Abstract
Recent technological advances have helped scientists understand early human development. However, scientists’ ability to fully explore their potential comes in conflict with national and state-level policies in the USA. In 2016, for the first time, researchers were able to grow human embryos in culture up to 14 days but stopped because of scientific and legal limits. Other researchers have used stem cells in culture to create organized models of early human development, known as embryoids or cell-based embryo models. In this paper, we review federal and state laws that affect US human embryo and embryoid research. While federal policies focus on funding, state laws are often associated with human embryonic stem cells, abortion, fetal tissue research, and reproductive cloning. Of the 29 states with laws impacting human embryo research, only 11 states ban it, and none address embryoids directly, although five states limit aspects of this research. Overall, this complicated landscape suggests that additional national guidance would help scientists and the public navigate these controversial areas of research, however, it is unlikely to happen, considering the lack of past progress determining embryo research policy.
Keywords: cloning, embryo, embryoid, embryonic stem cells, embryo models, state laws
I. INTRODUCTION
Human embryo research often has been viewed in terms of the first 14 days after fertilization before the formation of the primitive streak, which denotes the end of implantation and the beginning of gastrulation. As Lewis Wolpert famously once put it, ‘It is not birth, marriage or death, but gastrulation which is truly the important event in your life’.1 Traditionally, research on human embryos was permitted prior to Day 14. This work has helped improve in vitro fertilization (IVF) techniques, our understanding of early human development, and the isolation of human embryonic stem cells (hESCs). By contrast, research has been proscribed after Day 14 or the development of the primitive streak, whichever comes first. This 14-day limit was a scientific norm until recently,2 a limit set by scientific and professional societies as well as within laws in some countries.3
A 14-day limit was first recommended in the 1979 report on IVF from the US Department of Health, Education, and Welfare (DHEW).4 But it is better known from the UK Warnock Report from 1984, which led to the UK Human Fertilisation and Embryology Act of 1990.5 Both reports recommended that human embryo research be allowed but only up to 14 days after fertilization.6
This specific limit was justified for several reasons during the debates associated with the US and UK reports as well as in discussions published afterward.7 The date approximately corresponds to the development of the primitive streak, an easily observable event that is one of the first signs of significant embryo organization. Fourteen days after fertilization is also believed to be the last point at which twinning can occur, which some scholars see as a point of individuation. When the limit was discussed in 1979 and 1984, this point was well beyond scientists’ ability to culture the human embryo. At the time, scientists were limited to less than 7 days postfertilization. While both groups came to the same consensus, it was not without controversy. For example, in the UK report, the recommendations for the 14-day limit were not a consensus but a majority opinion; the report includes a dissenting opinion written by several members who opposed some or all human embryo research.8
Until recently, many scientific and medical societies adopted this limit as a result.9 In May 2016, two independent research groups (one from the USA and the other from the UK) reported culturing human embryos in vitro to 14 days postfertilization at which point they stopped not because of technological limitations but because of national laws (in the UK) and the 14-day limit guideline (in the USA).10
After these groups halted their experiments in observance of the 14-day limit, some scientists and ethicists called for a reevaluation of this decades-old limit.11 Proponents of extending the guideline to later points in time cite the therapeutic possibilities that could arise from more research on early development as well as the arbitrary nature of the date chosen. Other scholars note that the limit was a hard-won compromise and urge that it should not be easily lifted.12 Many scholars also have suggested that science alone ought not be the justification for a change, pushing for a careful evaluation of the moral status of the embryo as well as of the ethical implications of allowing research beyond the 14th day before any policy decision is made.13
In addition to finally reaching Day 14 of development in the culture of human embryos, the limit has become challenged by another scientific advance—the embryoid. Embryoids are organized ESCs or induced pluripotent stem cells (iPSCs) that model early embryo development.14 Because embryoids use existing ESCs or iPSCs, they permit the study of early development without having to create (via fertilization) or destroy embryos. Furthermore, those developed from iPSCs do not involve any embryos whatsoever.15 Because they are lab-created and do not involve the direct destruction of a human embryo, they are often viewed as ethically less contentious than human embryo research. Embryoids can be obtained in large numbers and can be genetically manipulated to help researchers understand how different genes play roles in early development, which would be challenging to do with an embryo because of limited IVF sources. As a model, they allow scientists to test theories and hypotheses prior to working on human embryos, ultimately improving our knowledge of early human development while limiting the number of human embryos needed for research. However, as embryoids become more sophisticated and develop more features and characteristics of human embryos, especially integrated models, they will face ethical questions related to their potential and moral status.16
Embryoids also complicate the 14-day limit. For instance, gastruloids are an embryoid model of the gastrula stage, approximately, Day 17 after fertilization.17 Their development does not follow the same developmental progression as human embryos. While embryos grow from the one-cell stage through progressive steps of complexity, gastruloids instead jump in <14 days to a later stage (Day 17) without developing the primitive streak prior to the gastrula stage.
As a result of these discussions, in 2021, the International Society for Stem Cell Research (ISSCR) removed the 14-day limit from the prohibited category in its guidelines for research, instead suggesting that the research be evaluated on a case-by-case basis if ‘broad public support [exists and] local policies and regulations permit [research, in any case] ensuring only a minimal number of embryos are used’.18 ISSCR was the first scientific society to publicly announce that the limit was no longer a scientific norm. The guidelines argued that with the technology to move beyond 14 days of development, further public discussions were warranted regarding removing the limit in local jurisdictions. And where there is public consensus, the research should be extended.19 In addition, ISSCR also removed the 14-day limit from embryoid research and stated that the research should not be regulated as an embryo. Instead, they placed the research in the ‘permitted without oversight’ category if the embryoids were non-integrated (mimic aspects but not the full embryo) and ‘with oversight’ if the embryoids were integrated (contain all relevant cell types to potentially undergo full development).
Regardless of professional guidelines, in the USA, the government is traditionally the source of biomedical research policy. In this paper, we will review US federal regulations and state laws related to human embryo and embryoids research. Previous work has described US state laws impacting hESC research, with most of this work focusing on laws developed prior to 2009 when President Obama changed federal guidelines. Other work focuses only on national embryo research policies with brief descriptions of US federal policies. This manuscript presents a more complete picture of US policy by analyzing US federal and state laws to determine their impact on both embryo and embryoid research. While the federal government holds significant influence over biomedical research policy, US state policies play an important role delimiting research into early human development since there are currently no existing federal laws specifically permitting or prohibiting human embryo or embryoid research.20 While US federal laws do include limitations on federal funding for embryo research, how this law is applied to embryoid research is determined on a ‘case-by-case basis’. The majority of states allow embryo and embryoid research, either by directly permitting it or by the absence of laws that would restrict it. Several states do have existing laws related to embryo, ESC or fetal tissue research, or abortion which limit or ban embryo and portions of embryoid research. As a result, the USA has developed over time a complicated patchwork of state laws. Little effort has been directed toward building a consensus between states or developing a federal policy. Instead, these science- and morality-related policies are left for each state to determine.
II. US FEDERAL LAWS AND GUIDELINES
Biomedical research policy traditionally has been a federal responsibility; however, US federal laws guiding human embryos and embryoid research are limited.21 There are no federal laws that cover both public and privately funded research, likely due to the controversial nature of the work.22
While federal regulations provide no definition of an embryo (instead, it is defined within a budget amendment, which will be discussed later), a fetus is defined as ‘the product of conception from implantation until delivery’.23 This is notable because it is significantly different from how scientists define the terms ‘embryo’ and ‘fetus’. Scientifically, the embryo stage spans from conception until 8 weeks of gestational age, while the fetal stage starts at the third month and ends at birth.24 Implantation, which ends around 14 days after fertilization, occurs within the embryonic stage per the scientific definition, but within the fetal stage, according to federal regulations. The definition of a fetus is linked with other federal statutes related to research on or funding for fetal research. Federal statutes related to human subjects research allow fetal research:
The Secretary may not conduct or support any research or experimentation, in the USA or in any other country, on a nonviable living human fetus ex utero or a living human fetus ex utero for whom viability has not been ascertained unless the research or experimentation—
(1) may enhance the well-being or meet the health needs of the fetus or enhance the probability of its survival to viability; or
(2) will pose no added risk of suffering, injury, or death to the fetus and the purpose of the research or experimentation is the development of important biomedical knowledge which cannot be obtained by other means.25
Together, the definition and statutes have been interpreted to impact all embryonic research post-implantation for human fetuses (or embryos) in utero.26 While institutions and individuals are governed by these human subject regulations, ex vivo embryos, embryos grown in vitro or in culture are not included in federal definitions or regulations as ‘human subjects’ for these purposes.27 Furthermore, no regulation or statute addresses or sets limits on in vitro embryo research nor is there a 14-day limit within the regulation.
