Abstract
Objectives
Although mRNA-based vaccines against SARS-CoV-2 induce a robust immune response and prevent infections and hospitalizations, there are limited data on the antibody response in individuals with humoral immunodeficiency. The aim of this study was to evaluate the humoral immune response after two vaccine doses with BNT162b2 or mRNA-1273 in patients with humoral immunodeficiency disease.
Methods
This cross-sectional study assessed 39 individuals with hypogammaglobulinemia under immunoglobulin replacement therapy. IgG anti-SARS-CoV-2 spike protein antibodies (anti-S) were measured 4 weeks to 4 months after two doses of an mRNA vaccine against SARS-CoV-2. The proportion of patients, who developed a humoral immune response to the spike protein were evaluated and compared to 19 healthy controls.
Results
After vaccination with two vaccine doses, 26/39 patients (66.7%) with humoral immunodeficiency disease and all healthy controls developed anti-S. In subjects with baseline IgG <3 g/l, only 1/5 (20%) showed a humoral immune response. 10 out of 26 with CVID (38.5%) and 7/9 under immunosuppressive drugs (77.8%) developed no immune response (13 subjects with no response) compared to 0/19 in healthy controls. Subgroup analysis in patients without immunosuppressive drugs revealed lower anti-S in patients with moderate to severe humoral immunodeficiency disease: baseline IgG <3 g/l: 12.0 AU/ml (95%CI 12.0–125.0), baseline IgG 3–5 g/l: 99.9 AU/ml (95%CI 14.4–400.0), baseline IgG >5 g/l: 151.5 AU/ml (95%CI 109.0–400.0), healthy controls 250.0 AU/ml (95%CI 209.0–358.0), p = 0.007.
Conclusion
In most patients with mild to moderate humoral immunodeficiency we found only slightly lower anti-S antibodies compared with healthy controls after two vaccine doses with BNT162b2 and mRNA-1273. However, in patients with a decreased baseline IgG below 3 g/l and/or under immunosuppressive drugs, we found severely impaired humoral immune responses.
Introduction
Following the global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccinations have been introduced since the end of 2020 to control the pandemic. In particular, mRNA-based vaccines are considered highly effective in prevention of infection and hospitalization, even against variants of concern [1,2]. To date, data on the humoral immune response to SARS-CoV-2 in patients with immunodeficiency disorders is limited [3]. Due to hypogammaglobulinemia and T- and B-cell impairment, the general immune response is reduced in these patients, which may explain severe or fatal Covid-19 infections in this population [3–5]. In Switzerland, this population was prioritized for vaccination early in 2021 with mRNA vaccines. In recent months, an increasing number of patient groups have been identified which do not have an optimal vaccine response to the Covid-19 vaccines. After two doses of Covid-19 vaccine, decreased immune responses are expected in the elderly and in subjects under dialysis, with central obesity, arterial hypertension, smoking, transplanted patients and patients under immunosuppressive drugs, especially anti-CD20 therapies [6–11]. It is known that vaccination response is reduced in CVID patients [12]. For example, influenza vaccination of individuals with a hypogammaglobulinemia only resulted in significant increase of IgG antibody-titers in 29% [13]. Other studies found an even lower fraction of immune responders after influenza vaccine [14]. Regarding mRNA based vaccines against SARS-CoV-2, several studies show a robust antibody response in the majority of individuals with an antibody deficiency [15–22]. All these findings may lead to the question which patients with humoral immunodeficiencies can be expected to have a good or an insufficient humoral vaccine response. The aim of this study was to further characterize the immune response to mRNA vaccination in relation to the severity of the immunoglobulin deficiency and immunosuppressive medications in an exploratory manner.
Methods
We studied the humoral immune response against the S1/S2 spike protein of SARS-CoV-2 in patients with humoral immunodeficiency disease. Subjects with a primary or secondary hypogammaglobulinemia (CVID, IgG deficiency, IgG subclass deficiency, drugs, lymphoproliferative disease) and treated by intravenous (IVIG) or subcutaneous immunoglobulin (SCIG) replacement therapy in our outpatient clinic were included if they had received two mRNA Covid-19 vaccine doses (BNT162b2 (Comirnaty®, Pfizer-BionTech) or mRNA-1273 (Spikevax®, Moderna)) 4–6 weeks apart between January and June 2021. CVID was defined with the following characteristics: significant reduced total IgG, reduced IgA or IgM values, poor vaccine response, recurrent infections and absent T-cell impairment [23]. As the production lead time of immunoglobulins is long, it can be assumed, that these products contained no significant amounts of anti-S at the time of the study.
