Abstract
Oxidation of branched-chain amino acids (BCAAs) is tightly regulated in mammals. We review here the distribution and regulation of whole-body BCAA oxidation. Phosphorylation and dephosphorylation of the rate-limiting enzyme, branched-chain α-ketoacid dehydrogenase complex directly regulates BCAA oxidation, and various other indirect mechanisms of regulation also exist. Most tissues throughout the body are capable of BCAA oxidation, and the flux of oxidative BCAA disposal in each tissue is influenced by three key factors: 1. tissue-specific preference for BCAA oxidation relative to other fuels, 2. the overall oxidative activity of mitochondria within a tissue, and 3. total tissue mass. Perturbations in BCAA oxidation have been implicated in many disease contexts, underscoring the importance of BCAA homeostasis in overall health.
Basic biochemistry of branched-chain amino acids
Intake and loss
The three branched-chain amino acids (BCAAs: leucine, valine, and isoleucine) are essential for animals, meaning they cannot be synthesized by the organism and must be obtained from dietary protein. BCAAs are synthesized by plants, fungi, and bacteria. Despite being essential, BCAAs are surprisingly abundant and make up ~35% of essential amino acids and 18% of all amino acids in animal protein [1-3]. Because BCAAs are essential, animals must balance their intake and loss. All intake comes from diet, and loss occurs through oxidation. The relative abundance of BCAAs in protein is almost always 2.2 : 1.6 : 1.0 leucine : valine : isoleucine, illustrating that the synthesis and oxidation of each individual BCAA are linked to one another [4]. Circulating plasma levels of BCAAs in mammals remain consistent at ~200 μM valine, 100 μM leucine, and 60 μM isoleucine in the fasted state; after feeding, these levels rise briefly but fall back to baseline after a few hours. Together this indicates homeostatic regulation of BCAAs [5].
In the fasted state, protein breakdown releases BCAAs from tissues into circulation [6-8]. The average rate of BCAA appearance in a fasted human is ~0.75 g/kg/day, reflecting a rate of protein breakdown of ~250 g/day for a 70 kg man. The main source of this protein breakdown is not known, and >75% of it is likely recycled into protein synthesis, while the remainder is oxidized [9-11]. This rate of oxidation, ~150 mg/kg/day, is on par with the recommended minimum daily intake of BCAAs [12]. In the fed state, BCAAs are taken up by tissues and both protein synthesis and oxidation are increased [4].
Both rates of intake and loss can be altered under physiological or pathological conditions. For example, BCAAs have become a popular exercise supplement [4] because protein intake coupled with exercise promotes muscle protein synthesis, a process likely largely driven by leucine. Numerous studies, typically using infusions of amino acids, BCAAs, or leucine during rest versus acute resistance exercise, have shown that the ensuing hyperaminoacidemia or hyperleucinemia boosts amino acid transport into skeletal muscle and muscle protein synthesis, and that repeated exercise training coupled with protein ingestion leads to skeletal muscle hypertrophy [13]. It is important to note, however, that the magnitude of protein/amino acid/leucine’s effects on stimulating muscle protein synthesis is much smaller than the effect of acute exercise itself. Conversely, limiting BCAA intake has received increasing attention as a potential mediator of the widely recorded benefits of calorie restriction on lifespan and healthspan. Recent work has strengthened the notion that BCAAs are a key component of these benefits [14]. Finally, rates of BCAA loss can also change significantly and are typically tied to rates of overall protein catabolism, as can occur in the often dramatic increases in protein catabolism that occur in prolonged fasting, or cachexia associated with for example AIDS, sepsis, heart failure, or cancer.
Oxidation overview
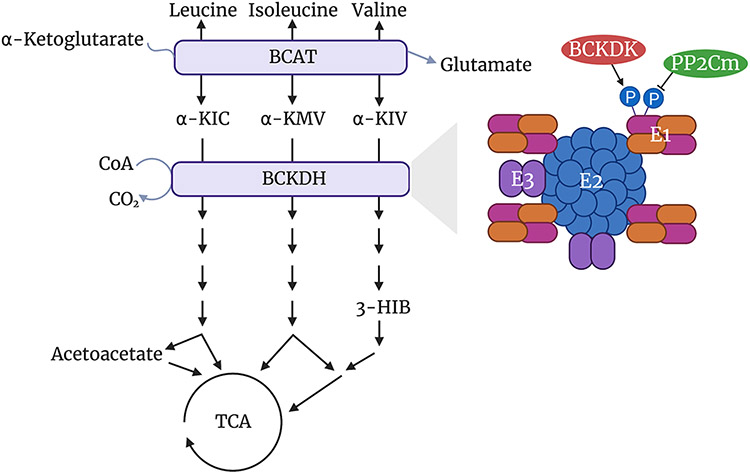
The BCAA oxidative pathway is conserved in animals, plants, fungi, and bacteria. BCAAs are initially transaminated by branched-chain amino transferases (BCATs 1 and 2) to form branched-chain α-ketoacids (BCKAs: α-KIC, α-KMV, and α-KIV), and this reaction is reversible. BCAT1 encodes a protein that localizes to the cytoplasm and is expressed primarily in the brain, while BCAT2 encodes a protein that localizes to mitochondria and is expressed in most tissues. BCKAs are then decarboxylated and dehydrogenated by the branched-chain α-ketoacid dehydrogenase (BCKDH) complex [15-17] (Figure 1). BCKDH is the rate-limiting enzyme in this pathway, and it lies on the inner mitochondrial membrane. Its oxidative decarboxylation of BCKAs releases carbon dioxide, while covalently linking a coenzyme A (CoA) group to the oxidized product (Figure 1). The bulky hydrophilic CoA group traps oxidized BCKAs and all downstream intermediates inside the mitochondria [18-20]. Only at two steps is the CoA moiety transiently removed, enabling exit from the mitochondria: 3-hydroxyisobutyrate (3-HIB) [21], a product of valine, and acetoacetate [4], a product of leucine (Figure 1). 3-HIB can be significantly secreted from cells and tissues [21], while, except in the liver, acetoacetate is likely not [4]. After decarboxylation and dehydrogenation by BCKDH, intermediate products from the three BCAAs each follow different catabolic pathways, with each terminating at the tricarboxylic acid (TCA) cycle either via acetyl-CoA (leucine and isoleucine) or succinyl-CoA (isoleucine and valine) (Figure 1) [4,22]. Rarely, BCAA oxidation does not terminate in the TCA cycle, generating instead monomethyl branched-chain fatty acids (mmBCFAs) from the CoA-bound intermediates of mitochondrial BCAA oxidation and exporting them to the cytoplasm. This process occurs mostly in white and brown adipocytes [23,24].
Figure 1. Basic BCAA oxidative pathway, including a simplified structure showing the regulation of the rate-limiting enzyme, BCKDH.
Figure created with BioRender.com.
