Abstract
The nanotechnology revolution is developing daily all over the world. Soil-borne fungi cause a significant yield loss in mung beans. Our study was performed to identify the impact of different concentrations of MgO nanoparticles (MgONPs) and to assess the prevalence of Fusarium solani (F. solani) and Fusarium oxysporum (F. oxysporum) in mung bean plants under in vivo conditions and, subsequently, the remaining impacts on soil health. In vitro studies revealed that MgONPs could inhibit fungal growth. Mung bean plants treated with MgONPs showed a promotion in growth. The obtained MgONPs were applied to the roots of 14-day-old mung bean plants at a concentration of 100 µg/mL. The application of MgONPs at a concentration of 100 µg/mL caused an increase in mung bean seedlings. Compared to the control treated with water, plants exposed to MgONPs at 100 µg/mL showed improvements (p < 0.05) in shoot fresh weight (28.62%), shoot dry weight (85.18%), shoot length (45.83%), root fresh weight (38.88%), root dry weight (33.33%), root length (98.46%), and root nodule (70.75%). In the greenhouse, the severity of disease caused by F. solani decreased from approximately 44% to 25% and that by F. oxysporum from 39% to 11.4%, respectively. The results of this study confirm that the temporal growth of the soil microbial biomass was partially reduced or boosted following the nanoparticle drenching addition and/or plant infections at higher concentrations of 50 and 100 µg/mL while there was no significant decrease at the lowest concentration (25 µg/mL). The current research helps us to better understand how nanoparticles might be used to prevent a variety of fungal diseases in agricultural fields while avoiding the creation of environmental hazards to soil health.
Keywords: antifungal activity, MgO, nanoparticles, mung bean, soil health
1. Introduction
Mung bean (Vigna radiata L.), commonly known as green gram, is an essential part of people’s daily diet. It is rich in protein and fiber; thus, it is as an important source of nutrients all over the world. In addition to being a major source of human food, it plays a significant role in maintaining soil fertility by enhancing soil physical properties and fixing nitrogen in soils from the atmosphere [1]. Mung bean is widely cultivated in Asia and is believed to be a native crop from India; it later reached the USA, Australia, and Africa because it is a short-term leguminous crop that can grow successfully under different environmental conditions [2]. Mung bean is suitable as a catch crop in the triple-cropping systems of intensive agriculture in Egypt (Nile Valley and Delta lands) due to its short life cycle (65–75 days). Moreover, it breaks the insect-pest and disease cycle and thereby enhances the sustainability of soil health and overall agroecological farming systems. Twidwel et al. [3] recorded that the delay in the mung bean planting date from May to July produced increased forage, with 2.2 tons/ha. Under Egyptian conditions, Hozayn et al. [4] recorded that some genotypes of mung bean exhibited suitable vegetative growth and yields. The major farming method in Egypt is maize followed by wheat, and this cereal following cereal production strategy has several negative consequences for soil health and water reserves. In addition, the emergence of illnesses and insect pests has steadily raised environmental concerns. As a result, there is a pressing need to replace this cropping pattern with some short- or medium-term leguminous crops to effectively address the difficulties described.
Nanotechnology is one of the most essential technologies in modern science, although few attempts have been made to use it to boost agricultural output. Microorganisms can be developed as bio-nano factories for the production of agriculturally significant particles. Magnesium oxide nanoparticles (MgONPs) show promise as an effective nutrient source for plants, increasing biomass output and nutrient use by stimulating microbial activity [5,6]. Magnesium oxide nanoparticles (MgONPs) have been recognized as safe materials by the United States Food and Drug Administration (21CFR184.1431). Metal nanoparticles, in particular, have been extensively explored; as a consequence, they have been examined and shown to have strong antifungal characteristics [7]. Metal nanoparticles have been produced and utilized to suppress phytopathogenic fungi [8]. Antimicrobial characteristics have been demonstrated for a variety of nanoparticles, including silver nanoparticles, zinc oxide (ZnO) nanoparticles, and titanium oxide (TiO2) nanoparticles [9]. However, because of the hazards connected with heavy metal elements and their accumulation in the body, these nanoparticles raise major concerns about their toxicity. MgONPs are an appealing alternative to heavy metal-based nanoparticles such as silver and ZnO because they can be efficiently degraded and metabolized in the body. The released degradation products of Mg+2 and OH ions can be effectively eliminated from the body as long as the renal function is normal, eliminating concerns about excessive metal accumulation in the body [10]. Furthermore, the precise mechanism of MgONPs as bactericidal agents and their potential to anticipate plant disease management is yet unknown. Given the potential for nanoparticles to be used in agriculture, it is crucial to note that the growing worry about MgONPs’ toxicity in agroecosystems cannot be overlooked. The antibacterial activity of these nanoparticles and their capacity to impact as few plant cells as possible are intimately connected in all circumstances [11,12].
