Abstract
An emerging body of evidence suggests that changes in cognitive and emotional function are common aspects of stiff person spectrum disorders (SPSD). We sought to examine the pattern of cognitive impairment and psychiatric symptoms in SPSD.
Methods
A retrospective review of medical records was conducted for patients seen at the Johns Hopkins Stiff Person Syndrome (SPS) center from 1997 to January 1st, 2020. Individuals who had received formal cognitive testing as part of routine clinical care for patient-reported cognitive changes were included. Demographics, prevalence of cognitive impairment, psychoactive medication use, and clinically significant psychiatric symptoms were described.
Results
Out of 205 patients screened, 20 completed cognitive testing (75% female, mean age 47.4 years). The most common domains of impairment were verbal learning and recall memory (n = 14, 70%), verbal fluency (n = 10, 50%), processing speed (n = 8, 40%), and attention (n = 8, 40%). 9/11 patients assessed for depression reported clinically significant symptoms, and 4/9 patients assessed for anxiety reported clinically significant symptoms.
Conclusions
Screening for cognitive impairment in SPSD should utilize testing that assesses verbal learning and recall, phonemic verbal fluency, attention, and processing speed. Moreover, it is important to evaluate for co-existing depression and anxiety symptoms, as these are common in SPSD.
Keywords: stiff person syndrome, cognition, attention, verbal fluency, depression, anxiety
Introduction
Stiff person spectrum disorders (SPSD) are immune-mediated disorders most often characterized by rigidity, unpredictable and painful spasms, and heightened sensitivity to external stimuli (1). Anti-glutamic acid decarboxylase 65 (anti-GAD65) antibodies are thought to play a role in the GABAergic dysfunction in SPSD. While it is classified as a neurologic disorder, research is limited regarding the effects of stiff person syndrome (SPS) on cognitive and emotional function (1, 2).
SPSD has been associated with lower than expected performance on cognitive testing relative to estimated premorbid intelligence (3). Furthermore, the presence of anti-GAD65 antibody has been associated with cognitive impairment in patients with neurological conditions (4), type 2 diabetes (5), and in animal models (6). In addition to cognitive dysfunction, patients with SPSD are also more likely than the general population to report anxiety and depressive symptoms, and to regularly use prescription benzodiazepines and muscle relaxants (7), all of which may contribute to poor performance on cognitive testing (8–10). To our knowledge, only two prior studies have assessed cognitive symptoms in patients with SPSD (3, 11). While one also included measures of psychiatric symptoms (3), neither study reported on psychiatric symptoms or patterns of medication use in the context of cognitive performance.
The aims of this case series were to: (1) describe the pattern of cognitive impairment in patients with SPSD who reported concerns of cognitive impairment and participated in cognitive testing as part of routine clinical care; and (2) examine the frequency of mood symptoms and use of benzodiazepines and muscle relaxants in the most commonly impaired cognitive domains.
Methods
A retrospective review of medical records was conducted for patients seen at the Johns Hopkins SPS center from 1997 to January 1st, 2020. All patients had provided informed consent to participate in a longitudinal observational study of clinical characteristics in SPS, approved by the Johns Hopkins Institutional Review Board.
Medical records were reviewed for formal cognitive testing, performed by either a licensed psychologist or a speech and language pathologist, as part of routine clinical care for patient-reported cognitive changes. Information on demographics, clinical characteristics, medical comorbidities, and medications at the time of cognitive testing were extracted. Patients with limbic encephalitis, co-existing intractable epilepsy, and/or other neurological conditions known to affect cognitive performance (e.g., Alzheimer's disease, multiple sclerosis, etc.) were excluded.
As a retrospective review of cognitive testing performed as part of routine clinical care, cognitive testing batteries used were determined at the discretion of the provider and therefore not standardized. Details of cognitive testing reports were extracted; results were interpreted as “impaired” if records included descriptive labels of “abnormal”, “extremely low”, or “weak”. If no descriptive interpretation was offered, an adjusted percentile score of <2 or z-score of < −2 (e.g., more than 2 standard deviations below mean) was interpreted as “impaired” (12). If standardized instruments of psychological symptoms (e.g., depression and/or anxiety) were administered, the scores and descriptive labels (e.g., “clinically significant”) were extracted.