Structure and regulation of BCKDH
BCKDH is a member of the mitochondrial α-ketoacid dehydrogenase complex family, along with PDH and OGDH, which together share primary and tertiary structure, as well as likely evolutionary origin. BCKDH is a multienzyme complex made up of three components, E1, E2, and E3 (Figure 1) [19,20]. E1 is a heterotetramer composed of two α subunits and two β subunits, encoded by BCKDHA and BCKDHB, respectively. E1 functions as a thiamin-dependent decarboxylase. E2 forms BCKDH’s structural core, made up of 24 identical subunits, encoded by DBT. E2 functions as a dihydrolipoyl transacylase. E3 is a homodimer, encoded by DLD and shared with the other α-ketoacid dehydrogenase complexes, that functions as a dihydrolipoamide dehydrogenase. E1 decarboxylates BCKAs and transfers an acyl group to the lipoamide cofactor on E2. E2 transfers the acyl group from lipoamide to CoA, which reduces lipoamide and forms an acyl-CoA. Finally, E3 re-oxidizes lipoamide, using FAD as a cofactor and NAD+ as an electron acceptor to form NADH [4,19,20]. Crystal structures of the BCKDH complex have recently been described [19,20].
BCKDH activity is regulated by phosphorylation and dephosphorylation. Phosphorylation of E1 by the BCKDH kinase (BCKDK) inhibits BCKDH activity [25,26]. Human BCKDH has two phosphorylation sites targeted by BCKDK, Ser292-α and Ser302-α, that both reside in the E1 catalytic site. Phosphorylation of Ser292-α alone inactivates BCKDH, while phosphorylation of Ser302-α appears to have no impact [27]. Dephosphorylation of E1 is carried out by the protein phosphatase Mg2+/Mn2+ dependent 1K (PP2Cm, encoded by PPM1K), thereby activating BCKDH [28,29] (Figure 1). Numerous additional mechanisms of regulation of BCKDH exist. Product inhibition of BCKDH by NADH and acyl-CoA can occur, as well as allosteric inhibition of BCKDK by high levels of BCKAs, thereby promoting their oxidation [30-33]. Interestingly, BCAT2 and BCKDH can associate to form a metabolon, a supramolecular complex that allows substrates to channel from enzyme to enzyme [34]. BCAT2 binding to BCKDH also increases BCKDH activity, while phosphorylation of BCKDH destabilizes BCAT2’s interaction with BCKDH [34].
Whole-body oxidation of branched-chain amino acids
Foundational infusions of stable isotopes of BCAAs quantified systemic flux of transamination and oxidative decarboxylation in humans and rodents (Figure 2A,B). Transamination is faster than oxidation and rapidly reversible [35]. Oxidation accounts for 10–15% of BCAA disposal in the fasted state (the remainder is deposited into protein) [36]. Upon feeding, oxidation as much as triples and accounts for a greater fraction of BCAA disposal [37,38].
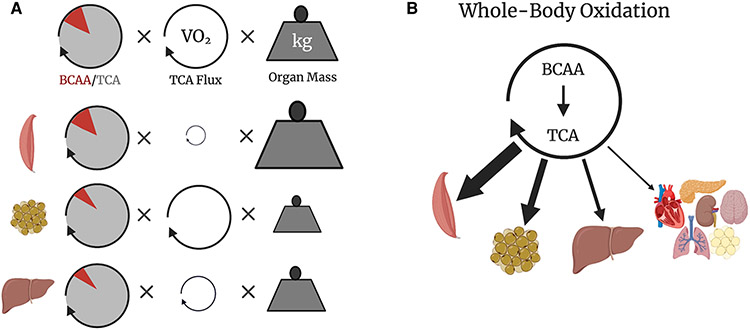
Figure 2. Quantifying Whole-Body BCAA Oxidation.
(A) BCAA oxidation flux into muscle, brown fat, and liver calculated from the fraction of TCA labeling from BCAAs, TCA turnover flux inferred from tissue oxygen consumption rate (VO2), and normalized to organ mass. (B) Whole-body distribution of measured BCAA oxidation. Figure created with BioRender.com.
Although most tissues are capable of BCAA oxidation, BCAAs only account for roughly 1% of total calories consumed. Tissues have a low preference for BCAA oxidation relative to other fuels like glucose, glutamine, lactate, and fatty acids [39]. An interesting exception is the pancreas, which, at least in mice, fills nearly a third of its TCA cycle with carbons derived from BCAAs [39,40]; the reason for this predilection is not known. In mice housed at normal room temperature, the greatest fraction of total BCAA oxidation occurs in skeletal muscle, liver, and brown fat [40] (Figure 2B). Skeletal muscle and liver are well-known sinks for BCAAs [41], but brown fat is a novel contributor to BCAA disposal. Genetic deletion in mice of BCKDHA or of a BCAA transporter specifically in brown fat is sufficient to raise plasma BCAAs [42]. Using similar genetic tools in mice, ongoing work will test the contribution of BCAA oxidation to systemic BCAA disposal in more tissues.
We have proposed a simple model of whole-body BCAA oxidation, highlighting three key factors influencing the flux of oxidative BCAA disposal in each tissue (Figure 2A):
First, the tissue-specific preference for BCAA oxidation relative to other fuels (Figure 2A). This factor is impacted by numerous tissue-specific variables, including transcriptional and post-translational mechanisms. Transcriptional regulation of the enzymatic machinery for BCAA oxidation can drive adaptive responses, as exemplified by PGC-1α or KLF15 driving increased BCAA oxidation [43-45]. Other transcription factors may regulate specific portions of the pathway, e.g. ChREBP regulating transcription of BCKDK and PPM1K [46], or KLF15 increasing transcription of BCAT2 in some contexts [44]. Acute and chronic regulation of BCAA oxidation is also mediated by phosphorylation of BCKDHA, as demonstrated by the rapid (within minutes) activation of BCAA oxidation by pharmacologic inhibition of BCKDK and the sustained changes upon whole-body or liver-specific knockouts of BCKDK in mice [40,46,47]. Phosphorylation status, abundance and activity of BCKDH measured by ex vivo enzyme preparations was the basis of previous estimates for tissue-specific BCAA oxidation [48]. Regulation beyond the BCKDH complex is almost certainly also at play, including cell- and organelle-specific BCAA transporters, transaminases, and catabolic enzymes downstream of BCKDH, although these aspects of regulation remain understudied.
Second, the overall oxidative activity of mitochondria within a tissue. To a first approximation, the mitochondrial oxidative activity can be represented by TCA flux and is proportional to oxygen consumption [40,49,50] (Figure 2A). The variable mitochondrial oxidative activity can have enormous impact on BCAA disposal. For example, the heart has the lowest preference for BCAA oxidation relative to other fuels, but its high mitochondrial activity makes the heart the highest oxidizer of BCAA per weight of tissue [40]. Tissue-specific modulation of mitochondrial oxidative activity will thus similarly impact BCAA oxidation, often accompanied by changes in specific regulation of BCKDH. For example, acute exercise, which can increase oxygen consumption of muscle by an order of magnitude, can also increase BCAA oxidation, and ex vivo assays for BCKDH activity show corresponding increases in activity [51]. Similarly, activation of thermogenesis in brown adipose tissue increases BCAA disposal in this tissue [42]. However, it remains to be tested if there is a specific increase in BCAA oxidation relative to other fuels during acute exercise or thermogenesis.
