Abstract
Background:
The effectiveness of hepatocellular carcinoma (HCC) surveillance is mitigated by underuse in clinical practice, highlighting a need for interventions. We evaluated the effectiveness of mailed HCC surveillance outreach to promote HCC surveillance in patients with cirrhosis
Methods:
We conducted a multi-center pragmatic randomized clinical trial comparing mailed outreach for surveillance ultrasound (n=1436) and usual care with visit-based surveillance (n=1436) among patients with cirrhosis at three health systems (tertiary care referral center, safety-net health system, and Veterans Affairs medical center) from April 2018 to December 2019. The primary outcome of this interim analysis was guideline concordant semi-annual HCC surveillance over a 12-month period and a secondary outcome was proportion time covered by surveillance. All patients were included in intention-to-screen analyses.
Results:
Compared to usual care, the outreach arm had significantly higher semi-annual surveillance (35.1% vs. 21.9%) and lower no-surveillance (29.8% vs. 43.5%) (p<0.001), resulting in significant increases in the proportion of time covered by surveillance (41.3% vs. 31.0%, p<0.001). The intervention increased HCC surveillance across most predefined subgroups; however, there were site-level differences in the intervention effect with significant increases in semi-annual surveillance at the Veterans Affairs and safety-net health systems but not the tertiary care referral center.
Conclusion:
Mailed outreach significantly increased semi-annual HCC surveillance versus usual care in patients with cirrhosis, with a consistent intervention effect across most examined subgroups. Continued follow-up is ongoing to determine if these increases in surveillance translate into improved downstream outcomes including early HCC detection and curative treatment receipt. NCT02582918 and NCT03756051
Keywords: screening, liver cancer, cirrhosis, intervention
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the few cancers with a rising mortality rate in the U.S., and it is projected to become the 3rd leading cause of cancer-related death if current trends continue.1 Over 90% of HCC occurs in the setting of chronic liver disease, and HCC is one of the leading causes of death in patients with compensated cirrhosis.2 However, prognosis varies widely by tumor stage, with 5-year survival exceeding 70% among patients with early-stage HCC compared to a median survival of 1–2 years in those detected at advanced stages.3
Professional societies, including the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL), recommend HCC surveillance using semiannual abdominal ultrasound with or without alpha fetoprotein (AFP) in at-risk patients, including those with cirrhosis from any etiology.4,5 In the absence of randomized clinical trial data in patients with cirrhosis, this practice is supported by several cohort studies demonstrating an association between HCC surveillance and improved survival, after adjusting for lead time and length time biases.6–8 However, the effectiveness of HCC surveillance in clinical practice has been mitigated by underuse.9–11
Although few studies have evaluated interventions to increase HCC surveillance, available data suggest promise for both inreach (e.g., provider-targeted electronic medical record (EMR) reminders at the time of a clinic visit) and outreach strategies (e.g., systematic patient-level invitations when overdue for surveillance).10,12 We previously demonstrated that mailed outreach significantly increased HCC surveillance among patients receiving care a safety-net health system.13,14 However, most interventions have been evaluated in single-center studies with limited small sample sizes, raising a question of generalizability to broader populations.10 Further, evaluation of most prior interventions focused on one-time screening, which may overestimate their effect compared to that on guideline-concordant semi-annual surveillance.
Herein, we report results of a pre-planned interim analysis examining semi-annual surveillance during the first year of a multi-center pragmatic randomized clinical trial of mailed outreach to promote HCC surveillance in a large racially, ethnically, and socioeconomically diverse population of patients with cirrhosis.
METHODS
Study Population
We conducted a pragmatic randomized clinical trial from March 2018 to September 2019 at three health systems: Parkland Health and Hospital System, UT Southwestern Medical Center, and Michael E. DeBakey Veterans Affairs (VA) Medical Center. Parkland is a publicly funded integrated safety-net health system in Dallas County comprised of a network of twelve primary care clinics, specialty hepatology clinics and radiology suites; UT Southwestern is an academic tertiary-care referral center with a robust liver transplant program; and the Michael E. DeBakey VA Medical Center is one of the largest VA systems in the U.S. and a designated VA liver transplant center.
