SUMMARY
Selenof (15-kDa selenoprotein; Sep15) is an endoplasmic reticulum (ER)-resident thioredoxin-like oxidoreductase that occurs in a complex with UDP-glucose:glycoprotein glucosyltransferase. We found that Selenof deficiency in mice leads to elevated levels of non-functional circulating plasma immunoglobulins and increased secretion of IgM during in vitro splenic B cell differentiation. However, Selenof knockout animals show neither enhanced bacterial killing capacity nor antigen-induced systemic IgM activity, suggesting that excess immunoglobulins are not functional. In addition, ER-to-Golgi transport of a target glycoprotein was delayed in Selenof knockout embryonic fibroblasts, and proteomic analyses revealed that Selenof deficiency is primarily associated with antigen presentation and ER-to-Golgi transport. Together, the data suggest that Selenof functions as a gatekeeper of immunoglobulins and, likely, other client proteins that exit the ER, thereby supporting redox quality control of these proteins.
In Brief
Yim et al. report that Selenof (15-kDa selenoprotein; Sep15) functions as a gatekeeper of immunoglobulins and, likely, other client proteins en route from the ER to the Golgi apparatus, thereby preventing secretion of dysfunctional proteins and supporting redox quality control.
Graphical Abstract

INTRODUCTION
Selenium is an essential trace element due to its occurrence in proteins in the form of selenocysteine (Sec, U). Sec is known as the 21st amino acid encoded by a UGA codon; it is inserted co-translationally into nascent polypeptides with the help of the Sec insertion sequence (SECIS) element (Berry et al., 1991). There are 25 selenoprotein genes in humans and 24 in mice and most other mammals (Kryukov et al., 2003). Functionally characterized selenoproteins serve thiol oxidoreductase functions with Sec located in enzyme active sites and are directly involved in catalysis. More than half of the mammalian selenoproteins are characterized by a thioredoxin fold, with Sec occupying the position of the catalytic redox cysteine (Cys) in thioredoxin (Labunskyy et al., 2014; Reeves and Hoffmann, 2009).
Selenof (Selenoprotein F; 15-kDa selenoprotein; Sep15) (Gladyshev et al., 1998) is one of the 25 selenoproteins encoded in the human genome. It consists of an endoplasmic reticulum (ER)-targeting signal peptide, an N-terminal Cys-rich domain, and a C-terminal thioredoxin-like domain (Labunskyy et al., 2009). While it is an ER-resident selenoprotein, Selenof lacks the ER retention signal. However, it binds UDP-glucose:glycoprotein glucosyltransferase (UGGT), an ER-resident glycoprotein folding sensor, through its Cys-rich domain. Thus, binding to UDP-glucose supports Selenof retention in the ER (Korotkov et al., 2001; Labunskyy et al., 2009). UGGT recognizes incompletely folded glycoproteins and targets them to the calnexin-calreticulin-ERp57 system by reglucosylation, thereby allowing another cycle of maturation (Caramelo and Parodi, 2008; Hammond et al., 1994; Trombetta and Helenius, 2000). This function prevents an ER-to-Golgi exit of folding intermediates, misfolded glycoproteins, and immature multimeric complexes, ensuring that only properly folded glycoproteins are targeted to other cellular compartments, reach the cell surface, and are secreted (Anelli and Sitia, 2008; Cabral et al., 2001; Sousa et al., 1992; Zuber et al., 2001). Prevention of secretion of dysfunctional proteins is complex and specific to particular protein classes, but it is not well defined (Frand et al., 2000; Spang, 2013).
The tight binding between Selenof and UGGT implies that Selenof may also be involved in ER quality control, but this possibility has been difficult to test. It was found that UGGT occurs in both Selenof-bound and Selenof-free forms, whereas the entire pool of Selenof binds UGGT (Korotkov et al., 2001). The enzyme activities of both of UGGT1 and UGGT2 are enhanced by the formation of a complex with Selenof (Ito et al., 2015; Takeda et al., 2014). Selenof exhibits a redox potential suggestive of a reductase or isomerase function, rather than an oxidase of Cys residues (Ferguson et al., 2006; Labunskyy et al., 2009). Thus, it is an attractive possibility that Selenof supports redox quality control for a subset of UGGT client proteins. In this regard, some secreted glycoproteins, such as immunoglobulins (Igs) that are exceptionally disulfide rich, are potential clients for the UGGT/Selenof sensor. These proteins may be exemplified by IgM, which is the major antibody produced upon primary immune response. It is expressed in B cells and circulates in the blood of all vertebrate species (Cenci and Sitia, 2007; Ehrenstein and Notley, 2010). IgM is often used to identify acute exposure to an immunogen or pathogen (Leijh et al., 1979). It triggers the opsonization of antigens (e.g., infectious microorganisms) and causes ingested microorganisms to be promptly destroyed by phagocytes. Glycosylation of IgM is vital to its B cell surface presentation and secretion (Arnold et al., 2007; Sitia et al., 1984). Secretory IgM consists of 21 subunits, has a total 51 N-glycans, and contains 98 intra- and inter-chain disulfide bonds (Hendershot and Sitia, 2005). Like glycosylation, disulfide formation in newly synthesized proteins destined for secretion is an errorprone process (Dejgaard et al., 2004; Hatahet and Ruddock, 2009). Protein disulfide isomerase and several other ER-resident thiol oxidoreductases have been extensively studied, but much of this research focused on the actual process of disulfide bond formation and isomerization. On the other hand, redox quality control of secreted glycoproteins has not been thoroughly addressed.
In the present study, by examining the consequences of Selenof deficiency in mice and primary plasma B cell differentiation models (Bertolotti et al., 2010), we observed elevated secretion of Igs, without evidence of enhanced immune function or phenotypes associated with the secretory cells. Mouse embryonic fibroblasts (MEFs) treated with chemicals that perturb ER-to-Golgi transport reduced Selenof protein expression, while tunicamycin treatment, which induces unfolded protein response, elevated Selenof protein levels. In a fluorescence-microscopy-based assay for ER-to-Golgi transport of the vesicular stomatitis virus (VSV) G protein, Selenof knockout (KO) MEFs exhibited a significantly delayed transport function. Proteomic analyses showed depletion of “antigen presentation” and enrichment of “ER-to-Golgi anterograde transport” in Selenof KO MEFs. Together with other analyses, the data suggested that Selenof serves a function as a gatekeeper for Igs and likely other disulfide-rich glycoproteins, thereby providing redox quality control of secreted glycoproteins.
RESULTS
Systemic Selenof Deficiency in Mice Leads to Elevated Ig Levels without Altering Immune Functions
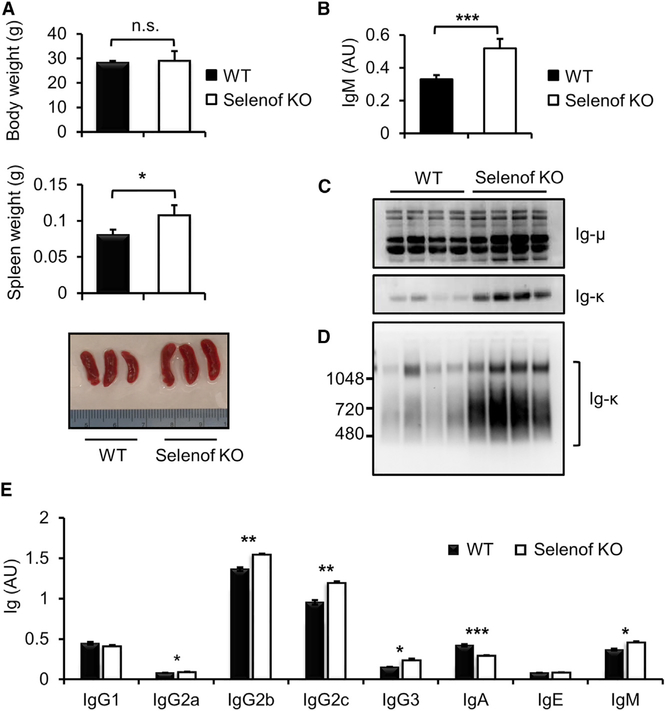
Selenof KO mice were fertile, grew and developed normally, and had no obvious pathologies. However, while wild-type (WT) and Selenof KO mice had similar body weights, the latter animals were characterized by mild splenomegaly (n = 6 per group, p = 0.02) (Figure 1A). By further examining littermate WT and Selenof KO mice (n = 8 per group, 12-week-old mice), we found that Selenof deficiency leads to elevated levels of IgM in serum (p = 0.0002), based on ELISAs (Figure 1B). Analysis of IgM components by western blotting under reducing (Figure 1C) and nonreducing conditions (Figure 1D) revealed higher levels of both Ig-μ and Ig-k chains in the serum of Selenof KO mice. In addition, global analyses of Ig abundance showed increased levels of IgG2a, IgG2b, IgG2c, IgG3, and IgM in Selenof KO mouse serum (n = 8 per group) (Figure 1E). In contrast, serum protein levels and their patterning, as analyzed by SDS-PAGE, were not different between WT and Selenof KO mice (Figure S1), suggesting that Selenof deficiency preferentially leads to elevation of serum Igs and possibly other proteins.
