Abstract
The production of peptide siderophores and the variation in siderophore production among strains of Pseudomonas syringae and Pseudomonas viridiflava were investigated. An antibiose test was used to select a free amino acid-containing agar medium favorable for production of fluorescent siderophores by two P. syringae strains. A culture technique in which both liquid and solid asparagine-containing culture media were used proved to be reproducible and highly effective for inducing production of siderophores in a liquid medium by the fluorescent Pseudomonas strains investigated. Using asparagine as a carbon source appeared to favor siderophore production, and relatively high levels of siderophores were produced when certain amino acids were used as the sole carbon and energy sources. Purified chelated siderophores of strains of P. syringae pv. syringae, P. syringae pv. aptata, P. syringae pv. morsprunorum, P. syringae pv. tomato, and P. viridiflava had the same amino acid composition and spectral characteristics and were indiscriminately used by these strains. In addition, nonfluorescent strains of P. syringae pv. aptata and P. syringae pv. morsprunorum were able to use the siderophores in biological tests. Our results confirmed the proximity of P. syringae and P. viridiflava; siderotyping between pathovars of P. syringae was not possible. We found that the spectral characteristics of the chelated peptide siderophores were different from the spectral characteristics of typical pyoverdins. Our results are discussed in relation to the ecology of the organisms and the conditions encountered on plant surfaces.
The species Pseudomonas syringae contains all of the phytopathogenic and oxidase-negative fluorescent pseudomonads except Pseudomonas viridiflava (27, 38). P. syringae is divided into 57 pathovars that are pathogenic for numerous monocot and dicot crops (13). P. syringae strains are well adapted to conditions on plant surfaces. A better understanding of the ecological benefits of these pathogens is necessary if new and efficient methods of biological control are to be developed. One of these benefits could be the production of peptide siderophores in iron-deficient environments (8, 33). In general, the peptide siderophores produced by fluorescent Pseudomonas strains are pyoverdins (2, 3). All pyoverdins contain the same quinoline chromophore, a peptide chain, and a dicarboxylic acid (or the corresponding amide) connected to the chromophore. The peptide chain is always the same for a given strain but is different in different strains and species (2). Two partially characterized peptide siderophores of P. syringae that have been described (8, 33) have Fe(III)-binding constants at pH 7.0 of about 1 × 1025. These values are 10 times higher than the values obtained for pyoverdins produced by saprophytic fluorescent Pseudomonas strains (8, 33). Therefore, it would be interesting to know whether production of these molecules is common in P. syringae strains and whether the molecules are effectively produced on plant surfaces.
A global study of peptide siderophore production in P. syringae should take the heterogeneity of the species into account. P. syringae pathovars have been divided into two distantly related genomic clusters (22). The first cluster is homogeneous and contains P. syringae pv. tomato and related pathovars. It is genetically more similar to P. viridiflava than to the second genomic cluster of P. syringae pathovars. The second cluster is less homogeneous and more distantly related to P. viridiflava. P. syringae pv. syringae and P. syringae pv. morsprunorum belong to two different subclusters in the second cluster (22). The complexity of the group and the presence of three different peptide siderophores in the species (4, 8, 33) raise questions about the differences in siderophore production by different P. syringae pathovars, particularly if they are distantly related.
In this paper, we describe culture conditions that favor siderophore production by strains of P. syringae and also describe the amino acid compositions of siderophores produced by pathotype and field strains of P. syringae pv. tomato, P. syringae pv. syringae, P. syringae pv. aptata, P. syringae pv. morsprunorum, and P. viridiflava. The biological activities of purified siderophores were verified for both siderophore-producing and non-siderophore-producing strains. The spectral characteristics of the siderophore molecules and of a pyoverdin purified from a culture of P. fluorescens were also compared in this study. The results are discussed in relation to the ecology of the organisms and the conditions encountered on plant surfaces.
MATERIALS AND METHODS
Bacterial strains and precultures.
The characteristics of the strains used in this study are described in Table 1. All precultures were grown on medium 2, as previously described (6).
TABLE 1.
Characteristics of strains used in this study
| Straina | RFLP clusterb | RFLP groupb | Genospeciesb | Host or origin | Country | Fluorescence on King's medium Bc | Growth stimulation on EDDHA-supplemented King's Bd | Source or referencee |
|---|---|---|---|---|---|---|---|---|
| P. syringae pv. tomato LMG 5093T | S1 | A | III | Tomato | England | + | + | LMG |
| P. syringae pv. morsprunorum strains | S2A | K | II | |||||
| LMG 2222 | Cherry | England | + | + | LMG | |||
| LMG 5075 t1T | Plum | Unknown | + | + | LMG | |||
| PmC14 | Cherry | Belgium | + | + | 6 | |||
| PmC22 | Cherry | Belgium | − | + | This study | |||
| PmC29 | Cherry | Belgium | − | + | This study | |||
| PmC36 | Cherry | Belgium | − | + | 6 | |||
| PmC62 | Cherry | Belgium | − | + | This study | |||
| P. syringae pv. syringae strains | S2B | M | I | |||||
| B301D | Pear | England | + | + | 6 | |||
| PsP2 | Pear | Belgium | + | + | 6 | |||
| LMG 5141 | Pear | England | + | + | LMG | |||
| LMG 5191 | Cherry | Switzerland | + | + | LMG | |||
| LMG 1247T | Lilac | England | + | + | LMG | |||
| PsM17 | Corn | Belgium | + | + | This study | |||
| P. syringae pv. aptata strains | S2B | M | I | |||||
| LMG 5059T | Sugar beet | United States | + | + | LMG | |||
| UPB 110 | Sugar beet | Belgium | − | + | 6 | |||
| UPB 165 | Sugar beet | France | − | + | 6 | |||
| P. syringae pv. atrofaciens strains | S2B | O | I | |||||
| PaBG2a | Wheat | Belgium | + | + | This study | |||
| PaBF7a | Wheat | Belgium | + | + | This study | |||
| P. viridiflava LMG 2352T | V | Q | IX | Bean | Switzerland | + | + | LMG |
| P. fluorescens strains | ||||||||
| LMG 1794T | Water | England | + | —f | LMG | |||
| LMG 5822 | Creamery waste | Unknown | + | − | LMG |
T = pathotype or type strain.