Demographic and clinical characteristics were evaluated using descriptive statistics, t-test for continuous variables and chi-squared test for dichotomous variables using R Studio Version 1.2.5033 (13). Significance was set at p < 0.05. Frequency of domain-specific cognitive impairment across individuals with cognitive testing was examined. For the 4 most commonly impaired cognitive domains, frequency of prescription antidepressants (e.g., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors), benzodiazepines (e.g., lorazepam, diazepam, clonazepam) and non-benzodiazepine muscle relaxants (e.g., cyclobenzaprine, baclofen, dantrolene), and clinically significant depression and anxiety were assessed.
Results
Out of 205 patients, 66 reported cognitive concerns, of which 20 completed cognitive testing (Table 1). There was no statistically significant difference in gender, age, or duration of illness in individuals included in this case series vs. the remainder of the cohort, or between those included in the case series vs. those who reported cognitive concerns but did not have cognitive testing (all p > 0.05). Three participants completed testing with a speech and language pathologist using the Repeatable Battery for the Assessment of Neuropsychological Status [RBANS; (20)], and 17 completed testing with a psychologist using a wide array of instruments (Supplementary Table 1). Our cohort was mostly female (n = 15, 75%), had a mean age at time of cognitive testing of 47.4 years (SD = 12.4), and mean duration of illness of 10.1 years (SD = 7.6). Most had anti-GAD65 antibodies (17/20, 75%), and classic SPS phenotype (15/20, 75%). Three (15%) had a history of seizures, none of which were intractable or poorly controlled. Common classes of medications prescribed included benzodiazepines (n = 14, 70%), antidepressants (n = 13, 65%), non-benzodiazepine muscle relaxants (n = 10, 50%), and opioids (n = 4, 20%). Nine out of eleven (82%) patients assessed for depression reported clinically significant symptoms, and 4 out of 9 (44%) patients assessed for anxiety reported clinically significant symptoms.
Table 1.
Clinical and laboratory features of patients with stiff person syndrome spectrum disorders who received formal cognitive testing as part of routine clinical care for patient-reported cognitive changes.
| Patient number | Baseline characteristics | Cognitive testing results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at testing | Years with SPSf | SPS phenotypes | Anti GAD-65 titer | Relevant medical comorbidities | Psychiatric comorbiditiesa | Psychoactive and immune-based medications | Areas of impairment | Psychiatric symptoms g | |
| 1 | 59 | 2 | GAD+SPS Cerebellar predominant | 63,525 IU/mL | Vitiligo B12 deficiency Remote Intestinal Ca Remote Testicular Ca |
None | Clonazepam | Processing speed Verbal phonemic fluency |
GDS-15: 8/15 NPI-Q: agitation, depression, apathy, irritability, nighttime behaviors, appetitive changes |
| 2 | 39 | <1 | GAD -SPS | 39 U/mL e | T2DM B12 deficiency Vit D deficiency Narcolepsy Small fiber neuropathy Mild OSA |
Depression Anxiety PTSD ADHD |
Oxymorphone Oxycodone Pregabalin Metaxalone Baclofen Clonazepam Alprazolam Armodafinil Certirizine |
No areas of impairment | PHQ-9 = 15 (moderately severe depressive symptoms) |
| 3 | 74 | 8 | GAD+SPS Cerebellar predominant | 6.3 U/mL | Coronary artery disease | Depression | IVIG Duloxetine |