Patients with cirrhosis were initially identified via the EMR using a validated set of ICD-9/ICD-10 codes for cirrhosis or cirrhosis complications (571.2, 571.5, 456.0, 456.1, 456.2, 456.21, 567.23, 572.2, 572.3, 572.4; K70.30, K74.6, K65.2, K72.9, K72.91, K76.6, K76.7, I85.0, I85.1) and/or FIB-4 >3.25.15 We also identified patients with suspected but not documented cirrhosis (i.e., elevated FIB-4 but no ICD-9/ICD-10 codes) given some patients fail to receive HCC surveillance due to unrecognized cirrhosis.16,17 The presence of cirrhosis was then confirmed via chart review by two authors (A.S. or R.H.), with cirrhosis diagnosis based on consistent histology, non-invasive markers of fibrosis demonstrating F4 fibrosis, or imaging showing a cirrhotic-appearing liver with signs of portal hypertension including splenomegaly, varices, or thrombocytopenia. Patients were required to have at least one outpatient clinic visit in the prior year to demonstrate that the health system was their medical home. We excluded patients in whom HCC surveillance is not recommended including those with Child Pugh Turcotte C cirrhosis, uncontrolled hepatic encephalopathy, personal history of HCC, and history of liver transplantation. Exclusion criteria were first applied using the EMR and then confirmed by chart review among potentially eligible patients. We also excluded patients without a phone number or address on file or language other than English or Spanish. The study was approved by the IRB of UT Southwestern Medical Center and Michael E. DeBakey VA Medical Center. We obtained a waiver of informed consent to avoid volunteer bias, in which patients interested in surveillance are selectively included. The trial protocol is registered on clinicaltrials.gov (NCT02582918 and NCT03756051). All authors had access to study data and approved the final manuscript.
HCC Surveillance Interventions
Eligible patients were randomly assigned to receive mailed outreach invitations for surveillance ultrasound and AFP or usual care with opportunistic, visit-based surveillance in a 1:1 ratio using a computer-generated randomization sequence. Randomization was stratified by health system and documented vs. suspected cirrhosis because intervention effect could differ between the subgroups.18 Research staff conducted all mailings and reminder telephone calls; thus, patients, primary care and subspecialty providers were all blinded to study arm assignment. We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits. The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.
The outreach intervention included a one-page letter with basic information regarding HCC risk and a recommendation to undergo HCC surveillance. Mailings, provided in English and Spanish, were written at a low-literacy level with assistance from health communication experts and underwent cognitive testing with English and Spanish speakers. With waiver of consent, all patients randomized to the outreach arm were mailed invitations. Patients who did not respond to mailed invitations within two weeks then received reminder calls to participate. Trained research staff conducted calls in English or Spanish, based on patients’ preferred language of communication, using standardized scripts. Feedback from patient advocates was incorporated into both mailings and call scripts. Attempts were stopped for patients with non-working phone numbers and those who could not be reached after three attempts. As part of the mailed outreach intervention, research staff also called patients 5–7 days prior to ultrasound appointments to remind them of the appointment. Outreach was discontinued at the time of liver cancer (Liver Reporting and Data System (LI-RADS) 5 or LI-RADS M) diagnosis, liver transplantation, or death.
Primary and Secondary Outcomes
Primary Outcome: HCC Surveillance Completion
Our primary outcome was adherence to semi-annual HCC surveillance during 12 months after randomization, for which we classified participants into three categories based on frequency and interval of imaging studies: semi-annual surveillance (≥1 imaging study during each 6-month period), annual surveillance (≥1 imaging study during the 12-month period but not meeting criteria for semi-annual surveillance), and no surveillance (no imaging during the one-year period). For patients randomized to mailed outreach, we included imaging completed through outreach and usual care. To ascertain surveillance for all patients, research staff members who did not deliver interventions and were blinded to intervention status queried the EMR for completed ultrasounds, contrast-enhanced CT, or contrast-enhanced MRI – including those exams done at outside institutions with results recorded in the EMR. Non-contrast CT or MRI exams were not included as possible surveillance exams given insufficient sensitivity for the exclusion of HCC. Although patients were invited to complete AFP testing at time of the ultrasound, it was not required for the outcome of surveillance participation given AASLD guidelines recommend ultrasound with or without AFP.4 As another measure of surveillance adherence, we also assessed proportion of time covered (PTC), which provides a more continuous measure of surveillance coverage.19 For this analysis, patients were assigned six months of covered time for normal abdominal imaging and three months for indeterminate or abnormal imaging results. Ultrasounds were classified as abnormal if there was a suspicious liver mass ≥1 cm and indeterminate if there was a liver mass <1 cm or visualization limitations precluded evaluation for liver masses. AFP results were considered positive if ≥20 ng/mL, the most common cut-off used for HCC surveillance in clinical practice, and indeterminate if ≥11 ng/mL, the upper limit of normal, but <20 ng/mL. For both measures of HCC adherence, patients were censored at time of liver cancer (LI-RADS 5 or LI-RADS M) diagnosis,20 death, liver transplantation, or end of follow-up.
