Abstract
The relationship between nutrients leached onto the leaf surface and the colonization of plants by bacteria was studied by measuring both the abundance of simple sugars and the growth of Pseudomonas fluorescens on individual bean leaves. Data obtained in this study indicate that the population size of epiphytic bacteria on plants under environmentally favorable conditions is limited by the abundance of carbon sources on the leaf surface. Sugars were depleted during the course of bacterial colonization of the leaf surface. However, about 20% of readily utilizable sugar, such as glucose, present initially remained on fully colonized leaves. The amounts of sugars on a population of apparently identical individual bean leaves before and after microbial colonization exhibited a similar right-hand-skewed distribution and varied by about 25-fold from leaf to leaf. Total bacterial population sizes on inoculated leaves under conditions favorable for bacterial growth also varied by about 29-fold and exhibited a right-hand-skewed distribution. The amounts of sugars on leaves of different plant species were directly correlated with the maximum bacterial population sizes that could be attained on those species. The capacity of bacteria to deplete leaf surface sugars varied greatly among plant species. Plants capable of supporting high bacterial population sizes were proportionally more depleted of leaf surface nutrients than plants with low epiphytic populations. Even in species with a high epiphytic bacterial population, a substantial amount of sugar remained after bacterial colonization. It is hypothesized that residual sugars on colonized leaves may not be physically accessible to the bacteria due to limitations in wettability and/or diffusion of nutrients across the leaf surface.
Aerial plant surfaces are usually colonized by large numbers of microorganisms. Although some of these microorganisms may not multiply after their arrival on plants (30), many saprophytic and pathogenic microorganisms are capable of growth on healthy leaves, where they can reach large population sizes (9, 12, 19, 33). In order for microbial colonization to occur, a carbon source for energy generation and growth, a nitrogen source, and certain essential inorganic molecules must be present on leaves. Exogenous nutrient sources, such as aphid honeydew and pollen, have been associated with a dramatic increase in the microbial carrying capacities of some leaves (12, 40). However, in the common absence of such obvious and abundant nutrient sources, plants are still usually colonized by high numbers of bacteria, which can reach 105 to 107 CFU per g of leaf under favorable environmental conditions, such as when high relative humidity or free water is present (16, 17, 33). This indicates that nutrients released from the plant to its intact surfaces are adequate to support large microbial populations. Molecules leached from plant leaves include a variety of organic and inorganic compounds, such as sugars, organic acids, amino acids, methanol, and various salts (5, 8, 10, 34, 38, 41). The abundance of such nutrients can vary with plant species, leaf age, and growing conditions (5, 10, 34, 38, 41).
The depletion of nutrients from the leaf surface by microorganisms has been demonstrated. For example, yeasts can reduce the abundance of aphid honeydew on wheat leaves (9, 12). Bacteria were also shown to remove radiolabeled amino acids and sugars from the leaf surface (6, 7, 35). This suggests that microorganisms growing on plant surfaces could be competing for a limited amount of nutrients, which in turn would determine the microbial carrying capacity of the leaf. Depending on the system studied, carbon compounds alone or both carbon and nitrogen compounds were shown to be limiting factors for bacterial and yeast populations on leaves (2, 9, 44, 45). Furthermore, Wilson et al. (43) have shown that plants genetically modified to produce opines supported higher population sizes of opine-utilizing microorganisms than nonmodified plants. Likewise, the application of salicylic acid selectively increased the population size of salicylate-utilizing bacterial strains on leaves (42). Such results suggest that microbial populations of plants could be manipulated by changing the nutrient status of the plant surface. This has many implications, especially for the biological control of foliar plant pathogens, where nutrient competition is a likely mechanism of inhibition of pathogens which have a saprophytic growth phase or require a nutrient source to infect plants (6, 11–13).
A notable feature of epiphytic bacterial populations is their variation in size on different leaves of the same plant species. The population sizes of total epiphytic bacteria vary by over 10-fold from one leaf to another, even when leaves of a given species of identical appearance and similar age are examined (16–18, 20, 21). The population sizes of individual bacterial species among such a collection of leaves can vary by over 1,000-fold (16–21). More importantly, even when grown under similar environmental conditions, different plant species support greatly different total epiphytic bacterial populations (24, 31, 33). The factors that lead to such great differences in epiphytic population sizes have not been elucidated. If epiphytic bacterial communities are limited in size by the presence of available and utilizable carbon and/or nitrogen sources, as suggested by recent studies (2, 9, 43–45), then it might be expected that the availability of utilizable nutrient sources would vary greatly among leaves of the same plant and also among leaves of different plant species. There has been no detailed examination of the variation in leaf surface nutrient availability among leaves that would enable us to determine its contribution to epiphytic bacterial populations. No study has quantitatively investigated the relationship between nutrients leached from a leaf and microbial colonization of that leaf. In this study we test the hypothesis that the availability of major utilizable carbon sources, such as sugars, on leaves determines the population size of epiphytic bacteria. As a test of this hypothesis we investigated the relationship between the abundance of sugars on individual leaves of well-fertilized bean plants and bacterial population size during and after colonization. The availability of sugar on plant species differing in the number of cells of Pseudomonas fluorescens that they could support was also determined in order to ascertain whether the abundance of such nutrients was associated with differential colonization potential.
