Abstract
Medicinal plants are an important source of therapeutic compounds used in the treatment of many diseases since ancient times. Interestingly, they form associations with numerous microorganisms developing as endophytes or symbionts in different parts of the plants. Within the soil, arbuscular mycorrhizal fungi (AMF) are the most prevalent symbiotic microorganisms forming associations with more than 70% of vascular plants. In the last decade, a number of studies have reported the positive effects of AMF on improving the production and accumulation of important active compounds in medicinal plants.
In this work, we reviewed the literature on the effects of AMF on the production of secondary metabolites in medicinal plants. The major findings are as follows: AMF impact the production of secondary metabolites either directly by increasing plant biomass or indirectly by stimulating secondary metabolite biosynthetic pathways. The magnitude of the impact differs depending on the plant genotype, the AMF strain, and the environmental context (e.g., light, time of harvesting). Different methods of cultivation are used for the production of secondary metabolites by medicinal plants (e.g., greenhouse, aeroponics, hydroponics, in vitro and hairy root cultures) which also are compatible with AMF. In conclusion, the inoculation of medicinal plants with AMF is a real avenue for increasing the quantity and quality of secondary metabolites of pharmacological, medical, and cosmetic interest.
Keywords: Arbuscular mycorrhiza fungi, Medicinal plants, Secondary metabolites, Hydroponics, Aeroponics, Hairy root cultures
Introduction
Medicinal plants have been a valuable source of therapeutic agents to treat various ailments and diseases such as diarrhea, fever, colds, and malaria since ancient times (Dambisya and Tindimwebwa 2003; Ghiaee et al. 2014; Mathens and Bellanger 2010; Titanji et al. 2008). Nowadays, they also represent a source for the development of new drugs to cure important diseases such as cancer (Newman and Cragg 2007; Beik et al. 2020). Their therapeutic value often is attributed to the presence and richness of active compounds belonging to the secondary metabolism, such as alkaloids, flavonoids, terpenoids, and phenolics (Hussein and El-Anssary 2018). Today, up to 80% of people in developing countries are totally dependent on herbal drugs for their primary healthcare (Ekor 2014), and over 25% of prescribed medicines in developed countries have been derived from plants collected in the wild (Hamilton 2004).
Numerous methods, such as isolation from plants and other natural sources, synthetic chemistry, combinatorial chemistry, and molecular modeling, have been used for drug discovery (Ley and Baxendale 2002; Geysen et al. 2003; Lombardino and Lowe 2004). However, natural products, and particularly medicinal plants, remain an important source of new drugs, new drug leads, and new chemical entities (Newman et al. 2000, 2003; Butler 2004) because of their cultural acceptability, high compatibility, and adaptability with the human body compared to synthetic chemicals (Garg et al. 2021). According to the International Union for Conservation of Nature and the World Wildlife Fund (Chen et al. 2016), an estimate of as many as 80,000 flowering plant species are used for medicinal purposes. For several thousands of plants worldwide, the activity or composition in bioactive compounds remains poorly documented, requiring further in-depth analysis to fully exploit their medicinal potential (Ali 2019).
In nature, plants are associated with an overwhelming number of beneficial microorganisms (e.g., endophytic or symbiotic bacteria and fungi) that play a significant role in plant health, development, and productivity, and in the modulation of metabolite synthesis (Berendsen et al. 2012; Panke-Buisse et al. 2015; Mendes et al. 2011; Castrillo et al. 2017; de Vries et al. 2020; Brader et al. 2014; Compant et al. 2021). Among these are the arbuscular mycorrhizal fungi (AMF), a ubiquitous group of soil microorganisms, forming symbiosis with more than 70% of vascular plants (Brundrett and Tedersoo 2018). Arbuscular mycorrhizas are characterized by the formation of finely branched structures called arbuscules within root cortical cells of host plants (Coleman et al. 2004), which are the site of bidirectional transport, i.e., minerals from the fungal cell to the plant cell and carbon compounds in the opposite direction.
The establishment of the AMF symbiosis requires recognition between the two partners. Lipochitooligosaccharides, the so-called Myc factors, are perceived by the plant in response to signaling molecules (i.e., strigolactones) released by the roots (Akiyama and Hayashi 2006). After reciprocal recognition, AMF hyphae form a hyphopodium on the root epidermis and colonize the root cortex. At the same time, fungal hyphae spread into the surrounding soil as an extensive extraradical mycelium, representing 9 to 55% of the total soil microbial biomass (Olsson et al. 1999). This dense extraradical mycelium considerably enhances the access of roots to water and mineral nutrients (e.g., P, N, K, Ca, S, Zn, Cu), often increasing plant biomass (Smith and Read 2008; Bowles et al. 2016) and quality of crops (Baum et al. 2015; Bona et al. 2016; Noceto et al. 2021). Moreover, this extraradical mycelium modifies the soil structure (Chen et al. 2018), which improves soil quality and fertility (Zou et al. 2016; Thirkell et al. 2017). AMF also are well known to improve plant resistance or tolerance to stress conditions, such as drought, salinity, nutrient deprivation, extreme temperatures, heavy metals, pests, and diseases (Ahanger et al. 2014; Salam et al. 2017; Porcel et al. 2012; Cicatelli et al. 2014). In addition to these benefits, they also quantitatively and qualitatively could affect the production of secondary metabolites produced by their hosts (Ahanger et al. 2014; Salam et al. 2017; Porcel et al. 2012; Cicatelli et al. 2014; Kaur and Suseela 2020).
Taber and Trappe (1982) were the first to document the presence of AMF in a medicinal plant (in their study conducted on ginger growing in the Fiji Islands and Hawaii). Since then, most medicinal plants were found capable of associating with mycorrhizal fungi (Chen et al. 2014). Recently, single or combinations of AMF have been inoculated to various medicinal plants to investigate their impact on plant biomass as well as on phytochemical constituents in seeds, fruits, leaves, shoots, and roots (e.g., Rydlová et al. 2016; Kapoor et al. 2004; Selvaraj et al. 2009; Dave et al. 2011; Zubek et al. 2012). The majority of studies revealed that AMF were able to enhance plant biomass as well as to promote the accumulation of several active compounds. For example, Lazzara et al. (2017) reported an increased above- and belowground biomass in Hypericum perforatum associated with a mixture of nine different AMF species. Interestingly, the concentrations of pseudohypericin and hypericin, two anthraquinone derivatives that exhibit important photodynamic, antiviral, antiretroviral, antibacterial, antipsoriatic, antidepressant, and antitumoral biological activities (Zubek et al. 2012; Bombardelli and Morazzoni 1995; Gadzovska et al. 2005; Guedes and Eriksson 2005), were increased by 166.8 and 279.2% in the AMF-colonized plants as compared to non-mycorrhizal controls (Lazzara et al. 2017). However, these results should not obviate other studies in which no effects on biomass were reported. For instance, Nell et al. (2010) found that AMF colonization decreased the biomass of rhizomes and roots of Valeriana officinalis, while significantly increasing the levels of sesquiterpenic acids. Another study by Engel et al. (2016) reported an increased content of rosmarinic acid and lithospermic acid A isomer (two phenolic compounds) in Melissa officinalis, while both compounds were diminished in Majorana hortensis, in the presence of three mixtures of AMF. More recently, Duc et al. (2021) showed that a mixture of different AMF species improved the salt stress tolerance of Eclipta prostrata, inducing major changes in polyphenol profile.
In this publication, we provide a thorough review of the literature on AMF mediation of secondary metabolites production in medicinal plants. We also review the different methods that are used to increase/stabilize the production of secondary metabolites. Indeed, the quantity and quality of secondary metabolites obtained from plants grown in natural habitats are critically influenced by various abiotic and biotic stresses (e.g., drought, extreme temperatures, and pathogen attack). This results in high variability of bioactive substances and influences the metabolic pathways responsible for the accumulation of the related natural compounds (Dayani and Sabzalian 2017; Giurgiu et al. 2017; Ramakrishna and Ravishankar 2011). Therefore, we additionally review the most widely used methods of cultivation (i.e., greenhouse, hydroponics, aeroponics, in vitro and hairy root cultures (HRCs)) of medicinal plants, and we investigate their possible application to AMF to further increase the quantity and quality of secondary metabolites produced.
Effect of AMF on growth and secondary metabolite production of medicinal plants
Since the pioneer work of Wei and Wang (1989, 1991), reporting the positive effect of AMF inoculation of Datura stramonium and Schizonepeta tenuifolia on the production of active compounds, numerous studies have been conducted. The literature focusing on AMF in medicinal plants involves 81 plant species belonging to 28 families (Table 1). These medicinal plants present different characteristics to be studied with AMF: important medicinal herbs to treat certain disease such as Artemisia annua producing artemisin to treat malaria in developing countries (Domokos et al. 2018); important condiment plants such as Allium sativum in India (Borde et al. 2009); rare plant species difficult to culture such as Arnica montana (Jurkiewicz et al. 2011); aromatic plants to produce essential oil widely used in the pharmaceutical, cosmetic, and food industries such as most plant species from the Apiaceae (Anethum graveolens and Coriandrum sativum (Rydlová et al. 2016)) and the Lamiaceae (Origanum vulgare (Karagiannidis et al. 2012)); and health foods such as Dioscorea spp. yam (Lu et al. 2015). For the majority of these plants, studies were focused on the effects of AMF on biomass increase and production of bioactive compounds simultaneously (45 studies) or only focused on bioactive compound production (30 studies). For a few other studies, the attention was focused on AMF community composition (four studies) or on the effects of AMF on plant growth under different conditions (15 studies) (Table 1). Among the AMF species tested, Funneliformis mosseae1 is the most investigated one (25 studies), followed by Rhizophagus intraradices (16), Claroideoglomus etunicatum (14), Rhizophagus fasciculatus (14), Rhizophagus irregularis and Rhizophagus clarus (six studies each), and Gigaspora margarita (five studies) (Table 1). Only a few medicinal plant species were inoculated with AMF present in the soil native to those plants, while the vast majority were inoculated with commercial inoculants.
Table 1.
Detailed summary of studies on the relationship between AMF and medicinal plants
| Plant family | Plant species | AMFa | Secondary metabolites group and active ingredients | Medicinal value of the plant | Plant organ studied | Change in secondary metabolite production | Change in plant growth/biomass | Reference |
|---|---|---|---|---|---|---|---|---|
| Alliaceae | Allium sativum | Rhizophagus fasciculatus | Alliin | Antibacterial, antifungal, antiviral, antiprotozoal, antioxidative, and anticancerogenic properties; against arteriosclerosis and high blood pressure | Leaves, flowers, and cloves | Significant increase | Increase in plant height, total biomass and bulb diameters, bulb weight, and yield | Borde et al. (2009) |
| Amaranthaceae | Achyranthes aspera | Rhizophagus fasciculatus | Flavonoids | Treatment of cough, bronchitis, rheumatism, malarial fever, dysentery, asthma, hypertension and diabetes (Bhosale et al. 2012) | ______b | Increased the contents of active principles | Positive effect on plant growth parameters | Tejavathi and Jayashree (2011) |
| Anacardiaceae |
Myracrodruon urundeuva |
Acaulospora longula | Total phenols and flavonoids |
Anti-inflammatory, anti-ulcer, astringent, anti-allergic, and antidiarrheal activities (Teixeira et al. 2020) |
Leaves | 81.03% increased | Promote plant growth | Oliveira et al. (2013) |
| Apiaceae |
Angelica dahurica |
Glomus spp. | Imperatorin and total coumarins |
Treatment for colds, headache, dizziness, toothache, supraorbital pain, nasal congestion, acne, ulcer, carbuncle, and rheumatism (Lee et al. 2015) |
Root, seed, and fruit | Significant increase | Plant growth and biomass promoted | Zhao and He (2011) |
| Angelica archangelica | Funneliformis mosseae | Monoterpenoid and coumarin | Dyspeptic complaints such as mild gastrointestinal spasms, sluggish digestion, flatulence and feeling of fullness, loss of appetite, anorexia and bronchitis | Rhizome and roots | A marked increase in yield | Biomass increased | Zitterl-Eglseer et al. (2015) | |
| Anethum graveolens | Glomus macrocarpum, Rhizophagus fasciculatus | Anethole |
Treatment for abdominal discomfort and colic and also for promoting digestion (Jana and Shekhawat 2010) |
Seed |
90% increased |
Improved the growth | Kapoor et al. (2002) | |
| Bupleuruin scorzonerifolium | ______ | Flavonoids | Treating cold, fever, chest pain, irregular menstruation, uterine fall off and rectocele | ______ | ______ | ______ | Teng and He (2005) | |
|
Coriandrum sativum |
Glomus hoi | β-Caryophyllene, p-cymene, geraniol |
Antioxidant, antidiabetic, hepatoprotective, antibacterial, and antifungal activities (Asgarpanah and Kazemivash 2012) |
Seeds or leaves | Significant improvement | ______ | Rydlová et al. (2016) | |
| Foeniculum vulgare |
Glomus macrocarpum and Rhizophagus fasciculatus |
Essential oil concentration |
Used for digestive, endocrine, reproductive, and respiratory systems (Badgujar et al. 2014) |
Seeds | Significantly increased | Improved plant growth | Kapoor et al. (2004) | |
| Trachyspermum ammi | Rhizophagus fasciculatus | Thymol |
Antifungal, antioxidant, antimicrobial properties and used for antinociceptive, hypolipidemic, antihypertensive, antispasmodic, broncho-dilating actions, antilithiasis, and diuretic (Bairwa et al. 2012) |
Fruits | 72% increased | ______ | Kapoor et al. (2002) | |
| Apocynaceae | Catharanthus roseus | Glomus spp. | Vinblastine and vincristine, rutin, quercetin, and kaempferol |
Treatment of diuretic, hemorrhagic, wound healing, coughs, sore throats, lung infections, and diabetes (Gupta et al. 2017) |
Aerial part | Significant increase | ______ | Andrade et al. (2013) |
| Gymnema sylvestre |
Rhizophagus fascuculatus and Funneliformis mosseae |
Gymnemic acid | Control diabetes mellitus | Shoots and leaves | Positive increased | Higher shoot and root length and fresh and dry weight | Zimare et al. (2013) | |
| Araliaceae | Panax ginseng | ______ | ______ |
Reinforcing vital energy and restoring physiological weakness and possess antioxidation, anti-inflammatory, antiallergic, antidiabetic, and anticancer properties (Kim et al. 2018) |
______ | ______ | Plant seedlings biomass Significantly increased | Cho et al. (2009) |
| Rhizophagus intraradices | Ginsenosides | Roots | Increased total content | ______ | Tian et al. (2019) | |||
| Panax notoginseng | ______ | ______ |
Used to staunch bleeding, and invigorating and supplementing blood (Yang et al. 2014) |
______ | ______ | Only AMF community study from plant | Ren et al. (2007) | |
| Araceae | Pinellia ternate |
Rhizophagus intraradices, Funneliformis mosseae |
l-Ephedrine and guanosine | Treating cough and vomiting | Tubers | Significant increase | Increasing fresh weight and dry weight | Guo et al. (2010) |
| Acorus calamus |
Funneliformis mosseae and Acaulospora laevis |
______ | Anti-spasmodic and anti-anthelmintic properties and also used for treatment of epilepsy, mental ailments, chronic diarrhea, dysentery, bronchial catarrh, intermittent fevers, and tumors | ______ | ______ | Significant increase in plant height, plant spread, number of leaves per plant, and leaf area | Yadav et al. (2011) | |
| Asteraceae | Atractylodes macrocephala | Funneliformis mosseae | Atractylol |
Strengthening the spleen, benefiting vital energy, eliminating dampness, hidroschesis, and soothing fetuses (Gu et al. 2019) |
Rhizome | Significant increase | ______ | Lu and He (2005) |
| Atractylodes lancea | Funneliformis mosseae | ______ |
Used to treat rheumatic diseases, digestive disorders, night blindness, and influenza and also exert anti-cancer, anti-obesity, and anti-inflammatory effects (Jun et al. 2018) |
______ | No effect on essential oil contents | Improved plant growth | Guo et al. (2006) | |
| Claroideoglomus etunicatum, Glomus tortuosum, and Funneliformis mosseae | Essential oils, hinesol, β-eudesmol, and atractylodin | ______ | Increased | Increased the survival rate of seedlings, plant height, root length, and leaf number significantly increased | Liang et al. (2018) | |||
| Artemisia annua | Rhizophagus irregularis | Artemisinin content | Treat fever, inflammation, malaria, cough, stomach and intestinal upset | Leaves | 17% increased | Significant increase in fresh and dry plant biomass | Domokos et al. (2018) | |
| Arnica montana | Funneliformis geosporum, Funneliformis constrictum | Sesquiterpene lactones | Stimulate blood flow, promote healing, and soothe arthritic pains | Fresh or dried flower | Significant increase | ______ |
Jurkiewicz et al. (2011) |
|
| several Glomus strains | Phenolic acids | Roots | Increased concentration | ______ |
Jurkiewicz et al. (2011) |
|||
| Artemisia umbelliformis | Planticonsortium tenue, Rhizophagus intraradices, Claroideoglomus claroideum/etunicatum, and a new Acaulospora species |
Essential oil E-β-ocimene |
Against coughs | Shoots | Significantly increased | Increase of P concentration in shoots | Binet et al. (2011) | |
| Baccharis trimera | Rhizophagus clarus | Phenolics |
Antioxidant, anti-microbial, anti-fungal, anti-parasitic and anti-inflammatory properties, and used for gastric and hepatic-protector (Rabelo and Costa 2018) |
______ | Marked increases | Dry weight of the aerial part and height of plants increased | Freitas et al. (2004) | |
| Cynara cardunculus | Rhizophagus intraradices and Funneliformis mosseae | Phenolics | Prevent carcinogenesis and atherosclerosis | Leaves and flowers | Marked increases | ______ | Ceccarelli et al. (2010) | |
| Total phenolic content | ______ | No impact | Significantly increased plant yield | Colonna et al. (2016) | ||||
| Echinacea purpurea |
Rhizophagus intraradices |
Phenolics and cichoric acid |
Treatment of toothache, bowel pain, snake bite, skin disorders, seizure, chronic arthritis, and cancer (Grimm and Muller 1999) |
Root and aerial parts | Significant increase | Plant growth increased | Araim et al. (2009) | |
| Eclipta alba |
Glomus aggregatum, Funneliformis mosseae, and Rhizophagus fasciculatus |
Flavonoids |
Treatment of gastrointestinal disorders, respiratory tract disorders (including asthma), fever, hair loss and graying of hair, liver disorders (including jaundice), skin disorders, spleen enlargement, and cuts and wounds (Jahan et al. 2014) |
______ | Increased | Positive effect on plant growth | Tejavathi and Jayashree (2011) | |
| Eclipta prostrata | Rhizophagus irregularis, Funneliformis mosseae, Claroideoglomus etunicatum, Claroideoglomus claroideum, Rhizoglomus microaggregatum, and Funneliformis geosporum | Scopolamine | treatment of diabetes type II, dizziness, hemoptysis, and liver diseases | Leaves |
0.34% increased |
______ | Vo et al. (2019) | |
| Quercetin | Whole plant |
0.87% increased |
______ | Vo et al. (2019) | ||||
| Inula ensifolia | Rhizophagus clarus | Thymol derivatives | Possess antiproliferative activity against human cancer | Roots | Increased | ______ | Zubek et al. (2010) | |
| Stevia rebaudiana | Rhizophagus fasciculatus | Stevioside, rebaudioside-A |
Used as a substance strengthening the heart, the circulatory system, and regulating blood pressure (Marcinek and Krejpcio 2016) |
Leaves | Significant increase | ______ | Mandal et al. (2013) | |
| Rhizophagus irregularis | ______ | ______ | Positive increase | Leaf dry biomass increased | Tavarini et al. (2018) | |||
| Spilanthes acmella |
Funneliformis mosseae and Acaulospora laevis |
______ | Antiseptic, antibacterial, antifungal, and antimalarial properties and used as remedy for toothache, flu, cough, rabies diseases, and tuberculosis | ______ | ______ | Improved the survival rate, plant growth, and biomass yield of micropropagated plantlets | Yadav et al. (2012) | |
| Tagetes erecta | ______ | ______ |
Used as antiseptic and in kidney troubles, muscular pain, and piles, and applied to boils and carbuncles (Singh et al. 2020) |
