Abstract
Cells may naturally proceed or be forced to transition to a state with a radically lower biological age, i.e. be rejuvenated. Examples are the conversion of somatic cells to induced pluripotent stem cells and rejuvenation of the germline with each generation. We posit that these processes converge to the same “ground zero”, the mid-embryonic state characterized by the lowest biological age where both organismal life and aging begin. It may also be related to the phylotypic state. The ground zero model clarifies the relationship between aging, development, rejuvenation, and de-differentiation, which are distinct throughout life. By extending the rejuvenation phase during early embryogenesis and editing the genome, it may be possible to achieve the biological age at the ground zero lower than that achieved naturally.
Keywords: ground zero, aging, rejuvenation, development, lifespan, biological age
The advent of rejuvenation
It has recently been realized that cells can be rejuvenated, i.e. they can naturally proceed to or be experimentally induced to transition to the states characterized by lower biological ages that their original states [1,2]. This understanding has the potential to transform what we know about the aging process and life itself. It is known that aging is malleable, as its long-term course may be adjusted by numerous genetic and environmental interventions, which can decelerate or accelerate the aging process. In addition, various studies established the regulation of the organismal biological age by metabolic manipulation, senescent cell ablation, immune interventions, and other approaches, i.e. by applying the strategies that target systems that globally affect the organismal state [1] (Fig. 1). Yet, all these approaches slow down aging or affect the biological age of the tested organ systems (e.g. as assessed by aging hallmarks [3] or biomarkers [4]) only marginally, and their effects on other organ systems are typically not assessed. Moreover, for most of these approaches, the long-term effects on healthspan (see Glossary) and lifespan remain unknown. Thus, although these approaches are extremely important and they may somewhat decrease the biological age, they represent fragmentary or partial rejuvenation strategies.
Fig. 1. Partial and complete rejuvenation.
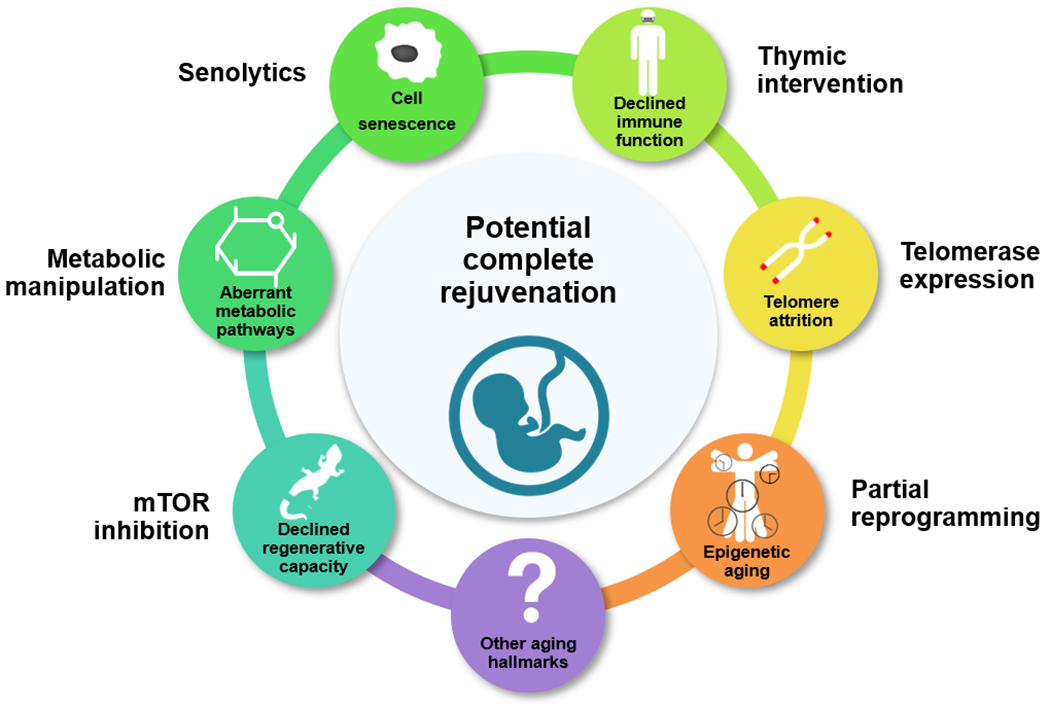
At advanced ages, aging is associated with several hallmarks (shown by 7 circles), which may be targeted by certain candidate interventions (shown next to each circle), thereby slightly reducing the biological age of an organism. However, since these approaches affect only some pathways, cells or organ systems, without affecting others, they may only lead to fragmentary or partial rejuvenation. In contrast, complete rejuvenation (shown by a large circle in the middle of the figure) should reset the age of all cells and systems, such that the organism becomes essentially indistinguishable from the same organism in the much younger state.
