Abstract
Background
A phase 1, clinical trial to evaluate FINLAY-FR-1A vaccine in COVID-19 convalescent individuals was completed. Here, we report results of the phase 2, clinical trial.
Methods
We studied 450 convalescent participants with a history of asymptomatic, mild, or moderate COVID-19 at the National Institute of Hematology and Immunology and the National Centre for Sexual Education in Havana, Cuba. The study included adults aged 19–78 years who had recovered from COVID-19 and had had a negative PCR test at least 2 months before the initiation of the study. Phase 2 was done sequentially in two stages. The first stage to assess safety comprised an open, non-controlled phase 2a study in participants aged 60–78 years who received a single dose of the FINLAY-FR-1A vaccine (50 μg of recombinant dimeric receptor binding domain [RBD]). The second stage comprised the placebo-controlled, double-blind, phase 2b trial in participants aged 19–78 years, where participants were randomly assigned (4:1) into two groups: an experimental group vaccinated with a single dose of the FINLAY-FR-1A vaccine, and a control (placebo) group injected with vaccine excipient. The primary outcomes were safety, evaluated 28 days after vaccination by the occurrence of serious adverse events in all participants, and successful immune response, assessed by neutralising antibody ELISA, and defined as half-maximal surrogate virus neutralisation titres of 250 or more. Secondary endpoints included vaccine immunogenicity assessed by ELISA anti-RBD and live-virus neutralisation test. All randomly assigned participants were included in the safety analysis (safety population), and immunogenicity was evaluated in participants without study interruptions (per-protocol population). The trial is registered with the Cuban Public Registry of Clinical Trials, RPCEC00000366-En and WHO-ICTRP and is complete.
Findings
From April 9, 2021, to April 17, 2021, 663 COVID-19 convalescent participants were enrolled in the study; 213 participants did not meet the selection criteria and 450 volunteers were recruited. 20 participants aged 60–78 years were included in the open, single-group, phase 2a study and 430 participants were randomly assigned to the experimental (n=344) or control groups (n=86) in the phase 2b study of participants aged 19–78 years. 19 (95%) of 20 phase 2a volunteers achieved a successful immune response after vaccination. No vaccine-associated serious adverse events were reported in the whole study population. Minor adverse events were found, the most common being pain at the injection site (105 [29%] of 364 in the intervention group; 13 [15%] of 86 in the placebo group). A successful immune response was found in 289 (81%) of 358 participants 28 days after vaccination. The vaccine elicited a greater than 31-times increase in anti-RBD-IgG antibodies compared with prevaccination rates, and the seroconversion rate was 302 (84%) of 358 on day 28 after vaccination; the geometric mean titres of live-virus neutralisation test increased from 15·4 (95% CI 10·3–23·2) to 400·3 (272·4–588·1) and high response was found against alpha, beta, and delta variants of concern.
Interpretation
A single dose of the FINLAY-FR-1A vaccine against SARS-CoV-2 strengthened the pre-existing natural immunity, with excellent safety profile.
Funding
Cuba's Ministry of Science, Technology, and Environment.
Introduction
Over time, the number of people who have recovered from COVID-19 is increasing. By the end of 2021, from about 300 million cases reported worldwide, the number of individuals recovered from SARS-CoV-2 infection had surpassed 250 million.1
Research in context.
Evidence before this study
Immunity against SARS-CoV-2 is highly dependent on the concentration and quality of neutralising antibodies, although the T-cell response plays an important role in COVID-19 mitigation. People recovered from COVID-19 might be reinfected, particularly those with low neutralising antibody titres and facing new SARS-CoV-2 variants of concern. Severe SARS-CoV-2 reinfections with delta variant have been reported, and evidence suggests an increased risk of reinfection with new omicron variant. A phase 1 clinical trial of FINLAY-FR-1A vaccine done in COVID-19 convalescent individuals showed that SARS-CoV-2 infection induces long-term memory immune cells that are activated by a single vaccine dose. We searched PubMed using the terms “Clinical trial” [Publication Type] AND “COVID-19 vaccines” [MeSH Terms] OR “SARS-CoV-2” [Text Word] OR “COVID-19” [Text Word] AND “convalescent” [Text Word] OR “infected” [Text Word] OR “recovered” [Text Word]. The only restriction was language (English) and no time limit was used. Only four post-licensing studies of COVID-19 vaccines in previously infected individuals were reported, all involving a small number of individuals. MedRxiv (subject area, infectious diseases) was also searched (search terms as described for PubMed): three additional trials were reported (seven, in total), all studies with a different design than our clinical trial and reporting a secondary antibody response induced by vaccination
Added value of this study
This is a randomised, placebo-controlled, phase 2, clinical trial of an anti-SARS-CoV-2 vaccine, specifically designed for COVID-19 convalescent individuals. The vaccine was shown to be safe with good tolerability, evidenced by the fact that most local and systemic reactions were mild. Receptor binding domain binding inhibitory antibodies (RBD:hACE2) were induced in most volunteers after a single vaccine dose, which proved its immunogenicity. There was also an increase in live-virus neutralising titres against the alpha, beta and delta variants of concern. The results confirm that natural infection leads to the production of long-term memory B cells that respond quickly to a single dose of FINLAY-FR-1A vaccine.
Implications of all the available evidence
An RBD vaccine can be used to trigger immunity against SARS-CoV-2 in COVID-19 convalescent individuals, including those with low concentrations of neutralising antibodies. Immunisation with a single dose of FINLAY-FR-1A vaccine triggered a rapid induction of high humoral immune response, suggesting a protective immunity against SARS-CoV-2, and a decrease in severe reinfection by SARS-CoV-2 variants of concern.
The efficiency and duration of protection elicited by viral infection is not well known, but they probably depend on the quality and intensity of the specific immune response.2, 3, 4, 5, 6 On the other hand, there is evidence of reinfection, especially since the emergence of variants of concern (VOCs). Severe SARS-CoV-2 reinfections with delta variant have been reported after recovery from COVID-19,7, 8, 9, 10 and evidence suggests an increased risk of reinfection with new omicron VOCs.10
Vaccine candidates based on the receptor-binding domain (RBD) developed on different platforms, have shown safety and immunogenicity.11, 12, 13 FINLAY-FR-1A (Soberana Plus, Finlay Vaccine Institute and the Centre of Molecular Immunology, in Havana, Cuba) vaccine is based on a recombinant protein antigen, a dimer of RBD with sequence 319–541 obtained in genetically modified Chinese hamster ovary cells. RBD is dimerised (d-RBD) through a Cys5p8-Cys538 interchain disulphide bridge.
