Abstract
An important question is whether consensus mechanisms for copper monooxygenase enzymes such as peptidylglycine monooxygenase (PHM) and dopamine β-monooxygenase (DBM) generated via computational and spectroscopic approaches account for important experimental observations. We examine this question in the light of recent crystallographic and QMMM reports which suggest that alternative mechanisms involving an open to closed conformational cycle may be more representative of a number of experimental findings that remain unaccounted for in the canonical mononuclear mechanisms. These include (i) the almost negligible reactivity of the catalytic copper site (CuM) with oxygen in the absence of substrate, (ii) the carbonyl chemistry and in particular the substrate-induced activation exemplified by the lowered CO stretching frequency, (iii) the peroxide shunt chemistry which demands an intermediate that facilitates equilibrium between a Cu(II)-peroxo state and a Cu(I)-dioxygen state, and (iv) clear evidence for both closed and open conformational states in both PHM and DBM. An alternative mechanism involving a dinuclear copper intermediate formed via an open to closed conformational transition appears better able to accommodate these experimental observations, as well as being shown by QMMM methodologies to be energetically feasible. This suggests that future experiments should be designed to distinguish between these competing mechanisms and the factors that govern the oxygen reactivity of the copper centers. In particular, determining how oxygen reactivity is activated by binding of substrate, should be considered an important new challenge.
Keywords: copper, monooxygenase, peptidylglycine, PHM, dopamine, DBM, oxygen, superoxide, mononuclear copper, dinuclear copper
Graphical Abstract

Introduction
Interest in copper dioxygen chemistry derives at least in part from the implied function of Cu(I)-O2 complexes as intermediates in copper monooxygenase mechanisms [1, 2]. In this short review we examine the reactivity of the active sites of the so-called mononuclear copper monooxygenases towards dioxygen, choosing the peptidylglycine monooxygenase (PHM) and dopamine β-monooxygenase (DBM) family as primary examples [3–5]. At issue is the assumption that the Cu(I) forms of these enzymes are reactive towards dioxygen in their resting states, such that their crystal structures can be used as starting points for modeling copper-dioxygen reactivity. In many cases this assumption ignores a crucial aspect of the reactivity of the enzymes, namely that their oxygen chemistry is completely coupled to substrate hydroxylation, indicating that Cu(I)-dioxygen reactivity is triggered by binding of substrate. PHM and DBM are excellent examples of this mechanistic paradigm.
Monooxygenases catalyze a reaction where a single atom of molecular oxygen is inserted into a C-H bond to form a C-OH entity, with the other atom of the oxygen molecule being reduced to water. The energy required to break the C-H bond is of the order of 104 kcals mol−1 while only some 84 kcal mol−1 is recovered on hydroxylation. The energy deficit is made up from the favorable energetics of oxygen reduction to water, provided that the initial unfavorable spin-restricted one-electron reduction of O2 to superoxide can be overcome. The enzymes are believed to work their catalytic magic via the formation of an initial copper-dioxygen complex which overcomes the endergonic step via internal electron transfer to generate a Cu(II)-superoxide species capable of further reactivity without constraints of spin restriction. Thus the nature and reactivity of the initial copper-dioxygen species is crucial to understanding the monooxygenase mechanism.
A major factor determining the reaction chemistry is the separation between metal centers in monooxygenase enzymes. The ‘dinuclear’ enzymes exemplified by tyrosinases have an active site with two copper ions in close (< 4 Å) proximity and capable of binding oxygen via an η2- or “side-on” bridging mode between the metal ions. Because these active sites can transfer two electrons to oxygen, they generate the μ-peroxo di-Cu(II) species immediately and reversibly upon reaction of the di-Cu(I) forms with oxygen. Mononuclear monooxygenases on the other hand either have a single copper center per protomer as is the case in the lytic polysaccharide monooxygenase family (LPMO), or bind their copper centers in separate subunits (particulate methane monooxygenase, pMMO) or in different sub-domains (PHM, DBM). For these enzymes, the two-electron transfer to form the peroxo species is not immediately available, and the first product is a Cu(II)-superoxo species. To complete the monooxygenase mechanism a second electron must be furnished, and how this is achieved represents a major unresolved question for all three mononuclear classes.
PHM and DBM
PHM and DBM are members of a small group of mononuclear monooxygenases which also include the insect analogue of DBM, tyramine β-monooxygenase, as well as a human homologue of unknown function called monooxygenase X or MOX [3, 5, 6]. PHM is widely distributed in plant and animal kingdoms and catalyzes the C-terminal amidation of glycine-extended pro-peptides [4, 7]. The amidation proceeds in two parts, the first involving stereospecific hydroxylation of the pro-S hydrogen of the glycine in a copper/ascorbate/O2 dependent reaction within the PHM domain [8, 9], while the second involves hydrolysis of the carbinolamide intermediate to form amidated peptide and glyoxalate in a Zn dependent process within the PAL domain [10–12] (Figure 1). DBM and TBM catalyze hydroxylation of the pro-S β-carbon of catecholamines dopamine and tyramine to form nor-epinephrine and octopamine respectively. Although more complex in their tertiary and quaternary structure, these enzymes share a common catalytic domain with PHM and appear to have identical active site copper coordination [13, 14]. Crystal structures are available for this catalytic core which shows a pair of copper atoms separated by 11 Å across a solvent-filled cleft between the two sub-domains [8, 9, 15, 16] (Figure 2). The individual copper sites have been termed CuH and CuM based on the residues that they coordinate [17, 18]. Thus CuH is coordinated by three His residues (H107, H108 and H172 PHM numbering) while CuM, named for its Met ligation, is coordinated by residues H242, H244, and M314. In addition to crystallography, PHM has been studied extensively by spectroscopic [17–21], kinetic [22–27], and computational [28–31] approaches which have identified many aspects of the structure-reactivity relationships that govern catalysis. These studies have led to a canonical mechanism (Figure 3) which we will briefly describe here and in more detail later in the text. The reaction is proposed to be initiated by dioxygen binding at the reduced Cu(I)M center which forms a Cu(II)-superoxo species. Since the two Cu centers are 11 Å apart, this copper-superoxide species is inhibited from picking up an electron from the second copper, rather seeking to resolve its enhanced electrophilicity by abstracting the pro-S H atom from the peptide substrate to form Cu(II)-peroxide and a peptidylglycyl Cα-based radical. At this point in the mechanism an additional electron needs to be transferred to break the O-O bond and generate products, but here consensus diverges as to whether this electron is supplied directly by CuM to form a Cu(III)-OH/Cu(II)-O•- entity and hydroxide ion, or via long-range proton coupled electron transfer (PCET) from CuH. Other mechanistic details, such as which oxygen is the initial recipient of the H atom, have also been debated. In this review we concentrate more on the initial stages of the chemistry, specifically the formation of the initial oxygen complex at the CuM site. Here, a crystal structure of what has been termed a pre-catalytic dioxygen adduct has served as a guide to the oxygen reactivity of the M center [32] (Figure 2(c)). The structure shows a diatomic molecule with a short 2.23 Å O-O bond coordinated to CuM together with a bound “slow” substrate iodotyrosyl-D-threonine. The Cu-O-O angle is 110° as expected, but the distal O points away from the pro-S H in a seemingly unproductive conformation.
Figure 1.
Reactions catalyzed by copper monooxygenases. (a) dopamine β-monooxygenase, DBM (b) peptidylglycine monooxygenase PHM, showing only the monooxygenase reaction (c) lytic polysaccharide monooxygenase LPMO: oxidation occurs at either C1 or C4 as shown (d) particulate methane monooxygenase pMMO. Oxygen atoms derived from molecular oxygen are shown in red.
Figure 2.
Structures of PHM (a) catalytic core residues 42 – 356 with the CuM subdomain on the left and the CuH subdomain on the right. Copper atoms M and H are shown as cyan spheres. The substrate di-iodotyrosylglycine is shown in slate; structure taken from RCSB entry 1OPM (b) close up of the active site with metal-binding residues numbered; the di-iodiotyrosylglycine substrate (raspberry red) is shown H-bonded via its C-terminal carboxylate to R240 (c) structure of the M center with dioxygen (red) and bound peptide (iodotyrosyl-D-threonine (slate)) with the C that is hydroxylated in green; structure taken from RCSB entry 1 SDW. All structures were rendered in PyMOL version 2.3.3 (Schrodinger LLC).
