Abstract
Breast cancer (BC) is the second leading cause of death among women, and it has become a global health issue due to the increasing number of cases. Different treatment options, including radiotherapy, surgery, chemotherapy and anti-estrogen therapy, aromatase inhibitors, anti-angiogenesis drugs, and anthracyclines, are available for BC treatment. However, due to its high occurrence and disease progression, effective therapeutic options for metastatic BC are still lacking. Considering this scenario, there is an urgent need for an effective therapeutic strategy to meet the current challenges of BC. Natural products have been screened as anticancer agents as they are cost-effective, possess low toxicity and fewer side effects, and are considered alternative therapeutic options for BC therapy. Natural products showed anticancer activities against BC through the inhibition of angiogenesis, cell migrations, proliferations, and tumor growth; cell cycle arrest by inducing apoptosis and cell death, the downstream regulation of signaling pathways (such as Notch, NF-κB, PI3K/Akt/mTOR, MAPK/ERK, and NFAT-MDM2), and the regulation of EMT processes. Natural products also acted synergistically to overcome the drug resistance issue, thus improving their efficacy as an emerging therapeutic option for BC therapy. This review focused on the emerging roles of novel natural products and derived bioactive compounds as therapeutic agents against BC. The present review also discussed the mechanism of action through signaling pathways and the synergistic approach of natural compounds to improve their efficacy. We discussed the recent in vivo and in vitro studies for exploring the overexpression of oncogenes in the case of BC and the current status of newly discovered natural products in clinical investigations.
Keywords: breast cancer, natural products, transcription factors, signaling pathways, treatment, therapeutic agents, combination therapy
1. Introduction
Cancer is considered as one of the most common causes of death worldwide, but no permanent treatment is available yet. Among cancers, breast cancer (BC) is the second leading cause of mortality among women, and it has become a global health challenge. It is estimated that about 7.8 million women were diagnosed in 2021 [1]. The global burden of BC is increasing every year in both developing and developed countries [2]. Many exogenous factors increased the risk and progression of BC associated with DNA damage, poor lifestyle, excessive alcohol consumption, nulliparity, diabetes, and estrogen replacement therapy [3,4,5]. About 5–10% of BC incidence occurs due to gene mutation events. BC progression is accelerated by elevated estrogens; leptin; and inflammatory mediators that promote BC cell proliferation, migration, and invasion [6].
Current treatment options for BC include radiotherapy, surgery, chemotherapy [7,8], anti-estrogen therapy, aromatase inhibitors, anti-angiogenesis drugs, and anthracyclines. However, long-term use of anti-estrogen therapy can cause serious health issues. Aromatase inhibitors increase the risk of cardiotoxicity. Anti-angiogenesis drugs also increased the risk of ischemic death. Anthracyclines can cause serious damage to the heart and nail tissues [9,10,11]. Moreover, these therapies are expensive and could cause toxic health effects. On the other hand, hormone therapy is another option at an early stage. However, patients are unable to show a response to hormone therapy. The excessive use of chemical drugs leads to increasing drug resistance and has become a major issue in the modern era. Nevertheless, chemical drugs are also causing serious health complications, such as liver cirrhosis, nausea, vomiting, and an increased risk of kidney failure (as toxic metabolites of drugs can cause serious cellular toxicities) [12]. The major challenges for BC treatment include chemoresistance, and there is a lack of effective therapeutic options for metastatic BC. Limited therapeutic options are currently available for triple-negative breast cancer (TNBC), such as chemotherapy. Due to poor responses to chemotherapy and aggressive behavior, there is an urgent need for a therapeutic strategy to meet the current challenges of TNBC. In order to overcome increasing drug resistance or side effects, natural products should be considered as viable therapeutic options with fewer side effects against TNBC. Therefore, there is a need for urgent BC treatment with no side effects [13,14].
Attention towards the safe use of natural products for BC treatment has increased over the past few years because they are cost-effective, low in toxicity, high in efficacy, and overcome drug resistance. Natural compounds are a rich source of bioactive compounds targeting tumor growth and cell invasion during BC progression [15,16]. The majority of therapeutic agents (>60%) for BC treatment are direct sources of natural products. In vitro and in vivo studies showed that consumption of an adequate amount of natural products obtained from plants, fruits, and vegetables is also helpful in the recurrence and reduction rate of BC, significantly increasing the survival rate among high-risk populations [17,18]. Various experimental studies showed that natural products showed inhibitory potentials for BC prevention through inhibiting angiogenesis, cell migrations, proliferations, and arresting the cell cycle by inducing apoptosis and cell death [19,20].
Recently, various natural products have been discovered and tested as anti-BC agents. These natural products are viridiflorol, verminoside, novel phloroglucinol derivatives, genistein, vulpinic acid, calcitrinone A, kaempferol, protopanaxadiol, thymoquinone, arctigenin, glycyrrhizin, 25-OCH3-PPD, oridonin, apigenin, wogonin, fisetin, curcumin, berberine, cimigenoside, and resveratrol [21,22,23,24,25,26,27,28,29,30]. These natural products are alkaloids, antioxidants, phenolic compounds, flavonoids, and polyphenols in nature, and are obtained from herbal products and vegetables. Consumption of these natural products in adequate amounts could target tumor cells, thus significantly reducing BC. Natural products also work synergistically to improve their efficacy against BC. Therefore, natural products could be used as a complementary therapy to improve the existing chemotherapy and reduce its side effects [31,32].
Natural products and derived bioactive compounds showed anticancer activities against BC by interacting with estrogen receptors, protein kinases, and the downstream regulation of signaling pathways (such as Notch, NF-κB- PI3K/Akt/mTOR, MAPK/ERK, and MDM2 pathways) by inducing apoptosis and cell death [23,33,34,35,36]. These pathways are misregulated following the invasion of BC and can be controlled through the action of natural products. Therefore, natural products have been used as an alternative therapeutic option for BC therapy. This review focuses on the emerging roles of novel natural products and derived bioactive compounds as therapeutic agents for BC therapy. The present review also discusses the mechanism of action through signaling pathways and the synergistic approach of natural compounds to improve efficacy. We have discussed the recent in vivo and in vitro studies for exploring the overexpression of oncogenes in the case of BC and the current status of newly discovered natural products in clinical investigations.
2. Pathogenesis of BC
BC is the most common cause of malignant tumors worldwide. BC can occur from any mutational defect in breast ducts [37]. Generally, BC is categorized on the basis of estrogen receptors into ER-positive and ER-negative breast cancers [38]. Based on specific biomarkers, BC is further categorized into subtypes, such as luminal A, B, and basal-like. TNBC is the basal-like and most severe form (see Figure 1) [39].
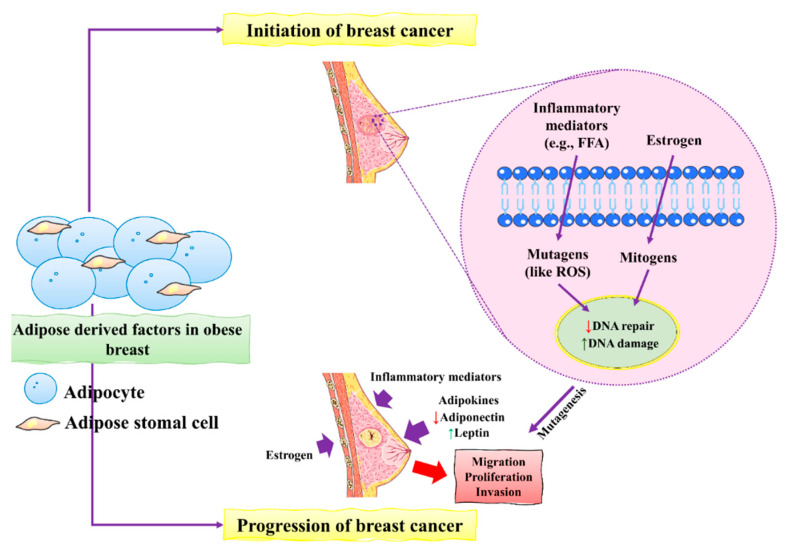
Figure 1.
The initiation and progression of BC by obese breast adipose-derived factors. Factors released by obese breast adipose tissue may operate as mutagens, for example by activating intracellular reactive oxygen species (ROS), which can lead to DNA damage in normal breast epithelial cells and other inflammatory mediators. DNA damage may occur as a result of estrogen’s mitogenic actions, which can lead to replication stress and stress. Unresolved DNA damage, which is associated with mutagenesis and the onset of cancer, may result from increased DNA damage and possible estrogen-induced defective DNA repair. An obese breast adipose tissue microenvironment promotes BC proliferation, migration, and invasion by releasing inflammatory mediators, increasing leptin, decreasing adiponectin, and increasing estrogen levels. This figure is reproduced from Bhardwaj et al. [40] (Creative Commons Attribution License (CC BY 4.0)).
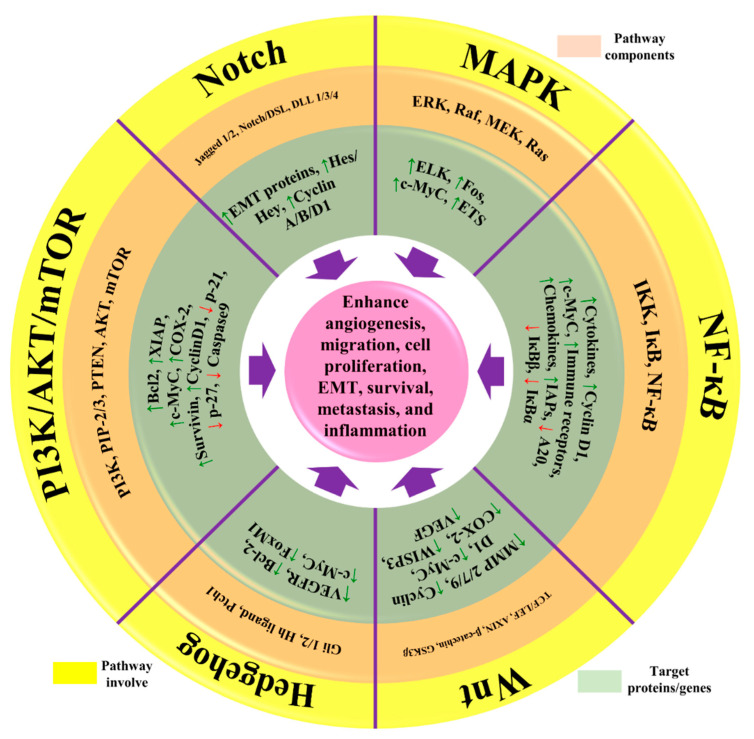
Many environmental and genetic factors increase the risk of BC. These factors include increased damage to DNA, unusual hereditary mutations, exposure to estrogen, and a lifestyle that can increase the development of BC. Patients with a family history already in the malignant phase can also increase the chances of developing BC [11]. Most of the patients inherit their susceptible genes viz p53, BRCA1, and BRCA2 [41]. Unusual mutation in CDH1 and overexpression of p53 can also increase the risk of developing BC [42]. RAS/MEK/ERK, PI3K/AKT, and RAS/MEK/ERK are the main pathways that help the normal cells to defend against cell death. Sometimes, mutational gene events in these pathways increase the chances of BC, as normal cells are incapable of committing cell suicide. For example, mutations in the PTEN gene activate the PI3K/AKT pathway, and cancerous cells are unable to go in commit suicide (see Figure 2) [43].
Figure 2.
The representation of aberrant signaling pathways involved in BC. The green arrow represents the upregulation/activation, while the red arrow represents downregulation/inhibition. This figure is reproduced from Varghese et al. [44] (Creative Commons Attribution License (CC BY 4.0)).
During BC development, epithelial cells change from neoplastic cells into cancerous cells. Adipose tissues mainly contribute to the initiation and progression of BC [40]. The main factors include the inflammatory mediators and mutagens in the form of estrogens that stimulate the production of ROS that ultimately causes severe damage to DNA in epithelial cells of the breast. Increased damage to DNA induced by estrogen causes severe defects and might lead to dysfunctional DNA repair. These unusual changes in DNA increased the risk of mutagenesis of BC. BC progression is accelerated by excess estrogens leptin and inflammatory mediators which promote BC cell proliferation, migration, and invasion [45].
In BC, chronic inflammation is mediated by tumor-infiltrating lymphocytes, cancer-associated fibroblasts, tumor cells, and tumor-associated macrophages. Inflammatory actions are triggered by either necrotic cells, such as damage-associated molecular patterns (DAMPS), or products released by microorganisms, such as pathogen-associated molecular patterns (PAMPS), in the case of breast cancer. As a result, innate and adaptive immune cells secreted the chemokines and cytokines that mediate inflammatory responses [46]. Angiogenesis is initiated by the activation of angiogenesis regulators IL-6, TNF-α, NF-κB, VEGF, Jun/Fos, and LPO. Different pathways are linked with chronic inflammation in BC, such as Notch, NF-κB, PI3K/Akt/mTOR, MAPK/ERK, and NFAT-MDM2 signaling pathways, as well as the regulation of EMT processes [47]. NF-κB is the most critical mediator during chronic inflammation in breast cancer. In NF-κB pathway, activation of different genes such as IER3L, COX2, CXCL12, and CCND3 is a critical inflammatory mediator in patients with BC [48]. Various signaling pathways are activated during the inflammation of BC, which then leads to the activation of transcription factors, such as the signal transducer and activator of transcription signaling (STAT) and the activator protein 1 (AP-1) transcription factor [49].
Chronic inflammation in BC represented the seventh hallmark of cancer as compared to other malignancies. Various cellular events are major consequences of tumor progression, proliferation, and survival. Chronic inflammation indicates different events of BC, such as initiation and progression stages. Identifying different events would be helpful as an important strategy for controlling the BC among high-risk populations and prevention [50,51].
3. Downstream Regulation of Signaling Pathways by Natural Products
Natural products and derived bioactive compounds showed anticancer activities against BC by interacting with estrogen receptors through inhibiting tumor growth and protein kinases. This then helps to induce apoptosis and cell death using Bcl-2, caspases, p53, and p21; arrest the M/G2 phase of the cell cycle; increase the levels of CDK and cyclins, and regulate EMT processes. The downstream regulation of signaling pathways involves Notch, NF-κB, PI3K/Akt/mTOR, and MAPK/ERK pathways [24,52,53].
3.1. PI3K/Akt/mTOR Signaling Pathway
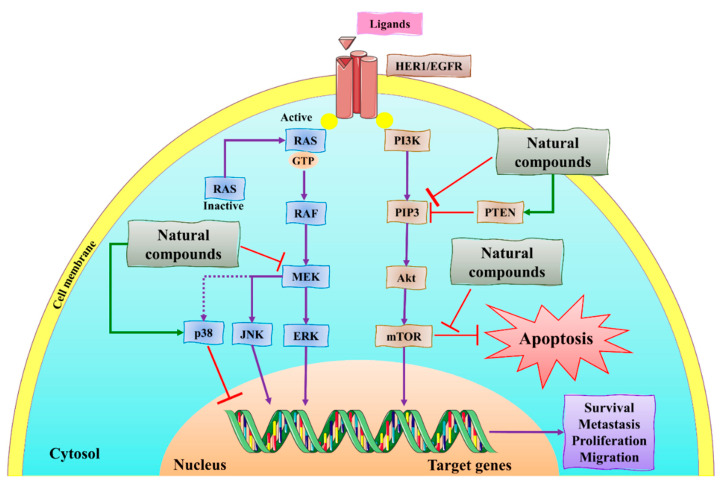
The PI3K/AKT/mTOR pathway is one of the most important signaling pathways activated in BC and participates in cellular activities, such as cell proliferation, invasion, and cell migration [52]. This pathway also suppresses apoptosis and thus increases the growth of abnormal breast tissues. It is also essential to overcome the increasing resistance to drugs used against BC. Suppressing the activation of different genes involved in this pathway is helpful in developing a novel strategy for BC prevention [53]. Activation of PI3K promotes the activation and phosphorylation of AKT, which is the main constituent in tumorigenesis. On the other hand, mTOR regulates cell proliferation and induces apoptosis in BC cells under exposure to chemotherapy. One of the potential strategies used to prevent BC is suppressing or downregulating the PI3K/Akt/mTOR pathway through natural products (see Figure 3) [54,55].
