Abstract
Purpose of Review
The effect of the transforming growth factor beta (TGFβ) signaling pathway on joint homeostasis is tissue-specific, non-linear, and context-dependent, representing a unique complexity in targeting TGFβ signaling in joint disease. Here we discuss the variety of mechanisms that TGFβ signaling employs in the synovial joint to maintain healthy joint crosstalk and the ways in which aberrant TGFβ signaling can result in joint degeneration.
Recent Findings
Osteoarthritis (OA) epitomizes a condition of disordered joint crosstalk in which multiple joint tissues degenerate leading to overall joint deterioration. Synovial joint tissues, such as subchondral bone, articular cartilage, and synovium, as well as mesenchymal stem cells, each demonstrate aberrant TGFβ signaling during joint disease, whether by excessive or suppressed signaling, imbalance of canonical and non-canonical signaling, a perturbed mechanical microenvironment, or a distorted response to TGFβ signaling during aging.
Summary
The synovial joint relies upon a sophisticated alliance among each joint tissue to maintain joint homeostasis. The TGFβ signaling pathway is a key regulator of the health of individual joint tissues, and the subsequent interaction among these different joint tissues, also known as joint crosstalk. Dissecting the sophisticated function of TGFβ signaling in the synovial joint is key to therapeutically interrogating the pathway to optimize overall joint health.
Keywords: TGF beta, Joint crosstalk, Osteoarthritis, Subchondral bone, Articular cartilage
Introduction
The synovial joint consists of multiple tissues, including articular cartilage, subchondral bone, meniscus, and synovium, that interact biologically and mechanically to maintain joint health [1]. During joint degeneration, the disruption of one tissue can lead to overall deterioration by shifting biomechanical loads, increasing inflammation, altering paracrine factors, or inducing aberrant cell-intrinsic signaling. These interactions among joint tissues are referred to as joint crosstalk. Though joint crosstalk is critical for joint health, it can also exacerbate disease. Unravelling the mechanisms and causal relationships among these tissues has been challenging and has limited the development of therapies that modify osteoarthritis (OA) progression.
The transforming growth factor beta (TGFβ) signaling pathway plays an intricate role in maintaining healthy joint crosstalk by carefully regulating homeostasis of each tissue. The delicate balance of TGFβ signaling throughout the joint is non-linear and relies upon control of effector selection, mechanical cues, and spatial localization. Here, we discuss the multifaceted function of TGFβ signaling in joint homeostasis and disease.
Overview of the TGFβ Signaling Pathway
The TGFβ signaling pathway controls cell behavior through hierarchical, context-dependent, and coordinated regulation [2–4]. The expression pattern of the three TGFβ ligand isoforms, TGFβ1, β2, and β3, is spatially and temporally regulated throughout the lifespan. Each joint tissue expresses ligands capable of activating the TGFβ signaling pathway, as well as the receptors and effectors needed to respond. The expression pattern and relative activity of each component are tightly regulated, which contributes to the diverse effects of TGFβ in the joint.
The TGFβ ligands are secreted as inactive ligands, consisting of the latency-associated peptide and the mature peptide. This complex is then secured to the extracellular matrix (ECM) through latent TGFβ binding proteins (LTBP) and other proteins, such as biglycan, fibrillin, and thrombospondin, many of which have also been implicated in joint homeostasis or disease [5]. Not only do these mechanisms function to locally sequester TGFβ in the ECM, but they also add multiple layers of regulation to TGFβ activation, to be discussed later. Briefly, latent TGFβ is activated through a variety of mechanisms, including those that are sensitive to the mechanical or chemical microenvironment [2]. This ECM-dependent control of TGFβ activity is especially important in the joint. First, tissues with abundant ECM, such as hyaline cartilage and bone, are reservoirs for latent TGFβ. Second, ECM changes that typify joint disease can indirectly disrupt the tight local control of TGFβ sequestration and activity.
Once latent TGFβ ligand becomes activated, it binds to two type II serine/threonine kinase receptors (TβRII), which then recruit and phosphorylate two type I serine/threonine kinase receptors (TβRI) to form a heterotetrameric transmembrane receptor complex [6]. The type I receptors, also termed activin receptor-like kinases (ALKs), calibrate ligand binding affinity and effector selection. Upon ligand binding, the receptors are phosphorylated, which permits effectors to be recruited and activated, including the canonical SMAD2/3 protein and multiple non-canonical effectors, such as SMAD1/5/8, Erk, JNK, Akt, and p38 [5]. Canonically, SMAD2/3-dependent TGFβ signaling is mediated by the ALK5 type I receptor, and SMAD1/5/8-dependent TGFβ signaling is mediated by the ALK1 type I receptor, illustrating one mechanism of effector selection in TGFβ signaling [5]. Once activated, phosphorylated SMAD3 (pSMAD3) forms a complex with the co-Smad SMAD4 and translocates to the nucleus where it binds directly to Smad-binding elements in the promotor, or interacts indirectly with other transcription factors, to alter gene expression [6].
TGFβ signaling exhibits exquisite internal control through negative feedback by a variety of mechanisms. Inhibitory Smads SMAD6 and SMAD7 are induced by TGFβ, activin, and BMP signaling and regulate signaling by competitively interfering with the receptor-Smad complex to prevent Smad activation [7]. Additionally, SMAD6 and SMAD7 inhibit TGFβ signaling by recruiting Smurf E3 ubiquitin ligases, SMURF1 and SMURF2, which ubiquitinate the TGFβ receptors and Smads, tagging them for degradation [7]. Multiple other mechanisms of transcriptional and post-transcriptional control, including targeting components of the TGFβ pathway through microRNAs (miRNAs), sumoylation, and regulated localization, also tune the functional activity of this pathway [5, 7]. Another level of complexity in effector regulation is that, in certain contexts, non-canonical effectors can themselves regulate the activity of SMAD2/3, thus impacting downstream signaling and gene expression [2].
Here, we focus on the function of TGFβ in skeletal cells and tissues in joint health and disease. Spatial and temporal control of TGFβ signaling is exerted at multiple levels of the pathway and is context dependent. Disrupting this balance can lead to joint degeneration.
Overview of Joint Health and Disease
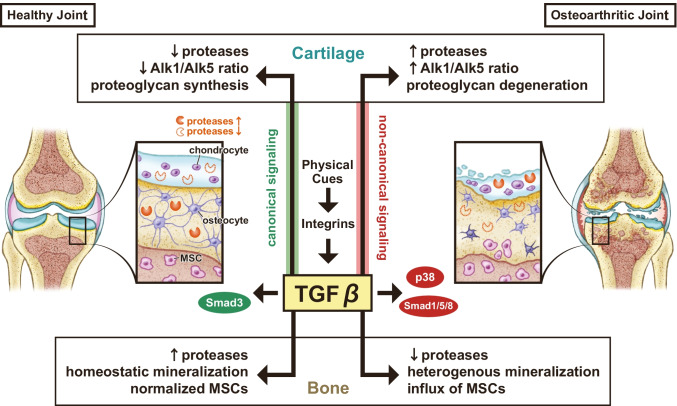
While there are a variety of joint diseases, this review will focus primarily on the impact of TGFβ signaling in OA. OA is a chronic and debilitating joint disease that diminishes mobility and causes severe pain. OA affects 30 million Americans [8] and is a leading cause of disability globally [9]. There is no currently available pharmacologic agent that can delay or prevent the development of OA, and end-stage OA often deteriorates to the point that a joint replacement is necessary. Given the dearth of treatment options for those suffering from OA, there is a pressing need to understand the cellular mechanisms that contribute to joint homeostasis. As mentioned previously, hierarchical regulation of the TGFβ signaling pathway indicates that differential function in various levels of the pathway could result in dysregulated TGFβ signaling (Fig. 1). Importantly, either diminished or excessive TGFβ signaling can perturb joint health.
Fig. 1.