______ | ______ | Positively improved plant growth, and flower quality under drought stress | Asrar and Elhindi (2010) | |
| Wedilia chinensis | Rhizophagus fasciculatus | Total phenols, ortho dihydroxy phenols, flavonoids, alkaloids, tannins, and saponins |
Treatment of bites, stings, fever, infection, kidney dysfunction, cold, wounds, and amenorrhea problems (Rehana and Nagarajan 2018) |
Seedlings | Increased | ______ | Nisha and Kumar (2010) | |
| Burseraceae | Commiphora leptophloeos |
Gigaspora albida and Claroideoglomus etunicatum (native) |
Total phenols and tannins | Treatment of bronchitis, cough, renal problems, general inflammation, and stomachache | Seedling, leaves | Significant increased | ______ | Lima et al. (2017) |
| Caprifoliaceae | Valeriana jatamansi |
Rhizophagus intraradices |
Gallic acid, chlorogenic acid, catechin, hydroxyl benzoic acid |
Possess sedative, neurotoxic, cytotoxic, antidepressant, antioxidant, and antimicrobial activities (Jugran et al. 2019) |
Rhizome and root | Significant increase | Significant increase in aboveground fresh and dry weight, and belowground fresh and dry weight | Jugran et al. (2015) |
| Valeriana officinalis | Rhizophagus intraradices | Valerenic acid |
Possess sedative and antispasmodic and sleep-inducing effects (Mungali and Tripathi 2021) |
Roots | Relative increasing | Biomass of rhizomes and roots negatively effected |
Nell et al. (2010) |
|
| Colchicaceae | Gloriosa superba | Funneliformis mossae, Rhizophagus fasciculatus, Gigaspora margarita, and Gigaspora gilmorei | Colchicine content | Treatment of gout, rheumatic arthritis, diseases of the skin and liver | Tubers | Increased | Improved plant growth |
Pandey et al. (2014) |
| Dioscoreaceae | Dioscorea spp. yam |
Rhizophagus clarus, Claroideoglomus etunicatum, Rhizophagus fasciculatus, Gigaspora sp., Funneliformis mosseae, and Acaulospora sp. |
Polyphenols, flavonoids, and anthocyanin | Anti-oxidative property to inhibit lipid peroxidation, resist the attack of free radicals, diminish low-density lipoproteins (LDLs), and reduce the occurrence of cardiovascular diseases | Bulbils | Significantly increased | Tube weights significantly increased | Lu et al. (2015) |
| Euphorbiaceae | Euphorbia hirta | Funneliformis mosseae | Phenols, flavonoids, alkaloids, and terpenoids |
Treatment for respiratory ailments (cough, coryza, bronchitis, and asthma), worm infestations in children, dysentery, jaundice, pimples, gonorrhea, digestive problems, and tumors (Kumar et al. 2010) |
______ | Increased | Positive effect on plant growth parameters | Tejavathi and Jayashree (2011) |
| Fabaceae | Astragalus membranaceus | ______ | ______ |
Increasing telomerase activity and posing antioxidant, anti-inflammatory, immunoregulatory, anticancer, hypolipidemic, antihyperglycemic, hepatoprotective, expectorant, and diuretic effects (Liu et al. 2017) |
______ | ______ | AMF community study | Liu and He (2008) |
|
Anadenanthera colubrina |
Acaulospora longula and Gigaspora albida | Catechin |
Treatment for respiratory problems and inflammations (Monteiro et al. 2006) |
Bark and leaves | Significant increase | Proteins and carbohydrates were significantly increased |
Pedone- Bonfim et al. (2013) |
|
| Castanospermum austral |
Rhizophagus intraradices and Gigaspora margarita |
Castanospermine | Possess anti-cancer and anti-inflammatory properties and as HIV inhibitors and treatment of AIDS | Seeds | Significant increase with R. intraradices | Increased the growth and P contents |
Abu-Zeyad et al. (1999) |
|
| Glycyrrhiza inflata | ______ | ______ | Clearing away toxic materials, eliminating phlegm, and relieving cough | ______ | ______ | Study under water stress | Liu and He (2009) | |
| Glycine max | Funneliformis mosseas | Isoflavonoids |
Reduction of different types of cancer, cardiovascular diseases, postmenopausal problems, diabetes, and some neurodegenerative disorders (Ahmad et al. 2014) |
Roots, seeds, leaves, and flowers | Significant increase | ______ |
Morandi and Bailey (1984) |
|
| Glycyrrhiza glabra |
Glomus hoi, Claroideoglomus etunicatum, Claroideoglomusclaroideum, Rhizophagus irregularis, and Acaulospora delicata |
Glycyrrhizic acid | Antiviral effects and act as a multifunctional drug carrier | Roots | Increased | ______ | Johny et al. (2021) | |
| Glycyrrhiza uralensis | Funneliformis mosseae | Contents of glycyrrhizic acid, liquiritin, isoliquiritin, and isoliquiritigen |
Having immune-modulating and anti-tumor potential (Ayeka et al. 2016) |
Roots | Significantly enhanced | Significantly increased the shoot and root biomass | Chen et al. (2017) | |
| Libidibia ferrea | Claroideoglomus etunicatum | Total flavonoids | Posing antiulcerogenic, antiinflammatory, anti-cancerogenic, anti-histaminic, antimicrobial, anti-coagulant, and cicatrizing properties | Leaves | Increased | Improving the production of seedlings, a larger stem diameter, higher chlorophyll a leaf content | Silvia et al. (2014) | |
|
Claroideoglomus etunicatum and Acaulospora longula |
Flavonoids | Stems, bark, and leaves | Significantly increased | ______ | Dos Santos et al. (2017) | |||
| Acaulospora longula | Tannins | ______ | Significantly increased | ______ |
Dos Santos et al. (2017) |
|||
| Medicago sativa |
Rhizophagus intraradics |
Formononetin |
Antioxidant, anti-inflammatory, immunomodulatory, and anticancer properties (Zagórska-Dziok et al. 2020) |
Roots | Significant increase | ______ | Volpin et al. (1994) | |
|
Prosopis laevigata |
Gigaspora rosea | Trigonelline |
Cardioprotection potential and treatment of heart diseases, throat infections, dysentery, and eye inflammations (Matta et al. 2017) |
Roots and leaves | 1.8-fold increase in roots | ______ | Rojas-Andrade et al. (2003) | |
| Ginkgoaceae | Ginkgo biloba | Funneliformis mosseae, Rhizophagus intraradices, and Diversispora epigaea | ______ |
Regulating cerebral blood flow, protection against free radicals, and delaying the progress of dementia and diabetes (Isah 2015) |
______ | ______ | Plant seedling growth significantly increased | Qi et al. (2002, 2003) |
| Hypericaceae |
Hypericum perforatum |
Rhizophagus intraradices alone or mixture of Funneliformis constrictum, Funneliformis geosporum, Funneliformis mosseae, and Rhizophagus intraradices | Naphthodianthrone-es, hypericin, and pseudohypericin | Possess sedative and astringent properties and utilized for excitability, neuralgia, anxiety, and depression | Shoots | Higher concentration | No impact on shoot biomass | Zubek et al. (2012) |
| Hypoxidaceae | Curculigo orchioides | Crude consortium of AMF spores isolated from rhizosphere soil of C. orchioides | ______ | Anticancerous properties | ______ | ______ | Increase biomass production, number of leaves and roots per plant, and higher concentrations of photosynthetic pigments as well as minerals | Sharma et al. (2008) |
| Lamiaceae | Coleus forskohlii |
Glomus bagyarajii and Scutellospora calospora |
Forskolin |
Treatment of eczema, asthma, psoriasis, cardiovascular disorders, and hypertension (Kavitha et al. 2010) |
Roots | Increased | Positive effect on plant growth | Sailo and Bagyaraj (2005) |
| Leucas aspera | Funneliformis mosseae | Alkaloids |
Carminative, antihistaminic, antipyretic, and antiseptic properties to treat jaundice, anorexia, dyspepsia, fever, helminthic manifestation, respiratory and skin diseases (Nirmala and Kanchana 2018) |
______ | Increased | Enhanced growth and total biomass | Tejavathi and Jayashree (2011) | |
| Mentha arvensis | Rhizophagus fasciculatus | Terpenes content |
Used for stomach problems, allergy, liver and spleen disease, asthma, and jaundice (Thawkar et al. 2016) |
Aerial parts | Significantly increased | Significantly increasing plant height, fresh herbage and dry matter yield | Gupta et al. (2002) | |
| Mentha spicata | Commercial AMF consortium “Rhizagold” | ______ | Antiseptic, restorative, carminative, and antispasmodic properties | ______ | ______ | Significantly positive effect of increasing various plant growth parameters | Birje and Golatkar (2016) | |
|
Melissa officinalis |
Claroideoglomus etunicatum, Claoideoglomus claroideum, and Rhizophagus intraradices | Citronellal and neral | To treat nervous disturbances (anxiety, insomnia, and stress) and gastrointestinal disorders and possess sedative, spasmolytic, antimicrobial, antioxidant, and antitumoral actions | Leaves | Increased | No impact | Engel et al. (2016) | |
|
Ocimum basilicum |
Gigarpora margarita and Gigaspora rosea | Linalool and geraniol |
Treatment for headaches, coughs, diarrhea, constipation, warts, worms, and kidney malfunctions (Joshi 2014) |
Seeds | Significant increase | Plant growth parameters and yield increased |
Rasouli- Sadaghiani et al. (2010) |
|
| Funneliformis caledonium | Rosmarinic and caffeic acids | Shoots | Increased | ______ | Toussaint et al. (2007) | |||
| Origanum onites | Claroideoglomus etunicatum | Total essential oil production | Treatment of indigestion, coughs, and toothache, and to stimulate menstruation | Leaves | Increased | Significantly higher shoot and root dry weight | Karagiannidis et al. (2012) | |
| Origanum vulgare | Claroideoglomus etunicatum | Essential oil composition of p-cymene, and γ-terpinene | Treatment for indigestion, coughs, and toothache, and to stimulate menstruation | Leaves | Increased | Significantly higher shoot and root dry weight | Karagiannidis et al. (2012) | |
| Plectranthus amboinicus | Rhizophagus clarus | Carvacrol, trans-caryophyllene, α-Bergamotene and α-humulene | Possess digestive, expectorant, antispasmodic, healing, and antiseptic actions | Shoots | Significant improvement | Improved shoot dry matter, root dry matter and total dry matter | Merlin et al. (2020) | |
| Pogostemon cablin | Claroideoglomus etunicatum | Essential oils | Used to treat nausea, diarrhea, colds, and headaches | ______ | Increased essential oil content | Greater plant height, number of branches and spread, biomass | Arpana et al. (2008) | |
| Acaulospora laevis, Funneliformis mosseae, and Scutellospora calaspora | Patchoulol | Leaves | Significant Improvement | ______ | Singh et al. (2012) | |||
| Satureja macrostema | Rhizophagus irregularis | β-Linalool, menthone, pulegone, and verbenol acetate | Antimicrobials | Aerial parts | Significantly increased | Significantly increased biomass, shoot and root length | Carreón-Abud et al. (2015) | |
| Salvia officinalis | Rhizophagus clarus | Essential oil camphor, α-humulene, viridiflorol, manool, α-thujone, and β-thujone |
Treatment of different kinds of disorders including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremor, paralysis, diarrhea, and hyperglycemia (Ghorbani and Esmaeilizadeh 2017) |
Shoots | Increased | Plant biomass increased | Sete da Cruz et al. (2019) | |
| Salvia miltiorrhiza |
Funneliformis geosporum or Acaulospora laevis |
Total phenolic acids | Treatment of menstrual disorders, cardiovascular, and cerebrovascular disease | Roots | Significant increase | Roots biomass, fresh and dry weight of the plant effectively increased | Wu et al. (2021) | |
| Scutelleria integrifolia | ______ | ______ | A strong emmenagogue and as a female medicinal herb | ______ | ______ | Positive effects on micropropagated plantlet growth, particularly root development | Joshee et al. (2007) | |
| Schizonepeta tenuifolia | ______ | Essential oil |
Used for headaches, colds, allergies, and eczema (Jeon et al. 2019) |
______ | Increased | ______ | Wei and Wang (1991) | |
| Thymus daenensis |
Funneliformis mosseae and Rhizophagus intraradices |
Essential oils |
Possess digestive, carminative, antitussive, antispasmodic, and expectorant attributes (Elahian et al. 2021) |
______ | Improve essential oil under drought stress | ______ | Arpanahi et al. (2020) | |
| Thymus vulgaris | Funneliformis mosseae | Thymol, p-cymene, and γ-terpinene | Possess antiseptic, antibacterial, antifungal, antispasmodic, antitussive, expectorant, and analgesic properties | Increased | Improved yield under drought condition | Machiani et al. (2021) | ||
| Leguminosae | Puerraria lobata | ______ | ______ |
To relieve body heat, eye soring, dry mouth, headache associated with high blood pressure, and stiff neck problems (Liu et al. 2019) |
______ | ______ | AMF community study | Wang et al. (2006) |
| Oleaceae | Forsythia suspense | Rhizophagus fasciculatus and Funneliformis constrictum | ______ |
Anti-inflammatory, antioxidant, antibacterial, anti-cancer, anti-virus, anti-allergy, and neuroprotective effects (Wang et al. 2018) |
______ | ______ | Strengthen the anti-drought of the seeding | Zhao et al. (2007) |
| Poaceae | Cymbopogon citratus | Funneliformis mosseae |
Essential oils Geranial, neral, and β-pinene |
To treat cough, cold, rheumatism, digestive problems, bladder issues, toothache, and swollen gums | Aerial Parts | Enhanced | ______ | Mirzaie et al. (2020) |
| Coix lachrymal-jobi | ______ | ______ |
Diuretic, anti-rheumatic, antispasmodic, anti-inflammatory, antidiarrheal, anthelmintic, antipyretic, antispasmodic, diuretic, hypoglycemic, anti-cancer, and tonic properties (Patel et al. 2017) |
______ | ______ | Plant growth study | Li (2003) | |
| Passifloraceae | Passiflora alata |
Claroideoglomus etunicatum, Rhizophagus intraradices |
Total phenols content |
Treatment of several diseases, such as insomnia, anxiety, and hysteria (Simao et al. 2018) |
Shoots | ______ | Dry mass of shoot and leaf number were greater | Riter et al. (2014) |
| Rhizophagus clarus and Glomus spurcum | ______ | Significant increase | Higher plant height | Riter et al. (2014) | ||||
| Rutaceae | Phellodendron amurense |
Funneliformis mosseae, Claroideoglomusetunicatum, Diversispora epigaea, and Glomus diaphanum |
Berberine, jatrorrhizine, palmatine |
Treatment of jaundice, dysentery, hypertension, inflammation, and liver-related diseases (Kuete 2014) |
Barks | Significant increase | ______ | Fan et al. (2006) |
| Phellodendron chinense | ______ | Berberine | Treating dysentery, detoxicating, and curing furuncles | ______ | ______ | ______ | Zhou and Fan (2007) | |
| Citrus aurantium | ______ | ______ |
Possess antiseptic, antioxidant, antispasmodic, aromatic, astringent, carminative, digestive, sedative, stimulant, stomachic and tonic properties Treatment of gastrointestinal disorders, insomnia, headaches, cardiovascular diseases, and cancer (Suryawanshi 2011) |
______ | Plant growth and root antioxidative enzymes study | Wu et al. (2010) | ||
| Solanaceae | Datura stramonium |
Funneliformis mosseae and Glomus epigaeum |
Hyoscine and hyoscyamine |
Treatment of stomach and intestinal pain from worm infestation, toothache, and fever from inflammation (Soni et al. 2012) |
Seeds And Fruits | Significant Increase | ______ | Wei and Wang (1989) |
| Solanum viarum |
Glomus aggregatum and bacteria Bacillus coagulans and Trichoderma harzianum |
Flavonoids | Used for cancer, patients with Addison’s disease and rheumatic arthritis treatment | Seedlings | Increased | ______ |
Hemashenpagam and Selvaraj (2011) |
|
| Withania somnifera | Rhizophagus irregularis | Withaferin-A | Treatment of cancer | Root | Significantly increased | ______ | Johny et al. (2021) | |
| Taxaceae | Taxus chinensis | ______ | ______ |
Anticancer effect (Jian et al. 2016) |
______ | ______ | AMF infection and colonization study | Ren et al. (2008) |
| Violaceae | Viola tricolor | Rhizophagus irregularis | Caffeic acid concentration | Treatment of various skin disorders and upper respiratory problems | Aerial part | Significant increase | No impact on root mass and negative impact on shoot biomass | Zubek et al. (2015) |
| Zingiberaceae | Curcuma longa |
Glomus, Gigaspora, and Acaulospora sp. |
Curcumin | A natural antioxidant with antitumor activity, an inhibitor of arachidonic acid metabolism, and a good antiinflammatory agent | Rhizomes | Increased | ______ | Dutta and Neog (2016) |
| Gigaspora margarita | Curcumin | ______ | No impact on curcumin content (field) | No impact on plant growth parameters, biomass production, nutrient uptake | Yamawaki et al. (2013) | |||
| Gigaspora margarita | Curcumin | ______ | Concentration of curcumin increased (greenhouse) | Higher biomass production and nutrient uptake | Yamawaki et al. (2013) |
aThe column “AMF” shows the current names, not the one at the time of publication
bThere are no studies or available data found online
A direct relationship has been highlighted between the biomass of AMF-colonized plants and the concentration of secondary metabolites for several medicinal plants, such as Chlorophytum borivilianum, Dioscorea spp., Gymnema sylvestre, Glycyrrhiza uralensis, Libidibia ferrea, Ocimum basilicum, Satureja macrostema, and Salvia miltiorrhiza (Dave et al. 2011; Lu et al. 2015; Zimare et al. 2013; Chen et al. 2017; Silvia et al. 2014; Zolfaghari et al. 2013; Carreón-Abud et al. 2015; Yang et al. 2017). Conversely, in Cynara cardunculus colonized by R. intraradices and F. mosseae, a significant increase in yield was noticed, but the concentrations of phenolics decreased (Colonna et al. 2016). Other studies conducted with Hypericum perforatum inoculated with R. intraradices or a mixture of Funneliformis constrictum, Funneliformis geosporum, F. mosseae, and R. intraradices reported no increase in shoot biomass, while in Valeriana officinalis inoculated with R. intraradices or a mixture of six AMF species (F. mosseae, R. intraradices, Glomus cladoideum, Rhizoglomus microaggregatum, Funneliformis caledonium, and C. etunicatum) a negative effect on rhizome and root biomass was noticed (Zubek et al. 2012; Nell et al. 2010). However, an increased concentration of active compounds (e.g., hypericin and pseudohypericin and sesquiterpenic acids, respectively) was noticed for both plants (Zubek et al. 2012; Nell et al. 2010).
Another beneficial aspect of AMF is their capability to improve plant nutrient uptake (Bowles et al. 2016), influencing directly or indirectly the concentration of secondary metabolites (Yamawaki et al. 2013). For instance, F. mosseae improved shoot and root biomass, root system architecture, and flavonoid accumulation in Glycyrrhiza uralensis growing under P-deficient nutrient conditions (Chen et al. 2017).
A number of studies also have reported enhanced survival and increased growth of micropropagated medicinal plants at the transfer stage from in vitro to ex vivo conditions (Rai 2001). For instance, with F. mosseae, the survival rate of micropropagated Spilanthes acmella and Glycyrrhiza glabra plantlets was 100%, and plant growth and development were improved under glasshouse and greenhouse conditions (Yadav et al. 2012, 2013) while in the absence of AMF, the survival rate was only 60–70%. Similarly, height and fresh weight of shoots, roots, and seeds of Scutelleria integrifolia seedlings inoculated with C. etunicatum were significantly increased in pots following micropropagation (Joshee et al. 2007).
These studies clearly evidenced the potential of using AMF inoculants for improving the yield of raw materials (e.g., roots, shoots) of medicinal plants, thus potentially increasing the quantity of active compounds.
Different groups of secondary metabolites whose production was enhanced by AMF inoculation are detailed below.
Alkaloids
Alkaloids are nitrogen-containing organic compounds produced by plants constitutively or in response to pests, diseases, or other external stimuli (Jan et al. 2021). They are found in different organs of important medicinal plants (Table 1) and are characterized by a diverse array of pharmacological properties including analgesia, local anesthesia, cardiac stimulation, respiratory stimulation and relaxation, vasoconstriction, muscle relaxation, antineoplastic, and hypertensive and hypotensive properties (Hussein and El-Anssary 2018).
Since Wei and Wang (1989) first observed that AMF symbiosis can increase the total content of hyoscyamine and scopolamine in Datura stramonium, numerous studies have reported a positive role of AMF in the accumulation of alkaloids. For example, a positive correlation was found between AMF colonization (a mixture of R. intraradices and G. margarita) of Castanospermum australe tree and the castanospermine content (which was reported to inhibit the HIV virus) of leaves (Abu-Zeyad et al. 1999). The contents of some commonly used “heat-clearing” herb compounds, such as berberine, jatrorrhizine, and palmatine, were increased in seedlings of Phellodendron amurense inoculated with AMF (Fan et al. 2006). Other active compounds were increased in the presence of AMF: trigonelline in roots and leaves of Prosopis laevigata colonized by Gigaspora rosea under in vitro conditions; colchicine in tubers of Gloriosa superba colonized by F. mosseae growing under glasshouse conditions, and scopolamine in leaves of Eclipta prostrata colonized by a mixture of C. etunicatum, Claroideoglomus claroideum, F. mosseae, F. geosporum, R. irregularis, and Rhizoglomus microaggregatum growing in climate chamber conditions (Rojas-Andrade et al. 2003; Pandey et al. 2014; Vo et al. 2019).