And third, total tissue mass. Simply put, the more of a given tissue, the more whole-body BCAA oxidation will be apportioned to that tissue (in the absence of changes in the two factors above) (Figure 2A). Thus whole-body BCAA oxidation can change when organs increase or decrease in mass. For example, any exercise that builds muscle mass (e.g. anabolic weight lifting) will increase the relative importance of skeletal muscle in systemic BCAA oxidation. In contrast, interestingly, dramatic expansion of white adipose tissue may not significantly impact total body BCAA disposal, in part because the metabolic rate in this tissue is low but also because the overall expression of BCAA catabolism enzymes is decreased in obesity [52-54]. This example underscores that all three components of this simple model of BCAA oxidation must be considered when making predictions about how physiological or pathological events will impact systemic BCAA disposal.
These factors should be considered when investigating mechanisms behind observed changes in BCAA oxidation flux. As mentioned earlier, acute exercise and thermogenesis in brown fat increase BCAA oxidation. Nutritional supply of protein and other macronutrients has complex effects on systemic BCAA oxidation. Compared with a normal overnight fasted state, starvation transiently increases BCAA oxidation, presumably to support gluconeogenesis until the body fully transitions to reliance on ketones [55]. BCAA oxidation is reduced after chronic dietary protein restriction [56], presumably to conserve BCAAs for protein synthesis. In normal conditions, BCAA oxidation increases after a meal [37]. All of this regulation of BCAA oxidation could be mediated or influenced by various hormones: insulin may contribute to increased oxidation during feeding by increasing the preference for BCAA oxidation in striated muscle [40], and other hormones like glucocorticoids and thyroid hormones are known to increase systemic BCAA oxidation [57,58]. In most of these cases, however, the molecular mechanisms behind these changes remain poorly understood, and studies have to date been limited to ex vivo measurements of BCKDH phosphorylation status and activity [59].
An important assumption of this model, which is based on tracing labeled BCAAs into the tissue TCA cycle, is that intermediate products of BCAA catabolism do not exchange between tissues, often referred to as secondary labeling [60]. This could be considered at three levels: secondary labeling from BCKAs, secondary labeling from metabolites produced after the BCKDH step but before the TCA cycle, and finally secondary labeling from metabolites derived from the TCA cycle. The first of these, i.e. interchange of BCKAs between tissues, undoubtedly occurs. For example, arterio-venous measurements of consumption and release across skeletal muscle and hepatic beds indicate that the liver does not transaminate BCAAs, instead, it only oxidizes BCKAs released by other tissues (mostly skeletal muscle) [22,61,62]. Isotopic tracing comparing the tissue-specific oxidation of valine vs α-KIV was consistent with this conclusion [40]. It is important to note, however, that the BCAA/BCKA transamination step does not involve oxidation. Therefore, the likely significant (and difficult to quantify) exchange of BCKAs between tissues does not impact conclusions in the above model as to where BCAA oxidation occurs.
Under physiological conditions, secondary labeling from products produced after the BCKDH step but before the TCA cycle is minimal, because nearly all intermediates are trapped in the mitochondria by covalent bonding to CoA. The exceptions are 3-HIB [21] (from valine) and acetoacetate [4] (from leucine), and possibly the mmBCFAs [23,24]. These mmBCFAs synthesized in adipose tissue are likely mixed with a very large pool of lipids within the adipose tissue, and therefore the labeled carbons from BCAAs may effectively ‘disappear’ during the course of a typical infusion experiment. Secondary labeling from mmBCFAs is thus not likely. Acetoacetate is avidly consumed by most tissues, and no 13C-labeled ketones are detectable in plasma of 13C-BCAA-infused animals, strongly arguing against the escape of acetoacetate from tissues. In contrast, 3-HIB likely does contribute to some tissue-exchange and secondary labeling, and 3-HIB secretion may acutely correlate with BCAA oxidation, but precise quantification of this exchange will require studies with infused labeled 3-HIB [63,64]. Under rare pathological conditions such as inborn errors of metabolism affecting enzymes in the BCAA catabolic pathway, massive accumulation of upstream intermediates can also lead to their CoA-free escape from mitochondria, possibly leading to some secondary labeling in other tissues, although these intermediates are likely mostly secreted in the urine. Finally, the impact of secondary labeling from metabolites derived from intermediates of the TCA cycle is also likely minimal, because escape of TCA intermediates into the circulation is low, and little of it is derived from BCAAs [39,40].
System perturbations in disease
Understanding how BCAA metabolism in one tissue impacts systemic BCAA levels is important for studying why BCAAs become elevated in specific diseases.
Maple syrup urine disease
Maple syrup urine disease (MSUD) is caused by autosomal recessive mutations in either the E1 (BCKDHA/BCKDHB) or E2 (DBT) subunits of BCKDH. Because the E3 (DLD) subunit of BCKDH is shared with PDH and OGDH, mutations in DLD cause more complex disease, and though sometimes labeled MSUD, is more appropriately labeled dihydrolipoamide dehydrogenase deficiency. Mutations in PPM1K can cause a mild variant of MSUD. Overall, disease severity is usually inversely related to residual enzyme activity. These mutations lead to elevated plasma BCAA and BCKA levels, as well as elevated urine levels of sotolone, an otherwise rare byproduct of excess leucine and isoleucine that gives urine a maple syrup-like odor [65-67]. If left untreated, MSUD can cause cerebral edema, encephalopathy, and ultimately death, underscoring the importance of tight homeostatic regulation of BCAAs [68,69]. Initial treatment requires patients to eat a protein-restricted diet to maintain BCAAs at a more physiologic level [70]. MSUD can be cured by liver transplantation, which restores BCAA homeostasis, presumably be reintroducing sufficient whole-body BCAA oxidation capacity [71].
Cancer
Alterations in plasma BCAA levels and BCAA metabolism have been noted in a variety of cancers. Plasma BCAAs are increased in mice and humans with pancreatic cancer [72] or hepatocellular carcinoma (HCC) [73]. The metabolism of the tumor itself is unlikely to account for systemic changes in plasma BCAA levels [74], suggesting that cancer can drive a change in whole-body BCAA metabolism. However, few studies have focused on the question of how cancer affects whole-body BCAA metabolism. Instead, most studies have focused on BCAA metabolism in the tumor itself, whether in tumor cells or in surrounding stroma. The majority of these studies focused specifically on BCAT1, which is found to be increased in breast cancer [75], both acute and chronic myeloid leukemia (AML and CML [76]), glioblastoma [77], ovarian cancer [78], and hepatocellular cancer [73]. Pro-tumorigenic mechanisms likely differ in different contexts. In AML, for example, by virtue of transferring nitrogen from BCAAs to α-ketoglutarate (α-KG), BCAT1 decreases the availability of α-KG needed as a cofactor for cellular diogenases, including histone and DNA demethylases [76,79]; BCAT1 overexpression thus mimics mutations in IDH1, which also inhibit demethylases via the production of the potent a-KG-like inhibitor (R)-2-hydroxy-glutarate (2HG), as well as mutations in TET2, a key target demethylase. Similar mechanisms are likely at play in glioblastoma, which bear similar genetic profiles [77]. In contrast, in pancreatic cancer, BCAT1 has recently been suggested to be required in stromal cancer-associated fibroblasts, serving to provide BCKAs to the tumor cells [80]. Other members of BCAA catabolism have received less attention. HCC is associated with low BCAA catabolic capacity, and pharmaceutical or nutritional reduction in plasma BCAAs is protective in a mouse model of HCC; in this setting, reduced BCAA catabolism in the tumor is hypothesized to increase leucine, in turn, activating mTOR [73]. And three recent reports have implicated BCAT2 in pancreatic cancer progression, although the mechanism of action is not clear [81-83]. In sum, BCAA metabolism has profound effects on multiple cancers and cell types, in ways that are only beginning to be understood. For further details, we refer the reader to Sivanand and Vander Heiden [74].