Data collection and blood sampling
All data on disease, treatment, and vaccinations were collected by the attending physicians based on the medical records. All individual vaccination dates and the vaccines administered were obtained from the original vaccination records in each study participant. Healthy volunteers were recruited among the private and professional surroundings of the investigators without performing a matching procedure. Single serum samples for analysis of vaccine antibodies were collected 2 weeks to 4 months after the second Covid-19 vaccination in all study participants and healthy controls.
IgG antibody assays
Blood samples were run on an Abbott ARCHITECT i2000 instrument using the Abbott SARS-CoV-2 nucleoprotein assay and the DiaSorin LIAISON® SARS-CoV-2 S1/S2 IgG assay following the manufacturer’s instructions. The Abbott assay is a chemiluminescent microparticle immunoassay for qualitative detection of IgG in human serum or plasma against the SARS-CoV-2 nucleoprotein. The Liaison SARS-CoV-2 S1/S2 is a chemiluminescence assay, that uses paramagnetic microparticles coated with S1 and S2 fragments of the viral surface spike protein (positive cut-off of >12 AU/ml). According to the manufacturer’s information, the Liaison SARS-CoV-2 S1/S2 IgG assay of >15 AU/ml reached a plaque-reduction neutralization test (PRNT) titer of 1:40 in 17/18 patients and a PRNT titer of >1:160 if SARS-CoV-2 S1/S2 IgG of 80 AU/ml were analysed [24]. Presence of antibodies against the nucleocapsid protein (anti-N) were evaluated to capture convalescent persons. The primary endpoint was the proportion of patients, who developed a humoral immune response to SARS-CoV-2 spike protein, compared to healthy controls.
Ethic approval
This study was approved by the local ethics committee (Kantonale Ethikkommission Bern, ID 2021–01355). All subjects included signed informed consent.
Statistical analysis
Statistical analysis were performed using Graphpad Prism 9 (GraphPad Software, Inc, La Jolla, Calif). Patient characteristics are summarized with descriptive statistics. Values are median and interquartile ranges (IQR) for continuous variables, categorical variables reported as n (%). The 95% confidence intervals (95%CI) from the proportions of patients with positive anti-S values in a specific group were calculated as Wilson/Brown interval. Anti-S titers were compared by Kruskal-Wallis or Mann-Whitney test, the median 95%CI was calculated for all groups.
Results
Patient characteristics
47 patients with a humoral immunodeficiency treated by immunoglobulin replacement therapy and vaccinated twice with one of the Covid-19 mRNA vaccines were identified in a single center. Eight subjects were excluded (4 refused to participate, two had missing anti-S values and two subjects had an interval from the second vaccination to the blood sampling of over 4 months). Of the 39 patients included, 28 were female (71.8%) compared to 15/19 healthy controls (79.0%). The median age was 61 years (IQR 51.0; 73.0) in the study group and 53 years (IQR 43.0; 63.0) in the control group (Table 1). Characteristics of healthy controls are summarized in S1 Table (supporting information). 26/39 subjects (66.7%) fulfilled the criteria of CVID, 2/39 had an IgG deficiency (5.1%), 9/39 an IgG subclass deficiency (mostly combined IgG1/IgG3) (23.1%), one person had a lymphoproliferative disorder and one subject developed a secondary humoral immunodeficiency after ocrelizumab treatment. 36 patients (92.3%) were under IVIG, 3 (7.7%) under SCIG. The median monthly immunoglobulin dose was 25.0 g (IQR 20.0; 30.0). Median total baseline IgG value before initiating immunoglobulin replacement therapy was 5.1 g/l (IQR 3.6; 6.3), median actual IgG trough levels under treatment were 8.9 g/l (IQR 8.1; 10.2). All included patients were twice vaccinated in 4 to 6 weeks intervals, 29/39 (65.6%) with BNT162b2 (Pfizer-Biontech) and 10/39 (31.7%) with mRNA-1273 (Moderna). Anti-N values were negative in all patients, even in two persons with past Covid-19 infection (patient 4 and 27, Table 2). 9/39 patients (23.1%) were under immunosuppressive treatment or had rituximab/ocrelizumab treatment in their past (Table 2).
Table 1. Patient characteristics.