On the other hand, complete rejuvenation may be defined by an exhaustive reset of every age-related feature of a cell or organism, so that they become essentially indistinguishable from those of younger cells or organisms. This age reset represents the transition that is conceptually (but not necessarily mechanistically) opposite to the aging process and is not limited to a reversal of a single or few parameters (e.g. protein activity, metabolite level or gene expression) or aging hallmarks (e.g. DNA damage, epigenetic alterations, telomere attrition, protein aggregation or accumulation of aberrant mitochondria). If a parameter, molecule or cell state change with age, this change may lead to a multiplicity of possible effects: (i) damaging when they result in a by-product of metabolism, mutation, or a posttranslational modification that alters protein function; (ii) protective when they lead to an increased expression of stress-response system components, such as damage repair; or (iii) neutral when their changes bear no functional consequences [5]. Therefore, it is difficult to infer the functional impacts of age-related changes merely from the fact that they occur. On the other hand, a complete rejuvenation is a biological process of turning back time that involves the reset of all age-related changes. Rejuvenation is also the process that distinguishes living organisms from inanimate objects, e.g. mechanical systems such as cars and gadgets irreversibly age, whereas life continually renews itself with each generation.
In this Opinion piece, we propose the ground zero model of aging. We will first build the model, which is based mainly on in vitro data as applied to organismal aging and rejuvenation, and then on this backdrop discuss the relation between development, aging, and rejuvenation. We will further consider the origin of the ground zero and its relationship to other embryonic models and finally discuss rejuvenation strategies to reset or lower the ground zero state.
Aging and rejuvenation of germline and somatic cells
It is often discussed that, since the germline is immortal, it does not age [6,7]; this notion dates to the 19th century, when August Weismann proposed the separation of ageless germline and aging soma. However, at the time of conception, the contributing germline has typically been maintained in a metabolically active state for two or more decades and must have accumulated damage, such as metabolic by-products, epimutations and modified irreplaceable proteins. In other words, it is biologically older compared to its earlier, embryonic state. While the germline biological age at the time of conception is expected to be much younger than that of somatic tissues of the same organism, and while some of the accumulated damage may be removed by designated molecular systems, rejuvenation in the pre-zygotic state could only be partial, as in the absence of cell division (as in the oocyte) there are always more damage forms than the means of protecting against them [24]. Also, while some germ cells may accumulate more damage than others and therefore may lead to early mortality and abnormalities in the offspring (this damage will also increase with the age of the host), all germ cells unavoidably accumulate some damage. Thus, for the new life to begin in the same young state as in the previous generation, the zygote must somehow remove this damage and decrease its biological age to the level of the germline age in the previous generation. In other words, it appears that the germline ages during development and adult life, and then it is rejuvenated in the offspring after conception.
The rejuvenation conceptualized above, i.e. the transition from a state characterized by a higher biological age to the state with lower biological age, is not limited to early embryogenesis. Yamanaka’s groundbreaking discovery of induced pluripotent stem cells (iPSCs) [8] has made it clear that somatic cells may also be rejuvenated. The conversion of somatic cells to iPSCs, which correspond to the state of embryonic stem cells, is accompanied by incremental cell heterogeneity, with many cells in the population acquiring different cell states and some becoming rejuvenated [6]. Mechanistic details of somatic rejuvenation are not fully clear, e.g. it is not known how damage is removed during this process, if it is a gradual and coordinated process, and if different damage forms are cleared according to their own temporal trajectories.
A model of the ground zero of organismal life
By convention, the age of a person is counted from the day he/she is born. But when does his/her aging begin? To consider this question, one needs to ask another question: when does organismal life begin? There are several common answers: conception, first neural activity, first heartbeat, first breath, or simply at birth. However, the beginning of organismal (as opposed to cellular) life at conception contradicts the observations that an early embryo (i) may be naturally or experimentally split generating two (i.e. twins) or more organisms [9]; (ii) can be combined with other embryos of the same species [10] and even with embryonic stem cells/iPSCs of other species [11] generating chimeric organisms; (iii) initially relies on maternal gene products rather than its own [12]; (iv) gradually extends telomeres (from early cleavage to blastocyst through a recombination-based mechanism and subsequently using telomerase) [13]; (v) gradually removes epigenetic marks [14]; (vi) decreases structural entropy [15]; (vii) gradually inactivates chromosome X and develops biased paternal/material monoallelic gene expression [16]; (viii) is unable to distinguish self-from non-self (its acquired immune system is formed later) [17]; and (ix) may become another individual by swapping its genetic material via somatic cell nuclear transfer. Together, this suggests gradual rejuvenation during early embryogenesis as opposed to the aging of the newly formed organism starting from zygote or early cleavage. However, the rejuvenation process is reversed at some point during development, wherein telomeres again begin to shorten, self-recognition is established, biological age starts to increase, etc. [12-17]. It is unclear if all these changes during early embryogenesis and their subsequent reversal closer to mid-embryogenesis occur simultaneously, gradually or in waves, or whether each follows its own temporal trajectory, as highly resolved measurements are currently lacking for many of them. However, the direction of changes is already clear from the current data.