Figure 3.
Representation of the time-dependent density functional theory (TD-DFT) generated “canonical” mechanism for PHM hydroxylation adapted from reference [30].
Mutagenetic analysis of the copper ligands has established that no residue at either copper center can be removed or substituted without almost complete loss of activity (>95%) [24, 33–37]. With the exception of H242A, all of the studied mutants bind copper at both sites in solution. Of particular interest is the Met residue M314, which appears absolutely essential: even mutation to histidine causes a 95% decrease in activity, which is surprising given the large literature showing that CuHis3 sites are generally reactive towards molecular oxygen [37]. Another interesting variant is the H-center H172A, which does not prevent copper binding but diminishes the rate of the chemical step (H-atom abstraction) by 1000 fold [24].
The mutagenesis data emphasize that PHM chemistry is very specific for the ligand set and coordination geometry of the copper ligands. However it also provides important constraints on any valid mechanism. Most PHM variants studied to date, with the exception of H242A, retain two to five percent of activity which has allowed the extent of coupling between oxygen reduction and substrate hydroxylation to be measured. This approach first applied to the DBM system by Klinman and coworkers [38] determines the ratio of product formed (usually by HPLC) to oxygen consumed. Wild-type and all variants of PHM and DBM exhibit 100 percent coupling, i.e. the molar ratio of oxygen consumed is exactly equal to that of hydroxylated product generated. One exception to this finding occurred for the active site Met to Cys mutation in tyramine β-monooxygenase where reaction inactivation decreased the amount of product generated [39]. The almost universal coupling of product to oxygen reduction implies that no unproductive side reactions occur, and specifically that no reduced oxygen species (peroxide) are allowed to form via redox cycling of the copper atoms and ascorbate.
While the enzyme provides complete substrate triggering of hydroxylation activity, it is still possible that this is the result of rapid irreversible reaction of the Cu(I)-dioxygen initial adduct with nearby bound substrate. In this scenario, we would expect that even in the absence of substrate this robust oxygen reactivity of the CuM site should be manifest by stoichiometric oxidation of ascorbate, catalyzed by the CuM center to generate dehydroascorbate and peroxide. The reaction would proceed via formation of the Cu(II)-superoxo (as suggested in the canonical mechanism), followed by dissociation and protonation of the superoxide, and finally disproportionation to form peroxide and oxygen. Ascorbate would then re-reduce the Cu(II)-M center allowing subsequent cycles to continue. This type of uncoupled reactivity does occur in monooxygenases such as the lytic polysaccharide monooxygenases discussed later [40]. Multiple pieces of evidence suggest that this reaction does not occur in PHM. Mixtures of ascorbate and PHM consume oxygen slowly in the absence of substrate in a process that is completely inhibited by catalase, showing that any ascorbate oxidation is propagated by peroxide-induced radical chemistry. Additionally, when ascorbate-free reduced PHM is exposed to oxygen, only 5 – 10 percent of the expected EPR signal of the Cu(II) form of the enzyme is generated in 2 minutes (unpublished observation). It should also be noted that fast oxygen reactivity of the Cu(I)-His2Met site is inconsistent with complete coupling with very slow variants, as some superoxide leakage would be anticipated. This discussion emphasizes that PHM has the property of being able to suppress CuM site oxygen reactivity until the enzyme has committed to catalysis. Computational approaches must recapitulate this aspect of catalysis in any valid description of the mechanism.
CO Chemistry Provides a Window on Oxygen Reactivity.
One approach to studying dioxygen binding and activation by copper monooxygenases is to examine the reaction chemistry of O2-binding surrogates such as carbon monoxide. CO binds to Cu(I) complexes via a combination of strong sigma donation from the lone-pair of electrons resident on the C atom coupled to back-donation of metal electron density from d-orbitals of appropriate symmetry to interact with the empty π*-orbitals of the CO. This π-back-bonding from metal to ligand decreases the C≡O triple bond order, which, in the case of a dioxygen adduct, would be equivalent to electron transfer or partial oxygen reduction. The degree of back-bonding is dependent on the overall electron density on the Cu(I) ion, which in turn depends on the amount of sigma donation from the other ligands in the complex. Decrease in bond order is directly observable via red-shifts in the infrared stretching frequency of the carbon-oxygen bond of CO complexes which occurs in the 2150–2000 cm−1 region of the IR spectrum, a region devoid of other vibrational modes of the protein. Binding of CO to copper monooxygenases and their active site variants monitored by FTIR is thus a powerful method for estimating how copper ligands contribute to the stability and/or the activation of bound diatomics.
Recognizing this possibility, we started our studies on copper monooxygenases examining the CO binding ability of DBM. We first developed a binding assay to determine which of the two coppers bound CO [41, 42]. A sample containing deoxyhemoglobin was titrated with aliquots of a CO-saturated solution of fully reduced DBM where the CO bound to DBM plus that dissolved in the buffer were captured by deoxyHb and the concentration determined by absorbance at 420 nm. The amount of CO dissolved in the buffer was next determined by an enzyme-free titration, allowing the stoichiometry of CO bound to the enzyme to be determined by subtraction. We found that DBM bound CO at only one of its two copper centers. Next we constructed a half-apo derivative by gently dialyzing the CO-bound enzyme against KCN under a CO atmosphere, which removed the copper that was not “protected” by the stability of its CO complex. Subsequent extended x-ray absorption fine structure (EXAFS) analysis of the product showed that the Cu-S interaction from the coordinated Met residue was still present in the half-apo CO complex allowing assignment of the CO reactivity to CuM [43]. We later applied this chemistry to PHM and showed identical CO reactivity [18, 44]. CO binding was also studied by Fourier transform infrared (FTIR) spectroscopy, and frequencies of 2089 cm−1 and 2093 cm−1 were determined for the DBM and PHM M-centers, respectively. When compared with known CO frequencies for other copper proteins (hemocyanin, cytochrome c-oxidase) and with the extended literature of model Cu(I)-CO compounds (Table 1), these frequencies were entirely consistent with the His2Met ligand set. Although few CO complexes with a His2Met ligand set have been reported, the large literature of CO complexes of the His3 ligand set (vide infra and Table 1) indicated lower frequencies were expected for His3 in the 2080– 2060 cm−1 region. The poor donor properties of the thioether ligand is responsible for decreasing the electron density available for back-donation in the His2Met ligand set thereby increasing the CO frequency.
Table 1.
Carbon Monoxide stretching frequencies for Cu(I) complexes of copper proteins and selected model compounds.
