The SARS-CoV-2 omicron variant (B.1.1.529) is less sensitive to neutralising antibody responses induced by vaccination and prior infection than previous variants.1, 2 Less is known regarding omicron-induced serological and T-cell responses after breakthrough infection of vaccinated individuals with and without prior infection.
In this prospective cohort study, we analysed serological and T-cell responses following omicron infection in 56 triple-vaccinated health-care workers in Sweden with and without prior SARS-CoV-2 infection. A surrogate virus neutralisation test (sVNT) was used to assess neutralisation of SARS-CoV-2 variants. Immune responses of all participants had been regularly assessed since April, 2020, in the ongoing Swedish COMMUNITY study.3, 4 For this sub-study, participants were screened with qPCR twice a week for 4 weeks,5 with additional qPCR tests every other day for 14 days if positive. Blood samples were collected 1 week, 2 weeks, 3 weeks, 5 weeks, and 7 weeks after the first positive qPCR sample. For information on study design, demographic characteristics of the study population, and vaccination histories see appendix pp 4–5.
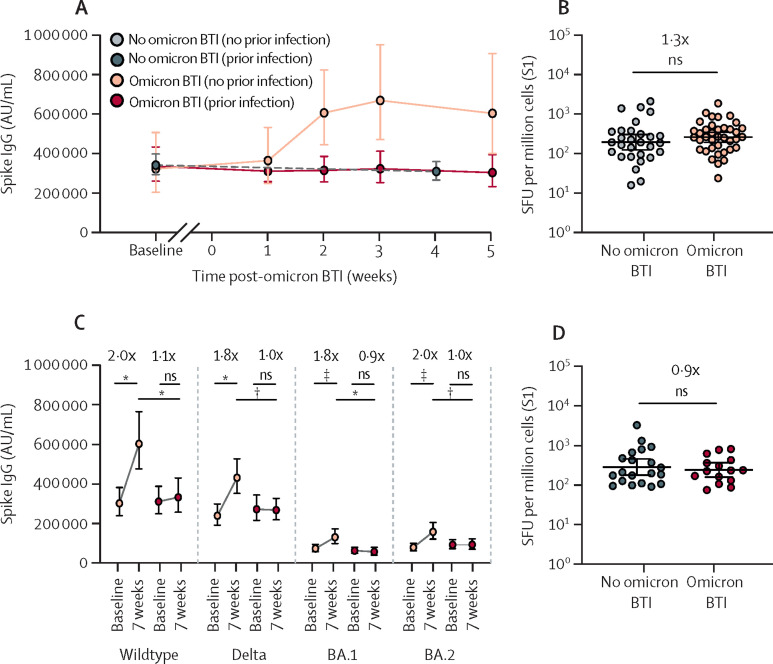
Overall, we observed a two-fold increase in anti-spike IgG and sVNT titres against wildtype, delta (B.1.617.2), BA.1, and BA.2 variants 2–5 weeks after omicron breakthrough infection (appendix pp 6–7). Strikingly, however, post-omicron serological responses were significantly higher in previously non-infected (triple-vaccinated with no history of SARS-CoV-2 infection; n=40) than in previously SARS-CoV-2-infected (triple-vaccinated with a confirmed SARS-CoV-2 wildtype infection before primary vaccination; n=16) participants (figure A,C ; appendix pp 8–9). The magnitude of serological responses correlated with nadir cycle threshold (Ct) values (appendix pp 8–9). Notably, nadir Ct value and symptomatology5 were similar in participants with and without previous SARS-CoV-2 infection (appendix pp 8–9). The magnitude of serological responses correlated inversely with pre-infection titres in both previously non-infected and previously infected participants (appendix pp 10–11).
Figure.
Immune responses following omicron BTI in triple-vaccinated health-care workers with and without prior SARS-CoV-2 infection
(A) GMTs (with 95% CIs) of anti-wildtype spike IgG at baseline and up to 5 weeks post-omicron BTI in participants without (n=20) and with (n=10) previous SARS-CoV-2 infection. The grey dots and dashed line represent participants who remained qPCR negative throughout the study period (n=69). (B) T-cell responses against SARS-CoV-2 S1 protein in participants without omicron BTI and 7 weeks post-infection in participants with omicron BTI; participants had no history of SARS-CoV-2 infection. Individual-participant data (dots) and GMTs (with 95% CIs; lines) are shown. (C) GMTs (with 95% CIs) of anti-spike IgG against wildtype, delta, and omicron BA.1 and BA.2 variants at baseline and 7 weeks after omicron BTI in participants without (n=40) and with (n=16) previous SARS-CoV-2 infection. (D) T-cell responses against SARS-CoV-2 S1 protein in participants without omicron BTI and 7 weeks post-infection in participants with omicron BTI; participants had a history of SARS-CoV-2 infection. Individual-participant data (dots) and GMTs (with 95% CIs; lines) are shown. BTI=breakthrough infection. GMT=geometric mean titre. ns=not significant. SFU=spot-forming units. *p<0·001. †p<0·01. ‡p<0·0001.
There were no differences in spike-specific T-cell responses between participants 7 weeks after omicron breakthrough infection and participants without omicron infection, regardless of previous SARS-CoV-2 infection status (figure B,D). A significant increase in specific T-cells against nucleocapsid and membrane proteins was observed in omicron-infected individuals without past SARS-CoV-2 infection, showing that omicron breakthrough infection can prime specific T-cells (appendix p 11). Higher serological responses against both BA.1 and BA.2, but similar T-cell responses, were observed in BA.1-infected compared with BA.2-infected individuals (appendix p 12).
This study is limited by the use of sVNT, which is based on the capacity of antibodies to block binding of variant-specific spike protein to ACE2. It is possible that other factors are also involved in neutralisation,6 which might be better reflected in live microneutralisation assays. However, when analysing a subset of samples we observed a strong correlation between live microneutralising titres and sVNT titres for both wildtype and BA.1 (appendix p 13), mirroring other reports4, 7 suggesting that sVNT can be used as a surrogate method for live virus neutralisation.
These findings suggest that previous SARS-CoV-2 infection, as well as high pre-infection antibody titres, might impact omicron-induced spike-specific serological responses in triple-vaccinated individuals. Close monitoring of immune responses following repeated antigenic exposures through infection or booster doses is needed.
We declare no competing interests. KB, UM, SHa, JK, and CT contributed equally. This research was funded by grants from the Knut and Alice Wallenberg Foundation (to CT and JK), the Jonas and Kristina af Jochnick Foundation (to CT), the Leif Lundblad Family Foundation (to CT), Region Stockholm (to CT), and Center for Innovative Medicine (to KB and JK).
Supplementary Material
References
- 1.Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399:234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudberg A-S, Havervall S, Månberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havervall S, Marking U, Greilert-Norin N, et al. Impact of SARS-CoV-2 infection on vaccine-induced immune responses over time. Clin Transl Immunology. 2022;11 doi: 10.1002/cti2.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marking U, Havervall S, Norin NG, et al. High rate of BA.1, BA.1.1 and BA.2 infection in triple vaccinated. medRxiv. 2022 doi: 10.1101/2022.04.02.22273333. published online April 3. [DOI] [Google Scholar]
- 6.Lustig Y, Gonen T, Meltzer L, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol. 2022 doi: 10.1038/S41590-022-01212-3. published online May 9. [DOI] [PubMed] [Google Scholar]
- 7.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.