Abstract
Background
Vaccination of children and young people against SARS-CoV-2 is recommended in some countries. Scarce data have been published on immune responses induced by COVID-19 vaccines in people younger than 18 years compared with the same data that are available in adults.
Methods
COV006 is a phase 2, single-blind, randomised, controlled trial of ChAdOx1 nCoV-19 (AZD1222) in children and adolescents at four trial sites in the UK. Healthy participants aged 6–17 years, who did not have a history of chronic respiratory conditions, laboratory-confirmed COVID-19, or previously received capsular group B meningococcal vaccine (the control), were randomly assigned to four groups (4:1:4:1) to receive two intramuscular doses of 5 × 1010 viral particles of ChAdOx1 nCoV-19 or control, 28 days or 84 days apart. Participants, clinical investigators, and the laboratory team were masked to treatment allocation. Study groups were stratified by age, and participants aged 12–17 years were enrolled before those aged 6–11 years. Due to the restrictions in the use of ChAdOx1 nCoV-19 in people younger than 30 years that were introduced during the study, only participants aged 12–17 years who were randomly assigned to the 28-day interval group had received their vaccinations at the intended interval (day 28). The remaining participants received their second dose at day 112. The primary outcome was assessment of safety and tolerability in the safety population, which included all participants who received at least one dose of the study drug. The secondary outcome was immunogenicity, which was assessed in participants who were seronegative to the nucleocapsid protein at baseline and received both prime and boost vaccine. This study is registered with ISRCTN (15638344).
Findings
Between Feb 15 and April 2, 2021, 262 participants (150 [57%] participants aged 12–17 years and 112 [43%] aged 6–11 years; due to the change in the UK vaccination policy, the study terminated recruitment of the younger age group before the planned number of participants had been enrolled) were randomly assigned to receive vaccination with two doses of either ChAdOx1 nCoV-19 (n=211 [n=105 at day 28 and n=106 at day 84]) or control (n=51 [n=26 at day 28 and n=25 at day 84]). One participant in the ChAdOx1 nCoV-19 day 28 group in the younger age bracket withdrew their consent before receiving a first dose. Of the participants who received ChAdOx1 nCoV-19, 169 (80%) of 210 participants reported at least one solicited local or systemic adverse event up to 7 days following the first dose, and 146 (76%) of 193 participants following the second dose. No serious adverse events related to ChAdOx1 nCoV-19 administration were recorded by the data cutoff date on Oct 28, 2021. Of the participants who received at least one dose of ChAdOx1 nCoV-19, there were 128 unsolicited adverse events up to 28 days after vaccination reported by 83 (40%) of 210 participants. One participant aged 6–11 years receiving ChAdOx1 nCoV-19 reported a grade 4 fever of 40·2°C on day 1 following first vaccination, which resolved within 24 h. Pain and tenderness were the most common local solicited adverse events for all the ChAdOx1 nCoV-19 and capsular group B meningococcal groups following both doses. Of the 242 participants with available serostatus data, 14 (6%) were seropositive at baseline. Serostatus data were not available for 20 (8%) of 262 participants. Among seronegative participants who received ChAdOx1 nCoV-19, anti-SARS-CoV-2 IgG and pseudoneutralising antibody titres at day 28 after the second dose were higher in participants aged 12–17 years with a longer interval between doses (geometric means of 73 371 arbitrary units [AU]/mL [95% CI 58 685–91 733] and 299 half-maximal inhibitory concentration [IC50; 95% CI 230–390]) compared with those aged 12–17 years who received their vaccines 28 days apart (43 280 AU/mL [95% CI 35 852–52 246] and 150 IC50 [95% CI 116–194]). Humoral responses were higher in those aged 6–11 years than in those aged 12–17 years receiving their second dose at the same 112-day interval (geometric mean ratios 1·48 [95% CI 1·07–2·07] for anti-SARS-CoV-2 IgG and 2·96 [1·89–4·62] for pseudoneutralising antibody titres). Cellular responses peaked after a first dose of ChAdOx1 nCoV-19 across all age and interval groups and remained above baseline after a second vaccination.
Interpretation
ChAdOx1 nCoV-19 is well tolerated and immunogenic in children aged 6–17 years, inducing concentrations of antibody that are similar to those associated with high efficacy in phase 3 studies in adults. No safety concerns were raised in this trial.
Funding
AstraZeneca and the UK Department of Health and Social Care through the UK National Institute for Health and Care Research.
Introduction
The COVID-19 vaccine ChAdOx1 nCoV-19 (AZD1222 [Covishield]; AstraZeneca) has been approved for use in more than 180 countries1 following phase 2/3 trials reporting acceptable safety, tolerability, immunogenicity, and efficacy of a two-dose schedule in adults aged 18 years and older.2, 3, 4, 5 In the UK setting, real-world data show 91% efficacy against hospitalisation-associated infection by the delta (B.1.617.2) variant, 74% efficacy against symptomatic infection by the alpha (B.1.1.7) variant, and 67% efficacy against symptomatic infection by the delta variant.6, 7 WHO currently advises that children aged 12 years and older who are at risk of severe COVID-19 be offered BNT162b2 (Pfizer-BioNTech).8 An increasing number of countries have approved COVID-19 vaccination with mRNA vaccines to children aged 5 years and older (such as Canada, Germany, Italy, the UK, and the USA).
ChAdOx1 nCoV-19 has not previously been evaluated in a paediatric population, but chimpanzee adenoviral (ChAd)-vectored malaria vaccines have shown acceptable tolerability profiles in infants and children aged between 10 weeks and 6 years in trials of ChAd63 ME-TRAP.9, 10, 11, 12 The role that children have in the transmission of SARS-CoV-2 in the wider community and the role of COVID-19 vaccination in reducing transmission from children to vulnerable adults remain uncertain,13, 14 but some countries have included transmission reduction in the rationale for vaccine introduction for children. Furthermore, the effect of school exclusions or closures on the education and mental health of children as a result of SARS-CoV-2 infection has also been cited as a reason for paediatric vaccination programmes.15 Although the estimated case fatality rate in children is much lower than it is in adults (approximately 0·0008% in children based on UK data16 compared with 0·05% in adults based on global data17), approximately 4% of children admitted to hospital with SARS-CoV-2 infection could require intensive care admission and intubation in a high-income country setting. Although reporting of global mortality rates from COVID-19 in children is challenging due to varying availability of PCR and serological testing, mortality might be higher in low-income and middle-income countries than in high-income countries.18 The estimated vaccine efficacy of BNT162b2 against hospital admission in adolescents in a US setting is estimated to be 93%.19
Research in context.
