Early hepatocellular cancer (HCC) detection is associated with curative treatment and improved survival.1 The American Association for the Study of Liver Diseases recommends semiannual ultrasound and α-fetoprotein (AFP) in patients with cirrhosis, and those with abnormal results should undergo diagnostic multiphase computed tomography (CT) or magnetic resonance imaging (MRI).2 The Liver Imaging Reporting and Data System (LI-RADS) was devised to standardize reporting of liver observations in at-risk individuals, ranging from LR1 (“definitely benign”) to LR-5 (“definitely HCC”), with indeterminate observations classified as LR-3 (“intermediate probability of malignancy”).3 A study among 999 cirrhosis patients found that indeterminate liver observations are common, being reported on diagnostic CT or MRI in 98 (38.3%) of 256 patients with abnormal ultrasound results.4 Prior studies have reported a wide range in HCC risk, from 4% to 31%, for LR-3 observations, so there is insufficient evidence to recommend a standardized strategy for monitoring LR-3 observations.5,6
To address this need, we conducted a retrospective cohort study from 3 health systems in the North American Liver Cancer Consortium: UT Southwestern Medical Center, Parkland Health and Hospital System, and the University of Michigan.7 We included patients who underwent multiphasic CT or MRI with ≥1 LR-3 observation between March 2015 and September 2018. Individuals with LR-4, LR-5, or LR-M observations on index imaging, prior history of HCC or cholangiocarcinoma, or liver transplantation were excluded (see Supplementary Methods).
Patients were followed per institutional routine from index imaging until progression to liver cancer, death, liver transplantation, or end of follow-up, whichever occurred earliest. On follow-up imaging, we recorded change in number, size, and imaging features of the LR-3 observations as well as development of any LR-4, LR-5, or LR-M observations. We performed Kaplan-Meier and Cox regression analyses to characterize time to HCC development and identify factors associated with progression, respectively. Variables with P < .10 in univariable analyses were retained in multivariable models, using a backward selection process with a significance threshold of P < .05. We also retained age, sex, maximum LR-3 diameter, and index imaging modality (CT vs MRI) in multivariable models, given a priori clinical importance. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Patient characteristics (n = 458) are in Supplementary Table 1. The median age was 59.0 years and 271 (59.2%) were men. The cohort was diverse regarding race, ethnicity, and liver disease etiology. On index imaging, 257 (61.8%) patients had a single LR-3 observation, 86 (20.7%) had 2 observations, and 73 (17.5%) had ≥3 observations. Median maximum diameter of the largest LR-3 observation was 1.0 (inter-quartile range, 0.8–1.5) cm, with 244 (59.7%) patients having an LR-3 observation ≥1.0 cm.
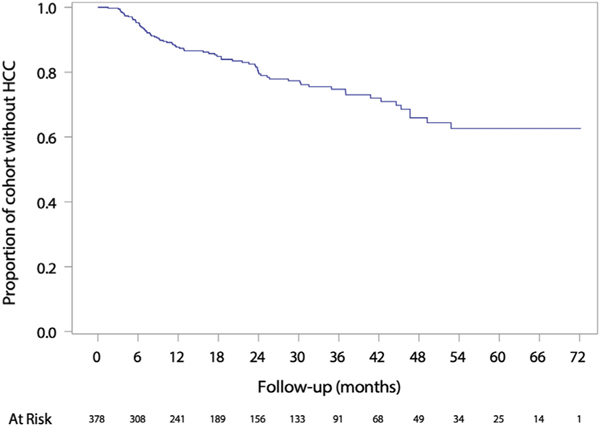
Over a median of 17.9 mxonths, at least 1 follow-up CT or MRI exam was performed in 311 patients: 133 (42.8%) had 1, 82 (26.4%) had 2, and 96 (30.9%) had ≥3 CT or MRI exams. The median interval to first followup CT or MRI was 5 (interquartile range, 3–9) months. An additional 29 patients were followed by semiannual ultrasound without CT or MRI, and 118 patients had no follow-up imaging. Among those with ≥1 follow-up CT or MRI, 75 (24.1%) progressed to HCC, yielding an incidence of 84 cases per 1000 person-years (Figure 1). Sixteen (5.1%), 39 (12.5%), and 46 (14.8%) patients developed HCC within 6, 12, and 18 months of index imaging, respectively. Of those who developed HCC, 51 (68.0%) occurred in the LR-3 observation and 24 (32.0%) occurred elsewhere in the liver. In multivariable analysis, age >60 years (hazard ratio [HR], 1.82; 95% confidence interval [CI], 1.06–3.13), male sex (HR, 1.83; 95% CI, 1.02–3.30), AFP >10 ng/mL (HR, 3.11; 95% CI, 1.80–5.36), and LR-3 diameter ≥1.0 cm (HR, 1.86; 95% CI, 1.05–3.31) were associated with HCC development (Supplementary Table 2).
Figure 1.
Time to HCC diagnosis among patients with ≥1 LR-3 observation.