Synovial joint homeostasis relies upon intricate control of TGFβ signaling across multiple joint tissues. Disruption of the complex hierarchical regulation of TGFβ signaling, whether through physical cues, receptor recruitment, or effector selection, impairs synovial joint health and results in joint degeneration in a tissue-specific manner. Proteases, such as MMP13, have differential TGFβ-dependent regulation within each tissue in joint disease, such that elevated proteases in subchondral bone improve bone quality and joint health, yet elevated proteases in cartilage leads to cartilage degeneration
One genetic syndrome vividly demonstrates the importance of the TGFβ pathway on joint health. Aneurysms osteoarthritis syndrome (AOS) is a familial syndromic form of aortic aneurysm and early-onset OA that is the result of loss-of-function mutations of SMAD3. For example, one familial form involves a truncating mutation that removes the MH2 domain, while another familial form involves missense mutations that result in conformational changes of the SMAD3 protein, disrupting protein trimerization [10, 11]. Other musculoskeletal anomalies can also be present in patients with AOS, including intervertebral disc degeneration, osteochondritis dissecans, and meniscal anomalies [11]. Most mutations that lead to AOS are located in the MH2 domain, which is important for SMAD association with TGFβ receptors, SMAD3/SMAD4 oligomerization, and SMAD-dependent transcriptional control [11]. Despite the loss-of-function mutation in SMAD3, patients with AOS paradoxically demonstrate increased total levels of SMAD3 protein, excessive activation and nuclear localization of SMAD2 and SMAD3, and higher levels of TGFβ1 in the aortic wall [10]. Taken together, these observations suggest a role for enhanced TGFβ signaling in AOS. While the level of TGFβ and downstream effectors was not assessed in musculoskeletal tissues, it is possible that early-onset OA in these patients coincides with excessive TGFβ signaling in the joint.
Dysregulated TGFβ signaling has been found in various synovial joint tissues in OA. Bone from patients with OA exhibits increased expression of TGFβ1 ligand and TGFB1 and SMAD3 gene expression, even at sites distant to joint degeneration [12–14]. Other changes observed in OA bone include altered collagen I production, RUNX2 expression, osteocalcin, and WNT signaling targets, each of which plays a role in osteoblast differentiation [12, 14, 15]. In human cartilage, SMAD3 mRNA expression is higher in OA cartilage than in non-arthritic control tissue [16], providing evidence that in some cases, the TGFβ/SMAD3 pathway may be overactive in OA cartilage leading to degeneration. In synovium and synovial fluid from patients with OA, elevated levels of TGFβ1 ligand are a strong predictor of OA progression [17].
Genome-Wide Association Studies (GWAS) provide compelling evidence of the multi-level genetic role of TGFβ signaling in joint disease. Single nucleotide polymorphisms (SNPs) in introns or exons of the SMAD3 gene have been associated with increased risk of OA [18–21]. More specifically, a missense mutation of an exon of the SMAD3 gene correlates with increased serum levels of MMP2 and MMP9, enzymes that play a role in articular cartilage degeneration [18]. Genetic variation in intronic SNPs of SMAD3 also increases risk of hip OA and knee OA, although the specific function of the SNPs is unclear given their intronic location in the SMAD3 gene [19–21]. In concert with the increased risk of OA with SMAD3 mutations, SMAD3 may have a pleiotropic effect on bone mineral density [20]. At the level of the ligand, a SNP within the LTBP3 gene, encoding a latent TGFβ binding protein that can regulate the availability and activity of TGFβ ligand, was associated with increased risk and clinical severity of hip OA [22]. Noncoding mutations in GDF5, a member of the TGFβ super family, or p38 MAP kinase-mediated signaling, a “non-canonical” effector of the TGFβ pathway, are causal variants in OA development [23••]. A single variant within the TGFB1 gene has been postulated as causal for OA development, a finding that coincides with significant genome-wide enrichment for other genes implicated in TGFβ-signaling, such as LTBP1, LTBP3, SMAD3, and RUNX2 [24••]. Evidence of a genetic basis for TGFβ mutations in OA development motivates the mechanistic study of TGFβ in joint health to identify appropriate therapeutic targets.
Mechanoregulation of TGFβ Signaling
At the cellular level, changes in the mechanical microenvironment can alter the effect of TGFβ signaling at different hierarchical levels of the signaling pathway in each joint tissue (Table 1) [25]. Cellular forces resulting from changes in ECM stiffness or cytoskeletal tension can directly activate the TGFβ ligand, rendering it available for downstream signaling [26]. Colocalization of TGFβ receptors TβRI and TβRII to form heteromeric complexes capable of activating downstream signaling is sensitive to cytoskeletal tension in chondrocytes and fluid flow shear stress in osteocytes [27, 28]. Similarly, cytoskeletal tension primes the activity of the chondrogenic transcription factor SOX9 in response to TGFβ or mechanical stimulation [29]. A discrete cell substrate stiffness that is consistent with the physiologic stiffness of healthy articular cartilage stimulates maximal TGFβ-dependent SMAD3 phosphorylation, nuclear localization, and transactivation of chondrogenic genes; substrates that are more or less stiff than healthy articular cartilage diminish the ability of TGFβ signaling to induce chondrogenesis [30].
Table 1.
Mechanoregulation of TGFβ signaling in the synovial joint
| Reference | |
|---|---|
| Bone | |
| Colocalization of TβRI and TβRII in osteocytes is sensitive to fluid shear stress | [28] |
| Loss of osteocytic TβRII impedes subchondral bone response to changes in load | [43•] |
| Cartilage | |
| Colocalization of TβRI and TβRII in chondrocytes is sensitive to cytoskeletal tension | [27] |
| Chondrogenic activation of SOX9 in response to TGFβ is sensitive to cytoskeletal tension | [29] |
| ECM stiffness affects chondrogenic potential of TGFβ signaling | [30] |
| Joint motion activates latent TGFβ in superficial zone of articular cartilage | [31] |
| Cartilage loading reduces Alk1 and increases Alk5 mRNA expression | [34, 35] |
| Aged cartilage has reduced ability to induce canonical TGFβ signaling with load | [38] |
| Synovium | |
| Mechanical shearing activates synovial fluid latent TGFβ1 | [32] |
| Mesenchymal stem cells | |
| Changes in load increases ALK5 in subchondral bone MSCs without increasing ALK1 | [39] |
Joint loading presents a tissue-level mechanical cue that regulates the function of TGFβ ligand to modulate metabolic activity of chondrocytes. Both the superficial zone chondrocytes and the synoviocytes secrete latent TGFβ into the synovial fluid, which becomes activated with joint motion, and accumulates in the superficial zone of articular cartilage [31]. Mechanical shearing within the joint activates latent TGFβ1 in the synovial fluid, rendering it available to stimulate TGFβ signaling in chondrocytes [32]. Though active TGFβ accumulates in the superficial zone, it is unable to penetrate deeper into middle and deep zones of cartilage [31]. In this way, superficial chondrocytes may receive adequate active TGFβ from synovial fluid to maintain homeostatic TGFβ signaling. In fact, physiologic dynamic compression of devitalized cartilage explants does not directly activate ECM-bound latent TGFβ in the deep zone of articular cartilage [33]. Therefore, activation of local TGFβ stores within the deep zone requires chondrocyte-dependent mechanisms. Taken together, articular cartilage relies upon synovial and cartilage-derived TGFβ, activated by mechanical and chondrocyte-dependent mechanisms, to sustain TGFβ signaling across the different articular cartilage zones.
Both physiologic and excessive loading of articular cartilage results in rapid induction of the canonical TGFβ signaling pathway [34]. In response to dynamic compression of either physiologic or excessive force, articular cartilage explants increase the mRNA levels for established TGFβ-inducible genes Serpine1 and Smad7, and production of Tgfb1 mRNA through an ALK5-dependent mechanism, demonstrating that TGFβ signaling and TGFβ production respond to cartilage loading [34]. The loss of compressive loading of cartilage explants results in a rapid decrease in TGFβ signaling, as shown by the reduction of pSMAD2 in chondrocytes and decreased expression of TGFβ-responsive genes Serpine1, Smad7, and Alk5, that is restored upon re-loading [35]. As a consequence of the unloading-dependent loss of TGFβ signaling, increased expression of Col10a1 mRNA, a marker of chondrocyte hypertrophy, is observed, suggesting a TGFβ-dependent mechanism that couples joint loading to cartilage homeostasis [35].