MATERIALS AND METHODS
Plant materials.
The plant species used in this study are listed in Table 1. All plants were grown in a greenhouse in 15-cm-diameter plastic pots containing UC-Mix, as described in a previous study (33). In the cases of tomato and tobacco, a single plant was grown in each pot, while five bean, corn, or pea plants and two or three cucumber plants were grown in a single pot. Microbial colonization and leaf surface sugars on leaves of a particular age were analyzed, as described in Table 1. The plants were fertilized daily with a modified Hoaglands nutrient solution as in previous studies (33). The temperature in the greenhouse was maintained between 18 and 22°C at night and between 20 and 26°C during the day.
TABLE 1.
Plant material used in this study
| Plant species | Leaves used | Growth stage |
|---|---|---|
| Bean (Phaseolus vulgaris L. cv. Bush Blue Lake) | Primary leaves | First trifoliate leaf emerging |
| Corn (Zea mays L. cv. Paymaster) | Third leaf | Fourth leaf emerging |
| Cucumber (Cucumis sativus L. cv. Slice Master) | First true leaf | Second true leaf emerging |
| Pea (Pisum sativum L. cv. Alaska) | Second and third leaves | Plants with four or five leaves |
| Tobacco (Nicotiana tabacum L. cv. Turk) | Leaf 4 or 5 | Plants with seven leaves |
| Tomato (Lycopersicon esculentum Mill. cv. Large Red Cherry) | Terminal leaflet from leaf 4 or 5 | Plants with six leaves |
Bacterial colonization of leaves.
Plants were sprayed with a suspension (106 cells/ml) of P. fluorescens A506 (46) and then enclosed in plastic bags to maintain 100% relative humidity and free moisture on the leaves. A subset of the leaves were stained by topical application of acridine orange shortly after inoculation, and bacteria were visualized by epifluorescence microscopy. Bacterial cells were quite uniformly present on all parts of the leaf, and every field of view revealed several bacterial cells. Yeast cells or fungal spores or hyphae were never observed on these greenhouse-grown plants. In experiments to assess the possible nitrogen limitation of bacterial growth on leaves, bacterial cells were suspended in a solution (2.0 g/liter) of ammonium sulfate before inoculation. The plants were kept on a laboratory bench at about 21°C during the course of the experiment. At each sampling time, 10 to 15 primary leaves of bean were removed randomly from among 10 replicate pots. A similar inoculation and incubation strategy with different numbers of potted plants was used for other plant species.
Quantification of sugars on leaf surfaces.
Pure water (ca. 50 to 300 μl/leaf) was lightly sprayed to wet the surfaces of 50 to 100 leaves 5 min before they were washed. The leaves were detached from the plant and immediately washed individually over a petri dish with a small volume of water (0.7 to 1.5 ml, depending on leaf size) and a Pasteur pipette. Care was taken not to touch the leaf either with a hand or with the pipette. The water was repeatedly applied until all parts of both sides of the leaf had been thoroughly exposed several times to the gentle flow of water. The washings were filter-sterilized (0.22-μm pore size) and evaporated to dryness under vacuum at 20°C. The dry samples were stored at −10°C until sugar analysis was performed. Sugars in the samples were analyzed by high-performance liquid chromatography (HPLC) with a Carbo Pac PA1 analytical column connected to an ED40 pulse amperimetric detector (Dionex Corporation, Sunnyvale, Calif.). An NaOH concentration gradient (30 to 90 mM) was used as an eluent, with a run time of 22 min and a flow rate of 1.0 ml/min. These conditions allowed the resolution of monosaccharides and sucrose. The abundance of sugars was determined by integrating the peak area and interpolating the measured peak area to that corresponding to known amounts of sugars determined separately in each experiment. Sugar abundance was expressed as glucose equivalents; the area under each peak was considered equivalent to the quantities from an equal amount of glucose.
Measurement of bacterial population sizes on leaves.
