Summary
Background
Testosterone is the standard treatment for male hypogonadism, but there is uncertainty about its cardiovascular safety due to inconsistent findings. We aimed to provide the most extensive individual participant dataset (IPD) of testosterone trials available, to analyse subtypes of all cardiovascular events observed during treatment, and to investigate the effect of incorporating data from trials that did not provide IPD.
Methods
We did a systematic review and meta-analysis of randomised controlled trials including IPD. We searched MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Epub Ahead of Print, Embase, Science Citation Index, the Cochrane Controlled Trials Register, Cochrane Database of Systematic Reviews, and Database of Abstracts of Review of Effects for literature from 1992 onwards (date of search, Aug 27, 2018). The following inclusion criteria were applied: (1) men aged 18 years and older with a screening testosterone concentration of 12 nmol/L (350 ng/dL) or less; (2) the intervention of interest was treatment with any testosterone formulation, dose frequency, and route of administration, for a minimum duration of 3 months; (3) a comparator of placebo treatment; and (4) studies assessing the pre-specified primary or secondary outcomes of interest. Details of study design, interventions, participants, and outcome measures were extracted from published articles and anonymised IPD was requested from investigators of all identified trials. Primary outcomes were mortality, cardiovascular, and cerebrovascular events at any time during follow-up. The risk of bias was assessed using the Cochrane Risk of Bias tool. We did a one-stage meta-analysis using IPD, and a two-stage meta-analysis integrating IPD with data from studies not providing IPD. The study is registered with PROSPERO, CRD42018111005.
Findings
9871 citations were identified through database searches and after exclusion of duplicates and of irrelevant citations, 225 study reports were retrieved for full-text screening. 116 studies were subsequently excluded for not meeting the inclusion criteria in terms of study design and characteristics of intervention, and 35 primary studies (5601 participants, mean age 65 years, [SD 11]) reported in 109 peer-reviewed publications were deemed suitable for inclusion. Of these, 17 studies (49%) provided IPD (3431 participants, mean duration 9·5 months) from nine different countries while 18 did not provide IPD data. Risk of bias was judged to be low in most IPD studies (71%). Fewer deaths occurred with testosterone treatment (six [0·4%] of 1621) than placebo (12 [0·8%] of 1537) without significant differences between groups (odds ratio [OR] 0·46 [95% CI 0·17–1·24]; p=0·13). Cardiovascular risk was similar during testosterone treatment (120 [7·5%] of 1601 events) and placebo treatment (110 [7·2%] of 1519 events; OR 1·07 [95% CI 0·81–1·42]; p=0·62). Frequently occurring cardiovascular events included arrhythmia (52 of 166 vs 47 of 176), coronary heart disease (33 of 166 vs 33 of 176), heart failure (22 of 166 vs 28 of 176), and myocardial infarction (10 of 166 vs 16 of 176). Overall, patient age (interaction 0·97 [99% CI 0·92–1·03]; p=0·17), baseline testosterone (interaction 0·97 [0·82–1·15]; p=0·69), smoking status (interaction 1·68 [0·41–6·88]; p=0.35), or diabetes status (interaction 2·08 [0·89–4·82; p=0·025) were not associated with cardiovascular risk.
Interpretation
We found no evidence that testosterone increased short-term to medium-term cardiovascular risks in men with hypogonadism, but there is a paucity of data evaluating its long-term safety. Long-term data are needed to fully evaluate the safety of testosterone.
Funding
National Institute for Health Research Health Technology Assessment Programme.
Research in context.
Evidence before this study
Testosterone treatment is most often given to men aged 40–65 years. Testosterone has potentially favourable effects on cardiovascular risk such as increased lean-to-fat body mass and improved insulin sensitivity and glycaemia. Conversely, testosterone treatment increases haematocrit, might lower high-density lipoprotein (HDL) cholesterol, and some studies have observed increased cardiovascular event risk. The US Food & Drugs Administration (FDA) has mandated a box label warning of potential cardiovascular risks for all testosterone products. Uncertainty regarding the safety of testosterone might unduly influence decision making regarding the management of men with hypogonadism who could otherwise derive substantial benefits from treatment. We designed highly sensitive search strategies to identify reports of published, ongoing, and unpublished randomised controlled trials assessing the clinical effectiveness of testosterone treatment in men with hypogonadism. Searches were restricted to reports published in English from 1992. We searched major electronic databases (MEDLINE, Embase, Science Citation Index, and CENTRAL), clinical trial registries, and contacted clinical experts. We focused on trials with at least 3-month treatment duration and mean baseline total testosterone of 12 nmol/L or less (or equivalent) before treatment. We established a collaborative group of investigators of all identified trials (35 trials) and collected individual patient data (IPD) from 17 trials (3431 participants in total). In general, the risk of bias of IPD trials was low.
Added value of this study
This individual IPD meta-analysis allowed us to conduct a reliable assessment of the frequency of mortality and cardiovascular events (including subtypes) during testosterone treatment in men with hypogonadism. Few deaths have occurred during trials of testosterone in men. Furthermore, testosterone treatment is not associated with an increased risk of any recorded cardiovascular event subtype in the short to medium term. The only detected adverse effects of testosterone were oedema and a modest lowering of HDL cholesterol.
Implications of all the available evidence
Men with hypogonadism should be counselled that there is no current evidence that testosterone treatment increases cardiovascular risk in the short to medium term. Long-term safety of testosterone is not yet established; an FDA-mandated study is ongoing.
Introduction
The steroid hormone testosterone is fundamental to male physical development and sexual behaviour. Deficiency of testosterone causes male hypogonadism, including diminished secondary sexual characteristics, sexual dysfunction, muscle wasting and weakness, osteoporosis, and reduced quality of life. Testosterone treatment is the standard of care for reversing the consequences of hypogonadism. Testosterone sales increased 12-fold globally from USD$150 million in 2000 to $1·8 billion in 2011.1 During this period, testosterone has been used increasingly in men aged 40–65 years, and has been over-prescribed by some clinicians.2, 3 Despite the increasing use of testosterone, the USA Endocrine Society, American College of Physicians, and Endocrine Society of Australia have independently concluded that the cardiovascular safety of testosterone has not been adequately established.4, 5, 6 Furthermore, the European Urology Association (EAU) and the European Academy of Andrology (EAA) have recommended the assessment of cardiovascular risk before initiation of testosterone therapy.7, 8
Testosterone exerts diverse effects on cardiovascular physiology. Some physiological testosterone effects could potentially reduce cardiovascular risk, including coronary vasodilatation and increased coronary blood flow, improved vascular reactivity, increased muscle mass, reduced whole body and visceral fat mass, shorter QTc interval, and normalisation of glycaemia during lifestyle interventions for prediabetes.4, 9 Other testosterone actions could increase cardiovascular risk, including increased haematocrit, reduced high density lipoprotein (HDL) cholesterol, induction of platelet aggregation by stimulation of thromboxane A2, sodium and water retention, and smooth muscle proliferation and increased expression of vascular cell adhesion molecules.4, 10, 11
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone compared with non-users, but the study designs have been widely criticised.12, 13, 14 Furthermore, a placebo-controlled trial was stopped early by its data and safety monitoring board following increased cardiovascular events in men aged 65 years and older who received 6 months of testosterone treatment.15 Other controlled trials have not observed significant effects of testosterone on cardiovascular events, but none were sufficiently powered to detect excess cardiovascular risks.16, 17 Nevertheless, the US Food and Drug Administration (FDA) mandated box label warnings of potential cardiovascular risks for all testosterone products. The FDA also restricted testosterone approval to hypogonadism caused by documented pituitary or testicular disease, specifically excluding age-related hypogonadism.18 Following the FDA's advisory about potential cardiovascular risk, testosterone prescription sales have declined in the USA.2 Conversely, the European Medicines Agency, EAU, and EAA have concluded that when hypogonadism is properly diagnosed and managed, there is currently no consistent evidence that testosterone therapy causes increased cardiovascular risk.7, 8, 19 Uncertainty about the cardiovascular safety of testosterone might be unduly influencing decision making regarding the management of men with hypogonadism who might otherwise derive substantial benefits from the treatment.