The antigen is adsorbed on aluminium hydroxide gel, and it is produced under Good Manufacturing Practice at The Finlay Vaccine Institute and The Centre of Molecular Immunology, in Havana, Cuba. It was evaluated in a phase 1 clinical trial in naive individuals and in a phase 1 trial carried out in COVID-19 convalescents (people who have recovered from SARS-CoV-2 infection).14, 15
Convalescent individuals of mild COVID-19 and individuals with subclinical infection received a single 50 μg intramuscular injection of the FINLAY-FR-1A vaccine. The vaccine was safe; minor adverse events only were found. High humoral and cellular immune responses were detected. Live-virus neutralisation titres higher than 160 were found in 80% of participants. Also, the correlation between the live-virus neutralisation test and in-vitro techniques was shown, especially with the half-maximal surrogate virus neutralisation titres.15
There is evidence that natural infection leads to the production of long-term memory cells that can respond quickly to a single dose of FINLAY-FR-1A vaccine.15 Here, we aim to study the response of memory B cells after a single dose of the vaccine in individuals with past SARS-CoV-2 infection.
Methods
Study design and participants
This phase 2a–2b clinical trial was carried out at the National Institute of Haematology and Immunology and the National Centre for Sexual Education (as vaccination facilities), both located in Havana, Cuba. 450 convalescent participants of both sexes aged 19–78 years with a history of asymptomatic, mild, or moderate COVID-19 were recruited in Havana, Cuba, among COVID-19 convalescent individuals who fulfilled the selection criteria (appendix pp 3, 5).
Owing to safety concerns, and in accordance with the requirements of the Cuban protocol for convalescent individuals,16 COVID-19 convalescents had been discharged from hospitals at least 2 months before beginning the study. The time elapsed from hospital discharge to vaccination was computed (according to Cuban regulations, all individuals with positive-PCR tests, including those asymptomatic, were admitted to hospitals). A negative PCR test at least 2 months before the initiation of the study was required.
Participants were randomly assigned to experimental or control groups: the experimental group was vaccinated with a single dose of FINLAY-FR-1A vaccine, the control (placebo) group received vaccine excipient. Adverse events and the humoral immune response were evaluated as will be described later in this Article.
A two-stage seamless trial was done. Phase 2a was an open, non-controlled stage with a single experimental group in adults aged 60–78 years; participants of this age subgroup would be included in phase 2b if the vaccine-associated serious adverse events rate was lower than 0·05, and the probability of achieving a successful immune response (defined later in this Article) greater than 50% was not less than 0·1. Phase 2b was a randomised, placebo-controlled, and double-blind stage; on the basis of phase 2a results, phase 2b included convalescent individuals aged 19–78 years, randomly assigned into two groups: the experimental group receiving the intervention, and the control group.
All participants underwent a screening visit (full medical history, rapid pregnancy test in women of childbearing potential, SARS-CoV-2 rapid antigen test (Hoffmann-La Roche, Switzerland). Full blood count, kidney, and liver function tests were done only in phase 2a). Exclusion criteria were: history of severe COVID-19, hospitalisation due to COVID-19 during the last 2 months, any severe disease or decompensated chronic disease, immunodeficiency, history of severe allergy, pregnancy, breastfeeding, positive SARS-CoV-2 test, immunological treatment during the last 30 days, history of having received any vaccine against SARS-CoV-2 (appendix p 5).
The Cuban Ministry of Public Health established a medical care programme for COVID-19 convalescent individuals,16 and approved the trial and the procedures. The National Institute of Hematology and Immunology (the main clinical site of the trial), the National Centre for Sexual Education (secondary clinical site), the Independent Ethics Committee for Studies on Human Subjects, and the Cuban National Regulatory Agency (Centre for State Control of Medicines and Medical Devices, CECMED), approved the trial and the procedures (CECMED, authorization date April 9, 2021, reference number 110/05·008·21BA). It was done according to the Declaration of Helsinki and Good Clinical Practice.
The clinical trial was monitored by the National Coordinating Centre of Clinical Trials. In addition, an Independent Data Monitoring Committee specialised in clinical trials and data monitoring, independent from the sponsors and clinical investigators, did an interim data analysis of safety, reactogenicity, and early immunogenicity on day 14 post-vaccination in phase 2a. It provided supervision during the whole trial. The final analysis of safety, reactogenicity, and immunogenicity in phases 2a and phase 2b were done by the statistician responsible for the design and statistical analysis. All participants were followed up on day 14 (interim analysis, phase 2a), and on day 28 for final analysis (both trial phases).
During recruitment, investigators provided potential participants with extensive oral and written information. All questions were clarified. The decision to participate in the study was completely voluntary and non-remunerated. Written informed consent was obtained from all participants. During the study, the committees assessed the trial's risk:benefit ratio and assured the rights, health, and privacy of volunteers, including information confidentiality.
Vaccine antigen, SARS-CoV-2 RBD (sequence 319–541 amino acid residues with a poly-histidine fusion tag at its C-terminus), was expressed in Chinese hamster ovary cells. RBD was dimerised through a Cys538-Cys538 interchain disulphide bridge. The composition per dose (0·5 mL) of FINLAY-FR-1A vaccine was: d-RBD 50 μg, NaCl 4·250 mg, Na2HPO4 0·03 mg, NaH2PO4 0·02 mg, thiomersal 0·05 mg, injection water, aluminium hydroxide gel 1·25 mg, pH 6·0–7·2. The control group was injected with vaccine excipient. Vaccine and placebo were manufactured according to Good Manufacturing Practice by the Finlay Vaccine Institute and the Centre of Molecular Immunology in Havana, Cuba. The study protocol is included in the appendix.
Randomisation and masking
After medical screening of volunteers with history of COVID-19, eligible participants aged 19–78 years old were recruited. Participants aged 60–78 years were included in the open, single-group, phase 2a. They were randomly selected among this age subgroup in the recruited population. In phase 2b, participants were randomly allocated 4:1 to two groups: experimental (vaccine) and control (placebo). Stratified random masked sampling proportionally divided participants into two age subgroups: 19–59 years and 60–78 years to ensure a representation of each age subgroup according to national reports of COVID-19 age incidence. Allocation of participants in each group was done by simple random masked sampling by means of centralised technology. Each participant received an identification code, which matched the vial label code. Study participants were enrolled by the research team. The research product management specialist generated the random allocation sequence and assigned participants to interventions.
All study staff, investigators, sponsors, and participants, remained masked until the conclusion of the study (28 days after the vaccine was applied to all volunteers). All vials had the same characteristics: R2 vial, single dose, volume, and pink cap.
