Abstract
Amyloidomas are focal solitary amyloid masses without systemic involvement that have been observed to occur in various body locations. When presenting intracranially, they pose a challenging diagnostic and therapeutic course given their location and rarity. We report a case of a 62-year-old man with a 4-year history of seizure and headaches. Magnetic resonance imaging was initially inconclusive but revealed an ill-defined right temporal lobe lesion. Biopsy later confirmed a cerebral amyloidoma. We also review the current literature on the pathogenesis, imaging findings, prognosis, and treatment of cerebral amyloidomas.
Keywords: Amyloidoma, Cerebral, Brain, Intracranial, Temporal lobe
Case report
A 62-year-old male with a past medical history of hypertension, hypercholesterolemia, and gastroesophageal reflux disease, presented to his local emergency department after being found on the ground in an obtunded state. He was noted at the hospital to have had a generalized convulsive seizure lasting several minutes which resolved with lorazepam; a thorough neurological exam once the patient had returned to baseline was unremarkable. Further investigation into the patient's history revealed that he may had been symptomatic for at least the past 4 years before seeking medical help. Episodes consistent with complex partial seizures occurred roughly every other month but was becoming more frequent. He also admitted to intermittent headaches which tended to be worse after these episodes.
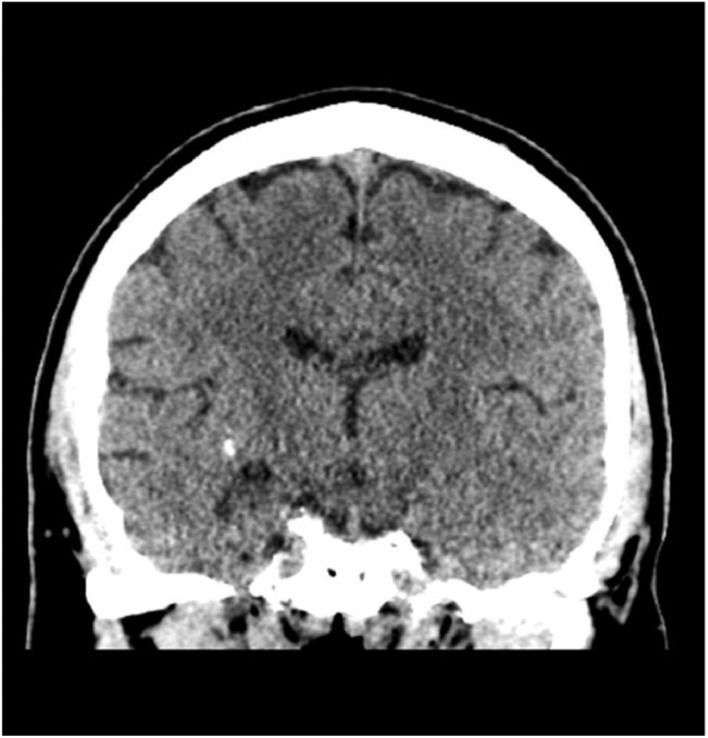
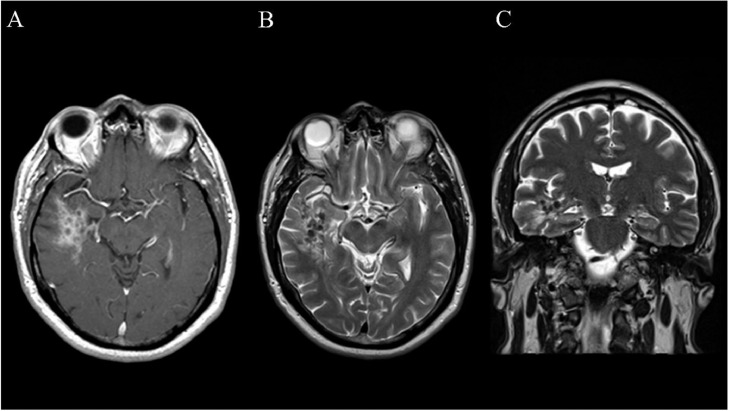
Initial brain imaging revealed an intraparenchymal right temporal lobe lesion. Non-contrast computed tomography (CT) scan revealed a hyperdense region with subcentimeter focus of calcification in the right temporal lobe without significant mass effect (Fig. 1). T1-weighted magnetic resonance imaging (MRI) showed a heterogeneous, ill-defined lesion with gadolinium contrast enhancement; this enhancement surrounded nodular areas of non-enhancement and seemed to involve the ependymal lining of the right lateral ventricle (Fig. 2A). On the corresponding T2-weighted imaging, there was an irregular area of hyperintensity surrounding nodular areas of hypointensity (Figs. 2B and C). There were no signs of mass effect or cerebral amyloid angiopathy. Given the contrast enhancement, the differential included central nervous system lymphoma, metastasis, and glioma. However, they were less likely given the lack of mass effect and the hypointensities seen on T2-weighted imaging. The patient was started on levetiracetam and was referred to neurosurgery.
Fig. 1.
