Abstract
Objective
This study examined changes due to COVID-19 lockdown in young and older adults’ self-reported sleep quality and dysfunctional sleep-related beliefs.
Methods
Adults involved in studies prior to the pandemic were contacted during the COVID-19 lockdown. Seventeen young adults (age range: 18–35 years) and 21 older adults (age range: 65–90 years) agreed to participate. Participants were interviewed by phone (between 27th April and 4th May, 2020) to complete the Pittsburgh Sleep Quality Index (PSQI) and the Dysfunctional Beliefs About Sleep (DBAS) questionnaire they had been administered before the pandemic.
Results
In terms of mean changes, the results showed null effect sizes for changes in self-reported sleep quality for both age groups. In young adults, a medium effect size emerged for changes in sleep latency, which increased during lockdown. No changes in any of the self-reported sleep quality dimensions emerged in older adults. In both age groups, the effect sizes for changes in dysfunctional sleep-related beliefs were negligible. In older adults, however, changes in self-reported sleep quality were largely associated with changes in dysfunctional sleep-related beliefs.
Conclusions
Our results suggest that self-reported sleep quality and dysfunctional sleep-related beliefs were not affected by the COVID-19 lockdown in young or older adults. They also suggest that it might be useful to consider changes in dysfunctional sleep-related beliefs to better capture the impact of stressful events (such as a period of quarantine) on sleep quality, especially where older adults are concerned.
Keywords: COVID-19 lockdown, Sleep quality, Dysfunctional sleep-related beliefs, Older adults
1. Introduction
Sleep is a well-established marker of an individual's health and a crucial factor in their behavioral and emotional functioning throughout their life [1]. Negative changes in sleeping patterns and sleep quality are known to affect physical health (e.g, impairing immune function and metabolism) and mental wellbeing (eg, contributing to depression and anxiety), as well as quality of life in a broad sense (eg., [2] ). It has been suggested that exposure to stressful life events can affect sleep quality (eg., [3]). The restrictions imposed on our daily routines (eg., being obliged to work/study at home and to minimize leisure, social and outdoor physical activities) due to lockdown measures taken in recent months to reduce the spread of COVID-19 could certainly be described as stressful life events. They are among the factors that have contributed to making the general population's home confinement an unexpected and prolonged stressful situation, giving rise to atypical lifestyles (eg, less exposure to daylight, limited activity levels, excessive use of technologies) that are likely to affect sleep quality and exacerbate sleep disturbances [[4], [5], [6]]. It therefore comes as no surprise that several studies have attempted to shed light on how home confinement prompted by COVID-19 has influenced people's perceptions of their sleep quality (see Table 1 ). Some such studies describe changes in perceived sleep quality as a whole (ie, self-reported sleeping difficulties), which are not always confirmed [6,11,12]. Most studies report changes in several sleep quality dimensions, including: a delayed bedtime and waketime [5,12,[14], [15], [16]]; a prolonged sleep latency [5,8,11,12,15] a lower sleep efficiency [5,8,16] and a longer sleep duration [10,12,13,16]. There have also been reports of people having more trouble sleeping, and sleep disturbances [[7], [8], [9],11,12,14,16] while confined at home due to the COVID-19 outbreak compared with before the pandemic.
Table 1.
Summary of findings in studies on changes in sleep quality under COVID-19 lockdown compared with the previous period.
| Study | Country | Sample | Method |
Key results on changes in sleep quality (T0 vs T1) | |||
|---|---|---|---|---|---|---|---|
| Data collection | Baseline assessment (T0) | Lockdown assessment (T1) | Sleep quality measures | ||||
| Chandra et al., 2020 [7] | Nepal | Young adults (Mage = 29.5 ±9.77 years) | Web-based survey | Retrospectively self-reported (time period not specified) | Completed between 20th April and 2nd May 2020 (after pandemic) | ISI | Increase in ISI total score (more insomnia symptoms) at T1 than at T0. Longer SOL, worse sleep maintenance, lower satisfaction with current sleep pattern, and stronger worries about current sleep problem at T1 than at T0. No significant differences/changes on awakening problems, impairment of quality of life, interference with daily functioning between T1 and T0. |
| Barrea et al. [8] | Italy | Adults (age range = 18–65 years; Mage = 44.90 ± 13.30 years) | Telephone interview | Retrospectively self-reported (time period not specified) | After 40 days of lockdown (started on 12th March 2020) | PSQI | Increase in PSQI total score (more sleeping difficulties) at T1 than at T0. Worse sleep quality, longer SOL, lower sleep efficiency, greater sleep disturbance, and daytime dysfunction at T1 than at T0. No significant differences/changes in sleep duration and hypnotic drugs between T1 and T0. |
| Beck et al. [9] | France | 26% < 35 years old 64% > 35 years old | Web-based survey | Data from the French Health Barometer Survey (2017) | A subsample of the panel interviewed in 2017 completed the survey between 3rd March and 2nd April 2020 | Ad-hoc questions on sleep quality | Greater trouble sleeping during the past 8 days at T1 than at T0. Greater use of sleeping pills at T1 than at T0. |
