Dear editor,
We have read with great interest the study by Martin-Blondel et al.1 Reporting the outcome of neutralizing antibodies in Omicron and Delta-infected patients. Currently, there is few specific treatments recommended for COVID-19 particularly its variant strains, most of which are under emergency use authorization (EUA).2, 3, 4 We generated potent SARS-CoV-2 neutralizing antibodies, IBI314A and IBI314B, against distinct and non-overlapping epitopes on the S protein from convalescent patients.5 Herein, we reported the efficacy and safety of IBI314, consisting of 2 antibodies IBI314A and IBI314 at a 1:1 ratio, in two cohorts of Chinese COVID-19 patients who were infected with the Delta and Omicron variants.
Since November 2021, COVID-19 outbreaks caused by variant of SARS-CoV-2 emerged in multiple locales in China and is still ongoing. Two cohorts of patients infected with the variant of SARS-CoV-2 from Qinghai and Henan Province were hospitalized and given IBI314, intravenously at a dose of 1500 mg under compassionate use. Oropharyngeal and nasopharyngeal swabs were obtained prior to, on the day of (Day 0), and after IBI314 administration. Blood samples were collected for laboratory tests, including oxygenation index (PaO2/FiO2), peripheral oxygen saturation (SpO2), C-reactive protein (CRP), IgG and IgM. CT scans were evaluated by two independent radiologists. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used and provided a cycle threshold (Ct) value, which is the number of cycles required for the fluorescent signal to cross the threshold for a positive test. A Ct value is a surrogate for viral load. A Ct value>40 denotes a negative test and a CT value≤40 denotes a positive test.
A total of 61 cases included 10 cases from Qinghai Province and 51 cases from Henan Province received the compassionate use of IBI314. Table 1a and b summarized the baseline characteristics of patients in these two cohorts. Notably, there were 40% and 27.5% of severe cases in Qinghai and Henan cohort, respectively. Patients’ symptoms and comorbidities reported at the time of hospitalization were described in Supplementary Tables 1 and 2.
Table 1a.
Patient demographics and baseline characteristics in Qinghai cohort.
| All Cases(N = 10) | Mild(N = 2) | Moderate(N = 4) | Severe(N = 4) | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 1 (10%) | 0 (0%) | 0 (0%) | 1 (25%) |
| Female | 9 (90%) | 2 (100%) | 4 (100%) | 3 (75%) |
| Age (years) | ||||
| Mean ± SD | 41.9 ± 16.64 | 31.5 ± 0.5 | 31 ± 2.55 | 58 ± 15.92 |
| Median | 33 | 31.5 | 31.5 | 58.5 |
| Min: Max | 27:80 | 31:32 | 27:34 | 35:80 |
| Age group, n (%) | ||||
| ≤ 65 years | 9 (90%) | 2 (100%) | 4 (100%) | 3 (75%) |
| > 65 years | 1 (10%) | 0 (0%) | 0 (0%) | 1 (25%) |
| Vaccination to disease onset (days) | ||||
| Mean ± SD | 194.78 ± 88.54 | 245 ± 57 | 240 ± 77.62 | 101 ± 10.71 |
| Median | 170.5 | 245 | 278.5 | 99 |
| Min: Max | 89:302 | 188:302 | 107:296 | 89:115 |
| Symptom onset to treatment (days) | ||||
| Mean ± SD | 5.8 ± 3.31 | 3.5 ± 1.5 | 3.75 ± 1.79 | 9 ± 2.45 |
| Median | 5 | 3.5 | 3.5 | 10 |
| Min: Max | 2:11 | 2:5 | 2:6 | 5:11 |
*The most severe stage experienced by patient was used as the diagnostic type (mild, moderate, severe).
Table 1b.
Patient demographics and baseline characteristics in Henan cohort.
| All Cases (N = 51) | Mild (N = 12) | Moderate (N = 25) | Severe (N = 14) | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 21(41.18%) | 5(41.67%) | 10(40%) | 6(42.86%) |
| Female | 30(58.82%) | 7(58.33%) | 15(60%) | 8(57.14%) |
| Age (years) | ||||
| Mean ± SD | 67.63±17.21 | 70.25±17.35 | 60.08±16.25 | 78.86±12.05 |
| Median | 69 | 73.5 | 65 | 81 |
| Min: Max | 25:98 | 25:89 | 32:88 | 48:98 |
| Age group, n (%) | ||||
| <65 years | 16(31.37%) | 3(25%) | 12(48%) | 1(7.14%) |
| 65≤age<75 | 16(31.37%) | 4(33.33%) | 9(36%) | 3(21.43%) |
| 75≤age<85 | 12(23.53%) | 3(25%) | 3(12%) | 6(42.86%) |
| ≥85 years | 7(13.73%) | 2(16.67%) | 1(4%) | 4(28.57%) |
| Variant strains, n (%) | ||||
| Delta | 47(92.16%) | 11(91.67%) | 23(92%) | 13(92.86%) |
| Omicron | 4(7.84%) | 1(8.33%) | 2(8%) | 1(7.14%) |
| Symptom onset to treatment (days) | ||||
| Number of cases | N = 41 | N = 9 | N = 22 | N = 10 |
| Mean ± SD | 8.41±3.75 | 8.22±3.67 | 8.36±4.19 | 8.7±3.09 |
| Median | 8 | 8 | 7 | 9 |
| Min: Max | 2:15 | 3:14 | 2:15 | 3:13 |
*The most severe stage experienced by patient was used as the diagnostic type (mild, moderate, severe).