Previous meta-analyses of cardiovascular safety of testosterone treatment have been restricted to published, aggregate data, limiting the ability to confirm quality and categorisation of source data, or analyse whether specific clinical benefits or adverse effects are associated with distinct subgroups such as patient age, baseline total and free testosterone, smoking, and diabetes status.20, 21, 22, 23, 24, 25, 26, 27, 28
To address ongoing uncertainly about the safety of testosterone, the Testosterone Efficacy and Safety Consortium was established as a global collaboration of principal investigators of testosterone trials. We report results of the most extensive individual participant dataset (IPD) of testosterone trials available and aimed to analyse subtypes of all cardiovascular events observed during treatment, and analyse the effect of incorporating data from trials not providing IPD.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, placebo-controlled trials evaluating the effects of at least 3 months of testosterone treatment in men with low testosterone were considered for inclusion. The following criteria were used for study selection: (1) men aged 18 years and older with a screening testosterone concentration of 12 nmol/L (350 ng/dL) or less. Studies restricted to conditions not resulting from hypogonadism likely to affect cardiovascular or thrombotic risk (eg, cancer, HIV, cirrhosis, Klinefelter syndrome, type 1 diabetes), or studies restricted to men with congenital hypogonadotrophic hypogonadism were not deemed suitable for inclusion. (2) The intervention of interest was treatment with any testosterone formulation, dose frequency, and route of administration, for a minimum duration of 3 months. Studies in which participants received non-testosterone drugs to increase androgen levels (eg, human chorionic gonadotropin, selective oestrogen receptor modulators) or concomitant interventions were not included. (3) A comparator of placebo treatment. (4) Studies assessing the pre-specified primary or secondary outcomes of interest.
Highly sensitive search strategies were applied by an information specialist on Aug 27, 2018, to the following databases: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Epub Ahead of Print, Embase, Science Citation Index, and the Cochrane Controlled Trials Register, Cochrane Database of Systematic Reviews, and Database of Abstracts of Review of Effects (appendix pp 1–2). Furthermore, the Health Technology Assessment databases were searched for evidence syntheses. Recent conference proceedings of key professional organisations in the fields of endocrinology, cardiology, and men's health were also searched. Searches were restricted to reports published from 1992. Articles published in languages other than English were translated when possible. Reference lists of included studies were checked, and expert panellists assembled for this review were contacted for further potentially relevant reports. We did not consider unpublished evidence or evidence published in a non-commercial form.
Two reviewers independently screened titles and abstracts of all citations identified by the search strategies (MC and MB or MA-M). All potentially relevant reports were retrieved in full and assessed by one reviewer (MC) with 10% independently checked by a second reviewer (MA-M). Additionally, all selected reports were independently assessed by a clinical expert (CNJ or RQ). Any disagreements during the selection process were resolved by consensus.
This study was done according to Centre for Reviews and Dissemination guidance for undertaking reviews in health care and the Cochrane Handbook for Systematic Reviews of Interventions.29, 30 Results were reported according to the PRISMA IPD checklist.31 Methods were pre-specified in a research protocol.
Data collection and risk of bias assessment
MC extracted details of study design, interventions, participants, and outcome measures from published articles using a bespoke data extraction form. In cases of multiple duplications, the most recent or complete article was selected for data extraction. MA-M cross-checked a random sample of 10% of selected studies. Extracted data were further checked for accuracy by the project statistician (JH). Anonymised IPD was requested from investigators of all identified trials, following the completion of a Data Sharing Agreement. A Standard Operating Procedure ensured secure receipt and storage of all IPD. Data sets received were checked for accuracy with published data and discrepancies were clarified with the collaborator. If this was not possible, the research team discussed discrepancies and decided whether data should be included. When applicable, variables were standardised to the same scale.
Primary outcomes were all-cause mortality and cardiovascular or cerebrovascular events, or both, at any time during the study period, irrespective of whether they were assessed as primary or secondary outcomes in the individual trials. Physiological markers were reported as secondary outcomes (appendix p 3). At baseline, data on age, body-mass index (BMI), ethnicity, hormone concentrations, cardiovascular history, and other medical history were extracted. We also collected data on additional outcomes including diabetes and prostate cancer (appendix p 4). Many secondary outcomes (eg, blood pressure) were measured serially. All but two eligible studies had durations of 12 months or less; to aid data comparison between studies, secondary outcomes were assessed at 12 months or the time-point closest to 12 months. Primary outcomes and additional secondary outcomes were categorised independently by two clinical review authors (CJ, RQ). All American College of Cardiology (ACC) cardiovascular endpoints for clinical trials (death, heart failure, myocardial infarction, unstable angina, coronary intervention, and peripheral vascular disease)32 were assessed; we also assessed any other cardiovascular endpoints reported within disclosed IPD. Stroke was the only reported cerebrovascular event. For simplicity, in the text of this Article, a reference to cardiovascular events indicates both cardiovascular and cerebrovascular events. A full list of secondary outcomes is available in the appendix (p 3).
The risk of study bias was assessed independently by MC and MA-M using the original version of the Cochrane Collaboration's risk of bias tool for randomised controlled trials.33 Follow-up enquiries were made with collaborators providing IPD for cases in which details required were unclear or not reported. The following domains were assessed: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Other bias was judged to be high if the study was sponsored or done by a pharmaceutical company. The overall risk of bias was considered high if one or both key domains (selection bias, detection bias) were judged at high risk of bias; was considered unclear if either key domain was judged as unclear; or considered low if both domains were judged at low risk of bias. Reviewer disagreements were resolved by consensus. Risk of bias was presented using RevMan software (version 5.4.1).
Data analysis
All analyses were done according to intention-to-treat and at the participant level, in accordance with a pre-specified statistical analysis plan (appendix pp 5–17). Both one-stage and two-stage meta-analyses were undertaken as IPD were not available from all included studies. For the one-stage meta-analysis, we used a fixed-effects logistic regression model accounting for clustering and allowing a separate intercept per study, with treatment effects presented as odds ratios (ORs) for the primary outcomes due to non-converge of a random-effects analysis. Secondary continuous outcomes were analysed used a random-effects linear regression accounting for clustering and allowing separate baseline adjustment per study as well as a separate residual variance using restricted maximum likelihood (REML). Effect estimates were presented as mean differences. Estimated between-study variance, τ2, is reported to assess heterogeneity. For the two-stage meta-analysis, IPD were analysed separately for each study. For the first stage, primary outcomes were analysed with logistic regression (while linear regression was adjusted for baseline value) and with REML for secondary outcomes. For studies without IPD, we obtained effect estimates and standard errors according to current methodological recommendations.33 The second stage pooled the effect estimates using a random-effects model with REML. For models not converging using REML, we used a random-effects model using the DerSimonian and Laird method.34 No adjustment for zero events was required due to the use of a parametric model and because both the one-stage and two-stage analysis approaches use information from across all the studies.
Heterogeneity was assessed by use of the I2 statistic. Counter-enhanced funnel plots and Peters' test for asymmetry were used for primary outcomes to assess small-study effects and publication bias.35 A χ2 test was used to assess additional secondary outcomes. For cardiovascular (or cerebrovascular) events, pre-specified subgroup analyses according to current methodological recommendations were done to assess effects of diabetes diagnosis, smoking status, testosterone, and free testosterone concentrations.
A post-hoc subgroup analysis was done for age and baseline cardiovascular or cerebrovascular event status.36 We also did sensitivity analyses according to age (<50, 50–75, >75 years), testosterone concentrations (<8, 8–10, >10 nmol/L), and free testosterone concentrations (<180, 180–220, >220 pmol/L). Analysis of mortality was unfeasible due to the limited number of total recorded deaths. Due to low numbers of mortality events, we did a sensitivity analysis using the Mantel-Haenszel method and including unknown cause of death for cardiovascular (or cerebrovascular) events.
