Abstract
Cylindrospermopsis raciborskii is a toxic-bloom-forming cyanobacterium that is commonly found in tropical to subtropical climatic regions worldwide, but it is also recognized as a common component of cyanobacterial communities in temperate climates. Genetic profiles of C. raciborskii were examined in 19 cultured isolates originating from geographically diverse regions of Australia and represented by two distinct morphotypes. A 609-bp region of rpoC1, a DNA-dependent RNA polymerase gene, was amplified by PCR from these isolates with cyanobacterium-specific primers. Sequence analysis revealed that all isolates belonged to the same species, including morphotypes with straight or coiled trichomes. Additional rpoC1 gene sequences obtained for a range of cyanobacteria highlighted clustering of C. raciborskii with other heterocyst-producing cyanobacteria (orders Nostocales and Stigonematales). In contrast, randomly amplified polymorphic DNA and short tandemly repeated repetitive sequence profiles revealed a greater level of genetic heterogeneity among C. raciborskii isolates than did rpoC1 gene analysis, and unique band profiles were also found among each of the cyanobacterial genera examined. A PCR test targeting a region of the rpoC1 gene unique to C. raciborskii was developed for the specific identification of C. raciborskii from both purified genomic DNA and environmental samples. The PCR was evaluated with a number of cyanobacterial isolates, but a PCR-positive result was only achieved with C. raciborskii. This method provides an accurate alternative to traditional morphological identification of C. raciborskii.
Cyanobacterial blooms have become an increasing worldwide problem in aquatic habitats such as lakes, rivers, estuaries, and oceans and in man-made water storage systems. These occurrences can be partially attributed to gradual eutrophication of waterways. Certain species of cyanobacteria produce toxins, and as a result, blooms create major threats to animal and human health, tourism, recreation, and aquaculture.
A range of cyanobacterial toxins have been described in detailed reviews (8, 17). Hepatotoxins (liver damaging), neurotoxins (nerve damaging), cytotoxins (cell damaging), and toxins responsible for allergenic reactions have all been isolated from cyanobacteria. We are particularly interested in the toxic-bloom-forming cyanobacterium Cylindrospermopsis raciborskii. This species produces the alkaloid hepatotoxin cylindrospermopsin, which is also produced by the cyanobacteria Aphanizomenon ovalisporum (3) and Umezakia natans (13).
C. raciborskii is infamous for its association with a human poisoning incident on Palm Island, Australia, in 1979 (14). The illness, which produced hepatitis-like symptoms, lasted between 4 and 26 days and required the hospitalization of most of the 148 reported victims (7). The outbreak occurred immediately after treatment of a dense cyanobacterial bloom in the domestic water supply reservoir with copper sulfate. Copper sulfate is known to cause lysis of cyanobacteria and the release of any toxic cellular components into the water. Although the organisms in the original bloom were not identified before treatment with copper sulfate, follow-up studies indicated C. raciborskii as the most likely causative agent of this outbreak (6, 14). More recently, toxic C. raciborskii blooms have also been implicated in the death of cattle in regions of northern Australia (39).
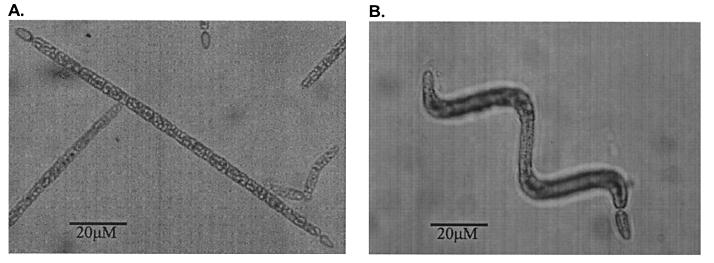
C. raciborskii is a cosmopolitan species found in tropical, subtropical, and temperate climatic regions (2). C. raciborskii is identified by the presence of gas vacuoles and by the shape and dimensions of terminal heterocysts, vegetative cells, and trichomes (20). However, the microscopic identification of C. raciborskii by morphological characteristics can sometimes be influenced by selective culturing techniques. In addition, descriptions of C. raciborskii encompass two distinct morphotypes, both straight (Fig. 1A) and coiled (Fig. 1B) trichomes. The morphological taxonomy of C. raciborskii is not supported by any genetic information, and little is known about the level of genetic similarity between the morphotypes and their phylogenetic relationship to other closely related taxa. A molecular test to identify this toxic cyanobacterial species would therefore be advantageous.
FIG. 1.
Morphotypes of C. raciborskii at ×268 magnification. (A) Straight; (B) coiled.
The use of DNA sequences for the taxonomic and phylogenetic analysis of cyanobacterial isolates has been reviewed (43). A number of genes have been used as evolutionary markers in the delineation of cyanobacterial taxonomy, with the 16S rRNA gene analyzed most extensively because of its ubiquitous distribution throughout prokaryotic phylogenetic groups (11, 27, 36). Although this technique is well established, the DNA-dependent RNA polymerase (rpoC1) gene is suggested to be more discriminatory than 16S rRNA gene analysis at the species level (30). The cyanobacterial rpoC1 gene encodes the γ subunit of RNA polymerase and exists as a single copy in the genome (5).