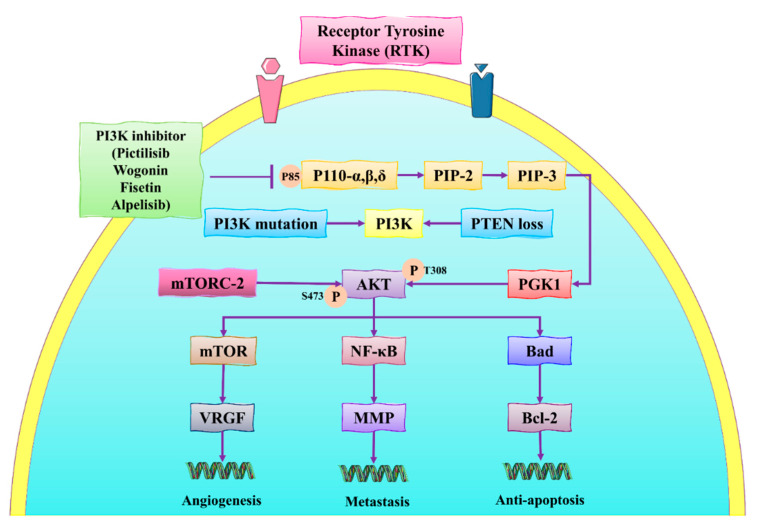
Figure 3.
The schematic representation of the PI3K/Akt/mTOR signaling pathway in BC. VEGF: vascular endothelial growth factor; NF-κB: nuclear factor kappa-B; EGF(R): epidermal growth factor (receptor); mTOR: mammalian target of rapamycin; PDK1/2: 3-phosphoinositide-dependent kinase-1/2; PI3K: phosphatidylinositol 3-kinase; MMP: matrix metalloprotein; PIP-3: phosphatidylinositol (3,4,5)-trisphosphate; Bcl-2: B-cell lymphoma 2; mTORC-2: mTOR complex 2; VRGF: vascular endothelial growth factor; Bad: Bcl-2 antagonist of cell death. This figure is reproduced from Dong et al. [56] (Creative Commons Attribution License (CC BY 4.0)).
Mutations in the PIK3CA gene lead to an increase in the risk of BC. The mutated PIK3CA gene activates the PIK3, initiating the tumor growth in breast tissues that comprised p85 and p110 [57,58]. PIK3 interacts with the VEGFR and promotes angiogenesis. PIK3 converts the phosphatidylinositol 3,4-bisphosphate (PIP2) into 3,4,5-triphosphate (PIP3), interacts with phosphoinositide-dependent kinase-1 (PDK1), and catalyzes the phosphorylation of AKT at Thr 308. AKT also helps in the phosphorylation of mTOR and MPP which activates NF-κB, thus promoting metastasis [59,60]. Hong et al. [61] conducted in vitro and in vivo studies for investigating the role of ginsenoside Rk1 against BC cells (MDA-MB-231). They demonstrated that ginsenoside Rk1 inhibited the PI3K/Akt pathway, stimulated ROS production, increased the expression of Bax, reduced Bcl-2 levels, and activated the release of cytochrome-c from the mitochondrial membrane, and activated caspase 3/8. These molecular events induced apoptosis in MDA-MB-231. Another recent in vivo study conducted by Liu et al. [62] showed that the administration of ginsenoside Rg5 (20 mg/kg) for 30 days significantly inhibited the PI3K/Akt pathway during BC treatment in a BALB-c nude mice model. Therefore, these pathways can be regulated through the action of natural products.
Voacamine (VOA) is a bis-indole alkaloid that is isolated from V. africana and showed anticancer activities against BC [63]. Zuo et al. [34] conducted an in vitro study to investigate the role of VOA in BC treatment by downregulating the PI3K/Akt/mTOR. VOA showed its usefulness against MCF-7 and 4T1 cells with an IC50 value of 1.48 μM. VOA also significantly inhibited the phosphorylated AKT and mTOR in BC cells and also decreased the expression of CDK2 and cyclin A/E. It also induced apoptosis and cell death in MCF-7 and 4T1 cells by arresting the S phase of the cell cycle. VOA induced mitochondrial apoptosis by inhibiting the activity of MMP and increased the level of cytochrome-c in the cytoplasm. Cytochrome-c interacts with protease-activating factors that cleave the assembly of caspase 8/9 and stimulate their activation. It also induced apoptosis which underlies the mitochondrial apoptotic pathway in BC cells [64].
Fisetin is a flavonoid-based natural product that showed anticancer potential against BC. It is found in cucumber, apple, strawberry, and onion. It induced apoptosis in BC cells, inhibiting the cell migrations and suppressing the tumor growth. It also inhibited the expression of Bcl-2 in BC cell lines (MDA-MB-231) [22,65]. Sun et al. [66] conducted in vitro and in vivo (BALB/c mice) studies to investigate fisetin’s role in BC. They found that fisetin induced apoptosis in MCF-7, 4T1, and MDA-MB-231 at 40 and 80 μM. They also found that fisetin acted as an inhibitor of PI3K/Akt/mTOR signaling and inhibited the proliferation and dysregulation of this signaling pathway. The low availability of fisetin in vivo studies limits their use in clinical investigations [67].
Wogonin (WG) is a natural product with a flavonoid nature and is found in the root of S. baicalensis. WG is also used for BC therapy, as it showed inhibitory potentials against BC cell lines (MCF-7 and MDA-MB-231) [68]. It also plays an important role in the downregulation of PI3K/Akt/mTOR pathway. Several studies showed that WG is also used in BC to overcome drug resistance [69]. Zhao et al. [70] conducted in vitro and in vivo studies and showed that WG can have inhibitory effects on MCF-7/MDA-MB-231 and the chicken chorioallantoic membrane (CAM) model, respectively, at 20 and 40 μM. They also found that WG acted as an inhibitor of PI3K/Akt/mTOR signaling and showed inhibition of the proliferation and downregulation of this signaling. 1,3,4,9-tetrahydropyran [3,4-b]-indoles showed anticancer activity against MDA-MB-231 cells with IC50 (2.29 μM). It also induced apoptosis and cell death of MDA-MB-231 cells by arresting the G0/G1 phase of the cell cycle. These recently discovered natural products are involved in downregulating the PI3K/Akt/mTOR pathway, thus acting as promising candidates for BC treatment.
3.2. NF-κB Signaling Pathway
Nuclear factor-kappa B (NF-κB) is one of the most important transcription factors that are activated during inflammation, tumor growth, and proliferation of BC cells. Activation of NF-κB in BC is crucial, and its regulation can be a therapeutic strategy for BC therapy [71]. Natural compounds showed interaction with NF-κB and blocked their activities through dephosphorylation that consecutively deactivates p50. IκB can be activated through the IκB kinase which promotes its phosphorylation and is helpful for the activation of p50, initiating transcription by entering into the nucleus via nuclear pores. This activation stimulates NF-κB for the genetic expression of sacral genes that cause inflammatory responses [71,72].
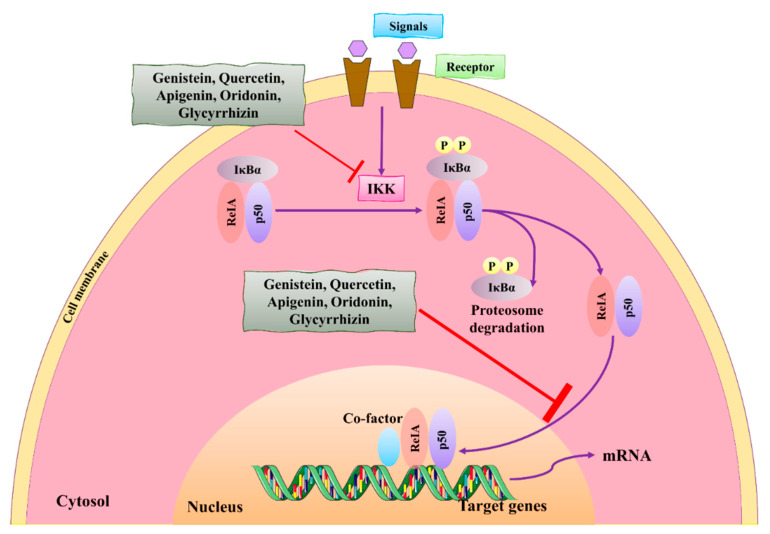
Natural compounds showed anticancer and antitumor activities by downregulating and suppressing the NF-κB pathway. These compounds, genistein and quercetin, can inhibit the phosphorylation of IκBα in MCF-7 HER2 cell lines, thus playing a significant role in the regulation of IκBα to the p50 (see Figure 4) [73]. These compounds also inhibited the NF-κB pathway by blocking the phosphorylation of p65 in the nucleus, thus inhibiting the nuclear translocation [74]. These events also inhibited the functions of NF-κB-targeted genes. Another study found that the application of genistein could control the activation of NF-κB and showed maximum potential at an IC50 value of 20 µM against MDA-MB-231 cells [74,75].
Figure 4.
The role of natural compounds in the treatment of BC by regulating the NF-κB signaling pathway and by suppressing the transcription of NF-κB-targeted genes. IKK: IκB kinase. This figure is reproduced from Ganesan et al. [76] after gaining permission from Elsevier (license no. 5310141484610).
Apigenin (AP) is another natural product with a flavone nature isolated from A. cepa and C. sinensis. It exhibited anticancer activity against BC cell lines (MDA-MB-231) [21]. It influenced the NF-κB pathway by suppressing the COX-2 and downregulating the gene expression of NF-κB. It also inhibited cell proliferation and migration by arresting the cell cycle at the G2/M phase. It also suppresses the cyclin A, B, and CDK1 which can control the G2/M phase. Bauer et al. [77] investigated the anticancer activity of AP against BC cells (MDA-MB-231) and found that it influenced the NF-κB pathway by suppressing the VEGF through deactivating progesterone receptors in BC cells.
Ginsenosides and their derivatives are anticancer-based natural products extracted from Panax ginseng with great potential against BC. Kim et al. [78] conducted in vitro and in vivo studies to investigate the role of ginsenoside Rg3 against BC cells (MDA-MB-231). They found that ginsenoside Rg3 inhibited the NF-κB pathway by inhibiting the phosphorylated AKT and ERK, and induced apoptosis and cell death in MDA-MB-231.
Glycyrrhizin (GLA) is a terpenoid-based natural product isolated from G. glabra. It showed anticancer activity against MDA-MB-231 by inhibiting invasion and cell proliferation, and also inhibited the E-cadherin [25]. These novel natural products are involved in downregulating the NF-κB pathway, thus acting as promising candidates for BC treatment.
3.3. MAPK/ERK Signaling Pathway
The MAPK/ERK signaling pathway is another molecular cascade used for the activation of several genes in BC. These genes are ERK 1/2 and JNK 1/2, which play a significant role in cell proliferation by activating transcription factors. The inactivation of genes through natural products can inhibit tumor growth and invasion in carcinogenic mechanisms in this signaling pathway. Natural compounds showed their ability to interact with MAPK/ERK transcription factors, thus downregulating the signaling pathway (see Figure 5). Arctigenin (ATG) showed maximum potential at 200 μM and was directly involved in suppressing the MAPK pathway by inhibiting the phosphorylation of JNK and ERK in MCF-7 and MDA-MB-231 cells [24]. Other studies showed that delphinidin, a natural compound of anticancer activity, was also involved in suppressing the MAPK pathway by inhibiting the phosphorylation of JNK 1/2 and ERK in MDA468 and MCF-7 cells [79]. Thymoquinone also downregulated the MAPK pathway by inhibiting the activation of p38 and JNK [80]. Protopanaxadiol (PPD) targeted BC cell lines by suppressing the MAPK pathway through deactivation of ERK1/2, p38, and JNK, and showed maximum activity against MDA-MB-231 below 20 μM [81].
Figure 5.
The schematic representation of the mechanism of action of natural compounds on MAPK/ERK and PI3K/Akt/mTOR signaling pathways can inhibit migration, survival, cell proliferation, and metastasis. PTEN: phosphatase and tensin homolog; MEK: mitogen-activated protein kinase; JNK: c-Jun N-terminal kinase; Akt: protein kinase B; ERK: extracellular signal-regulated kinase; mTOR: mammalian target of rapamycin; PIP-3: phosphatidylinositol (3,4,5)-trisphosphate. This figure is reproduced from Ganesan et al. [76] after gaining permission from Elsevier (License No. 5310141484610).
Kaempferol exhibited anticancer activities against BC (MDA-MB-453) by arresting the G2/M phase of the cell cycle [11]. It bound with CDK1 and blocked their activities. Zhu et al. [82] conducted an in vitro (BT474 and MDA-MB-231) study to investigate the role of kaempferol in treating BC and found that the number of cancerous cells decreased from 85.2% to 50.32% in the G1 phase of the cell cycle. These studies showed that kaempferol can significantly inhibit the BC cells by blocking the critical phases of the cell cycle. It induces apoptosis and ultimately leads to cell death [82]. The growth and proliferation of BC cells can depend on glucose utilization. Inhibiting glucose decreased the survival rate of cancerous cells, thus making it a promising therapy for early-stage BC treatments [83]. Azevedo et al. [84] conducted in vitro and in vivo studies to investigate the uptake of glucose and the absorption of lactate with MCF-7 BC cells. They found that kaempferol inhibited the proliferation of tumor cells, thus minimizing the utilization of glucose by MCF-7 BC cells. On the other hand, kaempferol also decreased the absorption of lactate to MCF-7 BC cells that cannot survive, thus leading to cell death.
Hung et al. [85] investigated the anticancer role of kaempferol and found that binding with estradiol can degrade ERα and inhibit the proliferation of MCF-7 cells. Kim et al. [33] also conducted an in vivo study in a mice model (BALB/c nu/nu) to discover the anticancer potentials against MCF-7 BC cells. They also found that the binding of kaempferol with triclosan causes the suppression of the ER signaling pathway. These events activate the RAS protein that induces cell proliferation and causes cell death in BC cells. Thus, natural compounds showed inhibitory actions against different breast cell lines by inhibiting the expression of genes involved in the MAPK/ERK pathway.
3.4. Notch Signaling Pathway
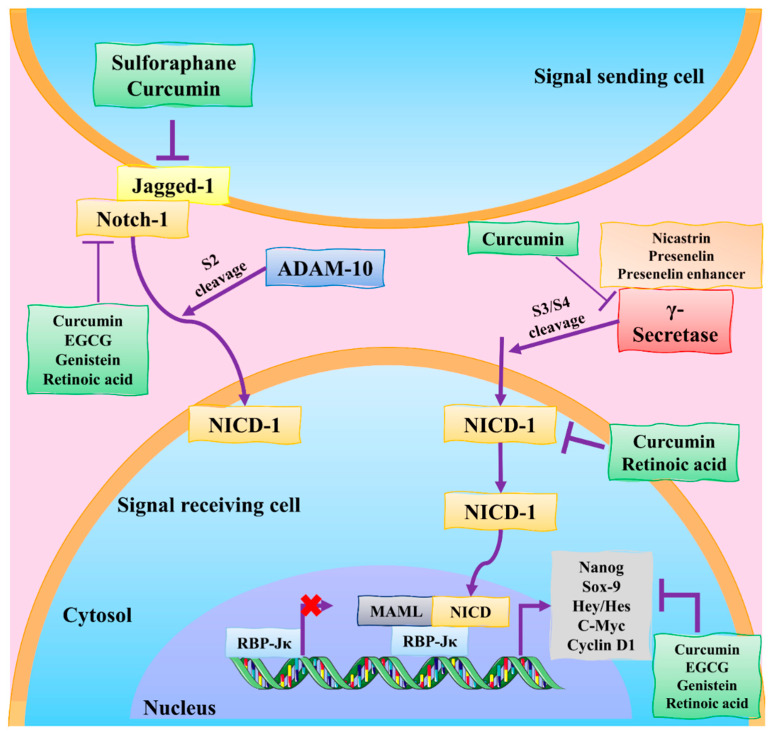
Various anti-apoptotic proteins (Bcl-2) are involved in regulating the Notch pathway and thus play a significant role in maintaining mitochondrial permeability [86]. Gamma secretase is the critical enzyme involved in the Notch intracellular domain (NICD) transcription via the Notch pathway (see Figure 6). NICD moves from the cytoplasm to the nucleus and binds with it to regulate transcriptional complexes containing DNA-binding protein CBF1/RBPjk/Su(H)/Lag1 (CSL) which downregulate the Notch pathway [87]. Cimigenoside, a novel natural compound isolated from C. dahurica, also inhibited the release of gamma–secretase from the transmembrane region by inhibiting their catalytic core PSEN-1, ultimately inhibiting the binding of NICD to CSL, and thus marinating the regulation of the Notch pathway. Cimigenoside showed its usefulness by inducing apoptosis and inhibiting Bcl-2, which ultimately then inhibited the Notch pathway [23,88,89].
