Abstract
The increased use of plastics has led to severe environmental pollution, particularly by microplastics—plastic particles 5 mm or less in diameter. These particles are formed by environmental factors such as weathering and ultraviolet irradiation, thereby making environmental pollution worse. This environmental pollution intensifies human exposure to microplastics via food chains. Despite potential negative effects, few toxicity assessments on microplastics are available. In this study, two sizes of polytetrafluoroethylene (PTFE) microplastics, approximately 5 μm and 10–50 μm, were manufactured and used for single and four-week repeated toxicity and pharmacokinetic studies. Toxicological effects were comprehensively evaluated with clinical signs, body weight, food and water consumption, necropsy findings, and histopathological and clinical-pathological examinations. Blood collected at 15, 30 60, and 120 min after a single administration of microplastics were analyzed by Raman spectroscopy. In the toxicity evaluation of single and four-week repeated oral administration of PTFE microplastics, no toxic changes were observed. Therefore, the lethal dose 50 (LD50) and no-observed-adverse-effect-level (NOAEL) of PTFE microplastics in ICR mice were established as 2000 mg/kg or more. PTFE microplastics were not detected in blood, so pharmacokinetic parameters could not be calculated. This study provides new insight into the long-term toxicity and pharmacokinetics of PTFE microplastics.
Keywords: microplastics, polytetrafluoroethylene, toxicity evaluation, pharmacokinetics
1. Introduction
Globally, the amount of plastic used is steadily increasing, with production rising from 1.5 million tons in the 1950s to 3.35 billion tons in 2016 [1]. Some plastics are recycled, but most are discarded, often causing environmental pollution. Plastic particles with a size of less than 5 mm are called microplastics [2,3]. These small particles are designated as one of the world’s four major environmental issues, along with environmental changes, ozone layer destruction, and marine oxidation [4]. Microplastics are classified as primary microplastics made for use in personal care products, cosmetics, toothpaste, detergents, sunscreens, and drug vectors [5,6,7]. Secondary microplastics are formed by environmental factors, such as UV oxidation, light degradation, and physical ablation [5,8,9,10]. Primary microplastics are mainly spherical in keeping with their uses, and secondary microplastics are mostly fractured or fibrous forms due to weathering and other environmental factors. The latter particles cause environmental pollution worldwide [11,12]. Studies on rivers and seas detect large amounts of microplastics [13,14,15]. In addition, secondary microplastics are small, making them difficult to remove during sewage treatment [16]. Thus, particles are released into receiving waters and eventually flow into the seas. These microplastics may stick to the gills of aquatic organisms during oxygen exchange or when particles are mistaken for food [17]. Subsequently, microplastics accumulate in these aquatic organisms and can be detected in various ways [18,19]. The consumption of aquatic organisms that have accumulated microplastics might be harmful to humans, making toxicological research essential. Further, many studies report microplastics of various sizes, colors, and types, such as polyethylene (PE), polypropylene (PP), and polyrthylene terephthalate (PET), in easily accessible foods (mineral water, salt, beer, wine, canned foods, milk, seaweed) [20,21,22,23,24,25,26,27]. Exposure to microplastics can occur via inhalation, dermal absorption, or ingestion, with the ingestion route being the most important [28]. In vitro, microplastics show increased accumulation and toxicity through the inhibition of ATP-binding cassette transporter activity in Caco-2 cells [29] and significantly increase the production of ROS in human-derived HDFs, PBMCs, and Raw 264.7 cells [30]. Oxidative stress increased in human cerebral cells (T98G) and epitaxial cells (HeLa) after exposure to microplastics [31]. These impacts at the cellular level suggest that microplastics might exhibit toxicity in humans. Experiments at this cell level suggest that microplastics can potentially exhibit influence or toxicity in humans. Recently, in vivo experiments using microplastics are on the rise. In aquatic organisms, PE and PP caused increased mortality and gut clearance times and decreased growth of Hyalella azteca [32]. Polystyrene (PS) caused reduced body weight, body length, and body mass index and increased inflammatory cytokine and chemokine gene expression in zebrafish [33]. In oysters, a decrease in the number and diameter of female oocytes, the speed and activity of male sperm, and the growth rate of larval were observed [34]. In experiments using mammals, PE also altered intestinal microbial populations in C57BL/6 mice and cause inflammatory cell infiltration and loosening of glands in the colon [35]. PS caused inflammation of testicles, fibrosis of ovaries, adipose metabolic disorders in the liver, a reduction of mucin secretion in the colon, and a change in the intestinal microbial population in intestinal microorganisms [36,37,38,39]. PS is distributed to the liver, kidney, and gastrointestinal tract, and affects energy metabolism, lipid metabolism, oxidative stress, and neurological function [40]. Additionally, our previous study showed that polyethylene microplastics can induce granulomatous inflammation in the lungs after four-week repeated oral administrations [41]. Thus, microplastics may be toxic to various tissues, including reproductive and nervous systems. PTFE is a plastic with a density of 2.0 g/m3 and 2.3 g/m3 for amorphous and crystalline portions, respectively [42]. Unlike general plastics, it is used to coat kitchen utensils applied via spraying. Most of these kitchenware coatings can peel off during cooking through heating or friction with utensils [43]. Released PTFE can be incorporated into cooked food and subsequently ingested. Despite this high potential for human exposure, the number of animal experiments using microplastics is still small, and most animal experiments used microplastics composed of polystyrene and polyethylene. PTFE is rarely studied in vivo for toxicity and kinetics in mammals. We manufactured two sizes of PTFE microplastics to confirm whether the manufactured microplastics conform to the commonly used definition of microplastics, under the size of 5 mm. A typical toxicity test was conducted using manufactured microplastics to establish LD50 and NOAEL, and a pharmacokinetic experiment was conducted to determine the organs in which microplastics are widely distributed in the body.
2. Materials and Methods
2.1. Preparation of Polytetrafluoroethylene Microplastics
Two sizes of PTFE microplastics (approximately 5 μm and 10–50 μm) were made from PTFE raw materials (TF1641, DyneonTM, Aston, PA, USA). For the preparation of PTFE 10~50 μm particles, PTFE was frozen at −78 °C with dry ice and ground using a blade-type homogenizer for 4–5 h. Particles were filtered sequentially through 63 μm and 10 μm mesh filters and washed 4–5 times with ethanol. Particles were then dried in an oven at 50 °C for 48 h. For the preparation of approximately 5 μm PTFE particles, 10~50 μm particles of PTFE were dispersed in ethanol and ground with a high-pressure homogenizer 4 times with 600 bar. Then we separated particles sequentially using a 15 μm mesh filter and a 5 μm mesh filter and washed particles with ethanol 4~5 times. Finally, the particles were dried for 48 h in a 50 °C oven.
2.2. Characterization of Polytetrafluoroethylene Microplastics
The average particle size was measured using a Particle Size Analyzer based on light scattering (PSA, ELS-Z2Plus, Otsuka Electronics, Osaka, Japan). This method can be used to obtain the hydrodynamic size of a particle using the Stokes–Einstein relationship by analyzing the fluctuation of scattered light by suspended particles when illuminated with a laser to determine the rate of Brownian motion. The shape of microplastics was confirmed using a 3D-laser profile (Confocal microscopy, Keyence, Itasca, IL, USA) and scanning electron microscopy (SEM, JSM-6701F, JEOL Inc., Akishima, Tokyo, Japan). Finally, chemical identity was confirmed using a Raman microscope (RAMANtouch, Nanophoton, Osaka, Japan) equipped with a 532 nm-laser diode. The morphology of microplastics was examined with a 20× objective lens (Nikon LU Plan Flour 20×/0.45), then Raman spectra were collected in the 45.3–4273.8 cm−1 range using 300 lines per mm grating with a 50 µm slit width. Spectra were measured over a 16-bit dynamic range with Peltier cooled charge-coupled device detectors. The laser power was adjusted to 2 mW for each scan to obtain a sufficient signal. The acquisition time and the number of accumulations were adjusted for each measurement to obtain sufficient data for library searching. The spectrometer was calibrated with silicon at a line of 520 cm−1 before spectral gaining. Raman spectra were analyzed using the SLOPP Library of Microplastics and the spectral library in KnowItAll software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Similarities above the Hit Quality Index of 80 were considered satisfactory.
2.3. Animal and Ethics Statement
Five-week-old specific-pathogen-free ICR mice, 128 male and 104 female, were purchased from KOATECH Inc., Pyeongtaek, Gyunggi-do, Korea. Mice were housed in a standard SPF facility in ventilated IVC cages (395W × 346D × 213H) at 22 ± 1 °C, relative humidity of 50 ± 10%, a ventilation time of 10–15 h, light for 12 h per day, and illumination of 150–300 lux. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Center of the DGMIF (IACUC; approval No. DGMIF-21031601-00) and were in accordance with their guidelines.
2.4. Single Oral Dose Toxicity Study
Two groups of 12 male and 12 female mice were acclimatized for 1 week to evaluate the different sizes of PTFE microplastics. Each group of animals was divided into four subgroups (control, low-dose (500 mg/kg), mid-dose (1000 mg/kg), and high-dose (2000 mg/kg)) with three male and three female animals for each subgroup. A single dose of corn oil was administered to control mice, and microplastics suspended in corn oil were administered for each of three microplastic doses. The volume of corn oil was constant at 10 mL/kg. The study was conducted with reference to OECD guideline 423. The observation of clinical signs, the presence of moribund or dead animals, and the measurement of body weight were performed once a day, twice a day, and once a week, respectively, during the two-week observation period. All animals were anesthetized with CO2 at the end of the study and exsanguinated through the abdominal aorta. Complete gross postmortem examination was performed on all mice.
