Abstract
IMPORTANCE
Crohn disease, a chronic gastrointestinal inflammatory disease, is increasing in incidence and prevalence in many parts of the world. Uncontrolled inflammation leads to long-term complications, including fibrotic strictures, enteric fistulae, and intestinal neoplasia. Therefore, early and effective control of inflammation is of critical importance.
OBSERVATIONS
The optimal management approach for Crohn disease incorporates patient risk stratification, patient preference, and clinical factors in therapeutic decision-making. First-line therapy generally consists of steroids for rapid palliation of symptoms during initiation of anti–tumor necrosis factor α therapy. Other treatments may include monoclonal antibodies to IL-12/23 or integrin α4β7, immunomodulators, combination therapies, or surgery. Effective control of inflammation reduces the risk of penetrating complications (such as intra-abdominal abscesses and fistulae), although more than half of patients will develop complications that require surgery. Adverse reactions to therapy include antibody formation and infusion reactions, infections, and cancers associated with immune modulators and biologics and toxicity to the bone marrow and the liver. Both Crohn disease and corticosteroid use are associated with osteoporosis. Vaccinations to prevent infections, such as influenza, pneumonia, and herpes zoster, are important components of health maintenance for patients with Crohn disease, although live vaccines are contraindicated for patients receiving immune suppression therapy.
CONCLUSIONS AND RELEVANCE
The treatment of patients with Crohn disease depends on disease severity, patient risk stratification, patient preference, and clinical factors, including age of onset and penetrating complications, and includes treatment with steroids, monoclonal antibody therapies, immunomodulators, and surgery. Physicians should be familiar with the advantages and disadvantages of each therapy to best counsel their patients.
Inflammatory bowel disease is an autoimmune disorder that manifests as a chronic, intractable activation of the intestinal immune system. There are 2 types of inflammatory bowel disease (IBD): Crohn disease and ulcerative colitis. These subtypes are differentiated based on disease distribution and histologic findings, although considerable overlap can occur.
The purpose of this review is to provide an evidence-based update of management options for Crohn disease. In the past several decades, the therapeutic armamentarium has significantly expanded. Previously, therapy options were limited to corticosteroids, immune modulators, and surgery. However, more recently, multiple classes of biologic therapies have been approved for the management of Crohn disease. Each treatment strategy has risks and benefits, and appropriate patient counseling and surveillance are necessary.
Methods
We searched PubMed for clinical trials, meta-analyses, systematic reviews, observational studies, and practice guidelines related to the natural history and management of Crohn disease. Articles published through August 1, 2020, were eligible for inclusion. Articles selected for inclusion were chosen by the authors based on the best available data. Priority was given to clinical trials, meta-analyses, Cochrane reviews, and practice guidelines. Included articles were restricted to English-language publications.
Six case-control studies, 17 cohort studies, 3 cross-sectional studies, 7 guidelines, 2 ideas/editorials/opinions, 14 meta-analyses, 14 narrative reviews, 18 randomized controlled trials, and 7 systematic reviews were included in the review.
Epidemiology and Pathogenesis
The peak incidence of Crohn disease occurs among patients aged 15 to 25 years, but all ages are affected. In North America, the incidence of Crohn disease is between 6.3 and 23.8 per 100 000 person-years, and the prevalence is between 96.3 and 318.5 per 100 000 person-years.1 The incidence of Crohn disease is increasing globally, notably in countries with increasing industrialization, air pollution, and Westernization of their diets. Examples include Brazil, with an annual incidence increase of 11.1% from 1988 to 2012, and Taiwan, with an annual incidence increase of 4.0% from 1998 to 2008.1
The pathogenesis of Crohn disease is multifactorial contributing factors, including genetics; the host microbiome; and environmental factors, such as smoking, medications, and diet.2–5 To date, more than 100 genetic markers have been associated with Crohn disease, highlighting the polygenic nature of the disease. Implicated genes suggest that immunoreactivity to gut bacteria is a critical component in the risk of developing Crohn disease.2–4 Interactions between genes and environment may facilitate the pathogenesis of Crohn disease via damage to the lining of the intestine or perturbation of immune defenses, which increase exposure of the primed immune system to intestinal bacteria.
Smoking is a well-described environmental risk factor for both the development of Crohn disease as well as many adverse disease outcomes. Smoking has been associated with an increased risk of Crohn disease (odds ratio [OR], 1.76 [95% CI, 1.40–2.22]), disease flare (OR, 1.56 [95% CI, 1.21–2.01]), and need for surgery (OR, 1.68 [95% CI, 1.33–2.12]).6,7 Furthermore, in a retrospective cohort study of patients with Crohn disease in Spain, smokers (n = 1535), compared with nonsmokers (n = 1689), had an increased need for steroids (92% vs 86%; P < .05), immunosuppressive medications (74% vs 64%; P < .05), and anti–tumor necrosis factor (TNF) biologic therapies (31% vs 25%; P < .05).8 Therefore, smoking cessation is advised in all patients with Crohn disease.
Clinical Presentation
Patients with Crohn disease often have symptoms for several years before the correct diagnosis is confirmed. The cardinal symptoms (Box 1) include fatigue, fever, abdominal pain, diarrhea, and weight loss; rectal bleeding is less common, but can occur when the distal colon is involved. Symptoms can fluctuate over time and are often misdiagnosed as irritable bowel syndrome. The average median diagnostic delay is 9 to 18 months from the onset of symptoms,9–11 and longer delays are associated with more intestinal obstruction and surgery. Isolated small bowel disease can present with protean constitutional symptoms. Up to 20% of patients can initially present with complications of Crohn disease, including intestinal strictures, abscesses, or fistulas.12
Box 1. Symptoms and Signs of Crohn Disease.
Symptoms
Fatigue
Fever
Abdominal pain and cramping
Diarrhea
Mouth sores
Lack of appetite
Thirst and lightheadedness
Urgent bowel movements
Redness or pain in the eyes from uveitis, iritis, or episcleritis
Swollen and painful joints, especially knees, hips, and elbows
Skin inflammation, including eczema, psoriasis, erythema
nodosum, and pyoderma gangrenosum
Signs
Weight loss
Slowed growth in children
Anemia (both iron deficiency and anemia of inflammation)
Mucus in the stool
Blood in the stool
Drainage of serum, pus, or stool from an opening near the anus
Dry mucus membranes
Psoas sign (consider abscess adjacent to terminal ileum)
Orthostatic signs
Elevated markers of inflammation (C-reactive protein, fecal
calprotectin, erythrocyte sedimentation rate)
Elevated alkaline phosphatase is associated with primary
sclerosing cholangitis
Diagnosis and Assessment
Many patients with Crohn disease have signs of anemia; iron deficiency; low vitamin D levels; and elevated inflammatory markers, including C-reactive protein, erythrocyte sedimentation rate, and fecal calprotectin. Fecal calprotectin is the most sensitive screening test for IBD (with estimated sensitivity of 0.87 [95% CI, 0.82–0.91] and specificity of 0.67 [95% CI, 0.58–0.75]), but results can be negative in patients with isolated small bowel disease.13 Patients with systemic symptoms, bowel symptoms, and evidence of inflammation should be evaluated endoscopically with biopsies. The hallmark findings of Crohn disease are discontinuous areas of inflammation characterized by ulceration, erythema, mucosal edema, and/or luminal narrowing (Figure 1). Tissue histology remains the cornerstone of diagnosis and is characterized by transmural inflammation with architectural distortion, lymphoid infiltrates, and/or granulomas (Figure 2). In patients who do not have rectal bleeding, it is often helpful to perform cross-sectional imaging with magnetic resonance enterography to identify locations of inflammation in the intestinal tract to target the endoscopic approach. Radiologic findings include stratified mural enhancement and luminal narrowing. Furthermore, cross-sectional imaging can also detect complications of Crohn disease, such as intra-abdominal abscesses and fistulae. Some patients with small bowel disease out of reach of the standard colonoscope may need a capsule endoscopy to identify ulcerative lesions followed by a balloon enteroscopy to obtain biopsies. Histologic evidence is required for a Crohn disease diagnosis given the costs and risks of biologic therapy.