| Sample | Ligand Set (excluding CO) | Coord. No (including CO) | Frequency (cm−1) | Reference |
|---|---|---|---|---|
| DBM | 2N(His) 1S(Met) | 4 | 2089 | [41] |
| PHM | 2N(His) 1S(Met) | 4 | 2093 | [18] |
| PHM + AcYVG | 2N(His) 1S(Met) | 4 | 2093, 2063 | [18] |
| PHM + Benzoylglycine | 2N(His) 1S(Met) | 4 | 2093, 2075 | [18] |
| PHM M314H | 3N(His) | 4 | 2075 | [37] |
| PHM M314H + AcYVG | 3N(His) | 4 | 2051 | [37] |
| CusF W44AM49H (M-site model) | 2N(His)S(Met) | 4 | 2089 | [57] |
| SeM CusF W44AM49H (M-site model) | 2N(His)Se(Met) | 4 | 2087 | [58] |
| Aβ(10–14) YEVHH | 2N(His) | 3 | 2110 | [69] |
| Histidylhistidine (Nδ coordinated) | 2N(His) | 3 | 2110–2105 weak | [67] |
| Histidylhistidine + N-methylimidazole | 2N(His)N(imid) | 4 | 2075 strong | [67] |
| HisXHis (X=Gly) (Nε coordinated) | 2N(His) | 3 | 2092 strong | [88] |
| Hc (mollusk) | 3N(His) + Cu-Cu | 4 | 2062 | [45] |
| Hc (limulus) | 3N(His) + Cu-Cu | 4 | 2053 | [45] |
| Hc (arthropod) | 3N(His) + Cu-Cu | 4 | 2043 | [45] |
| aa3-cytochrome oxidase | 3N(His) + Cu-Fe | 4 | 2066, 2054, 2039 | [89] |
| ba3-cytochrome oxidase | 3N(His) + Cu-Fe | 4 | 2054 | [89] |
| bis-dimethylimidazole ([Cu-(Me2imid)2]+) | 2N(imid) | No reaction | [90] | |
| tris-dimethylimidazole ([Cu-(Me2imid)3]+) | 3N(imid) | 4 | 2069 | [90] |
| tris-(3,5-dimethylpyrazolyl)borate | 3N(pyrazole) | 4 | 2081 | [91] |
| tris-(2-methylpyridyl)amine (TMPA) | 3N(py) and 4N(py) | 4 and 5 | 2094, 2075 | [92] |
| 1H-imidazol-4-yl)-N,N-bis((pyridin-2-yl)methyl)ethanamine | 2N(py)N(imid) | Probably 4 | 2082 | [92] |
| 2-Ethylthio-N,N-bis(pyridin-2-yl)methylethanamine | 2N(py)Sthioether or 3N(py) | 4 | 2094 | [93] |
An interesting twist to this story arose when the fully metallated PHM-CO complex was titrated with its substrate peptide N-Acetyl-Tyr-Val-Gly (Ac-YVG) [18]. Here, in addition to the 2093 cm−1 peak, a new peak was observed at 2063 cm−1, where the lower frequency suggested that substrate binding induced a flow of electron density onto the CO, driving activation of the diatomic molecule. This peak was shown to be due to a CO complex by isotope labeling, but was also substrate dependent. The complex with benzoylglycine, a slower substrate than Ac-YVG, gave a peak at 2075 cm−1. Initially it was speculated that the new feature might be due to a CO complex of the His3-ligated CuH center, which in turn suggested that CuH, rather than CuM, might be induced to bind oxygen by substrate binding and might indeed be the site of catalytic oxygen chemistry. Following on from this finding we proposed a “superoxide channeling” mechanism whereby CuH was activated by substrate to bind O2 and the superoxide generated then channeled across the solvent filled cleft to react with CuM and form a CuM-peroxo.
While this mechanism was disproved by later studies, particularly the substrate/dioxygen coupling work on slow mutants described above (which predicted superoxide leakage and uncoupling), the substrate activation of CO was and still remains a fascinating and as yet unresolved aspect of PHM mechanism. In later work we were able to unambiguously assign the 2063 cm−1 peak to a substrate-induced Cu(I)M carbonyl via studies on the M314H variant [37]. Mutation of the M314 residue to histidine creates a His3 ligand set (validated by EXAFS) which binds CO with a frequency of 2075 cm−1. The decrease in ν(CO) from 2093 cm−1 in the wild-type enzyme is expected on the basis of substitution of the weak thioether donor for the stronger imidazole ligand. The most interesting result of this study was the frequency shift upon addition of substrate Ac-YVG. First, the ν(CO) red-shifted to 2051 cm−1, allowing definite assignment to CuM since the substrate-induced shift was sensitive to mutation at CuM. Second, the observed frequency was well below what is usually found for mononuclear Cu(I)His3CO complexes and is one of the lowest frequencies ever observed for a Cu(His)3 ligand set (Table 1). It falls in the range observed for the CO complexes of the coupled dinuclear hemocyanins (2062 – 2043) cm−1 which have the same His3 coordination at each copper center. However, the interaction of the O atom of the CO ligand with the positive charge of the second Cu(I) of the binuclear site is believed to induce the red-shifted frequency [45]. Red-shifted IR frequencies have also been found between 2060 and 2036 cm−1 in the CuB-CO complexes of cytochrome-c-oxidase produced by flash photolysis of the Fe(II)-CO complex in the Fe-Cu binuclear site [46, 47], and, like hemocyanin, appear to correlate with the presence of a second positively charged metal ion in the vicinity of the CO binding site. This raised the question: could the substrate induced CO chemistry signal a conformational change to a state where the copper centers are closer than the 11 Å inter-metal distance observed by crystallography?
The Open and Closed Conformations of DBM and PHM.
One of the recent and thought-provoking observations on the structures of monooxygenase enzymes came into focus when a crystal structure of the catalytic domain of DBM was published in 2016 [14]. One of the two molecules in the unit cell was in the apo state and very similar to the structure of apo-PHM [48]. Modeling the two copper atoms into their respective H and M sites gave an inter-metal distance of around 14 Å. The second molecule in the unit cell exhibited a markedly different conformation. While the N-terminal H-site domain was roughly equivalent, the C-terminal M-site domain was rotated 18° about a hinge point so as to bring the two subdomains into close contact with a CuM-CuH distance of about 4 Å (Figure 4). The H-site of this conformer was empty, but the M-site was partially occupied by a single copper atom. The authors discussed whether this “closed” conformation was an artifact of expression or crystallization but also floated the possibility that domain movement from open to closed conformation might be a feature of the catalytic mechanism, generating an intermediate state with a binuclear copper center that could bind and reduce oxygen via established 2-electron transfer mechanisms common to tyrosinase [29, 49], NspF from Streptomyces murayamaensis [50] catechol oxidase [51] and many dinuclear model complexes [52–55]. For this mechanism to be credible, a similar “closed” conformation would need to exist for PHM, since the two proteins share a common catalytic mechanism [3, 22]. Recently a “closed” structure has indeed been described for a PHM variant [48]. When the H108A variant is crystallized in the presence of citrate, a closed conformer is formed with almost identical topology to the closed conformer of DBM, as well as the same 18° rotation of the M-domain towards the H-domain (Figure 5). This structure contains only a single copper atom coordinated by two M-site histidine ligands (H242, H244), by one H-site histidine (H107) and two oxygens from a carboxylate and the α-OH of citrate. Intriguingly, the citrate molecule appears to connect the H to the M site via three contacts: the coordination to the Cu center and two H-bonds to R240 and H172. The two H-bonding interactions take on added significance when the roles of these residues are considered. R240 binds the C-terminal carboxylate of peptidylglycine substrates, while the H172 ligand has been identified as critical to the chemical (H-atom abstraction) step of the mechanism [24]. Structural similarities between citrate and the physiological PHM reductant ascorbate may suggest that the latter could fulfil a similar role in inducing an open to closed conformational change.
Figure 4.
Open (left) and closed (right) conformations of DBM (pdb file 4EZL). Modeled Cu locations are shown as brown spheres
Figure 5.
Alignment of the closed crystal structure of H108A in the presence of citrate (PDB file 6ALA) with the open structure of oxidized PHM (PDB file 1PHM). (a) Alignment of the whole molecules to the C-alpha backbone of the N-terminal H-domain (RMSD 0.41 Å) (b) C-terminal M-domain showing the 18° rotation or hinge motion of the M-domain about residue 201 and (c) alignment of the metal binding region. The H108A-citrate and the oxidized native PHM main chains are depicted by pink and slate ribbons respectively. To emphasize the hinge motion, the first β-strands of the M-domains are colored green (closed: H108A citrate) and dark blue (open: oxidized PHM). The single copper atom of the closed H108A-citrate structure which coordinates M-site residues H242 and H244 and H-site residue H107 is shown in pink, while the citrate molecule is shown as a green carbon backbone with oxygen atoms in red. The positions of the two copper sites in the open PHM structure are shown as transparent slate spheres. Differences between the open and closed structures shown in the Figure are discussed in the text.
For an open to closed transition to be mechanistically feasible, it would have to proceed with an activation energy lower than that required for hydrogen atom abstraction (HAA) chemistry. Wang and coworkers [31] have recently explored the energy landscape for such a mechanism using a QMMM approach. These calculations suggested an energetically feasible reaction pathway via a closed conformer binuclear intermediate with an energy barrier for HAA significantly less than that calculated for the mononuclear superoxo pathway [30]. They also reported a surprising low (2 kcal mol−1) energetic difference between the open and closed conformer. The authors make the interesting observation that abstraction of a H atom from ascorbate, always present in large excess, also has a lower energy barrier than for peptidyl C-H HAA chemistry. Their study suggests that, in the presence of excess ascorbate, formation of an ascorbate radical and peroxide is more favorable than a Cα peptidyl radical, and that the Cu(II)M hydroperoxo species (formed only in the presence of ascorbate) drives the conversion of open to closed conformer. They postulate that a μ-oxo-, μ-hydroxo- mixed-valence Cu(I)-Cu(II) entity is the active intermediate; as yet, no direct evidence exists for the existence of such a species. A modified version of their postulated mechanism is shown in Figure 6.
