Abstract
Subclinical mastitis is a common intramammary disease in sheep production systems. Expenses associated with compromised animal performance, therapeutic interventions, and decreased ewe longevity make efforts to minimize its prevalence worthwhile. The objectives of this study were to 1) quantify the prevalence of subclinical mastitis throughout lactation, 2) evaluate the impact of bedding treatments on subclinical mastitis during early lactation, 3) evaluate the efficacy of prophylaxis and feed restriction during weaning on subclinical mastitis cure rates, and 4) identify levels and types of antimicrobial resistance in milk-derived bacteria. Ewe milk samples were collected at days 1, 2, and 28 post-partum, weaning, and 3-d post-weaning for bacterial identification via culture-based methods. Staphylococcus spp. and Streptococcus spp. isolates were subjected to in vitro antimicrobial susceptibility testing. The overall prevalence of subclinical mastitis defined by culture growth ranged between 22% and 66% and differences were observed between post-weaning and days 1 and 28 milk samples. Commonly isolated bacteria include coagulase-negative staphylococci (CoNS; 59%), Bacillus spp. (35%), Mannheimia haemolytica (10%), Staphylococcus aureus (8%), Streptococcus spp. (5%), and Corynebacterium spp. (5%). Early milk samples (days 1 and 2) were compared between jug bedding treatment: jugs were recently vacated, cleaned, and dusted with barn lime before adding fresh straw (CLEAN) or jugs were previously vacated and fresh straw was added atop soiled bedding (SOILED). Jug bedding treatment did not affect the prevalence of subclinical mastitis, though CoNS had greater sulfadimethoxine resistance in SOILED isolates than CLEAN isolates (P = 0.03). Three different weaning treatments were used: ewes were injected with penicillin at weaning (PENN), ewes had restricted feed access 48 h prior to and 72 h post-weaning (FAST), or a combination of these treatments (COMBO). Weaning treatment did not affect the prevalence of subclinical mastitis or cure rate from weaning to 3-d post-weaning, though all PENN and no FAST milk S. aureus isolates were resistant against tetracycline (P = 0.08). Subclinical mastitis prevalence tended to decrease from weaning to post-weaning (P = 0.08). These data show that subclinical mastitis is common throughout lactation and the levels of antimicrobial resistance of bacteria isolated from ewe milk are generally low against commonly used antimicrobials.
Keywords: antimicrobial susceptibility, ewe management, mastitis, milk
Our data show that subclinical mastitis is generally prevalent throughout lactation and the levels of antimicrobial resistance of bacteria isolated from ewe milk are generally low against antimicrobials commonly used in livestock production.
Introduction
Mastitis is commonly defined as the inflammation of mammary tissue largely resulting from microbial infection (Kahn and Line, 2010). Mastitis is prevalent throughout lactation and often categorized into clinical and subclinical states. Clinical mastitis is easily diagnosable, as the infected ewes often express milk that is abnormal in odor, color, and consistency, and have swollen, firm, and feverish udders. Ewes with subclinical mastitis have no visually apparent signs in the milk, udder, or overall health; therefore, on-farm diagnosis is difficult and laboratory methods are necessary to diagnose subclinical mastitis by isolating microorganisms from a milk sample or detecting an inflammatory response in the mammary gland (Fragkou et al., 2014).
Subclinical mastitis is an economically important disease in both dairy and meat-type sheep production systems due to reduced milk yields (Torres-Hernandez and Hohenboken, 1979; McCarthy et al., 1988; Fthenakis and Jones, 1990; Dario et al., 1996; Saratsis et al., 1999). In lamb-rearing ewes, milk yield reduction may cause suboptimal lamb growth, though creep feeding may partially overcome these effects (Fthenakis and Jones, 1990; Ahmad et al., 1992). Although a thorough and recent estimation of the cost of subclinical mastitis in meat-type sheep production systems in the United States has not been completed, earlier work has suggested an estimate between $20 and 25 million annually (Ahmad et al., 1992).
Commonly isolated mastitis-causing pathogens in ewe milk include Bacillus spp., Mannheimia haemolytica, Staphylococcus spp. (including S. aureus and coagulase-negative staphylococci, CoNS), and Streptococcus spp. (Arsenault et al., 2008; van den Crommenacker-Konings et al., 2021; Knuth et al., 2021) and may survive in the ewe’s environment. Little research has investigated how animal husbandry and facility management during critical time points, such as early lactation and weaning, affect the prevalence and etiology of subclinical mastitis. Although recommendations state fresh bedding should be provided daily to ewes in confinement (Binns et al., 2002; American Sheep Industry Association Inc., 2015; Shiels et al., 2022), some producers first remove soiled contents before adding fresh bedding, whereas others add fresh bedding on top of soiled contents. During early lactation, bacteria that survive in the environment may infect the mammary gland and cause subclinical mastitis, and the effects of these facility management strategies on subclinical mastitis are largely unknown.
Another critical time to prevent mastitis is at weaning while animals are moved through sheep handling facilities to separate lambs and ewes. During this time, there is heightened risk of mastitis as the udder transitions through the involution process (Petridis and Fthenakis, 2019). Although weaning husbandry practices have not been well researched, withholding feed from ewes for 48 h following weaning, and an additional 2 wk on a low-energy, low-protein diet, is recommended (American Sheep Industry Association Inc., 2015) to reduce milk production and risk of mammary gland infection (Aaron et al., 1997).
Antimicrobials are commonly used to control mastitis, especially at dry-off in dairy systems, as well as other bacterial diseases in livestock (Sawant et al., 2005; Raymond et al., 2006), and their potential over-usage raises concerns of antibiotic resistant strains affecting human health care via the food chain or environmental pollution (Skovgaard, 2007; Thanner et al., 2016). Although types and levels of antimicrobial resistance within dairy ewe milk bacteria have varied across reports, the most common resistance is to penicillin and ampicillin (Azara et al., 2017). Antimicrobials are used less routinely in meat- and wool-type sheep in semi-extensive production settings, which may lead to increased antimicrobial susceptibility of bacteria than in dairy sheep systems. Our research team hypothesized that subclinical mastitis prevalence would be generally high but that it would be greater in more soiled jugs, be predominantly associated with a small number of bacteria, penicillin injection at weaning would provide a greater cure rate than fasting, and antimicrobial resistance would be low in the absence of prolonged treatment with antimicrobials. Thus, the objectives of this research project were to: 1) quantify the prevalence of subclinical mastitis in early, mid, and late lactation, 2) evaluate the impact of animal bedding treatments on subclinical mastitis during early lactation, 3) evaluate the efficacy of prophylaxis and feed restriction during weaning on subclinical mastitis cure rates, and 4) identify levels and types of antimicrobial resistance in bacterial isolates from milk.
Materials and Methods
The University of Wyoming (UW) Institutional Animal Care and Use Committee (20200214HC00408-01) approved all experimental procedures.
Animal management
Primiparous (n = 20) and multiparous (n = 22) ewes were selected from the UW Laramie Research and Extension Center flock prior to lambing during March and April of 2020. Before lambing, ewes were randomly assigned to one of three early lactation bedding treatment groups that began in ewe-lamb bonding pens (jugs) shortly after parturition and continued through 48 h post-parturition. All ewes lambed in sheltered group drop-pens (6.8 × 13.4 m) with outdoor feed access (6.8 × 12.8 m) and then were moved with their lamb(s) to jugs. Prior to parturition, ewes were fed grass hay [nutrient composition: crude protein (CP) = 8.2%, neutral detergent fiber (NDF) = 58.2%, acid detergent fiber (ADF) = 34.3%; total digestible nutrients (TDN) = 63.4%; dry matter (DM) basis; Ward Laboratories, Inc., Kearney, NE] and alfalfa hay (CP = 12.1%, NDF = 57.0%, ADF = 45.9%, TDN = 47.9%) to meet their requirements (National Research Council, 2007), whereas in the jugs, ewes had ad libitum access to alfalfa hay. Ewes and lambs remained in jugs for approximately 48 h before being moved to a small indoor group pen (≈ 4 ewe/lamb pairs; 4.3 × 4.3 m) for less than 24 h then a larger sheltered group pen (7.6 × 8.5 m) with outdoor access (7.6 × 18.6 m) where they remained until weaning (68.7 ± 5.8 d of age). In the group pens, ewes were fed approximately 1.2 and 2.5 kg hd−1 d−1 of alfalfa and grass hays, respectively, and 0.8 kg hd−1 d−1 shelled corn (CP = 8.5%, NDF = 7.5%, ADF = 2.1%, TDN = 88.9%; DM basis). All ewes reared at least one lamb to weaning.