From 50 to 100 leaves were placed individually in 50-ml tubes containing 20 ml of washing buffer (33), and the tubes were sonicated for 7 min and then vortexed for 20 s, as in a previous study (33). An aliquot of 50 μl of the leaf washing was then plated on King's B medium (KB) with a spiral diluter and plater (model D; Spiral Systems, Inc., Bethesda, Md.) to estimate the total bacterial population. Some samples were also plated onto KB containing 100 μg of rifampin ml−1 to estimate the population of strain A506. The medium was amended with cycloheximide (100 μg ml−1) and benomyl (50 μg ml−1) in order to prevent fungal growth. The plates were incubated for 48 h at 28°C, and the bacterial colonies were then counted. When both sugar abundance and bacterial population size were determined on the same leaf, the sugars were first washed off the leaves as described above before the leaves were sonicated. In order to estimate the total bacterial population size on such leaves, a 10-μl aliquot of the initial washing liquid was also plated on KB before filtration. The numbers of bacteria found in the initial washings and from the subsequently sonicated leaves were then summed to estimate the total bacterial population size of an individual leaf.
Statistical methods.
All statistical calculations were performed with the SAS program (version 6.03) (SAS Institute Inc., Cary, N.C.). Analysis of variance, regression, and correlation analyses were done on log-transformed estimates of population size (log CFU per gram) or estimates of sugar abundance (micrograms of sugar/gram of leaf). The goodness of fit of the normal distribution to the log-transformed, square root-transformed, and nontransformed estimates of bacterial populations and measurements of sugar abundance on individual leaves was tested by the Shapiro-Wilk W statistic or the Kolomogorov D statistic by using the univariate procedure in SAS.
RESULTS AND DISCUSSION
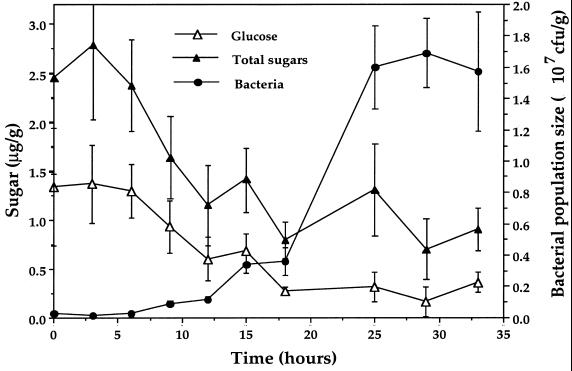
The abundance of glucose and other sugars was measured on leaves both before and during the process of colonization by P. fluorescens A506 to determine the extent to which available sugars are depleted during bacterial multiplication on plants. Averages of about 2.5 μg of total sugar and 1.4 μg of glucose were detected per g of uncolonized bean leaves (Fig. 1). Glucose, fructose, and sucrose were the predominant sugars detected, although small amounts of other sugars, such as galactose and an unknown sugar with a retention time of 15 min, were also occasionally detected. After inoculation with P. fluorescens A506 and incubation under moist conditions, there was a rapid reduction in the amounts of sugars on the leaf surface, concomitant with bacterial growth (Fig. 1). The amount of sugars remaining on the leaf surface decreased to about 0.25 μg/g of leaf after 20 h and changed little afterward, while the total bacterial population reached and maintained a size of about 1.7 × 107 CFU/g (Fig. 1); over 90% of the total bacteria on the inoculated leaves were strain A506. A similar depletion of glucose alone was observed (Fig. 1).
FIG. 1.
Changes in amounts of glucose and total sugars, as well as bacterial population sizes, on the surfaces of moist bean leaves at various times after inoculation with P. fluorescens A506. The vertical bars represent the standard errors of the mean of estimates of sugars or bacterial populations.
In one experiment in which bacterial colonization was allowed to continue for 4 days, the available glucose on the leaves also decreased upon bacterial multiplication from about 1.4 to about 0.2 μg/g (about 15% of the initial abundance on uncolonized plants) during the first 24 h without further change during the remaining time of the experiment (data not shown). Such a substantial decrease in nutrient abundance should make a highly colonized leaf an unfavorable colonization site for newly arrived microbial propagules, in comparison to a less colonized leaf which is likely richer in nutrients. The preemptive exclusion by pseudomonads of pathogenic or ice-nucleating bacteria on leaves and other plant surfaces (26–29, 36, 44, 46) is most easily explained by nutrient resource depletion by initial colonists of the leaf surface. The finding that 80% or more of the nutrients potentially available to epiphytes on bean leaves are depleted by the growth of P. fluorescens (Fig. 1) supports the model of resource depletion in preemptive competitive exclusion (26–29, 44, 46).