Secondary Outcome: Incidental findings
Abnormal extrahepatic findings from surveillance or diagnostic imaging were abstracted and classified by organ system (e.g., pancreatic, renal) and type of finding. We also recorded subsequent evaluation for incidental findings, including endoscopy, repeat cross-sectional imaging, biopsy, or surgical evaluation. Incidental findings were then categorized as high, medium, or low clinical importance. Findings of high importance included those requiring time-sensitive medical or surgical evaluation, e.g., solid organ masses; medium importance findings included conditions that would require non-urgent evaluation, e.g., adrenal adenoma; and findings of low importance were those considered benign and unlikely to require further evaluation, e.g., cholelithiasis or diverticulosis.
Statistical Analysis
We summarized patient characteristics across the two arms and calculated 95% confidence intervals using the binomial Clopper-Pearson exact method. We used Pearson Chi-Square to compare surveillance adherence between the outreach and usual care arms. We performed moderation analyses to evaluate if the intervention’s effect to increase surveillance adherence varied across pre-defined subgroups including the type of health system, race/ethnicity, Child Pugh Turcotte class, and receipt of hepatology subspecialty care. With 1400 patients randomly assigned to each arm, we had 90% power to detect a difference of at least 6% in semi-annual surveillance between the groups, assuming baseline surveillance of 25% and pre-specified alpha of 0.05. We used the intent-to-screen principle to guide analyses. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
A total of 2872 patients were selected for randomization. Although 330 (23.0%) patients in the outreach arm could not be contacted or had non-working phone numbers for reminder calls, all patients were included in intent-to-screen analyses (Figure 1). Baseline characteristics of the two groups were similar (Table 1). Median age was 61.2 years and 67.7% were men. The cohort was racially and ethnically diverse with 37.0% non-Hispanic White, 31.9% Hispanic, and 27.6% Black patients. Underlying liver disease etiology was hepatitis C virus (HCV) in 56.4%, alcohol 18.1%, nonalcoholic steatohepatitis (NASH) 14.5%, and hepatitis B virus (HBV) in 2.4% of patients. Most patients had compensated cirrhosis, with 36.7% having ascites and 17.1% having hepatic encephalopathy. Median Child Pugh Turcotte score was 6, with 59.3% of patients having Child Pugh A cirrhosis. Most patients were