Current nanomaterials research is focused on determining the effects of nanoparticles on plant development and productivity to improve their application in agricultural production. MgONPs, which have the advantages of being non-toxic, safe, and easy to obtain, are an excellent fungicide against Fusarium spp. and show great potential in preventing root and stem infections [13,14]. These findings suggest that MgONPs could be a good alternative to chemical fungicides in crop protection, although more research on their effects on plants, particularly on mung beans, is needed. As a result, understanding the interactions between MgONPs and plants is critical. The underlying mechanism of metal nanoparticles against bacterial infections appears to be the accumulation of reactive oxygen species (ROS) in cells, especially because the created ROS directly impair the cell’s ability to multiply [15]. Microorganism disinfection is thought to be based on direct contact between nanoparticles and biological cells [16]. Considering this, little study has been done on the effects of MgONPs on fungal infections and complex antimycotic systems. Based on past studies, we hypothesized that MgONPs may be antifungal by acting directly on fungal cells [17] based on earlier research. An ideal agricultural microbicide would have no phytotoxicity to plants, which is critical for green and sustainable agriculture. The application of MgONPs as nanoscale fertilizers or light absorption boosters stimulates the growth of numerous crops [18]. Typically, root rot caused by Fusarium solani and Fusarium oxysporum is widespread around the world and significantly decreases mung bean quality and yield [19,20,21]. Furthermore, despite MgONPs’ strong toxicity against a variety of phytopathogens, concrete evidence for their function in pathogen infection control in vivo is still lacking. As a result, the use of MgONPs as fungicidal agents should be investigated. In this work, the antifungal mechanisms of MgONPs against phytopathogenic fungi were studied in greater depth. These findings provide a new avenue for investigating metal nanoparticles’ substantial potential as a revolutionary disease management method in agricultural applications. The impact of root application of MgONPs on mung bean plants, and its residual influence on soil health in terms of the total count of bacteria and fungus and the soil resistance index, were assessed in this study. The researchers concentrated their attention on how MgONPs affected the growth of mung bean seedlings; to our knowledge, this is the first study to examine the impacts of MgONPs on mung bean plants in depth.
2. Results
2.1. In Vitro Inhibitory Effect of MgONPs on F. solani and F. oxysporum
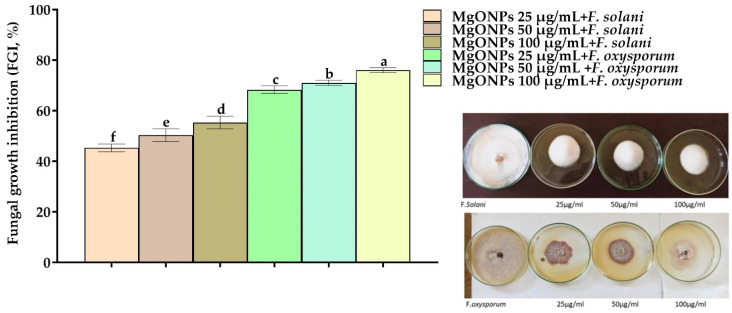
The inhibitory effect of MgONPs on the growth of F. solani and F. oxysporum in NB media was determined by calculating the percentage of inhibition, as shown in Figure 1. Compared to the control, MgONPs at the final concentrations of 25, 50, and 100 μg/mL showed an inhibition rate of 45.3, 50.3, and 55.3% for F. solani and 66, 70.3, and 75%, for F. oxysporum, respectively. The suppression of fungal development of F. solani in the petri dish was calculated as a percentage compared to the fungus grown in the nutrient media without any treatments. We observed that the lowest concentration was similar to the highest concentration in terms of the impact, and the same was true for the second fungus (F. oxysporum).
Figure 1.
The fungal growth inhibition (FGI%) of MgONPs at different concentrations (25, 50, and 100 µg/mL) on F. solani and F. oxysporum on NA media. a–f Columns with different superscripts are significantly different at p < 0.05.
2.2. Impacts of MgONPs on Mung Bean Plants
In the absence of the fungal pathogens, the results demonstrated a significantly higher plant growth in mung bean seedlings that were treated by MgONPs (Table 1, and Figure 2). The application of MgONPs at a concentration of 100 µg/mL caused an increase in mung bean seedlings. Comparing to the control treated with water, plants exposed to MgONPs at 100 µg/mL showed significant improvements (p < 0.05) in the shoot fresh weight (28.62%), shoot dry weight (85.18%), shoot length (45.83%), root fresh weight (38.88%), root dry weight (33.33%), root length (98.46%), and root nodule (70.75%). Furthermore, in the presence of the fungal pathogens, the plant growth parameters were dramatically improved compared with the previously infected plants. The results suggest that MgONPs can promote the growth of mung bean seedlings in the absence and presence of fungal infection.
