Abstract
Background
Several passive surveillance systems reported increased risks of myocarditis or pericarditis, or both, after COVID-19 mRNA vaccination, especially in young men. We used active surveillance from large health-care databases to quantify and enable the direct comparison of the risk of myocarditis or pericarditis, or both, after mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) vaccinations.
Methods
We conducted a retrospective cohort study, examining the primary outcome of myocarditis or pericarditis, or both, identified using the International Classification of Diseases diagnosis codes, occurring 1–7 days post-vaccination, evaluated in COVID-19 mRNA vaccinees aged 18–64 years using health plan claims databases in the USA. Observed (O) incidence rates were compared with expected (E) incidence rates estimated from historical cohorts by each database. We used multivariate Poisson regression to estimate the adjusted incidence rates, specific to each brand of vaccine, and incidence rate ratios (IRRs) comparing mRNA-1273 and BNT162b2. We used meta-analyses to pool the adjusted incidence rates and IRRs across databases.
Findings
A total of 411 myocarditis or pericarditis, or both, events were observed among 15 148 369 people aged 18–64 years who received 16 912 716 doses of BNT162b2 and 10 631 554 doses of mRNA-1273. Among men aged 18–25 years, the pooled incidence rate was highest after the second dose, at 1·71 (95% CI 1·31 to 2·23) per 100 000 person-days for BNT162b2 and 2·17 (1·55 to 3·04) per 100 000 person-days for mRNA-1273. The pooled IRR in the head-to-head comparison of the two mRNA vaccines was 1·43 (95% CI 0·88 to 2·34), with an excess risk of 27·80 per million doses (–21·88 to 77·48) in mRNA-1273 recipients compared with BNT162b2.
Interpretation
An increased risk of myocarditis or pericarditis was observed after COVID-19 mRNA vaccination and was highest in men aged 18–25 years after a second dose of the vaccine. However, the incidence was rare. These results do not indicate a statistically significant risk difference between mRNA-1273 and BNT162b2, but it should not be ruled out that a difference might exist. Our study results, along with the benefit–risk profile, continue to support vaccination using either of the two mRNA vaccines.
Funding
US Food and Drug Administration.
Introduction
In the USA, the BNT162b2 (Pfizer–BioNTech) COVID-19 vaccine is available for individuals aged 5 years and older and the mRNA-1273 (Moderna) COVID-19 mRNA vaccine is available for people aged 18 years and older. In the spring and summer of 2021, a potential association of myocarditis or pericarditis, or both, with COVID-19 mRNA vaccines was reported in passive surveillance systems in Europe,1 Israel,2 Canada,3 and the USA,4 and in case series5, 6, 7, 8, 9 and a few observational studies (some published10, 11, 12 and some unpublished13, 14). The US Food and Drug Administration (FDA) updated the Emergency Use Authorization Fact Sheets for BNT162b215 and mRNA-127316 in June, 2021, on the basis of an ongoing evaluation of US Vaccine Adverse Event Reporting System reports suggesting an increased risk of myocarditis and pericarditis. The updates indicated that the observed risk was higher among men younger than 40 years for BNT162b2 and in men aged 18–24 years for mRNA-1273.
As part of the effort to monitor COVID-19 vaccine safety in the USA, this study evaluates the safety signals for myocarditis or pericarditis, or both, using four large US health plan claims databases that cover more than 100 million people. The magnitude of the increased risk of myocarditis or pericarditis after COVID-19 mRNA vaccination and the difference in risk between the two vaccine brands, if any, are still not well understood. Peer-reviewed estimates of myocarditis and pericarditis incidence rates and incidence rate ratios (IRRs) after receiving a COVID-19 mRNA vaccine in the USA have not been published. Moreover, there are few large cohort studies that present direct comparisons of the post-vaccination risk of myocarditis or pericarditis between the two available COVID-19 mRNA vaccines. Accordingly, the FDA Center for Biologics Evaluation and Research conducted a retrospective cohort study (presented here) using administrative claims data sources within its active surveillance programme called the Biologics Effectiveness and Safety Initiative to: (1) evaluate the association between myocarditis, or pericarditis, or both, and each COVID-19 mRNA vaccine by comparing the risks (rates) of myocarditis, or pericarditis, or both, in people vaccinated with BNT162b2 or mRNA-1273 with risks (rates) in historical cohorts before the availability of the vaccines; (2) estimate the risks (rates) of myocarditis, or pericarditis, or both, after vaccination with BNT162b2 or mRNA-1273, with adjustment for potential confounding; and (3) establish if the risks (rates) differed between the two mRNA vaccine brands via a direct head-to-head comparison of the risk of myocarditis, or pericarditis, or both, for those vaccinated with mRNA-1273 versus BNT162b2. The results from this retrospective cohort study will further contribute to the current understanding of the risk of myocarditis, or pericarditis, or both, after vaccination with COVID-19 mRNA vaccines in the USA.
Research in context.
Evidence before this study
An association between myocarditis, pericarditis, or myopericarditis, or both, and COVID-19 mRNA vaccination has been reported in passive surveillance systems, case series, and observational studies. However, the magnitude of the increased risk of myocarditis or pericarditis after COVID-19 mRNA vaccination and the difference in risk between the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines, if any, are still not well understood. In one of the largest observational studies for COVID-19 mRNA vaccine recipients in the USA, we estimated the incidence rates of myocarditis or pericarditis, or both, after vaccination with BNT162b2 or mRNA-1273. Furthermore, our study directly compares the risks of post-vaccination myocarditis or pericarditis, or both, between mRNA-1273 and BNT162b2.