Nongenotypic approaches to cyanobacterial strain typing have been used (4, 9, 10, 23, 38), with the major limitation of these techniques being the phenotypic variations under different culture conditions. Improved molecular approaches to study cyanobacterial diversity at the strain level have been described (22, 25, 28, 42), but they require the use of axenic cultures, which are difficult to obtain. Cyanobacterium-specific strain-genotyping methods that do not require axenic cultures have also been described. DNA polymorphisms within the intergenic spacer region of the phycocyanin gene locus have been used to infer the genetic relatedness and evolution of toxic and bloom-forming cyanobacteria (26). Short tandemly repeated repetitive (STRR) sequences found to occur at high frequency in the genomes of filamentous, heterocystous cyanobacteria (24) have also been used to establish strain-specific DNA fingerprints. STRR sequences have been used either as oligonucleotide probes (34) or as primers in the generation of PCR-amplified DNA profiles (32).
The present study examines the level of diversity among Australian isolates of C. raciborskii. Strains isolated from geographically diverse populations are compared with respect to their rpoC1 sequences and STRR sequence-generated PCR fingerprints. The phylogenetic relationship of C. raciborskii to other taxonomic groups of cyanobacteria is also presented, based on rpoC1 gene sequences. In addition, this paper describes the design of a PCR test specific for the identification of C. raciborskii. The PCR test targets sequences unique to the C. raciborskii rpoC1 gene, and its robustness is evaluated with laboratory isolates and environmental samples known to contain C. raciborskii.
MATERIALS AND METHODS
Strains and growth conditions.
The cyanobacterial strains examined in this study are listed in Table 1, including information on their countries of origin and trichome morphologies. The strains were grown under constant light intensity (20 μM m−1 s−1) for up to 14 days at 25°C in ASM-1 medium (12), with the exception that a nitrogen source was omitted and Na2MoO4 was added to a final concentration of 0.01 mg ml−1. Environmental samples from Fred Haigh Dam, Queensland, Australia (courtesy of Glenn McGregor, Queensland Department of Health), and Currency Creek, South Australia, Australia, were frozen until they were required.
TABLE 1.
Strains used in this study
| Strain | Origin | Morphologya | Reference or source |
|---|---|---|---|
| C. raciborskii | |||
| AWT205 | NSWd, Australia | Straight | 15 |
| CYP003A | Victoria, Australia | Coiled | AWQCb |
| CYP003K | Victoria, Australia | Straight | AWQC |
| CYP005E | NSW, Australia | Coiled | AWQC |
| CYP005F | Australia | Coiled | AWQC |
| CYP010A | Australia | Coiled | AWQC |
| CYP010C | Australia | Coiled | AWQC |
| CYP014A | Queensland, Australia | Straight | AWQC |
| CYP015A | Queensland, Australia | Straight | AWQC |
| CYP020A | Palm Island, Australia | Straight | AWQC |
| CYP020B | Palm Island, Australia | Straight | AWQC |
| CYP023A | Queensland, Australia | Straight | AWQC |
| CYP023B | Queensland, Australia | Straight | AWQC |
| CYP023D | Queensland, Australia | Coiled/Straight | AWQC |
| CYP023E | Queensland, Australia | Straight | AWQC |
| CYP024C | Queensland, Australia | Straight | AWQC |
| CYP025B | Queensland, Australia | Coiled | AWQC |
| CYP025E | Queensland, Australia | Coiled | AWQC |
| CYP026J | NSW, Australia | Straight | AWQC |
| brazil 1 | Brazil | Unknown | S. Azevado (personal communication) |
| brazil 2 | Brazil | Unknown | S. Azevado (personal communication) |
| A. circinalis | |||
| ANA118C | Australia | AWQC | |
| ANA173A | Australia | AWQC | |
| Microcystis aeruginosa PCC7806 | The Netherlands | 33 | |
| Microcystis aeruginosa | Australia | AWQC | |
| N. spumigena PCC73104 | Canada | 33 | |
| A. bergii ANA283A | Australia | AWQC | |
| Anabaenopsis circularis | Japan | NIESc |
Refers to C. raciborskii trichome structure.
Australian Water Quality Centre culture collection.
National Institute of Environmental Studies culture collection.
NSW, New South Wales.
DNA extraction.
DNA techniques were carried out according to standard procedures (35). Genomic DNA was extracted from cyanobacterial cells essentially as described by Porter (31). Briefly, 50-ml cell cultures were pelleted by centrifugation and resuspended in 0.5 ml of lysis solution (25% sucrose, 50 mM Tris-HCl, 100 mM EDTA). Three freeze-thaw cycles were performed at −80°C and room temperature. The cells were treated with 5 mg of lysozyme for 30 min at 37°C. Sodium dodecyl sulfate and proteinase K (Sigma) were added to final concentrations of 1% and 100 μg ml−1, respectively, and the samples were incubated at 45°C overnight. The DNA was extracted three times with phenol-chloroform and twice with chloroform. The DNA was precipitated, washed with 70% ethanol, resuspended in 100 μl of Tris-EDTA buffer, and stored at −20°C. For environmental samples, DNA was extracted with the InstaGene matrix (Bio-Rad). Briefly, 10-ml samples were pelleted by centrifugation and resuspended in 200 μl of solution containing 90% InstaGene matrix and 10% Triton X-100. The cells were incubated at 55°C for 30 min, vortexed for 1 min, then heated to 95°C for 10 min. Following centrifugation, DNA was extracted once with an equal volume of phenol-chloroform and once with chloroform. The DNA was precipitated, resuspended in 50 μl of water, and used directly in PCRs.