Figure 1.
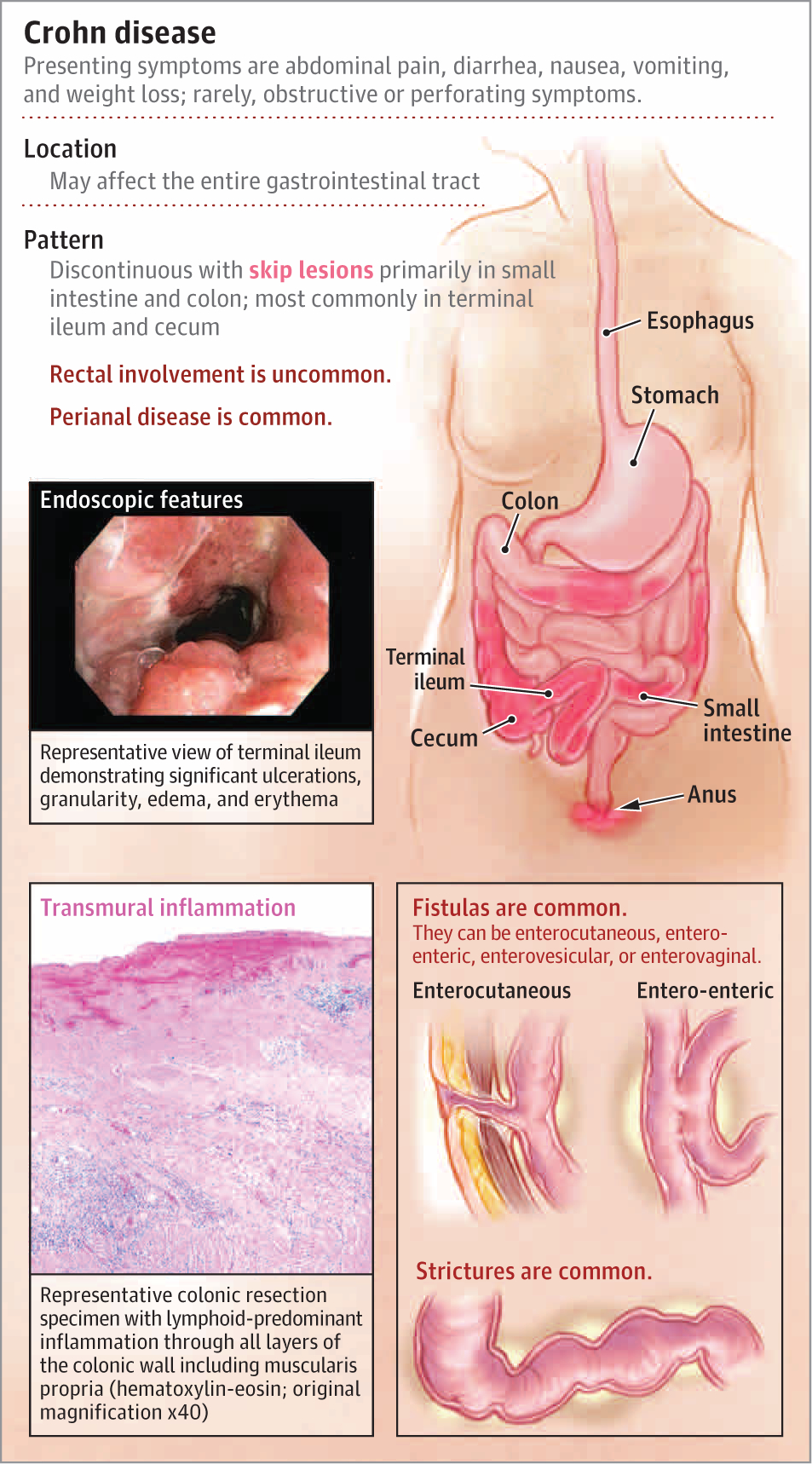
Characteristics of Crohn Disease
Figure 2. Helical Computed Tomography Imaging of Abdomen and Pelvis.
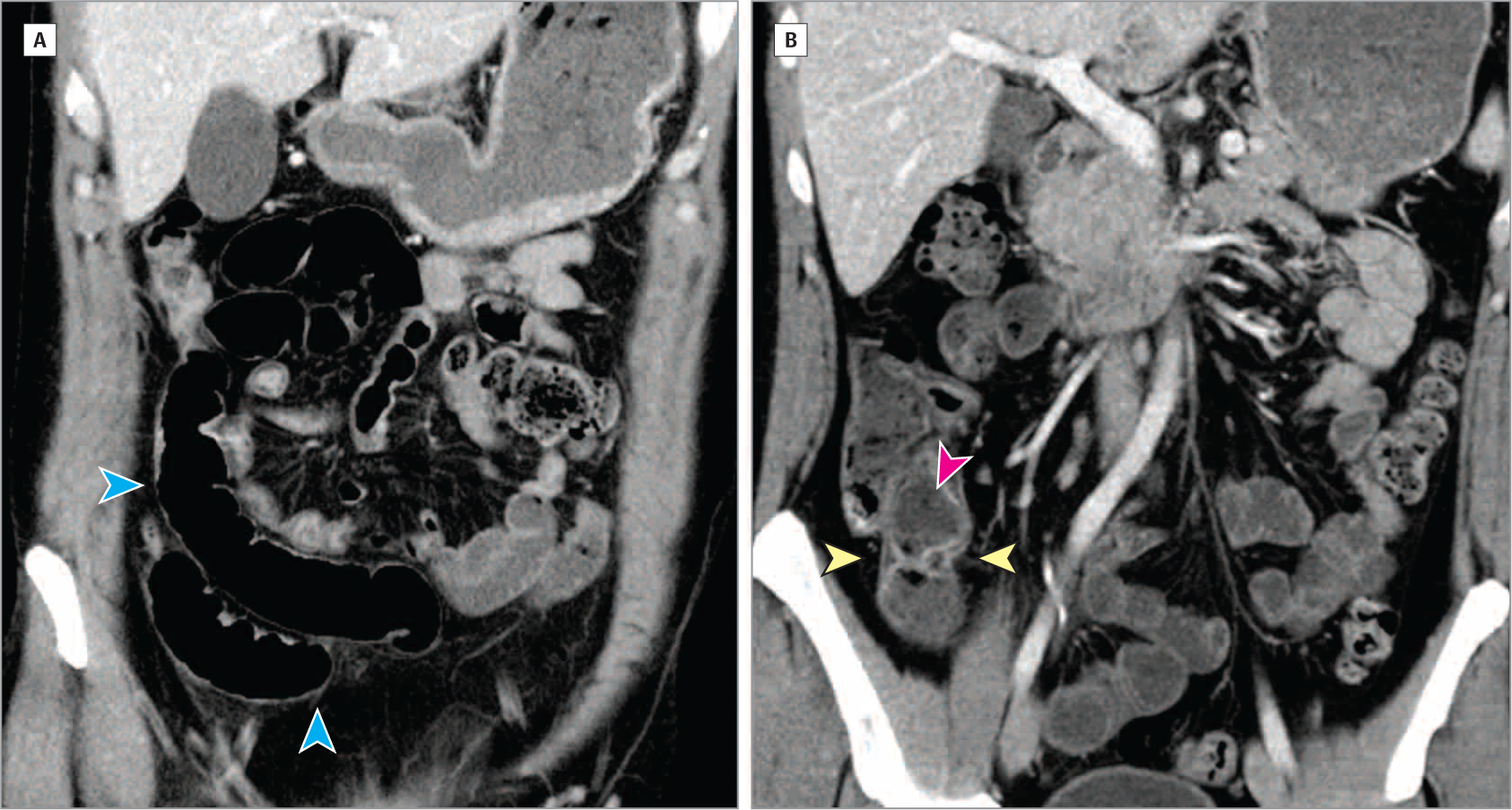
Coronal computed tomography views show slightly increased enhancement in the mucosa of the terminal ileum near the ileocecal valve with areas of slight wall thickening and mural stratification with predominantly intramural fat (yellow arrowheads), as well as feculent luminal contents (pink arrowhead). More proximal loops of ileum are slightly dilated (blue arrowheads). From Cheifetz AS. Management of acute Crohn disease. JAMA. 2013;309(20):2150–2158. doi:10.1001/jama.2013.4466.