BCAAs and insulin resistance
Elevated plasma BCAAs have been observed in obese, insulin-resistance patients compared with age- and sex-matched controls since the 1960s [84,85]. More recently, metabolite profiles taken from the blood of over 2000 individuals with normal glucose homeostasis who were followed for 12 years revealed that elevated BCAAs correlated with future type 2 diabetes development [86]. A similar study evaluated metabolite profiles and insulin resistance at baseline and after 6 years, revealing a strong association between BCAAs and the development of insulin resistance, especially in men [87]. A systematic review of multiple metabolomics studies including a total of 8000 people revealed that the risk of type 2 diabetes development increased by ~35% per standard deviation increase in circulating BCAA [88]. Another study demonstrated through metabolomic profiling that a BCAA-related metabolite signature was suggestive of increased BCAA catabolism in obese, insulin-resistant people compared with lean controls [89]. Together, these studies leave little doubt that elevated BCAAs and/or increased BCAA catabolism correlate with future insulin resistance and type 2 diabetes, strongly suggesting a causal role of BCAAs in the development of insulin resistance.
A genetic Mendelian randomization study further supported the conclusion that BCAAs cause type 2 diabetes [90]. This type of study proposes that if a biomarker, e.g. elevated BCAAs, is causal in the development of a disease, e.g. type 2 diabetes, then genetic variants associated with the biomarker should also associate with the disease. Metabolomic measurements were coupled with genome-wide association studies (GWASs) to obtain genetic signatures that associate with elevated plasma BCAAs, and these signatures were then shown also to associate with type 2 diabetes. The SNP with the strongest association with type 2 diabetes lay near, and alters the expression of, PPM1K, the gene that encodes the BCKDH phosphatase, adding mechanistic plausibility to the observation [90].
Finally, studies involving either infusion [91-93] or ingestion [64] of BCAAs followed by assessment of insulin resistance using hyperinsulinemic-euglycemic clamps also support a causal role for BCAAs in the development of insulin resistance. In these studies, healthy patients given BCAAs became acutely insulin resistant in less than 6 h [64,91-93]. Similarly, supplementing a high-fat diet with BCAAs worsens insulin resistance in rats [94], while restricting BCAAs improves insulin resistance in mice and rats [95,96]. Together, these numerous epidemiological studies, Mendelian randomization studies, and intervention studies, strongly support a causal role for BCAAs in the development of insulin resistance and type 2 diabetes. It is important to note that insulin resistance could also, conversely, drive elevations in BCAA levels. A different Mendelian randomization study than the one discussed above suggested the converse conclusion, i.e. that insulin resistance may drive elevated BCAA levels [97]. Similar conclusions were made in a rodent study of gastric bypass in which surgery improved glucose handling independent of various BCAA challenges [98]. Importantly, these relationships between BCAAs and insulin sensitivity are not mutually exclusive, and may in fact create a disease-worsening positive feedback loop.
The mechanism by which BCAAs drive insulin resistance remains unclear and the subject of active investigation. In 2016, White et al. demonstrated in Zucker-fatty rats (ZFRs) that BCKDH activity was higher in skeletal muscle and lower in liver from obese rats compared with lean controls [96]. Others have also noted elevated phosphorylation, and lower activity, of liver BCKDH in various models of diabetes [99,100]. The same group later found that inhibition of BCKDK, either systemically with the small-molecule BCKDK inhibitor 3,6-dichlorobrenzo(b)thiophene-2-carboxylic acid (BT2) or primarily in the liver with adenovirus-mediated delivery of PPM1K, increased BCKDH activity in the livers of ZFRs, lowered hepatic triacylglyceride levels, and improved insulin resistance [46]. These data further support the causality of aberrant BCAA metabolism in insulin resistance, and strongly implicated suppressed liver BCAA oxidation in this process.
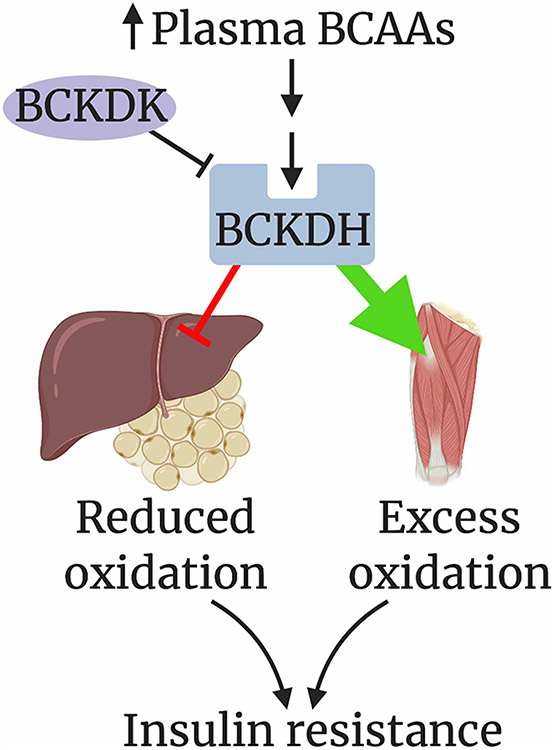
How might the suppression of liver BCAA catabolism promote whole-body insulin resistance? Work by our laboratory has implicated whole-body redistribution of BCAA oxidation towards skeletal muscle as a driver of insulin resistance (Figure 3). Steady-state in vivo heavy isotopic tracing has shown that the majority of BCAA oxidation occurs in muscle, and that, in db/db mice, a model of severe insulin resistance, BCAA oxidation is significantly further increased in skeletal muscle and decreased in the liver and adipose tissue [40]. The decreased adipose BCAA oxidation in models of insulin resistance appears to be mediated by suppressed transcriptional expression of BCAA catabolic genes [101], perhaps driven by hypoxic activation of HIF-1a [23], while the decreased liver BCAA oxidation is likely mediated by post-translational inhibition of BCKDH via phosphorylation [25-27]. Thus, in states of insulin resistance, BCAAs appear to be shunted away from the liver and adipose tissue and towards skeletal muscle [4]. We and others have suggested that alleviating this shunt via activation of BCKDH with BT2 improves insulin sensitivity [40,46,101]. Decreased BCAA metabolism in adipose tissue or the liver may also relate to reduced insulin sensitivity in other more indirect ways as well. For example, BCKDK and PPM1K in the liver have been suggested to target metabolic enzymes outside of BCAA metabolism [46]. And it is possible that the near complete loss of mmBCFA synthesis in adipose tissue may somehow promote insulin resistance.
