Abstract
Erismadelphus exsul Mildbr bark is widely used in Gabonese folk medicine. However, little is known about the active compounds associated with its biological activities. In the present study, phytochemical profiling of the ethanolic extract of Erismadelphus exsul was performed using a de-replication strategy by coupling HPLC-ESI-Q/TOF with a molecular network approach. Eight families of natural compounds were putatively identified, including cyclopeptide alkaloids, esterified amino acids, isoflavonoid- and flavonoid-type polyphenols, glycerophospholipids, steroids and their derivatives, and quinoline alkaloids. All these compounds were identified for the first time in this plant. The use of molecular networking obtained a detailed phytochemical overview of this species. Furthermore, antioxidant (2,2-diphenyl-1-picryl-hydrazylhydrate (DPPH) and ferric reducing capacity (FRAP)) and in vitro antimicrobial activities were assessed. The crude extract, as well as fractions obtained from Erismadelphus exsul, showed a better reactivity to FRAP than DPPH. The fractions were two to four times more antioxidant than ascorbic acid while reacting to FRAP, and there was two to nine times less antioxidant than this reference while reacting to DPPH. In addition, several fractions and the crude extract exhibited a significant anti-oomycete activity towards the Solanaceae phytopathogen Phytophthora infestans in vitro, and, at a lower extent, the antifungal activity against the wheat pathogen Zymoseptoria tritici had growth inhibition rates ranging from 0 to 100%, depending on the tested concentration. This study provides new insights into the phytochemical characterization and the bioactivities of ethanolic extract from Erismadelphus exsul bark.
Keywords: antimicrobial activity, antioxidant activity, de-replication, Erismadelphus exsul, molecular networks
1. Introduction
Erismadelphus exsul Mildbr (E. exsul), known as ‘Essang-Afane’ by local people (Fang) of northern Gabon, is a Gabonese medicinal plant. Its bark is used in folk Gabonese medicine to treat several microbial diseases, such as urinary tract infections, bronchitis, pneumonia, and sexually transmitted diseases—especially HIV opportunistic diseases [1]. E. exsul belongs to the family Vochysiaceae, which is commonly distributed across America and tropical Africa [2]. Indeed, Vochysiaceae is an important plant family in the neotropical plant formation [3]. Generally, plants belonging to this family are known for their numerous pharmacological properties, such as the case of E. exsul. However, despite being well known, E. exsul has not been the subject of any phytochemical or biological studies. Nevertheless, a few studies have been conducted on the Vochysiaceae family. For example, Weniger and coll. showed that several species of the Vochysia genus are used by traditional communities in South America to alleviate conditions related to inflammation, such as skin wounds, asthma, and pulmonary congestion [4]. The work of Franco and coll. also demonstrated the antioxidant and anti-glycation capacity of plant extracts from the Vochysiaceae family [5]. In addition, Dornelo and coll. reported that some hyphomycetes (fungi) could live in association with plants belonging to this plant family [6]. The literature studies reported here show the importance of searching for bioactive compounds in plants from the Vochysiaceae family, specifically E. exsul. Until now, the step of screening and identifying bioactive compounds from a plant extract was often very time consuming and tedious, as it consisted of purifying, testing, and individually identifying several compounds from a rich extract. In this context, new innovative bioinformatics approaches, such as molecular network (MN) and in-silico fragmentation tools, are currently being developed to make the search for molecules of biological interest less tedious [7,8]. Here we propose a de-replication strategy based on combining MN and liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) data to rapidly target and identify chemical constituents present in the ethanolic extract of E. exsul. The de-replication information was then collected by MN, which compares all MS/MS spectra of studied compounds and groups them according to their spectral similarity, thus allowing the visualization of all structurally related molecules within the same cluster and structurally different molecules distributed in different clusters [9]. This approach thus provides new ways to navigate the metabolome of biological samples by providing key information about the analogies between detected metabolites [10]. The main interest of molecular networks is that they can be used to exploit numerous MS/MS spectra without prior knowledge of the chemical composition of the samples, and the data obtained can be directly correlated to antimicrobial and antioxidant activity.
2. Results
One of the major focuses of the modern discovery of new compounds based on natural products is to apply an efficient strategy for the evaluation of secondary metabolites essential for human use. In this work, we demonstrate the usefulness of a new approach, molecular networking combined with LC-MS/MS, which is applied to the ethanolic extract of E. exsul in order to identify bioactive specialized metabolites of this extract.
2.1. Metabolite Profiling of E. exsul Determined via LC-MS/MS
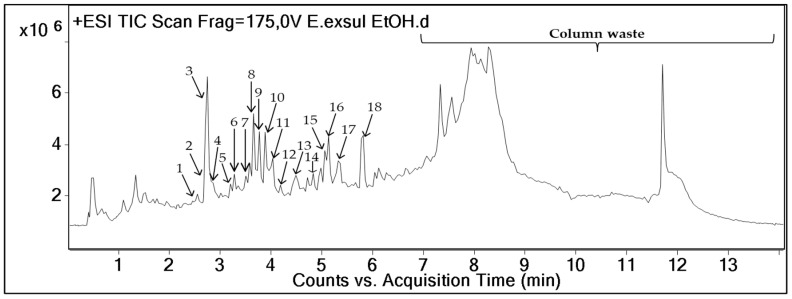
LC-HRMS/MS analyses of the crude extract and fractions of E. exsul were performed in a positive ionization mode using the data-dependent acquisition mode. A TIC chromatogram of the crude extract is presented in Figure 1. Next, an auto-MS2 was carried out in which the most predominant MS1 ions are selected for MS2 fragmentation. A manual inspection of the resulted MS/MS spectra led to the putative identification of chemical constituents of the crude extracts and fractions of E. exsul; they are listed in Table 1.
Figure 1.
Total ion chromatogram (TIC) in positive ion mode of the ethanolic extract of Erismadelphus exsul obtained on an Agilent 6530 Q/ToF (Scan rang m/z 100–1700). The number above each peak corresponds to the peak numbers in Table 1.
Table 1.