While federal regulations do not prohibit research on ex vivo embryos, an annual appropriation amendment (or rider) known as the Dickey-Wicker Amendment (DWA) does apply to both human embryo research and potential embryoid research.28 Named after the authors of the bill, Rep. Jay Dickey (R-AK) and Rep. Roger Wicker (R-MS), this appropriation amendment bans federal funding for human embryo research and includes a definition of a human embryo:
Sec. 508 (a). None of the funds made available in this Act may be used for—
(1) the creation of a human embryo or embryos for research purposes; or
(2) research in which a human embryo or embryos are destroyed, discarded, or knowingly subjected to risk of injury or death greater than that allowed for research on fetuses in utero under 45 CFR 46.204(b) and section 498(b) of the Public Health Service Act (42 U.S.C. 289g(b)).
(b) For the purposes of this section, the term ‘human embryo or embryos’ includes any organism, not protected as a human subject under 45 CFR 46 as of the date of the enactment of this Act, that is derived by fertilization, parthenogenesis, cloning or any other means from one or more human gametes or human diploid cells.29
As a result of the definition and the language of the Act, the DWA prohibits human embryo research projects from obtaining federal funds. The NIH interprets the DWA as a ban on funding for the derivation of hESCs, but which allows research using existing hESCs (created previously using non-federal funds). This interpretation was upheld in the 2010 case Sherley v Sebelius.30 However, the interpretation of the DWA with respect to embryoids has not been clarified by the NIH. Embryoid models use existing ESCs lines or iPSCs, which the DWA allows. But it is unclear whether or not they create an embryo. The DWA definition includes embryos created through ‘any other means from one or more human gametes or human diploid cells’, which could be interpreted to include ESCs and iPSCs. NIH has not ruled—nor has Congress—whether embryoids in general or specific types of embryoids are indeed an embryo. The law does not discuss or qualify within the definition that an embryo must have organismal potential to become a human being.
Unlike when ESCs were first isolated in 1998 and the Department of Health and Human Services (DHHS) lawyers ruled within 1 year how NIH and researchers should interpret DWA, the status of embryoids is yet to be determined despite the publication of the first models in 2014.31 Officially, NIH states they look at the potential of the model and ask specific questions related to the stage of embryo development being modeled, cell types present, and spatial orientation to determine whether a model is eligible for funding.32 These grant decisions are made on an ad hoc basis and likely by NIH staff prior to the grant being peer-reviewed externally. As a result, embryoid research is being reviewed on a case-by-case basis at NIH without clear or consistent guidelines prior to grant submissions.
Of note, unlike the definitions and statutes associated with fetal research, the DWA is not permanent legislation. Instead, it is passed annually attached to the funding bill associated with the DHHS.33 It has been replicated almost verbatim every year since its first appearance in the 1996 fiscal budget bill. 34 The amendment impacts all agencies within the DHHS, including NIH, the Centers for Disease Control and Prevention, and the Food and Drug Administration (FDA). With an annual budget of >$50 billion, the NIH is responsible for the vast majority of biomedical research in the USA and is the largest funder for biomedical research in the world. As a result, the DWA’s ban on human embryo and potential embryoid research has a significant impact on research. Scientists involved in banned research cannot receive federal grants for their work. They are also prohibited from using materials purchased using funds from federally funded projects, such as equipment and reagents. Therefore, scientists must use non-federally funded facilities, equipment, reagents, and other materials to conduct human embryo research lawfully.
In addition, the US government indirectly bans using any genetically modified human embryo, or embryoid, for reproductive purposes by limiting FDA’s ability to review protocols. Similar to the DWA, this ban is contained within the annual appropriation bill as an amendment:
Sec. 740. None of the funds made available by this Act may be used to notify a sponsor or otherwise acknowledge receipt of a submission for an exemption for investigational use of a drug or biological product under section 505(i) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 355(i)) or section 351(a)(3) of the Public Health Service Act (42 U.S.C. 262(a)(3)) in research in which a human embryo is intentionally created or modified to include a heritable genetic modification. Any such submission shall be deemed to have not been received by the Secretary, and the exemption may not go into effect.35
The amendment first appeared in the 2016 appropriations bill and has appeared annually since.36 The FDA has previously stated that it would oversee any genetically modified embryo, including those created via reproductive cloning, mitochondrial replacement therapy, and CRISPR-Cas9 modification of specific genes. While this authority has not been challenged in the courts, the amendment not only prohibits the FDA from not only approving any application but also acknowledging that a submission to use a genetically modified embryo was received.
While human embryoid research can be funded by NIH (although this is variable and still being reviewed on a case-by-case basis), human embryo research is funded using primarily private and state funds. For example, human embryo research and the creation of hESC lines have been funded by state agencies, such as the California Institute for Regenerative Medicine (CIRM), or private foundations like the Juvenile Diabetes Research Foundation. Private research is only constrained by laws of the state in which it is conducted, if any exist.37
III. THE STATE LANDSCAPE AND SURVEY
State legislatures play an important role in regulating human embryo and embryoid research, as the US federal policies (including regulation and statutes as well as the DWA) only address what can and cannot be federally funded. To determine the landscape of state legislation related to and impacting human embryo and embryoid research, we searched all 50 state legislative online databases for statutes and laws containing the following terms: ‘embryo’, ‘cloning’, ‘somatic cell nuclear transfer’, ‘fetus’, ‘stem cell’, and ‘in vitro fertilization’. Search terms were appended using the boolean search operator ‘*’ to capture word variations with the same root (eg ‘embryo*’ returned legislation containing ‘embryos’ and ‘embryonic’). Results were then reviewed by both authors to determine if they were applicable or were deemed irrelevant. For example, laws related to animal or adult stem cell research were excluded. The statutory interpretations were limited to the plain language of said statutes and excluded legislative history and case law, which are outside of the scope of this manuscript. Restricting the analysis to the plain text of the laws, we acknowledge, does overlook the implications of how some actors within the state and/or court rulings interpret the laws. Laws identified were compared to existing literature discussing state policies, especially articles related to ESC research, to verify interpretation and identify any missing statutes. From the review, we identified 29 states with laws that impacted human embryo and/or embryoid research.
Embryoid research is a new field with the first publications starting in 2014, with only minimal public discussions; as a result, as of 2022, there are no laws that directly address embryoids, but perhaps this could change in the future. Any restrictions on this research are a result of laws affecting research using human embryos and/or ESCs.
While the majority of states do not have explicit laws related to human embryo research, some individual states have chosen to implement policies within their own borders as to what research concerning human embryos, hESCs, and, indirectly, embryoids, is allowed. These laws vary significantly from state to state, resulting in a complicated patchwork of laws.38 A few human embryo laws are several decades old, some dating to the 1970s and linked with abortion laws. Other laws restricting fetal tissue research or prohibiting human cloning (either for research or reproductive purposes) were enacted as a result of Dolly, the first mammal being cloned.39 After President George W. Bush’s administration limited federally funding for ESC research in 2001, there was continued public discussions about hESCs from 1999 to 2008.40 This resulted in increased state engagement on the topic with >450 bills filed in state legislatures related to hESC during this period. 41 A portion, 67 bills, or 14 per cent, became law.42
State legislation can be organized into three broad categories with regard to human embryo research: (i) states without laws; (ii) prohibitive states that ban most or all research; and (iii) permissive states that allow research (Table 1 and Figure 1). In many cases, laws affecting human embryo research also impact human embryoid research but not always. Several states’ laws display different stances toward embryoid research compared to human embryo research. Finally, we located a few states regulating somatic cell nuclear transfer (SCNT), but these laws often have little to no impact on human embryo or embryoid research.43
Table 1.
