Abstract
Objective:
Depression is a prevalent (24–30%) and significant comorbidity in patients with systemic lupus erythematosus (SLE). In the present study, we leveraged the longitudinal SLE cohort at the Washington University Lupus Clinic to address: 1) what is the longitudinal course of depressed affect among outpatients with SLE? 2)what is the longitudinal relationship between SLE disease activity and depressed affect?
Methods:
Longitudinal data from patients with ACR or Systemic Lupus International Collaborating Clinics (SLICC)-classified SLE were analyzed. Depressed symptoms were assessed at each visit using the Center for Epidemiologic Studies Depression Scale, Revised (CESD-R) while SLE disease activity was measured via the SLEDAI2K Responder Index-50 (S2K RI-50). Group-based trajectory modeling (GBTM) and linear mixed models were used for analysis.
Results:
The sample (n=144) was 56.3% Black, and 38.9% White. GBTM revealed five distinct groups of patients who demonstrated consistent trends in depression overtime. Members of groups 4 (n=44, 30.6%) and 5 (n=44, 30.6%) demonstrated CESD-R scores consistent with depression. Of note, Black patients were much more common in Group 5 (n=32, 72.7%, p<0.02). Analyses identified an association between SLEDAI disease activity and depression scores in multivariate analysis but did not show significance in GBTM and univariate analysis.
Conclusions:
The majority (61.2%) of patients had CESD-R scores consistent with persistent depressed affect or major depression over a period of up to four years. The lack of a consistent relationship of CESD-R with SLE disease activity highlights the need to regularly monitor, treat and better understand the causes behind this comorbidity.
In 2018, an estimated 204,295 people in the United States met American College of Rheumatology (ACR) criteria for Systemic Lupus Erythematosus (SLE) [1]. Among patients with SLE, depression is a prevalent and significant comorbidity occurring in 24–30% of patients [2]. The impact of depression on patients with SLE is substantial: health-related quality of life (HRQoL) is negatively affected [2, 3], treatment nonadherence and emergency room visits are more common [2, 4], and increased prevalence of cardiovascular disease, cognitive impairment and suicidal ideation have been reported [5–8].
In 1999, the ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature classified mood disorders, which includes depression, as neuropsychiatric manifestations of SLE (NPSLE) [9]. Supporting this association, ribosomal P antibodies and the presence of antiphospholipid antibodies have been reported to correlate with severity of depression [10, 11]. A cross-sectional study noted that SLE patients with depression diagnosed using structured interviews had an average SLEDAI score of 7.38 compared to 3.44 in patients without depression [12]. Cardiovascular disease is a well-established comorbidity in SLE, which has been associated with depression [5].
By contrast, other observations have shown a lack of association of depression with SLE disease activity, suggesting that depression is experienced independently from the pathophysiology of this process [9, 13]. While as many as 23% of patients newly diagnosed with SLE experience a new onset of mood disorder (depression, depressive symptoms, manic or mixed features) at the time of diagnosis, only 4–12% of these events are linked to SLE disease activity [13]. These observations were confirmed in the Johns Hopkins SLE cohort, where no correlation between depression diagnosis and SLE disease activity was found [14]. Furthermore, depression assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) instrument did not correlate with physician-reported SLE disease activity measured using the Systemic Lupus Activity Measure (SLAM), European Consensus Lupus Activity Measure (ECLAM), SLEDAI, or physician global assessment (PGA) [15]. Conversely, using patient-reported SLE disease activity instruments such as the Systemic Lupus Activity Questionnaire (SLAQ), depression was associated with worse SLAQ scores while physician reported disease activity was not [16]. While the SLAQ has been noted by some to lead to over-reporting of disease activity as compared to the SLEDAI [17], the discordance between provider- and patient-reported assessment of disease activity suggest that certain symptoms such as depression may be distinct entities. One hypothesis proposed by investigators at Duke University defines Type I symptoms as those measured with provider-reported disease activity instruments while Type II symptoms are assessed with patient-reported disease activity instruments [17, 18]. This adds to the complexity of evaluating the etiologies of depression in SLE [18]. Whether or not this proposed idea of Type I and Type II symptoms fully captures the complexities of SLE symptoms, it is necessary to identify all possible causes of depression in SLE to optimize treatment practices and thereby address the complete needs of these patients. Always attributing depression to SLE disease activity may result in misguided treatment.
To examine the relationship between depression and disease activity, longitudinal data is needed to capture intermittent disease flares and variations in symptoms of depression. Depression has been noted to persist overtime in the general population [19], but this same longitudinal analysis is needed in patients with SLE to better understand the impact of this dynamic disease on mental health. There is a paucity of longitudinal studies examining depressed affect overtime in SLE patients. In the present analyses, we described the longitudinal course of depressed affect among patients with SLE using the Center for Epidemiologic Studies Depression Scale Revised (CESD-R). We also explored the relationship between depressed affect and physician-reported SLE disease overtime by using the SLEDAI-2K Responder Index-50 (S2K RI-50) after adjusting for confounders that may contribute to depression.
MATERIALS AND METHODS
Participants
Individuals eligible for participation in this study were the 256 patients with ACR or SLICC-classified SLE treated in the Washington University Lupus Clinic between February 2015 and January 2020. Several clinical measures were routinely collected at each clinic visit. Patients with chronic illnesses (e.g., hepatitis A, B, or C, HIV, cirrhosis, end-stage renal disease, or pregnancy) were excluded from the analysis due to these conditions being known independent risk factors for depression [20–24]. Individuals who completed a depression symptomology instrument during at least 3 visits over a maximum of 48 months were included in the final analytic sample (n=144). Characteristics of individuals included in the final sample were not meaningfully different from those not included.