Data from reference 22. S1, first P. syringae cluster; S2A, first subcluster in the second P. syringae cluster; S2B, second subcluster in the second P. syringae cluster; V, P. viridiflava cluster. RFLP, restriction fragment length polymorphism.
+, positive; −, negative.
Growth stimulation was observed after 18 h or less following the application of a 125-μg/ml solution of chelated siderophore of P. syringae pv. syringae B301D. +, positive; −, negative.
LMG, Laboratorium voor Microbiologie van Gent, Belgian Coordinated Collections of Microorganisms, Ghent, Belgium.
This strain was able to grow on EDDHA-supplemented King's medium B under the conditions used in the experiment.
Selection of a medium for siderophore production.
P. syringae pv. syringae LMG 5191 and LMG 5141 were used to compare the effects of the 20 amino acids common in proteins on siderophore production. These strains are not able to produce toxic lipodepsipeptides in culture (6). The autoclaved media used contained (per liter) 4 g of an amino acid, 5 g of glucose, 0.96 g of Na2HPO4, 0.44 g of KH2PO4, 0.2 g of MgSO4 · 7H2O, and 8 g of agar. All of the components were high quality in order to ensure that the levels of contaminating iron were low. Four cultures (6) were incubated for 24 h at 28°C. They were then sprayed with a cell suspension of the yeast Rhodotorula pilimanae MUCL 3039 and incubated for 4 days at 20°C. The maximal zones of inhibition between the organisms were measured, and production of fluorescent compounds was estimated by using UV light (wavelength, 360 nm). l-Aspartic acid and l-asparagine monohydrate were subsequently tested in media to which the filter-sterilized amino acid was added after autoclaving. The experiments were replicated three times.
Siderophore production in a liquid medium.
The agar medium containing asparagine was modified slightly and contained (per liter) 2 g of l-asparagine monohydrate, 7 g of glucose, 0.96 g of Na2HPO4, 0.44 g of KH2PO4, and 0.2 g of MgSO4 · 7H2O (GASN medium). The pH was adjusted to 7.0 with HCl, and the medium was autoclaved. Liquid cultures were started in 100-ml Erlenmeyer flasks containing 25 ml of GASN medium. The inoculum used consisted of 1 ml of a suspension of P. syringae pv. syringae LMG 1247 in water (approximately 108 CFU/ml). The cultures were incubated unshaken or shaken (200 rpm) at 20°C. The two other techniques which we used involved petri dishes; each petri dish contained either 10 ml of a liquid medium or a block of GASN agar (length, 30 mm; width, 10 mm; thickness, 5 mm) and 10 ml of a liquid medium. In each case the inoculum consisted of a pellet of cells obtained from a preculture. The cultures were incubated unshaken at 20°C. The experiment was conducted for 3 days, and five cultures were independently analyzed each day. Aliquots of culture medium that were diluted three times were examined with a Lambda 5 UV-VIS spectrophotometer (Perkin-Elmer). Bacterial growth was estimated by measuring the absorbance at 610 nm. Two measurements were obtained for each culture in a petri dish that contained an agar block; for one measurement the bacteria on the agar block were not included, and for the other these bacteria were included. The bacteria were removed by centrifugation (12 min, 10,000 × g), and siderophore production was estimated by measuring the absorbance at 380 nm. The preparations resulting from the five repetitions of each treatment were then combined and filtered, and the pH was measured. After the pH was adjusted to 7.0, the absorbance at 380 nm was measured again. Some techniques were tested by using NM medium (34) supplemented with a dilute salts solution (8) (NM-salts medium) and modified GASN medium that contained (per liter) 6 g of Na2HPO4 and 3 g of KH2PO4; both of these media were buffered in the same way.
Modification of GASN medium.
Asparagine was replaced by 1.4 g of NH4Cl per liter in GNH4 medium. In GASN-0.5 medium, the l-asparagine monohydrate concentration was reduced to 0.5 g per liter, which corresponded to the concentration used to detect the fluorescence of phytopathogenic Pseudomonas strains (34). Asparagine was then considered as a nitrogen source (34). Asparagine minimal medium (ASN-M medium) contained no glucose. The pH of each medium was adjusted to 7.0, and the media were autoclaved; ASN-M medium was also tested at pH 4.0 and 5.0. The experimental procedures used have been described previously, but the measurements were obtained after 4 days.