US state laws that impact research on human embryos, hESCs, and/or human embryoids. States were categorized based on the impact of laws (stated or implicit) on human embryo research. Permissive states (+) had laws explicitly encouraging or had laws which did not prohibit human embryo research. Prohibitive states (−) restrict most or all human embryo research, generally through statewide prohibition or bans on use of state funds.a State laws limiting embryoid research are noted, if present, otherwise states with no laws impacting research were labeled as ‘N/A’. State cloning laws were noted, if present, identifying if they banned only reproductive cloning (Rep) or if this ban extends to all SCNT (All).
| States | hER | Embryoidb | Cloning | Statute(s) |
|---|---|---|---|---|
| Arizona | + | N/A | All | AZ Rev. Stat. § 35-196.04, 36-2302, -2311, -2312, -2313 |
| Arkansas | − | N/A | All | AR Code §5-1-102, 16-62-102, 20-8-502, 20-16-1003, -2202, -2203, -2204, -2205, 20-17-801 |
| California | + | CIRM 12-day limit | Rep | CIRM guidelines per CA Health & Safety Code § 125118; CA Health & Safety Code § 24185, 125292.10, 125300 |
| Connecticut | + | 17-day limit | Rep | CT Stat. § 32-41jj |
| Florida | + | N/A | N/A | FL Stat. § 390.0111-6, 742.13-12 |
| Illinois | + | N/A | Rep | IL Executive Order 6 (2005) and Executive Order 3 (2006); 410 IL Code § 110 |
| Indiana | + | N/A | All | IN Code § 16-18-2-56.5, 16-34.5-1-1, 35-46-5-2, -3(f) |
| Iowa | + | N/A | Rep | IA Code § 707C.3, -4 |
| Kansas | N/A | Limit on state funds/facilitiesb | N/A | KS Stat. § 76-839 |
| Kentucky | − | N/A | N/A | KY Rev. Stat. § 311.715 |
| Louisiana | − | Ban on hESCs prior to 2001b | All | LA Rev. Stat. 9 §122; 40 § 1300 |
| Maine | + | N/A | N/A | ME Rev. Stat. Tit. 22, 263-B §1593, -1595 |
| Maryland | + | N/A | Rep | MD Economic Development Code Ann. § 10-430, -431, -438, -439, -440 |
| Massachusetts | + | N/A | Rep | MA Gen. Laws Part I Title XVI Ch 111L §8, Ch 112 §12 J |
| Michigan | + | N/A | All | MI Constit. Art. I §27; MI Comp. Laws § 333.16274, -16275; MI Pub Health Code Act 368 of 1978 § 333.2685–.2692 |
| Minnesota | − | N/A | N/A | MN Stat. § 145.421, -422 |
| Missouri | + | N/A | All | MO Const. Art. III, § 38(d) |
| Montana | + | N/A | Rep | MT Code § 50-11-101, -102, -103 |
| Nebraska | _ | Ban on state fundsb | All | NE Rev. Stat. § 71-7606, -8806 |
| New Jersey | + | N/A | Rep | NJ Rev. Stat. § 26:2Z-2, § 2C:11A-1 |
| New Mexico | − | N/A | N/A | NM Stat. §24-9A-1, -2, -3 |
| New York | + | N/A | N/A | NY Consolidated Laws, PBH §265-a |
| North Dakota | − | Yes | All | ND Cent. Code § 12.1-39, § 14-02 |
| Ohio | + | N/A | N/A | OH Rev. Code § 2919.14 |
| Oklahoma | − | Ban on hESCs prior to 2001b | All | OK Stat. tit. 63, § 1-270.2, § 63-1-727 |
| Pennsylvania | − | N/A | N/A | PA Cons. Stat. Tit. 18 Ch. 32 § 3216 |
| Rhode Island | − | N/A | All | RI Gen. Laws § 11-54-1, § 23–16.4 |
| South Dakota | − | Bans hESC researchb | All | SD Codified Laws §34.14.16, -17, -18, -19, -20, -27, -28 |
| Utah | + | N/A | N/A | UT Code § 76-7-301, -301.1, -310 |
| Virginia | + | N/A | Rep | VA Code Ann. § 32.1-162.22, -31 |
Notes: All = bans reproductive cloning and SCNT; hER = human embryo research; N/A = no legislation; Rep = bans only reproductive cloning; + = permissive; − = prohibitive.
aNo law impacting human embryo or embryoid research was located in the following states: Alabama, Alaska, Colorado, Delaware, Georgia, Hawaii, Idaho, Mississippi, Nevada, New Hampshire, North Carolina, Oregon, South Carolina, Tennessee, Texas, Vermont, Washington, West Virginia, Wisconsin, and Wyoming.
bLaws limit research using embryoids created from hESC, but not from other sources.
Figure 1.
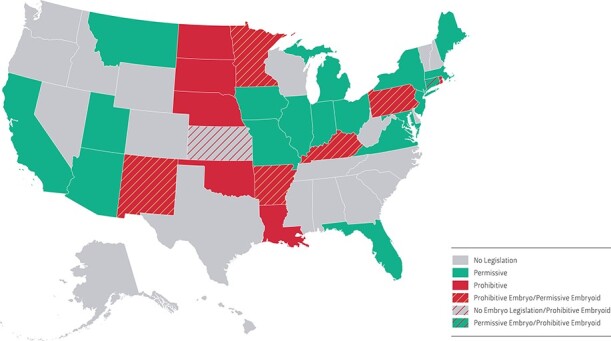
US map of state human embryo research policies. Human embryo research legislation falls into one of three categories: ‘no’ legislation (gray), ‘prohibitive’ (red), and ‘permissive’ (green). A few state laws differ between human embryo and embryoid research (stripes). Connecticut has a permissive human embryo law but restricts embryoids (<17 days). Kansas has no embryo law but does have a restriction on hESC research, including embryoid research. Six states (Arkansas, Kentucky, Minnesota, New Mexico, Pennsylvania, and Rhode Island) have restrictions on embryo research but not on embryoid research.
III.A. No Human Embryo Research Legislation
Twenty-one states lack any specific law on human embryo research, relying instead on federal law, policies, and guidelines: Alabama, Alaska, Colorado, Delaware, Georgia, Hawaii, Idaho, Kansas, Mississippi, Nevada, New Hampshire, North Carolina, Oregon, South Carolina, Tennessee, Texas, Vermont, Washington, West Virginia, Wisconsin, and Wyoming. In this category, there are no laws nor agencies overseeing human embryo research at the state level. The states opting out of passing laws to address this issue do not necessarily have much in common politically; they might have chosen not to pass legislation for various reasons, such as the topic not being a priority within the state, or not having the majority needed to push related legislation through. Politically, these states range from conservative (eg Texas) to more liberal (eg Oregon).44 Of these 21 states, only Kansas has legislation on hESC research that impacts embryoid research, which will be described in more detail in a later section.
III.B. Prohibitive State Legislation on Human Embryo Research
Eleven states have laws that are ‘prohibitive’, banning human embryo research: Arkansas, Kentucky, Louisiana, Minnesota, Nebraska, New Mexico, North Dakota, Oklahoma, Pennsylvania, Rhode Island, and South Dakota.45 Several themes emerge among these states’ laws. Some define a fetus to include conception through birth in prohibitions of fetal tissue research. Others only allow research that does not harm an embryo, which excludes the creation of hESCs as well as research on human embryos beyond IVF. Four states ban the use of public funds for human embryo research.
Five states prohibit fetal research and define a fetus broadly to include conception through birth: Minnesota, New Mexico, North Dakota, Pennsylvania, and Rhode Island. New Mexico law defines a fetus as ‘the product of conception from the time of conception until the expulsion or extraction of the fetus’.46 Similarly, Pennsylvania defines an unborn child or fetus as ‘an individual organism of the species homo sapiens from fertilization until live birth’.47 North Dakota’s legislation declares that ‘a person may not use any live human fetus, whether before or after expulsion from its mother’s womb, for scientific, laboratory, research, or other kind of experimentation’ and defines a fetus to include ‘an embryo or neonate’.48 In Rhode Island, the statutes also specify that ‘the word “fetus” includes an embryo or neonate’ in the law prohibiting fetal research.49 Many of these definitions do not specifically address the culturing of embryos in vitro, but hint enough at a ban on human embryo research—especially in combination with an additional law specifically banning all human cloning—to classify these states as being ‘prohibitive’.
Minnesota, New Mexico, and Pennsylvania frame their regulations to reflect a ‘do no harm’ approach. For example, Minnesota’s statute prohibits any manipulation ‘except to protect life or health’ of what it terms a ‘human conceptus’, that is, ‘any human organism, conceived either in the human body or produced in an artificial environment other than the human body, from fertilization through the first 265 days thereafter’.50 Pennsylvania bans ‘any type of nontherapeutic experimentation or nontherapeutic medical procedure’ on human embryos.51 Considering the risks to the human embryo associated with this research, these laws effectively ban any form of experimentation on them. Similarly, this approach proscribes the derivation of hESCs from human embryos for research—or any other—purposes. However, this does not restrict the use of existing hESCs, nor does it affect embryoid research.
