Abstract
Background
The potential effects of cardiovascular comorbidities on the clinical outcomes in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection remain unclear. Identification of the coronary and non-coronary cardiovascular findings may help to stratify the patients' prognosis. Therefore, we aimed to evaluate the prognostic impact of the coronary and the non-coronary cardiovascular findings in SARS-CoV-2 patients.
Methods
We studied a total of 594 SARS-CoV-2 patients who were hospitalized and performed a non-cardiac gated thoracic computed tomography. Two blinded radiologists assessed the coronary artery calcification segment involvement score (CACSIS) and non-coronary atherosclerosis cardiovascular findings (NCACVF). The baseline characteristics of the patients and CT findings were evaluated according to survival status. Logistic regression analyses were performed to identify the independent predictors of mortality.
Results
At a mean follow-up of 8 (4–12.5) days, 44 deaths occurred (7.4%). Compared to survivors, non-survivors had increased CACSIS [27.3% (CACSIS = 0) vs 25% (CACSIS 1–5) vs 47.7% (CACSIS >5), p < 0.001]. Similarly, on NCACVF, non-survivors had much more major findings compared to survivors (29.5% vs. 2.7%, respectively, p < 0.001). At multivariable analysis, age (p = 0.009), creatinine (p < 0.001), hs-cTnI (p = 0.004) and NCACVF (HR 1.789; 95% CI 1.053–3.037; p = 0.031) maintained a significant independent association with in-hospital mortality.
Conclusion
Our study shows that coronary and non-coronary cardiovascular findings on non-cardiac gated thoracic CT may help to predict mortality in patients with SARS-CoV-2 infection.
Keywords: SARS-CoV-2 infection, Coronary artery calcification, Non-coronary atherosclerosis cardiovascular findings, Mortality
1. Introduction
A new enveloped non-segmented RNA coronavirus called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) leads to a frightening pandemic outbreak [1]. Although most SARS-CoV-2 infection manifests as mild or self-limiting respiratory illness, up to 30% of patients may experience acute respiratory distress syndrome, multiple organ failure, and death [2]. Severe complications more frequently occur in advanced age, male gender, smoking, and patients with any comorbidity, including cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, cardiovascular disease, cardiac arrhythmia, hypertension, diabetes mellitus, dementia, cancer, and dyslipidemia 3., 4., 5.. Thoracic computed tomography (CT) has cornerstone importance in diagnosing SARS-CoV-2 infection and determining the lung involvement that causes morbidity and mortality. Multifocal ground-glass opacities, consolidations, and reticulations close to the visceral pleural surface with a posterior predominant are the typical thoracic CT findings for SARS-CoV-2 pneumonia 6., 7., 8..
Coronary artery calcification (CAC) is a marker of coronary atherosclerosis and a crucial cardiac risk predictor [9]. The presence and extension of CAC assessed by cardiac gated computed tomography (CT) and has been widely used as a predictor of major cardiovascular events 10., 11.. Except for cardiac gated thoracic CT scans, CAC can be identified and quantified with non-cardiac gated thoracic CT scans. Different scoring systems are defined to investigate the burden of coronary artery calcification and coronary artery disease risk. Moreover, thoracic CT scans give us much more information beyond the CAC. Non-coronary atherosclerosis cardiovascular findings (NCACVF) such as; aortic, valvular, and myocardial calcifications, myocardial tissue disturbances, pericardial effusion, dilatations, and cardiac anomalies can be identified during non-gated thoracic CT scans. However, their clinical relevance has remained unearthed yet.
This study aimed to investigate the association of CAC, NCACVF, and in-hospital mortality of SARS-CoV-2 patients. To the best of our knowledge, this is the first study to evaluate CAC and NCACVF in predicting mortality in SARS-CoV-2 patients using non-cardiac gated thoracic CT scans.
2. Methods
2.1. Study population
We conducted a single-center, retrospective, observational study between December 2020 and February 2021. All consecutive patients who were diagnosed with SARS-CoV-2 infection and underwent non-cardiac gated thoracic CT scans on admission were incorporated into the study. In this study, we included solely hospitalized patients (both service and intensive care unit), and the study's primary endpoint was in-hospital mortality. Patients who had a percutaneous coronary intervention, coronary artery bypass graft surgery, heart valve surgery, aortic endograft, cardiac pacemaker, implantable cardiac defibrillator implantation, or multiple trauma were excluded.