PCR and DNA sequence analysis.
All PCRs were performed on a Perkin-Elmer GeneAmp 2400 PCR system. Each 50-μl reaction mixture contained 1 to 10 ng of genomic DNA, 20 pmol of each PCR primer, 200 μM deoxynucleoside triphosphates, 250 μM magnesium chloride, 1× PCR buffer II, and 2.5 U of Ampli Taq Gold (Perkin-Elmer). Oligonucleotides were purchased from GeneWorks Pty. Ltd. For amplification of the rpoC1 gene from cyanobacterial strains, the following primers were used: rpoC1-1 (5′-GAGCTCYAWNACCATCCAYTCNGG) and rpoC1-T (5′-GGTACCNAAYGGNSARRTNGTTGG) (30). Thermal-cycling conditions for the PCR were 95°C for 10 min, 1 cycle; 92°C for 90 s, 58°C for 1 min, and 72°C for 2 min, 35 cycles; and holding of the sample at 4°C.
The rpoC1 PCR products were sequenced either directly or following ligation into the PCR cloning vector pCR 2.1 (Invitrogen). DNA sequencing was performed on both strands with the Taq DyeDeoxy Terminator cycle-sequencing kit and an automated model 373A DNA sequencer (Applied Biosystems) according to the manufacturer's instructions. Sequences were analyzed with a GeneJockeyII sequence processor (Biosoft), and homology searches were performed in the National Center for Biotechnology Information database with the BLAST network service. Sequence alignments were performed with ClustalX (40).
STRR sequence profiles.
A method that identified genetic variation among C. raciborskii strains was developed by using primers derived from previously identified cyanobacterial repeat sequences (24). The following primers were used: STRR1F (5′-CCCCARTCCCCART), STRR1R (5′-GGGGAYTGGGGAYT), STRR2F (5′-TTGGTCATTGGTCA), STRR2R (5′-TGACCAATGACCAA), STRR3F (5′-CAACAGTCAACAGT), and STRR3R (5′-ACTGTTGACTGTTG). Thermal-cycling conditions were 94°C for 10 min, 1 cycle; 94°C for 30 s, 40°C for 1 min, and 65°C for 4 min, 35 cycles; 65°C for 7 min, 1 cycle; 4°C, hold. All PCRs were performed in at least two independent experiments. STRR profiles were converted to binary data by scoring the presence or absence of bands for each isolate as one or zero. These data were used to calculate total character differences, which were subsequently used to construct a neighbor-joining tree with PAUP* (37).
C. raciborskii-specific PCR.
A PCR test was developed for the specific identification of C. raciborskii. The primers cyl2 (5′-GGCATTCCTAGTTATATTGCCATACTA), cyl4 (5′-GCCCGTTTTTGTCCCTTTCGTGC), and cyl-int (5′-TATTGCCATACTACCTGGTAATGCTGACACACTCG) were used. An internal control fragment (ICF) was produced with the primer cyl-int to spike into PCRs. The cyl-int primer was designed to match a contiguous 22-base sequence 63 bp downstream of primer cyl2. A 13-base sequence at the 3′ end of cyl2 exactly matched a 13-nucleotide overhang at the 5′ end of cyl-int. The ICF was constructed by performing PCRs with cyl-int and cyl4, and the PCR product was used in a final PCR with cyl2 and cyl4 to give a 247-bp ICF. Each 50-μl PCR mixture contained 100 ng of genomic DNA, 20 pmol of cyl2, 20 pmol of cyl4, 200 μM deoxynucleoside triphosphates, 250 μM magnesium chloride, 1× PCR buffer II, 2.5 U of Ampli Taq Gold, and 20 fg of ICF. The thermal-cycling conditions were 95°C for 10 min, 1 cycle; 94°C for 30 s, 45°C for 30 s, and 72°C for 30 s, 35 cycles; 72°C for 15 min, 1 cycle; 4°C, hold.
Phylogenetic analysis.
Phylogenetic analysis of the DNA sequence data was performed with the MEGA analysis platform (21). Briefly, pairwise distances were calculated by the Jukes-Cantor method, and a tree was constructed with the neighbor-joining algorithm. The pairwise-deletion option was used for missing data and gaps in the alignment. Bootstrap analyses were performed with 500 replicates.
Nucleotide sequence accession number.
The nucleotide sequences obtained in this work have been deposited with the GenBank database under the following accession numbers. C. raciborskii consensus rpoC1, AF159371; Nodularia spumigena PCC73104 rpoC1, AF159372; Anabaena circinalis ANA118C rpoC1, AF159373; Anabaenopsis circularis rpoC1, AF159374; and Anabaena bergii ANA283A rpoC1, AF159375.
RESULTS
rpoC1 gene sequence analysis.
