Abstract
Alzheimer’s disease (AD) causes progressive memory loss and cognitive dysfunction. It is triggered by multifaceted burdens such as cholinergic toxicity, insulin resistance, neuroinflammation, and oxidative stress. Syzygium plants are ethnomedicinally used in treating inflammation, diabetes, as well as memory impairment. They are rich in antioxidant phenolic compounds, which can be multi-target neuroprotective agents against AD. This review attempts to review the pharmacological importance of the Syzygium genus in neuroprotection, focusing on anti-cholinesterase, anti-diabetic, anti-inflammatory, and antioxidant properties. Articles published in bibliographic databases within recent years relevant to neuroprotection were reviewed. About 10 species were examined for their anti-cholinesterase capacity. Most studies were conducted in the form of extracts rather than compounds. Syzygium aromaticum (particularly its essential oil and eugenol component) represents the most studied species owing to its economic significance in food and therapy. The molecular mechanisms of Syzygium species in neuroprotection include the inhibition of AChE to correct cholinergic transmission, suppression of pro-inflammatory mediators, oxidative stress markers, RIS production, enhancement of antioxidant enzymes, the restoration of brain ions homeostasis, the inhibition of microglial invasion, the modulation of ß-cell insulin release, the enhancement of lipid accumulation, glucose uptake, and adiponectin secretion via the activation of the insulin signaling pathway. Additional efforts are warranted to explore less studied species, including the Australian and Western Syzygium species. The effectiveness of the Syzygium genus in neuroprotective responses is markedly established, but further compound isolation, in silico, and clinical studies are demanded.
Keywords: Syzygium, medicinal plants, Alzheimer’s disease, multi-target, neuroprotection, anti-cholinesterase, anti-diabetic, anti-inflammatory, antioxidant
1. Introduction
Alzheimer’s disease (AD) is the most prevalent type of dementia worldwide, constituting 70–80% of cases, primarily among the elderly [1]. This irreversible neurodegenerative disorder progressively impairs memory and other cognitive functions. It is characterized by the formation of neurofibrillary tangles and amyloid ß (Aß) protein plaques in the brain, leading to complete brain failure and eventually death [2]. The aggregation of hyperphosphorylated tau microtubule-associated proteins is responsible for the abnormal intracellular tangles, whereas the discharge of Aß peptide produced by the cleavage of the amyloid precursor protein (APP) results in the extracellular senile plaques. Family heredity and genetic factors can play a definite role in AD. However, aging still becomes one of the leading risk factors, although AD is not a normal part of aging [3].
The underlying mechanism of AD remains to be elucidated, but the evidence gathered through clinical and multidisciplinary studies has depicted AD as multifactorial [4]. Both early-onset (a rare form) and late-onset (the most prevalent form of AD) manifest multifaceted toxicity associated with their multifactorial nature [2]. More than 20 various pathological events have been described to give rise to multifactorial AD, including well-known amyloid toxicity, tau burden, cholinergic toxicity, oxidative stress, the role of cholesterol, insulin resistance, and diabetes [4]. Metal ion toxicity, neurovascular toxicity, α-synuclein mediated toxicity, membrane toxicity, biomolecular damage, immune outrage, glucose hypometabolism, and lymphatic dysfunction can also contribute to AD progression [4]. These pathological events are highly connected, leading from one cause to another. The currently approved drugs can only delay the progression as they may target certain pathological burdens while the others remain accelerating.
Natural products are a convincing starting point for exploring neuroprotective agents. Galantamine is currently an AD licensed drug derived from a natural product that helps to alleviate cognitive symptoms [5]. Huperzine A is among the alkaloid-based lead candidates for anti-AD, originating from the herb Huperzia serrata [5]. Natural products, especially from medicinal plants, provide inexhaustible sources of bioactive compounds with diverse biological effects if used appropriately. They are widely used in rural communities and studied for biological potentials, considering their cheaper option and accessibility. For example, Ginkgo biloba is a Chinese herbal medicine extensively investigated in AD preclinical and clinical trials [6]. It is rich in flavonoids and terpenoids responsible for anti-inflammatory, antioxidant, anti-apoptosis, and protection against Aß aggregation and mitochondrial dysfunction [6]. Multiple large-scale clinical trials are still entailed, but it is noteworthy that G. biloba is a versatile multitarget agent against AD.
In principle, multifactorial AD entails remedies that can deliver multiple effects to slow down the progression effectively. Medicinal plants meet the criteria to provide diverse bioactive constituents with low toxicity either in extract or compound form. Syzygium is a relatively understated plant genus; it is a functional food that has long been used as complementary medicine but is not fully utilized [7,8]. Syzygium species exert various biological activities, including antioxidant, anti-inflammatory, anti-diabetic, and anti-cholinesterase, and are deemed suitable as neuroprotective agents [7,8]. This review aimed to summarize the neuroprotective effects of Syzygium species for the first time, focusing on anti-cholinesterase, anti-diabetic, anti-inflammatory, and antioxidant properties. Their current trend, future direction, and limitations as an alternative source for the anti-AD remedies were discussed.
2. Multifactorial AD and Multitarget Agents as a Strategy for Neuroprotection
The multifactorial nature of AD has given rise to various hypotheses based on the pathophysiological and molecular observations in the AD brain. Nevertheless, the amyloid hypothesis is the most widely accepted, given that its deposition is the prime event found in the etiology of AD [9]. The amyloid burden is highly neurotoxic and inflicts other pathological events, including inflammation, tau phosphorylation, oxidative stress, and mitochondrial damage [4]. The ongoing tests on new drug candidates against Aß deposition and tau aggregation show favorable results in animal models but cannot deliver promising effects in clinical trials [9].
Choline acetyltransferase (ChAT) is responsible for the synthesis of the neurotransmitter acetylcholine (ACh) from acetyl-CoA, choline, and ATP, whereas acetylcholinesterase (AChE) tightly controls the hydrolysis of ACh [10]. During AD progression, ChAT activity is significantly decreased in the brain [11]. The reduction in the level of ChAT activity leads to a significant decline in ACh that triggers cholinergic toxicity caused by the overactivity of AChE. The inhibition of AChE helps to restore ACh function and synaptic transmission. Tacrine was the first approved AChE inhibitor by the FDA but was discontinued due to hepatotoxicity [12]. Donepezil, galantamine, and rivastigmine are the current anti-AChE drugs commercialized to reduce cholinergic toxicity. These drugs are capable of ameliorating memory deficit but can only satisfy the limited symptomatic effects of AD.
Diabetes Mellitus is pathophysiologically linked to AD, whereby insulin resistance and hyperinsulinemia related to the central nervous system can lead to cognitive deterioration [13]. According to the meta-analysis of merged scientific results, individuals with type 2 diabetes have a risk of exhibiting AD symptoms at 56% [4]. The reduction in both the levels of insulin and sensitivity of the insulin receptors in the brain can trigger Aß toxicity and tau burdens. Type 3 diabetes describes the correlation between diabetes and AD patients manifesting diabetes-related symptoms [4].
Lipid and protein oxidations are also evident within AD patients’ neurons or brain cell membranes due to the overproduction of reactive immediate species (RIS) [14]. The RIS comprise reactive oxygen species (ROS) and reactive nitrogen species (RNS) that appear from several pathological events, especially mitochondrial dysfunction [4]. During the respiratory chain reaction, an array of redox enzymes in mitochondria transmits electrons to molecular oxygen to generate ROS. The interaction of fusion dynamin-related protein 1 (Drp1) (responsible for mitochondrial dynamics) with Aß and tau will impair mitochondria. The excessive creation of RIS leads to oxidative stress that damages biomolecules and initiates neuronal death [4,14].
The multiple complex toxicities, including endoplasmic reticulum stress and telomerase malfunction, can trigger neuroinflammation and inflammatory proteins that deteriorate cognitive functions [4,15]. Microglial cells play a crucial role in initiating inflammation as a response to the immune system. Oxidative stress, the accumulation of Aß, and other neurotoxicities activate microglia to release cytokines and other inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12), and lipopolysaccharide (LPS) that elicit acute neuroinflammation [4]. Anti-inflammatory and antioxidant drugs are good candidates to reduce immune outrage and oxidative stress.
A few plant genera have been assessed for their neuroprotective capacity. For example, the evidence of the genus Pistachia in neuroprotection is based on motor function, behavior, memory impairment, antioxidant, inflammatory markers, neural toxicity, Aß aggregation, and AChE studies in animal models [16]. Their related molecular mechanisms were gathered based on in vitro and in vivo models. Berry fruits such as blueberry, strawberry, mulberry, blackberry, blackcurrant, and bilberry were also reviewed for their neuroprotective effects [17]. Owing to their phytochemicals such as anthocyanin, kaempferol, caffeic acid, catechin, quercetin, and tannin, these bioactive principles are essential in modulating signaling pathways to enhance neurotransmission, neuroplasticity, and cell survival via their anti-inflammatory and antioxidant efficacies.
In this review, a literature survey related to neuroprotection in Syzygium was conducted via a bibliographic databases assessment, including PubMed, ScienceDirect, Wiley Online Library, SpringerLink, and Google Scholar. The search was performed within five recent years until March 2022 for all biological activities except for anti-cholinesterase, anti-diabetic, and in vivo memory-related studies. Keywords “cholinesterase”, “anti-cholinesterase”, “AChE”, “diabetes”, “anti-diabetic” “inflammation”, “anti-inflammatory”, “neuroinflammation”, “antioxidant”, “neuroprotection”, “neuroprotective”, “memory”, “cognitive”, “Alzheimer’s”, “phytoconstituent”, and “compound” along with the word “Syzygium” were used.
3. Ethnobotany and Ethnomedicinal Properties of Syzgium
Syzygium constitutes a wide range of species that occurs naturally in subtropical and tropical regions of Africa and Madagascar, Asia, and throughout Oceania and the Pacific regions [7,8]. Belonging to the Myrtaceae family, Syzygium represents the biggest genus of flowering plants worldwide, encompassing about 1200–1800 species [8]. The largest diversity of Syzygium is found in Australia and Southeast Asia. Despite its variety, many species from this region have not been taxonomically classified properly [8]. New species will likely be cataloged from time to time. From medium to large evergreen shrubs and trees, the Syzygium genus has a long history of ethnomedicinal usage in Iranian folk medicine and the Ayurveda medicinal system [18,19,20,21]. It is also considered one of the functional foods, given that the leaves and fruits of certain species are consumed for nutrients and health benefits.
Most of the species are edible depending on different plant parts. Syzygium cumini has purplish-black fruits containing high nutritional values, which are often commercialized as food products, including jelly, jam, ice cream, and yogurt [22]. Syzygium aromaticum is well known for its unopened flower buds that comprise a high amount of clove oil [8]. Their strong aroma enables them to be widely used as spices and flavoring agents for curry and other cuisines in Southern Asia [23]. In Thailand, the young reddish leaves of Syzygium antisepticum, having a somewhat astringent and sour taste, are consumed as local vegetables [24]. In Indonesia, the leaves of Syzygium polyanthum known as salam, are used in salads (ulam) and cooking the same way as lime leaves are used [25]. In Malaysia, the bright pinkish-red fruits from Syzygium aqueum are cut into pieces and eaten with other fruits such as guavas, pineapples, and young mangos as fruit salads (rojak) along with spicy dips. Many young leaves, shoots, and fruits of other Syzygium species are consumed, such as Syzygium jambos, Syzygium samarangense, Syzygium alliiligneum, and Syzygium malaccense [26].
S. aromaticum is ethnopharmacologically important in treating fever, stomachache, colds, flu, diabetes, hypertension, bacterial and fungal infections in the throat, urinary, vaginal tract, skin infections, candidiasis, dyspepsia, and pain and inflammation related to rheumatism [27,28]. Its clove oil is applied as a stimulant and cognitive enhancer in Ayurveda and Iranian traditional practices [18]. Various applications of S. cumini have been recorded in the Ayurveda and Unani systems, including the treatment of digestive, diuretic, bronchitis, carminative, wound, antiulcer, antiasthma, antiallergic, and antiscorbutic [19,20,21]. Additionally, the seeds are traditionally exercised to treat memory impairment in India [29]. In Brazil, the leaf infusion from S. jambos is traditionally used to treat diabetes [30]. The leaves are decocted for diuretic, rheumatism, and sore eyes reliever, while the seeds are administered to treat diarrhea, dysentery, diabetes, and catarrh. A decoction of the bark of S. jambos is applied to relieve asthma and bronchitis [31].