Jug bedding treatments
Ewes were blocked by parity and randomly assigned to jug bedding treatments divergent in soiled bedding removal and dry-time of jug after barn lime application; however, due to a prolonged lambing interval, the dry-time no longer varied between groups and groups were only divergent in removal of soiled bedding. Therefore, two of the three treatments were combined and analysis was conducted between two groups: 1) jugs (2.3 × 1.2 or 1.7 × 1.2 m) recently vacated, removed of soiled bedding, dusted with barn lime before adding fresh straw (CLEAN; n = 29) and 2) jugs (2.3 × 1.2 m) previously vacated and fresh straw added on top of soiled bedding (SOILED, n = 13).
Weaning treatments
Prior to weaning, study ewes were blocked by jug bedding treatment into one of three weaning treatment groups: 1) intramuscular injection of penicillin (PENN; durvet; penicillin G procaine injectable suspension; 300,000 units mL−1; 1 mL per 45 kg BW; n = 13) at weaning, 2) restricted feed access 48 h prior to and 72 h post-weaning (FAST; n = 14), and 3) a combination of the administration of penicillin and fasting treatments (COMBO; n = 15). Ewes were pen-fed, and FAST and COMBO ewes were feed restricted by % BW. FAST and COMBO ewes received 1.30% to 1.72% grass hay only and their calculated nutrient intake was below recommended levels. PENN ewes were fed between 1.68% and 2.85%, 1.07% and 2.47%, and 0% and 1.17% BW grass hay, alfalfa hay, and shelled corn, respectively (multiparous ewes stopped being fed shelled corn 7 d prior to weaning due to limited feed resources).
Sample collection
Ewe milk samples were collected at 24 h (22.8 ± 6.9 h), 48 h (46.8 ± 4.7 h), 28 d (27.9 ± 1.0 d), weaning (68.02 ± 5.8 d), and post-weaning (71.6 ± 5.8 d). Prior to each collection, lambs were separated from their dams for approximately 15 min, and then ewes were hand-restrained. Milk was collected using aseptic techniques using gloved hands and by scrubbing the teat with 70% ethanol then discarding the first three streams of residual milk. Approximately 15 to 20 mL of milk was then obtained between the two udder halves. Two 1.5-mL milk samples for microbial analyses were aliquoted, flash frozen on dry ice, and stored at −80 °C until microbial analyses. An additional 10 mL of milk was used to perform an on-site California Mastitis Test (CMT; Whiteside, 1939; Schalm and Noorlander, 1957) that indirectly measures elevated somatic cell counts using the premises of the Whiteside phenomenon and modified Whiteside test. The CMT was subjectively scored following manufacturer (California Mastitis Test Kit; ImmuCell; Portland, ME) instructions: mixture remained liquid with no evidence of a precipitate (CMT score = −1); a slight precipitate was observed which tended to disappear as the paddle was swirled (CMT score = 0); a distinct precipitate forms without a tendency to gel (CMT score = 1); the mixture thickened immediately with some gel formation (CMT score = 2); and a distinct gel formed that adhered to the bottom of the well (CMT score = 3). Elevated CMT scores can be indicative of subclinical mastitis. Milk samples collected from ewes with clinical mastitis presenting abnormal milk or udders at the time of sample collection were excluded from analyses (n = 10).
Bacterial culturing and identification
Thawed milk samples (n = 204) were briefly vortexed before a sterilized cotton-tip applicator was used to streak the sample onto compartmentalized plates (Y-plate; Thermo Fisher Scientific Inc.; Waltham, MA) containing one each of three microbiological media: MacConkey agar (HiMedia Laboratories Pvt. Ltd.; Mumbai, India); Trypticase soy agar (TSA; Becton, Dickinson and Company; Sparks, MD); and TSA + 5% sheep blood agar (Hardy Diagnostics; Santa Maria, CA). These media were selected to isolate the following targets: MacConkey agar suppresses the growth of Gram-positive bacteria, thus favoring growth of Gram-negative and enteric bacilli; TSA is a general-purpose and nonselective medium to cultivate a majority of bacteria present; and TSA + 5% sheep blood contains added nutrients and growth factors to improve recovery of fastidious bacteria compared to TSA. Plates were incubated aerobically at 37 °C for 24 h, and plates that exhibited no growth were incubated for an additional 24 h. Following incubation and observed presence of bacteria, the number of colonies and their morphologies were recorded. In this study, we defined subclinical mastitis as the presence of ≥ 1 colonies of major pathogens (i.e., S. aureus, CoNS, and M. haemolytica) or the presence of ≥ 5 colonies of other pathogens. Samples with ≥ 3 colony types were considered contaminated and excluded from statistical analysis. This definition is similar to what (Dore et al., 2016) used in Italian dairy flocks, though CoNS are commonly considered primary mastitis pathogens in non-dairy ewes. Following initial incubation, colonies were sub-cultured for isolation (37 °C for 24 h). Single colonies were transferred into a sterile 15-mL tube containing 10 mL of brain heart infusion broth and incubated (37 °C for 24 h). To make a preserved freezer stock, tubes were vortexed for 10 s and 750 µL were transferred into a cryogenic storage vial containing 750 µL of sterile 40% glycerol, which was then frozen and stored at −80 °C until further analyses.
Cultures were reactivated on TSA plates for identification via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The extended direct colony transfer method was used by transferring purified colonies with a toothpick onto a 96-well steel-target plate. Then, 1 µL of 70% formic acid and subsequently 1 µL of matrix HCCA (α-cyano-4-hydroxycinnamic acid) were added to the center of each well. Each plate also included a bacteria test standard (Escherichia coli) before MALDI-TOF MS identification. This method uses a Bruker Microflex LRF instrument for data acquisition and Bruker Daltonics Biotyper Compass software (v. 4.1.100.10) and library for data analysis and species level identification (Proteomics and Metabolomics Facility, Colorado State University, Fort Collins, CO; Research Resource ID: SCR_011758).
Antimicrobial susceptibility testing
Staphylococcus spp. and Streptococcus spp. with a high quality score (≥ 2.0) for a MALDI-TOF MS identification match were subjected to in vitro antimicrobial susceptibility testing to identify base levels of susceptibility and evaluate any effects of antimicrobial usage at weaning. These genera were selected as they are considered to be major pathogens of mastitis in livestock (Al-Majali and Jawabreh, 2003; Makovec and Ruegg, 2003; Arsenault et al., 2008). After reactivation of cultures using previously stated methods, colonies were added to demineralized water (Thermo Scientific) to achieve a 0.5 McFarland standard. For staphylococci and streptococci, 30 µL were added to Mueller-Hinton broth (Thermo Scientific) and 10 µL were added to Mueller-Hinton broth with lysed horse blood (Thermo Scientific), respectively. Using a Sensititre AIM instrument, 50 µL were then inoculated into each well of the Sensititre Vet Mastitis CMV1AMAF plate (Thermo Scientific). This plate tests the antimicrobial susceptibility compounds of non-fastidious Gram-positive and Gram-negative bacterial isolates of veterinary origin against FDA-approved food animal compounds. These antimicrobial compounds include ampicillin, penicillin, erythromycin, oxacillin + 2% NaCl, pirlamycin, penicillin/novobiocin, tetracycline, cephalothin, ceftiofur, and sulfadimethoxine. The plate was sealed and incubated at 35 °C for 24 h and then analyzed via a Sensititre Vizion instrument (Thermo Scientific).