A study of the distribution of amounts of leaf surface sugars before and after bacterial colonization as well as bacterial carrying capacity in a population of bean leaves was done to evaluate the relationship between the two variables. Each set of bean plants was divided into two subsets. In the first subset, 100 leaves were washed to quantify the amount of sugars on their surfaces in the absence of substantial bacterial colonization. In the other subset, the maximum total bacterial population size on individual leaves, as well as the amount of sugars remaining on the leaves, was evaluated after the plants were colonized by P. fluorescens for 48 h. The total epiphytic bacterial population size on uninoculated leaves was only about 103 cells/g. In the population of uncolonized bean leaves, 65% had amounts of total sugars (glucose, fructose, and sucrose combined) that were less than 4 μg/g (Fig. 2). However, 25% of the leaves had amounts higher than 6 μg/g, and some harbored as much as 12 μg/g. Because some of the leaves had much higher amounts of sugars than the median, the distribution of total sugars in the population of leaves was strongly right hand skewed (Fig. 2). The abundance of sugars among the population of uncolonized bean leaves (Fig. 2) was best described by a log-normal distribution (W = 0.96; P < 0.12). The total epiphytic bacterial population size on inoculated leaves that were incubated until bacterial populations had reached a high and stable size ranged from 2 × 105 to 5.8 × 106 CFU/g (Fig. 3); over 95% of these bacteria were strain A506, and very few fungi were detected. The frequency distribution of total epiphytic bacterial populations among these leaves also was strongly right hand skewed (19–21) and was also described by a log-normal distribution (W = 0.93; P < 0.001). Colonized leaves that supported large epiphytic population sizes were markedly depleted in sugar, with 73% having less than 1 μg of total sugar/g remaining (Fig. 4). Interestingly, 16% of colonized leaves still had 3 μg/g or more remaining. On some leaves, there was as much as 8 to 10 μg of sugar/g remaining, resulting in a strongly right-hand-skewed distribution (Fig. 4). The abundance of sugar on the collection of colonized bean leaves was best described by the log-normal distribution (W = 0.79; P < 0.001). Similar distributions of bacterial populations and sugar abundances on colonized leaves were observed in replicate experiments (data not shown).
FIG. 2.
Distribution of total amounts of sugars on the surfaces of a population of individual bean leaves in the absence of bacterial colonization. The combined amounts of monosaccharides and disaccharides washed from individual leaves and measured by HPLC are shown.
FIG. 3.
Distribution of total bacterial population sizes in a population of individual bean leaves. The plants were inoculated with P. fluorescens A506 and incubated under moist conditions for 48 h until high and stable bacterial population sizes were reached.
FIG. 4.
Distribution of total amounts of sugars on the surfaces of a population of bean leaves after bacterial colonization. The plants were inoculated with P. fluorescens A506 and incubated for 48 h under moist conditions. The sums of monosaccharides and disaccharides washed from individual leaves and measured by HPLC are shown.
The amount of sugars on the surfaces of bean leaves of the same age, physical environment, and genotype clearly varied greatly in this study (Fig. 2), as did bacterial populations in this and other greenhouse and field studies (14, 15, 17, 21, 24). It is noteworthy that the range of amounts of sugar measured on a collection of different leaves (25-fold) also closely matched the range of total bacterial population size observed on similar leaves after colonization (28-fold) (Fig. 2 and 3). Although it is not possible to say that the leaves that supported the largest bacterial population sizes originally harbored the highest amount of nutrients, it could be expected that this would be the case. A direct test of this model is not possible, unfortunately, since the sampling of nutrients and bacterial populations is inherently destructive and thus does not allow the measurement of the initial amount of sugar and subsequent bacterial population sizes on the same leaf.