Since the Unani and Ayurveda medical systems, Syzygium species holds significant value in traditional medicine to treat ailments related to AD. As mentioned before, the seeds of S. cumini are attributed to memory enhancement [29], while its fruit possesses anti-diabetic activity that can benefit Alzheimer’s treatment [32]. Apart from that, S. jambos leaves are traditionally used to treat diabetes [33]. The wide range of medicinal properties exerted by S. aromaticum also includes anti-diabetic, anti-inflammatory, and cognitive enhancement properties that have been recorded in old manuscripts written by Avicenna [27,28]. The traditional medicinal properties of Syzygium species in neuroprotection are indeed well established [8], which has driven many researchers to conduct pharmacological studies in the last decades.
4. Neuroprotective Agents from Syzygium
4.1. Anti-Cholinesterase Activity
Anti-cholinesterase activity remains the primary test used to screen anti-AD drug candidates. Table 1 summarizes the anti-cholinesterase activities reported from Syzygium species. They are often tested in vitro based on Ellman’s method using AChE extracted from electric eel and butyrylcholinesterase (BChE) from equine serum. Nonetheless, efforts were made to determine in vitro activity using the parasite Cotylophoron cotylophorum [34] and the fruit fly Drosophila melanogaster [35]. Ex vivo AChE activity was also measured, revealing no significant activity from S. cumini [36]. Apart from that, in vivo AChE and BchE activities were determined in alloxan-induced diabetic rats [37] and scopolamine-induced memory-impaired rats [29]. Both studies showed a significant reduction in enzyme activity.
Table 1.
Summary of anti-cholinesterase activities exerted from Syzygium species.
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | Syzygium cumini (L.) Skeels. | Ethanol leaf extract | In vitro AChE | 44.54 µg/mL of IC50 | [36] |
| Ex vivo AChE | No significant effect | ||||
| Leaf essential oil | In vitro AChE | 32.9 µg/mL of IC50 | [22] | ||
| Polyphenol-rich leaf extract | In vitro AChE and BChE | Significant reduction in cholinesterase activities; bound polyphenolic extract showed better inhibitory activity than free polyphenolic extract | [37] | ||
| Polyphenol-rich leaf extract | In vivo AChE and BChE from alloxan-induced diabetic rats | Enzyme activities were significantly reduced after 14 days (400 mg/kg oral dose) | [37] | ||
| Methanol seed extract | In vivo AChE from scopolamine-induced rats | Significant reduction in AChE activity (400 mg/kg oral dose) | [29] | ||
| Leaf extract | In vitro AChE | No significant activity | [38] | ||
| 2 | Syzygium aqueum Alston | Methanol leaf extract | In vitro ACHE and BChE | 16.04 µg/mL and 13.95 µg/mL of IC50, respectively | [43] |
| 3 | Syzygium polyanthum (Wight) Walp. | Methanol and ethyl acetate extracts from leaves | In vitro ACHE | 47.30 and 45.10 µg/mL of IC50, respectively | [38] |
| Methanol leaf and stem extracts | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (8.28 and 6.54 µg/mL of IC50 in the leaf extract, respectively) |
[41] | ||
| 4 | Syzygium aromaticum (L.) Merrill and Perry | Methanol, ethyl acetate, and hexane extracts from leaves; methanol bud extract | In vitro ACHE | 42.10, 55.9, and 62.05 µg/mL of IC50, respectively (leaves); 45.25 µg/mL of IC50 (bud) |
[38] |
| Methanol extract, clove oil, and eugenol | In vitro ACHE and BChE using TLC bioautography | Eugenol (42.44 and 63.51 µg/mL of IC50) showed better inhibition than extract (61.5 and 103.53 µg/mL of IC50) and oil (49.73 and 88.14 µg/mL of IC50), respectively | [18] | ||
| Clove bud essential oil | In vitro ACHE and BChE | 1.5 μL/L and 18.2 μL/L of IC50, respectively | [27] | ||
| Ethanol extract | HPTLC-densitometry | Showed efficiency in AChE inhibition | [44] | ||
| Ethanol bud extract | In vitro AChE isolated from human erythrocytes | No inhibitory effect | [45] | ||
| Ethanol bud extract | In vitro AChE of parasite C. cotylophorum | 86.86% inhibition at 0.5 mg/mL after 8 hr exposure | [34] | ||
| Clove oil (eugenol) encapsulated with a nanostructured lipid carrier | In vitro ACHE and BChE from D. melanogaster tissue | 4.3 and 3.5 mM of IC50, respectively | [35] | ||
| Aqueous and hydroalcoholic extract of clove buds | In vitro AChE | 253.29 µg/mL of IC50 in aqueous extract | [40] | ||
| Clove oil | In vitro AChE from AlCl3-induced rats | Significant reduction in AChE activity | [46] | ||
| Ethanol bud extract | In vivo AChE from CeCI3-induced memory-impaired rats | Corrected the AChE rate caused by CeCI3 toxicity and improved cholinergic neural transmission | [42] | ||
| Eugenol derivatives | In vitro ACHE and BChE | 4-Allyl-2-methoxyphenyl-4-ethyl benzoate inhibited AChE with 5.64 µg/mL of IC50 | [47] | ||
| Isoeugenol | In vitro ACHE | 77 nM of IC50 | [48] | ||
| 5 | Syzygium antisepticum (Blume) Merr. and L.M.Perry | Methanol leaf extract; ursolic acid; gallic acid | In vitro ACHE | 61.9% at 300 µg/mL concentration; 81.64% at 200 µg/mL concentration; 73.39% at 200 µg/mL concentration | [24] |
| 6 | Syzygium samarangense (Blume) Merr. and L.M.Perry | Essential oil | In vitro ACHE and BChE | 4.83 and 5.69 mg GALAE/g, respectively | [39] |
| Dihydrochalcone | In vitro ACHE and BChE | 98.5% inhibition at 0.25 mM and 68% inhibition at 0.20 mM, respectively | [49] | ||
| 7 | Syzygium coriaceum Bosser and J. Guého | Essential oil | In vitro ACHE and BChE | 4.79 and 7.10 mg GALAE/g, respectively | [39] |
| 8 | Syzygium jambos (L.) Alston | Aqueous leaf extract | In vitro ACHE from homogenized tissue of rat brain | No significant activity | [30] |
| Methanol stem and leaf extracts | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (16.05 and 15.25 µg/mL of IC50 from stem extract, respectively) | [41] | ||
| 9 | Syzygium grande (Wight) Walp. | Methanol leaf extract | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration | [41] |
| 10 | Syzygium lineatum (DC.) Merr. and L.M.Perry | Methanol leaf extract | In vitro ACHE and BChE | >80% inhibition at 200 µg/mL concentration (20.69 µg/mL of IC50 for BChE) | [41] |
To our best knowledge, about 10 Syzygium species were investigated for their anti-cholinesterase potential. S. aromaticum (clove) represents the most studied Syzygium species, followed by S. cumini for this activity. Not only alcohol extracts were explored, but essential oils were also examined. Methanol and ethanol were widely used to extract Syzygium species, while leaves were the most studied plant part for this bioactivity. Eugenol compound from clove demonstrated more potent inhibitory activity than its essential oil and extract, indicating this compound might provide major inhibition from this plant [18]. Darusman et al. reported no significant activity from the leaf extract of S. cumini [38], but other studies showed a disagreement. A reduction in cholinesterase activity was demonstrated from the essential oil, polyphenol-rich leaf extract as well as the seed extract of S. cumini [22,29,37]. The inhibitory activity observed from essential oils of Syzygium coriaceum, S. cumini, S. aromaticum, and S. samarangense proposes their economic importance in therapy and nutrition [22,27,39].
The aqueous extract of S. jambos leaves revealed no significant activity [30]. Similarly, the aqueous extract of clove buds exhibited very weak activity with >250 µg/mL of IC50 [40], suggesting the unsuitability of water as a solvent for this analysis. Amir Rawa et al. reported the highest number of Syzygium species (Syzygium grande, Syzygium lineatum, S. jambos, and S. polyanthum) exhibiting above 80% cholinesterase inhibition at 200 µg/mL concentration in one experiment [41]. Among them, S. polyanthum leaf extract showed the lowest IC50 at 8.28 and 6.54 µg/mL against AchE and BchE, respectively. Further fractionation revealed that polar bioactive constituents (tannins and polyphenols) were accountable for the enzyme inhibition. It is noteworthy that the ethanol bud extract from S. aromaticum corrected the AchE rate in cerium chloride (CeCl3)-induced memory-impaired mice [42]. This study revealed a clear association between memory and AchE activity, where improved cholinergic neural transmission alleviated the state of memory in mice. Additional studies are required to confirm the bioactivity from other less known species of Syzygium, but the potency of inhibiting cholinesterase from this genus is well established.
4.2. Anti-Diabetic Activity
The most common form of diabetes, type 2 diabetes, is generally associated with hyperinsulinemia and insulin resistance. Insulin resistance causes neurodegeneration and impairment in the brain glucose metabolism and cognition, which are also observed in AD patients [4]. Hyperinsulinemia increases tau phosphorylation and Aß accumulation. Furthermore, neuroinflammation, oxidative stress, mitochondrial dysfunction, and advanced glycation products are evident in diabetic and AD patients [4]. Both disorders share similar features; medicinal plants that can stimulate insulin secretion would benefit diabetic as well as AD patients.
Zulcafli et al. extensively reviewed the anti-diabetic potential of eight Syzygium species [50]. The review reported that the inhibition of enzymes involving carbohydrate metabolisms such as α-glucosidase, maltase, and α-amylase is the most studied mechanism of action in the anti-diabetic potential of Syzygium [50]. Pertaining to type 2 diabetes, the ethanolic seed extract of S. cumini was shown to stimulate insulin secretion produced by pancreatic-ß cells in alloxan-induced mild and severely diabetic rabbits [51]. The hydroethanolic extract from S. cumini leaves also improved hyperinsulinemia and insulin resistance by modulating ß-cell insulin release in monosodium L-glutamate (MSG)-induced obese rats [52]. Additionally, Sahana et al. demonstrated that a reduction in insulin resistance was evident in 30 newly diagnosed type 2 diabetic patients when S. cumini seed powder was administered [53]. The treatment of high-fat diet/streptozotocin (HFD/STZ)-induced diabetic rats with the aqueous seed extract of S. cumini at 400 mg/kg decreased the levels of serum glucose, insulin, and other diabetic markers [54].
A study by Shen et al. demonstrated a clear connection between insulin resistance and inflammation in TNF-α-treated FL83B cells [55]. The suppression of c-Jun N-terminal kinase (JNK) inhibited an inflammatory response as the cells were treated with the fruit extract of S. samarangense. As a result, insulin resistance induced by TNF-α was alleviated via the activation of phosphatidylinositol-3 kinase–protein kinase B (PI3K–Akt/PKB) signaling [55]. S. aqueum leaf extract, on the other hand, reduced glucose levels, increased insulin secretion, and decreased the collagen deposition associated with its anti-inflammatory and antioxidant responses in STZ-induced diabetic rats [56]. It decreased the levels of toll-like receptor 4 (TLR-4), myeloid differentiation primary response 88 (MYD88), TNF receptor-associated factor 6 (TRAF-6), and TNF-α correlated to pancreatic inflammatory cell infiltration. Malondialdehyde, a sensitive biomarker of ROS-induced lipid peroxidation, was also reduced [56].
In another study, vescalagin isolated from S. samarangense ameliorated insulin resistance in high-fructose diet-induced hyperglycemic rats [57]. Myricitrin isolated from the S. malaccense leaf extract exhibited insulin-like effects by enhancing lipid accumulation, glucose uptake, and adiponectin secretion via the activation of the insulin signaling pathway [58]. The aqueous extract of Syzygium paniculatum fruits alleviated hepatic insulin resistance at a 100 mg/kg dose by reducing the blockage of the insulin signaling pathway via the improvement of insulin receptor (IR and IRS-1) function in HFD-induced diabetic rats [59]. In addition, the IR mRNA levels were restored to the control level in type-2 diabetic rats treated with Syzygium jambolanum homeopathic remedies, suggesting improvement in insulin secretion [60].