All this leads to a model, wherein early embryos are gradually rejuvenated, e.g. by extending their telomeres, erasing epigenetic marks, clearing up and diluting molecular damage, etc., and this continues up to a particular time during early development. Conception represents a starting point for this process, culminating in the state of the lowest biological age, the ground zero of organismal life and aging (Fig. 2). In effect, the period from conception to this stage may be viewed as a preparatory stage associated with damage clearance and rejuvenation for subsequent development of the organism. This suggests that organismal life begins after this preparatory stage and is associated with the formation of the body plan, immune system, neural activity, etc. With regard to the beginning of life, this argument clearly distinguishes conception (beginning of cellular life) from the ground zero (beginning of organismal life).
Fig. 2. The ground zero model or organismal life and aging.
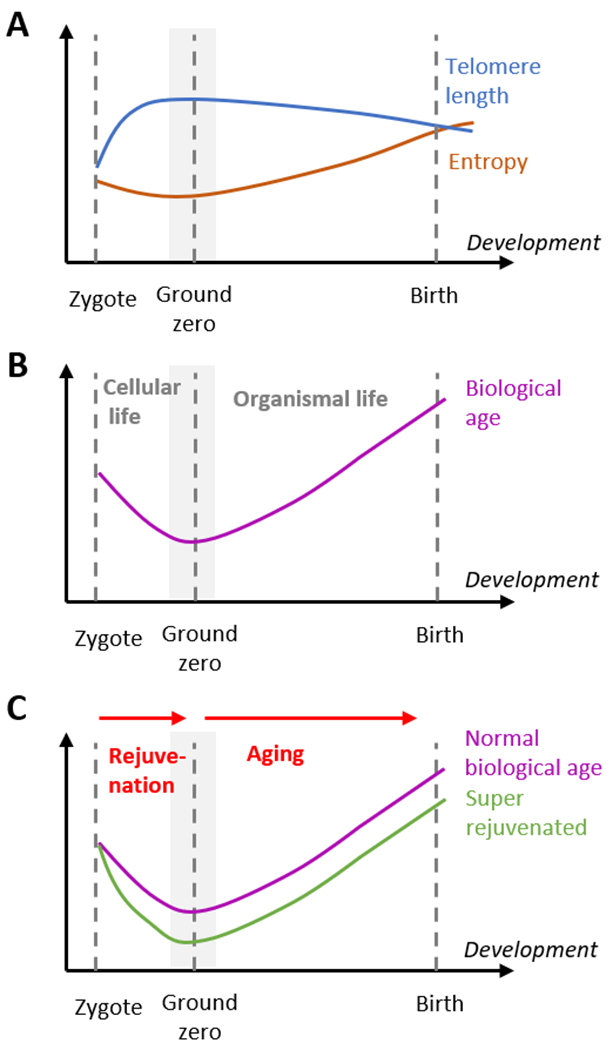
(A) Molecular features associated with aging are U-shaped. During early embryogenesis, telomeres are extended, and entropy is decreased, suggesting a transition from an older to a younger state, but after the inflection point, defined as the ground zero state (or period), their trajectories are reversed. The actual changes of features associated with aging during development may be more complex, e.g. be asynchronized or in waves. (B) Ground state as the beginning of organismal life and aging. The biological age of an organism decreases in early embryogenesis and increases starting at mid-embryogenesis. The ground zero corresponds to the lowest biological age of an organism. In this model, the zygote defines the beginning of cellular life, and the ground zero represents the beginning of organismal life. (C) The beginning of aging at mid-embryogenesis suggests approaches to achieve a lower biological age than that naturally possible. In the ground zero model, the period from the zygote to the ground zero represents rejuvenation, and aging begins at the ground zero. Extending or enhancing the rejuvenation period (as well as reducing the load of damaging mutations) may lead to a lower biological age (green) than naturally achieved (purple) at the ground zero, and therefore a reduced biological age throughout life, leading to an extended lifespan and healthspan.