The baseline demographic and clinical characteristics and laboratory findings on admission were obtained from the hospital's electronic database system. Complete blood counts and biochemical parameters, including blood glucose, creatinine, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), c-reactive protein (CRP), ferritin, and high-sensitive cardiac troponin I (hs-cTnI), were procured upon admission. In addition to laboratory data, hemodynamic parameters such as; heart rate, systolic blood pressure, oxygen saturation, and fever were also recorded. According to the Declaration of Helsinki, the present study was reviewed and approved by the Republic of Turkey Ministry of Health and Local Ethics Committee (Ethics Committee of the Kocaeli Derince Training and Research Hospital).
2.2. Thoracic CT imaging and data analysis
Thoracic CT imaging was performed using a 64-slice CT scanner (Aquilion 64, Toshiba Medical Systems, Japan) with 3-mm reconstructed slice thickness. All patients were examined in the supine position, end of inspiration, and hands raised by the side. Tube current and tube voltages were 300 mA, 120 kV, respectively, and gantry rotation time 0.4 s. All images were unenhanced and non-gated.
Thoracic CT scans were reviewed by two independent radiologists blinded to the study with experience in cardiovascular imaging. PACS software (Carestream Vue PACS version 11.4, New York, USA) was used to analyze the data. Axial, coronal, and sagittal planes were used to assess CAC and NCACVF. The CAC was classified by the 16-segment modified American Heart Association classification [12], called coronary artery calcification segment involvement score (CACSIS). The CACSIS represented the total numbers of involved segments and classified according to the points 0 to 16. CACSIS 0: Absence of the coronary calcification, CACSIS 1–5: Mild coronary calcification, CACSIS >5 Extensive coronary calcifications (Fig. 1 ). Patients were classified according to the number of vessels with any calcification, and the left main coronary artery was considered as two-vessel CAC. Thoracic CT of the patients was analyzed pursuant to NCACVF and stratified as; none or minimal; minor or major findings (the criterias of the NCACVF were given in Table 1 , and image examples were given in Fig. 2 ).
Fig. 1.
Examples of coronary calcifications. A: Mild coronary calcification. B: Extensive coronary calcification.
Table 1.
Non-coronary atherosclerosis cardiovascular findings classification (NCACVF) [27]
| None or minimal | No cardiovascular findings or mild thoracic aorta calcification or aortic valve calcification or mild right ventricular adipose tissue or minimum recess or posterior pericardial fluid. |
| Minor | Calcification of the thoracic aorta and cardiac valves; or diffuse aortic or cardiac valve calcification; or aortic dilatation; pericardial cyst, mild pericardial effusion; isolated right aortic arch, aberrant right subclavian artery; significant adipose tissue in the right ventricle; left atrium dilatation; lipomatous interatrial septum; coronary artery anomalies; pulmonary artery dilatation; mild endocardial calcification or mild isolated left ventricular adipose tissue. |
| Major | Chronic myocardial infarction (lipomatous metaplasia or calcification); intracavitary mass/calcification; cardiomegaly; significant adipose tissue in both right and left ventricles; pericardial calcification, moderate to severe pericardial effusion; aortic aneurysm. |
Fig. 2.
Examples of the non-coronary atherosclerosis cardiovascular findings. A: Mild thoracic aorta calcification (arrow). B: Diffuse aortic calcification (arrow). C: Lipomatous metaplasia of the left ventricular anterior wall and diffuse adipose tissue in the right ventricular wall and regions of the left ventricular apex (arrows). D: Cardiomegaly.