Since leaves of different plant species support different numbers of epiphytic bacteria (33), the maximum epiphytic-bacterial population size achieved on leaves of various species was compared with the amounts of sugars initially present on the leaves before colonization by bacteria. Differences in the total amounts of sugars found on leaves of various plant species, as well as the abilities of those leaves to support bacterial growth, were observed (Fig. 5). Pea and corn, which had the lowest amounts of leaf surface sugar, also had lower bacterial carrying capacities in comparison to bean and tomato, which harbored the highest amounts of sugars. There was a significant relationship between the amount of sugar detected on leaves before colonization and the maximum bacterial population size attained on the plant species (Fig. 5). Thus, again, the initial sugar abundance on an uncolonized leaf seems to determine the subsequent total bacterial population size that it can support. The population size of a particular bacterial species can vary by over 100,000-fold from one leaf to another (18–20, 24). It seems unlikely that the 15-fold variation in major nutrients, such as sugars, seen in our study could account for such high levels of variation, if they are typical of other systems. Instead, it seems more likely that other factors, such as patterns of immigration and subsequent competition among a diverse bacterial microflora for limiting resources and/or differential tolerance of environmental stresses, lead to the great variation in abundance of particular bacterial species on a collection of plants. Also, plants differed greatly in the amounts of sugars that were removed during bacterial colonization (Table 2). A relatively higher percentage of leaf surface sugars was depleted by bacterial growth on species such as bean and tomato, which initially harbored larger amounts of sugar than plants such as pea or corn, which had little leaf surface sugar (Table 2). For example, only 28 and 25% of the sugar originally present on bean and tomato, respectively, remained after bacterial colonization, while little reduction in sugar abundance occurred on pea and corn (Table 2).
FIG. 5.
Relationship between initial amounts of total sugars on uncolonized leaves of various plant species and the bacterial population achieved (2 days after inoculation with P. fluorescens A506) on leaves of each of these plant species. (A) Experiment 1. The line represents the linear regression y = −4.1176 × 107 + 8.4741 × 107 X (R2 = 0.94; P = 0.01). (B) Experiment 2. Data are from Table 2. The line represents the linear regression y = −2.5525 × 105 + 1.4583 × 107 X (R2 = 0.75; P = 0.01).
TABLE 2.
Amounts of leaf surface sugars before and after bacterial colonization and capacity of various plant species to support bacterial growth
| Plant species | Bacterial carrying capacity (log CFU/g)a | Total sugars (μg/g)
|
|
|---|---|---|---|
| Before colonization | After colonization | ||
| Bean | 7.19 ± 0.04b | 1.08 ± 0.22 | 0.31 ± 0.06 |
| Corn | 6.29 ± 0.13 | 0.08 ± 0.02 | 0.07 ± 0.02 |
| Cucumber | 7.04 ± 0.04 | 1.38 ± 0.22 | 1.46 ± 0.19 |
| Pea | 5.81 ± 0.12 | 0.06 ± 0.02 | 0.14 ± 0.03 |
| Tomato | 7.48 ± 0.03 | 1.55 ± 0.38 | 0.40 ± 0.05 |
Bacterial carrying capacity is defined as the large and stable bacterial population size achieved after 2 days of incubation of plants under saturated relative humidity following inoculation with P. fluorescens A506.
Standard error of the mean.
The presence of residual sugars on leaves that are apparently fully colonized by bacteria suggests that complex temporal or spatial patterns of nutrient leakage onto the leaf may be occurring. While sugars are depleted on leaves during periods of bacterial growth, a substantial reservoir of sugars remains after growth ceases (Fig. 1 and Table 2). In some cases, these leaves can harbor sizable amounts of sugars, as much as 10 μg/g (Fig. 4). While sugars that cannot be catabolized by bacteria would be expected to remain on leaves after bacterial multiplication, we would not expect that glucose, a sugar readily used by P. fluorescens and most epiphytic bacteria, would remain on leaves if the sugar was freely diffusible on the leaf surface. It is clear that about 20% of the glucose persists on moist bean leaves despite extensive bacterial colonization following inoculation (Fig. 1). This residual level of glucose could be due to two main causes. First, it is possible that nutrients continually leak onto the leaf surface from the interior of the plant, preventing total depletion. Tukey et al. (39) showed that repeated washings of bean leaves over time could yield additional, although decreasing, amounts of sugars; such leaves never become completely depleted of nutrients. Purnell and Preece (34) showed that sugars were partially replaced on washed leaves of Brassica napus within 5 days after their initial removal. In plants incubated under saturated moisture conditions, the presence of free water on the leaves could contribute to the enhanced leaching of nutrients, which is a passive process and was shown to be associated with rain in the field (38). It seems unlikely that such continual efflux of sugar could account for the substantial pool of glucose on leaves, however. On moist bean leaves having high bacterial populations such efflux should be readily consumed, likely leading to continued bacterial multiplication. This was not observed here; bacterial populations reached a high and stable size (Fig. 1), as in other studies (4, 26, 29, 33, 44, 45). Another possibility, which does not exclude the first one, is that sites on the leaf suitable for bacterial colonization are not always coincident spatially with sites where sugars occur, making the sugars inaccessible to the bacteria and preventing their depletion. This possibility is supported by the data on the variable colonization of different plant species by bacteria (Fig. 5 and Table 2). On plant species capable of supporting high numbers of bacteria, the majority of sugar is apparently accessible to the bacteria and is largely depleted during bacterial colonization. On the other hand, on plants harboring much smaller maximum bacterial population sizes, the growth of the bacteria did not greatly affect the nutrient availability on the leaves (Table 2), as the nutrients were apparently less accessible to epiphytic bacteria. Possibly, the very waxy surface of those leaves prevents the nutrients from being available. Leaves have been shown to have very irregular surfaces, which in turn are unevenly colonized (3, 23, 25). We hypothesize that at the small scale at which bacterial colonization of plants occurs, leaves are not uniformly wetted and diffusion of nutrients is restricted on the leaf. The leaf thus might be considered to be “compartmentalized” in such a way that local sources of nutrients support only local communities of bacterial epiphytes.