Figure 1. Selenof Deficiency Leads to Elevated Immunoglobulin Levels.
(A) Body and spleen weights of WT and Selenof KO mice (n = 4 per group). Spleen weights are different between WT and Selenof KO mice (p = 0.02). 13-week old WT and Selenof KO littermates were used.
(B) IgM analyses in WT and Selenof KO mouse sera by ELISA. The sera were diluted to 1:12,000, and anti-IgM antibody was used as a capture antibody. 13-week-old littermates (n = 4 per group) were used.
(C and D) Aliquots of sera from WT and Selenof KO mice were resolved under reducing SDS-PAGE (C) or 4–16% non-reducing NativePAGE (D), and blots were analyzed with anti-Ig-μ or anti-Ig-κ antibodies. 10 mg serum protein per lane was loaded.
(E) Immunoglobulins were quantified in WT and Selenof KO sera by ELISA. Blood was withdrawn from the submandibular vein of 12-week-old male littermates (n = 8 per group). Sera were diluted to 1:50,000, and anti-Ig (H+L) was used as a capture antibody. Two-tailed probability values of a Student’s t test are as follows: IgG2a, p = 0.03; IgG2b, p = 0.006; IgG2c, p = 0.002; IgG3, p = 0.02; IgA, p = 0.0004; and IgM, p = 0.02.
Data are expressed as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant
Complete blood count (CBC) using Hemavet Systems showed that all 24 measured and reportable CBC parameters, except platelet count, were not different between WT and Selenof KO animals (n = 10 per group, 12-week-old male mice). Platelet counts of Selenof KO mice were approximately 20% lower than those of WT littermates (p < 0.01). In addition, flow-cy-tometric analysis showed that the proportion of splenic CD4+ and CD8+ T cells, as well as of CD19+ B cells, were not altered by Selenof deficiency (Figure S2). Overall, the data suggested that Selenof deficiency increases Ig levels but does not lead to recognizable systemic changes in protein abundance or immune defects.
Selenof Deficiency Leads to Elevated Secretion of IgM during Plasma B Cell Differentiation
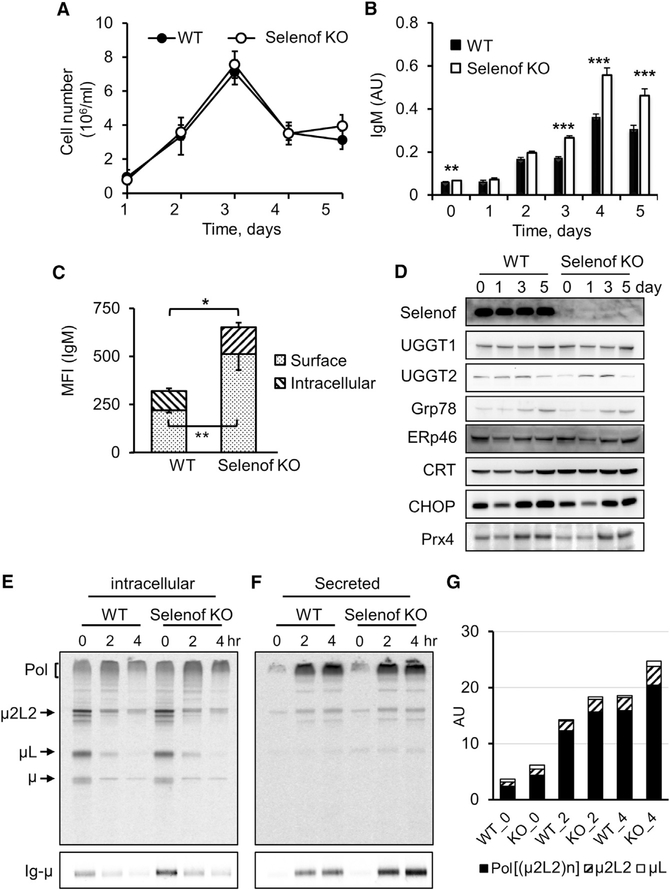
High Ig levels in the serum of Selenof KO mice may be the result of elevated production and/or decreased degradation of Igs. To characterize how Selenof deficiency alters serum IgM levels, we analyzed the rate of IgM secretion by activated plasma B cells. The proliferation rate of B cells, determined by the total number of cells per milliliter of media, during plasma B cell differentiation upon lipopolysaccharide (LPS) stimulation, was not affected by Selenof deficiency (Figure 2A). Without LPS stimulation, IgM secretion by naive B cells was higher in Selenof KO mice (day 0). Upon LPS treatment, IgM was secreted at a higher level during differentiation of splenic B cells isolated from Selenof KO mice starting from differentiation day 3, and increased IgM production continued until termination of the experiment at day 5 (Figure 2B). Using flow cytometry, expression levels of surface and intracellular IgM were measured. Activated B cells, 4 days after the LPS treatment, showed significantly higher levels of surface IgM and intracellular IgM in the absence of Selenof compared to controls (WT cells) (Figure 2C). To determine whether Selenof deficiency and the resulting excess IgM secretory phenotype resulted in increased ER stress or altered levels of ER stress-associated proteins during plasma B cell differentiation, we analyzed relevant protein expression by western blotting (Figure 2D). Selenof expression did not change in WT B cells during differentiation, and, as expected, this protein was not detected in KO cells. The expression of UGGT1 and UGGT2 proteins, which are the binding partners of Selenof, also was not affected, and Selenof deficiency did not alter their expression. As expected, ER stress response markers GRp78 and CHOP and the antioxidant Prx4 increased, but Selenof deficiency did not modify their expression patterns. In addition, no clear changes were observed in the expression of ERp46 and calreticulin (CRT). Thus, Selenof deficiency resulted in elevated IgM secretion but did not lead to (or change the level of) ER stress during B-to-plasma cell differentiation.
Figure 2. Elevated IgM Secretion by Selenof KO LPS-Activated B Cells.
(A) Growth of LPS-stimulated B splenocytes isolated from WT and Selenof KO mice. The graph shows B cell growth during differentiation.
(B) IgM secretion measured during plasma B cell differentiation by ELISA. Two-tailed probability values of a Student’s t test from day 0 to day 5 are as follows: day 0, p = 0.0091; day 1, p = 0.3891; day 2, p = 0.18; day 3, p = 5E–06; day 4, p = 0.0003; and day 5, p = 0.0013.
(C) Surface and intracellular IgM expression levels were measured in plasma B cells at differentiating day 4 using flow cytometry. MFI, median fluorescence intensity of the fluorescently labeled IgM.
(D) Expression of ER proteins in WT and Selenof KO B cells. Western blotting of Selenof and other ER proteins with relevance to Selenof function during B cell differentiation.
(E-G) Kinetics of IgM biosynthesis and transport were measured by radioactive pulse-chase assays (E and F) and quantified with ImageJ (G). The polymerization status of intracellular (E) and secreted (F) IgM was visualized in non-reducing conditions (E and F, upper panels), and Ig-μ levels were quantified in reducing blots (E and F, lower panels). At chase time 0, the levels of intracellular polymerization intermediates ((μ2L2)n) were increased by ~20%, and the Ig-μ levels were ~50% only slightly higher in Selenof KO plasma cells. Secreted IgM was more abundant (30–45%) in the spent media of Selenof KO LPS-activated splenocytes during the chase.