Louisiana and South Dakota implement explicit bans of human embryo research. In Louisiana’s legislation, ‘the use of a human ovum fertilized in vitro is solely for the support and contribution of the complete development of human in utero implantation’.52 It further states that ‘no in vitro fertilized human ovum will be farmed or cultured solely for research purposes or any other purposes’. South Dakota’s laws prohibit ‘research that destroys a human embryo’ as well as ‘research that subjects a human embryo to substantial risk of injury or death’.53 It goes further to prohibit research on ‘cells or tissues that the person knows were obtained’ from embryos.
Arkansas, Kentucky, Nebraska, and Oklahoma policies ban the use of state funds and facilities for human embryo research and therapeutic cloning. These laws only cover public funds but do not represent state-wide prohibitions. Research can be conducted using non-state funds and most allow research using hESCs and just not their creation. Arkansas passed a law in 2019 banning public funding of ‘human cloning or destructive embryo research, including destructive embryonic stem cell research’, stating that ‘the moral justification of medical and scientific research cannot be based upon the dehumanizing and utilitarian premise that the ends justify any means’.54 Kentucky’s law only allows the use of public funds for human embryo research ‘as long as such procedures do not result in the intentional destruction of a human embryo’.55 Nebraska law states: ‘No state facilities, no state funds, fees or charges and no investment income on state funds shall be used to destroy human embryos for the purpose of research. In no case shall state facilities, state funds, fees, or charges, or investment income on state funds be used to create a human embryo by somatic cell nuclear transfer for any purpose’.56 This prohibits the use of public funds for research ‘involving the use of embryonic stem cells’,57 or for procedures that result in the destruction of a human embryo.58 In Oklahoma, state funding is not allowed for research using a human embryo and it defines an embryo as ‘a living organism of the species Homo sapiens at the earliest stage of development, including the single-cell stage, that is not located in the body of a woman’.59
Most of the laws, with the exception of Nebraska, South Dakota, and Oklahoma, do not restrict the use of existing hESC lines or the development of embryoids (as described later in more detail related to state variations). Embryoids created from iPSCs also seem to be permissible.
III.C. Permissive State Legislation: Laws that Allow for Human Embryo Research
Eighteen additional states permit human embryo research.60 Legislation in five of these specifically allows it: California, Connecticut, Michigan, Montana, and New York. The other 13 states allow research on embryos by virtue of either vague or overly specific legislation, focused, for example, on the creation of hESCs for research or research using fetal tissue from abortions. These states are Arizona, Florida, Illinois, Indiana, Iowa, Maine, Maryland, Massachusetts, Missouri, New Jersey, Ohio, Utah, and Virginia.
Of the states with expanded human embryo research opportunities (associated with permissive hESC research policies), the most well-known case is California. It was the first state to pass a law (Proposition 71) that specifically allowed and funded stem cell research, including human embryo and hESC research.61 Initially passed in 2004 and later renewed in 2020, Proposition 71 allocated $3 billion to fund stem cell research over 10 years and established the CIRM, a new state agency, that would oversee the research. The CIRM’s ‘Guidelines for Human Stem Cell Research’ mandate that projects utilizing human embryos are eligible for funding as long as they are only cultured up to 12 days postfertilization or the appearance of the primitive streak, whichever comes first.62 This restriction does limit human embryo research but not embryoid research.
After California passed Proposition 71, several other states followed suit by passing laws that support but regulate human embryo and hESC research, including Connecticut, Illinois, Maryland, and New York.63 In 2005, Connecticut announced state funding for stem cell research and experimentation on human embryos. This law requires that all human embryo research be conducted before gastrulation occurs, which is typically around 17 days postfertilization.64 Maryland also created a stem cell fund that encourages adult stem cell research65 and in addition expressly permits hESC and embryo research within the state: ‘nothing in this part may be construed to prohibit the creation of stem cell lines to be used for therapeutic research purposes’.66
New York does not have an explicitly permissive legal statute, but the New York State Stem Cell Science program (NYSTEM), within the state’s Department of Health, has a 14-day limit spelled out in the consent form for donating embryos for research: ‘Embryos will not be used to create a pregnancy, and will not be allowed to develop beyond 14 days’.67 However, the program was defunded in the 2022 state budget.68
Many state laws affirm their support for research. Michigan law is categorized as supportive because it explicitly orients regulations toward ensuring that its ‘citizens have access to stem cell therapies and cures [and its] physicians and researchers can conduct the most promising forms of medical research’.69 Similarly, the Missouri Stem Cell Research and Cures Initiative stipulates similar aims, noting its intent ‘to ensure that Missouri patients have access to stem cell therapies and cures’.70 Iowa’s law is identical in its purpose—and phrasing—‘to ensure that Iowa patients have access to stem cell therapies and cures’.71 Montana law further states that ‘nothing in this section prohibits embryonic stem cell research using embryonic stem cell lines of uncloned origin’.72 New Jersey law explicitly permits ‘research involving the derivation and use of human embryonic stem cells, human embryonic germ cells and human adult stem cells, including somatic cell nuclear transplantation’.73 Virginia does not have a law related to embryo or hESC research, but its law banning human cloning explicitly permits SCNT.74
Some state laws that sanction human embryo research limit the sources of embryos, however. Illinois, Massachusetts, Michigan, and Missouri prohibit the creation of embryos solely for the purpose of research while allowing experimentation on leftover embryos from IVF. The IVF process often produces an excess of embryos that are frozen for later use. These embryos can be donated for research purposes, given to another couple, or destroyed. Research on embryos left over from IVF is often seen as less morally controversial because they might otherwise be discarded. Massachusetts law bans embryo creation for research purposes, stating that ‘no person shall knowingly create an embryo by the method of fertilization with the sole intent of donating the embryo for research’.75 Illinois and Missouri ban the creation of embryos solely to produce hESCs. Missouri law states that ‘no human blastocyst may be produced by fertilization solely for the purpose of stem cell research’.76 Illinois bans creating human embryos for research but funds hESC creation and research. The Illinois governor signed Executive Order 6 (2005) and Executive Order 3 (2006), which established the Illinois Regenerative Institute for Stem Cell Research and allocated $10 million in research funding. The orders prohibit the use of these funds for the creation of embryos for research purposes but allows research on embryos left over from IVF procedures. In addition, the Illinois state legislature passed a law prohibiting human reproductive cloning but explicitly permitting therapeutic cloning (cloning to develop cells but not using them to create a human), hESC, and human embryo research.77 Following a different approach, Michigan legislation does not include a ban on a portion of research but instead stipulates the desire to advance hESC research and therapies and a subsequent authorization to conduct human embryo research on IVF embryos not used or suitable for implantation.78
In Arizona and Indiana, the laws prohibit the creation of hESCs from embryos but not in vitro embryo research or research using hESCs that have already been developed. In Arizona, ‘a person shall not intentionally or knowingly engage in destructive human embryonic stem cell research’.79 Indiana’s law states the use of ‘a human embryo created with an ovum provided to a qualified egg bank under this section for purposes of embryonic stem cell research commits unlawful use of an embryo, a Level 5 felony’.80
Several states have laws limiting or banning fetal tissue research, but this does not cover the embryonic stage. In the case of Maine, existing legislation is a prohibition of fetal research, defining a fetus as ‘a product of conception after complete expulsion or extraction from its mother’. This law does not affect in vitro human embryo research nor does it specifically address hESC nor embryoid research.81 Florida law notes both the terms embryo and fetus, banning only the ‘use [of] any live fetus or live, premature infant for any type of scientific, research, laboratory or other kind of experimentation’.82
Other related laws focus specifically on abortions and research using aborted fetuses, which does not apply to in vitro embryo research. Utah’s Criminal Code states that ‘live unborn children may not be used for experimentation’, where ‘live unborn child’ is to be interpreted as a fertilized ovum after implantation.83 Since implantation implies in vivo, this ban can be viewed as permissive of in vitro human embryo research. Ohio’s laws only ban research on ‘the product of human conception which is aborted’.84
Language in none of these state laws prohibit embryoid research, with the exception of Connecticut, which will be discussed below. All allow hESC and iPSC research and therefore allow embryoids to be developed and researched.