4.3. Anti-Inflammatory Activity
Nearly all pathological events, including endoplasmic reticulum stress and autophagy dysfunction, can trigger inflammatory responses in AD [4]. Moroever, insulin resistance and diabetes have been shown to correlate well with inflammation. For example, the bark extract of S. jambos improved the insulin receptor substrate-2/protein kinase B/glucose transporter-4 (IRS-2/AKT/GLUT4) insulin signaling pathway in the liver while improving glycemic parameters by suppressing inflammation, oxidative stress, and apoptosis in STZ-induced rats [61]. Inflammation is defined as a physiological defense mechanism by the immune system to combat health hazards, causing pain to occur [4]. It was demonstrated that microglia accumulate in higher quantities near Aß plaques than in the healthy brain. Amyloid plaques and other factors can activate microglia to initiate neuroinflammation [4]. Anti-inflammatory drugs enable the central nervous system (CNS) to impede pain signaling in the brain, therefore, reducing inflammation in AD pathogenesis. Recent anti-inflammatory activities reported from Syzygium were summarized in Table 2.
Table 2.
Summary of anti-inflammatory activities reported from Syzygium species.
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | S. malaccense (L.) Merr. and L.M. Perry | Methanol leaf extract | In vitro LPS-induced neuroinflammatory assay on murine BV-2 microglial cells; in vivo croton oil-induced ear edema test | Neuroprotective activity by a reduction in nitric oxide production in vitro; decreased mice ear edema in vivo | [63] |
| 2 | S. cumini | Methanol fruit extract | In vitro membrane stabilization, egg albumin denaturation, and bovine serum albumin denaturation assays; in vivo murine models of carrageenan, formaldehyde, and PGE2 induced paw edema. | Showed inflammatory activities both in vitro and in vivo | [64] |
| Betulinic acid | In vivo Fx1A antiserum-induced passive Heymann nephritis (PHN) in Sprague-Dawley rats | Ameliorated mRNA and protein expression of NF-κB, iNOS, TNF-α, Nrf2, HO-1, and NQO1 in the kidney, reducing inflammation | [65] | ||
| Polyphenol-rich leaf extract | In vivo Alloxan-induced diabetic rats | NF-κB and inflammatory cytokines such as TNF-α and IL-1α were regulated | [37] | ||
| Anthocyanins di-glucosides from pulp | In vitro determination of cytokine production in LPS-induced RAW264.7 macrophages | Inhibited pro-inflammatory mediators such as IL-6, IL-1β, and TNF-α | [66] | ||
| Aqueous seed extract | In vivo high cholesterol diet-streptozotocin-induced diabetes in rats | Exhibited significant anti-inflammatory and β-cell salvaging activity via overexpression of PPARγ and PPARα activity and a significant decrease in TNF-α levels when treated with 100, 200, 400 mg/kg/day doses | [67] | ||
| Methanol seed extract | In vitro high glucose (HG) diabetic cardiomyopathy in H9C2 cardiomyoblast cells |
HG-induced activation of NF-κB, TNF-α, and IL-6 was remarkably reduced | [68] | ||
| Seed extract | In vivo Aβ1-40-infused AD model rats | Reduced the levels of Aß burdens and oligomers by suppressing the levels of TNFα and LPO in the corticohippocampal tissues | [62] | ||
| 3 | Syzygium caryophyllatum (L.) Alston | Aqueous root extract | In vitro anti-inflammatory test using heat-induced albumin denaturation assay | 6.229 µg/mL of IC50 | [69] |
| 4 | S. aqueum | Polyphenol-rich leaf extract | In vitro lipoxygenase inhibitor screening assay, membrane stabilizing activity (hypotonic solution-induced hemolysis), and in vivo carrageenan-induced hind-paw edema in rats | Inhibited LOX, COX-1, and COX-2 with higher COX-2 selectivity reduced the extent of lysis of erythrocytes and markedly reduced leukocyte numbers in rats challenged with carrageenan. | [70] |
| Leaf extract | In vivo STZ-induced oxidative stress and inflammation in pancreatic beta cells in rats | Significantly decreased levels of TLR-4, MYD88, pro-inflammatory cytokines TNF-α, and TRAF-6 in pancreatic tissue homogenates, which correlated well with minimal pancreatic inflammatory cell infiltration | [56] | ||
| 5 | Syzygium mundagam (Bourd.) Chithra | Methanol bark extract | In vivo carrageenin- and egg albumin-induced paw edema, cotton pellet implanted granuloma in rats | Effective anti-inflammation at 200 mg/kg dose | [71] |
| 6 | Syzygium calophyllifolium (Wight) Walp. | Methanol bark extract | In vivo carrageenin- and egg albumin-induced paw edema, cotton pellet implanted granuloma | 200 mg/kg dose significantly reduced the paw edema in carrageenan (96.71%) and egg albumin models (54.24%) compared to the control. Chronic inflammation was also inhibited by up to 70.46% | [72] |
| 7 | S. aromaticum | Ethanol/water extract | In vivo carrageenan-induced paw edema inflammatory in rats | Pretreatment at different doses (100, 200, and 400 mg/kg) produced a significant (p < 0.001) reduction in paw inflammation up to 5 h of carrageenan injection | [73] |
| Essential oil | In vivo formalin-induced and carrageenan-induced paw edema inflammation in rats | 26.9 ± 2.5 μg/paw of EC50 | [74] | ||
| Aqueous clove extract | In vivo LPS-induced lung inflammation in mice. | Inhibited matrix metalloproteinases: MMP-2 (15%) and MMP-9 (18%) activity in lung homogenates, reducing inflammation |
[75] | ||
| Ethanol extract | In vitro TNF-α induced inflammation in dental pulp stem cells | Prevented the increase in IL-6 levels | [76] | ||
| Eugenol | Cytochrome c reduction assay to measure superoxide anion generation in human neutrophils | Inhibited the generation of superoxide anion by neutrophils via the inhibition of Raf/MEK/ERK1/2/p47phox-phosphorylation pathway | [77] | ||
| Eugenol | In vivo ethanol-induced ulcer in rats | Decreased TNF-α and IL-6 cytokine concentrations responsible for inflammation | [78] | ||
| Essential oil | Isbolographic study using the formalin test in rats | S. aromaticum in combination with ketorolac, showed an antinociceptive effect in the treatment of inflammatory pain | [79] | ||
| 8 | S. samarangense | Polyphenol vescalagin | In vivo methylglyoxal-induced inflammation in diabetic rats | The pancreatic levels of NF-κB, ICAM-1, and TNF-α protein, were reduced | [80] |
| Lyophilized fruit powder | In vivo STZ-induced pancreatic beta cells apoptosis in rats | Pancreatic ß-cell apoptosis was alleviated with significantly down-regulated cleaved caspase-3 and Bax and upregulated Bcl-2 and Bcl-xl protein expression | [81] | ||
| 9 | S. polyanthum | Leaf extract | In vivo coronary artery ligation-induced myocardial infarction in rats | Reduced levels of C-reactive protein (CRP) and myeloperoxidase (MPO) in the rats started from day 4 after the induction of myocardial infarction. | [82] |
| 10 | S. jambos | Bark extract | In vivo streptozotocin-induced inflammation in diabetic rats | Significantly reduced TNF-α and increased IL-10 (p < 0.05) in pancreatic tissues | [61] |
The levels of pro-inflammatory mediators such as IL-6, IL-1β, and TNF-α were generally measured to determine the anti-inflammatory activity (Table 2). The inflammation was induced by a toxic chemical or drug such as alloxan and STZ to stimulate inflammatory diabetes in model rats. LPS- or HFD-induced inflammation in diabetic rats was also conducted to observe the anti-inflammatory potential of Syzygium. So far, one study has demonstrated a close correlation between inflammation and memory loss in AD model rats. The memory-related learning ability of Aβ1-40-infused AD model rats was improved as pro-inflammatory TNF-α and lipid peroxide (LPO) were suppressed when S. cumini seed extract was administered [62]. The leaf, fruit, pulp, and seed extracts of S. cumini exerting anti-inflammatory activity suggested that almost all parts are bioactive (Table 2).
S. malaccense leaf extract exerted neuroinflammatory protection against LPS-induced neuroinflammation on murine BV-2 microglial cell lines by reducing nitric oxide production [63]. Nitric oxide (NO) is one of the pro-inflammatory mediators released by microglia; reducing the NO levels can minimize immune outrage caused by microglia [4]. Other less studied Syzygium species have also been observed to exert anti-inflammatory activities, including Syzygium caryophyllatum, Syzygium mundagam, Syzygium calophyllifolium, and S. samarangense (Table 2).
4.4. Antioxidant Activity
Oxidative stress is highly apparent when redox circuitry is disrupted, and macromolecular damage occurs, leading to an imbalance in the pro-oxidant and antioxidant levels [83]. The overproduction of RIS, such as hydrogen peroxide (H2O2), hydroxyl radical (HO), and NO observed in the AD brain, can trigger severe oxidative stress. Various factors can contribute to excessive RIS, including mitochondrial dysfunction, high levels of cytochrome oxidase, and Aß peptide chelation by redox-active metal ions [4,83]. Moreover, the downregulation of the expression and activity of antioxidant enzymes such as dehydrogenase complexes is evident in AD, causing biomolecular damage (lipids, proteins, and DNA) and neuronal death [4]. Antioxidants help scavenge free radicals and balance the production of RIS to reduce oxidative damage [84].
Syzygium is undeniably a great source of antioxidant agents due to the presence of polyphenols, tannins, and flavonoids [84]. The evidence of antioxidant activities from Syzygium species was summed up in Table 3. Compared to other biological activities, the antioxidant potential is the most comprehensively explored in various Syzygium species, including less studied Syzygium cymosum, S. paniculatum, and S. caryophyllatum. They not only scavenge free radicals but also provide protective effects against other toxicities. For example, CeCl3-induced neurotoxicity in the brains of rats was improved by the antioxidant capacity of S. aromaticum to restore RIS levels, which alleviated the cholinergic neural transmission and the state of memory in mice [42]. In another study, the ability of antioxidants to maintain genomic stability and slow down aging was demonstrated in the Caenorhabditis elegans nematode model [85]. The essential oil of S. aromaticum exerted antioxidant potential by inducing the expression of SOD-3 or GST-4 (antioxidant enzymes) and DAF-16/FOXO nuclear translocation from the cytoplasm to promote longevity in C. elegans [85].
Table 3.
Summary of plant parts or compounds examined for antioxidant activity from Syzygium species.
| Species | Plant Part/Compound | Reference | |
|---|---|---|---|
| 1 | S. cumini | Leaf | [36,37,89] |
| Fruit | [64,88,90] | ||
| Bark | [91] | ||
| Polyphenol-rich extract | [92,93] | ||
| Seed kernels powder | [94] | ||
| 2 | S. polyanthum | Leaf | [38] |
| 3 | S. aromaticum | Flower | [86] |
| Bud | [42,95] | ||
| Bud essential oil | [27,86,87,96,97,98] | ||
| Eugenol | [87] | ||
| All parts | [99] | ||
| 4 | S. antisepticum | Leaf | [24] |
| Gallic acid, myricitrin, and quercitrin | [24] | ||
| 5 | S. caryophyllatum | Leaf | [100] |
| Fruit | [100,101] | ||
| Fruit pulp healthy snack | [102] | ||
| 6 | Syzygium paniculatum Gaertn. | Leaf | [103] |
| Fruit | [104] | ||
| Volatile oil from the aerial part | [105] | ||
| 7 | S. malaccense | Leaf | [63,88,106] |
| Myricetin derivatives | [107] | ||
| 8 | S. aqueum | Stem | [108] |
| Bark | [108] | ||
| 9 | S. polyanthum | Leaf | [109] |
| 10 | S. jambos | Fruit | [110] |
| Bark | [61] | ||
| 11 | S. samarangense | Vescalagin | [80] |
| 12 | Syzygiumcymosum (Lam.) DC. | Leaf | [111] |
S. aromaticum (clove) is indeed the most studied Syzygium species for its antioxidant capacity. Its economically important essential oil source has brought many interests toward its applications. Alfikri et al. reported that clove produced the best essential oil ingredient at the flowering stage and the most efficient source of antioxidants when the trees are young. [86]. Meanwhile, Teles et al. demonstrated that eugenol (the primary compound of clove oil) exhibited higher antioxidant activity than its essential oil [87]. Various plant parts of the clove have been examined, especially its bud essential oil (Table 3). As for S. cumini and S. malaccense, their dried peel powders showed higher phenolic compounds, anthocyanin content, and antioxidant activity than their freeze-dried extracts, which can be pharmacologically relevant to their food applications [88].