Figure 6.
Natural products regulate the modulation of the Notch signaling pathway in BC. The Notch receptor is cleaved by Notch ligands, including Jagged-1. ADAM-10 initially cleaves the Notch extracellular domain; the gamma–secretase complex then cleaves the Notch intracellular domain (NICD). NICD moves into the nucleus and initiates transcription. MAML: mastermind-like protein; EGCG: epigallocatechin-3-gallate; RBP-Jκ: recombinant signal binding protein for immunoglobulin kappa J region; DATS: diallyl trisulfide; ADAM-10: a disintegrin and metalloproteinase domain-containing protein 10; NICD: Notch receptor intracellular domain. This figure is reproduced from Kiesel et al. [87] (Creative Commons Attribution License (CC BY 4.0)).
Unusual abnormalities in the form of malignant tumors in the Notch signaling pathway lead to a variety of cancers, such as BC. Cimigenoside inhibited the Notch signaling pathway by interrupting various proteins. It tightly binds with the PSEN-1 and inhibits their activity [90,91]. Cimigenoside also degraded NICD in the nuclear region. It does not affect the expression of PSEN-1 in the cytoplasm. Thus, cimigenoside is directly involved in the inactivation of PSEN-1 and decreases the level of NICD in the nuclear region. Bcl-2 inhibited apoptosis, thus regulating the Notch signaling pathway. Cimigenoside also inhibited the activity, or interrupted the balance, of Bax and Bcl-2 and induced apoptosis in BC cells [92].
Jia et al. [23] performed in vivo (Balb/C Nude Crlj mice) and in vitro (MDA-MB-231 and MCF-7) studies on C. dahurica isolated to investigate the role of cimigenoside as an anticancer agent in BC treatment. The isolated cimigenoside showed maximum anticancer activity against BC cell lines (MDA-MB-231 and MCF-7) with IC50 (12.6 ± 1.47, 15.6 ± 2.47 μM). Cimigenoside induced apoptosis in BC cells by arresting the G2/M phase of the cell cycle. Another in vitro study screened the isolated compound, β-d-allopyranosyl-3-methoxyphenyl, from Cimicifuga dahurica. This isolated compound exhibited excellent anticancer potentials against BC cell lines (MCF-7 cells) with an IC50 value of 30 μM. They showed that this compound induced apoptosis by decreasing the expression of Bcl-2 and Bcl-XL which acted as anti-apoptotic proteins with anti-proliferation effects by inhibiting c-Myc and cyclin D1, and also arrested the cell cycle at G0/G1-S [90]. Oridonin is a diterpenoid-based natural product that was isolated from R. rubescens. By blocking the Notch signaling system and inhibiting angiogenesis and EMT linked to VEGF-A, this drug may be effective in the fight against breast cancer development and spread [88]. Xia et al. [89] conducted an in vivo experiment in BALB/C athymic nude mice and reported that oridonin successfully induced apoptosis in human BC cells. They also found that Notch 1−4 protein expression was also lowered by oridonin therapy, which hindered cancer cell migration and invasion. Thus, natural compounds showed inhibitory properties against different BC cell lines for regulating the Notch pathway.
3.5. MDM2 Signaling Pathway
MDM2 signaling pathway is another pathway activated during BC progression. p53 is a tumor suppressor protein that plays a significant role in the regulation of the cell cycle. It is encoded by the TPp53 gene. It is important for various cellular events occurring during the cell cycle, such as apoptosis. Overexpression of the p53 gene leads to an increase in the risk of BC. Its expression can be controlled by murine double minute 2 (MDM2) through a negative feedback mechanism in two ways [93,94]. In the case of non-stress environments, MDM2 tightly binds to the p53 via the transactivation domain. After binding with p53, it starts gradual degradation in the presence of ubiquitination. Without ubiquitination, degradation of p53 becomes slow. Under stress environments, in case of damage to DNA, a complex of MDM2-p53 has stabilized as stress facilities this process. Any defect in MDM2 can decrease the binding and degradation activities, thus losing negative feedback [36,95,96].
25-OCH3-PPD is one of the most active ginsenosides isolated from P. notoginseng and acts as a therapeutic agent for treating BC. Wang et al. [36] conducted in vitro and in vivo studies to demonstrate its anticancer activities against MDA-MB-231 and nu/nu mice, respectively. They found that 25-OCH3-PPD acted as an inhibitor of MDM2 at the transcriptional level. They observed that 25-OCH3-PPD was also involved in arresting the G1 phase of the cell cycle and induced apoptosis in BC cells. 25-OCH3-PPD also plays an essential role in inhibiting tumor growth, cell invasion, and the MDM2 pathway in BC. 25-OCH3-PPD could serve as a potential anticancer agent for BC therapy. Ginsenosides, a natural product, targeted the tumor cells and induced apoptosis and cell differentiation in BC. It also acts as an inhibitor of MDM2, which might be a potential target in BC therapy. These novel natural products are involved in downregulating the MDM2 pathway, thus acting as promising candidates for BC treatment [97].
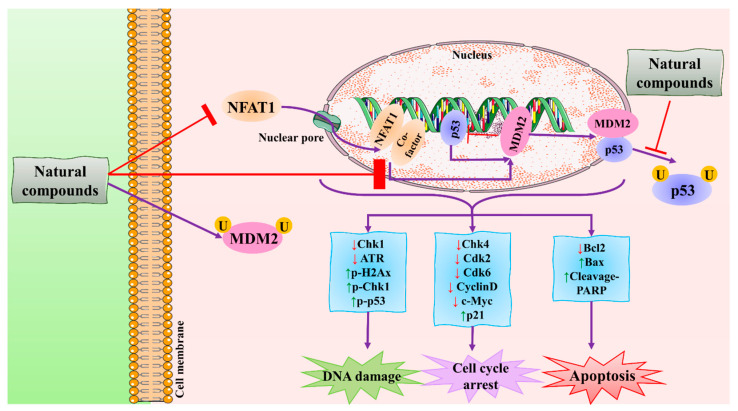
Lineariifolianoid A (Lin A) is another sesquiterpenoid-based natural product isolated from I. lineariifolia which could be used against BC [98,99]. The nuclear factor of activated T-cells (NFAT) and MDM2 are oncogenes that play an important role in BC cell proliferation, invasion, and migration. Both oncogenes are overexpressed in the case of BC. Several therapeutic agents have been used as an inhibitor of NFAT and MDM2. One of the important targets of NFAT and MDM2 is Lin A [100]. Qin et al. [95] conducted in an vitro study to investigate Lin A’s role in inhibiting the NFAT-MDM2 pathway. They found that Lin A arrested the cell cycle at the G2/M phase and inhibited cell invasion and cell proliferation in BC cells. They also observed that Lin A induced apoptosis at a higher concentration of 50% in BC cells (MCF7 and MDA-MB-231 with an IC50 value of 4.5 ± 0.3 and 7.8 ± 0.6 µM, respectively. Therefore, Lin A acted as a novel inhibitor of NFAT-MDM2 pathways and can be used as a therapeutic agent for treating BC therapy (see Figure 7). Detailed information about the individual and synergistic natural products with better anti-cancer activities is presented in Table 1 with a proper mechanism of action.
Figure 7.
The schematic representation of the mechanism of action of natural compounds against BC using the MDM2 signaling pathway. Natural compounds induce apoptosis by inhibiting the NFAT1-MDM2 signaling pathway to reduce cancer. NFAT1: nuclear factor of activated T cells 1; MDM2: mouse double minute 2 homolog; CDK: cyclin-dependent kinase; Chk: checkpoint kinase; Bcl-2: B-cell lymphoma 2; Bax: BCL2-associated X protein; PARP: poly (ADP-ribose) polymerase. This figure is reproduced from Qin et al. [95] (Creative Commons Attribution License (CC BY 4.0)).
Table 1.
The in vitro and in vivo studies on the role of natural products in downregulating the signaling pathways against various types of BC models.
| Extracted Compound | Biochemical Structure | Biochemical Nature | Source | Study Type | BC Type | Animal Model | Key Finding | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|---|---|---|
| VOA |
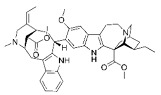
|
Alkaloid | V. africana | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | Downregulating the PI3K/Akt/mTOR. VOA showed its usefulness against MCF-7 and 4T1 cells with IC50 values (0.99, 1.48 μM). | VOA significantly inhibits the phosphorylated AKT and mTOR in BC cells and also decreases the expression of CDK2, cyclin A, E. It also induces apoptosis and cell death in MCF-7 and 4T1 cells by arresting the S phase of the cell cycle. | [34] |
| Lin A |
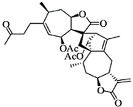
|
Sesquiterpenoid | I. lineariifolia | In vitro | TNBC, and HER2-positive BC | --- | Lin A induced apoptosis at a higher concentration of 50% in BC cells (MCF7 and MDA-MB-231 with IC50 (4.5 ± 0.3, 7.8 ± 0.6). | Lin A arrests the cell cycle at the G2/M phase, and inhibits cell invasion and cell proliferation in BC cells. | [100,95] |
| Fisetin |
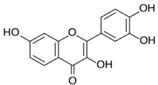
|
Flavonol | Cucumber, apple, strawberry | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | BALB/c mice | Fisetin induced apoptosis in MCF-7, 4T1, and MDA-MB-231 at 40 and 80 μM. | Fisetin acts as an inhibitor of PI3K/Akt/mTOR signaling and inhibits the proliferation and dysregulation of this signaling pathway. | [66] |
| WG |
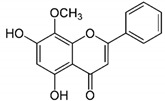
|
Flavone | S. baicalensis | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | Chicken chorioallantoic membrane (CAM) | WG showed inhibitory effects on MCF-7 and MDA-MB-231 at 20 and 40 μM. | WG acts as an inhibitor of PI3K/Akt/mTOR signaling and shows inhibition in cell proliferation. | [70] |
| AP |
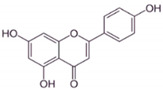
|
Flavone | A. cepa, C. sinensis | In vitro | ER-positive, HER2-positive BC | --- | It influenced the NF-κB pathway by suppressing the VEGF through deactivating progesterone receptors in BC cells. | It inhibits cell proliferation and migrations by arresting the cell cycle at the G2/M phase. It also suppresses the cyclin A, B, and CDK1 which controls the G2/M phase. | [77] |
| Oridonin |
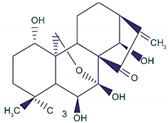
|
Diterpenoid | R. rubescens | In vivo | --- | BALB/C athymic nude mice | It induced apoptosis and cell death in BC cells. | Notch 1-4 protein expression is lowered by oridonin therapy, which hinders cancer cell migration and invasion. | [89] |
| Genistein |
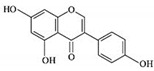
|
Isoflavones | Soy-based foods | In vitro | ER-positive, TNBC | --- | Activation of NF-κB showed potential against MCF-7 at an IC50 value of 20 µM. | It inhibits the phosphorylation of IκBα in MCF-7/T47D/MDA-MB-231 cell lines, thus playing a significant role in the regulation of IκBα to the p50. | [73] |
| GLA |
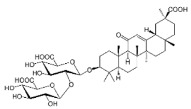
|
Terpenoid | G. glabra | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | It showed anticancer activity against MDA-MB-231/BT549. | It inhibits invasion and cell proliferation, as well as promote the expression of E-cadherin. | [25] |
| ATG |
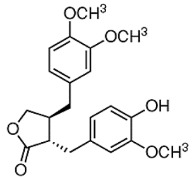
|
Isoflavones | S. heteromalla | In vitro and in vivo | ER-positive, TNBC | BALB/cA-nu | It showed anticancer potentials in MDA-MB-231 cells at 200 μM. | Inhibiting the phosphorylation of MAPK/ERK in MDA-MB-231 cells. | [24] |
| PPD |
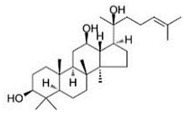
|
Glycoside | P. notoginseng | In vitro and in vivo | TNBC, and HER2-positive BC | BALB/C nude mice | It showed maximum activity below 20 μM against MDA-MB-231. | PPD targets BC cell lines by suppressing the MAPK pathway through the deactivation of ERK1/2, p38, and JNK. | [81] |
| Kaempferol |
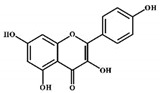
|
Flavonols | Onions, lettuce | In vitro | ER-positive, TNBC | --- | The number of cancerous cells decreased from 85.2% to 50.32% in the G1 phase of the cell cycle. Kaempferol significantly inhibited the BC cells (BT474 and MDA-MB-231) by blocking the critical phases of cell cycles. | Inhibitory actions against different breast cell lines can inhibit the expression of genes involved in MAPK/ERK. This shows that binding with estradiol causes degradation of Erα. | [82] |
| Cimigenoside |
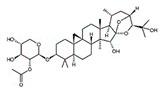
|
Glycoside | C. dahurica | In vitro and in vivo | ER-positive, TNBC | BALB/C nude Crlj mice | Cimigenoside showed maximum anticancer activity against BC cell lines (MDA-MB-231, MCF-7) with IC50 (12.6 ± 1.47, 15.6 ± 2.47 μM). | Cimigenoside induces apoptosis in BC cells by arresting the G2/M phase of the cell cycle. An in vitro study of cimigenoside also inhibits/attenuates BC cell proliferation and invasion. An in vivo study inhibited the growth of tumor growth in mice models. | [23,90] |
| Ginsenosides |
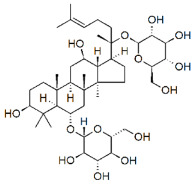
|
Glycosides | P. notoginseng | In vitro and in vivo | ER-positive, TNBC | Nu/nu mice | It showed maximum anticancer activity against BC cell lines (MDA-MB-231). | 25-OCH3-PPD is involved in arresting the G1 phase of the cell cycle and induces apoptosis in BC cells by downregulating MDM2. | [36] |
| BA |
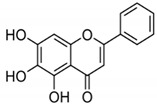
|
Flavonoid | Scutellaria baicalensis | In vitro and in vivo | ER-positive, HER2-positive BC | BALB/c mice | It showed suppression of the NF-κB pathway in the development of human breast epithelial cells (MCF10A). | Suppress the NF-κB signaling pathway, as well as IL-1β, Bcl-2, and VEGF. | [26,96] |
| VMS |
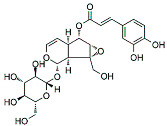
|
Monoterpenoids | P. rotundum | In vitro and in vivo | ER-positive, TNBC, and HER2-positive BC | PyMT/FP635 mouse | It showed maximum activity against MDA-MB-231 and MCF7 cells with IC50 value (10 µM). | VMS suppresses the growth of epithelial lining and the transition of mesenchymal breast cells. | [101] |
| Calcitrinone A |
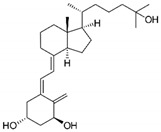
|
Phloroglucinol | C. citrinus | In vivo and in vitro | ER-positive | Chick chorioallantoic membrane (CAM) | Calcitrinone A induced apoptosis and cell death in MDA-MB-231 cells. | Calcitrinone A interferes with mitochondrial function by blocking succinate coenzyme Q reductase and ultimately inhibits the complex II that increases the production of ROS. | [29] |
| Vulpinic acid |
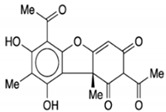
|
Butenolide | Lichens | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | Vulpinic acid induced apoptosis in MCF-7. | Elevate the levels of FOXO-3 and Bax, and suppress the expression of Bcl-2 and procaspase-3/9 to enhance the activity of tumor suppressor miRNAs. | [28] |
| Genistein |
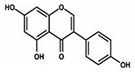
|
Isoflavone | Fabaceae family | In vitro | TNBC, and HER2-positive BC | --- | It induced apoptosis and cell death in MCF-7 and MDA-MB-231 cells. It also inhibited cell proliferation and progression in BC. | Arresting the cell cycle at G2/M phase, downregulating CDK-1, and inhibiting the expression of Bcl-2 and the function of DNA polymerase II. | [102,103] |
| CUR + BBR |
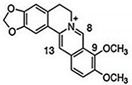
|
Diarylheptanoid, isoquinoline alkaloid | Curcuma longa, berberine from Rhizoma coptidis | In vitro | ER-positive, TNBC, and HER2-positive BC | --- | It showed effects against BC cell lines (MDA-MB-231 and MDA-MB-468) at p ≤ 0.010. | The EMT process in the case of BC is impaired. | [104,105] |
| BA + 5-FU |
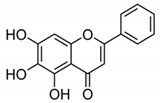
|
Flavonoid (BA) | Scutellaria baicalensis | In vivo | --- | Swiss albino mice | It showed inflammation by inhibiting the VEGF, IL-1β, and NF-κB. | Inflammation is inhibited by the VEGF, IL-1β, and NF-κB, which play significant roles in preventing BC. | [106] |
| MG + 5-FU |
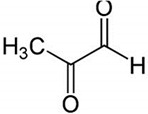
|
Polyphenolic (MG) | Coffee, wine | In vivo and in vitro | ER-positive, TNBC, and HER2-positive BC | BALB/c mice and Swiss albino mice | It showed anticancer activity against the BC cell line (MCF-7). | Arresting the cell cycle at MG G0/G1 phase induces apoptosis and cell death by increasing Bac-2 and caspase 9. | [30] |
| RSVL + SAL |
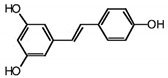
|
Polyphenol | Red grapes | In vivo and in vitro | ER-positive, TNBC, and HER2-positive BC | Swiss albino mice | It showed anticancer activity against MDA-MB-231. | Arresting the S1 phase of the cell cycle also induces apoptosis in BC cells. Other activities include inhibition and cell proliferation. It acts as an antioxidant by preventing the DNA dame and suppressing the tumor growth. SAL inhibits the epithelial mesenchymal transition, and suppresses p53, COX-2, and Beclin. | [107,108] |
Note: VOA: voacamine; Lin A: lineariifolianoid A; ATG: arctigenin; WG: wogonin; AP: apigenin; GLA: glycyrrhizin; PPD: protopanaxadiol; BA: baicalin; VMS: verminoside; CUR: curcumin; 5-FU: 5-fluorouracil; BBR: berberine; RSVL: resveratrol; SAL: salinomycin; MG: methylglyoxal; TNBC: triple-negative breast cancer; ER: estrogen receptor.