Measures
SLE Disease Activity
SLE disease activity was assessed using the SLEDAI-2000 (S2K) instrument at the patients’ first clinic visit and the SLEDAI-2k Responder Index-50 (S2K RI-50) at follow-up visits [25, 26]. The S2K and S2K RI-50 are validated measures that allow providers to assess overall SLE disease activity and severity [25]. All providers passed standardized S2K RI-50 training. S2K and S2K-RI50 scores above 4 reflect active SLE [27]. Several blood tests that are reflective of SLE disease activity were also collected at baseline, including erythrocyte sedimentation rate (ESR), anti-double-stranded DNA antibody titers (dsDNA), and C3 and C4 complement component levels [28].
Depression Symptomology
Depression symptomology was assessed at each visit using the CESD-R. The CESD-R is a 20-item questionnaire with high internal consistency (Cronbach’s ⍺ = 0.923) [9] for depression screening, with scores ranging from 0–60. CESD-R scores of < 16, 16–21, and > 21 were used to define no depression, depressed affect, and major depression, respectively.
Baseline Characteristics
Baseline characteristics obtained at the patients’ first clinic visit included age, biological sex, race, educational attainment, marital status, employment status, and obesity (see Table 1). The PROMIS anxiety Short Form 8a was collected as a measure of anxiety symptomology, since depression and anxiety are highly correlated [29]. The patients’ comorbid health conditions and medication regimen were also recorded. When information was incomplete, the electronic health record immediately preceding and following the baseline visit was reviewed and relevant data was extracted.
Table 1.
Demographic and clinical characteristics at study entry of participants, overall and by CESD-R trajectory group
| Variable | Total (n = 144) | Group 1 (n=17) | Group 2 (n=27) | Group 3 (n=12) | Group 4 (n=44) | Group 5 (n=44) | p-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographic Characteristics | |||||||
| Age (SD) | 40.4(12.6) | 38.9(15.4) | 38.7(14.7) | 37.1(12.0) | 38.5(11.2) | 44.2(11.4) | 0.168 |
| Sex (% female) | 131(91.0) | 14(82.4) | 24(88.9) | 11(91.7) | 42(95.5) | 40(90.9) | 0.516 |
| Race | 0.020* | ||||||
| White | 56(38.9) | 8(47.1) | 9(33.3) | 6(50.0) | 22(50.0) | 11(25.0) | |
| Black | 81(56.2) | 6(35.3) | 18(66.7) | 5(41.7) | 20(45.5) | 32(72.7) | |
| Other | 7(4.9) | 3(17.7) | 0 | 1(8.3) | 2(4.6) | 1(2.3) | |
| Highest Education | 0.053 | ||||||
| Attainment | |||||||
| Unknown | 42(29.2) | 4(23.5) | 5(18.5) | 3(25.0) | 13(29.6) | 17(18.6) | |
| Less than 12th grade | 3(2.1) | 0(0.0) | 0(0.0) | 0(0.0) | 1(2.3) | 2(4.6) | |
| GED/High School/Some | 69(47.9) | 9(52.9) | 14(51.9) | 7(58.3) | 17(38.6) | 22(50.0) | |
| College/Over college | 30(20.8) | 4(23.5) | 8(29.6) | 2(16.7) | 13(29.6) | 3(6.8) | |
| Marital Status | 0.691 | ||||||
| Unknown | 22(15.3) | 2(11.8) | 2(7.4) | 2(16.7) | 7(15.9) | 9(20.5) | |
| Not married | 77(53.5) | 8(47.1) | 19(70.4) | 6(50.0) | 21 (47.7) | 23(52.3) | |
| Married | 45(31.2) | 7(41.2) | 6(22.2) | 4(33.3) | 16(36.4) | 12(27.3) | |
| Employment | 0.015* | ||||||
| Status | |||||||
| Unknown | 47(32.6) | 6(35.3) | 6(22.2) | 4(33.3) | 16(36.4) | 15(34.1) | |
| Employed | 51(35.4) | 7(41.2) | 13(48.2) | 6(50.0) | 19(43.2) | 6(13.6) | |
| Unemployed | 8(5.6) | 0 | 3(11.1) | 1(8.3) | 1(2.3) | 3(6.8) | |
| Other1 | 38(26.4) | 4(23.5) | 5(18.5) | 1(8.3) | 8(18.2) | 20(45.5) | |
| Clinical Characteristics | |||||||
| SLEDAI at baseline (n=136) | 0.217 | ||||||
| Active (>4) | 50(36.8) | 5(33.3) | 7(28.0) | 3 (27.3) | 22 (51.2) | 13(31.0) | |
| Inactive (≤ 4) | 86(63.2) | 10(66.7) | 18 (72.0) | 8 (72.7) | 21 (48.8) | 29(69.1) | |
| CESD-R score at baseline (SD) | 20.1(14.9) | 3.1(4.4) | 11.9(8.1) | 2.2(4.5) | 20.8(8.3) | 36.0(10.5) | 0.000* |
| Anxiety (SD, n=130) | 55.1(12.9) | 40.5(5.5) | 49.6(10.5) | 42.5(7.6) | 55.5(9.4) | 67.9(7.6) | 0.000* |