Treatment effects were presented with 95% CIs apart from in the subgroup analyses, for which stricter levels of significance (99% CIs) were used. No adjustment for multiple secondary outcomes was performed. To allow direct comparison, SF-36 and SF-12 scores were transformed into T-scores.37 All statistical analyses were done with Stata software (version 16). The study is registered on the PROSPERO database, CRD42018111005.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Results
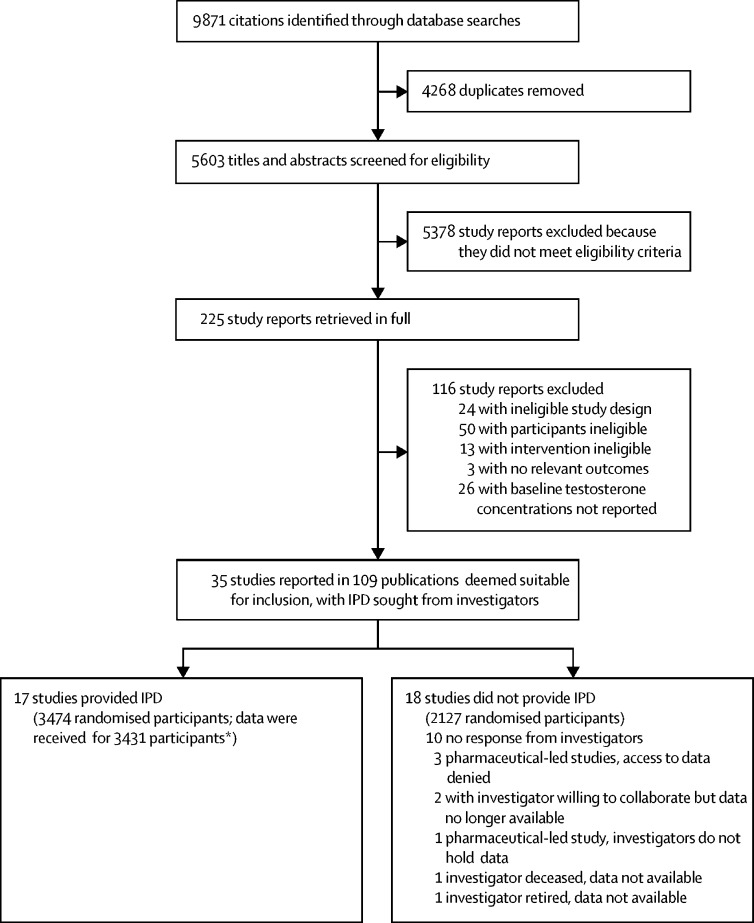
9871 citations were identified through all database searches, and following the removal of 4268 duplicates, 5603 titles and abstracts were screened for inclusion; 5378 study reports were subsequently excluded as they did not meet the eligibility criteria, and 225 were retrieved for full-text assessment. A total of 116 studies were then excluded because they did not meet the inclusion criteria in terms of study design and characteristics of the intervention and 35 primary studies (5601 participants) reported in 109 peer-reviewed publications were deemed suitable for inclusion. IPD were sought from the investigators of the 35 clinical studies. 17 studies (49%) from nine countries provided IPD (3431 participants; figure 1; appendix pp 18–24) and the remaining 18 studies did not provide IPD.
Figure 1.
Study selection
IPD=individual participant dataset. *Reasons for discrepancies listed in the appendix (pp 18–24).
Among the 17 studies that provided IPD, 1750 participants were allocated to the testosterone group and 1681 to the placebo group. Mean participant age was 65 years (SD 11) and most participants were White (testosterone, 915 [87·5%] of 1046; placebo, 888 [87·6%] of 1014) and non-smokers (testosterone, 838 [88·9%] of 943; placebo, 756 [87·2%] of 867). Mean BMI was 30 kg/m2 (SD 5). At baseline, 432 (27·4%) of 1574 men had diabetes, 5 (23·8%) of 21 had angina, and 81 (8·4%) of 970 had previous myocardial infarction in the testosterone group and 402 (26·9%) of 1492 had diabetes, 5 (26·3%) of 19 had angina, and 83 (8·6%) of 964 had previous myocardial infarction in the placebo group (table 1; appendix pp 25–27). Across studies, the mean duration of testosterone treatment was 9·5 months (range, 12 weeks–3 years). The rate of cardiovascular or cerebrovascular events was not a primary endpoint in any of the included trials. Overall, the risk of bias was judged to be low for 12 (71%) of the 17 IPD studies and unclear for the remaining five studies. For the 18 non-IPD studies, the overall risk of bias was judged to be low for three (17%) studies, unclear for 13 (72%) studies, and high for two (11%) studies (appendix pp 28–34).
Table 1.
Baseline characteristics of the participants enrolled in the 17 individual participant dataset studies
| Number of studies | Testosterone treatment group | Placebo group | ||
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 16 | 64·5 (11·0); 1724 | 65·3 (10·8); 1656 | |
| Body-mass index, kg/m2 | 17 | 30·3 (4·7); 1746 | 30·2 (4·5); 1677 | |
| Ethnicity | 6 | .. | .. | |
| Caucasian | .. | 915/1046 (87·5%) | 888/1014 (87·6%) | |
| Asian | .. | 63/1046 (6·0%) | 62/1014 (6·1%) | |
| Black or African American | .. | 16/1046 (1·5%) | 12/1014 (1·2%) | |
| Other | .. | 9/1046 (0·9%) | 7/1014 (0·7%) | |
| Missing | .. | 43/1046 (4·1%) | 45/1014 (4·4%) | |
| Smoking status | 10 | .. | .. | |
| No | .. | 838/943 (88·9%) | 756/867 (87·2) | |
| Yes | .. | 103/943 (10·9%) | 107/867 (12·3%) | |
| Missing | .. | 2/943 (0·2%) | 4/867 (0·5%) | |
| Hormone concentrations | ||||
| Albumin, g/L | 9 | 42·6 (3·2); 817 | 42·7 (3·1); 783 | |
| Estradiol, pmol/L | 8 | 80·8 (38·6); 782 | 77·1 (33·6); 710 | |
| Follicle stimulating hormone, IU/L | 8 | 14·7 (16·7); 711 | 14·2 (16·0); 683 | |
| Luteinising hormone, IU/L | 8 | 6·0 (5·6); 435 | 6·3 (5·6); 362 | |
| Sex hormone binding globulin, nmol/L | 15 | 33·8 (16·6); 1256 | 32·7 (16·2); 1190 | |
| Cardiovascular reported medical history | ||||
| Unspecified | 1 | 13/45 (28·9%) | 5/43 (11·6%) | |
| Angina | 1 | 5/21 (23·8%) | 5/19 (26·3%) | |
| Coronary heart disease | 7 | 95/803 (11·8%) | 82/771 (10·6%) | |
| Myocardial infarction | 6 | 81/970 (8·4%) | 83/964 (8·6%) | |
| Arrhythmia | 6 | 36/713 (5·0%) | 25/677 (3·7%) | |
| Peripheral vascular disease | 4 | 12/500 (2·4%) | 9/472 (1·9%) | |
| Atherosclerosis | 3 | 16/531 (3·0%) | 7/527 (1·3%) | |
| Heart failure | 6 | 13/624 (2·1%) | 3/591 (0·5%) | |
| Valvular heart disease | 4 | 2/586 (0·3%) | 9/55 (16·4%) | |
| Stable angina | 3 | 4/530 (0·8%) | 8/533 (1·5%) | |
| Aortic aneurysm | 2 | 2/379 (0·5%) | 5/376 (1·3%) | |
| Unstable angina | 2 | 0/513 | 1/508 (0·2%) | |
| Cardiac arrest | 1 | 0/113 | 1/110 (0·9%) | |
| Other medical conditions | ||||
| Cerebrovascular reported medical history | 8 | 37/1139 (3·2%) | 58/1085 (5·3%) | |
| Diabetes* | 12 | 432/1574 (27·4%) | 402/1492 (26·9%) | |
| Prostate cancer | 17 | 0/1750 | 0/1681 | |