Figure 6.
Representation of the open-to closed mechanism for PHM/DBM hydroxylation adapted from reference [31]
Reactivity of the Mononuclear Cu(I)His2Met site towards O2.
We now return to the question of the apparent low reactivity of fully reduced PHM with molecular oxygen. As discussed above, this feature of PHM chemistry derives from the complete coupling of dioxygen consumption to peptidylglycine substrate hydroxylation, and the extremely slow re-oxidation of fully reduced ascorbate-free enzyme by O2 as detected by recovery of the Cu(II) EPR signal. We note that while the enzyme exhibits this sluggish reactivity towards O2, an N3S thioether-containing model Cu(I) complex has recently been shown to react with oxygen to form a Cu(II)-superoxo species capable of HAA chemistry at cryogenic temperatures in organic solvents [56]. However, at room temperature, dissociation of O2 regenerates the parent Cu(I) complex, suggesting entropic destabilization at ambient temperatures potentially recapitulating the enzyme reactivity at its normal operating temperature. To further probe this chemistry, we constructed models of the PHM CuM site by rational design of this site into a small protein scaffold, CusF [57, 58]. CusF is a metallochaperone that transfers Cu(I) to both CusB and CusA as part of the CusBCAF exporter machinery [59–63]. The native protein has a Met2His Cu(I) binding site capped by a tryptophan π-cation interaction. We created the double mutant W44A, M49H in a M8IM59I background, the latter to remove the non-coordinating Met residues, such that the single Met was a ligand to Cu(I). The resulting His2Met ligand set of the protein variant now accurately mimicked the PHM M-site, which was demonstrated by EPR, EXAFS, and FTIR of the CO complexes [57]. The CO chemistry of the model was particularly instructive, showing coordination of a single CO, and recapitulating the ν(CO) of the M-site of DBM exactly, and that of PHM within 4 cm−1. Other attributes of the PHM M-site were also accurately reproduced, such as the larger than expected Debye-Waller factor for the Cu-S(Met) bond, further validated from the Se edge EXAFS of the selenomethionine derivative [58]. Of further relevance, when the single Met residue was mutated to His to emulate the M314H variant of PHM, the resulting CO derivative exhibited a red-shifted ν(CO) of 2075 cm−1, identical to its enzyme counterpart. The structural data were further validated via DFT calculations to determine optimized geometries as shown in Figure 7.
Figure 7.
Rational design of the PHM M-site using the CusF W44AM49H double mutant in the M8IM59I Met-deletion background. The figure shows in silico generated geometry optimized structures for the unligated and CO-bound sites. Geometry optimization was carried out in ORCA 3.0 [94] using the BP86 functional [95] and TZVP basis set [96]. Alpha carbon positions were constrained to X-ray crystallographically determined coordinates (PDB 2VB2). Distances from the Cu(I) center to the ligands are as follows with EXAFS derived distances from reference [57] listed in parentheses, Cu-N(His) distances reported are an average of the two His ligands. M49H: Cu-S(Met) 2.27A (2.28A); Cu-N(His) 1.99A (1.94A);. M49H-CO: Cu-S(Met) 2.42A (2.33A); Cu-N(His) 2.05A (1.99A); Cu-CO 1.83A (1.84A).
The oxidized proteins all formed azido- adducts with strong azide to Cu(II) charge transfer bands centered at 390 nm, again emblematic of PHM chemistry in the Cu(II) state. The rapid reduction of these azido- adducts by ascorbate could be monitored using stopped flow detection at 390 nm; this revealed the unexpected result that Cu(I)His3 is reduced more rapidly than Cu(I)His2Met. Thus the role of the fully active Met314 in the enzyme cannot be to solely stabilize the Cu(I) state, since the inactive M314H clearly has a more positive redox potential.
A second unexpected and important result was the complete inertness of the Cu(I) forms of the model towards O2. In this experiment, ascorbate was removed by desalting after reduction of the protein models and the resulting Cu(I) complexes exposed to oxygen in the presence of sodium azide, which forms the chromophoric Cu(II)-azido adduct upon oxidation. No reaction was observed over a period of 2 hours, confirming the lack of reactivity of the Cu(I)His2Met site towards oxygen in the absence of other site perturbations.
Distinguishing Between Mechanisms.
While elegant in their ability to predict reaction intermediates and the associated transition states for their formation and decay, computationally derived mechanisms must also account for experimental observations, and in particular the effects of mutations on catalytic rates. In the case of PHM, they must (i) explain how the enzyme converts a seemingly inactive Cu(I)M site to an active one upon binding peptidyl substrate, and provide a rational assignment of the 2063 cm−1 Cu(I)-CO species; (ii) predict the absolute requirement for methionine at position 314 and dramatic loss of activity in the M314H mutant [37]: this is significant since Cu(I)His3 systems are known to be reactive towards O2 in biomimetic models [64]; (iii) provide a rationale for the observation that the H172A variant decreases the rate of H-atom abstraction 1000 fold yet is positioned far from the Cα-H bond [24]; and finally (vide infra) the fact that substrate hydroxylation can be driven by hydrogen peroxide from the oxidized Cu(II) enzyme (peroxide shunt) in a slow yet still catalytic reaction in which the 18O of labeled peroxide is scrambled 60–70 percent with ambient 16O2 in air [65].
The hypothesis supported by Wang’s recent QMMM study [31], that an open to closed conformational transition generates the reactive entity in which HAA chemistry can proceed, provides a rationale for all of the above experimental observations. In preceding sections we have discussed how the binuclear mechanism rationalizes the substrate triggering and associated induction of the 2063 cm−1 M-site carbonyl. The remaining observations can also be rationalized within the context of the closed structures exemplified by Figures 4 and 5. With respect to the H108A structure (Fig. 5) the citrate molecule connects the H and M centers via 3 interactions: (i) bidentate coordination to the single Cu atom, (ii) H-bond to the guanidinium N of Arg240 which typically binds the C-terminal peptidyl carboxylate, and (iii) a H-bond to the Nδ of H172. To accommodate the binding of both M-site and H-site histidine residues, the copper atom has moved some 3Å such that the M314 thioether S to Cu distance has increased to 5.6 Å. The latter is particularly significant since mutation of residue M314 to histidine could frustrate the required movement on account of its stronger sigma donor properties. This could explain the perplexing observation that the weakly coordinating Met residue is essential to catalysis, perhaps as the result of the weak, fluxional character of a single Cu(I)-Met bond as recently demonstrated in our studies of the SMet and SeMet complexes of the PHM CusF model (vide supra) [57, 58].
We also note that an uncoordinated H172 Nδ position is required for the citrate H-bond. We have previously shown that in reduced forms of M-site deletion mutants such as H242A CuH is 2-coordinate with one His ligand dissociated [66]. While EXAFS could not detect which His residue was dissociated, it is reasonable to propose H172 as the most likely candidate, since H107 and H108 coordinate in a near linear fashion via their Nδ imidazole donor atoms in a configuration known from model histidylhistidine complexes to be particularly stable in the Cu(I) state [67]. Alternatively, as suggested from both our own stopped flow studies on PHM reduction [68] and from studies on the Cu(I) complexes of the Aβ amyloid peptide, the site could be 2-coordinate yet fluxional, with two out of three His residues coordinated at a time [69–71]. While the closed PHM H108A appears to require citrate binding, one may speculate that other hydroxyl or keto acids might react in a similar fashion. Therefore, a reasonable extension of these ideas is that ascorbate could fulfil a bifunctional role in site closure, via reductive dissociation of H172 and subsequent H-bonding of one of its free hydroxyl or keto moieties to the H172 Nδ. Since the H172 ligand would thus be critical in forming the binuclear intermediate, this reconciles its critical role in HAA chemistry.