2.5. Four-Week Repeated Oral Dose Toxicity Study
Two groups of 40 male and 40 female mice were acclimatized for 1 week to evaluate different sizes of PTFE microplastics. Each group of animals was divided into four subgroups (control, low-dose (500 mg/kg), mid-dose (1000 mg/kg), and high-dose (2000 mg/kg)) with ten male and ten female animals for each subgroup. Four-week repeated administration of corn oil for the control group and microplastics suspended in corn oil for each of three microplastic doses were performed. Animals in the remaining groups received microplastics suspended in corn oil to achieve the above doses for each particle size. The volume of corn oil was constant at 10 mL/kg. This study was conducted with reference to OECD guideline 408. The observation of clinical signs and the presence of moribund or dead animals were recorded once a day and twice a day, respectively. The measurement of body weight, food consumption, and water consumption were all measured once a week. After four weeks, blood was collected from the abdominal aorta under isoflurane (Hana Pharm, Co., Ltd., Seoul, Korea) anesthesia. Blood samples collected from animals were analyzed using a blood cell analyzer (ADVIA 2120i, SIEMENS, Muenchen, Germany) and a serum biochemistry analyzer (TBA 120-FR, Toshiba, Tokyo, Japan). Complete gross postmortem examinations were performed on all animals and tissues. The adrenal glands, brain, cecum, colon, duodenum, epididymis, esophagus, heart, ileum, jejunum, kidney, liver, lungs, ovary, pancreas, parathyroid glands, pituitary gland, rectum, spinal cord, spleen, stomach, testis, thymus, thyroid gland, trachea, and uterus were harvested. Organ weights were recorded for the brain, spleen, heart, kidney, liver, testis, epididymis, and ovary. Tissues were fixed in 10% neutral buffered formalin (BBC Biochemicals, Mount Vernon, WA, USA), except for testes, which were fixed in Davison’s fixative followed by storage in 10% neutral buffered formalin. A tissue processor (Thermo Fisher Scienctific, Inc., Runcorn, UK) was used to prepare fixed tissues. Four-micrometer sections were cut from paraffin-embedded tissue blocks and mounted onto glass slides. Sections were then stained with hematoxylin and eosin using an autostainer (Dako Coverstainer; Agilent, Santa Clara, CA, USA). Personnel that performed histopathological evaluations were blind to the sources of tissues.
2.6. Pharmacokinetics Study
Pharmacokinetics of PTFE was characterized in 24 male mice acclimatized for 1 week. Animals were divided into 2 groups with social housing of three mice per cage. Animals were administered a single oral dose of 2000 mg/kg PTFE microplastics, followed by blood collection after 15, 30, 60, and 120 min. Groups of animals were administered one of the two sizes of PTFE particles.
2.7. Quantitative Evaluation of Polytetrafluorethylene Microplastics in Blood
Whole blood collected at predetermined times was pooled, and a 10 wt% aqueous KOH solution (20 times sample weight) was added. These samples were incubated at 37 °C for 48 h with shaking at 250 rpm. Sample solutions were filtered stepwise through stainless-steel (47 mm disk, 45 µm pore size) and silicon filters (1 cm × 1 cm, 1 um pore size) provided by Nanophoton. The Raman microscope used RAMANtouch to assess the number of PTFE microplastics collected on silicon filters.
2.8. Data Analysis
A non-compartmental analysis was performed to calculate pharmacokinetic parameters for PTFE using Phoenix Winnonlin 8.1 software. The area under the plasma concentration–time curve (AUClast) from time zero to infinity (AUC∞) was calculated with linear trapezoidal and standard area extrapolation methods. Terminal half-life (t1/2) was calculated as 0.693/λ, where λ represents the terminal slope of the log-linear of larotrectinib concentration–time profile. Total clearance (CL) for PTFE was calculated as dose/AUC∞ and steady-state volume of distribution (Vss) was then calculated as MRT × CL. The observed maximum concentration (Cmax) and time to reach Cmax (Tmax) were obtained directly from individual PTFE plasma concentration–time profiles.
2.9. Human NOAEL Dose
The human NOAEL dose was estimated using the HED (human equivalent dose) converting table for mice. A 2000 mg/kg dose, the assumed NOAEL, was used for the calculation (Equation (1)) because PTFE plasma concentrations were not recorded within the range of 500~2000 mg/kg.
| Dosehuman (mg/kg) = Dosemouse (mg/kg) × (Kmmouse/Kmhuman) | (1) |
2.10. Statistical Analysis
All hematology, serum biochemistry, and body weight data are presented as means ± standard deviation. The statistical significance of the differences between treated and control animals was evaluated using Student’s t-tests and one-way analysis of variance with the SAS program (version 9.4, SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Characterization of Polytetrafluoroethylene Microplastics
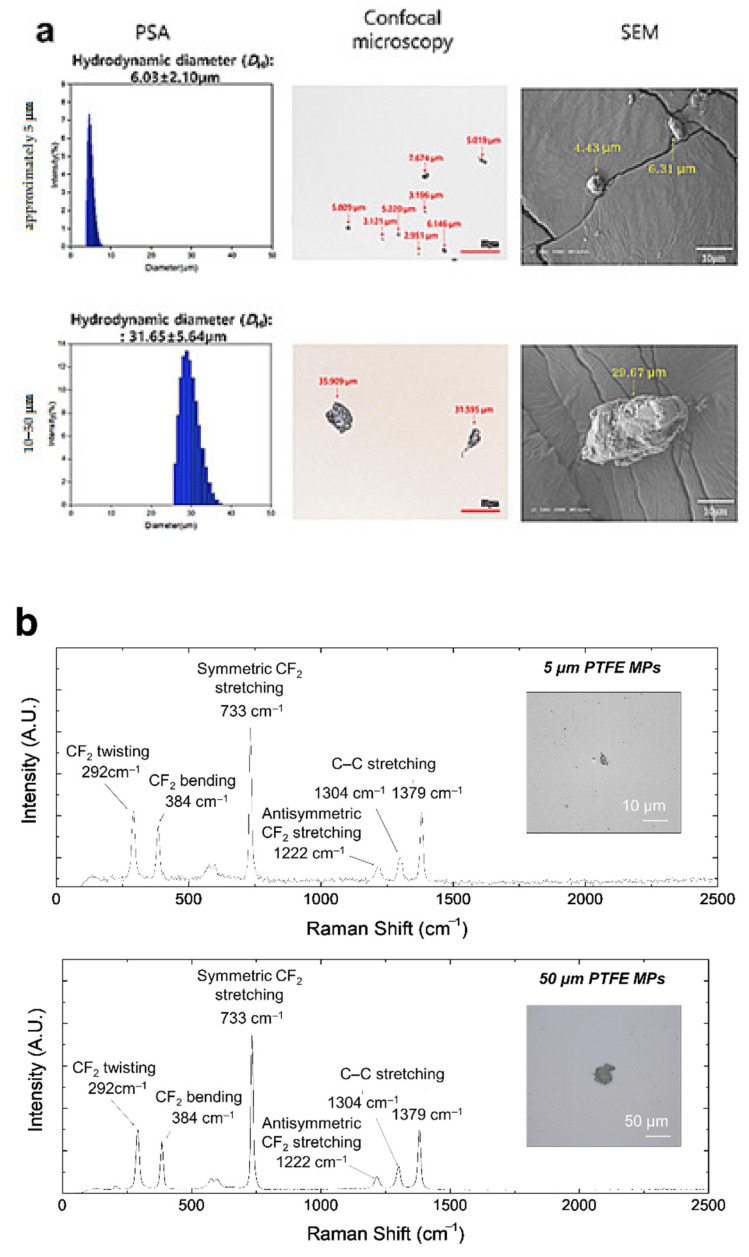
PSA, Confocal microscopy, SEM, and Raman microscope analysis were performed to characterize prepared PTFE microplastics and assess the intended size. PSA confirmed the two sizes of microplastics were approximately 5 μm (6.03 ± 2.10 μm) and 10–50 μm (31.65 ± 5.64 μm) (Figure 1a). The actual size of microplastics was measured with confocal microscopy and SEM (Figure 1a). Representative Raman spectra obtained from filtered microparticles identified PTFE based on peaks at 290 cm−1 to 1400 cm−1, presenting symmetric stretch CF2 and C–C peaks at 733 cm−1 and 1379 cm−1, and CF2 twisting and CF2 bending peaks at 292 cm−1 and 384 cm−1, respectively [44]. In addition, lines of medium intensity at 1222 cm−1 and 1304 cm−1 were ascribed to the antisymmetric stretch peak of CF2 [45] (Figure 1b). The physical and chemical properties of the particles prepared through these analytical methods were confirmed to be suitable for the conditions of PTFE microplastics.
Figure 1.
Characterization of PTFE microplastics. (a) Analysis with PSA, confocal microscopy, and scanning electron microscopy, (b) Raman spectra of approximately 5 μm and 10–50 μm-sized PTFE microplastics captured on silicon filters. Inset images show representative PTFE microplastics. Scale bar = 10 μm and 50 μm.
3.2. Toxicity Study
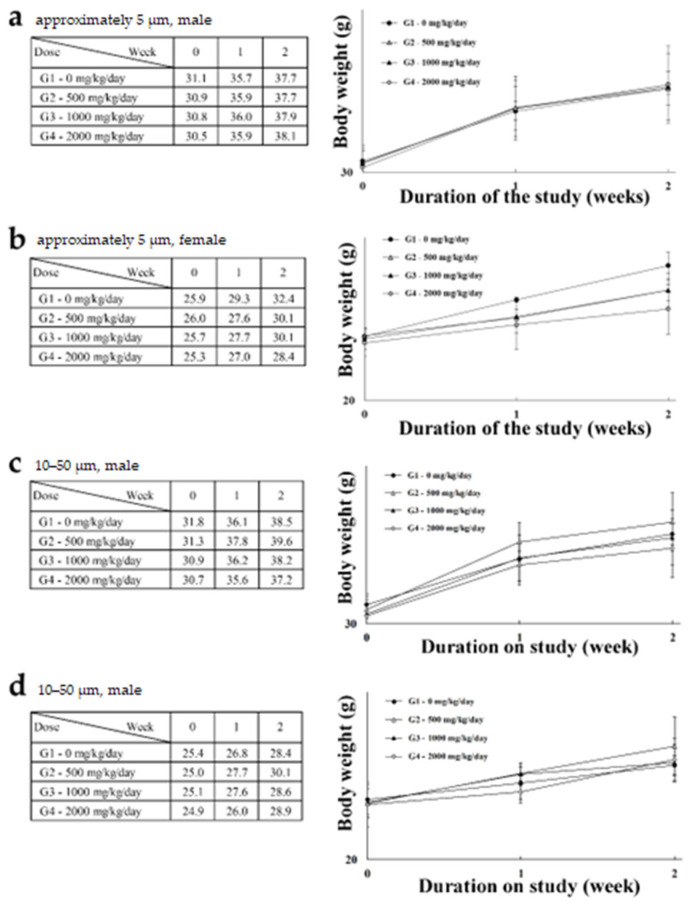
A single-dose toxicity study was performed to identify an approximate lethal dose of PTFE microplastics in two size ranges. No morbidity or death in mice was observed, and no specific clinical symptoms were recorded. Further, no significant weight changes were associated with exposure to microplastics when compared to control animals (Figure 2a–d). At necropsy, macroscopic examinations did not reveal changes associated with PTFE microplastic administration (data not shown). Thus, the approximate lethal dose for both sizes of PTFE microplastics was 2000 mg/kg or greater. The above LD50 and NOAEL values suggest that the prepared PTFE microplastics show no harm to mice in vivo at a dose of less than 2000 mg/kg.