Evidence before this study
Eight licensed vaccines to prevent COVID-19 have been approved by WHO for Emergency Use Listing (EUL): seven for use in adults aged 18 years and older, and one for use in adults aged 16 years and older. A further 28 vaccines have been approved for use in at least one country. Peer-reviewed clinical trial data describing safety and immunogenicity of EUL vaccines in children and adolescents have been published for four of these vaccines (CoronaVac [SinoVac], BBIBP-CorV [SinoPharm], BNT162b2 [Pfizer–BioNtech], and mRNA-1273 [Moderna]). Immunobridging studies have shown that two standard adult doses of either BNT162b2 or mRNA-1273 generated neutralising antibody titres in adolescents aged 12–15 years comparable with adults aged 18–25 years and a formal phase 3 study of BNT162b2 in adolescents is now underway. Use of fractional dosing of 10 μg of BNT162b2 in children aged 5–11 years has an acceptable reactogenicity profile and induces neutralising antibody titres comparable with those in adolescents at the age of 12–15 years receiving an adult dose. Similarly, phase 1/2 data of the BBIBP-CorV vaccine supports phase 3 evaluation of the adult regimen (two 4 μg doses, four weeks apart) in children. We searched ClinicalTrials.gov and ISRCTN.com on Feb 22, 2022, for all active studies of WHO-listed COVID-19 vaccines that had enrolled, or were enrolling, participants younger than 16 years. 34 trials were found, of which 17 are evaluating BNT162b2, ten evaluating mRNA-1273, ten evaluating CoronaVac, six evaluating ChAdOx1 nCoV-19, three evaluating Ad26.COV2.S (Janssen), and one evaluating each of BBIBP-CorV, NVX-CoV2373 (Novavax), and Covaxin (Bharat Biotech). Fourteen trials are taking place in Asia, 11 in North America, six in Europe, and five in South America. Trial start dates ranged from April, 2020, to Feb, 2022. Chimpanzee adenoviral (ChAd) vectored vaccines have previously been administered to more than 400 children from infancy to 6 years of age in trials of a malaria vaccine ChAd63 ME-TRAP. More recently, trials of an Ebola vaccine ChAd3-EBO-Z have shown acceptable safety and tolerability profiles in children aged 1–17 years at a dose similar to, or greater than, that of the currently licensed adult dose of ChAdOx1 nCoV-19: 5 X 1010 viral particles.
Added value of this study
We provide the first report of a paediatric clinical study of ChAdOx1 nCoV-19 in 262 children aged 6–17 years. The vaccine was well tolerated when given in a two-dose schedule of 5 X 1010 viral particles at either 28 days or 112 days apart. Adverse events following immunisation were short-lived. No serious adverse events related to vaccine administration occurred. A stronger humoral response was observed when the two doses were administered at the longer interval than the shorter interval and greater responses were seen in participants aged 6–11 years of age than in children aged 12–17 years. Humoral responses measured by anti-spike IgG and virus pseudoneutralisation were of a similar magnitude to those detected in adult studies of ChAdOx1 nCoV-19. Spike-specific T-cell responses were observed after a first dose.
Implications of all the available evidence
There are several active paediatric trials investigating COVID-19 vaccines. This is the first report on ChAdOx1 nCoV-19. Our study shows ChAdOx1 nCoV-19 is well tolerated and generates both cellular and humoral immunity in children aged 6–17 years. No safety concerns were raised in this study. Immunogenicity in those aged 12–17 years increased with a longer dosing interval and immunogenicity was greater in those aged 6–11 years than in children aged 12–17 years. The data support the further evaluation of ChAdOx1 nCoV-19 for use in paediatric populations.
Rarely, young children infected by SARS-CoV-2 might subsequently develop paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C) in the 4–8 weeks following infection.20 As of December, 2021, the US Centers for Disease Control and Prevention has reported 5973 patients meeting MIS-C case definition,21 and mortality rates are estimated to be 1–2%.22 The estimated vaccine efficacy of BNT162b2 against MIS-C in the US setting is currently 91%.23
Given the importance of having multiple options for immunisation of children and adolescents against SARS-CoV-2, we aimed to determine the safety, tolerability, and immunogenicity of ChAdOx1 nCoV-19 in children aged 6–17 years.
Methods
Study design and participants
This phase 2 single-blind, randomised, controlled trial was done at four study sites in the UK (Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, Oxford; University Hospital Southampton NHS Foundation Trust, Southampton; St George's University of London, London; and University Hospitals Bristol and Weston NHS Foundation Trust, Bristol). Healthy participants aged 6–17 years were recruited through social media, websites, and local and national media. All participants, or their parents on their behalf, initially completed an online screening form, and any queries were clarified by telephone conversation before attendance at their first clinic visit. Participants who had a history of chronic respiratory conditions, laboratory-confirmed COVID-19 (either a history of a positive result on a validated test or known seropositivity before enrolment), or previously received capsular group B meningococcal vaccine were excluded (full inclusion and exclusion criteria are in the protocol, which is available on the ISRCTN website).
A detailed medical history was obtained from each participant at the first visit, an examination done if clinically indicated, and written informed consent obtained from participants aged 16 years or older, or from parents or guardians if aged between 6 and 15 years. Written assent was obtained from participants aged 11 years or older. This study was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) and the South Central Berkshire Research Ethics Committee. The protocol and amendments were approved by the UK National Health Service Health Research Authority before implementation. The trial was done in compliance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines.
The trial was designed as an age de-escalation study, and participants aged 12–17 years were enrolled first. After an independent data safety and monitoring committee and the MHRA reviewed 7 days of safety data in this age group, the participants aged 6–11 years were enrolled.
Randomisation and masking
Computer-generated randomisation lists were prepared by the study statisticians (XL and NGM). Participants were randomly assigned (4:1:4:1) to receive a two-dose series of ChAdOx1 nCoV-19 or capsular group B meningococcal vaccine as a control with a 28-day interval, or a two-dose series of ChAdOx1 nCoV-19 or capsular group B meningococcal vaccine with an 84-day interval. An active control was used to maintain masking of participants who had local or systemic side-effects. Randomisation was stratified by age group (6–11 years and 12–17 years) and study site using a block size of 10, chosen to align with the study group sizes. The trial staff administering the vaccine prepared vaccines out of sight of the participants and syringes were covered with an opaque material until ready for administration to ensure masking of participants. Participants were observed for 30 min after vaccination. Clinical investigators involved in endpoint assessment and the laboratory team remained masked to ChAdOx1 nCoV-19 or capsular group B meningococcal vaccine group allocation.
Amendment to the study design
During the recruitment window for participants aged 6–11 years, the UK Government was advised by the Joint Committee on Vaccination and Immunisation that individuals younger than 30 years who had not yet received a first dose of the ChAdOx1 nCoV-19 vaccine should be given an alternative COVID-19 vaccine following safety concerns of vaccine-induced thrombosis and thrombocytopenia syndrome. This recommendation led to the cessation of further recruitment of participants aged 6–11 years, although recruitment targets for participants aged 12–17 years had already been met. Second-dose vaccination was then paused pending an MHRA review. Only participants aged 12–17 years randomly assigned to the 28-day interval groups had received second doses by this time. By April, 2021, further safety data in adults who had received second doses of ChAdOx1 nCoV-19 in the UK became available for review by the MHRA. The MHRA then authorised administration of second vaccine doses to those participants aged 12–17 years randomly assigned to 84-day interval groups and to all those aged 6–11 years. As the originally intended day 84 window for second doses had passed, the second dose of vaccine was given to those participants aged 12–17 years randomly assigned to 84-day interval groups and to all those aged 6–11 years at day 112. Hereafter, 84-day interval refers to the planned interval group to which participants were randomly assigned and 112-day interval to the interval at which participants in this group received the doses.