Table 1.
The impact of MgONPs on the shoot fresh weight, shoot dry weight, shoot length root fresh weight, root dry weight, root length, and the number of root nodules of mung bean seedlings.
| Treatments | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Shoot Length (cm) | Root Fresh Weight (g) | Root Dry Weight (g) | Root Length (cm) | Root Nodule (n) |
|---|---|---|---|---|---|---|---|
| Control | 5.10 ± 0.08 c | 0.81 ± 0.01 d | 24.00 ± 0.73 e | 1.80 ± 0.08 d | 0.60 ± 0.08 e | 9.10 ± 0.08 ed | 8.00 ± 0.81 fg |
| MgONPs 25 µg/mL | 5.50 ± 0.32 c | 1.03 ± 0.12 c | 27.66 ± 0.47 c | 2.00 ± 0.08 c | 0.65 ± 0.01 c | 10.86 ± 0.18 cd | 11.66 ± 0.47 d |
| MgONPs 50 µg/mL | 5.93 ± 0.73 b | 1.12 ± 0.08 b | 30.00 ± 0.81 b | 2.21 ± 0.01 b | 0.71 ± 0.08 b | 14.20 ± 0.21 b | 13.33 ± 0.47 b |
| MgONPs 100 µg/mL | 6.56 ± 0.49 a | 1.50 ± 0.01 a | 35.00 ± 0.81 a | 2.50 ± 0.08 a | 0.80 ± 0.01 a | 18.06 ± 0.17 a | 13.66 ± 0.47 a |
| F. solani | 2.43 ± 0.09 g | 0.313 ± 0.09 i | 14.5 ± 0.40 i | 0.75 ± 0.04 g | 0.25 ± 0.08 i | 4.80 ± 0.08 g | 3.33 ± 0.47 i |
| MgONPs 25 µg/mL + F. solani | 3.80 ± 0.08 e | 0.586 ± 0.01 g | 20.16 ± 0.62 g | 1.59 ± 0.04 e | 0.50 ± 0.08 g | 8.00 ± 0.12 e | 7.66 ± 0.47 g |
| MgONPs 50 µg/mL + F. solani | 3.91 ± 0.08 e | 0.650 ± 0.09 f | 22.00 ± 0.81 f | 1.70 ± 0.08 ed | 0.55 ± 0.04 f | 9.03 ± 0.04 ed | 8.33 ± 0.47 f |
| MgONPs 100 µg/mL + F. solani | 4.20 ± 0.08 de | 0.680 ± 0.01 ef | 24.00 ± 0.40 e | 1.74 ± 0.04 cd | 0.59 ± 0.01 ef | 9.76 ± 0.08 d | 9.33 ± 0.47 e |
| F. oxysporum | 3.00 ± 0.16 f | 0.41 ± 0.01 h | 18.00 ± 0.40 h | 1.10 ± 0.08 f | 0.30 ± 0.04 h | 5.10 ± 0.08 f | 4.00 ± 0.81 h |
| MgONPs 25 µg/mL + F. oxysporum | 4.36 ± 0.12 de | 0.700 ± 0.01 e | 22.00 ± 0.62 f | 1.77 ± 0.04 cd | 0.60 ± 0.01 e | 10.00 ± 0.32 d | 9.34 ± 0.47 e |
| MgONPs 50 µg/mL + F. oxysporum | 4.50 ± 0.08 de | 0.730 ± 0.01 ed | 25.00 ± 0.81 ed | 1.82 ± 0.08 cd | 0.63 ± 0.04 d | 11.00 ± 0.08 c | 11.33 ± 0.47 d |
| MgONPs 100 µg/mL + F. oxysporum | 4.70 ± 0.08 d | 0.753 ± 0.01 ed | 26.00 ± 0.81 d | 1.91 ± 0.08 cd | 0.64 ± 0.01 d | 11.50 ± 0.08 c | 12.66 ± 0.47 c |
a–i Columns with different superscripts are significantly different at p < 0.05.
Figure 2.
The impact of MgONPs on the growth of mung bean seedlings: A: a concentration of 100 µg/mL; B: a concentration of 50 µg/mL; C: a concentration of 25 µg/mL; and D: only water.
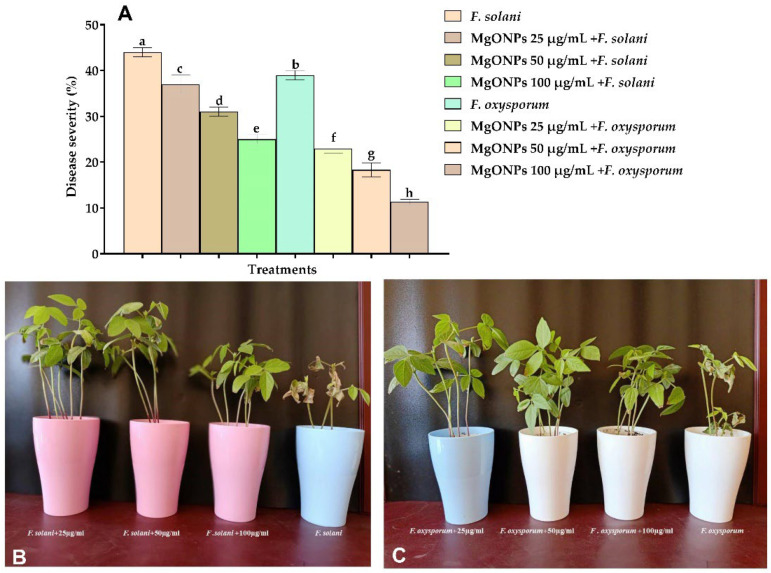
2.3. The Impact of MgONPs on the Disease Severity of Fusarium solani and Fusarium oxysporum in Mung Bean Seedlings
The present study showed MgONPs with strong antifungal activity against the mung bean pathogen of F. solani and F. oxysporum. The results from Figure 3A–C show the disease severity as a percentage of the mung bean plants reduced to 25% with plants infected with F. solani and to 11.33% with plants infected with F. oxysporum at a concentration of 100 µg/mL.
Figure 3.
The impact of MgONPs on the disease severity of (A) F. solani (B) and F. oxysporum (C) in mung bean plants. a–h Columns with different superscripts are significantly different at p < 0.05.
2.4. Soil Microbial Biomass and Resistance Index after Mung Bean Harvest
The different application rates of MgONPs with and without Fusarium plant infection’s impact on the soil microbial biomass counting of bacteria and fungi are shown in Table 2. The trend in the counts of bacteria and fungi among treatments were in the order of control > infection with Fusarium alone > foliar application of MgONPs with Fusarium infection > MgONPs foliar application alone. The means of the bacteria or fungi counts were significantly higher in the control treatments compared to the Fusarium infection alone and MgONPs foliar application alone or the integrated application of both fungi at different concentrations (25, 50, and 100 µg/mL). After soil incubation, significant differences were observed between soil infected with Fusarium alone, soil foliar application of MgONPs with Fusarium infection, and MgONPs foliar application alone in the counts of bacteria or fungi, reflecting that soil microbiological biomass (SMB) activities were provisionally boosted or rendered by each treatment.
Table 2.