Added value of this study
In a large study in several US administrative health plan claims databases of people aged 18–64 years, the occurrence of myocarditis or pericarditis, or both, after COVID-19 mRNA vaccination was rare (with only 411 events occurring in 15 million recipients of 16 912 716 doses of BNT162b2 and 10 631 554 doses of mRNA-1273) but higher compared with pre-COVID-19 background rates. The risk was highest in men aged 18–25 years, 1–7 days after receipt of the second dose. The 95% CI for the incidence rate ratio comparing the vaccine brands (mRNA-1273 to BNT162b2 [reference]) ranged from 0·80 to 1·94 for this highest risk group.
Implications of all the available evidence
Men aged 18–25 years had the highest risk of myocarditis or pericarditis, or both, after vaccination, but the incidence was low. In our data, the risk for men aged 18–25 years appeared to be similar between the two mRNA vaccine brands; however, a difference cannot be ruled out. There is uncertainty in the risk estimates because of the low number of events; accordingly, additional studies and data are needed to verify these findings.
Methods
Study design and participants
We did a retrospective cohort study using a study population generated from four administrative claims databases (Optum, HealthCore, Blue Health Intelligence, and CVS Health) and included all individuals who received at least one dose of a COVID-19 mRNA vaccine (either BNT162b2 or mRNA-1273) and were 18–64 years at the time of their first vaccination. Patients who had myocarditis, or pericarditis, or both, during the pre-vaccination clean window (ie, 365 days before COVID-19 mRNA vaccination) were excluded from the analysis. Included individuals were continuously enrolled in the insurer's health plan at least 365 days before vaccination and had no diagnosis of a myocarditis or pericarditis, or both, composite event in any health-care setting during the same period. Health plan names (ie, each database) are labelled as Data Partner (DP)1–4 throughout this report.
This retrospective study was a secondary analysis of administrative claims data. The study involved no personally identifiable information and the data used in this study were deidentified and anonymised before use. This study was conducted as a public health mandate and not as a research activity. Our study practices were performed in accordance with the Declaration of Helsinki guidelines.
Procedures and outcomes
MH, KLA, DCB, YW, ZW, SA, YJ, YC, CNM-W, RPO, JDS, and DAD each had access to at least one of the four databases for claims data from Dec 14, 2021, for DP1 and DP3, from Dec 14 to 31, 2021, for DP4, and from Dec 14, 2021, to Jan 1, 2022, for DP2. COVID-19 mRNA vaccination and myocarditis, or pericarditis, or both, were identified in the claims data using reimbursement codes during the study period starting on Dec 18, 2020, until Sept 30, 2021 (DP1), until Oct 31, 2021 (DP2), until Nov 4, 2021 (DP3), or until Dec 25, 2021 (DP4). Receipt of a COVID-19 vaccination was identified via the Healthcare Common Procedure Coding System or Current Procedural Terminology codes (used with the permission of the American Medical Association) and National Drug Codes (appendix p 2). Only the first and second doses of the vaccination were included in analyses, and any dose refers to either dose 1 or dose 2.
The primary outcome of myocarditis, or pericarditis, or both, was identified using the International Classification of Diseases (tenth revision, clinical modification) diagnosis codes (B33.22, B33.23, I30.0, I30.1, I30.8, I30.9, I32, I41, I40.0, I40.1, I40.8, I40.9, and I51.4) in claims from any health-care setting comprising inpatient or outpatient facilities and professional services. The secondary outcome of myocarditis, or pericarditis, or both, restricted to inpatient facilities or emergency department settings was added as a post-hoc analysis. Incident myocarditis, or pericarditis, or both, were defined as the first event occurring in the risk window of 1–7 days after an mRNA vaccination. Sensitivity analyses using risk windows of 1–21 days and 1–42 days were also conducted.
Statistical analysis
To assess the association of myocarditis or pericarditis with each COVID-19 mRNA vaccine, we compared the observed (O) rates of myocarditis or pericarditis after vaccination with the expected (E) rates estimated from historical cohorts from the same databases in 2019—one calendar year before the availability of vaccines—as a proxy of the myocarditis or pericarditis rates in the absence of a COVID-19 vaccination. Cumulative annual rates were estimated according to a prespecified protocol.17 We estimated the O/E ratios for myocarditis or pericarditis, or both, in three databases (DP1 did not have the expected rates and thus was not included) where the expected rates were available. O/E ratios and the corresponding 95% CIs of observed post-vaccination incidence rates versus the expected incidence rates in each database were estimated by age group and sex. Expected incidence rates were calculated using vaccine-exposed person-time, age group (18–25 years, 26–35 years, 36–45 years, 46–55 years, and 56–64 years), and sex-specific background rates and were adjusted for claims processing delays estimated from the historical data (appendix pp 17–18); all these data were obtained from the claims database.