One obvious exception from this principle (rejuvenation during early embryogenesis until a certain point during development) is the organismal genome. In contrast to the gradual acquisition of younger features during early embryogenesis, the genome is formed at conception and cannot be rejuvenated (i.e. mutations are irreversible). Instead, the genome is “rejuvenated” at the level of species, i.e. germline acquires mutations during the life of an organism, but post-conception the most deleterious genotypes are eliminated due to early-life mortality and decreased fitness during adult life [18]. In other words, purifying selection supports mutation-selection balance and may be viewed as rejuvenation of the genome at the level of species. Advances in genome editing [19] provide additional opportunities for rejuvenation of the genome by removing damaging mutations, which may be utilized when these tools further improve with regard to off-target effects [20].
Starting from the ground zero state, to begin a new life cycle, some cells of the organism need to (i) erase age-related (and also cell type-specific) somatic epigenetic patterns through a cell reprogramming process, generating primordial germ cells, (ii) establish sex-specific epigenetic patterns in these cells, which subsequently enable meiotic maturation and fertilization, (iii) remove these epigenetic patterns after fertilization, jumpstarting the developmental program [21], and (iv) establish the ground zero epigenetic patterns and beginning of a new life cycle. The specific solutions for these innovations differ among animals, whereas mid-embryogenesis, the timing that generally corresponds to the ground zero state is considered the most conserved state that characterizes metazoans that age, and even other eukaryotes [22]. It is known as the phylotypic state (Box 1).
Box 1. Relationship between the ground zero, phylotypic state, entropic minimum and the ancestral state of animals.
The notion of the ground zero reverberates with other embryonic models. At the level of gametes, zygotes, and early embryos, various vertebrates look very different, but during development they acquire a common state (phylotypic state), wherein they can be virtually indistinguishable from one another, and then they diverge again. In this hourglass model, the phylotypic state is defined by the expression of evolutionarily old genes and constrained variance in gene expression [42,43]. It is possible that the phylotypic state matches the ground zero state. Likewise, some metrics of entropy, such as structural entropy, may be lowest during mid-embryogenesis, and this might correspond to the lowest biological age. The period approximately related to the phylotypic state may also be associated with the loss of regeneration and the embryo-fetal transition [44]. Interestingly, mortality is also highest during early embryogenesis, wherein the species genome is rejuvenated by eliminating embryos inviable due to a combination of damaging alleles, which would be consistent with the use of genes with higher selection coefficients during this stage. Overall, all these milestones in embryonic life may converge to the same state, corresponding to the beginning of organismal life. This state may correspond to the ancestral state, perhaps ancestor of all animals, with innovations supported by new genes and functions extending to both before and after this state.
Relationship between aging, rejuvenation, and development
The ground zero model has several implications, and the one that should be addressed first is how aging, rejuvenation, and development are related to one another.
Aging and rejuvenation
The relationship between aging and rejuvenation is straightforward – they are essentially opposite to each other: the former makes an organism older, while the latter makes it younger. They are naturally linked by the ground zero, the timing when early life rejuvenation ends, and aging begins (Fig. 2), but experimentally (e.g. artificially in a test tube) they may be induced at other life stages. For example, somatic cells may be converted to iPSCs and thus be rejuvenated, whereas unfavorable environmental conditions (high oxygen, lack of nutrients, DNA damage) may lead to aging of the cells, which otherwise would not age. Mechanistically, aging and rejuvenation do not seem to proceed through the same trajectory in the opposite direction. The same mechanistic trajectory for these processes was famously conceived by Fitzgerald to describe the reverse aging of Benjamin Button [23], but this is not observed in real life.
Development and rejuvenation
Rejuvenation is also different from development. Development is a genetic program that begins at conception and ends roughly at the age 20 (although some developmental processes are completed earlier and some later), and its aim is to build a fit organism. Even during the period from conception to the ground zero, rejuvenation is different from development, as its essence is to remove damage and decrease the biological age rather than build an organism. After the ground zero, development is not opposite to rejuvenation, as disassembling what was built is not the same as decreasing the biological age.
Development and aging
Development and aging are different from each other in timing and purpose (although achieving a specific milestone during development may require a certain biological age of the cells). The former is a genetically programmed process (i.e. there are genes whose purpose is to support development) that begins at conception and ends with a functional adult organism. In contrast, aging begins at the ground zero and continues until an organism dies. Also, aging likely has no purpose, and there are no genes that evolved with the sole purpose to cause aging. Aging is a consequence of being alive, a by-product of metabolism that involves accumulation of age-related changes and proceeds from the ground zero state until the death of the organism.