Procedures
All participants received a single deltoid intramuscular injection (0·5 mL) of the vaccine or placebo. Volunteers were closely observed for 1 hour post-vaccination. After vaccination, active surveillance by health-care professionals was carried out on days 1, 2, 3, 7, and 28, plus day 14 in phase 2a. Participants were instructed to complete a diary record of solicited local and systemic adverse reactions during the 28 days follow-up period.
Solicited and protocol-defined local site reactions (injection site pain, warmth, redness, swelling, and induration) and systemic symptoms (general malaise, rash, and fever defined as an axillary temperature ≥38°C) were recorded for 7 days. All other events were recorded throughout the 28 days follow-up period. The intensity of expected and protocol-defined local and systemic adverse events were graded as mild, moderate, and severe, according to Brighton Collaboration definition and the Common Terminology Criteria for Adverse Events version 5.0. Intensities of unsolicited adverse events were graded as mild (transient or mild discomfort, no interference with activity), moderate (mild to moderate limitation in activity), or severe (marked limitation in activity).17, 18 All adverse events were reviewed for causality, and events were classified according to WHO: inconsistent causal association to immunisation, consistent causal association to immunisation, indeterminate, unclassifiable.19
Blood samples were taken on days 0 (before vaccination), 14, and 28 in phase 2a, and on days 0 and 28 in phase 2b. Humoral immune response at baseline and following vaccination was evaluated by three methods.
First, with UMELISA SARS-CoV-2 ANTI-RBD (Immunoassay Centre, Havana, Cuba), a commercial quantitative IgG anti-RBD ultra-micro ELISA, based on d-RBD as coating antigen and streptavidin-biotin technology (biotin-conjugated anti-human-γ, streptavidin-alkaline phosphatase conjugate, and 4-methylumbelliferyl phosphate as fluorometric substrate). A standard curve from 0 to 64 units (U)/mL was used for the quantitative determination of IgG anti-RBD. The IgG anti-RBD concentration was established by interpolating the fluorescence of serum samples in the standard curve constructed by means of ultramicroanalytic (SUMA) software.20 Seroconversion rates for IgG anti-RBD antibodies (≥4-times increase in antibody titres over preimmunisation titres) were calculated for all participants.
Second, with a SARS-CoV-2 neutralising antibody ELISA, which is based on antibody-mediated blockage of RBD:hACE2 interaction, and can be considered an in-vitro surrogate of the live-virus neutralisation test. It uses recombinant RBD-mouse-Fc (RBD-Fcm) and the host cell receptor hACE2-Fc (ACE2-Fch) as coating antigen. Human antibodies against RBD can block the interaction of RBD-Fcm with ACE2-Fch. The RBD-Fcm that was not inhibited can bind to ACE2-Fch, and it is recognised by a monoclonal antibody anti-γ murine conjugated to alkaline phosphatase. The inhibition ratio of RBD:hACE2 interaction at a serum dilution of 1:100 and the half-maximal surrogate virus neutralisation titres (sVNT50) were calculated; sVNT50 is the serum dilution inhibiting 50% of RBD:hACE2 interaction.15, 21 A successful immune response was considered if sVNT50 was at least 250; a value six times higher than the geometric mean of sVNT50 of the Cuban Convalescent Serum Panel (CCSP)15 and four times higher than the upper limit of the 95% CI, and correlating with live-virus neutralisation titres higher than 80. All participants were evaluated with this neutralising antibody ELISA.
And third, with the conventional live-virus neutralisation test, an assay that is the gold standard for establishing antibody efficacy against SARS-CoV-2. It is a colorimetric assay based on antibody neutralisation of SARS-CoV-2 cytopathic effect on Vero E6 cells.15, 21 The viral neutralisation titres (cVNT) against the D614G variant were assessed in all phase 2a participants and a subsample of 10% in phase 2b, randomly selected from participants with a successful immune response. Among them, ten samples were selected by simple random sampling and evaluated against alpha, beta, and delta VOCs in the Hospital Amedeo di Savoia, Turin, Italy.
The vaccine-elicited humoral immune response was compared with that of the CCSP,15 composed of 68 serum samples from asymptomatic individuals (25), and those recovered from mild or moderate (30) and serious (13) COVID-19.15 This panel was previously characterised by ELISA, in-vitro inhibitory assay, and live-virus neutralisation test.15
Outcomes
Full details of outcomes are available in the appendix (p 4). The primary outcome for phase 2a was safety, measured by the occurrence of serious adverse events over 28 days after vaccination; and for phase 2b, immunogenicity, evaluated by the successful immune response [sVNT50 ≥250]). It was assessed on days 0, 14, and 28 in phase 2a, and on days 0 and 28 in phase 2b.
Clinical laboratory tests done on day 14 were compared with prevaccination values.
The secondary outcomes were reactogenicity and immunogenicity. Reactogenicity was assessed by the occurrence of solicited and protocol-defined local and systemic reactions, daily for 7 days after vaccination, as well as unsolicited adverse events, daily for 28 days after vaccination. Vaccine immunogenicity was estimated after vaccination and compared with baseline. The IgG anti-RBD ELISA and the SARS-CoV-2 neutralising antibody ELISA were done on days 0, 14 and 28 in phase 2a, and on days 0 and 28 in phase 2b. Seroconversion rates and the inhibition ratio of RBD:hACE2 interaction were respectively estimated. The conventional live-virus neutralisation test was done on samples collected in both phases on days 0 and 28.
Statistical analysis
Calculation of the sample size for phase 2a was based on a serious adverse events rate lower than 5%. Two-sided 95% CIs for one proportion were calculated, with a precision (target width) of 0·250. In phase 2b, the calculation of the sample size was based on a successful immune response of 50%; a lower limit of the CI for the difference with respect to the control greater than 30%, and a randomisation ratio of 4:1. Two-sided 95% CIs for the difference between two proportions with a target width of 0·200 were calculated. Finally, 5% of the sample was added considering possible study withdrawals.
Safety and reactogenicity endpoints were described as frequencies (%). The following values were reported: mean, SD, median, IQR, and range for the demographic characteristics and adverse events; median, 25th–75th percentile, geometric mean titres (GMT) and 95% CIs for immunological endpoints. Seroconversion rates for IgG anti-RBD antibodies were calculated.
Spearman's rank correlation was used to assess relationships between techniques used to evaluate the immune response. Student's t test or the Mann-Whitney U test were used for before–after statistical comparison.
The assumption of normal distribution was checked by Kolmogorov-Smirnov test.
A stepwise logistic regression model was used to assess the influence of covariates on the successful immune response. A χ2 test was used to establish the association between two variables: the successful immune response induced by vaccination and independent variables (sex, race, age group, COVID-19 classification, hospital discharge time, and inhibitory antibodies prevaccination), and between treatment and solicited adverse events.