We assessed the post-vaccination incidence rates for myocarditis or pericarditis, or both, after each COVID-19 mRNA vaccine and IRRs for the head-to-head comparison of the two mRNA vaccines per the publicly posted protocol.18 The incidence rates and IRRs were estimated in multivariate Poisson regression models and adjusted by age, age by vaccine interaction, week of vaccination relative to the study start date, COVID-19 diagnosis before vaccination, and urban or rural residency, where the sample size permitted. Person-time started at the date of the first record of vaccination and was censored on the basis of disenrolment from the health plan, administration of a subsequent dose, or the study end date (Sept 30, 2021, for DP1, Oct 31, 2021, for DP2, Nov 4, 2021, for DP3, and Dec 25, 2021, for DP4); estimates were adjusted for database-specific delays in claims processing. The post-vaccination incidence rates were stratified by age group, sex, and dose (any dose, post-dose 1, and post-dose 2). The adjusted IRRs comparing the mRNA-1273 and BNT162b2 (which was used as the reference) vaccines were stratified by sex and dose. We pooled adjusted incidence rates and IRRs for any dose, first dose, and second dose across the databases using meta-analysis with a random-effects model and assessed between-database heterogeneity via an I2 statistic with corresponding 95% uncertainty interval and Cochran's Q test p value.19 For post-hoc sensitivity analyses, pooled IRRs comparing mRNA-1273 and BNT162b2 (reference) were estimated for (1) men aged 18–35 years, (2) the secondary outcome in men aged 18–25 years, and (3) the secondary outcome in men aged 18–35 years.
We used the same protocol, definitions, scripts, and analysis for all four databases in this study. Statistical analyses were conducted with SAS 9.4, R.4.0.2, and R.4.0.3.
Role of the funding source
The funder led the design of the study, interpretation of the results, writing of the manuscript, decision to submit, and made contributions to the coordination of data collection and analysis of the data.
Results
Across the four large health plan claims databases, 15 148 369 people aged 18–64 years (appendix p 4) received 16 912 716 doses of BNT162b2 and 10 631 554 doses of mRNA-1273, between Dec 18, 2020, and Dec 25, 2021. We observed 411 myocarditis or pericarditis events after any dose of either mRNA vaccine, with 33–42% in people aged 18–25 years, 58–73% in men, and 6–13% in people with a history of COVID-19 diagnosis (table 1 ). In comparison, among the recipients of COVID-19 mRNA vaccines, 12–14% events were in people aged 18–25 years, 52–53% were in men, and 7% were in people with a history of COVID-19 diagnosis in their insurance claims in the past year (appendix p 4).
Table 1.
Descriptive characteristics of myocarditis and pericarditis events after mRNA-1273 and BNT162b2 vaccination among people aged 18–64 years in four health plan claims databases
|
DP1 |
DP2 |
DP3 |
DP4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Total myocarditis or pericarditis events | 154 | 100% | 64 | 100% | 94 | 100% | 99 | 100% | |
| Age (years) | |||||||||
| 18–25 | 64 | 42% | 21 | 33% | 34 | 36% | 34 | 34% | |
| 26–35 | 15 | 10% | 10 | 16% | 16 | 17% | 21 | 21% | |
| 36–45 | 24 | 16% | 11 | 17% | 14 | 15% | 14 | 14% | |
| 46–55 | 26 | 17% | 9 | 14% | 13 | 14% | 14 | 14% | |
| 56–64 | 25 | 16% | 13 | 20% | 17 | 18% | 16 | 16% | |
| Sex | |||||||||
| Female | 43 | 28% | 27 | 42% | 25 | 27% | 32 | 32% | |
| Male | 111 | 72% | 37 | 58% | 69 | 73% | 67 | 68% | |
| Type of area* | |||||||||
| Urban | NA | NA | 60 | 94% | 80 | 85% | 94 | 95% | |
| Rural | NA | NA | 4 | 6% | 14 | 15% | 5 | 5% | |
| Previous COVID-19 diagnosis since April 1, 2020† | 15 | 10% | 8 | 13% | 6 | 6% | 10 | 10% | |
Percentages might not add up to 100% due to rounding. DP=data partner. NA=not available.
Urban versus rural status was defined on the basis of five-digit zip codes for enrolled members' physical addresses. This information was not available in the DP1 database.
A previous COVID-19 diagnosis was identified via the International Classification of Diseases (tenth revision, clinical modification) code U07.1.