In response to load in either cartilage or bone, relative Alk5 expression increases, but the consequences of this change for joint homeostasis depend upon the cell compartment. In cartilage, loading reduces Alk1 expression and increases Alk5 expression to support canonical TGFβ signaling, unveiling one mechanism by which the balance between canonical and non-canonical TGFβ signaling in cartilage is preserved [34, 35]. In aging cartilage, the relative ratio of ALK1/ALK5 receptors is increased, thus favoring a non-canonical TGFβ signaling pathway [36, 37]. In response to either physiologic or excessive mechanical load, aged cartilage has a reduced ability to induce canonical TGFβ signaling, with reduced overall levels of phosphorylated SMAD2 and diminished nuclear localization of phosphorylated SMAD2 [38]. Thus, the ability of loading to sustain chondrocyte homeostasis may decline with age, and other avenues to promote cartilage health may become relevant, while canonical TGFβ signaling becomes less favored. Interestingly, perturbed joint loading due to transection of the anterior cruciate ligament (ACL) increases ALK5 in subchondral bone mesenchymal stem cells (MSCs) without increasing ALK1 [39]. The extent to which the MSC response to ACL injury reflects cellular exposure to excessive or diminished loads, or a cell type–specific response to altered joint loading, remains to be determined.
Subchondral bone, likewise, is dynamically loaded during joint motion and provides mechanical support to the overlying cartilage. The load-induced response of bone is largely mediated by osteocytes, the primary mechanosensors of bone [40]. Osteocytic TGFβ signaling is required for bone mechanosensation, as well as the control of bone quality [41, 42]. We found that increased loading on the articular cartilage and underlying subchondral bone, as a result of meniscal injury in mice, represses the expression of TβRII in subchondral bone plate osteocytes [43•]. Likewise, subchondral bone from mice with a genetic deficiency in osteocytic TβRII fails to respond to injury-induced load. Therefore, TGFβ signaling is both regulated by and required for osteocytic mechanosensitivity [43•]. In the same model of osteocytic TGFβ deficiency, articular cartilage deteriorates, suggesting that the mechanosensitive function of the subchondral bone plate requires osteocytic TGFβ signaling in order to maintain the health of cartilage [43•].
Tissue-Specific Function of TGFβ Signaling in Joint Disease
TGFβ plays a tissue-specific role in joint homeostasis and joint disease, contributing to the sophisticated function of TGFβ in the joint (Table 2). For example, inhibiting TGFβ within the joint can prevent osteophyte formation but simultaneously exacerbate cartilage degeneration [44]. Tissue-level evidence in clinical specimens offers insight into the role of TGFβ in joint disease, and transgenic mouse models of joint disease with a tissue-specific ablation of key receptors or effectors within the TGFβ signaling pathway provide powerful tools for isolating the tissue- or cell-type–specific functions of TGFβ in joint disease.
Table 2.
Tissue-specific differences of TGFβ signaling leading to OA
| Reference | ||
|---|---|---|
| Bone | ||
| Excessive: | Increased TGFB1 mRNA, TGFβ1 ligand, and SMAD3 mRNA in human OA bone | [12–14] |
| Increased TGFB1 mRNA, TGFβ1 ligand, and SMAD3 mRNA in primary OA osteoblasts | [45–49] | |
| Camurati-Engelmann disease with excessive levels of TGFβ1 secreted by osteoblasts develop OA | [39] | |
| Repressed: | Loss of osteocytic TβRII leads to cartilage degeneration | [43•] |
| Cartilage | ||
| Excessive: | Increased TGFβ1 and β2 ligands in human OA cartilage | [58] |
| SMAD3 mRNA higher in human OA cartilage | [16] | |
| Repressed: | Loss of chondrocyte-intrinsic SMAD3, TβRII, or ALK5 in mice induces OA | [71–73] |
| Downregulation of TGFβ ligand, reduced TGFβ-induced proteoglycan synthesis, and decreased TβRI and TβRII in aging cartilage | [85, 91] | |
| Altered canonical/non-canonical TGFβ signaling: | Increased ALK1/ALK5 receptor ratio increases MMP13 in OA cartilage | [36] |
| TβRI competitively antagonizes BMP signaling | [67•] | |
| SMAD1/5/8 signaling promotes hypertrophic chondrocyte differentiation during chondrogenesis | [74] | |
| Increased ALK1/ALK5 receptor ratio in aging murine and bovine cartilage | [36, 37] | |
| Synovium | ||
| Excessive: | Increased TGFβ ligand in synovial fluid induces OA | [17, 64–66] |
| Repressed: | Reduced TGFβ signaling due to dominant-negative mutation of TβRII exhibit synovial hyperplasia | [68] |
| Synovial fluid TGFβ ligand antagonizes degenerative effects of IL-1 on cartilage | [87, 88] | |
| Mesenchymal stem cells | ||
| Excessive: | Excessive TGFβ signaling in MSCs after injury results in OA | [39, 89] |
| Repressed: | Higher concentrations of TβRI inhibitor induce proteoglycan loss in cartilage | [39] |
Bone-Intrinsic Role of TGFβ in Joint Health and Disease
The association of altered bone mass and mineralization with OA progression [1], coupled with differences in expression of genes critical for TGFβ signaling in OA bone [12–14], has led to an interest in understanding the role of TGFβ in bone during joint degeneration. Disrupted TGFβ signaling may participate in several possible mechanisms by which bone may contribute to cartilage degeneration in joint disease. These mechanisms include altering joint mechanics, disrupting nutrient and vasculature exposure through the osteocytic lacunocanalicular network, or changing levels of paracrine factors that disrupt cartilage health. All three bone-cell types — osteoblasts, osteoclasts, and osteocytes — are distinctly regulated by TGFβ signaling in joint health and disease.
Studies of TGFβ signaling in whole-bone samples from OA human joints have found increased TGFβ ligand production and TGFB1 and SMAD3 gene expression, relative to non-arthritic joints, even in bone specimens taken from sites distant from the OA joint [12–14]. Microarray gene expression of bone from OA patients demonstrates significant increases in expression of SMAD3 [12]. These disruptions in TGFβ signaling occur in the context of altered WNT/β-catenin signaling [12, 14]. Taken together, the differential gene expression patterns in OA bone are consistent with perturbed osteoblast differentiation, altered bone formation, and heterogenous mineralization. Given the mixture of bone-cell types within whole bone, it is necessary to further delineate the specific contributions of each cell type to TGFβ signaling in joint homeostasis to better characterize the bone-intrinsic role.
The evaluation of primary osteoblasts isolated from human OA specimens supports an osteoblast-intrinsic role of TGFβ in OA. Similar to findings in whole bone extracts, primary osteoblasts obtained from OA bone express increased levels of TGFB1 mRNA, TGFβ1 ligand, and SMAD3 mRNA [45–49]. OA osteoblasts exhibit a TGFβ-dependent shift from the normal ratio of COL1A1/COL1A2 mRNA of approximately 2:1 to levels 2–threefold higher than that in normal osteoblasts, which blunts the ability of osteoblasts to generate a mineralized ECM in vitro [15, 47]. When TGFβ1 is inhibited, OA osteoblasts correct the abnormal COL1A1/COL1A2 ratio at the mRNA level and mineralize normally [47]. In murine osteoblasts, SMAD3 represses the function of RUNX2 [50], a key regulator of osteoblast differentiation and mineralization. Increased SMAD3 in human OA osteoblasts is associated with the calcium:phosphorous ratio in bone mineral [45], likely through its regulation of RUNX2-dependent mineralization.