Effects of free amino acids on siderophore production.
All of the free amino acids that favored production of fluorescent compounds on the agar medium were used as sole nitrogen and carbon sources in modified ASN-M media. The minimal media contained (per liter) 2 g of l-aspartic acid (ASP-M), 2 g of l-glutamic acid (GLU-M), 2 g of l-glutamine (GLN-M), 2 g of l-proline (PRO-M), 2 g of l-serine (SER-M), 2 g of l-alanine (ALA-M), 2 g of l-glycine, or 2 g of l-arginine as a replacement for l-asparagine monohydrate. The filter-sterilized amino acids were added after autoclaving. Measurements were obtained after 4 and 5 days because of different growth rates.
Siderophore production and purification.
Twenty cultures were started in petri dishes containing agar blocks using GASN medium or NM-salts medium and were incubated for 72 h at 20°C. The liquid fractions were then combined, centrifuged (22 min, 10,000 × g), and filtered through a 0.2-μm-pore-size membrane filter. After the pH was adjusted to 7.0, 800 μl of a regularly renewed FeCl3 solution (1 M) was added, and the medium was stirred for 20 min. After the pH was adjusted to 5.0, the medium was passed through an octadecylsilane column made up in a 50 mM NaOH–acetic acid buffer (pH 5.0). The dominant product was collected with water-methanol (1:1, vol/vol). Cation-exchange chromatography was carried out with a type CM C25 Sephadex column made up in a 30 mM NaOH–formic acid buffer (pH 4.2) and eluted with the same buffer. Anion-exchange chromatography was carried out with a DEAE-Sephadex column made up in a 150 mM NaOH–acetic acid buffer (pH 5.0) and eluted first with 600 ml of the same buffer and then with a linear gradient of the same buffer (0.15 to 1 M; 1.8 liters overall). The dominant product was collected at 404 nm and desalted by using an octadecylsilane column. Purity was assessed at 214, 256, and 403 nm by performing analytical high-performance liquid chromatography (HPLC) with a Waters model 2690 system combined with a Waters model 996 detector. The method described by Demange et al. (12) was used, but NaOH was used instead of pyridine. Siderophores were purified in this way for strains LMG 1247, B301D, and PsP2 of P. syringae pv. syringae, strains LMG 2222, LMG 5075tl, and PmC14 of P. syringae pv. morsprunorum, strain LMG 5059 of P. syringae pv. aptata, strain LMG 5093 of P. syringae pv. tomato, and strain LMG 2352 of P. viridiflava. In order to compare the peptide siderophores produced by P. syringae strains with a typical pyoverdin, a slightly modified anion-exchange chromatography method was used to purify a chelated pyoverdin of Pseudomonas fluorescens LMG 1794.
Amino acid analysis.
Purified ferric siderophores were hydrolyzed for 24 and 48 h at 110°C by using 6 N HCl supplemented with 1% (wt/vol) phenol and then were analyzed by HPLC by using a Pharmacia Alpha+ amino acid analyzer. Authentic dl-threo-hydroxyaspartic acid was used as the control.
Growth stimulation tests.
The method described by Meyer et al. (23) was modified slightly and used for growth stimulation tests. Bacteria were grown at 28°C for approximately 24 h in glass tubes containing 4 ml of nutrient broth. The bacterial suspensions were diluted 10 times, and plates containing 10 ml of King's medium B agar (18) and 1 mg of ethylenediaminedihydroxyphenylacetic acid (EDDHA) per ml were homogeneously inoculated with 100-μl portions of the diluted cell suspensions. Sterile nonimpregnated 6-mm-diameter antibiotic discs (Difco) were impregnated with solutions of purified ferric siderophores (250 and 125 μg/ml) and placed on the surfaces of the agar plates. Sterile blanks impregnated with ultrapure water were used as controls. The plates were incubated at 28°C and observed for the following 24 h. We tested the ability of the strains listed in Table 1 to use the purified ferric siderophore of strain B301D. The purified siderophores of strains LMG 5075t1 and PmC14 of P. syringae pv. morsprunorum, strain LMG 5059 of P. syringae pv. aptata, strain PsP2 of P. syringae pv. syringae, P. syringae pv. tomato LMG 5093, and P. viridiflava LMG 2352 were also tested by using the producing strain.
Spectral analyses.
The chelated siderophores of strains of P. syringae, P. viridiflava, and P. fluorescens were purified and were dissolved (37 μg/ml) in a 100 mM NaOH-phosphonic acid buffer adjusted to pH 7.0. The solutions were analyzed by using a model UV-2101PC UV-visible light spectrophotometer (Shimadzu).
RESULTS
Selection of a medium for siderophore production.