| BMI Category (n=138) | 0.314 | ||||||
| Normal BMI (<25.0 kg/m2) | 39(28.3) | 6(35.3) | 9(33.3) | 1(8.3) | 11(27.5) | 12(28.6) | |
| Overweight (25.0–29.9 kg/m2) | 40(29.0) | 3(17.7) | 6(22.2) | 6(50.0) | 16(40.0) | 9(21.4) | |
| Obese (≥30.0 kg/m2) | 59(42.7) | 8(47.1) | 12(44.4) | 5(41.7) | 13(32.5) | 21(50.0) | |
| Hypertension (n=143) | 62(43.4) | 6(35.3) | 11(40.7) | 6(50.0) | 18(40.9) | 21(48.8) | 0.849 |
| Hyperlipidemia(n=144) | 24(16.7) | 2(11.8) | 4(14.8) | 1(8.3) | 5(11.4) | 12(27.3) | 0.322 |
| Diabetes Mellitus Type 2 (n=139) | 12(8.6) | 2(11.8) | 2(7.4) | 1(8.3) | 2(4.9) | 5(11.9) | 0.780 |
| Hypothyroidism (n=139) | 18(13.0) | 3(17.7) | 4(14.8) | 1(8.3) | 7(17.1) | 3(7.1) | 0.616 |
| APLS (n=143) 2 | 14(9.8) | 1(5.9) | 3(11.1) | 0 | 5(11.4) | 5(11.6) | 0.889 |
| C3 (n=132, SD) | 113.4(36.8) | 104.4(35.2) | 115.5(33.1) | 99.3(36.8) | 110.6(36.2) | 122.3(39.2) | 0.255 |
| C4 (n=131, SD) | 22.6(9.9) | 19.8(7.9) | 20.4(7.5) | 21.0(7.5) | 22.6(10.1) | 25.7(11.9) | 0.154 |
| dsDNA (n=64, SD) | 134.0(168.1) | 178.1(197.2) | 77.6(87.4) | 184.1(251.1) | 169.0(189.1) | 67.7(70.7) | 0.222 |
| ESR (n=127, SD) | 29.0(25.1) | 33.1(27.2) | 32.2(26.3) | 27.7(27.5) | 25.6(22.1) | 29.1(264) | 0.825 |
| Medications | |||||||
| Narcotics (%) | 40(27.8) | 2(11.8) | 10(37.0) | 0 | 11(25.0) | 17(38.6) | 0.030* |
| Prednisone dose > 7.5mg/d (%) | 33(22.9) | 1(5.9) | 7(25.9) | 1(8.3) | 6(13.6) | 18(40.9) | 0.006* |
| SSRI or SNRI (%)3 | 32(22.2) | 1(5.9) | 4(14.8) | 1(8.3) | 10(22.7) | 16(36.4) | 0.000* |
| Benzodiazepines (%) | 12(8.3) | 0 | 3(11.1) | 1(8.3) | 4(9.1) | 4(9.1) | 0.806 |
| Hypnotics (%) | 11(7.6) | 0 | 1(3.7) | 0 | 5(11.4) | 5(11.4) | 0.466 |
| TCA (%)4 | 9(6.3) | 3(17.7) | 0 | 0 | 3(6.8) | 3(6.8) | 0.197 |
Indicates statistically significant at p < 0.05
Other category includes individuals who are students, disabled, or retired
APLS = Anti-phospholipid syndrome
SSRI = selective serotonin reuptake inhibitor; SNRI = Serotonin-norepinephrine reuptake inhibitor
TCA = Tricyclic antidepressant
Statistical Methods
Descriptive characteristics of the overall sample were reported as mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. In order to identify unique trajectories of depression symptomology over time, group based trajectory modeling (GBTM) was used [30]. This method allowed for the identification of groups of individuals who followed similar patterns of depression symptoms using a statistical approach, as opposed to a theoretical approach. The method then classified individuals into their most likely trajectory, based on the predicted probability of membership in each identified trajectory group. The analysis was performed using the add-on TRAJ procedure in SAS 9.4 (SAS Institute, Cary, NC) [31]. Because of the skewed distribution of CESD-R scores, the values were log-transformed prior to the GBTM analysis. The final model was selected based on the Bayesian Information Criteria (BIC), where smaller values indicate better model fit, and theoretical understanding of the data. Baseline characteristics of individuals in each identified depression trajectory group were compared using chi-square tests, Fisher’s exact tests, or one-way ANOVA.
To further understand the relationship between SLE disease activity and depression over time, linear mixed models (LMMs) were used. The CESD-R was the continuous outcome and the S2K or S2K RI-50 was the primary predictor. The LMMs accounted for the multiple measurements of depression and disease activity from individuals over time (Table 3). Univariable and multivariable logistic regressions were used to determine the relationship between baseline characteristics and individuals’ classification into trajectory groups reflecting either elevated or normal depression symptomology. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC) and α=0.05 was used to determine statistical significance.
Table 3.