2.3. Statistical analysis
Data were analyzed using SPSS 22.0 version (SPSS Inc., Chicago, Illinois). Descriptive statistics were given as mean ± standard deviation and median with minimum-maximum values for continuous variables depending on their distribution. Numbers and percentages were used for categorical variables. The normal distribution of the numerical variables was analyzed by the Shapiro-Wilk, Kolmogorov-Smirnov, and Anderson-Darling tests. The Independent Samples t-test was used in comparing two independent groups where numerical variables had a normal distribution. The One-Way ANOVA test compared more than two independent groups where numerical variables had a normal distribution. For variables without normal distribution, the Kruskal Wallis test was applied. Pearson Chi-Square and Fisher's Exact tests were used to compare the differences between categorical variables. For the analyses in which parametric tests were used, the differences between the groups were evaluated with the Tukey or LSD tests when data was homogeneous based on its distribution. Cox regression analysis was performed to assess the relationship between CACSIS, NCACVF, and death as the outcome, summarized by hazard ratios (HR) and associated 95% confidence intervals. Survival analysis was performed by the Kaplan-Meier method, and differences in survival parameters were evaluated using the log-rank test. The significance level (p-value) was set at 0.05 in all statistical analyses.
3. Results
3.1. Baseline characteristics
A total of 594 SARS-CoV-2 patients hospitalized and had a non-cardiac gated thoracic CT performed were enrolled in the study. Five hundred fifty patients were discharged from the hospital, and 44 patients were deceased (7.4%) in the hospital. Baseline characteristics and clinical and laboratory parameters of the study population are demonstrated in Table 2 according to survival status (survival and non-survival). The median age was 45 (34–58), and 263 patients (44.3%) were female. One hundred eighty-six patients (31.3%) were smokers, 82 patients (13.8%) had diabetes mellitus, 141 patients (23.7%) had hypertension, 14 patients (2.4%) had congestive heart failure and 69 patients (11.6) had chronic obstructive pulmonary disease.
Table 2.
Baseline characteristics of the patients admitted with SARS-CoV-2 infection.
| Survivors (n = 550) | Non-survivors (n = 44) | Total (n = 594) | p value | ||
|---|---|---|---|---|---|
| Age | 44 (33–55) | 72 (63–80) | 45 (34–58) | <0.001 | |
| Gender (Female), n (%) | 243 (44.2) | 20 (45.5) | 263 (44.3) | 0.870 | |
| Smoking, n (%) | 167 (30.4) | 19 (43.2) | 186 (31.3) | <0.001 | |
| Diabetes mellitus, n (%) | 74 (13.5) | 8 (18.2) | 82 (13.8) | 0.382 | |
| Hypertension, n (%) | 119 (21.6) | 22 (50.0) | 141 (23.7) | <0.001 | |
| Congestive heart failure, n (%) | 6 (1.1) | 8 (18.2) | 14 (2.4) | <0.001 | |
| Chronic obstructive pulmonary disease, n (%) | 54 (9.8) | 15 (34.1) | 69 (11.6) | <0.001 | |
| Heart rate on admission, bpm | 96 ± 14 | 109 ± 19 | 97 ± 14 | 0.190 | |
| Systolic blood pressure on admission, mmHg | 115 ± 9 | 105 ± 17 | 115 ± 10 | 0.01 | |
| Oxygen saturation on admission, % | 96 ± 2 | 87 ± 8 | 95 ± 3 | <0.001 | |
| Fever on admission, °C | 37 (36.6–38) | 38 (37.7–38.6) | 37 (36.6–38) | 0.002 | |
| Fasting blood glucose, mg/dL | 97 (87–115) | 134 (106–235) | 99 (88–118) | <0.001 | |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 1.2 (0.8–2.2) | 0.8 (0.7–0.9) | <0.001 | |