Procedures
All participants had blood tests taken at baseline to determine the presence of anti-spike IgG and anti-nucleocapsid IgG. The vaccine ChAdOx1 nCoV-19 was supplied by AstraZeneca (Investigational Medicinal Product Dossier, version 1.0; Jan 28, 2021). ChAdOx1 nCoV-19 was administered intramuscularly at a dose of 5 × 1010 viral particles, as per the standard adult dose. Capsular group B meningococcal vaccine was supplied by the UK Health Security Agency (previously known as Public Health England) and administered intramuscularly at a standard dose of 0·5 mL. Participants completed electronic diaries for solicited adverse events for the first 7 days after vaccination and for unsolicited adverse events until 28 days after vaccination. Safety blood samples were taken for a subset of up to 40 participants aged 6–11 years at day 2 and day 7 following the second dose (up to 20 participants per timepoint). Laboratory and clinical adverse events were graded on a scale of 0–4, which is described in the protocol. Study follow-up was 12 months from date of first vaccination.
For participants aged 12–17 years, immunogenicity blood samples were taken at days 0, 28, and 56 in the 28-day interval groups and days 0, 84, 112, and 140 in those assigned to the 84-day interval groups. For participants aged 6–11 years, immunogenicity blood samples were taken at days 0, 84, 112, and 140 in those originally randomly assigned to the 84-day interval group. Because the study design amendments affected blood sampling visits for participants aged 6–11 years who were originally randomly assigned to the 28-day interval groups, immunogenicity blood samples were taken at days 0, 28, 84, and 112 for these participants and only a subset at day 140 to minimise blood sampling.
Baseline and post-vaccination humoral responses were measured via the following assays: a multiplexed electrochemiluminescence immunoassay against both SARS-CoV-2 spike protein and receptor-binding domain done at Pharmaceutical Product Development (PPD) laboratories (Richmond, VA, USA), an in-house standardised total IgG ELISA against trimeric SARS-CoV-2 spike protein, and a SARS-CoV-2 pseudovirus neutralisation assay done by Monogram Biosciences (South San Francisco, CA, USA), as previously described.2, 4 Anti-nucleocapsid IgG status at baseline was done at PPD laboratories (Zaventern, Belgium), by ECLIA (Cobas platform, Roche Diagnostics). Cellular responses at baseline and after vaccination were assessed in freshly isolated peripheral blood mononuclear cells with an ex-vivo interferon-γ (IFNγ) enzyme-linked immunospot (ELISpot [Jenner Institute, Oxford, UK]) assay based on a protocol previously described,2 modified to account for the lower volume of blood collected from participants aged 6–17 years than with adults. In this study, the 253 synthetic peptides (15mers overlapping by ten amino acids) spanning the SARS-CoV-2 spike insert were pooled into four pools, and peptide pools were tested in duplicates. Anti-ChAdOx neutralising antibodies were measured using a secreted embryonic alkaline phosphatase-reporter assay, as previously described.3 All participants receiving the study vaccine ChAdOx1 nCoV-19 will be offered a full two-dose course of the active comparator capsular group B meningococcal vaccine at the end of the study. This vaccine was introduced as an infant vaccination in the UK in 2015 and therefore was not available as a routine vaccination to participants.
Outcomes
The primary objective of this study was to assess the tolerability of the ChAdOx1 nCoV-19 vaccine in children aged 6–17 years, in a two-dose regimen with either a 28-day or 84-day dosing interval. This objective was assessed by recording the occurrence of solicited local and systemic adverse events up to 7 days after each vaccination, unsolicited local and systemic adverse events up to 28 days after each vaccination, serious adverse events throughout the study, and the occurrence of atypical laboratory findings (grade 3 or worse) on days 2 and 7 after the second dose in a subset of up to 20 participants aged 6–11 years. Secondary outcomes were humoral and cellular immunogenicity of ChAdOx1 nCoV-19, assessed by anti-spike pseudoneutralisation and ELISpot assays; and cell analysis by flow cytometry, which will be reported at a later date.
Statistical analysis
There was no formal hypothesis testing in this trial, and the sample size was determined on the basis of practical considerations with regards to site recruitment capacity. With 60 participants in each ChAdOx1 nCoV-19 group, the study provides more than 90% probability of observing at least one participant with an adverse event if the underlying adverse event incidence is 5%.
The population of trial participants included in our safety analysis consisted of randomly assigned participants who received at least one dose of study vaccine. The secondary immunogenicity analyses were done in baseline seronegative (defined by anti-nucleocapsid IgG negativity at the time of first vaccination) participants who received both doses of study vaccine for whom immunogenicity data were available. Both safety and immunogenicity analyses were done according to the actual vaccine schedule received. Frequencies and percentages are presented for safety endpoints, and geometric mean and 95% CIs were reported for immunogenicity endpoints. The effect of the interval between first and second dose of ChAdOx1 nCoV-19 on immunogenicity was evaluated by the geometric mean ratio (GMR). The GMR was calculated as the antilogarithm of the difference between the mean of the log10-transformed titre (to render a normal distribution) in the 112-day interval group and the 28-day interval group as the reference. Similarly, the GMRs and 95% CIs were reported to compare immunogenicity measured in participants aged 6–11 years and 12–17 years in the 112-day interval group. Further sensitivity analyses were done in the ChAdOx1 nCoV-19 groups after excluding participants who had an increase in anti-nucleocapsid IgG (by the multiplexed electrochemiluminescence immunoassay at PPD laboratories) of at least two times, or self-reported COVID-19, between baseline and 28 days after the second dose. To assess the relationship between neutralising antibody titres against the ChAdOx vector before the second dose and immune response at 28 days after the second dose, Pearson correlation coefficients were reported after log transformation. Censored data reported to be below the lower limit of quantification were imputed with a value equal to half of the threshold before transformation. All statistical analyses were done using R (version 4.1.1). An independent data and safety monitoring board provided safety oversight for this trial. This study is registered with ISRCTN (15638344).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. AstraZeneca reviewed the final manuscript.
Results
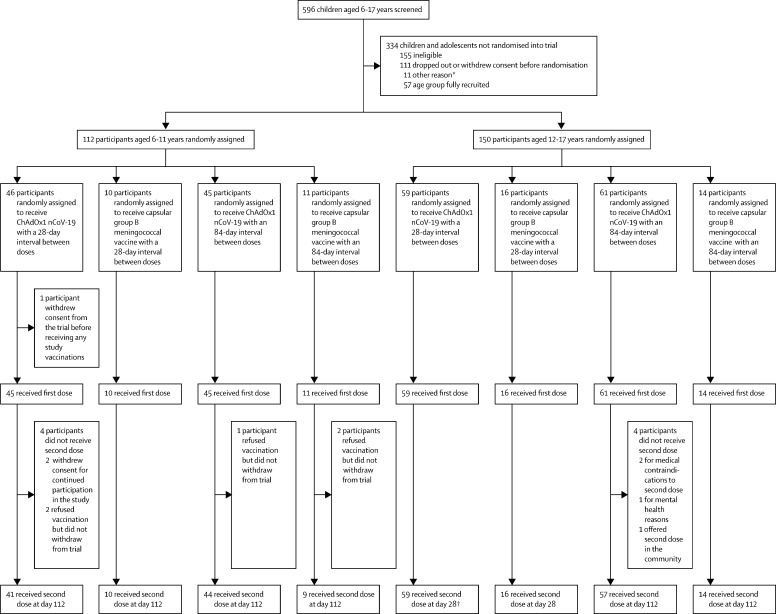
Between Feb 16 and April 2, 2021, 262 participants aged 6–17 years were enrolled into the study and assigned to vaccination with either ChAdOx1 nCoV-19 or capsular group B meningococcal vaccine at a 28-day or 84-day interval. Participants aged 12–17 years were recruited between Feb 16 and Feb 26, 2021, and participants aged 6–11 years were recruited between March 13 and April 2, 2021. Study cohorts included 150 (57%) participants aged 12–17 years and 112 (43%) participants aged 6–11 years. The median age of participants in the 12–17-year age group was 15·1 years (range 12·0–17·7; IQR 13·8–16·6); 74 (49%) participants were girls, and ten (7%) participants were from a minority ethnic background (table 1 ). The 150 older participants were randomly assigned to receive either ChAdOx1 nCoV-19 (59 [39%] participants) at a 28-day interval, capsular group B meningococcal vaccine (16 [11%]) at a 28-day interval, ChAdOx1 nCoV-19 (61 [41%]) at an 84-day interval, or capsular group B meningococcal vaccine (14 [9%]) at an 84-day interval. One participant in the ChAdOx1 nCoV-19 28-day interval group received their second dose 115 days after their first due to temporary exclusion criteria being met and was treated as a 112-day interval participant for the remaining duration of the trial and in the analyses.