Terpenoids
The largest and most diverse group of secondary metabolites are terpenoids, which are primary constituents of essential oils (Cox-Georgian et al. 2019). Essential oils are volatile lipophilic mixtures of secondary metabolites, consisting mostly of monoterpenes, sesquiterpenes, and phenylpropanoids, which often are used as flavors and fragrances, as antimicrobials and antioxidants, and as medicines (Deans and Waterman 1993).
Several studies have reported an AMF impact on the production of essential oils by medicinal and aromatic plants (Table 1). For instance, the production of these compounds was increased in Corianderum sativum, Trachyspermum ammi, Atractylodes lancea, Inula ensifolia, Artemisia umbelliformis, Plectranthus amboinicus, Satureja macrostema, Salvia officinalis, Origanum vulgare and Origanum onites, Thymus daenensis, Thymus vulgaris, and Foeniculum vulgare colonized by AMF (Rydlová et al. 2016; Kapoor et al. 2002; Liang et al. 2018; Zubek et al. 2010; Binet et al. 2011; Merlin et al. 2020; Carreón-Abud et al. 2015; Sete da Cruz et al. 2019; Karagiannidis et al. 2012; Arpanahi et al. 2020; Machiani et al. 2021; Kapoor et al. 2004). The content of artemisinin, an important sesquiterpene lactone compound found in Artemisia annua and well known for its effects on malaria and more recently on cancer (Krishna et al. 2008), was increased in leaves of plants colonized by F. mosseae or a combination of Glomus macrocarpum and R. fasciculatus or Diversispora epigaea and R. irregularis grown in pots or under field conditions (Huang et al. 2011; Chaudhary et al. 2008; Domokos et al. 2018). The forskolin content, a diterpene extensively used to treat heart diseases, glaucoma, asthma, and certain types of cancers (Kavitha et al. 2010), was significantly increased in roots of Coleus forskohlii inoculated with Glomus bagyarajii growing under greenhouse conditions (Sailo and Bagyaraj 2005). Similarly, Singh et al. (2013) reported an increased content of forskolin in tubers of Coleus forskohlii associated with R. fasciculatus growing under organic field conditions.
Researchers also have studied the impact of AMF symbiosis on medicinal plants derived from tissue cultures. An example is the increased content of the essential oil carvacrol, a phenolic monoterpenoid with antimicrobial, antioxidant, and anticancer activities (Sharifi-Rad et al. 2018) in micropropagated Origanum vulgare subsp. hirtum after association with the AMF Septoglomus viscosum (Morone Fortunato and Avato 2008).
Phenolics
Phenolics represent a wide group of compounds, sharing one or more phenol groups (Hussein and El-Anssary 2018), among which are flavonoids, curcuminoids, coumarins, tannins, stilbenes, lignans, phenolic acids, and quinones (Cosme et al. 2020).
Arbuscular mycorrhizal fungi have been shown to increase the content of phenols in medicinal plants (Table 1). For instance, the production of formononetin (an antimicrobial, antioxidant, antilipidemic, antidiabetic, antitumor, and neuroprotective compound) (Vishnuvathan et al. 2016), was increased in Medicago sativa grown in the presence of R. intraradices (Volpin et al. 1994). The production of curcumin (an anti-inflammatory, antioxidant, anticancer, antiseptic, antiplasmodial, astringent, digestive, diuretic compound) was increased by circa 26% in Curcuma longa colonized by AMF species belonging to the genera Glomus/Rhizophagus, Gigaspora, and Acaulospora sp., under greenhouse conditions (Dutta and Neog 2016). The concentration of total tannins, used to treat tonsillitis, pharyngitis, hemorrhoids, and skin eruptions (Britannica 2021), was increased by 40% in the fruits of Libidibia ferrea inoculated with Acaulospora longula under field conditions (Santos et al. 2020). Additionally, the concentrations of cichoric acid in Echinacea purpurea colonized by R. intraradices (Araim et al. 2009) and p-hydroxybenzoic acid and rutin in Viola tricolor colonized by R. irregularis (Zubek et al. 2015), and the total content of flavonoids in Libidibia ferrea colonized by Gigaspora albida and gallic acid in Valeriana jatamansi colonized by a consortium of three different isolates of R. intraradices spp. (Silvia et al. 2014; Jugran et al. 2015) were increased by the AMF symbiosis.
Saponins
Saponins are characterized by a polycyclic aglycone moiety with either a steroid (steroidal saponins) or triterpenoid (triterpenoidal saponins) attached to a carbohydrate unit (a monosaccharide or oligosaccharide chain) (Hussein and El-Anssary 2018). Among these compounds, a few have demonstrated pharmacological properties, such as antitumor, sedative, expectorant, analgesic, and anti-inflammatory (Hussein and El-Anssary 2018). Arbuscular mycorrhizal fungi were reported to enhance the production of saponins in medicinal plants (Table 1). For instance, the content of glycyrrhizic acid, a triterpenoid saponin used to alleviate bronchitis, gastritis, and jaundice (Pastorino et al. 2018), was increased by 0.38–1.07-fold and by 1.34–1.43-fold after 4 and 30 months, respectively, in Glycyrrhiza glabra (liquorice) plants colonized by F. mosseae and D. epigaea alone or in combination, grown in sand under greenhouse conditions (Liu et al. 2007). Similarly, Johny et al. (2021) reported an increase of glycyrrhizic acid concentration in Glycyrrhiza glabra inoculated with C. etunicatum under greenhouse conditions.
Other chemical compounds
Hypericin and pseudohypericin are naphthodianthrones (anthraquinone derivatives) mainly extracted from Hypericum species (Ayan and Cirak 2008). They have many pharmaceutical properties, such as sedatives, antiseptics, and antispasmodics (Baytop 1999). Zubek et al. (2012) reported an increased content of hypericin and pseudohypericin in Hypericum perforatum colonized by R. intraradices alone or by a mixture of F. constrictum, F. geosporum, F. mosseae, and R. intraradices, under greenhouse conditions.
Withaferin-A, a steroidal lactone, traditionally used in ayurvedic medicine (Mirjalili et al. 2009), has a wide range of pharmacological activities including cardioprotective, anti-inflammatory, immuno-modulatory, anti-angiogenesis, anti-metastasis, and anti-carcinogenic properties. Johny et al. (2021) reported that association between the medicinal plant Withania somnifera and R. irregularis increased the concentration of withaferin-A as compared to non-inoculated plants under greenhouse conditions.
It should be noted, however, that AMF showed a neutral or decreased effect on the production of certain secondary metabolites. For example, Nell et al. (2010) found that F. mosseae has no effect on the total concentrations of phenolic and rosmarinic acid in the roots of Salvia officinalis; and Geneva et al. (2010) showed that R. intraradices decreased total phenol and flavonoid contents in the leaves of Salvia officinalis. Similarly, Zubek et al. (2010) reported significant differences in the effectiveness of different AMF species tested in Inula ensifolia. An increased production of thymol derivatives was found in plant roots inoculated with Rhizophagus clarus, while a decreased production of these metabolites was reported in roots inoculated with R. intraradices under greenhouse conditions (Zubek et al. 2010). Moreover, changes in secondary metabolite composition have been observed in medicinal plants inoculated with AMF. For instance, Geneva et al. (2010) observed a modified composition of essential oils and promotion of the relative quantities of bornylacetate, 1,8-cineole, α-thujones, and β-thujones in Salvia officinalis associated with R. intraradices. Similarly, Artemisia umbelliformis inoculated with an alpine microbial community containing Planticonsortium tenue (formerly Glomus tenue), R. intraradices, G. claroideum/etunicatum, and a new Acaulospora species showed a significant increase of E-ocimene concomitant with a decrease of E-2-decenal and (E, E)-2–4-decadienal (Binet et al. 2011). Therefore, the selection of the most effective AMF strains for improving the accumulation of desirable active compounds needs to be taken into account.
Effect of AMF on biomass and production of secondary metabolites in medicinal plants under biotic and abiotic stress conditions
Drought, salinity, heavy metals, pests, and diseases can impact plant growth, reducing their biomass (Hashem et al. 2014; Alwhibi et al. 2017) and consequently affecting the production of secondary metabolites. Arbuscular mycorrhizal fungi can increase the tolerance/resistance of plants against those abiotic and biotic stresses, potentially influencing secondary metabolites production (Hashem et al. 2018).
Several studies have shown that AMF symbiosis can improve the growth and secondary metabolite production of medicinal plants under water deficit conditions. For example, a recent study by Machiani et al. (2021) showed that inoculation with F. mosseae significantly improved biomass and essential oil content (mainly thymol, p-cymene and γ-terpinene) of Thymus vulgaris plants grown in a 2-year field experiment in intercropping with soybean under water deficit conditions. Similarly, Mirzaie et al. (2020) reported that inoculation with F. mosseae significantly increased geranial and β-pinene (both belong to oxygenated monoterpenes essential oils) yields of Cymbopogon citratus grown in a greenhouse pot experiment under moderate water stress conditions (50% field capacity).
Salt stress stimulates the accumulation of phenolic compounds in plants as a general defense mechanism to stress (Parvaiz and Satyawati 2008). Intriguingly, this abiotic stress is a principal elicitor influencing synthesis of compounds in many herbs (e.g., cinnamic, gallic, and rosmarinic acids in Thymus vulgaris; glycyrrhizin in Glycyrrhiza glabra; quinic, gallic, and protocatechuic acids in Polygonum equisetiforme) (Bistgani et al. 2019; Behdad et al. 2020; Boughalleb et al. 2020). A recent study by Amanifar and Toghranegar (2020) reported that moderate salt stress stimulated higher production of valerenic acid in Valeriana officinalis than a situation without salt stress. Interestingly, this increase was significant when the plants were colonized by F. mosseae. Duc et al. (2021) found that a mixture of six AMF species (C. etunicatum, C. claroideum, F. mosseae, F. geosporum, Rhizoglomus microaggregatum, and R. intraradices) increased the tolerance of Eclipta prostrata under moderate salt stress in a pot experiment under controlled conditions, inducing major changes in its polyphenol profile.
Minerals, such as cadmium (Cd) and zinc (Zn), also were reported to impact secondary metabolite production in medicinal plants colonized by AMF. For instance, Hashem et al. (2016) observed that an AMF mixture comprising C. etunicatum, F. mosseae, and R. intraradices enhanced the chlorophyll and protein content and considerably reduced lipid peroxidation in Cassia italica plants under Cd stress in a pot experiment. Moreover, AMF inoculation caused a further increase in proline and phenol content ensuring improved plant growth under stress conditions.
Arbuscular mycorrhizal fungi symbiosis improved the disease tolerance of medicinal plants through the mediation of secondary metabolites. For instance, Jaiti et al. (2007) reported that a complex of native AMF species increased the tolerance of Phoenix dactylifera (a plant characterized by high nutritional and therapeutic value of its fruits (Al-Alawi et al. 2017)) against bayoud disease (the most damaging vascular disease of date palm caused by Fusarium oxysporum f. sp. albedinis) by increasing the enzymatic activities of peroxidases and polyphenoloxidases, which are associated with an increase of phenolic compounds in the cell wall.
Mechanisms by which AMF symbiosis promotes secondary metabolism in medicinal plants
It is often considered that the increased concentrations of various secondary metabolite groups (e.g., flavonoids, phenolics) in AMF-colonized plants are a result of the elicitation of several defense response pathways as reviewed by Zeng et al. (2013). For instance, terpenoids in the carotenoid pathway, flavonoids, phenolic compounds, and some alkaloids (such as hyoscyamine and scopolamine) in the phenylpropanoid pathway are often increased in AMF-colonized plants (Kaur and Suseela 2020). These pathways play different roles in the plant-AMF symbiosis, such as signaling, stress tolerance, nutrient uptake, and resistance against biotic and abiotic stresses. However, it is still not totally clear how AMF trigger changes in the concentrations of phytochemicals in plant tissues (Toussaint et al. 2007).
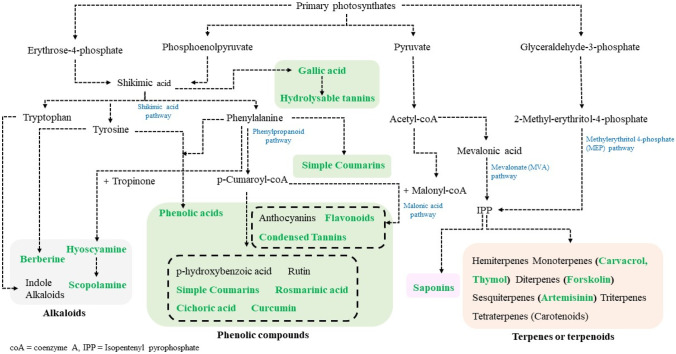
Many studies have focused on the mechanisms by which AMF modulate the production of terpenoids, phenolic compounds, and alkaloids in plants. Terpenoids are synthesized from isoprene units in the methyleritrophosphate (MEP) and the mevalonic acid (MVA) pathways (Zhi et al. 2007). Phenolic compounds (e.g., phenols, flavonoids, protanthocyanidins, tannins) are synthesized in the shikimic acid pathway where phenylpropanoids are formed and in the malonic acid pathway (Oksana et al. 2012). Most of the alkaloids are synthesized from various biological precursors (most amino acids) such as tyrosine and tryptophane in the shikimic acid pathway (Facchini 2001) (Fig. 1).
Fig. 1.
Main pathways of secondary plant metabolism resulting in the production of alkaloids, phenolics, saponins, and terpenes (in gray, green, pink, and brown shaded portions, respectively) mentioned in this review. Examples of upregulated compounds or classes of compounds in medicinal plants associated with AMF are highlighted with green type. This figure is modified from Dos Santos et al. (2021)
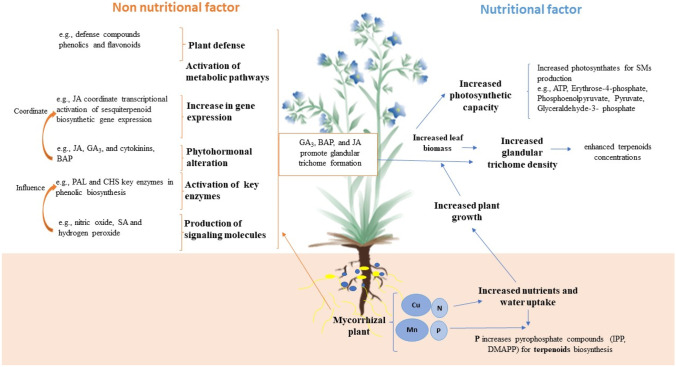
Several common nutritional and non-nutritional factors have been proposed to explain the increased production of secondary metabolites in AMF-colonized plants (Kapoor et al. 2017; Sharma et al. 2017; Dos Santos et al. 2021) (Fig. 2).
Fig. 2.
Non-nutritional and nutritional factors influencing the production of secondary metabolites (i.e., terpenoids, phenolics, and flavonoids) in AMF-colonized plants. Non-nutritional factors (leftside in orange): AMF colonization results in the activation of plant defense mechanisms with the production of phenolics and flavonoids. Change in phytohormone levels, such as jasmonic acid (JA), gibberellic acid (GA3), and 6-benzylaminopurine (BAP), increases the number and size of glandular trichomes and leads to transcriptional activation of sesquiterpenoid biosynthetic gene expression. AMF induce the production of signaling molecules, such as nitric oxide, salicylic acid (SA), and hydrogen peroxide, which influence the activation of key enzymes such as l-phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS), for the biosynthesis of phenolic compounds. Nutritional factors (rightside in blue): AMF colonization increases plant nutrients and water uptake leading to increased plant growth and leaf biomass. This results in enhanced plant photosynthetic capacity and increased production of photosynthates which are precursors of different secondary metabolites. Increased leaf biomass leads to an increased density of glandular trichomes in which terpenoids are synthesized and stored. This figure is adapted with permission from Springer Nature Customer Service Centre GmbHS: Springer Nature, Phytochemistry Reviews. Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza (Kapoor et al. 2017). We thank Evangelia Tsiokanou (National and Kapodistrian University of Athens, Greece) for graciously providing the picture of the plant used in this figure
Regarding nutritional factors, the increase was first attributed to the enhanced uptake of nutrients by AMF-colonized plants (Lima et al. 2015; Oliveira et al. 2015; Riter et al. 2014). For example, the role of phosphorus in the synthesis of terpenoids precursors via the MVA (acetyl-CoA, ATP, and NADPH) as well as the MEP (glyceraldehyde phosphate and pyruvate) pathways is widely recognized (Kapoor et al. 2017). Phosphorus enhances terpenoid biosynthesis by increasing the concentration of pyrophosphate compounds, such as isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Kapoor et al. 2002, 2004; Zubek et al. 2010), which contain high-energy phosphate bonds. However, Khaosaad et al. (2006) found that the concentration of essential oils significantly increased in two Origanum sp. genotypes colonized by F. mosseae, while the levels of essential oils in plants treated with P did not change. This suggests that the increased concentration of essential oils in AMF-colonized Origanum sp. plants may directly depend on the association with the fungus. In another study by Zubek et al. (2012), AMF colonization improved hypericin and pseudohypericin concentrations in Hypericum perforatum, probably because of an improved plant P and/or N nutrition in presence of the fungi. The increased growth through improved nutrients and water uptake of AMF-colonized plants also explains the enhanced production of these compounds in plants. It is well known that the AMF symbiosis increases shoot biomass, shoot length, and number of nodes in Ocimum basilicum (Gupta et al. 2002; Khaosaad et al. 2008; Rasouli-Sadaghiani et al. 2010; Copetta et al. 2006). Elevated leaf biomass results in increased photosynthetic capacity (Dave et al. 2011; Zubek et al. 2010), thus increasing the production of total photosynthates (e.g., ATP, carbon substrate, glyceraldehyde-3-phosphate, pyruvate, phosphoenolpyruvate, or erythrose-4-phosphate) required for terpenoids, phenolics, and alkaloid biosynthesis (Cao et al. 2008; Hofmeyer et al. 2010; Niinemets et al. 2002).
Regarding non-nutritional factors, alterations in the levels of phytohormones in AMF-colonized plants may reflect their enhanced production (Mandal et al. 2013, 2015a; Zubek et al. 2012). Indeed, it has been shown that the AMF symbiosis changes the concentrations of phytohormones, such as jasmonic acid (JA), gibberellic acid (GA3), and cytokinins (Allen et al. 1980, 1982; Hause et al. 2002; Shaul-Keinan et al. 2002) in plants. Moreover, it has been reported that phytohormones play a role in the secondary metabolism of plants (An et al. 2011; Maes and Goossens 2010; Maes et al. 2008). For instance, JA has been reported to coordinate transcriptional activation of sesquiterpenoid biosynthetic gene expression in Artemisia annua (Maes et al. 2011). Furthermore, the phytohormonal alterations of GA3, BAP (6-benzylaminopurine), and JA have been reported to promote the formation of glandular trichomes (Maes et al. 2011) which is positively correlated with an enhanced concentration of terpenoids in plant leaves. Glandular trichomes are the epidermal secretory structures in which terpenoids are synthesized and stored in plants (Covello et al. 2007). The enhanced concentration of terpenoids (essential oils) and increased glandular trichome density has been observed in a number of plants (e.g., Mentha x piperita, Phaseolus lunatus, and Lavendula angustifolia) (Ringer et al. 2005; Bartram et al. 2006; Behnam et al. 2006). Thus, an increase in trichome density upon mycorrhization often has been linked with an enhanced concentration of terpenoids (Copetta et al. 2006; Kapoor et al. 2007; Morone-Fortunato and Avato 2008). The modification of these secondary metabolite concentrations in AMF-plants also may be due to signaling mechanisms between host plants and the fungi (Larose et al. 2002; Rojas-Andrade et al. 2003; Xie et al. 2018). For example, Zhang et al. (2013) have reported that F. mosseae associated with Trifolium repens promoted changes in the concentration of signaling molecules, such as nitric oxide, salicylic acid (SA), and hydrogen peroxide, which influence the activation of key enzymes in phenolics biosynthesis (e.g., l-phenylalanine ammonia lyase (PAL), and chalcone synthase (CHS)). Moreover, AMF may increase the expression of genes encoding enzymes leading to the biosynthesis of these compounds in mycorrhizal plants (Andrade et al. 2013; Battini et al. 2016; Mandal et al. 2015a, b; Xie et al. 2018). For example, induction of terpene synthase (TPS) family genes TPS31, TPS32, and TPS33 has been observed in AMF-colonized tomato plants and probably can explain the changes in their terpenoid profile (Zouari et al. 2014). Mandal et al. (2015a) reported the increase of artemisinin in leaves of Artemisia annua inoculated with R. intraradices. This result was correlated with a higher expression of key biosynthesis genes (such as an allene oxidase synthase gene encoding one of the key enzymes for JA production) via enhanced JA levels. In addition, AMF may enhance the biosynthesis of these compounds either by increasing the production of precursors through the induction of metabolic biosynthetic pathways (Lohse et al. 2005; Zimare et al. 2013; Dos Santos et al. 2021) and/or by induction of key synthase enzymes (Mandal et al. 2013; Shrivastava et al. 2015; Sharma et al. 2017; Dos Santos et al. 2021). For example, mycorrhizal colonization (R. intraradices) has been found to elevate the transcript levels of two of the pivotal enzymes of the MEP pathway, 1-deoxy-D-xylulose 5-phosphate synthase (DXS), and 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) in wheat roots (Walter et al. 2000). DXS is an enzyme that catalyzes the initial step of the MEP pathway, where many isoprenoids are biosynthesized, and DXR is an enzyme that is immediately downstream from DXS in the MEP pathway (Walter et al. 2000). In another study by Walter et al. (2002), DXS2 transcript levels were strongly stimulated in Medicago truncatula roots upon colonization by AMF (a mixture of F. mosseae and R. intraradices), and were correlated with the accumulation of carotenoids and apocarotenoids. Finally, alterations in these secondary metabolites’ production also can result from plant defense responses to AMF colonization (Mechri et al. 2015; Zubek et al. 2012, 2015; Torres et al. 2015).