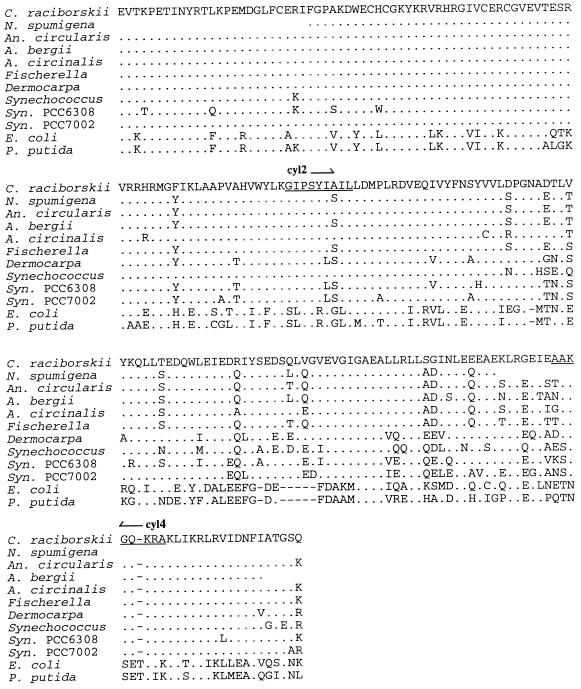
A 609-bp fragment of the rpoC1 gene was amplified and sequenced from each of 19 C. raciborskii isolates. The nucleotide sequences differed at only two sites, with 99 to 100% nucleotide sequence identity observed among strains of C. raciborskii. At position 351, 3 strains of 19 showed a synonymous substitution of T for C. At position 427, 7 strains of 19 showed a change from C to A that caused a change from glutamine to lysine. Neither sequence change could be associated with the trichome morphology or the geographic origin of the isolate. Importantly, the rpoC1 gene in C. raciborskii was found to be highly conserved, indicating that all isolates examined are the same species, including both coiled and straight morphotypes. The rpoC1 gene fragment was also amplified from a selection of other cyanobacteria, namely, A. bergii, A. circinalis, Anabaenopsis circularis, and N. spumigena, and the corresponding amino acid sequences were compared to a selection of cyanobacterial rpoC1 sequences obtained from the GenBank database (Fig. 2). At the amino acid level, the C. raciborskii rpoC1 sequence exhibited 84 to 93% identity to other cyanobacterial species, indicating sufficient variation in the rpoC1 sequences among species to examine the phylogenetic position of C. raciborskii.
FIG. 2.
Amino acid alignment of C. raciborskii, N. spumigena PCC73104, Anabaenopsis circularis, A. bergii ANA283A, and A. circinalis ANA118C rpoC1 sequences and additional rpoC1 sequences obtained from the GenBank database: Fischerella sp. strain PCC7414 (accession no. FSPRPOC1), Synechocystis (Syn.) sp. strain PCC6308 (accession no. U52344), Synechocystis sp. strain PCC7002 (accession no. U52345), Synechococcus sp. strain PCC7942 (accession no. SRPOC1), Dermocarpa sp. (accession no. U52341), E. coli (accession no. ECRPOBC), and P. putida (accession no. M38319). Amino acids identical to those of C. raciborskii are indicated by dots; the dashes represent gaps introduced into the alignment. The relative locations of primers cyl2 and cyl4 are also indicated (underlined).
Phylogenetic analysis of C. raciborskii.
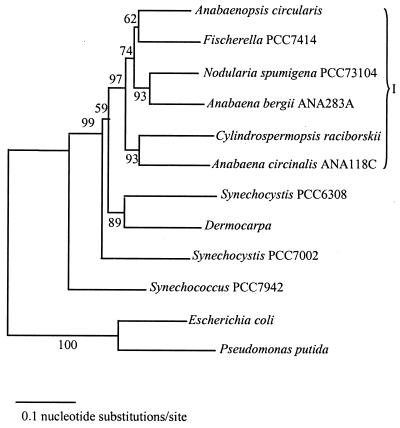
Jukes-Cantor distances, generated by pairwise comparisons of the isolates, were used to create a phylogenetic tree by neighbor-joining analysis (Fig. 3). In this analysis, Escherichia coli and Pseudomonas putida were included as outgroup taxa to root the tree. The phylogenetic relationships inferred from rpoC1 sequence comparison generally supported the traditional classification of cyanobacteria based on morphological criteria. One distinct cluster was apparent (cluster I [Fig. 3]), dominated by the filamentous forms containing heterocysts representing the orders Nostocales and Stigonematales. Genera representing simple coccoid forms which lack differentiation into specialized cell types were external to cluster I. Fischerella sp. strain PCC7414 is grouped within the Nostocales cluster despite significant morphological disparities, such as multiseriate trichomes. In addition, further anomalies are evident by the paraphyly of the two Anabaena species and the higher affinity between A. circinalis and C. raciborskii. Morphological support for the latter appears to be lacking (16).
FIG. 3.
Phylogenetic position of C. raciborskii in relation to other cyanobacteria (sequences resulting from this work and obtained from the GenBank database) and to E. coli and P. putida based on analysis of aligned rpoC1 nucleotide sequences. The neighbor-joining tree was constructed by using corrected Jukes-Cantor distances. Boostrap percentages (calculated from 500 resamplings) are indicated for the nodes. I, cluster I.
STRR sequence profiles.