Data are expressed as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
Next, we evaluated the kinetics of IgM synthesis and secretion during the course of B cell differentiation at day 3 by pulse-chase analyses (Figures 2E–2G and S3). Primary B splenocytes isolated from WT and Selenof KO mice were pulse-labeled with 35S-labeled amino acids, washed, and chased for 0, 2, or 4 hr. The total cell lysates (Figure S3A) or immunoprecipitates (Figures 2E and 2F) were resolved by SDS-PAGE under non-reducing (Figures 2E and 2F, upper panels) or reducing (Figures 2E and 2F, lower panels) conditions, visualized by autoradiography, and quantified with ImageJ (Figure 2G). Both intracellular (Figure 2E) and secreted (Figure 2F) IgM levels were higher in Selenof KO B cells. Secreted IgM polymers were quantified from the non-reducing SDS-PAGE (Figure 2G). Intracellular and secreted polymerized IgM ((μ2L2)n) levels were increased by 20% at 2 hr of chase in Selenof KO B cells, compared to WT controls. In addition, intracellular Ig-μ was measured under reducing SDS-PAGE conditions and found to be increased 50% at time 0 in Selenof B cells, and the levels of secreted Ig-μ were increased 25–45% during different chase time points (Figures 2E and 2F, lower panels, and S3B and S3C). These findings further support the selective elevated secretion of Igs as a consequence of Selenof deficiency.
Increased Ig Levels in Selenof KO Mice Are Not Associated with Increased Phagocytosis and Bacterial Killing Potential
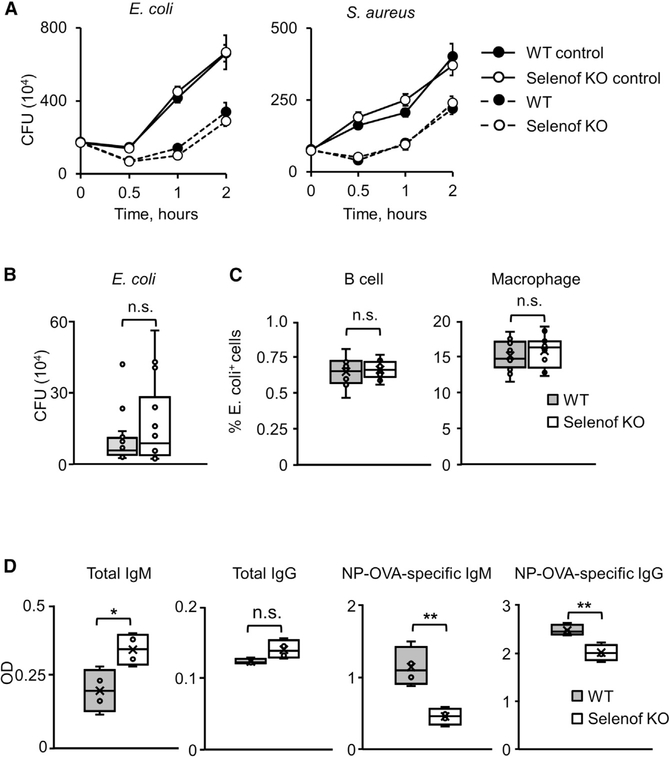
We considered the possibility that elevated Igs in Selenof KO mice might be functional and might better protect mice from antigen challenges such as bacterial infection. Since a complete blood test and lymphocyte levels of Selenof KO mice were not indicative of major changes in the immune function (Figure S2), we used a mouse model of acute peritoneal inflammation to examine Ig-neutrophil-mediated bacteria killing. We challenged Selenof KO and WT mice in vivo by intraperitoneal E. coli injection and performed in vitro assays using E. coli and S. aureus opsonized with sera obtained from WT and Selenof KO mice and then phagocytosed by neutrophils isolated from WT animals. Additionally, pHrodo Red E. coli bioparticles were opsonized with WT or Selenof KO sera and tested in phagocytosis assays.
The sera from WT and Selenof KO mice were equally efficient in opsonizing, leading to the killing of both tested bacterial strains (Figure 3A). If anything, the Selenof KO serum appeared to be less efficient, rather than more efficient, in clearing E. coli than the WT serum, although this effect did not reach statistical significance. In vivo clearance of bacterial infection was also not different between WT and Selenof KO mice (Figure 3B), but again, Selenof KO mice cleared the E. coli infection slightly less efficiently than control mice, although this difference was not statistically significant. In addition, bioparticles with a pH-sensitive fluorophore were used to quantify particle uptake by primary B cells or macrophages after opsonization with sera from WT or Selenof KO mice. As the bioparticles turn red after internalization, we used flow cytometry to quantify the number of cells that successfully ingested bioparticles, and we detected no significant differences between the sera from WT and Selenof KO mice (Figure 3C).
Figure 3. Elevated Immunoglobulins in Selenof Deficiency Are Partially Nonfunctional.
(A) In vitro clearance of bacterial cells by WT and Selenof KO serum. Shown are data for E. coli (left) and S. aureus (right).
(B) In vivo clearance of bacterial infection by WT and Selenof KO mice. In vivo killing of E. coli by WT and Selenof KO mice, assessed as the number of remaining E. coli cells.
(C) Uptake of pHrodo E. coli bioparticles by B cells (left) and macrophages (right) from WT mice that were opsonized by WT or Selenof KO serum.
(D) Total and NP-specific IgM and IgG antibody production in WT or Selenof KO mice after immunization with NP-OVA.
Data are expressed as mean ± SD. *p < 0.05; **p < 0.01; n.s., not significant.
To further test the functionality of antibodies in WT versus Selenof KO sera, we immunized WT and KO animals with the model antigen NP-OVA (4-hydroxy-3-nitrophenylacetyl hapten conjugated [NP] to ovalbumin protein [OVA]) and determined the amount of total as well as NP-specific IgM and IgG antibodies. Selenof KO mice had significantly higher levels of total IgM and IgG in comparison of the control mice. The amounts of NP-specific IgM and IgG antibodies, however, were significantly reduced in Selenof KO mice (Figure 3D). Overall, the data showed that elevated Ig levels in Selenof KO mice did not result in elevated clearance of bacterial infection or phagocytosis of pathogenic bioparticles in vivo. An in vitro bacterial killing assay further confirmed that this might not be due to the killing capacity of neutrophils of Selenof KO mice. Taken together, these data suggest that the excess of antibodies present in the sera of Selenof KO mice confer no obvious advantage in the functional assays utilized.
Selenof-Deficient Cells Are Not More Vulnerable to ER Stress but Show Changes Associated with ER-to-Golgi Transport
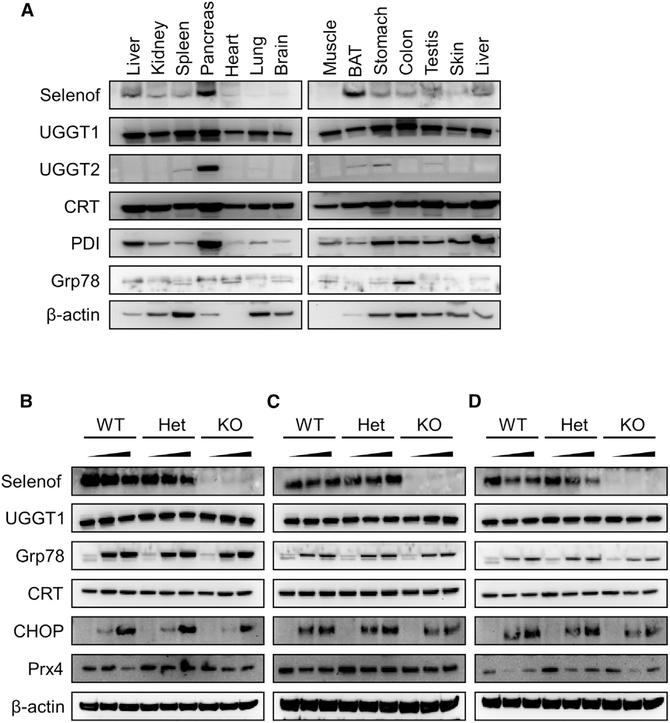
Since protein synthesis and secretion are linked to ER function (the compartment where Selenof resides), we further examined Selenof expression in mouse tissues in comparison with other proteins relevant to its function. Selenof was expressed in various organs, with the highest levels in the pancreas and brown adipose tissue (Figure 4A). A somewhat similar pattern was observed for UGGT2, whereas UGGT1 was expressed at high levels across mouse tissues.