Various studies have reported increased production of alkanin/shikonin and their derivatives (A/S) in cell cultures of Boragenaceous plants (e.g., Lithospermum erythrorhizon, Alkanna tinctoria, and Arnebia euchroma) after applying exogenous jasmonate (Gaisser and Heide 1996; Urbanek et al. 1996; Bychkova et al. 1993). Alkanin/shikonin are naphtoquinone compounds with a broad spectrum of biological activities, such as wound healing, anti-inflammatory, and anticancer (Kheiri et al. 2017; Kourounakis et al. 2002; Andújar et al. 2013). Interestingly, AMF colonization of various other plants, such as Hordeum vulgare, Cucumis sativus, Medicago truncatula, and Glycine max, has resulted in the increase of endogenous levels of jasmonates within roots (Hause et al. 2002; Vierheilig and Piché 2002; Stumpe et al. 2005; Meixner et al. 2005). Jasmonic acid and its derivatives, commonly termed jasmonates, are hormonal regulators involved in plant responses to abiotic and biotic stresses as well as in plant development (Creelman and Mullet 1997; Wasternack 2007). The level of endogenous jasmonate was shown to increase after wounding or pathogen attack. There is no direct study on the effects of AMF on A/S production in these Boragenaceous medicinal plants. However, these findings suggest that AMF could be a potential factor enhancing A/S production in mycorrhizal Boraginaceae plants through the regulation of jasmonate.
Cultivation techniques for secondary metabolite production in mycorrhiza-associated medicinal plants
Plant secondary metabolites are often extracted from individuals grown in nature. For instance, around 95% of the medicinal plants used in the Indian herbal industry today are collected from the wild (Lakshman 2016). However, the quantity and quality of secondary metabolites from plants grown in nature are erratic, often influenced by abiotic and biotic factors, such as extreme temperatures, drought, alkalinity, salinity, and plant pathogens, impacting the metabolic pathways responsible for the accumulation of bioactive substances (Dayani and Sabzalian 2017; Giurgiu et al. 2017; Ramakrishna and Ravishankar 2011). Furthermore, overharvesting of medicinal plant species in nature could place them at a high risk of extinction (Roberson 2008). Finally, growing medicinal plants under field conditions may be time consuming, especially for woody plants (e.g., Taxus brevifolia and Lithospermum erythrorhizon) and slow-growing perennial plants (e.g., Panax ginseng), which can take several years to reach the desired metabolites production (Malik et al. 2011; Chandran et al. 2020; Yazaki 2017; Murthy et al. 2014). Therefore, there is a need for alternative production systems.
Production of medicinal herbs in controlled environments provides opportunities for improving the quality, purity, consistency, bioactivity, and biomass production of the raw material (Hayden 2006). In order to secure the commercial production of secondary metabolites, several cultivation techniques have been developed, potentially compatible with AMF application.
Substrate-based cultivation systems
Greenhouse cultivation
Greenhouses are widely used for crop production all-year round. Environmental parameters (e.g., temperature, humidity) are controlled, providing optimal growth conditions to the target crop or plant, favoring development, and thus safeguarding the yield and consistent production of high-quality bioactive compounds (Panwar et al. 2003).
Many medicinal plant species, such as Echinacea angustifolia, Echinacea purpurea, Ocimum basilicum, Withania somnifera, and Psoralea croylifolia, have been grown under greenhouse conditions (Zheng et al. 2006; Panwar et al. 2003). Similarly, many, such as Artemisia annua, Curcuma longa, Coleus forskohlii, Glycyrrhiza glabra, and Gloriosa superba, have been associated with AMF under greenhouse conditions with high production of bioactive compounds reported (Huang et al. 2011; Dutta and Neog 2016; Sailo and Bagyaraj 2005; Liu et al. 2007; Pandey et al. 2014). Therefore, growing medicinal plants in association with AMF under greenhouse conditions could represent a suitable method for improving the quality and production of bioactive compounds at large scale.
Substrate-free cultivation systems
Aeroponics
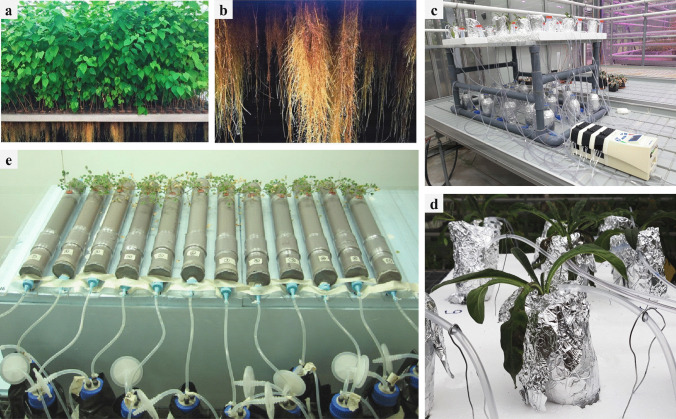
In the aeroponics cultivation system, the roots of plants are hung inside a sealed container in darkness and exposed to a water nutrient-rich spray through atomizers (Lakhiar et al. 2018) (Fig. 3a). This technique has been developed for the cultivation of many different plants, such as horticultural crops (e.g., Lactuca sativa, Cucumis sativus, and Solanum lycopersicum) (Movahedi and Rostami 2020), medicinal herbs (e.g., Anemopsis californica, Crocus sativus, and Valeriana officinalis) (Hayden 2006; Souret and Weathers 2000; Tabatabaei 2008), and medicinal crops (e.g., Arctium lappa and Zingiber officinale) used to extract secondary metabolites from their roots (Hayden et al. 2004a, b). It has been reported that Ocimum basilicum grown under aeroponic conditions had a higher yield, comparable phenolic and flavonoid contents, and antioxidant properties compared to plants grown in a solid substrate (Chandra et al. 2014). Similarly, Cichorium intybus, Withania coagulans, and Echinacea sp. grown in an aeroponic system had higher yields compared to the same plants grown in soil (Movahedi and Rostami 2020). This system was efficient for the production of bioactive molecules from roots of medicinal crops, such as chlorogenic acid in A. lappa and β-sitosterol in Cannabis sativa (Hayden 2006; Ferrini et al. 2021). For various medicinal plants, root apices constitute the main sites where active substances are produced and stored (Watson et al. 2015). However, these active substances are almost impossible to harvest through conventional farming methods. By using the Plant Milking Technology (Plant milking®) (https://www.plantadvanced.com/home) for Morus alba (an emblematic tree of traditional Chinese medicine, rich in alkaloids and flavonoids), Chajra et al. (2020) obtained an extract enriched in prenylated flavonoids that was 18-fold higher than commercial root extracts (Fig. 3a, b).
Fig. 3.
(a) Morus alba trees cultivated in aeroponic conditions and (b) close-up view of Morus alba roots grown aeroponically (Chajra et al. 2020). (c) Anchusa officinalis associated with Rhizophagus irregularis MUCL 41,833 growing in a semi-hydroponic cultivation system and (d) close-up view of a plant (UCLouvain, greenhouse). (e) Plant-based bioreactor system for the mass production of AMF as described in Declerck et al. (2009) (WO/2009/ 090,220)
Interestingly, aeroponic cultivation systems also have been developed and used for the production of AMF inoculum in which roots (and AMF) were bathed in a nutrient solution mist (Zobel et al. 1976; Hung and Sylvia 1988). For the production of AMF, plants are precolonized prior to their introduction into the system, through preculturing plant seedlings and AMF propagules (both preferably surface-sterilized) in pots containing a substrate (e.g., mixture of sand and perlite). Then the precolonized plants are transferred to the aeroponic container where the roots (and AMF) develop. The container is usually protected from light to prevent the development of algae (Jarstfer and Sylvia 1995). The mist can be applied by various techniques that differ mainly in the size of the fine droplets produced (e.g., atomizing disk, pressurized spray through a microirrigated nozzle, an ultrasonically generated fog of nutrient solution with droplets of 3–10-μm diameter, and ultrasonic nebulizer technology resulting into microdroplets of 1 μm in diameter) (IJdo et al. 2011; Jarstfer and Sylvia 1995; Mohammad et al. 2000). Mohammad et al. (2000) reported a high number of viable AMF propagules obtained in aeroponic culture, and such inoculum was used in a field experiment (Mohammad et al. 2004). Thus, aeroponic systems could potentially be used for growing medicinal plants associated with their AMF partners in order to obtain substantial biomass and production of secondary metabolites.
Hydroponics
Hydroponic systems include all systems that deliver nutrients in liquid, with or without a solid medium to anchor plant roots (Hayden 2006) (Fig. 3c). Such systems have been applied to several medicinal plants, such as Echinacea angustifolia, Ocimum basilicum, Leonurus quinquelobatus, Mentha piperita, Salvia officinalis, Achillea millefolium, Bidens tripartite, Leonurus sibiricus, Linum usitatissimum, Hypericum perforatum, and Tanacetum parthenium (Maggini et al. 2012; Mairapetyan et al. 2018; Simeunovic 2002). Thanks to these systems, the biosynthesis of active compounds, such as tropane alkaloids in Datura innoxia, total phenols and rosmarinic acid in Ocimum basilicum, and oil production in Valeriana officinalis, has been obtained (Gontier et al. 2002; Sgherri et al. 2010; Tabatabaei 2008).
Different hydroponic culture systems also exist for the mass production of AMF. They mainly differ in the mode of aeration and application of the nutrient solution (IJdo et al. 2011). For instance, in the static hydroponic culture system, the nutrient solution is not flowing and needs to be aerated via an aeration pump to prevent roots of mycorrhizal plants from suffering oxygen deprivation (IJdo et al. 2011). Via this system, Dugassa et al. (1995) obtained large quantities of mycorrhizal Linum usitatissimum plant roots as well as extramatrical mycelium and chlamydospores free of residues from solid substrate components. In another nutrient film technique (NFT) hydroponic system, a thin nutrient solution (i.e., film) flows into inclined channels (also called gulls) where the plant roots and AMF develop (IJdo et al. 2011). This technique has been used to culture AMF since the 1980s with the production of many sporocarps by F. mosseae (Elmes and Mosse 1984). Later, IJdo et al. (2011) developed an innovative low-cost in vitro plant-based bioreactor system for the mass production of AMF. In this system, Medicago truncatula roots and AMF (Glomus sp.) were grown in a sterilized tube connected at both extremities to a reservoir containing sterilized liquid culture medium. This nutrient solution circulates across the mycorrhizal root system, feeding the plant/fungus associates, while the plant shoot develops in open-air conditions inside a controlled growth chamber (Fig. 3e). The hydroponic system also has been developed for studying the effect of the AMF symbiosis (e.g., P uptake) on maize plants (Garcés-Ruiz et al. 2017). Therefore, hydroponic or semi-hydroponic systems could potentially be combined with medicinal plants and AMF in order to obtain increased production of secondary metabolites. In a recent study, Cartabia et al. (2021) showed how the R. irregularis modified the primary and secondary metabolism and the root exudates of the medicinal plant Anchusa officinalis growing under a semi-hydroponic cultivation system (Fig. 3c). Moreover, permeabilization treatments can be conducted in these cultivation systems, in order to extract the compounds exuded by roots in a non-destructive process that “milks” the same plants several times a year. For example, in the study by Gontier et al. (2002), Datura innoxia plants were cultivated in hydroponic conditions (no AMF were involved) and the plant roots subsequently permeabilized with Tween 20. As a result, a high concentration of tropane alkaloids (TA) (e.g., hyoscyamine and scopolamine) was detected in the nutrient solution. Interestingly, all the plants were able to survive after being rinsed and replaced in the hydroponic system. This approach allows the permeabilization of the plant multiple times without loss of viability (Gontier et al. 2002). Moreover, different permeabilization treatments (e.g., doses and duration of Tween 20, addition of TA precursors) can be chosen to release additional bioactive compounds in the nutrient solution (i.e., TA precursors (phenylalanine and ornithine) leading to 10–80 mg/l TA in the nutrient solution) (Gontier et al. 2002). This study, however, did not include association with AMF.
In vitro production systems
Micropropagation or in vitro propagation is the clonal propagation of plants by tissues, cells, or organs. It involves the aseptic culture of explants of tissues or organs in closed vessels using defined culture media in a controlled environment (Debnath and Arigundam 2020).
Whole plant in vitro culture
In vitro cultivation of whole plants is widely used for mass propagation, conservation of germplasm, production of bioactive compounds, and genetic improvement of a large number of medicinal plant species (Nalawade and Tsay 2004). For instance, protocols have been developed for the in vitro mass propagation of Limonium wrightii, Adenophora triphylla, Gentiana davidii, Anoectochilus formosanus, Scrophularia yoshimurae, Pinellia ternata, Bupleurum falcatum, Zingiber zerumbet, Dendrobium linawianum, and Fritillaria hupehensis via shoot morphogenesis, for Angelica sinensis and Corydalis yanhusuo via somatic embryogenesis, and for Taxus mairei, Angelica dahurica, Angelica sinensis, Dioscorea doryophora, Gentiana davidii, and Bupleurum falcatum via cell suspension cultures (Nalawade and Tsay 2004).
The association of AMF with whole plants in vitro has been described in several studies (e.g., Dupré de Boulois et al. 2006; Voets et al. 2005; Lalaymia and Declerck 2020). For instance, using the mycorrhizal donor plant in vitro cultivation system, Voets et al. (2009) obtained fast and homogenous mycorrhization of Medicago truncatula seedlings by placing the plantlets in an actively growing mycelial network arising from a mycorrhizal donor plant (Fig. 4a). In another system, called the half-closed arbuscular mycorrhizal plant in vitro culture system, roots of micropropagated potato plantlets were associated with actively growing AMF propagules, while the shoots developed in open-air conditions (Voets et al. 2005) (Fig. 4c). Applying both systems, several thousand spores of R. intraradices were produced on an extensive extraradical mycelium and abundant root colonization has been obtained. Hence, they could be extended to medicinal plants to enhance secondary metabolite production (Fig. 4).
Fig.4.
(a) A 145-mm mycorrhizal donor plant in vitro culture system. (i) The donor plant is Crotalaria spectabilis growing in a root compartment (RC) in close association with the arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 and (ii) the receiver plants are Alkanna tinctoria growing under a lid in a hyphal compartment (HC) in which only a profuse, active extraradical mycelium network proliferates; (b) close-up view of extensive development of extraradical mycelium and spores in the HC; (c) a 90-mm half-closed arbuscular mycorrhizal plant in vitro culture system allowing the growth of the roots of Lithospermum erythrorhizon in close association with R. irregularis MUCL 41833; (d) close-up view of the reddish roots due to shikonin production; (e) a 90-mm root organ culture in vitro system allowing the growth of Ri T-DNA transformed A. tinctoria hairy root (Rat et al. 2021) in assocation with R. irregularis MUCL 41833 in the RC; (f) close-up view of the red AMF spores produced in the RC (arrows). We thank Alicia Varela Alonso (Institut für Pflanzenkultur, Germany) for graciously providing the pictures c and d and Angélique Rat (Ghent University, Belgium) for providing the Alkanna tinctoria hairy roots used in this figure. The system (a) starts with a donor plant (Crotalaria spectabilis) introduced into the RC of a bi-compartmented system (a small Petri dish indicated by a dashed circle (RC) (90 mm diameter)) placed in a large Petri dish (HC) (145 mm diameter). A hole is made in both Petri dishes allowing the shoot to extend outside the system. Approximately 500 spores from an AMF in vitro culture are placed in contact with the roots. The roots and AMF are kept in the dark during the whole growth period, while shoots remain under light. Once the donor plant is well colonized, the extraradical mycelium starts to cross the partition wall separating the RC from the HC, developing profusely in the HC. At that time, one or several receiver micropropagated plants (Alkanna tinctoria) are placed in the HC with their roots in contact with the extraradical mycelium. The plants are planted inside the HC under a lid. Briefly, the base of a cylinder (150 mm high, 100 mm diameter) matches a hole made in the lid of the 145-mm Petri dish. The cylinder top is glued to a 100-mm Petri dish lid. The culture dishes containing the A. tinctoria plants are sealed and covered, up to the base of the cylinder, by black plastic bags. The systems are incubated in a growth chamber to allow plant and AMF growth (detailed procedures of this system can be found in Lalaymia and Declerck (2020)). For system (c), homogenously chopped agar containing AMF propagules from an AMF in vitro culture is inoculated to the newly growing roots of a micropropagated seedling of Lithospermum erythrorhizon. After a few days, the new hyphae growing from the spores colonize the roots of L. erythrorhizon. In system (e), fine root structures of Ri T-DNA transformed Alkanna tinctoria hairy roots are cut and placed in the RC part of a bi-compartmental Petri dish. Chopped agar containing AMF propagules is spread on the young parts of the hairy roots. After a few days, new hyphae growing from spores colonize the A. tinctoria hairy root, producing new spores and extensive mycelium after several months. All these three techniques should be conducted under a laminar flow hood with sterilized laboratory materials
Hairy root cultures (HRCs)
Hairy root cultures are cultures raised after the infection of explants/cultures by the gram-negative soil bacterium Agrobacterium rhizogenes2 (Tepfer and Casse-Delbart 1987). This bacterium leads to the neoplastic growth of roots which are characterized by high growth rates in hormone-free media and genetic stability (Pistelli et al. 2010). Ri T-DNA transformed hairy roots grow faster than adventitious roots, or even conventional cultures in which plants are growing in soil or substrate (Paek et al. 2009; Yu et al. 2005). For instance, Panax ginseng, a valuable medicinal plant originating from Asia, has been used as a healing drug and health tonic since ancient times (Tang and Eisenbrand 1992) due to its production of triterpene saponins, collectively called ginsenosides (Dewick 1997; Huang 1999). However, it generally takes 5 to 7 years in the field to attain maturity and to reach the harvesting stage for extraction of bioactive compounds (Murthy et al. 2014). To solve this problem, many different techniques have been explored, such as culture of callus tissues, suspended cells, adventitious roots, and HRCs (Furuya et al. 1973, 1983; Paek et al. 2009; Shi et al. 2021). It has been reported that HRCs grow more rapidly and produce a higher level of ginsenosides than the suspended cells and adventitious root cultures (Inomata et al. 1993; Yoshikawa and Furuya 1987). Moreover, HRCs produce the same phytochemical patterns as the wild-type root organs and accumulate higher levels of certain valuable compounds compared with adventitious roots and native-grown plant roots (Kai et al. 2011; Hao et al. 2020; Miao et al. 2017). For instance, the total tanshinone content reached 15.4 mg/g dry weight (DW) in transgenic Salvia miltiorrhiza hairy roots, while only 1.7–9.7 mg/g DW tanshinone was produced in roots of field-grown plants (Kai et al. 2011; Hao et al. 2020). High stability and productivity, high biomass production, and efficient biosynthetic capacity make HRCs valuable biotechnological tools for the production of plant secondary metabolites (Pistelli et al. 2010; Gutierrez-Valdes et al. 2020). Some examples of metabolites produced using HRCs are tropane alkaloids, such as scopolamine and hyoscyamine (Jouhikainen et al. 1999; Häkkinen et al. 2016; Guo et al. 2018; Khezerluo et al. 2018), catharanthine (Hanafy et al. 2016), ginsenosides (Woo et al. 2004; Ha et al. 2016), solanoside (Putalun et al. 2004), and anthraquinones (Perassolo et al. 2017). The studies mentioned here, however, did not involve inoculation with AMF.