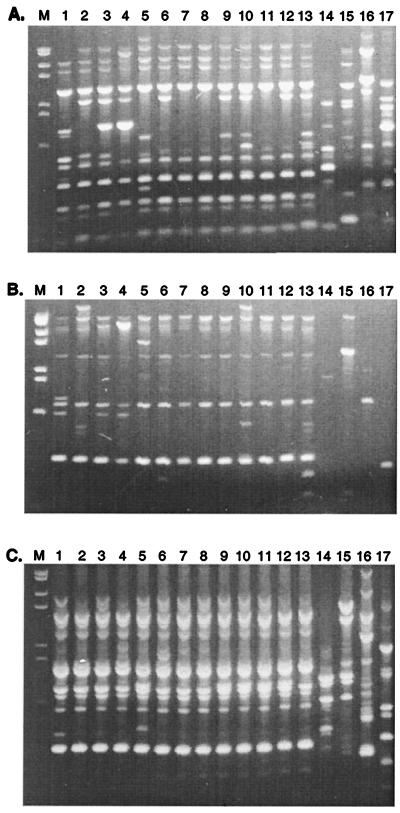
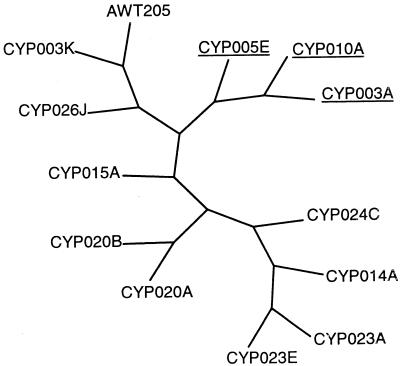
Since rpoC1 sequences did not reveal significant differences among isolates of C. raciborskii, STRR sequence profiles were used to examine the level of genetic diversity. Thirteen isolates produced growth to sufficient density for use in STRR sequence analysis. PCR amplification with primers derived from cyanobacterium-specific STRR sequences were used. Various combinations of these primers established a DNA fingerprint pattern for each isolate (Fig. 4). This analysis revealed unique band profiles among each of the different cyanobacterial genera examined, with the extent of this variation dependent on the primer combination used. The amplified PCR products ranged in size from approximately 0.1 to 2.5 kb. Combinations of STRR1R and STRR3R (Fig. 4C) yielded very similar patterns for all C. raciborskii isolates, while the greatest variation was seen with the primer combination of STRR1F and STRR3F (Fig. 4A). Differences were also observed among C. raciborskii strains isolated from the same population, i.e., strains CYP003A and CYP003K (Fig. 4, lanes 1 and 2) and strains CYP023A and CYP023E (Fig. 4A and B, lanes 9 and 10). Interestingly, two of the primer combinations yielded fragments unique to the three C. raciborskii isolates with coiled trichomes (Fig. 4A and B, lanes 1, 3, and 4). Information from the STRR sequence banding patterns was represented graphically by converting the bands to binary data, which was then used to construct a tree (Fig. 5). The tree shows similarities among different isolates according to their banding patterns. Interestingly, all of the isolates examined that had coiled trichomes grouped together.
FIG. 4.
STRR sequence profile analysis of 13 isolates of C. raciborskii and other representative genera obtained by PCR amplification with primers STRR1F and STRR3F (A), STRR1F and STRR3R (B), and STRR1R and STRR3R (C). Lanes 1, CYP003A; lanes 2, CYP003K; lanes 3, CYP005E; lanes 4, CYP010A; lanes 5, CYP014A; lanes 6, CYP015A; lanes 7, CYP020A; lanes 8, CYP020B; lanes 9, CYP023A; lanes 10, CYP023E; lanes 11, CYP024C; lanes 12, CYP026J; lanes 13, AWT205; lanes 14, A. circinalis ANA173A; lanes 15, A. circinalis ANA118C; lanes 16, Microcystis aeruginosa PCC7806; lanes 17, N. spumigena PCC73104. Lane M, molecular size marker: 2,027, 1,904, 1,584, 1,375, 947, 831, and 564 bp.
FIG. 5.
Graphical representation of the branching pattern of C. raciborskii STRR sequence profiles derived from total character differences by the neighbor-joining method. The isolates showing coiled morphology are underlined.
C. raciborskii-specific PCR.
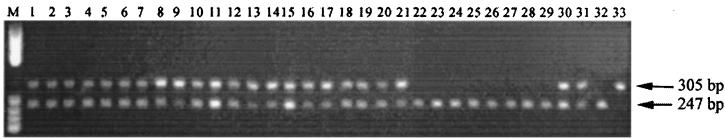
Although little difference was found among the rpoC1 sequences of C. raciborskii isolates, there was sufficient difference between the rpoC1 sequence of C. raciborskii and those of other species to design a specific PCR test for C. raciborskii. Primers cyl2 and cyl4 (Fig. 2) were used to amplify a 305-bp diagnostic PCR product from the rpoC1 genes of C. raciborskii isolates. Twenty femtograms of ICF per reaction was found to be the minimum amount required to yield a discernible product following gel electrophoresis of PCR products. The PCR test was used to screen all of the strains listed in Table 1, and the results are shown in Fig. 6. All C. raciborskii isolates produced positive reactions, with amplification of both the diagnostic 305-bp product and the ICF. Chromosomal DNA extracted from two Brazilian isolates, which had previously been tentatively identified as C. raciborskii (Table 1), also tested positive by PCR. The diagnostic product was absent in all other cyanobacterial strains tested, from which only the ICF was amplified. The PCR test was then applied to the direct analysis of two environmental samples, obtained from South Australia and Queensland, that were known to contain C. raciborskii. Significantly, both the diagnostic product and the ICF were amplified from both samples. The test described here is the first report of a rapid method for the identification of C. raciborskii directly from environmental samples.