Total counts of bacteria and fungi and the soil resistance index (SRI) as impacted by different treatments.
| Soil Resistance Index (SRI) and Total Counts of Bacteria and Fungi | ||||
|---|---|---|---|---|
| Treatment | Total Counts of Bacteria (×106 cfu g−1) | SRI | Total Counts of Fungi (×104 cfu g−1) | SRI |
| Control | 62.63 a | 1.00 | 46.30 a | 1.00 |
| MgONPs 25 µg/mL | 59.87 a | 0.926 | 44.73 ab | 0.935 |
| MgONPs 50 µg/mL | 44.60 cd | 0.553 | 26.23 d | 0.395 |
| MgONPs 100 µg/mL | 38.77 e | 0.448 | 16.33 e | 0.214 |
| F. solani | 47.23 c | 0.605 | 38.77 bc | 0.720 |
| F. oxysporum | 55.23 b | 0.789 | 40.03 abc | 0.762 |
| MgONPs 25 µg/mL + F. solani | 40.70 de | 0.481 | 19.83 de | 0.273 |
| MgONPs 50 µg/mL + F. solani | 45.97 cd | 0.580 | 37.67 c | 0.686 |
| MgONPs 100 µg/mL + F. solani | 46.33 c | 0.587 | 33.97 c | 0.579 |
| MgONPs 25 µg/mL + F. oxysporum | 47.23 c | 0.622 | 39.17 bc | 0.731 |
| MgONPs 50 µg/mL + F. oxysporum | 54.73 b | 0.779 | 41.13 abc | 0.757 |
| MgONPs 100 µg/mL + F. oxysporum | 46.23 c | 0.603 | 37.77 bc | 0.710 |
| L.S.D 0.05 | 7.331 | 0.304 | 7.512 | 0.321 |
a–e Columns with different superscripts are significantly different at p < 0.05.
The values of the resistance index (SRI) for soil bacteria and fungi were positive throughout the experiment but differed according to the MgONPs dose and Fusarium infection type applied (Table 2). Across all treatments, the soil resistance index (SRI) ranged between 0.448 and 1.00 for bacteria and 0.214 and 1.00 for fungi and was greatest in the control treatment followed by the integrated treatments.
The means of the SRI were significantly higher in treatments with the control compared to all treatments of the MgO-NPs doses and the application of both Fusarium infection types. Lower values of SRI indicate an inhibited influence of the treatment type on the microbial biomass activity and lower microbial activity and assimilation balance. Higher SRI values of bacteria and fungi were prominent in the control and MgONPs doses alone, indicating higher microbial activity than the other treatments of the MgONPs doses combined with Fusarium infections. The SRI for bacteria and fungi in the MgONPs doses combined with Fusarium infection treatments, regardless of F. oxysporum or F. solani, decreased to a minimal extent and caused stronger disturbances for soil microorganisms than the control and MgONPs doses alone. The temporal effects of the different treatments were more prominent in the counts of fungi than the counts of bacteria for all treatments as indicated by the soil microbial biomass resistance index (SRI).
3. Discussion
Nanotechnology products are promising and environmentally friendly strategies for the control of fungal disease. In particular, significant attention has been paid to the development of the plant-based synthesis of metal nanoparticles [22]. Green synthesis has attracted attention for the synthesis of various metal and metal oxide nanoparticles since chemical synthesis techniques result in the presence of harmful compounds adsorbed on the surface of nanoparticles [23]. Metal nanoparticles such as silver are considered the most reported antifungal agents followed by cupper nanoparticles; however, there are still few studies on magnesium nanoparticles that demonstrate the antifungal mechanism. Metallic nanoparticles were used to suppress phytopathogenic increases in our study of the green synthesis of Fe nanoparticles in green and black tea, with concentrations of 102, 550, 100 ppm against Aspergillus flavus and Aspergillus parasiticus observed in vitro. The results showed a 51.6% inhibition with black tea extract while with green tea extract was 43.5% at 100 ppm [24]. Moreover, another study of Ahmed et al. (2016) [8] on two species of Fusarium oxysporum using nickel nanoparticles at a concentration of 50 and 100 ppm in vitro showed that a concentration of 100 ppm significantly reduced mycelial development. Moreover, Ni nanoparticles reduced disease severity in lettuce and tomato plants by 58.4% and 57.0%, respectively, when used in vivo at a concentration of 50 ppm. This study is consistent with the study of Raliya et al. [25], which recorded that up to 250 mg/Kg TiO2 promoted the plant height, root length, and biomass. There are a few studies that suggest the positive effect of metal and metal oxide nanoparticles in agriculture, but the mechanisms are mostly unknown, and the data are inconsistent. As a result, much research is required prior to deploying nanoparticles in the field. Most of the research shows that the morphological diversity in plants is a result of metal nanoparticles and metal oxide. A drenching application of MgONPs on tomato plants resulted in significant bacterial wilt in tomato plants [18]. Metal oxide nanoparticles are thought to be a viable alternative for the control of phytopathogenic fungus in agriculture. Several metal nanoparticles (e.g., Ag, Zn, Cu, Au, Se, Ni, Mg, Fe, and Mn) have been produced and tested as antifungal agents. In accordance with our study, the use of Al2O3 NPs on tomato plants in vitro and in vivo suppressed the fungus Fusarium oxysporum [26]. According to the study of Pham et al. [27], Cu nanoparticles’ antifungal activity was determined using tests against Fusarium sp. CuNps at a 450 ppm concentration prevented 93.98% of the fungal growth. The study of Akpinar et al. [28] on phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici used a treatment of silver nanoparticles (AgNPs) of various nanosizes (3, 5, 8, and 10 nm) and concentrations (12.5–100 ppm). The AgNPs treatment with 3 nm diameters at 25, 37.5, and 50 ppm concentrations inhibited mycelium development by 50%, 75%, and 90%, respectively. The possibility of the antifungal activity of metal nanoparticles could be illustrated as the released ions bind to groups of proteins, which disrupts the function of membrane proteins and results in the permeability of the cell. Moreover, the ions of nanoparticles have a toxic impact on DNA, which causes cell death [16]. The results of this study suggest that the temporal growth of soil microbial biomass may either be partially inhibited or completely facilitated following foliar addition of nanoparticles and Fusarium infections, depending on the application rate [29,30,31]. In agreement with the previous study on chickpea (Cicer arietinum L), molybdenum nanoparticles enhanced root nodules and promoted the positive activity of microbiological processes in the soil [32].