In order to upscale production and commercialize secondary metabolites, various conventional bioreactors, broadly classified as liquid phase, gas phase, or hybrid reactors, have been employed for the mass production of HRCs, which permit the growth of interconnected tissues normally unevenly distributed throughout the vessel. For instance, increased production of terpenoid indole alkaloid and artemisinin was obtained with HRCs of Rauwolfia serpentina and Artemisia annua grown in different reactors (no AMF involved) (Mehrotra et al. 2015; Patra and Srivastava 2014). Interestingly, the in vitro large-scale production of AMF is mostly based on HRCs (Declerck et al. 1996; Declerck 2006) which involve the association of AMF propagules with transformed hairy roots on synthetic mineral media. Arbuscular mycorrhizal fungi species grown in HRCs produce viable and contaminant-free spores (Fortin et al. 2005) (Fig. 4e). From these studies, we can suggest HRCs of medicinal plants associated with AMF in bioreactors for the commercial production of secondary metabolites.
Conclusions
Arbuscular mycorrhizal fungi may confer several benefits to medicinal plants, such as growth promotion and improved tolerance to stress conditions. Interestingly, AMF also may enhance the accumulation of active substances in those plants. This makes mycorrhizal technology a potential and sustainable tool for improving the growth and secondary metabolite production of medicinal plants. Factors such as light, temperature, humidity, soil fertility, and cultivation techniques also could influence secondary metabolite production by medicinal plants (Szakiel and Pączkowski 2011a, b). It is thus essential to consider these parameters in fine-tuning the conditions for optimal production of plants and associated secondary metabolites. In order to guarantee the quality of the bioactive substances produced by mycorrhizal medicinal plants, different substrate or substrate-free systems were described: aeroponic and hydroponic or semi-hydroponic systems, micropropagated medicinal plants in half-closed arbuscular mycorrhizal-plant or mycorrhizal donor-plant in vitro culture systems, and HRCs. These systems may provide adequate environmental conditions to the plants, resulting in improved crop yield and production of bioactive compounds (Nazari Deljou et al. 2014; Dayani and Sabzalian 2017).
Whatever the system considered, high yields of secondary metabolites are dependent on the AMF strain, the plant, and the environmental growth conditions. Arbuscular mycorrhizal fungi are not host specific, but their affinity to a particular host can be preferential (Cesaro et al. 2008). Early studies have documented that different AMF species may induce differences in secondary metabolite production in the same host or genotype (Zeng et al. 2013). For example, Glomus caledonium increased rosmarinic acid and caffeic acid production in Ocimum basilicum, whereas F. mosseae enhanced only caffeic acid production (Toussaint et al. 2007). In a recent study, Frew (2020) showed that inoculation with four commercial AMF species (C. etunicatum, Funneliformis coronatum, F. mosseae, and R. irregularis) had stronger effects on cereal crop plant allometric partitioning, foliar nutrient, and phenolic concentrations than inoculation with a single commercial AMF species (R. irregularis). Interestingly, the results also showed that the effects of inoculating with these four commercial AMF species were not different from the effects of applying a native AMF inoculant extracted from field soil, suggesting that commercial AMF assemblages may provide little to no additional benefit compared with a resident AMF community (Frew 2020). Thus, a thorough study of AMF species native to medicinal plants should be conducted before choosing the most effective AMF species (native or not; single or combinations of different AMF strains) to inoculate target plant species.
The use of commercial inoculants is an option which should be considered with caution. Indeed, a number of studies have shown that the absence of a regulatory context for the industry of AMF inoculants may have contributed to inoculants of questionable quality. For instance, Salomon et al. (2022) reported that over 80% of tested commercial inoculants contained no viable propagules when screened in sterilized soil. Moreover, when preparing AMF inoculum, adequate phytosanitary controls must be implemented to avoid proliferation of unwanted microbes which may subsequently contaminate plant production. The use of root organs in vitro may provide a solution by avoiding the presence of such contaminants. However, genetically modified plants may represent a drawback for field application and thus for commercial mass production, and the number of species grown in this system remains limited (Ijdo et al. 2011).
Finally, whatever system is used, environmental parameters should be considered seriously. For instance, light intensity is known to strongly impact the development of AMF. Konvalinková and Jansa (2016) reported that a sudden decrease of light availability to an AMF-colonized plant resulted in a rapid decrease of P transfer from the AMF to the plant, and when arbuscular mycorrhizal plants were exposed to long-periods of shading (weeks to months), positive mycorrhizal growth responses often declined. Ballhorn et al. (2016) also reported that under light limited conditions, vegetative and reproductive traits were inhibited in AMF inoculated Phaseolus lunatus plants relative to non-colonized plants. Thus, in controlled conditions, light intensity and quality (e.g., blue/red ratio) should be modulated to improve and guarantee the symbiosis between AMF and plants of interest (Konvalinková and Jansa 2016). Adequate timing and harvestable plant parts also are crucial factors to increase the production of secondary metabolites. The best time to harvest (quality peak season/time of day) should be determined according to the quality and quantity of biologically active constituents rather than the total vegetative yield of targeted medicinal plant parts. Taking into consideration all these environmental factors would help optimize plant-AMF associations, increasing biomass and secondary metabolite production.
Funding
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No 721635.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
The species names of AMF shown in this review follow the current nomenclature and not that of the original publications.
Now called Rhizobium rhizogenes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Zeyad R, Khan A, Khoo C. Occurrence of arbuscular mycorrhiza in Castanospermum australe A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza. 1999;9:111–117. [Google Scholar]
- Ahanger MA, Tyagi SR, Wani MR, Ahmad P. Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients. In: Ahmad P, Wani MR, editors. Physiological mechanisms and adaptation strategies in plants under changing environment. New York, NY: Springer; 2014. pp. 25–55. [Google Scholar]
- Ahmad A, Hayat I, Arif S, Masud T, Khalid N, Ahmed A. Mechanisms involved in the therapeutic effects of soybean (Glycine Max) Int J Food Prop. 2014;17:6. [Google Scholar]
- Akiyama K, Hayashi H. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot (lond) 2006;97:925–931. doi: 10.1093/aob/mcl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi JSM, Al-Shihi BI, Baqi Y (2017) Date palm tree (Phoenix dactyliferaL.): natural products and therapeutic options. Front Plant Sci 8:845 [DOI] [PMC free article] [PubMed]
- Ali HM. Importance of medicinal plants. Res Pharm Healt Sci. 2019;5(2):151. [Google Scholar]
- Allen MF, Moore TS Jr, Christensen M (1980) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae: I. Cytokinin increases in the host plant. Can J Bot 58(3):371–374
- Allen MF,Moore TS Jr,Christensen M (1982) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae. II. Altered levels of gibberellin-like substances and abscisic acid in the host plant. Can J Bot 58(3):468–471
- Alwhibi MS, Hashem A, Abd-Allah EF, Alqarawi AA, Soliman DWK, Wirth S, Egamberdieva D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J Integ Agri. 2017;16(8):1751–1757. doi: 10.1016/S2095-3119(17)61695-2. [DOI] [Google Scholar]
- Amanifar S, and Toghranegar Z (2020) The efficiency of arbuscular mycorrhiza for improving tolerance ofValeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress.Ind. Crops Prod. 147:112234
- An L, Zhou Z, Yan A, Gan Y. Progress on trichome development regulated by phytohormone signaling. Plant Signal Behav. 2011;6(12):1959–1962. doi: 10.4161/psb.6.12.18120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade SAL, Malik S, Sawaya ACHF, Bottcher A, Mazzafera P. Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant. 2013;35:867–880. doi: 10.1007/s11738-012-1130-8. [DOI] [Google Scholar]
- Andújar I, Recio MD, Giner RM, Ríos JL. Traditional Chinese medicine remedy to jury: the pharmacological basis for the use of shikonin as an anticancer therapy. Curr Med Chem. 2013;20:2892–2898. doi: 10.2174/09298673113209990008. [DOI] [PubMed] [Google Scholar]
- Araim G, Saleem A, Arnason JT, Charest C (2009) Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J Agric Food Chem 57(6):2255–2258 [DOI] [PubMed]
- Arpana J, Bagyaraj DJ, Prakasa Rao EVS, Parameswaran TN, Abdul Rahiman BA (2008) Symbiotic response of patchouli [Pogostemon cablin (Blanco) Benth.] to different arbuscular mycorrhizal fungi. Adv Environ Biol 2(1):20–24
- Arpanahi AA, Feizian M, Mehdipourian G, Khojasteh DN (2020) Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur J Soil Biol 100:103217
- Asgarpanah J, Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. Afr J Pharmacy Pharmacol. 2012;6(31):2340–2345. [Google Scholar]
- Asrar AWA, Elhindi KM. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J of Biol Sci. 2010;18(1):93–98. doi: 10.1016/j.sjbs.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayan AK, Cirak C. Hypericin and pseudohypericin contents in some Hypericum species growing in Turkey. Pharm Biol. 2008;46(4):288–291. doi: 10.1080/13880200701741211. [DOI] [Google Scholar]
- Ayeka PA, Bian Y, Mwitari PG,Zhang YJ, Otachi E, Bian YH, Chu XQ, Uzayisenga R (2016) Immunomodulatory and anticancer potential of Gan cao(Glycyrrhiza uralensisFisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement Altern Med 16:206 [DOI] [PMC free article] [PubMed]
- Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int M. 2014;2014:842674. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairwa R, Sodha RS, Rajawat BS. Trachyspermum Ammi Pharmacogn Rev. 2012;6(11):56–60. doi: 10.4103/0973-7847.95871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballhorn DJ, Schädler M, Elias JD, Millar JA, Kautz S (2016) Friend or foe—light availability determines the relationship between mycorrhizal fungi, rhizobia and lima bean (Phaseolus lunatus L.). PLoS ONE 11(5):e0154116 [DOI] [PMC free article] [PubMed]
- Bartram S, Jux A, Gleixner G, Boland W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry. 2006;67(15):1661–1672. doi: 10.1016/j.phytochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Battini F, Bernardi R, Turrini A, Agnolucci M, Giovannetti M. Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza. 2016;26:699–707. doi: 10.1007/s00572-016-0707-2. [DOI] [PubMed] [Google Scholar]
- Baum C, El-Tohamy W, Gruda N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci Hortic. 2015;187:131–141. doi: 10.1016/j.scienta.2015.03.002. [DOI] [Google Scholar]
- Baytop T (1999) Therapy with medicinal plants in Turkey pp 66–167
- Behdad A, Mohsenzadeh S, Azizib M, Moshtaghi N (2020) Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabraL. populations. Phytochemistry 171:112236 [DOI] [PubMed]
- Behnam S, Farzaneh M, Ahmadzadeh M, Tehrani AS (2006) Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post- harvest phytopathogens. Commun Agric Appl Biol Sci 71(3 Pt B):1321–1326 [PubMed]
- Beik A, Joukar S, Najafipour H. A review on plants and herbal components with antiarrhythmic activities and their interaction with current cardiac drugs. J Tradit Complement Med. 2020;10(3):275–287. doi: 10.1016/j.jtcme.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bhosale UA, Yegnanarayan R, Pophale P, Somani R. Effect of aqueous extracts of Achyranthes aspera Linn. on experimental animal model for inflammation. Ancient Sci Life. 2012;31:202–206. doi: 10.4103/0257-7941.107362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet MN, van Tuinen D, Deprêtre N, Koszela N, Chambon C, Gianinazzi S. Arbuscular mycorrhizal fungi associated with Artemisia umbelliformis Lam, an endangered aromatic species in Southern French Alps, influence plant P and essential oil contents. Mycorrhiza. 2011;21:523–535. doi: 10.1007/s00572-010-0354-y. [DOI] [PubMed] [Google Scholar]
- Birje R, Golatkar VV. Effect of AM fungi on Mentha spicata L. Int J Life Sciences Special Issue. 2016;A7:33–40. [Google Scholar]
- Bistgani ZE, Hashemib M, DaCostab M, Crakerb L, Maggic F, Morshedloo MR. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind Crops Prod. 2019;135:311–320. doi: 10.1016/j.indcrop.2019.04.055. [DOI] [Google Scholar]
- Bombardelli E, Morazzoni P. Hypericum perforatum. Fitoterapia. 1995;66:43–68. [Google Scholar]
- Bona E, Lingua G, Todeschini V (2016) Effect of bioinoculants on the quality of crops. In: Arora NK, Mehnaz S, Balestrini R (eds) Bioformulations: for sustainable agriculture. Springer India, New Delhi, pp 93–124
- Borde M, Dudhane M, Jite PK (2009) Role bioinoculant (AM fungi) increasing in growth, flavor content and yield in Allium sativum L. under field condition. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 37(2):124–128
- Boughalleb F, Abdellaoui R, Mahmoudi M, Bakhshandeh E. Changes in phenolic profile, soluble sugar, proline, and antioxidant enzyme activities of Polygonum equisetiforme in response to salinity. Turk J Bot. 2020;44:25–35. doi: 10.3906/bot-1908-2. [DOI] [Google Scholar]
- Bowles TM, Barrios-Masias FH, Carlisle EA, Cavagnaro TR, Jackson LE. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci Total Environ. 2016;566–567:1223–1234. doi: 10.1016/j.scitotenv.2016.05.178. [DOI] [PubMed] [Google Scholar]
- Brader G, Compant S, Mitter B, Trognitz F. Metabolic potential of endophytic bacteria. Curr Opin Biotechnol. 2014;27:30–37. doi: 10.1016/j.copbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britannica (2021) The editors of encyclopaedia. Tannin. Encyclopedia Britannica. 27 Jan. 2021, https://www.britannica.com/science/tannin. Accessed 9 May 2022
- Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018;220:1108–1115. doi: 10.1111/nph.14976. [DOI] [PubMed] [Google Scholar]
- Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67(12):2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- Bychkova TP, Nanenina EB, Berzin VB, Miroshnikov AI. Influence of jasmonic acid and of 12-oxophytodienoic acid on the biosynthesis of shikonin in a cell culture of Arnebia euchroma. Bioorg Khim. 1993;19:1008–1012. [Google Scholar]
- Cao B, Dang QL, Yu¨ X, Zhang S, Effects of [CO2] and nitrogen on morphological and biomass traits of white birch (Betula papyrifera) seedlings. Forest Ecol Manag. 2008;254(2):217–224. doi: 10.1016/j.foreco.2007.08.002. [DOI] [Google Scholar]
- Carreón-Abud Y, Torres-Martínez R, Farfán-Soto B, Hernández-García A, Ríos-Chávez P, BelloGonzález MÁ, Martínez-Trujillo M, Salgado-Garciglia R (2015) Arbuscular mycorrhizal symbiosis increases the content of volatile terpenes and plant performance in Satureja macrostema (Benth.) Briq. Bol Latinoam Caribe Plant Med Aromat 14(4):273–279
- Cartabia A, Tsiokanos E, Tsafantakis N, Lalaymia I, Termentzi A, Miguel M, Fokialakis N, Declerck S (2021) The arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 modulates metabolites production of Anchusa officinalis L. under semi-hydroponic cultivation. Front Plant Sci 12:724352 [DOI] [PMC free article] [PubMed]
- Castrillo G, Teixeira PJ, Paredes SH, Law TF, de Lorenzo L, Feltcher ME, Finkel OM, Breakfield NW, Mieczkowski P, Jones CD, Paz-Ares J, Dangl JL. Root microbiota drive direct integration of phosphate stress and immunity. Nature. 2017;543:513–518. doi: 10.1038/nature21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, Giovannetti M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil. 2010;335:311–323. doi: 10.1007/s11104-010-0417-z. [DOI] [Google Scholar]
- Cesaro P, van Tuinen D, Copetta A, Chatagnier O, Berta G, Gianinazzi S, Lingua G (2008) Preferential colonization of Solanum tuberosum L. roots by the fungus Glomus intraradices in arable soil of a potato farming area. Appl Environ Microbiol 74(18):5776–5783 [DOI] [PMC free article] [PubMed]
- Chajra H, Salwinski A, Guillaumin A, Mignard B, Hannewald P, Duriot L, Warnault P, Guillet-Claude C, Fréchet M, Bourgaud F. Plant milking technology—an innovative and sustainable process to produce highly active extracts from plant roots. Molecules. 2020;25(18):4162. doi: 10.3390/molecules25184162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Khan S, Avula B, Lata H, Yang MH, Elsohly MA, Khan IA (2014) Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Alternat Med 2014 [DOI] [PMC free article] [PubMed]
- Chandran H, Meena M, Barupal T, Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep (amst) 2020;26:e00450. doi: 10.1016/j.btre.2020.e00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary VL, Kapoor R, Bhatnagar AK. Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua. Appl Soil Ecol. 2008;40:174–181. doi: 10.1016/j.apsoil.2008.04.003. [DOI] [Google Scholar]
- Chen YL, Guo PL, Li JX, He XH (2014) Application of AM Fungi to improve the value of medicinal plants. In: Solaiman ZM, Abbott LK, Varma A (eds) Mycorrhizal fungi: use in sustainable agriculture and land restoration. Springer. Chapter 10, pp 171−187
- Chen M, Arato M, Borghi L, Nouri E, Reinhardt D. Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Front Plant Sci. 2018;9:1270. doi: 10.3389/fpls.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Yang G, Sheng Y, Li PY, Qiu HY, Zhou XT, Huang LQ, Chao Z. Glomus mosseae inoculation improves the root system architecture, photosynthetic efficiency and flavonoids accumulation of liquorice under nutrient stress. Front Plant Sci. 2017;8:931. doi: 10.3389/fpls.2017.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Lee DJ, Wee CD, Kim HL (2009) Effects of AMF inoculation on growth of Panax ginseng C.A. Meyer seedlings and on soil structures in mycorrhizosphere. Sci Hortic 122(4):633–637
- Cicatelli A, Torrigiani P, Todeschini V, Biondi S, Castiglione S, Lingua G (2014) Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects. iForest 7:333–341
- Coleman DC, Crossley DA, Hendrix PF (2004) Primary production processes in soils: roots and rhizosphere associates. In: Coleman DC, Crossley DA, Hendrix PF (eds) Fundamentals of Soil Ecology (2nd Edition). Academic Press. pp 23–46
- Colonna E, Rouphael Y, De Pascale S, Barbieri G. Effects of mycorrhiza and plant growth promoting rhizobacteria on yield and quality of artichoke. Acta Hortic. 2016;1147:43–50. doi: 10.17660/ActaHortic.2016.1147.6. [DOI] [Google Scholar]
- Compant S, Cambon MC, Vacher C, Mitter B, Samad A, Sessitsch A. The plant endosphere world – bacterial life within plants. Environ Microbiol. 2021;23(4):1812–1829. doi: 10.1111/1462-2920.15240. [DOI] [PubMed] [Google Scholar]
- Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16(7):485–494 [DOI] [PubMed]
- Cosme P, Rodríguez AB, Espino J, Garrido M. Plant phenolics: bioavailability as a key determinant of their potential health-promoting applications. Antioxidants. 2020;9:1263. doi: 10.3390/antiox9121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello PS, Teoh KH, Polichuk DR, Reed DW, Nowak G. Functional genomics and the biosynthesis of artemisinin. Phytochemistry. 2007;68(14):1864–1871. doi: 10.1016/j.phytochem.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Cox-Georgian D, Ramadoss N, Dona C, Basu C (2019) Therapeutic and medicinal uses of terpenes. J Med Plant Res 333–359