Verbal learning and recall Motor speed Executive function (Set-shifting) Processing speed Verbal phonemic fluency |
BAI 8 (minimal anxiety) PHQ-9 = 0 (no symptoms) |
| 4 | 22 | 2 | GAD+SPS | 30 U/mL | Hypothyroidism Sickle cell anemia Asthma CVA partial seizures |
Generalized anxiety disorder b Major depressive disorder, recurrent, moderate b Adjustment disorder due to medical condition b |
Baclofen Diazepam Benzonatate Diphenhydramine |
Executive functioning (inhibition) Attention Verbal phonemic fluency Verbal recall Motor speed |
PAI: severe depressive symptoms |
| 5 | 60 | 8 | GAD-Possible SPS | Not available | B12 deficiency Vit D deficiency Ankylosing spondylitis Hypertension OSA |
None | Clonazepam Methotrexate Bupropion Tramadol |
Verbal recall | Not assessed |
| 6 | 29 | 17 | GAD+SPS | 250 IU/mL | Hypothyroidism Primary Immune deficiency Orthostatic hypotension Crohn's disease Chiari Malformation |
None | Adalimumab Tacrolimus Clonidine Duloxetine Modafinil Prednisone Topriamate |
Verbal learning and recall Executive functioning (set shifting) Attention Verbal phonemic fluency Verbal semantic fluency Working memory |
Not assessed |
| 7 | 54 | 12 | GAD+SPS | 21,888 U/mL | SLE | Depression Anxiety |
Baclofen Diazepam Clonazepam IVIG Duloxetine Buspirone Doxylamine Melatonin |
Verbal phonemic fluency Visual recall Executive function (set shifting) Attention Processing speed |
BDI: 29 (moderate depression) PAI: significant depression and anxiety |
| 8 | 43 | 6 | GAD+SPS Plus | 25,000 U/mL | Insulin dependent diabetes Epilepsy, sickle cell trait, migraines |
None | Clonazepam Cyclobenzaprine Lacosamide Levitracetam Oxycodone |
Language (verbal and reading comprehension, naming, spelling) Visual learning and recall |
PAI: significant anxiety |
| 9 | 36 | 4 | GAD+SPS | 320 IU/mL | Neuropathy Migraine |
Anxiety Depression PTSD |
Baclofen IVIG Clonazepam Diazepam Gabapentin Paroxetine |
Verbal phonemic fluency Verbal semantic fluency Verbal recall Motor speed |
PAI: significant anxiety, depression, anxiety related to past trauma and stress |
| 10 | 59 | 20 | GAD-Possible SPS | Not available | Cervical stenosis Migraines |
None | Carbamazepine Tizanidine |
Verbal recallc | Not assessed |
| 11 | 49 | 12 | GAD+SPS | 117 IU/mL | None | Major Depressive Disorder, recurrent b | Baclofen Bupropion Buspirone Clonazepam Diazepam IVIG Rituximab |
Processing speed Attention Verbal recall Visuospatial judgement |
Not assessed |
| 12 | 59 | 3 | GAD+SPS | 615 nmol/L | None | Paranoid schizophreniab | Fluoxetine Levitracitam Diazepam Olanzapine Memantine |
Verbal learning and recall c Visual learning and recall Language (expression) Verbal phonemic fluency Motor speed Executive function (inhibition) Processing speed |
BDI and BAI within normal limits (score not reported) |
| 13 | 49 | 24 | GAD+SPS | 207,650 U/mL | Diabetes Mellitus Epilepsy (s/p temporal lobectomy) Hypothyroidism Pernicious anemia SLE |
None | Baclofen Diazepam Lacosamide Levetiracetam Pregabalin |
Processing speed Executive function (Set shifting) Verbal learning and recall Language (naming) Verbal phonemic fluency |
PHQ-9 = 7 (mild depression) GAD-7 = 12 (moderate anxiety) |
| 14 | 54 | 22 | GAD+SPS | 6.6 U/mL | Insomnia Postural orthostatic tachycardia syndrome Migraines |
Generalized anxiety disorderb Major depressive disorderb |
Doxepin SCIG Pregabalin |
Processing speedc Verbal semantic fluency |
PHQ-9 = 27 (severe depression) GAD-7 = 19 (severe anxiety) |