4. Materials and Methods
4.1. Preparation of Nanoparticles
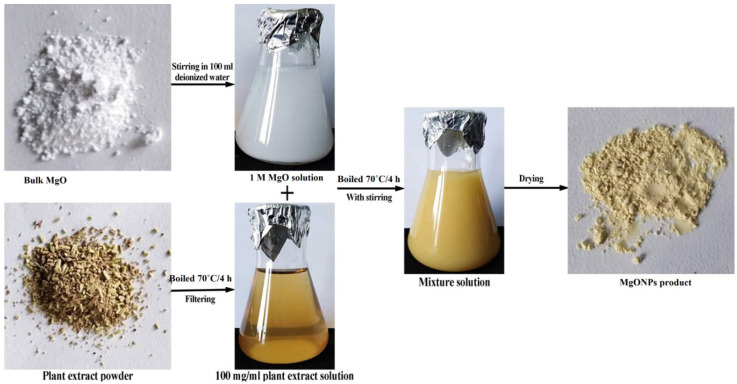
The green synthesis of MgO nanoparticles using rosemary extract is shown in Figure 4 as previously prepared and characterized through the biological method described by Abdallah et al. [33]. In brief, rosemary flowers were obtained from a local market, and then dried and ground in a domestic blender. The ground flowers were stored under a vacuum and maintained in a domestic freezer at 10 °C. In total, 1 g of ground rosemary was boiled at 70 °C for 4 h with 100 mL of distilled water. The extract was filtered through Whatman filter paper No. 1; then, the yellow-brown extract was collected for further experimental procedures. In total, 100 mL of MgO aqueous solution (1.0 mM) was mixed with 100 ml of rosemary extract and stirred continuously at 600 rpm at 70 °C for 4 h using a magnetic stirrer (Magnetic Stirrer, Jiangsu, China). Then, the mixture was separated by centrifugation at 5000 rpm for 15 min, and the precipitate was washed with distilled water and dried in an ALPHA 1-2/LD-Plus vacuum.
Figure 4.
Flowchart diagram of the green synthesis of MgO-NPs using rosemary flower extract (source: [33]).
4.2. Fungi Associated with Wilted Mung Bean Plants
For this study on mung bean plants, F. solani and F. oxysporum were provided by the department of plant pathology, college of agriculture, Minia university.
4.3. Isolation of Root Nodule Bacterium
The root nodule bacterium was isolated from root nodules according to Marques-Pinto et al. [34] using yeast extract mannitol Agar [35]. The root nodule bacterium was purified using yeast extract mannitol Agar Supplemented with Congo red as described by Hammad [36]. The isolated root nodule bacterium was kindly identified using the biolog detection technique (Biolog unit EPCRS 018) by Cairo MIRCEN, Fac. Agric., Ain Shams University as Bradyrhizobium sp.
4.4. Analysis of Soil Properties
The investigated soil had a clay texture and was classified as alluvial soil in accordance with Abd El-Azeim et al. [37]. Previous to the in vitro and greenhouse experimental procedures, the clay soil presented in Table 3 was obtained, then air-dried, sieved to <2.0 mm, and the prepared sample was used to determine the soil properties using the standard methods of Jackson [38], and Page et al. [39].
Table 3.
Estimated soil physicochemical characteristics.
| Soil Property | |||
|---|---|---|---|
| Soil Chemical Properties | Soil Physical Properties | ||
| pH (1:2.5 water) | 7.7 | F.C% | 42.45 |
| EC (dS m−1 at 25 °C) | 1.35 | PWP% | 13.78 |
| CEC (cmolc kg−1) | 37.87 | WHC% | 48.76 |
| O.M (g kg−1) | 28.61 | A.V (F.C-PWP) % | 28.67 |
| Total N (g kg−1) | 1.29 | Clay (%) | 56.45 |
| Total C/N Ratio | 22.18 | Sand (%) | 17.76 |
| S.O.C g kg−1 | 18.48 | Silt (%) | 25.79 |
| Total P (g kg−1) | 0.56 | Soil texture | Clay |
FC = field capacity, WHC = water holding capacity, PWP = wilting point, AV = available water, SOC = soil organic carbon, PWP = permanent wilting point, RS = resistance index, EC = electric conductivity, CEC = cation exchange capacity and OM = organic matter.
4.5. In Vitro Evaluation of the Antifungal Activity of MgONPs against Fusarium solani and Fusarium oxysporum
4.5.1. Linear Growth
The mycelial growth inhibition (FGI) of F. solani and F. oxysporum was evaluated using agar diffusion on Nutrient Agar media (NA) media and was sterilized at 121 °C for 15 min. Then, the stock nanoparticle solution was transferred to the NA media to obtain different initial nano MgO concentrations (to make up 25, 50, and 100 µg/mL). The mycelium growth in the PDA medium was measured by inoculating a disk (5 mm in diameter) of 5-day-old fungus in the middle of a dish. Three plates for each treatment were used and then incubated at 25 °C for 7 days in an incubator under dark conditions. After the incubation, the colony diameters were measured in millimeters. Data were recorded by measuring the diameter of the fungal growth (two diameters) and the means where the mycelia growth of any dish reached the edge of the plate. F. solani and F. oxysporum were also grown in NA without nanoparticles and evaluated as a positive control:
| FGI (%) = (dc − dt/dc) × 100 | (1) |
The percentage of mycelial growth inhibition (FGI) was calculated according to the following formula [40], where dc (mm) is the mean fungal growth diameter for the controls and dt (mm) is the mean fungal growth diameter for each group treated with MgONPs.