Linear Mixed Model
| Variable | Univariate Analysis | p-values | Multivariate Analysis | p-values |
|---|---|---|---|---|
| Age (baseline) | 0.16 (−0.01, 0.32) | 0.070 | - | - |
| Gender (reference: Male) | ||||
| Female | 1.17 (−6.25, 8.59) | 0.758 | - | - |
| Race (reference: White) | 0.006 | 0.013 | ||
| Black | 5.80 (1.50, 10.11) | 0.008 | 5.53 (1.14, 9.93) | 0.014 |
| Other | −5.38 (−15.33, 4.57) | 0.289 | −4.88 (−14.97, 5.20) | 0.342 |
| SLEDAI1 (reference: Inactive) | ||||
| Active2 | 1.44 (0.05, 2.82) | 0.042 | 1.41 (0.03, 2.79) | 0.045 |
| Month3 | −0.06 (−0.10, −0.02) | 0.003 | - | - |
| Prednisone | 0.08 (0.02, 0.15) | 0.010 | 0.10 (0.03, 0.18) | 0.010 |
Indicates statistically significant at p < 0.05
SLEDAI: Systemic Lupus Erythematosus Disease Activity Index
SLEDAI score greater than 4 is defined as active
The linear mixed model examines the overall relationship between the variables of interest and the CESD-R scores over multiple points in time. The month variable reflects the time in months from baseline at which each CESD-R t-score was collected. The SLEDAI active versus inactive indicator, month, and prednisone dose are also time-varying variables that have the potential to change at each measurement of the CESD-R score
Results
Baseline characteristics
The sample (n=144) was 91.0% female, 56.3% African American, and 38.9% White, with a mean age of 40.4 years (SD: 12.6; range: 19–74; Table 1). Patients had paired CESD-R and SLEDAI scores over an average time period of 30.2 months (SD: 13.3; range: 2.6–48.0). The average number of visits per patient was 6.9 (SD: 3.2; range: 3–16) and the mean interval between visits was 150.7 days (SD: 101.5; range: 21–1036). Information regarding educational attainment, employment status and marital status was recorded for only a subset of patients. For patients with information available, 47.9% reported completing GED/High School/some college, while 20.8% completed a college degree/graduate school, 35.4% of participants reported employment while 5.6% were unemployed, and over half of the cohort was married (53.5%). The patient’s recorded BMI data showed that 71.7% of the cohort was overweight or obese. Disease activity at baseline showed that 63.2% had inactive SLE disease as measured by SLEDAI disease activity scoring (SLEDAI </= 4) and 36.8% had active disease (SLEDAI > 4). Baseline CESD-R score for the patient cohort was 20.1 (SD: 14.9).
Measurement of comorbid diseases showed that while hypertension was noted in nearly half of the cohort (42.1%), diabetes mellitus type 2, hyperlipidemia, hypothyroidism, and antiphospholipid antibody syndrome (APLS) were not as prevalent (8.6%, 16.7%, 13.0%, 9.8%, respectively). At baseline, prednisone > 7.5 mg/day was prescribed for 24.3% of the sample and nearly one third of the cohort was on narcotic pain medications (27.8%). Selective serotonin reuptake inhibitors (SSRI)/serotonin-norepinephrine reuptake inhibitors (SNRI) were the most commonly prescribed psychotropic medications (22.2%) followed by benzodiazepines, hypnotics and tricyclic antidepressants (TCA).
Comparison of individuals with only 3 visits (the minimum number required for inclusion in the sample) versus those with more than 3 visits indicated that the degree of missing data for education, employment, and marital status was significantly higher among patients with fewer visits. However, all other clinical characteristics were not meaningfully different (Supplemental Table 1).
Depression Trajectory Group Identification
GBTM revealed five distinct groups of patients who demonstrated consistent trends in depression overtime (Figure 1). The 5-group model best represented the data when compared to both the 4-group and 6-group models that were also assessed, and was preferred despite the lower BIC in the 6-group model (Supplemental Figure 1). Posterior probabilities of group membership further supported the good overall fit of the 5-group model (Supplemental Table 2). Members of groups 1 (n=17, 12%) and 2 (n=27, 19%) had consistently normal CESD-R scores. The average CESD-R score at baseline of groups 1 and 2 were 3.1 and 11.9 respectively. Group 3 (n=12, 8%) had rising CESD-R scores over time and an average baseline score of 2.2. Groups 4 (n=44, 31%) and 5 (n=44, 31%) demonstrated symptom patterns consistent with depressed affect and major depression, respectively. The baseline CESD-R score of group 4 was 20.8 while the baseline of group 5 was 36. While visit-to-visit scores did vary, patterns remained durable over time.
Figure 1.
