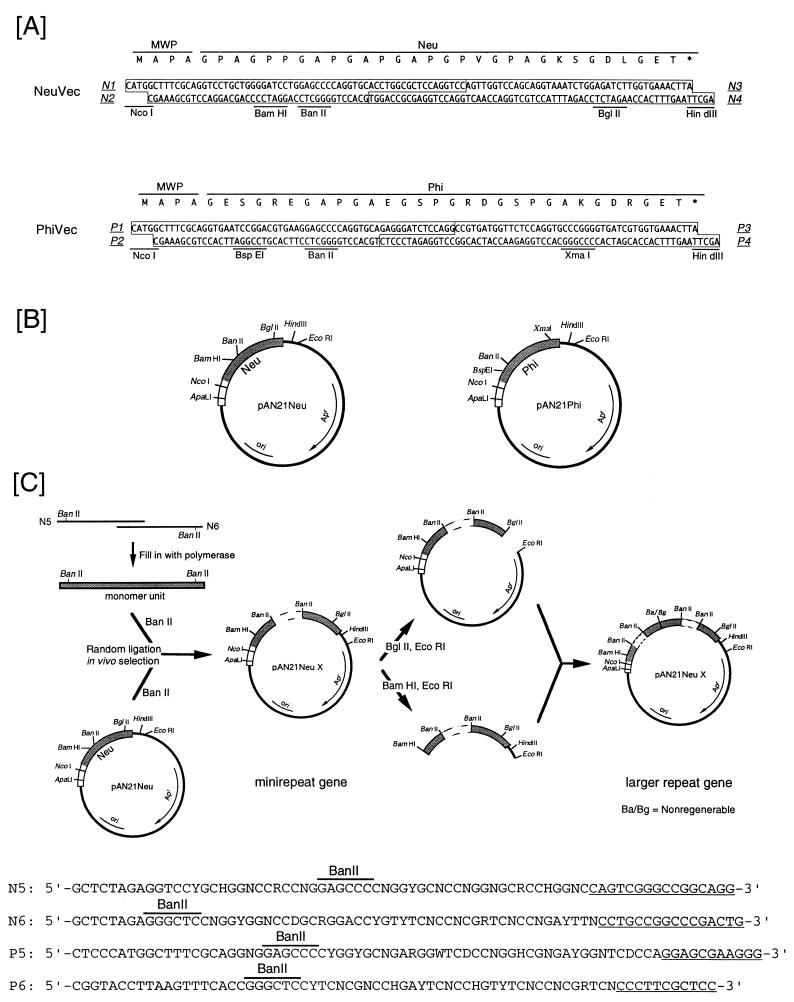
On the basis of the hydropathy of human αI collagen, two units were selected from a hydrophilic region, namely, PHI, and a moderately hydrophilic region, NEU, for the construction of artificial gelatins. PHI and NEU each comprise 30 amino acids of human αI collagen, as follows: PHI, 1010-GESGREGAPGAEGSPGRDGSPGAKGDRGET-1039, and NEU, 1040-GPAGPPGAPGAPGAPGPVGPAGKSGDRGET-1069. The DNAs encoding NEU and PHI were designed by considering the codon usage of B. brevis in accordance with these two 30-amino-acid unit sequences attached to the C-terminal end of the MWP signal peptide for secretion. NcoI and HindIII restriction sites were positioned at the 5′ and 3′ ends of the DNAs, respectively, for insertion into the plasmid. BanII sites were used for random oligomerization. The BspEI and XmaI site pair in the Phi gene and the BamHI and BglII site pair in the Neu gene were arranged for step-by-step tandem connection (Fig. 1A). The DNAs described above, NeuVec and PhiVec, were each synthesized from four fully overlapping oligonucleotides, N1 to N4 and P1 to P4, respectively, which were initially in two pieces with overhangs, though the two gene pieces were then combined. The N-terminal ends of the artificial gelatins were fused in frame to the C-terminal end of the MWP signal peptide in the constructs (Fig. 1A). Each resulting construct was inserted between the NcoI and HindIII sites in pAN21, yielding the plasmids pAN21Neu and pAN21Phi (Fig. 1B). These plasmids were used for the construction of repetitive genes. To construct the repetitive genes of Neu and Phi, the monomer units encoding NEU and PHI were initially synthesized by taking into consideration the codon usage of B. brevis, and then ligated at random into the BanII sites of pAN21Neu and pAN21Phi, respectively. The resulting ligates were transformed into E. coli. However, no transformant was obtained even after 10 trials. Therefore, the repeat unit was synthesized as a mixture of every possible sequence encoding an artificial gelatin. Two pairs of oligonucleotides, N5 and N6 and P5 and P6, were synthesized to construct the repeat units for the Neu and Phi genes, respectively (Fig. 1C). Each pair of oligonucleotides was designed so as to hybridize to each other in the complementary region (Fig. 1C) at the 3′ end of each fragment.