FIG. 6.
C. raciborskii-specific PCR. Genomic DNA from laboratory cultures and environmental samples was amplified with primers cyl2 and cyl4 in a PCR spiked with ICF. Lane 1, AWT205; lane 2, CYP003A; lane 3, CYP003K; lane 4, CYP005E; lane 5, CYP005F; lane 6, CYP010A; lane 7, CYP010C; lane 8, CYP014A; lane 9, CYP015A; lane 10, CYP020A; lane 11, CYP020B; lane 12, CYP023A; lane 13, CYP023B; lane 14, CYP023D; lane 15, CYP023E; lane 16, CYP024C; lane 17, CYP025B; lane 18, CYP025E; lane 19, CYP026J; lane 20, brazil 1; lane 21, brazil 2; lane 22, A. circinalis ANA118C; lane 23, A. circinalis ANA173A; lane 24, Microcystis aeruginosa PCC7806; lane 25, M. aeruginosa; lane 26, N. spumigena PCC73104; lane 27, N. spumigena; lane 28, A. bergii ANA283A; lane 29, Anabaenopsis circularis; lane 30, environmental sample (Fred Haigh Dam, Queensland, Australia); lane 31, environmental sample (Currency Creek, South Australia, Australia); lane 32, ICF only; lane 33, CYP014A only. Lane M, molecular size marker: 587, 540, 504, 458, 434, 267, 234, 213, 192, and 184 bp.
DISCUSSION
In this study the level of genetic diversity in a selection of Australian C. raciborskii isolates was examined. Although rpoC1 sequences were unable to distinguish among several strains of C. raciborskii, STRR sequence-generated PCR fingerprints were able to discriminate coiled from straight trichomes of C. raciborskii. A comparison of C. raciborskii rpoC1 profiles with those of other cyanobacteria resulted in a more comprehensive analysis of its cyanobacterial phylogeny than was previously available. As a consequence of this study, a rapid method has also been developed for the specific identification of C. raciborskii directly from environmental samples.
The 16S rRNA gene represents the most highly studied gene for identification and phylogenetic analysis. rpoC1 gene analysis has been shown to be more discriminatory than 16S rRNA analysis (30). PCR primers designed from conserved regions of the cyanobacterial rpoC1 gene (30) were used to analyze the C. raciborskii isolates. In a previous study, these primers were used in PCRs for strain-level identification of a number of taxonomic groups (41). In addition, they have been used to examine the phylogenetic relationship of prochlorophytes to each other and to the green chloroplasts (29) and to study the diversity of the cyanobacterial genus Synechococcus (41). We therefore hypothesized that sequence analysis of the rpoC1 gene might enable differentiation among strains of C. raciborskii isolated from both mixed cyanobacterial communities and monospecific blooms over a 10-year period from tropical and temperate regions in Australia and might even identify a genetic difference between coiled and straight morphotypes. However, although there was sufficient sequence variation at the amino acid level to distinguish and group other cyanobacteria in relation to C. raciborskii (Fig. 2), there was insufficient discrimination even at the nucleotide level to distinguish among C. raciborskii isolates.
Phylogenetic analysis of the partial rpoC1 gene sequence among those cyanobacterial species selected in this study indicated one distinct cluster (Fig. 3) and agreed with an earlier phylogenetic classification of cyanobacteria based on both the rpoC1 gene and the 16S rRNA gene (26, 29, 43). Cluster I (Fig. 3) consisted mostly of representative genera of the order Nostocales (Anabaenopsis circularis, N. spumigena, A. circinalis, A. bergii, and C. raciborskii). Genera of the order Chroococcales (Synechocystis, Dermocarpa, and Synechococcus) were placed external to this cluster. Unlike the simple aggregation of vegetative cells in the Chroococcales, representative taxa of the Nostocales are characterized by differentiation of cells with a specialized function (e.g., heterocysts). Within the Nostocales cluster there was no apparent grouping of common phenotypic features, such as the position and mode of heterocyst differentiation or trichome morphology. The grouping of Fischerella (order Stigonematales) within the Nostocales cluster does not reflect the significant morphological differences that separate them in the traditional classification hierarchy (i.e., Fischerella is characterized by the production of multiseriate branched filaments), although taxa of the Stigonematales are morphologically more similar to the Nostocales than to the Chroococcales (1, 18, 19). Further studies would be required to genetically characterize other species of the order Stigonematales and validate their phylogenetic relationship to the cluster of Nostocales taxa. The paraphyletic distribution of both Synechocystis and Anabaena species also contradicted the traditional classification system.