4.5. In Vivo Neuroprotection Studies
The roles of Syzygium in neuroprotection were validated in animal models (Table 4). Aß1-40 and Aß1-42 are the major amyloid species observed in the buildups of AD brains. A study by Hossain et al. investigated the amyloid deposition and neurobehavioral symptoms in Aß1-40-infused AD rats when the seed extract of S. cumini was administered [62]. The extract elevated the memory-related learning ability by suppressing the levels of TNF-α and LPO, suggesting that anti-inflammatory and antioxidative actions took place in reducing the amyloid deposits [62]. In another study, the clove oil combined with exercise increased the levels of antioxidant-related enzymes peroxiredoxin-6 (PRDX6) and general control of amino acid synthesis 5-like 1 (GCN5L1) in Aß1-42-infused spatial memory-impaired AD rats. This observation specifies the importance of S. aromaticum oil as an antioxidant agent [28].
Table 4.
Reported in vivo studies related to neuroprotection or aging from Syzygium species.
| Species | Plant Part/Compound | Test | Activity | Reference | |
|---|---|---|---|---|---|
| 1 | S. cumini | Seed extract | Eight-arm radial maze task for learning-related memory | Improved learning-related memory through the antioxidative defense by a reduction in corticohippocampal levels of lipid peroxide | [112] |
| Seed extract | Aß1-40-infused AD model rats | Significantly increased the memory-related learning ability of the AD model rats with reductions in the levels of corticohippocampal Aβ1-40-burden and Aβ1-40-oligomers, and increased the levels of brain cognition and memory-related proteins, including BDNF, TrKB, PSD-95 and SNAP-25 | [62] | ||
| 2 | S. aqueum | Methanol leaves | POBCCA surgery in rats | Improved short- and long-term recognition memory in NOR test, improved spatial learning in MWM test at 200 mg/kg dose | [43] |
| 3 | S. aromaticum | Aqueous bud extract | AlCl3-induced neurotoxic rats | Restored the parameters (Al, Ca2+, MDA, nitrite/nitrate, Mg+, Na+, GSH, GPx) to the near-normal levels, significantly normalized expression of the SOD1 gene | [46] |
| Clove oil | Amyloid1-42-induced spatial memory-impaired rats | Improved spatial memory in Shuttle box test and apoptosis, PRDX6, and GCN5L1 levels were recovered through swimming training and clove consumption | [28] | ||
| Clove oil | MCAO-stroke-induced rats | The pre-treated and post-treated groups with clove oil showed improvement in neurological deficit score | [96] | ||
| Ethanol bud extract | CeCI3-induced memory-impaired mice | Symptoms of retracted neurons with condensed chromatin undergoing necrosis or apoptosis and vacuolated space were alleviated, which improved the state of memory in mice | [42] | ||
| Clove essential oil | C. elegans model | Extended lifespan and promoted production and health of C. elegans by inducing DAF-16/FOXO nuclear translocation from the cytoplasm and causing apoptosis of germ cells in ACEP-1 and DAF-16 | [85] | ||
| 4 | S. malaccense | Freeze-dried fruit | HFD-induced cognitive impaired rats | Improved AKT signaling in the hippocampus that prevented the activation of GSK3-β, lowered tau phosphorylation, and improved brain antioxidant enzyme activities. | [114] |
Indeed, oxidative stress and its impacts on AD pathogenesis have been largely concentrated in several other cognitive-impaired rat models. Significant improvement in the neurological deficit score was observed via the clove oil administration by which endogenous antioxidants such as SOD, CAT, and GSH were enhanced [96]. In aluminium chloride (AlCl3)-induced neurotoxic rats, the bud extract restored the brain ions homeostasis and oxidative level [46]. The bud extract of S. aromaticum also restored oxidative stress biomarkers, reduced AChE activity, and improved memory impairment in CeCl3-induced AD rats [42]. An improvement in the symptoms of retracted neurons with condensed chromatin undergoing necrosis or apoptosis and vacuolated space was observed in the brain tissues.
In addition, the seed extract from S. cumini ameliorated the learning-related memory of hypoxia-induced old male rats based on the reduction in the level of LPO [112]. The oxidative injury triggered by hypoxia caused the presence of high microglial cells around the hippocampus as a result of immune outrage. The antioxidative defense of the seed extract on the hypoxic group decreased the level of LPO that reduced inflammation caused by microglial invasion in the corticohippocampal brain tissue [112]. As a result, the brain cells retained their normal cellular structures with less swelling and rupture.
HFD-induced obesity can result in insulin resistance that can enhance pathophysiological processes related to AD in the brain [113]. The fruit of S. malaccense improved the learning and memory tasks of HFD-induced cognitive-impaired rats by alleviating AKT signaling in the hippocampus, preventing the activation of GSK3-ß, and lowering tau phosphorylation [114]. It stimulated peripheral insulin activity and brain antioxidant enzyme activities, promoting an anti-AD effect in this diabetic model. In alloxan-induced diabetic rats, the polyphenol-rich extract of S. cumini leaves showed a neuroprotective effect via suppression of the cholinesterase activity and reduction in the lipid peroxidation and hydroperoxide concentration [37]. This study justified the presence of AD symptoms in a diabetic model.
Kumaran et al. developed chronic hypoperfusion (CCH)-induced rats via a permanent occlusion of bilateral common carotid arteries (POBCCA) surgery to evaluate the effect of S. aqueum leaves on dementia [43]. This plant extract improved short- and long-term recognition memories and spatial learning based on the behavioral tests, including automated open field test, novel object recognition (NOR), and a Morris water maze (MWR) test. Additionally, the plant extract exhibited good anti-AChE and anti-BChE activities, although the antioxidant and anti-inflammatory parameters were not determined.
5. Bioactive Phytoconstituents
In general, Syzygium species are rich in flavonoids, tannins, phenols, steroids, as well as alkaloids [7,50] (Table 5). The polyphenol-rich extract of S. cumini has shown potent anti-cholinesterase, anti-inflammatory, and antioxidant activities [37,92,93]. Betulinic acid from this plant ameliorated mRNA and protein expressions such as NF-κB, iNOS, TNF-α, Nrf2, HO-1, and NQO1 to reduce inflammation in Fx1A antiserum-induced passive PHN in Sprague-Dawley rats [65]. It restored antioxidant activities and malondialdehyde levels effectively. The cholinesterase inhibitor, eugenol from S. aromaticum, inhibited the generation of superoxide anion via the suppression of the Raf/MEK/ERK1/2/p47phox-phosphorylation pathway and decreased cytokine concentrations attributed to its antioxidant and anti-inflammatory activities [18,77,78]. Its derivative, isoeugenol, exhibited potent inhibition against AChE, α-amylase, and α-glucosidase at 77.00, 411.5, and 19.25 nM of IC50, respectively [48].
Table 5.
Reported bioactive principles for neuroprotection from Syzygium species.
| Compound Name | Chemical Structure | Biological Activity | Reference | |
|---|---|---|---|---|
| 1 | Betulinic acid (in powder) |
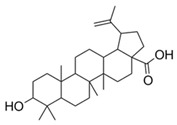
|
Anti-inflammatory and antioxidant | [65] |
| 2 | Eugenol (in MeOH) |
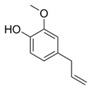
|
Anti-cholinesterase, anti-inflammatory, and antioxidant | [18,77,78] |
| 3 | Isoeugenol (in EtOH) |
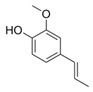
|
Anti-cholinesterase, and anti-diabetic | [48] |
| 4 | Vescalagin (in lyophilized powder) |
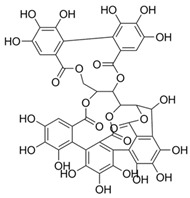
|
Anti-inflammatory, antioxidant, and anti-diabetic | [57,80] |
| 5 | 2′,4′-Dihydroxy-6′- methoxy-3′,5′-dimethyl-dihydrochalcone |
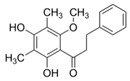
|
Anti-cholinesterase | [49] |
| 6 | Ursolic acid (in DMSO, tween 20, or MeOH) |
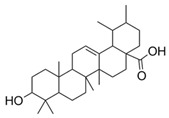
|
Anti-cholinesterase | [24] |
| 7 | Gallic acid (in DMSO, tween 20, or MeOH) |
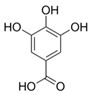
|
Anti-cholinesterase | [24] |
| 8 | Myricitin (in DMSO, tween 20, or MeOH) |
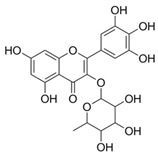
|
Antioxidant and anti-diabetic | [24,58] |
| 9 | Quercitin (in DMSO, tween 20, or MeOH) |
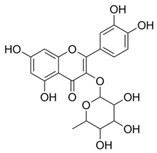
|
Antioxidant | [24] |
| 10 | 6-Heptadeca-8Z,11Z,14Z-trienyl salicylic acid (in DMSO) |

|
Anti-cholinesterase | [115] |
| 11 | 6-Heptadeca-9Z,12Z-dienyl salicylic acid (in DMSO) |
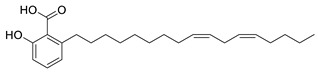
|
Anti-cholinesterase | [115] |
| 12 | (E)-β-Caryophyllene (in essential oil) |
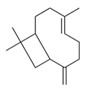
|
Anti-cholinesterase and anti-diabetic | [22] |
| 13 | ß-Pinene (in essential oil) |
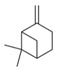
|
Anti-cholinesterase and anti-diabetic | [39] |
| 14 | (E)-ß-Ocimene (in essential oil) |
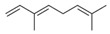
|
Anti-cholinesterase and anti-diabetic | [39] |
DMSO: dimethyl sulfoxide; EtOH: ethanol; MeOH: methanol.
Vescalagin isolated from S. samarangense ameliorated insulin resistance in high-fructose diet-induced hyperglycemic rats and reduced the pancreatic levels of NF-κB, ICAM-1, and TNF-α proteins [57,80]. Its antioxidant capacity helped to elevate the antioxidant contents in methylglyoxal (MG)-induced diabetic rats. 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethyl-dihydrochalcone from this species inhibited 98.5% at 0.25 mM and 68.0% at 0.20 mM against AChE and BChE, respectively [49]. The antioxidant agents, myricetin derivatives (with 77% of myricitrin) from S. malaccense exhibited insulin-like effects by enhancing lipid accumulation, glucose uptake, and adiponectin secretion via the activation of the insulin signaling pathway [58]. In S. antisepticum, ursolic acid and gallic acid showed fair inhibition against AChE [24]. Myricitin and quercitrin isolated from this species suppressed the production of ROS and catalase in HEK-293 cells [24]. An anti-AChE bioassay-guided isolation of S. jambos afforded two anacardic acid derivatives, 6-heptadeca-8Z,11Z,14Z-trienyl salicylic acid (SB-202742) and 6-heptadeca-9Z,12Z-dienyl salicylic acid (anacardic acid C) [115]. Both compounds inhibited AChE at 0.54 and 2.4 µM of IC50, respectively.