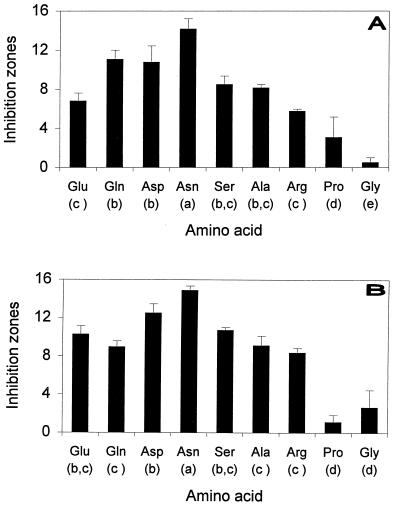
The observation that several autoclaved media containing peptone could be used for siderophore production (unpublished results) was confirmed in our experiments. The inhibition zones observed are shown in Fig. 1. In all instances, fluorescence under UV light was detected in the zones of inhibition. Autoclaving aspartic acid significantly reduced the size of the inhibition zones induced by both of the strains used. This was not the case for asparagine.
FIG. 1.
Maximal zones of inhibition (millimeters) between the yeast R. pilimanae MUCL 3039 and the bacteria P. syringae pv. syringae LMG 5191 (A) and LMG 5141 (B) in various amino acid-containing agar media and statistical grouping. An analysis of variance was performed by using the general linear models of SAS (SAS Institute). Means were compared by using the Newman-Keuls test (α = 0.05).
Production of siderophores in liquid medium.
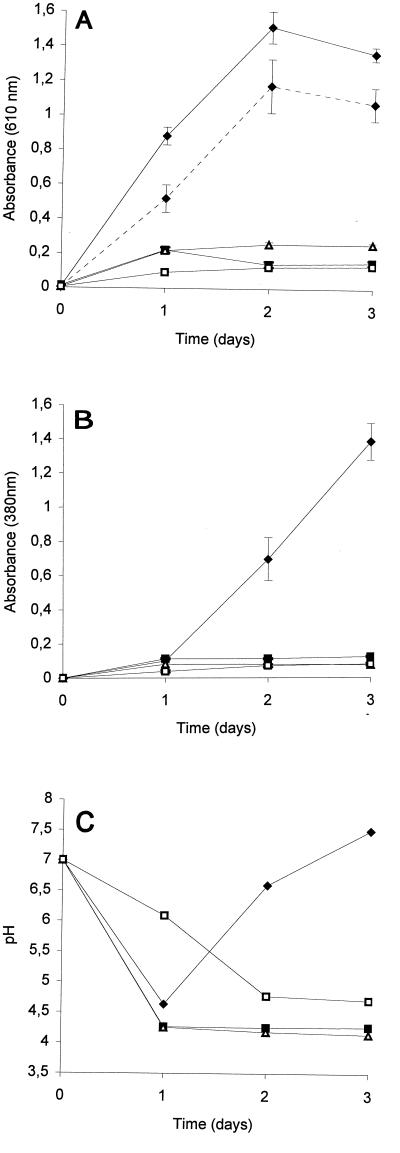
Figure 2 shows the results of production of siderophores in liquid medium. The best bacterial growth occurred in cultures in petri dishes that contained agar blocks (Fig. 2A). Siderophore production was also best in cultures in petri dishes that contained agar blocks, but this was evident only after at least 2 days of incubation (Fig. 2B). Similar results were obtained for all techniques by combining the five preparations used and adjusting the pH to 7.0 before measurements were obtained. Using strongly buffered medium (GASN medium or NM-salts medium) did not improve the results obtained in Erlenmeyer flasks, and there was a threefold reduction in siderophore production by the cultures in petri dishes containing agar blocks.
FIG. 2.
Absorbance (A and B) and pH (C) of GASN medium cultures of P. syringae pv. syringae LMG 1247 in petri dishes containing (⧫) and not containing (■) agar blocks and in shaken (▵) and unshaken (□) Erlenmeyer flasks. The absorbance data are means ± standard deviations based on five replications for each treatment; absorbance was measured at 610 nm (A) and, after bacteria were eliminated, at 380 nm (B) by using thrice-diluted individual cultures. The dotted line in panel A indicates the absorbance at 610 nm determined without the bacteria present on the agar blocks for cultures in petri dishes that contained agar blocks. The pH data are mean pH values based on five replications.
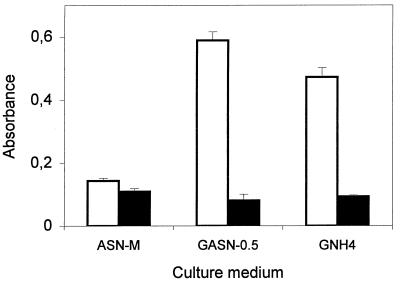
Marked changes in the pH of the culture medium were observed with cultures grown in GASN medium (Fig. 2C). In cultures in petri dishes containing agar blocks, the pH decreased to 4.6 after 1 day of incubation. It then increased to 6.6 on the second day and to 7.5 on the third day. The increase in pH visible after 2 days of incubation was accompanied by an abrupt increase in the siderophore concentration (Fig. 2B). Continuous decreases in pH were observed with the other techniques, and the pH values ranged from 4.25 to 4.77 after 3 days of incubation (Fig. 2C). Reducing the concentration of asparagine or replacing asparagine with NH4Cl resulted in appreciable bacterial growth but weak siderophore production (Fig. 3). In addition, acidification of both media used occurred, and the final pH values were 5.35 in GASN-0.5 medium and 4.33 in GNH4 medium. Asparagine was the source of carbon, nitrogen, and energy in ASN-M medium. Weak bacterial growth and siderophore production occurred in cultures grown in ASN-M medium when the pH was adjusted to 5.0 or 7.0 before autoclaving. However, the level of siderophore production was relatively high compared to the amount of bacterial growth (Fig. 3), and alkalization of the culture medium occurred. The absence of growth in cultures whose pH values were adjusted to 4.0 probably reflected the general inability of Pseudomonas strains to grow at pH 4.0 (26).