This study further supports the concept that the growth of epiphytic bacteria on plants is limited by the amount of carbon-containing compounds rather than by other limiting factors, such as nitrogen availability. The maximum total bacterial population size on bean leaves (primarily strain A506) inoculated with P. fluorescens A506 with and without added ammonium sulfate was log 7.12 and 7.11 per g, respectively, and did not differ statistically. Similar results were obtained in replicate experiments (data not shown). These results confirm earlier studies (44, 45) and indicate that carbon compounds and not inorganic nitrogen are the limiting factor for bacterial growth on bean plants under the greenhouse growing conditions used in these experiments. In addition to the similarity in variability of nutrients and bacterial populations among leaves (Fig. 2 and 3), the finding that the amounts of both sugars and bacterial populations vary directly on different plant species (Fig. 5) also supports the model of nutrient-limited epiphytic bacterial populations. We can estimate the number of bacterial cells which could develop on leaves, provided that all the sugar that we have measured on the leaf surface is accessible to the bacteria and is transformed into bacterial biomass. The dry yield of P. fluorescens grown in batch culture is about 0.47 g of cell per g of glucose when provided as the sole carbon source (1). Thus, using this conversion factor, a leaf with 1.5 μg of glucose should yield 0.70 μg of dry bacterial cells, equivalent to about 3.5 μg of wet bacteria, assuming that the cells contain about 80% water. If the mass of a single wet P. fluorescens cell is 1.2 × 10−6 μg, then the yield of P. fluorescens cells from this leaf would be about 3.0 × 106 cells. Since the total amount of sugar is usually about twofold higher than the amount of glucose alone (Fig. 1), yields of P. fluorescens of ca. 4 × 106 to 8 × 106 cells per leaf could be expected from the total sugar present on leaves; this closely matches the measured population sizes on these leaves in our experiments (Fig. 1).
The availability of major carbon-containing compounds such as sugars would place constraints on the population size of epiphytic bacteria that could be attained on plants, assuming that the physical environment on leaves was not limiting. In light of these results, it appears that under conditions favorable for microbial growth, nutrients on the leaf surface are a major determinant of population sizes of bacteria. However, the availability of these nutrients varies greatly at the scale of entire leaves and probably at much smaller scales as well. As nutrient accumulation and microbial colonization are not static processes but probably occur discontinuously at a rate influenced by environmental factors (15–18, 37), the factors that influence the availability of nutrients on the leaf surface and, in turn, microbial populations may be rather complex. Further insight into the factors determining nutrient availability to epiphytic bacteria in situ will require the development of tools such as biological sensors (32) that are responsive to such compounds.