| Study participants | Healthy controls | |
|---|---|---|
| N = 39 | N = 19 | |
| Demographics | ||
| Age | 61.0 (52.5; 73.5) | 53.0 (43.0; 63.0) |
| Gender (female) | 29 (70.7%) | 15 (79.0%) |
| Median time from 2. vaccine to sampling (days) | 45.0 (33.0; 65.0) | 70.5 (46.8; 85.8) |
| Clinical | ||
| CVID (n) | 26 (66.7%) | n/a |
| IgG deficiency (n) | 2 (5.1%) | n/a |
| Secondary immunodeficiency (drugs, neoplastic disease) (n) |
2 (5.1%) | n/a |
| IgG subclass deficiency (n) | 9 (23.1%) | n/a |
| Immunosuppressive drugs (n) | 9 (23.1%) | n/a |
| IVIG (n) | 36 (92.3%) | n/a |
| SCIG (n) | 3 (8.3%) | n/a |
| Monthly dose (g) | 25.0 (20.0; 30.0) | n/a |
| Laboratory | ||
| IgA (g/l) | 0.58 (0.3; 1.2) | n/a |
| Baseline IgG before IVIG (g/l) | 5.1 (3.6; 6.3) | n/a |
| IgG nadir value under IVIG/SCIG (g/l) | 8.9 (8.1; 10.2) | n/a |
| SARS-CoV-2-IgG spike protein (AU/ml) | 78.9 (12.0; 276.0) | 250.0 (209.0; 358.0) |
Values are median and interquartile ranges (IQR) for continuous variables. Categorical variables reported as n (%). Laboratory reference values: IgG 7.0–16.0 g/l, IgA 0.7–4.0 g/l, SARS-CoV-2-IgG spike protein cutoff 12 AU/ml.
Common variable immunodeficiency disease (CVID), intravenous immunoglobulin substitution (IVIG), subcutaneous immunoglobulin substitution (SCIG).
Table 2. Individual data of included patients.
| Age | Immuno-suppressive drugs | Immuno-deficiency disease | IgA (g/l) | Baseline IgG (g/l) | IgG nadir value under IVIG/SCIG (g/l) | Monthly dose IVIG/SCIG | Vaccine | Time from 2. vaccine to blood analysis (d) | SARS-CoV-2-IgG spike protein (AU/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | No | CVID | <0.05 | 0.07 | 8.3 | 30 | BNT162b2 | 71 | <12 |
| 2 | 51 | No | CVID | 0.07 | 0.7 | 7.2 | 50 | mRNA-1273 | 22 | <12 |
| 3 | 55 | No | CVID | <0.05 | 0.8 | 13.4 | 28 | BNT162b2 | 39 | <12 |
| 4 | 56 | No | CVID | 1.67 | 1.9 | 6.7 | 25 | BNT162b2 | 40 | 125 |
| 5 | 57 | No | CVID | 0.07 | 1.9 | 8.2 | 40 | BNT162b2 | 76 | <12 |
| 6 | 56 | No | CVID | 0.06 | 3.1 | 8.2 | 30 | BNT162b2 | 66 | >400 |
| 7 | 72 | Ibrutinib / rituximab | secondary | 0.7 | 3.2 | 10.6 | 25 | mRNA-1273 | 22 | <12 |
| 8 | 86 | No | CVID | 0.32 | 3.4 | 4.9 | 10 | BNT162b2 | 59 | 20.4 |
| 9 | 61 | No | CVID | <0.05 | 3.5 | 10.2 | 25 | BNT162b2 | 49 | 14.4 |
| 10 | 49 | No | CVID | 0.47 | 3.6 | 10.6 | 24 | BNT162b2 | 74 | 99.9 |
| 11 | 26 | No | CVID | 0.54 | 3.8 | 7.2 | 70 | BNT162b2 | 22 | >400 |
| 12 | 78 | Rituximab | CVID | 0.49 | 4.2 | 8.0 | 25 | BNT162b2 | 88 | <12 |
| 13 | 54 | Certolizumab | IgG deficiency | 0.83 | 4.2 | 6.8 | 25 | BNT162b2 | 28 | 37 |
| 14 | 69 | No | CVID | 11.15 | 4.3 | 8.7 | 25 | mRNA-1273 | 40 | 372 |