The signs and symptoms of Crohn disease may overlap with other inflammatory or malignant conditions of the gastrointestinal tract (Box 1). Therefore, careful attention should be paid to the clinical history, which can often assist in identifying alternative etiologies (Figure 3 and Box 2).
Figure 3. Establishing the Diagnosis of Crohn Disease.
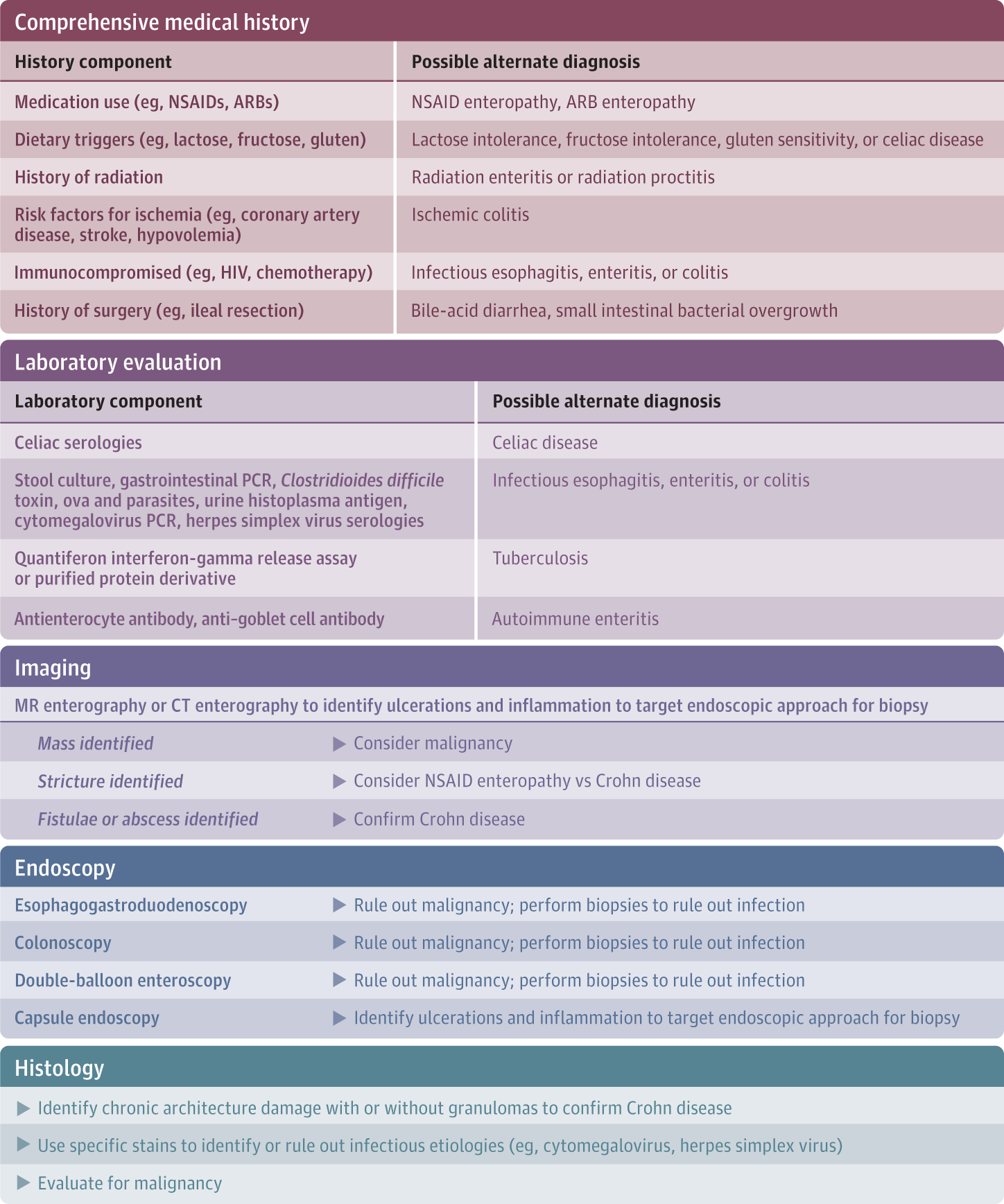
Essential components of a proper workup include a comprehensive history, laboratory evaluation, imaging, endoscopy, and biopsy. These elements can assist the clinician in ruling out alternative diagnoses. ARB indicates angiotensin II receptor blocker; CT, computed tomographic; MR, magnetic resonance; NSAID, nonsteroidal anti-inflammatory drug; PCR, polymerase chain reaction.
Box 2. Differential Diagnosis for Suspected Crohn Disease.
Upper Gastrointestinal Tract
Esophageal ulcers: herpes simplex virus, HIV, pill esophagitis
Small Intestine
Celiac sprue
Lactose intolerance
Small intestinal bacterial overgrowth
Autoimmune enteritis
Intestinal cancer, carcinoid
Angiotensin II receptor blocker
Meckel diverticulum (obstruction of small bowel)
Nonsteroidal anti-inflammatory drug–induced ulceration
Behçet disease
Endometriosis
Graft-versus-host disease
Leukemic infiltrate of the intestine
Common variable immunodeficiency
Kaposi sarcoma of the intestine
Large Intestine
Infectious colitis, including Shigella, Salmonella, Campylobacter, Escherichia coli, Yersinia, Clostridioides difficile, amebiasis, tuberculosis, histoplasmosis, cytomegalovirus, herpes simplex virus, lymphogranuloma venereum, and numerous parasites
Diverticular colitis, including segmental colitis associated with diverticulosis in the sigmoid colon, which can mimic a segmental sigmoid Crohn colitis
Diverticulitis
Ischemic colitis
Solitary rectal ulcer syndrome
Immune-activating cancer therapy targeting PD-1 or PD-L1 (eg, nivolumab, pembrolizumab)
Irritable bowel syndrome
Bile acid diarrhea
Nonsteroidal anti-inflammatory drug–induced ulceration
Behçet disease
Endometriosis
Graft-versus-host disease
Leukemic infiltrate of the intestine
Common variable immunodeficiency
Kaposi sarcoma of the intestine
Risk stratification for complicated Crohn disease should be part of the initial assessment. Patients with early-onset Crohn disease; small bowel location; perianal involvement; previous intestinal obstruction, fistula, or abscess; previous intestinal resections; and early steroid use and those who are current smokers are at increased risk of future complications.14,15 These patients especially require early effective therapy and close monitoring to maintain control of inflammation to prevent future disease complications.