REFERENCES
- 1.Atkinson B, Mavituna F. Biochemical engineering and biotechnology handbook. 2nd ed. New York, N.Y: Stockton Press; 1991. pp. 115–167. [Google Scholar]
- 2.Bashi E, Fokkema N J. Environmental factors limiting growth of Sporobolomyces roseus on wheat leaves. Trans Br Mycol Soc. 1977;68:17–25. [Google Scholar]
- 3.Beattie G A, Lindow S E. The secret life of bacterial colonists of leaf surfaces. Annu Rev Phytopathol. 1995;33:145–172. doi: 10.1146/annurev.py.33.090195.001045. [DOI] [PubMed] [Google Scholar]
- 4.Beattie G A, Lindow S E. Survival, growth, and localization of epiphytic fitness mutants of Pseudomonas syringae on leaves. Appl Environ Microbiol. 1994;60:3790–3798. doi: 10.1128/aem.60.10.3790-3798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakeman J P. Effect of plant age on inhibition of Botrytis cinerea spores by bacteria on beetroot leaves. Physiol Plant Pathol. 1972;2:143–152. [Google Scholar]
- 6.Brodie I D S, Blakeman J P. Competition for exogenous substrates in vitro by leaf surface micro-organisms and germination of conidia of Botrytis cinerea. Physiol Plant Pathol. 1976;9:227–239. [Google Scholar]
- 7.Brodie I D S, Blakeman J P. Competition for carbon compounds by a leaf surface bacterium and conidia of Botrytis cinerea. Physiol Plant Pathol. 1975;6:125–135. [Google Scholar]
- 8.Corpe W A, Rheem S. Ecology of the methylotrophic bacteria on living leaf surface. FEMS Microbiol Ecol. 1989;62:243–250. [Google Scholar]
- 9.Dik A J, Fokkema N J, Van Pelt J A. Consumption of aphid honeydew, a wheat yield reduction factor, by phyllosphere yeasts under field conditions. Neth J Plant Pathol. 1991;97:209–232. [Google Scholar]
- 10.Fiala V, Glad C, Martin M, Jolivet E, Derridj S. Occurrence of soluble carbohydrates on the phylloplane of maize (Zea mays L.): variations in relation to leaf heterogeneity and position on the plant. New Phytol. 1990;115:609–615. [Google Scholar]
- 11.Fokkema N J. The role of saprophytic fungi in antagonism against Drechslera sorokiana (Helminthosporium sativum) on agar plates and on rye leaves with pollen. Physiol Plant Pathol. 1973;3:195–205. [Google Scholar]
- 12.Fokkema N J, Riphagen I, Poot R J, de Jong C. Aphid honeydew, a potential stimulant of Cochliobolus sativus and Septoria nodorum and the competitive role of saprophytic mycoflora. Trans Br Mycol Soc. 1983;81:355–363. [Google Scholar]
- 13.Fokkema N J, den Houter J G, Kroterman Y J C, Nelis A L. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans Br Mycol Soc. 1979;72:19–29. [Google Scholar]
- 14.Hirano S S, Rouse D I, Clayton M K, Upper C D. Pseudomonas syringae pv. syringae and bacterial brown spot of bean: a study of epiphytic phytopathogenic bacteria and associated disease. Plant Dis. 1995;79:1085–1093. [Google Scholar]
- 15.Hirano S S, Upper C D. Dynamics, spread, and persistence of a single genotype of Pseudomonas syringae relative to those of its conspecifics on populations of snap bean leaflets. Appl Environ Microbiol. 1993;59:1082–1091. doi: 10.1128/aem.59.4.1082-1091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano S S, Upper C D. Population biology and epidemiology of Pseudomonas syringae. Annu Rev Phytopathol. 1990;28:155–177. [Google Scholar]
- 17.Hirano S S, Upper C D. Diel variation in population size and ice nucleation activity of Pseudomonas syringae on snap bean leaflets. Appl Environ Microbiol. 1989;55:623–630. doi: 10.1128/aem.55.3.623-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano S S, Upper C D. Ecology and epidemiology of foliar plant pathogens. Annu Rev Phytopathol. 1983;21:243–269. [Google Scholar]
- 19.Hirano S S, Nordheim E V, Arny D C, Upper C D. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl Environ Microbiol. 1982;44:695–700. doi: 10.1128/aem.44.3.695-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishimaru C, Eskridge K M, Vidaver A K. Distribution analysis of naturally occurring epiphytic populations of Xanthomonas campestris pv. phaseoli on dry beans. Phytopathology. 1991;82:262–268. [Google Scholar]
- 21.Kinkel L, Wilson M, Lindow S E. Effects of sampling scale on the assessment of epiphytic bacterial populations. Microb Ecol. 1995;29:283–297. doi: 10.1007/BF00164891. [DOI] [PubMed] [Google Scholar]
- 22.Kinkel L, Lindow S E. Invasion, exclusion, and coexistence among intraspecific bacterial epiphytes. Appl Environ Microbiol. 1993;59:3447–3454. doi: 10.1128/aem.59.10.3447-3454.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinkel L L. Microbial population dynamics on leaves. Annu Rev Phytopathol. 1997;35:327–347. doi: 10.1146/annurev.phyto.35.1.327. [DOI] [PubMed] [Google Scholar]
- 24.Kinkel L L, Wilson M, Lindow S E. Utility of microcosm studies for predicting phylloplane bacterium population sizes in the field. Appl Environ Microbiol. 1996;62:3413–3423. doi: 10.1128/aem.62.9.3413-3423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Last F T. Factors associated with the distribution of some phylloplane microbes. Neth J Plant Pathol. 1970;76:140–143. [Google Scholar]