4.5.2. Pathogenicity Test
The pathogenic ability of F. solani and F. oxysporum to induce wilt in mung bean plants was evaluated under greenhouse conditions. The two fungal species were grown separately on autoclaved sorghum grain sand medium (100 g washed dried sorghum grains, 100 g washed dried coarse sand, and 65 mL tap water per bottle) in 500 mL glass bottles. Inoculation was carried out using uniform agar discs (5 mm diameter) bearing 4-day-old fungal growth of any of the tested isolates. The bottles were incubated at 28 °C for 2 weeks to obtain sufficient growth of the fungal isolates. The fertile soil was taken from the surface layer of the soil of the experimental farm Faculty of Agric. Minia Univ. and was sterilized using formalin solution (5%). Formalin disinfested clay pots (30 cm diameter) were filled with sterilized soil at a rate of 3 kg/pot. The potting soil was then artificially infested with the desired inoculum prepared at a rate of 3% (w/w), and then watered 2 times 1 week before planting. In the check treatments, equal amounts of uninoculated substrate were added to the pots [41]. Mung bean seedlings grown for 30 days in seed boxes filled with autoclaved peat-moss vermiculite (1:1 w/w) were uprooted and transplanted to the pots at a rate of 3 seedlings/pot. Three replicate pots were used for each fungal isolate. Pots were irrigated directly after transplanting and subsequently when necessary.
4.5.3. Disease Index of Foliar Browning
The degree of foliar yellowing disease was measured by grading each leaf on the severity of wilt symptoms and yellowing on a 0–4 scale and the average grade for the entire plant was calculated using the formula: (Sum of foliar yellowing value/(4 × Total number of the leaves) × 100 = percent of foliar yellowing [42]. In the present study, the following numerical grades were used: 0 = healthy plants; 1 = 1—less than 25% of the plant leaflets are yellow (slight chlorosis, wilting, or stunting); 2 = 25—less than 50% of the plant leaflets are yellow (moderate chlorosis, wilting, or stunting); 3 = 50—less than 75% of the plant leaflets are yellow (severe chlorosis, wilting, or stunting); 4 = 75—less than 100% of the plant leaflets are yellow (very severe chlorosis, complete wilting, or dead plant).
4.6. In Vivo Effect of MgONPs on the Development of Vascular Wilt in Mung Bean Seedlings Inoculated with Fusarium oxysporum and Fusarium solani
Plants were harvested four weeks after the soil application to examine the plant’s morphology state. Shoots were cut at the soil surface, and roots were gently shaken to remove extra soil, clumps of soil caught between roots were removed, and the number of nodules and root length were measured.
4.7. Soil Incubation Experiment after Mung Bean Harvest
After the mung bean plant harvest, a sample of 1 kg of soil was taken from each experimental pot to be incubated under controlled conditions for 10 days at 30 °C under a 65% soil water holding capacity to estimate the impacts of MgONPs and Fusarium infection on soil health in terms of the soil biological properties.
4.7.1. Analyses of Soil Biological Properties
Total Bacteria and Total Fungi and Soil Resistance Index (SRI)
After soil aerobic incubation for 10 days, soil samples collected for the determination of soil biological properties were sieved to pass through a 1.5 mm mesh. After the end of the experiment, the plate count technique in accordance with Alef [43] was used to determine the total counts of bacteria and fungi in soil samples. The colony-forming units (CFUs) of total bacteria were counted on nutrient agar while total fungi (CFU) were counted on potato dextrose agar media. The total counts of bacteria or fungi that withstood each treatment were determined using the soil resistance index (SRI) calculated by the equation established by Orwin [44]:
| (2) |
The index anticipated for the soil resistance index (SRI) was calculated as (D0) the difference between the untreated and undisturbed soil control (C0) and the soil treated with MgONPs or Fusarium (F0) at different rates at the end of the experiment time (t0) (i.e., time 0 or t0 at the end of the experiment). This takes into account differences in the amount of change in the soil’s total counts of bacteria and fungi that an added disturbance could cause, considering that the foliar application of MgONPs and/or Fusarium infection are soil disturbance factors. This index of soil resistance is between +1 and −1, with +1 indicating the treatment had no disturbance effect (greatest resistance), and inferior data showing stronger effects (low resistance).
4.8. Statistical Analysis
Data were subjected to analysis of variance (ANOVA) using SAS 2003 software (SAS Institute, Cary, NC, USA). A general linear model (GLM) procedure was used to check the significant differences among the main treatments. Individual comparisons between the mean values were performed using Duncan’s method (p < 0.05).
5. Conclusions
The effect of MgONPs on the mung bean plant was revealed in this study. Plant growth was promoted at various concentrations. This study found that 100 µg/mL MgONPs was the most efficient therapy for improving growth among the various concentrations studied. Furthermore, the current study demonstrated the importance of MgONPs in plant disease management. MgONPs exhibited antifungal activity against F. solani and F. oxysporum in vitro and in vivo successfully suppressed the infection in mung bean plants. Under both in vitro and in vivo conditions, MgONPs were shown to have promising biocontrol potential. In addition, MgONPs are also less damaging to soil microbiomes and health. In this respect, our findings substantiate the use of MgONPs in the treatment of plant diseases, indicating the promising prospects of nanofertilizers and fungicide. However, more study is needed to confirm the effect of MgONPs on several phytopathogens that cause substantial crop losses in the field.