Extraintestinal Manifestations
Extraintestinal manifestations of Crohn disease can include enteropathic arthritis (usually of the knees, hips, and shoulders; prevalence, 10%-20%), sacroiliitis (prevalence, 13%-50%), ankylosing spondylitis (prevalence, 1%-10%), pyoderma gangrenosum (prevalence, 2%), erythema nodosum (prevalence, 6%), uveitis (prevalence, <1%-17%), episcleritis (prevalence, up to 29%), scleritis (prevalence, up to 18%), primary sclerosing cholangitis (prevalence, 1%), and mouth ulcers (prevalence, 10%).16–19 The inflammatory arthritides usually manifest as stiffness early in the morning or after long periods of inactivity, and can improve with heat, a warm bath, or movement, which is a different pattern from that of osteoarthritis, which tends to worsen with movement. Pyoderma gangrenosum is an autoimmune phenomenon, not an infection, and vigorous debridement will make pyoderma gangrenosum worse. Patients with Crohn disease and recurrent elevations of alkaline phosphatase could have early-stage primary sclerosing cholangitis, which can be reliably diagnosed with a magnetic resonance cholangiopancreatography.20
Treatment
Medical therapies for the management of Crohn disease work by suppressing an overly active intestinal immune system. Treatment consists of 2 phases: induction and maintenance. Induction involves a higher dose of a steroid-sparing medication during the initial weeks to months of therapy to rapidly induce clinical remission. Steroids may be used during the induction phase of treatment to achieve symptom control. Budesonide can be used in mild to moderate cases because it has significant first-pass metabolism in the liver and thus reduced systemic absorption. Maintenance therapy involves using a lower dose of a steroid-sparing medication, such as an immune modulator or a biologic, for the remainder of the patient’s life to keep a patient in remission and prevent disease flares. Steroids are not effective maintenance therapies. Further, inappropriate and excessive use of steroids increases the risk of complications (such as osteopenia and infections) and puts physicians at risk of malpractice claims.21
Many options are available for the medical treatment of patients with Crohn disease (Box 3). Long-term treatment strategies target various immune signaling pathways. Therapy options include immune modulators, such as azathioprine, 6-mercaptopurine, and methotrexate, as well as biologic medications, such as antibodies to TNF-α, IL-12/23, and integrin α4β7. Before initiating any biologic therapy, patients should be tested for latent tuberculosis and hepatitis B. Positive test results warrant specialty consultation for appropriate treatment prior to medication initiation.
Box 3. Maintenance Medications for Crohn Disease.
5-Aminosalicylic Acid
Sulfasalazine
Immune Modulator
Azathioprine
6-Mercaptopurine
Methotrexate
Anti-Tumor Necrosis Factor
Infliximab
Adalimumab
Certolizumab
Anti-Integrin
Vedolizumab
Natalizumab
Anti-IL-12/23
Ustekinumab
Mesalamine therapies, which are often used in ulcerative colitis, are ineffective in the medical management of Crohn disease. A systematic review that included 20 randomized clinical trials that evaluated the efficacy of aminosalicylates in mild to moderate Crohn disease found that high-dose mesalamine was not superior to placebo in induction of remission (relative risk, 2.02 [95% CI, 0.75–5.45]).22 Immunomodulators, including methotrexate and thiopurines (azathioprine and 6-mercaptopurine), can be used as maintenance medications for Crohn disease, but are less effective than biologic therapies or combination therapies with biologics.23–26 The SONIC trial25 evaluated the comparative efficacy of thiopurine (n = 170), anti-TNF (n = 169), and combination therapy with an anti-TNF and a thiopurine (n = 169) in 508 patients with moderate to severe Crohn disease. Results showed 30% steroid-free remission at week 26 in those receiving thiopurine monotherapy, compared with 44% for those receiving anti-TNF monotherapy (P = .006) and 56% for those receiving combination anti-TNF and thiopurine therapy (P < .001).25 Furthermore, trials of thiopurines have suggested minimal benefit over placebo in early Crohn disease.27,28 A prospective blinded trial of 131 participants (68 randomized to receive azathioprine and 63 randomized to receive placebo) found no difference in steroid-free remission throughout the duration of the study (ie, 76 weeks; 44% vs 36%; P = .47).27 Infliximab, adalimumab, and certolizumab are medications in the anti–TNF-α class.29–32 Ustekinumab is an anti–IL-12/23 monoclonal therapy33 and vedolizumab is a gut-specific anti–integrin α4β7 monoclonal therapy.34 Selection of therapy is tailored to the individual based on treatment history, disease location, disease severity, and patient comorbidities.
As described above, combination therapy (anti-TNF and thiopurine) is an effective management strategy for moderate to severe Crohn disease.25 The SONIC trial showed superiority of combination therapy (infliximab and azathiprine) over anti-TNF and thiopurine monotherapy in moderate to severe Crohn disease. Specifically, those receiving combination therapy had higher rates of steroid-free remission at week 26 (56%) compared with those receiving infliximab monotherapy (44%; P = .02) or azathioprine monotherapy (30%; P < .001).25 Therefore, this combination is considered more efficacious than either monotherapy, and is considered first-line therapy in the context of severe disease. The authors also found significantly higher levels of antibodies to infliximab in the infliximab monotherapy group (14%) compared with the combination therapy group (1%).25 Therefore, thiopurines are thought to add efficacy and reduce the immunogenicity of biologic therapy.
Monitoring on Therapy
The management of Crohn disease requires close monitoring of inflammation and careful assessment and adjustment of medical therapy to achieve clinical and endoscopic remission.35 Particularly after a change in therapy, or a change in the dose or frequency of therapy, objective markers of inflammation should be checked to ensure remission. Such markers may include C-reactive protein, fecal calprotectin, cross-sectional imaging, or endoscopy, depending on the disease location and patient’s medical comorbidities. The importance of following objective measures of disease control was illustrated in the CALM study, which showed that among 244 patients with Crohn disease, the use of biomarkers, compared with clinical management alone, was associated with improved rates of mucosal healing at 48 weeks (46% vs 30%; risk difference, 16.1% [95% CI, 3.9%-28.3%]; P = .01).36 Control of inflammation is strongly associated with reduced complications of Crohn disease, including intestinal cancers, strictures, fistulas, and abscesses, along with fewer steroid courses, hospitalizations, and surgical procedures.36–38
Symptoms are a sensitive, but not specific, assessment of inflammation. Minimally symptomatic smoldering inflammation can lead to significant complications and surgery over time. Patients often adjust to, adapt their diets to, or come to accept chronic symptoms that are indicators of clinically important inflammation.
Disease Flares
When a patient has a flare of symptoms, it is important to assess for infection because Escherichia coli, Norovirus, and Clostridioides difficile are all common causes of changes in symptoms that resemble Crohn disease flares. If infection is not found, inflammation should be assessed and localized. Intestinal inflammation can be measured with biomarkers (C-reactive protein, fecal calprotectin), imaging (computed tomography or magnetic resonance enterography), and/or endoscopy with biopsy. Magnetic resonance enterography should be the imaging modality of choice for patients younger than 35 years to minimize cumulative radiation exposure early in life.39,40 In chronic, long-standing Crohn disease, symptoms may not represent inflammation, but may be caused by long-term bowel damage. Chronic inflammation, scarring, and surgical procedures can lead to dysmotility, bile acid diarrhea, small intestinal bacterial overgrowth, and visceral hypersensitivity, which cause symptoms despite minimal current inflammation. Therefore, clinicians should determine whether persistent symptoms are from disease activity or an alternative etiology prior to adjusting or changing therapy.
Mild to moderate flares can often be managed with the gut-selective steroid budesonide, which has fewer adverse effects than systemic corticosteroids. Repeated courses of steroids (>2 per year) suggests that adjustment of therapy or surgical resection is needed. Severe flares will often require intravenous steroids for hospitalized patients. After induction of remission, a rigorous assessment of the previous maintenance therapy and medication adherence should be performed. One response measure is to check a drug trough and antibiologic antibody level. Drug-level testing is available for many biologic therapies, including infliximab, adalimumab, certolizumab, vedolizumab, and ustekinumab, but optimal levels are still being determined for many of the newer biologic therapies. Patients who have developed high-titer antibiologic antibodies with undetectable drug levels (ie, immune-mediated drug failure) or therapeutic levels without antibiologic antibodies (ie, mechanistic failure) will need to change to a new maintenance therapy.35 At this time, there is insufficient evidence to recommend proactive monitoring of drug trough levels and antibodies.