Figure 4. Selenof Deficiency Does Not Lead to ER Stress.
(A) Expression pattern of Selenof and other relevant proteins across mouse tissues.
(B-D) Expression of Selenof and other relevant proteins in MEFs from WT, heterozygous, and Selenof KO mice subjected to ER stressors thapsigargin (B), tunicamycin (C), and brefeldin A (D). WT, heterozygous, and KO MEFs were treated with two concentrations of stressors along with control (DMSO treated): thapsigargin (5 nM and 50 nM), tunicamycin (50 ng/mL and 500 ng/mL), and brefeldin A (0.5 μM and 5 mM). Proteins assayed are shown on the left.
We further developed MEFs from WT, Selenof heterozygous, and Selenof KO littermate mice and examined how protein expression is altered in these cells in response to ER stress. All examined ER stressors-thapsigargin (Figure 4B), tunicamycin (Figure 4C), and brefeldin A (Figure 4D)-induced abundant expression of GRp78/BiP and CHOP but not of UGGT1 and calreticulin. Brefeldin A (0.5 μM and 5 μM) and thapsigargin (5 nM and 50 nM) treatment reduced the expression of Selenof more than tunicamycin (50 ng/mL and 500 ng/mL). Thus, Selenof is not a part of the canonical, chemical-induced ER stress response pathway but may be involved in later steps of protein synthesis and trafficking, such as ER-to-Golgi transport.
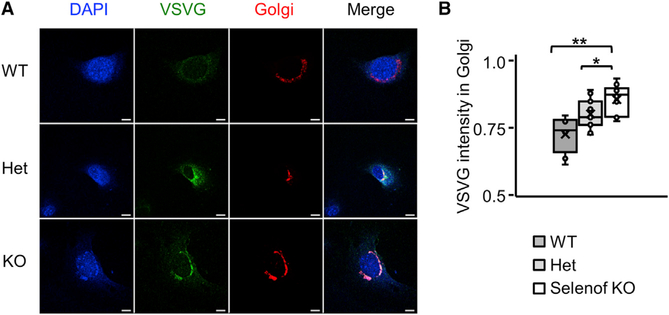
To further examine the role of Selenof in ER-to-Golgi transport, an in vivo transport assay using the temperature-sensitive variant of VSVG protein (ts045-VSVG)-EGFP was performed (Hirschberg et al., 1998; Vanhoutte et al., 2016). WT, heterozygous (Het), and Selenof KO MEFs were transduced with the ts045-VSVG-EGFP-expressing adenovirus, and the fluorescence intensity of VSVG in whole cells and the ER-to-Golgi positive area was measured. Expression of VSVG in MEFs was synchronized prior to the VSVG crossing the Golgi by compartment progression. We found that the transport vesicles exiting the ER and trafficking to the ER-to-Golgi intermediate compartment (ERGIC) were delayed in Selenof KO MEFs, compared to WT cells (Figures 5A and 5B). Ten min after the cells shifted from non-permissive temperature to 32°C, VSVG trafficking in Selenof KO MEFs was delayed approximately 20% (p < 0.01), compared to WT cells. It is possible that the delayed complete clearance of the VSVG in Selenof KO MEF is a consequence of the abundant VSVG that is partially misfolded. These findings support the notion that Selenof plays a role as a gatekeeper and is involved in protein folding and sorting, and ER-to-Golgi transport.
Figure 5. Delayed ER-to-Golgi Trafficking in Selenof KO MEFs.
(A) Immunofluorescence micrographs of WT, heterozygous, and Selenof KO MEFs expressing adenoviral ts045-VSVG-EGFP and a Golgi marker. Representative images show the overlapping area where the VSVG-EGFP is retained in the ER-to- Golgi area, 10 min after the cells were transferred from the restrictive temperature (40°C) to the permissive temperature (32°C). Scale bars: 10 mm.
(B) Quantification of VSVG fluorescence retained in the ER-to-Golgi area. Three cell lines per each genotype and more than 10 cells per each field were evaluated. Immunofluorescence microscopy images were analyzed by ImageJ. *p < 0.05; **p < 0.01.
Because Selenof KO animals were characterized by mild splenomegaly (Figure 1A), we also analyzed gene expression changes in this organ, followed by Ingenuity Pathway Analysis (IPA). Comparison of Selenof KO mice and WT littermate controls revealed several IPA “associated diseases and biofunctions” that included the categories “metabolism of protein” (198 molecules), “synthesis of protein” (91 molecules), “catabolism of protein” (121 molecules), and “ER stress response” (42 molecules, Z score: −0.887; p = 0.00002) (Data S1). Among the gene changes that contributed, the top canonical pathway identified within the ER stress response related to the “unfolded protein response.” These findings support the role of Selenof in ER functions.
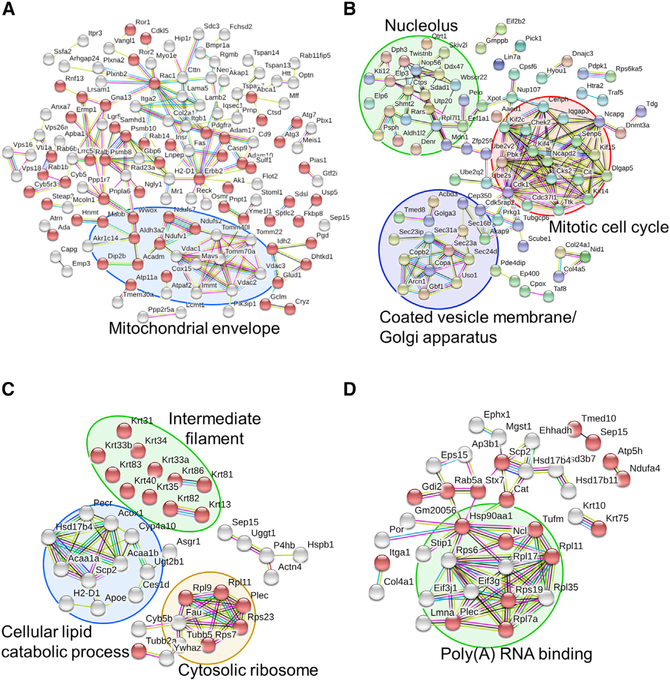
Inducers of ER stress, such as nutrient starvation, lead to the accumulation of unfolded proteins within the ER lumen and affect the ER-to-Golgi secretory pathway. Energy deprivation blocks the Golgi complex and the trans-Golgi network through a response mainly triggered by impaired coat protein-1 (COPI)- dependent retrograde transport from the Golgi to the ER (Chen et al., 2014). Hence, we tested whether Selenof impacted the ER-to-Golgi secretory pathway under conditions of ER stress induced by serum starvation (Figure S4). Quantitative global proteomic analyses in WT and Selenof KO MEFs were carried out, and the associated biological pathways were evaluated (Data S2). A total of 7,467 proteins were quantified by tandem mass spectrometry (MS/MS) in MEFs maintained in a serum-free culture condition. At a p value of < 0.05, 198 proteins were found to be decreased and 138 proteins increased (n = 3) in Selenof KO MEFs. The differentially expressed proteins (p < 0.05) were further analyzed and visualized using Reactome, STRING, and DAVID. Proteins decreased in Selenof KO cells (Figure 6A) were associated with “membrane part” (Gene Ontology [GO]: 0011125; false discovery rate [FDR], 4.45E–17) and “regulation of cellular localization” (GO: 0032879; FDR, 9.43E–05). Seventy-three of these proteins were also associated with “catalytic activity” (GO: 0003824; FDR, 0.0004189). “Porin activity” (GO: 0015288) was also enriched (FDR, 0.000114). Proteins with elevated levels in Selenof KO MEFs were associated with several pathways, most notably with “coated vesicle membrane” (GO: 0030662; FDR, 0.000196) and “mitotic cell cycle” (GO: 0000278; FDR, 0.0194) (Figure 6B).
Figure 6. Network Analysis of Enriched Pathways and Interactions.