In a previous study, primers based on the STRR1 repeat sequence were used in PCRs to fingerprint symbiotic cyanobacterial isolates from the angiosperm Gunnera (32). These results demonstrated both high genetic diversity and distinct clustering of symbiotic Nostoc isolates. Our attempts to fingerprint C. raciborskii isolates with the same primers did not generate any PCR products, a result which may reflect the number, position, and orientation of these repeat sequences within the C. raciborskii genome. In order to overcome this problem, we used different combinations of the three known STRR cyanobacterial sequences as primers in PCRs. The STRR primer combinations described here produced clear and reproducible PCR banding patterns among our C. raciborskii isolates. Only minor or no PCR products were obtained from control bacterial strains, including E. coli, Pseudomonas, Bacillus subtilis, and Klebsiella pneumoniae (data not shown). Our results reveal genetic heterogeneity among Australian C. raciborskii isolates and demonstrate that this strain variation also exists within a single cyanobacterial population or bloom.
The efficient management of water bodies currently relies on obtaining an accurate identification of problematic cyanobacterial species. With traditional microscopic methods, identification by morphological criteria can result in errors of subjective judgment by operators, and in the past isolates have been assigned to the wrong genus. This inconsistency in identification highlights the requirement for better identification methods. The rpoC1 gene sequence data presented here showed sufficient variation between C. raciborskii and closely related cyanobacteria to design primers specific to the C. raciborskii rpoC1 gene. A PCR test was developed to amplify a 305-bp C. raciborskii-specific rpoC1 fragment from both laboratory and environmental samples. It was necessary to pretreat environmental samples with phenol and chloroform to reduce the level of inhibitors to PCR present in the samples. As an additional control, the ICF served to verify that negative reactions were indeed negative and not due to PCR inhibition by some other factor. This test is the first report of a molecular method to identify C. raciborskii directly from environmental samples without the need for axenic culture conditions. Our PCR test will allow early detection of C. raciborskii, thus enabling efficient management before a bloom occurs and tracking of C. raciborskii throughout a water body.
ACKNOWLEDGMENTS
We thank Jenny House and Renate Velzeboer for expert technical assistance, Paul Monis for assistance with phylogenetic tree construction, and Peter Hawkins, Glenn McGregor, and Maria Runnegar for provision of strains. We also thank Brett Neilan for provision of DNA from Brazilian isolates.
K.M.W. and M.A.S. were supported by a grant from the Cooperative Research Centre for Water Quality and Treatment awarded to C.P.S.
REFERENCES
- 1.Anagnostidis K, Komarek J. Modern approach to the classification system of cyanophytes. 5-Stigonematales. Algol Stud. 1990;59:1–73. [Google Scholar]
- 2.Baker P D, Humpage A R. Toxicity associated with commonly occurring cyanobacteria in surface waters of the Murray Darling Basin, Australia. Aust J Mar Freshwater Res. 1994;45:773–786. [Google Scholar]
- 3.Banker R S, Carmeli S, Hadas O, Teltsch B, Porat R, Sukenik A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Isr. J Phycol. 1997;33:613–616. [Google Scholar]
- 4.Ben-Amotz A, Tornabene T G, Thomas W H. Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol. 1985;21:72–81. [Google Scholar]
- 5.Bergsland K J, Haselkorn R. Evolutionary relationships among eubacteria, cyanobacteria, and chloroplasts: evidence from the rpoC1 gene of Anabaena sp. strain PCC 7120. J Bacteriol. 1991;173:3446–3455. doi: 10.1128/jb.173.11.3446-3455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourke A T C, Hawes R B, Neilson A, Stallman N D. An outbreak of hepatoenteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon. 1983;3:45–48. [Google Scholar]
- 7.Byth S. Palm Island mystery disease. Med J Aust. 1980;2:40–42. doi: 10.5694/j.1326-5377.1980.tb131814.x. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael W W. The cyanotoxins. Adv Bot Res. 1997;27:211–256. [Google Scholar]
- 9.Caudales R, Wells J M. Differentiation of free-living Anabaena and Nostoc cyanobacteria on the basis of fatty acid composition. Int J Syst Bacteriol. 1992;42:246–251. doi: 10.1099/00207713-42-2-246. [DOI] [PubMed] [Google Scholar]
- 10.Erhard M, van Dohren H, Jungblut P. Rapid typing and elucidation of new secondary metabolites of intact cyanobacteria using MALDI-TOF mass spectrometry. Nat Biotech. 1997;15:906–909. doi: 10.1038/nbt0997-906. [DOI] [PubMed] [Google Scholar]
- 11.Fan L, Devi S K, Chitnis V P, Chitnis P R. Molecular genetics of cyanobacteria: new adventures in biotechnology. J Sci Ind Res. 1996;55:555–563. [Google Scholar]
- 12.Gorham P R, McLachlan J, Hammer U T, Kim W K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) deBreb. Verh Int Ver Limnol. 1964;15:796–804. [Google Scholar]