Tentative identification of compounds from crude extract of E. exsul by LC−ESI-MS/MS in the positive ion mode.
| Peak | RT (min) | m/z [M+H]+ | Molecular Formulas [M] | MS2 | Ions Tentative Identification | Confidence Level |
|---|---|---|---|---|---|---|
| 1 | 2.571 | 477.250 | C27H32N4O4 | 477.24, 460.74, 359.33, 265.95, 186.09, 120.08 | Unknown | 4 |
| 2 | 2.707 | 562.302 | C31H39N5O5 | 562.30, 477.24, 378.18, 174.09, 134.06, 120.08 | Mauritine F [13] | 3 |
| 3 | 2.745 | 576.319 | C32H41N5O5 | 576.31, 477.24, 378.18, 174.09, 134.06, 120.08 | Mauritine A [13] | 3 |
| 4 | 2.885 | 592.313 | C32H41N5O6 | 592.31, 477.24, 378.18, 174.09, 134.06, 120.08 | Mauritine A N-oxide [13] | 3 |
| 5 | 3.088 | 433.113 | C21H20O10 | 283.06, 313.07, 121.02, 165.01 | Vitexin | 3 |
| 6 | 3.206 | 599.330 | C30H38N12O2 | 336.21, 458.27 | Unknown | 4 |
| 7 | 3.282 | 313.086 | C17H12O6 | 315.08, 244.37, 272.80, 312.92, 103.07 | Aflatoxin B2 | 3 |
| 8 | 3.282 | 579.171 | C26H28O15 | 579.17, 152.01, 285.07, 460.87 | Rhoifolin | 3 |
| 9 | 3.661 | 439.357 | C30H46O2 | 393.35, 339.15, 313.07 | Ganodermenonol | 3 |
| 10 | 3.774 | 453.336 | C30H44O3 | 329.10, 264.23 | Kulactone | 3 |
| 11 | 4.646 | 322.23 | C19H31NO3 | 322.23, 163.063, 288.198 | 8-Dihydroantidesmone [14] | 3 |
| 12 | 4.987 | 306.243 | C19H31NO2 | 290.98, 296.48, 178.086, 163.063 | 8-Deoxoantidesmone [14] | 3 |
| 13 | 5.138 | 398.265 | C20H35N3O5 | 306.24, 194.11 | Unknown | 4 |
| 14 | 5.365 | 320.222 | C19H29NO3 | 278.24, 220.59, 222.11, 234.13, 163.063 | Antidesmone [14] | 3 |
| 15 | 5.782 | 256.133 | C16H17NO2 | Unknown | 4 | |
| 16 | 5.061 | 445.212 | C27H28N2O4 | 283.09, 398.26, 194.11 | Asperglaucide | 3 |
| 17 | 5.782 | 507.228 | C32H30N2O4 | 327.23, 256.13 | Asperphenamate | 3 |
| 18 | 6.123 | 317.196 | C16H28O6 | 2-Bornanol (1S,2R)-O-d-glucopyranoside | 3 |
The confidence level of compound annotations was performed with respect to the guidelines published by the Metabolomics Society [15]. Level 0: Unambigous 3D structure: isolated, pure compound, including full stereochemistry; Level 1: Confident 2D structure: uses reference standard match or full 2D structure elucidation; Level 2: Probable structure: matched to literature data or databases by diagnostic evidence; Level 3: Possible structure or class: most likely structure, isomers possible, substance class or substructure match; Level 4: Unkown feature of interest.
Known and unknown compounds were annotated using databases such as: Dictionary Natural Product (https://dnp.chemnetbase.com/, accessed on 5 May 2022) [11,12], Computer software review: Scifinder (https://scifinder-n.cas.org, accessed on 5 May 2022), and PubChem (https://pubchem.ncbi.nlm.nih.gov/compound, accessed on 5 May 2022). As shown in Table 1, a total of 18 compounds were putatively identified. These metabolites were reported to belong to eight (8) families of natural compounds: cyclopeptide alkaloids, isoflavonoids and flavonoids (polyphenols), glycerophospholipids, esterified amino acids, steroids and their derivatives, and quinoline alkaloids
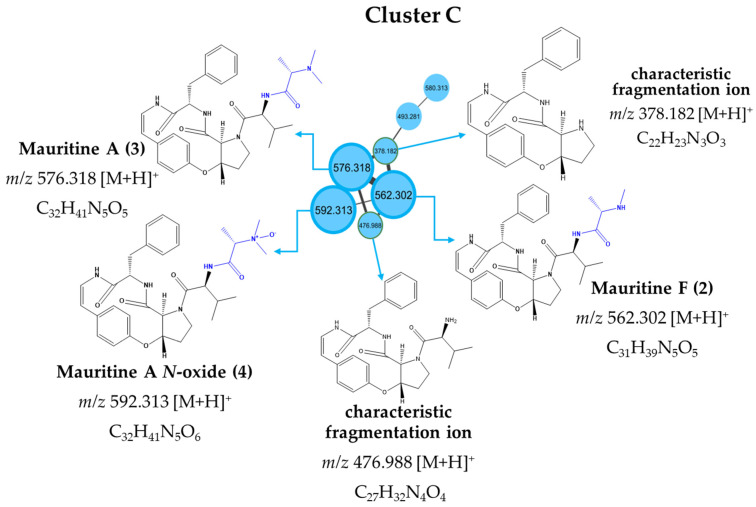
Among the cyclopeptide alkaloids, Mauritine F (2), Mauritine A (3), and Mauritine A N-oxide (4) at m/z 562.302 [M+H]+, m/z 576.319 [M+H]+, and m/z 592.313 [M+H]+, respectively, were putatively identified. Compounds 2, 3, and 4 were previously isolated by Han and coll. [13] from the stems of Ziziphus apetala. An analysis of the exact masses of the three alkaloids showed a mass difference of -14 Da between Mauritine F and Mauritine A, indicating a loss of one methylene (CH2) by Mauritine A; and a difference of +16 Da between Mauritine A and Mauritine A N-oxide, indicating one more oxygen than Mauritine A. Furthermore, the three compounds share the same MS/MS fragment ions 120.08 [M+H]+; 134.06 [M+H]+, and 174.09 [M+H]+, which were previously described by Kapadia and coll. [16]. Based on the literature, most cyclopeptide alkaloids differ only in their substituents in the asymmetric C34 position. Cyclopeptide alkaloids from epimeric pairs, wherein the two epimers can be transformed into each other, has the C34 position configured as (R) or (S) [13]. The available MS and MS/MS data do not allow us to assign with any certainty the epimer pairs present in the EtOH extract of E. exsul.