Among these many aberrant observations relative to the canonical mononuclear mechanism, the hydrogen peroxide reactivity is the most perplexing. We showed nearly a decade and a half ago that peptidylglycine hydroxylation could be driven from the fully oxidized enzyme using hydrogen peroxide as the source of oxygen atoms [65]. This reaction technically conforms to a simple peroxide shunt and does not require a supply of additional electrons from a reductant. We showed that product was generated equivalent to at least 40 turnovers and, when external oxygen was strictly eliminated using the glucose/glucose oxidase reaction as the source of anaerobic H2O2, the product was 100% coupled to peroxide consumption. One might conclude from these data that a Cu(II)-peroxo species must be a reactive intermediate, which is not inconsistent with the mononuclear mechanism provided that electron transfer occurs prior to HAA from substrate such that the Cu(II)M-peroxo is the reactive species. However, product isotope distribution ratios threw a wrench into this simplistic interpretation, as 60 % of the 18O label was scrambled with ambient 16O2 in air when H218O2 was used as the source of peroxide. This implied formation of an intermediate that allowed exchange of isotopically labeled dioxygen or in other words an intermediate where a Cu(II)-peroxo species was in equilibrium with a Cu(I)-dioxygen species. In an attempt to explain the isotope ratios, we postulated that peroxide could reduce the Cu(II)H to Cu(I) and superoxide, and that the superoxide generated migrated to the Cu(II)M site where it bound as Cu(II)-superoxo in equilibrium with Cu(I)-O2. This chemistry was in many ways equivalent to the previously proposed superoxide channeling mechanism [18], in which superoxide generated by reaction of Cu(I)H with O2 migrated to the M site to form the Cu(II)M-superoxo intermediate; this mechanism was discredited on the grounds that, with slow substrates or slow site-directed variants, superoxide must leak from the catalytic cleft leading to uncoupling [24]. In truth, an identical criticism can be levied at the peroxide-induced superoxide channeling from Cu(II)H-peroxo to CuM since, under strict anaerobic conditions, the reaction is also fully coupled. Thus some alternative mechanism is necessary to explain the isotope scrambling. A binuclear intermediate formed within the closed conformer can readily explain the data due to the well-known equilibrium between diCu(II)-peroxo and diCu(I)-O2 species. Finally, we note that reaction of peroxide with Cu(II) PHM led to a 25% decrease in EPR detectable Cu(II), which was interpreted as evidence for the reduction of the H center to the Cu(I) state yet could equally be the result of formation of a spin coupled diCu(II) intermediate.
We cannot leave this section without offering some alternative explanation for substrate triggering in PHM. Cowley and Solomon, on the basis of TD-DFT calculations, concluded that the formation of the Cu(II)-superoxide at the M site was energetically favorable only if the dioxygen displaced a hydroxide ligand from the Cu(I) center [30]. While Cu(I)-OH complexes are rare, crystal structures of the reduced enzyme do show water/hydroxide density near the CuM center which appears to be a coordinated solvent species. It is possible that substrate binding in the reduced state deprotonates the coordinated water to a hydroxide, which destabilizes the Cu(I)M site and induces reaction with oxygen. Such reactivity involving solvent ionization at the reduced Cu(I)M center is yet to be demonstrated experimentally but remains an interesting possibility for the origin of substrate triggering. However, the fact that the catalytic rate decreases with increasing pH above pH 6 would appear to mitigate against M-site water deprotonation as the driver of substrate triggering.
Cu(I)-Dioxygen Complexes in Other Monooxygenases
It is not surprising that the PHM and DBM catalytic reactions are completely coupled, showing no reactivity in the absence of peptide substrate, since mammalian hosts are generally unable to synthesize ascorbate, and would avoid squandering this precious molecular resource in a non-productive reaction. Indeed, uncoupled reactions involving oxidation of a reductant would be counterproductive for any organism where much energy is spent generating reducing equivalents (NADH production driven by water oxidation coupled to photosynthesis is one example). The lytic polysaccharide monooxygenases (LPMOs) however do react with molecular oxygen in their ascorbate-reduced Cu(I) forms both in the presence and absence of their substrates [40]. LPMOs catalyze the oxidative cleavage of β 1,4-glycosidic bonds in oligosaccharides by hydroxylation of either the C1 or the C4 C-H bond [72–74] (Figure 1). They are used in monooxygenase reactivity with cellulases to break down biomass to form fermentable sugars. These enzymes are divided into a variety of sub-classes (which differ largely in the identity of their natural substrate), with the A9 and A10 classes being most highly studied with respect to the reactivity of the copper sites. The enzymes bind a single copper coordinated by the N-terminal His residue in a bidentate configuration called the histidine brace, where the terminal amino and an imidazole N coordinate the copper (Figure 8 (a)). An imidazole group from an internal histidine provides a third equatorial ligand while a tyrosine binds along one axis. The fourth equatorial position is open and assumed to be the site of oxygen binding in the Cu(I) form. A number of proposals have been advanced for LPMO catalysis (see reference [73] for details) but the major issues are (i) where the necessary second electron comes from and (ii) whether the co-substrate is oxygen or peroxide. Cu(I) to Cu(II) oxidation furnishes only one of the 2 electrons necessary to complete the substrate hydroxylation. The surface of the protein in the vicinity of the copper site is flat, suggesting that the oligosaccharide substrate could dock above the copper and occlude it from solvent, facilitating high-valent intermediates such as Cu(III), hydroxyl radical, or both in the form of a Cu(II)-oxyl or its protonated form (Cu(III)-OH) to form the reactive species [75]. However, the debate is complicated by studies that show substrate hydroxylation can use peroxide rather than oxygen [76]. A further complexity arises when the reactivity of other members of the “histidine brace” family is considered. Bim1, a fungal protein from Cryptococcus neoformans which causes lethal meningitis, does not exhibit monooxygenase reactivity but rather functions in copper uptake in concert with Ctr1, establishing a critical role in fungal copper acquisition [77]. The structure of a homologue LaX325 from Laetisaria arvalis contains the same histidine brace/internal histidine coordination as LPMOs, but the active site differs in the use of an Asp ligand, rather than Tyr, to occupy the equatorial position left vacant in LPMOs [40, 78] (Figure 8(b)). Studies using ascorbate reduction have shown that Bim1 does not undergo redox cycling, but loses copper on reduction, indicating that its coordination environment stabilizes the Cu(II) form [40]. Yet another member of the histidine brace family is the periplasmic metallochaperone CopC, which has a similar ligand set to Bim1/LaX325 containing the N-terminal plus internal His ligands and an Asp, but the imidazole groups are cis to one another rather than trans as seen in the other proteins [78] (Figure 8(c)). This change renders CopC inert to ascorbate reduction, but also allows it to retain its copper, signifying greater stability of the Cu(II) state. An attempt to transform CopC into a Bim1-like protein via swapping the position of the internal His and Asp residues was unsuccessful, generating instead a protein that strongly bound Cu(I).
Figure 8.
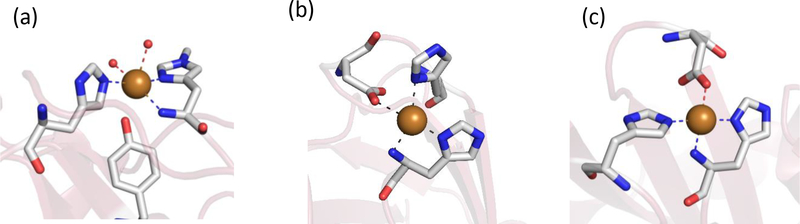
Structures of the active sites of histidine brace proteins. (a) Thermoascus LPMO AA9 pdb file 2YET (b) Pseudomonas fluorescens CopC pdf file 6NFQ (c) Laetisaria arvalis (LaX325) copper binding protein pdb file 6IBJ.