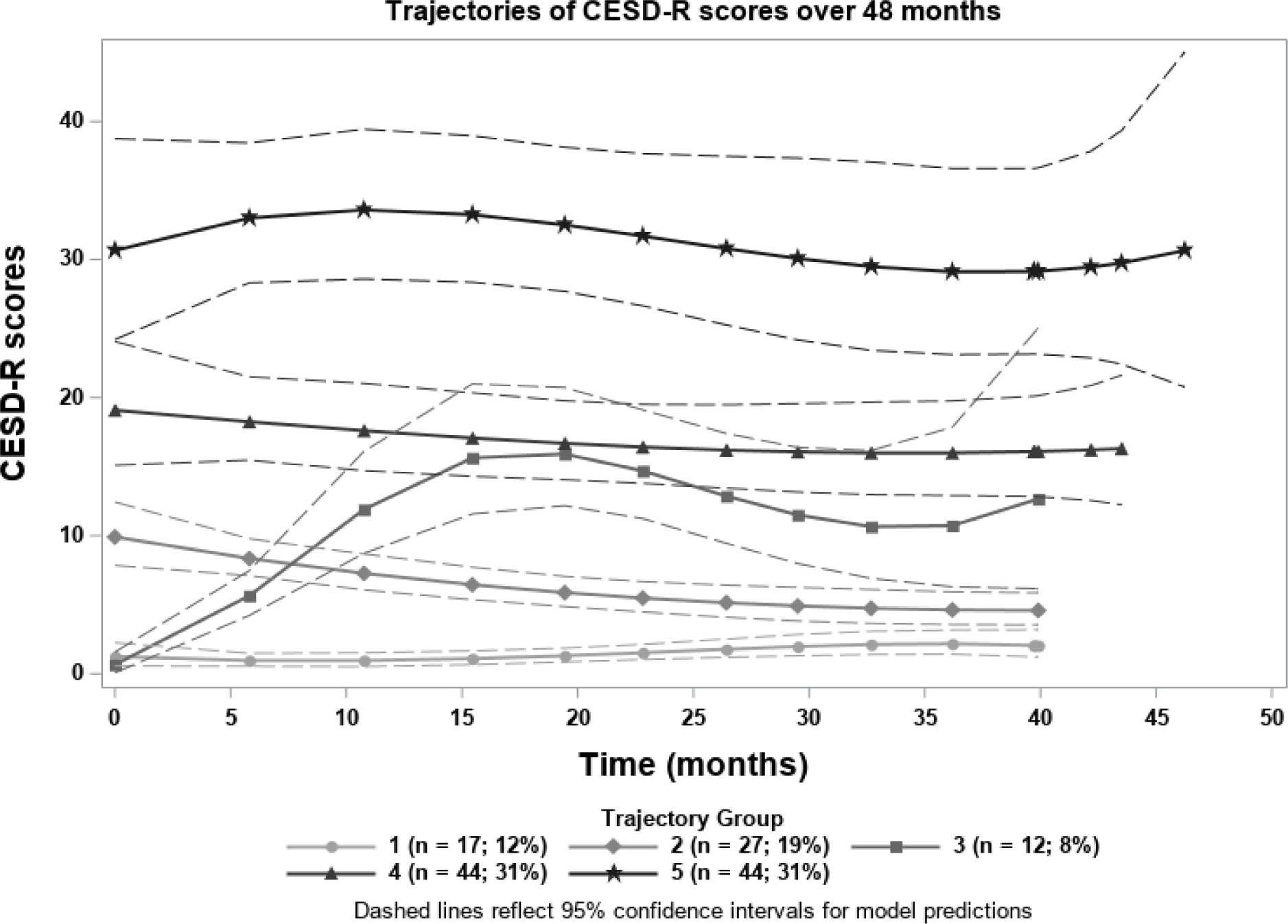
Identified trajectories of depression scores over time
Baseline Characteristics of the Depression Trajectory Groups
The characteristics of the participants in each of the groups identified through GBTM are displayed in Table 1. The most notable characteristic identified within the groups was the prevalence of Black patients in group 5 (n=32, 72.7%, p<0.02) as compared to White patients (n=11, 25.0%, p<0.02).
While there was noted statistical significance with employment status, this data may be biased as employment status at enrollment was not recorded for nearly half of the participants. Age, sex, educational attainment, and marital status were not found to be significantly different across trajectory groups. SLE duration, SLEDAI score, ESR, dsDNA, C3, and C4 did not meaningfully differ across the groups. The prevalence of hyperthyroidism, CHF, CAD, HTN, DMII, peripheral neuropathy, antiphospholipid syndrome, and hypothyroidism were also similar across the identified trajectory groups.
Narcotic use (including long acting and/or immediate release) was found to be differentially distributed across the groups (p = 0.030). Narcotic usage was noted in each of the groups with the highest prevalence being in group 5 (n=17, 38.6%) closely followed by group 2 with a similar prevalence (n=10, 37.0%) of the patients on either a long acting or immediate release narcotic. One-quarter of group 4 contained participants on narcotics (n=11, 25.0%).
Prednisone use was also found to be distributed unequally across the groups (p = 0.003). In group 5 prednisone > 7.5 mg use was the most prevalent (n=19, 43.2%). Groups 1 and 2 showed a prevalence of 5.9% and 29.6% respectively.
Baseline Predictors of Depression Trajectory Group Membership
To better understand the association between the patients’ baseline characteristics and trajectory of depression, logistic regression analysis was performed. The outcome variable used for this analysis was membership in either the depressed or non-depressed group as identified by CESD-R scores for the identified trajectories (CESD-R > 16 indicating depression or depressed affect). Specifically, individuals classified into trajectories 4 and 5 were considered the depressed group, as these individuals exhibited CESD-R scores persistently above a score of 16, whereas those assigned to trajectories 1, 2, and 3 were the non-depressed group, as these trajectories indicated CESD-R scores below 16. The associations from this analysis, in which all variables were assessed in separate univariate models are displayed in Table 2. These analyses showed that SSRI/SNRI was the only characteristic associated with the depressed group (OR: 3.27, 95% CI: [1.23, 8.70], p = 0.017).
Table 2.