A few molecular docking studies have been performed investigating the effect of compounds from Syzygium against cholinesterase and diabetic enzymes. (E)-β-caryophyllene, one of the major compounds of S. cumini, revealed the best docking scores at −6.75, −5.61, and −7.75 kcal/mol for AChE, α-amylase, and α-glucosidase, respectively, implying its ability to form an enzyme–ligand complex [22]. Sharmeen Jugreet et al. identified ß-pinene and (E)-ß-ocimene from the essential oils of S. samarangense and S. coriaceum, respectively [39]. These compounds showed molecular docking scores within a range of −3.8 to −6.4 kcal/mol against AChE, BChE, α-amylase, and α-glucosidase. SB-202742 and anacardic acid C from S. jambos formed molecular interactions with residues TRP 84, GLY 118, and GLY 119 of Torpedo californica AChE [115]. An additional polar H-bond with ALA 201 and a π–alkyl non-polar interaction with TYR 121 from anacardic acid C were observed, justifying its superior activity than SB-202742 due to the conformational flexibility of its saturated alkyl chain.
6. Syzygium aromaticum
The flowering bud, known as clove, from S. aromaticum can undoubtedly be found in almost every household. This common spice is largely used as a flavoring or as preservative [116]. Clove, historically, originated from the “Spice Island” of Maluku in Eastern Indonesia but is also cultivated in other parts of Asia, including India, Malaysia, and Sri Lanka [117]. Madagascar, Tanzania, and the West Indies also harvest cloves in high quantities. This aromatic spice plant is also known as Eugenia caryophyllus, Myrtus caryophyllus, Jambosa caryophyllus, Caryophyllus aromaticus, and Caryophyllus silvestris [118]. Due to different harvest seasons across countries with different climates, clove is available throughout the year. It requires well-distributed rainfall with high humidity and temperatures around 25 to 35 °C.
The clove bud contains almost 18% of essential oil, consisting of eugenol (the main bioactive constituent), eugenol acetate, and β-cariofilen [119]. Clove is indeed rich in phenolic compounds, including flavonoids, hydroxybenzoic acids, hydroxyphenyl propens, hydroxycinnamic acids, and gallic acid derivatives such as hydrolyzable tannins [117]. Flavonoids such as quercetin and kaempferol and phenolic acids such as ferulic, caffeic, ellagic, and salicylic acids are among the compounds reported in clove. These phenolic compounds are accountable for various biological activities such as the anti-microbial and antioxidant properties that have positioned clove as one of the best natural food preservatives among other spices.
Clove receives the most attention for the Syzygium species, and its pharmacological studies are constantly reported. Its pre-clinical and clinical studies in AD remain to be conducted. However, its enhancement of memory and cognitive functions has been immensely demonstrated in animal models. The mechanisms of action in the anti-AD effect reported from the clove are via its antioxidant capacity, protection against neuroinflammation, restoration of cholinergic and mitochondrial functions, and hypocholesterolemic activity. It increased the levels of antioxidant-related enzymes such as PRDX6 and GCN5L1 and the potential mediators of oxidative pathways such as SIRT1 to maintain oxidative balance [28,95,120]. The clove oil restored mitochondrial function by increasing the activities of the mitochondrial respiratory enzyme complex (I–IV) [121]. The brain cholinesterase activity and total cholesterol level were also significantly decreased, which enhanced cholinergic transmission and reversed amnesia [122]. This improvement in the learning and memory parameters was confirmed by the decreased levels of TL and TRC and the increased values of SDL.
7. Conclusions
This review has highlighted the pharmacological importance of Syzygium species in neuroprotection, which justified their ethnomedicinal relevance in relation to memory and cognitive enhancement. Despite much interest and rigorous scientific research toward this genus, S. aromaticum and S. cumini have gained the most attention due to their widely medical applications in the Ayurveda, Unani, and Iranian medicinal systems. These two species are well documented from both ethnomedicinal and pharmacological perspectives. The lack of traditional practices for the other species in other traditional systems such as Western, Southern, Central African, and Australian Aboriginal medicinal systems concerning memory and cognitive functions may have influenced the current pharmacological studies to concentrate on particular Syzygium species only. Other species, including Western and Australian species, remain largely unstudied. There is an urge to conduct more neuroprotective studies toward less studied species, although their ethnobotanical records seem inadequate.
In short, S. aromaticum and S. cumini deliver excellent neuroprotective effects against AD. There were a fair number of studies for S. jambos, S. malaccense, S. samarangense, and S. aqueum in neuroprotection, and these are worthy of further investigation. Eugenol represents the most studied compound in the Syzygium genus. As far as we are concerned, recent studies were more inclusive toward in vitro and in vivo models, with only one clinical study involving 30 newly diagnosed type 2 diabetic patients to observe the efficacy of S. cumini seed powder. Other species are far from getting tested for human trials, but the in vivo evidence gathered for S. aromaticum and S. cumini has proved that these species require clinical studies in the future. Nearly all of the studies examined Syzygium species in the form of extracts, which lacked the compound isolation part. Partially purified extracts such as essential oils and polyphenol-rich extracts were assessed, but the bioactive components remain unidentified. Furthermore, only two or three in-silico studies were performed to observe the molecular interactions between isolated compounds and protein targets. Future studies involving isolation, in vivo testing of bioactive compounds, and in silico molecular characterization are essential as broader research areas could be explored.
Correlations between diabetes, inflammation, and oxidative stress are well established, where the suppression of inflammation, pro-inflammatory cytokines, and ROS production stimulates the insulin signaling pathway to promote insulin secretion when Syzygium extracts are administered. Additional studies are needed that measureme brain-cognition parameters, memory-related proteins, and the levels of AChE activity in diabetic rat models. Similarly, in memory-impaired rat models, assessing the levels of diabetic parameters and AChE activity is warranted. Incorporating the parameters of all four activities into one experiment or animal model may help to understand their pathophysiological relations better. Overall, the molecular mechanisms of Syzygium species in neuroprotection involve the inhibition of AChE to correct cholinergic transmission, the suppression of pro-inflammatory mediators, oxidative stress markers, and ROS production and an enhancement of antioxidant enzymes, the restoration of brain ions homeostasis, the inhibition of microglial invasion, the modulation of ß-cell insulin release, and an enhancement of lipid accumulation, glucose uptake, and adiponectin secretion via the activation of the insulin signaling pathway. It is noteworthy that the Syzygium species stand out as functional foods due to their edible parts and nutritional benefit. The pharmacological values of their essential oils, fruits, and leaves in commercial exploitation deserve more recognition; additional studies are warranted focusing on the seeds, stems, and flowers to utilize the genus for the prevention of AD fully.
Author Contributions
Conceptualization, M.S.A.R., H.A.W. and T.N.; writing—original draft preparation, M.S.A.R., M.K.N.M. and R.A.; writing—review and editing, M.S.A.R., T.N. and H.A.W.; funding acquisition, H.A.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Ministry of Higher Education Malaysia for Transdisciplinary Research Grant Scheme (TRGS) for the project titled Elucidating the Mechanism of THICAPA and POET in Different Genetic Variants Using Structural Bioinformatics (TRGS/1/2020/USM/02/3/2).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Rajasekhar K., Govindaraju T. Current Progress, Challenges and Future Prospects of Diagnostic and Therapeutic Interventions in Alzheimer’s Disease. RSC Adv. 2018;8:23780–23804. doi: 10.1039/C8RA03620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samanta S., Rangra N., Pradhan K. A Comprehensive Review on Phytopharmacological Investigations of Acacia auriculiformis A.Cunn. Ex Benth. Asian Pac. J. Trop. Biomed. 2019;9:1–11. doi: 10.4103/2221-1691.250263. [DOI] [Google Scholar]
- 4.Samanta S., Ramesh M., Govindaraju T. Alzheimer’s Disease: Recent Findings in Pathophysiology, Diagnostic and Therapeutic Modalities. Royal Society of Chemistry; Cambridge, UK: 2022. Alzheimer’s Is a Multifactorial Disease. [Google Scholar]
- 5.Li X.-T. Alzheimer’s Disease Therapy Based on Acetylcholinesterase Inhibitor/Blocker Effects on Voltage-Gated Potassium Channels. Metab. Brain Dis. 2022;37:581–587. doi: 10.1007/s11011-022-00921-w. [DOI] [PubMed] [Google Scholar]
- 6.Xie L., Zhu Q., Lu J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells. 2022;11:479. doi: 10.3390/cells11030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aung E.E., Kristanti A.N., Aminah N.S., Takaya Y., Ramadhan R. Plant Description, Phytochemical Constituents and Bioactivities of Syzygium Genus: A Review. Open Chem. 2020;18:1256–1281. doi: 10.1515/chem-2020-0175. [DOI] [Google Scholar]
- 8.Cock I.E., Cheesman M. Bioactive Compounds of Medicinal Plants. Apple Academic Press; Waretown, NJ, USA: 2018. Plants of the Genus Syzygium (Myrtaceae): A Review on Ethnobotany, Medicinal Properties and Phytochemistry; pp. 75–124. [DOI] [Google Scholar]
- 9.Karran E., De Strooper B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022;21:306–318. doi: 10.1038/s41573-022-00391-w. [DOI] [PubMed] [Google Scholar]
- 10.Hampel H., Mesulam M.-M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain. 2018;141:1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies P., Maloney A.J.F. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet. 1976;308:1403. doi: 10.1016/S0140-6736(76)91936-X. [DOI] [PubMed] [Google Scholar]