2.2. MS/MS-Molecular Networking-Based Dereplication
In addition, we also performed an analysis of the crude extract and fractions of E. exsul using the molecular network (MN) approach by the GNPS website (http://gnps.ucsd.edu, accessed on 5 May 2022) [17]. All obtained HRESIMS/MS spectra were preprocessed via MZmine2 [18] software following the feature-based molecular networking workflow [19]. These arrayed MS/MS spectra were then searched in an automated batch mode against the spectral libraries hosted by GNPS and the results were annotated as part of this de-replication strategy. A re-examination of the molecular network that had previously been obtained from the organization of the LC-MS/MS data of the crude extract and fractions of E. exsul revealed the presence of a total of 585 precursor ions visualized in Cytoscape 3.51 [20] as nodes in the molecular map, which included 8 clusters (node ≥ 2) and 346 single nodes (Supplementary Materials Figure S1). An exploration of the relative contributions of peak areas at nodes in the network revealed that the compounds were grouped based on structural similarities and three main clusters emerged: Cluster A corresponded to steroids and derivatives (red), Cluster B grouped glycerophospholipids (purple), Cluster D displayed quinoline alkaloids (blue), and Cluster C, of particular interest to us, was composed of seven nodes, three of which were at m/z 576.319, 562.302, and 592.313 (previously annotated as Mauritine A, Mauritine F, and Mauritine A N-oxide, respectively). The presence of characteristic ions at m/z 378.182 and m/z 476.988 in cluster C provided additional information on the identity of the above compounds [13,16] (Figure 2). Based on chemotaxonomic data, the distribution of cyclopeptide alkaloids shows that they are mainly present in Rhamnaceae (Genus Ziziphus) [21], Malvaceae (Genus Melochia) [22,23], and Euphorbiaceae (Genus Antidesmone) [24], and all Angiosperms (Dicotyledons) evolved in the Rosideae as well as the Vochysiaceae family of E. exsul. Finally, polyphenols such as Vitexin at m/z 433.113, Formononetin at m/z 269.083, the glycerophospholipid on the example of 1,2-Dilinoleoyl-sn-glycero-3-phosphocholine at m/z 784.588, terpenoids such as Sarmentoside B at m/z 663.463, and quinoline alkaloids such as Antidesmone at m/z 320.222, 8-Deoxoantidesmone at m/z 306.243 and 8-Dihydroantidesmone at m/z 322.238 were also annotated (Supplementary Materials Figure S2). It is important to highlight the fact that some compounds detected in this plant are described in fungi so far. This is the case of Asperphenamate, Asperglaucide, Formononetin, and Aflotoxin B2. The most certain hypothesis until now that explains this strong presence of the molecules of the fungal type in this plant are found in the work done in Brazil by Dornelo and coll. (2017) [6]. Indeed, they showed plants of the Vochysiaceae family, of which Erismadelphus is a member, have the ability to live in association (symbiosis) with hyphomycetes. Given the similarity in terms of climate (equatorial climate) between Gabon and Brazil, it seems important to consider this hypothesis without rejecting the fact that these molecules can come from the plant since the harvests were made on several sites. In order to assess the likelihood of a possible contamination of the samples, thorough molecular biology investigations will be performed in due course to get more insight into the chemical diversity of this plant.
Figure 2.
Putative annotation of cluster C with three cyclopeptide alkaloids compounds.
The results show that the combination of molecular networks and LC-HRMS/MS data greatly improves the efficiency of the identification of chemical components in the targeted plant extracts. About seventeen compounds were putatively identified in the ethanolic extract of E. exsul bark (Table 2). The MN approaches also allowed us to support and confirm the conclusions from the interpretation of the MS/MS data for the three cyclopeptide alkaloid derivatives that we had previously identified. This network was carried out in a strategy of exploration of the metabolites present in the crude extract and fractions of E. exsul.
Table 2.
Annotated compounds on the molecular networking of E. exsul ethanolic extract.
| m/z [M+H]+ | Molecular Formulas [M] |
Adducts Types | Cosine Score | Compound |
|---|---|---|---|---|
| 758.570 | C42H82NO8P | [M+H]+ | 0.92 | 1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine |
| 784.588 | C44H80NO8P | [M+H]+ | 0.77 | 1,2-Dilinoleoyl-sn-glycero-3-phosphocholine |
| 772.582 | C43H85NO8P | [M+H]+ | 0.70 | 1-Heptadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine |
| 275.201 | C19H30O2 | [M+H−H2O]+ | 0.73 | Androstenediol |
| 309.207 | C19H30O3 | [M+H]+ | 0.76 | Epioxandrolone |
| 321.242 | C21H34O2 | [M+H]+ | 0.73 | Methasteron |
| 454.919 | C24H36O8 | [M+H]+ | 0.71 | [(1S,2R,4aR,8aR)-1-Acetyloxy-1,4a-dimethyl-6-oxo-7-propan-2-ylidene-2,3,4,5,8,8a-hexahydronaphthalen-2-yl]-3_acetyloxy-2-hydroxy-2-methylbutanoate |
| 439.840 | C21H22O9 | [M+Na]+ | 0.78 | Isoliquiritin |
| 437.341 | C24H32O6 | [M+Na]+ | 0.73 | [1S,3R,3aS,4S,8aR)-1-Acetyloxy-3-hydroxy-6,8a-dimethyl-3-propan-2-yl-1,2,3a,4,5,8-hexahydroazulen-4-yl]4-hydroxybenzoate |
| 406.259 | C20H30O6 | [M+K]+ | 0.81 | 3-[2-(6,7-Dihydroxy-1,2,4a-trimethylspiro [3,4,6,7,8,8a-hexahydro-2H-naphthalene-5,2′-oxirane]-1-yl)ethyl]-2-hydroxy-2H-furan-5-one |
| 369.263 | C27H46O | [M+H−H2O]+ | 0.82 | Cholesterol |
| 410.258 | C22H32O7 | [M+H]+ | 0.74 | [6,10a-dihydroxy-4-(hydroxymethyl)-4,7,11b-trimethyl-9-oxo-1,2,3,4a,5,6,6a,7,11,11a-decahydronaphtho[2,1-f][1]benzofuran-5-yl] acetate |
| 507.232 | C32H30N2O4 | [M+H]+ | 0.83 | Asperphenamate |
| 269.083 | C16H12O4 | [M+H]+ | 0.87 | Formononetin |