Figure 2.
Male and female bodyweight changes after a single dose of approximately 5 μm (a,b) and 10–50 μm (c,d) PTFE microplastics.
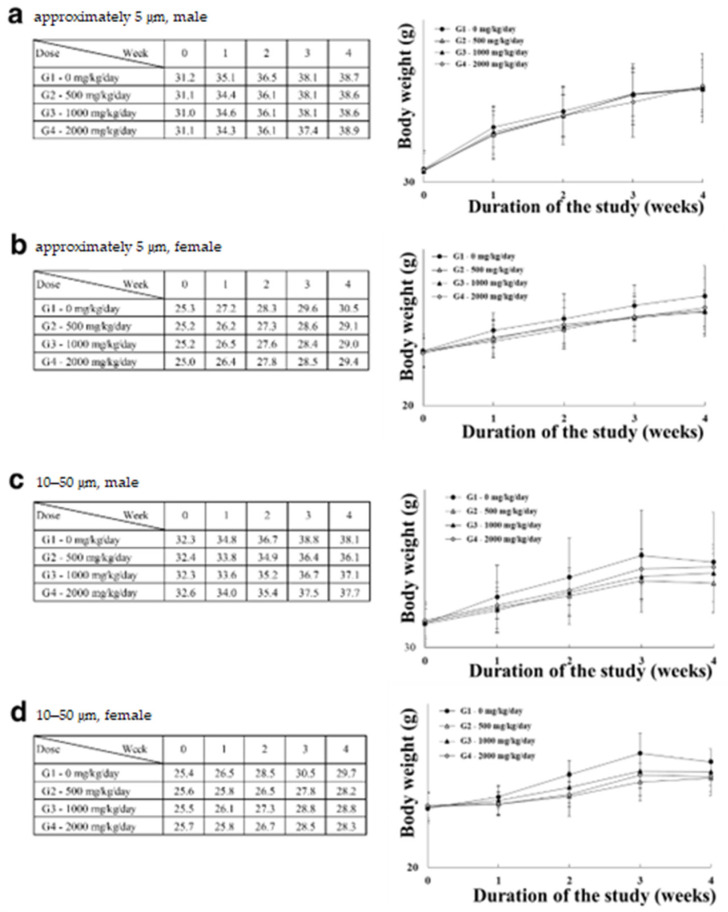
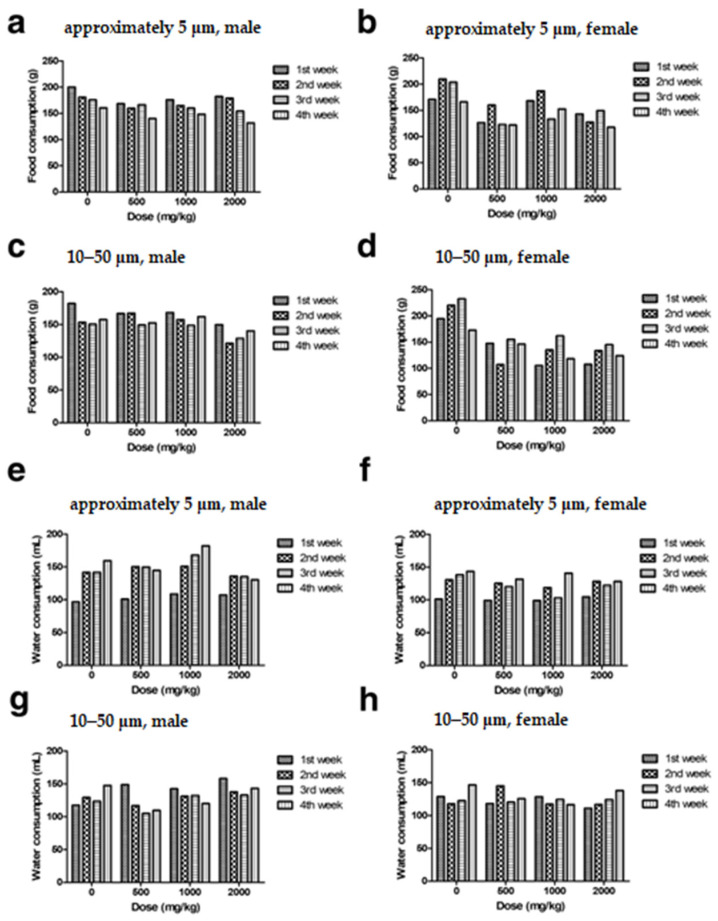
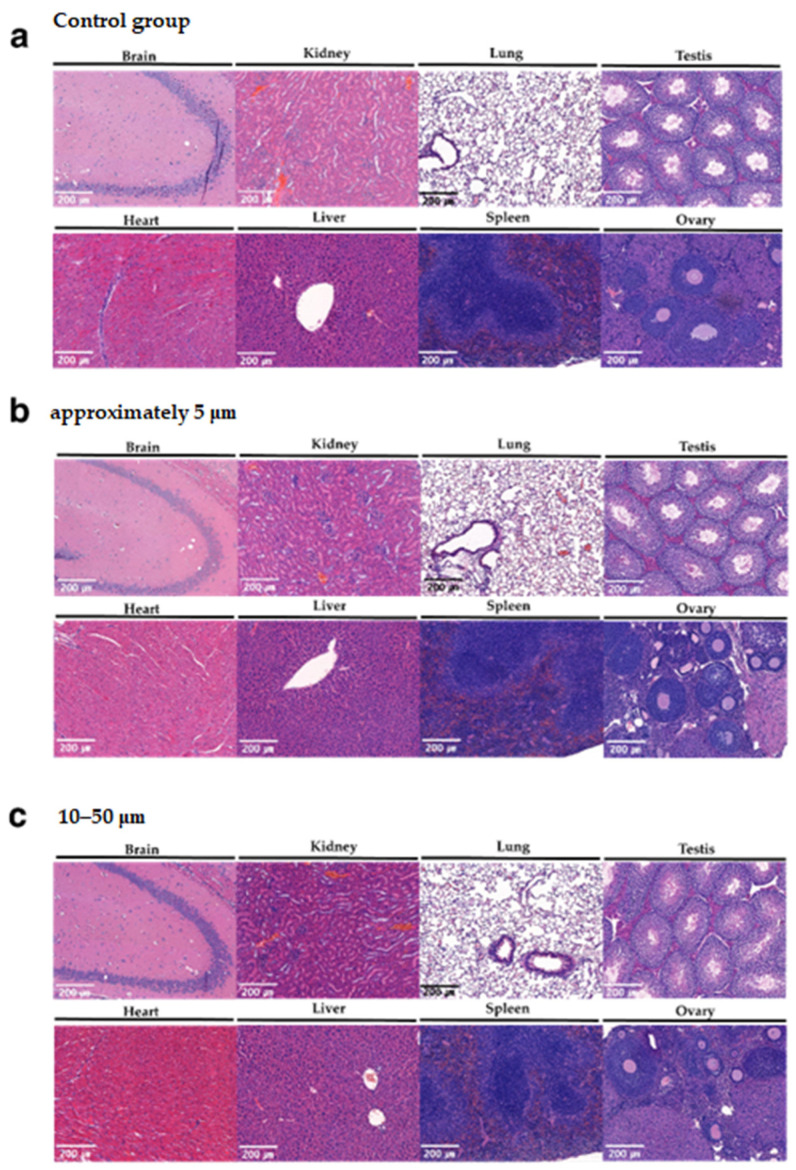
Four-week repeated-dose toxicity study based on the single-dose toxicity study and OECD guideline No. 408 was then used to establish a NOAEL for chronic exposure. No morbidity or death of animals was observed, and no specific clinical signs were recorded. Body and organ weights (Figure 3a–d, Supplementary Table S1), food (Figure 4a–d) and water (Figure 4e–h) consumption, clinical pathology (Table 1), and histopathological changes (Figure 5a–c) due to PTFE administration were not observed. Thus, NOAELs for two sizes of PTFE microplastics were 2000 mg/kg or greater.
Figure 3.
Male and female bodyweight changes after four weeks of repeated dosing with approximately 5 μm (a,b) and 10–50 μm (c,d) PTFE microplastics.
Figure 4.
Male and female food and water consumption changes during the four-week repeated-dose toxicity study of approximately 5 μm (a,b,e,f) and 10–50 μm (c,d,g,h) PTFE microplastics.
Table 1.