- 13.Harada K, Ohtani K, Iwamoto K, Suzuki M, Watanabe M F, Watanabe M, Terao K. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon. 1994;32:73–84. doi: 10.1016/0041-0101(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins P R, Runnegar M T, Jackson A R, Falconer I R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl Environ Microbiol. 1985;50:1292–1295. doi: 10.1128/aem.50.5.1292-1295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins P R, Chandrasena N R, Jones G J, Humpage A R, Falconer I R. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon. 1997;35:341–346. doi: 10.1016/s0041-0101(96)00185-7. [DOI] [PubMed] [Google Scholar]
- 16.Horecka M, Komarek J. Taxonomic position of three planktonic blue-green algae from the genera Aphanizomenon and Cylindrospermopsis. Preslia. 1979;51:289–312. [Google Scholar]
- 17.Hunter P R. Cyanobacterial toxins and human health. J Appl Microbiol Symp Suppl. 1998;84:35S–40S. doi: 10.1046/j.1365-2672.1998.0840s135s.x. [DOI] [PubMed] [Google Scholar]
- 18.Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes. 2-Chroococcales. Arch Hydrobiol Suppl. 1986;73(Algol. Stud. 43):157–226. [Google Scholar]
- 19.Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes. 4-Nostocales. Arch Hydrobiol Suppl 82 (Algol Stud) 1989;56:247–345. [Google Scholar]
- 20.Komarek J, Kling H. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa) Algol Stud. 1991;61:21–45. [Google Scholar]
- 21.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis, version 1.0. University Park: The Pennsylvania State University; 1993. [Google Scholar]
- 22.Lu W, Evans H, McColl S M, Saunders V A. Identification of cyanobacteria by polymorphisms of PCR-amplified ribosomal DNA spacer region. FEMS Microbiol Lett. 1997;153:141–149. [Google Scholar]
- 23.Lyra C, Hantula J, Vainio E, Rapala J, Rouhiainen L, Sivonen K. Characterization of cyanobacteria by SDS-PAGE of whole-cell proteins and PCR/RFLP of the 16S rRNA gene. Arch Microbiol. 1997;168:176–184. doi: 10.1007/s002030050485. [DOI] [PubMed] [Google Scholar]
- 24.Mazel D, Houmard J, Castets A M, Tandeau de Marsac N. Highly repetitive DNA sequences in cyanobacterial genomes. J Bacteriol. 1990;172:2755–2761. doi: 10.1128/jb.172.5.2755-2761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neilan B A. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl Environ Microbiol. 1995;61:2286–2291. doi: 10.1128/aem.61.6.2286-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan B A, Jacobs D, Goodman A E. Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl Environ Microbiol. 1995;61:3875–3883. doi: 10.1128/aem.61.11.3875-3883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilan B A, Hawkins P R, Cox P T, Goodman A E. Towards a molecular taxonomy for the bloom-forming cyanobacteria. Aust J Mar Freshwater Res. 1994;45:869–873. [Google Scholar]
- 28.Nishihara H, Miwa H, Watanabe M, Nagashima M, Yagi O, Takamura Y. Random amplified polymorphic DNA (RAPD) analyses for discriminating genotypes of Microcystis cyanobacteria. Biosci Biotech Biochem. 1997;61:1067–1072. doi: 10.1271/bbb.61.1067. [DOI] [PubMed] [Google Scholar]
- 29.Palenik B, Swift H. Cyanobacterial evolution and prochlorophyte diversity as seen in DNA-dependent RNA polymerase gene sequences. J Phycol. 1996;32:638–646. [Google Scholar]
- 30.Palenik B, Haselkorn R. Multiple evolutionary origins of prochlorophytes, the chlorophyll-containing prokaryotes. Nature. 1992;355:265–267. doi: 10.1038/355265a0. [DOI] [PubMed] [Google Scholar]
- 31.Porter R D. DNA transformation. Methods Enzymol. 1988;167:703–712. doi: 10.1016/0076-6879(88)67081-9. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen U, Svenning M M. Fingerprinting of cyanobacteria based on PCR with primers derived from short and long tandemly repeated repetitive sequences. Appl Environ Microbiol. 1998;64:265–272. doi: 10.1128/aem.64.1.265-272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rippka R, Herdman M. Pasteur culture collection (PCC) of cyanobacterial strains in axenic culture. 1. Catalogue of strains. Paris, France: Institut Pasteur; 1992. [Google Scholar]
- 34.Rouhiainen L, Sivonen K, Buikema W, Haselkorn R. Characterization of toxin-producing cyanobacteria by using an oligonucleotide probe containing a tandemly repeated heptamer. J Bacteriol. 1995;177:6021–6026. doi: 10.1128/jb.177.20.6021-6026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Shaw G, Sukenik A, Livne A, Chiswell R, Smith M J, Seawright A A, Norris R, Eaglesham G K, Moore M R. Blooms of the cylindrospermopsin containing cyanobacterium, Aphanizomenon ovalisporum (Forti), in newly constructed lakes, Queensland, Australia. Environ Toxicol. 1999;14:167–177. [Google Scholar]
- 37.Swofford D L. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), version 4.0b2a. Sunderland, Mass: Sinauer Associates; 1999. [Google Scholar]
- 38.Tatsumi K, Watanabe M F, Watanabe M. Allozyme divergence in Anabaena (Cyanophyceae) and its taxonomic inference. Arch Hydrobiol Suppl. 1991;92(Algol. Stud. 64):129–140. [Google Scholar]
- 39.Thomas A D, Saker M L, Norton J H, Olsen R D. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust Vet J. 1998;76:592–594. doi: 10.1111/j.1751-0813.1998.tb10233.x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toledo G, Palenik B. Synechococcus diversity in the California current as seen by RNA polymerase (rpoC1) gene sequences of isolated strains. Appl Environ Microbiol. 1997;63:4298–4303. doi: 10.1128/aem.63.11.4298-4303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilmotte A. Molecular evolution and taxonomy of the cyanobacteria. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–25. [Google Scholar]