| 15 | 45 | 9 | GAD+SPS | 213 IU/mL | Tublerculosis (1 yo) Coronary artery disease Dyslipidemia HTN Hypothyroidism |
None | Clonazepam Diazepam Hydralazine IVIG |
Verbal recall Verbal semantic fluency Executive functioning (Set-shifting) Processing speed Motor speed |
Not assessed |
| 16 | 45 | 23 | GAD+SPS | 174.2 U/mL | Anemia Anticardiolipin antibody positive T1DM Hepatitis Rheumatoid arthritis SLE |
Major depressive disorder Anxiety |
IVIG Baclofen Clonazepam Escitalopram Prednisone |
Attentiond Verbal learning and recall Visual learning and recall |
Not assessed |
| 17 | 41 | 9 | GAD+SPS | 174.2 U/mL | Dysautonomia Idiopathic small fiber sensory neuropathy T1DM |
None | Baclofen Clonazepam Diazepam Pregabalin Modafinil Oxycodone Roxicodone Paroxetine IVIG Tizanidine |
Verbal phonemic fluency Motor speed |
GAD-7 = 1 PHQ-9 = 19 |
| 18 | 41 | 3 | GAD+SPS | 53,650 U/mL | Anemia (iron deficiency) Eczema Asthma Seizures |
Anxiety | Baclofen Diazepam Mirtazapine |
Attentiond Visuospatial skills Verbal phonemic fluency Verbal learning and recall |
Not assessed |
| 19 | 33 | 7 | GAD+SPS | 34 IU/mL | Seizures Ataxia Nystagmus |
None | Baclofen Escitalopram IVIG Levetiracetam Rituximab |
Attentiond Verbal phonemic fluency Verbal recall Visual recall Visuospatial skills |
Not assessed |
| 20 | 56 | 1 | GAD+SPS | Not available | SLE Psoriatic arthritis T1DM B12 deficiency Autoimmune thyroiditis Hypertension Sjogren's syndrome |
Depression | Amlodipine Apremilast Diazepam Escitalopram Estradiol Clonazepam |
Attention Verbal phonemic fluency Verbal recall Motor speed |
Not assessed |
Psychiatric diagnoses are based only on patient report unless noted otherwise;
psychiatric diagnoses confirmed by psychiatric or psychologist notes;
no raw score or percentiles from neuropsychological batteries were reported for these patients. Domains of impairment were only based on testing interpretation summary;
only results of Repeatable Battery for Assessment of Neuropsychological Status (RBANS) completed by a Speech and Language Pathologist available;
While this patient had one positive anti-GAD65 antibody test, at the time closest to neuropsychological testing, prior and subsequent tests have been negative, thus categorization of phenotype is GAD-;
duration of illness calculated from time of symptom onset to time of cognitive testing;
Psychiatric symptoms were considered clinically significant based on previously established cut-offs of >9 for the Generalized Anxiety Disorder Assessment (GAD-7) (14) and >4 for the Patient Health Questionnaire-9 (PHQ-9) (15), >9 for Beck Depression Inventory (BDI) (16), >7 for the Beck Anxiety Inventory (BAI) (17). For individuals whose psychiatric symptoms were only assessed using the PAI, clinically significant symptoms were identified based on interpretation in the neuropsychiatric report. GAD -, anti-glutamic acid decarboxylase antibody negative; GAD +, anti-glutamic acid decarboxylase antibody positive; SLE, systemic lupus erythematosus; T1DM, type 1 diabetes; OSA, Obstructive Sleep Apnea; PTSD, Post-traumatic stress disorder; ADHD, Attention Deficit Hyperactivity Disorder; CVA, Cerebrovascular accident; Ca, Cancer; s/p, status-post; IVIG= Intravenous immunoglobulin; SCIG= Subcutaneous immunoglobulin; NPI-Q, Neuropsychiatric Inventory – Questionnaire (18); PHQ-9, Patient health questionnaire-9 (15); GAD-7, Generalized anxiety disorder-7 (14); PAI, Personality assessment inventory (19); BDI, Beck depression inventory (16); BAI, Beck anxiety inventory (17).