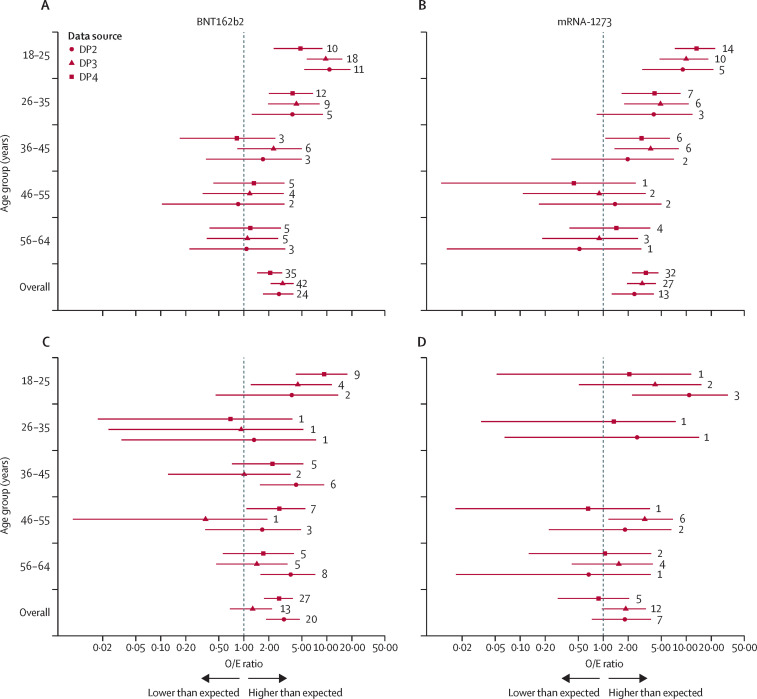
The observed rates of myocarditis, or pericarditis, or both, were higher than the expected rates that were based on the pre-COVID-19 vaccination period for both BNT162b2 and mRNA-1273 among younger men, particularly those younger than 35 years (figure 1 ) and after receipt of the second dose of the vaccine in the three databases included in the analysis (appendix p 5). The O/E ratio was highest among men aged 18–25 years, post-dose 2, ranging from between 16·13 and 21·53 for mRNA-1273 and between 8·57 and 21·85 for BNT162b2 (appendix p 8). The age-specific O/E ratio estimates for women suggest higher myocarditis or pericarditis post-vaccination rates compared with the expected rates for some age groups, with wide CIs and a low number of events in each database (appendix p 5).
Figure 1.
O/E ratios of myocarditis and pericarditis in the first 1–7 days of receipt of any dose of COVID-19 mRNA vaccine by age group and sex in three large health plan databases
O/E ratios of myocarditis, or pericarditis, or both, in men during the first 1–7 days of receipt of BNT162b2 (A) or mRNA-1273 (B). O/E ratios of myocarditis, or pericarditis, or both, in women during the first 1–7 days of receipt of BNT162b2 (C) or mRNA-1273 (D). Estimates and 95% CIs for O/E ratios are not displayed for age and sex groups with zero events. The numbers on the furthest right of each figure are the number of events for myocarditis, or pericarditis, or both, corresponding to each O/E ratio. DP=data partner. O/E=observed versus expected.
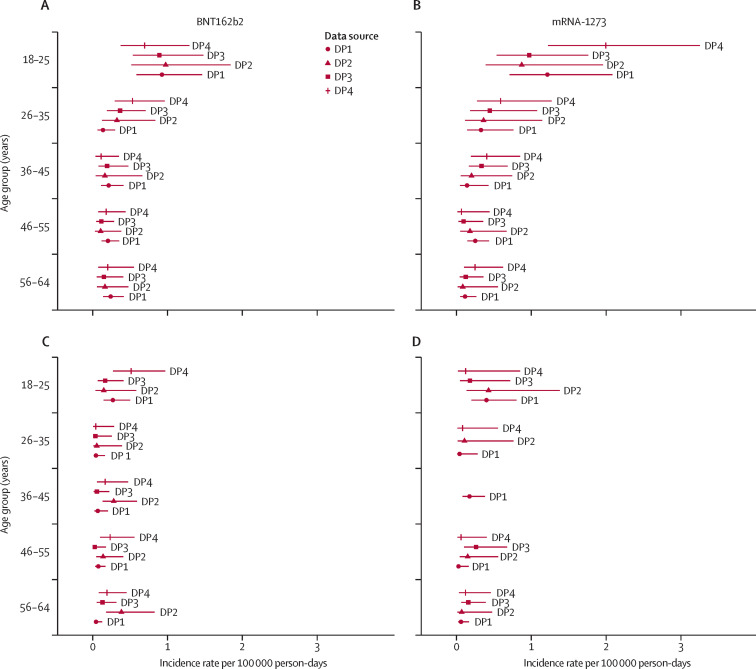
The adjusted incidence rates were the highest in the age group of 18–25 years compared with all other age groups and in men compared with women (figure 2 ) in the four databases included in this analysis. Among men aged 18–25 years, the risk was highest after receipt of the second dose (table 2 ). The pooled incidence rates for men aged 18–25 years post-dose 2 were 2·17 (95% CI 1·55–3·04) per 100 000 person-days for mRNA-1273 and 1·71 (1·31–2·23) per 100 000 person-days for BNT162b2 (table 2). The pooled incidence rates for men aged 18–25 years were lower in the 1–21 day and 1–42 day windows compared with the 1–7 day window (appendix pp 14–15).
Figure 2.
Adjusted incidence rates of myocarditis and pericarditis within 1–7 days of receipt of any dose of COVID-19 mRNA vaccines by age group and sex, in four large health plan databases
Adjusted incidence rate of myocarditis, or pericarditis, or both, in men within 1–7 days of receipt of BNT162b2 (A) or mRNA-1273 (B). Adjusted incidence rate of myocarditis, or pericarditis, or both, in women within 1–7 days of receipt of BNT162b2 (C) or mRNA-1273 (D). Estimates and 95% CIs for incidence rates are not displayed for age and sex groups with zero events. DP=data partner.
Table 2.