- 12.Marucci G., Buccioni M., Ben D.D., Lambertucci C., Volpini R., Amenta F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 13.Akter K., Lanza E.A., Martin S.A., Myronyuk N., Rua M., Raffa R.B. Diabetes Mellitus and Alzheimer’s Disease: Shared Pathology and Treatment? Br. J. Clin. Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterfield D.A., Lauderback C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid β-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 15.McGeer P.L., Schulzer M., McGeer E.G. Arthritis and Anti-Inflammatory Agents as Possible Protective Factors for Alzheimer’s Disease. Neurology. 1996;47:425–432. doi: 10.1212/WNL.47.2.425. [DOI] [PubMed] [Google Scholar]
- 16.Moeini R., Memariani Z., Asadi F., Bozorgi M., Gorji N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019;85:1326–1350. doi: 10.1055/a-1014-1075. [DOI] [PubMed] [Google Scholar]
- 17.Subash S., Essa M.M., Al-Adawi S., Memon M.A., Manivasagam T., Akbar M. Neuroprotective Effects of Berry Fruits on Neurodegenerative Diseases. Neural Regen. Res. 2014;9:1557–1566. doi: 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalai M.K., Bhadra S., Chaudhary S.K., Bandyopadhyay A., Mukherjee P.K. Anti-Cholinesterase Activity of the Standardized Extract of Syzygium aromaticum L. Pharmacogn. Mag. 2014;10:S276. doi: 10.4103/0973-1296.133275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayyanar M., Subash-Babu P. Syzygium cumini (L.) Skeels: A Review of Its Phytochemical Constituents and Traditional Uses. Asian Pac. J. Trop. Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhikara N., Kaur R., Jaglan S., Sharma P., Gat Y., Panghal A. Bioactive Compounds and Pharmacological and Food Applications of Syzygium cumini—A Review. Food Funct. 2018;9:6096–6115. doi: 10.1039/C8FO00654G. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S., Chandra D. Pharmacological Potentials of Syzygium cumini: A Review. J. Sci. Food Agric. 2013;93:2084–2093. doi: 10.1002/jsfa.6111. [DOI] [PubMed] [Google Scholar]
- 22.El-Nashar H.A.S., Eldehna W.M., Al-Rashood S.T., Alharbi A., Eskandrani R.O., Aly S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Egypt: Chemical Characterization and Molecular Docking Studies. Molecules. 2021;26:6984. doi: 10.3390/molecules26226984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S., Panda C.K., Das S. Clove (Syzygium aromaticum L.), a Potential Chemopreventive Agent for Lung Cancer. Carcinogenesis. 2006;27:1645–1654. doi: 10.1093/carcin/bgi372. [DOI] [PubMed] [Google Scholar]
- 24.Mangmool S., Kunpukpong I., Kitphati W., Anantachoke N. Antioxidant and Anticholinesterase Activities of Extracts and Phytochemicals of Syzygium antisepticum Leaves. Molecules. 2021;26:3295. doi: 10.3390/molecules26113295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail A., Mohamed M., Sulaiman S.A., Wan Ahmad W.A.N. Autonomic Nervous System Mediates the Hypotensive Effects of Aqueous and Residual Methanolic Extracts of Syzygium polyanthum (Wight) Walp. Var. Polyanthum Leaves in Anaesthetized Rats. Evid.-Based Complement. Altern. Med. 2013;2013:716532. doi: 10.1155/2013/716532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohanty S., Cock I.E. Bioactivity of Syzygium jambos Methanolic Extracts: Antibacterial Activity and Toxicity. Pharmacogn. Res. 2010;2:4. doi: 10.4103/0974-8490.60577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adefeghaa S., Oboha G., Odubanjoa T., Ogunsuyia O. A Comparative Study on the Antioxidative Activities, Anticholinesterase Properties and Essential Oil Composition of Clove (Syzygium aromaticum) Bud and Ethiopian Pepper (Xylopia Aethiopica) Riv. Ital. Sostanze Grasse. 2015;92:257–268. [Google Scholar]
- 28.Panahzadeh F., Mirnasouri R., Rahmati M. Exercise and Syzygium aromaticum Reverse Memory Deficits, Apoptosis and Mitochondrial Dysfunction of the Hippocampus in Alzheimer’s Disease. J. Ethnopharmacol. 2022;286:114871. doi: 10.1016/j.jep.2021.114871. [DOI] [PubMed] [Google Scholar]
- 29.Alikatte K.L., Akondi B.R., Yerragunta V.G., Veerareddy P.R., Palle S. Antiamnesic Activity of Syzygium cumini against Scopolamine Induced Spatial Memory Impairments in Rats. Brain Dev. 2012;34:844–851. doi: 10.1016/j.braindev.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Bonfanti G., Bitencourt P.R., de Bona K.S., da Silva P.S., Jantsch L.B., Pigatto A.S., Boligon A., Athayde M.L., Gonçalves T.L., Moretto M.B. Syzygium jambos and Solanum guaraniticum Show Similar Antioxidant Properties but Induce Different Enzymatic Activities in the Brain of Rats. Molecules. 2013;18:9179–9194. doi: 10.3390/molecules18089179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devakumar J., Keerthana V., Sudha S.S. Identification of Bioactive Compounds by Gas Chromatography-Mass Spectrometry Analysis of Syzygium jambos (L.) Collected from Western Ghats Region Coimbatore, Tamil Nadu. Asian J. Pharm. Clin. Res. 2017;10:364–369. [Google Scholar]
- 32.Chagas V.T., de Sousa Coelho R.M., Gaspar R.S., da Silva S.A., Mastrogiovanni M., de Jesus Mendonca C., de Souza Ribeiro M.N., de Andrade Paes A.M., Trostchansky A. Protective Effects of a Polyphenol-Rich Extract from Syzygium cumini (L.) Skeels Leaf on Oxidative Stress-Induced Diabetic Rats. Oxid. Med. Cell. Longev. 2018;2018:5386079. doi: 10.1155/2018/5386079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira C.C., Fuchs F.D., Blotta R.M., Knijnik J., Delgado I.C., Netto M.S., Ferreira E., Costa A.P., Müssnich D.G., Ranquetat G.G. Effect of Tea Prepared from Leaves of Syzygium jambos on Glucose Tolerance in Nondiabetic Subjects. Diabetes Care. 1990;13:907–908. doi: 10.2337/diacare.13.8.907. [DOI] [PubMed] [Google Scholar]
- 34.Dhanraj K.M., Veerakumari L. In Vitro Effect of Syzygium Aromaticum on the Motility and Acetylcholinesterase of Cotylophoron Cotylophorum. Ind. J. Vet. Anim. Sci. Res. 2014;43:187–194. [Google Scholar]
- 35.De Meneses A.C., Marques E.B.P., Leimann F.V., Gonçalves O.H., Ineu R.P., de Araújo P.H.H., de Oliveira D., Sayer C. Encapsulation of Clove Oil in Nanostructured Lipid Carriers from Natural Waxes: Preparation, Characterization and in Vitro Evaluation of the Cholinesterase Enzymes. Colloids Surf. A Physicochem. Eng. Asp. 2019;583:123879. doi: 10.1016/j.colsurfa.2019.123879. [DOI] [Google Scholar]
- 36.Borba L.A., Wiltenburg V.D., Negri G., Ibe M.B., dos Santos L., Mendes F.R. In Vitro Inhibition of Acetylcholinesterase and Monoamine Oxidase by Syzygium cumini Leaves Extract and Preliminary Assessment in Animal Models. S. Afr. J. Bot. 2022;146:553–563. doi: 10.1016/j.sajb.2021.11.041. [DOI] [Google Scholar]
- 37.Basiru O.A., Ojo O.A., Akuboh O.S., Okesola M.A., Idowu O.T., Talabi J.Y. The Protective Effect of Polyphenol-Rich Extract of Syzygium cumini Leaves on Cholinesterase and Brain Antioxidant Status in Alloxan-Induced Diabetic Rats. Jordan J. Biol. Sci. 2017;11:163–169. [Google Scholar]
- 38.Darusman L.K., Wahyuni T.W., Alwi F. Acetylcholinesterase Inhibition and Antioxidant Activity of Syzygium cumini, S. aromaticum and S. polyanthum from Indonesia. J. Biol. Sci. 2013;13:412–416. doi: 10.3923/jbs.2013.412.416. [DOI] [Google Scholar]
- 39.Sharmeen Jugreet B., Kouadio Ibrahime S., Zengin G., Abdallah H.H., Fawzi Mahomoodally M. GC/MS Profiling, In Vitro and In Silico Pharmacological Screening and Principal Component Analysis of Essential Oils from Three Exotic and Two Endemic Plants from Mauritius. Chem. Biodivers. 2021;18:e2000921. doi: 10.1002/cbdv.202000921. [DOI] [PubMed] [Google Scholar]
- 40.Saeedi M., Babaie K., Karimpour-Razkenari E., Vazirian M., Akbarzadeh T., Khanavi M., Hajimahmoodi M., Shams Ardekani M.R. In Vitro Cholinesterase Inhibitory Activity of Some Plants Used in Iranian Traditional Medicine. Nat. Prod. Res. 2017;31:2690–2694. doi: 10.1080/14786419.2017.1290620. [DOI] [PubMed] [Google Scholar]
- 41.Rawa M.S.A., Hassan Z., Murugaiyah V., Nogawa T., Wahab H.A. Anti-Cholinesterase Potential of Diverse Botanical Families from Malaysia: Evaluation of Crude Extracts and Fractions from Liquid-Liquid Extraction and Acid-Base Fractionation. J. Ethnopharmacol. 2019;245:112160. doi: 10.1016/j.jep.2019.112160. [DOI] [PubMed] [Google Scholar]
- 42.Kadri Y., Nciri R., Bardaa S., Brahmi N., Saber S., Harrath A.H., Aldahmash W., Alwasel S., Mohany M., El Feki A., et al. Syzygium aromaticum Alleviates Cerium Chloride-Induced Neurotoxic Effect In The Adult Mice. Toxicol. Mech. Methods. 2019;29:26–34. doi: 10.1080/15376516.2018.1506849. [DOI] [PubMed] [Google Scholar]
- 43.Kumaran K.R., Wahab H.A., Hassan Z. In Vitro Anti-Cholinesterase Activity and in Vivo Screening of Coccoloba Uvifera, Mimusops Elengi and Syzygium aqueum Extracts on Learning and Memory Function of Chronic Cerebral Hypoperfusion Rat. Neurosci. Res. Notes. 2021;4:1–13. doi: 10.31117/neuroscirn.v4i2.71. [DOI] [Google Scholar]
- 44.Affonso R.S., Lima J.A., Lessa B.M., Caetano J.V.O., Obara M.T., Nóbrega A.B., Nepovimova E., Musilek K., Kuca K., Slana G.B.C.A. Quantification through TLC-densitometric Analysis, Repellency and Anticholinesterase Activity of the Homemade Extract of Indian Cloves. Biomed. Chromatogr. 2018;32:e4096. doi: 10.1002/bmc.4096. [DOI] [PubMed] [Google Scholar]
- 45.Güller U., Güller P., Çiftci M. Radical Scavenging and Antiacetylcholinesterase Activities of Ethanolic Extracts of Carob, Clove, and Linden. Altern. Ther. Health Med. 2021;27:33–37. [PubMed] [Google Scholar]
- 46.Kassab R.B., Bauomy A.A. The Neuroprotective Efficency of the Aqueous Extract of Clove (Syzygium aromaticum) in Aluniniuminduced Neurotoxicity. Int. J. Pharm. Pharm. Sci. 2014;6:503–508. [Google Scholar]
- 47.Zamli K.M., Asari A., Khaw K.Y., Murugaiyah V., Al-Rashida H.M., Yusoff H.M., Hasnah N.H.A.W. Cholinesterase Inhibition Activity and Molecular Docking Study of Eugenol Derivatives. Sains Malays. 2021;50:1037–1045. doi: 10.17576/jsm-2021-5004-14. [DOI] [Google Scholar]
- 48.Topal F. Anticholinergic and Antidiabetic Effects of Isoeugenol from Clove (Eugenia Caryophylata) Oil. Int. J. Food Prop. 2019;22:583–592. doi: 10.1080/10942912.2019.1597882. [DOI] [Google Scholar]
- 49.Amor E.C., Villaseñor I.M., Nawaz S.A., Hussain M.S., Choudhar I. A Dihydrochalcone from Syzygium samarangense with Anticholinesterase Activity. Philipp. J. Sci. 2005;134:105. [Google Scholar]
- 50.Zulcafli A.S., Lim C., Ling A.P., Chye S., Koh R. Focus: Plant-Based Medicine and Pharmacology: Antidiabetic Potential of Syzygium Sp.: An Overview. Yale J. Biol. Med. 2020;93:307. [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S.B., Rajpoot R., Nasir A., Prabhu K.M., Murthy P.S. Ameliorative Effect of Active Principle Isolated from Seeds of Eugenia jambolana on Carbohydrate Metabolism in Experimental Diabetes. Evid.-Based Complement. Altern. Med. 2011;2011:789871. doi: 10.1093/ecam/nep233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanches J.R., França L.M., Chagas V.T., Gaspar R.S., Dos Santos K.A., Gonçalves L.M., Sloboda D.M., Holloway A.C., Dutra R.P., Carneiro E.M. Polyphenol-Rich Extract of Syzygium cumini Leaf Dually Improves Peripheral Insulin Sensitivity and Pancreatic Islet Function in Monosodium L-Glutamate-Induced Obese Rats. Front. Pharmacol. 2016;7:48. doi: 10.3389/fphar.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahana D.A., Shivaprakash G., Baliga R., MR A.P., Ganesh J., Pai M. Effect of Eugenia jambolana on Plasma Glucose, Insulin Sensitivity and HDL-C Levels: Preliminary Results of a Randomized Clinical Trial. J. Pharm. Res. 2010;3:1268–1270. [Google Scholar]