Measuring biological age
Another issue intimately related to progression through aging and rejuvenation is the concept of biological age. Biological age is an integrative measure of deleterious changes that occur during organismal life [24]. When we look at a person, we can estimate his/her age rather accurately. But some people age slightly faster than the average person and some age slower. There are now molecular tools available to assess the biological age, mostly notably epigenetic clocks. The first such clocks were developed for humans [4,25,26], and more recently for mice [27-32]. Other clocks are based on gene expression, metabolite patterns and other features of cells and organisms. The transitions through aging and rejuvenation can be tracked by these biomarkers, and early embryogenesis is not an exception (Fig. 3). In fact, it was shown that the epigenetic clock developed for human adults [4] tracks the aging process as early as 45 days post-conception [33]. It is important to note that the basis for aging clock applications to study early embryogenesis lies in the method researchers used to build it. By performing regression toward the broad range of chronological ages as well as across tissues, the clock readout tracks the aging process. More specifically, the DNA methylation clock quantifies the levels of errors in the DNA methylome. In the reproduction and early embryogenesis, these epigenetic errors, along with other errors, are sufficiently decreased to ensure the normal lifespan of the next generation. The ground zero is the time when an embryo is expected to feature the most “perfect” epigenome naturally accessible, which can be quantified by the clock as the youngest epigenetic age. Therefore, these assays, preferably in combination with other assays, may be used to define the exact timing of the ground zero, which is currently unclear. It probably lies somewhere between blastocyst (as its inner cell mass may be divided without aging) and pharyngula (the most conserved state of vertebrate development). It may also be related to gastrulation -as famously stated by Lewis Wolpert: “It is not birth, marriage, or death, but gastrulation which is truly the most important time in your life” [34].
Fig. 3. Defining the ground zero by following the biological age using DNA methylation clocks.
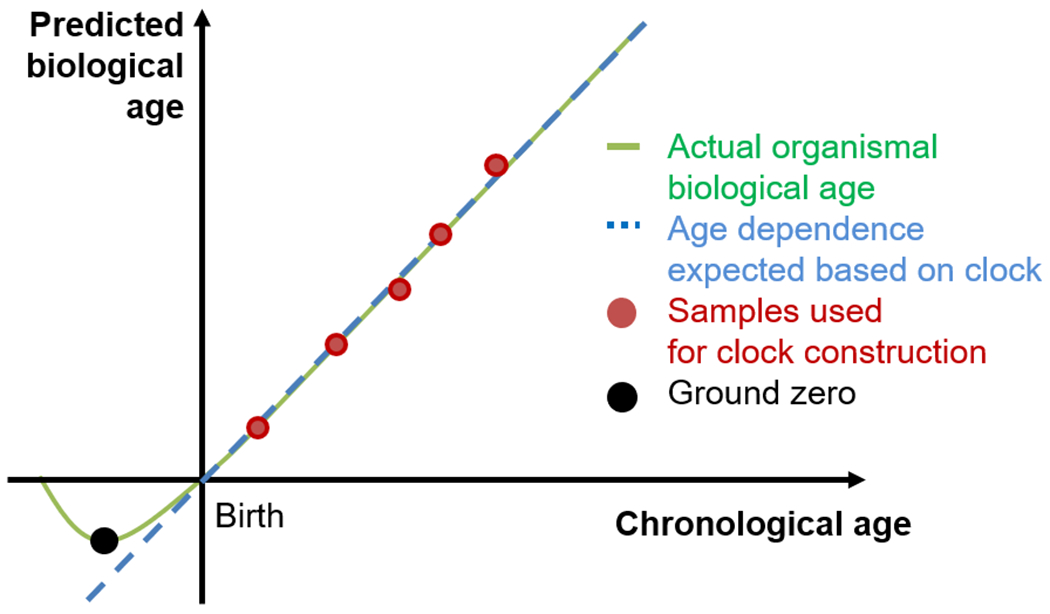
A hypothetical dependence of chronological age versus predicted biological age is shown. DNA methylation clocks trained based on the chronological age of samples (red dots) may track the aging process even before birth. Instead of the linear dependence (dashed blue line), a minimal biological age (green line) is expected, defined by the ground zero (black dot).
It is also possible that some processes are rejuvenated earlier during development, some later, and some in multiple bouts. This asynchronization can be illustrated by changes in DNA methylation, which is remodeled differently for paternal and maternal DNA during cleavage and is remodeled again later during embryonic development [14]. Likewise, it may be that some processes are rejuvenated prior to fertilization, at the onset of zygotic transcription, in the blastula or at onset of neurulation. In this sense, the ground zero state may be viewed as the ground zero period. The ground zero timing will also depend on methods used to assess age reversal of various cellular components. Perhaps, some integrative, entropy-like measure is needed to determine the lowest biological age during development.