A likelihood ratio—Bayes factor—was used to carry out the risk–benefit analysis. Benefit was measured by the proportion of participants with successful immune response induced by vaccination; risks were calculated by the serious and severe adverse events associated with the vaccine (appendix p 13).
All randomly assigned participants were included in the safety analysis (safety population), and immunogenicity was evaluated in participants without study interruptions ie excluding patients who withdrew or did not have serum samples available for analysis (per-protocol population).
Statistical analyses were done by means of SPSS version 25.0; EPIDAT version 4.1, Prism GraphPad version 6.0. A type I error of 0·05 was used. The study was registered at the Cuban Public Registry of Clinical Trials, RPCEC00000366-En, and is included in the WHO International Clinical Registry Trials Platform.
Role of the funding source
The funder of the study had a role in study design, data analysis, data interpretation, and writing of the report.
Results
From April 9, 2021, to April 17, 2021, 663 COVID-19 convalescent participants were enrolled in the study; 213 participants were excluded for not meeting selection criteria and 450 volunteers were recruited. 20 participants aged 60–78 years were allocated into the open, non-controlled phase 2a, and received a single dose of FINLAY-FR-1A vaccine. Serious adverse events were not found, and successful immune response (sVNT50 ≥250) was found in 19 (95%) of 20 participants; therefore, inclusion of this age group in phase 2b was approved.
430 participants aged 19–78 years were randomly assigned 4:1 to the experimental (n=344) or control groups (n=86) in phase 2b and received a single dose of the vaccine or placebo respectively. There were three voluntary dropouts in the experimental group. Immunological results of eight participants—three in the experimental group and five in the control group—could not be obtained; they could not be repeated, as not enough serum was available. All randomly assigned participants were included in the safety analysis (safety population), and the immunogenicity was evaluated in participants without study interruptions (per-protocol population; figure 1 ). The study ended on June 14, 2021.
Figure 1.
Trial profile
The study was done sequentially in two stages, phase 2a, open, non-controlled; phase 2b, randomised, placebo-controlled, and double-blind..
Table 1 summarises the demographic and baseline characteristics of the participants. There were no differences between the experimental and control groups. The mean time from hospital discharge to vaccination was 4·5 months (SD 3·3) in the experimental group, and 4·8 months (SD 3·9) in the control group. Mild COVID-19 predominated in both groups.
Table 1.
Baseline characteristics from the phase 2a and 2b studies
| FINLAY-FR-1A vaccine group (n=364)* | Vaccine excipient group (n=86) | ||
|---|---|---|---|
| Sex | |||
| Female | 204 (56%) | 47 (55%) | |
| Male | 160 (44%) | 39 (45%) | |
| Race | |||
| White | 224 (62%) | 55 (64%) | |
| Black | 54 (15%) | 11 (13%) | |
| Mixed race | 85 (23%) | 20 (23%) | |
| East Asian | 1 (<1%) | 0 | |
| Age, years | |||
| Mean (SD) | 46·0 (14·3) | 45·0 (14·3) | |
| Median (IQR) | 49·0 (24·0) | 45·0 (23·0) | |
| Range | 19–78 | 21–78 | |
| 19–59 years age group | 305 (84%) | 77 (90%) | |
| 60–78 years age group | 59 (16%) | 9 (10%) | |
| Weight, kg | |||
| Mean (SD) | 74·5 (15·0) | 73·7 (14·6) | |
| Median (IQR) | 74·0 (21·0) | 73·0 (21·1) | |
| Range | 44·0–130·0 | 44·0–105·0 | |
| Height, cm | |||
| Mean (SD) | 166·0 (9·0) | 165·6 (10·0) | |
| Median (IQR) | 165·0 (12·0) | 166·0 (1·3) | |
| Range | 147–198 | 145–190 | |
| Body-mass index, kg/m2 | |||
| Mean (SD) | 26·9 (4·3) | 26·8 (4·2) | |
| Median (IQR) | 27·0 (6·5) | 27·0 (6·4) | |
| Range | 18·4–35·3 | 18·3–34·7 | |
| Time from hospital discharge with negativeCOVID-19 PCR test to vaccination, months | |||
| Mean (SD) | 4·5 (3·3) | 4·8 (3·9) | |
| Median (IQR) | 3·1 (1·3) | 3·0 (1·4) | |
| Range | 1·8–15·9 | 2·0–15·5 | |
| COVID-19 classification | |||
| Asymptomatic | 85 (23%) | 25 (29%) | |
| Mild | 245 (67%) | 38 (44%) | |
| Moderate | 34 (9%) | 23 (27%) | |
Data are n (%) unless stated otherwise.
n=364 comprises 20 participants from the phase 2a study and 344 participants from the phase 2b study.
In participants who received the experimental vaccine, site pain was the most frequent (105 [29%] of 364) vaccine-associated adverse event, followed by swelling (16 [4%] of 364); the main solicited systemic reactions were general malaise (24 [7%] of 364) and headache (15 [4%] of 364; table 2 ). The frequency of local and systemic reactions was higher during the first 24 h after vaccination in both groups; they generally disappeared within the first 3 days (appendix p 6).
Table 2.
Frequency of treatment-associated adverse events in the phase 2a and 2b studies
| FINLAY-FR-1A vaccine group (n=364)* | Vaccine excipient group (n=86) | ||
|---|---|---|---|
| Subjects with TAAEs | 117 (32%) | 18 (21%) | |
| Subjects with serious TAAEs | 0 | 0 | |
| Subjects with severe TAAEs | 1 (<1%)* | 0 | |
| Solicited local TAAEs | |||
| Site pain | 105 (29%) | 13 (15%) | |
| Swelling | 16 (4%) | 4 (5%) | |
| Local heat | 14 (4%) | 0 | |
| Induration | 11 (3%) | 1 (1%) | |
| Redness | 8 (2%) | 0 | |
| Solicited systemic TAAEs | |||
| General malaise | 24 (7%) | 7 (8%) | |
| Headache | 15 (4%) | 1 (1%) | |
| Somnolence | 8 (2%) | 1 (1%) | |
| Fever | 2 (1%) | 1 (1%) | |
| Limitation of activity | 0 | 1 (1%) | |
| Unsolicited systemic TAAEs | |||
| Dizziness | 1 (<1%) | 0 | |
| Diarrhoea | 1 (<1%) | 0 | |
| Asthenia | 0 | 1 (1%) | |
| Nasal discharge | 1 (<1%) | 1 (1%) | |
| Fatigue | 1 (<1%) | 0 | |
| Cough | 1 (<1%) | 0 | |
| Dyspnoea | 1 (<1%) | 0 | |
| Bilateral conjunctival injection | 1 (<1%) | 0 | |
| Chills | 1 (<1%) | 0 | |
| Number of TAAEs per subject | |||
| Mean (SD) | 0·6 (1·0) | 0·4 (0·8) | |
| Median (IQR) | 0 | 0 | |
| Range | 0–5 | 0–4 | |
Data are n (%) unless stated otherwise. TAAE=treatment-associated adverse event.
n=364 comprises 20 participants from the phase 2a study and 344 participants from the phase 2b study.