A systematic review found HCC risk in LR-3 observations was 38% (95% CI, 31%–45%) but only included 391 LR-3 observations across studies.8 Follow-up differed across studies, creating heterogeneity and complicating interpretation of the pooled estimate. In our multicenter cohort study, cirrhosis patients with LR3 observations had an 8.4% annual incidence of HCC. Our data suggest a sufficiently high risk to warrant monitoring with CT or MRI, particularly in older patients, those with elevated AFP, and those with LR-3 observations ≥1.0 cm.
Notably, 32% of HCC occurred outside the LR-3 observation, which is similar to a study of 154 patients with dysplastic liver nodules, in which 49 developed HCC elsewhere.9 These findings may reflect a “field effect” in cirrhosis, whereby the inflammatory and carcinogenic microenvironment predisposes the entire liver to HCC development. Thus, LR-3 observations may be a manifestation of the carcinogenic milieu and a marker for HCC risk, rather than the observation from which HCC develops. These data have implications when considering chemoprevention strategies and need to treat more than the LR-3 observation.
Maximum LR-3 observation diameter and baseline AFP level were associated with progression to HCC. However, over 30% of patients who developed HCC had an LR-3 diameter <1.0 cm and over 66% had AFP level ≤10 ng/mL, suggesting that neither can be used alone to guide management. Other emerging strategies, such as high-throughput radiomics and blood-based risk stratification biomarkers, warrant evaluation in patients with LR-3 observations.10
We acknowledge study limitations. First, variable follow-up among patients may have resulted in ascertainment bias for HCC diagnoses. Second, reliance on interpreting radiologists is liable to misclassification errors, particularly given imperfect interrater reliability for LI-RADS interpretations. Third, assignment of LI-RADS may depend on technical factors, such as contrast timing and motion, so an upgrade in LI-RADS category could be due to technical factors, as opposed to true disease progression. Finally, LR-3 observations were identified and monitored using 2 imaging modalities (ie, CT and MRI) with different sensitivities and specificities for detecting LI-RADS features. We believe that these limitations are balanced by the study’s strengths including our large, contemporary multicenter cohort with detailed clinical and imaging data.
In summary, patients with cirrhosis and LR-3 observations have a high annual risk for progression to HCC, particularly older patients and those with an observation ≥1 cm on index imaging. While awaiting accurate risk-stratification tools, our results suggest these patients likely warrant close observation using CT or MRI imaging.
Supplementary Material
Funding
This study was conducted with support from National Institutes of Health Grant Nos. U01 CA230694 and R01 CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
North American Liver Cancer (NALC) Consortium
Sruthi Yekkaluri1, Takeshi Yokoo4, David Fetzer4, Travis Browning4, Nicole E. Rich1,3, Gaurav Khatri4, Hao Zhu1, Yujin Hoshida1, Jorge A. Marrero5, Adam C. Yopp6, Purva Gopal7, Carrie Manwaring1
1Department of Internal Medicine, 4Department of Radiology, 6Department of Surgery, and 7Department of Pathology, UT Southwestern Medical Center, Dallas, TX 2Department of Internal Medicine, University of Michigan, Ann Arbor, MI 3Department of Internal Medicine, Parkland Health and Hospital System, Dallas, TX 5Department of Medicine, University of Pennsylvania, Philadelphia, PA
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.11.042.
Conflicts of interest
These authors disclose the following: Neehar D. Parikh has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Glycotest, and Freenome. Amit Singal has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL. The remaining authors disclose no conflicts.
Contributor Information
ASHWINI ARVIND, Department of Internal Medicine UT Southwestern Medical Center Dallas, Texas.
SAGAR JOSHI, Department of Internal Medicine University of Michigan Ann Arbor, Michigan.
TIMOTHY ZAKI, Department of Internal Medicine UT Southwestern Medical Center Dallas, Texas.
DANIEL BURKHOLDER, Department of Internal Medicine University of Michigan Ann Arbor, Michigan.
NEEHAR D. PARIKH, Department of Internal Medicine University of Michigan Ann Arbor, Michigan.
AMIT G. SINGAL, Department of Internal Medicine UT Southwestern Medical Center Dallas, Texas; Department of Internal Medicine Parkland Health and Hospital System Dallas, Texas.
References
- 1.Singal AG, et al. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero JA, et al. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 3.Tang A, et al. Clin Gastroenterol Hepatol 2019;17:1228–1238. [DOI] [PubMed] [Google Scholar]
- 4.Konerman MA, et al. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnello F, et al. Clin Imaging 2020;68:169–174. [DOI] [PubMed] [Google Scholar]
- 6.Hong CW, et al. Eur Radiol 2019;29:5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolen SA, et al. Clin Gastroenterol Hepatol 2022; 20(1):204–215.e6. 10.1016/j.cgh.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Pol CB, et al. Gastroenterology 2019;156:976–986. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, et al. Cancer 2006;106:636–647. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara N, et al. Med (N Y) 2021;2:836–850.34318286 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.