| 15 | 84 | No | CVID | 0.57 | 4.3 | 9.1 | 30 | BNT162b2 | 112 | <12 |
| 16 | 62 | Etanercept | IgG deficiency | 0.8 | 4.4 | 11.4 | 40 | BNT162b2 | 24 | <12 |
| 17 | 74 | No | CVID | 0.3 | 4.5 | 9.3 | 20 | mRNA-1273 | 63 | 276 |
| 18 | 86 | Rituximab | CVID | <0.05 | 4.7 | 8.4 | 25 | BNT162b2 | 92 | <12 |
| 19 | 64 | No | CVID | 0.53 | 4.8 | 8.9 | 25 | BNT162b2 | 48 | 59.9 |
| 20 | 59 | No | CVID | 1.31 | 5.1 | 9.6 | 15 | mRNA-1273 | 22 | >400 |
| 21 | 72 | No | CVID | 1.25 | 5.3 | 8.5 | 50 | BNT162b2 | 34 | 141 |
| 22 | 91 | No | IgG 1 and 3 deficiency | 1.23 | 5.6 | 7.3 | 10 | BNT162b2 | 57 | 109 |
| 23 | 72 | Ibrutinib | CVID | 0.29 | 5.7 | 12 | 60 | mRNA-1273 | 37 | <12 |
| 24 | 31 | No | CVID | 0.42 | 5.9 | 12.1 | 35 | BNT162b2 | 47 | >400 |
| 25 | 31 | No | IgG 1 and 3 deficiency | 0.92 | 5.9 | 9.3 | 40 | BNT162b2 | 45 | >400 |
| 26 | 31 | No | CVID | 0.58 | 6 | 10.0 | 30 | BNT162b2 | 14 | >400 |
| 27 | 72 | No | IgG 1 deficiency | 1.7 | 6.1 | 9.2 | 25 | BNT162b2 | 62 | >400 |
| 28 | 49 | No | CVID | 0.4 | 6.2 | 9.9 | 40 | BNT162b2 | 22 | 78.9 |
| 29 | 62 | Ocrelizumab | secondary | 1.16 | 6.3 | 7.8 | 15 | mRNA-1273 | 33 | <12 |
| 30 | 57 | Methotrexat / prednisolone | CVID | 1.26 | 6.3 | 8.3 | 25 | BNT162b2 | 37 | <12 |
| 31 | 23 | No | IgG 2 deficiency | 0.75 | 6.5 | 7.6 | 15 | mRNA-1273 | 37 | 136 |
| 32 | 57 | No | IgG 1 and 3 deficiency | 2.62 | 6.6 | 9.6 | 20 | BNT162b2 | 65 | 161 |
| 33 | 45 | No | CVID | 0.33 | 6.6 | 8.8 | 25 | BNT162b2 | 39 | 248 |
| 34 | 79 | No | IgG 1 deficiency | 1.18 | 6.8 | 9.6 | 25 | mRNA-1273 | 56 | 20 |
| 35 | 87 | No | IgG 2 and 3 deficiency | 2.7 | 7.5 | 8.1 | 10 | mRNA-1273 | 50 | >400 |
| 36 | 73 | Mycophenolat-mofetile | CVID | 0.3 | 8.3 | 15.6 | 30 | BNT162b2 | 43 | 12.8 |
| 37 | 41 | No | IgG 2 deficiency | 0.82 | 8.6 | 11.2 | 20 | BNT162b2 | 99 | 142 |
| 38 | 75 | No | IgG 1 and 3 deficiency | 2.49 | 9.1 | 11.5 | 20 | BNT162b2 | 71 | 129 |
| 39 | 58 | No | CVID | 0.58 | 5.7 | 8.7 | 20 | BNT162b2 | 18 | <12 |
Laboratory reference values: IgG 7.0–16.0 g/l, IgA 0.7–4.0 g/l, SARS-CoV-2-IgG spike protein cutoff 12 AU/ml.
Common variable immunodeficiency disease (CVID), intravenous immunoglobulin substitution (IVIG), subcutaneous immunoglobulin substitution (SCIG).
SARS-CoV-2 S1/S2 IgG values after two mRNA vaccine doses
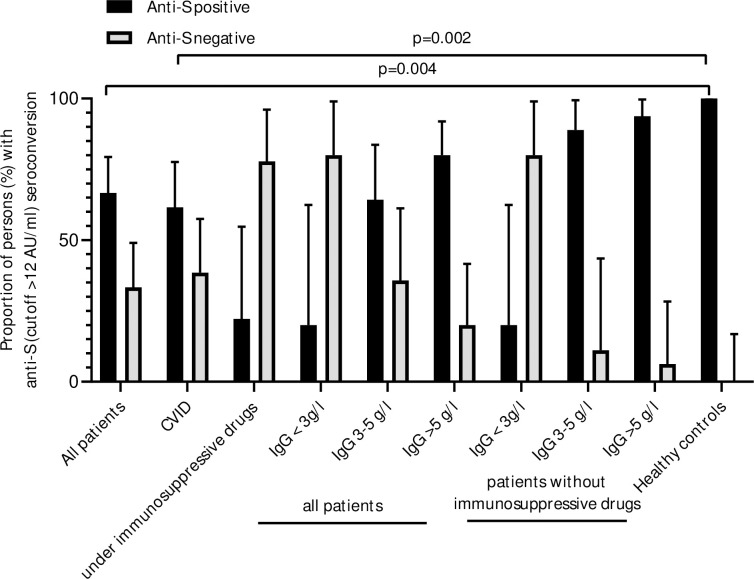
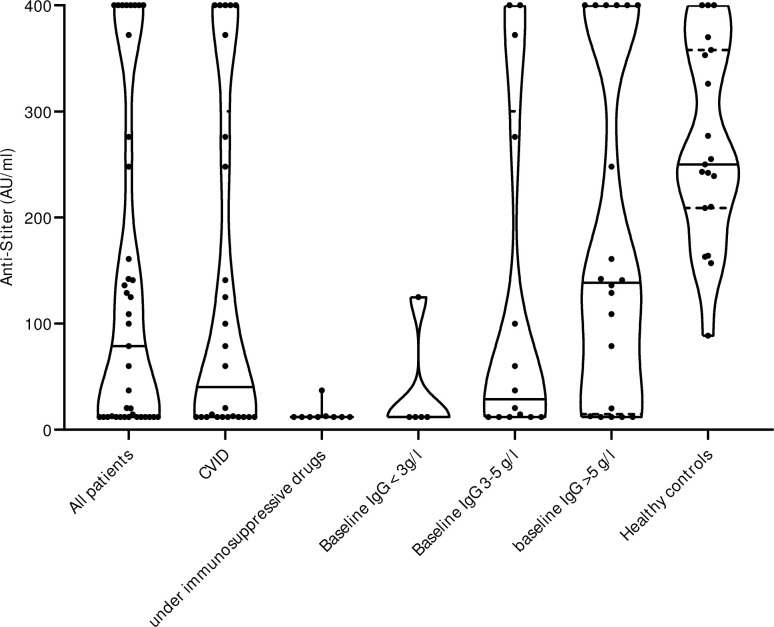