1 participant experienced headache that impeded activities.
A significant association was detected between experimental treatment and the occurrence of solicited adverse events (calculated with data in table 2; p=0·041, where pain at the injection site was highly predominant (p<0·01). No association was shown between treatment (vaccine or placebo) and the other adverse events.
Serious vaccine-associated adverse events were not found. The intensity of the solicited adverse events was generally mild; only one individual (<1%) reported a severe adverse vaccine associated event (headache) but recovered within 1 h after vaccination (table 2). Five participants in the experimental group (1%) had moderate vaccine associated adverse events: local pain at the vaccination site (3), general malaise (1) and headache (1). Unsolicited adverse events were predominantly mild and resolved spontaneously during the follow-up period. Abnormal laboratory parameters related to vaccination were not found for the phase 2a study (appendix p 7).
Successful immune response was present in 289 (81%) of 358 participants immunised with FINLAY-FR-1A, versus only four (5%) of 81 in the control group (p<0·0001) and nine (13%) of 68 in the CCSP (p<0·0001) (table 3 ). High titres of sVNT50 were detected on day 28 post-vaccination: the GMT of sVNT50 on day 28 represented a 21-times increase over the CCSP value (p<0·0001), a 51-times increase over the pre-vaccination value (p<0·0001) and a 45-times increase over the control group (for all comparisons; figure 2 ). The sVNT50 of at least 250 was used to define successful immune response for the primary endpoint. Most non-responders (69) had a history of asymptomatic COVID-19 42 (61%) and 27 (39%) had a history of mild disease
Table 3.
Humoral immune response induced by a single dose of FINLAY-FR-1A vaccine
|
FINLAY-FR-1A vaccine group (n=358) |
Vaccine excipient group (n=81) |
Cuban Convalescent Serum Panel (n=68) | |||
|---|---|---|---|---|---|
| Prevaccination | 28 days post-vaccination | Prevaccination | 28 days post-vaccination | ||
| Anti-RBD IgG U/mL | |||||
| Median | 9·7 | 301·0 | 10·2 | 6·6 | 50·8 |
| 25–75 percentile | 3·0–28·8 | 103·0–819·2 | 2·5–25·7 | 1·9–17·1 | 23·8–94·0 |
| Anti-RBD IgG seroconversion | |||||
| n (%) | NA | 302 (84%) | NA | 0 | NA |
| 95% CI | NA | 80–88 | NA | 0–1 | NA |
| RBD:hACE2 inhibition % | |||||
| Median | 11 | 94 | 12 | 13 | 32 |
| 25–75 percentile | 4–27 | 89–95 | 5–26 | 6–22 | 17–62 |
| sVNT50 | |||||
| GMT | 17·4 | 884·0 | 20·1 | 19·6 | 41·8 |
| 95% CI | 15·0–20·1 | 682·1–1145·7 | 14·8–27·4 | 13·3–28·8 | 27·7–63·2 |
| sVNT50≥250 | |||||
| n (%) | 13 (4%) | 289 (81)% | 6 (7%) | 4 (5%) | 9 (13%) |
| 95% CI | 2–6 | 76–85 | 3–15 | 1–12 | 6–24 |
| cVNT* | |||||
| GMT | 15·4 | 400·3 | NA | NA | 46·4 |
| 95% CI | 10·3–23·2 | 272·4–588·1 | NA | NA | 31·5–68·4 |
RBD=receptor binding domain.
Evaluated in 57 participants. Anti-RBD IgG seroconversion=≥4-times increase in antibody titres over pre-immunisation titres. RBD:hACE2 inhibition %=RBD:hACE2 inhibition % at a dilution 1/100. sVNT50=serum dilution inhibiting 50% of RBD:hACE2 interaction. sVNT50 ≥250=successful immune response. cVNT=conventional live-virus neutralisation titre. GMT=geometric mean titre. NA=not applicable. RBD=receptor binding domain. sVNT50=half-maximal surrogate virus neutralisation titre.
Figure 2.
Half-maximal surrogate virus neutralisation titre
sVNT50 is the reciprocal serum dilution giving 50% inhibition of RBD:hACE2 interaction, measured by competitive ELISA at days 0 (prevaccination) and 28. CCSP=Cuban Convalescent Serum Panel. A successful immune response was found in 81% of participants (p<0.0001) Horizontal lines between error bars=geometric mean titre. Error bars=95% CI.
A significant increase in RBD antibodies was detected after vaccination (median: 301·0 U/mL [103·0–819·2] 28 days post-vaccination), such that the median value was six-times higher than that of CCSP15 (50·8 U/mL [IQR 23·8–94·0]), 31-times higher than the prevaccination concentration (9·7 U/mL [3·0–28·8]), and 46-times higher than the control group [6·6 U/mL [1·9–17·1]) (p<0·0001 for all comparisons). Anti-RBD IgG seroconversion occurred in 302 (84%) of 358 participants 28 days after the experimental vaccine (table 3; appendix p 9).
We measured the inhibition ratio of RBD:hACE2 interaction at a serum dilution of 1:100. On day 28 after FINLAY-FR-1A vaccination, the concentrations of inhibitory antibodies were significantly higher than their pre-vaccination titres (p<0·0001). The median of inhibitory antibody titres (94% [IQR 89–95]) was three times greater than that of the CCSP (32% [17–62]; p<0·0001) and seven times greater than that of the control group (13% [6–22]; p<0·0001; table 3; appendix p 10).
We found an association between successful immune response and disease classification, as well as with time elapsed after hospital discharge. A significantly higher number of vaccinated participants with a successful immune response was found in moderate COVID-19 cases and in those with more than 4 months after hospital discharge (p<0·0001 for all comparisons). No association with a successful immune response was found with sex, race, age, and RBD:hACE2 inhibition rate before vaccination (p>0·05; appendix pp 8, 10).