Data analyses
The prevalence of subclinical mastitis (i.e., the prevalence of milk samples with culture growth used to define subclinical mastitis) and MALDI-TOF MS identifications was analyzed within and across day, within and across jug bedding treatment from days 1 and 2 milk samples, and within and across weaning treatment from weaning and post-weaning milk samples. Binomial proportions and 95% confidence intervals of MALDI-TOF MS identifications within culture-positive samples were estimated using the binom package of R (Dorai-Raj, 2014; RStudio Team, 2021). The glm, anova, and glht (multcomp package; Hothorn et al., 2021) procedures of R were used to estimate the effects of jug bedding treatment, weaning treatment, day of lactation, parity, and CMT score on subclinical mastitis prevalence and prevalence of bacilli, staphylococci, streptococci, or Mannheimia haemolytica. The survival package of R (Therneau et al., 2021) was used to perform a survival analysis of bacterial isolates following antimicrobial susceptibility testing using similar methods of Pol and Ruegg (2007). Briefly, antimicrobial concentrations present in plate wells were used as “time” and inhibition of bacterial growth was used as “event”. Effects of jug bedding treatment (days 1 and 2 milk samples), weaning treatment (weaning and post-weaning milk samples), day, parity, and CMT on antimicrobial susceptibility were assessed. Kaplan–Meier survival curves were created, and log-rank and Wilcoxon tests were used to analyze each effect. Statistical significance and tendency were considered for P ≤ 0.05 and 0.05 < P ≤ 0.10, respectively.
Results
Prevalence and etiological agents of mastitis
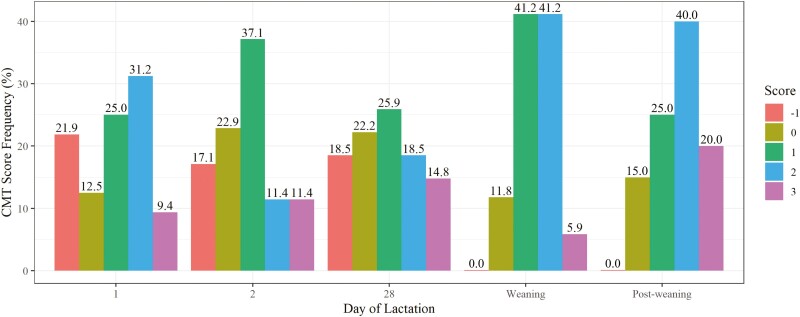
Subclinical mastitis was prevalent throughout lactation based on culture-data (Table 1) and CMT scores (Figure 1). Based on culture data, subclinical mastitis frequencies were 57%, 49%, 66%, 41%, and 22% at days 1, 2, 28, weaning, and post-weaning, respectively (overall = 45%). There was a tendency for a day of lactation effect for subclinical mastitis (P ≤ 0.01). Post-weaning milk samples had the lowest subclinical mastitis prevalence, which was statistically different from days 1 and 28 samples (P ≤ 0.02). Statistical significance was observed for day of lactation differences in CMT score (P < 0.01), and CMT score distribution varied by day. In general, greater CMT scores were observed at weaning and post-weaning compared to days 1, 2, and 28 (P ≤ 0.10; Figure 1).
Table 1.
Number of milk samples within and across day of lactation and estimated species frequency (95% confidence interval) within culture-positive samples identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)
| Day of lactation | Overall | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 28 | Weaning | Weaning + 72 h | ||
| Item | ||||||
| No. of samples | 35 | 35 | 29 | 34 | 41 | 174 |
| No. of positive (%) | 20 (57%) | 17 (49%) | 19 (66%) | 14 (41%) | 9 (22%) | 79 (45%) |
| Bacteria1 | ||||||
| Unidentified2 | 0.05 [0.00, 0.25] | – | – | – | – | 0.01 [0.00, 0.07] |
| Acinetobacter lwoffi | – | – | 0.05 [0.00, 0.26] | – | – | 0.01 [0.00, 0.07] |
| Advenella spp.3 | 0.10 [0.01, 0.32] | – | – | – | – | 0.03 [0.00, 0.09] |
| Aerococcus viridians | 0.05 [0.00, 0.25] | – | – | – | – | 0.01 [0.00, 0.07] |
| Bacillus spp.4 | 0.25 [0.09, 0.49] | 0.18 [0.04, 0.43] | 0.32 [0.13, 0.57] | 0.93 [0.66, 1] | 0.11 [0.00, 0.48] | 0.35 [0.25, 0.47] |
| Corynebacterium spp.5 | 0.10 [0.01, 0.32] | – | 0.11 [0.01, 0.33] | – | – | 0.05 [0.01, 0.12] |
| Enterococcus mundtii | – | – | – | – | – | 0.01 [0.00, 0.07] |
| Escherichia coli | – | – | – | – | – | 0.01 [0.00, 0.07] |
| Mannheimia haemolytica | 0.05 [0.00, 0.25] | 0.06 [0.00, 0.29] | 0.05 [0.00, 0.26] | 0.29 [0.08, 0.58] | 0.11 [0.00, 0.48] | 0.10 [0.04, 0.19] |
| Pantoea agglomerans | – | – | 0.05 [0.00, 0.26] | – | – | 0.01 [0.00, 0.07] |
| Staphylococcus aureus | – | 0.06 [0.00, 0.29] | 0.11 [0.01, 0.33] | 0.07 [0.00, 0.34] | 0.22 [0.03, 0.60] | 0.08 [0.03, 0.16] |
| CoNS6 | 0.80 [0.56, 0.94] | 0.76 [0.50, 0.93] | 0.42 [0.20, 0.67] | 0.29 [0.08, 0.58] | 0.67 [0.30, 0.93] | 0.59 [0.48, 0.70] |
| S. auricularis | 0.05 [0.00, 0.25] | – | – | – | – | 0.01 [0.00, 0.07] |
| S. capitis | – | – | – | 0.07 [0.00, 0.34] | – | 0.01 [0.00, 0.07] |
| S. chromogenes | – | – | – | 0.07 [0.00, 0.34] | – | 0.01 [0.00, 0.07] |
| S. epidermidis | 0.10 [0.01, 0.32] | 0.12 [0.01, 0.36] | 0.21 [0.06, 0.46] | – | 0.11 [0.00, 0.48] | 0.11 [0.05, 0.21] |
| S. equorum | 0.15 [0.03, 0.38] | 0.12 [0.01, 0.36] | – | 0.14 [0.02, 0.43] | 0.33 [0.07, 0.70] | 0.13 [0.06, 0.22] |
| S. hominis | 0.05 [0.00, 0.25] | – | – | – | – | 0.01 [0.00, 0.07] |
| S. microti | – | – | 0.11 [0.01, 0.33] | – | – | 0.03 [0.00, 0.09] |
| S. sciuri | – | 0.06 [0, 0.29] | – | – | – | 0.01 [0.00, 0.07] |
| S. succinus | 0.30 [0.12, 0.54] | 0.12 [0.01, 0.36] | 0.05 [0.00, 0.26] | – | – | 0.11 [0.05, 0.21] |
| S. vitulinus | – | 0.18 [0.04, 0.43] | – | – | 0.11 [0.00, 0.48] | 0.05 [0.01, 0.12] |
| S. xylosus | 0.45 [0.23, 0.68] | 0.35 [0.14, 0.62] | 0.21 [0.06, 0.46] | 0.07 [0.00, 0.34] | 0.11 [0.00, 0.48] | 0.27 [0.17, 0.38] |
| Streptococcus spp. | – | 0.06 [0.00, 0.29] | – | 0.14 [0.02, 0.43] | 0.11 [0.00, 0.48] | 0.05 [0.01, 0.12] |
| Str. gallolyticus | – | – | – | 0.14 [0.02, 0.43] | 0.11 [0.00, 0.48] | 0.04 [0.01, 0.11] |
| Str. pluranimalium | – | 0.06 [0.00, 0.29] | – | – | – | 0.01 [0.00, 0.07] |
Only bacteria genera or species identified at an overall frequency ≥ 0.01 are presented.