Of the 20 patients who completed cognitive testing, 19 performed in the “impaired” range in at least one cognitive domain. The most common domains of impairment were verbal learning and recall memory (n = 14, 70%), verbal fluency (n = 11, 55%), processing speed (n = 8, 40%), attention (n = 8, 40%), motor speed (n = 7, 35%), semantic verbal fluency (n = 6, 30%), visual learning and recall memory (n = 5, 25%), set-shifting (n = 5, 25%), inhibition control (n = 3, 15%), and visuospatial processing (n = 3, 15%).
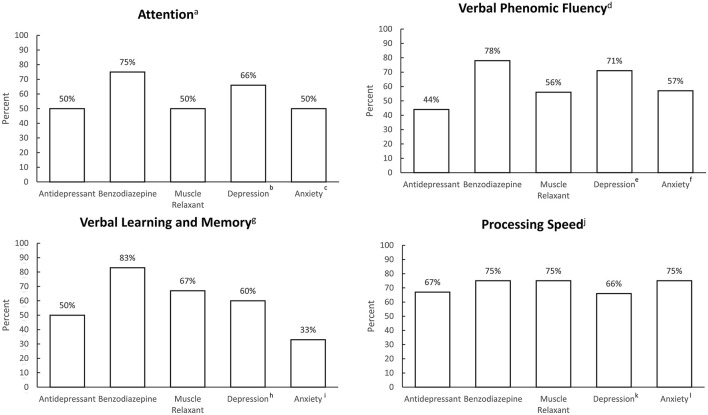
Patterns of medication use and clinically significant depressive and anxiety symptoms are described in Figure 1.
Figure 1.
Frequency of antidepressant use, benzodiazepine use, non-benzodiazepine muscle relaxant use, and clinically significant depression and anxiety symptoms, grouped by most commonly impaired cognitive domains. an = 8, bn = 3, cn = 2; dn = 11, en = 7, fn = 7; gn = 14, hn = 11, in = 6; jn = 8, kn = 6; ln = 4.
Discussion
To our knowledge, this is the first detailed examination of cognitive and mood profiles in patients with SPSD who present with cognitive concerns. The most common cognitive domains exhibiting impairment were verbal recall, processing speed, attention, and phonemic verbal fluency. Additionally, results suggest an overlap of cognitive impairment with use of SPSD medications and presence of mood and anxiety symptoms. Reduced GABA levels have been associated with anxiety and depression (21), as well as cognitive impairment in schizophrenia (22), multiple sclerosis (23), and Alzheimer's disease (24). Metabolic abnormalities in the frontal cortex, temporal cortex, thalamus, and cingulate cortex (25) have been reported in classic SPS, regions that have previously been associated with psychiatric symptoms in cognitive disorders (26). Thus, there is a biological plausibility that cognitive impairment and mood and anxiety disorders are intrinsic to the disease process.
Our results expand on previously published work by Budhram et al. (11). Though cognitive findings specific to SPS phenotype were not reported separately, they found that 18% (n = 38) of their cohort with various anti-GAD65 associated neurological disorders had cognitive impairment as diagnosed by the Kokmen short test of mental status (11, 27). Consistent with our findings, the predominant cognitive domains impacted were verbal learning and recall memory (29/38, 76%), followed by working memory/attention (6/38, 16%), and verbal fluency/language processing (3/38, 8%). Similarly, another study of cognitive profiles in 21 patients with anti-GAD65-positive diabetes (without a co-existing neurological condition, severe psychiatric disorders or use of psychotropic medications) reported that performance on recall memory and phonemic verbal fluency tasks were significantly lower in anti-GAD65-positive individuals than in the control group (5). Psychiatric symptoms, however, were not evaluated in either study in relation to cognition.
Among the 20 patients included in our case series, 65% were prescribed antidepressants, and approximately half of those assessed for depression and anxiety reported clinically significant symptoms. This is consistent with prior studies (3, 28, 29), and a recent systematic review which found that the relative risk of psychiatric comorbidity in SPS was higher than that of the general population (7). Mood and anxiety disorders are associated with deficits in learning and memory, executive function, and attention—areas also impaired in SPSD and anti-GAD65 associated diseases (8, 9). Although the present findings are observational and cannot confirm causation, bidirectional pathways of mood and cognition have been established in longitudinal studies of other patient populations (30, 31).