4. Synergistic Approach of Natural Products against BC
Several chemotherapeutic drugs have been used to treat BC. The development of chemotherapeutic drugs has increased the resistance rate in BC cells and remained a challenge for breast therapy [109,110]. Drug resistance and tumor growth are critical factors for increased mortality risk among women. The prolonged use of a single drug is not effective for the treatment of BC [111]. Therefore, the clinical use of these drugs has become critical for BC. Therefore, urgent therapy with a safe mode on breast tissues can overcome this issue.
For the complete eradication of BC, combinations of natural products with drugs are an effective strategy to overcome the side effects and minimize the risk of recurrence of BC cases [112]. Therefore, combining the effects of natural products is an important therapeutic strategy for overcoming drug resistance and controlling the BC in high-risk populations.
4.1. Synergistic Effects of Curcumin and Berberine
Some natural products have been used as combinations with synergistic effects to improve their efficacy against BC. For example, curcumin (CUR) and berberine (BBR) have been used as potential sources of natural products to treat BC. CUR and BBR were isolated from the root of Curcuma longa and Rhizoma coptidis respectively in the search for secondary metabolites with anticancer, antitumor, and anti-inflammatory properties [113,114]. CUR and BBR are of great interest in clinical investigations because of their low toxicity. Recently, the synergistic effects of CUR and BBR have shown excellent anticancer properties against BC (see Figure 8) [115,116].
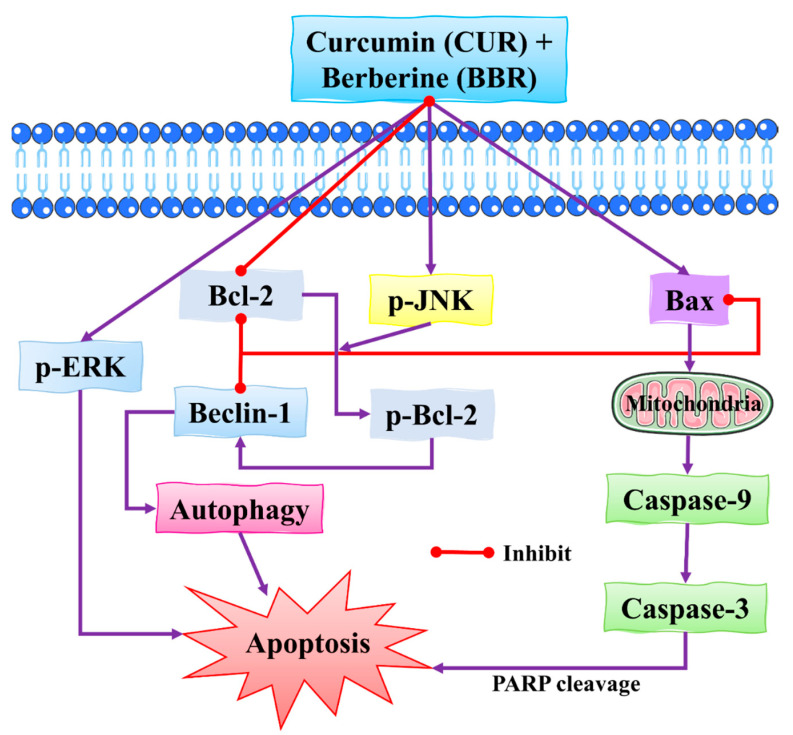
Figure 8.
The synergistic effect of CUR and BBR against BC cells by inducing apoptosis. Apoptosis in BC cells is induced by a combination of CUR and BBR, mediated through the activation of the ERK signaling pathway. These two compounds work together to enhance JNK activation, phosphorylation of Bcl-2, and dissociation of the Beclin1/Bcl-2 complex in BC cells, eventually resulting in autophagic cell death. Bcl-2: B-cell lymphoma 2; Bax: BCL2-associated X protein; PARP: poly (ADP-ribose) polymerase; JNK: c-Jun N-terminal kinase; ERK: extracellular signal-regulated kinase. This figure is reproduced from Wang et al. [104] (Creative Commons Attribution License (CC BY 4.0)).
CUR is a diarylheptanoid-based natural compound, and its chemical structure reveals the presence of carbonyl and phenolic groups. CUR is mainly composed of turmeric compounds (2–8%) and is an excellent source of yellow turmeric [117]. It is also used in TNBC as it interferes with the EMT process and inhibits BC cell migration [118]. Its anticancer activities induced apoptosis and cell death in BC cells in order to prolong the survival of cells [119]. BBR is an isoquinoline alkaloid that exhibits anticancer activities and induced apoptosis and cell death in BC cells. Its anticancer activities are reflected by its binding ability with DNA topoisomerase, as well as the ability to induce apoptosis in BC cells [120]. Genus Berberis is an excellent source of BBR [121]. It is widely used for the treatment of TNBC due to inhibition and migration of BC cell MDA-MB-231. BBR is also used as a potential agent for breast therapy due to its ability to reverse MDR. Therefore, combinations of natural products showed robust effects in reducing invasion, migration, and the EMT process [122,123].
Wang et al. [104] conducted an in vitro study to investigate the combined effects of CUR and BBR on the MCF-7 and MDA-MB-231 BC cells. The synergistic effects of both natural compounds induced apoptosis and cell death via the activation of the ERK and JNK signaling pathways which regulated the phosphorylation of JNK and decreased the phosphorylation of Bcl-2. The combined effects of CUR and BBR played a significant role in preventing BCs; thus, they can be used for BC therapy.
CUR and BBR are widely used for the treatment of BC. They exhibit a polyphenol nature, and affect various cellular processes, including EMT, as in the case of BC. Thus, the synergistic effects of these natural compounds are helpful in combating BC as compared to traditional chemotherapies [122,124]. Another recent in vitro study conducted by Kashyap et al. [105] demonstrated that combinations of CUR + BBR impaired the EMT process, thus showing effects against BC cell lines (MDA-MB-231 and MDA-MB-468) with p ≤ 0.010. Thus, synergistic combinations of CUR and BBR could be used as potential therapeutic agents for BC therapy.
4.2. Synergistic Effects of Baicalin and Methylglyoxal with 5-Fluorouracil
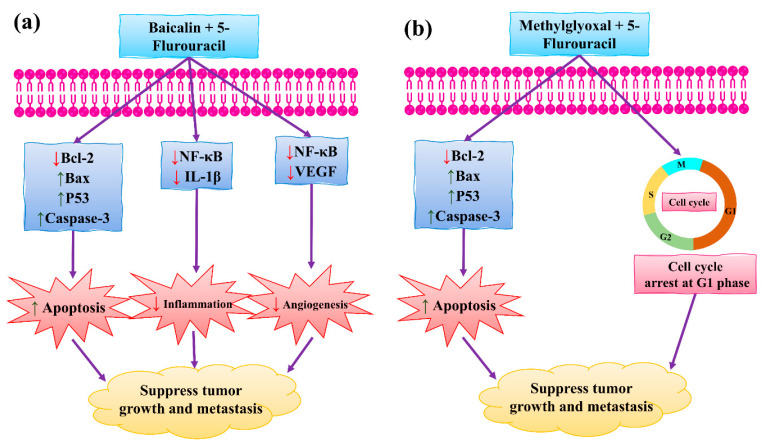
Baicalin (BA) is a flavonoid-based natural compound isolated from Scutellaria baicalensis that exhibited excellent anticancer, anti-proliferative, and antioxidant properties [125]. BA is non-toxic for humans. It regulates the normal development of the breast by inhibiting the proliferation and invasion of cancer cells, thus triggering apoptosis which ultimately leads to cell death (see Figure 9). Chung et al. [26] have demonstrated that BA can suppress the NF-κB pathway in the development of human breast epithelial cells. Other studies showed that BA significantly prevents BC, including inhibited tumor growth, angiogenesis, fibrosis, invasion, and apoptosis [126]. Methylglyoxal (MG) showed anticancer activity against BC by inhibiting ATP depletion, and by suppressing glycolysis and mitochondrial respiration [127,128]. 5-fluorouracil (5-FU) has also been used as a chemotherapeutic drug to treat BC. It binds with DNA by blocking the activity of thymidylate synthase. It is also involved in targeting invasion cells and in inhibiting their proliferation. Other roles include the activation of p53 by inducing apoptosis [129,130]. It is known that toxic metabolites released from 5-FU metabolism also block DNA synthesis [131]. The side effects of 5-FU have been reduced through combinations with natural products. For example, 5-FU itself is a toxic agent, but combinations positively impacted BC prevention [106]. 5-FU is widely used for the treatment of BC. It inhibited the invasion and progression of cancer cells by inducing apoptosis, ultimately leading to cell death. Clinical trials in combination therapy with other natural products are needed to overcome chemoresistance in BC [131].
Figure 9.
The synergistic effect of (a) BA and 5-FU, and (b) MG and 5-FU in BC therapy. BCL2: B-cell lymphoma two protein; NF-κB: nuclear factor kappa B; Bax: Bcl-2-associated X protein; p53: inducible gene 3; IL-1β: interleukin 1 beta; VEGF: vascular endothelial growth factor. This figure is reproduced from Shehatta et al. [106] (Attribution Non-Commercial No. Derivatives 4.0 International (CC BY-NC-ND 4.0)).
Shehatta et al. [106] demonstrated the combined effect of 5-FU and BA on in vivo animal models (Swiss albino mice) and revealed that they significantly suppress the NF-κB signaling pathway. The surviving (IL-1β, Bcl-2, and VEGF) and upregulating (p53, caspase-3, and Bax) genes were involved in this suppression. Another study conducted by Miao et al. [132] demonstrated that the combined effect of 5-FU and BA reduced inflammation by inhibiting VEGF, IL-1β, and NF-κB. They found that it induced apoptosis by caspase-3 and Bax. In view of the above, BA acts as a promising candidate for improving BC therapy.
4.3. Synergistic Effects of Resveratrol and Salinomycin
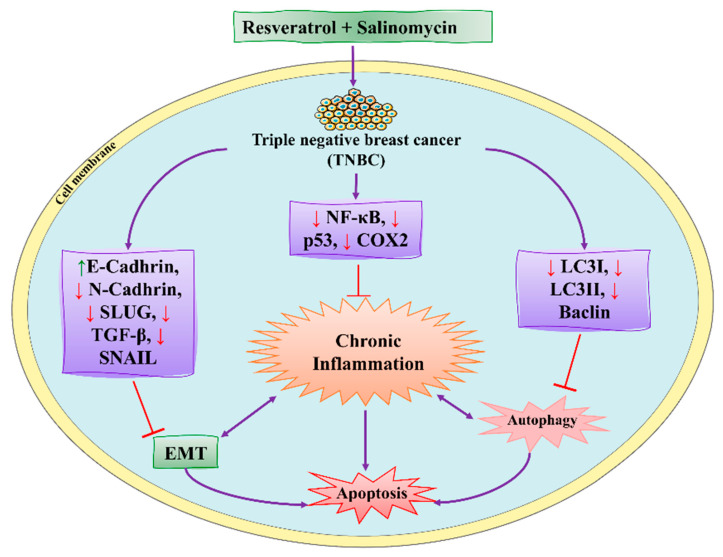
Resveratrol (RSVL) is a polyphenolic compound and showed anticancer activity against BC cells (MDAMB-231 and MCF-7). It is obtained from dietary sources, such as peanuts, grapes, and berries. RSVL activities include arresting the S1 phase of the cell cycle and inducing apoptosis in BC cells [133]. It acts as an antioxidant by preventing DNA damage and suppressing tumor growth (see Figure 10) [108]. RSVL also inhibited ATP in MCF-7. Thus, RSVL, as a natural product, decreases MDAMB-231 and MCF-7 cells [134]. Salinomycin (SAL) is a potent antibiotic used for the treatment of BC. It is derived from Streptomyces albus and was useful for inhibition, cell proliferation, and the suppression of tumor growth [135]. SAL also induced apoptosis and cell death in metastatic BC cells by promoting DNA damage and elevating ROS production. Some in vivo studies showed that SAL significantly suppressed tumor growth [136].
Figure 10.
RSVL and SAL synergistic effect against BC cells by inducing apoptosis. NF-κB: nuclear factor kappa B; p53: inducible gene 3; TGF-β: transforming growth factor-beta; EMT: epithelial to mesenchymal transition; COX2: cyclooxygenase-2. This figure is reproduced from Rai et al. [107].
RSVL and SAL acted synergistically to improve the efficacy of BC therapy. Rai et al. [107] investigated the role of RSVL and SAL in vitro and in vivo studies against MDA-MB-231 BC cells and Swiss albino mice, respectively. They found that synergistic combinations of RSVL and SAL inhibited the epithelial–mesenchymal transition and suppressed p53, COX-2, and Beclin. They also found that RSVL and SAL induced apoptosis in TNBC. Dewangan et al. [137] investigated the role of SAL and RSVL against BC. They found that this combination induced apoptosis and cell death in MCF-7 cells by promoting ROS production, leading to mitochondrial dysfunction. This combination also cleaved the PARP network and activated the caspases. SAL and RSVL synergistically decreased Bcl-2 and downregulated the MAPK pathway by activating p38, increasing the oxidative stress and apoptosis in MCF-7 cells [35]. The combination of RSVL with SAL is also helpful as a novel therapy for TNBC to overcome drug resistance.
5. Recent Discoveries and Developments of Natural Products against BC
5.1. Novel Verminoside and Their Derivatives
Various adjuvant therapies have been used, including combinations of natural products, in order to improve their susceptibility to BC. These novel products have replaced or limited the use of conventional therapies due to increasing drug resistance. Different natural products have been reported influenced positive effects and could be used as adjuvant therapy by targeting tumors or affecting the EMT process [138]. For example, verminoside (VMS) from Pseudolysimachion rotundum interferes with the EMT process by suppressing the invasion and tumor growth; thus, it is widely used for BC treatment [101].
VMS has been used as an anticancer agent and exhibited inflammatory properties. Its anticancer activities have been evaluated in in vivo and in vitro models. Lee et al. [101] carried out an in vivo study in animal models (PyMT/FP635 mouse model) to investigate the anticancer potentials of VMS and showed maximum activity against MDA-MB-231 and MCF7 cells with an IC50 value of 10 µM. They found that VMS suppresses epithelial lining growth and the transition of mesenchymal breast cells without activating the ERK signaling pathway (see Table 1). Therefore, VMS can be used as a chemo-adjuvant for the treatment of BC.