Lowering the ground zero
Natural rejuvenation during early embryogenesis decreases the biological age (i.e. reduces damage, extends telomeres, rebuilds the epigenome, etc.) to a certain state sufficiently low to avoid damage overload before completion of development. There is no selective pressure to decrease it even further, as the damage would only be relevant much later in life when the organism already passed its genes to the next generation. Therefore, the biological age at the ground zero must be above zero. Moreover, it is increased by the existing genomic damage in the form of mutations and may be related to the primitive ancestor multi-cellular organisms, if not to simple cell aggregates, which must have lived at a certain biological age (Box 2). In addition, it is important to examine heritability of the biological age at the ground zero - variance is expected among different individuals within a population, between sexes and across species. An additional variance may be related to the method used to assess the ground zero age, e.g. DNA methylation age may be different from gene expression age or metabolome age.
Box 2. Origin of the ground zero.
Origin of the ground zero may be traced to pre-metazoan ancestor populations, which existed as patterned agglomerates of cells. The addition of earlier developmental stages, such as the egg, happened later in evolution and likely independently for various phyla [45]. Apparently, zygote cleavage, guided by cytoplasmic patterning, could generate the ancestor cell agglomerates. In addition, the earliest transcribed zygotic genes are short, newly evolved, and different across species [46]. This explains the diversity of egg shapes and strategies for early development, which converge to similar morphologies in mid-embryogenesis. It may be speculated that the ancestor agglomerates represented non-aging organisms, and that the addition of developmental phases preceding and following them coincided with the origin of aging in these organisms. In this regard, the ground zero state may be viewed as a non-aging state, which is consistent with the unlimited cell division potential of ES cells.
It may be possible to manipulate this rejuvenation process experimentally, achieving a lower ground zero age, so that organismal life begins at a lower biological age than it does naturally (Fig. 2). One idea is to extend the phase that is associated with rejuvenation. For example, early embryos may be bisected: a single blastomere of an 8-cell embryo can develop into an organism, whereas several 8-cell embryos can be combined into a single organism. Recurrent splitting of early embryos, i.e. separating blastomeres might alter the process of rejuvenation. Another approach may be to support cell division of the inner cell mass from blastocysts. In addition, it is known that primed and naïve iPSCs can transition to each other and differ in the biological age [35]. Finally, development of genome editing technologies may allow to decrease the genomic damage. Human genome on average have six ultra-rare highly damaging (protein-truncating) mutations, each accounting for a decrease of 6 months of life [36], and a large variation of such mutations could commonly affect lifespan. Their removal would decrease the genomic damage. The use of these techniques for heritable genome editing is currently restricted, but future advances may make this research possible [20].
I speculate that, if a lower ground zero is achieved, the organism whose organismal life begins at a lower biological age may be expected to maintain consistently lower damage than control organisms with the same chronological age. They may also have a better fitness and lower incidence of age-related diseases. Naturally, a super-rejuvenated organism might not have the phenotypic advantages at young ages, as lower biological age will have little impact during the early stages of life. However, this effect may be detected in later life in the form of a lower incidence of age-related diseases and reduced susceptibility to diseases.
Testing the ground zero model
How does the model fare against the existing experimental data? The direct proof is currently lacking, but there are several clues. For example, it was found, based on DNA methylation clock analyses, that the male germline ages [37] and thus the age must be reset after conception. Aging of the germline is also seen in C.elegans, e.g. in the form of protein aggregates. Interestingly, in the oocytes of hermaphrodite nematodes these aggregates are cleared in response to signals from sperm [38], suggesting that the preparatory stage for the ground zero may begin even before fertilization. It was also found that continuous growth and passaging of mouse inner cell mass-derived ES cells leads to an extension of their telomeres, suggesting a reduced biological age compared to the original ES cells. Remarkably, mice made from these passaged ES cells live longer, have lower cancer incidence, and improve glucose and insulin tolerance compared to the ES cells that were not passaged [39]. Conversion of fibroblasts to iPSCs is also associated with a reduced biological age, based on DNA methylation clocks [40], as was observed in both mice and humans. In addition, continuous culturing of iPSCs was fond to extend their telomeres [39]. Thus, reduction in biological age is associated with the dividing cells being in a particular metabolic state.
Concluding Remarks
One of most profound revelations from recent advances in science is that biological systems can be completely rejuvenated [41]. Indeed, just a few years ago, reversing the deleterious changes that accumulate with age in their entirety was simply unimaginable. Yet, we now know this is possible, whether we consider the conversion of somatic cells to iPSCs or the natural reversal of age of the germline with each generation. These two processes converge somewhere during early development at the point here proposed to be termed the ground zero. It is here that both organismal life and aging begin. The ground zero model extends and modifies the Weismann’s notion of heritable immortal germline and non-inheritable aging soma by positing that (i) both soma and germline can age; (ii) both soma and germline can be rejuvenated; (iii) soma and germline can be brought to a common state characterized by the lowest biological age, the ground zero; and (iv) age can be reversed without the need to have separate soma and germline. The proposed model is currently based on in vitro experiments and application of clocks to assess the biological age, and should be extended to experimental organismal biology. Understanding the nature and mechanisms of rejuvenation and defining the exact point of the ground zero and ways to manipulate the lowest age may provide opportunities for dramatic advances in human biology.
