Abstract
Woody tissue carbon (C) concentration is a key wood trait necessary for accurately estimating forest C stocks and fluxes, which also varies widely across species and biomes. However, coarse approximations of woody tissue C (e.g., 50%) remain commonplace in forest C estimation and reporting protocols, despite leading to substantial errors in forest C estimates. Here, we describe the Global Woody Tissue Carbon Concentration Database (GLOWCAD): a database containing 3,676 individual records of woody tissue C concentrations from 864 tree species. Woody tissue C concentration data—i.e., the mass of C per unit dry mass—were obtained from live and dead woody tissues from 130 peer-reviewed sources published between 1980–2020. Auxiliary data for each observation include tissue type, as well as decay class and size characteristics for dead wood. In GLOWCAD, 1,242 data points are associated with geographic coordinates, and are therefore presented alongside 46 standardized bioclimatic variables extracted from climate databases. GLOWCAD represents the largest available woody tissue C concentration database, and informs studies on forest C estimation, as well as analyses evaluating the extent, causes, and consequences of inter- and intraspecific variation in wood chemical traits.
Subject terms: Carbon cycle, Plant ecology, Carbon cycle, Forest ecology
| Measurement(s) | wood carbon concentrations |
| Technology Type(s) | elemental analyzer |
| Factor Type(s) | species |
| Sample Characteristic - Organism | Plant |
| Sample Characteristic - Environment | terrestrial biome |
| Sample Characteristic - Location | Globe |
Background & Summary
Forests play a critical role in the global carbon (C) cycle, with the world’s forests storing an estimated 861 ± 66 Pg C across tropical (~471 Pg C), boreal (~272 Pg C), and temperate forest ecosystems (~119 Pg C)1. At the same time, C cycling in forested biomes is highly dynamic and transient, with estimates indicating that forests sequester between ~2.15 to 2.4 Pg C y−1 globally on average1,2. Throughout the 2000s, structurally intact old-growth forests accounted for ~0.85 Pg C y−1, while C sequestration was ~1.30 Pg C y−1 in secondary forests2. Tropical regions are particularly important in sequestering atmospheric carbon dioxide (CO2) in both regenerating3–5 and intact forests1,6,7. Nevertheless, recent analyses from both temperate8 and tropical regions7 have indicated that the magnitude of C sinks in old-growth forests are declining.
The amount of C stored within, and transferred to and from, trees and forests have been estimated from field- or remote-sensing-based observations of tree attributes, which are used to obtain estimates of tree- or forest aboveground biomass (AGB)1,9–12. Estimates of AGB are then converted into C estimates by multiplying these values by a woody tissue C concentration, commonly referred to in the literature as a C fraction13–16 (i.e., the mass of C per unit dry mass). Accurate woody tissue C concentration data are therefore critical in (1) accurately estimating terrestrial forest C budgets and sequestration rates17, (2) estimating the C emissions associated with land-use change18, and ultimately (3) informing decision-making related to the identification of forests with high C storage capacity11. Indeed, the Intergovernmental Panel on Climate Change’s (IPCC) Tier 3 C accounting protocols suggests that a “specific carbon fraction…should also be incorporated” when estimating C stocks and fluxes in AGB13. Moreover, woody tissue C concentration data can be employed in studies on the abiotic or biotic predictors of variation in – and possible adaptive significance of – wood chemical traits across tree species19,20, as well as evaluating the role that different sample extraction, preparation, and analytical methods have on wood C fractions17. Owing at least in part to a lack of large woody tissue C datasets, these research areas have received relatively little attention in comparison to other suites of plant traits21.
To date, most C estimation and reporting protocols use generic approximations of woody tissue C concentrations (namely, an assumption that 50% of AGB is comprised of C13), which has led to substantial systematic errors in forest C estimates. For example, our recent analyses indicated that generic woody tissue C fractions overestimate C stocks by approximately 8.9% in tropical forests19. Similar issues exist for the accounting of C stocks and fluxes in dead wood, with recent analyses indicating that generic dead wood C fractions may result in dead wood C pools being overestimated by ~3.0 Pg C globally22. Although multiple studies evaluating woody tissue C concentrations in trees globally through field- or meta-analyses now exist19,23–25, there is no single woody tissue C data repository to aid researchers in accessing and using these data.
To address these issues, we created and describe here the “Global Woody Tissue Carbon Concentration Database” (hereafter GLOWCAD26), which contains woody tissue C concentrations measured on live and dead tree tissues, spanning all forested biomes. By organizing and standardizing data from a range of taxonomic groups and woody tissue-types (described below), GLOWCAD represents a resource that helps improve our understanding of both global forest C dynamics and inter- and intraspecific variability in wood chemical traits. GLOWCAD only includes data from peer-reviewed sources. In addition to associated information on the taxonomic identities and woody tissue types for each woody tissue C data point, GLOWCAD includes geographical and associated bioclimatic data obtained from climate databases27.