Figure 3. Proposed model of how the redistribution of BCAA oxidation towards skeletal muscle drives insulin resistance. Figure created with BioRender.com.
Several theories have been proposed to explain how excess BCAA oxidation in skeletal muscle may link to insulin resistance. Insulin resistant patients and type 2 diabetics often have increased free fatty acid levels, as well as higher triglyceride content within their skeletal muscle, suggesting defects in lipid metabolism. An accumulation of incompletely oxidized lipid species, known as lipotoxicity, has been implicated in the development of insulin resistance, but how lipotoxicity occurs is incompletely understood [102,103]. One recently proposed mechanism directly implicates BCKA as inhibitors of insulin signaling [104]. Another hypothesis, supported by the observation of increased circulating levels of downstream catabolites of BCAA oxidation, such C3 and C5 acylcar-nitines, in patients with insulin resistance [89], is that increased BCAA oxidation may out-compete fatty acid oxidation, causing the accumulation of lipotoxic, incompletely oxidized lipid species [99,105]. Support for this notion includes studies in ZFRs that demonstrate that when fed a BCAA-restricted diet, fatty acyl-CoA content in the skeletal muscle of the rats decreased and insulin resistance was improved [96]. The same group also proposed the intriguing additional hypothesis that BCAA overload in muscle also presents a nitrogen burden, which is relieved by pyruvate conversion to alanine, at the expense of glycine conversion to pyruvate [106]. The consequent decrease in glycine may prevent the buffering of the excess of acyl (especially acetyl) CoAs as acyl-glycines to be secreted in urine, thus contributing to a bottleneck in lipid metabolism [96]. This hypothesis is consistent with the established observation that levels of glycine are inversely correlated with high circulating BCAA levels in cohorts with insulin resistance [107,108]. Glycine supplementation did not, however, improve glucose tolerance in HF-fed ZFRs, nor decrease muscle long-chain acyl CoAs [106]. The interesting connection between BCAAs, glycine, and lipid metabolism thus needs further investigation.
Work from our laboratory also supports the model that excess BCAA oxidation alters lipid metabolism and drives insulin resistance. We found that BCAA catabolism in skeletal muscle causes the secretion of 3-HIB, a catabolic intermediate of valine, which in turn promotes fatty acid uptake and transport to the muscle by the endothelium. Mice treated with 3-HIB were more insulin resistance than vehicle-treated controls [21]. Increased BCAA oxidation can thus promote lipid accumulation in skeletal muscle via paracrine effects on the vasculature. 3-HIB levels are elevated in db/db mice, as well as human subjects with type 2 diabetes compared with healthy controls [21]. How FA transport by the endothelium is mediated and regulated by 3-HIB remains unclear, but appears to involve a small family of ATP-dependent acyl-CoA synthetases and the production of ATP specifically from mitochondria [109].
BCAAs and heart failure
Elevations in plasma BCAAs are also associated with heart failure [45]. The expression of BCAA catabolic genes has been shown to be reduced in tissue from rodent and human failing hearts [110-112], suggesting that a BCAA catabolic defect exists in heart failure, although this has not yet been demonstrated directly. As noted above, the heart accounts for <4% of whole-body BCAA oxidation in wildtype mice and therefore defective BCAA oxidation in the heart is unlikely to explain elevated BCAA levels observed in heart failure, suggesting that this increase in plasma BCAAs may be due to a defect in BCAA metabolism elsewhere — e.g. skeletal muscle or liver. Heart failure is recognized to be, in part, caused by a failure of the heart to produce the energy it needs to pump blood, i.e. that the heart is ‘an engine out of fuel’ [113]. It has thus been suggested that catabolic defect of BCAAs in heart failure may contribute to this fuel insufficiency. However, numerous studies, including our measurements in rodents with isotopic infusions in vivo, and our more recent studies in humans, indicate the loss of BCAA oxidation would starve the heart of less than 2% of its energy production [39,114-116]. It may be that, instead, the healthy heart uses BCAA oxidation either to regulate BCAA levels per se, or to produce BCAA-derived signaling metabolites. No matter the mechanism, it is striking that BT2 treatment in multiple different mouse models of heart failure markedly improves outcomes [111,112,115], indicating that systemic BCAA metabolism is somehow critical in heart failure. Considering the diverse literature surrounding BCAAs and heart failure, we hypothesize that BT2 might improve outcomes in heart failure either by activating oxidation in another tissue or by impacting signaling from BCAA-derived metabolites within the heart.
Future directions
In conclusion, BCAA oxidation is distributed among most organs in healthy mice, and likely humans. Oxidation is regulated by both transcriptional and post-translational mechanisms (Figure 1). The impact of oxidation in a given tissue on systemic BCAA levels can be modeled by the tissue-specific relative preference for BCAA oxidation over other fuels, the metabolic rate of the tissue, and the total tissue mass in the organism (Figure 2). Alterations in BCAA metabolism have been implicated in a variety of diseases. Overexpression of BCAT1 is associated with a variety of cancers. In models of insulin resistance, BCAA oxidation is altered in adipose tissue, liver, and skeletal muscle. Increased oxidation in skeletal muscle and defective oxidation in the liver and/or adipose tissue likely contribute to insulin resistance (Figure 3). Altered oxidation of BCAAs have also been noted in heart failure, but the implications remain poorly understood. Future work is needed to test the contribution of altered BCAA oxidation in muscle, liver, and adipose tissue to the development of insulin resistance, as well as the role of BCAA oxidation in heart failure.