If LPMOs are indeed monooxygenases rather than peroxidases, then the accepted paradigm would be initial attack of the Cu(I) site by dioxygen to generate a Cu(II)-superoxide species. EXAFS spectroscopy of the Cu(I) form in association with geometry optimization by DFT suggested a 3-coordinate T-shaped structure with the two imidazole ligands at a short distance (1.90 Å) and the N-terminal amine at a longer distance of 2.3 Å [79]. The rate of oxidation of this reduced LPMO species by O2 was rapid (> 0.15 s−1), and based on correlations of redox potential and rate constant the authors argued that the oxidation must involve an inner sphere mechanism initiated through a discreet Cu(I)-dioxygen/Cu(II)-superoxo complex, although no signals attributable to a Cu(II)-superoxo species were detected. Attempts have also been made to characterize the Cu(II)-superoxo via crystallography [80–82] but although diatomic species have been observed close to the copper center, many of these do not appear close enough to be considered copper complexes. One structure derived from neutron diffraction did show an end-on Cu-oxygen species with Cu-O1 of 1.9 Å and a O-O bond length of 1.4 Å, suggestive of a peroxide rather than a superoxide species, but was otherwise well fit to the Cu(II)-superoxo suggested by the DFT modeling [82]. Notwithstanding, the absence of substrate in either the crystallography or the spectroscopy of these reduced oxygen species limit direct correlation to the hydroxylation chemistry and their relevance may be limited to the “uncoupled reaction” in which LPMOs catalyze the oxidation of ascorbate to dehydroascorbate and hydrogen peroxide via a Cu(I)-Cu(II) redox shunt [40].
Particulate methane monooxygenase (pMMO) is another copper-dependent monooxygenase that contains a histidine brace motif. After many years of controversy some clarity is emerging as to the role of the multiple copper centers in the enzyme (Figure 9). Originally, three distinct copper centers were identified, two in the soluble subunit B and one in membrane associated subunit C located near the interface of the membrane and the soluble domain. Of the two centers in the soluble domain, one was not conserved leading to the designation of the second pMMOB copper center (CuB) as the probable active site. Of interest here, this site bound copper via a histidine brace from His33 which formed the N-terminal residue of the B subunit. Two additional His residues were clustered in this region and, while crystallography showed density attributable to only one copper, EXAFS studies on pMMOs from a variety of organisms suggested a dinuclear site with short 2.6 Å Cu-Cu distance. The occurrence of the His brace motif at the active site of both LPMOs and pMMOs suggested an important role in monooxygenation chemistry, such as to stabilize a necessary Cu(III)-OH/Cu(II)-O•− intermediate via deprotonation of the terminal amine or restricting geometry changes during redox, thereby lowering the reorganizational energy for electron transfer.
Figure 9.
Structure of particulate methane monooxygenase from Methylocystis sp. ATCC 49242 (Rockwell). Insert on the right shows an expanded view of the copper sites from the B and C subunits respectively.
New data has led to a re-evaluation of the nuclearity of the pMMOB copper center and its role in catalysis. EXAFS data collected on a pMMO from Methylomicrobium alcaliphilum 20 Z prepared in bicelles showed only mononuclear copper [83], while high energy resolution fluorescence detected XAS (HERFD) and partial yield fluorescence EXAFS data collected on the M. capsulatus (Bath) enzyme provided strong evidence that the Cu-Cu interaction prominent in the earlier EXAFS data was artifactual, associated with a metallic copper contaminant arising from degradation products [84]. Mononuclearity was also established for the pMMOB CuB center using native top-down mass spectrometry [85] and corroborated by EPR and electron nuclear double resonance (ENDOR) [86]. Importantly, the EPR and pulsed ENDOR studies establish that the site is tetragonal Cu(II) with the His brace and two additional His residues as equatorial ligands and a water in the axial position. The present consensus denotes the strictly conserved copper center in the pMMOC subunit (CuC) as the probable active site where crystallography, EPR and ENDOR have established His133, His146 and Asp129 as the equatorial ligands to the Cu(II) center together with a more distant water molecule which is absent in a nanodisc environment and presumably also in the membrane [87].
Conclusions
In this review we set out to explore whether the consensus mechanisms for the reactions of copper monooxygenases with molecular oxygen, and the subsequent prediction of catalytic steps, were consistent with experimental observations on the enzymes themselves. The result of this deep dive into the catalytic mechanisms of PHM and DBM identified a significant number of experimental observations that were not well represented by the canonical mechanism, involving oxygen reaction at mononuclear CuM followed by HAA chemistry and long-range electron transfer over the 11 Å intersite distance between the H and M centers. Of particular note were (i) the almost negligible reactivity of CuM with oxygen in the absence of substrate, (ii) the carbonyl chemistry and in particular the substrate induced activation exemplified by the lowered CO stretching frequency, (iii) the peroxide shunt chemistry which demands an intermediate that facilitates equilibrium between a Cu(II)-peroxo state and a Cu(I)-dioxygen state, and (iv) clear evidence for both closed and open conformational states in both PHM and DBM. An alternative mechanism involving a dinuclear copper intermediate formed via an open to closed conformational transition appeared better able to accommodate these experimental facets, as well as being shown by QMMM methodologies to be energetically feasible.
Similar considerations may apply to LPMOs and pMMOs. For LPMOs, the concept of monooxygenase activity linked to the histidine brace motif is challenged by the lack of reactivity of CopC and Bimm1 towards oxygen and their obvious preference for the Cu(II) state. Indeed the ascorbate oxidase activity of LPMOs observed in the absence of substrate appears to be the exception to the rule, as the reduced states of the other monooxygenases and their protein-derived models exhibit no such reactivity. However, it is possible that the ascorbate oxidation cycle catalyzed by LPMOs in the absence of substrate is required for generating the hydrogen peroxide co-substrate of what is essentially a peroxidase rather than a monooxygenase enzyme. Finally, the discovery that CuC, not CuB, is the active site of pMMO introduces a completely new paradigm in copper-dioxygen chemistry, introducing a His2Asp ligand set into the family of Cu(I) sites that catalyze alkyl and peptidyl hydroxylation. Future studies must therefore embrace both the metal-based oxygen chemistry and exploration of how binding of substrate in a non-coordinating nearby site can itself lead to oxygen activation and reactivity at the copper center.
Acknowledgements.
The work was supported by a grant from the National Institutes of Health GM R35 136239 to NJB. We acknowledge use of the SSRL computing cluster for ORCA calculations.
Footnotes
Author Conflict Statement. The authors declare no conflicts associated with any work described in this review.