The Importance of Continuing Maintenance Therapy
Many patients want to discontinue their therapy after achieving remission because of concern for adverse effects or desire to limit medication use. However, medication discontinuation increases the risk of relapse, need for surgery, and risk of other disease-related complications. In a meta-analysis of 3 studies that included 334 patients, thiopurine continuation was associated with a decreased risk of disease relapse (OR, 0.35 [95% CI, 0.21–0.6]) up to 18 months.41 Additionally, a meta-analysis of 23 studies showed that approximately 50% of patients in remission who discontinued anti-TNF therapy relapsed in the long term (>25 months).42 For these reasons, medication discontinuation is generally not advised. De-escalation of therapy can be considered in select situations, such as for patients receiving combination therapy (anti-TNF and thiopurine). In an open-label randomized clinical trial of 50 patients who achieved at least 6 months of clinical remission while receiving combination therapy, there was no significant difference in subsequent rates of clinical remission at 52 weeks between those who continued combination therapy (95% [95% CI, 76.5%-99.1%]) and those who continued anti-TNF therapy alone (93% [95% CI, 72%-98.9%]). Therefore, patients receiving combination therapy may be considered for thiopurine de-escalation. Ultimately, decisions about de-escalation are individualized and involve discussion between the patient and the gastroenterologist. Considerations such as age of the patient, risks of therapy, depth of remission, previous treatment course, and drug levels influence the decision-making process.
Many patients wish to experiment with complementary therapy (ie, acupuncture, meditation, dietary changes, herbal supplements), which should only be used as an adjunct to established, effective therapies, and can occasionally cause significant drug interactions. Adherence to effective therapies, especially biologic therapies, is essential because skipping doses of biologics greatly increases the rate of neutralizing antibody formation and loss of efficacy.43 Many patients may wish to consider stopping or delaying biologic therapy during a viral upper respiratory infection. For the vast majority of outpatient infections, biologic therapy for Crohn disease should be continued, and any discussion of stopping therapy or delaying doses should include the prescribing gastroenterologist.
Many patients want to stop therapy for Crohn disease management during pregnancy because of concern of adverse effects on the fetus. Large cohort studies have shown that maintaining control of inflammation during the pregnancy is the most important factor in having a healthy outcome.44 A Swedish cohort study that included 1220 patients with Crohn disease demonstrated an increased risk of adverse outcomes, such as preterm birth (adjusted OR, 2.66 [95% CI, 1.89–3.74]), low birth weight (OR, 3.30 [95% CI, 2.29–4.74]), and stillbirth (adjusted OR, 4.48 [95% CI, 1.67–11.9]), with active disease.45 Furthermore, a meta-analysis of 5 studies that included 1216 patients suggested no increased risk for abortions (OR, 1.53 [95% CI, 0.97–2.41]), preterm birth (OR, 1.00 [95% CI, 0.62–1.62]), low birth weight (OR, 1.05 [95% CI, 0.62–1.78]), or congenital malformations (OR, 1.10 [95% CI, 0.58–2.09]) with anti-TNF exposure, highlighting the safety of continued medical therapy.46 Therefore, it is recommended that individuals who are pregnant continue biologic therapy during pregnancy to reduce adverse outcomes associated with disease activity.47 In contrast, methotrexate is contraindicated in those attempting to become and those who are pregnant due to teratogenic effects.
Most patients with Crohn disease will require intestinal surgery at some point in their life. A timely operation is important to prevent disease-related complications (eg, intra-abdominal abscesses, fistulae) and reduce postoperative complications. In a meta-analysis involving 15 studies (N = 3807 patients), hypoalbuminemia (OR, 1.93 [95% CI, 1.36–2.75]) and preoperative steroid use (OR, 1.99 [95% CI, 1.54–2.57]) were associated with an increased risk of postoperative complications, such as anastomotic leak, intra-abdominal abscess, and enterocutaneous fistula.48 Therefore, prompt recognition of medically refractory disease is important to prevent prolonged steroid exposure and worsening nutritional status, which negatively effect postoperative surgical course. Although timely surgery is important, repeated surgical procedures can result in short bowel syndrome and dependence on total parenteral nutrition, emphasizing the important of postoperative disease control.49 Previous surgical procedures, penetrating disease (eg, intra-abdominal abscesses, fistulae), and smoking are risk factors for postoperative recurrence and should guide postoperative medication planning.50 In a randomized clinical trial of 297 patients with Crohn disease who had undergone ileocolonic resection in the past 45 days, postoperative treatment with infliximab was associated with reduced endoscopic recurrence (30.6 vs 60%; absolute risk reduction, 29.4% [95% CI, 18.6%-40.2%]).51 Therefore, anti-TNF therapy is an effective option for prevention of postoperative disease recurrence. Patients who have active inflammation despite multiple therapies or who require repeated surgical procedures are well served by specialty referral for consideration of novel medications in clinical trials.
Adverse Effects of Therapy
Patients with Crohn disease have an increased risk of intestinal cancer, which is attributed to disease-related inflammation.52,53 The relative risk of small bowel adenocarcinoma in Crohn disease is 31.2 (95% CI, 15.9–60.9) and of colon cancer in Crohn disease is 2.5 (95% CI, 1.3–4.7), with the risk of colon cancer related to colonic Crohn disease.54 Therefore, colorectal cancer surveillance is an important part of health maintenance in patients with colonic Crohn disease (please refer to the Health Maintenance section below for additional details). In addition, an independent association between IBD and melanoma has also been demonstrated, and a meta-analysis of 12 studies that included 179 individuals with melanoma showed that the risk of melanoma was increased by almost 2-fold (relative risk, 1.80 [95% CI, 1.17–2.75]) in patients with Crohn disease.55
Patients with Crohn disease are also at risk for many medication-related cancers, such as skin cancer and lymphoma. The risk of nonmelanoma skin cancers (by thiopurines) and melanoma (by biologic therapies) are increased almost 2-fold (nonmelanoma skin cancer: OR, 1.85 [95% CI, 1.66–2.05]; melanoma: OR, 1.88 [95% CI, 1.08–3.29]).56 Patients with IBD also have an increased risk of lymphoma, attributable to thiopurines and anti-TNF medications. Data from a national French claims registry showed that thiopurines (adjusted hazard ratio [HR], 2.60 [95% CI, 1.96–3.44]) and anti-TNF medications (adjusted HR, 2.41 [95% CI, 1.60–3.64]) were associated with a 2-fold to 3-fold increase in risk of lymphoma when used alone and more than a 6-fold increase when used in combination.57 The risk of lymphoma increases with age and, in the French claims registry study, the highest risk was observed among those aged at least 65 years.57 Hepatosplenic T cell lymphoma is a rare, but aggressive, type of lymphoma, which is more common in young men, and the incidence is increased by the use of thiopurines.58 For these reasons, thiopurine as a maintenance medication is used less often in young patients and older patients.