FIG. 3.
Means ± standard deviations for absorbance of thrice-diluted 4-day cultures of P. syringae pv. syringae LMG 1247 grown in different media. Absorbance was measured at 610 nm (□) and 380 nm (■).
Considerable, reproducible siderophore production occurred with all of the fluorescent strains listed in Table 1 when we combined GASN medium and the technique involving growth in petri dishes containing agar blocks. However, when this technique was used, we could not induce production of fluorescent siderophores by the three nonfluorescent strains tested, strains PmC36, UPB 110, and UPB 165 (Table 1).
Influence of free amino acids on siderophore production.
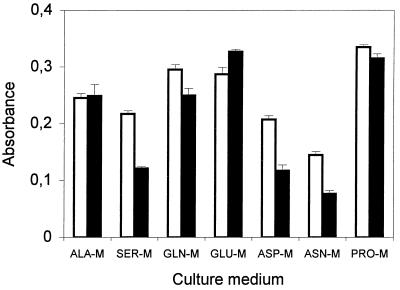
Except for glycine and arginine, which were not used as carbon sources by strain LMG 1247, all of the amino acids tested induced relatively high levels of siderophore production in the absence of glucose after 4 or 5 days (Fig. 4). Alkalization of the culture medium occurred with all media. The final pH values ranged from 8.28 in cultures in PRO-M to 8.83 in cultures in GLU-M.
FIG. 4.
Means ± standard deviations for absorbance of thrice-diluted 4- or 5-day cultures of P. syringae pv. syringae LMG 1247 grown in different amino acid-containing minimal media. Absorbance was measured at 610 nm (□) and 380 nm (■).
Siderophore production and purification.
Passing the medium through an octadecylsilane column was a far more efficient way of extracting siderophores than the previously described chloroform-phenol technique (8). The chelated dominant siderophore stayed adsorbed on the resin at pH 5.0, regardless of the culture medium used. Generally, GASN medium was used for purification because it induced better siderophore production than NM-salts medium. One dominant siderophore was obtained after 72 h with all of the strains of P. syringae or P. viridiflava investigated. Incubating cultures for more than 72 h resulted in alkalinization of the medium and in increase in the level of secondary compounds or appearance of secondary compounds. The two ion-exchange techniques were used separately or together to purify the dominant products up to 98%, as determined by HPLC. No trace of unchelated siderophores was detected in these analyses. In some cases, more than 20 mg of purified siderophores was obtained from 200 ml of culture medium. Since P. fluorescens LMG 1794 is identical to P. fluorescens ATCC 13525, whose pyoverdins have been comprehensively described (19), the dicarboxylic amide of a purified pyoverdin produced by this strain has been identified as succinamide (unpublished results).
Amino acid analyses.
The siderophore produced by P. syringae pv. syringae B301D in GASN medium and NM-salts medium contained two hydroxyaspartic acid residues, two serine residues, two threonine residues, and one lysine residue. The amino acid compositions of the siderophores produced by the P. syringae and P. viridiflava strains investigated in this study were identical to the amino acid composition of the siderophore produced by strain B301D, regardless of the pathovar or species considered.
Growth stimulation tests.
All strains of P. syringae and P. viridiflava were able to use the siderophore of strain B301D and reversed iron starvation on King's medium B supplemented with EDDHA (Table 1). This occurred regardless of the ability of a strain to produce a fluorescent pigment on King's medium B. The purified siderophores were indiscriminately used by the strains. Identical results were obtained when the siderophores of individual strains were tested with the producing strains.
Spectral analyses.
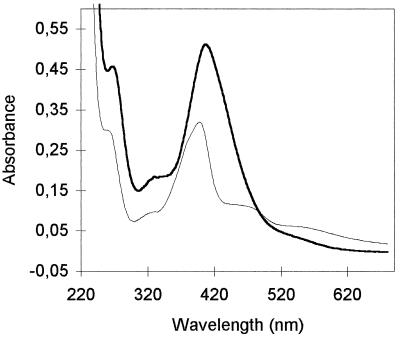
Figure 5 shows the spectral characteristics at pH 7.0 of the succinamide form of the chelated pyoverdin produced by P. fluorescens LMG 1794. Maxima occurred in the vicinity of 230 and 399 nm, and two shoulders were present at about 270 and 320 nm. Broad charge transfer bands occurred at about 470 and 550 nm. These characteristics are characteristics of pyoverdins (2, 12).
FIG. 5.
Differences in absorption spectra in 100 mM phosphate buffer (pH 7.0) between the Fe(III)-complexed peptide siderophore of P. syringae pv. morsprunorum LMG 2222 (dark line) and the succinamide form of the Fe(III)-complexed typical pyoverdin of P. fluorescens LMG 1794 (light line).