| ALT, U/L | 22 (16–35) | 22 (15–37.5) | 22 (16–35) | 0.897 | |
| AST, U/L | 22 (17–30) | 31.5 (23–46.5) | 23 (17–31) | <0.001 | |
| CRP, mg/L | 7.4 (2–22.6) | 93.2 (43.8–192) | 7.5 (2.2–30.1) | <0.001 | |
| Ferritin, ng/mL | 98 (41–220.1) | 401 (153.5–585) | 107.1 (43–262.7) | <0.001 | |
| Hemoglobin, g/dL | 13.6 ± 1.6 | 11.5 ± 2.6 | 13.4 ± 1.8 | <0.001 | |
| White blood cell count, x10 [3]/μl | 6.6 ± 2.6 | 11.8 ± 6.5 | 7.0 ± 3.3 | <0.001 | |
| Platelet count, x10 [3]/μl | 220 (178–269) | 194 (155–338) | 218 (175–272) | 0.374 | |
| hs-cTnI, pg/mL | 1 (0.1–3) | 30 (9–132) | 1.3 (0.2–4) | <0.001 | |
| CACSIS, n (%) | 0 | 464 (84.4) | 12 (27.3) | 476 (80.1) | <0.001 |
| 1–5 | 59 (10.7) | 11 (25) | 70 (11.8) | ||
| >5 | 27 (4.9) | 21 (47.7) | 48 (8.1) | ||
| NCACVF, n (%) | None or minimal | 438 (79.6) | 12 (27.3) | 450 (75.8) | <0.001 |
| Minor | 97 (17.6) | 19 (43.2) | 116 (19.5) | ||
| Major | 15 (2.7) | 13 (29.5) | 28 (4.7) | ||
| Follow-up (day) | 5 (3–7) | 8 (4–12.5) | 5 (3–8) | <0.001 | |
Abbreviations: ALT: Alanine aminotransferase, AST: Aspartate transaminase, CRP: C-reactive protein, hs-cTnI: High-sensitivity cardiac troponin I, CACSIS: Coronary artery calcification segment involvement score, NCACVF: Noncoronary atherosclerosis cardiovascular findings classification.
3.2. Comparison of survivors and non-survivors
Non-survivors were older [median age 72 (63–80) vs 44 (33–55), p < 0.001] and had a higher prevalence of smoking (43.2% vs 30.4%, p < 0.001), hypertension (50% vs 21.6%, p < 0.001), cognestive heart failure (CHF) (18.2% vs 1.1%, p < 0.001), and chronic obstructive pulmonary disease (COPD) (34.1% vs 9.8%, p < 0.001).
According to the hemodynamic parameters and laboratory assays on admission there were significant differences between two groups. Compared to survivors, non-survivors had higher fever [38.0 (37.7–38.6) vs 37.0 (36.6–38) oC, p = 0.002] and lower systolic blood pressure [105 ± 17 mmHg vs 115 ± 9 mmHg, p = 0.01]. On laboratory examination, non-survivors had higher fasting blood glucose [134 (106–235) vs 97 (87–115) mg/dL, p < 0.001], creatinine [1.2 (0.8–2.2) vs 0.8 (0.7–0.9) mg/dL, p < 0.001], aspartate aminotransferase (AST) [31.5 (23–46.5) vs 22 (17–30) U/L, p < 0.001], c-reactive protein (CRP) [93.2 (43.8–192) vs 7.4 (2–22.6) mg/L, p < 0.001], ferritin [401 (153.5–585) vs 98 (41–220.1) ng/mL, p < 0.001], white blood cell count (11.8 ± 6.5 vs 6.6 ± 2.6 × 10 [3]/μl, p < 0.001], and high-sensitivity cardiac troponin I (hs-cTnI) [30 (9–132) vs 1 (0.1–3) pg/mL, p < 0.001]. However, hemoglobulin levels (11.5 ± 2.6 vs 13.6 ± 1.6 g/dL, p < 0.001) were lower in non-survivors, significantly.
3.3. Analysis of the thoracic CT
Patients were evaluated according to CACSIS and NCACVF. Compared to survivors, non-survivors had increased coronary calcification burden and this was reflected to CACSIS [27.3% (CACSIS = 0) vs 25% (CACSIS 1–5) vs 47.7% (CACSIS >5), p < 0.001]. Regarding to NCACVF, 13 (29.5%) patients had major findings in non-survivors group, while only 15 (2.7%) patients had major findings in the survivors group (p < 0.001). ROC analysis revealed that CACSIS ≥1 predicted in-hospital mortality with a sensitivity of 72.7% and specificity of 84.3% (AUC: 0.805, 95% CI: 0.771–0.836; p < 0.001) and NCACVF major findings predicted in-hospital mortality with a sensitivity of 72.7% and specificity of 79.6% (AUC: 0.782, 95% CI: 0.747–0.815; p < 0.001).