TGFβ signaling in OA osteoblasts exhibits an epistatic relationship with the Wnt signaling pathway, an important regulatory pathway in bone formation [45, 48, 49]. TGFβ antagonizes the Wnt signaling pathway in OA osteoblasts by increasing the mRNA expression of Dickkopf-2 (DKK2), which diminishes osteoblastic mineralization in response to BMP-2 [48]. Increased hepatocyte growth factor (HGF) mRNA and HGF protein in OA osteoblasts increase TGFB1 expression, which subsequently inhibits the Wnt signaling pathway and blunts BMP-2-dependent mineralization [49]. The loss of HGF, therefore, favors BMP-2 signaling through SMAD1/5/8 and stimulates the canonical Wnt signaling pathway [49].
Camurati-Engelmann disease (CED) is a disease of poor bone quality due to excessive levels of TGFβ1. A mouse model of CED, in which the Col1A1 promotor drives expression of Tgfb1 with the point mutation H222D, results in higher levels of active TGFβ1 secreted by osteoblasts [39]. Coupled with the disrupted subchondral bone, these mice develop thin articular cartilage with relative hypocellularity, thick calcified cartilage, and excessive subchondral bone vascularity [39]. The subchondral bone in these mice exhibits increased levels of nestin-positive MSCs and osterix-positive osteoprogenitors, suggesting that excessive TGFβ signaling alters the joint environment to increase recruitment of MSCs. This, in turn, increases the number of available osteoprogenitors [39]. This MSC-dependent mechanism illustrates how excessive TGFβ secreted by subchondral bone osteoblasts can exacerbate OA.
On the other hand, inadequate subchondral bone TGFβ signaling also has negative consequences for joint homeostasis. TGFβ signaling in osteocytes is essential for function of these cells, regulating their ability to maintain the lacunocanalicular network through osteocytic perilacunar/canalicular remodeling (PLR) [41, 42]. In addition to their role in maintaining bone quality, osteocytes have recently emerged as cellular contributors to OA [43•, 51•]. More specifically, osteocytic TGFβ signaling is required for the response of subchondral bone to joint injury [43•]. Without this key signaling pathway, subchondral bone homeostasis is disturbed, resulting in arthritic cartilage degeneration [43•].
As mediators of bone homeostasis, osteoclasts are inevitably involved in joint health. The altered mass and mineralization in OA subchondral bone suggest an imbalance in bone remodeling by osteoblasts and osteoclasts, for which TGFβ is a critical coupling factor [52]. Osteoclasts can play a direct or indirect role on the effect of TGFβ within the joint. Directly, osteoclasts require TGFβ for osteoclastogenesis. Osteoclasts also locally control the level of active TGFβ by liberating and activating latent TGFβ from the bone matrix [53–55]. In an ACLT model of OA, osteoclast suppression with alendronate reduces local TGFβ activation, with a corresponding reduction in matrix metalloproteinase 13 (MMP13) in articular cartilage and osteophyte formation [56]. Indirectly, osteoclasts are sensitive to aberrant TGFβ signaling within the joint because it can alter Rankl expression by osteocytes and interfere with osteoblast-osteoclast coupling during bone remodeling [41, 57]. Therefore, the activity of osteoclasts can impact the level of active TGFβ signaling, and vice versa, with broader impacts throughout the joint.
Cartilage-Specific Role of TGFβ in Joint Health and Disease
Human osteoarthritic cartilage shows a complex disruption of homeostatic TGFβ signaling at various levels involving ligand, receptor, effector, and negative regulators, underscoring the importance of studying the hierarchy of altered TGFβ signaling in OA. There are at least three ways that altered TGFβ signaling in human cartilage is implicated in OA. First, excessive activity of the pathway is observed in human OA cartilage: the expression of genes encoding TGFβ1 and β2 ligands is positively correlated with cartilage degeneration [58]. Likewise, SMAD3 mRNA levels are significantly higher in OA cartilage, in a manner that is unexplained by DNA methylation in the SMAD3 promoter region [16]. Second, there is a shift from canonical to non-canonical signaling: the mRNA expression of ALK1 in human OA cartilage is correlated with chondrocytic MMP13 mRNA expression, whereas ALK5 mRNA correlates with aggrecan and type II collagen mRNA expression. Since ALK1 preferentially activates SMAD1/5/8, and ALK5 preferentially activates SMAD2/3, this suggests a role for TGFβ receptor and effector selection in expression of genes that are protective or deleterious for cartilage [36]. Third, there is aberrant negative regulation of the signaling pathway: in human OA cartilage, higher levels of SMURF2, a multi-level negative regulator of TGFβ signaling, can be observed compared to healthy cartilage [59]. SMURF2 ubiquitinates SMADs and the TβRI receptor to promote degradation and reduce TGFβ signaling. Therefore, high levels of SMURF2 indicate a general reduction in TGFβ signaling.
In addition to direct regulation of the signaling pathway, TGFβ signaling can regulate, and be regulated by, microRNAs (miRNAs) to affect downstream signaling and OA gene expression. The expression of miR-140, for instance, is repressed by TGFβ signaling and significantly reduced in human OA cartilage [60–62], whereas miR-455, which promotes TGFβ/SMAD3 signaling, is also repressed in OA cartilage [63]. Overall, these observations demonstrate an association of cartilage degeneration with aberrant TGFβ signaling, whether by suppressed or excessive activity, a shift from canonical to non-canonical signaling, or altered negative regulation. While these associations in human cartilage from end-stage OA show clinical relevance and motivate further study of TGFβ signaling in chondrocytes, other approaches are needed to establish causal mechanisms that operate earlier in the disease process.
Findings in animal models reinforce the importance of an optimal level and type of TGFβ signaling in the joint. Increased TGFβ in the synovial fluid, whether by intra-articular administration of TGFβ or overexpression of TGFβ by the synovium, induces cartilage degeneration and osteophyte formation [64–66]. Elevated TGFβ signaling activates a positive feedback loop that further increases expression of Tgfb1, b2, and b3 mRNAs in the synovium and cartilage, relative to vehicle-injected controls [64–66]. Conversely, inhibition of endogenous TGFβ by systemic injection of scavenging soluble TβRII prevents osteophyte formation but, likewise, induces proteoglycan degeneration in cartilage [44]. At the chondrocyte-level, although the expression of both receptors declines with age, the number of cells positive for ALK5 in OA cartilage decreases more rapidly than those positive for ALK1. This consequently increases the ALK1/ALK5 ratio and shifts the function from ALK5-dependent collagen II and aggrecan expression and, instead, favoring SMAD1/5/8 signaling and MMP13 production [36]. In cartilage development at the growth plate, TβRI competitively antagonizes BMP signaling mediated by ALK1, thus shifting the balance of TGFβ signaling [67•]. Together, these results highlight the importance of an optimal quantity and quality of TGFβ signaling, since excessive or inhibited TGFβ activity, or a shift in the balance of canonical and non-canonical TGFβ signaling, can exacerbate cartilage degeneration.
Transgenic mouse models with disrupted TGFβ signaling in multiple tissues have likewise demonstrated the requirement of TGFβ signaling in cartilage health. Mice with a dominant-negative mutation of the gene encoding TβRII in the articular cartilage, synovium, periosteum, and perichondrium exhibit a severe OA phenotype with substantial cartilage loss and osteophyte formation [68]. This mutation suppresses the responsiveness to TGFβ ligand, thus promoting terminal differentiation of chondrocytes, reducing proteoglycan synthesis, and increasing type X collagen [68]. Mice with a global loss of canonical TGFβ effector SMAD3 similarly develop severe OA [69, 70]. Taken together, these two mouse models illustrate the chondroprotective role of TGFβ in synovial joint tissues, motivating mechanistic study of the tissue-specific effects of TGFβ in articular cartilage.