| 413.380 | C27H42O3 | [M+H]+ | 0.83 | 3-Oxocholest-4-en-26-oic acid |
| 579.177 | C26H28O15 | [M+H]+ | 0.76 | Rhoifolin |
| 663.463 | C34H48O13 | [M+H]+ | 0.73 | Sarmentoside B |
2.3. Antioxidant Activity
The antioxidant activity of crude extract and ethanolic fractions of E. exsul was evaluated by DPPH and FRAP methods using Trolox as a standard and ascorbic acid for comparison. Both methodologies react to different antioxidant mechanisms, including free radical scavenging for DPPH and iron reduction for FRAP. Overall, the crude extract and fractions obtained from E. exsul showed a better reactivity to FRAP than to DPPH (Table 3). The E. exsul fraction was two to four times more antioxidant than ascorbic acid while reacting to FRAP, whereas it was two to nine times less antioxidant than this reference while reacting to DPPH. These results are in agreement with the way E. exsul peel was used in traditional medicine. Indeed, ingestion in the form of herbal tea could allow for better assimilation of the metabolites and thus their passage through the blood system (where iron is located) than another type of traditional use such as cataplasm (where radical scavenging activity will be more efficient). Focusing on the crude extract responsiveness to both tests may seem surprising. Regarding FRAP, the antioxidant property of the crude extract is equivalent to those of fractions F4 to F8, and four times more antioxidant than ascorbic acid. This could be explained by a threshold effect or by the presence in those fractions of the same metabolite that reacts to FRAP. Regarding DPPH, the antioxidant property of the crude extract is 20 times lower than those of fractions F4 to F8, and 50 times less antioxidant than ascorbic acid. This result can be interpreted in three ways: (i) an antagonistic effect of metabolites present in the crude extract and separated in the fractions regarding free radical scavenging activity; (ii) the presence in the crude extract of a compound inhibiting radical scavenging activity of the compounds from fraction 3 to fraction 8; and less probably, (iii) nevertheless it cannot be excluded that the quantity and/or proportion of each metabolite in the crude extract led to this weak antioxidant activity. It has been widely demonstrated that polyphenols have a great capacity to scavenge free radicals and to chelate transition metals such as iron [25]. The high reactivity in the FRAP assay can be linked to the presence of several flavonoids and isoflavonoids such as Formononetin, Biochanin A, Rhoifolin, and Vitexin. Indeed, many studies have shown the strong antioxidant activity of Formononetin [26,27], Rhoifolin [28], and Vitexin.
Table 3.
Antioxidant properties of the crude extract and fractions (F1–F8) of Erismadelphus exsul.
| Test Sample | FRAP (µM TE/g DW) |
DPPH (µM TE/g DW) |
|---|---|---|
| EE crude extract | 2173.40 ± 13.92 | 29.04 ± 6.01 |
| EE fraction 1 | 234.37 ± 12.21 | 42.47 ± 4.19 |
| EE fraction 2 | 1959.24 ± 7.33 | 39.58 ± 1.95 |
| EE fraction 3 | 1369.14 ± 83.57 | 145.51 ± 6.69 |
| EE fraction 4 | 2173.40 ± 7.33 | 253.67 ± 50.50 |
| EE fraction 5 | 2246.52 ± 44.42 | 498.03 ± 23.17 |
| EE fraction 6 | 2307.54 ± 48.40 | 464.62 ± 8.57 |
| EE fraction 7 | 2329.42 ± 47.90 | 565.51 ± 9.29 |
| EE fraction 8 | 2187.22 ± 85.49 | 574.03 ± 7.94 |
| Ascorbic Acid | 631.99 ± 39.08 | 1261.79 ± 150.68 |
The results are expressed as mean ± standard deviation (S.D.), EE = Erismadelphus exsul, DW = dry weight, TE = Trolox-Equivalent.
These results are in agreement with those obtained by R.R Franco and coll. (2019) who showed strong antioxidant activities of several plants belonging to the Vochysiaceae family [5]. In fact, they are even more interesting because all of the activities observed by Franco and colleagues (2019) are lower than the antioxidant capacity of ascorbic acid regarding the FRAP test, while the extract and fractions obtained from Erismadelphus exsul are four times more antioxidant than ascorbic acid, suggesting a putative interest for industrial valorization such as nutraceutical potential.
2.4. Antimicrobial Assay
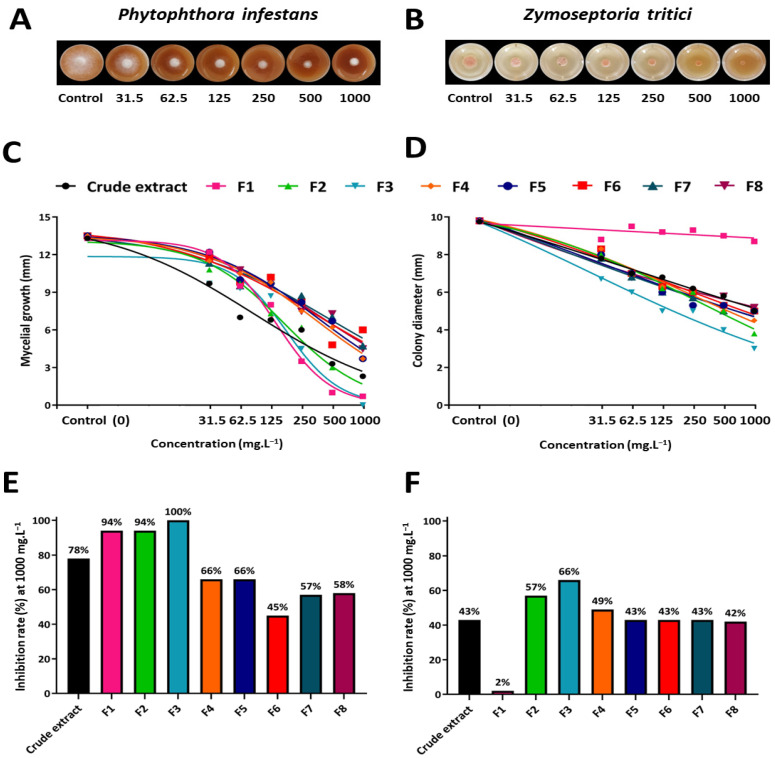
The in vitro antimicrobial activity of E. exsul bark was measured on both Phytophthora infestans, an oomycete responsible for late blight on numerous Solanaceae such as tomato and potato, and Zymoseptoria tritici, a fungus responsible for Septoria tritici blotch of wheat. Two independent experiments with three biological replicates were performed for each pathogen and both experiments provided similar results (Supplementary Materials Table S1). Overall, close patterns of antimicrobial activity were observed for all fractions and the crude extract on each pathogen (Figure 3A). Dose-response curves revealed that all fractions, as well as the crude extract, inhibit the mycelial growth of P. infestans, especially at the highest tested concentration of 1000 mg·L−1 (Figure 3A,C). The most active fraction was F3, with 100 % mycelial growth inhibition at 1000 mg.L−1 (Figure 3E). Regarding Z. tritici, the fractions and the crude extract showed lower overall antimicrobial activities when compared to those obtained for P. infestans (Figure 3B,D). No fraction was able to totally inhibit the fungal growth, even at the highest tested concentration (Figure 3F). Interestingly, F3, the most effective fraction at 1000 mg·L−1 on P. Infestans, was also the most active one at this concentration on Z. tritici (Figure 3E,F). However, F1, one of the most active fractions on P. Infestans, displayed no activity on Z. tritici (Figure 3C–F). The difference in activity highlighted among the two targeted phytopathogens can be explained by the fact that the compounds responsible for the anti-oomycete activity occur in higher concentrations in the