The spectral characteristics of the chelated siderophore produced by P. syringae pv. morsprunorum LMG 2222 were different (Fig. 5). The maximum in the vicinity of 400 nm occurred at 408 nm rather than at 399 nm. Other maxima occurred at about 234 and 267 nm, and the shoulder at about 320 nm was more pronounced. Broad charge transfer bands at 470 and 550 nm were not observed, and the molecule disappeared faster than pyoverdin disappeared. All of the chelated siderophores purified from cultures of P. syringae and P. viridiflava in this study had identical spectral characteristics at pH 7.0.
DISCUSSION
Siderophore production in a shaken liquid medium can be irregular or difficult to obtain with some phytopathogenic fluorescent Pseudomonas strains (Fig. 2B) (34; unpublished results). In addition, these growth conditions are very unlike those encountered on plant surfaces. Indeed, P. syringae cells grown on solid media were better able to survive on plants immediately after inoculation than cells grown in liquid media were (35). For these reasons, using alternative methods of siderophore production was considered. Fe(III) has been shown to gradually repress siderophore production by strain B301D at concentrations of >1 to 10 μM, but low concentrations of Fe(III) (between 0 and 1 μM) similarly induced siderophore production (8). As an absence of iron tends to suppress bacterial growth (2, 8), low levels of iron are generally recommended for siderophore production (2, 30). In this study, the culture media were not deferrated because low levels of iron did not repress siderophore production (unpublished results). Thus, GASN medium cultures in petri dishes containing agar blocks were easily reproducible and adaptable for inducing siderophore production by all of the fluorescent strains investigated. P. syringae and P. viridiflava obtain their nutrients in the phyllosphere. The leachates from plant foliage contain almost all of the free amino acids common in proteins (25). Asparagine, which was always found in such leachates (25), is used as a source of both carbon and nitrogen by almost all fluorescent pseudomonads (26). In this study, the decreases in the pH values of cultures in GASN medium observed after 1 day of incubation (Fig. 2C) indicated that glucose was used by the bacteria and acids were produced from glucose (10, 15). However, the subsequent increases in the pH values of cultures to more than pH 7.0 observed during the subsequent days when bacteria were grown in petri dishes that contained agar blocks (Fig. 2C) indicated that asparagine was used as a carbon and energy source. This metabolism was accompanied by an abrupt increase in the siderophore concentration (Fig. 2B and C). The relatively high levels of siderophore production in GASN and ASN-M media (Fig. 2 and 3) compared to GASN-0.5 and GNH4 media showed that this metabolism of asparagine favored siderophore production. The weak bacterial growth observed in ASN-M medium indicated that siderophore production was activated under nutritionally poor conditions, when bacteria had to use asparagine and other amino acids (Fig. 4) as carbon and energy sources. There was apparently no correspondence between these amino acids and the constituents of the siderophore. P. syringae strains are nutrient limited in the phyllosphere, and the carbon source is the limiting environmental resource (36, 37). Asparagine and the free amino acids that favored siderophore production improved the growth of a P. syringae strain in the phyllosphere because of the strain's ability to use these amino acids as carbon sources (36). In addition, the levels of iron on plant surfaces allowed the expression of an iron-regulated gene involved in siderophore production (21). Consequently, the findings of this study suggest that siderophore production is probably activated in the phyllosphere when certain amino acids are used as carbon sources.
The eight P. syringae strains investigated in this study, which included carefully chosen distantly related strains (Table 1), apparently produce the same peptide siderophore. Therefore, this siderophore can be considered the essential peptide siderophore produced in the species. As this siderophore has a high Fe(III)-binding constant and is probably produced on plants, this information should be noted by phytopathologists studying biological control of P. syringae. Our results also indicate that a clear-cut peptide siderophore-based classification is not possible for the essential clusters of the species. This observation is surprising considering that more than 20 different pyoverdins have been found in P. fluorescens (3) and that P. syringae pathovars differ genetically (13, 22), in their host plants (26), and in the toxins which they produce (14). However, the descriptions of two other P. syringae siderophores (4, 33) indicate that variant strains could differ in terms of their siderophores. One molecule is produced by a strain of an undetermined pathovar and resembles the siderophore encountered in this study (33). However, a typical pyoverdin is produced by two strains of P. syringae pv. aptata isolated from sugar beet (4, 31). This molecule is completely different from the siderophore produced by the pathotype strain of this pathovar, which is closely related to P. syringae pv. syringae (6, 22, 28). Further investigations are necessary before a conclusion can be reached concerning this. Even more surprising are the identical characteristics of siderophores produced by strains of P. syringae and P. viridiflava. Production of identical pyoverdins by different Pseudomonas species has been reported previously for borderline species (3, 5, 7, 16). Similar results were obtained in this study since the genomic cluster containing P. syringae pv. tomato is more closely related to P. viridiflava than to the second genomic cluster of P. syringae (22). These results confirm that siderophores provide information concerning the similarity of strains. However, the variation in siderophore composition in fluorescent pseudomonads is limited in the two phytopathogenic species investigated compared to the saprophytic species. These results raise questions about the ecological causes of the different evolutionary processes.