| Blume et al. [10] | Austria, Switzer-land, Germany | Young adults (median age range = 26–35 years) | Web-based survey | Retrospective self-reported sleep quality in relation to the time before the lockdown (Austria on 13 March; Switzerland on 16 March, Germany on 23 March) | Completed between March and April 2020 | Ad-hoc questions on sleep quality | Lower sleep quality (slightly decreased), but longer sleep duration (∼13 min more under lockdown than before lockdown) at T1 than at T0. |
| Casagrande et al. [11] | Italy | Young adults (age range: 18–89 years; Mage = 30.00 ± 11.50 years) | Web-based online survey | Data from general population (derived from previous published studies) | Completed between 18th March and 2nd April 2020 | PSQI | No significant differences/changes between respondents under restrictions (T1) than general population (T0) on PSQI total score and its subscales, except for PSQI Sleep latency and PSQI Daytime dysfunctions. |
| Cellini et al. [5] | Italy | Young adults (age range = 18–35 years; Mage = 23.91 ± 3.60 years) | Web-based survey | Retrospective self-reported sleep quality in relation to the time before the lockdown (period from 3rd to 10th February) | Completed between 24th and 28th March 2020, in relation to the second week of lockdown (17th-23rd March 2020) | PSQI | Increase in PSQI total score at T1 compared with T0. Prolonged Bedtime (∼41 min later under lockdown) and Waketime, and longer TIB at T1 than at T0. |
| Gao and Scullin [12] | USA | Adults (Mage = 38.04 ± 11.65 years) | Web-based survey | 86 participants completed a survey on 17 February 2020 All participants were asked to retrospectively estimate their sleep habits before lockdown (time period not specified) |
Completed between 25–27 March 2020, after two weeks of US lockdown and social distancing |
PSQI; FIRST; SSS |
Baseline vs Quarantine: No significant differences/changes in PSQI total score, FIRST total score, and SSS total score between T1 and T0. Increase in sleep duration, delayed bedtime and waketime, and fewer sleep disturbances at T1 than at T0. Retrospective recall of sleep prior to the quarantine phase: Greater sleep onset latency, fewer awakenings in the middle of the night, earlier bedtime, later waketime, and feeling more rested prior to the COVID-19 quarantine (T0) than at T1. No significant differences/changes in sleep duration between T1 and T0. |
| Hisler and Twenge [13] | USA | Adults (age range = 18–60+ y old; Mage = 43.35±14.88 years) | Web-based survey | NHIS 2018 (nationally representative sample of internet users) | Completed on 27th April 2020 (sample with similar demographic characteristics to the NHIS 2018 sample) | Ad-hoc questions on sleep quality and habits | Slightly shorter sleep duration at T1 than in 2018. Greater prevalence of insufficient sleep duration, difficulty staying asleep, and higher number of days with difficulty falling asleep and not feeling rested at T1 than at T0. |
| Gupta et al. [14] | India | Adults (Mage = 37.32 ±13.39 years) | Web-based survey | Retrospectively self-reported for sleep patterns (time period not specified) | Completed between 28th April and 10th May 2020 | Ad-hoc questions on sleep quality and habits; ISI | Prolonged bedtime and waketime, longer SOL, increased daytime napping and not refreshed days at T1 than at T0. Just over 10% of the sample met the criteria for clinically significant insomnia according to ISI, however (% similar to that of the general population before lockdown). |
| Leone et al. [6] | Argentina | Adults (age range = 20–70 years) | Web-based survey | Participants who had completed the Crono Argentina survey (www.cronoargentina.org) either on February to May 2018 or 2019, or in February 2020. | April 2020 | PSQI | No significant differences/changes in PSQI total score between T1 and T0. The effects of lockdown on sleep quality and patterns were greater for younger people and those whose working status changed. Those who did not work away from home during lockdown slept better, but their chronotype became more delayed. |
| Marelli et al. [15] | Italy | Young adults (age range: 19–67 years; Mage = 22.84 ± 2.68 years) | Web-based survey | Retrospectively self-reported (time period not specified) | Completed between 24th March and 3rd May 2020 | PSQI; ISI | Increase in PSQI total score and ISI total score at T1 compared with T0. Prolonged bedtime and waketime, and longer sleep latency at T1 compared with T0. No differences/changes in TST and TIB between before and during COVID-19 emergency. |
| Salehinejad et al. [16] | Germany | Young adults (Mage = 25.79±7.31 years) | Web-based survey | Retrospectively self-reported (time period not specified) | Completed between 20th and 28th April 2020 | PSQI | Higher PSQI total score at T1 than at T0. Lower self-reported sleep quality, and higher daily disturbances at T1 than at T0. No differences/changes in sleep efficiency between T1 and T0. Prolonged bedtime and waketime, and longer sleep duration at T1 than at T0. |
Although these studies offer important insight on how sleep patterns and sleep quality may have changed in response to the COVID-19 lockdown, most of them (8 studies) use a retrospective assessment of sleep quality. The researchers asked participants to think back to their sleep quality before the pandemic as compared with during their confinement at home [8,10]; Chandra et al., 2020, [5,12,[14], [15], [16]]. Respondents are therefore likely to have overestimated their self-reported sleeping difficulties during lockdown (see also [12]). Another two studies drew a comparison with data actually collected before the pandemic from normative samples [11,13]. Only three studies [6,9,12] compared a sample's assessment of their sleep quality prior to the pandemic (ie, a real baseline in a “typical” situation) and during COVID-19 lockdown. In these cases, no marked changes emerged - in overall self-reported sleep quality at least [6,11,12].