Direct head-to-head comparison of incidence rates of mRNA-1273 and BNT162b2 for myocarditis or pericarditis in the first 1–7 days after COVID-19 mRNA vaccination, for men aged 18–25 years by type, dose number, and database
|
BNT162b2 |
mRNA-1273 |
mRNA-1273 vs BNT162b2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of vaccine doses | Number of observed events | Incidence rate per 100 000 person-days (95% CI) | Number of vaccine doses | Number of observed events | Incidence rate per 100 000 person-days (95% CI) | Incidence rate ratio (95% CI) | Excess risk* (95% CI) | |
| DP1 | ||||||||
| Any dose | 449 020 | 29 | 0·93 (0·59 to 1·46) | 211 821 | 17 | 1·21 (0·71 to 2·08) | 1·31 (0·64 to 2·67) | 20·14 (−27·56 to 67·84) |
| Dose 1 | 250 271 | 4 | 0·23 (0·09 to 0·63) | 117 192 | 4 | 0·52 (0·20 to 1·39) | 2·23 (0·55 to 8·92) | 20·08 (−29·14 to 69·30) |
| Dose 2 | 198 749 | 25 | 1·87 (1·26 to 2·79) | 94 629 | 13 | 2·10 (1·22 to 3·62) | 1·12 (0·57 to 2·19) | 15·90 (−82·62 to 114·42) |
| DP2 | ||||||||
| Any dose | 159 435 | 11 | 0·98 (0·52 to 1·85) | 84 586 | 5 | 0·87 (0·39 to 1·96) | 0·89 (0·32 to 2·50) | −7·43 (−82·38 to 67·53) |
| Dose 1 | 87 769 | 1 | 0·16 (0·02 to 1·17) | 47 301 | 1 | 0·31 (0·04 to 2·24) | 1·95 (0·12 to 31·30) | 10·71 (−107·08 to 128·50) |
| Dose 2 | 71 666 | 10 | 2·09 (1·11 to 3·91) | 37 285 | 4 | 1·62 (0·61 to 4·33) | 0·78 (0·24 to 2·48) | −32·42 (−202·58 to 137·75) |
| DP3 | ||||||||
| Any dose | 262 536 | 18 | 0·89 (0·54 to 1·48) | 139 732 | 10 | 0·97 (0·53 to 1·76) | 1·09 (0·49 to 2·41) | 5·54 (−49·52 to 60·61) |
| Dose 1 | 145 109 | 5 | 0·45 (0·18 to 1·11) | 78 572 | 3 | 0·54 (0·17 to 1·68) | 1·19 (0·28 to 4·99) | 5·95 (−55·37 to 67·28) |
| Dose 2 | 117 427 | 13 | 1·46 (0·83 to 2·57) | 61 160 | 7 | 1·52 (0·71 to 3·24) | 1·04 (0·41 to 2·61) | 3·95 (−100·80 to 108·70) |
| DP4 | ||||||||
| Any dose | 209 473 | 10 | 0·69 (0·37 to 1·29) | 106 271 | 14 | 2·00 (1·22 to 3·26) | 2·88 (1·30 to 6·36) | 91·13 (6·74 to 175·51) |
| Dose 1 | 116 908 | 2 | 0·24 (0·06 to 0·96) | 59 925 | 4 | 0·99 (0·37 to 2·65) | 4·13 (0·76 to 22·58) | 52·39 (−34·71 to 139·48) |
| Dose 2 | 92 565 | 8 | 1·28 (0·64 to 2·57) | 46 346 | 10 | 3·23 (1·74 to 6·02) | 2·52 (0·99 to 6·39) | 136·40 (−28·22 to 301·03) |
| Meta-analysis | ||||||||
| Any dose | 1 080 464 | 68 | 0·88 (0·67 to 1·15) | 542 410 | 46 | 1·27 (0·88 to 1·84) | 1·43 (0·88 to 2·34) | 27·80 (−21·88 to 77·48) |
| Dose 1 | 600 057 | 12 | 0·30 (0·17 to 0·53) | 302 990 | 12 | 0·62 (0·35 to 1·10) | 2·07 (0·91 to 4·71) | 22·72 (−6·27 to 51·70) |
| Dose 2 | 480 407 | 56 | 1·71 (1·31 to 2·23) | 239 420 | 34 | 2·17 (1·55 to 3·04) | 1·25 (0·80 to 1·94) | 32·20 (−33·90 to 98·30) |
DP=data partner.
Excess risk is the difference in incident cases per million doses between mRNA-1273 and BNT162b2 (reference) based on adjusted incidence rates within 7 days of vaccination. Any dose models were adjusted for urban or rural residency status (DP-3 and DP-4), COVID-19 diagnosis before vaccination (all databases), and week of vaccination (all databases). Dose 1 models were adjusted for urban or rural residency status (DP-3), COVID-19 diagnosis before vaccination (DP-1, DP-3, and DP-4), and week of vaccination (all databases). Dose 2 models were adjusted for urban or rural residency status (DP-3 and DP-4), COVID-19 diagnosis before vaccination (all databases), and week of vaccination (all databases).