` WQS. Male and female serum biochemistry hematology data of approximately 5 μm (a–d) and 10–50 μm (e–h). The results are expressed as the mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. 0.
| a. Approximately 5 μm, Male, Serum Biochemistry Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Total Protein (g/dL) | Albumin (g/dL) |
Blood Urea Nitrogen
(mg/dL) |
Creatinine (mg/dL) | Glucose (mg/dL) |
| G1 0 | 150.3 ± 1.9 | 7.6 ± 2.0 | 113.0 ± 2.7 | 5.0 ± 0.3 | 3.1 ± 0.3 | 22.4 ± 4.4 | 0.2 ± 0.0 | 93.0 ± 28.9 |
| G2 500 | 149.7 ± 1.7 | 7.7 ± 1.8 | 114.8 ± 3.3 | 5.1 ± 0.1 | 3.3 ± 0.1 | 19.2 ± 4.7 | 0.2 ± 0.0 | 90.1 ± 22.1 |
| G3 1000 | 151.7 ± 0.7 | 7.3 ± 1.1 | 114.7 ± 1.1 | 5.2 ± 0.2 | 3.2 ± 0.2 | 22.6 ± 4.9 | 0.2 ± 0.0 | 78.6 ± 15.6 |
| G4 2000 | 150.7 ± 2.7 | 6.9 ± 1.0 | 113.9 ± 4.3 | 5.3 ± 0.2 * | 3.3 ± 0.1 | 23.1 ± 6.7 | 0.2 ± 0.0 | 93.5 ± 51.1 |
|
Group/
Dose (mg/kg/Day) |
Total Bilirubin (mg/dL) | Calcium (mg/dL) | Phosphate (mg/dL) | Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | Aspartate Aminotransferase (U/L) | Alanine Aminotransferase (U/L) | Alkaline Phosphatase (U/L) |
| G1 0 | 0.1 ± 0.0 | 8.9 ± 0.4 | 7.9 ± 1.1 | 137.2 ± 23.0 | 167.9 ± 53.6 | 69.4 ± 28.2 | 23.9 ± 6.5 | 248.6 ± 92.5 |
| G2 500 | 0.1 ± 0.0 | 8.9 ± 0.3 | 7.1 ± 1.3 | 139.9 ± 20.6 | 136.6 ± 40.1 | 71.8 ± 35.6 | 27.1 ± 8.6 | 222.6 ± 90.3 |
| G3 1000 | 0.1 ± 0.0 | 9.0 ± 0.2 | 7.8 ± 0.9 | 152.7 ± 23.7 | 140.5 ± 45.5 | 64.8 ± 9.8 | 22.9 ± 3.2 | 191.6 ± 65.8 |
| G4 2000 | 0.1 ± 0.1 | 9.2 ± 0.5 | 7.5 ± 0.8 | 150.8 ± 26.1 | 140.7 ± 47.6 | 81.9 ± 53.9 | 30.4 ± 11.0 | 223.0 ± 72.4 |
| b. Approximately 5 μm, Female, Serum Biochemistry Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Total Protein (g/dL) | Albumin (g/dL) |
Blood Urea Nitrogen
(mg/dL) |
Creatinine (mg/dL) | Glucose (mg/dL) |
| G1 0 | 148.7 ± 1.4 | 6.4 ± 1.2 | 112.7 ± 1.4 | 5.1 ± 0.2 | 3.6 ± 0.1 | 17.7 ± 2.2 | 0.2 ± 0.0 | 85.1 ± 21.9 |
| G2 500 | 150.3 ± 1.3 * | 6.4 ± 0.7 * | 114.4 ± 1.7 | 5.0 ± 0.2 | 3.6 ± 0.2 | 18.0 ± 3.4 | 0.2 ± 0.0 | 76.8 ± 21.5 |
| G3 1000 | 151.6 ± 1.3 *** | 6.5 ± 1.3 * | 115.3 ± 3.0 | 5.0 ± 0.2 | 3.6 ± 0.2 | 19.2 ± 2.1 | 0.2 ± 0.0 | 74.5 ± 17.6 |
| G4 2000 | 153.1 ± 1.5 *** | 6.3 ± 0.5 *** | 116.2 ± 1.6 | 5.1 ± 0.2 | 3.5 ± 0.1 | 18.4 ± 1.7 | 0.2 ± 0.0 | 91.2 ± 21.0 |
|
Group/
Dose (mg/kg/Day) |
Total Bilirubin (mg/dL) | Calcium (mg/dL) | Phosphate (mg/dL) | Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | Aspartate Aminotransferase (U/L) | Alanine Aminotransferase (U/L) | Alkaline Phosphatase (U/L) |
| G1 0 | 0.0 ± 0.0 | 9.1 ± 0.3 | 7.4 ± 1.0 | 113.0 ± 19.0 | 97.9 ± 27.5 | 88.0 ± 42.6 | 22.8 ± 5.0 | 291.1 ± 84.1 |
| G2 500 | 0.0 ± 0.0 | 9.1 ± 0.3 | 7.6 ± 0.8 | 100.8 ± 15.7 | 101.7 ± 38.3 | 91.4 ± 37.6 | 22.6 ± 4.3 | 604.4 ± 78.3 |
| G3 1000 | 0.0 ± 0.0 | 9.2 ± 0.4 | 7.5 ± 1.0 | 106.5 ± 21.1 | 70.5 ± 22.6 * | 106.8 ± 43.1 | 24.5 ± 4.5 | 295.7 ± 78.2 |
| G4 2000 | 0.0 ± 0.0 | 9.1 ± 0.2 | 7.8 ± 0.8 | 120.7 ± 21.7 | 108.5 ± 30.9 | 93.5 ± 39.8 | 24.5 ± 5.9 | 329.7 ± 99.8 |
| c. Approximately 5 μm, Male, Hematology Parameter s | ||||||||
|
Group/
Dose (mg/kg/Day) |
White blood cell (×103 cells/uL) | Red blood cell (×106 cells/uL) |
Hemoglobin
(g/dL) |
Hematocrit
(%) |
Mean Corpuscular Volume
(fL) |
Mean Corpuscular Hemoglobin
(pg) |
Mean Corpuscular Hemoglobin Concentration
(g/dL) |
Red Cell Distribution Width
(%) |
| G1 0 | 3.52 ± 1.58 | 9.23 ± 1.27 | 14.5 ± 1.7 | 46.6 ± 6.0 | 50.5 ± 0.4 | 15.7 ± 0.3 | 31.1 ± 0.6 | 12.5 ± 0.3 |
| G2 500 | 3.41 ± 0.65 | 9.25 ± 0.23 | 13.8 ± 0.1 | 45.0 ± 0.3 | 48.7 ± 1.5 | 15.0 ± 0.3 | 30.7 ± 0.4 | 12.3 ± 0.5 |
| G3 1000 | 4.25 ± 1.23 | 9.03 ± 0.17 | 14.0 ± 0.4 | 45.4 ± 1.0 | 50.2 ± 0.3 | 15.5 ± 0.2 | 30.8 ± 0.4 | 13.5 ± 0.4 |
| G4 2000 | 4.90 ± 0.98 | 8.74 ± 0.17 | 13.4 ± 0.4 | 43.1 ± 1.0 | 49.6 ± 1.0 | 15.4 ± 0.5 | 31.0 ± 0.6 | 12.5 ± 0.4 |
|
Group/
Dose (mg/kg/Day) |
Hemoglobin Distribution Width
(g/dL) |
Platelet (×103 cells/uL) |
Mean Platelet Volume
(fL) |
Neutrophil
(%) |
Lymphocyte
(%) |
Monocyte
(%) |
Eosinophil
(%) |
Basophil
(%) |
| G1 0 | 2.35 ± 0.14 | 844 ± 62 | 5.3 ± 0.2 | 26.5 ± 1.7 | 39.5 ± 9.3 | 3.4 ± 0.9 | 30.2 ± 7.0 | 0.2 ± 0.1 |
| G2 500 | 2.32 ± 0.06 * | 1042 ± 60 | 4.9 ± 0.1 | 21.7 ± 6.6 | 60.2 ± 10.3 | 2.9 ± 1.9 | 14.9 ± 16.3 | 0.1 ± 0.1 |
| G3 1000 | 2.44 ± 0.21 | 1150 ± 229 | 4.7 ± 0.3 | 28.7 ± 3.3 | 60.9 ± 3.4 | 3.1 ± 1.0 | 6.8 ± 3.1 | 0.1 ± 0.0 |
| G4 2000 | 2.34 ± 0.12 | 1146 ± 91 | 4.8 ± 0.4 | 18.9 ± 4.4 | 67.0 ± 3.9 | 1.7 ± 0.5 | 11.8 ± 8.5 | 0.1 ± 0.0 |
|
Group/
Dose (mg/kg/Day) |
Neutrophil
(×103 cells/uL) |
Lymphocyte
(×103 cells/uL) |
Monocyte (×103 cells/uL) | Eosinophil (×103 cells/uL) | Basophil (×103 cells/uL) | Reticulocyte (×109 cells/L) | Reticulocyte (%) | |
| G1 0 | 0.92 ± 0.40 | 1.46 ± 0.84 | 0.11 ± 0.04 | 1.00 ± 0.39 | 0.01 ± 0.01 | 427.3 ± 75.7 | 4.61 ± 0.18 | |
| G2 500 | 0.91 ± 0.09 | 2.58 ± 0.43 | 0.13 ± 0.07 | 0.81 ± 1.06 | 0.00 ± 0.01 | 312.2 ± 56.5 * | 3.37 ± 0.58 * | |
| G3 1000 | 1.35 ± 0.29 | 2.93 ± 0.90 | 0.15 ± 0.05 | 0.35 ± 0.25 | 0.01 ± 0.01 | 388.4 ± 53.7 | 4.30 ± 0.52 | |
| G4 2000 | 1.02 ± 0.25 | 3.60 ± 0.38 | 0.10 ± 0.04 | 0.63 ± 0.43 | 0.01 ± 0.00 | 332.6 ± 46.2 | 3.81 ± 0.61 | |
| d. Approximately 5 μm, Female, Hematology Parameter s | ||||||||
|
Group/
Dose (mg/kg/Day) |
White Blood Cell (×103 cells/uL) | Red Blood Cell (×106 cells/uL) |