otherwise healthy, with over 60% of patients having CirCom scores of 0–1. Most (>90%) patients had documented cirrhosis. All patients had at least one outpatient clinic visit in the year preceding randomization, including 56.3% having at least one primary care and 45.8% at least one gastroenterology/hepatology visit during that time. Fifty percent of patients had completed surveillance imaging within 6 months before randomization, with no significant difference between the two groups.
Figure 1:
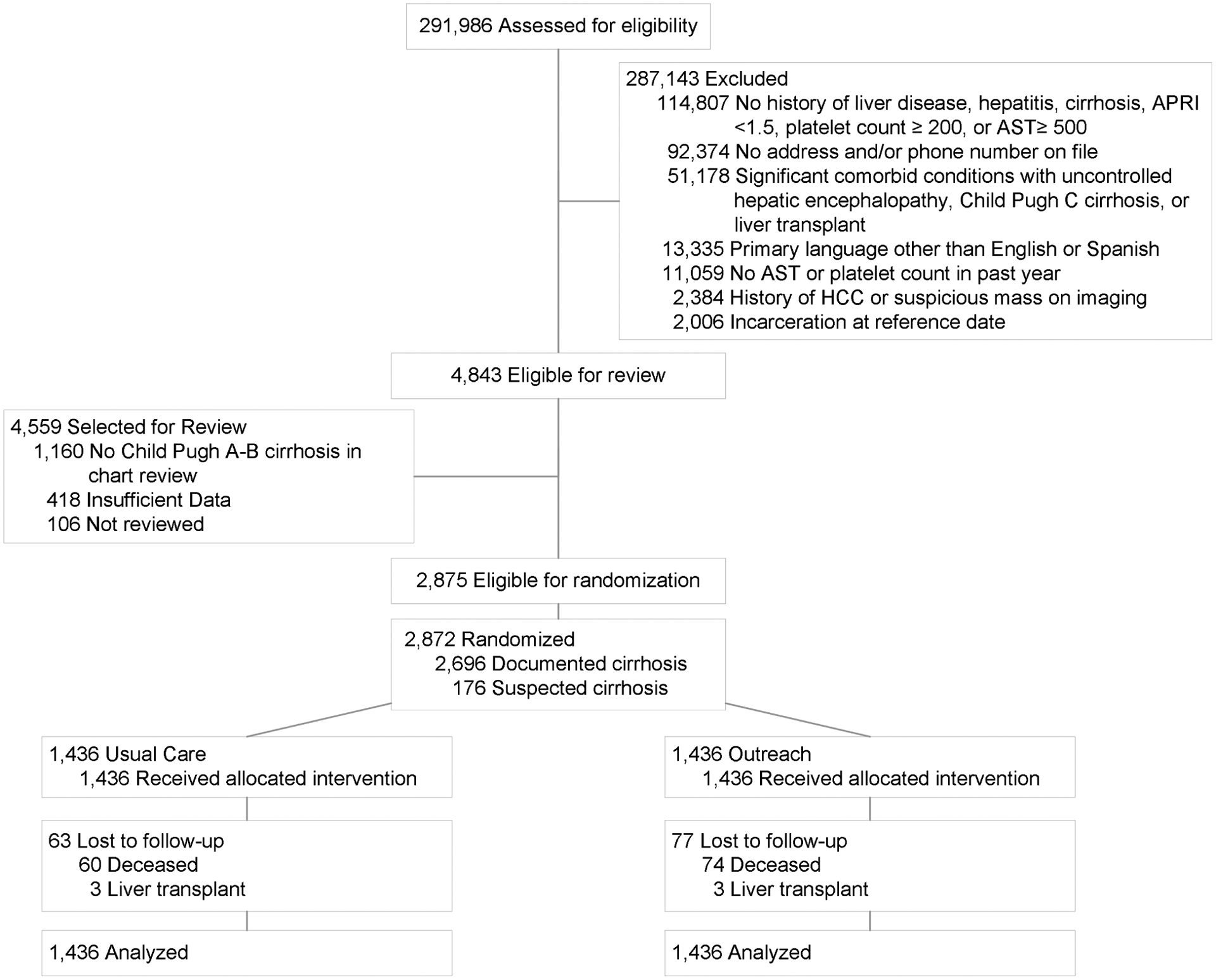
Study Consort Diagram
Table 1:
Patient characteristics at time of randomization
| Usual Care (n=1436) | Outreach (n=1436) | |
|---|---|---|
| Site | ||
| Parkland Health & Hospital System | 858 (59.8) | 857 (59.7) |
| Michael E Debakey VA | 365 (25.4) | 365 (25.4) |
| UT Southwestern Medical Center | 213 (14.8) | 214 (14.9) |
| Age (years) | 61.7 (55.2 – 67.5) | 61.0 (54.7 – 66.8) |
| Male sex (%) | 953 (66.4) | 991 (69.0) |
| Race/Ethnicity (%) | ||
| Non-Hispanic White | 530 (36.9) | 532 (37.0) |
| Hispanic White | 469 (32.6) | 449 (31.2) |
| Non-Hispanic Black | 383 (26.7) | 409 (28.5) |
| Other/Unknown | 54 (3.8) | 46 (3.2) |
| Language | ||
| English | 1169 (81.4) | 1199 (83.5) |
| Spanish | 265 (18.4) | 235 (16.4) |
| Etiology of Liver Disease (%) | ||
| Hepatitis C | 809 (56.3) | 811 (56.5) |
| Alcohol-related | 271 (18.9) | 248 (17.3) |
| Nonalcoholic fatty liver disease | 198 (13.8) | 218 (15.2) |
| Hepatitis B | 38 (2.6) | 32 (2.2) |
| Other | 120 (8.4) | 127 (8.8) |
| Presence of documented cirrhosis (%) | 1348 (93.9) | 1348 (93.9) |
| Presence of ascites (%) | 522 (36.3) | 532 (37.0) |
| Presence of hepatic encephalopathy (%) | 240 (16.7) | 251 (17.5) |
| Child Pugh score | 6 (5 – 7) | 6 (5 – 7) |
| Cirrhosis Comorbidity (CirCom) Index (%) | ||
| 0 | 494 (34.4) | 476 (33.1) |
| 1 | 391 (27.2) | 393 (27.4) |
| 2 | 250 (17.4) | 250 (17.4) |
| 3+ | 301 (21.0) | 317 (22.1) |
| Number of primary care visits* | 2 (0 – 3) | 2 (0 – 3) |
| Receipt of gastroenterology care* | 660 (46.0) | 655 (45.6) |
Continuous variables are expressed as median (P25 – P75) and categorical as n(%)
Year prior to randomization
HCC Surveillance Receipt
Semiannual surveillance was performed in 35.1% (95%CI 32.6 – 37.6%) of outreach patients, compared to 21.9% (95%CI 19.8 – 24.2%) of usual care patients, a difference of 13.2%; (95%CI 11.5 – 15.1%; p<0.001) (Table 2) (Figure 2). Similarly, the proportion of time covered was significantly higher in the mailed outreach group compared to usual care (41.3% vs. 31.0%, p<0.001).