| Sexual function | ||||
| IIEF-15 | 5 | .. | .. | |
| Total | .. | 33·47 (20·65); 800 | 31·11 (20·84); 818 | |
| Erectile function | .. | 13·12 (10·03); 814 | 12·02 (10·00); 838 | |
| Orgasmic function | .. | 5·28 (3·91); 820 | 4·76 (4·02); 841 | |
| Sexual desire | .. | 5·18 (2·12); 819 | 5·03 (2·12); 839 | |
| Intercourse satisfaction | .. | 5·27 (5·00); 818 | 4·65 (4·96); 844 | |
| Overall satisfaction | .. | 4·65 (2·48); 808 | 4·59 (2·52); 826 | |
| IIEF-5 | 5 | 14·66 (7·16); 273 | 14·74 (7·01); 206 | |
| Androgen deficiency in the aging men | 1 | 4·06 (2·21); 113 | 3·69 (2·43); 110 | |
| Physiological marker | ||||
| Testosterone, nmol/L | 16 | 9·21 (2·85); 1387 | 9·21 (2·83); 1318 | |
| Free testosterone, pmol/L | 12 | 196·02 (66·46); 120 | 198·92 (70·87); 116 | |
| Fasting glucose, mmol/L | 12 | 6·55 (2·18); 1421 | 6·66 (2·36); 1353 | |
| Cholesterol, mmol/L | 15 | 4·71 (1·12); 1670 | 4·73 (1·10); 1606 | |
| Low-density lipoproteins, mmol/L | 15 | 2·81 (1·02); 1644 | 2·78 (1·00); 1584 | |
| High-density lipoproteins, mmol/L | 15 | 1·20 (0·36); 1664 | 1·21 (0·39); 1599 | |
| Triglycerides, mmol/L | 15 | 1·87 (1·39); 1653 | 1·91 (1·50); 1584 | |
| Haemoglobin, g/L | 14 | 145·26 (12·64); 160 | 144·30 (12·89); 151 | |
| HbA1c (%) | 10 | 6·35 (1·08); 1067 | 6·36 (1·12); 1059 | |
| Haematocrit (%) | 16 | 43·29 (3·68); 1694 | 42·99 (3·83); 1621 | |
| Systolic blood pressure, mm Hg | 12 | 133·13 (17·30); 130 | 133·52 (16·62); 127 | |
| Diastolic blood pressure, mm Hg | 12 | 77·21 (10·74); 1300 | 77·08 (10·72); 1274 | |
Data are mean (SD) with number of participants, or n/N (%). IIEF=International Index of Erectile Function.
Type 1, 2, and unknown type.
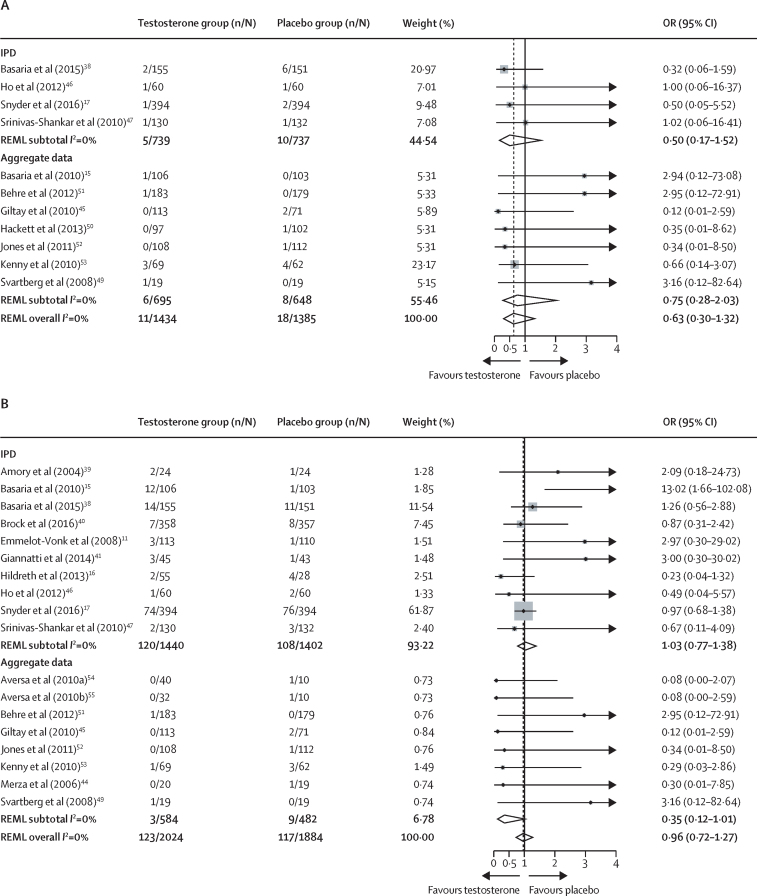
14 (82%) studies provided IPD on mortality. The one-stage analysis indicated that fewer deaths were reported in the testosterone group (six [0·4%] of 1621) than in the placebo group (12 [0·8%] of 1537) with no significant differences between groups (OR 0·46 [95% CI 0·17–1·24]; p=0·13). Causes of death included myocardial infarction, cancer, and ruptured aortic aneurysm; in three studies (seven deaths in total) cause was undetermined (table 2). The two-stage analysis (figure 2A) and Mantel-Haenszel sensitivity analysis showed similar results (appendix p 35). Both contour-enhanced funnel plots as well as Peters' test on small-study effects for IPD (Peters' test p=0·28), aggregate, and all studies combined (Peters' test p=0·46) showed no evidence of significant small-study bias (appendix p 36).
Table 2.
One-stage individual participants dataset meta-analysis for all-cause mortality and cardiovascular or cerebrovascular events
| Number of studies | Testosterone treatment group | Placebo group | OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| Mortality from any cause | ||||||
| Number of participants* | 14 | 6/1621 (0·4%) | 12/1537 (0·8%) | 0·46 (0·17–1·24) | 0·13 | |
| Myocardial Infarction | 3 | 2/6 (33·3%) | 2/12 (16·7%) | .. | .. | |
| Cancer | 1 | 0 | 3/12 (25·0%) | .. | .. | |
| Ruptured aortic aneurysm | 1 | 0 | 1/12 (8·3%) | .. | .. | |
| Constrictive pericarditis | 1 | 1/6 (16·7%) | 0 | .. | .. | |
| Multiple organ failure | 1 | 1/6 (16·7%) | 0 | .. | .. | |
| Venous thromboembolism | 1 | 0 | 1/12 (8·3%) | .. | .. | |
| Unknown | 3 | 2/6 (33·3%) | 5/12 (41·7%) | .. | .. | |
| Cardiovascular or cerebrovascular events | ||||||
| Number of participants† | 13 | 120/1601 (7·5) | 110/1519 (7·2) | 1·07 (0·81, 1·42) | 0.62 | |
| Total number of events | 13 | 182 | 183 | .. | .. | |
| Number of participants with a cardiovascular event | 11 | 107/120 (89·2%) | 105/110 (95·5%) | .. | .. | |
| Total number of cardiovascular events‡ | 11 | 166 | 176 | .. | .. | |
| Arrhythmia | 6 | 52/166 (31·3%) | 47/176 (26·7%) | .. | .. | |
| Coronary heart disease | 6 | 33/166 (19·9%) | 33/176 (18·8%) | .. | .. | |
| Heart failure | 6 | 22/166 (13·3%) | 28/176 (15·9%) | .. | .. | |
| Myocardial infarction | 7 | 10/166 (6·0%) | 16/176 (9·1%) | .. | .. | |
| Valvular heart disease | 2 | 18/166 (10·8%) | 12/176 (6·8%) | .. | .. | |
| Peripheral vascular disease | 4 | 8/166 (4·8%) | 14/176 (8·0%) | .. | .. | |
| Stable angina | 5 | 7/166 (4·2%) | 7/176 (4·0%) | .. | .. | |
| Aortic aneurysm§ | 5 | 6/166 (3·6%) | 7/176 (4·0%) | .. | .. | |
| New angina | 3 | 5/166 (3·0%) | 5/176 (2·8%) | .. | .. | |
| Unstable angina | 3 | 2/166 (1·2%) | 4/176 (2·3%) | .. | .. | |
| Aortic dissection | 1 | 2/166 (1·2%) | 0 | .. | .. | |
| Atherosclerosis | 1 | 1/166 (0·6%) | 1/176 (0·6%) | .. | .. | |
| Cardiac arrest | 2 | 0 | 2/176 (1·1%) | .. | .. | |
| Number of participants with a cerebrovascular event | 11 | 15/120 (12·5%) | 7/110 (6·4%) | .. | .. | |
| Total number of cerebrovascular events‡ | 11 | 16 | 7 | .. | .. | |
Data are n/N (%), unless otherwise specified. For one study38 outcomes were reported up to 3 years and the date of events could not be confirmed. OR=odds ratio.