Hemoglobin
(g/dL) |
Hematocrit
(%) |
Mean Corpuscular Volume
(fL) |
Mean Corpuscular Hemoglobin
(pg) |
Mean Corpuscular Hemoglobin Concentration
(g/dL) |
Red Cell Distribution Width
(%) |
| G1 0 | 5.31 ± 2.22 | 9.47 ± 0.27 | 15.1 ± 0.3 | 47.5 ± 1.5 | 50.1 ± 0.5 | 15.9 ± 0.2 | 31.7 ± 0.7 | 13.3 ± 0.7 |
| G2 500 | 3.75 ± 0.97 | 9.26 ± 0.22 | 14.5 ± 0.3 | 46.3 ± 0.7 | 50.0 ± 0.9 | 15.7 ± 0.5 | 31.4 ± 0.6 | 12.8 ± 0.8 |
| G3 1000 | 7.42 ± 3.21 | 9.17 ± 0.23 | 14.5 ± 0.7 | 45.5 ± 1.9 | 49.7 ± 1.0 | 15.8 ± 0.3 | 31.8 ± 0.5 | 13.2 ± 0.4 |
| G4 2000 | 5.18 ± 2.70 | 9.53 ± 0.35 | 14.9 ± 0.4 | 46.9 ± 1.4 | 49.2 ± 1.1 | 15.7 ± 0.7 | 31.8 ± 0.8 | 13.1 ± 0.2 |
|
Group/
Dose (mg/kg/Day) |
Hemoglobin Distribution Width
(g/dL) |
Platelet (×103 Cells/uL) |
Mean Platelet Volume
(fL) |
Neutrophil
(%) |
Lymphocyte
(%) |
Monocyte
(%) |
Eosinophil
(%) |
Basophil
(%) |
| G1 0 | 2.41 ± 0.12 | 872 ± 186 | 5.1 ± 0.3 | 15.6 ± 3.5 | 71.2 ± 8.6 | 1.9 ± 0.6 | 10.9 ± 6.5 | 0.1 ± 0.1 |
| G2 500 | 2.37 ± 0.10 | 968 ± 35 | 5.0 ± 0.2 | 14.9 ± 2.5 | 73.7 ± 3.4 | 1.6 ± 0.3 | 9.2 ± 5.2 | 0.1 ± 0.1 * |
| G3 1000 | 2.55 ± 0.13 | 903 ± 94 | 5.3 ± 0.6 | 21.6 ± 3.1 | 64.1 ± 10.0 * | 2.2 ± 0.5 | 11.4 ± 8.1 | 0.1 ± 0.1 |
| G4 2000 | 2.49 ± 0.08 | 849 ± 102 | 5.7 ± 0.2 | 18.0 ± 2.7 ** | 64.0 ± 9.9 | 1.1 ± 0.5 | 16.3 ± 8.2 | 0.3 ± 0.1 |
|
Group/
Dose (mg/kg/Day) |
Neutrophil
(×103 Cells/uL) |
Lymphocyte
(×103 cells/uL) |
Monocyte (×103 Cells/uL) | Eosinophil (×103 Cells/uL) | Basophil (×103 Cells/uL) | Reticulocyte (×109 Cells/L) | Reticulocyte (%) | |
| G1 0 | 0.96 ± 0.21 | 4.72 ± 2.31 | 0.13 ± 0.07 | 0.71 ± 0.45 | 0.01 ± 0.01 | 371.2 ± 82.3 | 3.91 ± 0.77 | |
| G2 500 | 0.64 ± 0.16 | 3.23 ± 0.95 | 0.07 ± 0.02 | 0.40 ± 0.20 | 0.01 ± 0.01 | 424.0 ± 20.6 | 4.58 ± 0.26 | |
| G3 1000 | 1.75 ± 0.25 | 5.42 ± 2.20 | 0.19 ± 0.10 | 0.88 ± 0.51 | 0.01 ± 0.01 | 350.8 ± 21.7 | 3.83 ± 0.27 | |
| G4 2000 | 1.13 ± 0.57 | 4.13 ± 2.25 | 0.07 ± 0.06 | 0.93 ± 0.58 | 0.02 ± 0.01 | 316.7 ± 26.2 | 3.32 ± 0.19 | |
| e. 10–50 μm, Male, Serum Biochemistry Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Total Protein (g/dL) | Albumin (g/dL) |
Blood Urea Nitrogen
(mg/dL) |
Creatinine (mg/dL) | Glucose (mg/dL) |
| G1 0 | 148.0 ± 1.1 | 7.8 ± 1.0 | 111.2 ± 2.1 | 5.1 ± 0.3 | 3.2 ± 0.2 | 23.2 ± 2.9 | 0.2 ± 0.0 | 119.4 ± 41.0 |
| G2 500 | 148.4 ± 1.2 | 7.3 ± 1.8 | 112.3 ± 2.1 | 5.3 ± 0.2 | 3.4 ± 0.2 | 21.8 ± 2.4 | 0.2 ± 0.0 | 178.9 ± 36.8 |
| G3 1000 | 149.2 ± 1.1 | 7.8 ± 1.2 | 113.5 ± 1.3 | 5.1 ± 0.2 | 3.1 ± 0.2 | 19.7 ± 3.7 | 0.2 ± 0.0 | 159.8 ± 49.5 |
| G4 2000 | 150.7 ± 1.6 | 7.3 ± 0.9 | 112.9 ± 1.8 | 5.2 ± 0.2 | 3.3 ± 0.2 | 26.1 ± 5.7 | 0.2 ± 0.0 | 123.7 ± 36.5 |
|
Group/
Dose (mg/kg/Day) |
Total Bilirubin (mg/dL) | Calcium (mg/dL) | Phosphate (mg/dL) | Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | Aspartate Aminotransferase (U/L) | Alanine Aminotransferase (U/L) | Alkaline Phosphatase (U/L) |
| G1 0 | 0.1 ± 0.0 | 8.5 ± 0.3 | 8.5 ± 1.1 | 158.8 ± 24.2 | 117.5 ± 26.9 | 81.9 ± 46.1 | 26.6 ± 8.9 | 188.5 ± 63.4 |
| G2 500 | 0.1 ± 0.0 | 8.6 ± 0.3 | 7.1 ± 1.5 | 151.5 ± 18.7 | 125.5 ± 51.5 | 64.4 ± 19.6 | 28.0 ± 12.5 | 235.6 ± 86.4 |
| G3 1000 | 0.1 ± 0.0 | 8.5 ± 0.2 | 7.5 ± 1.3 | 146.4 ± 23.0 | 127.2 ± 78.5 | 63.3 ± 31.5 | 21.3 ± 5.4 | 152.1 ± 53.1 |
| G4 2000 | 0.1 ± 0.1 | 8.6 ± 0.3 | 7.8 ± 1.2 | 155.0 ± 19.8 | 108.5 ± 26.5 | 78.2 ± 24.9 | 39.3 ± 30.8 | 200.2 ± 72.5 |
| f. 10– 50 μm, Female, Serum Biochemistry Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Total Protein (g/dL) | Albumin (g/dL) |
Blood Urea Nitrogen
(mg/dL) |
Creatinine (mg/dL) | Glucose (mg/dL) |
| G1 0 | 151.8 ± 3.4 | 6.3 ± 0.9 | 114.1 ± 2.9 | 5.0 ± 0.3 | 3.6 ± 0.2 | 18.7 ± 1.9 | 0.2 ± 0.0 | 115.6 ± 34.8 |
| G2 500 | 155.9 ± 3.7 * | 6.5 ± 0.6 | 117.9 ± 4.5 * | 5.0 ± 0.3 | 3.5 ± 0.2 | 16.1 ± 4.3 | 0.2 ± 0.0 | 147.4 ± 57.4 |
| G3 1000 | 159.1 ± 5.0 ** | 7.2 ± 1.9 | 120.5 ± 3.3 *** | 5.1 ± 0.3 | 3.7 ± 0.2 | 19.5 ± 3.3 | 0.2 ± 0.0 | 141.0 ± 48.4 |
| G4 2000 | 152.2 ± 2.7 | 7.5 ± 2.0 | 117.1 ± 2.3 * | 5.2 ± 0.4 | 3.6 ± 0.2 | 14.7 ± 3.2 ** | 0.2 ± 0.0 | 172.8 ± 30.5 ** |
|
Group/
Dose (mg/kg/Day) |
Total Bilirubin (mg/dL) | Calcium (mg/dL) | Phosphate (mg/dL) | Total Cholesterol (mg/dL) |
Triglyceride
(mg/dL) |
Aspartate Aminotransferase (U/L) |
Alanine Aminotransferase
(U/L) |
Alkaline Phosphatase (U/L) |
| G1 0 | 0.0 ± 0.0 | 9.4 ± 0.4 | 7.9 ± 1.2 | 105.4 ± 25.6 | 50.2 ± 14.6 | 82.1 ± 79.1 | 45.6 ± 69.4 | 301.6 ± 111.1 |
| G2 500 | 0.0 ± 0.0 | 9.7 ± 0.3 | 7.9 ± 0.8 | 113.8 ± 25.6 | 55.8 ± 19.3 | 53.8 ± 6.3 | 23.4 ± 12.8 | 262.4 ± 35.0 |
| G3 1000 | 0.0 ± 0.0 | 10.0 ± 0.5 * | 8.1 ± 1.4 | 114.6 ± 18.1 | 62.4 ± 27.0 | 54.2 ± 7.8 | 22.4 ± 8.6 | 333.0 ± 88.1 |
| G4 2000 | 0.0 ± 0.0 | 9.5 ± 0.4 | 8.4 ± 1.1 | 95.6 ± 25.3 | 34.4 ± 20.4 | 113.6 ± 115.1 | 24.6 ± 12.3 | 261.4 ± 55.2 |
| g. 10–50 μm, Male, Hematology Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
White Blood Cell (×103 cells/uL) | Red Blood Cell (×106 cells/uL) |
Hemoglobin
(g/dL) |
Hematocrit
(%) |
Mean Corpuscular Volume
(fL) |
Mean Corpuscular Hemoglobin
(pg) |
Mean Corpuscular Hemoglobin Concentration
(g/dL) |
Red Cell Distribution Width
(%) |
| G1 0 | 5.50 ± 1.88 | 9.25 ± 0.58 | 14.4 ± 0.9 | 47.0 ± 3.2 | 50.8 ± 1.33 | 15.6 ± 0.3 | 30.7 ± 0.2 | 12.9 ± 0.7 |