Table 2:
Completion of HCC surveillance over 12-month study period
| Overall cohort | Safety-net health system | Tertiary care referral center | Veterans Affairs health system | |||||
|---|---|---|---|---|---|---|---|---|
| Usual care (n=1436) | Outreach (n=1436) | Usual care (n=858) | Outreach (n=857) | Usual care (n=213) | Outreach (n=214) | Usual care (n=365) | Outreach (n=365) | |
| No surveillance | 624 (43.5%) (95%CI 40.9 – 46.1) |
428 (29.8%) (95%CI 27.5 – 32.3) |
399 (46.5%) (95%CI 43.1 – 49.9) |
275 (32.1%) (95%CI 29.0 – 35.3) |
81 (38.0) (95%CI 31.5 – 44.9) |
69 (32.2) (95%CI 26.0 – 39.0) |
144 (39.5) (95%CI 34.4 – 44.7) |
84 (23.0) (95%CI 18.8 – 27.7) |
| Annual surveillance | 497 (34.6%) (95%CI 32.1 – 37.1) |
504 (35.1%) (95%CI 32.6 – 37.6) |
294 (34.3) (95%CI 31.1 – 37.6) |
299 (34.9%) (95%CI 31.7 – 38.2) |
81(38.0%) (95%CI 31.5 – 44.9) |
88 (41.1) (95%CI 34.5 – 48.0) |
122 (33.4) (95%CI 28.6 – 38.5) |
117 (32.1) (95%CI 27.3 – 37.1) |
| Semiannual surveillance | 315 (21.9%) (95%CI 19.8 – 24.2) |
504 (35.1%) (95%CI 32.6 – 37.6) |
165 (19.2) (95%CI 16.6 – 22.0) |
283 (33.0) (95%CI 29.9 – 36.3) |
51 (23.9) (95%CI 18.4 – 30.3) |
57 (26.6) (95%CI 20.8 – 33.1) |
99 (27.1) (95%CI 22.6 – 32.0) |
164 (44.9) (95%CI 39.8 – 50.2) |
| Proportion time covered | 31.0% ± 33.3% | 41.3% ± 33.4% | 28.1 ± 32.6% | 39.2 ± 33.2% | 34.5 ± 33.5% | 39.1 ± 32.8% | 35.8 ± 34.6% | 47.9 ± 33.6% |
Figure 2:
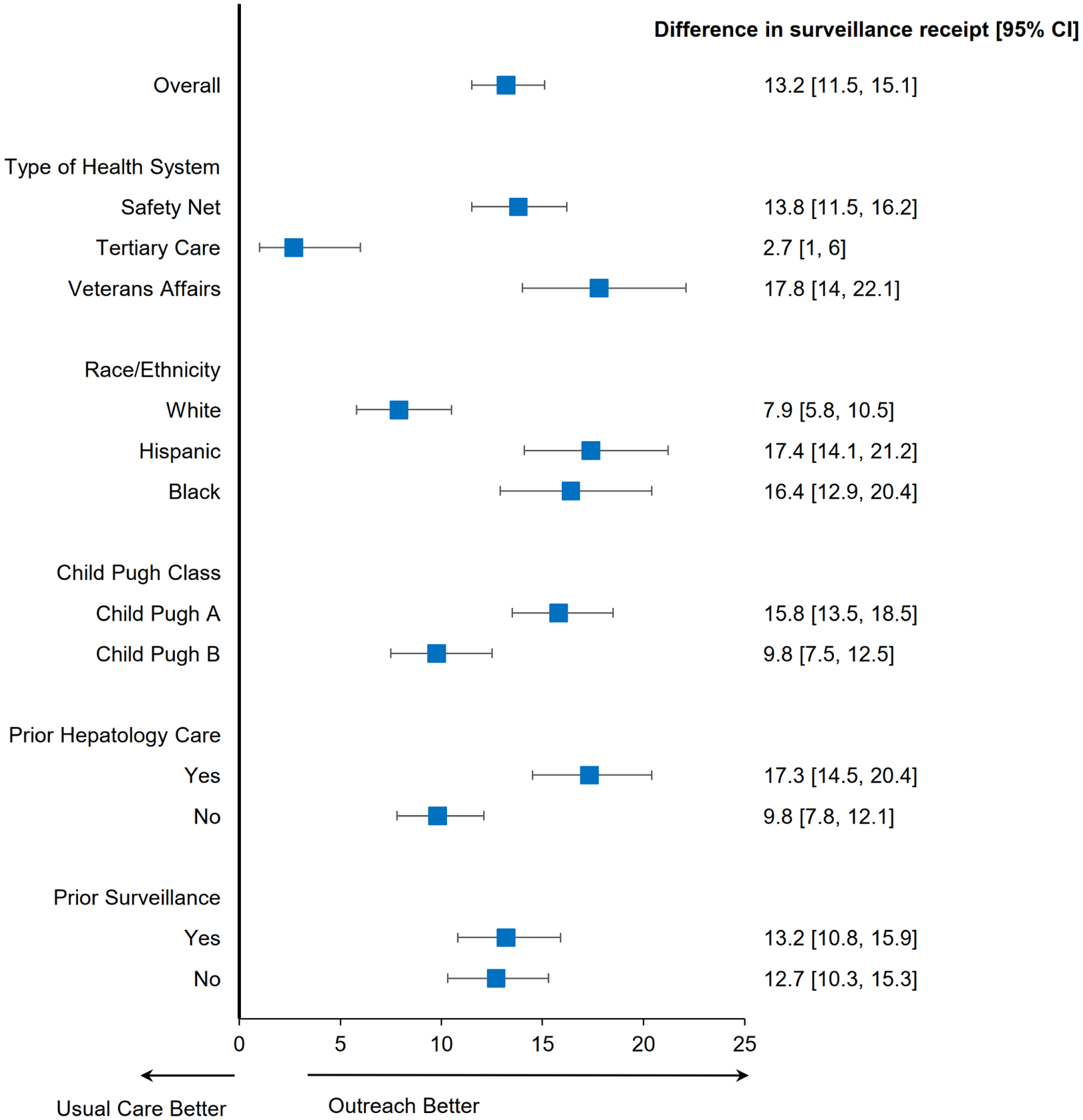
Subgroup Analyses of HCC Surveillance Completion, Mailed Outreach vs. Usual Care