(A and B) WT and Selenof KO MEFs were cultured in serum-free media and subjected to quantitative proteomics analysis. Enriched proteins (p < 0.05, 138 up- and 198 down-regulated in Selenof KO) were further analyzed and visualized with STRING. Proteins with catalytic activity are marked with red bubbles. The functions “membrane part” and “regulation of cellular localization” were the most decreased in Selenof KO cells. Mitochondrial envelope proteins were decreased in Selenof KO (A). Proteins with increased levels in Selenof KO MEFs belonged to the clusters “Coated vesicle membrane/Golgi apparatus” (circled in purple), “Nucleolus” (circled in green), and “Mitotic cell cycle” (circled in red) (B).
(C and D) Selenof and UGGT1 interacting proteins and the associated enriched pathways. Proteins from WT and Selenof KO livers were immunoprecipitated with antibodies against Selenof (C) or UGGT1 (D), followed by MS/MS analysis. Proteins identified (p < 0.05) were furtheranalyzed. Selenof antibodies enriched 44 proteins belonging to “structural molecule activity” (marked with red bubbles). Four protein clusters were identified and visualized: “cellular lipid catabolic process” (circled in blue), “Cytosolic ribosome” (circled in yellow), “Intermediate filament” (circled in green), and Selenof along with UGGT1 (C). UGGT1 antibodies enriched 63 proteins belonging to “extracellular exosome” (marked with red bubbles). A cluster with a function “Poly(A) RNA binding proteins” is circled in green in (D). Disconnected nodes are not shown.
To examine protein-protein interactions involving Selenof and UGGT1, immunoprecipitation was performed from liver lysates of WT and Selenof KO mice using antibodies against Selenof and UGGT1, followed by MS/MS analyses (Figure S5; Data S2). The use of Selenof antibodies revealed 44 proteins (p < 0.05) affected by Selenof deficiency, whereas the use of UGGT1 antibodies revealed 63 proteins. Five proteins (Hsd17b4, Plec, Rpl11, Scp2, and Selenof) were detected by using both antibodies. Forty-three proteins that were enriched with Selenof antibodies were further analyzed using STRING (Figure 6C). “Structural molecule activity” was the strongest association: 17 of the 43 proteins belonged to this functional category (GO: 0005198; FDR, 3.76E–15). In addition, cellular components associated with “intermediate filament” (FDR, 5.51E–14) and “extracellular exosome” (FDR, 8.3E–08) were enriched. Other notable enriched functions were “cellular lipid catabolic process” (GO: 0044242; FDR, 0.000532), and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway of “peroxisome” (KEGG: 04146; FDR, 2.69E–06). Among proteins differentially immunoprecipitated with UGGT1 antibodies from WT and Selenof KO livers, 30 proteins were associated with “extracellular exosome” (GO: 0070062; FDR, 3.41E–11) (Figure 6D). “Poly(A) RNA binding” (GO: 0044822; FDR, 7.21E–06) was also enriched. The Reactome-enriched biological pathways included “antigen presentation: folding, assembly, and peptide loading,” “COPII (Coat Protein 2)-mediated vesicle transport,” and “ER-to-Golgi transport and its associated pathways,” such as vesicle transport and ER-phagosome pathway (Tables 1, S1, S2, and S3). The DAVID functional annotation tool similarly identified pathways associated with the ER to the trans-Golgi network (Table S3), including “Golgi vesicle transport” (p = 4.20E–05), “cytoplasmic membrane-bounded vesicle” (p = 1.10E–04), and “membrane-bounded vesicle” (p = 1.70E–04).
Table 1.
Pathway Enrichment Analysis of 336 Proteins Differentially Expressed between Selenof KO and WT Mouse Embryonic Fibroblast Cells
| Pathway | Proteins Found | Proteins Total | Ratio | p Value | FDR |
|---|---|---|---|---|---|
| Antigen presentation: folding, assembly, and peptide loading of class I MHC | 14 | 118 | 0.011 | 1.46E–06 | 0.00115 |
| Laminin interactions | 6 | 31 | 0.003 | 1.21E–04 | 0.0476 |
| COPII (Coat Protein 2)-mediated vesicle transport | 8 | 75 | 0.007 | 5.36E–04 | 0.103 |
| Endosomal/vacuolarpathway | 9 | 97 | 0.009 | 6.60E–04 | 0.103 |
| Antigen processing-cross-presentation | 12 | 176 | 0.016 | 1.32E–03 | 0.162 |
| ER-phagosome pathway | 11 | 154 | 0.014 | 1.45E–03 | 0.162 |
| Class I MHC-mediated antigen processing and presentation | 18 | 368 | 0.033 | 3.87E–03 | 0.379 |
| ER-to-Golgi anterogradetransport | 10 | 157 | 0.014 | 5.22E–03 | 0.407 |
| Transport to the Golgi andsubsequent modification | 12 | 212 | 0.019 | 5.82E–03 | 0.414 |
| WNT5A-dependent internalization of FZD2, FZD5, and ROR2 | 2 | 5 | 0 | 6.66E–03 | 0.433 |
| Golgi cisternae pericentriolar stack reorganization | 3 | 17 | 0.002 | 8.35E–03 | 0.501 |
| Constitutive signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555) translocation mutant | 2 | 8 | 0.001 | 1.63E–02 | 0.558 |
| Signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555)translocation Mutant | 2 | 8 | 0.001 | 1.63E–02 | 0.558 |
| Non-integrin membrane-ECM interactions | 5 | 61 | 0.006 | 1.68E–02 | 0.558 |
| Interferon alpha/beta signaling | 8 | 138 | 0.013 | 1.97E–02 | 0.558 |
| 5-Phosphoribose 1-diphosphate biosynthesis | 2 | 9 | 0.001 | 2.03E–02 | 0.558 |
| CHL1 interactions | 2 | 10 | 0.001 | 2.46E–02 | 0.558 |
| Interferon gamma signaling | 9 | 174 | 0.016 | 2.64E–02 | 0.558 |
| Zinc influx into cells by the SLC39 gene family | 2 | 11 | 0.001 | 2.94E–02 | 0.558 |
| Sema4D-induced cell migration and growth-cone collapse | 3 | 28 | 0.003 | 3.08E–02 | 0.558 |
The Reactome pathway database was used for enrichment analysis. p < 0.05.
DISCUSSION
Selenof has been an enigmatic protein, many efforts to address its function notwithstanding (Hu et al., 2001; Kasaikina et al., 2011; Kumaraswamy et al., 2002; Labunskyy et al., 2005; Novoselov et al., 2006). Being a selenoprotein with a thioredoxin fold, and having Sec in the position of the redox-active Cys in thioredoxin, Selenof was expected to function as a thiol oxidoreductase. Its redox potential is −225 mV, which is between that of thioredoxin (−270 mV) and protein disulfide isomerase (−175 mV); therefore, Selenof is likely to act as a reductase or isomerase (Ferguson et al., 2006). All functionally characterized selenoproteins are oxidoreductases and the majority of thioredoxin-fold proteins are oxidoreductases. Selenof is localized in the ER due to its strong binding to UGGTs, whose function is to maintain glycoprotein quality control in the ER. Upon binding of Selenof to UGGT1 or UGGT2, the glucosyltransferase activities of these proteins are increased (Takeda et al., 2014). Together with the previously described oxidative stress-like phenotypes that characterize Selenof deficiency in cell culture and animal models (Ferguson et al., 2006; Kasaikina et al., 2011), a possible function of Selenof was thought to be related to redox quality control of UGGT targets. However, it was difficult to examine this function.
Our findings support the idea that Selenof participates in the complex secretome gatekeeping function, likely assisting newly synthesized glycoproteins as they exit the ER or during their trafficking between the ER and the Golgi. While mice lacking Selenof are viable and fertile and have no gross morphological abnormalities, Selenof deficiency led to elevated levels of serum Igs-in particular, IgM-as well as to mild splenomegaly. We found that the elevated serum IgM levels were due to increased secretion of these molecules. The data further suggest that Selenof is a gatekeeper of UGGT targets translocated from the ER to the Golgi apparatus.