Treatment with immunosuppressive therapy after a cancer diagnosis can generate anxiety in both patients and physicians. When possible, gut-selective therapy, such as vedolizumab, may be preferable. Potent chemotherapy often suppresses the immune system and the activity of IBD for an extended period. However, intestinal inflammation often returns. In a multicenter cohort study, Axelrad and colleagues59 demonstrated that 12 of 69 patients (17.4%) who were in remission at the time of cancer diagnosis eventually had a Crohn disease flare, and the median time to flare was 37 months. Increasing evidence suggests that immunosuppressive treatment, even with biologics, in patients with significant inflammation 3 or more years after receiving a cancer diagnosis is associated with low rates of new or recurrent cancer (ranging from 20.3 cases per 1000 person-years [95% CI, 15.2–26.7] to 66.0 cases per 1000 person-years [95% CI, 8.0–238.4]) and overall rates of cancer that are not increased compared with patients with IBD without cancer.60–62
Another adverse effect of therapy is an increased risk of serious infection. Therefore, it is recommended that patients with IBD undergo routine health maintenance and vaccination. Patients with Crohn disease have an increased risk of pneumonia compared with patients without IBD (151 per 10 000 person-years vs 74 per 10 000 person-years; adjusted HR, 1.71 [95% CI, 1.62–1.80]), which leads to the recommendation for pneumococcal vaccination. The risk of pneumonia is increased with immunosuppressive therapy, including corticosteroids (adjusted OR, 3.52 [95% CI, 3.10–4.01]) and biologic therapy (adjusted OR, 1.28 [95% CI, 1.05–1.56])63,64 Patients with IBD are also at increased risk of shingles compared with those without IBD (7.55 per 1000 person-years vs 3.22 per 1000 person-years; adjusted HR, 1.72 [95% CI, 1.51–1.96]), and this risk is significantly increased by thiopurines (11.73 per 1000 person-years; adjusted HR, 1.47 [95% CI, 1.31–1.65]).65 For this reason, inactivated zoster vaccine is recommended for patients with IBD starting this category of therapy. Furthermore, patients with IBD often receive antibiotics, which puts them at increased risk of C difficile colitis. This should be suspected in individuals with watery diarrhea with diffuse abdominal pain and minimal bleeding, especially if the patient reports that the symptoms feel different than their typical IBD flare. Oral vancomycin is preferred as first-line therapy for C difficile infection in patients with IBD.66
Patients with IBD are at risk of osteopenia and osteoporosis, particularly if they have long-term active inflammation, and this risk is increased by exposure to corticosteroids. Patients with more than 3 months of lifetime exposure to corticosteroids are recommended to have a dual-energy x-ray absorptiometry scan to assess their bone density.67
Health Maintenance
Patients with Crohn disease with involvement of more than one-third of the colon have an increased risk of colon cancer after 8 years of disease, and regular colonoscopic surveillance with biopsies is recommended every 1 to 3 years beginning at year 8, with adjustments based on family history, prior dysplasia, disease extent, disease duration, and primary sclerosing cholangitis.68 Patients with a concurrent diagnosis of primary sclerosing cholangitis are at higher risk for colorectal neoplasia and should undergo annual colorectal cancer surveillance.
In addition to cardiovascular, pulmonary, and cancer risks, smoking is associated with increased incidence of Crohn disease, more rapid recurrence of Crohn disease after surgery, increased risk of complications of Crohn disease, and reduced response to anti-TNF therapy.69 Cessation of smoking in individuals with Crohn disease is strongly recommended.70
The American Gastroenterological Association and the Crohn’s and Colitis Foundation have published lists of IBD quality measures (Box 4). Many of these quality measures are for outpatients and can be comanaged by gastroenterology and primary care, such as screening for tobacco abuse and counseling patients to quit if they are smoking, yearly inactivated influenza vaccination, pneumococcal vaccination, and assessment for bone loss with a bone density scan in patients exposed to systemic steroids for 3 months or more of the equivalent of 7.5 mg per day of prednisone. Up-to-date vaccination status for tetanus, diphtheria, acellular pertussis, human papilloma virus, hepatitis A and B, varicella, and zoster vaccination after 50 years of age should be confirmed. Live vaccinations should not be given to patients currently receiving immunosuppression therapy. Killed or recombinant vaccination alternatives are available for influenza, zoster, polio, and typhoid (Table).67,71,72 Patients receiving thiopurines, methotrexate, or anti-TNF medications should have regular skin checks for skin cancer and, in women receiving thiopurines, a yearly pap smear is recommended given the increased risk of cervical dysplasia.
Box 4. Inflammatory Bowel Disease (IBD) Quality Measuresa.
American Gastroenterological Association
Type, anatomic location, and activity of IBD all assessed annually
Corticosteroid-sparing maintenance therapy is used
Assessment for bone loss in patients receiving corticosteroids
Annual influenza vaccination
Vaccination for pneumococcus
Testing for latent tuberculosis before initiating anti–tumor necrosis factor therapy
Assessment of hepatitis B virus before initiating anti–tumor necrosis factor therapy
Testing for Clostridioides difficile in inpatients with diarrhea
Prophylaxis for venous thromboembolism in inpatients
Screening for and cessation of tobacco use
Crohn’s and Colitis Foundation
If a patient with IBD is initiating 6-mercaptopurine and azathioprine, thiopurine methyltransferase testing should be performed before starting therapy
If a patient with extensive ulcerative colitis or Crohn disease involving the colon has had the disease for 8 to 10 years, surveillance colonoscopy should be performed every 1 to 3 years
If a patient with IBD is receiving immunosuppressive therapy, patients should be educated about appropriate vaccinations, including annual inactivated influenza, pneumococcal vaccination with a 5-year booster, and general avoidance of live virus vaccines
If a patient with Crohn disease is an active tobacco smoker, smoking cessation should be recommended and treatment should be offered or suitable referral should be provided at least annually
Adapted from Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9(5):286–292.
Table.
Vaccinations in Patients With Inflammatory Bowel Disease (IBD)a
| Vaccination | Live or inactivated | Patient population | Comment(s) |
|---|---|---|---|
| HepatitisA | Inactivated | All patients with IBD | |
| Hepatitis B | Inactivated | All patients with IBD | |
| Influenza | Inactivated | All patients with IBD | An exception is the live attenuated intranasal vaccine, which is contraindicated in immunocompromised patients |
| Pneumonia | Inactivated | All patients with IBD | Vaccination includes pneumococcal conjugate vaccine and pneumococcal polysaccharide vaccine; timing of administration per national guidelines |
| Tetanus, diphtheria, pertussis | Inactivated | All patients with IBD | |
| Special considerations | |||
| Meningitis | Inactivated | Adolescents (per national guidelines) | |
| Human papillomavirus | Inactivated | Adolescents and young adults (per national guidelines) | |
| Herpes zoster | Inactivated | Adults older than 50 y (per national guidelines) | An exception is the live vaccine, which is contraindicated in immunocompromised patients |
| Live vaccines (contraindicated in immunocompromised patients) | |||
| Influenza (intranasal) | Live | Use injectable inactivated influenza vaccine | |
| Shingles | Live | Use the recombinant inactivated vaccine | |
| Measles, mumps, rubella | Live | ||
| Rotavirus | Live | Delay until 6 mo of age if mother is receiving biologic therapy for Crohn disease | |
| Varicella | Live | ||
| Yellow fever | Live | ||
| Smallpox | Live | ||
| Oral typhoid | Live | Use injectable inactivated typhoid vaccine | |
| Bacille Calmette-Guérin | Live | ||
| Adenovirus | Live | ||
| Oral cholera | Live | ||
Adapted from Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112(2):241–258. doi:10.1038/ajg.2016.537.
Nutrition and Diet
Many patients have questions about nutrition and concerns about nutritional deficiencies. Nutritional deficiencies are uncommon in patients with mild disease. The most common deficiencies are iron, vitamin D, and B12 (B12 particularly in patients with extensive ileal disease). It is recommended that testing for levels should be done before supplementation with goal-directed therapy. Many patients with moderate ileal disease have adequate absorption of oral B12. Patients who have had severe flares or have undergone surgical procedures should ensure adequate protein intake to support healing of the intestine and surgical wounds.
Avoidant/restrictive food intake disorder is an increasingly recognized gastroenterological condition in which patients selectively avoid certain categories of food, usually occurring in response to food-induced symptoms.73,74 For most patients with Crohn disease, a diverse diet is to be encouraged and food avoidance should be limited. An exception is for patients with intestinal strictures for whom a low residue diet is encouraged to limit obstructive symptoms. For patients with significant bloating, abdominal distention, and abdominal pain without significant detectable inflammation, a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet can prove benefit in reducing these symptoms.75,76
Emotional Care
The emotional aspects of Crohn disease are often underrecognized and undertreated. Anxiety (18%-35%) and depression (20%-34%) are quite common77–79 in patients with Crohn disease as patients adjust to lost opportunities and restrict their life opportunities because of concerns about future disease flares. The prevalence of these disorders is much higher during active disease. Many patients choose to be childless due to medication-related concerns or misinformation, including the perception that there is a high risk of genetic transmission.80–82 In a study that included 89 patients with Crohn disease, posttraumatic stress disorder occurred in 34 patients (38.2%), most frequently in those with difficult hospitalizations, severe symptoms, and ileostomy surgery for Crohn disease.83 Increasing evidence suggests that cognitive behavioral therapy and antidepressant therapy can be beneficial for symptomatic patients with Crohn disease.84,85
Limitations
The scope of this review was intended to provide a broad overview of the management of Crohn disease for an internal medicine audience. Therefore, several concepts were not reviewed in great depth, including the nuances of therapeutic drug monitoring, advanced endoscopic approaches in Crohn disease (eg, chromoendoscopy, balloon dilation), and postoperative management of Crohn disease. However, this article provides a breadth of information pertinent for internists who are treating patients with Crohn disease in collaboration with gastroenterology.