Unidentified, bacteria isolates cultivated but not identified by MALDI-TOF MS.
Advenella spp. identified include A. incenata and A. kashmirensis.
Bacillus spp. identified include B. altitudinis, B. atrophaeus, B. cereus, B. clausii (now Alkalihalobacillus clausii), B. licheniformis, B. megaterium, B. pumilus, B. safensis, B. sonorensis, B. subtilis, and B. thuringiensis.
Corynebacterium spp. identified include C. ammoniagenes, C. casei, C. freneyi, C. stationis, and C. xerosis.
CoNS, coagulase-negative staphylococci.
Figure 1.
California Mastitis Test (CMT) score distribution by day of lactation: days 1, 2, and 28 post-partum, weaning (day 68 post-partum), and 3-d post-weaning.
Forty bacterial species were identified via MALDI-TOF MS, and prevalent species across day of lactation are presented in Table 1. The most commonly identified bacterial genera and species include CoNS (59% of subclinical mastitis samples): S. xylosus = 27%, S. equorum = 13%, S. epidermidis = 11%, S. succinus = 11%; S. vitulinus = 5%), Bacillus spp. (35%), Mannheimia haemolytica (10%), S. aureus (8%), Streptococcus spp. (5%), and Corynebacterium spp. (5%).
Frequency of Bacillus spp. identification differed by day of lactation (P < 0.01), where weaning milk samples differed from day 2 and post-weaning milk (P ≤ 0.05). A day effect was observed for CoNS frequency (P < 0.01), with pairwise comparisons showing frequencies to differ between day 1 and weaning or post-weaning milk samples (P ≤ 0.03). Subclinical mastitis status and CMT score had a statistically significant relationship (P = 0.03). CMT score also tended to have a relationship with S. aureus (P = 0.10), where samples with S. aureus presence had elevated CMT scores. No statistically significant litter size effects were observed for prevalence of subclinical mastitis or bacterial genera (P > 0.27). Additionally, no jug bedding treatment effect was observed within days 1 and 2 milk samples for subclinical mastitis or bacteria genera frequencies (P > 0.10; Table 2). Parity affected the frequency of cultivating M. haemolytica (P = 0.03), where multiparous ewes had 6 times greater frequency of M. haemolytica than primiparous ewes.
Table 2.
Number of milk samples collected at days 1 and 2 post-parturition within jug bedding treatment and estimated species frequency (95% confidence interval) within culture-positive samples identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)
| Jug bedding treatment | ||
|---|---|---|
| CLEAN1 | SOILED2 | |
| Item | ||
| No. of samples | 51 | 19 |
| No. of positive (%) | 28 (55%) | 9 (47%) |
| Bacteria3 | ||
| Unidentified4 | 0.04 [0.00, 0.18] | – |
| Advenella spp.5 | 0.07 [0.01, 0.24] | – |
| Bacillus spp.6 | 0.25 [0.11, 0.45] | 0.11 [0.00, 0.48] |
| Corynebacterium spp.7 | – | 0.22 [0.03, 0.60] |
| Mannheimia haemolytica | 0.07 [0.01, 0.24] | – |
| CoNS8 | 0.75 [0.55, 0.89] | 0.89 [0.52, 1.00] |
| S. auricularis | – | 0.11 [0.00, 0.48] |
| S. epidermidis | 0.07 [0.01, 0.24] | 0.22 [0.03, 0.60] |
| S. equorum | 0.11 [0.02, 0.28] | 0.22 [0.03, 0.60] |
| S. hominis | – | 0.11 [0.00, 0.48] |
| S. succinus | 0.18 [0.06, 0.37] | 0.33 [0.07, 0.70] |
| S. vitulinus | 0.11 [0.02, 0.28] | – |
| S. xylosus | 0.43 [0.24, 0.63] | 0.33 [0.07, 0.70] |
| Streptococcus spp. | – | 0.11 [0.00, 0.48] |
| Str. pluranimalium | – | 0.11 [0.00, 0.48] |
CLEAN, jugs were recently vacated, removed of soiled bedding, dusted with barn lime before adding fresh straw.
SOILED, jugs were previously vacated and fresh straw was added on top of soiled bedding.
Only bacteria genera or species identified at a frequency > 0.05 within jug bedding treatment are presented.
Unidentified, bacteria isolates cultivated but not identified by MALDI-TOF MS.
Advenella spp. identified include A. kashmirensis and A. incenata.
Bacillus spp. identified include B. cereus, B. licheniformis and B. pumilus, and B. subtilis.
Corynebacterium spp. identified include C. ammoniagenes, C. casei, and C. stationis.
CoNS, coagulase-negative staphylococci.
Weaning and post-weaning samples were assessed within and across day and weaning treatment (Table 3). Weaning treatment by day of lactation did not affect the frequency of subclinical mastitis from weaning to post-weaning milk samples (P = 0.20). Although there was no evidence of a weaning treatment (P = 0.34), there was a day effect observed where subclinical mastitis prevalence tended to decrease from weaning to post-weaning (P = 0.07). An interaction between day and weaning treatment was observed for Bacillus spp. frequency (P < 0.01), where frequencies in COMBO milk samples at weaning were greater than those observed in COMBO milk samples post-weaning. There was also an interaction tendency observed for M. haemolytica isolation (P = 0.07), though no pairwise comparisons were significant. Samples collected post-weaning tended to have reduced subclinical mastitis (P = 0.07) as well as reduced Bacillus spp. and M. haemolytica (P ≤ 0.10) than samples collected at weaning. No weaning treatment effects were observed in post-weaning milk samples (P = 0.97).
Table 3.
Number of milk samples collected at weaning (68.02 ± 5.8 d) and post-weaning (71.6 ± 5.8 d) within weaning treatment and estimated species frequency (95% confidence interval) within culture-positive samples identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)
| Weaning | Post-weaning | |||||
|---|---|---|---|---|---|---|
| PENN1 | FAST2 | COMBO3 | PENN | FAST | COMBO | |
| Item | ||||||
| No. of samples | 14 | 9 | 11 | 15 | 13 | 13 |
| No. of positive (%) | 5 (36%) | 2 (22%) | 7 (64%) | 3 (20%) | 3 (23%) | 3 (23%) |
| Bacteria4 | ||||||
| Bacillus spp.5 | 0.80 [0.28, 0.99] | 1.00 [0.16, 1.00] | 1.00 [0.59, 1.00] | – | – | 0.33 [0.01, 0.91] |
| Mannheimia haemolytica | 0.20 [0.01, 0.72] | – | 0.43 [0.10, 0.82] | – | – | 0.33 [0.01, 0.91] |
| Staphylococcus aureus | 0.20 [0.01, 0.72] | – | – | 0.33 [0.01, 0.91] | 0.33 [0.01, 0.91] | – |
| CoNS6 | 0.20 [0.01, 0.72] | 0.50 [0.01, 0.99] | 0.29 [0.04, 0.71] | 0.67 [0.09, 0.99] | 0.67 [0.09, 0.99] | 0.67 [0.09, 0.99] |
| S. capitis | – | – | 0.14 [0.00, 0.58] | – | – | – |
| S. chromogenes | 0.20 [0.01, 0.72] | – | – | – | – | – |
| S. epidermidis | – | – | – | – | – | 0.33 [0.01, 0.91] |
| S. equorum | 0.20 [0.01, 0.72] | 0.5 [0.01, 0.99] | – | 0.67 [0.09, 0.99] | – | 0.33 [0.01, 0.91] |
| S. vitulinus | – | – | – | – | 0.33 [0.01, 0.91] | – |
| S. xylosus | – | – | 0.14 [0.00, 0.58] | – | 0.33 [0.01, 0.91] | – |
| Streptococcusspp. | 0.20 [0.01, 0.72] | – | 0.14 [0.00, 0.58] | – | – | 0.33 [0.01, 0.91] |
| Str. gallolyticus | 0.20 [0.01, 0.72] | – | 0.14 [0.00, 0.58] | – | – | 0.33 [0.01, 0.91] |
PENN, ewes received an intramuscular injection of penicillin at weaning.