Secreted disulfide-rich proteins are characterized by a high rate of incorrect disulfide formation, as well as significant variation in glycosylation (Buchberger et al., 2010; Sevier and Kaiser, 2002). Although the calnexin-calreticulin-ERp57 system permits incorrectly glycosylated proteins to pass through an additional round of maturation (Anelli and Sitia, 2008), it has been unclear how the disulfide quality control is maintained during this process, since many UGGT clients are rich in disulfides. Selenof nicely fits this role, although it may not be the exclusive oxidoreductase in this process. It appears that Selenof deficiency weakens glycoprotein redox quality control, allowing some glycoproteins with incorrect disulfides to be secreted. If so, a subset of secreted disulfide-rich glycoproteins may bypass the checkpoint, and the secreted pool would consist of a mixture of functional and non-functional molecules. In the absence of Selenof, the latter would bypass also the ERp44 quality control system that operates beyond the ER in a pH-dependent way (Anelli etal., 2015).
Our findings support this model. While the levels of IgM, a highly glycosylated molecule with numerous inter- and intradisulfide bridges, were elevated in Selenof KO mice, in vitro and in vivo clearance of bacterial infection was not affected. The fact that Selenof deficiency does not lead to measurable ER stress is also consistent with this possibility. In addition, Selenof protein expression was decreased upon brefeldin A and thapsigargin treatments that inhibit ER-to-Golgi transport and induce retrograde protein transport between these compartments. Also, confocal images of ts045-VSVG-EGFP-expressing MEFs confirmed that the VSVG-EGFP cargo exiting from the Golgi apparatus was delayed but, ultimately, could be completed in Selenof KO cells. Newly synthesized, secreted non-functional glycoproteins would pass through the ER and Golgi in the absence of Selenof, so the ER compartment itself would be subjected to similar levels of stress in WT and Selenof KO cells. It is unclear whether elevated levels of non-functional Igs directly cause splenomegaly, as this phenotype may also result from other processes, but at least this phenotype is consistent with the predicted function of Selenof. Nutrient-starvation-induced ER stress leads to the accumulation of unfolded proteins within the ER lumen and affects the ER-to-Golgi secretory pathway. Regulatory mechanisms preventing secretion of dysfunctional proteins from cells may be complex and involve multiple proteins or even pathways. Perturbations of ER homeostasis mediate ER stress through translational attenuation, upregulation of the ER chaperone functions, and degradation of misfolded proteins. It is also possible that some of the changes observed in Selenof KO mice are an outcome of the ER-stress-associated secondary or feedback mechanisms. Our quantitative microarray analyses in spleen indicated various mechanisms related to ER stress and protein synthesis affected by systemic Selenof deficiency in mice. Quantitative global proteomic analyses revealed that proteins differentially expressed between WT and Selenof KO MEFs were primarily enriched in the pathways associated with the ER-to-Golgi transport. Proteins that were differentially expressed and involved in antigen presentation and vesicle transport categories included Sec23a, Sec24d, Sec16b, Sec23ip, Uso1, Rab1b, and Mr1. These findings support the idea that Selenof functions as a gatekeeper and that disruption of this gene significantly affects the critical ER-to-Golgi transition.
Most previous research on thiol oxidoreduction in the ER dealt with the formation of disulfide bonds, whereas the redox gatekeeper function remained poorly characterized. Nevertheless, this function is expected to be selected during evolution (Sun et al., 2014), particularly with regard to secreted disulfide-rich proteins, as otherwise organisms would need to invest large amounts of energy in producing and then detecting and degrading secreted non-functional molecules. The gatekeeper function of Selenof is likely not limited to IgM or, more generally, to Igs. Selenof is expressed in all cells and appears to target a subset of secreted proteins. It is possible that the phenotype we observed in activated B cells was better exposed due to the specialized function of these cells in secreting large quantities of disulfide-rich glycoproteins.
Our work was performed using mouse models, which tolerate Selenof deficiency and exhibit relatively mild phenotypes, at least in the lab setting. Interestingly, based on the analysis of more than 60,000 human exomes (http://exac.broadinstitute.org/) (Lek et al., 2016), we found that the loss-of-function SELENOF alleles (functionally equivalent to heterozygous KO) are depleted 5-fold compared to the expected occurrence, whereas the occurrence of synonymous and missense mutations in SELENOF is as expected. This observation suggests that the SELENOF-supported function may be more important in long-lived humans than in short-lived mice. Selenof has a paralog, Selenom, in both mice and humans, and it is also a selenoprotein. However, Selenom does not bind UGGTs or increase the activity of UGGTs (Ito et al., 2015; Takeda et al., 2014). We speculate that it may serve some other subsets of disulfide-containing proteins that pass through the ER.
Overall, this study identifies a new function of the selenoprotein oxidoreductase Selenof: a gatekeeper of secreted disulfide-rich glycoproteins. The data suggest that it blocks these proteins from secretion when they possess incorrectly formed disulfides or have some other redox-related errors in their Cys residues that inhibit their functions, so the erroneous proteins are subjected to additional rounds of maturation in the ER.
EXPERIMENTAL PROCEDURES
Materials
Tunicamycin, brefeldin A, and thapsigargin were from Tocris Bioscience (Bristol, UK); and lipopolysaccharide (LPS; L2637), SIGMAFAST OPD (o-phenylenediamine), and 2-mercaptoethanol were from Sigma (St. Louis, MO, USA). Cell culture media (DMEM and RPMI), antibiotics, non-essential amino acids, sodium pyruvate, N-glutamine, insulin-transferrin-selenium (ITS-G, 100x), serum, and ammonium-chloride-potassium (ACK) lysis buffer were from Invitrogen. For western blot analysis, NuPAGE 10% Bis-Tris and NativePAGE 4–16% Bis-Tris Gel systems (Invitrogen) were used. The following antibodies for western blotting were used: anti-β-Actin (Sigma), anti-Selenof (NCIR128A, Abcam), anti-Peroxiredoxin 4 (Prx4, AbFrontier, Korea), anti-UDP-glucose:glycoprotein glucosyltransferase 1 and anti-UDP-glucose:glycoprotein glucosyltransferase 2 (UGGT1 and UGGT2, Novus Biologicals), anti-calreticulin (Cell Signaling Technology), anti-CCAAT/anti-enhancer-binding protein homologous protein (CHOP, Abcam), anti-46 kDa endoplasmic reticulum protein (ERp46, Abcam), anti-glucose regulated protein 78 (GRp78, Abcam), and rabbit anti-mouse IgM antibody (cat# 61–6800, Zymed). For ELISAs, goat anti-mouse IgG1-HRP (horseradish peroxidase), goat anti-mouse IgA-HRP, goat anti-mouse IgG2a-HRP, goat anti-mouse IgG2b-HRP, goat anti-mouse IgG2c-HRP, goat anti-mouse IgG3-HRP, goat anti-mouse IgE-HRP, and anti-IgM μ-HRP were from SouthernBiotech. CellLight Golgi-RFP, BacMam 2.0, and pHrodo Red E. coli BioParticles were from Thermo Fisher Scientific. Mouse B cell and neutrophil isolation kits were from Miltenyi Biotec. BD Microtainer serum separator tubes were from BD Biosciences. Bacterial strains Staphylococcus aureus (S. aureus, strain 10390) and E. coli (E. coli, strain 19138) were from the American Type Culture Collection (ATCC).
Mice
Selenof KO mice were generated as previously described (Kasaikina et al., 2011) and backcrossed to C57BL/6J background for at least 10 generations. WT and Selenof KO mice for the described experiments were produced by mating heterozygous animals. Genotyping was performed by PCR using Selenof gene-specific primers (forward [F]1: GCA GCT CTT GCG ATC TTC TT and reverse [R]1: TTT GGC CAG ATA CCA GGA AG) and inserted Neo-cassette specific primer (F2: TCG CCT TCT TGA CGA GTT CT). Littermate male mice, aged 8 to 14 weeks, were used for all experiments, except for the generation of Selenof MEF cells. Experimental animal protocols were approved by the Institutional Animal Care and Use Committees of Brigham and Women’s Hospital, Harvard University, and University of Nebraska-Lincoln.
Generation of MEFs and Cell Culture
Primary MEFs were generated by mating Selenof heterozygous animals. Pregnant females were sacrificed on day 13.5 post-coitum, embryos were surgically removed, and fibroblast cells were prepared in culture. Each embryo was cultured separately and was genotyped for the disruption of the Selenof gene. Selenof WT, heterozygous, and KO cells were generated from a single litter, and same-passage cells were used for the study. MEFs were cultured in DMEM (Invitrogen) supplemented with 1 mM non-essential amino acids, 10% fetal bovine serum, 1% penicillin-streptomycin (Pen/Strep), and 0.5 μmol/L 2-mercaptoethanol (β-ME). MEFs used in the experiments were in passages 3 to 7 without immortalization.