After two vaccine doses, we found detectable anti-S levels in 26/39 subjects (66.7% [95%CI 51.0–79.4%]) with a humoral immunodeficiency disease, and in all healthy controls (19/19, 100% [95%CI 83.2–100%]). In CVID, the proportion of patients with presence of anti-S was 16/26 (61.5% [95%CI 42.5–77.6%]), and thus lower than in non-CVID patients (3/10, 77.0% [95%CI 49.7–91.8]) and in the healthy control group (19/19, 100.0% [95%CI 83.2–100.0]), p = 0.002. All individuals with a humoral non-response to the vaccines (n = 13) were either under immunosuppressive drugs (including anti-CD20 therapy in the past) (7/13, 53.8%) and/or had a diagnosis of CVID (10/13, 76.9%). Two patients treated by immunosuppressive treatment showed a low anti-S response (Fig 1). Median anti-S concentration in individuals with a humoral immunodeficiency disease was 78.9 AU/ml (95%CI 12.0–142.0), and in the controls 250.0 AU/ml (95%CI 209.0–358.0) (p = 0.002). Patients with CVID had a median anti-S concentration of 40.15 AU/ml (95%CI 12.0–248.0). To assess the correlation of the vaccination response with the severity of the immunodeficiency disease, participating patients were sub-grouped according to baseline IgG levels before the start of immunoglobulin replacement therapy. In subjects without immunosuppressive drugs, only 1/5 patients (20% [95%CI 1.0–62.4%) with a baseline IgG of <3 g/l showed detectable anti-S antibodies, in subjects with baseline IgG between 3–5 g/l 8/9 (88.9% [95%CI 56.5–99.4%) and in the subgroup with IgG values >5 g/l 15/16 (93.8% [95%CI 71.7–99.7%) (Fig 2) showed detectable anti-S. Median anti-S values were significantly lower in patients with severe humoral immunodeficiency disease without immunosuppressive drugs: Baseline IgG <3 g/l: 12.0 AU/ml (95%CI 12.0–125.0), baseline IgG 3–5 g/l: 99.9 AU/ml (95%CI 14.4–400.0), baseline IgG >5 g/l: 151.5 AU/ml (95%CI 109.0–400.0), p = 0.007 (Fig 2). Results were similar when all patients were included in the subgroup analyses (p = 0.002) (Fig 2). Overall, in subjects with baseline IgG >5 g/l there was only a trend to lower anti-S values compared to healthy controls (250.0 AU/ml (95%CI 209.0–358.0)).
Fig 1. Proportion of patients with anti-S after two Covid-19 mRNA doses.
Proportion of patients with humoral immune response to SARS-CoV-2 spike protein (anti-S) are shown in total and for various subgroups (Anti-SARS-CoV-2 spike protein antibodies cutoff >12 AU/ml). Baseline IgG values indicate the severity of the humoral immunodeficiency disease at start of immunoglobulin replacement therapy. P values are calculated by Chi-square test. Anti-SARS-CoV-2 spike protein antibodies (anti-S).
Fig 2. Anti-S quantity subgroup analysis.
All included patients were divided into subgroups according to baseline total IgG levels at start of immunoglobulin replacement therapy. Kruskal-Wallis test was calculated for the comparison of the IgG subgroups and healthy control: p = 0.002. Anti-SARS-CoV-2 spike protein antibodies (anti-S).