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dambisya YM, Tindimwebwa G. Traditional remedies in children around Eastern Cape. South Africa East Afr Med J. 2003;80(8):402–405. doi: 10.4314/eamj.v80i8.8730. [DOI] [PubMed] [Google Scholar]
- Dave S, Das J, Tarafdar JC. Effect of vesicular arbuscular mycorrhizae on growth and saponin accumulation in Chlorophytum borivilianum. ScienceAsia. 2011;37(2):165–169. doi: 10.2306/scienceasia1513-1874.2011.37.165. [DOI] [Google Scholar]
- Dayani S, Sabzalian MR (2017) Production of secondary metabolites in medicinal plants through hydroponic system. In: Asaduzzaman M (ed) Controlled environmental agriculture -production of specialty crops providing human health benefits through hydroponics. Nova Science, Inc., New York. Chapter 2, pp 33–53
- Deans SG, Waterman PG. Biological activity of volatile oils. In: Waterman PG, editor. Volatile Oil Crops: Their Biology, Biochemistry and Pro-duction (Hay RKM. Longman Scientific and Technical: Essex, England; 1993. pp. 97–111. [Google Scholar]
- de Vries FT, Griffiths RI, Knight CG, Nicolitch O, Williams A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science. 2020;368:270–274. doi: 10.1126/science.aaz5192. [DOI] [PubMed] [Google Scholar]
- Debnath SC, Arigundam U (2020) In vitro propagation strategies of medicinally important berry crop, Lingonberry (Vaccinium vitis-idaea L.). Agronomy 10:744
- Declerck S (2006) Being sure of what you are buying. The BCPC International conference (Crop Science and technology) — Glasgow, Scotland, UK. Communication
- Declerck S, Strullu DG, Plenchette C. In vitro mass production of the arbuscular mycorrhizal fungus, Glomus versiforme, associated with Ri T-DNA transformed carrot roots. Myco Res. 1996;100:1237–1242. doi: 10.1016/S0953-7562(96)80186-9. [DOI] [Google Scholar]
- Declerck S, IJdo M, Fernandez K, Voets L, de la Providencia I (2009) Method and system for in vitro mass production of arbuscular mycorrhizal fungi. WO/2009/090220 [DOI] [PubMed]
- Dewick PM. Medicinal natural products. West Sussex, UK: Wiley; 1997. [Google Scholar]
- Domokos E, Jakab-Farkas L, Darkó B, Bíró-Janka B, Mara G, Albert C, Balog A. Increase in Artemisia annua plant biomass artemisinin content and guaiacol peroxidase activity using the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci. 2018;9:478. doi: 10.3389/fpls.2018.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos EL, Alves da Silva F, Barbosa da Silva FS. Arbuscular mycorrhizal fungi increase the phenolic compounds concentration in the bark of the stem of Libidibia Ferrea in field conditions. Open Microbiol J. 2017;11:283–291. doi: 10.2174/1874285801711010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos EL, Falcão EL, Barbosa da Silva FS. Mycorrhizal technology as a bioinsumption to produce phenolic compounds of importance to the herbal medicine industry. Res Soc Dev. 2021;10(2):e54810212856. doi: 10.33448/rsd-v10i2.12856. [DOI] [Google Scholar]
- Duc NH, Vo AT, Haddidi I, Daood H, Posta K (2021) Arbuscular mycorrhizal fungi improve tolerance of the medicinal plantEclipta prostrata(L.) and induce major changes in polyphenol profiles under salt stresses. Front Plant Sci 11:612299 [DOI] [PMC free article] [PubMed]
- Dugassa DG, Grunewaldt-StOcker G, Schonbeck F. Growth of Glomus intraradices and its effect on linseed (Linum usitatissimum L.) in hydroponic culture. Mycorrhiza. 1995;5:279–282. [Google Scholar]
- Dupré de Boulois H, Voets L, Delvaux B, Jakobsen I, Declerck S. Transport of radiocaesium by arbuscular mycorrhizal fungi to Medicago truncatula under in vitro conditions. Environ Microbiol. 2006;8:1926–1934. doi: 10.1111/j.1462-2920.2006.01070.x. [DOI] [PubMed] [Google Scholar]
- Dutta SC, Neog B. Accumulation of secondary metabolites in response to antioxidant activity of turmeric rhizomes co-inoculated with native arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Sci Hortic. 2016;204:179–184. doi: 10.1016/j.scienta.2016.03.028. [DOI] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahian F, Garshasbi M, Asiabar ZM, Dehkordi NG, Yazdinezhad A, Mirzaei SA (2021) Ecotypic variations affected the biological effectiveness of Thymus daenensis Celak essential oil. Evid Based Complement Alternat Med pp. 12 [DOI] [PMC free article] [PubMed]
- Elmes RP, Mosse B. Vesicular-arbuscular endomycorrhizal inoculum production II Experiments with maize (Zea mays) and other hosts in nutrient flow culture. Can J Bot. 1984;62:1531–1536. doi: 10.1139/b84-203. [DOI] [Google Scholar]
- Engel R, Szabó K, Abrankó L, Rendes K, Füzy A, Takács T. Effect of arbuscular mycorrhizal fungi on the growth and polyphenol profile of marjoram, lemon balm, and marigold. J Agric Food Chem. 2016;64(19):3733–3742. doi: 10.1021/acs.jafc.6b00408. [DOI] [PubMed] [Google Scholar]
- Facchini PJ. Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:29–66. doi: 10.1146/annurev.arplant.52.1.29. [DOI] [PubMed] [Google Scholar]
- Fan JH, Yang GT, Mu LQ, Zhou JH. Effect of AM fungi on the content of berberine, jatrorrhizine and palmatine of Phellodendron amurense seedings. Prot Forest Sci Technol. 2006;5:24–26. [Google Scholar]
- Ferrini F, Fraternale D, Donati Zeppa S, Verardo G, Gorassini A, Carrabs V, Albertini MC, Sestili P (2021) Yield, characterization, and possible exploitation of Cannabis sativa L. roots grown under aeroponics cultivation. Molecules 26:4889 [DOI] [PMC free article] [PubMed]
- Fortin JA, Declerck S, Strullu DG (2005) In vitro culture of mycorrhizas. In: Declerck S, Fortin JA, Strullu DG (eds) In vitro culture of mycorrhizas. Soil Biology, vol 4. Springer, Berlin, Heidelberg
- Freitas MSM, Martins MA, Carvalho AJC, Carneio RFV (2004) Growth and production of total phenols in carqueja [Baccharis trimera (Less.) A.D.] in response to inoculation with arbuscular mycorrhizal fungi, in the presence and in the absence of mineral manuring. Rev Bras de Plantas Medicinais 6(3):30–34
- Frew A (2020) Contrasting effects of commercial and native arbuscular mycorrhizal fungal inoculants on plant biomass allocation, nutrients and phenolics. Plants People Planet 00:1–5
- Furuya T, Yoshikawa T, Orihara Y, Oda H. Saponin production in cell suspension cultures of Panax ginseng. Plant Med. 1983;48:83–87. doi: 10.1055/s-2007-969892. [DOI] [PubMed] [Google Scholar]
- Furuya T, Kojima H, Syono K, Ishii T, Uotani K. Isolation of saponins and sapogenins from callus tissue of Panax ginseng. Chem Pharm Bull (tokyo) 1973;21(1):98–101. doi: 10.1248/cpb.21.98. [DOI] [PubMed] [Google Scholar]
- Gadzovska S, Maury S, Ounnar S, Righezza M, Kascakova S, Refregiers M, Spasenoski M, Joseph C, Hagège D. Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum. L. in vitro cultures. Plant Physiol Biochem. 2005;43:591–601. doi: 10.1016/j.plaphy.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Gaisser S, Heide L. Inhibition and regulation of shikonin biosynthesis in suspension cultures of Lithospermum. Phytochemistry. 1996;41(4):1065–1072. doi: 10.1016/0031-9422(95)00633-8. [DOI] [Google Scholar]
- Garcés-Ruiz M, Calonne-Salmon M, Plouznikoff K, Misson C, Navarrete-Mier M, Cranenbrouck S, Declerck S. Dynamics of short-term phosphorus uptake by intact mycorrhizal and non-mycorrhizal maize plants grown in a circulatory semi-hydroponic cultivation system. Front Plant Sci. 2017;8:1471. doi: 10.3389/fpls.2017.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Faheem M, Singh S. Role of medicinal plant in human health disease. Asian J Plant Sci Res. 2021;11(1):19–21. [Google Scholar]
- Geneva MP, Stancheva IV, Boychinova MM, Mincheva NH, Yonova PA. Effects of foliar fertilization and arbuscular mycorrhizal colonization on Salvia officinalis L. growth, antioxidant capacity, and essential oil composition. J Sci Food Agric. 2010;90:696–702. doi: 10.1002/jsfa.3871. [DOI] [PubMed] [Google Scholar]
- Geysen HM, Schoenen F, Wagner D, Wagner R. Combinatorial compound libraries for drug discovery: an ongoing challenge. Nat Rev Drug Discov. 2003;2(3):222–230. doi: 10.1038/nrd1035. [DOI] [PubMed] [Google Scholar]
- Ghiaee A, Naghibi F, Esmaeili S, Mosaddegh M. Herbal remedies connected to malaria like fever in Iranian ancient medicinal books. Iran J Parasitol. 2014;9(4):553–559. [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7(4):433–440. doi: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgiu RM, Morar G, Dumitraș A, Vlăsceanu G, Dune A, Schroeder FG. A study of the cultivation of medicinal plants in hydroponic and aeroponic technologies in a protected environment. Acta Hortic. 2017;1170:671–678. doi: 10.17660/ActaHortic.2017.1170.84. [DOI] [Google Scholar]
- Gontier E, Clément A, Tran TLM, Gravot A, Lièvre K, Guckert A, Bourgaud F. Hydroponic combined with natural or forced root permeabilization: a promising technique for plant secondary metabolite production. Plant Sci. 2002;163(4):723–732. doi: 10.1016/S0168-9452(02)00171-1. [DOI] [Google Scholar]
- Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138–143. doi: 10.1016/S0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- Gu SH, Li L, Huang H, Wang B, Zhang T (2019) Antitumor, antiviral, and anti-inflammatory efficacy of essential oils from Atractylodes macrocephala Koidz produced with different processing methods. Molecules 24(16):2956 [DOI] [PMC free article] [PubMed]
- Guedes RC, Eriksson LA. Theoretical study of hypericin. J Photochem Photobiol A Chem. 2005;172:293–299. doi: 10.1016/j.jphotochem.2004.12.025. [DOI] [Google Scholar]
- Guo LP, Wang HG, Hang LQ. Effects of arbuscular mycorrhizae on growth and essential oil of Atractylodes lancea. Chin J Chin Mater Med. 2006;31(8):1491–1495. [PubMed] [Google Scholar]
- Guo Q, Cheng L, Liu Z. Study on influence of arbuscular mycorrhizal fungi Pinellia ternata yield and chemical composition. Zhongguo Zhong Yao Za Zhi. 2010;35(3):333–338. doi: 10.4268/cjcmm20100317. [DOI] [PubMed] [Google Scholar]
- Guo ZY, Tan HX, Lv ZY, Ji Q, Huang YX, Liu JJ, Chen DD, Diao Y, Si JP, Zhang L. Targeted expression of Vitreoscilla hemoglobin improves the production of tropane alkaloids in Hyoscyamus niger hairy roots. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-36156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta ML, Prasad A, Ram M, Kumar S. Effect of the vesicular arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour Technol. 2002;81(1):77–79. doi: 10.1016/S0960-8524(01)00109-2. [DOI] [PubMed] [Google Scholar]
- Gupta M, Kaushik S, Tomar RS, Mishra RK. An overview of Catharanthus roseus and medicinal properties of their metabolites against important diseases. Eur Acad Res. 2017;5(2):1237–1247. [Google Scholar]
- Gutierrez-Valdes N, Häkkinen ST, Lemasson C, Guillet M, Oksman-Caldentey KM, Ritala A, Cardon F. Hairy root cultures—a versatile tool with multiple applications. Front in Plant Sci. 2020;11:33. doi: 10.3389/fpls.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha LT, Pawlicki-Jullian N, Pillon-Lequart M, Boitel-Conti M, Duong HX, Gontier E. Hairy root cultures of Panax vietnamensis, a promising approach for the production of ocotillol-type ginsenosides. Plant Cell Tiss Organ Cult. 2016;126:93–103. doi: 10.1007/s11240-016-0980-y. [DOI] [Google Scholar]
- Häkkinen ST, Moyano E, Cusidó RM, Oksman-Caldentey KM. Exploring the metabolic stability of engineered hairy roots after 16 years maintenance. Front Plant Sci. 2016;7:1486. doi: 10.3389/fpls.2016.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AC. Medicinal plants, conservation and livelihoods. Biodivers Conserv. 2004;13:1477–1517. doi: 10.1023/B:BIOC.0000021333.23413.42. [DOI] [Google Scholar]
- Hanafy MS, Matter MA, Asker MS, Rady MR. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. S Afr J Bot. 2016;105:9–18. doi: 10.1016/j.sajb.2016.01.004. [DOI] [Google Scholar]
- Hao X, Pu Z, Cao G, You D, Zhou Y, Deng C, Shi M, Nile SH, Wang Y, Zhou W, Kai G. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J Adv Res. 2020;23:1–12. doi: 10.1016/j.jare.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A, Abd Allah EF, Alqarawi A, Egamberdieva D (2018) Arbuscular mycorrhizal fungi and plant stress tolerance. In: Egamberdieva D, Ahmad P (eds) Plant microbiome: stress response. Microorganisms for Sustainability, vol 5. Springer, Singapore. pp 81–103
- Hashem A, Abd_Allah EF, Alqarawi AA, Egamberdieva D (2016) Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci 23(1):39–47 [DOI] [PMC free article] [PubMed]
- Hashem A, Abd-Allah EF, Alqarawi AA, El-Didamony G, Alwhibi Mona S, Egamberdieva D, Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak J Bot. 2014;46:2003–2020. [Google Scholar]
- Hause B, Maier W, Miersch O, Kramell R, Strack D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 2002;130:1213–1220. doi: 10.1104/pp.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden AL (2006) Aeroponic and hydroponic systems for medicinal herb, rhizome and root crops. Hortic Science 41(3)
- Hayden AL, Brigham LA, Giacomelli GA. Aeroponic cultivation of ginger (Zingiber officinale) rhizomes. Acta Hort. 2004;659:397–402. doi: 10.17660/ActaHortic.2004.659.52. [DOI] [Google Scholar]
- Hayden AL, Yokelsen T, Giacomelli G, Hoffmann J. Aeroponics: an alternative production system for high value root crops. Acta Hort. 2004;629:207–213. doi: 10.17660/ActaHortic.2004.629.27. [DOI] [Google Scholar]
- Hemashenpagam N, Selvaraj T. Effect of arbuscular mycorrhizal (AM) fungus and plant growth promoting rhizomicroorganisms (PGPR's) on medicinal plant Solanum viarum seedlings. J Environ Biol. 2011;32(5):579–583. [PubMed] [Google Scholar]
- Hofmeyer PV, Seymour RS, Kenefic LS. Production ecology of Thuja occidentalis. Can J for Res. 2010;40(6):1155–1164. doi: 10.1139/X10-070. [DOI] [Google Scholar]
- Huang KC (1999) Pharmacology of Chinese herbs. CRC, Boca Raton, Florida, USA
- Huang JH, Tan JF, Jie HK, Zeng RS. Effects of inoculating arbuscular mycorrhizal fungi on Artemisia annua growth and its officinal components. J Appl Ecol. 2011;22(6):1443–1449. [PubMed] [Google Scholar]
- Hung LLL, Sylvia DM. Production of vesicular–arbuscular mycorrhizal fungus inoculum in aeroponic culture. Appl Environ Microbiol. 1988;54:353–357. doi: 10.1128/aem.54.2.353-357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein RA, El-Anssary AA (2018) Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. In: Builders P (ed) Herbal Medicine. Intech Open. Chapter 2
- IJdo M, Cranenbrouck S, Declerck S, Methods for large-scale production of AM fungi: past, present, and future. Mycorrhiza. 2011;21:1–16. doi: 10.1007/s00572-010-0337-z. [DOI] [PubMed] [Google Scholar]
- Inomata S, Yokoyama M, Gozu Y, Shimizu T, Yanagi M. Growthpattern and ginsenoside production of Agrobacterium transformed Panax ginsengroots. Plant Cell Rep. 1993;12:681–686. doi: 10.1007/BF00233419. [DOI] [PubMed] [Google Scholar]
- Isah T (2015) Rethinking Ginkgo biloba L.: medicinal uses and conservation. Pharmacogn Rev 9(18):140–148 [DOI] [PMC free article] [PubMed]
- Jahan R, Al-Nahain A, Majumder S, Rahmatullah M (2014) Ethnopharmacological significance of Eclipta alba (L.) Hassk. (Asteraceae). Int Sch Res Notices 2014:385969 [DOI] [PMC free article] [PubMed]
- Jaiti F, Meddich A, Hadrami IE (2007) Effectiveness of arbuscular mycorrhizal fungi in the protection of date palm (Phoenix dactyliferaL.) against bayoud disease. Physiol Mol Plant Pathol 71 (4–6):166–173
- Jan R, Asaf S, Numan M, Lubna KKM. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- Jana S, Shekhawat GS. Anethum graveolens: an Indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4(8):179–184. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarstfer AG, Sylvia DM. Aeroponic culture of VAM fungi. In: Varma A, Hock B, editors. Mycorrhiza. Heidelberg: Springer; 1995. pp. 427–441. [Google Scholar]
- Jeon BR, Irfan M, Kim M, Lee SE, Lee JH, Rhee MH. Schizonepeta tenuifolia inhibits collagen stimulated platelet function via suppressing MAPK and Akt signaling. J Biomed Res. 2019;33(4):250–257. doi: 10.7555/JBR.32.20180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian ZY, Meng L, Wang N, Xu GF, Yu JB, Dai L, Shi YH. Characteristic and protection of rare and endangered Taxus chinensis var. mairei in the Taihang Mountains. Nutr Hosp. 2016;33:698–702. doi: 10.20960/nh.281. [DOI] [PubMed] [Google Scholar]
- Johny L, Cahill DM, Adholeya A. AMF enhance secondary metabolite production in ashwagandha, licorice, and marigold in a fungi-host specific manner. Rhizosphere. 2021;17:100314. doi: 10.1016/j.rhisph.2021.100314. [DOI] [Google Scholar]
- Joshee N, Yadav AK, Mentreddy R. Mycorhizal fungi and growth and development of micropropagated Scutelleria integrifolia plants. Ind Crops Prod. 2007;25(2):169–177. doi: 10.1016/j.indcrop.2006.08.009. [DOI] [Google Scholar]
- Joshi RK (2014) Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of Northwest Karnataka, India. Anc Sci Life 33(3):151–156 [DOI] [PMC free article] [PubMed]
- Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teeri TH, Oksman-Caldentey KM. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta. 1999;208:545–551. doi: 10.1007/s004250050592. [DOI] [Google Scholar]
- Jugran AK, Rawat S, Bhatt ID, Ranbeer S. Valeriana jatamansi: an herbaceous plant with multiple medicinal uses. Phytother Res. 2019;33(3):482–503. doi: 10.1002/ptr.6245. [DOI] [PubMed] [Google Scholar]
- Jugran AK, Bahukhandi A, Dhyani P, Bhatt ID, Rawal RS, Nandi SK, Palni LM (2015) The effect of inoculation with mycorrhiza: AM on growth, phenolics, tannins, phenolic composition and antioxidant activity in Valeriana jatamansi Jones. J Soil Sci Plant Nutr 15(4):1036–1049
- Jun X, Fu P, Lei Y, Cheng P (2018) Pharmacological effects of medicinal components of Atractylodes lancea (Thunb.) DC. Chin Med 13:59 [DOI] [PMC free article] [PubMed]
- Jurkiewicz A, Ryszka P, Anielska T, Waligórski P, Białońska D, Góralska K, Tsimilli-Michael M, Kai G, Xu H, Zhou C, Liao P, Xiao J, Luo X, You L, Zhang L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab Eng. 2011;13(3):319–327. doi: 10.1016/j.ymben.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kai G, Xu H, Zhou C, Liao P, Xiao J, Luo X, You L, Zhang L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab Eng. 2011;13(3):319–27. doi: 10.1016/j.ymben.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Chaudhary V, Bhatnagar AK. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza. 2007;17(7):581–587. doi: 10.1007/s00572-007-0135-4. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Giri B, Mukerji KG. Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour Technol. 2004;93(3):307–311. doi: 10.1016/j.biortech.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Giri B, Mukerji KG. Glomus macrocarpum: a potential bioinoculant to improve essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague) World J Microb Biot. 2002;18:459–463. doi: 10.1023/A:1015522100497. [DOI] [Google Scholar]
- Kapoor R, Anand G, Gupta P, Mandal S. Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem Rev. 2017;16:677–692. doi: 10.1007/s11101-016-9486-9. [DOI] [Google Scholar]
- Karagiannidis N, Thomidis T, Panou-Filotheou E, Karagiannidou C. Response of three mint and two oregano species to Glomus etunicatum inoculation. AJCS. 2012;6(1):164–169. [Google Scholar]
- Kaur S, Suseela V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites. 2020;10(8):335. doi: 10.3390/metabo10080335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha C, Rajamani K, Vadivel E. Coleus forskohlii: a comprehensive review on morphology, phytochemistry and pharmacological aspects. J Med Plant Res. 2010;4(4):278–285. [Google Scholar]
- Khaosaad T, Krenn L, Medjakovic S, Ranner A, Lossl A, Nell M, Jungbauer A, Vierheilig H. Effect of mycorrhization on the isoflavone content and the phytoestrogen activity of red clover. J Plant Physiol. 2008;165:1161–1167. doi: 10.1016/j.jplph.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16(6):443–446 [DOI] [PubMed]