Univariate logistic regression model, comparing demographic and clinical characteristics at study entry of depressed (Groups 4 and 5) to non-depression (Groups 1, 2, and 3) participants
| Baseline Characteristic | Odds ratio (95% CI) | p-value |
|---|---|---|
|
| ||
| Age | 1.01 (0.98, 1.04) | 0.455 |
| Gender | 0.216 | |
| Female | 0.43 (0.13,1.42) | |
| Male | Reference | |
| Race | 0.623 | |
| Black | 1.09 (0.53,2.27) | |
| Other | 0.44 (0.07,2.84) | |
| White | Reference | |
| BMI Category | 0.943 | |
| Overweight | 1.18 (0.46, 3.00) | |
| Obese | 1.08 (0.47, 2.47) | |
| Normal | Reference | |
| Narcotics | 1.68 (0.72, 3.92) | 0.227 |
| SSRI/SNRI | 3.27 (1.23,8.70) | 0.017* |
| Benzodiazepines | 1.32 (0.38, 4.65) | 0.661 |
| TCA | 1.08 (0.25, 4.73) | 0.918 |
| Hypnotics | 7.25 (0.90,58.45) | 0.063 |
| SLEDAI Active | 1.81 (0.86, 3.84) | 0.120 |
Indicates statistically significant at p < 0.05
Relationship Between SLE Disease Activity and Depression Over Time
The examination of the relationship between SLE disease activity and depression over time is presented in Table 3. Univariate analysis showed significant associations between race, active SLE, and supraphysiologic prednisone use (> 7.5 mg/day). Multivariate analysis showed that disease activity as measured by SLEDAI was significantly associated with depression over time (β = 1.41, 95% CI = [0.03, 2.79], p = 0.045). Prednisone dosage of >7.5 mg daily was also noted to be significant in multivariate analysis (β = 0.10, 95% CI = [0.03, 2.18], p = 0.010).
DISCUSSION
Here, we have presented a longitudinal analysis of depression in patients treated at the Washington University Lupus Clinic. Depressed affect and depression were noted to chronically persist in over half of the cohort. Analysis identified five unique trajectories with groups 1 and 2 showing no evidence of depression as defined by CESD-R score. Group 3 showed gradually increasing CESD-R scores over time. Groups 4 and 5 showed evidence of persistent depression.
Longitudinal analysis of depression in non-SLE patients has shown depression to be persistent over time [19, 32]. The longitudinal course of depression outside of a comorbid illness has been noted to have an association with lower socioeconomic status and less education [33]. In patients with non-SLE comorbid diseases, chronic pain is known to have a strong association with depression [34] as well as cancer, stroke and cardiovascular diseases [35–37].
In regards to association of depression with disease activity, an analysis of the longitudinal course of depression in rheumatoid arthritis noted that increased disease activity was associated with depression [38]. Though it has been noted in previous analyses that depression persists overtime in SLE [39] and may associate with disease activity [12], there is a scarcity of data characterizing the course of depression and the factors contributing to the chronicity of this mental health disorder in SLE patients.
Our analysis found that SLE disease activity was associated with depression score in multivariate analysis but did not show a significant association in univariate analysis. Since depression is considered as a manifestation of NPSLE, one would expect a strong association in each of these analyses. There are a couple possible explanations for this lack of strong association. First, mood disorders are not included in the SLEDAI instrument, which was used in this study. The SLEDAI was based on expert opinion using a predominantly White cohort [40]. We have found a strong relationship between Black race and higher rates and severity of depressed affect in our cohort, the racial composition of which is distinctly unique from what the SLEDAI has been based on. This suggests that certain critical symptoms may not be appropriately captured in the assessment of SLE disease activity.
Another more nuanced reason may lie in the complexity of appropriately attributing symptoms to SLE itself. It has been proposed that disease activity can be separated into two separate categories: Type I symptoms are typically physician measured and captured by SLE disease activity instruments (i.e. SLEDAI), while Type II symptoms are more subjective and come from patient report [18]. This idea is supported by a study by Ward et al., which showed a relationship between self-perception of disease activity and depression but did not show a relationship between physician-measured disease activity as measured by SLEDAI with depression [15]. Here, this nuance adds the component of symptoms that contribute to the overall experience of living with SLE but may not be treated using immunosuppressive medications. Nevertheless, this proposed disintegration of SLE symptoms remains a controversial topic needing further support before being widely accepted.
Prednisone and SSRI/SNRI medication use were noted to have a higher prevalence of use among the more depressed patients. Supraphysiologic dosing of prednisone was noted to be more prevalent in groups 4 and 5. Prednisone use in SLE patients may cause a synergistic effect on depression scores as steroids can independently contribute to depression, mania, hypomania and psychosis [41]. Prednisone dosing > 20 mg daily was associated with charted depression diagnosis in the John Hopkins Lupus cohort [14].
SSRI/SNRI use was highest in the cohort among patients in groups 4 and 5. This was documented at study entry, so one would have anticipated that these scores would decrease overtime due to therapeutic intervention. An unmeasured confounder may be medical non-adherence. In primary care patients, 41% of patients prescribed antidepressants were not taking these medications [42]. In patients with SLE, lack of adherence to SLE treatment regimens has been approximated in up to 46% of patients; one of the reasons attributed to this has been depression [4]. Another possible reason for the ineffectiveness of prescribed SSRI/SNRIs could be attributed to their effect on sleep quality. SSRI/SNRIs have a deteriorating effect on sleep quality [43], which is a common issue among at least half of patients with SLE [44] and may independently contribute to depression [45].
Narcotic pain medications had the highest documented usage in groups 2 and 5, and was not linearly related to depressive symptoms. While opioid analgesics have been noted to associate with the risk of major depressive disorder, increasing with dose and duration [46, 47], we found no such link in our data. Better understanding the level and persistence of pain in this cohort would be beneficial, as we did not gather patient-reported pain scores. Higher pain scores in patients with SLE have been associated with depression as well as anxiety and decreased HRQoL [3]. A weakness of this cohort was the paucity of pain score documentation. Chronic pain in its own right has been associated with depression and this information could lead to a better understanding of the complicated factors at play in these patients.