FAST, ewes had restricted feed access 48 h prior to and 72 h post-weaning.
COMBO, ewes were administered penicillin at weaning and had restricted feed access 48 h prior to and 72 h post-weaning.
Only bacteria genera or species identified at a frequency > 0.05 within weaning treatment by day are presented.
Bacillus spp. identified include B. altitudinis, B. atrophaeus, B. cereus, B. circulans, B. clausii, B. licheniformis, B. megaterium, B. pumilus, B. safensis, B. subtilis, and B. thuringiensis.
CoNS, coagulase-negative staphylococci.
Antimicrobial susceptibility of select isolates
Overall antimicrobial susceptibility of S. aureus, CoNS, and Streptococcus spp. is displayed in Figure 2. The S. aureus isolates (n = 20) were largely susceptible to ceftiofur, erythromycin, and penicillin at low concentrations of each antimicrobial compound, though 55% of isolates were resistant against tetracycline and 65% of isolates were resistant against sulfadimethoxine. Coagulase-negative staphylococci isolates (n = 87) were largely susceptible to ceftiofur, penicillin, and tetracycline, though some isolates exhibited resistance against erythromycin (40%) or sulphadimethoxine (44%). Streptococci isolates (n = 18) were largely susceptible to ceftiofur, erythromycin, penicillin, and tetracycline, albeit 33% of isolates were resistant against sulfadimethoxine.
Figure 2.
Antimicrobial susceptibility of Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and Streptococcus spp. isolated from ewe milk samples collected at days 1, 2, and 28 post-partum, weaning (day 68 post-partum), and 3-d post-weaning against common antimicrobial compounds.
Across day of lactation, sulfadimethoxine susceptibility of CoNS varied by day (P = 0.03), where greater resistance was observed in isolates cultivated from post-weaning milk samples (no. isolates = 9; Figure 3). Statistical tendencies were observed for day of lactation to effect CoNS isolate susceptibility of ceftiofur, erythromycin, and tetracycline and S. aureus isolate susceptibility of erythromycin (P ≤ 0.10).
Figure 3.
Antimicrobial susceptibility of select ewe milk-derived bacterial isolates against various antimicrobial compounds.
Across milk samples collected during early lactation (days 1 and d 2), jug bedding treatment affected CoNS isolate susceptibility towards sulfadimethoxine (P = 0.03) where isolates collected from SOILED jugs (no. of isolates = 19) had greater resistance than isolates collected from CLEAN jugs (no. of isolates = 33; Figure 3). A statistical tendency was observed for jug bedding treatment to effect streptococci isolate susceptibility of sulfadimethoxine (P = 0.10).
Weaning treatment affected S. aureus isolate resistance against tetracycline (P = 0.03) in milk samples collected around weaning, where all PENN isolates were resistant and all FAST isolates were susceptible at a low concentration (Figure 3). Additionally, penicillin resistance of CoNS was not impacted by weaning treatment (P = 0.40), and all S. aureus and Streptococcus spp. isolates had a minimum inhibitory concentration (MIC) equal to 0.12. In post-weaning milk sample isolates (n = 4), statistical tendencies were observed for S. aureus susceptibility to erythromycin and tetracycline where PENN isolates had greater resistance to each antimicrobial compound (P = 0.08).
Discussion
Prevalence of subclinical mastitis
Overall, the present data show a generally moderate incidence of subclinical mastitis throughout lactation, including at days 1, 2, and 28 as well as around weaning. These results are generally lower than the high prevalence of subclinical mastitis in flocks at Montana State University (Bozeman, MT) and the U.S. Sheep Experiment Station (Dubois, ID) from milk samples collected around days 1–5 and 30–45 (Knuth et al., 2021). Still, this previous report had a wide range (11%–74%) of subclinical mastitis prevalence within flock and year and the research group used a less strict definition of subclinical mastitis (≥ 1 colony of any type). The present data also show a variable prevalence (22%–66%) within the UW flock. Others have identified much lower prevalence of subclinical mastitis in non-dairy flocks using culture-based diagnostics (17% in Maisi et al., 1987; 14% in Watson et al., 1990; 12% in Watkins et al., 1991; 22%–25% in Ahmad et al., 1992; 29% in Arsenault et al., 2008; 30% in Persson et al., 2017). These differences could partially be attributed to differing definitions of subclinical mastitis, as past researchers have used values ≥ 1 (Knuth et al., 2021) to ≥ 5 colonies (Arsenault et al., 2008; Dore et al., 2016; Persson et al., 2017; van den Crommenacker-Konings et al., 2021) to ≥ 10 colonies (Fragkou et al., 2014). In the present study, we used the growth of ≥ 1 S. aureus, CoNS, or M. haemolytica colony or ≥ 5 colonies of other bacteria species in a pure or mixed culture to define subclinical mastitis. There are likely to be many additional factors that affect the prevalence of subclinical mastitis, including geographic region and presence of pathogenic bacteria in the environment, animal husbandry practices, facility management, parity, litter size, breed, and previous mastitis episode. However, in the present study, there was neither a significant jug bedding treatment effect nor weaning treatment effect on the prevalence of subclinical mastitis in early or late lactation.
In the present study, greater CMT scores were generally observed around weaning than in early or mid lactation, and there was a tendency for the relationship between CMT score and subclinical mastitis or S. aureus, which is considered a major pathogen of mastitis. Though CMT scores are subjective, they provide a quick on-farm test and are used to diagnose subclinical mastitis in a cost effective and practical manner. In non-dairy sheep, the use of CMT scores has been assessed with sensitivity and specificity metrics. Generally, the threshold for subclinical mastitis falls between 0 and 2 with inconsistent sensitivity and specificity measures ranging from 9% to 88% (Maisi et al., 1987; Persson et al., 2017). Although some groups have identified CMT to be correlated to intramammary infection, lysosomal N-acetyl-β-d-glucosaminidase (NAGase) or lamb weaning weights (Maisi et al., 1987; Arsenault et al., 2008; Persson et al., 2017), the inconsistency across studies has led us and others to conclude CMT is not the most reliable tool to assess subclinical mastitis in ewes (Maisi et al., 1987; Keisler et al., 1992).