In head-to-head comparisons among men aged 18–25 years, the rates of myocarditis or pericarditis after any dose of mRNA-1273 did not differ significantly compared with after any dose of BNT162b2 (pooled adjusted IRR 1·43; 95% CI 0·88 to 2·34; table 2). The IRR ranged from 0·89 to 2·88 for any dose of either vaccine among databases, with moderate between-database heterogeneity (I 2=30%, 95% uncertainty interval 0–75%; Cochran's Q test p value=0·23). The excess risk of myocarditis or pericarditis for this highest risk group of men aged 18–25 years was 27·80 (95% CI –21·88 to 77·48) additional myocarditis or pericarditis events per million doses for those vaccinated with any dose of mRNA-1273 compared with those vaccinated with BNT162b2 (table 2). The results for women aged 18–25 years showed a lower number of myocarditis or pericarditis events than men (appendix p 9). Results for other risk windows, such as 1–21 days (appendix p 14) and 1–42 days (appendix p 15), showed more captured events but similar IRR estimates (pooled adjusted IRR for 1–21 days with any dose, 1·43, 95% CI 0·98–2·10; pooled adjusted IRR for 1–42 days with any dose, 1·23; 95% CI 0·84–1·80).
The pooled IRRs for head-to-head comparisons differed in sensitivity analyses within 1–7 days post-vaccination after any dose. Among men aged 18–35 years, the IRR was reduced compared with the primary analyses of men aged 18–25 years (IRR 1·33; 95% CI 0·94 to 1·88; n=168) with 13·22 additional events (95% CI –7·95 to 34·39) per million doses in mRNA-1273 recipients (appendix p 11). When restricting the analyses to events occurring only in inpatient or emergency department settings, the IRR was attenuated firstly among men 18–25 years (IRR 1·25; 95% CI 0·79 to 1·98; n=77) with 11·40 additional events (95% CI –17·92 to 40·72) per million doses in individuals who received mRNA-1273 (appendix p 12) and secondly among men aged 18–35 years (1·30; 0·85 to 1·98; n=108) with 7·38 additional events per million doses in individuals who received mRNA-1273 (–6·19 to 20·96; appendix p 13).
Discussion
In our study of large cohorts aged 18–64 years in US health plan claims databases, the occurrence of myocarditis or pericarditis after receipt of each COVID-19 mRNA vaccine (mRNA-1273 or BNT162b2) was rare but increased compared with pre-COVID-19 background rates. The highest risk was among men aged 18–25 years after their second dose of either vaccine. Our results did not indicate a statistically significant difference in the risk of myocarditis or pericarditis between mRNA-1273 and BNT162b2. Additionally, we found that the risk might range from approximately 12% lower to 134% higher in the mRNA-1273 recipients compared with BNT162b2 recipients.
Our study results showing an increased risk of myocarditis or pericarditis after mRNA vaccination compared with expected rates reflect similar findings reported in passive surveillance systems, particularly among younger men in the USA,4 Canada,20 and Israel,2 as well as in observational studies in the UK,21 Denmark,10 and the USA.22 Our study estimated the incidence rates of myocarditis or pericarditis in the USA for various demographic groups using several large claims databases covering more than 100 million people. These databases represent four of the largest commercial health insurers in the USA, and the included insured members have a similar age and sex distribution as the overall US population. The use of these large databases provides statistical power to evaluate rare adverse events such as myocarditis and pericarditis. Our study provided more complete ascertainment of myocarditis, or pericarditis, or both, events that might not be identified through passive surveillance because of the potential under-reporting of events and uncertainty in the number of vaccinated individuals used in rate calculations. Although several studies, including ours, have identified an increased risk of myocarditis or pericarditis after COVID-19 mRNA vaccination, a UK self-controlled study found that the risk of myocarditis after vaccination was substantially lower than the risk after SARS-CoV-2 infection.11 Additionally, benefit–risk assessments from the FDA23 and the Advisory Committee on Immunization Practices24 concluded that the benefits of COVID-19 vaccination to individual people and at the population level clearly outweigh the risk of myocarditis and pericarditis after mRNA vaccination.
Our head-to-head comparisons of mRNA-1273 and BNT162b2 for men aged 18 to 25 years do not indicate a statistically significant difference in myocarditis or pericarditis risk between recipients of mRNA-1273 and BNT162b2. Accordingly, because of the rarity of these events, uncertainty in the risk difference ranged from approximately 22 fewer events to 77 more events per million doses after mRNA-1273 vaccination. In contrast, the few studies with head-to-head comparisons between the two mRNA vaccine brands reported from a 1·6 to 5·0-times increased risk of myocarditis or pericarditis with mRNA-1273 vaccination compared with BNT162b2 vaccination.20 One observational study in electronic health records in the US Centers for Disease Control and Prevention Vaccine Safety Data link reported an approximately 1·61-times higher risk of chart-confirmed myocarditis or pericarditis, or both, after either the first or second dose of mRNA-1273 than the risk after BNT162b2 vaccination in a group of men and women aged 18–39 years (relative risk [RR] 1·61; 95% CI 1·02–2·54; n=79) and an RR of 1·52 (0·93–2·48) when restricted to men aged 18–39 years.25 In the Canadian enhanced passive surveillance system, Abraham and colleagues20 reported an approximately 5-times higher risk of myocarditis or pericarditis, or both, 0–7 days after the second dose of the mRNA-1273 vaccine than the risk after BNT162b2 vaccination in men aged 18–29 years (RR 4·73; 3·19–7·20; n=137) after adjustment for one calendar week of administration.20 These studies evaluated myocarditis or pericarditis, or both, events that occurred in inpatient and emergency department settings, or assessed these events within a wider age group of either 18–29 or 18–39 years. In our study, restricting events to those that occurred (1) in an inpatient or emergency department setting, (2) within patients aged 18–35 years, or (3) both (patients aged 18–35 years in an inpatient or emergency department setting) resulted in smaller RRs but did not alter our interpretation of the observed associations. Further studies are needed, given the uncertainty around the current estimates.