III.D. Reproductive Cloning Bans
While no federal legislation bans reproductive cloning (cloning a human being), a type of human embryo research, we found 22 state laws banning it.85 Some of these bans also include the use of SCNT for research purposes in addition to reproductive cloning. Eight cloning laws are in states deemed as ‘prohibitive’ (Arkansas, Arizona, Louisiana, Nebraska, North Dakota, Oklahoma, Rhode Island, and South Dakota). Five states with permissive policies include the restriction (California, Connecticut, Massachusetts, Michigan, and Montana), as do a few other states with laws that do not explicitly permit or inhibit human embryo research (Illinois, Indiana, Iowa, Maryland, New Jersey, and Virginia).86 For example, Iowa law states that ‘a person shall not intentionally or knowingly do any of the following: a) perform or attempt to perform human reproductive cloning; b) participate in performing or in an attempt to perform human reproductive cloning’.87
Some states explicitly permit SCNT for research purposes, though. In Iowa law, ‘human reproductive cloning does not include somatic cell nuclear transfer [SCNT] performed for the purpose of creating embryonic stem cells’.88 Massachusetts bans both creating human embryos for research purposes through fertilization and experimentation on fetuses (which includes embryos), but its legislation explicitly allows the ‘creation of a pre-implantation embryo by somatic cell nuclear transfer [SCNT], parthenogenesis or other asexual means for research purposes’.89 California, Connecticut, Illinois, Maryland, Montana, and Virginia are other states with exceptions for SCNT within their ban of reproductive cloning.90 Rhode Island passed a similar law in 1998, but it expired in 2017.91
By contrast, Michigan’s laws banning cloning define human cloning as ‘the use of human somatic cell nuclear transfer [SCNT] technology to produce a human embryo’. Therefore, while it permits human embryo and embryonic stem cell research, it prohibits SCNT.92 Missouri law similarly bans creating a human embryo for research purposes as well as cloning a human being.93
III.E. Embryoid Research Variations: State with Differing Embryo and Embryoid Research Limits
Legislation in eight states impacts human embryo research and hESC-related research in contrasting ways (Table 1, Figure 1). In six states that are prohibitive of embryo research, the laws allow for ESC research and thereby permit embryoid research: Arkansas, Kentucky, Minnesota, New Mexico, Pennsylvania, and Rhode Island. Laws in Minnesota, New Mexico, Pennsylvania, and Rhode Island apply to human embryo research and not to embryoids. For example, Minnesota law specifically requires fertilization for an entity to be considered a ‘human conceptus’, which does not apply to embryoids, which are created from ESCs or iPSCs.94 Arkansas and Kentucky have restrictions on creating ESCs but not on using ESC lines created elsewhere. They also permit embryoids from lines created using iPSCs.
Kansas has no human embryo research legislation but does have laws addressing ESCs that also affect embryoid research. Kansas legislation prohibits the use of public funds and facilities allocated to the Midwest Stem Cell Therapy Center for research involving ESCs or fetal tissue cells.95 Funds and facilities can only be used for research using ‘adult, cord blood and related stem cells and non-embryonic stem cells’, thus also limiting the ability to use public (but not other) funds and facilities to conduct embryoid research for models derived from ESCs, although not from iPSCs.
Connecticut legislation is supportive of human embryo and ESC research, but is prohibitive of some embryoid research. While narrowly focused on hESC research (again allowing iPSC-based embryoids), the law states:
A person may conduct research involving embryonic stem cells, provided (1) the research is conducted with full consideration for the ethical and medical implications of such research, (2) the research is conducted before gastrulation occurs, (3) any human embryos, embryonic stem cells, unfertilized human eggs or human sperm used in such research have been donated voluntarily in accordance with the provisions of subsection (c) of this section, or if any embryonic stem cells have been derived outside the state of Connecticut, such stem cells have been acceptably derived as provided in the National Academies’ Guidelines for Human Embryonic Stem Cell Research, as amended from time to time, and (4) all activities involving embryonic stem cells are overseen by an embryonic stem cell research oversight committee.96
Since gastrulation occurs around 17-day postfertilization, this limits hESC-based embryoids to time periods before this point. This prohibits, for example, gastruloids, both two- and three-dimensional versions, which mimic development around this time.
IV. DISCUSSION
Human embryo and embryoid research is governed by limited federal policies and regulations and disharmonious state laws. The inconsistency among, as well as the ambiguous language within, states’ laws make it complicated to understand what research can and cannot be conducted. Moreover, the majority of the laws were developed specifically to address other issues, such as abortions, fetal tissue research, reproductive cloning, or hESC research. For these reasons, some laws overlap with and impact human embryo and embryoid research, while others leave them unaddressed.
These state laws can be viewed as a form morality policy.97 Many Americans tend to view embryo and related research (including hESCs) with strong emotions linked to their opinions about abortion and the moral status of the embryo. 98 By contrast, scientists have approached advocacy for research in this area based on another set of emotions, including (i) the anxieties, despair, and hope of patients who could benefit from this research; (ii) whether that research answers questions related to infertility; or (iii) whether said research might lead to potential therapies for severe diseases.99 Further complicating the policy landscape is the overall limited trust many Americans have of the government and governmental institutions. As a result, most US research is self-regulated by scientists and through locally based oversight committees such as institutional review boards (IRBs) instead of a national authority.100 Other factors affecting state policies include the political party in charge, prior morality policies, the policy environment of neighboring states, the strength of the scientific community, and discussions at the federal level bringing the topic to the forefront of policymaking, all of which have influenced whether states adopt supportive or restrictive policies.101 These overlapping and sometimes conflicting factors make it unlikely that we will see any consensus among states or developed at the federal level.
To conduct embryo and embryoid research in the USA, researchers must carefully navigate these laws as well as local politics. Creating additional difficulties, a number of state laws lack clarity, which creates an environment open to interpretation. For example, Pennsylvania’s law intended to restrict fetal experimentation defines a fetus as ‘an individual organism of the homo sapiens from fertilization until live birth’.102 By this definition, all human embryo research, including research on IVF, is banned. By contrast, Arizona law banning human fetal or embryo research specifically focuses on the use of products of abortion stating: ‘A person may not use a human fetus or embryo or any part, organ or fluid of the fetus or embryo resulting from an abortion in animal or human research, experimentation or study’.103 This law does not cover any research using IVF embryos, nor does it impact embryoid research.
While some states may have unclear laws, or laws with, perhaps, unintended consequences, other states are very precise. In South Dakota’s laws, there is no doubt that human embryo research is banned: ‘No person may knowingly conduct nontherapeutic research that subjects a human embryo to substantial risk of injury or death’.104 Another example can be found in the Michigan constitution, which is supportive of human embryo research: ‘any research permitted under federal law on human embryos may be conducted in Michigan’.105
We also discovered similar language and phrases in different state laws, suggesting a vertical diffusion of policies between states.106 For example, several states specify a ban on ‘destructive embryo research’, including Arkansas, Kentucky, Nebraska, and South Dakota.107 Both North Dakota and Rhode Island specifically define a fetus as ‘embryo or neonate’. Iowa and Missouri have identical language related to the purpose of their laws, ‘to ensure that [state] patients have access to stem cell therapies and cures [and] that [state] researchers may conduct stem cell research’.108 Similar but slightly different phrases were used in Michigan’s law as well.
While the 14-day limit is common in national policies outside of the USA, it was not alluded to in US federal or state laws.109 California and Connecticut laws used alternative limits, 12 and 17 days, respectively, while New York only had the 14-day limit embedded in the consent documentation for embryo research for hESCs but not in the law.110
There were no laws specifically addressing embryoid research. For the purpose of this type of research, all laws require interpretation. Overall, embryoid research limits were linked to how supportive a state is of hESC research. However, as noted, a few states with prohibitions for creating a hESC line still allow for the use of the cells in research and therefore also permit research with hESC-based embryoids. No laws limit research using iPSC-based embryoids.
Interestingly, laws banning reproductive cloning were straightforward compared to national laws abroad. Many were as explicit as Virginia law, which states that:
No person shall (i) perform human cloning or (ii) implant or attempt to implant the product of somatic cell nuclear transfer into a uterine environment so as to initiate a pregnancy or (iii) possess the product of human cloning or (iv) ship or receive the product of a somatic cell nuclear transfer in commerce for the purpose of implanting the product of somatic cell nuclear transfer into a uterine environment so as to initiate a pregnancy.111
By contrast, Australia’s reproductive cloning law includes broader and vaguer language: ‘any process that initiates organized development of a biological entity’.112 This law, passed originally in 2002, impacted both reproductive cloning as well as embryoids which can have the potential to be a human being (although limited). Currently, Australian policies regulate embryoids like embryo research, requiring researchers to obtain a license to conduct the work.113 Furthermore, none of the US state laws define the embryo in the detail we see in the DWA. As a result, embryoid research is permitted in all jurisdictions for those derived from iPSCs and any state without a complete hESC research ban.