Among most pre-defined subgroups including race and ethnicity, Child Pugh Turcotte class, and receipt of hepatology care, mailed outreach increased semi-annual surveillance compared to usual care (Figure 2). However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the Veterans Affairs and safety-net health systems (both p<0.001) but not the tertiary care referral center (p=0.52). In a post-hoc subgroup analysis among patients with at least one primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semi-annual surveillance compared to usual care (46.8% vs. 32.7%, p<0.001).
Incidental Findings
There were 140 patients with a total of 224 incidental findings on follow-up imaging – 88 patients with only one incidental finding, 33 with two findings, and 19 patients with three or more findings (Supplemental Table). Three-fourths (n=109) of patients had incidental findings of low clinical importance, with only 21 (15%) and 10 (7%) patients having incidental findings of medium and high clinical importance, respectively. The most common incidental findings included gallstones, renal cysts, and colonic diverticulosis. Incidental findings prompted further imaging for nine patients, and two patients required invasive evaluation – one patient with an incidental renal cell carcinoma requiring nephrectomy and one with pancreatic cancer requiring ERCP.
DISCUSSION
In this multi-center pragmatic randomized clinical trial, we found a mailed outreach intervention for HCC surveillance significantly increased adherence to semi-annual HCC surveillance compared to usual care. The effect of the intervention was consistent across most pre-defined subgroups but differed across sites, with greater improvements in surveillance observed at the safety-net health system and Veterans Affairs hospital compared to the tertiary care referral center. Despite the intervention, most patients failed to complete semi-annual surveillance across all three sites and nearly 30% did not complete any surveillance over the one-year period.