VMS is a monoterpenoid that also has been isolated from K. pinanta. The biochemical nature of VMS is greatly reflected due to the presence of two hydroxyl groups that are responsible for anticancer and inflammatory properties. It is widely used for inhibiting the EMT process in BC [139]. BC cells under the EMT process have increased mesenchymal characteristics and decreased the formation of epithelial cells and cell linings [109]. These events promoted invasion and migration among BC cells. In the previous studies, it was shown that the excessive use of chemotherapeutic drugs promoted the EMT process. As a result, chemoresistance is one of the major issues tackled through the safe use of natural products as they can inhibit invasion, migration, and EMT in BC development [101].
5.2. Novel Phloroglucinol and Derivatives
Phloroglucinols are phenolic compounds that exhibit anticancer and anti-inflammatory activity. Phloroglucinol is the most important novel natural product, and its chemical structure reveals that it has an aromatic ring surrounded by hydroxyl groups. It is composed of two phloroglucinol units linked by a methylene bridge. Calcitrinone A is a type of natural product, novel phloroglucinol, isolated from C. citrinus. Calcitrinone A showed potential against MDA-MB-231 BC cells [140].
Calcitrinone A inhibited cell proliferation, invasion, and tumor growth, and stimulated apoptosis in MDA-MB-231 cells. Calcitrinone A is less toxic as compared to other drugs used for the treatment of BC. Calcitrinone A bound with the succinate-coenzyme Q reductase located at complex II of the mitochondrial membrane and blocks their activity. It resulted in the depletion of ATP levels. Calcitrinone A is the most promising anticancer agent used for BC therapy as it interferes with complex II of the mitochondria [141].
An in vivo and in vitro study was conducted by Gaafary et al. [29] to investigate the role of calcitrinone A in the chick chorioallantoic membrane (CAM) and MDA-MB-231 cells, respectively. They found that calcitrinone A interferes with mitochondrial function by blocking succinate coenzyme Q reductase and inhibiting complex II, which can untimely increase ROS production. These events induced apoptosis and cell death in MDA-MB-231 cells (see Table 1). Kim et al. [142] found that phloroglucinol inhibited the epithelial–mesenchymal transition in BC; they also found that phloroglucinol increases ROS production. They found that phloroglucinol also induced apoptosis and cell death in metastatic BC cells.
5.3. Role of Viridiflorol against BC
Viridiflorol is a natural organic compound, and its chemical structure contains a cyclopropazulen in its carbon skeleton, responsible for anticancer activity against BC. Viridiflorol, which was isolated from S. algeriensis. Furthermore, the Lamiaceae family, including the Senecio rowleyanus Jacob, Mentha aquatica L, and Ballota undulata, are excellent sources of viridiflorol derivatives [143,144]. Previous studies showed that oil secretions from S. rowleyanus contained viridiflorol (11%) that possesses anticancer and anti-inflammatory activities [145]. The oil secretions from Salvia leriifolia showed anticancer activity against BC (MCF-7 and MBA-MD-231) [146]. Viridiflorol is a class of sesquiterpenoid that isolated various aromatic plants, such as M. quinquenervia and A. edulis. Extracts of viridiflorol from these plants exhibited anticancer activities against different BC cell lines (MCF-7 and MDA-MB-231) [147]. Essential oil from Blepharocalyx salicifolius and Cyperus longus is an excellent source of viridiflorol [148,149].
Akiel et al. [27] performed an in vitro study in searching for anticancer activities of viridiflorol against BC MCF-7 cells. The tested viridiflorol showed maximum anticancer activity against MCF-7 with IC50 value of 0.1 µM. Memariani et al. [148] conducted in vitro study to investigate the anticancer potentials and apoptosis of viridiflorol. The tested derivatives of viridiflorol showed maximum anticancer activity against MCF7 with IC50 value of 31.60% and induced apoptosis at concentrations of 78.23%. Furtado et al. [149] also conducted in vitro study and successfully isolated viridiflorol from Blepharocalyx salicifolius that showed anticancer potentials against MDA-MB-231 at concentrations of 46.60 µg/mL. The isolated viridiflorol is also involved in suppressing the cellular metabolism and biological activities of BC cells.
5.4. Effect of Vulpinic Acid against BC
Vulpinic acid is a natural product that was isolated from lichens and exhibited anticancer potentials against BC. Lichens secreted a large variety of secondary metabolites effective for the treatment of BC. For example, vulpinic acid is secreted by lichenized fungi which demonstrates anticancer and anti-proliferative activities [150]. Vulpinic acid is a safe and easily assessable metabolite in nature. Vulpinic acid inhibits cell proliferation and invasion, inhibits tumor growth, and stimulates apoptosis in MC-231 cells. Vulpinic acid interacts with the FOXO-3 gene and suppresses their activity, leading to decreased expression of miRNAs [151].
In the previous studies, vulpinic acid has been used for breast treatment as it induces apoptosis and suppresses Bcl-2 [152]. Cansaran-Duman et al. [28] conducted an in vitro study and investigated the role of vulpinic acid on BC by regulating the expression of miRNA levels. They found that vulpinic acid induced apoptosis in MCF-7 by elevating the level of FOXO-3 and Bax, and suppressing Bcl-2 and pro-caspase-3,9, thus altering the tumor suppressor miRNAs (see Table 1). Kilic et al. [153] investigated the anticancer potentials of vulpinic acid against MCF-7, BT-474, MDA-MB-231, and SK-BR-3. They found that vulpinic acid showed anti-proliferative and significant inhibitory effects on the MCF-7. They also found that vulpinic acid induced apoptosis and cell death in MCF-7.
5.5. Action of Genistein against BC
Genistein is a naturally occurring compound and possesses anticancer, anti-proliferative, and anti-tumor activities. Fabaceae families are rich in genistein and its derivatives. It is also found in soybeans. It is used to overcome the drug resistance caused by BC and control the reoccurrence of metastatic invasion. It also suppresses the activities of DNA polymerase II [154]. It also arrested the cell cycle at the G2/M phase and induced apoptosis and cell death in MCF-7 and MDA-MB-231 cells. It also inhibited the cell proliferation and progression of BC. Genistein has been used for clinical uses for the prevention of BC because it increases survival rates [102].
Other studies investigated the role of genistein against BC and found that it downregulated CDK-1 and inhibited the expression of Bcl-2, as well as the function of DNA polymerase II. They also found that genistein also increased the expression of p21 and p51 [103,155]. Genistein also showed inhibitory effects on tyrosine kinases and inhibited the cancer progression. Xie et al. [156] investigated that genistein is an effective therapeutic agent that inhibits DNA methylation in MCF-7 and MDA-MB-231 cells by blocking DNA methyltransferase activity. Liu et al. [102] studied that genistein showed inhibitory potentials in the deactivation of IGF-1R and p-Akt. They also found that it decreases the level of Bcl-2 by promoting apoptosis.
6. Clinical Trials
Clinical trials focus on the biochemical, pathological, and molecular events for controlling the cancers among the high-risk population. The studies showed that there is a very small number of clinical trials on natural compounds because most of the studies are still investigating in vivo and in vitro studies to check the proper mechanism of action of the natural compounds. However, some clinical trials of natural compounds used for the treatment of BC are under clinical investigation. The details of each clinical trial, i.e., the length of the clinical trial, the clinical phase, the number of participants, and the trial number, are shown in Table 2 [157,158].
Table 2.
The representation of clinical trial studies of some natural compounds against BC.
| Natural Compound | Nature | NCT No. | Number of Participants | Disease Type | Dose/Concentration | Duration of Trial | Trial Phase | Study Location | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Curcumin | Polyphenol | NCT01740323 | 30 | BC | 8 g | 8 weeks | Phase II | USA | www.clinicaltrials.gov (accessed on 27 April 2022) |
| Resveratrol | Stilbenoid | --- | 39 | Metastatic BC | 5 or 50 mg | 3 months | Phase I | USA | [157,158] |
| Berberine | Alkaloids | NCT03281096 | 1000 | Invasive BC, colorectal | 300 mg | 4 weeks | Phase II and III | China | www.clinicaltrials.gov (accessed on 27 April 2022) |
| Curcumin (iv) + Paclitaxel | Polyphenol | NCT03072992 | 75 | Metastatic BC | 300 mg | 12 weeks | Phase II | Armenia | www.clinicaltrials.gov (accessed on 27 April 2022) |
| Quercetin | Carotenoids | --- | 42 | Advanced BC | 200 mg | 2 weeks | Randomized crossover clinical trial | UK | [159,160] |
| Resveratrol | Stilbenoid | NCT04266353 | 50 | TNBC | 150 mg | 2–4 weeks | Suspended (due to COVID-19) | California | www.clinicaltrials.gov (accessed on 27 April 2022) |
| Curcumin | Polyphenol | NCT01975363 | 30 | BC (obese women) | 100 mg | 3 months | Pilot trial | USA | www.clinicaltrials.gov (accessed on 27 April 2022) |
| Genistein | Isoflavon | NCT00099008 | 30 | BC | 10 or 20 mg | 84 Days | Completed | US | www.clinicaltrials.gov (accessed on 27 April 2022) |
7. Current Challenges and Future Perspectives
Poor stability and low bioavailability of natural products decrease their therapeutic potential against BC treatment. These challenges could be tackled through a nanotechnology approach by employing the dendrimers to easily and more efficiently improve their delivery for BC therapy. For example, CUR is an excellent source of natural products, whereby nanosuspensions of centrosomes are made for targeting BCs. Further research is needed to improve the functionality of nano-formulated natural products for TNBC [15,161].
Recent challenges for BC treatment in the modern area mainly include drug resistance and high chances of recurrence. For example, it is evident that patients with early BC have a 30% risk of developing metastasis. There is an urgent need to develop novel therapeutics to control the high risk among women [162]. Traditional treatments for the treatment of BC are causing adverse health effects due to increasing drug resistance. For example, there is a high risk of skin cancer in those patients taking BC chemotherapy, which is inevitable. In order to overcome the adverse effects or drug resistance, natural products are reliable sources, as compared to traditional therapies that showed anticancer potentials against BCs [163]. Currently, the synergistic effects of natural compounds have significantly controlled the BC risk. These natural compounds are alternatives to traditional therapies for overcoming drug resistance for BC treatment (see Table 3). However, natural products are also successfully studied in tumor cells of other cancers [164,165,166].
Table 3.
The natural compounds that overcome drug resistance in BC.
| Natural Compound | Nature | Dose/Concentration | Target | Cell Mode | Chemo Drug | IDS (x-Fold) | Reference |
|---|---|---|---|---|---|---|---|
| Ginsenosides | Glycosides | 40 µM | MCF | ADM | Doxorubicin | 29.2 | [167,168] |
| Baicalin | Flavonoid | 150 µg/mL | MDR1 and MRP1 | MCF7/ADR | Doxorubicin | 6.5 | [169,170] |
| Quercetin | Carotenoids | 50 µM | MDR1 and MRP1 | MCF7/ADM | Cisplatin | 3.5 | [171,172,173] |
| Berberine | Alkaloids | 20 µM | MDR1 | MDR1 | Vincristine | 3.2 | [174,175] |
| Ginsenoside Rb1 | Glycosides | 80 µM | MDR1 | MCF-7/ADR | Vincristine | 2.5 | [176,177] |
| Apigenin | Flavone | 13 µM | MDR1 | HCT | 5-FU | 4.9 | [169,173] |
| Curcumin | Alkaloids | 25 µM | MDR1 | Various | Various | 4.5 | [178,179] |
| Oridonin | Diterpenoid | 3 µM | MDR1 | MCF7/ADM | Various | 8.5 | [180,181] |
| Ginsenoside Rg3 | Glycosides | 30 µg/mL | MDR1 and MRP1 | MCF7/ADR | Various | 8.5 | [182] |
Note: HCT: hematocrit; ADR: adverse drug reactions; MCF-7: Michigan Cancer Foundation 7; IDS: increase in drug sensitivity; MDR1: multidrug resistance 1; MRP1: multidrug resistance protein 1.
Patients under chemotherapy develop a high risk of chemoresistance caused by ATP-binding cassettes that delay the activity of anticancer drugs [183]. Several natural compounds have been isolated which helped in overcoming the multidrug resistance. For example, β-elemene showed its usefulness against MCF-7 cells [184]. 3,3′-diindolylmethane is also used as an anticancer agent against multidrug resistance. Scientists are also working on the mechanism of action of newly discovered natural compounds that can be used against multi-drug resistance [185].
TNBC has become a global health concern as a limited number of treatments are available. There is a need to isolate novel natural compounds as alternatives for TNBC [186]. For example, CUR and RSVL have been used as effective therapeutic agents for TNBC. These compounds inhibited the proliferation of tumors and induced apoptosis for cell survival [187]. Another prosing natural compound, carnosol, showed the anticancer activity against MDA-MB-231 by arresting the G2 phase of the cell cycle [188].
Several studies showed inconsistencies in the natural products for in vitro and in vivo experimental studies that lower the bioavailability in the experimental models. However, some epidemiological studies on the use of natural compounds for BC have surprising and contradictory effects. For example, soy products can cause serious cellular toxicities and hormonal imbalances [189]. It is necessary to authenticate their anticancer activities, toxicities, and effectiveness prior to using them for BC treatment [76]. Another challenge of using natural compounds is the lack of target specificity, as these compounds have multi-targeted potentials in triggering several signaling pathways rather than specific ones [190].
Oral delivery of natural products remained a major challenge in cancer therapy, and recent attempts have been made in order to improve the oral delivery of ginsengs through nanotechnology. Combining polylactic co-glycolic acid (PLGA) nanoparticles with ginsengs has improved its oral absorption and maximized its potential against MCF-7 cell lines. The nanotechnology approach has been applied to improve the oral absorption of 25-OCH3-PPD via polylactic co-glycolic acid (PLGA). Diameter less than 43 nm showed stability at a pH range of 6.5–7.4, which the FDA has approved for use in therapeutic devices. This approach is helpful for the efficient delivery of poorly soluble natural products that ultimately enhance their use in BC therapy [191]. Another nano-emulsion system coated/fabricated with phospholipids was designed to improve the bioavailability of 25-OCH3-PPD [192]. Cancer stem cells are a major cause of tumor growth initiation and the recurrence of BC due to developing drug resistance. Therefore, targeting the cancer stem cells is crucial for overcoming drug resistance. Natural products, such as therapeutic agents, have become an essential strategy for BC.
Most efficient and reliable therapies are urgently needed for future research of TNBC subtypes for discovering novel biomarkers in order to identify malignant tumor subtypes among high-risk populations. Many natural products from crude extracts have been discovered every year, paving the screening of novel drugs. Many of the plants found in the deep sea are a great source of unknown natural products. This isolation could be a hot spot for the discovery of anticancer-based compounds. Most research carried out for TNBC in vitro studies still lacks in vivo experiments that could explore the mechanism of newly discovered natural compounds [193].
8. Conclusions
Recently, attention toward natural products for BC treatment has increased. Natural products have been tested in vivo and in vitro studies for their safe use against BC. Bioactive compounds derived from natural products are the main source of targeting tumor invasion, inhibiting cell-proliferation and cyclins by arresting the cell cycle at M2/S phase, activating caspases, and inducing apoptosis in BC cells. Natural products also showed anticancer and antitumor activities against BC by suppressing NF-κB, PI3K/Akt/mTOR, and MAPK/ERK. Natural products and their respective bioactive compounds act synergistically to overcome drug resistance. Natural products possess low toxicity and are efficiently delivered to the targeted cells; thus, they are helpful for reducing the high risk of BC. The bioavailability issues of natural products can be solved through nanocarriers that are effective for delivering the natural products to the targeted tissues or site. However, several marine plants exhibit a variety of natural products. In the future, screening of more bioactive compounds from marine plants will be helpful to treat and prevent BC. In addition, the efficacy of natural products needs to explore at a clinical level.
Acknowledgments
We thank the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and the Kaohsiung Medical University Research Center Grant (KMU-TC109A01-1).