SARS-CoV-2 S1/S2 IgG values after three mRNA vaccine doses
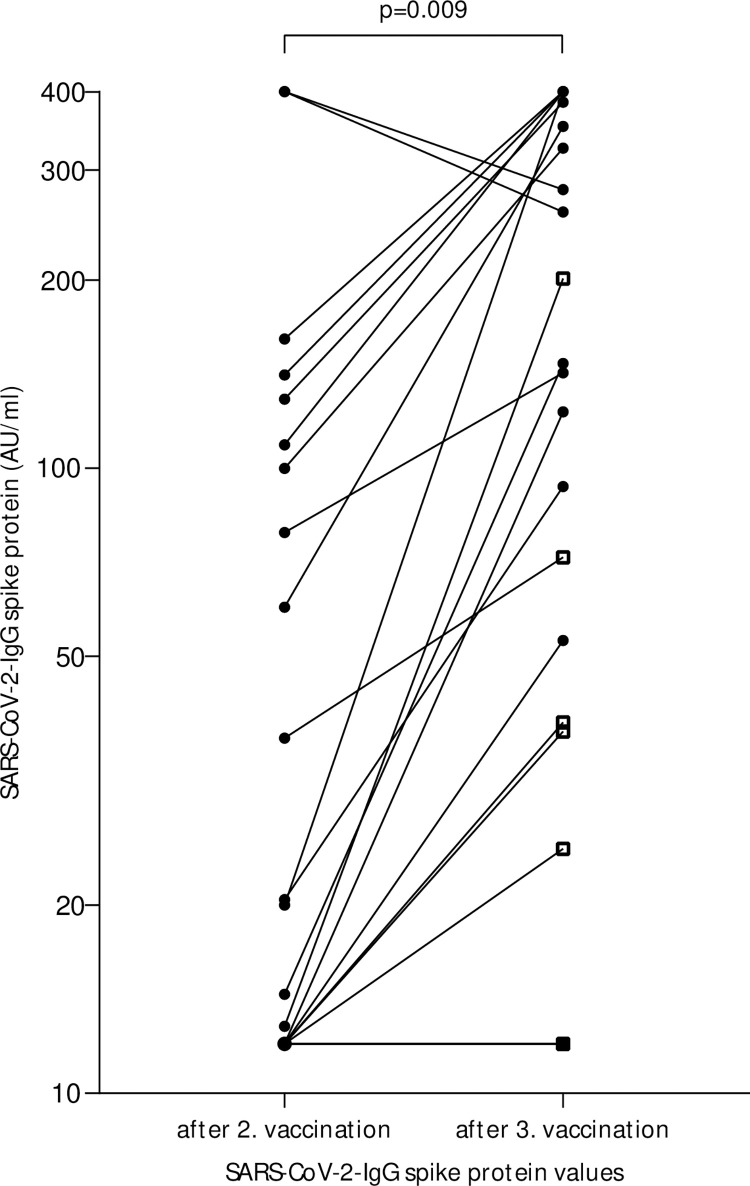
In a post-hoc analysis, we obtained anti-S levels from 22 patients after the third vaccination (Fig 3, S2 Table). A third mRNA vaccination was administered in 8/22 subjects with absent anti-S increase after the second vaccination, 7/22 of the patients with a third vaccination were on immunosuppressive drugs. The median anti-S levels significantly increased from 20.2 AU/ml (95%CI 12.0–109.0) to 144.5 (95%CI 39.2–352.0) after the third vaccination (p = 0.009). All but five cases showed an increase in anti-S. Two subjects with preexisting high IgG values showed a slight decrease, but maintained high anti-S antibody levels over time. Reasons for the lack of increase in the other three cases were treatment with immunosuppressive drugs (case 12 and 29) and a CVID disease state (case 39).
Fig 3. Anti-S quantity after 3. vaccination.
Data of SARS-CoV-2-IgG spike protein values of 22 patients with a third mRNA vaccination, including 7 persons under immunosuppressive drugs. In the two subjects with decreasing SARS-CoV-2-IgG spike protein levels despite third vaccination, laboratory analysis was not performed until 4 months after the third vaccination. Subjects under immunosuppressive treatment are marked with square. The p value was calculated by Mann-Whitney test.