Acknowledgments
The authors acknowledge the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization, Y.A.; Data curation, Y.A.; Formal analysis, Y.A., M.H., M.O.A.O. and R.M.S.E.; Funding acquisition, D.H.M.A. and W.N.H.; Investigation, Y.A., M.H., M.O.A.O. and R.M.S.E.; Methodology, Y.A., M.H., M.O.A.O. and R.M.S.E.; Resources, Y.A., M.H., M.O.A.O., R.M.S.E. and W.N.H.; Software, Y.A., M.O.A.O. and W.N.H.; Visualization, Y.A. and D.H.M.A.; Writing—original draft, Y.A. and M.H.; Writing—review and editing, Y.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ilyas N., Ambreen F., Batool N., Arshad M., Mazhar R., Bibi F., Saeed M. Contribution of Nitrogen Fixed by Mung Bean to the Following Wheat Crop. Commun. Soil Sci. Plant Anal. 2018;49:148–158. doi: 10.1080/00103624.2017.1421215. [DOI] [Google Scholar]
- 2.Nair R.M., Schafleitner R., Kenyon L., Srinivasan R., Easdown W., Ebert R.W. Genetic improvement of mung bean. SABRAO J. Breed. Genet. 2012;44:177–190. [Google Scholar]
- 3.Twidwell E.K., Boe A., Kephart K.D. Planting date effects on yield and quality of foxtail millet and three annual legumes. Can. J. Plant Sci. 1992;72:819–823. doi: 10.4141/cjps92-098. [DOI] [Google Scholar]
- 4.Hozayn M., El-Habbasha S.F., Abd El-Lateef E.M., Abd El-Monem A.A. Genetic variability in 16 exotic mung bean genotypes for late sowing under Egyptian conditions. J. Appl. Sci. Res. 2013;9:643–651. [Google Scholar]
- 5.El-Argawy E., Rahhal M.M.H., El-Korany A., Elshabrawy E.M., Eltahan R.M. Efficacy of some nanoparticles to control damping-off and root rot of sugar beet in El-behiera governorate. Asian J. Plant Pathol. 2017;11:35–47. doi: 10.3923/ajppaj.2017.35.47. [DOI] [Google Scholar]
- 6.Khan A., Shabbier D., Ahmad P., Khandaker M.U., Faruque MR I., Din I. Biosynthesis and antibacterial activity of MgONPs produced from Camellia-sinensis leaves extract. Mater. Res. Express. 2021;8:015402. doi: 10.1088/2053-1591/abd421. [DOI] [Google Scholar]
- 7.Kong F., Wang J., Han R., Ji S., Yue J., Wang Y., Ma L. Antifungal Activity of Magnesium Oxide Nanoparticles: Effect on the Growth and Key Virulence Factors of Candida albicans. Mycopathologia. 2020;185:485–494. doi: 10.1007/s11046-020-00446-9. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A.I., Yadav D.R., Lee Y.S. Applications of nickel nanoparticles for control of fusarium wilt on lettuce and tomato. Int. J. Innov. Res. Sci. Eng. Technol. 2016;5:7378–7385. [Google Scholar]
- 9.Dizaj S.M., Lotfpour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Fouda A., Eid A.M., Abdel-Rahman M.A., EL-Belely E.F., Awad M.A., Hassan S.E.-D., AL-Faifi Z.E., Hamza M.F. Enhanced antimicrobial, cytotoxicity, larvicidal, and repellence activities of brown algae, cystoseira crinita-mediated green synthesis of magnesium oxide nanoparticles. Front. Bioeng. Biotechnol. 2022;10:849921. doi: 10.3389/fbioe.2022.849921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen N.Y.T., Grelling N., Wetteland C.L. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, Yeasts, and Biofilms. Sci. Rep. 2018;8:16260. doi: 10.1038/s41598-018-34567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan M.I., Akhtar M.N., Ashraf N. Green synthesis of Magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020;10:2351–2364. doi: 10.1007/s13204-020-01414-x. [DOI] [Google Scholar]
- 13.Ouda S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2011;9:34. doi: 10.3923/jm.2014.34.42. [DOI] [Google Scholar]
- 14.Ammulu M.A., Viswanath K.V., Giduturi A.K., Vemuri P.K., Mangamuri U., Poda S. Phytoassisted synthesis of magnesium oxide nanoparticles from Pterocarpus marsupium rox.b heartwood extract and its biomedical applications. J. Genet. Eng. Biotechnol. 2021;19:21. doi: 10.1186/s43141-021-00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X., Zhou L., Rajoka M.S.R., Dongyan S., Jiang C., Shao D., Zhu J., Shi J., Huang Q., Yang H., et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2017;38:817–835. doi: 10.1080/07388551.2017.1414141. [DOI] [PubMed] [Google Scholar]
- 17.Jin T., He Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 2011;13:6877–6885. doi: 10.1007/s11051-011-0595-5. [DOI] [Google Scholar]
- 18.Imada K., Sakai S., Kajihara H., Tanaka S., Ito S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016;65:551–560. doi: 10.1111/ppa.12443. [DOI] [Google Scholar]
- 19.Sun F., Sun S., Zhu L., Duan C., Zhu Z. Confirmation of Fusarium oxysporum as a causal agent of mung bean wilt in China. Crop Protect. 2019;117:77–85. doi: 10.1016/j.cropro.2018.11.017. [DOI] [Google Scholar]
- 20.Lipșa F.-D., Ursu E.-L., Ursu C., Ulea E., Cazacu A. Evaluation of the Antifungal Activity of Gold–Chitosan and Carbon Nanoparticles on Fusarium oxysporum. Agronomy. 2020;10:1143. doi: 10.3390/agronomy10081143. [DOI] [Google Scholar]
- 21.Matei P.M., Iacomi B.M., Martín-Gil J., Pérez-Lebeña E., Ramos-Sánchez M.C., Barrio-Arredondo M.T., Martín-Ramos P. In vitro antifungal activity of composites of AgNPs and polyphenol inclusion compounds against Fusarium culmorum in different dispersion media. Agronomy. 2018;8:239. doi: 10.3390/agronomy8110239. [DOI] [Google Scholar]