In addition, the above-mentioned studies included samples over a broad range of ages (eg., people from 18 to 60+ years old), but the participants involved were relatively young (see Table 1). As a result, we still know little about any changes in self-reported sleep quality due to COVID-19 lockdown experienced by normally-aging older adults without any sleeping disorders. Since aging coincides with changes in some sleep patterns (eg., a shorter sleep duration or numerous awakenings during the night), and in the way older adults perceive their sleep quality (eg., Ref. [1], it is worth investigating whether the COVID-19 outbreak might have prompted a deterioration in the self-reported sleep quality of a vulnerable population like older adults.
Little is known also about the impact of COVID-19 lockdowns on the so-called subjective sleep-related factors, which refer (among others) to individuals’ own beliefs and behavior relating to their sleep quality and sleeping habits [21]. These factors have been shown to affect self-reported sleep quality not only in young and older people diagnosed with insomnia [21,22], but also in young and older individuals without any clinically-relevant sleeping disorders (ie, [23,24]. At the time of writing, the study by Ref. [25] is the only one to have examined the association between dysfunctional beliefs or attitudes regarding sleep and self-reported sleep quality (in terms of insomnia symptoms) in the general population during lockdown (without a baseline assessment, however). The Authors found strong correlations between the high prevalence of dysfunctional sleep-related beliefs and more severe insomnia symptoms under home confinement. No studies, to our knowledge at least, have examined whether such subjective sleep-related factors changed under lockdown in young and normally-aging older adults without any sleeping disorders (eg, insomnia), and the influence of any such changes on their self-reported sleep quality.
The main aim of the present study was therefore to identify any changes in self-reported sleep quality and dysfunctional sleep-related beliefs in young and older adults with no sleeping disorders (insomnia) or other clinically-relevant issues (eg., depression and/or anxiety) while they were confined to their homes to contain the COVID-19 outbreak. To achieve this aim, young and older adults who had taken part in previous studies - in which the well-known Pittsburgh Sleep Quality Index (PSQI [6]); was used to assess sleep quality, and the Dysfunctional Beliefs and Attitudes about Sleep (DBAS [21]); questionnaire was used to assess dysfunctional sleep-related beliefs (ie, [24,26]), - were contacted during lockdown and asked to repeat the same assessments.
We expected self-reported sleep quality to have deteriorated under lockdown in young adults, in line with previous findings [[7], [8], [9],5,10,13,[14], [15], [16]]. On the other hand, however, we might find that the COVID-19 lockdown made no difference to self-reported overall sleep quality, in young adults at least, in line with studies that also considered data collected before the pandemic [6,11,12]. Either way, some changes in particular dimensions of self-reported sleep quality (ie, sleep efficiency, sleep duration, sleep latency, daytime dysfunction) might be expected, in line with previous findings irrespective of the type of comparison drawn (see Table 1). We also explored whether older adults experienced any changes in their self-reported sleep quality and its dimensions, as previous studies had focused only on younger adults.
As for dysfunctional sleep-related beliefs, in line with [25], we might expect an increase in such beliefs during COVID-19 lockdown, in young adults at least. Changes in older adults’ dysfunctional beliefs and attitudes about sleep during the pandemic were also explored for the first time.
Finally, we explored whether any changes in self-reported sleep quality due to being confined at home by the COVID-19 lockdown were associated with changes in young and older adults’ dysfunctional sleep-related beliefs. People who perceive a poorer sleep quality tend to have dysfunctional beliefs and attitudes about sleep [21], so a worsening perceived sleep quality under lockdown might presumably be associated with more severely dysfunctional sleep-related beliefs.
2. Methods
2.1. Participants
Young and older adults who had been involved in previous research from the end of 2017 to mid-2018 ([26]; for older adults [24]; for young adults) were contacted - through the social clubs previously involved in recruiting them - during the COVID-19 lockdown and invited to take part in the present study. Of 70 young adults and 50 older adults contacted, 20 of the former and 23 of the latter agreed to participate in the study, and completed a single individual phone interview (from 27 April to 4 May 2020) while confined to their homes under the COVID-19 lockdown in Italy.1 Due to the above constraints, the sample size could not be determined a priori. We therefore avoided basing any conclusions on statistical inference, and focused instead on considering effect sizes and their uncertainty (95% confidence intervals) (see Results section below).
The inclusion criteria were as follows: (i) no depression, ie. scores under the clinical cut-off of 11 on the Geriatric Depression Scale (GDS [27]); for older adults, and under the cut-off of 14 on the Beck Depression Inventory II (BDI-II [28]); for young adults; (ii) no cognitive impairment or signs of incipient dementia, as tested with the Italian Multidimensional Assessment Checklist (SVAMA) [29], for older adults; (iii) no sleeping disorders, examined by means of an ad hoc semi-structured interview (see Ref. [24,26]).
Three young adults and two older adults were excluded due to an increase in their scores on the depression scales. The final sample thus included 17 young and 21 older adults. None of the participants were healthcare workers (eg, doctors or nurses), essential workers (eg, retailers, supermarket employees, or restaurant staff), or others on the frontline. Most of the younger participants were university students, while the older participants were mainly retired people. All participants were confined at home during the lockdown. None of them reported having been infected with COVID-19.