Race was found to be an important sociodemographic factor with a high proportion of Black patients in groups 4 and 5. Epidemiologic studies have noted a paradoxical relationship with Whites having a higher lifetime prevalence of documented major depressive disorder, but Blacks having a higher likelihood of severe and persistent depression [48]. Indeed, this is what we have observed in our cohort.
Unfortunately, there was not enough employment and education information in this patient cohort to analyze the relationship this may have with CESD-R scores. The reason for this paucity of data comes from many of the patients leaving this area blank on their initial visit paperwork. According to social rank theory, perceived inferiority to others with regards to social rank can lead to defensiveness and shame [49]. This theory presents the idea that emotions and moods are influenced by this feeling of being looked down upon and is one possible explanation for why patients did not divulge this information.
Strengths of this analysis include the relatively large sample size for a SLE longitudinal study, duration of data assessments, and high Black representation. An important weakness is missing data points. The GBTM method uses maximum likelihood estimation to incorporate all available data for individuals into the model, making it robust to missing data. However, this estimation assumes that data are missing at random [50], which may not be appropriate for our study, since patients with chronic illness and depression may be more likely to miss visits. A more formalized study structure, with more frequent assessments (i.e. weekly or monthly) performed within defined windows will provide more clarity to analyses such as those performed here. This could possibly be done using mobile applications in which patients periodically evaluate their SLE activity and complete various patient-reported outcome instruments. Furthermore, a more granular examination of the potential impact of social determinants of health on SLE disease activity and depression severity is certainly warranted given our results.
These findings indicate that in patients with SLE, depression is persisting despite interventions designed to treat pain, mood disorders and SLE disease. Further work is needed to better understand the causes of depression and improve outcomes for those who must daily live with both this chronic, unpredictable disease and the burdens that come with a chronic mood disorder.
Supplementary Material
Significance and Innovations.
Depression persists in patients with SLE despite the use of interventions designed to treat mood disorders and SLE disease.
Severe depression is more common in Black patients than White Patients.
Disclosures:
No specific funding was received for this work.
References
- 1.Izmirly PM, et al. , Prevalence of Systemic Lupus Erythematosus in the United States: Estimates from a Meta-Analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz B, et al. , Major Depression and Adverse Patient-Reported Outcomes in Systemic Lupus Erythematosus: Results From a Prospective Longitudinal Cohort. Arthritis Care Res (Hoboken), 2021. 73(1): p. 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldheim E, et al. , Health-related quality of life, fatigue and mood in patients with SLE and high levels of pain compared to controls and patients with low levels of pain. Lupus, 2013. 22(11): p. 1118–27. [DOI] [PubMed] [Google Scholar]
- 4.Julian LJ, et al. , Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum, 2009. 61(2): p. 240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greco CM, et al. , Association between depression and vascular disease in systemic lupus erythematosus. J Rheumatol, 2012. 39(2): p. 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisitsyna TA, et al. , [Prevalence of mental disorders in SLE patients: correlations with the disease activity and comorbid chronic conditions]. Ter Arkh, 2009. 81(6): p. 10–6. [PubMed] [Google Scholar]
- 7.Petri M, et al. , Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol, 2010. 37(10): p. 2032–8. [DOI] [PubMed] [Google Scholar]
- 8.Mok CC, et al. , Suicidal ideation in patients with systemic lupus erythematosus: incidence and risk factors. Rheumatology (Oxford), 2014. 53(4): p. 714–21. [DOI] [PubMed] [Google Scholar]
- 9.Ainiala H, et al. , Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum, 2001. 45(5): p. 419–23. [DOI] [PubMed] [Google Scholar]
- 10.Karimifar M, Sharifi I, and Shafiey K, Anti-ribosomal P antibodies related to depression in early clinical course of systemic lupus erythematosus. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences, 2013. 18(10): p. 860–864. [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna G, et al. , Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol, 2003. 30(5): p. 985–92. [PubMed] [Google Scholar]
- 12.Nery FG, et al. , Major depressive disorder and disease activity in systemic lupus erythematosus. Compr Psychiatry, 2007. 48(1): p. 14–9. [DOI] [PubMed] [Google Scholar]
- 13.Hanly JG, et al. , Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum, 2007. 56(1): p. 265–73. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Magder LS, and Petri M, Predictors of incident depression in systemic lupus erythematosus. J Rheumatol, 2014. 41(9): p. 1823–33. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM, Marx AS, and Barry NN, Psychological distress and changes in the activity of systemic lupus erythematosus. Rheumatology (Oxford), 2002. 41(2): p. 184–8. [DOI] [PubMed] [Google Scholar]
- 16.Julian LJ, et al. , Cardiovascular and disease-related predictors of depression in systemic lupus erythematosus. Arthritis Care Res (Hoboken), 2011. 63(4): p. 542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quimby KR, et al. , Comparison of the systemic lupus erythematosus activity questionnaire and the systemic lupus erythematosus disease activity index in a black barbadian population. Int J Rheumatol, 2013. 2013: p. 875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisetsky DS, et al. , A Novel System to Categorize the Symptoms of Systemic Lupus Erythematosus. Arthritis Care & Research, 2019. 71(6): p. 735–741. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR, et al. , Longitudinal Trajectories of Depression and Anxiety in a Prospective Community Study: The Zurich Cohort Study. Archives of General Psychiatry, 2003. 60(10): p. 993–1000. [DOI] [PubMed] [Google Scholar]