3.4. Clinical outcomes
After a mean follow-up of 8 (4–12.5) days, in-hospital mortality ensued in 44 (7.4%) patients. The vast majority of deceased patients were in the intensive care unit [34 patients (77.3%)]. Table 3 and Table 4 demonstrated the univariable and multivariable Cox regression analysis for factors associated with in-hospital mortality. In addition to the Cox regression model, Kaplan-Meier analysis was performed to evaluate the survival according to CACSIS and NCACVF.
Table 3.
Independent predictors of in-hospital mortality at Cox regression analysis and the effect of the coronary artery calcification segment involvement score.
| Univariable | p value | HR | 95% CI |
Multivariable | p value | HR | 95% CI |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Age | <0.001 | 1.065 | 1.044 | 1.086 | Age | 0.002 | 1.046 | 1.016 | 1.076 |
| Gender (Female) | 0.898 | 1.041 | 0.566 | 1.913 | – | – | – | – | – |
| Hypertension | 0.097 | 1.681 | 0.911 | 3.100 | – | – | – | – | – |
| DM | 0.904 | 0.953 | 0.440 | 2.064 | – | – | – | – | – |
| Smoking | 0.415 | 1.287 | 0.702 | 2.359 | – | – | – | – | – |
| COPD | 0.013 | 2.271 | 1.191 | 4.328 | – | – | – | – | – |
| CHF | 0.001 | 3.799 | 1.667 | 8.656 | – | – | – | – | – |
| Creatinine, mg/dl | <0.001 | 1.779 | 1.504 | 2.104 | Creatinine, mg/dl | 0.001 | 1.468 | 1.182 | 1.822 |
| hs-cTnI, pg/ml | <0.001 | 1.071 | 1.041 | 1.102 | hs-cTnI, pg/mL | 0.012 | 1.050 | 1.011 | 1.091 |
| CACSIS | <0.001 | 2.795 | 1.949 | 4.007 | CACSIS | 0.874 | 1.050 | 0.578 | 1.904 |
Abbreviations: DM: Diabetes mellitus, COPD: Chronic obstructive pulmonary disease, CHF: Congestive heart failure, hs-cTnI: High-sensitivity cardiac troponin I, CACSIS: Coronary artery calcification segment involvement score.
Table 4.
Independent predictors of in-hospital mortality at Cox regression analysis and the effect of the noncoronary atherosclerosis cardiovascular findings.
| Univariable | p value | HR | 95% CI |
Multivariable | p value | HR | 95% CI |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Age | <0.001 | 1.065 | 1.044 | 1.086 | Age | 0.009 | 1.034 | 1.008 | 1.060 |
| Gender (female) | 0.898 | 1.041 | 0.566 | 1.913 | – | – | – | – | – |
| Hypertension | 0.097 | 1.681 | 0.911 | 3.100 | – | – | – | – | – |
| DM | 0.904 | 0.953 | 0.440 | 2.064 | – | – | – | – | – |
| Smoking | 0.415 | 1.287 | 0.702 | 2.359 | – | – | – | – | – |
| COPD | 0.013 | 2.271 | 1.191 | 4.328 | – | – | – | – | – |
| CHF | 0.001 | 3.799 | 1.667 | 8.656 | – | – | – | – | – |
| Creatinine, mg/dl | <0.001 | 1.779 | 1.504 | 2.104 | Creatinine, mg/dl | <0.001 | 1.482 | 1.217 | 1.804 |
| hs-cTnI, pg/ml | <0.001 | 1.071 | 1.041 | 1.102 | hs-cTnI, pg/mL | 0.004 | 1.057 | 1.018 | 1.098 |
| NCACVF | <0.001 | 3.085 | 2.077 | 4.580 | NCACVF | 0.031 | 1.789 | 1.053 | 3.037 |
Abbreviations: DM: Diabetes mellitus, COPD: Chronic obstructive pulmonary disease, CHF: Congestive heart failure, hs-cTnI: High-sensitivity cardiac troponin I, NCACVF: Noncoronary atherosclerosis cardiovascular findings classification.