obtained fractions when compared to those responsible for the antifungal activity. On the other hand, this difference in the activity between P. infestans and Z. tritici might be explained by the physiology and the wall composition of the two species. Indeed, the composition of the wall plays a major physiological role in the exchanges between the intra- and extracellular environment. In fungi, the presence of chitin, glucans, and other polymers favors the maintenance of the flexibility of the wall, thus preventing certain molecules from penetrating the cell and reaching their cellular target [29]. This may, in part, explain the lower activity of molecules present in the extract and fractions of E. exsul on Z. tritici. Although several previous works have shown significant antimicrobial activities of a wide range of botanical extracts against both pathogens [30,31], this is the first report highlighting the antimicrobial activity of E. exsul extract against P. infestans and Z. tritici. The objective being to search for the molecules involved in these activities, the most active fractions were analyzed by mass spectrometry and the molecules of interest were identified and targeted using the molecular network approach. Thus, it was shown that cyclopeptide alkaloids (Mauritine A, Mauritine F, and Mauritine A N-oxide) and quinoline alkaloids (8-Deoxoantidesmone, 8-Dihydroantidesmone, and Antidesmone) are partly responsible for the antimicrobial activity of Erismadelphus exsul bark (Supplementary Materials Figures S3 and S4). This is consistent with several studies that have shown the antimicrobial activity of cyclopeptide and quinoline alkaloids. Among them, the work of Panseeta and coll. showed that cyclopeptide alkaloids have strong antimicrobial activities, especially against some fungi, mycobacteria, or protozoa [32]. Also, a study by Cretton and coll. showed that quinoline alkaloids exhibit antifungal activity against candida albicans [33]. In general, alkaloids play an important role in biological structures, they are known for their high antimicrobial power [34].
Figure 3.
Antimicrobial activity of crude ethanolic extract and fractions from Erismadelphus exsul bark on Phytophthora infestans (A,C,E) and Zymoseptoria tritici (B,D,F) at three and eleven days after 12-well microplate inoculation with the pathogens, respectively. (A,B) illustration of the in vitro growth of P. infestans (A) and Z. tritici (B) obtained on V8 and PDA mediums, respectively, amended or not with different concentrations of the fraction F3. (C,D) Dose-response curves obtained for P. infestans (C) and Z. tritici (D) using the crude extract and fractions at different concentrations. (E,F) Percentage of inhibitions scored for P. infestans (E) and Z. tritici (F) using the crude extract and fractions at the highest tested concentration of 1000 mg·L−1. The X-axis graduation in both (C) and (D) subfigures is presented in Log2 of the tested concentrations.
3. Materials and Methods
3.1. Plant Materials
Based on an ethnopharmacological survey of medicinal plants used in Gabonese traditional medicine, fresh plant samples were collected from the forests of Woleu-Ntem province in northern Gabon: (2°36′0″ N, 12°4′0″ E) (0°43′0″ N, 11°37′60″ E) in August 2018. Fresh bark from the trunks of E. exsul was cut using pruning shears, labeled, and transported in a UV-resistant plastic bag. Botanical identification was confirmed by Prof. Henri Paul Bourobou Bourobou and Mr. Raoul Niangadouma of the Institute of Pharmacopoeia and Traditional Medicine (IPHAMETRA) in Libreville. An herbarium reference specimen (M.E.M 001) has been deposited at the National Herbarium of Gabon (Libreville).
3.2. Extraction and Fractionation
The dried and pulverized barks of E. exsul (1500 g) were extracted by stirred maceration with 5 L of 70% ethanol at room temperature for 24 h. The resulting crude EtOH extract was vacuum filtered and concentrated by rotary evaporation to 3.64 g of an ethanolic fraction. All this residue was fractionated by flash chromatography on an 80 g Reveleris® Grace Silica PF-50SIHC-F0080 cartridge (Rheinland-Pfalz, Germany) using a gradient of CH2Cl2, thereby increasing the proportions of MeOH (100:0 to 0:100). The flow rate was fixed at 60 mL/min. Separation by flash chromatography yielded fractions based on their chromatographic profiles. Fractions of a similar nature were grouped by TLC thin layer chromatography, which were analyzed by HPLC-QTOF-HRES in order to localize the potential new compounds.
3.3. Data Dependent LC-HRMS2 Analyses
HPLC-QTOF-HRES analyses were achieved by coupling the LC system to a hybrid quadrupole time-of-flight mass spectrometer Agilent 6530 (Agilent Technologies, Massy, France) equipped with an ESI source, operating in positive ion mode. Source parameters were set as follows: capillary temperature at 320 °C, source voltage at 3500 V, and sheath gas flow rate at 10 L.min−1. The divert valve was set to waste for the first 3 min. MS scans were operated in full-scan mode from m/z 100 to 1700 (0.1 s scan time) with a mass resolution of 11 000 at m/z 922. A MS1 scan was followed by MS2 scans of the three most intense ions above an absolute threshold of 5000 counts. The selected parent ions were fragmented with one collision energy fixed at 45 eV and an isolation window of 1.3 amu. The calibration solution containined two internal reference masses (purine, C5H4N4, m/z 121.050873, and HP-921 [hexakis-(1H,1H,3H-tetrafluoropentoxy)phosphazene], C18H18O6N3P3F24, m/z 922.0098). A permanent MS/MS exclusion list criteria was set to prevent oversampling of the internal calibrant. LC-UV and MS data acquisition and processing were performed using MassHunter Workstation software (Agilent Technologies, Massy, France).