Abbreviations
- 3-HIB
3-hydroxyisobutyrate
- BCAAs
branched-chain amino acids
- BCKDH
branched-chain α-ketoacid dehydrogenase
- BCKDK
BCKDH kinase
- HCC
hepatocellular carcinoma
- MSUD
maple syrup urine disease
- TCA
tricarboxylic acid
- ZFRs
Zucker-fatty rats
- mmBCFAs
monomethyl branched-chain fatty acids
- BCKAs
branched-chain α-ketoacids
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Davis TA, Fiorotto ML and Reeds PJ (1993) Amino acid compositions of body and milk protein change during the suckling period in rats. J. Nutr 123, 947–956 10.1093/jn/123.5.947 [DOI] [PubMed] [Google Scholar]
- 2.Moura A, Savageau MA and Alves R R (2013) Relative amino acid composition signatures of organisms and environments. PLoS One 8, e77319 10.1371/journal.pone.0077319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweigert BS, Bennett BA and Guthneck BT (1954) Amino acid composition of organ meats. J. Food Sci 19, 219–223 10.1111/j.1365-2621.1954.tb17442.x [DOI] [Google Scholar]
- 4.Neinast M, Murashige D and Arany Z (2019) Branched chain amino acids. Annu. Rev. Physiol 81, 139–164 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agueusop I, Musholt PB, Klaus B, Hightower K and Kannt A (2020) Short-term variability of the human serum metabolome depending on nutritional and metabolic health status. Sci. Rep 10, 16310 10.1038/s41598-020-72914-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everman S, Mandarino LJ, Carroll CC and Katsanos CS (2015) Effects of acute exposure to increased plasma branched-chain amino acid concentrations on insulin-mediated plasma glucose turnover in healthy young subjects. PLoS One 10, e0120049 10.1371/journal.pone.0120049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louard RJ, Barrett EJ and Gelfand RA (1990) Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin. Sci 79, 457–466 10.1042/cs0790457 [DOI] [PubMed] [Google Scholar]
- 8.Wahren J, Felig P and Hagenfeldt L (1976) Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J. Clin. Invest 57, 987–999 10.1172/JCI108375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR and Bier DM (1980) Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-13C] leucine. Am. J. Physiol. Endocrinol. Metab 238, E473–E479 10.1152/ajpendo.1980.238.5.E473 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR, Goodenough RD, Wolfe MH, Royle GT and Nadel ER (1982) Isotopic analysis of leucine and urea metabolism in exercising humans. J. Appl. Physiol 52, 458–466 10.1152/jappl.1982.52.2.458 [DOI] [PubMed] [Google Scholar]
- 11.Short KR, Vittone JL, Bigelow ML, Proctor DN and Nair KS (2004) Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab 286, E92–E101 10.1152/ajpendo.00366.2003 [DOI] [PubMed] [Google Scholar]
- 12.Fulgoni VL (2008) Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr 87, 1554S–1557S 10.1093/ajcn/87.5.1554S [DOI] [PubMed] [Google Scholar]
- 13.Stokes T, Hector AJ, Morton RW, McGlory C and Phillips SM (2018) Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10, 180 10.3390/nu10020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC et al. (2021) Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 1, 73–86 10.1038/s43587-020-00006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichihara A (1975) Isozyme patterns of branched chain amino acid transaminase during cellular differentiation and carcinogenesis. Annals of the New York Academy of Sciences, 259, 347–354. 10.1111/j.1749-6632.1975.tb25431.x [DOI] [PubMed] [Google Scholar]
- 16.Goto M, Shinno H and Ichihara A (1977) Isozyme patterns of branched-chain amino acid transaminase in human tissues and tumors. GANN Jpn. J. Cancer Res 68, 663–667 [PubMed] [Google Scholar]
- 17.Ichihara A and Koyama E (1966) Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J. Biochem 59, 160–169 10.1093/oxfordjournals.jbchem.a128277 [DOI] [PubMed] [Google Scholar]
- 18.Johnson WA, Connelly JL and Glynn MT (1972) Cellular localization and characterization of bovine liver branched-chain α-keto acid dehydrogenases. Biochemistry 11, 1967–1973 10.1021/bi00760a036 [DOI] [PubMed] [Google Scholar]
- 19.Ævarsson A, Seger K, Turley S, Sokatch JR and Hol WG (1999) Crystal structure of 2-oxoisovalerate and dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nat. Struct. Biol 6, 785–792 10.1038/11563 [DOI] [PubMed] [Google Scholar]
- 20.Ævarsson A, Chuang JL, Wynn RM, Turley S, Chuang DT and Hol WG (2000) Crystal structure of human branched-chain α-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure 8, 277–291 10.1016/S0969-2126(00)00105-2 [DOI] [PubMed] [Google Scholar]
- 21.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC et al. (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med 22, 421–429 10.1038/nm.4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper AE, Miller RH and Block KP (1984) Branched-chain amino acid metabolism. Ann. Rev. Nutr 4, 409–454 10.1146/annurev.nu.04.070184.002205 [DOI] [PubMed] [Google Scholar]
- 23.Wallace M, Green CR, Roberts LS, Lee YM, McCarville JL, Sanchez-Gurmaches J et al. (2018) Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol 14, 1021–1031 10.1038/s41589-018-0132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green C, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP et al. (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol 12, 15–21 10.1038/nchembio.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxton R and Harris RA (1982) Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J. Biol. Chem 257, 14433–14439 10.1016/S0021-9258(19)45399-4 [DOI] [PubMed] [Google Scholar]
- 26.Harris RA, Popov KM, Shimomura Y, Zhao Y, Jaskiewicz J, Nanaumi N et al. (1992) Purification, characterization, regulation and molecular cloning of mitochondrial protein kinases. Adv. Enzyme Regul 32, 267–284 10.1016/0065-2571(92)90022-R [DOI] [PubMed] [Google Scholar]
- 27.Wynn RM, Kato M, Machius M, Chuang JL, Li J, Tomchick DR et al. (2004) Molecular mechanism for regulation of the human mitochondrial branched-chain α-ketoacid dehydrogenase complex by phosphorylation. Structure 12, 2185–2196 10.1016/j.str.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 28.Damuni Z and Reed LJ (1987) Purification and properties of the catalytic subunit of the branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney mitochondria. J. Biol. Chem 262, 5129–5132 10.1016/S0021-9258(18)61164-0 [DOI] [PubMed] [Google Scholar]
- 29.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P et al. (2009) Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest 119, 1678–1687 10.1172/JCI38151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowell PL, Block KP, Repa JJ, Torres N, Nawabi MD, Buse MG et al. (1990) High branched-chain α-ketoacid intake, branched chain α-keto acid dehydrogenase activity, and plasma and brain amino acid and plasma keto acid concentrations in rats. Am. J. Clin. Nutr 52, 313–319 10.1093/ajcn/52.2.313 [DOI] [PubMed] [Google Scholar]
- 31.Frick GP, Tai LR, Blinder L and Goodman HM (1981) L-leucine activates branched chain α-keto acid dehydrogenase in rat adipose tissue. J. Biol. Chem 256, 2618–2620 10.1016/S0021-9258(19)69657-2 [DOI] [PubMed] [Google Scholar]
- 32.Lau KS, Fatania HR and Randle PJ (1982) Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 144, 57–62 10.1016/0014-5793(82)80568-1 [DOI] [PubMed] [Google Scholar]
- 33.Waymack PP, DeBuysere MS and Olson MS (1980) Studies on the activation and inactivation of the branched chain α-keto acid dehydrogenase in the perfused rat heart. J. Biol. Chem 255, 9773–9781 10.1016/S0021-9258(18)43460-6 [DOI] [PubMed] [Google Scholar]
- 34.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT and Hutson SM (2010) Branched-chain amino acid metabolon, interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase (BCATm). J. Biol. Chem 285, 265–276 10.1074/jbc.M109.048777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haymond MW and Miles JM (1982) Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 31, 86–89 10.2337/diab.31.1.86 [DOI] [PubMed] [Google Scholar]