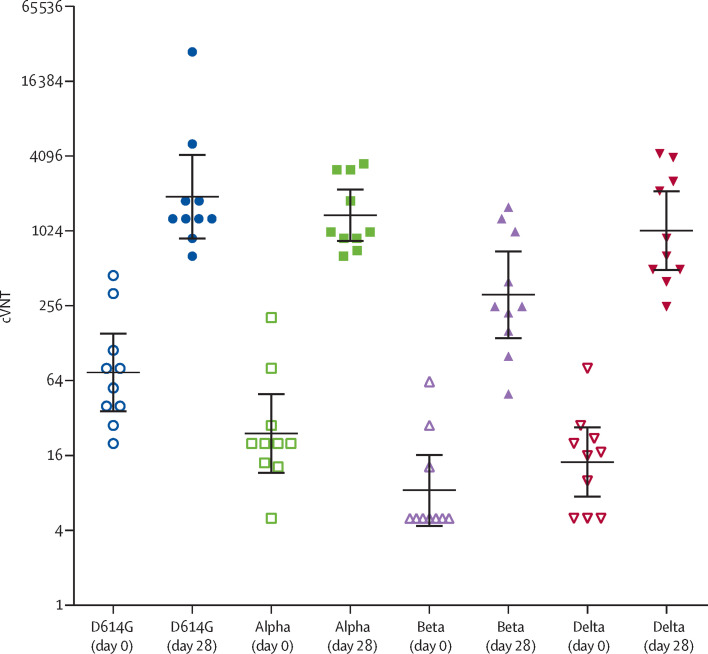
The conventional live-virus neutralisation test was evaluated in 57 participants: all participants of phase 2a and 37 participants of phase 2b. Most individuals (47 [82%] of 57) achieved cVNT greater than 160. The GMT was 400·3 (95% CI 272·4–588·1), this represents a nine-times increase over the CCSP and it was 26-times higher than prevaccination titres (p<0·0001; table 3; appendix p 11). The vaccine induced neutralising antibodies against the alpha, beta and delta variants of the virus (figure 3 ; appendix p 9).
Figure 3.
Titres of neutralising antibodies against four SARS-CoV-2 variants of concern at days 0 (prevaccination) and 28 (post-vaccination)
cVNT=conventional live-virus neutralisation titre. Horizontal lines between error bars=geometric mean titre. Error bars=95% CI.
There was a good correlation of cVNT with other variables (coefficients greater than 0·7), except with RBD:hACE2 inhibition at a dilution of 1:100. The sVNT50 and cVNT achieved the strongest correlation coefficient: 0·889; the correlation was 0·826 for cVNT and anti-RBD IgG concentration. Also, a strong correlation was found between sVNT50 and anti-RBD IgG concentration in 358 participants (0·934; appendix p 12).
The risk–benefit analysis showed strong evidence in favour of benefit. The odds were greater than 200, indicating that the probability of benefit is greater than the probability of risk (appendix pp 13, 14).
Discussion
FINLAY-FR-1A is a safe, effective, and immunogenic vaccine. No serious vaccine-associated adverse events were reported. Minor adverse events, especially local pain, were the most common. A strong booster immune response was also shown. Most individuals had a significant increase in functional antibodies, including live-virus neutralising activity.
We selected a convalescent serum panel representative of the various clinical manifestations of COVID-19 to evaluate vaccine-induced immune response versus disease-acquired immunity, taking into account that during convalescence, specific IgG antibodies contribute to immunological protection against SARS-CoV-2. Our results were superior to those of the convalescent serum panel, supporting vaccination in COVID-19 convalescent individuals.
COVID-19 vaccines have been designed by means of several platforms: mRNA vaccines and viral vector vaccines are very immunogenic; however, there is concern regarding their reactogenicity.13, 22, 23 The inactivated SARS-CoV-2 vaccines are less immunogenic, and concerns about their reactogenicity have been also reported.13, 24 Vaccines based on recombinant spike protein vaccines are also less immunogenic but provoke fewer adverse reactions.13, 15, 25
FINLAY-FR-1A vaccine (Soberana Plus) is based on recombinant d-RBD on aluminium hydroxide gel. It has been used as the third dose of a heterologous schedule in naive individuals, after two first doses of FINLAY-FR-2 (Soberana 02), a vaccine based on monomeric RBD units conjugated to tetanus toxoid as carrier protein.26 After successful clinical trials, the Cuban National Regulatory Agency issued an emergency use authorisation for this vaccination schedule in adults and children aged at least 2 years. FINLAY-FR-1A has also been studied as the third dose of a heterologous schedule in conjunction with the FINLAY-FR-1 (Soberana 01) vaccine, which is based on d-RBD adjuvanted with outer membrane vesicles of Neisseria meningitidis group B,14 (a vaccination schedule now under consideration by regulatory authorities). FINLAY-FR-1A has also been used as a booster dose after prime-vaccination, and it has been studied for the protection of COVID-19 convalescent individuals against emerging SARS-CoV-2 variants.15
A key concern is the safety and reactogenicity of vaccines used in COVID-19 convalescents. A single dose of mRNA vaccines in SARS-CoV-2 seropositive individuals elicits a very rapid immune response, but there is an increase in adverse events. One study reported that 73% of US health-care workers previously infected with SARS-CoV-2 had at least one adverse event.27 In another study, adverse events were 89% more frequent in vaccinees with pre-existing immunity than in naive participants.28, 29 this might be owing to a hypersensitivity reaction mediated by deposition of antigen-antibody immune complexes in tissues, which trigger an inflammatory reaction involving complement and leukocytes.
Here, only 32% of immunised individuals reported vaccine-associated adverse events, predominantly local and mild events. Serious vaccine-associated adverse events were not detected.
This evaluation was carried out in a fragile population, people who recently suffered from COVID-19, some with chronic disease, instead of in healthy naive volunteers—as is usual in clinical trials. The low rate of adverse events and the absence of serious events confirmed the safety of the experimental vaccine. We found fewer vaccine-associated adverse events than those reported in other studies.22, 23, 24, 30, 31
A 31-times increase in anti-RBD IgG was detected over the pre-vaccination concentration. A similar finding was reported in other studies, proving stimulation of a secondary antibody response.23, 27, 28, 32, 33, 34 Seroconversion was 84%, slightly higher than that found in phase 1 (80%).15
Functional antibodies blocking RBD:hACE2 interaction were assessed in an in-vitro surrogate assay of the conventional live-virus neutralisation test. The median inhibition value was 94%, the same value that we obtained in the phase 1 clinical trial done in COVID-19 convalescents.15
The successful immune response was defined as the half-maximal sVNT50 of at least 250, assessed 28 days post-vaccination. This assay showed the best correlation with the live-virus neutralisation test in the phase 1 clinical trial,15 and here (0·889). The correlation between both tests has been verified, suggesting that this in-vitro test could replace the complex live-virus neutralisation test.