The study was approved by the local University Research Ethics Committee and conducted in accordance with its recommendations.
2.2. Materials
2.2.1. Self-reported sleep quality
Pittsburg Sleep Quality Index (PSQI; [17]). This 19-item questionnaire was used to assess self-reported sleep quality and sleeping difficulties over the previous month (Cronbach's alpha = 0.83). As well as overall sleep quality, it covers seven dimensions of sleep: latency; duration; sleep disturbances; sleep medication use; daytime dysfunction; and sleep efficiency (derived from the proportion of time spent asleep while in bed). Each dimension is scored from 0 to 3, with higher scores indicating a worse sleep quality. Dependent variables were the PSQI total score (ie, self-reported overall sleep quality) and the subscores for the various dimensions measured, with higher scores indicating a worse self-reported sleep quality.
2.2.2. Sleep-related dysfunctional beliefs
Dysfunctional Beliefs and Attitudes about Sleep Questionnaire (DBAS; [21]). This tool comprises 30 items assessing maladaptive sleep-related beliefs, and respondents' expectations and attitudes regarding the causes, and consequences of sleeping issues (excessive intrusive sleep-related cognitive activity). Participants rate each statement on a 10-point scale from 0 (“strongly disagree”) to 10 (“strongly agree”). The final score is the mean of all items, with higher scores indicating a stronger endorsement of dysfunctional beliefs and attitudes about sleep (Cronbach's alpha = 0.80).
2.3. Procedure
All participants had completed the SVAMA, the BDI-2 or GDS, the STAI-Y2, the PSQI, and the DBAS before the lockdown (in 2017 and 2018), which gave us a baseline (T0) assessment of their perceived sleep quality and sleep-related beliefs. The lockdown coincided with a number of countrywide restrictions on people's movements from March 10th to May 3rd, 2020. During this period, people were only allowed to leave their homes to buy essential supplies (such as food and medicines), schools and workplaces remained closed, and all public gatherings (eg, bars and restaurants, clubs, gyms, cinemas) were strictly prohibited. While confined at home due to the COVID-19 outbreak (T1), participants were contacted by phone (between 27 April and 4 May, 2020) to complete a single interview lasting about 90 min, in which they repeated the screening measures (the SVAMA, the BDI-2 or GDS, the STAI-Y2), the PSQI, and the DBAS, referring to the lockdown period. To better measure their perception of sleep quality (overall and its various dimensions) during the COVID-19 lockdown, we specified that respondents should consider the previous month.
The experimenter interviewing participants recorded their answers in a Google Forms survey developed to make it easier to administer the screening measures.
3. Results
Table 2 shows the descriptive statistics for participants’ demographic characteristics, and the measures of interest by group. Given the limited sample size, we focused on effect size point estimates and their 95% confidence intervals (CI), rather than on statistical significance. All data analyses were performed using the R (R Core Team, 2020) free software and related packages.
Table 2.
Means (M) and standard deviations (SD) of the sample's demographic characteristics, sleep quality, and sleep-related beliefs by age group.
| Young adults (age range: 18–35 years)(N = 17; F = 52.90%) |
Older adults (age range: 65–90 years)(N = 21; F = 52.40%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| T0 (baseline) |
T1 (lockdown) |
T0 (baseline) |
T1 (lockdown) |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| Age | 25.83 | 2.70 | 28.81 | 2.58 | 73.62 | 7.64 | 76.80 | 7.76 |
| Education (years) | 17.83 | 2.07 | 18.05 | 1.95 | 10.43 | 4.14 | 10.43 | 4.14 |
| PSQI (total score) | 4.71 | 2.39 | 4.65 | 2.62 | 6.95 | 2.52 | 7.33 | 3.37 |
| PSQI_Overall sleep quality | 1.00 | 0.61 | 1.00 | 0.79 | 0.90 | 0.44 | 1.14 | 0.79 |
| PSQI_Sleep latency | 0.59 | 0.71 | 1.00 | 0.94 | 1.14 | 1.06 | 1.10 | 0.94 |
| PSQI_Sleep duration | 0.71 | 0.77 | 0.71 | 0.69 | 1.43 | 0.98 | 1.52 | 1.08 |
| PSQI_Sleep disturbance | 1.06 | 0.43 | 0.94 | 0.43 | 0.95 | 0.22 | 0.95 | 0.22 |
| PSQI_Sleep medication use | 0.18 | 0.73 | 0.12 | 0.33 | 0.86 | 1.32 | 0.81 | 1.29 |
| PSQI_Daytime dysfunction | 0.88 | 0.60 | 0.71 | 0.47 | 0.76 | 0.70 | 0.86 | 0.85 |
| PSQI_Sleep efficiency (%) | 94.23 | 11.40 | 93.80 | 9.82 | 85.01 | 17.18 | 81.36 | 14.87 |
| DBAS | 3.51 | 0.98 | 3.89 | 1.16 | 3.40 | 1.17 | 3.10 | 0.99 |
Note. F = female; PSQI = Pittsburgh Sleep Quality Index; DBAS = Dysfunctional Beliefs and Attitudes About Sleep.