Conclusions
Management of Crohn disease includes treatment with monoclonal antibody therapies, immunomodulators, and surgery. The optimal approach depends on patient risk stratification; patient preference; and clinical factors, including age of onset, penetrating complications, smoking, and severity of ulceration. Physicians should be familiar with the advantages and disadvantages of each therapy to best counsel their patients.
Footnotes
Conflict of Interest Disclosures: Dr Higgins reported receiving grant funding from the National Institutes of Health, the Crohn’s and Colitis Foundation, Pfizer, AbbVie, Eli Lilly, Twine Clinical Consulting, Target PharmaSolutions, Shire, Seres, Genentech, Janssen, Takeda, and UCB and consulting for Pfizer, Eli Lilly, Takeda, and Arena Pharmaceuticals. No other disclosures were reported.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Administrative, technical, or material support: Higgins.
Supervision: Higgins.
Other - Literature review: Cushing.
Submissions: We encourage authors to submit papers for consideration as a Review. Please contact Edward Livingston, MD, at Edward. livingston@jamanetwork.org or Mary McGrae McDermott, MD, at mdm608@northwestern.edu.
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390 (10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 2.McGovern DP, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology. 2015;149(5):1163–1176.e2. doi: 10.1053/j.gastro.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–986. doi: 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna S, Raffals LE. The microbiome in Crohn’s disease: role in pathogenesis and role of microbiome replacement therapies. Gastroenterol Clin North Am. 2017;46(3):481–492. doi: 10.1016/j.gtc.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81 (11):1462–1471. doi: 10.4065/81.11.1462 [DOI] [PubMed] [Google Scholar]
- 7.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther. 2016;43(5):549–561. doi: 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 8.Nunes T, Etchevers MJ, Domènech E, et al. ; Tobacco-Eneida Study Group of GETECCU. Smoking does influence disease behaviour and impacts the need for therapy in Crohn’s disease in the biologic era. Aliment Pharmacol Ther. 2013;38(7):752–760. doi: 10.1111/apt.12440 [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R, Pal P, Girish BG, Reddy DN. Risk factors for diagnostic delay in Crohn’s disease and their impact on long-term complications: how do they differ in a tuberculosis endemic region? Aliment Pharmacol Ther. 2018;47(10):1367–1374. doi: 10.1111/apt.14617 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Ren J, Wang G, et al. Diagnostic delay in Crohn’s disease is associated with increased rate of abdominal surgery: a retrospective study in Chinese patients. Dig Liver Dis. 2015;47(7):544–548. doi: 10.1016/j.dld.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen VQ, Jiang D, Hoffman SN, et al. Impact of diagnostic delay and associated factors on clinical outcomes in a U.S. inflammatory bowel disease cohort. Inflamm Bowel Dis. 2017;23(10):1825–1831. doi: 10.1097/MIB.0000000000001257 [DOI] [PubMed] [Google Scholar]
- 12.Lo B, Vester-Andersen MK, Vind I, et al. Changes in disease behaviour and location in patients with Crohn’s disease after seven years of follow-up: a Danish population-based inception cohort. J Crohns Colitis. 2018;12(3):265–272. doi: 10.1093/ecco-jcc/jjx138 [DOI] [PubMed] [Google Scholar]
- 13.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–819. doi: 10.1038/ajg.2015.120 [DOI] [PubMed] [Google Scholar]
- 14.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139(4):1147–1155. doi: 10.1053/j.gastro.2010.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zallot C, Peyrin-Biroulet L. Clinical risk factors for complicated disease: how reliable are they? Dig Dis 2012;30(suppl 3):67–72. doi: 10.1159/000342608 [DOI] [PubMed] [Google Scholar]
- 16.Karreman MC, Luime JJ, Hazes JMW, Weel AEAM. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11(5):631–642. [DOI] [PubMed] [Google Scholar]
- 17.Harbord M, Annese V, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239–254. doi: 10.1093/ecco-jcc/jjv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42(2):97–114. doi: 10.3109/07853890903559724 [DOI] [PubMed] [Google Scholar]
- 19.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106(1):110–119. doi: 10.1038/ajg.2010.343 [DOI] [PubMed] [Google Scholar]
- 20.Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256 (2):387–396. doi: 10.1148/radiol.10091953 [DOI] [PubMed] [Google Scholar]
- 21.Feld LD, Rubin DT, Feld AD. Legal risks and considerations associated with inflammatory bowel disease: a primer. Am J Gastroenterol. 2018;113(11): 1577–1579. doi: 10.1038/s41395-018-0204-7 [DOI] [PubMed] [Google Scholar]
- 22.Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2016;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feagan BG, Rochon J, Fedorak RN, et al. ; The North American Crohn’s Study Group Investigators. Methotrexate for the treatment of Crohn’s disease. N Engl J Med. 1995;332(5):292–297. doi: 10.1056/NEJM199502023320503 [DOI] [PubMed] [Google Scholar]
- 24.Feagan BG, Fedorak RN, Irvine EJ, et al. ; North American Crohn’s Study Group Investigators. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. N Engl J Med. 2000;342(22):1627–1632. doi: 10.1056/NEJM200006013422202 [DOI] [PubMed] [Google Scholar]
- 25.Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492 [DOI] [PubMed] [Google Scholar]
- 26.Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database of Syst Rev 2015(10):CD000067. doi: 10.1002/14651858.CD000067.pub3u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panés J, López-Sanromán A, Bermejo F, et al. ; AZTEC Study Group. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology. 2013; 145(4):766–74.e1. doi: 10.1053/j.gastro.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 28.Cosnes J, Bourrier A, Laharie D, et al. ; Groupe d’Etude Thérapeutique des Affections Inflammatoires du Tube Digestif (GETAID). Early administration of azathioprine vs conventional management of Crohn’s Disease: a randomized controlled trial. Gastroenterology. 2013;145(4):758–65.e2. doi: 10.1053/j.gastro.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 29.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–333. doi: 10.1053/j.gastro.2005.11.030 [DOI] [PubMed] [Google Scholar]
- 30.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 31.Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–238. doi: 10.1056/NEJMoa067594 [DOI] [PubMed] [Google Scholar]
- 32.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–250. doi: 10.1056/NEJMoa062897 [DOI] [PubMed] [Google Scholar]
- 33.Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s Disease. N Engl J Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 34.Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi: 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 35.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27 [DOI] [PubMed] [Google Scholar]
- 36.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390(10114): 2779–2789. doi: 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 37.Khanna R, Bressler B, Levesque BG, et al. ; REACT Study Investigators. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386(10006):1825–1834. doi: 10.1016/S0140-6736(15)00068-9 [DOI] [PubMed] [Google Scholar]
- 38.Colombel J-F, D’haens G, Lee W-J, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020; 14(2):254–266. doi: 10.1093/ecco-jcc/jjz131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(5):529–539. doi: 10.1111/j.1365-2036.2011.04975.x [DOI] [PubMed] [Google Scholar]
- 40.Council NR. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. National Academies Press; 2006. [PubMed] [Google Scholar]
- 41.French H, Mark Dalzell A, Srinivasan R, El-Matary W. Relapse rate following azathioprine withdrawal in maintaining remission for Crohn’s disease: a meta-analysis. Dig Dis Sci. 2011;56(7): 1929–1936. doi: 10.1007/s10620-011-1671-5 [DOI] [PubMed] [Google Scholar]
- 42.Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2016;111(5):632–647. doi: 10.1038/ajg.2016.54 [DOI] [PubMed] [Google Scholar]