3.4. Feature-Based Molecular Networking Parameters
The MS2 data files were converted from the .d (Agilent) standard data-format to .mzXML format using the MSConvert software, which is part of the ProteoWizard package [35]. All .mzXML were then processed using MZmine 2 v32 [18]. The mass detection was realized by keeping the noise level at 3.0 E3. The ADAP chromatogram builder was used using a minimum group size of four scans, a group intensity threshold of 3000, a minimum highest intensity of 4000, and m/z tolerance of 0.01 Da (or 20 ppm) [36]. The ADAP wavelets deconvolution algorithm was used with the following standard settings: S/N threshold = 8, minimum feature height = 3000, coefficient/area threshold = 8, peak duration range 0.02–2 min, and RT wavelet range 0.02–1.2. MS2 scans were paired using an m/z tolerance range of 0.03 Da and RT tolerance range of 1.0 min. Isotopologues were grouped using the isotopic peaks grouper algorithm with an m/z tolerance of 0.005 Da (or 15 ppm) and a RT tolerance of 0.3 min. Peak alignment was performed using the joint aligner module (m/z tolerance = 0.005 Da (or 15 ppm), weight for m/z = 2, weight for RT = 2.0, and absolute RT tolerance 0.3 min). The peak list was gap-filled with the same RT and m/z range gap filler module (m/z tolerance of 0.005 Da (or 15 ppm)). Next, the .mgf spectral data file and its corresponding .csv metadata file (for RT and areas) were exported using the dedicated “Export to GNPS-FBMN” built-in option.
3.5. Molecular Network Analysis
A molecular network was created using the online workflow at GNPS (http://gnps.ucsd.edu, accessed on 5 May 2022) with a parent mass tolerance of 0.02 Da and an MS/MS fragment ion tolerance of 0.02 Da. A network was then created where edges were filtered to have a cosine score above 0.65 and more than 6 matched peaks. Further edges between two nodes were kept in the network if and only if each of the nodes appeared in each other’s respective top ten most similar nodes. The spectra in the network were then searched against GNPS spectral libraries. All matches kept between network spectra and library spectra were required to have a score above 0.6 and at least 6 matched peaks. The molecular networking data were analyzed and visualized using Cytoscape software (ver. 3.9.0) [20].
3.6. Antioxidant Assay
The Ferric Reducing Power (FRAP) assay was performed according to the method of Benzie and Souche [37] with slight modifications. The working FRAP reagent was produced by mixing 300 mM of acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution, and 20 mM FeCl3 in a 10:1:1 ratio just before use. Trolox (Sigma-Aldrich) was used as a standard. A 750 µM stock solution (diluted in ethanol) was prepared for Trolox. Then, serial dilutions were performed to obtain a range from 50 µM to 375 µM. After this, 950 µL of FRAP solution was combined with 50 µL of each standard dilution or its appropriately diluted sample. Each sample was done in triplicate and incubated at 37 °C for 30 min. The absorbance was read at 593 nm using a UV spectrophotometer (Anthelie Advanced, SECONAM).
3.7. 2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH•) Assay
DPPH free radical scavenging assay was adapted from Brand-Williams and coll. (1995) [38]. A 100 µM solution of DPPH was diluted in absolute ethanol. A 1 mM stock solution (diluted in ethanol) was prepared for Trolox. Then, serial dilutions were performed to obtain a range from 5 µM to 25 µM. After this, 1950 µL of DPPH solution was combined with 50 µL of each standard dilution or the appropriately diluted plant extract. Each sample was done in triplicate and incubated at room temperature for 20 min. The absorbance was read at 517 nm using a UV spectrophotometer (Anthelie Advanced, SECONAM).
3.8. Antimicrobial Assays
The anti-oomycete activity of the crude extract and fractions was evaluated according to the method described by Banso and coll. (1999), with slight modifications [39]. V8 medium was prepared by autoclaving at 121°C and cooled to 45 °C. Then, crude extract and fractions were prepared at a concentration of 100 mg·mL−1 in dimethyl sulfoxide (DMSO) and then added to the medium to have a range of six concentrations (1000, 500, 250, 125, 62.5, and 31.5 mg·L−1). Two milliliters of each mixture were poured into sterile 12-well microplates and allowed to solidify. Three repetitions per extract were made. The 4-mm diameter mycelial pellets inoculum were collected from 10-days-old P. infestans culture using a sterilized cork borer and then placed in the center of each well on the microplate. The plates were incubated at 18 ± 1 °C for three days and the growth of the P. infestans strain was measured for each modality (control, DMSO control, extracts, and fractions). The percentage of inhibition was obtained using the following formula: Pi (%) = [(Dm−dm)/Dm] × 100; Dm = average of D1 + D2 + D3 [filament length (mm) of mycelial growth in the DMSO control], and dm = average of d1 + d2 + d3 [filament length (mm) of mycelial growth with extract]. Pi = percent reduction in mycelial growth.
The antifungal activity of the crude extract and fractions against Z. tritici was assessed in 12-well microplates containing PDA medium that was either amended or not with compounds at 1000, 500, 250, 125, 62.5, and 31.5 mg·L−1, according to the protocol described previously by Platel and coll. (2021) [40]. The fungal growth was recorded eleven days after microplate inoculation with fungal spore suspension by measuring the perpendicular diameter of each fungal colony. The percentage of inhibition was calculated following the same formula used above for P. infestans. Dose-response curves for both P. infestans and Z. tritici were performed on GraphPad Prism software version 9 (GraphPad Software Inc., San Diego, CA, USA). The bioassays for both pathogens were performed twice in two independent experiments with three biological repetitions each.
4. Conclusions
Ethnopharmacological and molecular networking approaches have provided good visibility, not only on the chemical composition of the E. exsul plant, but also highlighted its importance both in traditional medicine and in the search for bioactive natural substances. In total, 20 compounds were identified through GNPS, and their identity was further confirmed by previous works. The plant extract fractions, in particular F3, were active against the targeted phytopathogens, especially against P. infestans. The observed strong antioxidant activity (F8, F4, and F6) reinforces the importance of valorizing this plant in the human health area. Although this study represents the first exploration of chemistry of E. exsul, the results are very encouraging and pave the way for further analyses, and a possible isolation to characterize the compounds responsible for the observed biological activities.