The conservation of a siderophore in two species indicates the importance of the molecule. This was confirmed by the ability of nonfluorescent strains belonging to two pathovars to use this siderophore (Table 1); normally, this would imply that a specific iron-regulated outer membrane protein is produced (9). The strains might be able to produce the siderophore under certain conditions on a plant, or they could use the siderophore produced by other P. syringae strains. However, the siderophore's utility remains unclear. It is not essential for the growth and virulence of two P. syringae pv. syringae mutants (9, 20). Because of its high Fe(III)-binding constant (8), it might be useful in competition with other microorganisms (8, 9, 33) under nutritionally poor conditions. Many pyoverdins produced by saprophytic fluorescent Pseudomonas strains have been described, and their spectral characteristics are similar whatever peptide chain or dicarboxylic acid is connected to the chromophore (1, 2, 24, 29). This is due to the quinoline chromophore found in all pyoverdins (2). Several pyoverdin precursors (2, 4, 16, 31, 32) or by-products (11, 16, 17) differ in chromophore structure and spectral characteristics. The spectral characteristics of the siderophore produced by P. syringae and P. viridiflava resemble the spectral characteristics of pyoverdins, but the siderophore differs from all of these molecules (Fig. 5). This might explain the high Fe(III)-binding constant. The differences between siderophores produced by phytopathogenic and saprophytic fluorescent Pseudomonas strains are interesting at a systematic level, but they might also help improve our understanding of the ecology of these pathogens.
ACKNOWLEDGMENTS
We thank B. Wathelet for performing the amino acid analyses and E. De Hoffmann and R. Rozenberg for help in identifying the pyoverdin produced by P. fluorescens LMG 1794. We are grateful to R. Oger, and we thank the CRA of Gembloux for the support of this project.
This work was supported by the Ministère des Classes Moyennes et de l'Agriculture de Belgique.
REFERENCES
- 1.Briskot G, Taraz K, Budzikiewicz H. Siderophore vom Pyoverdin-Typ aus Pseudomonas aeruginosa. Z Naturforsch. 1986;41C:497–506. [PubMed] [Google Scholar]
- 2.Budzikiewicz H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol Rev. 1993;104:209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 3.Budzikiewicz H. Siderophores of fluorescent pseudomonads. Z Naturforsch. 1997;52C:713–720. [PubMed] [Google Scholar]
- 4.Budzikiewicz H, Schröder H, Taraz K. Zur Biogenese der Pseudomonas-Siderophore: der Nachweis analoger Strukturen eines Pyoverdin-Desferribactin-Paares. Z Naturforsch. 1992;47C:26–32. doi: 10.1515/znc-1992-1-206. [DOI] [PubMed] [Google Scholar]
- 5.Budzikiewicz H, Kilz S, Taraz K, Meyer J M. Identical pyoverdines from Pseudomonas fluorescens 9AW and from Pseudomonas putida 9BW. Z Naturforsch. 1997;52C:721–728. [Google Scholar]
- 6.Bultreys A, Gheysen I. Biological and molecular detection of toxic lipodepsipeptide-producing Pseudomonas syringae strains and PCR identification in plants. Appl Environ Microbiol. 1999;65:1904–1909. doi: 10.1128/aem.65.5.1904-1909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen H, Bye M, Poulsen L K, Rasmussen O F. Analysis of fluorescent pseudomonads based on 23S ribosomal DNA sequences. Appl Environ Microbiol. 1994;60:2196–2199. doi: 10.1128/aem.60.6.2196-2199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cody Y S, Gross D C. Characterization of pyoverdinpss, the fluorescent siderophore produced by Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1987;53:928–934. doi: 10.1128/aem.53.5.928-934.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cody Y S, Gross D C. Outer membrane protein mediating iron uptake via pyoverdinpss, the fluorescent siderophore produced by Pseudomonas syringae pv. syringae. J Bacteriol. 1987;169:2207–2214. doi: 10.1128/jb.169.5.2207-2214.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Ley J. Pseudomonas and related genera. Annu Rev Microbiol. 1964;18:17–46. doi: 10.1146/annurev.mi.18.100164.000313. [DOI] [PubMed] [Google Scholar]
- 11.Demange P, Bateman A, Dell A, Abdallah M A. Structure of azotobactin D, a siderophore of Azotobacter vinelandii strain D (CCM 289) Biochemistry. 1988;27:2745–2752. [Google Scholar]
- 12.Demange P, Bateman A, Mertz C, Dell A, Piemont Y, Abdallah M A. Bacterial siderophores: structures of pyoverdins Pt, siderophores of Pseudomonas tolaasii NCPPB 2192, and pyoverdins Pf, siderophores of Pseudomonas fluorescens CCM 2798. Identification of an unusual natural amino acid. Biochemistry. 1990;29:11041–11051. doi: 10.1021/bi00502a005. [DOI] [PubMed] [Google Scholar]
- 13.Gardan L, Shafif H, Grimont P A D. DNA relatedness among pathovars of P. syringae and related bacteria. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 445–448. [Google Scholar]