| G2 500 | 5.75 ± 0.94 | 8.93 ± 0.09 | 13..8 ± 0.3 | 44.4 ± 1.0 | 49.7 ± 1.4 | 15.4 ± 0.4 | 31.0 ± 0.7 | 12.9 ± 0.2 |
| G3 1000 | 6.30 ± 2.32 | 8.90 ± 0.49 | 13.6 ± 0.4 * | 44.0 ± 1.5 | 49.4 ± 1.1 | 15.3 ± 0.4 | 31.0 ± 0.4 | 12.9 ± 0.6 |
| G4 2000 | 4.68 ± 0.59 | 8.36 ± 1.10 | 13.3 ± 1.1 | 42.3 ± 3.9 | 50.8 ± 2.0 | 16.0 ± 0.9 | 31.4 ± 0.6 | 12.8 ± 0.1 |
|
Group/
Dose (mg/kg/Day) |
Hemoglobin Distribution Width
(g/dL) |
Platelet (×103 Cells/uL) |
Mean Platelet Volume
(fL) |
Neutrophil
(%) |
Lymphocyte
(%) |
Monocyte
(%) |
Eosinophil
(%) |
Basophil
(%) |
| G1 0 | 2.47 ± 0.21 | 1047 ± 86 | 5.1 ± 0.3 | 17.4 ± 3.1 | 66.6 ± 4.7 | 3.4 ± 0.3 | 12.0 ± 5.6 | 0.2 ± 0.2 |
| G2 500 | 2.34 ± 0.12 | 983 ± 54 | 4.9 ± 0.7 | 21.2 ± 6.6 | 65.3 ± 6.6 | 3.6 ± 1.2 | 9.4 ± 8.9 | 0.2 ± 0.2 |
| G3 1000 | 2.43 ± 0.06 | 1080 ± 232 | 4.9 ± 0.3 | 17.1 ± 4.3 | 70.4 ± 3.4 | 2.2 ± 1.0 | 9.8 ± 2.5 | 0.1 ± 0.0 |
| G4 2000 | 2.39 ± 0.15 | 1101 ± 167 | 4.9 ± 0.2 | 40.8 ± 36.4 | 41.5 ± 35.4 | 2.5 ± 1.0 | 14.9 ± 9.3 | 0.1 ± 0.0 |
|
Group/
Dose (mg/kg/Day) |
Neutrophil
(×103 Cells/uL) |
Lymphocyte
(×103 Cells/uL) |
Monocyte (×103 Cells/uL) | Eosinophil (×103 Cells/uL) | Basophil (×103 Cells/uL) | Reticulocyte (×109 Cells/L) | Reticulocyte (%) | |
| G1 0 | 4.33 ± 1.69 | 0.22 ± 0.06 | 0.69 ± 0.16 | 0.03 ± 0.01 | 0.01 ± 0.01 | 364.7 ± 78.9 | 3.92 ± 0.62 | |
| G2 500 | 1.48 ± 0.35 | 4.65 ± 1.04 | 0.26 ± 0.11 | 0.66 ± 0.59 | 0.02 ± 0.01 | 326.9 ± 59.5 | 3.66 ± 0.70 | |
| G3 1000 | 1.26 ± 0.31 | 5.52 ± 2.35 | 0.16 ± 0.07 | 0.81 ± 0.51 | 0.01 ± 0.00 | 333.6 ± 18.7 | 3.75 ± 0.28 | |
| G4 2000 | 1.85 ± 1.55 | 2.06 ± 1.90 | 0.12 ± 0.07 | 0.67 ± 0.36 | 0.00 ± 0.01 | 355.9 ± 26.9 | 4.33 ± 0.86 | |
| h. 10– 50 μm, Female, Hematology Parameters | ||||||||
|
Group/
Dose (mg/kg/Day) |
White Blood Cell (×103 Cells/uL) | Red Blood Cell (×106 cells/uL) |
Hemoglobin
(g/dL) |
Hematocrit
(%) |
Mean Corpuscular Volume
(fL) |
Mean Corpuscular Hemoglobin
(pg) |
Mean Corpuscular Hemoglobin Concentration
(g/dL) |
Red Cell Distribution Width
(%) |
| G1 0 | 4.95 ± 2.22 | 9.50 ± 0.66 | 15.1 ± 0.2 | 47.5 ± 0.7 | 50.1 ± 2.7 | 15.9 ± 0.9 | 31.8 ± 0.1 | 12.8 ± 1.0 |
| G2 500 | 7.77 ± 2.80 | 9.50 ± 0.90 | 14.8 ± 1.1 * | 47.2 ± 3.3 * | 49.8 ± 2.2 | 15.5 ± 0.5 | 31.2 ± 0.3 | 12.7 ± 0.6 |
| G3 1000 | 5.61 ± 0.64 | 9.04 ± 0.60 | 14.2 ± 0.9 | 45.6 ± 3.0 | 50.4 ± 0.9 | 15.7 ± 0.1 | 31.1 ± 0.5 | 13.6 ± 2.4 |
| G4 2000 | 3.49 ± 1.89 | 9.91 ± 0.60 | 15.6 ± 0.7 | 49.8 ± 2.6 | 50.2 ± 1.0 | 15.7 ± 0.3 | 31.4 ± 0.5 | 12.5 ± 0.2 |
|
Group/
Dose (mg/kg/Day) |
Hemoglobin Distribution Width
(g/dL) |
Platelet (×103 Cells/uL) |
Mean Platelet Volume
(fL) |
Neutrophil
(%) |
Lymphocyte
(%) |
Monocyte
(%) |
Eosinophil
(%) |
Basophil
(%) |
| G1 0 | 2.34 ± 0.02 | 915 ± 12 | 5.0 ± 0.2 | 15.2 ± 3.2 | 67.5 ± 9.6 | 1.8 ± 0.4 | 15.1 ± 8.4 | 0.1 ± 0.0 |
| G2 500 | 2.29 ± 0.11 | 942 ± 78 | 5.1 ± 0.3 | 18.3 ± 4.0 | 68.9 ± 3.1 | 1.5 ± 0.9 | 10.7 ± 6.4 | 0.2 ± 0.1 |
| G3 1000 | 2.45 ± 0.38 | 977 ± 193 | 4.9 ± 0.5 | 16.3 ± 2.8 | 74.5 ± 2.0 | 1.4 ± 0.3 | 7.1 ± 3.4 | 0.1 ± 0.0 |
| G4 2000 | 2.29 ± 0.10 | 939 ± 212 | 5.2 ± 0.5 | 17.4 ± 3.2 | 69.2 ± 13.0 | 1.3 ± 0.4 | 11.5 ± 10.3 | 0.1 ± 0.1 |
|
Group/
Dose (mg/kg/Day) |
Neutrophil
(×103 Cells/uL) |
Lymphocyte
(×103 Cells/uL) |
Monocyte (×103 Cells/uL) | Eosinophil (×103 Cells/uL) | Basophil (×103 Cells/uL) | Reticulocyte (×109 Cells/L) | Reticulocyte (%) | |
| G1 0 | 0.80 ± 0.39 | 3.75 ± 2.27 | 0.09 ± 0.04 | 0.65 ± 0.10 | 0.00 ± 0.01 | 396.3 ± 132.9 | 4.21 ± 1.58 | |
| G2 500 | 1.57 ± 0.74 | 5.75 ± 1.99 | 0.12 ± 0.04 | 0.78 ± 0.22 | 0.01 ± 0.01 | 425.9 ± 153.7 | 4.49 ± 1.48 | |
| G3 1000 | 1.05 ± 0.29 | 4.78 ± 0.81 | 0.09 ± 0.01 | 0.44 ± 0.16 | 0.01 ± 0.01 | 508.2 ± 397.6 | 5.82 ± 4.89 | |
| G4 2000 | 0.79 ± 0.42 | 3.08 ± 1.82 | 0.06 ± 0.04 | 0.54 ± 0.50 | 0.01 ± 0.01 | 325.0 ± 34.2 | 3.29 ± 0.42 | |
Figure 5.
Histopathological evaluation of tissues from mice after four weeks of repeated dosing. Representative images of the brain (hippocampus), heart, kidney, liver, lung, spleen, testis, and ovary of the control mice (a), approximately 5 μm PTFE-treated mice (b) and 10–50 μm PTFE-treated mice (c). Scale bar = 200 μm.
3.3. Pharmacokinetics Study
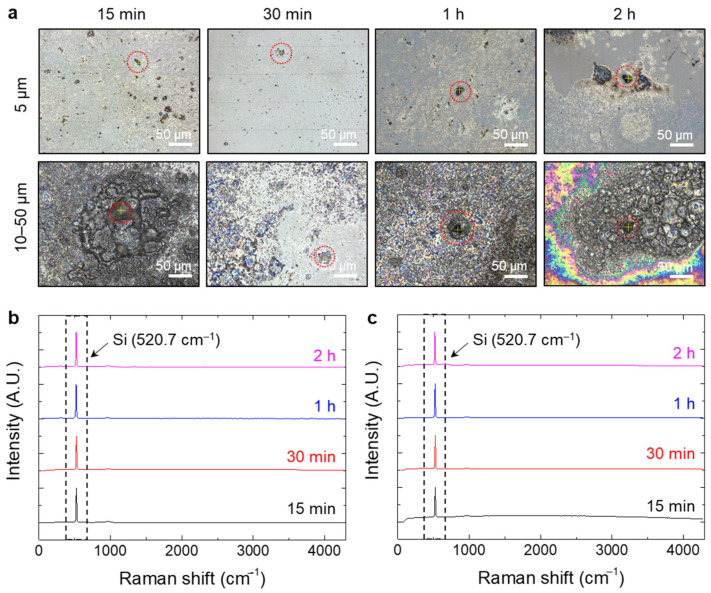
Pharmacokinetic experiments were performed to determine how PTFE microplastics were distributed in blood and organs after oral administration. The distribution of PTFE microplastics was analyzed at four time points (15, 30, 60, and 120 min) after a single oral dose of microplastics. However, PTFE plasma concentrations were not detected with Raman spectrometry (Figure 6a–c), and pharmacokinetic parameters for PTFE could not be calculated.
Figure 6.
Detection of PTFE microplastics in whole blood. (a) Microscopic images of microparticles captured on silicon filters from blood collected at 15, 30, 60, and 120 min after oral administration of PTFE microplastics (approximately 5 and 10–50 μm). (b,c) PTFE microplastics were not detected on filters. Silicon (Si) peaks are indicated by the arrow. Scale bar = 50 μm.
3.4. Human NOAEL Dose
The human NOAEL dose could be estimated using the HED converting table and Equation (1). Assuming the NOAEL in mice is 2000 mg/kg (i.e., the highest dose in mice pharmacokinetic studies), the human NOAEL was calculated to be 9720 mg per 60 kg.