Acknowledgments
MEM wishes to acknowledge Campus France for administrative support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11111505/s1, Figure S1: The molecular network of the ethanolic crude extract of Erismadelphus exsul obtained with the GNPS platform and visualized with the Cytoscape 3.9.0 software; Figure S2: Structures and names of compounds identified on the molecular network; Figure S3: Focus on the cluster of antimicrobial ions of interest contained in fractions F1 and F3; Figure S4: Mass spectra of the [M+H]+ ions of Mauritine F, Mauritine A, Mauritine A N-oxide, 8-Dihydroantidesmone, 8-Deoxoantidesmone, and Antidesmone obtained by LC–ESI–MS; Table S1: In vitro colony diameters (mm) obtained using two independent experiments for Phytophthora infestans and Zymoseptoria tritici at different concentrations of crude extract and eight fractions from Erismadelphus exsul bark.
Author Contributions
Conceptualization, B.K. and F.M.; Data curation, M.E.M., É.C., A.S., J.M. and M.A.B.; Formal analysis, M.E.M., A.S., R.M., R.R. and C.S.O.; Funding acquisition, F.M.; Investigation, M.E.M., E.O.N., J.J., R.M. and R.R.; Methodology, M.E.M., E.O.N., É.C., J.J., J.M. and D.C.; Project administration, B.K. and F.M.; Resources, J.-P.O.; Software, R.M.; Supervision, E.O.N., A.S. and F.M.; Validation, J.M.; Writing—original draft, M.E.M.; Writing—review & editing, M.A.B., B.K. and F.M.. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
MEM wishes to acknowledge the Agence Nationale des Bourses du Gabon for the attribution of a personal grant.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feuya Tchouya G.R., Souza A., Tchouankeu J.C., Yala J.-F., Boukandou M., Foundikou H., Nguema Obiang G.D., Fekam Boyom F., Mabika Mabika R., Zeuko’o Menkem E., et al. Ethnopharmacological Surveys and Pharmacological Studies of Plants Used in Traditional Medicine in the Treatment of HIV/AIDS Opportunistic Diseases in Gabon. J. Ethnopharmacol. 2015;162:306–316. doi: 10.1016/j.jep.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 2.Moya E., Brea M. A New Record of Fossil Wood of Vochysiaceae from the Late Pleistocene (Arroyo Feliciano Formation), Argentina, South America. Rev. Bras. Paleontol. 2015;18:83–90. doi: 10.4072/rbp.2015.1.05. [DOI] [Google Scholar]
- 3.Sajo M.G., Rudall P.J. Leaf and Stem Anatomy of Vochysiaceae in Relation to Subfamilial and Suprafamilial Systematics. Bot. J. Linn. Soc. 2002;138:339–364. doi: 10.1046/j.1095-8339.2002.00025.x. [DOI] [Google Scholar]
- 4.Weniger B., Lobstein A., Um B.-H., Vonthron-Sénéchau C., Anton R., Usuga N.J., Basaran H., Lugnier C. Bioactive Triterpenoids from Vochysia Pacifica Interact with Cyclic Nucleotide Phosphodiesterase Isozyme PDE4. Phytother. Res. 2005;19:75–77. doi: 10.1002/ptr.1613. [DOI] [PubMed] [Google Scholar]
- 5.Franco R.R., Justino A.B., Martins M.M., Silva C.G., Campana P.R.V., Lopes J.C.D., De Almeida V.L., Espindola F.S. Phytoscreening of Vochysiaceae Species: Molecular Identification by HPLC-ESI-MS/MS and Evaluating of Their Antioxidant Activity and Inhibitory Potential against Human α-Amylase and Protein Glycation. Bioorganic Chem. 2019;91:103122. doi: 10.1016/j.bioorg.2019.103122. [DOI] [PubMed] [Google Scholar]
- 6.Dornelo-Silva D., Dianese J.C. Hyphomycetes on the Vochysiaceae from the Brazilian Cerrado. Mycologia. 2003;95:1239–1251. doi: 10.1080/15572536.2004.11833032. [DOI] [PubMed] [Google Scholar]
- 7.Nothias L.-F., Nothias-Esposito M., da Silva R., Wang M., Protsyuk I., Zhang Z., Sarvepalli A., Leyssen P., Touboul D., Costa J., et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018;81:758–767. doi: 10.1021/acs.jnatprod.7b00737. [DOI] [PubMed] [Google Scholar]
- 8.Quinn R.A., Nothias L.-F., Vining O., Meehan M., Esquenazi E., Dorrestein P.C. Molecular Networking As a Drug Discovery, Drug Metabolism, and Precision Medicine Strategy. Trends Pharmacol. Sci. 2017;38:143–154. doi: 10.1016/j.tips.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Allard P.-M., Péresse T., Bisson J., Gindro K., Marcourt L., Pham V.C., Roussi F., Litaudon M., Wolfender J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016;88:3317–3323. doi: 10.1021/acs.analchem.5b04804. [DOI] [PubMed] [Google Scholar]
- 10.Mad Nasir N., Ezam Shah N.S., Zainal N.Z., Kassim N.K., Faudzi S.M.M., Hasan H. Combination of Molecular Networking and LC-MS/MS Profiling in Investigating the Interrelationships between the Antioxidant and Antimicrobial Properties of Curculigo Latifolia. Plants. 2021;10:1488. doi: 10.3390/plants10081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckingham J. Dictionary of Natural Products, Supplement 4. CRC Press; Boca Raton, FL, USA: 1997. [Google Scholar]
- 12.Buckingham J., Baggaley K.H., Roberts A.D., Szabo L.F., editors. Dictionary of Alkaloids with CD-ROM. 2nd ed. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 13.Han J., Ji C.-J., He W.-J., Shen Y., Leng Y., Xu W.-Y., Fan J.-T., Zeng G.-Z., Kong L.-D., Tan N.-H. Cyclopeptide Alkaloids from Ziziphus Apetala. J. Nat. Prod. 2011;74:2571–2575. doi: 10.1021/np200755t. [DOI] [PubMed] [Google Scholar]
- 14.Buske A., Schmidt J., Hoffmann P. Chemotaxonomy of the Tribe Antidesmeae (Euphorbiaceae): Antidesmone and Related Compounds. Phytochemistry. 2002;60:489–496. doi: 10.1016/S0031-9422(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 15.Blaženović I., Kind T., Ji J., Fiehn O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites. 2018;8:31. doi: 10.3390/metabo8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapadia G.J., Shukla Y.N., Morton J.F., Lloyd H.A. New Cyclopeptide Alkaloids from Melochia Tomentosa. Phytochemistry. 1977;16:1431–1433. doi: 10.1016/S0031-9422(00)88797-X. [DOI] [Google Scholar]