Despite states maybe allowing research, US researchers still need additional guidance on how to conduct human embryo and embryoid research. In the past, NIH has played a substantial role developing and enforcing federal guidance for other areas of biomedical research including research using human subjects, recombinant DNA, and hESCs.114 Using the hESC example, NIH considered hESC derivations outside of their scope until 2009 when it finally released finalized guidelines for NIH funding and clarified previously nongovernmental guidelines from the National Academies of Science, Engineering and Medicine (NASEM), and ISSCR.115 The NIH guidelines are now used by other entities to direct how hESCs are derived or used.
However, NIH considers embryo research outside of its jurisdiction (because the DWA bans funding from the agency) and has yet to set clear and precise guidelines for what embryoid research is eligible for funding. By not adequately and explicitly delineating what is permitted and prohibited at the federal level, the USA risks bad actors taking advantage and conducting questionable work.116 In addition, the USA loses efficiency that could be gained through a federal framework of uniformed standards for research.117
NASEM at times plays a role in developing these standards, especially related to ethical guidelines, when they are not present at the federal level. For example, NASEM created guidelines for hESC research and the guidelines for developing hESC lines, which can be applied to some embryoid work.118 However, they have not directly addressed human embryo or embryoid research beyond promoting a 14-day limit within their hESC guidelines.119
ISSCR also sets both US and international stem cell research standards. In their 2021 guidelines, they recommend what should and should not be permitted with regard to human embryo and embryoid research as well as the type of oversight needed.120 However, additional guidance is still necessary. For example, research on human embryos beyond Day 14 was designated as permissible in 2021 after having listed it as prohibited in their 2006 and 2016 guidelines. Research projects involving embryos are to be reviewed on a case-by-case basis, but it is less clear what level of evidence is needed and what would be an appropriate level of justification to warrant research on human embryos at later developmental points.121 In the case of embryoids, the ISSCR 2021 guidelines suggest these should be approached differently from human embryos because they are only models without full developmental capacity or the potential to become a human. They divide embryoids into two types of models: embryoids with extra-embryonic cells that have more development potential (integrated models), and embryoids without extra-embryonic cells which mimic specific aspects of development (non-integrated models). But why the extra-embryonic cells and tissues are significant enough to be the deciding factor to distinguish between which embryoids receive ethical oversight is not explained.
Both NASEM and ISSCR recommend oversight of some form for hESC research that could be applied to both embryoid and embryo research at the local level. NASEM recommends that hESC research be reviewed by a specialized board, an embryo stem cell research oversight (ESCRO) committee.122 While this covers embryoid research, it does not necessarily include human embryo research, which is only reviewed by an IRB for informed consent. ISSCR calls for a similar committee that would also review embryo, embryoid, and hESC research. However, unlike an IRB, there are no federal regulations requiring an ESCRO committee, describing how it must be staffed or providing rules for it to follow, just optional guidelines from ISSCR and NASEM to follow.123 Most ESCRO committees are voluntarily developed by universities, although some states and journals require them for funding and publication of research.124 Despite the lack of oversight or authority over these committees, many universities implemented ESCRO committees soon after the NASEM recommended them in 2005.125
Federal guidelines for embryo and embryoid research would allow for increased transparency in the research. It would also create more uniformity as many researchers in the public and private sector follow prescribed rules set by NIH in biomedical research to account for potential future funding as well as overall public support of their work. This was evident in 2009, when NIH set new guidelines for the development of hESC lines that were then adopted by other organizations. Finally, a federal policy can help prevent abuses by bad actors working outside of scientific norms. In order to properly self-regulate, scientists need to clearly understand the regulations and limits to what is and is not justifiable. While it is unlikely that Congress will pass new legislation related to embryo and embryoid research, NIH could play a role in defining more explicitly what embryoid research is impacted by the DWA prohibition and move beyond their current ‘case-by-case’ policy.
Overall, these issues with emerging biomedical technologies suggest a need to have a formal presidential ethics committee similar to those that were active during the Clinton, Bush, and Obama Administrations.126 These committees were populated by scientists, ethicists, and other scholars who would review and assess controversial topics and offer recommendations to the White House on how proceed. They also provided an opportunity for transparent and open discussions with the public on controversial topics. Past issues, including hESCs, cloning, and synthetic biology, were assessed in their time by a presidential committee and today help determine current policies.
There are certainly a few advantages with policies being state-based instead of being federal-based. In highly controversial areas of research with pluralistic views on questions, such as the moral status of an embryo and what research ought to be conducted, it is challenging to determine policies or develop compromises that would be acceptable nationally, especially in this highly partisan political culture we are now seeing.127 Moreover, with the size of the country, population, and geography, providing the public with opportunities to voice concerns and suggestions is challenging. State-based initiatives, such as CIRM in California, are able to conduct public and stakeholder engagements more effectively than similar initiatives based at the federal level.128 Allowing broad public and stakeholder participation in decision-making as it relates to the use of state monies for research, including having lay people involved in the governance structure of the institute, did not appear to have inhibited the research process at CIRM.129 By contrast, it helped obtain public support to extend CIRM for another 10 years.
V. CONCLUSIONS
Our analysis of the 50 US states laws related to human embryo and embryoid research found 29 laws that could impact human embryo research, and none directly addressing embryoid research, based on a plain text interpretation of the laws. However, we acknowledge that this interpretation excludes in some cases relevant court ruling or interpretations by actors within the states which could affect whether research is limited or banned. Despite these limitations, we uncovered a complex regulatory landscape, which suggests that additional national guidance may be needed to help scientists and the public navigate these controversial areas of research.
However, this national guidance on human embryo research and perhaps even embryoid research might not be possible. There has been continuous debate over the past 50 years regarding whether human embryo research should be conducted and, more recently, how embryoid research should be regulated as well as if limits should be placed on either research area and, if so, what enforcement would look like. Effective governance of controversial research calls for careful and coordinated development of funding and regulatory regimes. Unfortunately, this is not how embryo and embryoid research legislation is pursued in the USA and it is unlikely that this will change in the near future. The patchwork of policies between US states is morally inconsistent, with some research banned in one state but publicly funded in another. As a result of our current decentralized approach to human embryo and embryoid research, we find a highly pluralist approach to regulation which reflects geographic differences in perceptions and opinions about the research.130 In addition, these policies are arguably inefficient in terms of scientific and ethical standard development or economic competitiveness.131 The tensions between emerging biotechnological advances and morality issues will likely increase, especially as embryo and embryoid research expands.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the project team for the Spring 2017 Greenwall Foundation Making a Difference grant: Ana S. Iltis, Inmaculada de Melo-Martín, Jason S. Robert, Daniel S. Wagner, and Ali Brivanlou. We would also like to thank the Rice University staff and students who helped research and edit the manuscript, including Erin Yang and Rice undergraduate research intern Sam Lowe, for their help collecting data for the manuscript. Funding for this project was provided in part by The Greenwall Foundation ‘Making a Difference’ grant to KRWM, the Baker Institute Qatar Stem Cell Endowment, and the Baker Institute Center for Health and Biosciences.
Footnotes
Lewis Wolpert, Triumph of the Embryo (1991).
The ‘14-day limit’ is sometimes referred to as a rule or guideline. This depends on if it is a law or a recommendation from a national authority or a recommendation from a nongovernmental organization such as a scientific, medical, or professional society. For this article, we use the term ‘limit’ since it is only based on recommendations from nongovernmental organizations in most of the USA.
Kirstin RW Matthews & Daniel Morali, National Human Embryo and Embryoid Research Policies: A Survey of 22 Top Research-Intensive Countries, 15 Reg. Med. 1905 (2020).
DHEW Ethics Advisory Board, HEW Support of Research Involving Human in vitro Fertilization and Embryo Transfer: Reports and Conclusions (1979).
Mary Warnock, Report of the Committee of Inquiry into Human Fertilisation and Embryology (1984).; UK Human Fertilisation and Embryology Act 1990 c37. http://www.legislation.gov.uk/ukpga/1990/37/contents (accessed Jan. 21, 2022).
For more details related to how the limit was developed as well as debates and discussion related to the limit, see Natasha Hammond-Browning, Ethics, Embryos, and Evidence: A Look Back at Warnock, 23 Med. Law. Rev. 588 (2015); J. Benjamin Hurlbut, Experiments in Democracy: Human Embryo Research and the Politics of Bioethics (2017); Sheila Jasanoff & Ingrid Metzler, Borderlands of Life: IVF Embryos and the Law in the United States, United Kingdom, and Germany, 46 Sci. Technol. Hum. Val. 1001 (2018); Kirstin R.W. Matthews & Nuria Gallego Marquez, A Survey of National Human Embryo Research Policies and Use of the 14-day Guidelines, https://doi.org/10.25613/9cv5-w030; Kirstin R.W. Matthews & Erin Yang, Politics and Policies Guiding Human Embryo Research in the United States, https://doi.org/10.25613/vbe8-z426.