4. Discussion
Plastic use continues to increase worldwide. Physical properties, diversity, and low prices make the use of plastics attractive [46]. One report indicated that approximately 3% of plastic was recycled worldwide in 2017 and 67.8 million metric tons will be discarded in the environment by 2050, reflecting an increase in plastic use [47]. Discarded plastics are degraded to a size of less than 5 mm, called microplastics, via weathering and ultraviolet rays [48]. Their small size allows microplastics to move easily to contaminate soil, lakes, rivers, and seas [49,50,51,52,53,54]. In particular, many reports indicate that marine life accumulates microplastics on gills during oxygen exchange or plastics are ingested when mistaken for food [17]. Human exposure may occur when microplastic-loaded aquatic organisms are consumed [55]. Another means of human exposure is encountered when eating cooked food [56]. Microplastics are reported in tap water, beer, salt, and mineral water, and large amounts are detected in meat or cooked food in Thai markets [57,58,59]. Thus, the ingestion of contaminated food will cause human exposure to microplastics found in various aquatic organisms.
Many studies indirectly confirm such exposure to microplastics. Microplastics are detected in marine environments and aquatic organisms [60,61,62]. In vivo, aquatic organisms are often used as experimental models, and microplastics accumulate in zebrafish in the intestines, histopathological changes occur in vacuolization, and the existence of mast cells, the release of mobile cells, and other changes such as an increase in oxidative stress occur [63]. In addition, decreased movement, neurological changes such as seizures, and female reproductive dysfunction are also reported [62]. In experiments using minnows, changes such as decreased movement and increased oxidative stress and immune response are reported [64]. Female and male reproductive dysfunction appeared in oysters [34]. Experiments with mammals, especially rodents, report changes in the reproductive systems of female and male mice, behavioral changes, and liver inflammation [36,37,38]. Information is thus increasing on the risks associated with microplastics, but many knowledge gaps remain. In particular, toxicity studies have distinct limitations, such as observing only specific physiological systems or very low concentrations. Our work included a comprehensive evaluation of toxicity across all organs for PTFE particles, as recommended by OECD guidelines 408 and 423. In addition, a pharmacokinetic evaluation using Raman spectroscopy was completed to confirm in vivo behavior of PTFE.
Microplastics used in most experiments are spherical. Microplastics in the environment are more fragmented or fibrous [65]. We manufactured two sizes of fragmented microplastics, approximately 5 μm and 10–50 μm. These morphological characteristics were confirmed through PSA, confocal microscopy, and SEM (Figure 1a), and the chemical properties of PTFE were confirmed through Raman spectroscopy (Figure 1b). These analytical results suggest that manufactured particles are suitable for the physical and chemical conditions of the PTFE microplastics. Subsequently, single and four-week repeated-dose toxicity evaluations were performed using these microplastics. The single-dose toxicity test confirmed the LD50 of PTFE using doses of 500, 1000, and 2000 mg/kg. During the two-week observation period after a single administration, no deaths or clinical signs of toxicity were recorded (data not shown) and no weight changes (Figure 2a–d) due to PTFE microplastic administration were observed. Further, no changes due to microplastic administration were observed in necropsy. Thus, the LD50 for PTFE microplastics was 2000 mg/kg or more for both particle sizes.
Subsequently, no specific clinical sign was recorded during the 4-week observation period, and no significant differences were observed in body weight (Figure 3a–d), organ weight (Supplementary Table S1), or water and food consumption (Figure 4a–h) in the administration groups compared to the control group. As a result of the clinical pathological evaluation (a–h in Table 1), no dose-dependent and simultaneously significant difference was observed, and no degenerative changes such as inflammation, desquamation, or necrosis were observed in the histopathological evaluation (Figure 5a–c). Therefore, NOAEL for four-week repeated oral administration of PTFE microplastics was confirmed to be 2000 mg/kg or higher. The results provide a new understanding of acute and chronic oral exposure to PTEF microplastics.
In addition, Raman spectroscopy analysis of blood after a single dose of 2000 mg/kg showed no PTFE microplastics. The absorption of microplastics through oral administration is known to be very low [66]. In the case of weathered microplastics, if analyzed by Raman spectroscopy, the peak of microplastics may change, resulting in inaccurate results [67]. Additional experiments such as administering microplastics through IV or experiments to visually determine the bio-distribution of microplastics by labeling fluorescent substances on microplastics are necessary to overcome this limitation and directly confirm the kinetics of microplastics in vivo.
Our present study is the first to report single and repeated toxicity, along with pharmacokinetics using high concentrations of PTFE microplastics in mammals. These experiments provide new insight into the biological effects of microplastics. The difference between our previous study on the toxicity of polyethylene microplastics [41] and the results of the present study suggests that there may be differences in biological effects depending on the type of plastic. This hypothesis can be tested with in vivo experiments using other plastics, such as PS, PET, and polyamides.
5. Conclusions
The purpose of this study was to establish LD50 and NOAEL values, which are representative values of the toxicity study for two sizes of PTFE microplastics, and confirm the movement of microplastics in the body. In the single and four-week repeated toxicity study, no significant differences were observed in clinical sign, body weight, organ weight, water and food consumptions, macroscopic examination at necropsy, or clinical and histopathological evaluation. Both single and four-week repeated oral dose toxicity tests suggested LD50 and NOAEL values of more than 2000 mg/kg. A pharmacokinetic experiment using Raman spectroscopy analysis did not detect PTFE in blood, and pharmacokinetic parameters could not be calculated. To overcome the limitations of this experiment, pharmacokinetic evaluation using a different route of administration is required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14112220/s1; Table S1: Male and female organ weight in the 28-day repeated-oral-dose toxicity study.
Author Contributions
Conceptualization, M.-S.S. and K.K.; animal study, S.L. (Sijoon Lee); pathological analysis, K.-K.K., S.-E.S. and M.S.; Raman analysis, J.-H.C., K.-Y.S., J.L., S.K., S.L. (Sunjong Lee) and S.Y.Y.; pharmacokinetic analysis, K.-R.L.; writing—original draft preparation, S.L. (Sijoon Lee); writing—review and editing, M.-S.S. and K.K.; visualization, S.L. (Sijoon Lee); supervision, M.-S.S. and K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of the Institutional Animal Care and Use Committee of The Laboratory Animal Center of the Daegu-Gyeongbuk Medical Innovation Foundation (IACUC; approval No. DGMIF-21031601-00) and was in accordance with their guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Korea Environmental Industry and Technology Institute (KEITI) through the Measurement and Risk assessment Program for Management of Microplastics Project, funded by the Korea Ministry of Environment (MOE) (grant No. 2020003120002).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alimba C.G., Faggio C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019;68:61–74. doi: 10.1016/j.etap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Thompson R.C., Olsen Y., Mitchell R.P., Davis A., Rowland S.J., John A.W., McGonigle D., Russell A.E. Lost at sea: Where is all the plastic? Science. 2004;304:838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- 3.Moore C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008;108:131–139. doi: 10.1016/j.envres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Galloway T.S., Lewis C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA. 2016;113:2331–2333. doi: 10.1073/pnas.1600715113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Cole M., Lindeque P., Fileman E., Halsband C., Galloway T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015;49:1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S., Sharma S. Microplastics in our oceans and marine health, Field Actions Science Reports. J. Field Actions. 2019;2019:54–61. [Google Scholar]
- 8.Barnes D.K., Galgani F., Thompson R.C., Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita R., Takada H., Fukuwaka M.A., Watanuki Y. Physical and chemical effects of ingested plastic debris on short-tailed shearwaters, Puffinus tenuirostris, in the North Pacific Ocean. Mar. Pollut. Bull. 2011;62:2845–2849. doi: 10.1016/j.marpolbul.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Sighicelli M., Pietrelli L., Lecce F., Iannilli V., Falconieri M., Coscia L., di Vito S., Nuglio S., Zampetti G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018;236:645–651. doi: 10.1016/j.envpol.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu X., Tang N., Yang W., Chang J. Microplastics pollution in the soils of various land-use types along Sheshui River basin of Central China. Sci. Total Environ. 2022;806:150620. doi: 10.1016/j.scitotenv.2021.150620. [DOI] [PubMed] [Google Scholar]
- 13.Tsering T., Sillanpaa M., Sillanpaa M., Viitala M., Reinikainen S.P. Microplastics pollution in the Brahmaputra River and the Indus River of the Indian Himalaya. Sci. Total Environ. 2021;789:147968. doi: 10.1016/j.scitotenv.2021.147968. [DOI] [PubMed] [Google Scholar]
- 14.de Haan W.P., Sanchez-Vidal A., Canals M., Party N.S.S. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar. Pollut. Bull. 2019;140:523–535. doi: 10.1016/j.marpolbul.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J., Ran W., Teng J., Liu Y., Liu H., Yin X., Cao R., Wang Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018;640–641:637–645. doi: 10.1016/j.scitotenv.2018.05.346. [DOI] [PubMed] [Google Scholar]