- 26.Lindemann J, Suslow T V. Competition between ice nucleation active wild-type and ice nucleation deficient deletion mutant strains of Pseudomonas syringae and P. fluorescens biovar I and biological control of frost injury on strawberry blossoms. Phytopathology. 1987;77:882–886. [Google Scholar]
- 27.Lindow S E. Control of epiphytic ice nucleation active bacteria for management of plant frost injury. In: Gusta L, Warren G, Lee R, editors. Biological ice nucleation and its applications. St. Paul, Minn: American Phytopathological Society Press; 1994. pp. 239–256. [Google Scholar]
- 28.Lindow S E. Competitive exclusion of epiphytic bacteria by Ice− mutants of Pseudomonas syringae. Appl Environ Microbiol. 1987;53:2520–2527. doi: 10.1128/aem.53.10.2520-2527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindow S E. Ecology of Pseudomonas syringae relevant to the field use of Ice− deletion mutants constructed in vitro for plant frost control. In: Halvorson H O, Pramer D, Rogul M, editors. Engineered organisms in the environment: scientific issues. Washington, D.C.: American Society for Microbiology; 1985. pp. 23–25. [Google Scholar]
- 30.Lindow S E, Andersen G A. Influence of immigration in establishing epiphytic bacterial populations on navel orange. Appl Environ Microbiol. 1996;62:2978–2987. doi: 10.1128/aem.62.8.2978-2987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindow S E, Arny D C, Upper C D. Distribution of ice nucleation active bacteria on plants in nature. Appl Environ Microbiol. 1978;36:831–838. doi: 10.1128/aem.36.6.831-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loper J E, Lindow S E. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl Environ Microbiol. 1994;60:1934–1941. doi: 10.1128/aem.60.6.1934-1941.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien R D, Lindow S E. Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology. 1989;79:619–627. [Google Scholar]
- 34.Purnell T J, Preece T F. Effects of foliar washing on subsequent infection of leaves of swede (Brassica napus) by Erysiphe cruciferarum. Physiol Plant Pathol. 1971;1:123–132. [Google Scholar]
- 35.Rodger G, Blakeman J P. Microbial colonization and uptake of 14C label on leaves of sycamore. Trans Br Mycol Soc. 1984;82:45–51. [Google Scholar]
- 36.Seidler R J, Walter M V, Hern S, Fieland V, Schmedding D, Lindow S E. Measuring the dispersal and reentrainment of recombinant Pseudomonas syringae at California test sites. Microb Releases. 1994;2:209–216. [Google Scholar]
- 37.Timmer L W, Marois J J, Achor D. Growth and survival of xanthomonads under conditions nonconducive to disease development. Phytopathology. 1987;77:1341–1345. [Google Scholar]
- 38.Tukey H B., Jr The leaching of substances from plants. Annu Rev Plant Physiol. 1970;21:305–324. [Google Scholar]
- 39.Tukey H B, Jr, Wittwer S H, Tukey H B. Leaching of carbohydrates from plant foliage as related to light intensity. Science. 1957;126:120–121. doi: 10.1126/science.126.3264.120-a. [DOI] [PubMed] [Google Scholar]
- 40.Warren R C. The effect of pollen on the fungal leaf microflora of Beta vulgaris L. and on infection of leaves by Phoma betae. Neth J Plant Pathol. 1972;78:89–98. [Google Scholar]
- 41.Wildman H G, Parkinson D. Seasonal changes in water-soluble carbohydrates on Populus tremuloides leaves. Can J Bot. 1981;59:862–869. [Google Scholar]
- 42.Wilson M, Lindow S E. Enhanced epiphytic coexistence of near-isogenic salicylate-catabolizing and non-salicylate-catabolizing Pseudomonas putida strains after exogenous salicylate application. Appl Environ Microbiol. 1995;61:1073–1076. doi: 10.1128/aem.61.3.1073-1076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson M, Savka M A, Hwang I, Farrand S K, Lindow S E. Altered epiphytic colonization of mannityl opine-producing transgenic tobacco plants by a mannityl opine-catabolizing strain of Pseudomonas syringae. Appl Environ Microbiol. 1995;61:2151–2158. doi: 10.1128/aem.61.6.2151-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson M, Lindow S E. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl Environ Microbiol. 1994;60:4468–4477. doi: 10.1128/aem.60.12.4468-4477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson M, Lindow S E. Ecological differentiation and coexistence between epiphytic Ice+Pseudomonas syringae strains and an Ice− biological control agent. Appl Environ Microbiol. 1994;60:3128–3137. doi: 10.1128/aem.60.9.3128-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson M, Lindow S E. Interactions between the biological control agent Pseudomonas fluorescens A506 and Erwinia amylovora in pear blossoms. Phytopathology. 1993;83:117–123. [Google Scholar]