- 36.Matthews DE, Bier DM, Rennie MJ, Edwards RH, Halliday D, Millward DJ et al. (1981) Regulation of leucine metabolism in man: a stable isotope study. Science 214, 1129–1131 10.1126/science.7302583 [DOI] [PubMed] [Google Scholar]
- 37.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL and Beaufrère B (1997) Slow and fast dietary proteins differently modulate postprandial proteinaccretion. Proc. Natl Acad. Sci. U.S.A 94, 14930–14935 10.1073/pnas.94.26.14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P et al. (2001) The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab 280, E340–E348 10.1152/ajpendo.2001.280.2.E340 [DOI] [PubMed] [Google Scholar]
- 39.Hui S, Cowan AJ, Zeng X, Yang L, TeSlaa T, Li X et al. (2020) Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 10.1016/j.cmet.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ et al. (2019) Quantitative analysis of whole-body metabolic fate for branched-chain amino acids. Cell Metab. 29, 417–429 10.1016/j.cmet.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tessari P, Garibotto G, Inchiostro S, Robaudo C, Saffioti S, Vettore M et al. (1996) Kidney, splanchnic, and leg protein turnover in humans. Insight from leucine and phenylalanine kinetics. J. Clin. Invest 98, 1481–1492 10.1172/JCI118937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K et al. (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 10.1038/s41586-019-1503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatazawa Y, Tadaishi M, Nagaike Y, Morita A, Ogawa Y, Ezaki O et al. (2014) PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS One 9, e91006 10.1371/journal.pone.0091006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeyaraj D, Scheer FAJL, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA et al. (2012) Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 15, 311–323 10.1016/j.cmet.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z et al. (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133, 2038–2049 10.1161/CIRCULATIONAHA.115.020226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman J et al. (2018) The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 27, 1281–1293 10.1016/j.cmet.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi MA, Jeoung NH, Obayashi M, Hattab EM, Brocken EG, Liechty EA et al. (2006) Impaired growth and neurological abnormalities in branched-chain α-keto acid dehydrogenase kinase-deficient mice. Biochem. J 400, 153–162 10.1042/BJ20060869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE and Hutson SM (1998) A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr 68, 72–81 10.1093/ajcn/68.1.72 [DOI] [PubMed] [Google Scholar]
- 49.Randle PJ, England PJ and Denton RM (1970) Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem. J 117, 677–695 10.1042/bj1170677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malloy CR, Jones JG, Jeffrey FM, Jessen ME and Sherry AD (1996) Contribution of various substrates to total citric acid cycle flux and anaplerosis as determined by 13C isotopomer analysis and O2 consumption in the heart. MAGMA 4, 35–46 10.1007/BF01759778 [DOI] [PubMed] [Google Scholar]
- 51.Shimomura Y, Murakami T, Nakai N, Nagasaki M and Harris RA (2004) Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr 134, 1583S–1587S 10.1093/jn/134.6.1583S [DOI] [PubMed] [Google Scholar]
- 52.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H et al. (2008) Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 5, e51 10.1371/journal.pmed.0050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiklund P, Zhang X, Pekkala S, Autio R, Kong L, Yang Y et al. (2016) Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci. Rep 6, 24540 10.1038/srep24540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, Thapar D et al. (2011) Multi-tissue, selective PPARγ modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. Endocrinol. Metab 300, E164–E174 10.1152/ajpendo.00219.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahill GF (1970) Starvation in man. N. Engl. J. Med 282, 668–675 10.1056/NEJM197003052821026 [DOI] [PubMed] [Google Scholar]
- 56.Harris RA, Zhang B, Goodwin GW, Kuntz MJ, Shimomura Y, Rougraff P et al. (1990) Regulation of the branched-chain α-ketoacid dehydrogenase and elucidation of a molecular basis for maple syrup urine disease. Adv. Enzyme Regul 30, 245–263 10.1016/0065-2571(90)90021-S [DOI] [PubMed] [Google Scholar]
- 57.Burt MG, Gibney J and Ho KKY (2007) Protein metabolism in glucocorticoid excess: study in Cushing’s syndrome and the effect of treatment. Endocrinol. Metab 292, E1426–E1432 10.1152/ajpendo.00524.2006 [DOI] [PubMed] [Google Scholar]
- 58.Shimomura Y, Obayashi M, Murakami T and Harris RA (2001) Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr. Opin. Clin. Nutr. Metab. Care 4, 419–423 10.1097/00075197-200109000-00013 [DOI] [PubMed] [Google Scholar]
- 59.Gelfand RA, Hutchinson-Williams KA, Bonde AA, Castellino P and Sherwin RS (1987) Catabolic effects of thyroid hormone excess: the contribution of adrenergic activity to hypermetabolism and protein breakdown. Clin. Exp. Metab 36, 562–569 10.1016/0026-0495(87)90168-5 [DOI] [PubMed] [Google Scholar]
- 60.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W et al. (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloomgarden ZT, Liljenquist J, Lacy W and Rabin D (1981) Amino acid disposition by liver and gastrointestinal tract after protein and glucose ingestion. Am. Physiol. Soc 241, E90–E99 10.1152/ajpendo.1981.241.1.E90 [DOI] [PubMed] [Google Scholar]
- 62.Elia M and Livesey G (1983) Effects of ingested steak and infused leucine on forelimb metabolism in man and the fate of the carbon skeletons and amino groups of branched-chain amino acids. Clin. Sci. (Lond) 64, 517–526 10.1042/cs0640517 [DOI] [PubMed] [Google Scholar]
- 63.Lee S, Gulseth HL, Langleite TM, Norheim F, Olsen T, Refsum H et al. (2020) Branched-chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia 64, 410–423 10.1007/s00125-020-05296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris LLS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, Kelly SC et al. (2017) Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes 66, 1871–1878 10.2337/db16-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schadewaldt P, Bodner-Leidecker A, Hammen HW and Wendel U (1999) Significance of L-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin. Chem 45, 1734–1740 10.1093/clinchem/45.10.1734 [DOI] [PubMed] [Google Scholar]
- 66.Podebrad F, Heil M, Reichert S, Mosandl A, Sewell AC and Böhles H (1999) 4,5-Dimethyl-3-hydroxy- 2[5H]-furanone (sotolone)—the odour of maple syrup urine disease. J. Inherit. Metab. Dis 22, 107–114 10.1023/A:1005433516026 [DOI] [PubMed] [Google Scholar]
- 67.Sewell AC, Mosandl A and Böhles H (1999) False diagnosis of maple syrup urine disease owing to ingestion of herbal tea. N. Engl. J. Med 341, 769 10.1056/NEJM199909023411020 [DOI] [PubMed] [Google Scholar]
- 68.Yudkoff M (1997) Brain metabolism of branched-chain amino acids. Glia 21, 92–98 [DOI] [PubMed] [Google Scholar]
- 69.Yudkoff M, Nissim I, Kim S, Pleasure D, Hummeler K and Segal S (1983) [15n] leucine as a source of [15N] glutamate in organotypic cerebellar explants. Biochem. Biophys. Res. Commun 115, 174–179 10.1016/0006-291X(83)90985-3 [DOI] [PubMed] [Google Scholar]
- 70.Gambello MJ and Li H (2018) Current strategies for the treatment of inborn errors of metabolism. J. Genet. Genom 45, 61–70 10.1016/j.jgg.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 71.Díaz VM, Camarena C, de la Vega A, Martínez-Pardo M, Díaz C, López M et al. (2014) Liver transplantation for classical maple syrup urine disease: long-term follow-up. J. Pediatr. Gastroenterol. Nutr 59, 636–639 10.1097/MPG.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 72.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP et al. (2014) Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med 20, 1193–1198 10.1038/nm.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ et al. (2019) Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 29, 1151–1165 10.1016/j.cmet.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sivanand S and Vander Heiden MG (2020) Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 37, P147–P156 10.1016/j.ccell.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L and Han J (2017) Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun 486, 224–231 10.1016/j.bbrc.2017.02.101 [DOI] [PubMed] [Google Scholar]