FIG. 1.
Construction of plasmids to oligomerize an artificial gelatin unit. (A) Nucleotide and amino acid sequences of the artificial gelatin unit. The gene was designed with reference to the codon usage for cell wall proteins of B. brevis (16). The gelatin unit was in frame following the MWP signal peptide. The oligonucleotides synthesized are boxed and major restriction sites are indicated. (B) The plasmids for oligomerizing the artificial gelatin unit. The open and shadowed bars denote the signal sequence region of the MWP gene of B. brevis 47 and the structural gene for an artificial gelatin, respectively. Apr and ori represent the ampicillin resistance gene and replication origin, respectively. (C) Schematic diagram of the construction of a tandemly repeated gene. First, a pair of oligonucleotides, N5 and N6, was annealed, and then the rest of the bases were filled in with polymerase to synthesize a monomer unit. Second, minirepeat genes were obtained by ligating the monomer unit at random and in vivo selection. Third, larger repeat genes were constructed by connecting the minirepeat genes step by step. Dashed bars indicate the repeats of the artificial gelatin unit. X, the number of repeat units. The exact sequences of N5 and N6 and P5 and P6 are indicated below the figure.
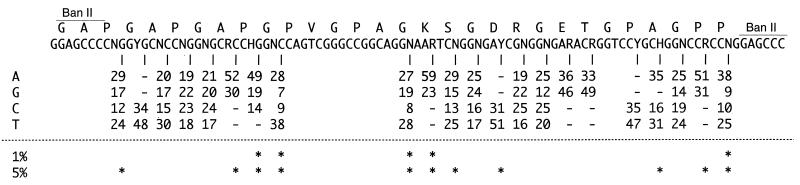
The rest of the bases were filled in with Vent polymerase (New England Biolabs, Beverly, Mass.) after annealing, yielding a double-stranded DNA which encoded the 30-amino-acid repeat unit of NEU (Fig. 1C). The resulting DNA was digested with BanII and then randomly ligated into the BanII site in pAN21Neu. E. coli was transformed with the resulting ligate (Fig. 1C). A total of 48 recombinant clones containing the tandem repeat of the Neu gene (the minirepeat gene) (2 clones with five repeats, 6 clones with four repeats, 16 clones with three repeats, and 24 clones with two repeats) were successfully obtained. The DNA sequences of the cloned genes revealed a bias of codon selection in the wobble base of nearly all codons (Fig. 2). Around the fixed sequence region, the codon bias was remarkable. This codon selection bias was obviously different from that of either E. coli or B. brevis. The stability of sequences selected by the in vivo selection method was evaluated as the efficiency of transformation into E. coli (Table 1). Although all three sequences (A, B, and C in Table 1) selected by the in vivo selection method were efficiently transformed and formed many colonies, the sequence (D in Table 1) artificially designed on the basis of the codon usage of E. coli could not form any colony. These findings suggest that the sequences selected once by the host cells are stable in the host.
FIG. 2.
Codon bias of the Neu gene repeat cloned in E. coli. A total of 82 Neu repeat units in 48 clones carrying the correct structured plasmid were sequenced. The codon usage for each amino acid of the repeat unit is shown. The codon bias was examined statistically by means of the chi-square test with the null hypothesis that the probabilities for codon selection are equal for all codons. Asterisks show the statistically significant codon bias.
TABLE 1.