Table 1.
Baseline characteristics by study group (4:1:4:1 randomisation)
|
Aged 12–17 years |
Aged 6–11 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1 nCoV-19 28-day interval (n=59) | MenB vaccine 28-day interval (n=16) | ChAdOx1 nCoV-19 84-day interval (n=61) | MenB vaccine 84-day interval (n=14) | ChAdOx1 nCoV-19 28-day interval (n=46) | MenB vaccine 28-day interval (n=10) | ChAdOx1 nCoV-19 84-day interval (n=45) | MenB vaccine 84-day interval (n=11) | ||
| Received 1st dose | 59 (100%) | 16 (100%) | 61 (100%) | 14 (100%) | 45 (98%) | 10 (100%) | 45 (100%) | 11 (100%) | |
| Received 2nd dose | 59 (100%)* | 16 (100%) | 57 (93%) | 14 (100%) | 41 (89%) | 10 (100%) | 44 (98%) | 9 (82%) | |
| Interval between 1st and 2nd dose, days | 31·0 (30·0–32·0) | 30·5 (29·8–32·0) | 116·0 (115·0–130·0) | 117·5 (115·2–131·0) | 117·0 (114·0–119·0) | 119·0 (119·0–144·8) | 117·0 (114·0–119·2) | 117·0 (114·0–119·0) | |
| Sex | |||||||||
| Female | 26 (44%) | 10 (62%) | 27 (44%) | 11 (79%) | 25 (54%) | 5 (50%) | 19 (42%) | 6 (55%) | |
| Male | 33 (56%) | 6 (38%) | 34 (56%) | 3 (21%) | 21 (46%) | 5 (50%) | 26 (58%) | 5 (45%) | |
| Age, years | 15·0 (13·8–16·6) | 14·6 (13·6–16·0) | 15·1 (13·8–16·6) | 15·8 (14·1–16·9) | 9·7 (8·2–10·2) | 9·5 (7·1–10·8) | 8·8 (7·2–10·9) | 9·1 (7·7–9·8) | |
| Ethnicity | |||||||||
| White | 55 (93%) | 13 (81%) | 58 (95%) | 14 (100%) | 39 (85%) | 9 (90%) | 39 (87%) | 9 (82%) | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asian | 2 (3%) | 0 | 1 (2%) | 0 | 1 (2%) | 0 | 0 | 1 (9%) | |
| Mixed | 2 (3%) | 3 (19%) | 2 (3%) | 0 | 6 (13%) | 1 (10%) | 6 (13%) | 1 (9%) | |
| Arab | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Serostatus at baseline | |||||||||
| Seropositive | 4/52 (8%) | 0 | 5/58 (9%) | 1/13 (8%) | 1/41 (2%) | 1/10 (10%) | 2/45 (4%) | 0 | |
| Seronegative | 48/52 (92%) | 14/14 (100%) | 53/58 (91%) | 12/13 (92%) | 40/41 (98%) | 9/10 (90%) | 43/45 (96%) | 9/9 (100%) | |
Data are n (%) or median (IQR). MenB=capsular group B meningococcal.
One participant received their second dose according to the long interval schedule.
Due to the change in the UK vaccination policy, the study terminated recruitment when 112 of the planned 150 participants aged 6–11 years were enrolled. The median age of participants in this age group was 9·3 years (range 6·1–12·0; IQR 7·7–10·6 years); 55 (49%) participants were girls, and 16 (14%) were from a minority ethnic background. Among the 112 participants, 91 (81%) were randomly assigned to receive ChAdOx1 nCoV-19 and 21 (19%) were randomly assigned to receive capsular group B meningococcal vaccine. One participant in the ChAdOx1 nCoV-19 group withdrew consent before receiving their first vaccine. All participants aged 6–11 years received their second dose at a 112-day interval. Nine (5%) participants in the ChAdOx1 nCoV-19 groups did not receive a second dose of vaccine (five declined a second dose, three did not receive a second dose for medical reasons, and one accepted a second dose offered in the community; table 1; figure 1 ). Of those participants with available serostatus at baseline who received ChAdOx1 nCoV-19, three (4%) of 86 participants aged 6–11 years had baseline seropositivity and nine (8%) of 110 participants aged 12–17 years had baseline seropositivity. No dosing errors were reported.
Figure 1.
CONSORT diagram
*One participant had postcode outside of catchment area, ten participants with unknown reason. †One participant who was originally randomly assigned to the short interval ChAdOx1 nCoV-19 group received a delayed second dose (at the long interval second dose timepoint) due to meeting a temporary exclusion criteria.
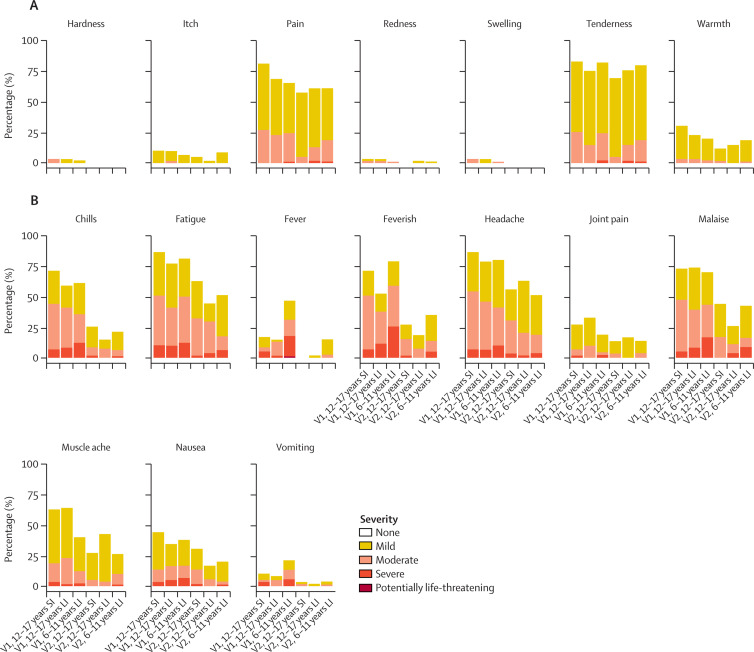
Pain and tenderness were the most common local solicited adverse events for all the ChAdOx1 nCoV-19 and capsular group B meningococcal groups following both doses (appendix pp 14–16). The proportion of participants reporting a moderate or severe local reaction up to day 7 after vaccination was higher in the capsular group B meningococcal groups than in the ChAdOx1 nCoV-19 groups for both first and second doses. Fatigue and headache were the most commonly reported systemic solicited adverse events, and higher proportions of systemic solicited adverse events were reported in the ChAdOx1 nCoV-19 groups than in the capsular group B meningococcal groups and the majority had self-resolved by 48 h after vaccination (appendix pp 14–16). 19 (16%) of 120 participants aged 12–17 years and 42 (47%) of 90 participants aged 6–11 years reported fever (temperature ≥38°C) after receiving the first dose, reducing to 1 (1%) of 113 in the older age group in both interval groups and 12 (15%) of 80 in the younger age group after receipt of the second dose at 112 days (appendix p 1). One participant aged 6–11 years receiving ChAdOx1 nCoV-19 reported a grade 4 fever of 40·2°C on day 1 following first vaccination, which resolved within 24 hours.