Because OA involves the coordinated disruption of multiple joint tissues, mouse models employing chondrocyte-intrinsic mutations in the TGFβ signaling pathway can diminish the apparently confounding effects of TGFβ signaling in joint crosstalk. Toggling this pathway experimentally by targeting the receptors, effectors, and negative regulators in chondrocyte-intrinsic models, for example, by driving transgenic expression of Cre recombinase or other genes using a Col2a1 promoter, isolates the relative chondroprotective nature of TGFβ. As mentioned previously, SMURF2 levels are elevated in human OA cartilage, disrupting homeostatic TGFβ signaling [59]. Mice with a chondrocyte-intrinsic overexpression of SMURF2 develop spontaneous OA with loss of articular cartilage, increased subchondral bone sclerosis, and increased mRNA expression of type X collagen and elevated levels of MMP13 protein in the articular cartilage [59], demonstrating the chondrocyte-intrinsic requirement of balanced TGFβ signaling. Likewise, mice with a chondrocyte-intrinsic loss of Smad3 develop OA and demonstrate decreased expression of genes encoding aggrecan and collagen II associated with increased MMP13 protein levels and enzyme activity in the articular cartilage, thus indicating that chondrocyte-intrinsic SMAD3 is essential for maintenance of healthy cartilage [71]. At the receptor level, chondrocyte-specific ablation of the gene encoding either TβRII [72] or ALK5 [73] promotes joint degeneration by inducing genes implicated in cartilage destruction. More specifically, the deletion of Tgfbr2 in chondrocytes exacerbates OA and induces Runx2, Mmp13, and Adamts5 gene expression in articular cartilage [72]. Interestingly, the deleterious effects of chondrocytic Tgfbr2 ablation were mediated by activities of proteinases encoded by Mmp13 and Adamts5, since deletion of either gene or treatment with an MMP13 inhibitor ameliorated the OA phenotype in this model [72]. Likewise, in chondrocyte culture, siRNA-mediated inhibition of TβRII upregulated Mmp13 and Col10a1 gene expression through a RUNX2-dependent mechanism [72]. Furthermore, chondrocyte-intrinsic deletion of Alk5 resulted in a spontaneous OA phenotype with articular cartilage degradation, synovial hyperplasia, osteophyte formation, and subchondral bone sclerosis, with reduced SMAD3 phosphorylation and increased Mmp13, Adamts5, and Col10a1 gene expression and elevated protein levels of MMP13 and ADAMTS5 [73].
Some of the effects of TGFβ signaling in cartilage are explained by its ability to control chondrocyte differentiation. Prior to chondrogenesis, MSCs require both ALK5-dependent SMAD2/3 activity and ALK1-dependent SMAD1/5/8 activity, in concert with SMAD4, to induce COL2AI gene expression and subsequently produce increased protein levels of collagen II [74–76]. As chondrogenesis proceeds, the function of SMAD1/5/8 shifts to promote terminal hypertrophic chondrocyte differentiation, such that it inhibits collagen II production and increases MMP13, collagen X, and alkaline phosphatase [74]. On the other hand, SMAD3 acts to expand the pool of chondrocytes by stimulating their differentiation and preventing their hypertrophy [77–79]. In MSCs, TGFβ increases the transcriptional activity of SOX9 and the gene expression of COL2A1 [78]. Through SMAD2/3-dependent signaling, TGFβ inhibits chondrocyte hypertrophy [77, 78] and decreases expression of the genes encoding collagen X and other terminal differentiation markers [79]. TGFβ tightly regulates its own signaling in chondrocytes, both by positive feedback that increases the expression of TGFβ receptors and SMAD3 [80] and by negative feedback that can reduce mRNA stability of TGFβ receptors, decrease SMAD3, and increase SMAD7 [80]. Decreased levels of TGFβ signaling shift chondrocytes from expression of chondroprotective genes such as SOX9 and COL2A1 to hypertrophic genes such as COL10A1 [80]. In addition to the availability of TGFβ ligand and the relative balance of SMAD2/3 signaling, the chondrogenic response to TGFβ depends on mechanical cues from the ECM [30], as mentioned previously.
Among the constituents of the TGFβ pathway that control chondrocyte homeostasis, the most well studied is SMAD3, motivated by the association of human OA and SMAD3 variants identified in GWAS [19–21]. In chondrocytes, SMAD3-dependent TGFβ signaling promotes cartilage health by repressing RUNX2-inducible Mmp13 and Col10 gene expression and tempering BMP signaling [71, 72, 81], each through mechanisms that diminish non-canonical TGFβ signaling. Chondrocytes exhibit a time-dependent response to exogenous TGFβ, with an early repression of Mmp13 mRNA and MMP13 protein requiring SMAD3 and a later induction of Mmp13 mRNA and MMP13 protein requiring p38 [71]. Importantly, in the absence of SMAD3, non-canonical TGFβ signaling through p38 is favored, inducing the cartilage-destructive enzyme MMP13 [71]. Primary chondrocytes from mice lacking SMAD3 show enhanced BMP-related gene expression by increasing Smad1, Smad5, BMP2, and BMP6 mRNA levels, resulting in increased phosphorylated SMAD1/5/8 and Col10 mRNA expression and accelerating chondrocyte maturation [81]. This aberrant signaling can be blocked by overexpressing Smad2 and Smad3 or inhibiting BMP signaling [81]. TGFβ likewise counteracts the effects of MMPs and protects the local cartilage ECM by inducing expression of tissue inhibitor of metalloproteinases-3 (TIMP-3) through SMAD2/3-dependent and ERK1/2-dependent mechanisms [82, 83], illustrating the multiple avenues by which SMAD3 functions to promote chondrocyte homeostasis.
TGFβ and Synovial Inflammation
As in cartilage, a balanced level of TGFβ signaling in the synovium and synovial fluid is required for healthy joint homeostasis, with insufficient or excessive TGFβ levels compromising joint health. The ability of TGFβ to induce a suppressive or inflammatory immune response within the synovium is context-dependent [84]. The synovium is a source of TGFβ ligand within the joint [32, 85], and the level of TGFβ in synovial fluid correlates positively with OA severity [17]. TGFβ is also a key mediator of synovial hyperplasia in rheumatoid arthritis [86]. Either excessive or suppressed TGFβ in the synovium or the synovial fluid can result in synovial hyperplasia. For instance, mice with reduced TGFβ signaling throughout the joint due to a dominant-negative mutation of the gene encoding TβRII in multiple joint tissues exhibit synovial hyperplasia [68]. Excessive levels of TGFβ within the joint, as a result of intra-articular injection of exogenous TGFβ, likewise promote synovial hyperplasia [64]. On the other hand, synovial inflammation with joint degeneration involves production of inflammatory cytokines, such as interleukin (IL)-1. TGFβ antagonizes the degenerative effects of IL-1 on cartilage [87, 88]. Together, TGFβ and the synovium can participate in bidirectional feedback, where synovial production of TGFβ can have broader effects on other joint tissues, and TGFβ signaling in other joint tissues can affect the synovium.
Mesenchymal Stem Cells and TGFβ in Joint Health and Disease
In addition to the role of subchondral bone osteoblasts, osteoclasts, and osteocytes in synovial joint homeostasis, there is evidence of an influx of bone marrow MSCs into the subchondral bone during OA progression, which induce angiogenesis and differentiate into osteoblasts to further perturb the subchondral bone environment [39]. Joint injury from ACL transection increases the level of nestin-positive MSCs in the subchondral bone marrow and osteoprogenitor clusters in the bone marrow [39, 89]. Systemic administration of a TβRI inhibitor can reduce the number of MSCs and osteoprogenitors, normalize the subchondral bone, and attenuate OA after injury [39]. The benefits of the TβRI inhibitor on joint health are tissue-dependent; higher concentrations of the TβRI inhibitor not only mitigate injury-induced changes in subchondral bone structure but also induce proteoglycan loss in cartilage, underscoring the tight control of TGFβ that is necessary for joint health [39]. The relative benefits of inhibiting TGFβ signaling within the subchondral compartment are mediated by nestin-positive MSCs. A nestin-positive MSC-specific ablation of the gene encoding TβRII recapitulates findings with the pharmacologic TβRI inhibitor, with protection of subchondral bone microarchitecture and cartilage proteoglycan levels after ACL transection [39].