Coronal non-contrast CT shows a hyperdense region with subcentimeter focus of calcification in the right temporal lobe without mass effect or sulcal effacement.
Fig. 2.
(A) Axial T1-weighted MR sequence with gadolinium contrast enhancement displaying the amyloidoma in the right temporal lobe. An ill-defined area of contrast enhancement surrounds several round hypointensities. (B) Axial T2-weighted sequence displaying round areas of hypointensity surrounded by hyperintensity. (C) Coronal T2-weighted sequence displaying the vertical extent of the lesion.
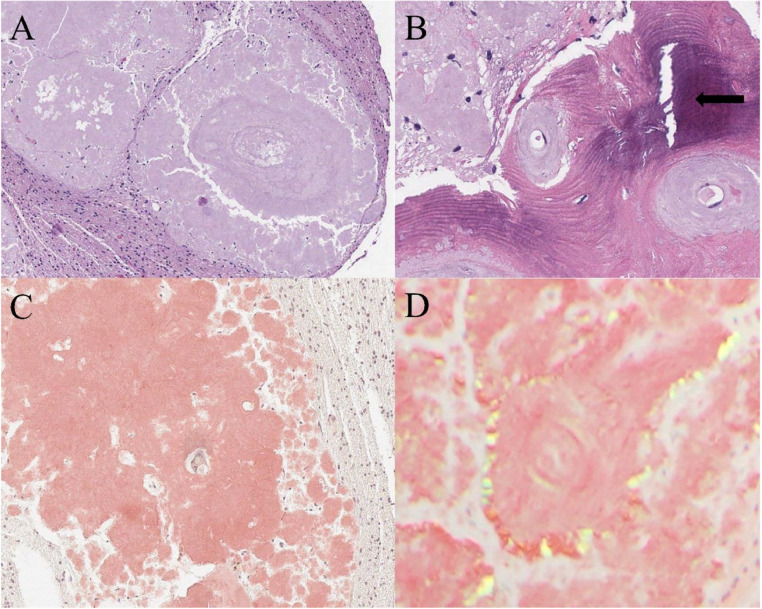
Neurosurgery performed a needle biopsy of the lesion. Final pathology demonstrated eosinophilic, amorphous, acellular deposits, positive for Congo red staining and apple-green birefringent under polarized light, with predominant lambda chain immunoreactivity consistent with amyloidoma (Fig. 3). This prompted subsequent evaluation for systemic amyloidosis. Laboratory tests including troponin, serum protein electrophoresis, serum immunoglobulin free light chains, and urine immunofixation were unremarkable. A bone marrow biopsy was normal without evidence of clonal plasma cells. Since then, the patient has followed up regularly with a local neurology office for continuing management of the amyloidoma. His seizures have been well controlled on levetiracetam and lacosamide.
Fig. 3.
Histology of cerebral amyloidoma. (A) Hematoxylin and Eosin (H&E) staining shows large deposits of amorphous eosinophilic material surrounded by glial tissue. No plasma cells or inflammatory cells were identified. (B) H&E staining showing focal area of bone metaplasia (arrow) associated with amyloid deposits around vessels and in surrounding brain parenchyma (C) Congo red staining highlights in orange-red the amorphous material. (D) Congo red staining under polarized light shows apple green birefringence.
Discussion
Amyloidomas are a rare form of amyloid deposition which can occur in various locations throughout the body and are distinct from more common presentations such as systemic amyloidosis in that they are organ restricted and present in a tumefactive manner [1,2]. Intracerebral amyloidomas represent an even rarer subset within this group of conditions. To the best of our knowledge, there have been only 67 case reports of cerebral amyloidoma since 1981 [3,4].
There have been multiple proposed hypotheses over the years regarding the etiology of amyloidomas. Glass et al [5] proposed that amyloidomas may derive from a plasmacytoma transformation. Cohen et al [6] proposed that genetically predisposed microglia were responsible for the production of amyloid precursor proteins. In recent years, there has been a growing body of evidence that suggests a low-grade lymphoid neoplasm underlies the etiology of these lesions [2]. A particular antigen triggers a local monoclonal B-cell response, which then produces antibodies that lead to amyloid deposition [2]. This hypothesis is further supported by the fact that in 31 of 65 recent patient case reports of cerebral amyloidomas, plasma cells or B cells were found proximal to amyloid deposits [3].
Due to their rarity, cerebral amyloidomas may be absent in the differential diagnostic consideration by radiologists on neuroimaging exams. A few imaging clues may help direct the radiologist in suggesting amyloidoma as a differential over more common brain tumors. Non-contrast CT typically shows hyperdense lesion(s) occasionally with calcification, such as in our patient [3]. Using MRI, amyloidomas may show variable signal on T1- and T2-weighted images: hypointensities on T2-weighted imaging may represent the dense amyloid protein deposits with the surrounding contrast enhancement due to disruption of the blood brain barrier [1,2,7]. In our case, nodules of T2 hypointensities and heterogeneous gadolinium contrast enhancement with lack of mass effect were present.