Abbreviations
| Abbreviation | Explanation |
| ADAM-10 | A disintegrin and metalloproteinase domain-containing protein 10 |
| ADR | Adverse drug reactions |
| Akt | Protein kinase B |
| AP | Apigenin |
| BA | Baicalin |
| Bad | Bcl-2 antagonist of cell death |
| Bax | Bcl-2-associated X protein |
| BBR | Berberine; BC: breast cancer |
| Bcl-2 | B-cell lymphoma 2 |
| CDK | Cyclin-dependent kinase |
| Chk | Checkpoint kinase |
| CUR | Curcumin |
| DATS | Diallyl trisulfide |
| EGCG | Epigallocatechin-3-gallate |
| EGF(R) | Epidermal growth factor (receptor) |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinase |
| 5-FU | 5-Fluorouracil |
| HCT | Hematocrit |
| IKK | IκB kinase |
| IL-1β | Interleukin 1 beta |
| IDS | Increase in drug sensitivity |
| JNK | C-Jun N-terminal kinase |
| Lin A | Lineariifolianoid A |
| MAML | Mastermind-like protein |
| MDM2 | Mouse double minute 2 homolog |
| MEK | Mitogen-activated protein kinase kinase |
| MCF-7 | Michigan Cancer Foundation 7 |
| MDR1 | Multidrug resistance 1 |
| MG | Methylglyoxal |
| MMP | Matrix metalloprotein |
| mTOR | Mammalian target of rapamycin |
| mTORC-2 | mTOR Complex 2 |
| MRP1 | Multidrug resistance protein 1 |
| NFAT1 | Nuclear factor of activated T cells 1 |
| NF-κB | Nuclear factor kappa B |
| NICD | Notch receptor intracellular domain |
| p53 | Inducible gene 3 |
| PARP | Poly (ADP-ribose) polymerase |
| PDK1/2 | 3-phosphoinositide dependent kinase-1/2 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP-3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PPD | Protopanaxadiol |
| PTEN | Phosphatase and tensin homolog |
| RBP-Jκ | Recombinant signal binding protein for Immunoglobulin kappa J region |
| RSVL | Resveratrol |
| SAL | Salinomycin |
| TNBC | Triple-negative breast cancer |
| VEGF | Vascular endothelial growth factor |
| VOA | Voacamine |
| VS | Verminoside |
| WG | Wogonin |
Author Contributions
Conceptualization, M.N. and A.H.; methodology, M.N. and A.H.; software, A.H. and M.M.A. (Muhammad Masood Ahmed); validation, M.O.I., M.F., and H.K.; formal analysis, M.N. and M.M.A. (Muhammad Moeen Aadil); investigation, A.H.; resources, A.H.; data curation, M.N. and A.H.; writing—original draft preparation, M.I.J., H.K., M.M.A. (Muhammad Masood Ahmed), M.M.A. (Muhammad Moeen Aadil), and M.N.; writing—review and editing, A.H., M.F., M.O.I., and W.-C.T.; visualization, W.-C.T. and M.I.J.; supervision, A.H. and W.-C.T.; project administration, W.-C.T.; funding acquisition, W.-C.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the NSYSU-KMU Joint Research Project (grant number: NSYSUKMU 111-I06) and the Kaohsiung Medical University Research Foundation (grant number: KMU-M111009).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jha V., Devkar S., Gharat K., Kasbe S., Matharoo D.K., Pendse S., Bhosale A., Bhargava A. Screening of Phytochemicals as Potential Inhibitors of Breast Cancer using Structure Based Multitargeted Molecular Docking Analysis. Phytomed. Plus. 2022;2:100227. doi: 10.1016/j.phyplu.2022.100227. [DOI] [Google Scholar]
- 2.Nassif A.B., Talib M.A., Nasir Q., Afadar Y., Elgendy O. Breast cancer detection using artificial intelligence techniques: A systematic literature review. Artif. Intell. Med. 2022;127:102276. doi: 10.1016/j.artmed.2022.102276. [DOI] [PubMed] [Google Scholar]
- 3.Rieder V., Salama M., Glöckner L., Muhr D., Berger A., Tea M.K., Pfeiler G., Rappaport-Fuerhauser C., Gschwantler-Kaulich D., Weingartshofer S. Effect of lifestyle and reproductive factors on the onset of breast cancer in female BRCA 1 and 2 mutation carriers. Mol. Genet. Genom. Med. 2016;4:172–177. doi: 10.1002/mgg3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sisti J.S., Collins L.C., Beck A.H., Tamimi R.M., Rosner B.A., Eliassen A.H. Reproductive risk factors in relation to molecular subtypes of breast cancer: Results from the nurses’ health studies. Int. J. Cancer. 2016;138:2346–2356. doi: 10.1002/ijc.29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma H., Ursin G., Xu X., Lee E., Togawa K., Duan L., Lu Y., Malone K.E., Marchbanks P.A., McDonald J.A. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: A pooled analysis. Breast Cancer Res. 2017;19:6. doi: 10.1186/s13058-016-0799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campeau P.M., Foulkes W.D., Tischkowitz M.D. Hereditary breast cancer: New genetic developments, new therapeutic avenues. Hum. Genet. 2008;124:31–42. doi: 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 7.Moulder S., Hortobagyi G. Advances in the treatment of breast cancer. Clin. Pharmacol. Ther. 2008;83:26–36. doi: 10.1038/sj.clpt.6100449. [DOI] [PubMed] [Google Scholar]
- 8.Cazzaniga M., Bonanni B. Breast cancer chemoprevention: Old and new approaches. J. Biomed. Biotechnol. 2012;2012:985620. doi: 10.1155/2012/985620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocanu M.-M., Nagy P., Szöllősi J. Chemoprevention of breast cancer by dietary polyphenols. Molecules. 2015;20:22578–22620. doi: 10.3390/molecules201219864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abotaleb M., Kubatka P., Caprnda M., Varghese E., Zolakova B., Zubor P., Opatrilova R., Kruzliak P., Stefanicka P., Büsselberg D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018;101:458–477. doi: 10.1016/j.biopha.2018.02.108. [DOI] [PubMed] [Google Scholar]
- 11.Akram M., Iqbal M., Daniyal M., Khan A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017;50:33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSantis C., Ma J., Bryan L., Jemal A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Wu Y., Cong W., Hu M., Li X., Zhou C. Experience of women with breast cancer undergoing chemotherapy: A systematic review of qualitative research. Qual. Life Res. 2021;30:1249–1265. doi: 10.1007/s11136-020-02754-5. [DOI] [PubMed] [Google Scholar]
- 14.Sartaj A., Baboota S., Ali J. Assessment of Combination Approaches of Phytoconstituents with Chemotherapy for the Treatment of Breast Cancer: A Systematic Review. Curr. Pharm. Des. 2021;27:4630–4648. doi: 10.2174/1381612827666210902155752. [DOI] [PubMed] [Google Scholar]
- 15.Yap K.M., Sekar M., Fuloria S., Wu Y.S., Gan S.H., Rani N.N.I.M., Subramaniyan V., Kokare C., Lum P.T., Begum M.Y. Drug delivery of natural products through nanocarriers for effective breast cancer therapy: A comprehensive review of literature. Int. J. Nanomed. 2021;16:7891–7941. doi: 10.2147/IJN.S328135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y., Tao Q., Zhou F., Si Y., Fu R., Xu B., Xu J., Li X., Chen B. The relationship between dairy products intake and breast cancer incidence: A meta-analysis of observational studies. BMC Cancer. 2021;21:1109. doi: 10.1186/s12885-021-08854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farvid M.S., Chen W.Y., Michels K.B., Cho E., Willett W.C., Eliassen A.H. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ. 2016;353:i2343. doi: 10.1136/bmj.i2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nechuta S.J., Caan B.J., Chen W.Y., Lu W., Chen Z., Kwan M.L., Flatt S.W., Zheng Y., Zheng W., Pierce J.P. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012;96:123–132. doi: 10.3945/ajcn.112.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Z.-D., Liu X.-P., Zhao W.-J., Dong Q., Li F.-N., Wang H.-B., Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014;7:2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 20.Bonofiglio D., Giordano C., De Amicis F., Lanzino M., Ando S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016;16:596–604. doi: 10.2174/1389557515666150709110959. [DOI] [PubMed] [Google Scholar]
- 21.Imran M., Aslam Gondal T., Atif M., Shahbaz M., Batool Qaisarani T., Hanif Mughal M., Salehi B., Martorell M., Sharifi-Rad J. Apigenin as an anticancer agent. Phytother. Res. 2020;34:1812–1828. doi: 10.1002/ptr.6647. [DOI] [PubMed] [Google Scholar]
- 22.Imran M., Saeed F., Gilani S.A., Shariati M.A., Imran A., Afzaal M., Atif M., Tufail T., Anjum F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021;9:3–16. doi: 10.1002/fsn3.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia H., Liu M., Wang X., Jiang Q., Wang S., Santhanam R.K., Lv C., Zhao Q., Lu J. Cimigenoside functions as a novel γ-secretase inhibitor and inhibits the proliferation or metastasis of human breast cancer cells by γ-secretase/Notch axis. Pharmacol. Res. 2021;169:105686. doi: 10.1016/j.phrs.2021.105686. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh C.-J., Kuo P.-L., Hsu Y.-C., Huang Y.-F., Tsai E.-M., Hsu Y.-L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic. Biol. Med. 2014;67:159–170. doi: 10.1016/j.freeradbiomed.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 25.He L., Wang X., Ma Q., Zhao W., Jia Y., Dong G., Zhu Y., Jia X., Tong Z. Glycyrrhizin inhibits the invasion and metastasis of breast cancer cells via upregulation of expressions of miR-200c and e-cadherin. Trop. J. Pharm. Res. 2020;19:1807–1813. doi: 10.4314/tjpr.v19i9.2. [DOI] [Google Scholar]
- 26.Chung H., Choi H.S., Seo E.-K., Kang D.-H., Oh E.-S. Baicalin and baicalein inhibit transforming growth factor-β1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem. Biophys. Res. Commun. 2015;458:707–713. doi: 10.1016/j.bbrc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 27.Akiel M.A., Alshehri O.Y., Aljihani S.A., Almuaysib A., Bader A., Al-Asmari A.I., Alamri H.S., Alrfaei B.M., Halwani M.A. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci. 2022;29:816–821. doi: 10.1016/j.sjbs.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cansaran-Duman D., Yangin S., Çolak B. The role of vulpinic acid as a natural compound in the regulation of breast cancer-associated miRNAs. Biol. Res. 2021;54:37. doi: 10.1186/s40659-021-00360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Gaafary M., Saber F.R., Mahrous E.A., Ashour R.M., Okba M.M., Jin L., Lang S.J., Schmiech M., Simmet T., Syrovets T. The phloroglucinol calcitrinone A, a novel mitochondria-targeting agent, induces cell death in breast cancer cells. Food Chem. Toxicol. 2022;162:112896. doi: 10.1016/j.fct.2022.112896. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S., Pal A., Ray M. Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition. Biomed. Pharmacother. 2019;114:108855. doi: 10.1016/j.biopha.2019.108855. [DOI] [PubMed] [Google Scholar]
- 31.Schuster C., Wolpert N., Moustaid-Moussa N., Gollahon L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants. 2022;11:591. doi: 10.3390/antiox11030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrijauskaite K., Wargovich M.J. Role of natural products in breast cancer related symptomology: Targeting chronic inflammation. Semin. Cancer Biol. 2020;380:370–378. doi: 10.1016/j.semcancer.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.-H., Hwang K.-A., Choi K.-C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016;28:70–82. doi: 10.1016/j.jnutbio.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Zuo Y., Zhang C.-Z., Ren Q., Chen Y., Li X., Yang J.-R., Li H.-X., Tang W.-T., Hing-Man H., Sun C. Activation of mitochondrial-associated apoptosis signaling pathway and inhibition of PI3K/Akt/mTOR signaling pathway by voacamine suppress breast cancer progression. Phytomedicine. 2022;99:154015. doi: 10.1016/j.phymed.2022.154015. [DOI] [PubMed] [Google Scholar]
- 35.Son Y., Cheong Y.-K., Kim N.-H., Chung H.-T., Kang D.G., Pae H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Zhang X., Qin J.-J., Voruganti S., Nag S.A., Wang M.-H., Wang H., Zhang R. Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. PLoS ONE. 2012;7:e41586. doi: 10.1371/journal.pone.0041586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y., Spezia M., Huang S., Yuan C., Zeng Z., Zhang L., Ji X., Liu W., Huang B., Luo W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis-Filho J.S., Pusztai L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet. 2011;378:1812–1823. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 39.Hudis C.A., Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16:1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 40.Bhardwaj P., Brown K.A. Obese adipose tissue as a driver of breast cancer growth and development: Update and emerging evidence. Front. Oncol. 2021;11:638918. doi: 10.3389/fonc.2021.638918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans D.G., van Veen E.M., Byers H.J., Evans S.J., Burghel G.J., Woodward E.R., Harkness E.F., Eccles D.M., Greville-Haygate S.L., Ellingford J.M. High likelihood of actionable pathogenic variant detection in breast cancer genes in women with very early onset breast cancer. J. Med. Genet. 2022;59:115–121. doi: 10.1136/jmedgenet-2020-107347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed S., Thomas G., Ghoussaini M., Healey C.S., Humphreys M.K., Platte R., Morrison J., Maranian M., Pooley K.A., Luben R. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23. 2. Nat. Genet. 2009;41:585–590. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh V., Kumar K., Purohit D., Verma R., Pandey P., Bhatia S., Malik V., Mittal V., Rahman M.H., Albadrani G.M. Exploration of therapeutic applicability and different signaling mechanism of various phytopharmacological agents for treatment of breast cancer. Biomed. Pharmacother. 2021;139:111584. doi: 10.1016/j.biopha.2021.111584. [DOI] [PubMed] [Google Scholar]
- 44.Varghese E., Samuel S.M., Abotaleb M., Cheema S., Mamtani R., Büsselberg D. The “yin and yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers. 2018;10:346. doi: 10.3390/cancers10100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marino N., German R., Rao X., Simpson E., Liu S., Wan J., Liu Y., Sandusky G., Jacobsen M., Stoval M. Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer. 2020;6:50. doi: 10.1038/s41523-020-00191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 47.Suman S., Sharma P.K., Rai G., Mishra S., Arora D., Gupta P., Shukla Y. Current perspectives of molecular pathways involved in chronic inflammation-mediated breast cancer. Biochem. Biophys. Res. Commun. 2016;472:401–409. doi: 10.1016/j.bbrc.2015.10.133. [DOI] [PubMed] [Google Scholar]
- 48.Huang A., Cao S., Tang L. The tumor microenvironment and inflammatory breast cancer. J. Cancer. 2017;8:1884. doi: 10.7150/jca.17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puar Y.R., Shanmugam M.K., Fan L., Arfuso F., Sethi G., Tergaonkar V. Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines. 2018;6:82. doi: 10.3390/biomedicines6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oshi M., Newman S., Tokumaru Y., Yan L., Matsuyama R., Endo I., Nagahashi M., Takabe K. Intra-tumoral angiogenesis is associated with inflammation, immune reaction and metastatic recurrence in breast cancer. Int. J. Mol. Sci. 2020;21:6708. doi: 10.3390/ijms21186708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michels N., van Aart C., Morisse J., Mullee A., Huybrechts I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. Hematol. 2021;157:103177. doi: 10.1016/j.critrevonc.2020.103177. [DOI] [PubMed] [Google Scholar]
- 52.Alzahrani A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Verret B., Cortes J., Bachelot T., Andre F., Arnedos M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. 2019;30:x12–x20. doi: 10.1093/annonc/mdz381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dandoti S. Mechanisms adopted by cancer cells to escape apoptosis–A review. Biocell. 2021;45:863–884. doi: 10.32604/biocell.2021.013993. [DOI] [Google Scholar]