Fewer solicited systemic adverse events were reported after the second dose of ChAdOx1 nCoV-19 than after the first dose (figure 2 ), regardless of interval between vaccinations or age group. Paracetamol use in the first 24 h after the second dose of ChAdOx1 nCoV-19 was similar between the 28-day interval groups and the 112-day interval groups in those aged 12–17 years (13 [23%] of 56 vs 13 [26%] of 50) and higher in children aged 6–11 years (36 [39%] of 93).
Figure 2.
Local (A) and systemic (B) solicited adverse events following each dose of ChAdOx1 nCoV-19, by age and interval groups
Data presented are maximum severity across the first 0–7 days following the respective dose. V1=first dose. V2=second dose. SI=short interval (28-day interval). LI=long interval (112-day interval).
61 unsolicited adverse events within 28 days of immunisation with ChAdOx1 nCoV-19 were reported by 41 (34%) of 120 participants aged 12–17 years, and 67 adverse events were reported in 42 (47%) of 90 participants aged 6–11 years (appendix p 3). Among these events, there were eight grade 3 adverse events, with four considered to be possibly, or probably, related to ChAdOx1 nCoV-19 (appendix p 4). Unsolicited adverse events were most commonly reported in the gastrointestinal disorders Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC) after a first dose of ChAdOx1 nCoV-19, and respiratory, thoracic, and mediastinal disorders MedDRA SOC after a second dose (appendix pp 5–7). Serious adverse events and adverse events of special interest were recorded separately from unsolicited adverse events. As of Oct 28, 2021, four adverse events of special interest (besides SARS-CoV-2 infection) were reported, with only one event (mild lymphadenopathy) considered probably related to the study vaccine (appendix p 8). Four serious adverse events were reported with none deemed related to the study vaccine (appendix p 9). The number of self-reported PCR-confirmed or lateral flow assay-confirmed SARS-CoV-2 infections regardless of symptoms was 16 (8%) of 211 in the ChAdOx1 nCoV-19 groups and five (10%) of 51 in the capsular group B meningococcal vaccine groups, none of which required hospital admission (appendix p 10). All infections in the ChAdOx1 nCoV-19 groups occurred after the second dose (ranging from 53 days to 211 days after the second dose) except for one case in which the participant did not receive a second dose and which occurred 216 days after first dose. Of the safety blood cohort in the younger group, one (8%) of 13 participants in the ChAdOx1 nCoV-19 group had a grade 3 D-dimer with normal platelet count at day 2. There were no atypical laboratory findings at day 7 following ChAdOx1 nCoV-19 vaccination (n=15).
Among the baseline seronegative participants, geometric mean concentrations (GMCs) of anti-spike IgG at 28 days after the second dose of ChAdOx1 nCoV-19 were higher in the 112-day interval group than in the 28-day interval group of participants aged 12–17 years (table 2 ). GMCs of anti-spike IgG were highest (108 924 arbitrary units [AU]/mL [95% CI 84 852–139 823]) in those aged 6–11 years, with a GMR of 1·48 (95% CI 1·07–2·07) compared with those aged 12–17 years in the 112-day interval group (table 2).
Table 2.
Immunogenicity at 28 days after the second dose among the seronegative participants with a blood sample taken at this timepoint
|
Aged 12–17 years, 28-day interval, GM |
Aged 12–17 years, 112-day interval, GM |
Aged 6–11 years, 112-day interval, GM |
GMR (95% CI)* | GMR (95% CI)† | ||||
|---|---|---|---|---|---|---|---|---|
| ChAdOx1 nCoV-19 (n=45) | MenB vaccine (n=13) | ChAdOx1 nCoV-19 (n=46) | MenB vaccine (n=10) | ChAdOx1 nCoV-19 (n=48) | MenB vaccine (n=9) | |||
| Anti-spike by PPD (AU/mL) | 43 280 (35 852–52 246); 45 | 73 (33–160); 13 | 73 371 (58 685–91 733); 45 | 227 (17–3072); 10 | 108 924 (84 852–139 823); 48 | 32 (16–61); 9 | 1·70 (1·27–2·26) | 1·48 (1·07–2·07) |
| Anti-spike in-house (EU/mL) | 1194 (908–1568); 45 | 4 (2–10); 13 | 1963 (1575–2448); 45 | 9 (1–106); 10 | 2377 (1773–3185); 48 | 2 (1–2); 9 | 1·64 (1·16–2·33) | 1·21 (0·84–1·74) |
| PseudoNA (IC50) | 150 (116–194); 45 | 20 (20–20); 13 | 299 (230–390); 44 | 57 (11–280); 10 | 885 (614–1275); 48 | 20 (20–20); 9 | 1·99 (1·39–2·86) | 2·96 (1·89–4·62) |
| ELISpot in-house (SFC per 106 PBMCs) | 270 (196–372); 39 | 22 (15–32); 10 | 135 (96–190); 36 | 30 (12–77); 8 | 104 (79–137); 39 | 19 (12–32); 6 | 0·50 (0·31–0·79) | 0·77 (0·50–1·19) |
| Anti-receptor-binding domain by PPD (AU/mL) | 49 764 (41 278–59 995); 45 | 150 (99–228); 13 | 92 520 (73 068–117 151); 45 | 445 (41–4805); 10 | 140 413 (108 182–182 247); n=48 | 102 (102–102); 9 | 1·86 (1·38–2·50) | 1·52 (1·07–2·15) |
Data are GM (95% CI); n or GMR (95% CI). GM=geometric mean. GMR=geometric mean ratio. MenB=capsular group B meningococcal. PPD=Pharmaceutical Product Development laboratories. AU=arbitrary units. EU=ELISA units. IC50=half-maximal inhibitory concentration. SFC=spot forming cell. PBMCs=peripheral blood mononuclear cells.
GMR between participants aged 12–17 years in the ChAdOx1 nCoV-19 long interval group and participants aged 12–17 years in the ChAdOx1 nCoV-19 short interval group.
GMR between participants aged 6–11 years in the ChAdOx1 nCoV-19 group and participants aged 12–17 years in the ChAdOx1 nCoV-19 long interval group.