The stability of sequences selected by in vivo selectiona
| Insert | Sequence design of insert | Transformation efficiency (CFU/μg of DNA) |
|---|---|---|
| A | In vivo selection | 1.2 ± 0.5 × 105 |
| B | In vivo selection | 0.9 ± 0.3 × 105 |
| C | In vivo selection | 3.1 ± 0.9 × 105 |
| D | Codon usage | <102 |
| None | 5.3 ± 1.3 × 106 |
Competent cells of E. coli HB101 (Nippon Gene, Toyama, Japan) have transformation ability of 108 CFU/μg of DNA (pBR322). Transformation was performed four times for each vector insert according to the protocol recommended by the manufacturer. Transformation efficiency values are the averages of the results of four transformation experiments. The DNA sequences of the inserts are as follows: A, GGAGCCCCGGGTGCTCCGGGTGCACCGGGTCCGGTTGGTCCGGCTGGTAAATCTGGTGACCGTGGTGAAACAGGTCCTGCAGGTCCGCCG; B, GGAGCCCCAGGTGCTCCAGGAGCACCGGGTCCAGTCGGGCCGGCAGGAAAATCAGGAGATCGCGGAGAAACAGGTCCTGCAGGACCACCA; C, GGAGCCCCAGGTGCTCCCGGAGCACCAGGACCAGTCGGGCCGGCAGGTAAATCTGGGGACCGCGGCGAGACGGGTCCTGCTGGACCACCA; and D, GGAGCCCCGGGCGCGCCCGGTGCGCCGGGACCAGTCGGGCCGGCAGGAAAATCAGGGGACCGTGGTGAAACGGGTCCTGCAGGACCACCA.
To construct larger repeat genes, the minirepeat genes obtained as described above were step by step connected homogeneously or heterogeneously (9, 10) (Fig. 1C). Thus, E. coli clones containing larger repeat genes (8, 10, and 12 repeats, named pAN21NEU8, pAN21Neu10, and pAN21Neu12, respectively) were obtained, and these larger repeat genes were also stable in the cells. A clone containing the Phi repetitive gene (6 repeats, named pAN21Phi6) was obtained in the manner described above for the Neu repeat gene except that a different pair of oligonucleotides, P5 and P6, and plasmid pAN21Phi were used.
Extracellular production of artificial gelatins by B. brevis.
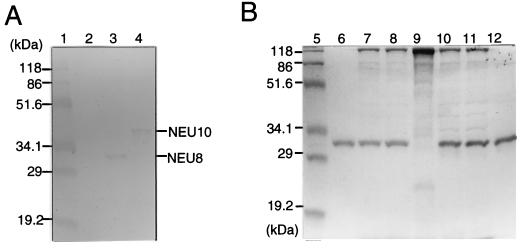
These tandemly repeated genes were excised from the plasmids by digestion with NcoI and HindIII and then inserted between the NcoI and HindIII sites in a B. brevis expression vector, pNU212, and transformed into B. brevis 31-OK. No B. brevis transformants containing larger repeat genes composed of single minirepeats were obtained. A B. brevis transformant carrying pNU212NEU8 or pNU212Neu10, which contained 8 or 10 repeats constructed by connecting a few minirepeat genes, respectively, was obtained, but one containing 12 repeats was not. In 6-day culture supernatants of B. brevis carrying pNU212NEU8 and pNU212Neu10, a protein which cross-reacted with antiserum to the synthesized NEU peptide was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 3A), indicating that these products were derived from the Neu gene. We tried to increase the yield of NEU8 by changing the expression vector to pNH326, which has a modified signal peptide (R2L6) and only two of the five promoters of the MWP promoter region. The amount of NEU8 produced by pNH326NEU8 was about 10 times greater than that produced by pNU212NEU8, reaching as much as 0.5 g of extracellular NEU8 protein per liter of culture on optimization of the culture conditions, as estimated from the intensity of the band compared with that of the purified NEU8 (Fig. 3B).
FIG. 3.
SDS-PAGE and immunoblot analysis of artificial gelatins secreted by B. brevis. B. brevis 31-OK carrying an expression vector was grown at 30°C. YC and YC-P2 media were used for pNU212 and pNH326, respectively. Culture supernatants were subjected to SDS-PAGE, followed by immunoblot analysis with anti-NEU antiserum (A) or staining with Coomassie brilliant blue (B). Lanes 1 and 5, molecular weight markers; lanes 2, 3, and 4, 5 μl of 6-day culture supernatants of B. brevis carrying pNU212NEU8, pNU212Neu10, and pNU212, respectively; lanes 6 and 12, 1 μg of purified NEU and PHI, respectively; lanes 7 and 8, 5 μl of 3-day and 6-day culture supernatants of B. brevis carrying pNH326NEU8; lane 9, 5 μl of a 6-day culture supernatant of B. brevis carrying pNH326; lanes 10 and 11, 5 μl of 3-day and 6-day culture supernatants of B. brevis carrying pNH326PHI6, respectively.