- 17.Wang M., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y., Nguyen D.D., Watrous J., Kapono C.A., Luzzatto-Knaan T., et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluskal T., Castillo S., Villar-Briones A., Orešič M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nothias L.F., Petras D., Schmid R., Dührkop K., Rainer J., Sarvepalli A., Protsyuk I., Ernst M., Tsugawa H., Fleischauer M., et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods. 2020;17:905–908. doi: 10.1038/s41592-020-0933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon P. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Maaiden E., El Kharrassi Y., Qarah N.A.S., Essamadi A.K., Moustaid K., Nasser B. Genus Ziziphus: A Comprehensive Review on Ethnopharmacological, Phytochemical and Pharmacological Properties. J. Ethnopharmacol. 2020;259:112950. doi: 10.1016/j.jep.2020.112950. [DOI] [PubMed] [Google Scholar]
- 22.Kapadia G.J., Shukla Y.N., Basak S.P., Sokoloski E.A., Fales H.M. The Melosatins—A Novel Class of Alkaloids from Melochia Tomentosa. Tetrahedron. 1980;36:2441–2447. doi: 10.1016/0040-4020(80)80221-3. [DOI] [Google Scholar]
- 23.Bhakuni R.S., Shukla Y.N., Thakur R.S. Cyclopeptide Alkaloids from Melochia Corchorifolia. Phytochemistry. 1986;26:324–325. doi: 10.1016/S0031-9422(00)81541-1. [DOI] [Google Scholar]
- 24.Arbain D., Taylor W.C. Cyclopeptide Alkaloids from Antidesma Montana. Phytochemistry. 1993;33:1263–1266. doi: 10.1016/0031-9422(93)85062-V. [DOI] [Google Scholar]
- 25.Mu H., Bai Y.-H., Wang S.-T., Zhu Z.-M., Zhang Y.-W. Research on Antioxidant Effects and Estrogenic Effect of Formononetin from Trifolium Pratense (Red Clover) Phytomedicine. 2009;16:314–319. doi: 10.1016/j.phymed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Ong S.K.L., Shanmugam M.K., Fan L., Fraser S.E., Arfuso F., Ahn K.S., Sethi G., Bishayee A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers. 2019;11:611. doi: 10.3390/cancers11050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado Dutra J., Espitia P.J.P., Andrade Batista R. Formononetin: Biological Effects and Uses—A Review. Food Chem. 2021;359:129975. doi: 10.1016/j.foodchem.2021.129975. [DOI] [PubMed] [Google Scholar]
- 28.Peng S., Hu C., Liu X., Lei L., He G., Xiong C., Wu W. Rhoifolin Regulates Oxidative Stress and Proinflammatory Cytokine Levels in Freund’s Adjuvant-Induced Rheumatoid Arthritis via Inhibition of NF-ΚB. Braz. J. Med. Biol. Res. 2020;53 doi: 10.1590/1414-431x20209489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elagbar Z.A., Shakya A.K., Barhoumi L.M., Al-Jaber H.I. Phytochemical Diversity and Pharmacological Properties of Rhus Coriaria. Chem. Biodivers. 2020;17:e1900561. doi: 10.1002/cbdv.201900561. [DOI] [PubMed] [Google Scholar]
- 30.Najdabbasi N., Mirmajlessi S.M., Dewitte K., Landschoot S., Mänd M., Audenaert K., Ameye M., Haesaert G. Biocidal Activity of Plant-Derived Compounds against Phytophthora Infestans: An Alternative Approach to Late Blight Management. Crop Prot. 2020;138:105315. doi: 10.1016/j.cropro.2020.105315. [DOI] [Google Scholar]
- 31.Bocquet L., Rivière C., Dermont C., Samaillie J., Hilbert J.-L., Halama P., Siah A., Sahpaz S. Antifungal Activity of Hop Extracts and Compounds against the Wheat Pathogen Zymoseptoria Tritici. Ind. Crops Prod. 2018;122:290–297. doi: 10.1016/j.indcrop.2018.05.061. [DOI] [Google Scholar]
- 32.Panseeta P., Lomchoey K., Prabpai S., Kongsaeree P., Suksamrarn A., Ruchirawat S., Suksamrarn S. Antiplasmodial and Antimycobacterial Cyclopeptide Alkaloids from the Root of Ziziphus Mauritiana. Phytochemistry. 2011;72:909–915. doi: 10.1016/j.phytochem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Cretton S., Dorsaz S., Azzollini A., Favre-Godal Q., Marcourt L., Ebrahimi S.N., Voinesco F., Michellod E., Sanglard D., Gindro K., et al. Antifungal Quinoline Alkaloids from Waltheria Indica. J. Nat. Prod. 2016;79:300–307. doi: 10.1021/acs.jnatprod.5b00896. [DOI] [PubMed] [Google Scholar]
- 34.Scazzocchio F., Cometa M.F., Tomassini L., Palmery M. Antibacterial Activity of Hydrastis Canadensis Extract and Its Major Isolated Alkaloids. Planta Med. 2001;67:561–564. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- 35.Chambers M.C., Maclean B., Burke R., Amodei D., Ruderman D.L., Neumann S., Gatto L., Fischer B., Pratt B., Egertson J., et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers O.D., Sumner S.J., Li S., Barnes S., Du X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017;89:8696–8703. doi: 10.1021/acs.analchem.7b00947. [DOI] [PubMed] [Google Scholar]
- 37.Benzie I.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 38.Brand-Williams W., Cuvelier M., Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 39.Banso A., Adeyemo S.O., Jeremiah P. Antimicrobial Properties of Vernonia Amygdalina Extract. J. Appl. Sci. Manag. 1999;3:9–11. [Google Scholar]
- 40.Platel R., Chaveriat L., Le Guenic S., Pipeleers R., Magnin-Robert M., Randoux B., Trapet P., Lequart V., Joly N., Halama P., et al. Importance of the C12 Carbon Chain in the Biological Activity of Rhamnolipids Conferring Protection in Wheat against Zymoseptoria Tritici. Molecules. 2021;26:40. doi: 10.3390/molecules26010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.