Selenof WT, heterozygous, and KO MEF cells were treated with ER-stress-inducing agents. For tunicamycin (TM), brefeldin A (BFA), and thapsigargin (TG) treatments, 1,000x stock solutions were prepared in DMSO, and an equal volume of DMSO alone (0.1%, v/v) was applied to cell culture as a control. MEF cells (WT, heterozygous, and KO) derived from a single litter and same passage number were plated at a concentration of 106 cells per 10-cm dish and allowed to attach overnight prior to application of ER-stress-inducing reagents. The cells were treated with tunicamycin (50 ng/mL or 500 ng/mL), TG (5 μM or 50 μM), brefeldin A (0.5 mM or 5 mM), or DMSO (0.1%, v/v). After 24 hr of treatment with ER-stress-inducing agents, cell culture plates were briefly washed twice with ice-cold PBS, and the attached cells were directly lysed with SDS sample buffer for protein analysis.
Liquid Chromatography-Tandem Mass Spectrometry Analysis and Pathway Enrichment Analysis
To subject cells to serum starvation, MEFs derived from littermate embryos (three WT and three Selenof KO) were seeded in 150-mm dishes for 24 hr and washed twice in PBS, and the culture media were replaced with phenol-red-free RPMI 1640 media supplemented with insulin-transferrin-selenium (1 g/L, 0.55 g/L, and 0.67 mg/L as sodium selenite, respectively). Three days later, adherent cells were lysed in ice-cold protein lysis buffer (50 mM HEPES [pH 7.5], 150 mM KCl, 2 mM EDTA, 1 mM NaF, 0.5% [v/v] NP-40, 0.5 mM DTT, and protease inhibitor cocktail [Roche, EDTA-free], and 10 mM N-ethylmaleimide). After enzymatic digestion, an aliquot of 100 μg peptide per condition was fractionated and then analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an Orbitrap Fusion Lumos (Thermo Fisher Scientific). Mass-spectrometry data were searched using Sequest and filtered to a 1% FDR at the protein level to generate the target protein list. Proteins that are differentially expressed with a p value less than 0.05 between Selenof WT and KO MEFs were further analyzed for pathway enrichment. Protein interaction databases Reactome (Fabregat et al., 2016), STRING (Szklarczyk et al., 2017), and DAVID (Huang et al., 2009) were used for network analyses. In a separate experiment, immunoprecipitation (IP) was performed from livers of WT and Selenof KO with antibodies against Selenof or UGGT1. Livers were perfused with PBS to remove blood prior to tissue collection. Antibodies were first conjugated to magnetic beads to prevent co-elution using a Dynabeads Antibody Coupling Kit prior to performing IP. Proteins eluted using each antibody were subjected for MS/MS analysis for protein identification and quantification.
Splenic B Cell Isolation and Differentiation
Spleens of WT and Selenof KO mice were removed aseptically and mashed through cell strainers. The splenocytes were prepared by lysing red blood cells using ACK lysis buffer. Splenic B cells were further purified by magnetic isolation with a mouse B cell isolation kit by depletion of non-B cells (Miltenyi Biotec) (Bertolotti et al., 2010). Pre-splenic B cells of WT and Selenof KO mice at a concentration of 2 × 106 cells per milliliter were cultured in a flask with RPMI medium containing 10% endotoxin-free fetal calf serum, 100 U/mL penicillin/streptomycin, 1 mM sodium pyruvate, 2 mM N-glutamine, and 50 μM β-ME. Primary B cells were activated by the addition of 20 μg/mL LPS in culture media. Daily, a one-third volume of culture media was replaced with fresh RPMI medium containing β-ME and LPS to avoid risk of starvation or oxidative stress. A portion of the cells was harvested daily for cell count and at days 0, 1, 3, and 5 for protein analyses. Cell proliferation curves were plotted by counting putative cell numbers (per milliliter of culture media) on each day after LPS-stimulated plasma B cell activation.
Immunoglobulin Production Assay
Secreted IgM levels during B cell activation were measured daily using ELISA. See the Supplemental Experimental Procedures for full details.
ELISA
Analysis of antigen-specific Ig titers in serum and B cell culture media was performed with capture ELISA. See the Supplemental Experimental Procedures for full details.
Flow Cytometry
Flow cytometry was performed on an LSR II instrument (BD Biosciences), and data were analyzed using FlowJo v10.1 (FlowJo). See the Supplemental Experimental Procedures for full details.
Neutrophil Isolation for Bacteria Killing Assay
Murine bone-marrow-derived neutrophils were isolated from 12-week-old C57BL/6J WT male animals with a Neutrophil Isolation Kit (Miltenyi Biotec). See the Supplemental Experimental Procedures for full details.
In Vitro and In Vivo Bacteria Killing Assays
Fresh overnight cultures of S. aureus (ATCC strain 10390) and E. coli (ATCC strain 19138) were used for in vitro bacteria killing assays, and E. coli cells were used for in vivo assay. See the Supplemental Experimental Procedures for full details.
pHrodo Red Bead Uptake
B cells and macrophages from WT mice were purified using magnetic isolation by depleting non-B cells or positively selecting for CD11b+ cells, respectively (Miltenyi Biotec). The purified cells were incubated in R10 medium with 10% sera collected from WT or Selenof KO mice, and pHrodo Red E. coli BioParticles were added. After 2 hr, the in vitro uptake of pHrodo Red beads by B cells and macrophages was determined using flow cytometry.
Mouse Immunization and NP-Specific Antibody Production
Six- to 12-week-old WT or Selenof KO mice were immunized with 100 μg NP16-OVA(Biosearch Technologies) in a 1:1 H37RA complete Freund’s adjuvant (CFA; DIFCO) emulsion. The emulsion was injected subcutaneously into the left and right flanks of each experimental mouse. 14 days after immunization, NP-specific antibody titers were measured by the incubation of serum for 1 hr in ELISA plates coated with NP16-BSA(Biosearch Technologies), followed by incubation with alkaline-phosphatase-conjugated IgG-detection and IgM-detection antibodies (1030–04; SouthernBiotech). The level of activity was measured as optical density (OD) units using a Spectramax ELISA plate reader (Molecular Devices).
VSVG Transport Assay
Anterograde transport of VSVG-EGFP in WT, heterozygous, and Selenof KO MEFs was analyzed using the ts045-VSVG-EGFP (kindly provided by Dr. Jeffery D. Molkentin) as described previously (Vanhoutte et al., 2016). Briefly, 48 hr after adenoviral transduction, cells were co-infected with CellLight Golgi-RFP Bacmmam 2.0 and incubated at 40°C for 20 hr to retain the VSVG-EGFP in the ER. To measure the co-localization of VSVG-EGFP during the ER-to-Golgi transit, cells were shifted to 32°C, allowing the VSVG-EGFP to traffic to the Golgi apparatus. Three independent MEF lines per each genotype were tested. Representative confocal images of VSVG-EGFP fluorescence overlapping with Golgi-RFP were captured (n = 10 per each MEF line). Fractions of VSVG-EGFP retained in the Golgi apparatus at 10 min after being released were further analyzed. All images were captured, analyzed, and quantified with the ImageJ program (NIH), and overlapping signals were measured.
Radioactive Pulse-Chase Assay
Kinetics of IgM biosynthesis and transport were measured with a radioactive pulse-chase assay (Cals et al., 1996; Mezghrani et al., 2001) in primary B cells isolated from WT and Selenof KO mice. See the Supplemental Experimental Procedures for full details.
Statistical Analyses
Data are expressed as the mean ± SD as indicated. Two-tailed Student’s t tests were used for comparing different groups of samples, and significant differences were defined as *p < 0.05, **p < 0.01, and ***p < 0.001, unless otherwise stated.
Supplementary Material
Highlights.