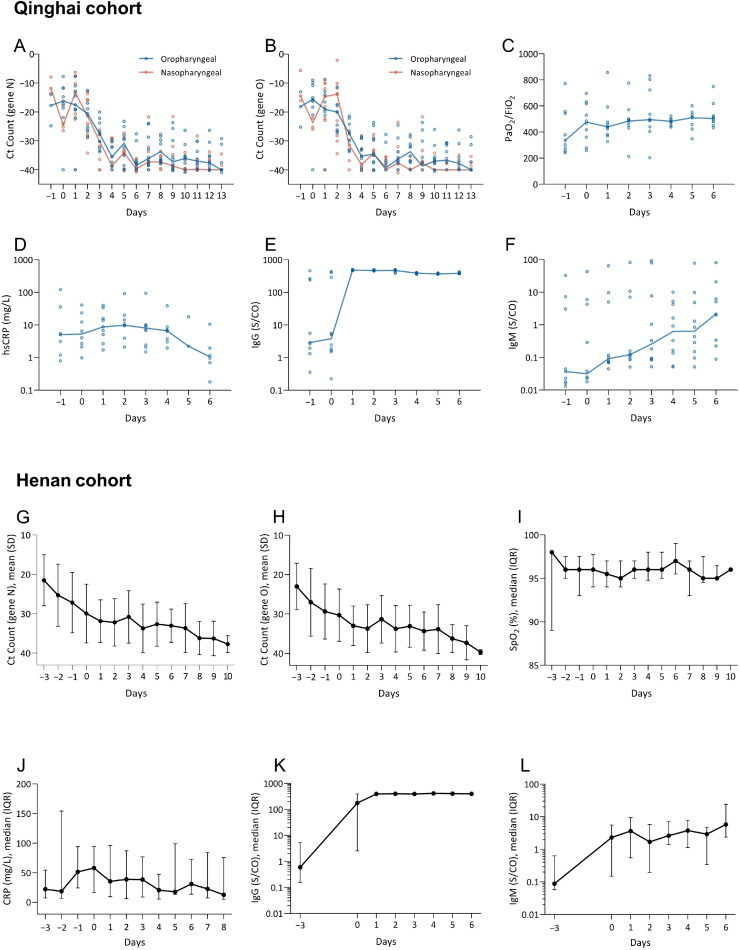
Ct values of N gene and O gene of oropharyngeal and nasopharyngeal samples were plotted (Fig. 1 A, B and 1G, H). After administration, the change of Ct values of both N and O genes exhibited a similar pattern, with a trend of increasing in Ct values, indicating a gradual viral clearance in all patients of both cohorts. A pooled analysis of partial pressure arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) referred to as oxygenation index (P/F) was performed in Qinghai cohort. A trend of increase in P/F was observed (Fig. 1C). Moreover, this indicator was replaced by SpO2, because of the incomplete data of patients’ oxygenation index (P/F) in Henan cohort, and showed a stable trend (Fig. 1I). Furthermore, we used CRP to reflect the level of inflammation. After the treatment of IBI314, 71.43% (5/7) and 30.77% (8/26) of patients converted to a normal CRP level, from an abnormal value prior to administration in the Qinghai and Henan cohort, respectively (Fig. 1D and J). We also investigated the level of specific IgG and IgM to SARS-CoV-2. In comparison to the baseline median level of 3.77S/CO and 190.89 S/CO in Qinghai and Henan cohort respectively, the level of IgG was drastically increased in almost all patients after treatment, reached the peak on Day 1, with a median level of 482.58 S/CO and 350.85 S/CO (Fig. 1E and K). Plateaued IgG levels were similar across all patients, and sustained after that. In Qinghai cohort, patients’ IgM level increased gradually over the time, and the plateau was not observed (Fig. 1F). By contrast, the IgM level of patients in Henan cohort increased after administration (Day1) and were relatively stable in the following days (Fig. 1L).
Fig. 1.
Qinghai cohort: Changes of N gene (A), O gene (B), PaO2/ FiO2 (C), High sensitivity -CRP (D), IgG (E) and IgM (F) were evaluated prior to, on the day of (Day 0), and after IBI314 administration. Y-axis denotes the level of specific parameter measure and X-axis denotes the time in relation to IBI314 administration. Medians of various indicators in each study day were connected by lines.