The synthetic gene encoding PHI6 was also excised with NcoI and HindIII, and inserted between the NcoI and HindIII sites of pNH326, resulting in pNH326PHI6 for the expression of PHI6. A specific band of approx. 31.7 kDa was detected by SDS-PAGE. The sequence of the five N-terminal amino acids of this protein matched that deduced from the DNA sequence of the Phi gene. Thus, we confirmed that the 31.7-kDa product was derived from the Phi gene. The amount of PHI6 in the culture supernatant of pNH326PHI6 was estimated to be 0.5 g/liter from the intensity of the band on SDS-PAGE gels (Fig. 3B). The amounts of NEU8 and PHI6 in the culture supernatant increased from the early stationary phase of growth (data not shown), and after 3 days the amounts of these gelatins had increased only slightly (Fig. 3B). These findings suggested that NEU8 and PHI6 secreted into the medium are stable throughout the cultivation.
Characterization of NEU8 and PHI6 produced by B. brevis.
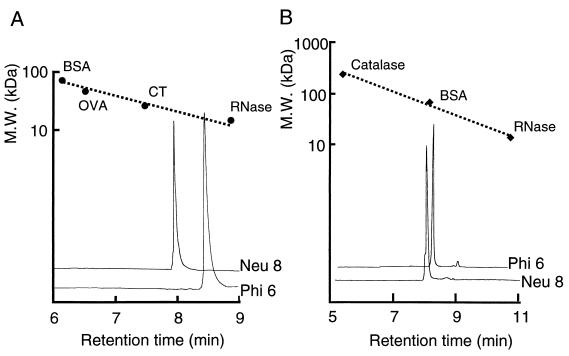
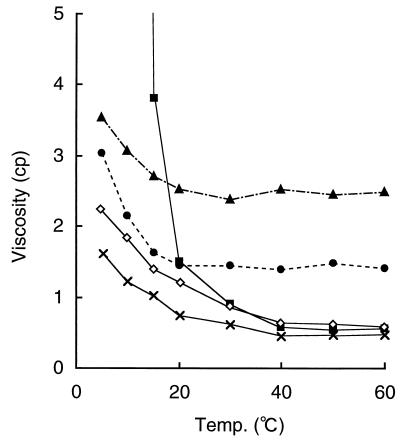
The artificial gelatins were purified, yielding almost single-band SDS-PAGE gels with one (NEU) or two (PHI) conventional steps, as described in Materials and Methods, the yields being 81 and 53% of total secreted NEU and PHI, respectively. The six N-terminal amino acid residues of the purified NEU8 and PHI6 were identical to those deduced from the DNA sequences, and this amino acid composition was also similar to that deduced for the DNA. Although the molecular masses of NEU8 and PHI6 estimated by SDS-PAGE were about 32.4 and 31.7 kDa, respectively, which were about 1.5 and 2 times larger than the values deduced from the DNA sequences, those in the presence of 5 M GnHCl were estimated to be 20.3 kDa and 16.3 kDa, respectively, by gel filtration chromatography (Fig. 4A). These values were in good agreement with those deduced from the DNA sequences (19.3 and 15.6 kDa). In the absence of 5 M GnHCl, the molecular masses of NEU8 and PHI6 were 59.6 and 50.3 kDa, respectively (Fig. 4B). Figure 5 shows the viscosity change with temperature. The viscosity of 1% solutions of NEU8 and PHI6 increased with decreasing temperature and decreased with increasing temperature, as in the case of the native gelatin solution. This reversible viscosity change was possible at least three times. These results suggested that the artificial gelatins have a solgel property like that of the native gelatin. But the threshold temperature of the solgel transition of an artificial gelatin was lower than that of the native gelatin by about 15°C, and the viscosity changes of 1% solutions of NEU8 and PHI6 from 60 to 5°C were similar to those of 0.3 and 0.1% solutions of the native gelatin, respectively.
FIG. 4.
Gel filtration of the purified artificial gelatins on a TSK gel G3000 SWXL column. Gel filtration chromatograms of the artificial gelatins in the presence (A) and absence (B) of 5 M GnHCl. Proteins were monitored as to the absorbance at 215 nm. BSA, bovine serum albumin; OVA, ovalbumin; CT, chymotrypsinogen; RNase, ribonuclease A.