- 16.Cozar A., Echevarria F., Gonzalez-Gordillo J.I., Irigoien X., Ubeda B., Hernandez-Leon S., Palma A.T., Navarro S., Garcia-de-Lomas J., Ruiz A., et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA. 2014;111:10239–10244. doi: 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steer M., Cole M., Thompson R.C., Lindeque P.K. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017;226:250–259. doi: 10.1016/j.envpol.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Collard F., Gasperi J., Gilbert B., Eppe G., Azimi S., Rocher V., Tassin B. Anthropogenic particles in the stomach contents and liver of the freshwater fish Squalius cephalus. Sci. Total Environ. 2018;643:1257–1264. doi: 10.1016/j.scitotenv.2018.06.313. [DOI] [PubMed] [Google Scholar]
- 19.Digka N., Tsangaris C., Torre M., Anastasopoulou A., Zeri C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018;135:30–40. doi: 10.1016/j.marpolbul.2018.06.063. [DOI] [PubMed] [Google Scholar]
- 20.Ossmann B.E., Sarau G., Holtmannspotter H., Pischetsrieder M., Christiansen S.H., Dicke W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018;141:307–316. doi: 10.1016/j.watres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Schymanski D., Goldbeck C., Humpf H.U., Furst P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Gundogdu S. Contamination of table salts from Turkey with microplastics. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:1006–1014. doi: 10.1080/19440049.2018.1447694. [DOI] [PubMed] [Google Scholar]
- 23.Liebezeit G., Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31:1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- 24.Prata J.C., Paco A., Reis V., da Costa J.P., Fernandes A.J.S., da Costa F.M., Duarte A.C., Rocha-Santos T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020;331:127323. doi: 10.1016/j.foodchem.2020.127323. [DOI] [PubMed] [Google Scholar]
- 25.Akhbarizadeh R., Dobaradaran S., Nabipour I., Tajbakhsh S., Darabi A.H., Spitz J. Abundance, composition, and potential intake of microplastics in canned fish. Mar. Pollut. Bull. 2020;160:111633. doi: 10.1016/j.marpolbul.2020.111633. [DOI] [PubMed] [Google Scholar]
- 26.Kutralam-Muniasamy G., Perez-Guevara F., Elizalde-Martinez I., Shruti V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020;714:136823. doi: 10.1016/j.scitotenv.2020.136823. [DOI] [PubMed] [Google Scholar]
- 27.Li Q., Feng Z., Zhang T., Ma C., Shi H. Microplastics in the commercial seaweed nori. J. Hazard. Mater. 2020;388:122060. doi: 10.1016/j.jhazmat.2020.122060. [DOI] [PubMed] [Google Scholar]
- 28.Cho Y.M., Choi K.H. The current status of studies of human exposure assessment of microplastics and their health effects: A rapid systematic review. Environ. Anal. Health Toxicol. 2021;36:e2021004. doi: 10.5620/eaht.2021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu B., Wu X., Liu S., Wang Z., Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2cells. Chemosphere. 2019;221:333–341. doi: 10.1016/j.chemosphere.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 30.Hwang J., Choi D., Han S., Choi J., Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 31.Schirinzi G.F., Perez-Pomeda I., Sanchis J., Rossini C., Farre M., Barcelo D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 32.Au S.Y., Bruce T.F., Bridges W.C., Klaine S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015;34:2564–2572. doi: 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- 33.Yang H., Lai H., Huang J., Sun L., Mennigen J.A., Wang Q., Liu Y., Jin Y., Tu W. Polystyrene microplastics decrease F-53B bioaccumulation but induce inflammatory stress in larval zebrafish. Chemosphere. 2020;255:127040. doi: 10.1016/j.chemosphere.2020.127040. [DOI] [PubMed] [Google Scholar]
- 34.Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M.E., le Goic N., Quillien V., Mingant C., Epelboin Y., et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA. 2016;113:2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B., Ding Y., Cheng X., Sheng D., Xu Z., Rong Q., Wu Y., Zhao H., Ji X., Zhang Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/j.chemosphere.2019.125492. [DOI] [PubMed] [Google Scholar]
- 36.Jin H., Ma T., Sha X., Liu Z., Zhou Y., Meng X., Chen Y., Han X., Ding J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021;401:123430. doi: 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- 37.Hou B., Wang F., Liu T., Wang Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021;405:124028. doi: 10.1016/j.jhazmat.2020.124028. [DOI] [PubMed] [Google Scholar]
- 38.An R., Wang X., Yang L., Zhang J., Wang N., Xu F., Hou Y., Zhang H., Zhang L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665. doi: 10.1016/j.tox.2020.152665. [DOI] [PubMed] [Google Scholar]
- 39.Lu L., Wan Z., Luo T., Fu Z., Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 40.Deng Y., Zhang Y., Lemos B., Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S., Kang K.K., Sung S.E., Choi J.H., Sung M., Seong K.Y., Lee S., Yang S.Y., Seo M.S., Kim K. Toxicity Study and Quantitative Evaluation of Polyethylene Microplastics in ICR Mice. Polymers. 2022;14:402. doi: 10.3390/polym14030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperati C.A., Starkweather H.W. Fortschritte Der Hochpolymeren-Forschung. Springer; Berlin/Heidelberg, Germany: 1961. Fluorine-containing polymers. II. Polytetrafluoroethylene. [Google Scholar]
- 43.Sajid M., Ilyas M. PTFE-coated non-stick cookware and toxicity concerns: A perspective. Environ. Sci. Pollut. Res. Int. 2017;24:23436–23440. doi: 10.1007/s11356-017-0095-y. [DOI] [PubMed] [Google Scholar]
- 44.Rastogi V., Rao U., Chaurasia S., Sijoy C.D., Mishra V., Chaturvedi S., Deo M.N. Time-Resolved Vibrational Spectroscopy of Polytetrafluoroethylene Under Laser-Shock Compression. Appl. Spectrosc. 2017;71:2643–2652. doi: 10.1177/0003702817726542. [DOI] [PubMed] [Google Scholar]
- 45.Firsov S.P., Zhbankov G., Bakhramov M., Abdukadyrov A.M., Gafurov A.G. Raman spectra and structure of polytetrafluoroethylene subjected to elastic deformation grinding. J. Appl. Spectrosc. 1993;59:644–647. doi: 10.1007/BF00661793. [DOI] [Google Scholar]
- 46.Andrady A.L., Neal M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1977–1984. doi: 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enyoh C.E., Verla A.W., Verla E.N., Ibe F.C., Amaobi C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019;191:668. doi: 10.1007/s10661-019-7842-0. [DOI] [PubMed] [Google Scholar]
- 48.Frias J., Nash R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019;138:145–147. doi: 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y., Wu Y., Ma J., An Y., Liu Q., Yang S., Qu Y., Chen H., Zhao W., Tian Y. Microplastics pollution in the soil mulched by dust-proof nets: A case study in Beijing, China. Environ. Pollut. 2021;275:116600. doi: 10.1016/j.envpol.2021.116600. [DOI] [PubMed] [Google Scholar]
- 50.Sruthy S., Ramasamy E.V. Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India. Environ. Pollut. 2017;222:315–322. doi: 10.1016/j.envpol.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 51.Lenaker P.L., Corsi S.R., Mason S.A. Spatial Distribution of Microplastics in Surficial Benthic Sediment of Lake Michigan and Lake Erie. Environ. Sci. Technol. 2021;55:373–384. doi: 10.1021/acs.est.0c06087. [DOI] [PubMed] [Google Scholar]
- 52.Campanale C., Stock F., Massarelli C., Kochleus C., Bagnuolo G., Reifferscheid G., Uricchio V.F. Microplastics and their possible sources: The example of Ofanto river in southeast Italy. Environ. Pollut. 2020;258:113284. doi: 10.1016/j.envpol.2019.113284. [DOI] [PubMed] [Google Scholar]
- 53.Park T.J., Lee S.H., Lee M.S., Lee J.K., Lee S.H., Zoh K.D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020;708:134535. doi: 10.1016/j.scitotenv.2019.134535. [DOI] [PubMed] [Google Scholar]
- 54.Pegado T., Brabo L., Schmid K., Sarti F., Gava T.T., Nunes J., Chelazzi D., Cincinelli A., Giarrizzo T. Ingestion of microplastics by Hypanus guttatus stingrays in the Western Atlantic Ocean (Brazilian Amazon Coast) Mar. Pollut. Bull. 2021;162:111799. doi: 10.1016/j.marpolbul.2020.111799. [DOI] [PubMed] [Google Scholar]
- 55.Fournier E., Etienne-Mesmin L., Grootaert C., Jelsbak L., Syberg K., Blanquet-Diot S., Mercier-Bonin M. Microplastics in the human digestive environment: A focus on the potential and challenges facing in vitro gut model development. J. Hazard. Mater. 2021;415:125632. doi: 10.1016/j.jhazmat.2021.125632. [DOI] [PubMed] [Google Scholar]
- 56.Kwon J.H., Kim J.W., Pham T.D., Tarafdar A., Hong S., Chun S.H., Lee S.H., Kang D.Y., Kim J.Y., Kim S.B., et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health. 2020;17:6710. doi: 10.3390/ijerph17186710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokhun N., Somparn A. Microplastic Contaminations in Buffet Food from Local Markets. Naresuan Univ. J. 2020;28:4. [Google Scholar]
- 58.Kosuth M., Mason S.A., Wattenberg E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE. 2018;13:e0194970. doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuccarello P., Ferrante M., Cristaldi A., Copat C., Grasso A., Sangregorio D., Fiore M., Conti G.O. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019;157:365–371. doi: 10.1016/j.watres.2019.03.091. [DOI] [PubMed] [Google Scholar]
- 60.Kanhai D.K., Gardfeldt K., Krumpen T., Thompson R.C., O’Connor I. Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep. 2020;10:5004. doi: 10.1038/s41598-020-61948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X., Qiang L., Shi H., Cheng J. Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Mar. Pollut. Bull. 2020;154:111079. doi: 10.1016/j.marpolbul.2020.111079. [DOI] [PubMed] [Google Scholar]
- 62.Mak C.W., Yeung K.C., Chan K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019;182:109442. doi: 10.1016/j.ecoenv.2019.109442. [DOI] [PubMed] [Google Scholar]
- 63.Qiao R., Deng Y., Zhang S., Wolosker M.B., Zhu Q., Ren H., Zhang Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334. doi: 10.1016/j.chemosphere.2019.07.065. [DOI] [PubMed] [Google Scholar]
- 64.Choi J.S., Jung Y.J., Hong N.H., Hong S.H., Park J.W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus) Mar. Pollut. Bull. 2018;129:231–240. doi: 10.1016/j.marpolbul.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 65.Uurasjarvi E., Paakkonen M., Setala O., Koistinen A., Lehtiniemi M. Microplastics accumulate to thin layers in the stratified Baltic Sea. Environ. Pollut. 2021;268:115700. doi: 10.1016/j.envpol.2020.115700. [DOI] [PubMed] [Google Scholar]
- 66.Carr K.E., Smyth S.H., McCullough M.T., Morris J.F., Moyes S.M. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012;46:185–252. doi: 10.1016/j.proghi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Nava V., Frezzotti M.L., Leoni B. Raman spectroscopy for the analysis of microplastics in aquatic systems. Appl. Spectrosc. 2021;75:1341–1357. doi: 10.1177/00037028211043119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.