References
- [1].Elwell CE, Gagnon NL, Neisen BD, Dhar D, Spaeth AD, Yee GM, Tolman WB, Chem Rev, vol. 117, 2017, pp. 2059–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Quist DA, Diaz DE, Liu JJ, Karlin KD, J. Biol. Inorg. Chem, vol. 22, 2017, pp. 253–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Klinman JP, J. Biol. Chem, vol. 281, 2006, pp. 3013–3016. [DOI] [PubMed] [Google Scholar]
- [4].Kumar D, Mains RE, Eipper E, Journal of molecular endocrinology, vol. 56 2016, pp. T63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prigge ST, Mains RE, Eipper BA, Amzel LM, Cell. Mol. Life Sci, vol. 57, 2000, pp. 1236–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xin X, Mains RE, Eipper BA, J. Biol. Chem, vol. 279, 2004, pp. 48159–48167. [DOI] [PubMed] [Google Scholar]
- [7].Kulathila R, Merkler KA, Merkler DJ, Nat. Prod. Rep, vol. 16, 1999, pp. 145–154. [DOI] [PubMed] [Google Scholar]
- [8].Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM, Science, vol. 278, 1997, pp. 1300–1305. [DOI] [PubMed] [Google Scholar]
- [9].Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM, Nature Struct. Biol, vol. 6, 1999, pp. 976–983. [DOI] [PubMed] [Google Scholar]
- [10].Bell J, Ash DE, Snyder LM, Kulathila R, Blackburn NJ, Merkler DJ, Biochemistry, vol. 36, 1997, pp. 16239–16246. [DOI] [PubMed] [Google Scholar]
- [11].Chufan EE, De M, Eipper BA, Mains RE, Amzel LM, Structure, vol. 17, 2009, pp. 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De M, Bell J, Blackburn NJ, Mains RE, Eipper BA, J. Biol. Chem, vol. 281, 2006, pp. 20873–20882. [DOI] [PubMed] [Google Scholar]
- [13].Blackburn NJ, Hasnain SS, Pettingill TM, Strange RW, J. Biol. Chem, vol. 266, 1991, pp. 23120–23127. [PubMed] [Google Scholar]
- [14].Vendelboe TV, Harris P, Zhao Y, Walter TS, Harlos K, El Omari K, Christensen HE, Sci. Adv, vol. 2, 2016, pp. E1500980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chufan EE, Prigge ST, Siebert X, Eipper BA, Mains RE, Amzel LM, J. Am. Chem. Soc, vol. 132, 2010, pp. 15565–15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rudzka K, Moreno DM, Eipper B, Mains R, Estrin DA, Amzel LM, J. Biol. Inorg. Chem, vol. 18, 2013, pp. 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blackburn NJ, Rhames FC, Ralle M, Jaron S, J. Biol. Inorg. Chem, vol. 5, 2000, pp. 341–353. [DOI] [PubMed] [Google Scholar]
- [18].Jaron S, Blackburn NJ, Biochemistry, vol. 38, 1999, pp. 15086–15096. [DOI] [PubMed] [Google Scholar]
- [19].Boswell JS, Reedy BJ, Kulathila R, Merkler DJ, Blackburn NJ, Biochemistry, vol. 35, 1996, pp. 12241–12250. [DOI] [PubMed] [Google Scholar]
- [20].Bauman AT, Broers BA, Kline CD, Blackburn NJ, Biochemistry, vol. 50, 2011, pp. 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen P, Bell J, Eipper BA, Solomon EI, Biochemistry, vol. 43, 2004, pp. 5735–5747. [DOI] [PubMed] [Google Scholar]
- [22].Francisco WA, Merkler DJ, Blackburn NJ, Klinman JP, Biochemistry, vol. 37, 1998, pp. 8244–8252. [DOI] [PubMed] [Google Scholar]
- [23].Francisco WA, Knapp MJ, Blackburn NJ, Klinman JP, J. Am. Chem. Soc, vol. 124, 2002, pp. 8194–8195. [DOI] [PubMed] [Google Scholar]
- [24].Evans JP, Blackburn NJ, Klinman JP, Biochemistry, vol. 45, 2006, pp. 15419–15429. [DOI] [PubMed] [Google Scholar]
- [25].Klinman JP, Biochim. Biophys. Acta, vol. 1757, 2006, pp. 981–987. [DOI] [PubMed] [Google Scholar]
- [26].Osborne RL, Zhu H, Iavarone AT, Blackburn NJ, Klinman JP, Biochemistry, vol. 52, 2013, pp. 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McIntyre NR, Lowe EW Jr., Belof JL, Ivkovic M, Shafer J, Space B, Merkler DJ, J. Am. Chem. Soc, vol. 132, 2010, pp. 16393–16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen P, Solomon EI, J. Am. Chem. Soc, vol. 126, 2004, pp. 4991–5000. [DOI] [PubMed] [Google Scholar]
- [29].Chen P, Solomon EI, Proc. Natl. Acad. Sci. U S A, vol. 101, 2004, pp. 13105–13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cowley RE, Tian L, Solomon EI, Proc. Natl. Acad. Sci. USA, vol. 113, 2016, pp. 12035–12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu P, Fan F, Song J, Peng W, Liu J, Li C, Cao Z, Wang B, J. Am. Chem. Soc, vol. 141, 2019, pp. 19776–19789. [DOI] [PubMed] [Google Scholar]
- [32].Prigge ST, Eipper BA, Mains RE, Amzel M, Science, vol. 304, 2004, pp. 864–867. [DOI] [PubMed] [Google Scholar]
- [33].Eipper BA, Quon ASW, Mains RE, Boswell JS, Blackburn NJ, Biochemistry, vol. 34, 1995, pp. 2857–2865. [DOI] [PubMed] [Google Scholar]
- [34].Jaron S, Mains RE, Eipper BA, Blackburn NJ, Biochemistry, vol. 41, 2002, pp. 13274–13282. [DOI] [PubMed] [Google Scholar]
- [35].Siebert X, Eipper BA, Mains RE, Prigge ST, Blackburn NJ, Amzel LM, Biophys. J, vol. 89, 2005, pp. 3312–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kline CD, Mayfield M, Blackburn NJ, Biochemistry, vol. 52, 2013, pp. 2586–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kline CD, Blackburn NJ, Biochemistry, vol. 55, 2016, pp. 6652–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Evans JP, Ahn K, Klinman JP, J. Biol. Chem, vol. 278, 2003, pp. 49691–49698. [DOI] [PubMed] [Google Scholar]
- [39].Hess CR, Wu Z, Ng A, Gray EE, McGuirl MA, Klinman JP, J. Am. Chem. Soc, vol. 130, 2008, pp. 11939–11944. [DOI] [PubMed] [Google Scholar]
- [40].Brander S, Horvath I, Ipsen JO, Peciulyte A, Olsson L, Hernandez-Rollan C, Norholm MHH, Mossin S, Leggio LL, Probst C, Thiele DJ, Johansen KS, Sci. Rep, vol. 10, 2020, pp. 16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blackburn NJ, Pettingill TM, Seagraves KS, Shigeta RT, J. Biol. Chem, vol. 265, 1990, pp. 15383–15386. [PubMed] [Google Scholar]
- [42].Pettingill TM, Strange RW, Blackburn NJ, J. Biol. Chem, vol. 266, 1991, pp. 16996–17003. [PubMed] [Google Scholar]
- [43].Reedy BJ, Blackburn NJ, J. Am. Chem. Soc, vol. 116, 1994, pp. 1924–1931. [Google Scholar]
- [44].Jaron S, Blackburn NJ, Biochemistry, vol. 40, 2001, pp. 6867–6875. [DOI] [PubMed] [Google Scholar]
- [45].Fager LY, Alben JO, Biochemistry, vol. 11, 1972, pp. 4786–4792. [DOI] [PubMed] [Google Scholar]
- [46].Dyer RB, Einarsdottir O, Killough PM, Lopez GJJ, Woodruff WH, J. Am. Chem. Soc, vol. 111, 1989, pp. 7657–7659. [Google Scholar]
- [47].Einarsdottir O, Killough PM, Fee JA, Woodruff WH, Biol J. Chem, vol. 264, 1989, pp. 2405–2408. [PubMed] [Google Scholar]
- [48].Maheshwari S, Shimokawa C, Rudzka K, Kline CD, Eipper BA, Mains RE, Gabelli SB, Blackburn N, Amzel LM, Commun. Biol. (Nature Publishing; ), vol. 1, 2018, pp. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wilcox DE, Porras AG, Hwang YT, Lerch K, Winkler ME, Solomon EI, J. Am. Chem. Soc, vol. 107, 1985, pp. 4015–4027. [Google Scholar]
- [50].Ginsbach JW, Kieber-Emmons MT, Nomoto R, Noguchi A, Ohnishi Y, Solomon EI, Proc. Natl. Acad. Sci. USA, vol. 109, 2012, pp. 10793–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Klabunde T, Eicken C, Sacchettini JC, Krebs B, Nature Struct. Biol, vol. 5, UNITED STATES, 1998, pp. 1084–1090. [DOI] [PubMed] [Google Scholar]