Table 3 showed age (HR 1.046; 95% CI 1.016–1.076; p = 0.002), creatinine (HR 1.468; 95% CI 1.182–1.822; p = 0.001), and hs-cTnI (HR 1.050; 95% CI 1.011–1.091; p = 0.012) levels were associated with in-hospital mortality. However, CACSIS (HR 1.050; 95% CI 0.578–1.904; p = 0.874) was not independently associated with in-hospital mortality, when these variables added to the model.
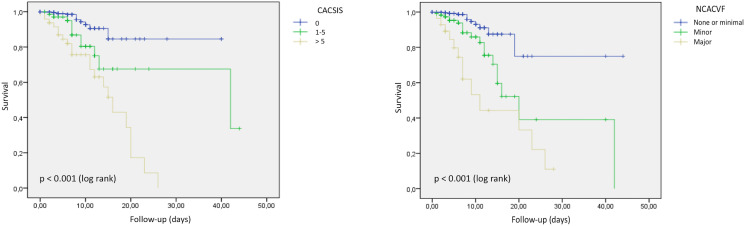
Table 4 indicated age (HR 1.034; 95% CI 1.008–1.060; p = 0.009), creatinine (HR 1.482; 95% CI 1.217–1.804; p < 0.001), and hs-cTnI (HR 1.057; 95% CI 1.018–1.098; p = 0.004) levels were associated with in-hospital mortality. When added to these variables, NCACVF (HR 1.789; 95% CI 1.053–3.037; p = 0.031) pursued independent association with in-hospital mortality. Kaplan-Meier curves showed significant differences in the probability of in-hospital mortality with CACSIS (p < 0.001), and NCACVF (p < 0.001) (Fig. 3 ).
Fig. 3.
Kaplan-Meier survival according to the presence of any coronary artery calcification, to the coronary artery calcification segment involvement score, and the non-coronary atherosclerosis cardiovascular findings classification.
4. Discussion
This study demonstrated that CAC and NCACVF, which can easily be accessible on admission, were associated with the short-term clinical outcome in SARS-CoV-2 patients. When these thoracic CT findings were evaluated after adjusting for significant clinical and laboratory variables, NCACVF was found as independently associated with in-hospital mortality (HR 1.789, p = 0.031). However, CACSIS was not significant when the clinical (especially older age) and laboratory parameters were added to the model (HR 1.050, p = 0.874).
Atherosclerosis, which results from chronic inflammation and immune system dysregulation, presents an ideal inflammatory environment for SARS-Cov2 infection. Endothelial cells are one of the best targets for the virus, which explains the multiorgan failure in SARS-Cov2 infection. Atherosclerosis is associated with endothelial dysfunction, which may cause aggressive viral replication, inflammatory response, and clinical manifestations. As a result, direct and indirect viral effects may lead to clinical or subclinical myocardial injury 13., 14., 15..
The essential coronary artery findings are varying degrees of CAC, which was detected on thoracic CT scans. CAC is a well-known marker to determine individuals for high cardiovascular risk 16., 17.. ECG-gated thoracic CT is the best option for CAC examination. However, some studies demonstrated that CAC assessment via non-gated thoracic CT scans is feasible and provided a significant prognostic value 18., 19., 20.. In the SARS-CoV-2 pandemic, non-gated thoracic CT is the preferred imaging modality in most emergency departments. Subclinical CAC on the thoracic CT was associated with a worse prognosis in SARS-CoV-2 patients 21., 22..
Gupta et al. demonstrated that SARS-CoV-2 patients with CAC were more likely to require intubation and had higher mortality rates than those without CAC. Also, increasing CAC and the number of affected arteries were associated with mortality [21]. Similar results were found by Dillinger et al., in which the presence and extent of CAC were associated with worse outcomes [22]. On the other hand, CAC burden and myocardial injury can be affected by many conditions. In a recent study, Bergström et al. included 25.182 individuals without known coronary artery disease and subclinical coronary artery atherosclerosis evaluated using coronary computed tomography angiography. They found that coronary atherosclerosis is more common in older individuals and male gender [13]. This close relationship between advanced age and CAC may also cause conflicting results in determining the mortality of SARS-CoV-2 patients. A study conducted by Ferrante et al. reported that patients with myocardial injury had a higher prevalence of a CAC. However, the CAC did not emerge as a predictor of myocardial injury and mortality in the multivariable logistic regression analysis. Advanced age and lower estimated glomerular filtration rate were independent predictors for both myocardial injury and death [23]. Our study found that the CAC was significantly higher in the deceased group and had an association with in-hospital mortality by univariable analysis only. However, when we added the variables into the Cox proportional-hazards model, the CAC didn't provide incremental value for in-hospital mortality independently.