The GMT of sVNT50 on day 28 was notably higher than the prevaccination value, the control group, and CCSP values, showing the strong secondary immune response induced by FINLAY-FR-1A vaccine. Most participants reached inhibitory antibody titres; 81% achieved a successful immune response.
The conventional live-virus virus neutralisation test is considered the gold standard to evaluate neutralising antibodies against SARS-CoV-2; a 26-times increase over baseline titres evidences the efficacy of this vaccine in producing protective functional antibodies. Most individuals (82%) achieved cVNT greater than 160, a value considered indicative of protection, similar to that of the phase 1 clinical trial,15 and higher than the reported in other clinical trials.24, 30, 31
A live-virus neutralisation test against the D614G variant was done on a subset of 57 participants. This variant was selected because it was the main circulating variant in the first two waves when participants in this study were infected.14, 15 As expected, most participants had neutralising antibodies before vaccination, which substantially increased post-vaccination, showing stimulation of memory B cells. A subsample of ten participants was further studied at the Hospital Amedeo di Savoia in Italy, with the inclusion of alpha, beta, and delta VOCs (appendix p 9). The omicron variant was not evaluated because it had not yet emerged at the time of the test. The alpha variant, initially reported in the UK, has been associated with severe disease and mortality. The beta variant, first documented in South Africa, has been associated with increases in hospitalisations and deaths, owing to its ability to evade the vaccine-induced antibody response. In Cuba, this variant predominated during the first months of 2021. Delta emerged in India and is characterised by its ability to spread more easily. It is the predominant variant in Cuba and worldwide, along with the rapidly spreading omicron variant.1, 7, 8, 9, 10, 26
The immunological protection provided by COVID-19 vaccines or natural infection is being intensively studied.7, 8, 9, 10 Although some studies reported natural protective immunity induced by SARS-CoV-2, reinfections have been reported in recovered participants,5, 7, 8, 9, 10, 11, 32, 35 which seem to increase with the emergence of new VOCs.
As expected, low concentrations of neutralising antibodies against alpha, beta, and delta VOCs were found before vaccination, especially the beta and delta VOCs, which increased considerably post-vaccination. Neutralising antibodies against conserved epitopes could explain the large protective immune response against mutated SARS-CoV-2 variants induced by a single dose of FINLAY-FR-1A.
More convalescent participants achieved a successful immune response when vaccinated beyond 4 months after hospital discharge with a negative PCR test, which could be related to lower concentrations of RBD inhibitory antibodies that would prevent clearance of the vaccine antigen. However, there is no statistical evidence of association between RBD:hACE2 inhibitory antibodies detected before vaccination and a successful immune response (appendix p 8).
95% of phase 2a volunteers achieved a successful immune response after vaccination, compared with 81% when considering both trial phases together; however, this difference is not statistically representative (p=0·60), and no differences were found between the two age subgroups in the full trial (appendix p 8). There is some imbalance concerning the number of participants vaccinated more than 4 months after hospital discharge: eight (40%) of 20 in phase 2a and 75 (23%) 328 considering the full trial. Although the difference is not significant (p=0·071), it might be influencing the results and should be re-evaluated in upcoming clinical trials.
Symptomatic COVID-19 has been related to a stronger immune response compared with asymptomatic individuals,4, 5, 32, 36 and to a higher number of long-term memory B cells; this could explain the association between COVID-19 severity and sVNT50 of at least 250.
Most non-responders were asymptomatic or had a history of very mild COVID-19. Natural immunity probably controlled their disease, with low involvement of the B cell-mediated response and an insufficient generation of memory B cells. However, we cannot rule out effector T-cell activation in these individuals, as shown in the phase 1 study of FINLAY-FR-1A in COVID-19 convalescent individuals.15
This study confirms—now in convalescent participants—the immunogenicity of the FINLAY-FR-1A vaccine. B cells were successfully stimulated 4·5 months on average after hospital discharge, with high concentrations of neutralising antibodies, showing that natural infection leads to the production of long-term memory B cells, and that a single dose induces a strong secondary immune response. Our results are in accordance with those of our phase 1 trial in convalescent individuals,15 as well as with another study, reporting that 1 year after infection, mRNA vaccines increase the immune response against SARS-CoV-2.37
The inclusion of a prime-vaccinated group in the study design would have been interesting for comparing the booster effect in this population with the response achieved in COVID-19 convalescent individuals. Additional studies deserve the finding of higher neutralising antibody titres in the 60–80 years age subgroup; owing to the natural age-related decline of the immune response, this result should be further investigated.
Including COVID-19 convalescent individuals with a history of severe disease should also be considered in further trials to evaluate potential association of the induced immune response with clinical severity of SARS-CoV-2 infection. The inclusion of younger age groups should also be considered in the design of upcoming clinical trials, as well as the evaluation of the omicron variant and future emerging VOCs.
Although there is evidence of memory B-cell stimulation, on the basis of a rapid induction of specific antibodies, we did not examine memory B cells and T cells and specific effector T cells, which should be studied by in vitro techniques.
The efficacy and duration of the immune response elicited after viral infection is still under study. In our view, vaccination of previously infected individuals is necessary to protect them against new circulating variants. FINLAY-FR-1A could be an important tool against COVID-19, especially to strengthen pre-existing immunity secondary to infection or vaccination.
Data sharing
Data about adverse events and immune response are shared in the appendix (pp 6, 8, 9). Some information is also available at the Cuban Public Registry of Clinical Trials, included in WHO International Clinical Trials Registry Platform (Soberana Plus). The study protocol is shared in the appendix. The individual immunological documents and safety data as well as other supporting clinical documents will be available after publication of this article. Proposals should be sent to ochoa@finlay.edu.cu or vicente.verez@finlay.edu.cu. These proposals must be reviewed and approved by the sponsor and the investigator. Finally, a data access agreement must be signed.
Declaration of interests
The Finlay Vaccine Institute and the Centre of Molecular Immunology manufacture the vaccine and have filed patent applications related to the vaccine's use in individuals with pre-existing SARS-CoV2 immunity. VV-B, YV-B, DG-R, RO-A, YC-R, BS-R, MD-H, IO-V, CM-A, AC-M, and MR-A are authors of these patent applications. RO-A, YC-R, LR-N, RG-M, YV-B, DG-R, VV-B, BS-R, TH-G, IO-V, and MD-H are researchers of the Centres that manufacture the vaccine. Partial funding for this study was received from Fondo de Ciencia e Innovación (FONCI) of Cuba's Ministry of Science, Technology and Environment (Project-2020-20). The other authors declare no competing interests. No authors received an honorarium for this paper.