Selenof deficiency elevates levels of plasma immunoglobulins
IgM secretion is higher in Selenof knockout B cells
Elevated IgM does not increase antimicrobial capacity or systemic IgM activity
Selenof facilitates antigen presentation via ER-to-Golgi transport
ACKNOWLEDGMENTS
We thank Drs. Davy Vanhoutte and Jeffery Molkentin for the generous gift of temperature-sensitive VSVG-EGFP adenovirus. This work was supported by NIH grant CA080946 (to V.N.G.) and AIRC IG-18624 (to R.S.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures, three tables, and three data files and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.04.009.
REFERENCES
- Anelli T, and Sitia R (2008). Protein quality control in the early secretory pathway. EMBO J. 27, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Sannino S, and Sitia R (2015). Proteostasis and “redoxtasis” in the secretory pathway: Tales of tails from ERp44 and immunoglobulins. Free Radic. Biol. Med 83, 323–330. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, and Dwek RA (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol 25, 21–50. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, and Larsen PR (1991). Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 353, 273–276. [DOI] [PubMed] [Google Scholar]
- Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, luchi Y, Fujii J, Vene R, Rubartelli A, et al. (2010). B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid. Redox Signal. 13,1133–1144. [DOI] [PubMed] [Google Scholar]
- Buchberger A, Bukau B, and Sommer T (2010). Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell 40, 238–252. [DOI] [PubMed] [Google Scholar]
- Cabral CM, Liu Y, and Sifers RN (2001). Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem. Sci.. 26, 619–624. [DOI] [PubMed] [Google Scholar]
- Cals MM, Guenzi S, Carelli S, Simmen T, Sparvoli A, and Sitia R (1996). IgM polymerization inhibits the Golgi-mediated processing of the mu-chain carboxy-terminal glycans. Mol. Immunol 33, 15–24. [DOI] [PubMed] [Google Scholar]
- Caramelo JJ, and Parodi AJ (2008). Getting in and out from calnexin/calreticulin cycles. J. Biol. Chem. 283, 10221–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, and Sitia R (2007). Managing and exploiting stress in the antibody factory. FEBS Lett. 581, 3652–3657. [DOI] [PubMed] [Google Scholar]
- Chen R, Zou Y, Mao D, Sun D, Gao G, Shi J, Liu X, Zhu C, Yang M, Ye W, et al. (2014). The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J. Cell Biol 206,173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard S, Nicolay J, Taheri M, Thomas DY, and Bergeron JJ (2004). The ER glycoprotein quality control system. Curr. Issues Mol. Biol 6, 29–42. [PubMed] [Google Scholar]
- Ehrenstein MR, and Notley CA (2010). The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol 10, 778–786. [DOI] [PubMed] [Google Scholar]
- Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, et al. (2016). The Reactome pathway Knowledgebase. Nucleic Acids Res. 44 (D1), D481–D487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AD, Labunskyy VM, Fomenko DE, Arac D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, and Deisenhofer J (2006). NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J. Biol. Chem 281, 3536–3543. [DOI] [PubMed] [Google Scholar]
- Frand AR, Cuozzo JW, and Kaiser CA (2000). Pathways for protein disulphide bond formation. Trends Cell Biol. 10, 203–210. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Jeang KT, Wootton JC, and Hatfield DL (1998). A new human selenium-containing protein. Purification, characterization, and cDNA sequence. J. Biol. Chem 273, 8910–8915. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, and Helenius A (1994). Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 91, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatahet F, and Ruddock LW (2009). Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 11, 2807–2850. [DOI] [PubMed] [Google Scholar]
- Hendershot L, and Sitia R (2005). Immunoglobulin assembly and secretion. In Molecular Biology of B Cells, Honjo T, Alt FW, and Neuberger MS, eds. (Elsevier Academic Press; ), pp. 261–273. [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, and Lippincott-Schwartz J (1998). Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Korotkov KV, Mehta R, Hatfield DL, Rotimi CN, Luke A, Prewitt TE, Cooper RS, Stock W, Vokes EE, et al. (2001). Distribution and functional consequences of nucleotide polymorphisms in the 3′-untranslated region of the human Sep15 gene. Cancer Res. 61, 2307–2310. [PubMed] [Google Scholar]
- Huang W, Sherman BT, and Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takeda Y, Seko A, Izumi M, and Kajihara Y (2015). Functional analysis of endoplasmic reticulum glucosyltransferase (UGGT): Synthetic chemistry’s initiative in glycobiology. Semin. Cell Dev. Biol. 41, 90–98. [DOI] [PubMed] [Google Scholar]
- Kasaikina MV, Fomenko DE, Labunskyy VM, Lachke SA, Qiu W, Moncaster JA, Zhang J, Wojnarowicz MW Jr., Natarajan SK, Malinouski M, et al. (2011). Roles of the 15-kDa selenoprotein (Sep15) in redox homeostasis and cataract development revealed by the analysis of Sep 15 knockout mice. J. Biol. Chem. 286, 33203–33212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, and Gladyshev VN (2001). Association between the 15-kDa selenoprotein and UDP-gluco- se:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J. Biol. Chem 276, 15330–15336. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, and Gladyshev VN (2003). Characterization of mammalian selenoproteomes. Science 300, 1439–1443. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy E, Korotkov KV, Diamond AM, Gladyshev VN, and Hatfield DL (2002). Genetic and functional analysis of mammalian Sep15 selenoprotein. Methods Enzymol. 347, 187–197. [DOI] [PubMed] [Google Scholar]
- Labunskyy VM, Ferguson AD, Fomenko DE, Chelliah Y, Hatfield DL, and Gladyshev VN (2005). A novel cysteine-rich domain of Sep15 mediates the interaction with UDP-glucose:glycoprotein glucosyltransferase. J. Biol. Chem 280, 37839–37845. [DOI] [PubMed] [Google Scholar]
- Labunskyy VM, Yoo MH, Hatfield DL, and Gladyshev VN (2009). Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry 48, 8458–8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy VM, Hatfield DL, and Gladyshev VN (2014). Selenoproteins: molecular pathways and physiological roles. Physiol. Rev 94, 739–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh PC, van den Barselaar MT, van Zwet TL, Daha MR, and van Furth R (1979). Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J. Clin. Invest. 63, 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. ; Exome Aggregation Consortium (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, and Sitia R (2001). Manipulation of oxidative protein folding and PDI redox state in mammalian cells. EMBO J. 20, 6288–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoselov SV, Hua D, Lobanov AV, and Gladyshev VN (2006). Identification and characterization of Fep15, a new selenocysteine-containing member of the Sep15 protein family. Biochem. J. 394, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MA, and Hoffmann PR (2009). The human selenoproteome: recent insights into functions and regulation. Cell. Mol. Life Sci 66, 2457–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier CS, and Kaiser CA (2002). Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol 3, 836–847. [DOI] [PubMed] [Google Scholar]
- Sitia R, Rubartelli A, and Hammerling U (1984). The role of glycosylation in secretion and membrane expression of immunoglobulins M and A. Mol. Immunol 21,709–719. [DOI] [PubMed] [Google Scholar]
- Sousa MC, Ferrero-Garcia MA, and Parodi AJ (1992). Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc: glycoprotein glucosyltransferase. Biochemistry 31, 97–105. [DOI] [PubMed] [Google Scholar]
- Spang A (2013). Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol 5, a013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Deng T, and Fu J (2014). Chicken 15-kDa selenoprotein plays important antioxidative function in splenocytes. Biol. Trace Elem. Res. 161, 288–296. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. (2017). The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45 (D1), D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Seko A, Hachisu M, Daikoku S, Izumi M, Koizumi A, Fujikawa K, Kajihara Y, and Ito Y (2014). Both isoforms of human UDP-glucose:glycoprotein glucosyltransferase are enzymatically active. Glycobiology 24, 344–350. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, and Helenius A (2000). Conformational requirements for glycoprotein reglucosylation in the endoplasmic reticulum. J. Cell Biol. 148, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte D, Schips TG, Kwong JQ, Davis J, Tjondrokoesoemo A, Brody MJ, Sargent MA, Kanisicak O, Yi H, Gao QQ, et al. (2016). Thrombospondin expression in myofibers stabilizes muscle membranes. eLife 5, e17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber C, Fan JY, Guhl B, Parodi A, Fessler JH, Parker C, and Roth J (2001). Immunolocalization of UDP-glucose:glycoprotein glucosyltransferase indicates involvement of pre-Golgi intermediates in protein quality control. Proc. Natl. Acad. Sci. USA 98, 10710–10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.