- 54.Sharma A.K., Bharti S., Kumar R., Krishnamurthy B., Bhatia J., Kumari S., Arya D.S. Syzygium cumini Ameliorates Insulin Resistance and β-Cell Dysfunction via Modulation of PPARγ, Dyslipidemia, Oxidative Stress, and TNF-α in Type 2 Diabetic Rats. J. Pharmacol. Sci. 2012;119:205–213. doi: 10.1254/jphs.11184FP. [DOI] [PubMed] [Google Scholar]
- 55.Shen S.-C., Chang W.-C., Chang C.-L. Fraction from Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Fruit Extract Ameliorates Insulin Resistance via Modulating Insulin Signaling and Inflammation Pathway in Tumor Necrosis Factor α-Treated FL83B Mouse Hepatocytes. Int. J. Mol. Sci. 2012;13:8562–8577. doi: 10.3390/ijms13078562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahmoud M.F., Abdelaal S., Mohammed H.O., El-Shazly A.M., Daoud R., Abdelfattah M.A.O., Sobeh M. Syzygium Aqueum (Burm.f.) Alston Prevents Streptozotocin-Induced Pancreatic Beta Cells Damage via the TLR-4 Signaling Pathway. Front. Pharmacol. 2021;12:769244. doi: 10.3389/fphar.2021.769244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang D.-W., Chang W.-C., Wu J.S.-B., Shih R.-W., Shen S.-C. Vescalagin from Pink Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Alleviates Hepatic Insulin Resistance and Ameliorates Glycemic Metabolism Abnormality in Rats Fed a High-Fructose Diet. J. Agric. Food Chem. 2016;64:1122–1129. doi: 10.1021/acs.jafc.5b05558. [DOI] [PubMed] [Google Scholar]
- 58.Arumugam B., Palanisamy U.D., Chua K.H., Kuppusamy U.R. Potential Antihyperglycaemic Effect of Myricetin Derivatives from Syzygium malaccense. J. Funct. Foods. 2016;22:325–336. doi: 10.1016/j.jff.2016.01.038. [DOI] [Google Scholar]
- 59.Konda P.Y., Chennupati V., Dasari S., Sharma N., Muthulingam M., Ramakrishnan R., Sade A., Jagadheeshkumar V., Natesan V., Jaiswal K.K. Ethno-Pharmacological Insulin Signaling Induction of Aqueous Extract of Syzygium paniculatum Fruits in a High-Fat Diet Induced Hepatic Insulin Resistance. J. Ethnopharmacol. 2021;268:113576. doi: 10.1016/j.jep.2020.113576. [DOI] [PubMed] [Google Scholar]
- 60.Sampath S., Narasimhan A., Chinta R., Nair K.R.J., Khurana A., Nayak D., Kumar A., Karundevi B. Effect of Homeopathic Preparations of Syzygium jambolanum and Cephalandra indica on Gastrocnemius Muscle of High Fat and High Fructose-Induced Type-2 Diabetic Rats. Homeopathy. 2013;102:160–171. doi: 10.1016/j.homp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Mahmoud M.F., Abdelaal S., Mohammed H.O., El-Shazly A.M., Daoud R., El Raey M.A., Sobeh M. Syzygium jambos Extract Mitigates Pancreatic Oxidative Stress, Inflammation and Apoptosis and Modulates Hepatic IRS-2/AKT/GLUT4 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2021;142:112085. doi: 10.1016/j.biopha.2021.112085. [DOI] [PubMed] [Google Scholar]
- 62.Hossain S., Islam J., Bhowmick S., Haque M., Rahaman A. Effects of Syzygium cumini Seed Extract on the Memory Loss of Alzheimer’s Disease Model Rats. Adv. Alzheimer’s Dis. 2017;6:53–73. doi: 10.4236/aad.2017.63005. [DOI] [Google Scholar]
- 63.Mendes R.F., Bellozi P.M.Q., Conegundes J.L., Fernandes M.F., Pinto N.C.C., Silva J.M., Costa J.C., Chedier L.M., Dias A.C.P., Scio E. In Vivo Anti-Inflammatory and Antinociceptive Effects, and in Vitro Antioxidant, Antiglycant and Anti-Neuroinflammatory Actions of Syzygium malaccense. An. Acad. Bras. Ciências. 2021;93:e20210457. doi: 10.1590/0001-3765202120210457. [DOI] [PubMed] [Google Scholar]
- 64.Qamar M., Akhtar S., Ismail T., Yuan Y., Ahmad N., Tawab A., Ismail A., Barnard R.T., Cooper M.A., Blaskovich M.A.T. Syzygium cumini (L.), Skeels Fruit Extracts: In Vitro and in Vivo Anti-Inflammatory Properties. J. Ethnopharmacol. 2021;271:113805. doi: 10.1016/j.jep.2021.113805. [DOI] [PubMed] [Google Scholar]
- 65.Sutariya B., Taneja N., Saraf M. Betulinic Acid, Isolated from the Leaves of Syzygium cumini (L.) Skeels, Ameliorates the Proteinuria in Experimental Membranous Nephropathy through Regulating Nrf2/NF-ΚB Pathways. Chem. Biol. Interact. 2017;274:124–137. doi: 10.1016/j.cbi.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Abdin M., Hamed Y.S., Akhtar H.M.S., Chen D., Chen G., Wan P., Zeng X. Antioxidant and Anti-Inflammatory Activities of Target Anthocyanins Di-Glucosides Isolated from Syzygium cumini Pulp by High Speed Counter-Current Chromatography. J. Food Biochem. 2020;44:1050–1062. doi: 10.1111/jfbc.13209. [DOI] [PubMed] [Google Scholar]
- 67.Sharma S., Pathak S., Gupta G., Sharma S.K., Singh L., Sharma R.K., Mishra A., Dua K. Pharmacological Evaluation of Aqueous Extract of Syzygium cumini for Its Antihyperglycemic and Antidyslipidemic Properties in Diabetic Rats Fed a High Cholesterol Diet-Role of PPARγ and PPARα. Biomed. Pharmacother. 2017;89:447–453. doi: 10.1016/j.biopha.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 68.Atale N., Mishra C.B., Kohli S., Mongre R.K., Prakash A., Kumari S., Yadav U.C.S., Jeon R., Rani V. Anti-Inflammatory Effects of S. cumini Seed Extract on Gelatinase-B (MMP-9) Regulation against Hyperglycemic Cardiomyocyte Stress. Oxidative Med. Cell. Longev. 2021;2021:8839479. doi: 10.1155/2021/8839479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heendeniya S., Ratnasooriya W.D., Pathirana R.N. In Vitro Investigation of Anti-Inflammatory Activity and Evaluation of Phytochemical Profile of Syzygium caryophyllatum. J. Pharmacogn. Phytochem. 2018;7:1759–1763. [Google Scholar]
- 70.Sobeh M., Mahmoud M.F., Petruk G., Rezq S., Ashour M.L., Youssef F.S., El-Shazly A.M., Monti D.M., Abdel-Naim A.B., Wink M. Syzygium aqueum: A Polyphenol-Rich Leaf Extract Exhibits Antioxidant, Hepatoprotective, Pain-Killing and Anti-Inflammatory Activities in Animal Models. Front. Pharmacol. 2018;9:566. doi: 10.3389/fphar.2018.00566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Chandran R., George B.P., Abrahamse H. Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract. Molecules. 2020;25:2900. doi: 10.3390/molecules25122900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandran R., Abrahamse H., Parimelazhagan T. Cytotoxic, Analgesic and Anti-Inflammatory Properties of Syzygium calophyllifolium Bark. Biomed. Pharmacother. 2018;103:1079–1085. doi: 10.1016/j.biopha.2018.04.067. [DOI] [PubMed] [Google Scholar]
- 73.Fatima H., Shahid M., Jamil A., Naveed M. Therapeutic Potential of Selected Medicinal Plants Against Carrageenan Induced Inflammation in Rats. Dose Response. 2021;19:15593258211058028. doi: 10.1177/15593258211058028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Déciga-Campos M., Beltrán-Villalobos K.L., Aguilar-Mariscal H., González-Trujano M.E., Ángeles-López G.E., Ventura-Martínez R. Synergistic Herb-Herb Interaction of the Antinociceptive and Anti-Inflammatory Effects of Syzygium aromaticum and Rosmarinus officinalis Combination. Evid.-Based Complement. Altern. Med. 2021;2021:8916618. doi: 10.1155/2021/8916618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chniguir A., Zioud F., Marzaioli V., El-Benna J., Bachoual R. Syzygium aromaticum Aqueous Extract Inhibits Human Neutrophils Myeloperoxidase and Protects Mice from LPS-Induced Lung Inflammation. Pharm. Biol. 2019;57:55–63. doi: 10.1080/13880209.2018.1557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mendi A., Yağci B.G., Kiziloğlu M., Saraç N., Yilmaz D., Uğur A., Uçkan D. Effects of Syzygium aromaticum, Cinnamomum zeylanicum, and Salvia triloba Extracts on Proliferation and Differentiation of Dental Pulp Stem Cells. J. Appl. Oral Sci. 2017;25:515–522. doi: 10.1590/1678-7757-2016-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chniguir A., Pintard C., Liu D., Dang P.M.-C., El-Benna J., Bachoual R. Eugenol Prevents FMLF-Induced Superoxide Anion Production in Human Neutrophils by Inhibiting ERK1/2 Signaling Pathway and P47phox Phosphorylation. Sci. Rep. 2019;9:18540. doi: 10.1038/s41598-019-55043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hobani Y.H., Mohan S., Shaheen E., Abdelhaleem A., Faruque Ahmad M., Bhatia S., Abou-Elhamd A.S. Gastroprotective Effect of Low Dose Eugenol in Experimental Rats against Ethanol Induced Toxicity: Involvement of Antiinflammatory and Antioxidant Mechanism. J. Ethnopharmacol. 2022;289:115055. doi: 10.1016/j.jep.2022.115055. [DOI] [PubMed] [Google Scholar]
- 79.Beltrán-Villalobos K.L., Déciga-Campos M., Aguilar-Mariscal H., González-Trujano M.E., Martínez-Salazar M.F., de Ramírez-Cisneros M.L.Á., Rios M.Y., López-Muñoz F.J. Synergistic Antinociceptive Interaction of Syzygium aromaticum or Rosmarinus officinalis Coadministered with Ketorolac in Rats. Biomed. Pharmacother. 2017;94:858–864. doi: 10.1016/j.biopha.2017.07.166. [DOI] [PubMed] [Google Scholar]
- 80.Chang W.-C., Wu J.S., Shen S.-C. Vescalagin from Pink Wax Apple (Syzygium samarangense (Blume) Merrill and Perry) Protects Pancreatic β-Cells against Methylglyoxal-Induced Inflammation in Rats. Plants. 2021;10:1448. doi: 10.3390/plants10071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khamchan A., Paseephol T., Hanchang W. Protective Effect of Wax Apple (Syzygium samarangense (Blume) Merr. & L.M. Perry) against Streptozotocin-Induced Pancreatic ß-Cell Damage in Diabetic Rats. Biomed. Pharmacother. 2018;108:634–645. doi: 10.1016/j.biopha.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 82.Hasan R., Lindarto D., Siregar G.A., Mukhtar Z. The Effect of Bay Leaf Extract Syzygium polyanthum (Wight) Walp. on C-Reactive Protein (CRP) and Myeloperoxidase (MPO) Level in the Heart of Rat Model of Myocardial Infarction. Med. Glas. Off. Publ. Med. Assoc. Zenica-Doboj Canton Bosnia Herzeg. 2020;17:41–45. doi: 10.17392/1068-20. [DOI] [PubMed] [Google Scholar]
- 83.Go Y.-M., Jones D.P. Redox Control Systems in the Nucleus: Mechanisms and Functions. Antioxid. Redox Signal. 2010;13:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uddin A.B.M.N., Hossain F., Reza A.S.M.A., Nasrin M.S., Alam A.H.M.K. Traditional Uses, Pharmacological Activities, and Phytochemical Constituents of the Genus Syzygium: A Review. Food Sci. Nutr. 2022:1–31. doi: 10.1002/fsn3.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L., Gu B., Wang Y. Clove Essential Oil Confers Antioxidant Activity and Lifespan Extension in C. Elegans via the DAF-16/FOXO Transcription Factor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021;242:108938. doi: 10.1016/j.cbpc.2020.108938. [DOI] [PubMed] [Google Scholar]