Discussion
26/39 of our patients with humoral immunodeficiency showed a detectable immune response against SARS-CoV-2 after mRNA vaccination. After the second Covid-19 vaccine, the majority of individuals with a mild to moderate IgG deficiency had a specific anti-S concentration that was slightly below the level of healthy controls. Even in most patients with CVID an immune response was detected after a second dose of the Covid-19 mRNA vaccine. Our findings are in line with recent studies, demonstrating an increase of anti-S levels in most individuals with humoral immunodeficiency without immunosuppressive drugs after two Covid-19 vaccinations [15–22]. In a recent study, an immune response to SARS-CoV-2 was detected only in one third of patients with CVID [25]. These data are similar to previous studies on CVID and influenza vaccination [13]. These inconsistent findings may be explained by the severity of the humoral immunodeficiency. Even though immunization with mRNA vaccines is very effective against SARS-CoV-2, it is important to consider confounding factors that may block or attenuate an immune response. In our study, most patients with absent anti-S increase either had a severe form of CVID with a very low IgG baseline titer and/or were currently co-treated by immunosuppressants. This constellation was also observed in a small case series of individuals with CVID by Kinoshita, although these patients developed a significant T-cell response [15]. Nevertheless, patients with humoral immunodeficiency could experience potentially severe breakthrough Covid-19 infections despite vaccination [26]. Importantly, in the majority of those cases with very low or undetectable anti-S levels, an increase was achieved with a third vaccination, in line with the finding of Barmettler et al. [27]. Many country guidelines now advise to administer a third dose in the primary series to patients with CVID, including Swiss guidelines [28].
Therefore, anti-S antibody determination might be useful to detect non-responders after completion of a two Covid-19 vaccination schedule in patients at risk, to enable a timely third vaccination. In patients under immunosuppressive drugs or humoral immunodeficiency, a full vaccination schedule should include three Covid-19 mRNA vaccinations followed by a booster vaccine [29]. If a deficient immune response persists, T-cell assays should be performed and a fourth vaccination should be considered [29].
Our study has limitations: Although both mRNA vaccines seem to be effective in a majority of patients with humoral immunodeficiencies, we have no long-time data since the observational period was very short. Blood samples were taken over a wide time interval (4 weeks to 4 months) in both study groups. Since antibody titers decrease over time, it is therefore likely, that the measured antibody levels underestimate the peak levels in these patients. Furthermore, it has to be assumed that patients with a humoral immunodeficiency have a faster decline of the IgG-anti-S titer than healthy controls. In addition, it is unclear whether the anti-S antibodies we measured are neutralizing in nature. We cannot make any statement about the T-cell response, which certainly plays a major role. Specific T-cell responses seem to be defective in about one third of CVID patients [25]. Lastly, our study groups were small, with both gender and age not completely balanced; this could be a confounding factor. Therefore, prospective cohorts with larger patient numbers and a longer observation period including humoral and cellular vaccine responses are warranted for a better understanding of the long-term effect and benefit of recurrent vaccination in humoral immunodeficiency.
Conclusion
Although individuals with humoral immunodeficiencies have impaired responses to vaccines, BNT162b2 and mRNA-1273 mRNA vaccines produce humoral immune responses in the majority of these patients. Most individuals with mild to moderate humoral immunodeficiency disease without immunosuppressive drugs did not have significantly reduced anti-S values compared to healthy individuals. Treatment with immunosuppressive drugs and a diagnosis of CVID are risk factors for reduced or even absent humoral response. In these individuals, a full vaccination schedule should include three Covid-19 mRNA vaccinations as primary series followed by a booster vaccine.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors acknowledge Dr. Benno Schnyder for editing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Bern University Hospital. The authors received no specific funding for this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Griffin JB, Haddix M, Danza P, Fisher R, Koo TH, Traub E et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status—Los Angeles County, California, May 1-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021. Aug 27;70(34):1170–1176. doi: 10.15585/mmwr.mm7034e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status—New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021. Aug 27;70(34):1150–1155. doi: 10.15585/mmwr.mm7034e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weifenbach N, Jung A, Lötters S. COVID-19 infection in CVID patients: What we know so far. Immun Inflamm Dis. 2021. May 12. doi: 10.1002/iid3.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakoc Aydiner E, Bilgic Eltan S, Babayeva R, Aydiner O, Kepenekli E, Kolukisa B et al. Adverse COVID-19 outcomes in immune deficiencies: Inequality exists between subclasses. Allergy. 2021. Jul 27. doi: 10.1111/all.15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol. 2021. Feb;147(2):520–531. doi: 10.1016/j.jaci.