Amyloidomas generally follow a stable course with little risk of transformation to either systemic or malignant disease, and they carry a relatively benign prognosis [2,8,9]. From our literature review, there has only been one patient with a cerebral amyloidoma who was eventually found to have additional tumors [3]. After ruling out systemic amyloidosis, treatment of localized amyloidomas should be evaluated on an individualized basis. Medical therapy in the past has not shown to be effective [10]. The appropriateness of neurosurgical removal remains a debated topic, specifically regarding its ability (or inability) to affect progression-free survival [8]. When surgical management is not amenable, a “wait and see” strategy can be adopted given the risk of iatrogenic neurological complications [11]. Recently, local radiation therapy has shown effectiveness for amyloidomas located at various body sites [4]. However, whole-brain radiation therapy was complicated by long-term neurocognitive effects [4]. Regardless of the treatment modality pursued, there is no doubt a less aggressive treatment approach is indicated when compared to intracranial malignancies.
Cerebral amyloidomas constitute an important diagnostic consideration for brain masses despite their rarity, especially in cases where imaging findings are not consistent with more common pathologies. Ultimately, histopathological confirmation of the amyloidoma, exclusion of systemic disease, and individualized therapy are critical in arriving at a final diagnosis and treatment plan.
Conclusion
Cerebral amyloidomas are rare, relatively low-risk, focal solitary masses of amyloid in the brain. They are challenging to diagnose on imaging due to variable findings, but disease can be confirmed with biopsy. Systemic disease should be ruled out prior to individualized treatment.
Patient consent statement
Patient consent was obtained from the patient. The patient gave permission to use his clinical course, CT, MRI, and pathology slide images.
Footnotes
Competing Interests: The authors have no conflicts of interest, financial or otherwise, to disclose. This manuscript has not been published elsewhere and is not under simultaneous consideration by another journal. Research adhered to ethical guidelines.
References
- 1.Parmar H, Rath T, Castillo M, Gandhi D. Imaging of focal amyloid depositions in the head, neck, and spine: amyloidoma. AJNR Am J Neuroradiol. 2010;31(7):1165–1170. doi: 10.3174/ajnr.A1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laeng RH, Altermatt HJ, Scheithauer BW, Zimmermann DR. Amyloidomas of the nervous system: a monoclonal B-cell disorder with monotypic amyloid light chain lambda amyloid production. Cancer. 1998;82(2):362–374. doi: 10.1002/(sici)1097-0142(19980115)82:2<375::aid-cncr18>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Fujimoto M, Yokogawa R, Tomoaki T, Ohara J, Ogata H, et al. Cerebral amyloidoma accompanied by Sjögren’s syndrome: a case report and literature review. NMC Case Rep J. 2021;8(1):781–786. doi: 10.2176/nmccrj.cr.2021-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiao JC, Wolf AB, Rabinovitch RA, Smith C, Kleinschmidt-DeMasters BK, Ney DE. Long-term control of primary cerebral ALH amyloidoma with focal radiation therapy. Adv Radiat Oncol. 2021;7(2) doi: 10.1016/j.adro.2021.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass R, Scheie HG, Yanoff M. Conjunctival amyloidosis arising from a plasmacytoma. Ann Ophthalmol. 1971;3(8):823–825. [PubMed] [Google Scholar]
- 6.Cohen M, Lanska D, Roessmann U, Karaman B, Ganz E, Whitehouse P, et al. Amyloidoma of the CNS. I. Clinical and pathologic study. Neurology. 1992;42(10):2019–2023. doi: 10.1212/wnl.42.10.2019. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor A, Manje Gowda A, Kaur S, Estifan E, Maroules M. A rare case of cerebral amyloidoma mimicking a hemorrhagic malignant central nervous system neoplasm. Cureus. 2020;12(3):e7245. doi: 10.7759/cureus.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heß K, Purrucker J, Hegenbart U, Brokinkel B, Berndt Rouven, Keyvani Kathy, et al. Cerebral amyloidoma is characterized by B-cell clonality and a stable clinical course. Brain Pathol. 2018;28(2):234–239. doi: 10.1111/bpa.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood S, Bridoux F, Venner CP, Sachchithanantham S, Gilbertson J, Rowczenio D, et al. Natural history and outcomes in localised immunoglobulin light-chain amyloidosis: a long-term observational study. Lancet Haematol. 2015;2(6):e241–e250. doi: 10.1016/S2352-3026(15)00068-X. [DOI] [PubMed] [Google Scholar]
- 10.van Gameren II, Lokhorst H, Hazenberg BP, Vellenga E. Therapeutic options in systemic AL amyloidosis. Neth J Med. 2004;62(4):106–113. [PubMed] [Google Scholar]
- 11.Fischer B, Palkovic S, Rickert C, Weckesser M, Wassmann H. Cerebral AL lambda-amyloidoma: clinical and pathomorphological characteristics. Review of the literature and of a patient. Amyloid. 2007;14(1):11–19. doi: 10.1080/13506120600960585. [DOI] [PubMed] [Google Scholar]