- 86.Alfikri F.N., Pujiarti R., Wibisono M.G., Hardiyanto E.B. Yield, Quality, and Antioxidant Activity of Clove (Syzygium aromaticum L.) Bud Oil at the Different Phenological Stages in Young and Mature Trees. Scientifica. 2020;2020:9701701. doi: 10.1155/2020/9701701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teles A.M., Silva-Silva J.V., Fernandes J.M.P., Abreu-Silva A.L., da Calabrese K.S., Mendes Filho N.E., Mouchrek A.N., Almeida-Souza F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid.-Based Complement. Altern. Med. 2021;2021:6663255. doi: 10.1155/2021/6663255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simas Frauches N., Montenegro J., Amaral T., Abreu J.P., Laiber G., Junior J., Borguini R., Santiago M., Pacheco S., Nakajima V.M., et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules. 2021;26:564. doi: 10.3390/molecules26030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco R.R., Ribeiro Zabisky L.F., Pires de Lima Júnior J., Mota Alves V.H., Justino A.B., Saraiva A.L., Goulart L.R., Espindola F.S. Antidiabetic Effects of Syzygium cumini Leaves: A Non-Hemolytic Plant with Potential against Process of Oxidation, Glycation, Inflammation and Digestive Enzymes Catalysis. J. Ethnopharmacol. 2020;261:113132. doi: 10.1016/j.jep.2020.113132. [DOI] [PubMed] [Google Scholar]
- 90.Koop B.L., Knapp M.A., Di Luccio M., Pinto V.Z., Tormen L., Valencia G.A., Monteiro A.R. Bioactive Compounds from Jambolan (Syzygium cumini (L.)) Extract Concentrated by Ultra- and Nanofiltration: A Potential Natural Antioxidant for Food. Plant Foods Hum. Nutr. 2021;76:90–97. doi: 10.1007/s11130-021-00878-8. [DOI] [PubMed] [Google Scholar]
- 91.Siddika A., Das P.K., Asha S.Y., Aktar S., Tareq A.R.M., Siddika A., Rakib A., Islam F., Khanam J.A. Antiproliferative Activity and Apoptotic Efficiency of Syzygium cumini Bark Methanolic Extract against EAC Cells In Vivo. Anticancer Agents Med. Chem. 2021;21:782–792. doi: 10.2174/1871520620666200811122137. [DOI] [PubMed] [Google Scholar]
- 92.De Morais Sousa M., de Lima R.M.T., de Lima A., Reis A.C., Cavalcante A.A.D.C.M., Sattler J.A.G., de Almeida-Muradian L.B., de Lima Neto J.S., Moreira-Araujo R.S.D.R., do Nogueira N. Antioxidant Action and Enzyme Activity Modulation by Bioaccessible Polyphenols from Jambolan (Syzygium cumini (L.) Skeels) Food Chem. 2021;363:130353. doi: 10.1016/j.foodchem.2021.130353. [DOI] [PubMed] [Google Scholar]
- 93.Ajiboye B.O., Ojo O.A., Oyinloye B.E., Akuboh O., Okesola M.A., Idowu O., Kappo A.P. In Vitro Antioxidant and Inhibitory Activities of Polyphenolic-Rich Extracts of Syzygium cumini (Linn) Skeels Leaf on Two Important Enzymes Relevant to Type II Diabetes Mellitus. Pak. J. Pharm. Sci. 2020;33:523–529. [PubMed] [Google Scholar]
- 94.Mahindrakar K.V., Rathod V.K. Valorization of Waste Syzygium cumini Seed Kernels by Three-Phase Partitioning Extraction and Evaluation of in Vitro Antioxidant and Hypoglycemic Potential. Prep. Biochem. Biotechnol. 2021;51:1036–1045. doi: 10.1080/10826068.2021.1894442. [DOI] [PubMed] [Google Scholar]
- 95.Shekhar S., Yadav Y., Singh A.P., Pradhan R., Desai G.R., Dey A.B., Dey S. Neuroprotection by Ethanolic Extract of Syzygium aromaticum in Alzheimer’s Disease like Pathology via Maintaining Oxidative Balance through SIRT1 Pathway. Exp. Gerontol. 2018;110:277–283. doi: 10.1016/j.exger.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 96.Jawed H., Shamim M., Sohail S., Firdous U., Iqbal Khan N. Antioxidative Activity of Clove (Syzygium aromaticum) Oil Administration in Middle Cerebral Artery Occlusion (Mcao) Models of Acute Focal Cerebral Ischemia. Pak. J. Neurol. Sci. 2019;14:10–15. [Google Scholar]
- 97.Radünz M., da Trindade M.L.M., Camargo T.M., Radünz A.L., Borges C.D., Gandra E.A., Helbig E. Antimicrobial and Antioxidant Activity of Unencapsulated and Encapsulated Clove (Syzygium aromaticum, L.) Essential Oil. Food Chem. 2019;276:180–186. doi: 10.1016/j.foodchem.2018.09.173. [DOI] [PubMed] [Google Scholar]
- 98.De Santos M.V.O., da Silva A.M., Praxedes É.A., Borges A.A., de Teles Filho A.C.A., Souza-Junior J.B.F., Bertini L.M., Silva A.R., Pereira A.F. Antioxidant Effects of the Essential Oil of Syzygium aromaticum on Bovine Epididymal Spermatozoa. Andrologia. 2019;51:e13448. doi: 10.1111/and.13448. [DOI] [PubMed] [Google Scholar]
- 99.Chang H.-J., Kim Y.-H., Kang Y.-H., Choi M.-H., Lee J.-H. Antioxidant and Antibacterial Effects of Medicinal Plants and Their Stick-Type Medicinal Concentrated Beverages. Food Sci. Biotechnol. 2020;29:1413–1423. doi: 10.1007/s10068-020-00793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wathsara H.P.T., Weeratunge H.D., Mubarak M.N.A., Godakumbura P.I., Ranasinghe P. In Vitro Antioxidant and Antidiabetic Potentials of Syzygium caryophyllatum L. Alston. Evid. Based. Complement. Alternat. Med. 2020;2020:9529042. doi: 10.1155/2020/9529042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumari G.U.W.U.P., Gunathilake K.D.P.P. In Vitro Bioaccessibility and Antioxidant Activity of Black Plum (Syzygium caryophyllatum) J. Food Biochem. 2020;44:e13499. doi: 10.1111/jfbc.13499. [DOI] [PubMed] [Google Scholar]
- 102.Kasunmala I.G.G., Bandara Navarathne S., Wickramasinghe I. Preparation of Liquid-Core Hydrogel Beads Using Antioxidant-Rich Syzygium caryophyllatum Fruit Pulp as a Healthy Snack. J. Texture Stud. 2020;51:937–947. doi: 10.1111/jtxs.12553. [DOI] [PubMed] [Google Scholar]
- 103.Kim S., Semple S.J., Simpson B.S., Deo P. Antioxidant and Antiglycation Activities of Syzygium paniculatum Gaertn and Inhibition of Digestive Enzymes Relevant to Type 2 Diabetes Mellitus. Plant Foods Hum. Nutr. 2020;75:621–627. doi: 10.1007/s11130-020-00858-4. [DOI] [PubMed] [Google Scholar]
- 104.Konda P.Y., Dasari S., Konanki S., Nagarajan P. In Vivo Antihyperglycemic, Antihyperlipidemic, Antioxidative Stress and Antioxidant Potential Activities of Syzygium paniculatum Gaertn. in Streptozotocin-Induced Diabetic Rats. Heliyon. 2019;5:e01373. doi: 10.1016/j.heliyon.2019.e01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okoh S.O., Okoh O.O., Okoh A.I. Seasonal Variation of Volatile Oil Composition and Antioxidant Property of Aerial Parts of Syzygium paniculatum Gaertn. Grown in the Eastern Cape, South Africa. Nat. Prod. Res. 2019;33:2276–2280. doi: 10.1080/14786419.2018.1497032. [DOI] [PubMed] [Google Scholar]
- 106.Itam A., Anna L. Antioxidant Activities, Cytotoxic Properties and Total Phenolic Content of Syzygium malaccense (L.) Merr. & L.M. Perry Leaves Extracts: A West Sumatera Indonesian Plant. Pak. J. Pharm. Sci. 2020;33:175–181. [PubMed] [Google Scholar]
- 107.Arumugam B., Palanisamy U.D., Chua K.H., Kuppusamy U.R. Protective Effect of Myricetin Derivatives from Syzygium malaccense against Hydrogen Peroxide-Induced Stress in ARPE-19 Cells. Mol. Vis. 2019;25:47–59. [PMC free article] [PubMed] [Google Scholar]
- 108.Itam A., Wati M.S., Agustin V., Sabri N., Jumanah R.A., Efdi M. Comparative Study of Phytochemical, Antioxidant, and Cytotoxic Activities and Phenolic Content of Syzygium aqueum (Burm. f. Alston f.) Extracts Growing in West Sumatera Indonesia. Sci. World J. 2021;2021:5537597. doi: 10.1155/2021/5537597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartanti L., Yonas S.M.K., Mustamu J.J., Wijaya S., Setiawan H.K., Soegianto L. Influence of Extraction Methods of Bay Leaves (Syzygium polyanthum) on Antioxidant and HMG-CoA Reductase Inhibitory Activity. Heliyon. 2019;5:e01485. doi: 10.1016/j.heliyon.2019.e01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed Yunus S.N., Abas F., Jaafar A.H., Azizan A., Zolkeflee N.K.Z., Abd Ghafar S.Z. Antioxidant and α-Glucosidase Inhibitory Activities of Eight Neglected Fruit Extracts and UHPLC-MS/MS Profile of the Active Extracts. Food Sci. Biotechnol. 2021;30:195–208. doi: 10.1007/s10068-020-00856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wahed T.B., Mondal M., Rahman M.A., Hossen M.S., Bhoumik N.C., Saha S., Tanvir E.M., Khalil M.I., Kundu S.K., Islam M.T., et al. Protective Role of Syzygium cymosum Leaf Extract Against Carbofuran-Induced Hematological and Hepatic Toxicities. Chem. Res. Toxicol. 2019;32:1619–1629. doi: 10.1021/acs.chemrestox.9b00164. [DOI] [PubMed] [Google Scholar]
- 112.Rahaman A., Hossain S., Rahman M., Hossain I., Nahar T., Uddin B., Khalil I. Syzygium cumini (L.) Seed Extract Improves Memory Related Learning Ability of Old Rats in Eight Arm Radial Maze. J. Pharmacogn. Phytochem. 2013;1:85–94. [Google Scholar]
- 113.Ledreux A., Wang X., Schultzberg M., Granholm A.-C., Freeman L.R. Detrimental Effects of a High Fat/High Cholesterol Diet on Memory and Hippocampal Markers in Aged Rats. Behav. Brain Res. 2016;312:294–304. doi: 10.1016/j.bbr.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Batista Â.G., Mendonça M.C.P., Soares E.S., da Silva-Maia J.K., Dionísio A.P., Sartori C.R., da Cruz-Höfling M.A., Maróstica Júnior M.R. Syzygium malaccense Fruit Supplementation Protects Mice Brain against High-Fat Diet Impairment and Improves Cognitive Functions. J. Funct. Foods. 2020;65:103745. doi: 10.1016/j.jff.2019.103745. [DOI] [Google Scholar]
- 115.Amir Rawa M.S., Nurul Azman N.A., Mohamad S., Nogawa T., Wahab H.A. In Vitro and In Silico Anti-Acetylcholinesterase Activity from Macaranga tanarius and Syzygium jambos. Molecules. 2022;27:2648. doi: 10.3390/molecules27092648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jessica Elizabeth D.L.T., Gassara F., Kouassi A.P., Brar S.K., Belkacemi K. Spice Use in Food: Properties and Benefits. Crit. Rev. Food Sci. Nutr. 2017;57:1078–1088. doi: 10.1080/10408398.2013.858235. [DOI] [PubMed] [Google Scholar]
- 117.Batiha G.E.-S., Alkazmi L.M., Wasef L.G., Beshbishy A.M., Nadwa E.H., Rashwan E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules. 2020;10:202. doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soh W.-K., Parnell J. A Revision of Syzygium Gaertn.(Myrtaceae) in Indochina (Cambodia, Laos and Vietnam) Adansonia. 2015;37:179–275. [Google Scholar]
- 119.Jirovetz L., Buchbauer G., Stoilova I., Stoyanova A., Krastanov A., Schmidt E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 120.Amber S., Shah S.A.A., Ahmed T., Zahid S. Syzygium aromaticum Ethanol Extract Reduces AlCl3-Induced Neurotoxicity in Mice Brain through Regulation of Amyloid Precursor Protein and Oxidative Stress Gene Expression. Asian Pac. J. Trop. Med. 2018;11:123. [Google Scholar]
- 121.Kumar A., Aggrawal A., Pottabathini R., Singh A. Possible Neuroprotective Mechanisms of Clove Oil against Icv-Colchicine Induced Cognitive Dysfunction. Pharmacol. Rep. 2016;68:764–772. doi: 10.1016/j.pharep.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 122.Milind P., Deepa K. Pro-Cholinergi, Hypo-Cholesterolemic and Memory Improving Effects of Clove. Int. Res. J. Pharm. 2011;2:119–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