Of the 14 studies, eight reported no deaths11, 16, 39, 40, 41, 42, 43, 44 and six reported deaths.15, 17, 38, 45, 46, 47 We were unable to confirm whether any deaths occurred for the remaining three studies;48, 49, 50 therefore, they were not included.
Of the 13 studies, two42, 43 reported no cardiovascular or cerebrovascular events. We were unable to confirm whether cardiovascular or cerebrovascular events occurred for the remaining four studies.44, 48, 49, 50
Some participants had more than one event.
One event was a ruptured aortic aneurysm.
Figure 2.
Two-stage IPD meta-analysis for all-cause mortality (A) and cardiovascular or cerebrovascular events (B)
IPD=individual participant dataset. OR=odds ratio. REML=restricted maximum likelihood.
13 studies (76%) provided IPD for cardiovascular or cerebrovascular events (table 2). There were 120 (7·5%) of 1601 events in the testosterone group and 110 (7·2%) of 1519 events in the placebo group; there was no significant difference between groups (OR 1·07 [95% CI 0·81–1·42]; p=0·62). Most reported events were arrhythmia, coronary heart disease, heart failure, myocardial infarction, and valvular heart disease (table 2). Sensitivity analysis, including unknown cause of death, did not change observed results (OR 1·05 [95% CI 0·79–1·38]; p=0·74). Results were not changed significantly by including follow-up time as a weight in the model (cardiovascular or cerebrovascular events unadjusted OR 1·07 [95% CI 0·81–1·42] to OR 1·02 [0·92–1·14]; death unadjusted OR 0·46 [0·17–1·24] to OR 0·42 [0·28–0·62]). Two-stage analysis showed differences between IPD (OR 1·03 [95% CI 0·77–1·38]) and aggregate data (OR 0·35 [0·12–1·01]) (figure 2B; appendix p 37). Both contour-enhanced funnel plots (appendix p 38), as well as Peters' test on small-study effects for IPD (Peters' test p=0·82), aggregate, and all studies combined (Peters' test p=0·70), showed no significant small-study bias. There was no evidence of treatment-covariate interaction for diabetes or smoking status, age, or baseline cardiovascular or cerebrovascular events (appendix p 37). Furthermore, pre-specified analysis showed no evidence that baseline total or free testosterone were associated with risks of cardiovascular or cerebrovascular event risk during testosterone treatment. However, post-hoc sensitivity analyses suggested that cardiovascular or cerebrovascular event risk favoured testosterone treatment when free testosterone was between 180 and 220 pmol/L (appendix p 40).
Regarding physiological markers, as expected, the one-stage analysis showed evidence of higher serum total testosterone concentrations in the testosterone group than the placebo group (mean difference 7·24 nmol/L [95% CI 5·07–9·41]; p<0·0001; τ2=17·01; table 3). Similar findings were observed for free testosterone but with substantial heterogeneity. Furthermore, the one-stage analysis showed evidence of lower HDL cholesterol in the testosterone group than the placebo group (mean difference –0·06 nmol/L [95% CI –0·08 to –0·04]; p<0·0001; τ2=0·0). Both serum total cholesterol and triglycerides were significantly lower in the testosterone group than the placebo group; there was evidence of some difference and a degree of homogeneity (mean difference for total cholesterol, –0·15 mmol/L [95% CI –0·20 to –0·10]; p<0·001; τ2=0·00 and mean difference for triglycerides, –0·09 nmol/L [95% CI –0·18 to –0·00]; p=0·04; τ2=0·01). Significant differences were observed for haemoglobin and haematocrit. For fasting glucose sensitivity, HbA1c, and blood pressure, there was no difference between treatment groups. However, fasting glucose and HbA1c analyses were not limited to patients with diabetes. Results for the one-stage analysis for the remaining physiological marker outcomes are presented in the appendix (p 41). The two-stage analysis showed similar results to the one-stage analysis; however, for some outcomes there was a difference between the studies with and without IPD (appendix p 42–61).
Table 3.
One-stage analysis for secondary outcome of physiological markers
| Number of studies | Testosterone treatment group | Placebo group | Mean difference (95% CI) | τ2 | |
|---|---|---|---|---|---|
| Testosterone, nmol/L | 16 | 17·27 (10·34); 1211 | 9·87 (3·98); 1156 | 7·24 (5·07 to 9·41) | 17·01 |
| Free testosterone, pmol/L | 12 | 426·70 (368·42); 1058 | 203·57 (86·24); 1027 | 186·40 (115·91 to 256·90) | 13 741·90 |
| Fasting glucose, mmol/L | 12 | 6·50 (2·09); 1259 | 6·75 (2·38); 1181 | −0·16 (−0·24 to −0·07) | 0·00 |
| Fasting glucose sensitivity*, mmol/L | 11 | 6·04 (1·69); 946 | 6·24 (2·04); 897 | −0·13 (−0·28 to 0·02) | 0·04 |
| Cholesterol, mmol/L | 14 | 4·51 (1·05); 1388 | 4·67 (1·11); 1314 | −0·15 (−0·20 to −0·10) | 0·00 |
| Low-density lipoproteins cholesterol, mmol/L | 14 | 2·69 (0·98); 1378 | 2·70 (0·98); 1299 | −0·03 (−0·08 to 0·01) | 0·00 |
| High-density lipoproteins cholesterol, mmol/L | 14 | 1·15 (0·33); 1384 | 1·21 (0·39); 1312 | −0·06 (−0·08 to −0·04) | 0·00 |
| Triglycerides, mmol/L | 14 | 1·73 (1·30); 1368 | 1·89 (1·51); 1297 | −0·09 (−0·18 to −0·00) | 0·01 |
| Haemoglobin, g/L | 13 | 153·53 (14·71); 1291 | 143·58 (12·67); 1206 | 10·87 (8·19 to 13·55) | 20·80 |
| Haematocrit (%) | 15 | 46·06 (4·37); 1399 | 42·94 (3·77); 1309 | 3·15 (2·42 to 3·88) | 1·77 |
| HbA1c (%) | 8 | 6·46 (1·12); 748 | 6·58 (1·21); 742 | −0·09 (−0·25 to 0·06) | 0·03 |
| HbA1c (%) sensitivity* | 7 | 6·14 (0·94); 519 | 6·24 (1·08); 523 | −0·89 (−2·43 to 0·64) | 4·29 |
| Systolic blood pressure, mmHg | 10 | 134·11 (17·14); 1069 | 133·31 (16·64); 1041 | 0·99 (−0·08 to 2·06) | 0·00 |
| Diastolic blood pressure, mmHg | 10 | 77·20 (11·03); 1069 | 76·84 (10·98); 1041 | 0·48 (−0·30 to 1·26) | 0·15 |
Data are mean (SD), unless otherwise specified. Outcomes were analysed using a random-effects model.
Participants with diabetes at baseline were excluded.