- 76.Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J et al. (2017) Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 545, 500–504 10.1038/nature22314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tönjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM et al. (2013) BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med 19, 901–908 10.1038/nm.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang ZQ, Faddaoui A, Bachvarova M, Plante M, Gregoire J, Renaud MC et al. (2015) BCAT1 expression associates with ovarian cancer progression: possible implications in altered disease metabolism. Oncotarget 6, 31522–31543 10.18632/oncotarget.5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Zhang Y, Ni M, Cao H, Signer RJA, Li D et al. (2017) Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol 19, 626–638 10.1038/ncb3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Z, Achreja A, Meurs N, Animasahun O, Owen S, Mittal A et al. (2020) Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat. Metab 2, 775–792 10.1038/s42255-020-0226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li JT, Yin M, Wang D, Wang J, Lei MZ, Zhang Y et al. (2020) BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol 22, 167–174 10.1038/s41556-019-0455-6 [DOI] [PubMed] [Google Scholar]
- 82.Lei MZ, Li XX, Zhang Y, Li JT, Zhang F, Wang YP et al. (2020) Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Sig. Transduct. Target Ther 5, 70 10.1038/s41392-020-0168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JH, Cho YR, Kim JH, Kim J, Nam HY, Kim SW et al. (2019) Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp. Mol. Med 51, 1–11 10.1038/s12276-019-0350-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adibi SA (1968) Influence of dietary deprivations on plasma concentration of free amino acids of man. J. Appl. Physiol 25, 52–57 10.1152/jappl.1968.25.1.52 [DOI] [PubMed] [Google Scholar]
- 85.Felig P, Marliss E and Cahill GF Jr (1969) Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med 281, 811–816 10.1056/NEJM196910092811503 [DOI] [PubMed] [Google Scholar]
- 86.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat. Med 17, 448–453 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M et al. (2013) Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655 10.2337/dc12-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J et al. (2016) Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 10.2337/dc15-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF et al. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T et al. (2016) Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a mendelian randomisation analysis. PLoS Med. 13, 1–22 10.1371/journal.pmed.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M et al. (2002) Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 51, 599–605 10.2337/diabetes.51.3.599 [DOI] [PubMed] [Google Scholar]
- 92.Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P et al. (2003) Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 46, 917–925 10.1007/s00125-003-1129-1 [DOI] [PubMed] [Google Scholar]
- 93.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E et al. (2005) Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54, 2674–2684 10.2337/diabetes.54.9.2674 [DOI] [PubMed] [Google Scholar]
- 94.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA et al. (2017) Restoration of metabolic health by decreased consumption of branched-chain amino acids: a low BCAA diet restores metabolic health. J. Physiol 596, 623–645 10.1113/JP275075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontana L, Cummings NE, Apelo SIA, Neuman JC, Kasza I, Schmidt BA et al. (2016) Decreased consumption of branched chain amino acids improves metabolic health. Cell Rep. 16, 520–530 10.1016/j.celrep.2016.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD et al. (2016) Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab 5, 538–551 10.1016/j.molmet.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahendra Y, Jonsson A, Have CT, Allin KH, Witte DH, Jørgensen ME et al. (2017) Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 60, 873–878 10.1007/s00125-017-4222-6 [DOI] [PubMed] [Google Scholar]
- 98.Kramer NB, Evers SS, Shin JH, Silverwood S, Wang Y, Burant CF et al. (2020) The role of elevated branched-chain amino acids in the effects of vertical sleeve gastrectomy to reduce weight and improve glucose regulation. Cell Rep. 33, 108239 10.1016/j.celrep.2020.108239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.She P, Van Horn C, Reid T, Hutson SM, Cooney RN and Lynch CJ (2007) Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab 293, E1552–E1563 10.1152/ajpendo.00134.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H et al. (2015) Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64, 49–59 10.2337/db14-0312 [DOI] [PubMed] [Google Scholar]
- 101.Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y et al. (2019) Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68, 1730–1746 10.2337/db18-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brøns C and Vaag A (2009) Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes. J. Physiol 587, 3977–3978 10.1113/jphysiol.2009.177758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Savage DB, Petersen KF and Shulman GI (2007) Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev 87, 507–520 10.1152/physrev.00024.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biswas D, Dao KT, Mercer A, Cowie AM, Duffley L, El Hiani Y et al. (2020) Branched-chain ketoacid overload inhibits insulin action in the muscle. J. Biol. Chem 295, 15597–15621 10.1074/jbc.RA120.013121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Newgard CB (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 10.1016/j.cmet.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.White PJ, Lapworth AL, McGarrah RW, Coulter Kwee L, Crown SB, Ilkayeva O et al. (2020) Muscle-liver trafficking of BCAA-derived nitrogen underlies obesity-related glycine depletion. Cell Rep. 33, 108375 10.1016/j.celrep.2020.108375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG et al. (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62, 639–648 10.2337/db12-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y et al. (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol 8, 615 10.1038/msb.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibrahim A, Yucel N, Kim B and Arany Z (2020) Local mitochondrial ATP production regulates endothelial fatty acid uptake and transport. Cell Metab. 32, 1–11 10.1016/j.cmet.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGarrah RW, Crown SB, Zhang GF, Shah SH and Newgard CB (2018) Cardiovascular metabolomics. Circ. Res. 122, 1238–1258 10.1161/CIRCRESAHA.117.311002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M et al. (2017) Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 25, 374–385 10.1016/j.cmet.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H et al. (2016) Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol 311, H1160–H1169 10.1152/ajpheart.00114.2016 [DOI] [PubMed] [Google Scholar]
- 113.Neubauer S (2007) The failing heart- an engine out of fuel. N. Engl. J. Med 356, 1140–1151 10.1056/NEJMra063052 [DOI] [PubMed] [Google Scholar]
- 114.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC et al. (2020) Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 10.1126/science.abc8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM et al. (2019) Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J. Am. Heart Assoc 8, e011625 10.1161/JAHA.118.011625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allard MF, Schönekess BO, Henning SL, English DR and Lopaschuk GD (1994) Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am. J. Physiol 267, H742–H750 10.1152/ajpheart.1994.267.2.H742 [DOI] [PubMed] [Google Scholar]