Hurlbut, supra note 6; Martin F. Pera, Human Embryo Research and the 14-Day Rule, 144 Development 1923 (2017); Kirstin R.W. Matthews, et al., Rethinking Human Embryo Research Policies, 51 Hasting Cent. Rep. 47 (2021); Kate Williams & Martin H. Johnson, Adapting the 14-Day Rule for Embryo Research to Encompass Evolving Technologies, 10 Reprod. Biomed. Soc. Online 1 (2020).
Hammond-Browning, supra note 6; Warnock, supra note 5.
Hurlbut, supra note 6; George Daley, et al., Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines, 6 Stem Cell Rep. 787 (2016); US National Academies of Sciences, Engineering, and Medicine (NASEM), Final Report of the National Academies’ Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research (2010).
Alessia Deglincerti, et al., Self-organization of the In Vitro Attached Human Embryo, 533 Nature 251 (2016); Marta. N Shahbazi, et al., Self-organization of the Human Embryo in the Absence of Maternal Tissues, 18 Nature Cell Biol. 700 (2016).
John B. Appleby & Annelien L. Bredenoord, Should the 14 day Rule for Embryo Research Become the 28 day Rule?, 10 EMBO Mol. Med. 9437 (2018); Sarah Chan. How and Why to Replace the 14-day Rule, 4 Curr. Stem Cell Rep. 228–34 (2018); J. Benjamin Hurlbut, et al., Revisiting the Warnock Rule, 35 Nature Biotech. 1029–42 (2017); Insoo Hyun, et al., Revisit the 14-day Rule, 533 Nature 169 (2016).
Hurlbut, et al., supra note 11; Giulia Cavaliere, A 14-Day Limit for Bioethics: The Debate over Human Embryo Research, 18 BMC Med. Ethics 38 (2017); Mary Warnock, Should the 14-day Limit on Human Embryo Research be Extended, https://www.bionews.org.uk/page_95833 (accessed Jan. 21, 2022); Magdalena Zernicka-Goetz & Roger Highfield, The Dance of Life: The New Science of How a Single Cell Becomes a Human Being (2020).
Sheila Jasanoff, ed., States of Knowledge: The Co-Production of Science and the Social Order (2004).
PSC models are known by various names, including references to developmental time points (ie blastoids and gastruloids), to the cells used to create the model (ie micropatterned hESC colonies or post-implantation amniotic sac embryoid) or general names to be used for the field (ie artificial embryos, embryoids, or synthetic entities with embryo-like features). See also Mijo Simunovic & Ali Brivanlou, Embryoids, Organoids, and Gastruloids: New Approaches to Understanding Embryogenesis, 144 Development 976 (2017) (for full descriptions of different embryoid models). For this article, we chose to use the term embryoid as a general term to include all PSC-derived models of early embryo development, whether they represent all or part of the developing embryo.
Marta. N. Shahbazi, et al., Self-organization of Stem Cells into Embryos: A Window on Early Mammalian Development, 366 Science 948 (2019)s; Mijo Simunovic & Ali Brivanlou, Embryoids, Organoids, and Gastruloids: New Approaches to Understanding Embryogenesis, 144 Development 976 (2017).
Matthews, et al., supra note 7.
Id.
ISSCR, Guidelines for Stem Cell Research and Clinical Translation (2021), http://isscr.org/guidelines (accessed Jan. 21, 2022); Robin Lovell-Badge, et al., ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update, 16 Stem Cell Rep. 1398 (2021).
Amander T. Clark, et al., Human Embryo Research, Stem Cell-Derived Embryo Models and In Vitro Gametogenesis: Considerations Leading to the Revised ISSCR Guidelines, 16 Stem Cell Rep. 1416 (2021).
For more on national policies, see Timothy Caulfield, et al. The Stem Cell Research Environment: A Patchwork of Patchworks, 5 Stem Cell Rev and Rep. 82 (2009).
Aaron D. Levine, Policy Considerations for States Supporting Stem Cell Research: Evidence from a Survey of Stem Cell Scientists, Public Admin Rev. 681 (2008).
Id.
45 C.F.R. § 46 (2016).
Gary C. Schoenwolf, et al., Larsen’s Human Embryology, 5th Edition (2015).
42 U.S.C. § 289 g (2003).
Carol A. Tauer, et al., Embryo Research, in Bioethics, 4th Edition, 923–35 (Bruce Jennings ed., 2014).
45 C.F.R. § 46 (2016); The President’s Council on Bioethics, Reproduction and Responsibility: The Regulation of New Biotechnologies (2004); Tauer, et al., supra note 26.
Kirstin R.W. Matthews & Maude L. Rowland, Stem Cell Policy in the Obama Age: UK and US Perspectives, 6 Reg. Med. 125 (2011); Levine, supra note 21; Aaron D. Levine, State Stem Cell Policy and the Geographic Preferences of Scientists in a Contentious Emerging Field, 39 Sci. Publ. Policy 530 (2012); Aaron D. Levine, T. Austin Lacy & James C. Hearn, The Origins of Human Embryonic Stem Cell Research Policies in US States, 40 Sci. Publ. Policy 544 (2013).
Consolidated Appropriation Act, H.R 133, 116th Cong. (2021).
Sherley v Sebelius, 776 F. Supp. 2d 1. (2011); Maude L. Cuchiara, et al., Defining ‘Research’ in the US and EU: Contrast of Sherley v. Sebelius and Brustle v. Greenpeace Rulings, 9 Stem Cell Rev. and Rep. 743 (2013).
Eric Anthony, Robin Lovell-Badge & Sean J. Morrison, New Guidelines for Stem Cell and Embryo Research from the ISSCR, 28 Cell Stem Cell 991 (2021); Matthews & Rowland, supra note 28.; Paola Nicolas, Fred Etoc & Ali H. Brivanlou, The Ethics of Human-Embryoids Model: A Call for Consistency, 99 J. Mol. Med. (Berl) 569 (2021); van den Brink et al., Symmetry Breaking, Germ Layer Specification and Axial Organisation in Aggregates of Mouse Embryonic Stem Cells, 141 Development 4231 (2014); Aryeh Warmflash, et al., A Method to Recapitulate Early Embryonic Spatial Patterning in Human Embryonic Stem Cells, 11 Nat. Methods 847 (2014).
Carrie D. Wolinetz, Sharing Our Current Thinking: Models Containing Aspects of Human Embryos, https://osp.od.nih.gov/2021/03/11/human-embryo-development/ (accessed Jan. 20, 2022).
Matthews & Rowland, supra note 28.
Note: The DWA remains relatively unchanged each year with the exception of the expansion of the definition of an embryo from the original version to include non-fertilization methods.
Consolidated Appropriation Act, H.R 133, 116th Cong. (2020).
I. Glenn Cohen & Eli Y. Adashi, The FDA is Prohibited from Going Germline, 353 Science 545 (2017).
John C. Fletcher, The Stem Cell Debate in Historical Context, in The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy, 27–34 (Suzanne Collins et al. eds., 2001).
Ruchir N. Karmali, Natalie M. Jones & Aaron D. Levine, Tracking and Assessing the Rise of State-funded Stem Cell Research, 28 Nature Biotech. 1246 (2010); National Conference of State Legislatures (NCSL), Embryonic and Fetal Research Laws, https://www.ncsl.org/research/health/embryonic-and-fetal-research-laws (accessed January 13, 2022).
Matthews & Rowland, supra note 28.
Karmali, Jones & Levine, supra note 38; Levine, supra note 21.
Richard O. Hynes, US Policies on Human Embryonic Stem Cells, 9 Nature Rev. 993 (2008); Andrew Karch. Vertical Diffusion and the Policy-Making Process: The Politics of Embryonic Stem Cell Research, 65 Polit Res Quart. 48 (2010); Karmali, Jones & Levine, supra note 38; Geoffrey Lomax, Rejuvenated Federalism: State-Based Stem Cell Research Policy, in Contested Cells: Global Perspectives on the Stem Cell Debate, 359–75 (Benjamin J. Capps & Alastair V. Campbell eds., 2010); Susan Stayn, A Guide to State Laws on hESC Research and a Call for Interstate Dialogue, 5 Med. Res. Law Policy Rep. 718 (2006).
Karch, supra note 41.
SCNT, also known as therapeutic cloning, is a procedure to create genetically identical cells using DNA from a mature cell and inserting it into an unfertilized egg cell, which is then encouraged to grow and hESCs are created from the resulting embryo.