Results of additional outcomes are presented in table 4. In the testosterone group, 14 (1·9%) of 752 men had new diabetes or diabetes complications, but there was no evidence of difference between groups (χ2 test, p>0·05). Similarly, there was no evidence of difference between groups in terms of incidence of prostate cancer, hypertension, venous thromboembolism, and non-stroke cerebrovascular pathology. More men treated with testosterone had oedema and high haematocrit than treated with placebo.
Table 4.
Summary of additional outcomes
| Number of studies | Testosterone treatment group | Placebo group | |
|---|---|---|---|
| Diabetes or diabetes complications | 2 | 14/752 (1·9%) | 19/751 (2·5%) |
| Prostate cancer | 8 | 10/1293 (0·8%) | 3/1059 (0·3%) |
| Oedema | 7 | 34/1301 (2·6%) | 17/1290 (1·3%) |
| Hypertension | 7 | 28/1195 (2·3%) | 20/1182 (1·7%) |
| High haematocrit | 7 | 30/1079 (2·8%) | 5/993 (0·5%) |
| Venous thromboembolism | 4 | 5/1037 (0·5%) | 7/1034 (0·7%) |
| Non-stroke cerebrovascular pathology* | 3 | 4/655 (0·6%) | 11/648 (1·7%) |
Data are n/N (%).
Examples include carotid occlusion and carotid stenosis.
Discussion
Our IPD meta-analysis of more than 3000 patients with hypogonadism from randomised placebo-controlled trials done by 17 research groups indicates that testosterone treatment is not associated with increased risk of various subtypes of cardiovascular events compared with placebo in the short to medium term. The small total number of deaths within our IPD analysis precluded a meaningful evaluation of the impact of testosterone treatment on mortality; furthermore, there was little available data evaluating the cardiovascular safety of testosterone beyond a 12-month duration of administration. Testosterone treatment did not have adverse effects on blood pressure or glycaemic markers compared with placebo; furthermore, it did not increase thrombotic events despite increased haematocrit. Testosterone treatment was associated with a modest lowering of total and HDL cholesterol and triglyceride concentrations compared with placebo.
Men with hypogonadism included in this IPD analysis had a higher prevalence of cardiovascular risk factors compared with the general population. Despite these risk factors, the overall incidence of cardiovascular events was not significantly higher during testosterone treatment than for placebo. Because most trials do not publish details of individual adverse events, the exact frequency of cardiovascular events occurring during testosterone treatment, up until this point, has been unclear. Two previous meta-analyses, which used different inclusion and exclusion criteria, have quantified the total numbers of reported cardiovascular events of any subtype, during testosterone therapy.21, 22 Xu and colleagues21 reported 180 (6·0%) cardiovascular-related events among 2994 men from 27 trials while Corona and colleagues22 reported 210 (3·8%) cardiovascular events among 5464 men from 75 trials. Within our IPD analysis, two masked clinical investigators identified a total of 342 cardiovascular events from the included IPD, which to date is the highest published rate of cardiovascular events. There is currently no consensus on the components of cardiovascular endpoint constituting a major adverse cardiovascular event, which might prohibit the comparison, replication, and aggregation of data.56 Here, we have reported every cardiovascular event (including those classified by ACC32) encountered within several clinical trials providing IPD. Focusing on all cardiovascular events has enabled us to evaluate all aspects of cardiovascular safety for clinicians and patients, without making assumptions about the mechanisms of any potential association between testosterone and cardiovascular disease.
The most frequently recorded cardiovascular event categories in the identified trials were arrhythmia, coronary heart disease (without further description provided), heart failure, cerebrovascular events, and myocardial infarction. We have also identified and reported frequencies of stable angina, peripheral vascular disease, aortic aneurysm, and aortic dissection, which have not been reported by any previous meta-analysis.5, 20, 21, 22, 28, 57, 58, 59 None of the cardiovascular event subtypes were significantly more common in patients assigned to testosterone treatment than in patients assigned to placebo. Neither patient age nor the previous diagnosis of cardiovascular events were associated with an increased risk of cardiovascular events. Several thresholds for serum total testosterone (ranging between 8 and 12 nmol/L) have been proposed for the diagnosis of hypogonadism.60 A post-hoc subgroup analysis showed a lower risk of cardiovascular events with testosterone treatment when calculated free testosterone was 180–220 pmol/L. However, no overall association was observed between cardiovascular events and either free or total serum testosterone at baseline or during testosterone treatment. No clear factors associated with cardiovascular risk were identified during testosterone treatment. Two previous studies (one of which is included in our IPD analysis) have reported that testosterone treatment is associated with reduced mortality in hypogonadal men with type 2 diabetes.61, 62 Our IPD analysis reported a non-significant increase in cardiovascular event risk in men with diabetes during testosterone treatment, suggesting potential heterogeneity in the results of participating studies. In summary, our findings indicate that testosterone treatment did not increase risks of any subtype of cardiovascular event in men with hypogonadism and did not identify any patient characteristics that were associated with a significantly increased risk of cardiovascular events during testosterone treatment. Furthermore, we observed a similar mortality rate during testosterone treatment when compared with placebo, which was reassuring. A meta-analysis of several observational studies63 reported an association between low endogenous testosterone concentrations and increased risk of cardiovascular events, suggesting, notwithstanding the possibility of reverse causality, that testosterone therapy might result in some beneficial effects on the cardiovascular system. According to the results of our analysis, the overall short to medium-term effect of testosterone seems neutral.
In view of the lack of consistent cardiovascular event classification, adjudication, or reporting within trials, we did a masked analysis of each individual adverse event by two independent clinicians to classify cardiovascular events from all IPD studies objectively. We successfully obtained data from 3431 (61·3%) of the 5601 participants included in eligible published trials, but IPD from some studies could not be included due to data loss, retirement or death of lead investigators, or unwillingness of two pharmaceutical sponsors (Bayer AG, Kyowa Kirin) to disclose them. To assess the effect of studies for which IPD were not available, we extracted appropriate aggregate study-level data and incorporated them alongside the IPD using two-stage IPD random-effect meta-analyses.64 Our aggregate meta-analysis suggested that outcome data were not significantly discrepant between our IPD and non-IPD studies. Nevertheless, we cannot exclude that a high number of unreported cardiovascular events in the non-IPD studies could ultimately change the conclusions of our analysis. The very small total number of deaths recorded during testosterone trials limits our ability to analyse why they occurred. The mean follow-up of included randomised controlled trials was 9·5 months, which might be too short for atherosclerotic plaque progression to accrue. This is important, since the Testosterone Trials observed that a 12-month duration of testosterone treatment was associated with a significantly greater increase in coronary artery non-calcified plaque volume versus placebo, in older men.65 This finding has led some to advocate coronary artery calcium scoring before treatment of high-risk individuals with underlying cardiovascular disease, due to the remaining possibility that testosterone might be riskier in such individuals. However, we observed no significant associations between existing (baseline) cardiovascular or cerebrovascular events and risks of future events during the first 9·5 months after initiation of testosterone treatment.
The secondary objective of this IPD analysis was to assess the physiological effects of testosterone treatment. Testosterone had no significant effect on HbA1c. Testosterone reduced serum fasting glucose concentrations in cases for which this was recorded, but this effect became non-significant when patients with known diabetes were excluded. Consistent with some published studies, minor reductions in serum total cholesterol, HDL cholesterol, and triglycerides were observed. Testosterone significantly elevated haematocrit and haemoglobin, which is in keeping with its role of suppressing hepcidin, a tonic, negative regulator of haematopoiesis. Furthermore, testosterone treatment was associated with a five-times higher risk of polycythemia. However, only five cases of deep vein thromboses were recorded during testosterone treatment, compared with seven in the placebo group. We observed no overall effect of testosterone on systolic or diastolic blood pressure.