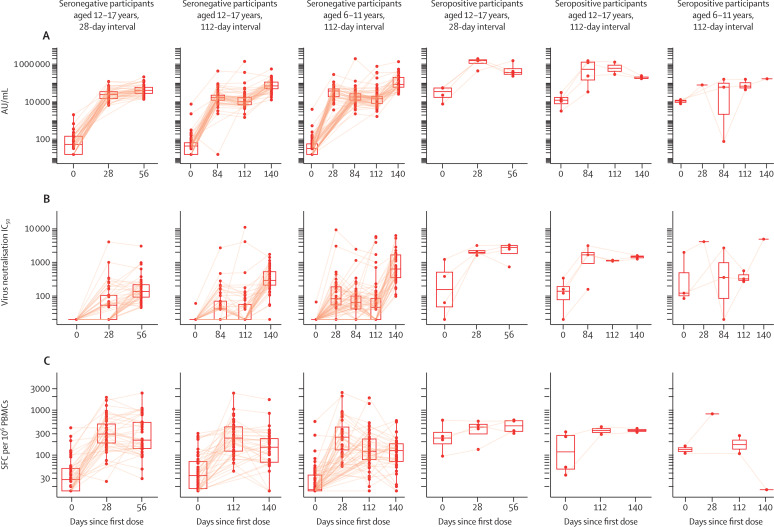
GMCs of anti-spike IgG at day 28 after the first dose of ChAdOx1 nCoV-19 were 24 816 AU/mL (95% CI 20 225–30 451) in participants aged 12–17 years and 32 709 AU/mL (24 381–43 882) in participants aged 6–11 years (appendix p 11). Anti-spike IgG concentrations declined between day 28 after the first dose and the time of the second dose (GMC 16 555 AU/mL [95% CI 12 883–21 273]) in those aged 6–11 years. A decline was also seen in those aged 12–17 years in the 112-day interval group, with a GMC of anti-spike IgG of 15 821 AU/mL (95% CI 10 830–23 113) at day 84 after the first dose and 13 791 AU/mL (9939–19 135) at day 112 (figure 3 A; appendix p 11). A similar kinetic was observed for the anti-receptor-binding domain IgG and in-house anti-spike IgG titres (appendix pp 17–18).
Figure 3.
Immunogenicity kinetics by serostatus among the participants in the ChAdOx1 nCoV-19 groups for anti-spike multiplexed ECL assay by PPD (A), pseudovirus SARS-CoV-2 neutralisation antibody assay by Monogram (B), cellular responses by in-house ELISpot (C)
Boxplots represent the median and IQRs. Each data point is one participant. Solid lines connect samples from the same participant at multiple timepoints. In participants aged 6–11 years, the day 28 samples were taken in half of the participants. AU=arbitrary units. IC50=half-maximal inhibitory concentration. SFC=spot forming cell. PBMCs=peripheral blood mononuclear cells. ECL=electrochemiluminescence. PPD=Pharmaceutical Product Development laboratories.
Similar to anti-spike IgG titres, pseudovirus SARS-CoV-2 neutralisation antibody titres peaked at day 28 after the second dose of ChAdOx1 nCoV-19, with a GMR of 1·99 (95% CI 1·39–2·86) between 112-day interval and 28-day interval groups in participants aged 12–17 years. Pseudovirus SARS-CoV-2 neutralisation antibody titres were higher in those aged 6–11 years than in those aged 12–17 years in the 112-day interval group, with a GMR of 2·96 (95% CI 1·89–4·62). Pseudovirus SARS-CoV-2 neutralisation antibody activity was measured in three participants in the capsular group B meningococcal vaccination groups that was attributed to unreported COVID-19 during the trial (appendix p 19). ChAdOx1 nCoV-19-induced spike-specific cellular responses were detected after a first dose by IFNγ ELISpot in all study vaccine groups. After the first dose, in participants aged 12–17 years, the cellular responses were 302 spot forming cells (SFCs) per 106 peripheral blood mononuclear cells (PBMCs; 95% CI 233–391) at 28 days and 243 SFCs per 106 PBMCs (188–314) at 112 days in the 112-day interval group (figure 3C; appendix p 11). The responses were 267 SFCs per 106 PBMCs (95% CI 189–377) at 28 days and 128 SFCs per 106 PBMCs (95% CI 101–162) at 112 days after the first dose in participants aged 6–11 years. Geometric mean cellular responses remained above baseline levels, but, by 28 days after the second dose, declined to 270 SFCs per 106 PBMCs in the 28-day interval group and 135 SFCs per 106 PBMCs in the 112-day interval groups of those aged 12–17 years (GMR between 112-day and 28-day interval groups: 0·50 [95% CI 0·31–0·79]). In participants aged 6–11 years, the response declined to 104 SFCs per 106 PBMCs (95% CI 79–137) at day 28 after the second dose (table 2; figure 3C; appendix p 11). Humoral and cellular immunogenicity results in the ChAdOx1 nCoV-19 groups were consistent between the primary and sensitivity analyses (appendix p 13).
Both humoral and cellular responses were higher 28 days after the first dose of ChAdOx1 nCoV-19 in the baseline seropositive participants than at 28 days after the second dose of ChAdOx1 nCoV-19 in the seronegative participants (figure 3; appendix p 17). Anti-spike IgG, T-cell, and pseudovirus SARS-CoV-2 neutralisation antibody activity in seropositive participants did not increase significantly after the second dose, and concentrations remained higher than concentrations induced with two doses of ChAdOx1 nCoV-19 in seronegative participants.
Similar titres of anti-ChAdOx neutralising antibody (anti-vector antibody) were observed before the second dose in the participants aged 12–17 years in the 28-day interval group receiving ChAdOx1 nCoV-19 vaccinations (geometric mean 757 [95% CI 541–1059] and 112-day interval group 581 [449–751]; GMR 0·77 [95% CI 0·51–1·16]). The anti-vector antibody titre before the second dose was significantly lower in those aged 6–11 years than in participants aged 12–17 years in the 112-day interval groups (geometric mean 314 [95% CI 258–381]; GMR 0·54 [95% CI 0·39–0·74]). Absolute correlation coefficients between anti-ChAdOx neutralising antibody titres before the second dose and humoral responses induced at 28 days after the second dose in ChAdOx1 nCoV-19 recipients ranged between 0·05 and 0·24 (figure 4 ). The absolute correlation coefficient between anti-vector titres before the second dose and cellular response 28 days after the second dose ranged between 0·01 and 0·37 in all ChAdOx1 nCoV-19 groups.
Figure 4.
Correlation between ChAdOx neutralising antibodies before the second dose and anti-spike IgG by PPD (A), in-house anti-spike IgG (B), pseudovirus SARS-CoV-2 neutralisation antibody titres by Monogram (C), cellular responses by in-house ELISpot (D), and anti-RBD IgG by PPD (E) at 28 days after the second dose by study groups among the baseline seronegative participants receiving ChAdOx1 nCoV-19
Each dot is one participant with paired data and solid lines indicate the linear regression within each interval group, with 95% CIs presented as the shaded area from the unadjusted model. Pearson correlation coefficients are shown with 95% CIs. AU=arbitrary units. IC50=half-maximal inhibitory concentration. SFC=spot forming cell. PBMCs=peripheral blood mononuclear cells. nAb= neutralising antibody. PPD=Pharmaceutical Product Development laboratories. RBD=receptor-binding domain.
Discussion
This is the first reported study of immunisation of children and adolescents with ChAdOx1 nCoV-19 at different intervals and shows that the vaccine is well tolerated and immunogenic in children and adolescents aged 6–17 years when given as a two-dose regimen. Reactogenicity was lower after the second dose than after the first dose regardless of dosing interval, a finding also observed in adult studies.2 Spike-specific cellular immune responses were observed following the first dose. The humoral immunity was greater in the 112-day dosing interval group than the 28-day interval group in participants aged 12–17 years, and greater in younger participants than older participants dosed 112 days apart.