- [52].Baldwin MJ, Root DE, Pate JE, Fujisawa K, Kitajima N, Solomon EI, J. Am. Chem. Soc, vol. 114, Amer Chemical Soc, 1155 Sixteenth St NW, Washington, DC 20036, 1992, pp. 10421–10431. [Google Scholar]
- [53].Kitajima N, Fujisawa K, Fujimoto C, Moro-oka Y, Hashimoto S, Kitagawa T, Toriumi K, Tatsumi K, Nakamura A, J. Am. Chem. Soc, vol. 114, 1992, pp. 1277–1291. [Google Scholar]
- [54].Tyeklar Z, Karlin KD, Acc. Chem. Res, vol. 22, 1989, pp. 241–248. [Google Scholar]
- [55].Kitajima N, Moro-oka Y, Chem. Rev, vol. 94, 1994, pp. 737–757. [Google Scholar]
- [56].Bhadra M, Transue WJ, Lim H, Cowley RE, Lee JYC, Siegler MA, Josephs P, Henkel G, Lerch M, Schindler S, Neuba A, Hodgson KO, Hedman B, Solomon EI, Karlin KD, J. Am. Chem. Soc, vol. 143, 2021, pp. 3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Alwan KB, Welch EF, Arias RJ, Gambill BF, Blackburn NJ, Biochemistry, vol. 58, 2019, pp. 3097–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Alwan KB, Welch EF, Blackburn NJ, Biochemistry, vol. 58, 2019, pp. 4436–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Loftin IR, Blackburn NJ, McEvoy MM, J. Biol. Inorg. Chem, vol. 14, 2009, pp. 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Loftin IR, Franke S, Blackburn NJ, McEvoy MM, Protein Sci, vol. 16, 2007, pp. 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xue Y, Davis AV, Balakrishnan G, Stasser JP, Staehlin BM, Focia P, Spiro TG, Penner-Hahn JE, O’Halloran TV, Nat. Chem. Biol, vol. 4, 2008, pp. 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chacon KN, Mealman TD, McEvoy MM, Blackburn NJ, Proc. Natl. Acad. Sci. U S A, vol. 111, 2014, pp. 15373–15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chacon KN, Perkins J, Mathe Z, Alwan K, Ho EN, Ucisik MN, Merz KM, Blackburn NJ, Commun. Biol. (Nature Publishing; ), vol. 1, 2018, pp. 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sanyal I, Karlin KD, Strange RW, Blackburn NJ, J. Am. Chem. Soc, vol. 115, 1993, pp. 11259–11270. [Google Scholar]
- [65].Bauman AT, Yukl ET, Alkevich K, McCormack AL, Blackburn NJ, J. Biol. Chem, vol. 281, 2006, pp. 4190–4198. [DOI] [PubMed] [Google Scholar]
- [66].Chauhan S, Kline CD, Mayfield M, Blackburn NJ, Biochemistry, vol. 53, 2014, pp. 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Himes RA, Park YG, Barry AN, Blackburn NJ, Karlin KD, J. Am. Chem. Soc, vol. 129, 2007, pp. 5352–5353. [DOI] [PubMed] [Google Scholar]
- [68].Chauhan S, Hosseinzadeh P, Lu Y, Blackburn NJ, Biochemistry, vol. 55, 2016, pp. 2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Himes RA, Park GY, Siluvai GS, Blackburn NJ, Karlin KD, Angew Chem Int Ed Engl, vol. 47, 2008, pp. 9084–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yako N, Young TR, Cottam Jones JM, Hutton CA, Wedd AG, Xiao Z, Metallomics, vol. 9, 2017, pp. 278–291. [DOI] [PubMed] [Google Scholar]
- [71].Young TR, Kirchner A, Wedd AG, Xiao Z, Metallomics, vol. 6, 2014, pp. 505–517. [DOI] [PubMed] [Google Scholar]
- [72].Ipsen JO, Hallas-Moller M, Brander S, Lo Leggio L, Johansen KS, Biochem. Soc. Trans, vol. 49, 2021, pp. 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Meier KK, Jones SM, Kaper T, Hansson H, Koetsier MJ, Karkehabadi S, Solomon EI, Sandgren M, Kelemen B, Chem Rev, vol. 118, 2018, pp. 2593–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Walton PH, Davies GJ, Curr Opin Chem Biol, vol. 31, 2016, pp. 195–207. [DOI] [PubMed] [Google Scholar]
- [75].Dhar D, Tolman WB, J. Am. Chem. Soc, vol. 137, 2015, pp. 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bissaro B, Rohr AK, Muller G, Chylenski P, Skaugen M, Forsberg Z, Horn SJ, Vaaje-Kolstad G, Eijsink VGH, Nat Chem Biol, vol. 13, 2017, pp. 1123–1128. [DOI] [PubMed] [Google Scholar]
- [77].Garcia-Santamarina S, Probst C, Festa RA, Ding C, Smith AD, Conklin SE, Brander S, Kinch LN, Grishin NV, Franz KJ, Riggs-Gelasco P, Lo Leggio L, Johansen KS, Thiele DJ, Nat. Chem. Biol, vol. 16, 2020, pp. 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ipsen JO, Hernandez-Rollan C, Muderspach SJ, Brander S, Bertelsen AB, Jensen PE, Norholm MHH, Lo Leggio L, Johansen KS, FEBS Lett, 2021. [DOI] [PubMed] [Google Scholar]
- [79].Kjaergaard CH, Qayyum MF, Wong SD, Xu F, Hemsworth GR, Walton DJ, Young NA, Davies GJ, Walton PH, Johansen KS, Hodgson KO, Hedman B, Solomon EI, Proc. Natl. Acad. Sci. USA, vol. 111, 2014, pp. 8797–8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bacik JP, Mekasha S, Forsberg Z, Kovalevsky AY, Vaaje-Kolstad G, Eijsink VGH, Nix JC, Coates L, Cuneo MJ, Unkefer CJ, Chen JC, Biochemistry, vol. 56, 2017, pp. 2529–2532. [DOI] [PubMed] [Google Scholar]
- [81].Li X, Beeson W.T.t., Phillips CM, Marletta MA, Cate JH, Structure, vol. 20, 2012, pp. 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].O’Dell WB, Agarwal PK, Meilleur F, Angew. Chem. Int. Ed. Engl, vol. 56, 2017, pp. 767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ro SY, Ross MO, Deng YW, Batelu S, Lawton TJ, Hurley JD, Stemmler TL, Hoffman BM, Rosenzweig AC, J. Biol. Chem, vol. 293, 2018, pp. 10457–10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cutsail GE 3rd, Ross MO, Rosenzweig AC, DeBeer S, Chemical Science, vol. 12, 2021, pp. 6194–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ro SY, Schachner LF, Koo CW, Purohit R, Remis JP, Kenney GE, Liauw BW, Thomas PM, Patrie SM, Kelleher NL, Rosenzweig AC, Nature communications, vol. 10, 2019, pp. 2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ross MO, MacMillan F, Wang J, Nisthal A, Lawton TJ, Olafson BD, Mayo SL, Rosenzweig AC, Hoffman BM, Science, vol. 364, 2019, pp. 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jodts RJ, Ross MO, Koo CW, Doan PE, Rosenzweig AC, Hoffman BM, J. Am. Chem. Soc, vol. 143, 2021, pp. 15358–15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Park GY, Lee JY, Himes RA, Thomas GS, Blackburn NJ, Karlin KD, J. Am. Chem. Soc, vol. 136, 2014, pp. 12532–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Einarsdottir O, Killough PM, Fee JA, Woodruff WH, J. Biol. Chem, vol. 264, 1989, pp. 2405–2408. [PubMed] [Google Scholar]
- [90].Sanyal I, Karlin KD, Strange RW, Blackburn NJ, J. Am. Chem. Soc, vol. 115, 1993, pp. 11259–11270. [Google Scholar]
- [91].Kealy S, White AJP, Gee AD, Long NJ, Eur. J. Inorg. Chem, 2014, pp. 1896–1905. [Google Scholar]
- [92].Lee Y, Park GY, Lucas HR, Vajda PL, Kamaraj K, Vance MA, Milligan AE, Woertink JS, Siegler MA, Narducci Sarjeant AA, Zakharov LN, Rheingold AL, Solomon EI, Karlin KD, Inorg. Chem, vol. 48, 2009, pp. 11297–11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lee DH, Hatcher LQ, Vance MA, Sarangi R, Milligan AE, Sarjeant AA, Incarvito CD, Rheingold AL, Hodgson KO, Hedman B, Solomon EI, Karlin KD, Inorg. Chem, vol. 46, 2007, pp. 6056–6068. [DOI] [PubMed] [Google Scholar]
- [94].Neese F, WIREs Comput. Mol. Sci, vol. 2, 2012, pp. 73–78. [Google Scholar]
- [95].Becke A, D., Phys. Rev. A, vol. 38, 1988, pp. 3098–3100. [DOI] [PubMed] [Google Scholar]
- [96].Weigend F, Ahlrichs R, Phys. Chem. Chem. Phys, vol. 7, 2005, pp. 3297–3305. [DOI] [PubMed] [Google Scholar]