3.1. Age-related differences at the baseline (T0)
In a preliminary step, we examined whether the two age groups differed at the baseline (T0) in the sleep quality (PSQI) and dysfunctional sleep-related beliefs (DBAS) measures. Cohen's d for independent samples was used, with Hedge's (1981) [30] correction for small samples. We interpreted Hedge's g = 0.20 as a “small” effect, g = 0.50 as a “medium” effect, and g = 0.80 or higher as a “large” effect [31]. We found a large effect size for the difference between young and older adults in PSQI total scores (Hedge's g = −0.89 [-1.57, −0.21]), and a negligible difference for the DBAS scores (Hedge's g = 0.10 [-0.55, 0.75]).
3.2. Mean changes
Then we examined how sleep quality (PSQI), and its dimensions, and dysfunctional beliefs and attitudes about sleep (DBAS), changed on average between the baseline (T0) and under lockdown (T1), both in the sample as a whole, and separately by age group (young and older adults).
Mean changes were examined by calculating standardized mean differences (Cohen's d). The measurements obtained at T0 and T1 represent data repeated by participant, so Cohen's d for paired data was used, with Hedge's (1981) [30] correction for small samples. Once again, we interpreted a g of about 0.50 as a “medium” effect, and a g of about 0.80 as a “large” effect. Effects clearly smaller than 0.50 were judged to be of little importance and not discussed. The same interpretation was applied to the comparison between effect sizes (ie, a difference of Δg = 0.50 was interpreted as a medium-sized difference between the two groups and judged to be worth mentioning).
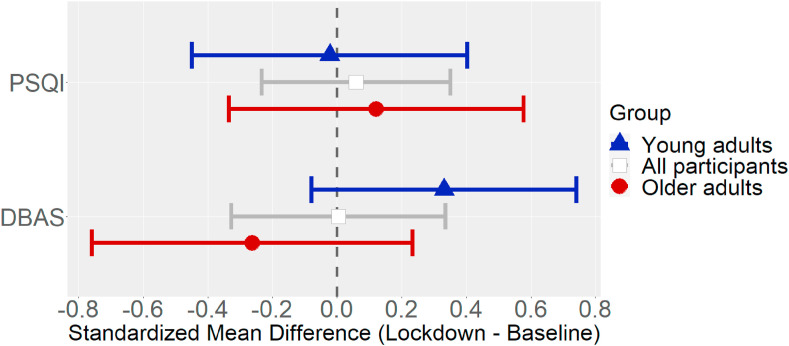
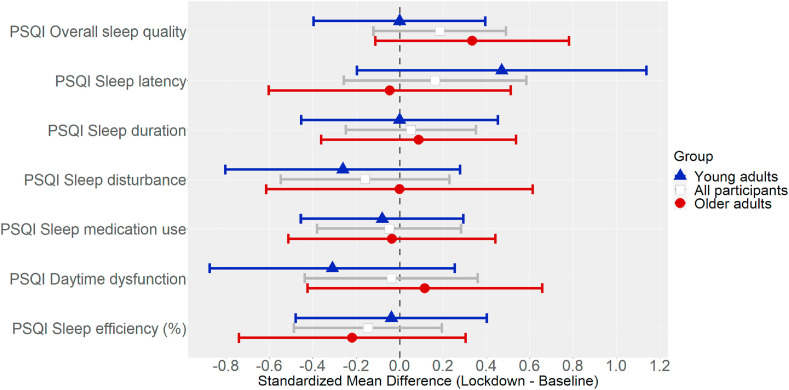
Fig. 1 shows the mean changes in PSQI total scores and DBAS scores. Fig. 2 shows the mean changes in the various PSQI dimensions.2
Fig. 1.
Standardized mean changes (Hedge's g) in self-reported sleep quality (PSQI total score) and dysfunctional sleep-related beliefs (DBAS), with their 95% CIs, by age group (young adults, n = 17; older adults, n = 21).
Fig. 2.
Standardized mean changes (Hedge's g) in dimensions of self-reported sleep quality (PSQI), with their 95% CIs.
To further investigate any changes in sleep quality, PSQI scores were analyzed qualitatively by ascertaining the percentages of younger and older participants who could be considered as having symptoms of insomnia according to the SOL (SOL≥30 min), and the percentages of those who could be labeled as “poor sleepers” according to their PSQI total score (PSQI>5) at T0 and T1.
At the baseline, there were 3 out of 17 young adults (18% [0%, 36%]) with insomnia symptoms (SOL≥30 min), and 7 out of 21 older adults (33% [13%, 53%]). Under lockdown, there were 5 out of 17 young adults (29% [8%, 51%]) with insomnia symptoms (SOL≥30 min), and 8 out of 21 older adults (38% [17%, 59%]). As for “poor sleepers”, there were 5 out of 17 young adults (29% [8%, 51%]) who had a PSQI score >5 at the baseline, and 15 out of 21 older adults (71% [52%, 91%]), while under lockdown, there were 7 out of 17 young adults (41% [18%, 65%]), and 14 out of 21 older adults (67% [47%, 87%]).