- 22.Mohammadlou M., Maghsoudi H., Jafarizadeh-Malmiri H. A review on green silver nanoparticles based on plants: Synthesis, potential applications and eco-friendly approach. Int. Food Res. J. 2016;232:446–463. [Google Scholar]
- 23.Rahimi H., Doostmohammadi M. Nanoparticle Synthesis, Applications, and Toxicity. In: Stoytcheva M., Zlatev R., editors. Applications of Nanobiotechnology. IntechOpen; London, UK: 2019. [DOI] [Google Scholar]
- 24.Asghar M.A., Zahir E., Shahid S.M., Khan M.N., Iqbal J., Walker G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. Lebensm.-Wiss. Technol. 2018;90:98–107. doi: 10.1016/j.lwt.2017.12.009. [DOI] [Google Scholar]
- 25.Raliya R., Nair R., Chavalmane S., Wang W.N., Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7:1584–1594. doi: 10.1039/C5MT00168D. [DOI] [PubMed] [Google Scholar]
- 26.Shenashen M., Derbalah A., Hamza A., Mohamed A., El Safty S. Antifungal activity of fabricated mesoporous alumina nanoparticles against root rot disease of tomato caused by Fusarium oxysporium. Pest Manag. Sci. 2017;73:1121–1126. doi: 10.1002/ps.4420. [DOI] [PubMed] [Google Scholar]
- 27.Pham V., Hai T.N., Thi M.C., Le V.H. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016;2016:1957612. doi: 10.1155/2016/1957612. [DOI] [Google Scholar]
- 28.Akpinar I., Unal M., Sar T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum F. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021;3:506. doi: 10.1007/s42452-021-04524-5. [DOI] [Google Scholar]
- 29.Song Y., Song C., Ren J., Ma X., Tan W., Wang X., Gao J., Hou A. Short-term response of the soil microbial abundances and enzyme activities to experimental warming in a boreal peatland in northeast china. Sustainability. 2019;11:590. doi: 10.3390/su11030590. [DOI] [Google Scholar]
- 30.VandeVoort A.R., Arai Y. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019;9:499. [Google Scholar]
- 31.Gupta C., Prakash D. Effect of soil fertilizers on soil microflora. Ann. Plant Sci. 2020;9:3846–3859. doi: 10.21746/aps.9.5.3. [DOI] [Google Scholar]
- 32.Taran N.Y., Gonchar O.M., Lopatko K.G. The effect of colloidal solution of molybdenum nanoparticles on the microbial composition in rhizosphere of Cicer arietinum L. Nanoscale Res. Lett. 2014;9:289. doi: 10.1186/1556-276X-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah Y., Ogunyemi S.O., Abdelazez A., Zhang M., Hong X., Ibrahim E., Fouad H., Bin L., Chen J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (rosemary) and the antibacterial activities against Xanthomonas oryzae pv. Oryzae. BioMed Res. Int. 2019;2019:5620989. doi: 10.1155/2019/5620989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques Pinto C., Yao P.Y., Vincent J.M. Nodulating competitiveness amonst Strains of Rizobium mellioti and R. trifolli. Aust. J. Agric. Res. 1974;25:317–329. doi: 10.1071/AR9740317. [DOI] [Google Scholar]
- 35.Skinner F., Lotz W., Lang D. Electron microscope study length and partial denaturation of Rizobium bacteriophage DNA. J. Virol. 1973;11:946–952. doi: 10.1128/jvi.11.6.946-952.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammad A.M.M. Ph.D. Thesis. Faculty of Agriculture, Minia University; El-Minya, Egypt: 1989. Acomparative study of Bacteriophage of Rizobium leguminosarum in Soil of Egypt and Scotland. [Google Scholar]
- 37.Abd El-Azeim M.M., Sherif M.A., Hussien M.S., Tantawy I.A.A., Bashandy S.O. Impacts of nano- and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 2020;40:388. doi: 10.1016/j.chnaes.2019.12.007. [DOI] [Google Scholar]
- 38.Jackson M.L. Soil Chemical Analysis. Prentice Hall Inc.; Harrisburg, PA, USA: 1973. [Google Scholar]
- 39.Page A.L., editor. Method of Soil Analysis, Part 2, Chemical and Microbiological Properties. 2nd ed. American Society of Agronomy, Inc.; Soil Science Society of America, Inc.; Madison, WI, USA: 1982. [Google Scholar]
- 40.Bomfim N.d., Nakassugi L.P., Oliveira J.F.P., Kohiyama C.Y., Mossini S.A.G., Grespan R., Nerilo S.B., Mallmann C.A., Filho B.A.A., Machinski M. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015;166:330–336. doi: 10.1016/j.foodchem.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Gabr M.R., Saleh O.L., Hussein N.A., Khalil M.A. Physiological studies and cell-wall degrading enzymes of Fusarium oxysporum f.sp sesame and Macrophomina phaseolina: The causal organisms of wilt and root rot diseases of sesame. Egypt. J. Microbiol. 1998;33:595–610. [Google Scholar]
- 42.Song W., Zhou L., Yang C., Cao X., Liu Z.X. Tomato Fusarium wilt and its chemical control strategies in hydroponic system. Crop. Prot. 2004;23:243–247. doi: 10.1016/j.cropro.2003.08.007. [DOI] [Google Scholar]
- 43.Alef K. 4—Enrichment, isolation and counting of soil microorganisms. Methods Appl. Soil Microbiol. Biochem. 1995;4:123–191. doi: 10.1016/B978-012513840-6/50019-7. [DOI] [Google Scholar]
- 44.Orwin K.H., Wardle D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbance. Soil Biol. Biochem. 2004;36:1907–1912. doi: 10.1016/j.soilbio.2004.04.036. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.