An important strength of this IPD meta-analysis is its large size compared with individual testosterone trials, which have provided limited and situation-dependent information on cardiovascular safety. This IPD meta-analysis draws data from multiple, geographically diverse studies with approximately five-times more participants than the largest single participating trial. Definitive conclusions about the long-term cardiovascular safety of testosterone therapy cannot be made without results of an adequately powered clinical trial. However, this study has allowed us to more precisely estimate the incidence of cardiovascular events associated with testosterone treatment, which might be generalisable to patients worldwide. Furthermore, utilising previously collected data, we have actively reduced research waste.66 We did not detect any significant funnel plot asymmetry in our analyses, suggesting that publication bias is not likely to be present in the overall IPD set. Several meta-analyses of published aggregate data have investigated the cardiovascular safety of testosterone treatment in men. Xu and colleagues analysed cardiovascular episodes of any type within the international statistical classification of diseases (ICD-10) and observed an increased cardiovascular risk during testosterone treatment.21 By contrast, Corona and colleagues and Diem and colleagues did three separate meta-analyses of five-point major adverse cardiovascular events as the primary outcome, and any cardiovascular event as a secondary outcome; testosterone treatment was neither associated with major adverse cardiovascular events nor other cardiovascular events in any meta-analysis.5, 22, 59 Fernandez-Balsells and colleagues reported that testosterone treatment did not significantly modify risks of myocardial infarction, all-cause mortality, coronary artery bypass, and arrhythmia, but did reduce HDL cholesterol concentrations.20 Both Alexander and colleagues and Elliot and colleagues analysed risks of discrete major adverse cardiovascular event subtypes during testosterone treatment; neither myocardial infarction, stroke, nor mortality risks were associated with testosterone therapy.28, 58 Guo and colleagues observed a reduction in total cholesterol during testosterone therapy, but did not analyse lipid subtypes, or cardiovascular event risk.57 Most of these meta-analyses failed to observe increased cardiovascular event risk with testosterone; however, many guidelines recommend that cardiovascular risk is considered when commencing testosterone treatment.
The current study has several strengths compared with all previous meta-analyses. Firstly, our access to unpublished cardiovascular events, which were independently adjudicated by investigators masked to the treatment allocation, allows for more robust scrutiny of cardiovascular safety. Secondly, we have been able to investigate whether subgroups of patients have distinct cardiovascular risk profiles during testosterone administration. Some previous meta-analyses of published data have comprised studies in which the relatively high baseline serum testosterone concentrations have allowed non-hypogonadal patients to be included.5, 20, 28, 57 By contrast, this IPD meta-analysis is restricted to patients with serum testosterone <12 nmol/L (350 ng/dL) using a validated mass spectrometry or immunoassays; this threshold was chosen after consideration that all current clinical guidelines on testosterone treatment recommend serum testosterone thresholds of between 8 and 12 nmol/L to ensure the inclusion of hypogonadal men exclusively. Variation among testosterone assay measurements limits the extent to which results from different studies can be compared.67 Our IPD approach was further strengthened by subgroup analyses to assess whether any observed effect of testosterone was consistent across subgroups of patients. We did not observe any significant association between baseline testosterone and risks of any adverse outcome. Unlike some other meta-analyses, we excluded studies of patient groups with distinct risk profiles such as cancer, HIV, and cirrhosis,22, 27 or those with less than 3 months of testosterone exposure.28 Finally, our analysis compared physiological markers in a more standardised manner compared with previous meta-analyses, by analysing the outcome at the timepoint closest to 12 months of testosterone treatment, regardless of whether that data had been previously published. Two meta-analyses have reported that testosterone improves glycaemic parameters in men.68, 69 Furthermore, Corona and colleagues reported an improvement in blood pressure during testosterone treatment.70 However, our study suggests that testosterone has no significant effects on either blood pressure or glycaemic indices.
Results of this meta-analysis have potentially important implications for the management of men with hypogonadism. Worldwide prescribing of testosterone for hypogonadism is increasing;2 however, conflicting messages on testosterone safety might have caused variations in treatment among patients. We have conducted the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism. Testosterone treatment did not increase cardiovascular event risk in the short term to medium term. Furthermore, we did not identify subgroups with high cardiovascular risk. An ongoing trial (NCT03518034) is investigating the longer-term safety of testosterone, and future studies are needed to analyse the risk–benefit and cost-effectiveness of testosterone therapy. However, the current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism.
Data sharing
The statistical analysis plan used for this study is included in the appendix (pp 5–17). All aggregate patient data are presented either in the manuscript or appendix. Individual patient data cannot be made publicly available because they are protected by a confidentiality agreement.
Declaration of interests
CNJ reports research grants from Logixx Pharma. SBhas reports research grants from AbbVie and National Institutes of Health; consulting fees from Aditum; participation on the Data Safety Monitoring board of OKPO; a leadership or fiduciary role in the Endocrine Society; and a patent (free T calculator based on the ensemble allosteric model, reference 20200174026). SSE reports research grants from AbbVie. MG reports research grants from Otsuka, Bayer, Lilly, Weight Watchers, Novartis, National Health and the Medical Research Council Australia; speaker honoraria from Besins Healthcare, Bayer, and Otsuka; consulting fees from Bayer; and Royalties from Walter and Eliza Hall Institute Melbourne Australia. KLH reports research grants from NIA K23 Career Development Award; a leadership or fiduciary role from Kavod Senior Life Board of Directors, Executive Committee; and stock or stock options from Medaware Systems. NO reports research grants from Dexcom, Roche Diabetes, and Medtronic Diabetes; speaker honoraria from Roche Diabetes; consulting fees from Roche Diabetes and Medtronic Diabetes; payment for expert testimony from London law firm Wilmer Hale; and an issued patent for automatic closed loop glucose control with an adaptive meal bolus calculator. SR reports research grants from North Staffordshire Medical Institute and support for attending meetings or travel from Besins Healthcare. PJS reports research grants from Abbvie and payment for expert testimony from Teva. RQ reports speaker honoraria and support for attending meetings or travel from Bayer. SBhat reports speaker honoraria from Obstetrical & Gynaecological Society of Singapore, Merck SMART Masterclass, and Merck FERRING Forum; leadership or fiduciary role at National Health Service Grampian; editorial role (Editor in Chief of Human Reproduction Open, Special Senior Editor of Cochrane Gynaecology and Fertility); membership of NHS Grampian Board; other financial or non-financial interests from Oxford University Press; and Royalties from Cambridge University Press (Royalties for book Reproductive Medicine for the MRCOG, Cambridge University Press). GBB reports speaker honoraria and consulting fees from Acerus Pharmaceutical. HMT reports consulting fees from Besin Healthcare. EJGil reports provision of study materials from Bayer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the National Institute for Health Research Health Technology Assessment (NIHR HTA) Programme (project no 17/68/01). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR HTA Programme, or the Department of Health and Social Care, UK. The funders were not actively involved in the research process at any stage. The study design; collection, analysis, and interpretation of data; writing of the manuscript; and decision to submit for publication were performed independent of the funders. The Health Services Research Unit at the University of Aberdeen is funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The Section of Endocrinology and Investigative Medicine at Imperial College London is funded by grants from the Medical Research Council, Biotechnology and Biological Sciences Research Council, NIHR, an Integrative Mammalian Biology Capacity Building Award, an FP7-HEALTH-2009-241592 EuroCHIP grant, and is supported by the NIHR Biomedical Research Centre Funding Scheme. The following authors are also funded as follows: NIHR Research Professorship (WSD), NIHR post-doctoral fellowship (CNJ). SBhasin receives National Institutes of Health research grant funding. The authors are grateful to Prakash Abraham, Alison Avenell, Craig Ramsay, Graham Scotland, Neil Scott, and Finlay MacKenzie for their advice; and to the many individuals from academia and industry who helped in the conduct of this study.