Acknowledgements
Supported by NIH grants. The author would like to thank Bohan Zhang for help with figures, and Bohan Zhang, Leonid Peshkin, Sergey Dmitriev, Csaba Kerepesi and Marc Kirschner for discussion.
Glossary
- Blastocyst
A multi-cellular embryonic structure formed during early development of mammals. It has the inner cell mass, which gives rise to an organism and is the source of embryonic stem cells, and the outer cell layer known as trophoblast, which gives rise to the placenta. In humans, blastocyst is formed approximately 5 days after conception, and two days later it implants into the endometrium in the uterus. Blastocysts are used in in vitro fertilization and generation of transgenic and chimeric animal models.
- Epimutations
Persistent aberrations that do not affect the DNA sequence but result in a change in DNA structure or gene expression. Examples are changes in DNA methylation and histone modification.
- Gastrulation
a phase in embryonic development, wherein a single-layer sphere of cells (blastula) is reorganized into a multilayer structure (gastrula)
- Healthspan
The period of life spent in good health, without chronic diseases and disabilities that impair a normal function of an organism. The exact meaning of this term is still being discussed in the research community.
- Induced pluripotent stem cells (iPSCs)
Pluripotent stem cells generated from different somatic cells. They are analogous to embryonic stem cells in that they can give rise to every other cell type in the body. They were discovered by Shinya Yamanaka, who prepared them by expressing four transcription factors, and are widely used in biology and regenerative medicine.
- Lifespan
A measure of how long an organism lives. The often-used categories of lifespan are the maximal lifespan (e.g. the age at which the last individual in a cohort dies) and mean lifespan.
- Pharyngula
A stage approximately in the middle of embryonic development of vertebrates. By this stage, all major organs are present. It follows blastula, gastrula and neurula.
- Phylotypic state
A mid-embryonic stage of a period during development, where embryos of different species within a phylum exhibit the highest morphological and molecular similarity. It is characterized by the expression of evolutionary oldest genes and genes under strongest purifying selection, and lower variance in gene expression among related species than during other stages of development.
- Zygote
A eukaryotic cell formed between two gametes upon a fertilization event. Its genome is a combination of the genomes of each gamete. In complex eukaryotes, the zygote marks the beginning of embryogenesis.
References
- 1.Mahmoudi S, Xu L, Brunet A (2019) Turning back time with emerging rejuvenation strategies. Nat Cell Biol. 21, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galkin F, Zhang B, Dmitriev SE, Gladyshev VN (2019) Reversibility of irreversible aging. Ageing Res Rev. 49, 104–114. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol. 14, R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Gladyshev VN (2020) How can aging be reversed? Exploring rejuvenation from a damage-based perspective. Genetics and Genomics Next, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiebinger G, Shu J, Tabaka M, Cleary B, Subramanian V, Solomon A, Gould J, Liu S, Lin S, Berube P, Lee L, Chen J, Brumbaugh J, Rigollet P, Hochedlinger K, Jaenisch R, Regev A, Lander ES (2019) Optimal-Transport Analysis of Single-Cell Gene Expression Identifies Developmental Trajectories in Reprogramming. Cell 176, 928–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepperdinger G (2009) Open-ended question: is immortality exclusively inherent to the germline? - A mini-review. Gerontology 55, 114–7. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 9.Hall JG (2003) Twinning. Lancet 362, 735–743. [DOI] [PubMed] [Google Scholar]
- 10.Moscona A (1957) The development in vitro and chimeric aggregates of dissociated embryonic chick and moues cells. Proc Natl Acad Sci USA 43, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Platero-Luengo A, Sakurai M, et al. (2017) Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 168, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peshkin L, Wühr M, Pearl E, et al. (2015) On the Relationship of Protein and mRNA Dynamics in Vertebrate Embryonic Development. Dev Cell 35, 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Bailey SM, Okuka M, et al. (2007) Telomere lengthening early in development. Nat Cell Biol. 9, 1436–1441. [DOI] [PubMed] [Google Scholar]
- 14.Smith ZD, Chan MM, Humm KC, et al. (2014) DNA methylation dynamics of the human preimplantation embryo. Nature 511, 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waites W, Davies JA. (2019) Emergence of structure in mouse embryos: Structural Entropy morphometry applied to digital models of embryonic anatomy. J Anat. 235, 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Q, Ramsköld D, Reinius B, Sandberg R. (2014) Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196. [DOI] [PubMed] [Google Scholar]