Voting America: United States Politics, 1840–2008, http://www.americanpast.org/voting/ (accessed Jan. 21, 2022).
NCSL, supra note 38.
NM Stat § 24-9A (1979).
PA Cons. Stat. Tit. 18 Ch. 32, § 3216 (1989).
ND Cent. Code § 14–02.2-01, −02 (2002).
RI Gen. Law § 11–54-1 (1981). Note: While a 1998 Rhode Island law passed that bans reproductive cloning and supportive of SCNT research on ‘human cells, genes, tissues, or organs’, the provision expired in 2017 (RI Gen. Law § 23–16.4-1, 2 (1998)).
MN Stat. § 145.421, 2 (1973).
PA Cons. Stat. Tit. 18 Ch. 32, § 3216 (1989).
LA-R.S. 9 § 122 (1986).
SD Stat. § 34-14-16, -17, -18 (2000).
AR A.C.A. § 20-16-2201-2208 (2019).
KY R.S. § 311.715 (1980) (amended 2017).
NE Code § 71-8806 (2008).
NE Revised Statute § 71-7606 (1998).
KY Revised Statute § 311.715 (1980) (amended 2017).; NE Code § 71-8806 (2008).
OK Stat. tit. 63 § 1-270.2 (2001).
NCSL, supra note 38.
Hynes, supra note 41; Karmali, Jones & Levine, supra note 38; Lomax, supra note 41; Stayn, supra note 41.
CA Health & Safety Code § 125118 (2005); Nidhi Subbaraman, California’s Vote to Revive Controversial Stem-Cell Institute Sparks Debate, 587 Nature 535 (2020).
Karmali, Jones & Levine, supra note 38; Levine, Lacy & Hearns, supra note 28.
CT Public Act No. 05-149 (2005).
MD Economic Development Code Ann. § 10-431 (2008).
Id.
NYSTEM, Form for Embryo Donation for Human Embryonic Stem Cell Research (in Excess of Clinical Need), https://stemcell.ny.gov/node/58 (accessed Jan. 21, 2022).
Sofia Moutinho, New York State Ends Stem Cell Research Funding, Science (Apr 16, 2021), https://www.sciencemag.org/news/2021/04/new-york-state-ends-stem-cell-research-funding.
MI Const. Art. I § 27 (2008).; MI Public Health Code § 333.16275 (1999).
MO Const. Art. 3, § 38(d) (2006).
IA Code § 707C.3, 707C.4 (2007).
MT Code § 50-11-103 (2009).
NJ Rev. Stat. § 26:2Z-2, § 2C:11A-1 (2003).
VA Code Ann. § 32.1-162.22 (2001), 162.31 (2005) (amended 2016).
MA Gen. Law Part I Title XVI Chapter 111L § 8 (2005).
MO Const. Art. 3, § 38(d) (2006).
IL 410 I.L.C.S 110 (2008).
MI Const. Art. I § 27 (2008); MI Public Health Code § 333.16275 (1999).
AZ Rev. Stat. § 36-2312 (1988).
IN Code § 35-46-3(f) (1987).
ME Rev. Stat. Tit. 22, 263-B § 1593, 1595 (1977).
FL Stat. § 390.0111-6, 742.13-12 (2019).
UT CC 76-7-310 (1974); While the Utah Criminal Code does not specifically define unborn child as a fertilized ovum after implantation, the preamble to Chapter 7, Part 3, states the ‘intent of the Legislature to protect and guarantee to unborn children their inherent and inalienable right to life’, defining ‘abortion’ as the ‘termination of human pregnancy after implantation of a fertilized ovum’.
OH Rev. Code § 2919.14 (1974).
Nefi D. Acosta & Sidney H. Golub, The New Federalism: State Policies Regarding Embryonic Stem Cell Research, 44 J. Law Med. Ethics 419 (2016).
Acosta & Golub, supra note 85; Levine, Lacy & Hearn, supra note 28.
IA Code § 707C.3, 707C.4 (2007).
Id.
MA General Laws Part I, Title XVI, Chapter 111L, §8 (2005).
CA Health & Safety Code § 125118 (2005); CT Stat. § 32-41jj (2005) (amended 2015); IL 410 ILCS 110 (2008); MD Code § 10-440 (2008); MT Code § 50-11-103 (2009); VA Code Ann. § 32.1-162.22 (2001), -31 (2005) (amended 2016).
RI Gen. Law § 23-16.4-1, 2 (1998)
MI Comp. Laws § 333.16274 (1998).
MO Const. Art. III, § 38(d).
MN Stat. § 145.421, 2 (1973).
KS Stat. § 76–839 (2013).
CT Stat. § 32-41jj (2005) (amended 2015).
Levine, Lacy, & Hearn, supra note 28; Michael Mintrom, Competitive Federalism and the Governance of Controversial Science, 39 Publius J. Federalism 606 (2009).
Herbert Gottweis & Barbara Prainsack, Emotion in Political Discourse: Contrasting Approaches to Stem Cell Governance in the USA, UK, Israel and Germany, 1 Reg Med. 823 (2006).
Id.
Id.
Karch, supra note 41; Levine, Lacy, & Hearn, supra note 28; Mintrom, supra note 97.
PA Cons. Stat § 3216 (1989).
AZ Rev. Stat. § 36-2302 (1988).
SD Stat. § 34-14-16, -17, -18 (2000).
MI Const. Art. 1, § 27 (2008).
Karch, supra note 41.
AR Code § 20-16-2204 (2019); KY R.S. § 311.715 (1980) (amended 2017); NE Code § 71-8806 (2008); SD Stat. § 34-14-16 (2000).
IA Code § 707C.3, -4 (2007); MO Const. Art. 3, § 38(d) (2006).
Matthews & Morali, supra note 3.
CA Health & Safety Code § 125118 (2005); CT Stat. § 32-41jj; NYSTEM, supra note 67.
VA Code Ann. § 32.1-162.22 (2001).
Matthews & Morali, supra note 3.
Rachel A. Ankeny, Megan J. Munsie & Joan Leach, Developing a Reflexive, Anticipatory, and Deliberative Approach to Unanticipated Discoveries: Ethical Lessons from iBlastoids, 22 AJOB. 36 (2022).
45 C.F.R. § 46 (2016); Recombinant DNA Advisory Committee, https://osp.od.nih.gov/biotechnology/recombinant-dna-advisory-committee (accessed Jan. 21, 2022); NIH, Guidelines for Human Stem Cell Research, https://stemcells.nih.gov (accessed Jan. 21, 2022).
NASEM, supra note 9; ISSCR, supra note 18.
Kirstin R.W Matthews & Ana S. Iltis, Are We Ready to Genetically Modify a Human Embryo? Or Is It Too Late to Ask?, 4 Account Res. 265 (2019).
Lomax, supra note 41.
NASEM, supra note 9.
Id.
ISSCR, supra note 18.
Clark, et al., supra note 19.
NASEM, supra note 9.
For more details comparing IRB regulation versus SCRO oversight, see Henry T. Greely, Assessing ESCROs: Yesterday and Tomorrow, 13 AJOB. 13, 44 (2013).
Greely, supra note 123; Nature, Editorial Policies: Ethics and Biosecurity, https://www.nature.com/nature/editorial-policies/ethics-and-biosecurity (accessed on Jan. 21, 2022).
Greely supra note 123.
Eli Y. Adashi & I. Glenn Cohen, An Overdue Executive Order: Reinstating the National Bioethics Commission,134 Amer J Med. 1199 (2021); Kirstin R.W. Matthews & Ana S. Iltis, Modern Biomedical Breakthroughs Require a Federal Ethics Commission, The Hill https://thehill.com/opinion/healthcare/548602-modern-biomedical-breakthroughs-require-a-federal-ethics-commission (accessed Jan. 24, 2022).
James W. Fossett, et al., Federalism & Bioethics: States and Moral Pluralism, 37 Hasting Cent. Rep. 24 (2007).
Lomax, supra note 41; Michael Mintrom & Rebecca Bollard, Governing Controversial Science: Lessons from Stem Cell Research, 28 Policy Soc. 301 (2009).
Id.
Mintrom & Bollard, supra note 128.
Lomax, supra note 41.
Contributor Information
Kirstin R W Matthews, Baker Institute for Public Policy Center for Health and Bioscience, Rice University, Houston, TX, 77005, USA.
Daniel Morali, Baker Institute for Public Policy Center for Health and Bioscience, Rice University, Houston, TX, 77005, USA.