Both benzodiazepines and muscle relaxants have been associated with increased risk of cognitive impairment (32–34). In particular, long-term benzodiazepine use has been associated with deficits in visuospatial processing, processing speed, and verbal learning (10). While we observed a high prevalence of these medications in individuals with cognitive impairment, future studies on the potential effects of these medications on cognition in SPSD are needed to establish causality. At a minimum, there should be increased consideration for their long-term use given the potentially harmful effects.
These findings should be interpreted within the context of their limitations. This was a convenience, retrospective sample of individuals who had completed cognitive testing following referral based on reported cognitive concerns. Testing was conducted at different sites and by different providers, without standardization of test selection or interpretation. Moreover, as previously noted, certain medications that are used in SPSD can influence cognitive function. Despite the aforementioned limitations, our present findings contribute to the limited literature on cognitive and mood profiles in patients with SPSD by identifying common domains of cognitive impairment and potential overlap of cognitive impairment with mood symptoms and medication use.
In summary, assessment of cognitive impairment in SPSD should include testing of verbal learning and recall, phonemic verbal fluency, attention, and processing speed. Cognitive screening tools that examine these domains, such as the Montreal Cognitive Test (MoCA), could be used in the clinical setting to help identify patients who may need additional cognitive evaluation. Psychiatric symptoms and use of medications that may affect cognition are common, and should be considered when evaluating cognitive impairment in this population. Further studies are needed to replicate these findings using longitudinal prospective study designs with consistent cognitive assessment tools and interpretive standards to further clarify the scope of neuropsychiatric disturbance in SPSD and their underlying mechanisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Johns Hopkins Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CC: conceptualization of the study, data analysis, data interpretation, drafting, and revision of manuscript. DP, YW, and DO: data acquisition, data interpretation, and revision of manuscript. AH: data interpretation and revision of manuscript. SN: conceptualization of the study, data acquisition, data interpretation, study supervision, and revision of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.865462/full#supplementary-material
References
- 1.Hadavi S, Noyce AJ, Leslie RD, Giovannoni G. Stiff person syndrome. Pract Neurol. (2011) 11:272–82. 10.1136/practneurol-2011-000071 [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. (2009) 11:102–10. 10.1007/s11940-009-0013-9 [DOI] [PubMed] [Google Scholar]
- 3.Ameli R, Snow J, Rakocevic G, Dalakas MC. A neuropsychological assessment of phobias in patients with stiff person syndrome. Neurology. (2005) 64:1961–3. 10.1212/01.WNL.0000163984.71993.FE [DOI] [PubMed] [Google Scholar]
- 4.Lohmann T, Hawa M, Leslie RD, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxylase 65 in Stiffman syndrome and type 1 diabetes mellitus. Lancet. (2000) 356:31–5. 10.1016/S0140-6736(00)02431-4 [DOI] [PubMed] [Google Scholar]
- 5.Takagi M, Ishigaki Y, Uno K, Sawada S, Imai J, Kaneko K, et al. Cognitive dysfunction associated with anti-glutamic acid decarboxylase autoimmunity: a case-control study. BMC Neurol. (2013) 13:76. 10.1186/1471-2377-13-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampe CS, Petrosini L, De Bartolo P, Caporali P, Cutuli D, Laricchiuta D, et al. Monoclonal antibodies to 65kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J Rare Dis. (2013) 8:82. 10.1186/1750-1172-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffrey D, Finn CT, Song SM, Burton F, Arsan C. Stiff-person syndrome and psychiatric comorbidities: a systematic review. J Acad Consult Liaison Psychiatry. (2021) 62:3–13. 10.1016/j.psym.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. (2005) 39:207–14. 10.1016/j.jpsychires.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Rock P, Roiser J, Riedel W, Blackwell A. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. (2013) 44: 2029–40. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- 10.Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. (2005) 66(Suppl 2):9−13. [PubMed] [Google Scholar]