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2021. May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moor MB, Suter-Riniker F, Horn MP, Aeberli D, Amsler J, Möller B et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021. Sep 7. doi: 10.1016/S2665-9913(21)00251-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achiron A, Mandel M, Dreyer-Alster S, Harari G, Dolev M, Menascu S et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J Neuroimmunol. 2021. Oct 9;361:577746. doi: 10.1016/j.jneuroim.2021.577746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021. Sep 14. doi: 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2021. Oct 22. doi: 10.1002/ana.26251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotugno N, Pighi C, Morrocchi E, Ruggiero A, Amodio D, Medri C, et al. BNT162B2 mRNA COVID-19 Vaccine in Heart and Lung Transplanted Young Adults: Is an Alternative SARS-CoV-2 Immune Response Surveillance Needed? Transplantation. 2022. Feb 1;106(2):e158–e160. doi: 10.1097/TP.0000000000003999 [DOI] [PubMed] [Google Scholar]
- 12.Milito C, Soccodato V, Collalti G, Lanciarotta A, Bertozzi I, Rattazzi M et al. Vaccination in PADs. Vaccines (Basel). 2021. Jun 9;9(6):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Assen S, Holvast A, Telgt DS, Benne CA, de Haan A, Westra J et al. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin Immunol. 2010. Aug;136(2):228–35. doi: 10.1016/j.clim.2010.03.430 [DOI] [PubMed] [Google Scholar]
- 14.Gardulf A, Abolhassani H, Gustafson R, Eriksson LE, Hammarström L. Predictive markers for humoral influenza vaccine response in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2018. Dec;142(6):1922–1931.e2. doi: 10.1016/j.jaci.2018.02.052 [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita H, Durkee-Shock J, Jensen-Wachspress M, Kankate VV, Lang H, Lazarski CA et al. Robust Antibody and T Cell Responses to SARS-CoV-2 in Patients with Antibody Deficiency. J Clin Immunol. 2021. Aug;41(6):1146–1153. doi: 10.1007/s10875-021-01046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagin D, Freund T, Navon M, Halperin T, Adir D, Marom R et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021. Jun 1:S0091-6749(21)00887-3. doi: 10.1016/j.jaci.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amodio D, Ruggiero A, Sgrulletti M, Pighi C, Cotugno N, Medri C et al. Humoral and Cellular Response Following Vaccination With the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front Immunol. 2021. Oct 4;12:727850. doi: 10.3389/fimmu.2021.727850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abo-Helo N, Muhammad E, Ghaben-Amara S, Panasoff J, Cohen S. Specific antibody response of patients with common variable immunodeficiency to BNT162b2 coronavirus disease 2019 vaccination. Ann Allergy Asthma Immunol. 2021. Oct;127(4):501–503. doi: 10.1016/j.anai.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squire J, Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann Allergy Asthma Immunol. 2021. Sep;127(3):383–384. doi: 10.1016/j.anai.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano C, Esposito S, Donnarumma G, Marrone A. Detection of neutralizing anti-severe acute respiratory syndrome coronavirus 2 antibodies in patients with common variable immunodeficiency after immunization with messenger RNA vaccines. Ann Allergy Asthma Immunol. 2021. Oct;127(4):499–501. doi: 10.1016/j.anai.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delmonte OM, Bergerson JRE, Burbelo PD, Durkee-Shock JR, Dobbs K, Bosticardo M et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021. Nov;148(5):1192–1197. doi: 10.1016/j.jaci.2021.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arroyo-Sánchez D, Cabrera-Marante O, Laguna-Goya R, Almendro-Vázquez P, Carretero O, Gil-Etayo FJ, et al. Immunogenicity of Anti-SARS-CoV-2 Vaccines in Common Variable Immunodeficiency. J Clin Immunol. 2022. Feb;42(2):240–252. doi: 10.1007/s10875-021-01174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016. Jan-Feb;4(1):38–59. doi: 10.1016/j.jaip.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiaSorin 2021 LIAISON® SARS-CoV-2 S1/S2 IgG REF 311450.
- 25.Salinas AF, Mortari EP, Terreri S, Quintarelli C, Pulvirenti F, Di Cecca S et al. SARS-CoV-2 Vaccine Induced Atypical Immune Responses in Antibody Defects: Everybody Does their Best. J Clin Immunol. 2021. Nov;41(8):1709–1722. doi: 10.1007/s10875-021-01133-0 Epub 2021 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Respir Med. 2022. Feb 25:S2213-2600(22)00042-X. doi: 10.1016/S2213-2600(22)00042-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barmettler S, DiGiacomo DV, Yang NJ, Lam T, Naranbhai V, Dighe AS, et al. Response to SARS-CoV-2 Initial Series and Additional Dose Vaccine in Patients with Predominant Antibody Deficiency. J Allergy Clin Immunol Pract. 2022. Apr 2:S2213-2198(22)00328-2. doi: 10.1016/j.jaip.2022.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swiss Federal Office of Puplic Health and “Eidgenössische Kommission für Impffragen (EKIF), Swiss Vaccination recommendation for mRNA vaccines against covid-19, https://www.bag.admin.ch/dam/bag/de/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/impfempfehlung-covid-19.pdf.download.pdf/Impfempfehlung%20f%C3%BCr%20mRNA-Impfstoffe%20gegen%20Covid-19.pdf.
- 29.Ameratunga R, Woon ST, Steele R, Lehnert K, Leung E, Edwards ESJ, et al. Common Variable Immunodeficiency Disorders as a Model for Assessing COVID-19 Vaccine Responses in Immunocompromised Patients. Front Immunol. 2022. Jan 18;12:798389. doi: 10.3389/fimmu.2021.798389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.