- 55.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front. Pharmacol. 2021;12:628690. doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dornan G.L., Burke J.E. Molecular mechanisms of human disease mediated by oncogenic and primary immunodeficiency mutations in class IA phosphoinositide 3-kinases. Front. Immunol. 2018;9:575. doi: 10.3389/fimmu.2018.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L., Meng T., Zheng X., Liu Y., Hao R., Yan Y., Chen S., You H., Xing J., Dong Y. Transgelin 2 promotes paclitaxel resistance, migration, and invasion of breast cancer by directly interacting with PTEN and activating PI3K/Akt/GSK-3β pathway. Mol. Cancer Ther. 2019;18:2457–2468. doi: 10.1158/1535-7163.MCT-19-0261. [DOI] [PubMed] [Google Scholar]
- 59.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cidado J., Park B.H. Targeting the PI3K/Akt/mTOR pathway for breast cancer therapy. J. Mammary Gland Biol. Neoplasia. 2012;17:205–216. doi: 10.1007/s10911-012-9264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong Y., Fan D. Ginsenoside Rk1 induces cell cycle arrest and apoptosis in MDA-MB-231 triple negative breast cancer cells. Toxicology. 2019;418:22–31. doi: 10.1016/j.tox.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Fan D. Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model. Food Funct. 2018;9:5513–5527. doi: 10.1039/C8FO01122B. [DOI] [PubMed] [Google Scholar]
- 63.Bing Z., Shi-ke Y., Chun-ling B., Ning L. Research progress of natural products targeting tumor angiogenesis. Nat. Prod. Res. Dev. 2021;33:510–520. [Google Scholar]
- 64.Condello M., Pellegrini E., Multari G., Gallo F.R., Meschini S. Voacamine: Alkaloid with its essential dimeric units to reverse tumor multidrug resistance. Toxicol. Vitr. 2020;65:104819. doi: 10.1016/j.tiv.2020.104819. [DOI] [PubMed] [Google Scholar]
- 65.Grynkiewicz G., Demchuk O.M. New perspectives for fisetin. Front. Chem. 2019;7:697. doi: 10.3389/fchem.2019.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun X., Ma X., Li Q., Yang Y., Xu X., Sun J., Yu M., Cao K., Yang L., Yang G. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018;42:811–820. doi: 10.3892/ijmm.2018.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirza-Aghazadeh-Attari M., Ekrami E.M., Aghdas S.A.M., Mihanfar A., Hallaj S., Yousefi B., Safa A., Majidinia M. Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: Implication for cancer therapy. Life Sci. 2020;255:117481. doi: 10.1016/j.lfs.2020.117481. [DOI] [PubMed] [Google Scholar]
- 68.Sharifi-Rad J., Herrera-Bravo J., Salazar L.A., Shaheen S., Ayatollahi S.A., Kobarfard F., Imran M., Imran A., Custódio L., López M.D. The therapeutic potential of wogonin observed in preclinical studies. Evid. Based Complement. Altern. Med. 2021;2021:9935451. doi: 10.1155/2021/9935451. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Gharari Z., Bagheri K., Khodaeiaminjan M., Sharafi A. Potential therapeutic effects and bioavailability of wogonin, the flavone of Baikal skullcap. J. Nutri. Med. Diet Care. 2019;5:039. [Google Scholar]
- 70.Zhao K., Song X., Huang Y., Yao J., Zhou M., Li Z., You Q., Guo Q., Lu N. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur. J. Pharmacol. 2014;737:57–69. doi: 10.1016/j.ejphar.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Ganesan K., Xu B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2017;19:13. doi: 10.3390/ijms19010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganesan K., Xu B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017;1401:102–113. doi: 10.1111/nyas.13446. [DOI] [PubMed] [Google Scholar]
- 73.Pons D.G., Vilanova-Llompart J., Gaya-Bover A., Alorda-Clara M., Oliver J., Roca P., Sastre-Serra J. The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 2019;70:941–949. doi: 10.1080/09637486.2019.1597025. [DOI] [PubMed] [Google Scholar]
- 74.Seo H.S., Choi H.S., Choi H.S., Choi Y.K., Um J.-Y., Choi I., Shin Y.C., Ko S.-G. Phytoestrogens induce apoptosis via extrinsic pathway, inhibiting nuclear factor-κB signaling in HER2-overexpressing breast cancer cells. Anticancer. Res. 2011;31:3301–3313. [PubMed] [Google Scholar]
- 75.Pan H., Zhou W., He W., Liu X., Ding Q., Ling L., Zha X., Wang S. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 2012;30:337–343. doi: 10.3892/ijmm.2012.990. [DOI] [PubMed] [Google Scholar]
- 76.Ganesan K., Du B., Chen J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol. Res. 2022;178:105974. doi: 10.1016/j.phrs.2021.105974. [DOI] [PubMed] [Google Scholar]
- 77.Bauer D., Mazzio E., Soliman K.F. Whole transcriptomic analysis of apigenin on TNFα immuno-activated MDA-MB-231 breast cancer cells. Cancer Genom. Proteom. 2019;16:421–431. doi: 10.21873/cgp.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim B.-M., Kim D.-H., Park J.-H., Surh Y.-J., Na H.-K. Ginsenoside Rg3 inhibits constitutive activation of NF-κB signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as potential upstream targets. J. Cancer Prev. 2014;19:23–30. doi: 10.15430/JCP.2014.19.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozbay T., Nahta R. Delphinidin inhibits HER2 and Erk1/2 signaling and suppresses growth of HER2-overexpressing and triple negative breast cancer cell lines. Breast Cancer Basic Clin. Res. 2011;5:143–154. doi: 10.4137/BCBCR.S7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imran M., Rauf A., Khan I.A., Shahbaz M., Qaisrani T.B., Fatmawati S., Abu-Izneid T., Imran A., Rahman K.U., Gondal T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018;106:390–402. doi: 10.1016/j.biopha.2018.06.159. [DOI] [PubMed] [Google Scholar]
- 81.Peng B., He R., Xu Q., Yang Y., Hu Q., Hou H., Liu X., Li J. Ginsenoside 20 (S)-protopanaxadiol inhibits triple-negative breast cancer metastasis in vivo by targeting EGFR-mediated MAPK pathway. Pharmacol. Res. 2019;142:1–13. doi: 10.1016/j.phrs.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Zhu L., Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019;27:629. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heiden M.G.V. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 84.Azevedo C., Correia-Branco A., Araújo J.R., Guimaraes J.T., Keating E., Martel F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer. 2015;67:504–513. doi: 10.1080/01635581.2015.1002625. [DOI] [PubMed] [Google Scholar]
- 85.Hung H. Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J. Cell. Physiol. 2004;198:197–208. doi: 10.1002/jcp.10398. [DOI] [PubMed] [Google Scholar]
- 86.Xu Y., Shu B., Tian Y., Wang G., Wang Y., Wang J., Dong Y. Oleanolic acid induces osteosarcoma cell apoptosis by inhibition of Notch signaling. Mol. Carcinog. 2018;57:896–902. doi: 10.1002/mc.22810. [DOI] [PubMed] [Google Scholar]
- 87.Kiesel V.A., Stan S.D. Modulation of Notch Signaling Pathway by Bioactive Dietary Agents. Int. J. Mol. Sci. 2022;23:3532. doi: 10.3390/ijms23073532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X., Zhang C.-T., Ma W., Xie X., Huang Q. Oridonin: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2021;12:1642. doi: 10.3389/fphar.2021.645824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia S., Zhang X., Li C., Guan H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017;25:638–643. doi: 10.1016/j.jsps.2017.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huyen C.T.T., Luyen B.T.T., Khan G.J., Oanh H.V., Hung T.M., Li H.-J., Li P. Chemical constituents from Cimicifuga dahurica and their anti-proliferative effects on MCF-7 breast cancer cells. Molecules. 2018;23:1083. doi: 10.3390/molecules23051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Einbond L.S., Wen-Cai Y., He K., Wu H.-A., Cruz E., Roller M., Kronenberg F. Growth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cells. Phytomedicine. 2008;15:504–511. doi: 10.1016/j.phymed.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rickels R., Shilatifard A. Enhancer logic and mechanics in development and disease. Trends Cell Biol. 2018;28:608–630. doi: 10.1016/j.tcb.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Vassilev L.T. MDM2 inhibitors for cancer therapy. Trends Mol. Med. 2007;13:23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Tan B.X., Khoo K.H., Lim T.M., Lane D.P. High Mdm4 levels suppress p53 activity and enhance its half-life in acute myeloid leukaemia. Oncotarget. 2014;5:933–943. doi: 10.18632/oncotarget.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin J.-J., Sarkar S., Voruganti S., Agarwal R., Wang W., Zhang R. Identification of lineariifolianoid A as a novel dual NFAT1 and MDM2 inhibitor for human cancer therapy. J. Biomed. Res. 2016;30:322–333. doi: 10.7555/JBR.30.20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng A., Liang X., Zhu S., Liu C., Luo X., Zhang Q., Song L. Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front. Pharmacol. 2020;11:879. doi: 10.3389/fphar.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin Y., Huynh D.T.N., Nguyen T.L.L., Jeon H., Heo K.-S. Therapeutic effects of ginsenosides on breast cancer growth and metastasis. Arch. Pharmacal Res. 2020;43:773–787. doi: 10.1007/s12272-020-01265-8. [DOI] [PubMed] [Google Scholar]
- 98.Qin J.-J., Jin H.-Z., Huang Y., Zhang S.-D., Shan L., Voruganti S., Nag S., Wang W., Zhang W.-D., Zhang R. Selective cytotoxicity, inhibition of cell cycle progression, and induction of apoptosis in human breast cancer cells by sesquiterpenoids from Inula lineariifolia Turcz. Eur. J. Med. Chem. 2013;68:473–481. doi: 10.1016/j.ejmech.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin J.-J., Huang Y., Wang D., Cheng X.-R., Zeng Q., Zhang S.-D., Hu Z.-L., Jin H.-Z., Zhang W.-D. Lineariifolianoids A–D, rare unsymmetrical sesquiterpenoid dimers comprised of xanthane and guaiane framework units from Inula lineariifolia. RSC Adv. 2012;2:1307–1309. doi: 10.1039/c2ra01014c. [DOI] [Google Scholar]
- 100.Wang W., Zafar A., Rajaei M., Zhang R. Two birds with one stone: NFAT1-MDM2 dual inhibitors for cancer therapy. Cells. 2020;9:1176. doi: 10.3390/cells9051176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C., Ryu H.W., Kim S., Kim M., Oh S.-R., Ahn K.-S., Park J. Verminoside from Pseudolysimachion rotundum var. subintegrum sensitizes cisplatin-resistant cancer cells and suppresses metastatic growth of human breast cancer. Sci. Rep. 2020;10:20337. doi: 10.1038/s41598-020-77401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y., Zou T., Wang S., Chen H., Su D., Fu X., Zhang Q., Kang X. Genistein-induced differentiation of breast cancer stem/progenitor cells through a paracrine mechanism. Int. J. Oncol. 2016;48:1063–1072. doi: 10.3892/ijo.2016.3351. [DOI] [PubMed] [Google Scholar]
- 103.Tarkowski M., Kokocińska M., Latocha M. Genistein in chemoprevention and treatment. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2013;34:54–57. [PubMed] [Google Scholar]
- 104.Wang K., Zhang C., Bao J., Jia X., Liang Y., Wang X., Chen M., Su H., Li P., Wan J.-B. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci. Rep. 2016;6:26064. doi: 10.1038/srep26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kashyap A., Umar S.M., Dev A., Jr., Mendiratta M., Prasad C.P. In vitro anticancer efficacy of a polyphenolic combination of Quercetin, Curcumin, and Berberine in triple negative breast cancer (TNBC) cells. Phytomed. Plus. 2022;2:100265. doi: 10.1016/j.phyplu.2022.100265. [DOI] [Google Scholar]
- 106.Shehatta N.H., Okda T.M., Omran G.A., Abd-Alhaseeb M.M. Baicalin; a promising chemopreventive agent, enhances the antitumor effect of 5-FU against breast cancer and inhibits tumor growth and angiogenesis in Ehrlich solid tumor. Biomed. Pharmacother. 2022;146:112599. doi: 10.1016/j.biopha.2021.112599. [DOI] [PubMed] [Google Scholar]
- 107.Rai G., Suman S., Mishra S., Shukla Y. Evaluation of growth inhibitory response of Resveratrol and Salinomycin combinations against triple negative breast cancer cells. Biomed. Pharmacother. 2017;89:1142–1151. doi: 10.1016/j.biopha.2017.02.110. [DOI] [PubMed] [Google Scholar]
- 108.Chimento A., Santarsiero A., Iacopetta D., Ceramella J., De Luca A., Infantino V., Parisi O.I., Avena P., Bonomo M.G., Saturnino C. A phenylacetamide resveratrol derivative exerts inhibitory effects on breast cancer cell growth. Int. J. Mol. Sci. 2021;22:5255. doi: 10.3390/ijms22105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang J., Li H., Ren G. Epithelial-mesenchymal transition and drug resistance in breast cancer. Int. J. Oncol. 2015;47:840–848. doi: 10.3892/ijo.2015.3084. [DOI] [PubMed] [Google Scholar]
- 110.Linn S.C., Pinedo H.M., van Ark-Otte J., van Der Valk P., Hoekman K., Honkoop A.H., Vermorken J.B., Giaccone G. Expression of drug resistance proteins in breast cancer, in relation to chemotherapy. Int. J. Cancer. 1997;71:787–795. doi: 10.1002/(SICI)1097-0215(19970529)71:5<787::AID-IJC16>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 111.Tang Y., Wang Y., Kiani M.F., Wang B. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin. Breast Cancer. 2016;16:335–343. doi: 10.1016/j.clbc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 112.Noel B., Singh S.K., Lillard J.W., Jr., Singh R. Role of natural compounds in preventing and treating breast cancer. Front. Biosci. 2020;12:137–160. doi: 10.2741/s544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghosh S., Banerjee S., Sil P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 114.Kumar A., Chopra K., Mukherjee M., Pottabathini R., Dhull D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 115.Park W., Amin A.R., Chen Z.G., Shin D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaboli P.J., Rahmat A., Ismail P., Ling K.-H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014;740:584–595. doi: 10.1016/j.ejphar.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 117.Batool A., Hazafa A., Ahmad S., Khan H.A., Abideen H.M., Zafar A., Bilal M., Iqbal H.M. Treatment of lymphomas via regulating the Signal transduction pathways by natural therapeutic approaches: A review. Leuk. Res. 2021;104:106554. doi: 10.1016/j.leukres.2021.106554. [DOI] [PubMed] [Google Scholar]
- 118.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Priyadarsini K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Czarny P., Pawlowska E., Bialkowska-Warzecha J., Kaarniranta K., Blasiak J. Autophagy in DNA damage response. Int. J. Mol. Sci. 2015;16:2641–2662. doi: 10.3390/ijms16022641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen C., Yu Z., Li Y., Fichna J., Storr M. Effects of berberine in the gastrointestinal tract—A review of actions and therapeutic implications. Am. J. Chin. Med. 2014;42:1053–1070. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 122.Umar S.M., Patra S., Kashyap A., Dev A., Jr., Kumar L., Prasad C.P. Quercetin Impairs HuR-Driven Progression and Migration of Triple Negative Breast Cancer (TNBC) Cells. Nutr. Cancer. 2021;74:1497–1510. doi: 10.1080/01635581.2021.1952628. [DOI] [PubMed] [Google Scholar]
- 123.Qian K., Tang C.-Y., Chen L.-Y., Zheng S., Zhao Y., Ma L.-S., Xu L., Fan L.-H., Yu J.-D., Tan H.-S. Berberine reverses breast cancer multidrug resistance based on fluorescence pharmacokinetics in vitro and in vivo. ACS Omega. 2021;6:10645–10654. doi: 10.1021/acsomega.0c06288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.George B.P., Chandran R., Abrahamse H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants. 2021;10:1455. doi: 10.3390/antiox10091455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Liu D.-K., Dong H.-F., Liu R.-F., Xiao X.-L. Baicalin inhibits the TGF-β1/p-Smad3 pathway to suppress epithelial-mesenchymal transition-induced metastasis in breast cancer. Oncotarget. 2020;11:2863–2872. doi: 10.18632/oncotarget.27677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Talukdar D., Chaudhuri B., Ray M., Ray S. Critical evaluation of toxic versus beneficial effects of methylglyoxal. Biochemistry. 2009;74:1059–1069. doi: 10.1134/S0006297909100010. [DOI] [PubMed] [Google Scholar]