- 20.Alian S, et al. , Depression in hepatitis B and C, and its correlation with hepatitis drugs consumption (interfron/lamivodin/ribaverin). Iranian journal of psychiatry and behavioral sciences, 2013. 7(1): p. 24–29. [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia MS and Munjal S, Prevalence of Depression in People Living with HIV/AIDS Undergoing ART and Factors Associated with it. Journal of clinical and diagnostic research : JCDR, 2014. 8(10): p. WC01–WC4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Liu X, and Yu Y, Depression and Chronic Liver Diseases: Are There Shared Underlying Mechanisms? Frontiers in molecular neuroscience, 2017. 10: p. 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmel PL, et al. , Depression in end-stage renal disease patients: a critical review. Adv Chronic Kidney Dis, 2007. 14(4): p. 328–34. [DOI] [PubMed] [Google Scholar]
- 24.Pearson RM, et al. , Prevalence of Prenatal Depression Symptoms Among 2 Generations of Pregnant Mothers: The Avon Longitudinal Study of Parents and Children. JAMA network open, 2018. 1(3): p. e180725–e180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gladman DD, Ibañez D, and Urowitz MB, Systemic lupus erythematosus disease activity index 2000. The Journal of Rheumatology, 2002. 29(2): p. 288. [PubMed] [Google Scholar]
- 26.Touma Z, et al. , Development and initial validation of the systemic lupus erythematosus disease activity index 2000 responder index 50. J Rheumatol, 2011. 38(2): p. 275–84. [DOI] [PubMed] [Google Scholar]
- 27.Yee CS, et al. , The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford), 2011. 50(5): p. 982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsokos GC, Systemic Lupus Erythematosus. New England Journal of Medicine, 2011. 365(22): p. 2110–2121. [DOI] [PubMed] [Google Scholar]
- 29.Pollack MH, Comorbid anxiety and depression. J Clin Psychiatry, 2005. 66 Suppl 8: p. 22–9. [PubMed] [Google Scholar]
- 30.Nagin DS, Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods, 1999. 4(2): p. 139–157. [DOI] [PubMed] [Google Scholar]
- 31.Jones BL, Nagin DS, and Roeder K, A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research, 2001. 29(3): p. 374–393. [Google Scholar]
- 32.Murphy JM, Trends in depression and anxiety: men and women. Acta Psychiatrica Scandinavica, 1986. 73(2): p. 113–127. [DOI] [PubMed] [Google Scholar]
- 33.Skipstein A, et al. , Trajectories of maternal symptoms of anxiety and depression. A 13-year longitudinal study of a population-based sample. BMC Public Health, 2010. 10(1): p. 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bair MJ, et al. , Depression and pain comorbidity: a literature review. Arch Intern Med, 2003. 163(20): p. 2433–45. [DOI] [PubMed] [Google Scholar]
- 35.Kang H-J, et al. , Comorbidity of depression with physical disorders: research and clinical implications. Chonnam medical journal, 2015. 51(1): p. 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peter RS, et al. , Long-term trajectories of anxiety and depression in patients with stable coronary heart disease and risk of subsequent cardiovascular events. Depression and Anxiety, 2020. 37(8): p. 784–792. [DOI] [PubMed] [Google Scholar]
- 37.Harshfield EL, et al. , Association Between Depressive Symptoms and Incident Cardiovascular Diseases. JAMA, 2020. 324(23): p. 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fragoulis GE, et al. , Depression and anxiety in an early rheumatoid arthritis inception cohort. associations with demographic, socioeconomic and disease features. RMD Open, 2020. 6(3): p. e001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touma Z, et al. , Cognitive function trajectories are associated with the depressive symptoms trajectories in systemic lupus erythematosus over time. Arthritis Care & Research. n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bombardier C, et al. , Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum, 1992. 35(6): p. 630–40. [DOI] [PubMed] [Google Scholar]
- 41.Brown ES and Chandler PA, Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Primary care companion to the Journal of clinical psychiatry, 2001. 3(1): p. 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson SK, et al. , Antidepressant treatment for primary care patients with depressive symptoms: Data from the diamond longitudinal cohort study. Australian & New Zealand Journal of Psychiatry, 2020. 54(4): p. 367–381. [DOI] [PubMed] [Google Scholar]
- 43.Wichniak A, et al. , Effects of Antidepressants on Sleep. Current psychiatry reports, 2017. 19(9): p. 63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palagini L, et al. , Sleep disorders and systemic lupus erythematosus. Lupus, 2014. 23(2): p. 115–23. [DOI] [PubMed] [Google Scholar]
- 45.Palagini L, et al. , Poor sleep quality in systemic lupus erythematosus: does it depend on depressive symptoms? Lupus, 2014. 23(13): p. 1350–7. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan MD, Depression Effects on Long-term Prescription Opioid Use, Abuse, and Addiction. Clin J Pain, 2018. 34(9): p. 878–884. [DOI] [PubMed] [Google Scholar]
- 47.Semenkovich K, et al. , Prescription Opioid Analgesics Increase Risk of Major Depression: New Evidence, Plausible Neurobiological Mechanisms and Management to Achieve Depression Prophylaxis. Missouri medicine, 2014. 111(2): p. 148–154. [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DR, et al. , Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry, 2007. 64(3): p. 305–15. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert P, The relationship of shame, social anxiety and depression: the role of the evaluation of social rank. Clinical Psychology & Psychotherapy, 2000. 7(3): p. 174–189. [Google Scholar]
- 50.Enders CK, A Primer on Maximum Likelihood Algorithms Available for Use With Missing Data. Structural Equation Modeling: A Multidisciplinary Journal, 2001. 8(1): p. 128–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