Consistent with previous clinical trials of ChAd-vectored vaccines in children, ChAdOx1 nCoV-19 had an acceptable tolerability profile. Fever rates of 16% in participants aged 12–17 years after a first dose are similar to those reported after routine childhood immunisations and in an adult trial of ChAdOx1 nCoV-19 in which fever was reported in 87 (18%) of 487 participants.2 No serious adverse events or adverse events of special interest related to study vaccine administration occurred. Other trials have also assessed safety and reactogenicity of COVID-19 vaccines in adolescents. A phase 2 trial of BNT162b2 in adolescents reported higher fever rates after a second dose than after a first dose, consistent with adult findings,24 but these events were not observed in our study. Fever rates of 4–5% were reported in a phase 2 trial of CoronaVac in adolescents25 and in 32 (13%) of 251 participants in a phase 2 trial of BBIBP-CorV (Sinopharm).26 However, in our study, fever rates were higher in younger participants aged 6–11 years than those aged 12–17 years who received ChAdOx1 nCoV-19 (42 [47%] of 90), which is consistent with a phase 2/3 study of BNT162b2 in which fever occurred in 4 (100%) of 4 participants aged 5–11 years after the administration of two adult doses.27 Fractional doses of BNT162b2, which have an acceptable reactogenicity profile for younger children, are now recommended for this age group.28
Participants in our trial generated at least as great a magnitude of anti-spike IgG response as adults aged 18–65 years after two doses of ChAdOx1 nCoV-19 given at an 8–12-week interval. However, there were insufficient data from adult participants aged 18–25 years matched for vaccination interval to allow a direct comparison of immune responses.3 As already described in adults,4 a greater humoral response was measured after a longer dosing interval of ChAdOx1 nCOV-19. Younger children aged 6–11 years produced greater anti-spike IgG titres and neutralising antibody concentrations than older children aged 12–17 years after both a first and second dose of vaccine with a 112-day interval. In seronegative participants, an increase in anti-spike IgG titres was measured after the second dose of ChAdOx1 nCoV-19 regardless of dosing interval, but no rise in titres was seen in seropositive participants after a second dose. This finding suggests that when vaccine supplies are scarce, receiving only one dose of ChAdOx1 nCoV-19 could be sufficient to confer protection in children and adolescents with previous COVID-19.4 The concentration of IgG titres observed in our trial are similar to those seen in a study done in Chile, Peru, and the USA, before the emergence of delta and omicron variants, in which receiving two adult doses 4 weeks apart was associated with 74% efficacy against symptomatic PCR-positive SARS-CoV-2 infection in adults aged 18–64 years.5
Immunogenicity data from paediatric trials of other COVID-19 vaccines have also been published. Two separate phase 1/2 trials of CoronaVac and BBIBP-CorV in children aged 3–17 years showed that two standard adult doses of vaccine given 28 days apart were as immunogenic in children as in adults, as assessed by neutralising antibody titres.25, 26 Greater immunogenicity in younger children aged 5–11 years has been observed after vaccination with adult doses of BNT162b2 than observed immunogenicity in the adult population, such that fractional doses of 10 μg are now recommended for use in this age group.27 Phase 2/3 trials of BNT162b2 in children aged 6–15 years24, 27 reported preliminary efficacy results similar to adult trials; however, emerging real-world data suggest that protection against mild disease conferred by fractional dosing regimens might be less than that with adult doses.29 ChAdOx1 nCoV-19 produced significant increases in spike-specific T-cell responses in our cohort, and this is the first study to describe the generation of cellular immunity in response to COVID-19 vaccination in healthy children. A direct comparison to responses measured in adults aged 18–65 years after ChAdOx1 nCoV-19 vaccination is not possible due to the different spike peptide pooling strategy used in the IFNγ ELISpot assay done in this study. Although humoral immunogenicity differed significantly between younger and older age groups, T-cell responses were similar between children aged 6–11 years and 12–17 years after both first and second doses. As previously observed in the adult population,3 a second dose of vaccine did not further increase spike-specific T-cell responses regardless of the interval between doses. Vaccination against the ancestral SARS-CoV-2 strain induces memory T cells that have the ability to recognise conserved epitopes in variants of concern.30 Vaccination-associated protection against severe COVID-19 including hospital admission caused by variants of concern persists despite falling concentrations of neutralising antibodies, suggesting that non-neutralising antibodies and T-cell responses might confer a degree of protection against severe disease.31 Additionally, early induction of SARS-CoV-2-specific T-cell activity correlates is associated with reduced severe disease in natural infection.4 Although these findings support a role for T-cell activity in conferring protection against severe disease, the role that cellular immunity has in the child and adolescent population, who are at low risk of severe disease, requires further investigation.
Anti-vector immunity has been hypothesised to negatively affect the immunogenicity of ChAd-vectored vaccines. In our study, similar concentrations of anti-vector immunity were seen regardless of vaccination interval and age group. Therefore, anti-vector immunity is unlikely to be the main reason explaining the observed difference of the ChAdOx1 nCoV-19 immunogenicity between groups. Consistent with findings in adults, there was no strong correlation between the concentration of anti-vector immunity measured immediately before administering a second dose and humoral and cellular measures of immunogenicity 28 days after the second vaccination.4
The current study allows comparison of both humoral and cellular immunity with results from adult studies and indicates that ChAdOx1 nCoV-19 is at least as immunogenic in the age groups studied and therefore could be expected to provide a degree of clinical protection similar to that observed in the adult efficacy trials and real-world effectiveness studies. However, this indication remains to be formally shown.
Following conditional approvals and emergency use authorisation of ChAdOx1 nCoV-19 in adults, rare cases of thrombosis with thrombocytopenia syndrome emerged in association with first-dose administration of ChAdOx1 nCoV-19, with an incidence rate of 8·1 per million population administered vaccine doses among adults.32 Thrombosis with thrombocytopenia syndrome is now listed as a rare side-effect of the vaccine, and several countries restrict the use of ChAdOx1 nCoV-19 in younger age groups. However, incidence of thrombosis with thrombocytopenia syndrome appears to be lower in non-northern European populations than in northern European populations, which could be important in a risk–benefit analysis.33 The risk of thrombosis with thrombocytopenia syndrome in children cannot be directly extrapolated from adult data as the epidemiology of thrombotic disorders differs between children and adults.34 Thrombosis with thrombocytopenia syndrome has also been reported following administration of the Ad26.COV2.S (Janssen) vaccine35, 36 and mRNA COVID-19 vaccines, as well as after SARS-CoV-2 infection.37 Other side-effects associated with COVID-19 vaccination include myocarditis, which has been observed predominantly in males after having received BNT162b2 or mRNA-1273 (Moderna) vaccines, and most commonly within 4 days of receiving a second dose of vaccine.38, 39 Policy makers will have to weigh up the risks of any paediatric COVID-19 vaccination programme against the probable benefits.
The majority of adolescents eligible for COVID-19 vaccination in high-income countries are currently offered BNT162b2 as advised by WHO.8 However, clinical trial data for other COVID-19 vaccines in children are needed because countries are resorting to the use of alternative vaccines due to supply constraints; BBIBP-CorV and CoronaVac are being administered to children younger than 16 years in low-income, middle-income, and high-income countries.40 This trial provides the first safety and immunogenicity data in children on a further COVID-19 vaccine.