In addition to the studies with head-to-head comparisons of mRNA-1273 and BNT162b2 vaccines, other studies have examined the RRs of myocarditis or pericarditis, or both, in individuals who were vaccinated compared with those who were unvaccinated for each mRNA vaccine brand, without formal comparisons between mRNA-1273 and BNT162b2; for example, observational studies in linked national immunisation databases with hospital electronic health records (English cohort)11 or patient registries (Danish cohort).10 Additionally, passive surveillance systems3, 26 have reported higher proportions of myocarditis or pericarditis, or both, cases among the doses of mRNA-1273 or BNT162b2 in the population than the RRs reported in active surveillance, but did not provide the corresponding CIs to aid the interpretation of the reported ratios. Indirect comparisons of mRNA vaccines without formal testing should be interpreted cautiously. This includes addressing the differences in the proportion of risk factors for myocarditis or pericarditis among individuals who received mRNA-1273 and BNT162b2 vaccines. For example, younger age groups with an increased risk of myocarditis or pericarditis are more prevalent among individuals who received an mRNA-1273 vaccine than among those who received a BNT162b2 vaccine, as reported in an English cohort (percentage of vaccinated cohort aged 16–29 years with myocarditis or pericarditis events: 41% in mRNA-1273 vs 25% in BNT162b2)11 and a Danish cohort (percentage of vaccinated cohort aged 12–39 years with myocarditis or pericarditis, or both, events: 40% in mRNA-1273 vs 21% in BNT162b2).10 Another consideration is risk factors that vary by calendar time, such as SARS-CoV-2 circulation and its association with an increased risk of myocarditis and pericarditis, as well as the differential availability of vaccine brands in some countries. Comparing reported ratios from passive surveillance systems might additionally be limited by the inaccurate estimates of the number of vaccinees or reported events.
Our study has several strengths. The study population comprises patients in large commercial claims databases that collectively cover every state in the USA. It is one of the largest observational studies of individuals who have received mRNA vaccines in the USA, with similar numbers of doses of each of the COVID-19 mRNA vaccine brands and available data within 3 weeks of authorisation dates (Dec 11, 2020, for BNT162b2 [Pfizer–BioNTech], and Dec 18, 2020, for mRNA-1273 [Moderna] in the USA). This enabled head-to-head comparisons of the myocarditis or pericarditis risks between mRNA-1273 and BNT162b2 vaccines within the highest risk group reported in the passive surveillance systems—namely, males aged 18–25 years—within 1–7 days after vaccination. The study results accounted for several potential confounders, including the week of vaccination, demographic factors, and previous history of a COVID-19 diagnosis.
Our study also has some limitations. The comparisons of O/E incidence rates (risks) accounted for age and sex difference but no other covariates. RRs in this analysis were not based on comparisons between vaccinated and unvaccinated individuals and instead relied on rates calculated from historical cohorts in a pre-COVID-19-vaccination period to approximate the expected rates in unvaccinated individuals. The adjusted incidence rates were based on myocarditis or pericarditis events identified via reimbursement codes rather than a medical chart review. Thus, the incidence rates (a measure of absolute risk) might be underestimated or overestimated compared with the true risk. However, the comparisons of the incidence rates between the vaccine brands (IRRs) were less likely to be affected, given the low probability of a difference in the misclassification of myocarditis or pericarditis events between the vaccine brands. A chart review of myocarditis or pericarditis events is underway. To conduct timely analyses, our study adjusted for claims observation delay under the assumption that events occurring after BNT162b2 and mRNA-1273 vaccination have a similar delay distribution. The pooled IRR across databases relied on rates that might have some heterogeneity. Because of the low number of databases in the meta-analysis, the statistics for measuring heterogeneity between studies could be biased. Because of the low number of myocarditis or pericarditis events observed by us and others, these estimates are subject to some uncertainty. These findings might not be generalisable to those not covered by similar health plans in the USA or those who are uninsured.
In conclusion, the risk of myocarditis or pericarditis events in people who received COVID-19 mRNA vaccines was elevated in younger populations; however, the incidence was rare. Our study showed that, in the period of 1–7 days after receipt of the second dose, the highest risk was in men aged 18–25 years. A head-to-head comparison of myocarditis and pericarditis risk for the mRNA-1273 and BNT162b2 vaccine brands did not indicate a statistically significant difference, but also could not rule out that a difference might exist. Studies with additional data sources are needed to further evaluate the risk.
Data sharing
The study protocol was publicly posted as referenced in the manuscript before data analyses18 and related documents can be made available where needed, by contacting the corresponding author. De-identified participant data will not be shared without approval from the data partners.