- 14.Gross D C. Molecular and genetic analysis of toxin production by pathovars of Pseudomonas syringae. Annu Rev Phytopathol. 1991;29:247–278. [Google Scholar]
- 15.Haynes W C, Burkholder W H. Genus I. Pseudomonas. In: Breed R S, Murray E G D, Smith N R, editors. Bergey's manual of determinative bacteriology. 7th ed. Baltimore, Md: The Williams & Wilkins Co.; 1957. pp. 89–152. [Google Scholar]
- 16.Hohlneicher U, Hartmann R, Taraz K, Budzikiewicz H. Pyoverdin, ferribactin, azotobactin—a new triad of siderophores from Pseudomonas chlororaphis ATCC 9446 and its relation to Pseudomonas fluorescens ATCC 13525. Z Naturforsch. 1995;50C:337–344. [Google Scholar]
- 17.Jacques P, Ongena M, Gwose I, Seinsche D, Schröder H, Delfosse P, Thonart P, Taraz K, Budzikiewicz H. Structure and characterization of isopyoverdin from Pseudomonas putida BTP 1 and its relation to the biogenetic pathway leading to pyoverdins. Z Naturforsch. 1995;50C:622–629. doi: 10.1515/znc-1995-9-1005. [DOI] [PubMed] [Google Scholar]
- 18.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 19.Linget C, Azadi P, MacLeod J K, Dell A, Abdallah M A. Bacterial siderophores: the structures of the pyoverdins of Pseudomonas fluorescens ATCC 13525. Tetrahedron Lett. 1992;33:1737–1740. [Google Scholar]
- 20.Loper J E, Lindow S E. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology. 1987;77:1449–1454. [Google Scholar]
- 21.Loper J E, Lindow S E. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl Environ Microbiol. 1994;60:1934–1941. doi: 10.1128/aem.60.6.1934-1941.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manceau C, Horvais A. Assessment of genetic diversity among strains of Pseudomonas syringae by PCR-restriction fragment length polymorphism analysis of rRNA operons with special emphasis on P. syringae pv. tomato. Appl Environ Microbiol. 1997;63:498–505. doi: 10.1128/aem.63.2.498-505.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer J M, Stintzi A, De Vos D, Cornelis P, Tappe R, Taraz K, Budzikiewicz H. Use of siderophores to type pseudomonads, the three Pseudomonas aeruginosa pyoverdin systems. Microbiology. 1997;143:35–43. doi: 10.1099/00221287-143-1-35. [DOI] [PubMed] [Google Scholar]
- 24.Mohn G, Taraz K, Budzikiewicz H. New pyoverdin-type siderophores from Pseudomonas fluorescens. Z Naturforsch. 1990;45C:1437–1450. [Google Scholar]
- 25.Morgan J V, Tukey H B., Jr Characterisation of leachate from plant foliage. Plant Physiol. 1964;39:590–593. doi: 10.1104/pp.39.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palleroni N J. Family I. Pseudomonadaceae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 141–199. [Google Scholar]
- 27.Palleroni N J, Kunisawa R, Contopoulou R, Doudoroff M. Nucleic acid homologies in the genus Pseudomonas. Int J Syst Bacteriol. 1973;23:333–339. [Google Scholar]
- 28.Pecknold P C, Grogan R G. Deoxyribonucleic acid homology groups among phytopathogenic Pseudomonas species. Int J Syst Bacteriol. 1973;23:111–121. [Google Scholar]
- 29.Poppe K, Taraz K, Budzikiewicz H. Pyoverdine type siderophores from Pseudomonas fluorescens. Tetrahedron. 1987;43:2261–2272. [Google Scholar]
- 30.Schäfer H, Taraz K, Budzikiewicz H. Zur Genese der amidisch an den Chromophor von Pyoverdinen gebundenen Dicarbonsäuren. Z Naturforsch. 1991;46C:398–406. [Google Scholar]
- 31.Schröder H, Adam J, Taraz K, Budzikiewicz H. Dihydropyoverdinsulfonsäuren-Zwischenstufen beider Biogenese. Z Naturforsch. 1995;50C:616–621. [Google Scholar]
- 32.Teintze M, Leong J. Structure of pseudobactin A, a second siderophore from plant growth promoting Pseudomonas B10. Biochemistry. 1981;20:6457–6462. doi: 10.1021/bi00525a026. [DOI] [PubMed] [Google Scholar]
- 33.Torres L, Perez-Ortin J E, Tordera V, Beltran J P. Isolation and characterization of an Fe(III)-chelating compound produced by Pseudomonas syringae. Appl Environ Microbiol. 1986;52:157–160. doi: 10.1128/aem.52.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidaver A K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967;15:1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson M, Lindow S E. Phenotypic plasticity affecting epiphytic survival in Pseudomonas syringae. Phytopathology. 1990;80:1058. [Google Scholar]
- 36.Wilson M, Lindow S E. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl Environ Microbiol. 1994;60:4468–4477. doi: 10.1128/aem.60.12.4468-4477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson M, Lindow S E. Ecological similarity and coexistence of epiphytic ice-nucleating (ice+) Pseudomonas syringae strains and a non-ice-nucleating (ice−) biological control agent. Appl Environ Microbiol. 1994;60:3128–3137. doi: 10.1128/aem.60.9.3128-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young J M. Changing concepts in the taxonomy of plant pathogenic bacteria. Annu Rev Phytopathol. 1992;30:67–105. [Google Scholar]