Acknowledgments
Acknowledgments
Partial funding for this study was received from Fondo de Ciencia e Innovación (FONCI) of Cuba's Ministry of Science, Technology and Environment (Project-2020-20). The Finlay Vaccine Institute sponsored the study. Researchers of the clinical sites, and other participating institutions were responsible for the clinical trial execution and data collection. They contributed to data analysis and interpretation. We thank the individuals enrolled in the study for their generosity, Lila Castellanos-Serra for reviewing the manuscript.
Contributors
RO-A and AC-M are joint first authors. RO-A, AC-M, CM-A, YC-R, and VV-B contributed equally. AC-M was the principal investigator and RO-A was the co-principal investigator of this trial. RO-A, CM-A, CV-S, YV-B, DG-R, and VV-B conceived the study, designed the trial, the study protocol, and were involved in data analysis and interpretation. YC-R, RO-A and PPG-C supervised and monitored the trial. AC-M, CM-A, MdlAG-G, YJ-B, YT-M, LR-V and LDR-P were responsible for the site work including the recruitment and data collection. They contributed to data analysis and interpretation. LR-N, BS-R, TH-G, IO-V, MD-H, MR-A, EN-R, JE-P, DO-L, IV-Á, AD-F, AP-D, FC, AC, and VG carried out immunological experiments and the analysis of results. AC-M, CV-S, RG-M, and RO-A had access to the raw data. CV-S and RO-A verified the data. CV-S and RG-M were involved in data curation and statistical analysis of data. RO-A and VV-B wrote the manuscript, and all authors provided paper feedback. RO-A has final responsibility for publication.
Supplementary Material
References
- 1.WHO . WHO; Geneva: 2021. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/table [Google Scholar]
- 2.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salazar E, Kuchipudi SV, Christensen PA, et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxiv. 2020 doi: 10.1101/2020.06.08.138990. published online June 9. (preprint). [DOI] [Google Scholar]
- 5.Brouwer PJM, Caniels TG, van der Straten K, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shastri J, Parikh S, Aggarwal V, et al. Severe SARS-CoV-2 Breakthrough Reinfection With Delta Variant After Recovery From Breakthrough Infection by Alpha Variant in a Fully Vaccinated Health Worker. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.737007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirola L. Genetic emergence of B.1.617.2 in COVID-19. New Microbes New Infect. 2021;43 doi: 10.1016/j.nmni.2021.100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra S, Mindermann S, Sharma M, et al. Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . WHO; Geneva: 2021. Tracking SARS-CoV-2 variants.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [Google Scholar]
- 11.Hotez PJ, Corry DB, Strych U, Bottazzi ME. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol. 2020;20:399–400. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Wang W, Chen Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 13.Alturki SO, Alturki SO, Connors J, et al. The 2020 Pandemic: Current SARS-CoV-2 Vaccine Development. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Rodríguez S, de la Caridad Rodríguez-González M, Ochoa-Azze R, et al. A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: Safety, reactogenicity and immunogenicity. Vaccine. 2022;40:2068–2075. doi: 10.1016/j.vaccine.2022.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang-Monteagudo A, Ochoa-Azze R, Climent-Ruiz Y, et al. A single dose of SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with excellent safety profile: an open-label phase 1 clinical trial. Lancet Reg Health Am. 2021 doi: 10.1016/j.lana.2021.100079. published online Sept 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministerio de Salud Pública . MINSAP; La Habana: 2020. Protocolo de actuación nacional para la COVID-19. Versión 1.5. (in Spanish). [Google Scholar]
- 17.Brighton Collaboration . BC; Basel: 2021. Case definitions.https://brightoncollaboration.us/category/pubs-tools/case-definitions/ [Google Scholar]
- 18.US Department of Health and Human Services . HHS; Washington: 2017. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 [Google Scholar]
- 19.WHO . 2nd edn. WHO; Geneva: 2018. Causality assessment of an adverse event following immunization (AEFI)https://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654-eng.pdf [Google Scholar]
- 20.Center for State Control of Medicines and Medical Devices . CECMED; Havana: 2021. UMELISA SARS-CoV-2 anti RBD. Sanitary registry D2107-11.https://www.cecmed.cu/registro/diagnosticadores [Google Scholar]
- 21.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 22.Funk CD, Laferrière C, Ardakani A. Target Product Profile Analysis of COVID-19 Vaccines in Phase III Clinical Trials and Beyond: An Early 2021 Perspective. Viruses. 2021;13:418. doi: 10.3390/v13030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathioudakis AG, Ghrew M, Ustianowski A, et al. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: an international vaccine-recipient survey. medRxiv. 2021 doi: 10.1101/2021.02.26.21252096. published online March 8. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toledo-Romani ME, García-Carmenate M, Valenzuela-Silva C, et al. Efficacy and safety of SOBERANA 02, a COVID conjugate vaccine in heterologous three doses combination. medRxiv. 2021 doi: 10.1101/2021.10.31.21265703. published online Nov 6. (preprint). [DOI] [Google Scholar]
- 27.Saadat S, Rikhtegaran-Tehrani Z, Logue J, et al. Single dose vaccination in healthcare workers previously infected with SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.01.30.21250843. published online Feb 18. (preprint). [DOI] [Google Scholar]
- 28.Krammer F, Srivastava K, the PARIS team. Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv. 2021 doi: 10.1101/2021.01.29.21250653. published online Feb 1. (preprint). [DOI] [Google Scholar]
- 29.Gobbi F, Buonfrate D, Moro L, et al. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses. 2021;13:422. doi: 10.3390/v13030422. https://doi.org/doi:10.3390/v13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samanovic MI, Cornelius AR, Wilson JP, et al. Poor antigen-specific responses to the second BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. medRxiv. 2021 doi: 10.1101/2021.02.07.21251311v1. published online Feb 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badano MN, Sabbione F, Keitelman I, et al. Humoral response to the BBIBP-CorV vaccine over time in healthcare workers with or without exposure to SARS-CoV-2. Mol Immunol. 2022;143:94–99. doi: 10.1016/j.molimm.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi R, Azzolini E, Pozzi C, et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data about adverse events and immune response are shared in the appendix (pp 6, 8, 9). Some information is also available at the Cuban Public Registry of Clinical Trials, included in WHO International Clinical Trials Registry Platform (Soberana Plus). The study protocol is shared in the appendix. The individual immunological documents and safety data as well as other supporting clinical documents will be available after publication of this article. Proposals should be sent to ochoa@finlay.edu.cu or vicente.verez@finlay.edu.cu. These proposals must be reviewed and approved by the sponsor and the investigator. Finally, a data access agreement must be signed.