With increased levels of TGFβ in circulation after injury, targeting the TGFβ signaling pathway at the level of the ligand, rather than receptor, could likewise normalize the aberrant environment after injury. Local treatment to the subchondral bone or systemic treatment with an inhibitor of TGFβ1, β2, and β3 reduces the number and mobilization of nestin-positive MSCs in the subchondral bone and diminishes angiogenesis after ACL transection [39, 89]. This effect is dose-dependent, such that lower and higher concentrations lead to proteoglycan loss [89]. As discussed below, when considering the benefits of inhibiting excess TGFβ signaling post-injury, care must be taken not to compromise the essential role of this growth factor in other joint cell types where it also supports joint homeostasis.
TGFβ in Aging and OA
Aging can have broad effects on TGFβ signaling in multiple joint tissues. Because the prevalence of OA increases with age, considering the shift in function of TGFβ with age could identify a distinct age-related pathophysiology of cartilage degeneration. Furthermore, studying spontaneous age-related OA allows for the evaluation of joint degeneration in the absence of the substantial mechanical and inflammatory changes that occur with joint injury in post-traumatic OA models.
Among the many roles of TGFβ in the joint is its interaction with IL-1, a pro-inflammatory cartilage destructive cytokine that is upregulated with age and following injury [90]. TGFβ and IL-1 interact, such that TGFβ can protect against the deleterious effects of IL-1 on proteoglycan synthesis [87, 88]. With age, however, IL-1-induced cartilage degeneration outpaces the protective effects of TGFβ [87, 88, 91], mediated in part by nitric oxide production [88]. While TGFβ blocks IL-1-induced nitric oxide production in young mice, TGFβ is unable to induce the same response in old mice, likely due to decreased expression of TGFβ receptors [88]. Interestingly, while TGFβ shows protective effects on cartilage synthesis in the presence of IL-1, it exacerbates the inflammatory response, generating a severe synovitis, underscoring the importance of delineating the tissue-specific effects of TGFβ [87]. A similar role for IL-1 and TGFβ crosstalk has been shown in equine cartilage, where TGFβ-induced proteoglycan synthesis is diminished by the addition of IL-1 [92].
Aging cartilage exhibits decreased levels of TGFβ1, β2, and β3 ligands, diminished TGFβ-induced proteoglycan synthesis, and reduced the number of cells positive for TβRI and TβRII protein [85, 91]. This age-related suppression of TGFβ ligands and receptors results in decreased phosphorylated SMAD2 without a reduction in overall SMAD2 expression, suggesting reduced active canonical TGFβ signaling [91]. Furthermore, a mouse model of spontaneous OA demonstrated lower levels of TGFβ3 ligand and phosphorylated SMAD2 over the course of OA progression with a complete loss by 1 year of age, coinciding with an increase in protein levels of BMP-2 with age [85].
Across species, articular cartilage exhibits a shift from canonical to non-canonical TGFβ signaling during aging [36, 37, 93]. In aging murine cartilage, increased ALK1/ALK5 ratio favors TGFβ-induced SMAD1/5/8 signaling and MMP13 production [36]. A similar increase in the ALK1/ALK5 ratio is observed in aged bovine cartilage [37], and aged chondrocytes from guinea pigs demonstrate a progressive shift of SMAD2/3 signaling to SMAD1/5/8 signaling [93], illustrating the age-related shift in TGFβ function across multiple species. Aged bovine cartilage demonstrates reduced activation of SMAD2/3 signaling and nuclear localization in response to either stimulation with TGFβ or mechanical activation [38]. These changes with age precede gross degeneration of cartilage and, therefore, may be early signs of OA [38]. Together, these findings emphasize the importance of the relative balance of canonical and non-canonical TGFβ signaling in cartilage health.
Interactions of TGFβ and Other Joint Tissues
Aging also impacts the TGFβ-induced collagen production in ligamental fibroblasts. With aging, the ability of medial collateral ligament (MCL)-derived fibroblasts to synthesize collagen in response to TGFβ is diminished relative to young controls [94]. While overall collagen synthesis decreases at all doses in MCL-derived fibroblasts from older rabbits, the sensitization to TGFβ is higher in aged animals, such that relative to controls lacking TGFβ, collagen synthesis increases with increasing doses of TGFβ [94]. These findings suggest that the diminished mechanical integrity and prolonged healing with injury of aged ligaments may be due to a weakened response to TGFβ. Coupled with the increased sensitization to TGFβ, treatment with TGFβ could, thus, improve the mechanical stability of the ligament.
One area of interest in the intersection of TGFβ and joint homeostasis is the extent of crosstalk with the nervous system. Nerve growth factor (NGF) is a key driver of the musculoskeletal pain response. Despite the lack of apparent sequence similarities, TGFβ and NGF demonstrate similarities in topological structure that place them in a common growth factor superfamily [5]. Anti-NGF therapies in OA reduce knee OA pain [95] but increase the incidence of rapidly progressive OA [96]. Therefore, understanding the extent to which TGFβ is involved in joint pain could provide further context for the complexity of targeting NGF.
Targeting TGFβ for Treatment of Joint Disease
Careful consideration must be taken when developing TGFβ-targeting treatments for OA due to the complex role of TGFβ in joint health and disease. Treatment strategies need exquisite control of multiple factors, including optimal dose, tissue-specific effects, selection of downstream TGFβ receptors and effectors, and local mechanical cues. For instance, injury can induce excessive levels of TGFβ ligand within the joint [39], suggesting that returning TGFβ to pre-injury levels would encourage joint health. However, injury also represses osteocytic TβRII in the subchondral bone, and reduced levels of TGFβ ligand may further reduce downstream osteocytic TGFβ signaling [43•]. Furthermore, in aging cartilage, the relative balance of canonical and non-canonical TGFβ signaling is disrupted [36, 37, 93], complicating the efforts of targeting this pathway by enhancing or inhibiting TGFβ.
Therapeutics have been developed that target different levels of the TGFβ signaling pathway in the clinical setting, primarily in the context of cancer or fibrotic disease [97, 98]. Recently, human chondrocytes virally transduced with a gene containing TGFβ1 have been employed in the setting of patients with OA in a phase II clinical trial. Patients with knee OA received an intra-articular injection of either placebo or transduced allogenic chondrocytes expressing TGFβ1, which significantly improved clinical pain scores [99, 100]. Further work is needed to fully uncover the relative benefit of delivering TGFβ1-producing chondrocytes, designed to be a cell-mediated cytokine gene therapy. Another critical consideration is the likelihood that OA originates through several distinct mechanisms, each of which may respond differently to changes in the level or type of TGFβ signaling. Overall, the complex nature of the TGFβ signaling pathway suggests that therapeutically regulating TGFβ would require a precise understanding of the underlying etiology of OA and the specific disruption in TGFβ to successfully use it as a therapeutic target of OA.
Conclusion
In conclusion, TGFβ signaling plays a sophisticated function in maintaining healthy joint crosstalk that is non-linear and depends upon effector selection, physical and mechanical cues, and tissue-specific function to support joint health. Synovial joints facilitate smooth motion and load transfer through integrated function of multiple tissues, including articular cartilage, bone, ligaments, tendons, synovium, and menisci. Under normal conditions, the biological and mechanical activities of these tissues are exquisitely coordinated, yet in the setting of aberrant TGFβ signaling, one or more of these joint tissues can deteriorate leading to overall joint destruction. Regulation of the TGFβ signaling pathway can occur at different levels, and disrupting the homeostatic TGFβ signaling at any level of the pathway can have broad effects through crosstalk among multiple joint tissues.
Acknowledgements
Illustration was kindly provided by M. Ouchida.
Funding
This work was supported by the National Institutes of Health (R01 DE019284), the Department of Defense (OR130191), and the National Science Foundation (1636331).
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/ experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards.