Etiological agents of subclinical mastitis
The most commonly isolated and identified bacteria from ewe milk were CoNS (59%), Bacillus spp. (35%), Mannheimia haemolytica (10%), S. aureus (8%), Streptococcus spp. (5%), and Corynebacterium spp. (5%). These bacteria are routinely identified in culture-based investigations of ovine subclinical mastitis. Although bacilli are commonly found in the environment and soil, they are also often detected in milk and teat microbial communities using both culture-dependent and culture-independent techniques (Arsenault et al., 2008; Fotou et al., 2011; Bhatt et al., 2012; Braem et al., 2013; Knuth et al., 2021). In cases of ovine clinical mastitis, Arsenault et al. (2008) identified Bacillus spp. in 3% of milk samples. Much greater frequencies of Bacillus spp. isolation have been identified in ovine subclinical mastitis, ranging between 6% and 29% (Arsenault et al., 2008; Fotou et al., 2011; Knuth et al., 2021). These results suggest that environmental bacteria such as bacillus may be associated with mastitis cases after the bacteria gain entry to the mammary gland, especially in immuno-compromised animals. Therefore, bacilli should not be always regarded as an environmental contaminant in cases of subclinical mastitis. Coagulase-negative staphylococci are common members of the skin and mucus membrane microbial communities and considered to be common (29%–71%) in non-dairy ewe subclinical mastitis (Clements et al., 2003; Hariharan et al., 2004; Arsenault et al., 2008). The frequency of CoNS differed by day of lactation in the present study, though frequencies followed no trend. Streptococci commonly inhabit the respiratory and urogenital tracts, and they have been isolated at variable frequencies in non-dairy ewes with subclinical mastitis, ranging from 1% to 42% (Watkins et al., 1991; Arsenault et al., 2008; Persson et al., 2017). Mannheimia haemolytica are common members of the respiratory tract but can also be a pathogen of pneumonia and mastitis (Watkins et al., 1991; Clements et al., 2003; Arsenault et al., 2008). In the present study, M. haemolytica frequency differed by parity, with multiparous ewes having a much greater frequency than primiparous ewes. This effect could result from multiparous ewes having reared more litters and thus more frequent exposure to M. haemolytica since lambs carry this specie in their mouth and respiratory tract and transfer the bacteria to the teat when they suckle, leading to a lessened immune response in the mammary gland (Scott and Jones, 1998). Staphylococcus aureus are found on the skin and in the environment and are among the most common mastitis-causing bacteria in dairy ewes (Lafi et al., 1998; Al-Majali and Jawabreh, 2003; Mørk et al., 2007) though are variable in non-dairy ewe subclinical mastitis (8%–40%; Watson et al., 1990; Watkins et al., 1991; Arsenault et al., 2008). In livestock, Corynebacterium spp. are most commonly associated with infection of the limbs, abdomen, and lymph nodes in sheep, cattle, and horses and have been identified in relatively few cases of non-dairy ewe subclinical mastitis (3%–5%; Watson et al., 1990; Watkins et al., 1991; Arsenault et al., 2008), which agree with our results. In the present study, neither jug bedding treatment nor weaning treatment effects were observed on the prevalence of these commonly isolated bacteria in ovine subclinical mastitis. With more animal microbiologists utilizing both culture-dependent and culture-independent methods to better characterize microbial communities, some cultivable pathogens are appearing to be normal members of the microbiomes of various organ systems. This may include M. haemolytica, which is common in the respiratory tract microbiome but also a causative agent of pneumonia and other respiratory diseases. Similar findings of pathogenic bacteria occurring in healthy animals have been reported in the dairy cow and ewe milk microbiomes (Oikonomou et al., 2014; Esteban-Blanco et al., 2020). This has notable repercussions as culture-independent methods improve genus-level identifications and more microbiomes are investigated in veterinary medicine for diagnostics of subclinical states of diseases.
Antimicrobial susceptibility of staphylococci and streptococci isolates
Antimicrobial compounds including cefapirin, pirlimycin, erythromycin, and penicillin are commonly used to treat livestock mastitis and other diseases both prophylactically and therapeutically (Sawant et al., 2005; Raymond et al., 2006). Animal health is more easily monitored in dairy systems with intensive management where caretakers handle animals several times per day than in extensive and semi-extensive sheep production systems in the Western United States. With the overutilization of antimicrobial compounds, concerns of antibiotic resistant strains of bacteria impacting human health care via the food chain and environmental pollution have arisen (Skovgaard, 2007; Thanner et al., 2016). Additionally, surveys of public opinion of consumers in the United States, Italy, and Germany show that the average consumer is wary of antibiotic use in livestock and believe their usage affects human health (Busch et al., 2020). Persuaded by consumer demands, some food companies are mandating their suppliers to source food products with restrictive production standards (i.e., “cage-free eggs,” “antibiotic-free meat,” “non-GMO”; Hobbs, 2019).
Researchers continue to investigate antimicrobial susceptibility of microbes related to livestock production. In dairy cattle systems, resistance against antimicrobial compounds has varied by compound and bacterium isolated from milk: 0%–57% for CoNS, 0%–40% for S. aureus, and 0%–68% for streptococci (Owens et al., 1997; Guérin-Faublée et al., 2003; Poutrel et al., 2018). Antimicrobial resistance levels for staphylococci isolates in sheep and goat milk vary by antimicrobial compound: 14%–43% against penicillin, 41%–43% against ampicillin, 7%–50% against tetracycline, 5%–6% against erythromycin, and 7%–22% against streptomycin (Concepción Porrero et al., 2012; Ergün et al., 2012; Azara et al., 2017). In the present study, S. aureus, CoNS, and streptococci isolates were generally susceptible to many antimicrobial compounds, though some resistance was observed against penicillin (18% for CoNS, 5% for streptococci), tetracycline (55% for S. aureus, 11% for CoNS, 50% for streptococci), erythromycin (40% for CoNS), and sulfadimethoxine (65% for S. aureus, 44% for CoNS, 33% for streptococci). These levels of antimicrobial resistance are generally similar to those reported in small ruminant dairies, though some differences exist within antimicrobial compound. These data contrast with differences in antimicrobial resistance between beef and dairy cattle operations. Greater resistance against penicillin and sulfathiazone was recorded in beef systems, whereas greater resistance against streptomycin, oxytetracycline, amoxicillin, and tetracycline was observed in dairy systems (Parveen et al., 2006). This study further illustrates other factors affecting types and levels of antimicrobial resistance, including geographic location, species, and season.
Our study is among the few investigating antimicrobial susceptibility in ewe milk bacteria and may be the first published research highlighting antimicrobial susceptibility in non-dairy ewe milk bacteria. Animal husbandry and facility management are also likely to impact levels of antimicrobial resistance, though our data show few differences across jug bedding treatment or weaning treatment groups. Jug bedding treatment only affected CoNS susceptibility against sulfadimethoxine and weaning treatment affected S. aureus susceptibility against tetracycline. Within the UW flock, antimicrobial compounds are not routinely used unless severe clinical signs of mastitis or respiratory diseases are observed and antimicrobial resistance is likely to be higher in sheep production systems that liberally treat livestock with antimicrobial compounds.
Conclusion
Subclinical mastitis is prevalent throughout lactation and can be associated with decreased ewe and lamb performance leading to reduced profits in wool- and meat-type ewes. Our data show that many potentially pathogenic bacteria are commonly isolated in ewe milk and some milk-derived bacterial isolates show resistance to antimicrobial compounds that are not extensively administered in this flock. As animal-product sources continue to restrict conventional livestock production practices within the United States and globally, farmers and ranchers must adapt their production systems to abide by new rules and evolving consumer preferences, likely with limited use of antimicrobial compounds as prophylactic and therapeutic treatments. Alternatives to antimicrobial compounds may allow farmers and ranchers to turn to more sustainable means of managing animal health and welfare, including improved cleaning of animal facilities and holistic/alternative veterinary medicine. Thus, future research is warranted to judiciously employ antimicrobial agents in the livestock industry and to identify effective methods to mitigate mastitis and other diseases to ensure animal health and welfare while maximizing efficient and effective production.
Acknowledgments
We thank Kalli Koepke (UW Assistant Farm Manager—Sheep Unit Manager) and the staff at the Sheep Unit of University of Wyoming for helping with animal care and management. Funding for this research was provided by the National Sheep Industry Improvement Center.
Glossary
Abbreviations
- ADF
acid detergent fiber
- CoNS
coagulase-negative staphylococci
- CP
crude protein
- DM
dry matter
- GMO
genetically modified organism
- HCCA
α-cyano-4-hydroxycinnamic acid
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MIC
minimum inhibitory concentration
- NAGase
lysosomal N-acetyl-β-d-glucosaminidase
- NDF
neutral detergent fiber
- TDN
total digestible nutrients
- TSA
Trypticase soy agar
Contributor Information
Ryan M Knuth, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Kelly L Woodruff, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Gwendolynn L Hummel, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Jordan D Williams, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Kathleen J Austin, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Whitney C Stewart, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Hannah C Cunningham-Hollinger, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Bledar Bisha, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Conflict of Interest Statement
Mention of commercial products was solely stated to provide specific information and does not imply recommendation. The authors confirm that the research was conducted in the absence of commercial or financial relationships that could have influenced the outcome of this study.