3.3. Association between changes in self-reported sleep quality and changes in dysfunctional sleep-related beliefs
We also examined how changes in the DBAS were associated with changes in the PSQI (total score) at individual level. This is because, although there was virtually no mean change in self-reported sleep quality from T0 to T1 in either group, and the mean change in beliefs was small (positive in young adults, negative in older adults), changes of relevance on an individual level might emerge from their association. We therefore tested whether changes in dysfunctional sleep-related beliefs were associated with changes in self-reported overall sleep quality (PSQI total score), in both young and older adults. The two groups differed considerably: in young adults, r = 0.10 [-0.40, 0.56]; in older adults, r = 0.55 [0.16, 0.79]. The intervals of uncertainty are very wide, however, because the two groups were very small, so this difference must be considered with caution.
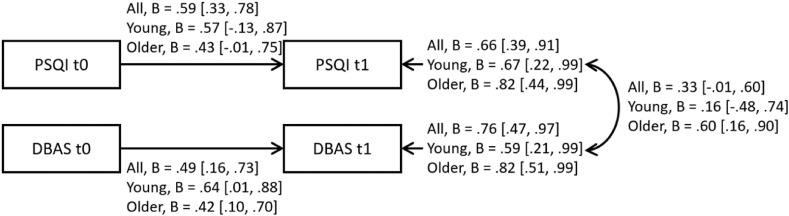
To model the associations between changes in dysfunctional sleep-related beliefs and changes in self-reported overall sleep quality more formally, we fitted a multivariate model in which these two variables (sleep quality and beliefs) at T1 (lockdown) autoregress on their values at T0 (baseline), and examined how the residuals (representing the variation from T0 to T1) correlated. The model is shown in Fig. 3 . The standardized coefficients were bootstrapped with 10,000 iterations to obtain the 95% CIs. The fit indices of the model were very good, χ2(2) = 1.08, p = 0.58, RMSEA = 0.00, SRMR = 0.06, CFI = 1.00, indicating that there were no relevant relationships (eg., cross-relations between self-reported sleep quality and beliefs from T0 to T1) missing from the model.
Fig. 3.
Multivariate model of the relationship between changes in self-reported sleep quality and changes in dysfunctional sleep-related beliefs, with standardized coefficients and their 95% CIs (ascertained with a bootstrap procedure).
4. Discussion
This study newly investigated changes in self-reported sleep quality and dysfunctional beliefs and attitudes about sleep brought on by the COVID-19 pandemic confining young and older adults with no sleep disorders to their homes. We also examined whether any more severely dysfunctional sleep-related beliefs, given their well-known association with sleep quality [21], were associated with a worsening self-reported sleep quality in young and older people. To our knowledge, this is one of only a few studies to have included a baseline, pre-COVID assessment of both self-reported sleep quality and dysfunctional sleep-related beliefs for comparison with the situation under lockdown.
Our results for self-reported sleep quality (PSQI total score and its dimensions) indicated that neither young nor older adults experienced, on average, a clearly worsening overall sleep quality during lockdown compared with beforehand, nor any large changes in sleep duration, sleep disturbances, daytime dysfunction, or sleep efficiency. Nor did our sample report any noticeable changes in the use of sleep medication under lockdown – though this is possibly because we only considered individuals without any sleeping disorders and in a good general state of health. Such a pattern of results was also reflected in the proportions of participants with insomnia symptoms (SOL≥30 min) or labelled as “poor sleepers” (PSQI>5) at the two assessment points: at T1 (under lockdown), these proportions changed to a negligible degree (becoming slightly worse) compared with the baseline (T0). Young and older adults differed, however, in terms of changes in sleep latency: only young adults, in line with previous studies [5,11,15], took longer to fall asleep during lockdown than before the pandemic, but the effect was medium. It may be that younger people's sleeping habits change when they are confined to their homes. For instance, they may make more use of technology before going to bed, which could particularly affect how long it takes to fall asleep, whereas older adults are more likely to retain their usual routines under home confinement. Though this is mere speculation, as we did not administer a questionnaire on these aspects, previous studies [5] showed that technology had a negative impact on young adults' sleep quality during the lockdown.
It is worth noting that, although our findings are not consistent with those of some previous studies involving the retrospective “recall” of sleep quality before the pandemic, they do seem to mirror those found by studies that included a baseline assessment obtained in normal times, as done here (relating to a general population: [11,13]; relating to their current sample: [6,9,12]). Retrospective assessments of sleep quality before the time of COVID (rather than assessments conducted already before the coronavirus came to light) seem to overestimate people's perceived sleeping difficulties under lockdown (see also [12]). This is an aspect worth considering in future studies: in the event of another lockdown, it would be of interest to obtain both types of “baseline” assessment - one actually collected in a period of no lockdown and one obtained in retrospect - to further clarify the impact of home confinement on sleep quality.
As for dysfunctional sleep-related beliefs, our results again reveal only small changes in the frequency of erroneous beliefs and attitudes about sleep during lockdown, in either of our age groups. However, since there was a tendency towards an increase in these aspects in young adults, and a decrease in older adults, a clearly divergent pattern emerged between the two groups (Δg = 0.59), which is worth investigating further. Such a pattern of results was partly unexpected. For young adults at least [25], found a strong prevalence of dysfunctional beliefs and attitudes about sleep in young adults under home confinement. It is worth noting, however, that their sample included people with chronic diseases and healthcare workers particularly exposed to the effects of the COVID-19 outbreak (and therefore more liable to have difficulty sleeping), whereas our young adult respondents were healthy individuals under lockdown. This might account for such divergent results.