- 11.Budhram A, Sechi E, Flanagan EP, Dubey D, Zekeridou A, Shah SS, et al. Clinical spectrum of high-titre GAD65 antibodies. J Neurol Neurosurg Psychiatry. (2021) 92:645–54. 10.1136/jnnp-2020-325275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. (1996) 10:120–4. 10.1037/0894-4105.10.1.120 [DOI] [Google Scholar]
- 13.RStudio Team,. RStudio: Integrated Development Environment for R . Boston, MA: RStudio, PBC; (2020). Available online at: http://www.rstudio.com/ [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Internal Med. (2006) 166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. (2001) 16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; (1996). [Google Scholar]
- 17.Steer RA, Beck AT. Beck anxiety inventory. In: Zalaquett CP, Wood RJ, editors. Evaluating Stress: A Book of Resources. Scarecrow Education; (1997). p. 23–40. [Google Scholar]
- 18.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12:233–9. 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- 19.Morey LC, Lowmaster SE. Personality assessment inventory. In: Irving B, Weiner W, editors. The Corsini Encyclopedia of Psychology. Hoboken, NJ: Edward Craighead John Wiley & Sons Inc; American Cancer Society; (2010). p. 1–4. 10.1002/9780470479216.corpsy0663 [DOI] [Google Scholar]
- 20.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–9. 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 21.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. (2007) 24:495–517. 10.1002/da.20262 [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Wong AHC. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol Sin. (2018) 39:733–53. 10.1038/aps.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao G, Edden RAE, Gao F, Li H, Gong T, Chen W, et al. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur Radiol. (2018) 28:1140–8. 10.1007/s00330-017-5064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solas M, Puerta E, Ramirez JM. Treatment options in Alzheimer's disease: the GABA story. Curr Pharmaceut Design. (2015) 21:4960–71. 10.2174/1381612821666150914121149 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Sadaghiani MS, Tian F, Fitzgerald KC, Solnes L, Newsome SD. Brain and muscle metabolic changes by FDG-PET in stiff person syndrome spectrum disorders. Front Neurol. (2021) 12:1479. 10.3389/fneur.2021.692240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegen. (2021) 16:38. 10.1186/s13024-021-00456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. (1987) 62:281–8. 10.1016/S0025-6196(12)61905-3 [DOI] [PubMed] [Google Scholar]
- 28.Gerschlager W, Schrag A, Brown P. Quality of life in stiff person syndrome. Movement Disord. (2002) 17:1064–7. 10.1002/mds.10235 [DOI] [PubMed] [Google Scholar]
- 29.Newsome SD. Other Proven and Putative Autoimmune Disorders of the CNS: Anti-GAD Associated Neurological Disorders-Stiff-Person Syndrome, Cerebellar Ataxia, Progressive Encephalopathy with Rigidity and Myoclonus, and Encephalitis. New York, NY: Oxford University Press; (2016). [Google Scholar]
- 30.Chruzander C, Johansson S, Gottberg K, Einarsson U, Fredrikson S, Holmqvist LW, et al. A 10-year follow-up of a population-based study of people with multiple sclerosis in Stockholm, Sweden: changes in disability and the value of different factors in predicting disability and mortality. J Neurol Sci. (2013) 332:121–7. 10.1016/j.jns.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 31.Petkus AJ, Gomez ME, Filoteo JV, Schiehser DM, Petzinger G. Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson's disease: a bidirectional analysis. Neuropsychology. (2018) 33:35–46. 10.1037/neu0000498 [DOI] [PubMed] [Google Scholar]
- 32.Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. (2004) 24:1177–85. 10.1592/phco.24.13.1177.38089 [DOI] [PubMed] [Google Scholar]
- 33.Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. (2018) 75:e6–12. 10.2146/ajhp160381 [DOI] [PubMed] [Google Scholar]
- 34.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. (2019) 179:1084. 10.1001/jamainternmed.2019.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.