This study has several limitations, including a small sample size and poor ethnic diversity; 93% of the trial participants were of White ethnicity. Although ChAdOx1 nCoV-19 efficacy does not vary significantly with ethnicity in adults, a higher prevalence of PIMS-TS or MIS-C in Black, Hispanic, and Asian and Pacific Islander children has been reported.41 Our cohort of healthy volunteers excluded children with pronounced medical comorbidities, who are at increased risk of severe COVID-1916 and might be prioritised for vaccination. We have included immunogenicity data up to 28 days after a second dose; however, long-term data on the persistence of immunity will be made available at a later date.
Data sharing
This online publication has been corrected. The corrected version first appeared at thelancet.com on June 30, 2022
Declaration of interests
AJP is Chair of the UK Department of Health and Social Care's Joint Committee on Vaccination and Immunisation but does not participate in policy advice on coronavirus vaccines and was a member of the WHO Strategic Advisory Group of Experts. AJP is a National Institute for Health and Care Research (NIHR) Senior Investigator and is Chief Investigator on clinical trials of Oxford University's COVID-19 vaccine funded by NIHR. TL is named as an inventor on a patent application covering the ChAdOx1 nCoV-19 vaccine and is an occasional consultant to Vaccitech, unrelated to this work. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM. He receives no personal financial payment for this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator or provides consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva. He receives no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, Moderna, and Valneva. He receives no personal financial payment for this work. All other authors declare no competing interests. AstraZeneca reviewed the final manuscript before submission, but the authors retained editorial control.
Acknowledgments
Acknowledgments
The study is funded by the UK Government through the NIHR and AstraZeneca. This research was supported by the NIHR Oxford Biomedical Research Centre. AJP, MDS, and SNF are NIHR Senior Investigators. The views expressed in this Article do not necessarily represent the views of the UK Department of Health and Social Care, the UK Joint Committee on Vaccination and Immunisation, NIHR, or WHO. We are grateful for the support and commitment from the participants and their families in the running of this trial and all of the teams at the four UK sites who contributed to the running of this study. We also thank the Clinical Trials Research Governance team and Department of Paediatrics at the University of Oxford for their support and guidance.
Acknowledgments
Contributors
AJP and TL conceived and designed the trial and AJP is the Chief Investigator. AJP, TL, MDS, RS, GL, XL, AL, and SR contributed to the protocol and design of the study. MRR, MDS, RS, PTH, and SNF were the study site principal investigators. EC, FC, and TL were responsible for laboratory testing and assay development. XL, SF, and NGM accessed and verified the data, and did the statistical analysis. AJP, TL, NGM, XL, FC, and GL contributed to the preparation of the report. HRobe, GL, PKA, RAn, DS, KT, and NS contributed to the implementation of the study. All other authors contributed to the implementation of the study and data collection. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Anonymised participant data will be made available when the trials are complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. All data will be made available for a minimum of 5 years from the end of the trial.
Contributor Information
the COV006 study team:
J Aboagye, W Ambihapathy, JF Baker, ER Beales, A Boon, R Brampton, NM Branch, R Cooper, EL Cornish, S Cuevas-Asturias, Z Danos, S Davies, L de Luna George, R Drury, A Flaxman, J Fowler, E Galiza, L Godfrey, L Haskell, K Hillson, CL Hultin, S Koleva, E Lees, R Mabbett, J Muller, APS Munro, AL Oliver, DR Owens, JP Pearce, M Rajan, H Ratcliffe, I Rowbotham, S Salter, H Sanders, SS Sapuan, H Sharpe, E Sheehan, N Sutton, G Thaygaraja, S Thomson-Hill, M Ulaszewska, D Woods, and Bristol Clinical Research Nurse Team
Supplementary Material
References
- 1.London School of Hygiene & Tropical Medicine COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ [PubMed]
- 2.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health England Effectiveness of COVID-19 vaccines against hospital admission with the delta (B.1·617·2) variant. 2021. https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view/479607266
- 8.WHO COVID-19 advice for the public: getting vaccinated. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice
- 9.Afolabi MO, Tiono AB, Adetifa UJ, et al. Safety and immunogenicity of ChAd63 and MVA ME-TRAP in west African children and infants. Mol Ther. 2016;24:1470–1477. doi: 10.1038/mt.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss CM, Drammeh A, Bowyer G, et al. Viral vector malaria vaccines induce high-level T cell and antibody responses in west African children and infants. Mol Ther. 2017;25:547–559. doi: 10.1016/j.ymthe.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensah VA, Roetynck S, Kanteh EK, et al. Safety and immunogenicity of malaria vectored vaccines given with routine expanded program on immunization vaccines in Gambian infants and neonates: a randomized controlled trial. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiono AB, Nébié I, Anagnostou N, et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polisena J, Ospina M, Sanni O, et al. Public health measures to reduce the risk of SARS-CoV-2 transmission in Canada during the early days of the COVID-19 pandemic: a scoping review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344–353. doi: 10.1016/S1473-3099(20)30882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masonbrink AR, Hurley E. Advocating for children during the COVID-19 school closures. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1440. [DOI] [PubMed] [Google Scholar]
- 16.Smith C, Odd D, Harwood R, et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat Med. 2022;28:185–192. doi: 10.1038/s41591-021-01578-1. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JPA. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Organ. 2021;99:19–33F. doi: 10.2471/BLT.20.265892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitano T, Kitano M, Krueger C, Jamal H, Al Rawahi H, Lee-Krueger R, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One. 2021;16:1–12. doi: 10.1371/journal.pone.0246326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 years - United States, June-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1483–1488. doi: 10.15585/mmwr.mm7042e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Health Department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance
- 22.Guimarães D, Pissarra R, Reis-Melo A, Guimarães H. Multisystem inflammatory syndrome in children (MISC): a systematic review. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14450. [DOI] [PubMed] [Google Scholar]
- 23.Zambrano LD, Newhams MM, Olson SM, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenck RW, Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han B, Song Y, Li C, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2021;3099:1–13. doi: 10.1016/S1473-3099(21)00462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Medicines Agency Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11. Nov 25, 2021. https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11
- 29.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg E. Risk of infection and hospitalisation among vaccinated and unvaccinated children and adolescents in New York after the emergency of the omicron variant. JAMA. 2022 doi: 10.1001/jama.2022.7319. published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell. 2022;185:847–859. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England—technical briefing 31. Dec 10, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1040076/Technical_Briefing_31.pdf
- 32.Bhuyan P, Medin J, da Silva HG, et al. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: a global safety database analysis. Lancet. 2021;398:577–578. doi: 10.1016/S0140-6736(21)01693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soboleva K, Shankar NK, Yadavalli M, et al. Geographical distribution of TTS cases following AZD1222 (ChAdOx1 nCoV-19) vaccination. Lancet Glob Health. 2022;10:e33–e34. doi: 10.1016/S2214-109X(21)00545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achey MA, Nag UP, Robinson VL, et al. The developing balance of thrombosis and hemorrhage in pediatric surgery: clinical implications of age-related changes in hemostasis. Clin Appl Thromb. 2020;26:1–12. doi: 10.1177/1076029620929092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahase E. AstraZeneca vaccine: blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ. 2021;373:n931. doi: 10.1136/bmj.n931. [DOI] [PubMed] [Google Scholar]
- 36.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374 doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 40.Reuters Countries vaccinating children against COVID-19. 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473–3099(21)00267-X/fulltext
- 41.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.