- 128.Mele L., Paino F., Papaccio F., Regad T., Boocock D., Stiuso P., Lombardi A., Liccardo D., Aquino G., Barbieri A. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018;9:572. doi: 10.1038/s41419-018-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Far A.H., Tantawy M.A., Al Jaouni S.K., Mousa S.A. Thymoquinone-chemotherapeutic combinations: New regimen to combat cancer and cancer stem cells. Naunyn-Schmiedeberg Arch. Pharmacol. 2020;393:1581–1598. doi: 10.1007/s00210-020-01898-y. [DOI] [PubMed] [Google Scholar]
- 130.Muthusamy T., Yadav L., Ramalingam S., Ramachandran I. Synergistic effect of 5-fluorouracil combined with naringin in MDA-MB-231 human breast cancer cells. Int. Res. J. Oncol. 2020;3:13–27. [Google Scholar]
- 131.Botticelli A., Scagnoli S., Roberto M., Lionetto L., Cerbelli B., Simmaco M., Marchetti P. 5-Fluorouracil degradation rate as a predictive biomarker of toxicity in breast cancer patients treated with capecitabine. J. Oncol. Pharm. Pract. 2020;26:1836–1842. doi: 10.1177/1078155220904999. [DOI] [PubMed] [Google Scholar]
- 132.Miao Y., Ishfaq M., Liu Y., Wu Z., Wang J., Li R., Qian F., Ding L., Li J. Baicalin attenuates endometritis in a rabbit model induced by infection with Escherichia coli and Staphylococcus aureus via NF-κB and JNK signaling pathways. Domest. Anim. Endocrinol. 2021;74:106508. doi: 10.1016/j.domaniend.2020.106508. [DOI] [PubMed] [Google Scholar]
- 133.Hazafa A., Iqbal M., Javaid U., Tareen M., Amna D., Ramzan A., Piracha S., Naeem M. Inhibitory effect of polyphenols (phenolic acids, lignans, and stilbenes) on cancer by regulating signal transduction pathways: A review. Clin. Transl. Oncol. 2021;24:432–445. doi: 10.1007/s12094-021-02709-3. [DOI] [PubMed] [Google Scholar]
- 134.Gomez L.S., Zancan P., Marcondes M.C., Ramos-Santos L., Meyer-Fernandes J.R., Sola-Penna M., Da Silva D. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie. 2013;95:1336–1343. doi: 10.1016/j.biochi.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 135.Wang H., Zhang H., Zhu Y., Wu Z., Cui C., Cai F. Anticancer mechanisms of salinomycin in breast cancer and its clinical applications. Front. Oncol. 2021;11:654428. doi: 10.3389/fonc.2021.654428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tyagi M., Patro B.S. Salinomycin reduces growth, proliferation and metastasis of cisplatin resistant breast cancer cells via NF-kB deregulation. Toxicol. Vitr. 2019;60:125–133. doi: 10.1016/j.tiv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 137.Dewangan J., Tandon D., Srivastava S., Verma A.K., Yapuri A., Rath S.K. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis. 2017;22:1246–1259. doi: 10.1007/s10495-017-1394-y. [DOI] [PubMed] [Google Scholar]
- 138.Aung T.N., Qu Z., Kortschak R.D., Adelson D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017;18:656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saini M., Sangwan R., Khan M.F., Kumar A., Verma R., Jain S. Specioside (SS) & verminoside (VS)(Iridoid glycosides): Isolation, characterization and comparable quantum chemical studies using density functional theory (DFT) Heliyon. 2019;5:e01118. doi: 10.1016/j.heliyon.2019.e01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh I.P., Sidana J., Bharate S.B., Foley W.J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 2010;27:393–416. doi: 10.1039/b914364p. [DOI] [PubMed] [Google Scholar]
- 141.Larayetan R., Ololade Z.S., Ogunmola O.O., Ladokun A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid. Based Complement. Altern. Med. 2019;2019:5410923. doi: 10.1155/2019/5410923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim R.K., Suh Y., Yoo K.C., Cui Y.H., Hwang E., Kim H.J., Kang J.S., Kim M.J., Lee Y.Y., Lee S.J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015;106:94–101. doi: 10.1111/cas.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vidic D., Maksimovic M., Cavar S., Solic M.E. Comparison of essential oil prof iles of Satureja montana l. and Endemic Satureja visianii Šilic. J. Essent. Oil Bear. Plants. 2009;12:273–281. doi: 10.1080/0972060X.2009.10643720. [DOI] [Google Scholar]
- 144.Esmaeili A., Rustaiyan A., Masoudi S., Nadji K. Composition of the essential oils of Mentha aquatica L. and Nepeta meyeri Benth. from Iran. J. Essent. Oil Res. 2006;18:263–265. doi: 10.1080/10412905.2006.9699082. [DOI] [Google Scholar]
- 145.Medjahed F., Merouane A., Saadi A., Bader A., Cioni P.L., Flamini G. Chemical profile and antifungal potential of essential oils from leaves and flowers of Salvia algeriensis (Desf.): A comparative study. Chil. J. Agric. Res. 2016;76:195–200. doi: 10.4067/S0718-58392016000200009. [DOI] [Google Scholar]
- 146.Loizzo M.R., Menichini F., Tundis R., Bonesi M., Nadjafi F., Saab A.M., Frega N.G., Menichini F. Comparative chemical composition and antiproliferative activity of aerial parts of Salvia leriifolia Benth. and Salvia acetabulosa L. essential oils against human tumor cell in vitro models. J. Med. Food. 2010;13:62–69. doi: 10.1089/jmf.2009.0060. [DOI] [PubMed] [Google Scholar]
- 147.Ghantous A., Gali-Muhtasib H., Vuorela H., Saliba N.A., Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 148.Memariani T., Hosseini T., Kamali H., Mohammadi A., Ghorbani M., Shakeri A., Spandidos D.A., Tsatsakis A.M., Shahsavand S. Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines. Oncol. Lett. 2016;11:1353–1360. doi: 10.3892/ol.2015.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Furtado F.B., Borges B.C., Teixeira T.L., Garces H.G., de Almeida Junior L.D., Alves F.C.B., da Silva C.V., Junior A.F. Chemical composition and bioactivity of essential oil from Blepharocalyx salicifolius. Int. J. Mol. Sci. 2018;19:33. doi: 10.3390/ijms19010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Solárová Z., Liskova A., Samec M., Kubatka P., Büsselberg D., Solár P. Anticancer potential of lichens’ secondary metabolites. Biomolecules. 2020;10:87. doi: 10.3390/biom10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sahin E., Dabagoglu Psav S., Avan I., Candan M., Sahinturk V., Koparal A.T. Vulpinic acid, a lichen metabolite, emerges as a potential drug candidate in the therapy of oxidative stress-related diseases, such as atherosclerosis. Hum. Exp. Toxicol. 2019;38:675–684. doi: 10.1177/0960327119833745. [DOI] [PubMed] [Google Scholar]
- 152.Kılıç N., Derici M.K., Buyuk I., Aydın S., Aras S., Duman D. Evaluation of in vitro anticancer activity of vulpinic acid and its apoptotic potential using gene expression and protein analysis. Indian J. Pharm. Educ. Res. 2018;52:46–54. doi: 10.5530/ijper.52.4.73. [DOI] [Google Scholar]
- 153.Kılıç N., Aras S., Cansaran-Duman D. Determination of vulpinic acid effect on apoptosis and mRNA expression levels in breast cancer cell lines. Anti-Cancer Agents Med. Chem. 2018;18:2032–2041. doi: 10.2174/1871520618666180903101803. [DOI] [PubMed] [Google Scholar]
- 154.Zhang L., Yang B., Zhou K., Li H., Li D., Gao H., Zhang T., Wei D., Li Z., Diao Y. Potential therapeutic mechanism of genistein in breast cancer involves inhibition of cell cycle regulation. Mol. Med. Rep. 2015;11:1820–1826. doi: 10.3892/mmr.2014.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nichols J.A., Katiyar S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie Q., Bai Q., Zou L.Y., Zhang Q.Y., Zhou Y., Chang H., Yi L., Zhu J.D., Mi M.T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom. Cancer. 2014;53:422–431. doi: 10.1002/gcc.22154. [DOI] [PubMed] [Google Scholar]
- 157.Zhu W., Qin W., Zhang K., Rottinghaus G.E., Chen Y.-C., Kliethermes B., Sauter E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ávila-Gálvez M.Á., Giménez-Bastida J.A., Espín J.C., González-Sarrías A. Dietary phenolics against breast cancer. A critical evidence-based review and future perspectives. Int. J. Mol. Sci. 2020;21:5718. doi: 10.3390/ijms21165718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beatty E.R., O’Reilly J.D., England T.G., McAnlis G.T., Young I.S., Halliwell B., Geissler C.A., Sanders T.A., Wiseman H. Effect of dietary quercetin on oxidative DNA damage in healthy human subjects. Br. J. Nutr. 2000;84:919–925. doi: 10.1017/S0007114500002555. [DOI] [PubMed] [Google Scholar]
- 160.Islam M.R., Islam F., Nafady M.H., Akter M., Mitra S., Das R., Urmee H., Shohag S., Akter A., Chidambaram K. Natural small molecules in breast cancer treatment: Understandings from a therapeutic viewpoint. Molecules. 2022;27:2165. doi: 10.3390/molecules27072165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li J., Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: The next generation. J. Am. Chem. Soc. 2020;143:538–559. doi: 10.1021/jacs.0c09029. [DOI] [PubMed] [Google Scholar]
- 162.Burguin A., Diorio C., Durocher F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021;11:808. doi: 10.3390/jpm11080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mitra S., Dash R. Natural products for the management and prevention of breast cancer. Evid. Based Complement. Altern. Med. 2018;2018:8324696. doi: 10.1155/2018/8324696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Buhrmann C., Shayan P., Brockmueller A., Shakibaei M. Resveratrol suppresses cross-talk between colorectal cancer cells and stromal cells in multicellular tumor microenvironment: A bridge between in vitro and in vivo tumor microenvironment study. Molecules. 2020;25:4292. doi: 10.3390/molecules25184292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Buhrmann C., Popper B., Kunnumakkara A.B., Aggarwal B.B., Shakibaei M. Evidence that Calebin A, a component of curcuma longa suppresses NF-κB mediated proliferation, invasion and metastasis of human colorectal cancer induced by TNF-β (Lymphotoxin) Nutrients. 2019;11:2904. doi: 10.3390/nu11122904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shakibaei M., Kraehe P., Popper B., Shayan P., Goel A., Buhrmann C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15:250. doi: 10.1186/s12885-015-1291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang H., Wang H.Q., Zhang H.L., Kong D., Wu X.Z. Ginsenoside Rh2 reverses P-glycoprotein-mediated multidrug resistance of MCF-7/ADM cells. Tumor. 2007;27:365–369. [Google Scholar]
- 168.Li X., Chu S., Lin M., Gao Y., Liu Y., Yang S., Zhou X., Zhang Y., Hu Y., Wang H. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020;203:112627. doi: 10.1016/j.ejmech.2020.112627. [DOI] [PubMed] [Google Scholar]
- 169.Wang P., Yang H.L., Yang Y.J., Wang L., Lee S.C. Overcome cancer cell drug resistance using natural products. Evid. Based Complement. Altern. Med. 2015;2015:767136. doi: 10.1155/2015/767136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang S. The multidrug-resistant mechanism study of reverse effect of Baicalin for hepatocellular carcinoma cell line (Bel 7402/ADM) Chin. J. Mod. Drug Appl. 2012;6:10–11. [Google Scholar]
- 171.Tang X., Tang Y. Efficiency and mechanisms of peimine reversing multi-drug resistance of A549/DDP cell line. Shandong Med. J. 2012;52:4–6. [Google Scholar]
- 172.Tang S.-M., Deng X.-T., Zhou J., Li Q.-P., Ge X.-X., Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020;121:109604. doi: 10.1016/j.biopha.2019.109604. [DOI] [PubMed] [Google Scholar]
- 173.Tian C., Liu X., Chang Y., Wang R., Lv T., Cui C., Liu M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr. J. Bot. 2021;137:257–264. doi: 10.1016/j.sajb.2020.10.022. [DOI] [Google Scholar]
- 174.Wu Y., Zeng Y., Liang S. The exploratory research on the berberine reverse the HCT-8/VCR multidrug resistance and its relationship with P-gp functional variance. Guide China Med. 2010;8:18–20. [Google Scholar]
- 175.Warowicka A., Nawrot R., Goździcka-Józefiak A. Antiviral activity of berberine. Arch. Virol. 2020;165:1935–1945. doi: 10.1007/s00705-020-04706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yan L., Zizheng W., Tengfei Y. In vitro study on the reversal of multidrug resistance (MDR) in HL60/VCR cell line with ginsenoside-Rb1. J. Radioimmunol. 2005;18:362–365. [Google Scholar]
- 177.Gao H., Kang N., Hu C., Zhang Z., Xu Q., Liu Y., Yang S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine. 2020;69:153197. doi: 10.1016/j.phymed.2020.153197. [DOI] [PubMed] [Google Scholar]
- 178.Qin Y., Fu Z., Lu W., Li L., Tang W. Experimental study of multidrug-resistance reversing of curcumin on human colon carcinoma HCT-8/VCR cells. Chin. J. Biochem. Pharm. 2011;32:172–173. [Google Scholar]
- 179.Kabir M., Rahman M., Akter R., Behl T., Kaushik D., Mittal V., Pandey P., Akhtar M.F., Saleem A., Albadrani G.M. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules. 2021;11:392. doi: 10.3390/biom11030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo J., Pan X., Feng C., Zhou J. Study on reversal effects of Oridonin on multidrug resistant cell line K562/A02. Shanghai Med. J. 2002;25:43–45. [Google Scholar]
- 181.Li J., Wu Y., Wang D., Zou L., Fu C., Zhang J., Leung G.P.-H. Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol. Res. 2019;146:104313. doi: 10.1016/j.phrs.2019.104313. [DOI] [PubMed] [Google Scholar]
- 182.Zhang W., Liu S., Wang J., Jiang W., Zhang Y., Liu Y., Wang Z. Reversal effect of ginsenoside Rg3 on cisplatin-resistant human lung adenocarcinoma cell line (A549DDP) and its mechanisms. Chin. J. Respir. Crit. Care Med. 2002;1:100–103. [Google Scholar]
- 183.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 184.Xu H.-B., Li L., Fu J., Mao X.-P., Xu L.-Z. Reversion of multidrug resistance in a chemoresistant human breast cancer cell line by β-elemene. Pharmacology. 2012;89:303–312. doi: 10.1159/000337178. [DOI] [PubMed] [Google Scholar]
- 185.Wang W., Lv M., Wang Y., Zhang J. Development of novel application of 3,3′-diindolylmethane: Sensitizing multidrug resistance human breast cancer cells to γ-irradiation. Pharm. Biol. 2016;54:3164–3168. doi: 10.1080/13880209.2016.1192198. [DOI] [PubMed] [Google Scholar]
- 186.Shindikar A., Singh A., Nobre M., Kirolikar S. Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J. Oncol. 2016;2016:750785. doi: 10.1155/2016/9750785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shao C., Wu J., Han S., Liu Y., Su Z., Zhu H.-L., Liu H.-K., Qian Y. Biotinylated curcumin as a novel chemosensitizer enhances naphthalimide-induced autophagic cell death in breast cancer cells. Eur. J. Med. Chem. 2022;228:114029. doi: 10.1016/j.ejmech.2021.114029. [DOI] [PubMed] [Google Scholar]
- 188.Al Dhaheri Y., Attoub S., Ramadan G., Arafat K., Bajbouj K., Karuvantevida N., AbuQamar S., Eid A., Iratni R. Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PLoS ONE. 2014;9:e109630. doi: 10.1371/journal.pone.0109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sukalingam K., Ganesan K., Das S., Thent Z.C. An insight into the harmful effects of soy protein: A review. Clin. Ter. 2015;166:131–139. doi: 10.7417/CT.2015.1843. [DOI] [PubMed] [Google Scholar]
- 190.Ahmad A., Li Y., Sarkar F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016;60:1251–1263. doi: 10.1002/mnfr.201500867. [DOI] [PubMed] [Google Scholar]
- 191.Kim D., Park M., Haleem I., Lee Y., Koo J., Na Y.C., Song G., Lee J. Natural product Ginsenoside 20 (S)-25-methoxyl-dammarane-3β, 12β, 20-triol in cancer treatment: A review of the pharmacological mechanisms and pharmacokinetics. Front. Pharmacol. 2020;11:521. doi: 10.3389/fphar.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang X., Zhang Y., Guo S., Bai F., Wu T., Zhao Y. Improved oral bioavailability of 20 (R)-25-methoxyl-dammarane-3β, 12β, 20-triol using nanoemulsion based on phospholipid complex: Design, characterization, and in vivo pharmacokinetics in rats. Drug Des. Dev. Ther. 2016;10:3707–3716. doi: 10.2147/DDDT.S114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen H., Yang J., Yang Y., Zhang J., Xu Y., Lu X. The natural products and extracts: Anti-triple-negative breast cancer in vitro. Chem. Biodivers. 2021;18:e2001047. doi: 10.1002/cbdv.202001047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.