We found underuse of HCC surveillance in patients randomized to usual care arm across all three sites with over one-third to nearly one-half of patients not receiving any surveillance during the study period, reinforcing that surveillance underuse continues to be a challenge in routine clinical practice.10 Prior studies evaluating interventions including nurse-based surveillance protocols, provider education, and electronic medical record reminders have demonstrated 25 – 60% relative increases in surveillance use.12,21,22 However, each of these interventions only benefits patients who are actively seen in clinic and may miss those who are less engaged in regular outpatient care. In our study, mailed outreach increased semiannual surveillance from 21.9 to 35.1% – a 60% relative increase. Despite this significant increase, it is noteworthy that only 35% of patients in the mailed outreach arm completed semi-annual surveillance and 30% continued to not have any surveillance. These data highlight the need for more intensive interventions to further increase surveillance. The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.23,24 Specifically, patient-reported transportation and financial barriers have been associated with failure to complete surveillance and may be improved with the addition of patient navigation.25 Further, validation of novel blood-based biomarkers with high accuracy for early HCC detection may better align with patient preferences and simplify surveillance logistics, facilitating surveillance to be done at the same time as clinic visits instead of requiring a separate ultrasound appointment.26,27
Although the intervention increased semi-annual surveillance overall, the intervention effect differed by site, with significant increases in surveillance completion at the safety-net and Veterans Affairs health systems but not the tertiary care referral center. Although we stratified randomization by site to account for this possibility, it’s unclear why the intervention failed to have a similar benefit among patients receiving care in a tertiary care referral center. The tertiary care site had a higher proportion of females, non-Hispanic Whites, patients with non-viral cirrhosis, patients with hepatic decompensation, and patients with Hepatology care. We did not observe any differences in intervention fidelity, such as returned outreach letters and patients with whom we were not able to contact for reminder calls. It is possible that patients at the tertiary care referral center are more strictly aligned to their provider recommendation and may be less open to population health management efforts. Identifying predictors of intervention effect can be helpful to best identify subgroups who may benefit from more intensive interventions. Further studies should continue to explore factors that may moderate intervention effect to best tailor intervention intensity and maximize the cost-effectiveness of population health management strategies.28,29 Continued follow-up of trial participants will also allow us to examine if these site differences persist when examined screening process completion over a three-year period.
During continued follow-up over a three-year period, our trial will evaluate downstream outcomes, including surveillance benefits (i.e., early HCC detection and curative treatment receipt), physical harms (diagnostic testing due to false positive or indeterminate surveillance results), financial harms, and psychological harms. These data are particularly important in light of evolving data about breast and prostate cancer screening benefits and harms, which created controversy about published screening guidelines and eroded trust between society groups, providers, and patients for clinical practice guidelines.30,31 We did not observe an increase in surveillance benefits and harms in the first year of the trial (data not shown); however, the lack of difference in surveillance benefits likely relates to the short duration of follow-up at the time of this interim analysis and the limited number of incident HCC.
Several limitations must be noted when considering study results. Although we enrolled patients from three types of health systems, our results may not generalize to other patients, such as those in community practices or those followed outside the United States. Second, we performed intent-to-screen analyses so some underuse of surveillance in both the usual care and mailed outreach groups may relate to patients being lost to follow-up or having contraindications to surveillance (e.g., development of significant medical comorbidity). Third, although we attempted to identify outside imaging via scanned records and Care Everywhere (i.e., EMR platform for record-sharing from other health systems), it is still possible that some patients may have received imaging at outside institutions that was not documented in the EMR. This may be particularly true for patients followed at the tertiary care referral center who often receive medical care from multiple healthcare systems; however, one would not expect ascertainment of outside of imaging to differ between the two groups. Finally, this pre-planned interim analysis evaluated outcomes over a one-year period, and longer follow-up is likely needed to determine cost-effectiveness of the intervention and observe differences in downstream outcomes such as early HCC detection. We feel these weaknesses are outweighed by the study’s strengths, including the enrollment of a large, racially diverse patient population across three types of health systems and its pragmatic design avoiding volunteer bias.
In summary, we demonstrated that mailed outreach significantly increased adherence to semi-annual HCC surveillance over a one-year period in a large multi-center cohort of patients with cirrhosis. Study follow-up is ongoing to determine if the intervention improves screening process completion over a three-year period and can improve downstream outcomes including early HCC detection and curative treatment receipt.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Hepatocellular carcinoma (HCC) surveillance effectiveness in at-risk patients is limited by underuse in practice, highlighting a need for interventions.
Findings:
Compared to usual care, mailed outreach significantly increased semi-annual surveillance (35.1% vs. 21.9%) and proportion of time covered by surveillance (41.3% vs. 31.0%, p<0.001). The intervention increased surveillance across most predefined subgroups.
Implications:
In this multi-site study, mailed outreach was an effective population health strategy to increase HCC surveillance among patients with cirrhosis. Continued follow-up is needed to determine if mailed outreach improves downstream outcomes including early HCC detection.