Even though the mean changes in self-reported sleep quality and dysfunctional sleep-related beliefs were small, there might have been relevant changes at the individual level. Results of correlations and our multivariate model showed that changes in dysfunctional beliefs and attitudes about sleep correlated significantly (and strongly) with changes in self-reported sleep quality under lockdown for older adults, but not for young adults. This pattern of findings might be because older people are more likely than young people to perceive their sleep quality as poor already in baseline conditions (before lockdown), as also reported in the literature [21,22,24,33]. Although our two age groups seemed to have similar sleep-related beliefs in normal times, a particular life event like the COVID-19 lockdown might have led some of our older adults to attribute any changes they perceived in their sleep quality to their having fostered any dysfunctional beliefs and attitudes about sleep more strongly than before. This would raise the association between sleep quality and sleep-related beliefs, which is also characteristic of individuals who perceive a poor sleep quality [21,22,24,33]. Since such beliefs and attitudes are maladaptive, and can lead to dysfunctional actions or behavior concerning one's own sleep (eg., counterproductive and inappropriate strategies to fall asleep, such as taking sleeping pills), a stressful life event like lockdown seems to contribute to making people (and especially older adults) more vulnerable to sleeping difficulties in the long run.
Despite the potentially interesting results, our study is limited by the small size of the sample in each age group, and consequent non-negligible intra-group variability, meaning that our findings should be considered with caution. In addition, this study did not take into account the psychological distress deriving from individuals’ perception of the constraints on their freedom under lockdown, and from their appraisal of the risks inherent in the pandemic – ie, their fear of contracting the virus and/or infecting their families and contaminating their homes. Considering these aspects would have enabled us to clarify the separate contributions of these diverse (but associated) stressors to changes in self-reported sleep quality, and dysfunctional sleep-related beliefs in young and older adults.
In conclusion, the present findings suggest that neither young nor older adults experienced any marked changes in their self-reported sleep quality when confined to their homes due to the COVID-19 outbreak. There were only changes in some dimensions of sleep quality (and sleep latency in particular), in young adults at least. Our results suggest that it could be useful to consider changes in other sleep-related factors too - such as dysfunctional sleep-related beliefs - to better capture the impact of stressful events like the COVID-19 pandemic on self-reported sleep quality, especially where older adults are concerned.
CRediT author statement
Enrico Sella: Investigation; Data Curation; Writing - original draft, Writing - review & editing; Elena Carbone: Data Curation; Writing - original draft, Writing - review & editing; Enrico Toffalini: Formal analysis; Writing - original draft, Writing - review & editing; Erika Borella: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Supervision.
Acknowledgments
This work was carried out within the scope of the “Use-inspired basic research” project for which the Department of General Psychology at the University of Padova has been recognized as a “Dipartimento di eccellenza” by the Italian Ministry for the University and Research.
The authors are grateful to the Volontari Amici degli Anziani (VADA) and Agora - Terza Età Protagonista associations, and to all the volunteers involved, for their cooperation and participation in this research.
Footnotes
Independent t-tests were run separately to compare demographic characteristics, self-reported measures of sleep quality, and dysfunctional beliefs and attitudes about sleep between participants (n = 43) who were assessed both at the baseline (T0) and under lockdown (T1) and those who did not take part at T1 (n = 77). The results confirmed negligible differences between the groups at the baseline for age [t(118) = −1.797, p = 0.073, Hedges' g = −0.342], education [t(118) = 1.769, p = 0.080, Hedges' g = 0.335], anxiety level [t(118) = −0.092, p = 0.927, Hedges' g = −0.017], depression (for young adults: BDI-2 [t(68) = −1.888, p = 0.685, Hedges' g = −0.107]; for older adults: GDS [t(48) = −0852, p = 0.398, Hedges' g = −0.238]), self-reported sleep quality [PSQI: t(118) = −0.935, p = 0.352, Hedges' g = −0.117], and dysfunctional beliefs and attitudes about sleep [DBAS: t(118) = −0.230, p = 0.352, Hedges' g = −0.044].
Since an excessive sleep latency is considered as a clinical symptom of insomnia for both young and older adults (eg Ref. [32], the raw sleep latency (in minutes) was also examined for descriptive purposes. The raw sleep latency (in minutes) in young adults was: 13.53 [8.75, 18.31] at T0, 16.94 [9.09, 24.79] at T1; and in older adults it was: 20.57 [16.27, 24.87] at T0, 22.52 [15.46, 29.58] at T1. Consistently with the content of Fig. 2, the results emerging from mean level changes for raw sleep latency (in minutes) revealed a very small effect size for the difference between T1 and T0 in both young adults (Hedge's g = 0.29 [-0.18, 0.77]), and older adults (Hedge's g = 0.12 [-0.30, 0.53]).
None declared.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.02.017.