- 43.Govani SM, Noureldin M, Higgins PDR, et al. Defining an optimal adherence threshold for patients taking subcutaneous anti-TNFs for inflammatory bowel diseases. Am J Gastroenterol. 2018;113(2):276–282. doi: 10.1038/ajg.2017.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahadevan U, McConnell RA, Chambers CD. Drug safety and risk of adverse outcomes for pregnant patients with inflammatory bowel disease. Gastroenterology. 2017;152(2):451–462.e2. doi: 10.1053/j.gastro.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 45.Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014;20(6):1091–1098. doi: 10.1097/MIB.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 46.Narula N, Al-Dabbagh R, Dhillon A, Sands BE, Marshall JK. Anti-TNFα therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20(10):1862–1869. doi: 10.1097/MIB.0000000000000092 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150(3):734–757.e1. doi: 10.1053/j.gastro.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 48.Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: a meta-analysis of observational studies. J Crohns Colitis. 2015;9(3):293–301. doi: 10.1093/ecco-jcc/jju028 [DOI] [PubMed] [Google Scholar]
- 49.Limketkai BN, Parian AM, Shah ND, Colombel JF. Short bowel syndrome and intestinal failure in Crohn’s disease. Inflamm Bowel Dis. 2016;22(5): 1209–1218. doi: 10.1097/MIB.0000000000000698 [DOI] [PubMed] [Google Scholar]
- 50.Barnes EL, Lightner AL, Regueiro M. Perioperative and postoperative management of patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(6):1356–1366. doi: 10.1016/j.cgh.2019.09.040 [DOI] [PubMed] [Google Scholar]
- 51.Regueiro M, Feagan BG, Zou B, et al. ; PREVENT Study Group. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150 (7):1568–1578. doi: 10.1053/j.gastro.2016.02.072 [DOI] [PubMed] [Google Scholar]
- 52.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–459. doi: 10.1053/j.gastro.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 53.Bojesen RD, Riis LB, Hogdall E, Nielsen OH, Jess T. Inflammatory bowel disease and small bowel cancer risk, clinical characteristics, and histopathology: a population-based study. Clin Gastroenterol Hepatol. 2017;15(12):1900–1907. doi: 10.1016/j.cgh.2017.06.051 [DOI] [PubMed] [Google Scholar]
- 54.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x [DOI] [PubMed] [Google Scholar]
- 55.Singh S, Nagpal SJ, Murad MH, et al. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12(2):210–218. doi: 10.1016/j.cgh.2013.04.033 [DOI] [PubMed] [Google Scholar]
- 56.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390–399. doi: 10.1053/j.gastro.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679–1686. doi: 10.1001/jama.2017.16071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9(1):36–41. doi: 10.1016/j.cgh.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 59.Axelrad JE, Fowler SA, Friedman S, Ananthakrishnan AN, Yajnik V. Effects of cancer treatment on inflammatory bowel disease remission and reactivation. Clin Gastroenterol Hepatol. 2012;10(9):1021–1027. doi: 10.1016/j.cgh.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 60.Shelton E, Laharie D, Scott FI, et al. Cancer recurrence following immune-suppressive therapies in patients with immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2016;151(1):97–109.e4. doi: 10.1053/j.gastro.2016.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mamtani R, Clark AS, Scott FI, et al. Association between breast cancer recurrence and immunosuppression in rheumatoid arthritis and inflammatory bowel disease: a cohort study. Arthritis Rheumatol. 2016;68(10):2403–2411. doi: 10.1002/art.39738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waljee AK, Higgins PDR, Jensen CB, et al. Anti-tumour necrosis factor-α therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: a nationwide, population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5(3):276–284. doi: 10.1016/S2468-1253(19)30362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(2):240–248. doi: 10.1038/ajg.2012.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long MD, Farraye FA, Okafor PN, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19(5):1018–1024. doi: 10.1097/MIB.0b013e3182802a9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan N, Patel D, Trivedi C, et al. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol. 2018;16(12):1919–1927. doi: 10.1016/j.cgh.2017.12.052 [DOI] [PubMed] [Google Scholar]
- 66.Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15(2):166–174. doi: 10.1016/j.cgh.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 67.Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112(2):241–258. doi: 10.1038/ajg.2016.537 [DOI] [PubMed] [Google Scholar]
- 68.Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81(5):1101–1121. doi: 10.1016/j.gie.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 69.Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8(8):717–725. doi: 10.1016/j.crohns.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 70.Nos P, Domènech E. Management of Crohn’s disease in smokers: is an alternative approach necessary? World J Gastroenterol. 2011;17(31):3567–3574. doi: 10.3748/wjg.v17.i31.3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tkacz J, Brady BL, Meyer R, Lofland JH, Ruetsch C, Coelho-Prabhu N. An assessment of the AGA and CCFA quality indicators in a sample of patients diagnosed with inflammatory bowel disease. J Manag Care Spec Pharm. 2015;21(11):1064–1076. doi: 10.18553/jmcp.2015.21.11.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9(5):286–292. [PMC free article] [PubMed] [Google Scholar]
- 73.Harer KN. Irritable bowel syndrome, disordered eating, and eating disorders. Gastroenterol Hepatol (N Y) 2019;15(5):280–282. [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmerman J, Fisher M. Avoidant/restrictive food intake disorder (ARFID). Curr Probl Pediatr Adolesc Health Care. 2017;47(4):95–103. doi: 10.1016/j.cppeds.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 75.Pedersen N, Ankersen DV, Felding M, et al. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23(18):3356–3366. doi: 10.3748/wjg.v23.i18.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cox SR, Lindsay JO, Fromentin S, et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158(1):176–188.e7. doi: 10.1053/j.gastro.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 77.Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. 2016;87:70–80. doi: 10.1016/j.jpsychores.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 78.Bhamre R, Sawrav S, Adarkar S, Sakaria R, J Bhatia S. Psychiatric comorbidities in patients with inflammatory bowel disease. Indian J Gastroenterol. 2018;37(4):307–312. doi: 10.1007/s12664-018-0870-9 [DOI] [PubMed] [Google Scholar]
- 79.Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22(3):752–762. doi: 10.1097/MIB.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 80.Selinger CP, Eaden J, Selby W, et al. Inflammatory bowel disease and pregnancy: lack of knowledge is associated with negative views. J Crohns Colitis. 2013;7(6):e206–e213. doi: 10.1016/j.crohns.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 81.Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(5):591–599. doi: 10.1002/ibd.20082 [DOI] [PubMed] [Google Scholar]
- 82.Mountifield R, Bampton P, Prosser R, Muller K, Andrews JM. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm Bowel Dis. 2009;15(5):720–725. doi: 10.1002/ibd.20839 [DOI] [PubMed] [Google Scholar]
- 83.Taft TH, Bedell A, Craven MR, Guadagnoli L, Quinton S, Hanauer SB. Initial assessment of post-traumatic stress in a US cohort of inflammatory bowel disease patients. Inflamm Bowel Dis. 2019;25(9):1577–1585. doi: 10.1093/ibd/izz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bennebroek Evertsz’ F, Sprangers MAG, Sitnikova K, et al. Effectiveness of cognitive-behavioral therapy on quality of life, anxiety, and depressive symptoms among patients with inflammatory bowel disease: a multicenter randomized controlled trial. J Consult Clin Psychol. 2017;85(9):918–925. doi: 10.1037/ccp0000227 [DOI] [PubMed] [Google Scholar]
- 85.Kristensen MS, Kjærulff TM, Ersbøll AK, Green A, Hallas J, Thygesen LC. The influence of antidepressants on the disease course among patients with Crohn’s disease and ulcerative colitis-a danish nationwide register-based cohort study. Inflamm Bowel Dis. 2019;25(5):886–893. doi: 10.1093/ibd/izy367 [DOI] [PMC free article] [PubMed] [Google Scholar]