FIG. 5.
Viscosity change of gelatin solutions with temperature. ■, gelatin from bovine skin (1%); ◊, gelatin from bovine skin (0.3%); ×, gelatin from bovine skin (0.1%); ▴, NEU8 (1%); ●, PHI6 (1%). cp, centipoise.
DISCUSSION
We have successfully produced large amounts of repetitive proteins, artificially designed uniform gelatins, with B. brevis. Codon selection according to the codon usage of the host strain is generally good for efficient production. The stability in the host cells of a designed DNA is another important factor for the efficient production of heterologous proteins. However, we do not know how to design a stable DNA in host cells because the sequences that are stable in the host strain, especially repetitive ones, are not known. In fact, tandem repeats composed of single units designed by taking into consideration the codon usage of E. coli could not be cloned into E. coli. However, we were able to obtain stable tandem repeat DNA sequences encoding repetitive proteins by allowing the host cells to select. The selected sequences exhibited a bias skewed strongly from the codon usage of the host strain in the wobble base, showing that stable sequences were selected and cloned by the host cells. Based on these results, we emphasize that it is critically important to select a stable sequence encoding a protein for expressing a repetitive protein, and it is only possible to do so by the random cloning method. Although six-unit repeats constructed from both single and dual three-unit repeats could be cloned into E. coli, only hetelorogous six-unit repeats derived from dual three-unit repeats could be cloned into B. brevis. These results suggest that B. brevis is more sensitive to repetitive sequences. We do not know any obvious reason for these results, but speculate that rec+ causes a phenotype of higher sensitivity to a repetitive sequence in B. brevis than in E. coli XL1-Blue, which is a rec mutant strain.
The artificial gelatin productivity became obviously higher with the use of pNH326, which contained a modified signal peptide and a weakened promoter. Although the modified signal peptide increased the productivity of many kinds of protein (7, 14, 15), in the case of gelatin no transformant could be obtained with the use of pNU211L4 or pNU211R2L4, which contained a modified signal peptide and strong multiple promoters. These results suggest that a weakened promoter is more favorable for the efficient production of repeative proteins like artificial gelatins.
By SDS-PAGE, the molecular masses of the purified artificial gelatins were estimated to be larger than those deduced from the DNA sequences. This may be due to the mobilities of collagen-related peptides on SDS-PAGE gels, which mobilities are lower than those of common proteins used as molecular weight markers (5). The molecular masses estimated in the presence of 5 M GnHCl, and the N-terminal amino acid sequences and amino acid composition analysis results also coincided with those of the gelatins produced by B. brevis. Gel filtration chromatography with or without 5 M GnHCl revealed that both NEU8 and PHI6 existed as aggregates and that these artificial gelatins might form a collagen-like triple helical structure in solution at room temperature.
The artificial gelatins showed a thermoreversible viscosity change similar to that of the native gelatin, suggesting that the artificial gelatins have a solgel property like that of the native gelatin. But the viscoelastic parameters of artificial gelatin and native gelatin solutions were distinct from each other. The amino acid sequence and/or composition of gelatin are well-known to affect the gelation property of a solution of it (2), and the hydroxylation of proline residues is also known to stabilize the gel structure (13). The differences in the numbers of proline residues and the deficiency of hydroxylation of proline residues in artificial gelatins seem to cause the variation in the viscoelastic property.
This is the first report of the efficient extracellular production of artificially designed gelatins, repetitive proteins. As the properties of artificial gelatins can be manipulated by means of the amino acid sequence, unique gelatins or collagen-related peptides having novel properties, e.g., photochemically active materials for holography or nonimmunogenic materials for medical use, can be produced by means of the B. brevis system. Furthermore, this study showed that the B. brevis system is useful for the production of other repetitive proteins, e.g., elastin and silk protein, and revealed the possible industrial application of artificially designed repetitive proteins.
ACKNOWLEDGMENTS
We are grateful to Shigezo Udaka of Tokyo University of Agriculture and Kunihiko Gekko of Hiroshima University for valuable advice and fruitful discussions.