Financial Support:
This work was conducted with support from National Cancer Institute R01 CA212008, U01 CA230694, and Cancer Prevention Research Institute of Texas (CPRIT) RP150587. The work is also supported in part by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, TX. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, Cancer Prevention Research Institute of Texas, or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Amit Singal has served as a consultant or on advisory boards for Bayer, FujiFilm Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL. None of the other authors have any relevant conflicts of interest to disclose
Clinical Trials number: NCT02582918 and NCT03756051
REFERENCES
- 1.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Network Open 2021; 4(4): e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Kim R, Coelho R, Mettler T, Benson JT, Sanderson S, et al. Cirrhosis Is Present in Most Patients with Hepatitis B and Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 2011; 9(1): 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet J, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nature Reviews Disease Primers 2021 [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik L, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018; 68(2): 723–750. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018; 69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 6.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014; 11(4): e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance with Hepatocellular Carcinoma Surveillance Guidelines Associated with Increased Lead-Time Adjusted Survival of Patients with Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018; 155(2): 431–442.e10. [DOI] [PubMed] [Google Scholar]
- 8.Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, et al. Hepatocellular Carcinoma Screening Is Associated with Increased Survival of Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2019; 17(5):976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra M, et al. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. American Journal of Medicine 2015; 128(1): 90.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021; 73(2):713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clinical Gastro and Hepatology 2021. 10.1016/j.cgh.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172–9. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Sanders J, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients with Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017; 152(3): 608–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal AG, Tiro JA, Murphy CC, Marrero JA, McCallister K, Fullington H, et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients with Cirrhosis: A Randomized Clinical Trial. Hepatology 2019; 69(1): 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehra M, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of Administrative Claims Data for Identifying Patients with Cirrhosis. J. Clinical Gastroenterology 2013; 47(5): e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Yopp A, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prevention Research 2012; 5(9): 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marqardt P, Liu P-H, Immergluck J, Olivares J, Arroyo A, Rich NE, et al. Hepatocellular carcinoma screening process failures in patients with cirrhosis. Hepatology Communications 2021. 10.1002/hep4.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero J, Yopp A, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival among Patients with Hepatocellular Carcinoma in the United States. Am J Medicine 2017; 130; 9: 1099–1106.e1 [DOI] [PubMed] [Google Scholar]
- 19.Murphy CC, Halm E, Skinner CS, Balasubramanian B, Singal AG. Challenges and approaches to repeat fecal immunochemical test for colorectal cancer screening. Cancer Epi Biomarker Prev 2020; 29(8): 1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh J, Bashir MR, et al. Accuracy of the Liver Imaging Reporting and Data System on Computed Tomography and Magnetic Resonance Imaging – A Systematic Review. Gastroenterology 2019; 156(4): 976–986. [DOI] [PubMed] [Google Scholar]
- 21.Del Poggio P, Olmi S, Ciccarese F, et al. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2015;27:1103–8. [DOI] [PubMed] [Google Scholar]
- 22.Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci 2013;58:1157–60. [DOI] [PubMed] [Google Scholar]
- 23.Farvardin S, Patel J, Khambaty M, Yerokun O, Mok H, Tiro JA, et al. Patient-Reported Barriers are Associated with Lower HCC Surveillance Rates in Patients with Cirrhosis. Hepatology 2017; 65(3): 875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons OL, Feng Y, Parikh ND, Singal AG. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clinical Gastroenterology Hepatology 2019; 17(4): 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singal AG, Tiro JA, Murphy CC, Blackwell J, Kramer KR, Khan A, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multi-center cohort of patients with cirrhosis. Clinical Gastroenterology Hepatology 2021; 19(5): 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolen SA, Singal AG, Davenport MS, Troost JP, Khalatbari S, Mittal S, et al. Patient Preferences for Hepatocellular Carcinoma Surveillance Parameters: A MultiCenter Conjoint Study. Clinical Gastroenterology Hepatology 2021. doi: 10.1016/j.cgh.2021.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, Hoshida Y, Pinato DJ, Marrero JA, Nault J-C, Paradis V, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021; 160(7): 2572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal AG, Chen Y, Sridhar S, Mittal V, Fullington H, Shaik M, et al. Novel Application of Predictive Modeling: A Tailored Approach to Promoting HCC Surveillance in Patients with Cirrhosis. Clinical Gastroenterology Hepatology 2021. doi: 10.1016/j.cgh.2021.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Lee JY, Sridhar S, Mittal V, McCallister K, Singal AG. Improving cancer outreach effectiveness through targeting and economic assessments: insights from a randomized field experiment. Journal of Marketing 2020; 84(3): 1–27. [Google Scholar]
- 30.Haas JS, Sprague BL, Klabunde CN, Tosteson AN, Chen JS, Bitton A, et al. Provider Attitudes and Screening Practices Following Changes in Breast and Cervical Cancer Screening Guidelines. J Gen Intern Med. 2016; 31(1): 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JD, Bluethmann SM, Sheets M et al. Women’s responses to changes in U.S. preventive task force’s mammography screening guidelines: results of focus groups with ethnically diverse women. BMC Public Health 2013; 13: 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