Conflict of interest
The following is the supplementary data related to this article:
Multimedia component 1
References
- 1.Ohayon M., Wickwire E.M., Hirshkowitz M., et al. National sleep foundation's sleep quality recommendations: first report. Sleep Health. 2017;3:6–19. doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Buysse D.J. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell B.A., Redeker N. Sleep and trauma: an overview. Issues Ment Health Nurs. 2005;26(7):721–738. doi: 10.1080/01612840591008294. [DOI] [PubMed] [Google Scholar]
- 4.Altena E., Baglioni C., Espie C.A., et al. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy. Journal of Sleep Research. 2020;29(4):e13052. doi: 10.1111/jsr.13052. [DOI] [PubMed] [Google Scholar]
- 5.Cellini N., Canale N., Mioni G., et al. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. Journal of Sleep Research. 2020;29(4):e13074. doi: 10.1111/jsr.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone M.J., Sigman M., Golombek D.A. Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr Biol. 2020;30(16):R930–R931. doi: 10.1016/j.cub.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandra A., Karki P., Prakash P., et al. Impact of Covid-19 pandemic on quality of sleep among nepalese residents. Sleep Sci Prac. 2020:1–10. doi: 10.21203/rs.3.rs-31619/v1. [DOI] [Google Scholar]
- 8.Barrea L., Pugliese G., Framondi L., et al. Does sars-cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Transl Med. 2020;18:318. doi: 10.21203/rs.3.rs-33081/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck F., Léger D., Fressard L., et al. Coconel Group Covid-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. Journal of Sleep Research. 2020;30(1):e13119. doi: 10.1111/jsr.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blume C., Schmidt M.H., Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R797. doi: 10.1016/j.cub.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casagrande M., Favieri F., Tambelli R. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. doi: 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao C., Scullin M.K. Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Med. 2020;73:1–10. doi: 10.1016/j.sleep.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisler G., Twenge J. 2020. Sleep health in US adults before and during the COVID-19 pandemic. [Google Scholar]
- 14.Gupta R., Grover S., Basu A., et al. Changes in sleep pattern and sleep quality during COVID-19 lockdown. Indian J Psychiatr. 2020;62(4):370. doi: 10.4103/psychiatry.IndianJPsychiatry_523_20. https://10.4103/psychiatry.IndianJPsychiatry_523_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marelli S., Castelnuovo A., Somma A., et al. Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J Neurol. 2020;1–8 doi: 10.1007/s00415-020-10056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sella E., Cellini N., Miola L., et al. The influence of metacognitive beliefs on sleeping difficulties in older adults. Appl Psychol: Health and Well-Being. 2019;11(1):20–41. doi: 10.1111/aphw.12140. https://doi:10.1111/aphw.12140 [DOI] [PubMed] [Google Scholar]
- 17.Buysse D.J., Reynolds C.F., III, Monk T.H., et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Morin C.M., Belleville G., Bélanger L., et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake C., Richardson G., Roehrs T., et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 20.Hoddes E., Zarcone V., Dement W. Enzyklopädie der Schlafmedizin; 1972. Stanford sleepiness scale; p. 1184. [Google Scholar]
- 21.Morin C.M., Stone J., Trinkle D., et al. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. doi: 10.1037/0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 22.Smith S., Trinder J. Detecting insomnia: comparison of four self-report measures of sleep in a young adult population. Journal of Sleep Research. 2001;10(3):229–235. doi: 10.1046/j.1365-2869.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 23.Vand H.D.A., Gharraee B., Farid A.A.A., et al. Prediction of insomnia severity based on cognitive, metacognitive, and emotional variables in college students. Explore. 2014;10(4):233–240. doi: 10.1016/j.explore.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Sella E., Carbone E., Toffalini E., et al. Personality traits and sleep quality: the role of sleep-related beliefs. Pers Indiv Differ. 2020;156:109770. doi: 10.1016/j.paid.2019.109770. [DOI] [Google Scholar]
- 25.Idrissi A.J., Lamkaddem A., Benouajjit A., et al. Sleep quality and mental health in the context of COVID-19 pandemic and lockdown in Morocco. Sleep Med. 2020;74:248–253. doi: 10.1016/j.sleep.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salehinejad M.A., Majidinezhad M., Ghanavati E., et al. Negative impact of COVID-19 pandemic on sleep quantitative parameters, quality, and circadian alignment: implications for health and psychological well-being. EXCLI Journal. 2020;19:1297–1308. doi: 10.17179/excli2020-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yesavage J.A., Brink T.L., Rose T.L., et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/00223956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 28.Beck A.T., Steer R.A., Ball R., et al. Comparison of beck depression inventories IA and II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Gallina P., Saugo M., Antoniazzi M., et al. Validazione della scheda per la valutazione multidimensionale dell'anziano (SVAMA) Tend Nuove. 2006;6:229–264. [Google Scholar]
- 30.Hedges L.V. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–128. doi: 10.3102/10769986006002107. [DOI] [Google Scholar]
- 31.Cohen J. Lawrence Erlbaum Associates; New York, NY: 1988. Statistical power Analysis for the behavioral sciences. [Google Scholar]
- 32.McCrae C.S., Wilson N.M., Lichstein K.L., et al. ‘Young old’and ‘old old’poor sleepers with and without insomnia complaints. J Psychosom Res. 2003;54(1):11–19. doi: 10.1016/S0022-3999(02)00543-3. [DOI] [PubMed] [Google Scholar]
- 33.Willis T.A., Yearall S.M., Gregory A.M. Self-reported sleep quality and cognitive style in older adults. Cognit Ther Res. 2011;35(1):1–10. doi: 10.1007/s10608-009-9270-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1