Literature Cited
- Aaron, D. K., D G. E., Deweese W. P., Fink E., and Gatke C. R.. . 1997. Reducing milk production in ewes at weaning using restricted feeding and methscopolamine bromide. J. Anim. Sci. 75:1434–1442. doi: 10.2527/1997.7561434x. [DOI] [PubMed] [Google Scholar]
- Ahmad, G., Timms L. L., Morrical D. G., and Brackelsberg P. O.. . 1992. Dynamics and significance of ovine subclinical intramammary infections and their effects on lamb performance. Sheep Res. J. 8:25–29. [Google Scholar]
- Al-Majali, A. M., and Jawabreh S.. . 2003. Period prevalence and etiology of subclinical mastitis in Awassi sheep in southern Jordan. Small Rumin. Res 47:243–248. doi: 10.1016/S0921-4488(02)00259-6. [DOI] [Google Scholar]
- American Sheep Industry Association Inc. 2015. SID: Sheep Production Handbook. ADS/Nightwing Publishing, Fort Collins, CO. [Google Scholar]
- Arsenault, J., Dubreuil P., Higgins R., and Bélanger D.. . 2008. Risk factors and impacts of clinical and subclinical mastitis in commercial meat-producing sheep flocks in Quebec, Canada. Prev. Vet. Med. 87:373–393. doi: 10.1016/j.prevetmed.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Azara, E., Piras M. G., Parisi A., and Tola S.. . 2017. Antimicrobial susceptibility and genotyping of Staphylococcus aureus isolates collected between 1986 and 2015 from ovine mastitis. Vet. Microbiol. 205:53–56. doi: 10.1016/j.vetmic.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Bhatt, V. D., Ahir V. B., Koringa P. G., Jakhesara S. J., Rank D. N., Nauriyal D. S., Kunjadia A. P., and Joshi C. G.. . 2012. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 112:639–650. doi: 10.1111/j.1365-2672.2012.05244.x. [DOI] [PubMed] [Google Scholar]
- Binns, S. H., Cox I. J., Rizvi S., and Green L. E.. . 2002. Risk factors for lamb mortality on UK sheep farms. Prev. Vet. Med. 52:287–303. doi: 10.1016/S0167-5877(01)00255-0. [DOI] [PubMed] [Google Scholar]
- Braem, G., De Vliegher S., Verbist B., Piessens V., Van Coillie E., De Vuyst L., and Leroy F.. . 2013. Unraveling the microbiota of teat apices of clinically healthy lactating dairy cows, with special emphasis on coagulase-negative staphylococci. J. Dairy Sci. 96:1499–1510. doi: 10.3168/jds.2012-5493. [DOI] [PubMed] [Google Scholar]
- Busch, G., Kassas B., Palma M. A., and Risius A.. . 2020. Perceptions of antibiotic use in livestock farming in Germany, Italy and the United States. Livest. Sci 241:104251. doi: 10.1016/j.livsci.2020.104251. [DOI] [Google Scholar]
- Clements, A. C. A., Taylor D. J., and Fitzpatrick J. L.. . 2003. Evaluation of diagnostic procedures for subclinical mastitis in meat-producing sheep. J. Dairy Res. 70:139–148. doi: 10.1017/S0022029903006022. [DOI] [PubMed] [Google Scholar]
- Concepción Porrero, M., Hasman H., Vela A. I., Fernández-Garayzábal J. F., Domínguez L., and Aarestrup F. M.. . 2012. Clonal diversity of Staphylococcus aureus originating from the small ruminants goats and sheep. Vet. Microbiol. 156:157–161. doi: 10.1016/j.vetmic.2011.10.015. [DOI] [PubMed] [Google Scholar]
- van den Crommenacker-Konings, L. W. J. H., van Dam P., Everts R., Shittu A., Nielen M., Lam T. J. G. M., and Koop G.. . 2021. Dynamics of intramammary infections in suckler ewes during early lactation. J. Dairy Sci. 104:5979–5987. doi: 10.3168/jds.2020-19865. [DOI] [PubMed] [Google Scholar]
- Dario, C., Laudadio V., Corsalini T., Bufano G., and Buonavoglia C.. . 1996. Subclinical mastitis in sheep occurrence, etiology, and milk production in different genetic types. Agr Mediterr. 126:320–325. [Google Scholar]
- Dorai-Raj, S. 2014. binom: binomial confidence intervals for several parameterizations. Available from: https://CRAN.R-project.org/package=binom
- Dore, S., Liciardi M., Amatiste S., Bergagna S., Bolzoni G., Caligiuri V., Cerrone A., Farina G., Montagna C. O., Saletti M. A., . et al. 2016. Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rumin. Res 141:91–93. doi: 10.1016/j.smallrumres.2016.07.010. [DOI] [Google Scholar]
- Ergün, Y., Aslantaş O., Kireçci E., Öztürk F., Ceylan A., and Boyar Y.. . 2012. Antimicrobial susceptibility, presence of resistance genes and biofilm formation in coagulase negative staphylococci isolated from subclinical sheep mastitis. Kafkas Univ. Vet. Fak. Derg. 18:449–456. doi: 10.9775/kvfd.2011.5643. [DOI] [Google Scholar]
- Esteban-Blanco, C., Gutiérrez-Gil B., Puente-Sánchez F., Marina H., Tamames J., Acedo A., and Arranz J. J.. . 2020. Microbiota characterization of sheep milk and its association with somatic cell count using 16s rRNA gene sequencing. J. Anim. Breed. Genet. 137:73–83. doi: 10.1111/jbg.12446. [DOI] [PubMed] [Google Scholar]
- Fotou, K., Tzora A., Voidarou C., Alexopoulos A., Plessas S., Avgeris I., Bezirtzoglou E., Akrida-Demertzi K., and Demertzis P. G.. . 2011. Isolation of microbial pathogens of subclinical mastitis from raw sheep’s milk of Epirus (Greece) and their role in its hygiene. Anaerobe 17:315–319. doi: 10.1016/j.anaerobe.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Fragkou, I. A., Boscos C. M., and Fthenakis G. C.. . 2014. Diagnosis of clinical or subclinical mastitis in ewes. Small Rumin. Res. 118:86–92. doi: 10.1016/j.smallrumres.2013.12.015. [DOI] [Google Scholar]
- Fthenakis, G. C., and Jones J. E. T.. . 1990. The effect of experimentally induced subclinical mastitis on milk yield of ewes and on the growth of lambs. Br. Vet. J. 146:43–49. doi: 10.1016/0007-1935(90)90075-E. [DOI] [PubMed] [Google Scholar]
- Guérin-Faublée, V., Carret G., and Houffschmitt P.. . 2003. In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Vet. Rec. 152:466–471. doi: 10.1136/vr.152.15.466. [DOI] [PubMed] [Google Scholar]
- Hariharan, H., Donachie W., Macaldowie C., and Keefe G.. . 2004. Bacteriology and somatic cell counts in milk samples from ewes on a Scottish farm. Can. J. Vet. Res. 68:188–192. [PMC free article] [PubMed] [Google Scholar]
- Hobbs, J. E. 2019. Heterogeneous consumers and differentiated food markets: Implications for quality signaling in food supply chains. Can. J. Agric. Econ. Can. Agroeconomie. 67:237–249. doi: 10.1111/cjag.12202. [DOI] [Google Scholar]
- Hothorn, T., Bretz F., Westfall P., Heiberger R. M., Schuetzenmeister A., and Scheibe S.. . 2021. multcomp: simultaneous inference in general parametric models. Available from: https://CRAN.R-project.org/package=multcomp [DOI] [PubMed]
- Kahn, C. M., and Line S.. . 2010. The Merck veterinary manual. Whitehouse Station, NJ. [Google Scholar]
- Keisler, D. H., Andrews M. L., and Moffatt R. J.. . 1992. Subclinical mastitis in ewes and its effect on lamb performance. J. Anim. Sci. 70:1677–1681. doi: 10.2527/1992.7061677x. [DOI] [PubMed] [Google Scholar]