Henan cohort: Changes of N gene (G) and O gene (H) in oropharyngeal swabs, SpO2 (I), CRP (J), IgG (K) and IgM (L) were evaluated prior to, on the day of (Day 0), and after IBI314 administration. Y-axis denotes the level of specific parameter measure and X-axis denotes the time in relation to IBI314 administration. Medians (or means) of various indicators in each study day were connected by lines.
The clinical symptoms of most patients were alleviated shortly after IBI314 treatment in both cohorts. In Qinghai cohort, all severe patients (N = 4) were transferred out of ICU after IBI314 treatment with a median time of 5 days, accompanying a significant improvement in CT scan (Supplementary Fig. 1). At the end of observation window, 3 patients (3/10) in Qinghai cohort and 28 patients (28/51) in Henan cohort achieved negative conversion for SARS-CoV-2, with a mean interval after administration of 11.7 and 5.8 days, respectively. IBI314 is generally well-tolerated with no serious adverse events or infusion-related reactions observed. Laboratory parameters including but not limited to white blood cell counts, lymphocyte counts, hemoglobulin, transaminases were assessed in every patient and no clinically significant abnormality related to IBI314 was observed. Only a few patients experienced elevation in ALT and AST, and leukocytes decreased (dropped below 4.0 × 109/L) after IBI 314 treatment. No death occurred.
The emergence of novel variants of SAR-CoV-2 presented a serious challenge for the development of therapeutic agents against COVID-19. Preclinical data indicated that IBI314 exhibited a high degree of mutation resistance and maintained potency against prevailing variants of concerns in pseudoviral neutralization and authentic virus neutralization in vitro.5 This study expounded the efficacy and safety of IBI314 under compassionate use in two cohorts with mild to severe COVID-19, infected with the Delta and Omicron variant of SARS-CoV-2. Most patients showed a significant increase in the Ct value (corresponding to a decrease in viral load) shortly after IBI314 treatment, suggesting a rapid viral clearance. Other parameters including oxygenation index, SpO2, and CRP were also improved. What's more, almost all patients showed significant improvement in clinical symptoms shortly after IBI314 treatment.
Collectively, we presented the first-in-human data on the efficacy of IBI314 in patients infected with Delta and Omicron variant of SARS-CoV-2, indicating that IBI314 have a satisfying potency to reduce viral load and alleviate clinical symptoms in any stage of cases with favorable safety. We also observed a trend that the earlier IBI314 was used, the better efficacy was achieved.
CRediT authorship contribution statement
Ling Sang: Conceptualization, Visualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Bo Cheng: Writing – review & editing, Conceptualization, Visualization, Data curation, Formal analysis, Writing – original draft. Yuheng Yu: Writing – review & editing, Data curation, Formal analysis, Writing – original draft. Yin Xi: Writing – review & editing, Data curation, Formal analysis. Yun Wang: Writing – review & editing, Data curation. Bingdong Fan: Writing – review & editing, Data curation. Jijie Li: Writing – review & editing, Data curation. Jingtao Dai: Writing – review & editing, Data curation. Guifen Gan: Writing – review & editing, Writing – original draft. Shijun Tong: Writing – review & editing, Data curation. Bin Sun: Writing – review & editing, Writing – original draft. Xiaojing Qi: Writing – review & editing, Data curation. Wenhua Liang: Writing – review & editing, Conceptualization, Visualization, Data curation. Jianxing He: Writing – review & editing, Conceptualization, Visualization, Data curation. Nanshan Zhong: Writing – review & editing, Conceptualization, Visualization.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
Ethics approval
The compassionate use of IBI314 was approved by the ethics committee of Qinghai Provincial Fourth People's Hospital and Zhengzhou First People's Hospital, with written informed content from all participants.
Funding
None
Acknowledgments
We thank all patients and their families. We also thank Innovent Biologics for providing IBI314.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.06.004.
Appendix. Supplementary materials
References
- 1.Martin-Blondel G., Marcelin A.G., Soulié C., Kaisaridi S., Lusivika-Nzinga C., Dorival C., et al. Outcome of very high-risk patients treated by Sotrovimab for mild-to-moderate COVID-19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study) J Infect. 2022;84(6):e101–e1e4. doi: 10.1016/j.jinf.2022.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA. COVID-19 Therapeutics-Types of EUA-authorized Products. https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs.
- 3.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385(23):e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Rodrigues Falci D., et al. Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou J., Li L., Zheng P., Liang W., Hu S., Zhou S., et al. Ultrapotent neutralizing antibodies against SARS-CoV-2 with a high degree of mutation resistance. J Clin Invest. 2022;132(4) doi: 10.1172/JCI154987. e154987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.