Footnotes
This article is part of the Topical Collection on Osteoarthritis
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;2016:632–44. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;2012:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGFbeta family: context dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;2016: 8(5), a021873. [DOI] [PMC free article] [PubMed]
- 4.Ayyaz A, Attisano L, Wrana JL. Recent advances in understanding contextual TGFbeta signaling. F1000Res. 2017;2017:749. doi: 10.12688/f1000research.11295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derynck R, Budi EH. Specificity, versatility, and control of TGFbeta family signaling. Sci Signal. 2019;2019: 12(570), eaav5183. [DOI] [PMC free article] [PubMed]
- 6.Wrana JL. Signaling by the TGFbeta superfamily. Cold Spring Harb Perspect Biol. 2013;2013:a011197. doi: 10.1101/cshperspect.a011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazawa K, Miyazono K. Regulation of TGF-beta family signaling by inhibitory smads. Cold Spring Harb Perspect Biol. 2016;2017: 9(3), a022095. [DOI] [PMC free article] [PubMed]
- 8.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res (Hoboken) 2015;2016:574–80. doi: 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;2012:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 10.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;2011:121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 11.van de Laar IM, van der Linde D, Oei EH, Bos PK, Bessems JH, Bierma-Zeinstra SM, et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet. 2011;2012:47–57. doi: 10.1136/jmedgenet-2011-100382. [DOI] [PubMed] [Google Scholar]
- 12.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res Ther. 2007;2007:R100. doi: 10.1186/ar2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dequeker J, Mohan S, Finkelman RD, Aerssens J, Baylink DJ. Generalized osteoarthritis associated with increased insulin-like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum. 1993;1993:1702–8. doi: 10.1002/art.1780361209. [DOI] [PubMed] [Google Scholar]
- 14.Kumarasinghe DD, Perilli E, Tsangari H, Truong L, Kuliwaba JS, Hopwood B, et al. Critical molecular regulators, histomorphometric indices and their correlations in the trabecular bone in primary hip osteoarthritis. Osteoarthritis Cartilage. 2010;2010:1337–44. doi: 10.1016/j.joca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Truong LH, Kuliwaba JS, Tsangari H, Fazzalari NL. Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res Ther. 2006;2006:R188. doi: 10.1186/ar2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aref-Eshghi E, Liu M, Razavi-Lopez SB, Hirasawa K, Harper PE, Martin G, et al. SMAD3 is upregulated in human osteoarthritic cartilage independent of the promoter DNA methylation. J Rheumatol. 2015;2016:388–94. doi: 10.3899/jrheum.150609. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh MF, Zhang X, Wellman SS, Bolognesi MP, Kraus VB. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. 2020;2020. [DOI] [PMC free article] [PubMed]
- 18.Yao JY, Wang Y, An J, Mao CM, Hou N, Lv YX, et al. Mutation analysis of the Smad3 gene in human osteoarthritis. Eur J Hum Genet. 2003;2003:714–7. doi: 10.1038/sj.ejhg.5201034. [DOI] [PubMed] [Google Scholar]
- 19.Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, et al. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. 2010;2010:2347–52. doi: 10.1002/art.27530. [DOI] [PubMed] [Google Scholar]
- 20.Hackinger S, Trajanoska K, Styrkarsdottir U, Zengini E, Steinberg J, Ritchie GRS, et al. Evaluation of shared genetic aetiology between osteoarthritis and bone mineral density identifies SMAD3 as a novel osteoarthritis risk locus. Hum Mol Genet. 2017;2017:3850–8. doi: 10.1093/hmg/ddx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Shu J, Han Y, Ren XY, Xu K, Fan H, et al. The polymorphism of SMAD3 rs1065080 is associated with increased risk for knee osteoarthritis. Mol Biol Rep. 2019;2019:4501–5. doi: 10.1007/s11033-019-04905-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Zhao J, Ma C, Wei J, Wei B, Liu J. Common variants in LTBP3 gene contributed to the risk of hip osteoarthritis in Han Chinese population. Biosci Rep. 2020; 2020: 40(6), BSR20192999. [DOI] [PMC free article] [PubMed]
- 23.Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. 2018;2018:549–58. doi: 10.1038/s41588-018-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;2019:230–6. doi: 10.1038/s41588-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rys JP, Monteiro DA, Alliston T. Mechanobiology of TGFbeta signaling in the skeleton. Matrix Biol. 2016;2016:413–25. doi: 10.1016/j.matbio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;2007:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rys JP, DuFort CC, Monteiro DA, Baird MA, Oses-Prieto JA, Chand S, et al. Discrete spatial organization of TGFbeta receptors couples receptor multimerization and signaling to cellular tension. Elife. 2015;2015:e09300. doi: 10.7554/eLife.09300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro DA, Dole NS, Campos JL, Kaya S, Schurman CA, Belair CD, et al. Fluid shear stress generates a unique signaling response by activating multiple TGFbeta family type I receptors in osteocytes. FASEB J. 2021;2021:e21263. doi: 10.1096/fj.202001998R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haudenschild DR, Chen J, Pang N, Lotz MK, D'Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2009;2010:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012;2012:3731–42. doi: 10.1091/mbc.e12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Alliston T, Hung CT, et al. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophys J. 2013;2013:1794–804. doi: 10.1016/j.bpj.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, et al. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis Cartilage. 2012;2012:1374–82. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Shim JJ, Hung CT, et al. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-beta. J Biomech. 2013;2013:1433–9. doi: 10.1016/j.jbiomech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madej W, van Caam A, Blaney Davidson EN, van der Kraan PM, Buma P. Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling. Osteoarthritis Cartilage. 2014;2014:1018–25. doi: 10.1016/j.joca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Madej W, van Caam A, Blaney Davidson E, Buma P, van der Kraan PM. Unloading results in rapid loss of TGFbeta signaling in articular cartilage: role of loading-induced TGFbeta signaling in maintenance of articular chondrocyte phenotype? Osteoarthritis Cartilage. 2016;2016:1807–15. doi: 10.1016/j.joca.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;2009:7937–45. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 37.van Caam A, Madej W, Thijssen E, Garcia de Vinuesa A, van den Berg W, Goumans MJ, et al. Expression of TGFbeta-family signalling components in ageing cartilage: age-related loss of TGFbeta and BMP receptors. Osteoarthritis Cartilage. 2016;2016:1235–45. doi: 10.1016/j.joca.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Madej W, van Caam A, Davidson EN, Hannink G, Buma P, van der Kraan PM. Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage. Osteoarthritis Cartilage. 2015;2016:146–57. doi: 10.1016/j.joca.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;2013:704–12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;2011:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;2017:2585–96. doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dole NS, Yee CS, Mazur CM, Acevedo C, Alliston T. TGFbeta Regulation of Perilacunar/Canalicular remodeling is sexually dimorphic. J Bone Miner Res. 2020;2020:1549–61. doi: 10.1002/jbmr.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.• Bailey KN, Nguyen J, Yee CS, Dole NS, Dang A. Alliston T. Mechanosensitive control of articular cartilage and subchondral bone homeostasis requires osteocytic TGFbeta signaling. Arthritis Rheumatol. 2020;2021: 73(3), 414-425. This study demonstrates that osteocytic TGFβ signaling is required for articular cartilage health and the mechanosensitive response to joint injury. [DOI] [PMC free article] [PubMed]
- 44.Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;2002:507–14. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 45.Kumarasinghe DD, Sullivan T, Kuliwaba JS, Fazzalari NL, Atkins GJ. Evidence for the dysregulated expression of TWIST1, TGFbeta1 and SMAD3 in differentiating osteoblasts from primary hip osteoarthritis patients. Osteoarthritis Cartilage. 2012;2012:1357–66. doi: 10.1016/j.joca.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, et al. Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002;2002:491–500. doi: 10.1053/joca.2002.0528. [DOI] [PubMed] [Google Scholar]
- 47.Couchourel D, Aubry I, Delalandre A, Lavigne M, Martel-Pelletier J, Pelletier JP, et al. Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheum. 2009;2009:1438–50. doi: 10.1002/art.24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan TF, Couchourel D, Abed E, Delalandre A, Duval N, Lajeunesse D. Elevated Dickkopf-2 levels contribute to the abnormal phenotype of human osteoarthritic osteoblasts. J Bone Miner Res. 2011;2011:1399–410. doi: 10.1002/jbmr.358. [DOI] [PubMed] [Google Scholar]