- Knuth, R. M., Stewart W. C., Taylor J. B., Bisha B., Yeoman C. J., Van Emon M. L., and Murphy T. W.. . 2021. Relationships among intramammary health, udder and teat characteristics, and productivity of extensively managed ewes. J. Anim. Sci. 99:1–14. doi: 10.1093/jas/skab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafi, S. Q., Al-Majali A. M., Rousan M. D., and Alawneh J. M.. . 1998. Epidemiological studies of clinical and subclinical ovine mastitis in Awassi sheep in northern Jordan. Prev. Vet. Med. 33:171–181. doi: 10.1016/S0167-5877(97)00048-2. [DOI] [PubMed] [Google Scholar]
- Maisi, P., Junttila J., and Seppänen J.. . 1987. Detection of subclinical mastitis in ewes. Br. Vet. J 143:402–409. doi: 10.1016/0007-1935(87)90017-0. [DOI] [PubMed] [Google Scholar]
- Makovec, J. A., and Ruegg P. L.. . 2003. Results of milk samples submitted for microbiological examination in Wisconsin from 1994 to 2001. J. Dairy Sci. 86:3466–3472. doi: 10.3168/jds.S0022-0302(03)73951-4. [DOI] [PubMed] [Google Scholar]
- McCarthy, F. D., Lindsey J. B., Gore M. T., and Notter D. R.. . 1988. Incidence and control of subclinical mastitis in intensively managed ewes. J. Anim. Sci. 66:2715. doi: 10.2527/jas1988.66112715x. [DOI] [PubMed] [Google Scholar]
- Mørk, T., Waage S., Tollersrud T., Kvitle B., and Sviland S.. . 2007. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Vet. Scand. 49:1–8. doi: 10.1186/1751-0147-49-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.), ed. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and New World camelids. National Academies Press, Washington, D.C. [Google Scholar]
- Oikonomou, G., Bicalho M. L., Meira E., Rossi R. E., Foditsch C., Machado V. S., Teixeira A. G. V., Santisteban C., Schukken Y. H., and Bicalho R. C.. . 2014. Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS One 9(1):e85904. doi: 10.1371/journal.pone.0085904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, W. E., C. H.Ray.,Watts J. L., and Yancey R. J.. . 1997. Comparison of success of antibiotic therapy during lactation and results of antimicrobial susceptibility tests for bovine mastitis. J. Dairy Sci. 80:313–317. doi: 10.3168/jds.S0022-0302(97)75940-X. [DOI] [PubMed] [Google Scholar]
- Parveen, S., Lukasik J., Scott T. M., Tamplin M. L., Portier K. M., Sheperd S., Braun K., and Farrah S. R.. . 2006. Geographical variation in antibiotic resistance profiles of Escherichia coli isolated from swine, poultry, beef and dairy cattle farm water retention ponds in Florida. J. Appl. Microbiol. 100:50–57. doi: 10.1111/j.1365-2672.2005.02773.x. [DOI] [PubMed] [Google Scholar]
- Persson, Y., Nyman A. K., Söderquist L., Tomic N., and Waller K. P.. . 2017. Intramammary infections and somatic cell counts in meat and pelt producing ewes with clinically healthy udders. Small Rumin. Res. 156:66–72. doi: 10.1016/j.smallrumres.2017.09.012. [DOI] [Google Scholar]
- Petridis, I. G., and Fthenakis G. C.. . 2019. Mammary involution and relevant udder health management in sheep. Small Rumin. Res. 181:66–75. doi: 10.1016/j.smallrumres.2019.07.001. [DOI] [Google Scholar]
- Pol, M., and Ruegg P. L.. . 2007. Relationship between antimicrobial drug usage and antimicrobial susceptibility of gram-positive mastitis pathogens. J. Dairy Sci. 90:262–273. doi: 10.3168/jds.S0022-0302(07)72627-9. [DOI] [PubMed] [Google Scholar]
- Poutrel, B., Bareille S., Lequeux G., and Leboeuf F.. . 2018. Prevalence of mastitis pathogens in France: antimicrobial susceptibility of Staphylococcus aureus, Streptococcus uberis, and Escherichia coli. J. Vet. Sci. Technol. 09:4–7. doi: 10.4172/2157-7579.1000522. [DOI] [Google Scholar]
- Raymond, M. J., Wohrle R. D., and Call D. R.. . 2006. Assessment and promotion of Judicious antibiotic use on dairy farms in Washington State. J. Dairy Sci. 89:3228–3240. doi: 10.3168/jds.S0022-0302(06)72598-X. [DOI] [PubMed] [Google Scholar]
- RStudio Team. 2021. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. Available from: http://www.rstudio.com/ [Google Scholar]
- Saratsis, P., Alexopoulos C., Tzora A., and Fthenakis G. C.. . 1999. The effect of experimentally induced subclinical mastitis on the milk yield of dairy ewes. Small Rumin. Res. 32:205–209. doi: 10.1016/S0921-4488(98)00189-8. [DOI] [Google Scholar]
- Sawant, A. A., Sordillo L. M., and Jayarao B. M.. . 2005. A survey on antibiotic usage in dairy herds in Pennsylvania. J. Dairy Sci. 88:2991–2999. doi: 10.3168/jds.S0022-0302(05)72979-9. [DOI] [PubMed] [Google Scholar]
- Schalm, O. W., and Noorlander D. O.. . 1957. Experiments and observations leading to the development of the California mastitis test. J. Am. Vet. Med. Assoc. 130:199–204. [PubMed] [Google Scholar]
- Scott, M. J., and Jones J. E. T.. . 1998. The carriage of Pasteurella haemolytica in sheep and its transfer between ewes and lambs in relation to mastitis. J. Comp. Pathol. 118:359–363. doi: 10.1016/S0021-9975(07)80011-9. [DOI] [PubMed] [Google Scholar]
- Shiels, D., Loughrey J., Dwyer C. M., Hanrahan K., Mee J. F., and Keady T. W. J.. . 2022. A survey of farm management practices relating to the risk factors, prevalence, and causes of lamb mortality in Ireland. Animals 12:30. doi: 10.3390/ani12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgaard, N. 2007. New trends in emerging pathogens. Int. J. Food Microbiol. 120:217–224. doi: 10.1016/j.ijfoodmicro.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Thanner, S., Drissner D., and Walsh F.. . 2016. Antimicrobial resistance in agriculture. F. Baquero, editor. mBio 7(2): e02227–15. doi: 10.1128/mBio.02227-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T. M., Lumley T., Atkinson E., and Crowson C.. . 2021. Survival: survival analysis. Available from: https://CRAN.R-project.org/package=survival
- Torres-Hernandez, G., and Hohenboken W.. . 1979. Genetic and environmental effects on milk production, milk composition and mastitis incidence in crossbred ewes. J. Anim. Sci. 49:410–417. doi: 10.2527/jas1979.492410x. [DOI] [PubMed] [Google Scholar]
- Watkins, G. H., Burriel A. R., and Jones J. E. T.. . 1991. A field investigation of subclinical mastitis in sheep in southern England. Br. Vet. J 147:413–420. doi: 10.1016/0007-1935(91)90083-Y. [DOI] [PubMed] [Google Scholar]
- Watson, D. L., Franklin N. A., Davies H. I., Kettlewell P., and Frost A. J.. . 1990. Survey of intramammary infections in ewes on the New England Tableland of New South Wales. Aust. Vet. J. 67:6–8. doi: 10.1111/j.1751-0813.1990.tb07381.x. [DOI] [PubMed] [Google Scholar]
- Whiteside, W. H. 1939. Observations on a new test for the presence of mastitis in milk. Can. Public Health J. 30:24–58. [Google Scholar]