Contributors
MB, CNJ, KG, LA, WSD, NO, RQ, and SBhat were involved in conceptualisation and study design. MC, CNJ, MB, FW, and RQ were involved in data collection and management. MC and MA-M extracted data. SBhas, PJS, SSE, MG, TGT, EJGil, YTvderS, MHE-V, EJGia, GH, SR, JS, KLH, KGA, GBB, JLT, HMT, CHCK, WST, LSM, RJR, RSS, SR, MSA, and LVM were involved in trial data collection and data transfer. JH formatted the data and did the statistical analyses. LA provided statistical advice. JH, MC, CNJ, MB, FW, WSD, NO, and RQ wrote the first draft of the manuscript. JH, MC, MB, CNJ, KG, LA, WSD, RH, NO, FW, RQ, SBhat, SBhas, PJS, SSE, MG, TGT, EG, YTS, MHE-V, EJGil, GH, and KGA revised the manuscript. PM did the literature searches and formatted the manuscript. JH and MB verified the underlying data. All authors have critically reviewed and approved the final manuscript version. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Supplementary Material
References
- 1.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–551. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 2.Baillargeon J, Kuo YF, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002–2016. JAMA. 2018;320:200–202. doi: 10.1001/jama.2018.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746–752. doi: 10.1097/MLR.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 5.Diem SJ, Greer NL, MacDonald R, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020;172:105–118. doi: 10.7326/M19-0830. [DOI] [PubMed] [Google Scholar]
- 6.Yeap BB, Grossmann M, McLachlan RI, et al. Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations. Med J Aust. 2016;205:228–231. doi: 10.5694/mja16.00448. [DOI] [PubMed] [Google Scholar]
- 7.Dohle G, Arver S, Bettocchi C, Jones T, Kliesch S. European Association of Urology guidelines: male hypogonadism. 2018. https://d56bochluxqnz.cloudfront.net/media/EAU-Guidelines-on-Male-Hypogonadism-2018-large-text.pdf
- 8.Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European Society of Endocrinology. Andrology. 2020;8:970–987. doi: 10.1111/andr.12770. [DOI] [PubMed] [Google Scholar]
- 9.Wittert G, Bracken K, Robledo KP, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9:32–45. doi: 10.1016/S2213-8587(20)30367-3. [DOI] [PubMed] [Google Scholar]
- 10.Walker RF, Zakai NA, MacLehose RF, et al. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2020;180:190–197. doi: 10.1001/jamainternmed.2019.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men—a randomized controlled trial. J Am Med Assoc. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 12.Vigen R, O'Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 13.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311:963. doi: 10.1001/jama.2014.395. [DOI] [PubMed] [Google Scholar]
- 15.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildreth KL, Barry DW, Moreau KL, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98:1891–1900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “age-related hypogonadism”—FDA Concerns. N Engl J Med. 2015;373:689–691. doi: 10.1056/NEJMp1506632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency No consistent evidence of an increased risk of heart problems with testosterone medicines. 2014. https://www.ema.europa.eu/en/news/no-consistent-evidence-increased-risk-heart-problems-testosterone-medicines
- 20.Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–1351. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 23.Borst SE, Shuster JJ, Zou B, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seftel AD, Kathrins M, Niederberger C. Critical update of the 2010 Endocrine Society Clinical Practice Guidelines for male hypogonadism: a systematic analysis. Mayo Clin Proc. 2015;90:1104–1115. doi: 10.1016/j.mayocp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf) 2016;85:436–443. doi: 10.1111/cen.13084. [DOI] [PubMed] [Google Scholar]
- 26.Huo S, Scialli AR, McGarvey S, et al. Treatment of men for “low testosterone”: a systematic review. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on International Index of Erectile Function Scores. Eur Urol. 2017;72:1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017;130:293–305. doi: 10.1016/j.amjmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Centre for Reviews and Dissemination Systematic reviews: CRD's guidance for undertaking systematic reviews in health care. 2009. http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm
- 30.Higgins J, Green S, Thomas J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021. https://training.cochrane.org/handbook
- 31.Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 32.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71:1021–1034. doi: 10.1016/j.jacc.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. http://handbook-5-1.cochrane.org/
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control ClinTrials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 36.Riley RD, Debray TPA, Fisher D, et al. Individual participant data meta-analysis to examine interactions between treatment effect and participant-level covariates: statistical recommendations for conduct and planning. Stat Med. 2020;39:2115–2137. doi: 10.1002/sim.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruish M. Third edn. QualityMetric Incorporated; Lincoln, RI: 2011. User's manual for the SF-36v2 Health Survey. [Google Scholar]
- 38.Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–581. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 39.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 40.Brock G, Heiselman D, Maggi M, et al. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men: results of a placebo controlled study. J Urol. 2016;195:699–705. doi: 10.1016/j.juro.2015.10.083. [DOI] [PubMed] [Google Scholar]
- 41.Gianatti EJ, Dupuis P, Hoermann R, Zajac JD, Grossmann M. Effect of testosterone treatment on constitutional and sexual symptoms in men with type 2 diabetes in a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99:3821–3828. doi: 10.1210/jc.2014-1872. [DOI] [PubMed] [Google Scholar]
- 42.Groti K, Žuran I, Antonič B, Foršnarič L, Pfeifer M. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169. doi: 10.1080/13685538.2018.1468429. [DOI] [PubMed] [Google Scholar]
- 43.Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund K, Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes Metab. 2016;18:980–989. doi: 10.1111/dom.12701. [DOI] [PubMed] [Google Scholar]
- 44.Merza Z, Blumsohn A, Mah PM, et al. Double-blind placebo-controlled study of testosterone patch therapy on bone turnover in men with borderline hypogonadism. Int J Androl. 2006;29:381–391. doi: 10.1111/j.1365-2605.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 45.Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJ, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7:2572–2582. doi: 10.1111/j.1743-6109.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 46.Ho CC, Tong SF, Low WY, et al. A randomized, double-blind, placebo-controlled trial on the effect of long-acting testosterone treatment as assessed by the Aging Male Symptoms scale. BJU Int. 2012;110:260–265. doi: 10.1111/j.1464-410X.2011.10755.x. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 48.Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism—a randomized controlled trial. J Am Med Assoc. 2006;296:2351–2361. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- 49.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 50.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10:1612–1627. doi: 10.1111/jsm.12146. [DOI] [PubMed] [Google Scholar]
- 56.Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21:241. doi: 10.1186/s12874-021-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo C, Gu W, Liu M, et al. Efficacy and safety of testosterone replacement therapy in men with hypogonadism: a meta-analysis study of placebo-controlled trials. Exp Ther Med. 2016;11:853–863. doi: 10.3892/etm.2015.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elliott J, Kelly SE, Millar AC, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Kwong JCC, Krakowsky Y, Grober E. Testosterone deficiency: a review and comparison of current guidelines. J Sex Med. 2019;16:812–820. doi: 10.1016/j.jsxm.2019.03.262. [DOI] [PubMed] [Google Scholar]
- 61.Hackett G, Cole N, Mulay A, Strange RC, Ramachandran S. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int. 2019;123:519–529. doi: 10.1111/bju.14536. [DOI] [PubMed] [Google Scholar]
- 62.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 63.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344 doi: 10.1136/bmj.d7762. [DOI] [PubMed] [Google Scholar]
- 65.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macleod MR, Michie S, Roberts I, et al. Biomedical research: increasing value, reducing waste. Lancet. 2014;383:101–104. doi: 10.1016/S0140-6736(13)62329-6. [DOI] [PubMed] [Google Scholar]
- 67.Travison TG, Vesper HW, Orwoll E, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Yang B, Xiao W, Li X, Li H. Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review. World J Urol. 2018;36:1315–1326. doi: 10.1007/s00345-018-2256-0. [DOI] [PubMed] [Google Scholar]
- 69.Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16:146–152. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corona G, Giagulli VA, Maseroli E, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39:967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
Uncited References
- 51.Behre HM, Tammela TL, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15:198–207. doi: 10.3109/13685538.2012.699562. [DOI] [PubMed] [Google Scholar]
- 52.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 Study) Diabetes Care. 2011;34:828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenny AM, Kleppinger A, Annis K, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–3503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 55.Aversa A, Bruzziches R, Francomano D, Spera G, Lenzi A. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010;33:776–783. doi: 10.1007/BF03350341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The statistical analysis plan used for this study is included in the appendix (pp 5–17). All aggregate patient data are presented either in the manuscript or appendix. Individual patient data cannot be made publicly available because they are protected by a confidentiality agreement.