- 49.Abed E, Bouvard B, Martineau X, Jouzeau JY, Reboul P, Lajeunesse D. Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein-2 and reduced mineralization capacity. Bone. 2015;2015:111–9. doi: 10.1016/j.bone.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;2005:2543–55. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.• Mazur CM, Woo JJ, Yee CS, Fields AJ, Acevedo C, Bailey KN, et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019;2019:34 This study demonstrates a causal role for osteocytes in osteoarthritis. [DOI] [PMC free article] [PubMed]
- 52.Tang SY, Alliston T. Regulation of postnatal bone homeostasis by TGFbeta. Bonekey Rep. 2014;2013:255. doi: 10.1038/bonekey.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneda T, Nojima T, Nakagawa M, Ogasawara A, Kaneko H, Sato T, et al. Endogenous production of TGF-beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF-kappa B ligand and macrophage-colony-stimulating factor. J Immunol. 2000;2000:4254–63. doi: 10.4049/jimmunol.165.8.4254. [DOI] [PubMed] [Google Scholar]
- 54.Oursler MJ. Osteoclast synthesis and secretion and activation of latent transforming growth factor beta. J Bone Miner Res. 1994;1994:443–52. doi: 10.1002/jbmr.5650090402. [DOI] [PubMed] [Google Scholar]
- 55.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGFbeta(1) in osteoclast differentiation and survival. J Cell Sci. 2000;2000:2445–53. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 56.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;2004:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 57.Weivoda MM, Ruan M, Pederson L, Hachfeld C, Davey RA, Zajac JD, et al. Osteoclast TGF-beta receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation. J Bone Miner Res. 2015;2016:76–85. doi: 10.1002/jbmr.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pombo-Suarez M, Castano-Oreja MT, Calaza M, Gomez-Reino J, Gonzalez A. Differential upregulation of the three transforming growth factor beta isoforms in human osteoarthritic cartilage. Ann Rheum Dis. 2008;2009:568–71. doi: 10.1136/ard.2008.090217. [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Kim KO, Sampson ER, Chen D, Awad H, O'Brien T, et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008;2008:3132–44. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tardif G, Pelletier JP, Fahmi H, Hum D, Zhang Y, Kapoor M, et al. NFAT3 and TGF-beta/SMAD3 regulate the expression of miR-140 in osteoarthritis. Arthritis Res Ther. 2013;2013:R197. doi: 10.1186/ar4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R, Inoue A, et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;2009:2723–30. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tardif G, Hum D, Pelletier JP, Duval N, Martel-Pelletier J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2009;2009:148. doi: 10.1186/1471-2474-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu S, Zhao X, Mao G, Zhang Z, Wen X, Zhang C, et al. MicroRNA-455–3p promotes TGF-beta signaling and inhibits osteoarthritis development by directly targeting PAK2. Exp Mol Med. 2019;2019:1–13. doi: 10.1038/s12276-019-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 1999;2000:25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 65.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, et al. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;2001:128–36. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 66.Itayem R, Mengarelli-Widholm S, Reinholt FP. The long-term effect of a short course of transforming growth factor-beta1 on rat articular cartilage. APMIS. 1999;1999:183–92. doi: 10.1111/j.1699-0463.1999.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Chun H, Baek J, Sadik JE, Shirazyan A, Razavi P, et al. The TGFbeta type I receptor TGFbetaRI functions as an inhibitor of BMP signaling in cartilage. Proc Natl Acad Sci U S A. 2019;2019:15570–9. doi: 10.1073/pnas.1902927116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;1997:541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;2001:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li TF, Gao L, Sheu TJ, Sampson ER, Flick LM, Konttinen YT, et al. Aberrant hypertrophy in Smad3-deficient murine chondrocytes is rescued by restoring transforming growth factor beta-activated kinase 1/activating transcription factor 2 signaling: a potential clinical implication for osteoarthritis. Arthritis Rheum. 2010;2010:2359–69. doi: 10.1002/art.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CG, Thuillier D, Chin EN, Alliston T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;2012:3278–89. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, et al. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;2013:3107–19. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q, Tan QY, Xu W, Qi HB, Chen D, Zhou S, et al. Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthritis Cartilage. 2017;2017:1868–79. doi: 10.1016/j.joca.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellingman CA, Davidson EN, Koevoet W, Vitters EL, van den Berg WB, van Osch GJ, et al. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng A. 2010;2011:1157–67. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 75.de Kroon LM, Narcisi R, Blaney Davidson EN, Cleary MA, van Beuningen HM, Koevoet WJ, et al. Activin receptor-like kinase receptors ALK5 and ALK1 are both required for TGFbeta-induced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. PloS One. 2016;2015:e0146124. doi: 10.1371/journal.pone.0146124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Kroon LM, Narcisi R, van den Akker GG, Vitters EL, Blaney Davidson EN, van Osch GJ, et al. SMAD3 and SMAD4 have a more dominant role than SMAD2 in TGFbeta-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Sci Rep. 2017;2017:43164. doi: 10.1038/srep43164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O'Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;2000:4728–35. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 78.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2004;2005:8343–50. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 79.Ionescu AM, Schwarz EM, Zuscik MJ, Drissi H, Puzas JE, Rosier RN, et al. ATF-2 cooperates with Smad3 to mediate TGF-beta effects on chondrocyte maturation. Exp Cell Res. 2003;2003:198–207. doi: 10.1016/S0014-4827(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 80.Bauge C, Cauvard O, Leclercq S, Galera P, Boumediene K. Modulation of transforming growth factor beta signalling pathway genes by transforming growth factor beta in human osteoarthritic chondrocytes: involvement of Sp1 in both early and late response cells to transforming growth factor beta. Arthritis Res Ther. 2011;2011:R23. doi: 10.1186/ar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2005;2006:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta. 2008;2008:1605–12. doi: 10.1016/j.bbamcr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Zhu Y, Tao H, Jin C, Liu Y, Lu X, et al. Interaction of ERK1/2 and Smad2/3 signaling pathways in TGF-beta1-induced TIMP-3 expression in rat chondrocytes. Arch Biochem Biophys. 2014;2014:229–36. doi: 10.1016/j.abb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;2017:9(6), a022236. [DOI] [PMC free article] [PubMed]
- 85.Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;2006:1414–21. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fennen M, Pap T, Dankbar B. Smad-dependent mechanisms of inflammatory bone destruction. Arthritis Res Ther. 2016;2016:279. doi: 10.1186/s13075-016-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994;1994:593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;2002:1095–8. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie L, Tintani F, Wang X, Li F, Zhen G, Qiu T, et al. Systemic neutralization of TGF-beta attenuates osteoarthritis. Ann N Y Acad Sci. 2016;2016:53–64. doi: 10.1111/nyas.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010;2011:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 91.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;2005:R1338–47. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun. 2000;2000:467–71. doi: 10.1006/bbrc.2000.3167. [DOI] [PubMed] [Google Scholar]
- 93.Zhao W, Wang T, Luo Q, Chen Y, Leung VY, Wen C, et al. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-beta signaling. J Orthop Res. 2015;2016:763–70. doi: 10.1002/jor.23079. [DOI] [PubMed] [Google Scholar]
- 94.Deie M, Marui T, Allen CR, Hildebrand KA, Georgescu HI, Niyibizi C, et al. The effects of age on rabbit MCL fibroblast matrix synthesis in response to TGF-beta 1 or EGF. Mech Ageing Dev. 1997;1997:121–30. doi: 10.1016/S0047-6374(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 95.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;2010:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karsdal MA, Verburg KM, West CR, Bay-Jensen AC, Keller DS, Arends R. Serological biomarker profiles of rapidly progressive osteoarthritis in tanezumab-treated patients. Osteoarthritis Cartilage. 2018;2019:484–92. doi: 10.1016/j.joca.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Colak S, Ten Dijke P. Targeting TGF-beta signaling in cancer. Trends Cancer. 2017;2017:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;2016:325–38. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 99.Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage. 2015;2015:2109–18. doi: 10.1016/j.joca.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 100.Lee B, Parvizi J, Bramlet D, Romness DW, Guermazi A, Noh M, et al. Results of a phase II study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1. J Knee Surg. 2019;2020:167–72. doi: 10.1055/s-0038-1676803. [DOI] [PubMed] [Google Scholar]