REFERENCES
- 1.Bhatnagar R S, Rapaka R S. Synthetic polypeptide models of collagen: synthesis and applications. In: Ramachandran G N, Reddi A H, editors. Biochemistry of collagen. New York, N.Y: Plenum Press; 1976. pp. 479–523. [Google Scholar]
- 2.Bhatnagar R S, Pattabiraman N, Sorensen K R, Langridge R, MacElroy R D, Renugopara-Krishnan V. Inter-chain proline: proline contacts contribute to the stability of the triple helical conformation. J Biomol Struct Dyn. 1988;6:223–233. doi: 10.1080/07391102.1988.10507709. [DOI] [PubMed] [Google Scholar]
- 3.Dreyer W, Bynum E. High-voltage paper electrophoresis. Methods Enzymol. 1967;11:32–39. [Google Scholar]
- 4.Goldberg I, Salerno A J, Patterson T, Williams J I. Cloning and expression of a collagen-analog-encoding synthetic gene in Escherichia coli. Gene. 1989;80:305–314. doi: 10.1016/0378-1119(89)90294-1. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Nagai Y. The anomalous behavior of collagen peptides on sodium dodecyl sulfate-polyacrylamide gel electrophoresis is due to the low content of hydrophobic amino acid residues. J Biochem. 1980;87:803–808. doi: 10.1093/oxfordjournals.jbchem.a132809. [DOI] [PubMed] [Google Scholar]
- 6.Heslot H. Artificial fibrous proteins. Biochimie. 1998;80:19–31. doi: 10.1016/s0300-9084(98)80053-9. [DOI] [PubMed] [Google Scholar]
- 7.Kajino T, Kato K, Miyazaki C, Asami O, Yamada Y, Hirai M, Udaka S. Isolation of a protease-deficient mutant of Bacillus brevis and efficient secretion of a fungal protein disulfide isomerase by the mutant. J Biosci Bioeng. 1999;87:37–42. doi: 10.1016/s1389-1723(99)80005-x. [DOI] [PubMed] [Google Scholar]
- 8.Kajino T, Saito Y, Hirai M, Asami O, Yamada Y, Udaka S. Extracellular production of an intact and biologically active human growth hormone by the Bacillus brevis system. J Ind Microbiol Biotechnol. 1998;19:227–231. doi: 10.1038/sj.jim.2900445. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Fujiwara Y, Okamoto A, Yoshikawa M, Chiba H, Udaka S. Efficient production of Casoxin D, a bradykinin agonist peptide derived from human casein, by Bacillus brevis. Biosci Biotechnol Biochem. 1995;59:2056–2059. doi: 10.1271/bbb.59.2056. [DOI] [PubMed] [Google Scholar]
- 10.Lewis R V, Hinman M, Kothakota S, Fournier M J. Expression and purification of a spider silk protein: a new strategy for producing repetitive proteins. Protein Expr Purif. 1996;7:400–406. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- 11.Martin S L, Vrhovski B, Weiss A S. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin. Gene. 1995;154:159–166. doi: 10.1016/0378-1119(94)00848-m. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto A, Kosugi A, Koizumi Y, Yanagida F, Udaka S. High efficiency transformation of Bacillus brevis by electroporation. Biosci Biotechnol Biochem. 1997;61:202–203. doi: 10.1271/bbb.61.202. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbloom J, Harsch M, Jimenez S A. Hydroxyproline content determines the denaturation temperature of chick tendon collagen. Arch Biochem Biophys. 1973;158:478–481. doi: 10.1016/0003-9861(73)90539-0. [DOI] [PubMed] [Google Scholar]
- 14.Sagiya Y, Yamagata H, Udaka S. Direct high-level secretion into the culture medium of tuna growth hormone in biologically active form by Bacillus brevis. Appl Microbiol Biotechnol. 1994;42:358–363. doi: 10.1007/BF00902742. [DOI] [PubMed] [Google Scholar]
- 15.Takimura Y, Kato M, Ohta T, Yamagata H, Udaka S. Secretion of human interleukin-2 in biologically active form by Bacillus brevis directly into culture medium. Biosci Biotechnol Biochem. 1997;61:1858–1861. doi: 10.1271/bbb.61.1858. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi A, Uchihi R, Adachi T, Sasaki T, Hayakawa S, Yamagata H, Tsukagoshi N, Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988;170:935–945. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udaka S, Yamagata H. High-level secretion of heterologous proteins by Bacillus brevis. Methods Enzymol. 1993;217:23–33. doi: 10.1016/0076-6879(93)17053-8. [DOI] [PubMed] [Google Scholar]