- 17.Golub ES and Green DR (1991) Immunology, a Synthesis, 2nd edition, Sunderland, MA: Sinauer. [Google Scholar]
- 18.Kinzina ED, Podolskiy DI, Dmitriev SE, Gladyshev VN (2019) Patterns of Aging Biomarkers, Mortality, and Damaging Mutations Illuminate the Beginning of Aging and Causes of Early-Life Mortality. Cell Rep. 29, 4276–4284. [DOI] [PubMed] [Google Scholar]
- 19.Cox DB, Platt RJ, Zhang F (2015) Therapeutic genome editing: prospects and challenges. Nat Med. 21, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander ES, Baylis F, Zhang F, et al. (2019) Adopt a moratorium on heritable genome editing. Nature 567, 165–168. [DOI] [PubMed] [Google Scholar]
- 21.Messerschmidt DM, Knowles BB, Solter D. (2014) DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 28, 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drost HG, Janitza P, Grosse I, Quint M. (2017) Cross-kingdom comparison of the developmental hourglass. Curr Opin Genet Dev. 45, 69–75. [DOI] [PubMed] [Google Scholar]
- 23.Scott Fitzgerald F (2008) The Curious Case of Benjamin Button and Other Jazz Age Stories. Penguin Classics. [Google Scholar]
- 24.Gladyshev VN (2016) Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 15, 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocklandt S, Lin W, Sehl ME, et al. (2011) Epigenetic predictor of age. PLoS One. 6, e14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannum G, Guinney J, Zhao L, et al. (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 49, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN (2017) Using DNA Methylation Profiling to Evaluate Biological Age and Longevity Interventions. Cell Metabolism 25, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meer MV, Podolskiy DI, Tyshkovskiy A, Gladyshev VN (2018) A whole lifespan mouse multi-tissue DNA methylation clock. eLife 7, e40675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Tsui B, Kreisberg JF, Robertson NA, Gross AM, Yu MK, Carter H, Brown-Borg HM, Adams PD, Ideker T (2017) Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 18, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbs TM, Bonder MJ, Stark AK, Krueger F; BI Ageing Clock Team, von Meyenn F, Stegle O, Reik W (2017) Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 18, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson MJ, Chwiałkowska K, Rubbi L, Lusis AJ, Davis RC, Srivastava A, Korstanje R, Churchill GA, Horvath S, Pellegrini M (2018) A multi-tissue full lifespan epigenetic clock for mice. Aging 10, 2832–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Lemos B (2019) Ribosomal DNA harbors an evolutionarily conserved clock of biological aging. Genome Res 29, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshino A, Horvath S, Sridhar A, Chitsazan A, Reh TA. (2019) Synchrony and asynchrony between an epigenetic clock and developmental timing. Sci Rep. 9, 3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpert L (2008) The triumph of the embryo. Courier Corporation. [Google Scholar]
- 35.Weinberger L, Ayyash M, Novershtern N, Hanna JH (2016) Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17, 155–69. [DOI] [PubMed] [Google Scholar]
- 36.Shindyapina AV, Zenin AA, Tarkhov AE, Santesmasses D, Fedichev PO, Gladyshev VN (2020) Germline burden of rare damaging variants negatively affects human healthspan and lifespan. eLife 9, e53449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins TG, Aston KI, Cairns B, Smith A, Carrell DT (2018) Paternal germ line aging: DNA methylation age prediction from human sperm. BMC Genomics 19, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohnert KA, Kenyon C. (2017) A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage Nature 551, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz-Lorente MA, Cano-Martin AC, Blasco MA (2019) Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat Commun. 10, 4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olova N, Simpson DJ, Marioni RE, Chandra T (2019) Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18, e12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Gladyshev VN (2020) How can aging be reversed? Exploring rejuvenation from a damage-based perspective. Advanced Genetics 1, e10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogdanović O, Smits AH, de la Calle Mustienes E, et al. (2016) Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat Genet. 48, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin M, Anavy L, Cole AG, et al. (2016) The mid-developmental transition and the evolution of animal body plans. Nature 531, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West MD, Sternberg H, Labat I, et al. (2019) Toward a unified theory of aging and regeneration. Regen Med. 14, 867–886. [DOI] [PubMed] [Google Scholar]
- 45.Newman SA (2011) Animal egg as evolutionary innovation: a solution to the “embryonic hourglass” puzzle. J Exp Zool B Mol Dev Evol. 316, 467–483. [DOI] [PubMed] [Google Scholar]
- 46.Heyn P, Kircher M, Dahl A, Kelso J, Tomancak P, Kalinka AT, Neugebauer KM (2014) The earliest transcribed zygotic genes are short, newly evolved, and different across species. Cell Rep. 6, 285–292. [DOI] [PubMed] [Google Scholar]