- Kheiri A, Amini S, Javidan AN, Saghafi MM, Khorasani G. The effects of Alkanna tinctoria Tausch on split-thickness skin graft donor site management: a randomized, blinded placebo-controlled trial. BMC Complement Altern Med. 2017;17:253. doi: 10.1186/s12906-017-1741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezerluo M, Hosseini B, Amiri J. Sodium nitroprusside stimulated production of tropane alkaloids and antioxidant enzymes activity in hairy root culture of Hyoscyamus reticulatus L. Acta Biol Hung. 2018;69:437–448. doi: 10.1556/018.69.2018.4.6. [DOI] [PubMed] [Google Scholar]
- Kim NH, Jayakodi M, Lee SC, et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol J. 2018;16(11):1904–1917. doi: 10.1111/pbi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinková T, Jansa J. Lights off for arbuscular mycorrhiza: on its symbiotic functioning under light deprivation. Front Plant Sci. 2016;7:782. doi: 10.3389/fpls.2016.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourounakis AP, Assimopoulou AAN, Papageorgiou AG, Gavalas A, Kourounakis PN. Alkanin and shikonin effect on free radicle process and on inflammation-A preliminary pharmacochemical investigation. Arch Pharm. 2002;6:262–266. doi: 10.1002/1521-4184(200208)335:6<262::AID-ARDP262>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Krishna S, Bustamante L, Haynes RK, Staines HM. Artemisinins: their growing importance in medicine. Trends Pharmacol Sci. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuete V (2014) Health effects of alkaloids from African medicinal plants. In book: Toxicological survey of African medicinal plants, pp 611–633
- Kumar S, Malhotra R, Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 2010;4(7):58–61. doi: 10.4103/0973-7847.65327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhiar IA, Gao JM, Syed TN, Chandio FA, Buttar NA. Modern plant cultivation technologies in agriculture under controlled environment: a review on aeroponics. J Plant Interact. 2018;13(1):338–352. doi: 10.1080/17429145.2018.1472308. [DOI] [Google Scholar]
- Lakshman CD. Biodiversity and conservation of medicinal and aromatic plants. Adv Plants Agric Res. 2016;5(4):561–566. [Google Scholar]
- Lalaymia I, Declerck S (2020) The mycorrhizal donor plant (MDP) in vitro culture system for the efficient colonization of whole plants. In: Ferrol N, Lanfranco L (eds) Arbuscular mycorrhizal fungi. Humana, New York, NY. Methods Mol Biol 2146:19–31 [DOI] [PubMed]
- Larose G, Chênevert R, Moutoglis P, Gagné S, Piché Y, Vierheilig H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J Plant Physiol. 2002;159:1329–1339. doi: 10.1078/0176-1617-00896. [DOI] [Google Scholar]
- Lazzara S, Militello M, Carrubba A, Napoli E, Saia S. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contr asting P availability in a highly organic substrate. Mycorrhiza. 2017;27:345–354. doi: 10.1007/s00572-016-0756-6. [DOI] [PubMed] [Google Scholar]
- Lee K, Shin MS, Ham I, Choi HY. Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement Altern Med. 2015;15:395. doi: 10.1186/s12906-015-0889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley SV, Baxendale IR. New tools and concepts for modern organic synthesis. Nat Rev Drug Discov. 2002;1(8):573–586. doi: 10.1038/nrd871. [DOI] [PubMed] [Google Scholar]
- Li CX. Effects of infecting vesicular-arbuscular mycorrhiza on growth and development of Coix Lachryma-jobi L. J Shanxi Agr Univers. 2003;23(4):351–353. [Google Scholar]
- Liang XF, Tang MJ, Lu LX, Zhao XY, Dai CC. Effects of three arbuscular mycorrhizal fungi (AMF) species on the growth, physiology, and major components of essential oil of Atractylodes Lancea. Chin J Plant Ecol. 2018;37(6):1871–1879. [Google Scholar]
- Lima CS, Santos HRS, Albuquerquec UP, Barbosa da Silva FS. Mycorrhizal symbiosis increase the level of total foliar phenols and tannins in Commiphora leptophloeos (Mart.) J.B. Gillett Seedlings Ind Crops Prod. 2017;104:28–32. doi: 10.1016/j.indcrop.2017.04.020. [DOI] [Google Scholar]
- Lima KB, Riter Netto AF, Martins MA, Freitas MSM. Crescimento, acúmulo de nutrientes e fenóis totais de mudas de cedro australiano (Toona ciliata) inoculadas com fungos micorrízicos. Ciência Florestal. 2015;25:853–862. doi: 10.5902/1980509820583. [DOI] [Google Scholar]
- Liu T, He XL (2008) Research on the formation course of arbuscular mycorrhizae from Astragalus membranaceus (Fisch.) Bunge seedlings. J Hebei Fores Orc Res 23(3):311–314
- Liu SL, He XL. Effects of AM fungi on growth of Glycyrrhiza inflata Bat under water stress. J Nuc Agr Sci. 2009;23(4):692–696. [Google Scholar]
- Liu J, Shi YC, Lee DY. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin Herb Med. 2019;11(2):141–149. doi: 10.1016/j.chmed.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis. 2017;8(6):868–886. doi: 10.14336/AD.2017.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JN, Wu LJ, Wei SL, Xiao X, Su CX, Jiang P, Song ZB, Wang T, Yu ZL. Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch) Plant Growth Regul. 2007;52:29–39. doi: 10.1007/s10725-007-9174-2. [DOI] [Google Scholar]
- Lohse S, Schliemann W, Ammer C, Kopka J, Strack D, Thomas Fester T. Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol. 2005;139:329–340. doi: 10.1104/pp.105.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino JG, Lowe JA., III The role of the medicinal chemist in drug discovery—then and now. Nat Rev Drug Discov. 2004;3(10):853–862. doi: 10.1038/nrd1523. [DOI] [PubMed] [Google Scholar]
- Lu YQ, He XL. Effects of AM fungi on the chemical composition and growth amount of Atractylodes macrocephala koidz seedling on diffetent N levels. J Hebei Univers. 2005;25(6):650–653. [Google Scholar]
- Lu FC, Lee CY, Wang CL (2015) The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. Peer J 3:e1266 [DOI] [PMC free article] [PubMed]
- Machiani MA, Javanmard A, Morshedloo MR, Aghaee A, Maggi F. Funneliformis mosseae inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci Rep. 2021;11:15279. doi: 10.1038/s41598-021-94681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L, Goossens A. Hormone-mediated promotion of trichome initiation in plants is conserved but utilizes species and trichome-specific regulatory mechanisms. Plant Signal Behav. 2010;5(2):205–207. doi: 10.4161/psb.5.2.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L, Inze´ D, Goossens A, Functional specialization of the TRANSPARENT TESTA GLABRA1 network allows differential hormonal control of laminal and marginal trichome initiation in Arabidopsis rosette leaves. Plant Physiol. 2008;148(3):1453–1464. doi: 10.1104/pp.108.125385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L, Van Nieuwerburgh FC, Zhang Y, Reed DW, Pollier J, Vande Casteele SR, Inze D, Covello PS, Deforce DL, Goossens A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011;189(1):176–189. doi: 10.1111/j.1469-8137.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- Maggini R, Kiferle C, Guidi L, Pardossi A, Andrea R. Growing medicinal plants in hydroponic culture. Acta Hort. 2012;952:697–704. doi: 10.17660/ActaHortic.2012.952.88. [DOI] [Google Scholar]
- Mairapetyan SK, Alexanyan JS, Tadevosyan AH, Tovmasyan AH, Stepanyan BT, Galstyan HM, Daryadar MK. The productivity of some valuable medicinal plants in conditions of water stream hydroponic. J Agri Sci Food Res. 2018;9:237. [Google Scholar]
- Malik S, Cusido RM, Mirjalili MH, Moyano E, Palazon J, Bonfill M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem. 2011;46(1):23–34. doi: 10.1016/j.procbio.2010.09.004. [DOI] [Google Scholar]
- Mandal S, Evelin H, Giri B, Singh VP, Kapoor R. Enhanced production of steviol glycosides in mycorrhizal plants: a concerted effect of arbuscular mycorrhizal symbiosis on transcription of biosynthetic genes. Plant Physiol Biochem. 2015;89:100–106. doi: 10.1016/j.plaphy.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Mandal S, Evelin H, Giri B, Singh VP, Kapoor R. Arbuscular mycorrhiza enhances the production of stevioside and rebaudioside-A in Stevia rebaudiana via nutritional and non-nutritional mechanisms. Appl Soil Ecol. 2013;72:187–194. doi: 10.1016/j.apsoil.2013.07.003. [DOI] [Google Scholar]
- Mandal S, Upadhyay S, Wajid S, Ram M, Jain DC, Singh VP, Abdin MZ, Kapoor R. Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza. 2015;25:345–357. doi: 10.1007/s00572-014-0614-3. [DOI] [PubMed] [Google Scholar]
- Marcinek K, Krejpcio Z. Stevia rebaudiana Bertoni: health promoting properties and therapeutic applications. J Verbr Lebensm. 2016;11:3–8. doi: 10.1007/s00003-015-0968-2. [DOI] [Google Scholar]
- Mathens A, Bellanger R. Herbs and other dietary supplements: current regulations and recommendations for use to maintain health in the management of the common cold or other related infectious respiratory illnesses. J Pharm Pract. 2010;23:117–127. doi: 10.1177/0897190009358711. [DOI] [PubMed] [Google Scholar]
- Matta D, Nanda H, Mahalingam G. Phytopharmaceutical potentials of Prosopis laevigate, symplocos cochinchinensis and nymphaea Alba: a review. Asian J Pharm Clin Res. 2017;10(10):63–68. doi: 10.22159/ajpcr.2017.v10i10.20316. [DOI] [Google Scholar]
- Mechri B, Tekaya M, Cheheb H, Attia F, Hammami M. Accumulation of flavonoids and phenolic compounds in olive tree roots in response to mycorrhizal colonization: a possible mechanism for regulation of defense molecules. J Plant Physiol. 2015;185:40–43. doi: 10.1016/j.jplph.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Goel M, Srivastava V, Rahman L. Hairy root biotechnology of Rauwolfia serpentina: a potent approach for the production of pharmaceutically important terpenoid indole alkaloids. Biotechnol Lett. 2015;37:253–263. doi: 10.1007/s10529-014-1695-y. [DOI] [PubMed] [Google Scholar]
- Meixner C, Ludwig-Mu¨ller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H, Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta. 2005;222:709–715. doi: 10.1007/s00425-005-0003-4. [DOI] [PubMed] [Google Scholar]
- Mendes R, Kruijt M, Bruijn I, Dekkers E. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332(6033):1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Merlin E, Melato E, Emerson LBL, Ezilda J, Arquimedes GJ, Rayane MSC, Joice KO, Camila S, Odair A (2020) Inoculation of arbuscular mycorrhizal fungi and phosphorus addition increase coarse mint (Plectranthus amboinicus Lour.) plant growth and essential oil content. Rhizosphere 15:100217
- Miao GP, Han J, Zhang JF, Zhu CS, Zhang X (2017) A MDR transporter contributes to the different extracellular production of sesquiterpene pyridine alkaloids between adventitious root and hairy root liquid cultures of Tripterygium wilfordii Hook.f. Plant Mol Biol 95(1–2):51–62 [DOI] [PubMed]
- Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14(7):2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaie M, Ladanmoghadam A, Hakimi Leila and Danaee E (2020) Water stress modifies essential oil yield and composition, glandular trichomes and stomatal features of lemongrass (Cymbopogon citratus L.) inoculated with arbuscular mycorrhizal fungi. J Agric Sci Technol 22(7):1575–1585
- Mohammad A, Khan AG, Kuek C. Improved aeroponic culture of inocula of arbuscular mycorrhizal fungi. Mycorrhiza. 2000;9:337–339. doi: 10.1007/s005720050278. [DOI] [Google Scholar]
- Mohammad A, Mirta B, Khan AG. Effects of sheared-root inoculum of Glomus intraradices on wheat grown at different phosphorus levels in the field. Agric Ecosyst Environ. 2004;103:245–249. doi: 10.1016/j.agee.2003.09.017. [DOI] [Google Scholar]
- Monteiro JM, de Almeida Cde F, de Albuquerque UP, de Lucena RF, Florentino AT, de Oliveira RL (2006) Use and traditional management of Anadenanthera colubrina (Vell.) Brenan in the semi-arid region of northeastern Brazil. J Ethnobiol Ethnomed 2:6 [DOI] [PMC free article] [PubMed]
- Morandi D, Bailey JA. Isoflavonoid accumulation in soybean roots infected with vesicular-arbuscular mycorrhizal fungi. Phys Plant Path. 1984;24:357–364. doi: 10.1016/0048-4059(84)90009-2. [DOI] [Google Scholar]
- Morone-Fortunato I, Avato P. Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tiss Organ Cult. 2008;93:139–149. doi: 10.1007/s11240-008-9353-5. [DOI] [Google Scholar]
- Movahedi Z, Rostami M. Production of some medicinal plants in aeroponic system. J Medicinal Plants by-Products. 2020;1:91–99. doi: 10.29252/jmp.1.73.91. [DOI] [Google Scholar]
- Mungali M, Tripathi A (2021) Valeriana officinalis (valerian) In book: naturally occurring chemicals against alzheimer's disease. pp. 283–291
- Murthy HN, Kim YS, Jeong CS, Kim SJ, Zhong JJ, Paek KY. Production of ginsenosides from adventitious root cultures of Panax ginseng. In: Paek KY, Murthy H, Zhong JJ, editors. Production of biomass and bioactive compounds using bioreactor technology. Dordrecht: Springer; 2014. pp. 625–651. [Google Scholar]
- Nalawade SM, Tsay HS. In vitro propagation of some important Chinese medicinal plants and their sustainable usage. In Vitro Cell Dev Biol Plant. 2004;40:143–154. doi: 10.1079/IVP2003504. [DOI] [Google Scholar]
- Nazari Deljou MJ, Marouf A, Jaberian Hamedan H. Effect of inoculation with arbuscular mycorrhizal fungi (AMF) on gerbera cut flower (Gerbera Jamesonii) production in soilless cultivation. Acta Hortic. 2014;1034:417–422. doi: 10.17660/ActaHortic.2014.1034.51. [DOI] [Google Scholar]
- Nell M, Wawrosch C, Steinkellner S, Vierheilig H, Kopp B, Lössl A, Franz C, Novak J, Zitterl-Eglseer K. Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med. 2010;76(4):393–398. doi: 10.1055/s-0029-1186180. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17(3):215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Seufert G, Steinbrecher R, Tenhunen JD. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol. 2002;153(2):257–275. doi: 10.1046/j.0028-646X.2001.00324.x. [DOI] [Google Scholar]
- Nirmala KA, Kanchana M. Leucas aspera – a review of its biological activity. Sys Rev Pharm. 2018;9(1):41–44. doi: 10.5530/srp.2018.1.8. [DOI] [Google Scholar]
- Nisha MC, Rajeshkumar S. Effect of arbuscular mycorrhizal fungi on growth and nutrition of Wedilia chinensis (Osbeck) Merril. Indian J Sci Technol. 2010;3(6):676–678. doi: 10.17485/ijst/2010/v3i6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noceto PA, Bettenfeld P, Boussageon R, Hériché M, Sportes A, van Tuinen D, Courty PE, Wipf D. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza. 2021;31(6):655–669. doi: 10.1007/s00572-021-01054-1. [DOI] [PubMed] [Google Scholar]
- Oksana S, Marian B, Mahendra R, Bo SH. Plant phenolic compounds for food, pharmaceutical and cosmetics production. J Med Plant Res. 2012;6:2526–2539. [Google Scholar]
- Oliveira MS, Campos MAS, Silva FSB. Arbuscular mycorrhizal fungi and vermicompost to maximize the production of foliar biomolecules in Passiflora alata Curtis seedlings. J Sci Food Agric. 2015;95:522–528. doi: 10.1002/jsfa.6767. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Campos M, Albuquerque U, Fábio S. Arbuscular mycorrhizal fungi (AMF) affects biomolecules content in Myracrodruon urundeuva seedlings. Ind Crops Prod. 2013;50:244–247. doi: 10.1016/j.indcrop.2013.07.041. [DOI] [Google Scholar]
- Olsson PA, Thingstrub I, Jakobsen I, Baath E. Estimation of the biomass of arbuscular mycorrhizal fungi in a linseed field. Soil Biol Biochem. 1999;31:1879–1887. doi: 10.1016/S0038-0717(99)00119-4. [DOI] [Google Scholar]
- Paek KY, Murthy HN, Hahn EJ, Zhong JJ. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol. 2009;113:151–176. doi: 10.1007/10_2008_31. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Malik T, Dey A, Singh J, Banik RM. Improved growth and colchicine concentration in Gloriosa superba on mycorrhizal inoculation supplemented with phosphorus-fertilizer. Afr J Tradit Complement Altern Med. 2014;11(2):439–446. doi: 10.4314/ajtcam.v11i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panke-Buisse K, Poole A, Goodrich J, Ley RE, Kao-Kniffin J. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2015;9:980–989. doi: 10.1038/ismej.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar NL, Kothar S, Rathore NS. Sustainability of greenhouse cultivation of medicinal plants. Agr Eng Today. 2003;27(5–6):52–61. [Google Scholar]
- Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ. 2008;54:89. doi: 10.17221/2774-PSE. [DOI] [Google Scholar]
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32(12):2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Patel P, Shah S, Parmar S. A review: Coix lacryma jobi L. J Pharmacogn Phytochem. 2017;9(4):248–252. [Google Scholar]
- Patra N, Srivastava AK. Enhanced production of artemisinin by hairy root cultivation of Artemisia annua in a modified stirred tank reactor. Appl Biochem Biotechnol. 2014;174(6):2209–2222. doi: 10.1007/s12010-014-1176-8. [DOI] [PubMed] [Google Scholar]
- Pedone‐ Bonfim MV, Lins MA, Coelho IR, Santana AS, Silva FS, Maia LC (2013) Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J Sci Food Agric 93(6):1479–1484 [DOI] [PubMed]
- Perassolo M, Cardillo AB, Mugas ML, Montoya SN, Giulietti AM, Talou JR. Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum. Ind Crops Prod. 2017;105:124–132. doi: 10.1016/j.indcrop.2017.05.010. [DOI] [Google Scholar]
- Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L. Hairy root cultures for secondary metabolites production. Adv Exp Med Biol. 2010;698:167–184. doi: 10.1007/978-1-4419-7347-4_13. [DOI] [PubMed] [Google Scholar]
- Porcel R, Aroca R, Ruiz-Lozano JM. Salinity stress alleviation using arbuscular mycorrhizal fungi. A Review Agron Sustain Dev. 2012;32:181–200. doi: 10.1007/s13593-011-0029-x. [DOI] [Google Scholar]
- Putalun W, Prasarnsiwamai P, Tanaka H, Shoyama Y. Solasodine glycoside production by hairy root cultures of Physalis minima Linn. Biotechnol Lett. 2004;26:545–548. doi: 10.1023/B:BILE.0000021953.50300.52. [DOI] [PubMed] [Google Scholar]
- Qi GH, Zhang LP, Yang WL, Lv GY (2003) The effects of abruscular mycorrhiza fungi on ginkgo (Ginkgo biloba L.) in the field. Hebei Fruits 19(1):40–42
- Qi GH, Zhang LP, Yang WL, Lu XR, Li CL (2002) Effects of arbuscular mycorrhizal fungi on growth and disease resistance of replanted ginkgo (Ginkgo biloba L.) seedlings. J Hebei Forest Orch Res 17(1):58–61
- Rai MK. Current advances in mycorrhization in micropropagation. In Vitro Cell Dev Biol Plant. 2001;37:158–167. doi: 10.1007/s11627-001-0028-8. [DOI] [Google Scholar]
- Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli-Sadaghiani M, Hassani A, Barin M, Danesh YR, Sefidkon F. Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. J Med Plants Res. 2010;4(21):2222–2228. [Google Scholar]
- Rat A, Naranjo HD, Krigas N, Grigoriadou K, Maloupa E, Alonso AV, Schneider C, Papageorgiou VP, Assimopoulou AN, Tsafantakis N, Fokialakis N, Willems A. Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria tausch (Boraginaceae): exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Front Microbiol. 2021;12:633488. doi: 10.3389/fmicb.2021.633488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehana B, Nagarajan N. Evaluation of in vitro antioxidant activity of a medicinal herb, Wedelia chinensis (Osbeck) Merrill. Asian J Pharm Clin Res. 2018;11(10):433–437. doi: 10.22159/ajpcr.2018.v11i10.25008. [DOI] [Google Scholar]
- Ren JH, Liu RX, Li YL. Study on arbuscular mycorrhizae of Panax notoginseng. Microbiology. 2007;34(2):224–227. [Google Scholar]
- Ren JH, Zhang JF, Liu RX, Li YQ (2008) Study on arbuscular mycorrhizae in Taxus chinensis var. mairei. Acta Bot Bor Occi Sin 28(7):1468–1473
- Ringer KL, Davis EM, Croteau R (2005) Monoterpene metabolism. Cloning, expression, and characterization of (-)- isopiperitenol/(-)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol 137(3):863–872 [DOI] [PMC free article] [PubMed]
- Riter Netto AF, Freitas MSM, Martins MA, Carvalho AJC, Vitorazi Filho JA. Efeito de fungos micorrízicos arbusculares na bioprodução de fenóis totais e no crescimento de Passiflora alata Curtis. Rev Bras De Plantas Medicinais. 2014;16(1):1–9. doi: 10.1590/S1516-05722014000100001. [DOI] [Google Scholar]
- Roberson E (2008) Medicinal plants at risk in nature’s pharmacy, our treasure chest: why we must conserve our natural heritage. A native plant conservation campaign report.