Coronary artery calcification alone provides limited information about the cardiovascular system, including myocardium, pericardium, great vessels, and heart valves. Some studies have explored the prognostic value of non-coronary findings measured during CAC scans 24., 25., 26.. These studies focused on thoracic aortic calcification, epicardial adipose tissue, left ventricular and left atrial size [26]. Rodriguez-Granillo et al. designed a new scoring system that includes almost whole cardiovascular findings on thoracic CT (Table 1) [27]. These findings were stratified according to cardiovascular involvement and were investigated for the prognostic value on malignancy. They found that major NCACVF findings were significantly associated with mortality in patients with malignancy and without malignancy. However, few studies were performed about the relationship between cardiovascular involvement and SARS-CoV-2 infection mortality, except CAC. The study by Giannini et al. is one of them, and they found that aortic valve and thoracic aorta calcifications on non-gated thoracic CT were significantly related to the SARS-CoV-2 patient mortality. Another study conducted by Slipczuk et al. indicated that epicardial adipose tissue thickness is a robust independent predictor of mortality from SARS-CoV-2 infection [28]. When the current literature was reviewed, it was seen that only a few specific findings in thoracic CT scans, other than CAC, were studied in SARS-CoV-2 patients. Therefore, our study used the NCACVF scoring system, which includes the broadest cardiovascular findings. Major NCACVF score was associated with in-hospital mortality, and this result showed a significant prognostic value.
4.1. Limitations of the study
The present study has several limitations. First, this study was a retrospective single-center study, and the results were retrospectively evaluated and adjudicated. Secondly, we only included the hospitalized patients; therefore, our study sample couldn't represent the SARS-CoV-2 population. Thirdly, CAC couldn't reach a significant statistical value to predict in-hospital mortality in our study, but the main reason may be the small sample size. Fourthly, although experienced radiologists evaluated the thoracic CT images (both CACSIS and NCACVF), scores were not quantitatively analyzed. Finally, we investigated only the in-hospital mortality of the SARS-CoV-2 patients. However, we didn't examine the other adverse events, including stroke, acute myocardial infarction, venous thromboembolism, and acute limb ischemia.
5. Conclusion
Non-cardiac gated thoracic CT scan is a cornerstone diagnostic tool for pulmonary involvement in SARS-CoV-2 infection. Apart from pulmonary parenchyma involvement, it also inholds many findings that can predict the patient's prognosis. In this study, CACSIS and NCACVF, obtained from non-cardiac gated thoracic CT scans, showed that these scores may help predict in-hospital mortality easily and quickly. Further studies are needed to explore more comprehensive and detailed scores in foreseeing SARS-CoV-2 infection damage.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., Bengolea A., Ceirano A., Espinosa F., Saavedra E., Sanguine V., Tassara A., Cid C., Catalano H.N., Agarwal A., Foroutan F., Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020:109009. doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR. 2020;214:1072–1077. doi: 10.2214/ajr.20.22976. [DOI] [PubMed] [Google Scholar]
- 8.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budoff M.J., Shaw L.J., Liu S.T., Weinstein S.R., Mosler T.P., Tseng P.H., Flores F.R., Callister T.Q., Raggi P., Berman D.S. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R., Guerci A.D., Carr J.J., Bild D.E., Burke G., Folsom A.R., Liu K., Shea S., Szklo M., Bluemke D.A., O'Leary D.H., Tracy R., Watson K., Wong N.D., Kronmal R.A. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 11.Sarwar A., Shaw L.J., Shapiro M.D., Blankstein R., Hoffmann U., Cury R.C., Abbara S., Brady T.J., Budoff M.J., Blumenthal R.S., Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Min J.K., Shaw L.J., Devereux R.B., Okin P.M., Weinsaft J.W., Russo D.J., Lippolis N.J., Berman D.S., Callister T.Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 13.Bergström G., Persson M., Adiels M. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144(12):916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J., Weber C., Bochaton-Piallat M.L., Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc. Res. 2020:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miedema M.D., Dardari Z.A., Nasir K., Blankstein R., Knickelbine T., Oberembt S., Shaw L., Rumberger J., Michos E.D., Rozanski A., Berman D.S., Budoff M.J., Blaha M.J. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J., Budoff M.J., Nasir K., Wong N.D., Yeboah J., Al-Mallah M.H., Shea S., Dardari Z.A., Blumenthal R.S., Blaha M.J., Cainzos-Achirica M. Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: the multi ethnic study of atherosclerosis (MESA) Atherosclerosis. 2017;257:1–8. doi: 10.1016/j.atherosclerosis.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes-Austin J.M., Dominguez A., 3rd, Allison M.A., Wassel C.L., Rifkin D.E., Morgan C.G., Daniels M.R., Ikram U., Knox J.B., Wright C.M., Criqui M.H., Ix J.H. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152–159. doi: 10.1016/j.jcmg.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiles C., Duan F., Gladish G.W., Ravenel J.G., Baginski S.G., Snyder B.S., Desjardins S.S., Munden R.F., DeMello S., NLST Study Team Association of Coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs P.C., Prokop M., van der Graaf Y., Gondrie M.J., Janssen K.J., de Koning H.J., Isgum I., van Klaveren R.J., Oudkerk M., van Ginneken B., Mali W.P. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high risk population of heavy smokers. Atherosclerosis. 2010;209(2):455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Gupta Y.S., Finkelstein M., Manna S., Toussie D., Bernheim A., Little B.P., Concepcion J., Maron S.Z., Jacobi A., Chung M., Kukar N., Voutsinas N., Cedillo M.A., Fernandes A., Eber C., Fayad Z.A., Hota P. Coronary artery calcification in COVID-19 patients: an imaging biomarker for adverse clinical outcomes. Clin Imaging. 2021;77:1–8. doi: 10.1016/j.clinimag.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., Hamzi L., Chauvin A., Leroy P., Gautier J.F., Sène D., Henry P. COVID research Group of Lariboisiere Hospital. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2468–2470. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante G., Fazzari F., Cozzi O., Maurina M., Bragato R., D'Orazio F., Torrisi C., Lanza E., Indolfi E., Donghi V., Mantovani R., Liccardo G., Voza A., Azzolini E., Balzarini L., Reimers B., Stefanini G.G., Condorelli G., Monti L. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc Res. 2020;116(14):2239–2246. doi: 10.1093/cvr/cvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong N.D., Gransar H., Shaw L., Polk D., Moon J.H., Miranda-Peats R., Hayes S.W., Thomson L.E., Rozanski A., Friedman J.D., Berman D.S. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009:319–326. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Yeboah J., Carr J.J., Terry J.G., Ding J., Zeb I., Liu S., Nasir K., Post W., Blumenthal R.S., Budoff M.J. Computed tomography derived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in nondiabetics: the multi-ethnic study of atherosclerosis. Eur J Prev Cardiol. 2014:1233–1241. doi: 10.1177/2047487313492065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahabadi A.A., Lehmann N., Mohlenkamp S., Pundt N., Dykun I., Roggenbuck U., Moebus S., Jöckel K.H., Erbel R., Kälsch H. Heinz nixdorf investigative group. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc Imaging. 2016:1177–1185. doi: 10.1016/j.jcmg.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Granillo G.A., Reynoso E., Capunay C., Garcia-Garcia H.M., Carrascosa P. Impact on mortality of coronary and non-coronary cardiovascular findings in non-gated thoracic CT by malignancy status. Eur J Radiol. 2017;93:169–177. doi: 10.1016/j.ejrad.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Slipczuk L., Castagna F., Schonberger A., Novogrodsky E., Sekerak R., Dey D., Jorde U.P., Levsky J.M., Garcia M.J. Coronary artery calcification and epicardial adipose tissue as independent predictors of mortality in COVID-19. Int J Cardiovasc Imaging. 2021;37(10):3093–3100. doi: 10.1007/s10554-021-02276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]