Declaration of interests
All Optum Epidemiology coauthors are employees of Optum. KLA and JDS own stock in UnitedHealth Group. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the US Food and Drug Administration. We thank Kristin A Sepúlveda and Tainya C Clarke of the US Food and Drug Administration; Kathryn Matuska, Xi Li, Henry Amir, Purva Shah, Mengjia Xu, Bradley Lufkin, Yuqin Wei, and Ellen Tworkoski of Acumen; Smita Bhatia, Anne Marie Kline, Nancy Sheffield, and Charlalynn Harris of CVS Health; C Robin Clifford, Michael Kirksey, Jennifer Song, Lisa Weatherby, and Vivian Wilt of Optum; Michael Goodman, Jim Frankfort, Michael Bruhn, and Ruth Weed of IQVIA; and Shiva Vojjala, Ramya Avula, Shiva Chaudhary, Shanthi P Sagare, Ramin Riahi, Navyatha Namburu, and Grace Stockbower of HealthCore, for their assistance with data validation, analysis, and project coordination. We acknowledge Blue Health Intelligence for their assistance in data acquisition.
Acknowledgments
Contributors
SAA and RF were responsible for the conception of the work. H-LW, MH, CKZ, PCL, YL, YC, RD, ASh, RF, and SAA reviewed the design of the work. MH, KLA, DCB, ASe, CR, YW, ZW, SA, YJ, YC, CNM-W, RPO, JDS, and DAD contributed to the acquisition, analysis, and curation of data. H-LW, MH, CKZ, PCL, KLA, DCB, ASe, YL, YW, RPO, ZW, JDS, YC, CNM-W, DAD, ASh, RF, and SAA contributed to the validation and interpretation of the data. All authors except RD, JH, and CR contributed to the writing and editing of the manuscript. H-LW, ASh, RF, and SAA provided oversight for the work and YC, JH, and JO contributed to project administration. Because of data-sharing policies, only authors affiliated with a specific data partner had access to individual-level data from that partner. MH, KLA, DCB, YW, ZW, SA, YJ, YC, CNM-W, RPO, JDS, and DAD each had access to at least one of the four databases and all four databases had at least one author with access to the database. All authors had access to summary-level data across data partners. MH, YW, ZW, YC, and YJ verified the data across data partners. All authors had final responsibility for the final decision to submit for publication.
Supplementary Material
References
- 1.European Medicines Authority Signal assessment report on myocarditis and pericarditis with Spikevax (previously COVID-19 Vaccine Moderna) 2021. https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-myocarditis-pericarditis-spikevax-previously-covid-19-vaccine-moderna-covid_en.pdf
- 2.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health Ontario Adverse events following immunization (AEFI) for COVID-19 in Ontario: December 13, 2020 to October 10, 2021. 2021. https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-aefi-report.pdf?sc_lang=en
- 4.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer–BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Vaccines and Related Biological Products Advisory Committee October 14–15, 2021 meeting presentation. 2021. https://www.fda.gov/media/153090/download
- 14.US Centers for Disease Control and Prevention COVID-19 vaccine safety updates. Advisory Committee on Immunization Practices (ACIP) October 21, 2021 meeting presentation. 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/08-COVID-Klein-508.pdf
- 15.US Food and Drug Administration Fact sheet for healthcare providers administering vaccine (vaccine providers) Emergency Use Authorization (EUA) of the Pfizer–BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
- 16.US Food and Drug Administration Fact sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019. 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
- 17.US Food and Drug Administration Background rates of adverse events of special interest for COVID-19 vaccine safety monitoring protocol. 2021. https://bestinitiative.org/wp-content/uploads/2021/01/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-2020.pdf
- 18.US Food and Drug Administration COVID-19 vaccine safety protocol: comparative risk of myocarditis or pericarditis following COVID-19 mRNA vaccination. 2021. https://bestinitiative.org/wp-content/uploads/2021/12/C-19-MyoPericarditis-mRNA-Comparative-Safety-Protocol-2021.pdf
- 19.Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand (1977) 1982;87:377–385. doi: 10.6028/jres.087.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham N, Spruin S, Rossi T, et al. Myocarditis and/or pericarditis risk after mRNA COVID-19 vaccination: a Canadian head to head comparison of BNT162b2 and mRNA-1273 vaccines. SSRN. 2021 doi: 10.1016/j.vaccine.2022.05.048. https://ssrn.com/abstract=3988612 published online Dec 28. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patone M, Mei XW, Handunnetthi L, et al. Risk of myocarditis following sequential COVID-19 vaccinations by age and sex. medRxiv. 2021 doi: 10.1101/2021.12.23.21268276. published online Dec 25. (preprint). [DOI] [Google Scholar]
- 22.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration Benefit–risk analysis. Food and Drug Administration Vaccines and Related Biological Products Advisory Committee October 26, 2021 meeting presentation. 2021. https://www.fda.gov/media/153507/download
- 24.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein N. Myocarditis analyses in the vaccine safety datalink: rapid cycle analyses and “head-to-head” product comparisons. Feb 4, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-04/10-COVID-Klein-508.pdf
- 26.Medicines & Healthcare products Regulatory Agency UK Coronavirus vaccine - weekly summary of Yellow Card reporting. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol was publicly posted as referenced in the manuscript before data analyses18 and related documents can be made available where needed, by contacting the corresponding author. De-identified participant data will not be shared without approval from the data partners.