- Rojas-Andrade R, Cerda-García-Rojas C, FríasHernández J, Dendooven L, Olalde-Portugal V, RamosValdivia A. Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza. 2003;13(1):49–52. doi: 10.1007/s00572-002-0201-x. [DOI] [PubMed] [Google Scholar]
- Rydlová J, Jelínková M, Dušek K, Dušková E, Vosátka M, Püschel D. Arbuscular mycorrhiza differentially affects synthesis of essential oils in coriander and dill. Mycorrhiza. 2016;26(2):123–131. doi: 10.1007/s00572-015-0652-5. [DOI] [PubMed] [Google Scholar]
- Sailo GS, Bagyaraj DJ. Influence of different AM-fungi on the growth, nutrition and forskolin content of Coleus forskohlii. Mycol Res. 2005;109(7):795–798. doi: 10.1017/S0953756205002832. [DOI] [PubMed] [Google Scholar]
- Salam EA, Alatar A, El-Sheikh MA. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J Biol Sci. 2017;25(8):1772–1780. doi: 10.1016/j.sjbs.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon MJ, Demarmels R, Watts-Williams SJ, Malaughlin MJ, Kafle A, et al. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl Soil Ecol. 2022;169:104225. doi: 10.1016/j.apsoil.2021.104225. [DOI] [Google Scholar]
- Santos EL, Ferreira MRA, Soares LAL, Sampaio EVSB, Silva WAV, Silva FA. Acaulospora longula increases the content of phenolic compounds and antioxidant activity in fruits of Libidibia ferrea. Open Microbiol J. 2020;14(1):132–139. doi: 10.2174/1874285802014010132. [DOI] [Google Scholar]
- Selvaraj T, Nisha MS, Rajeshkumar S. Effect of indigenous arbuscular mycorrhizal fungi on some growth parameters and phytochemical constituents of Pogostemon patchouli Pellet. Maejo Int J Sci Technol. 2009;3(1):222–234. [Google Scholar]
- Sete da Cruz RM, Dragunski DC, Gonçalves AC, Alberton O, Hulse S, Sete da Cruz GL. Inoculation with arbuscular mycorrhizal fungi alters content and composition of essential oil of sage (Salvia officinalis) under different phosphorous levels. Aust J Crop Sci. 2019;13(10):1617–1624. doi: 10.21475/ajcs.19.13.10.p1834. [DOI] [Google Scholar]
- Sgherri C, Pinzino C, Izzo R, Cecconami S, Navari-Izzo F. Antioxidant activity and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010;123(2):416–422. doi: 10.1016/j.foodchem.2010.04.058. [DOI] [Google Scholar]
- Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, Del Mar CM, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M, Sharifi-Rad J. Carvacrol and human health: a comprehensive review. Phytother Res. 2018;32(9):1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kapoor R, Bhatnagar AK. Arbuscular mycorrhizal (AM) technology for the conservation of Curculigo orchioides Gaertn: an endangered medicinal herb. World J Microbio Biotechnol. 2008;24:395–400. doi: 10.1007/s11274-007-9488-2. [DOI] [Google Scholar]
- Sharma E, Anand G, Kapoor R. Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann Bot. 2017;119:791–801. doi: 10.1093/aob/mcw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul-Keinan O, Gadkar V, Ginzberg I, Grunzweig JM, Chet I, Elad Y, Wininger S, Belausov E, Eshed Y, Atzmon N, Ben-Tal Y. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 2002;154(2):501–507. doi: 10.1046/j.1469-8137.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- Shi M, Liao P, Nile SH, Georgiev MI, Kai G. Biotechnological exploration of transformed root culture for value-added products. Trends Biotechnol. 2021;39(2):137–149. doi: 10.1016/j.tibtech.2020.06.012. [DOI] [PubMed] [Google Scholar]
- Shrivastava G, Ownley BH, Auge´ RM, Toler H, Dee M, Vu A, Kollner TG, Chen F, Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis. 2015;65(2):65–74. doi: 10.1007/s13199-015-0319-1. [DOI] [Google Scholar]
- Silveira Rabelo AC, Caldeira Costa D. A review of biological and pharmacological activities of Baccharis trimera. Chem Biol Interact. 2018;296:65–75. doi: 10.1016/j.cbi.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Silvia FA, Silva FSB, Maia LC (2014) Biotechnical application of arbuscular mycorrhizal fungi used in the production of foliar biomolecules in ironwood seedlings [Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz var. ferrea]. J Med Plant Res 8(20):814–819
- Simao MJ, Barboza TJS, Vianna MG, Garcia R, Mansur E, Ignacio ACPR, Pacheco G. A comparative study of phytoconstituents and antibacterial activity of in vitro derived materials of four Passiflora species. An Acad Bras Cienc. 2018;90(3):2805–2813. doi: 10.1590/0001-3765201820170809. [DOI] [PubMed] [Google Scholar]
- Simeunovic D (2002) Cultivation of medicinal plants in greenhouse hydroponics. Electronic Theses and Dissertations 1594. https://scholar.uwindsor.ca/etd/1594
- Singh R, Soni SK, Kalra A (2013) Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza 23(1):35–44 [DOI] [PubMed]
- Singh Y, Gupta A, Kannojia P (2020) Tagetes erecta (marigold) - a review on its phytochemical and medicinal properties. Curr Med Drug Res 4 (1): Article ID 201
- Singh R, Divya S, Awasthi A, Kalra A. Technology for efficient and successful delivery of vermicompost colonized bioinoculants in Pogostemon cablin (patchouli) Benth. World J Microbio Biotechnol. 2012;28(1):323–333. doi: 10.1007/s11274-011-0823-2. [DOI] [PubMed] [Google Scholar]
- Smith S, Read D (2008) Mycorrhizal Symbiosis, 3rd edition. Academic Press, Cambridge, pp 1–787
- Soni P, Siddiqui AA, Dwivedi J, Soni V (2012) Pharmacological properties of Datura stramonium L. as a potential medicinal tree: an overview. Asian Pac J Trop Biomed 2(12):1002–1008 [DOI] [PMC free article] [PubMed]
- Souret FF, Weathers PJ. The growth of saffron (Crocus sativus L.) in aeroponics and hydroponics. J Herbs Spices Med Plants. 2000;7:25–35. doi: 10.1300/J044v07n03_04. [DOI] [Google Scholar]
- Stumpe M, Carsjens JG, Stenzel I, Gobel C, Lang I, Pawlowski K, Hause B, Feussner I. Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry. 2005;66:781–791. doi: 10.1016/j.phytochem.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Suryawanshi JAS. An overview of Citrus aurantium used in treatment of various diseases. Afr J Plant Sci. 2011;5(7):390–395. [Google Scholar]
- Szakiel A, Pączkowski HM. Influence of environmental biotic factors on the content of saponins in plants. Phytochem Rev. 2011;10:493–502. doi: 10.1007/s11101-010-9164-2. [DOI] [Google Scholar]
- Szakiel A, Pączkowski H. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev. 2011;10:471–491. doi: 10.1007/s11101-010-9177-x. [DOI] [Google Scholar]
- Tabatabaei SJ. Effects of cultivation systems on the growth, and essential oil content and composition of Valerian. J Herbs Spices Med Plants. 2008;14:54–67. doi: 10.1080/10496470802341219. [DOI] [Google Scholar]
- Taber RA, Trappe JM. Vesicular-arbuscular mycorrhiza in rhizomes, scale-like leaves, roots, and xylem of ginger. Mycologia. 1982;74(1):156–161. doi: 10.1080/00275514.1982.12021485. [DOI] [Google Scholar]
- Tang W, Eisenbrand G (1992) Panax ginseng C. A. Mey.. In: Tang W, Eisenbrand G (eds) Chinese Drugs of Plant Origin. Springer, Berlin, Heidelberg. pp 711–737
- Tavarini S, Passera B, Martini A, Avio L, Sbrana C, Giovannetti M, Angelini LG. Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind Crops Prod. 2018;111:899–907. doi: 10.1016/j.indcrop.2017.10.055. [DOI] [Google Scholar]
- Teixeira MC, Lopes MJP, de Sousa-Júnior DL, et al. Evaluation of the healing potential of Myracrodruon urundeuva in wounds induced in male rats. Rev Bras Farmacogn. 2020;30:214–223. doi: 10.1007/s43450-020-00025-5. [DOI] [Google Scholar]
- Tejavathi DH, Jayashree D. Effect of AM fungal association on the growth performance of selected medicinal herbs. Indian J Appl Res. 2011;3(7):12–15. doi: 10.15373/2249555X/JULY2013/5. [DOI] [Google Scholar]
- Teng HR, He XL. Effects of different AM fungi and N levels on the flavonoid content of Bupleuruin scorzonerifolium Willd. J Shanxi Agr Sci. 2005;4:53–54. [Google Scholar]
- Tepfer M, Casse-Delbart F. Agrobacterium rhizogenes as a vector for transforming higher plants. Microbiological Sci. 1987;4:24–28. [PubMed] [Google Scholar]
- Thawkar BS, Jawarkar AG, Kalamkar PV, Pawar KP, Kale MK. Phytochemical and pharmacological review of Mentha arvensis. Int J Green Pharm. 2016;10(2):71. [Google Scholar]
- Thirkell TJ, Charters MD, Elliott AJ, Sait SM, Field KJ. Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J Ecol. 2017;105:921–929. doi: 10.1111/1365-2745.12788. [DOI] [Google Scholar]
- Tian L, Shi SH, Ma LN, Zhou X. The effect of Glomus intraradices on the physiological properties of Panax ginseng and on rhizospheric microbial diversity. J Ginseng Res. 2019;43(1):77–85. doi: 10.1016/j.jgr.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titanji VP, Zofou D, Ngemenya MN. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. Afr J Tradit Complement Altern Med. 2008;5(3):302–321. [PMC free article] [PubMed] [Google Scholar]
- Torres N, Goicoechea N, Antolín MC. Antioxidant properties of leaves from different accessions of grapevine (Vitis vinifera L.) cv. Tempranillo after applying bioticand/or environmental modulator factors. Ind Crops Prod. 2015;76:77–85. doi: 10.1016/j.indcrop.2015.03.093. [DOI] [Google Scholar]
- Toussaint JP, Smith FA, Smith SE. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza. 2007;17(4):291–297. doi: 10.1007/s00572-006-0104-3. [DOI] [PubMed] [Google Scholar]
- Urbanek H, Katarzyna Bergier K, Marian Saniewski M, Patykowski J. Effect of jasmonates and exogenous polysaccharides on production of alkannin pigments in suspension cultures of Alkanna tinctoria. Plant Cell Rep. 1996;15(8):637–641. doi: 10.1007/BF00232468. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Piche Y (2002) Signalling in arbuscular mycorrhiza: facts and hypothesis. In: Buslig BS, Manthey JA (eds) Flavonoids in cell function. Advances in experimental medicine and biology, vol 505. Springer, Boston, MA. pp 23–39 [DOI] [PubMed]
- Vishnuvathan VJ, Lakshmi KS, Srividya AR. Medicinal uses of formononetin- a review. J Ethnobiol Ethnomedicine. 2016;126:1197–1209. [Google Scholar]
- Vo AT, Haddidi I, Daood H, Mayer Z, Posta K. Impact of arbuscular mycorrhizal inoculation and growth substrate on biomass and content of polyphenols in Eclipta prostrata. Hortic Science. 2019;54(11):1976–1983. [Google Scholar]
- Voets L, de Boulois HD, Renard L, Strullu DG, Declerck S. Development of an autotrophic culture system for the in vitro mycorrhization of potato plantlets. FEMS Microbiol Lett. 2005;248(1):111–118. doi: 10.1016/j.femsle.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Voets L, de la Providencia IE, Fernandez K, IJdo M, Cranenbrouck S, Declerck S, Extraradical mycelium network of arbuscular mycorrhizal fungi allows fast colonization of seedlings under in vitro conditions. Mycorrhiza. 2009;19(5):347–356. doi: 10.1007/s00572-009-0233-6. [DOI] [PubMed] [Google Scholar]
- Volpin H, Elkind Y, Okon Y, Kapulnik Y. A vesicular arbuscular mycorrhizal fungus (Glomus intraradix) induces a defense response in alfalfa roots. Plant Physiol. 1994;104(2):683–689. doi: 10.1104/pp.104.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter MH, Fester T, Strack D. Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the ‘yellow pigment’ and other apocarotenoids. Plant J. 2000;21:571–578. doi: 10.1046/j.1365-313x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Walter MH, Hans J, Strack D. Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J. 2002;31:243–254. doi: 10.1046/j.1365-313X.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, He XL, Chen TS, Dou WF. Ecological research of arbuscular mycorrhizal fungi in rhizosphere of Puerraria lobata. J Hebei Univ. 2006;26(4):420–425. [Google Scholar]
- Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei X, Luo J, Shan M, Lin R, Zou D, Ma Z. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: a review. J Ethnopharmacol. 2018;210:318–339. doi: 10.1016/j.jep.2017.08.040. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100(4):681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BS, Bedair MF, Urbanczyk-Wochniak E, Huhman DV, Yang DS, Allen NS, Li W, Tang Y, Sumner LW. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015;167:1699–1716. doi: 10.1104/pp.114.253054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GT, Wang HG. Effects of VA mycorrhizal fungi on growth, nutrient uptake and effective compounds in Chinese medicinal herb Datura stramonium L. Sci Agr Sin. 1989;22(5):56–61. [Google Scholar]
- Wei GT, Wang HG. Effect of vesicular-arbuscular mycorrhizal fungi on growth, nutrient uptake and synthesis of volatile oil in Schizonepeta tenuifolia. Chin J Chin Mater Med. 1991;16(3):139–142. [PubMed] [Google Scholar]
- Woo SS, Song JS, Lee JY, In DS, Chung HJ, Liu JR, Choi DW. Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochemistry. 2004;65:2751–2761. doi: 10.1016/j.phytochem.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Wu QS, Liu W, Zhai HF, Ye XF, Zhao LJ. Influences of AM fungi on growth and root antioxidative enzymes of Trifoliate orange seedlings under salt stress. Acta Agr Univ Jiangxiensis. 2010;32(4):759–762. [Google Scholar]
- Wu YH, Wang H, Liu M, Li B, Chen X, Ma YT, Yan ZY. Effects of native arbuscular mycorrhizae isolated on root biomass and secondary metabolites of Salvia miltiorrhiza Bge. Front Plant Sci. 2021;12:617892. doi: 10.3389/fpls.2021.617892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Hao Z, Zhou X, Jiang X, Xu L, Wu S, Zhao A, Zhang X, Chen B. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza. 2018;28:285–300. doi: 10.1007/s00572-018-0827-y. [DOI] [PubMed] [Google Scholar]
- Yadav K, Aggarwal A, Singh N. Arbuscular mycorrhizal fungi induced acclimatization and growth enhancement of Glycyrrhiza glabra L.: a potential medicinal plant. Agric Res. 2013;2:43–47. doi: 10.1007/s40003-012-0047-1. [DOI] [Google Scholar]
- Yadav K, Singh N, Aggarwal A. Arbuscular mycorrhizal technology for the growth enhancement of micropropagated Spilanthes acmella Murr. Plant Protect Sci. 2012;48:31–36. doi: 10.17221/21/2011-PPS. [DOI] [Google Scholar]
- Yadav K, Singh N, Aggarwal A (2011) Influence of arbuscular mycorrhizal (AM) fungi on survival and development of micropropagated Acorus calamus L. during acclimatization. JAgricSci Technol 7(3):775–781
- Yamawaki K, Matsumura A, Hattori R, Tarui A, Hossain M, Ohashi Y, Daimon H (2013) Effect of inoculation with arbuscular mycorrhizal fungi on growth, nutrient uptake and curcumin production of turmeric (Curcuma longaL.). Agri Sci 4(2):66–71
- Yang X, Xiong X, Wang H, Wang J. Protective effects of Panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi: 10.1155/2014/204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ou XH, Yang G, Xia YS, Chen ML, Guo LP, Liu DH. Arbuscular mycorrhizal fungi regulate the growth and phyto-active compound of Salvia miltiorrhiza seedlings. Appl Sci. 2017;7(1):68. doi: 10.3390/app7010068. [DOI] [Google Scholar]
- Yazaki K. Lithospermum erythrorhizon cell cultures: present and future aspects. Plant Biotechnol (tokyo) 2017;34(3):131–142. doi: 10.5511/plantbiotechnology.17.0823a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Furuya T. Saponin productionby cultures of Panax ginseng transformed with Agrobacterium rhizogens. Plant Cell Rep. 1987;6:449–453. doi: 10.1007/BF00272780. [DOI] [PubMed] [Google Scholar]
- Yu KW, Murthy HN, Hahn EJ, Paek KY. Ginsenoside production by hairy root cultures of Panax ginseng: influence of temperature and light quality. Biochem Eng J. 2005;23(1):53–56. doi: 10.1016/j.bej.2004.07.001. [DOI] [Google Scholar]
- Zagórska-Dziok M, Ziemlewska A, Nizioł-Łukaszewska Z, Bujak T (2020) Antioxidant activity and cytotoxicity ofMedicago sativaL. seeds and herb extract on skin cells. Biores Open Access 9(1):229–242 [DOI] [PMC free article] [PubMed]
- Zeng Y, Guo LP, Chen BD, Hao ZP, Wang JY, Huang LQ, Yang G, Cui XM, Yang L, Wu ZX, Chen ML, Zhang Y. Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza. 2013;23(4):253–265. doi: 10.1007/s00572-013-0484-0. [DOI] [PubMed] [Google Scholar]
- Zhang RQ, Zhu HH, Zhao HQ, Yao Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J Plant Physiol. 2013;170(1):74–79. doi: 10.1016/j.jplph.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Zhao JL, He XL. Effects of AM fungi on drought resistance and content of chemical components in Angelica dahurica. Acta Agr Bor Occi Sin. 2011;20(3):184–189. [Google Scholar]
- Zhao PJ, An F, Tang M. Effects of arbuscular mycorrhiza fungi on drought resistance of Forsythia suspense. Acta Bot Bor Occi Sin. 2007;27(2):396–399. [Google Scholar]
- Zheng Y, Dixon M, Saxena P. Greenhouse production of Echinacea purpurea (L.) and E. angustifolia using different growing media, NO3 –/NH4 + ratios and watering regimes. Can J Plant Sci. 2006;86:809–815. doi: 10.4141/P05-167. [DOI] [Google Scholar]
- Zhi LY, Chuan CD, Lian QC. Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. Afr J Biotechnol. 2007;6:1266–1271. [Google Scholar]
- Zhou JH, Fan JH. Effects of AM fungi on the berberine content in Phellodendron chinense seedings. North Hortic. 2007;12:25–27. [Google Scholar]
- Zimare S, Borde M, Jite PK, Malpathak N (2013) Effect of AM fungi (Gf, Gm) on biomass and gymnemic acid content of Gymnema sylvestre (Retz.). Biol Sci 83(3):439–445
- Zitterl-Eglseer K, Nell M, Lamien-Meda A, Steinkellner S, Wawrosch C, Kopp B, Zitterl W, Vierheilig H, Novak J. Effects of root colonization by symbiotic arbuscular mycorrhizal fungi on the yield of pharmacologically active compounds in Angelica archangelica L. Acta Physiol Plant. 2015;37:21. doi: 10.1007/s11738-014-1750-2. [DOI] [Google Scholar]
- Zobel RW, Del Tredici P, Torrey JG. Method for growing plants aeroponically. Plant Physiol. 1976;57:344–346. doi: 10.1104/pp.57.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari M, Nazeri V, Sefidkon F, Rejali F. Effect of arbuscular mycorrhizal fungi on plant growth and essential oil content and composition of Ocimum basilicum L. Iran J Plant Physiol. 2013;3:643–650. [Google Scholar]
- Zou YN, Srivastava AK, Wu QS. Glomalin: a potential soil conditioner for perennial fruits. Int J Agric Biol. 2016;18:293–297. doi: 10.17957/IJAB/15.0085. [DOI] [Google Scholar]
- Zouari I, Salvioli A, Chialva M, Novero M, Miozzi L, Tenore GC, Bagnaresi P, Bonfante P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genomics. 2014;15:2215. doi: 10.1186/1471-2164-15-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubek S, Mielcarek S, Turnau K. Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza. 2012;22:149–156. doi: 10.1007/s00572-011-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubek S, Stojakowska A, Anielska T, Turnau K. Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza. 2010;20(7):497–504. doi: 10.1007/s00572-010-0306-6. [DOI] [PubMed] [Google Scholar]
- Zubek S, Rola K, Szewczyk A, Majewska ML, Katarzyna T. Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant Soil. 2015;390:129–142. doi: 10.1007/s11104-015-2388-6. [DOI] [Google Scholar]