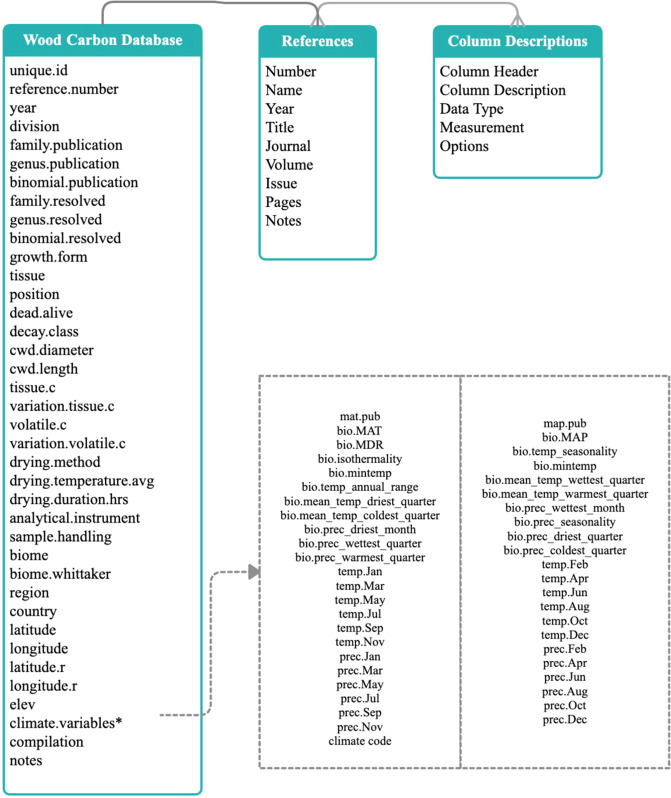
Data records in GLOWCAD are stored in 3 easy-to-use Comma Separated Values (.csv) spreadsheets (Fig. 1). All spreadsheets comprise plain text, with the first spreadsheet (titled “Wood Carbon Database”) containing the core data (i.e., woody tissue C concentrations and related information), while the other spreadsheets provide descriptive supporting information including references (titled “References”) and column descriptions (titled “Column Descriptions”). GLOWCAD has been made publicly available through the Dryad Digital Repository, with existing applications including studies on: (1) woody tissue C concentrations variation across live trees19,23,25; (2) variation in dead woody tissue C concentrations22; (3) relationships between woody tissue C concentrations and tree life-history strategies19,22; and (4) climate correlates of woody tissue C concentrations in trees28.
Fig. 1.
Structure of Global Woody Tissue Carbon Concentration Database (GLOWCAD). Teal boxes represent the three spreadsheets contained in GLOWCAD and include the column names of each record. Details for all measurements in the “Wood Carbon Database” worksheet are described in Supplementary Tables 1 and 2. Thick gray lines indicate links between worksheets, while gray dashed line indicates a sub-table containing sub-units of a primary variable.
Methods
Literature review
Data compilation expanded earlier versions of the GLOWCAD first initiated in 201225, and more recently published in 201819 and 202129. GLOWCAD is therefore based on a systematic search on primary literature of all peer-reviewed papers that cited previously published studies on woody tissue C concentrations19,23–25. We searched key terms “carbon”, “tree”, “wood carbon”, “coarse woody debris”, “dead wood”, and “wood nutrient”, as well as “carbon” alongside major tree tissue types (including “wood”, “bark”, “root” and “stem”), within four web-based platforms (Google Scholar, Web of Science, Web of Knowledge, and Scopus), in order to identify peer-reviewed publications that present species- specific woody tissue C concentration data.
In addition to peer-reviewed papers, other sources of data included in GLOWCAD include the TRY Plant Trait Database (v. 5.0)29 and the Global Root Traits (GRooT) Database28. The TRY datasets included are the Subarctic Plant Species Database (dataset ID 105), Plant Traits for Pinus and Juniperus Forests in Arizona (dataset ID 193), Plant Physiology Database (dataset ID 97), Panama Tree Traits Database (dataset ID 230), FRED database (dataset ID 339), and the ECOCRAFT Database (dataset ID 12). While most root C values in GRooT were included in the FRED database (v. 2.0)30, data from 10 papers in GRooT were added to GLOWCAD (n = 197 data points): Isaac et al.31; Liu et al.32; Wang et al.33; Minden et al.34; Alameda et al.35; Aubin et al.36; Fernández-García et al.37; Grechi et al.38; Ineson et al.39; and Pregitzer et al.40.
Wood C data attributes
To be included in GLOWCAD, the species-specific binomial nomenclature and tissue-specific information for each woody tissue C sample was required. A detailed field and lab methodology was also necessary, in order to maximize our sample size while permitting reliable species- and tissue-specific analysis. Where a single paper contained multiple tissue- and species-specific woody tissue C records, all the published values were recorded. In the majority of cases, woody tissue C data were extracted directly from published tables or from supplementary data of the articles. In instances where woody tissue C data were published as figures, the data was extracted using the WebPlotDigitizer v4.2 software41. If species-by-tissue-specific woody tissue C data were not published, the corresponding authors were contacted to provide data.
Each published woody tissue C record was then classified according to the forest biome in which it was sampled. A small number of studies (e.g.42) presented both boreal and temperate data, which were differentiated in our database based on the sampling location coupled with a consultation of species distribution maps. Species taxonomy was first recorded as presented as in published articles. A final list of taxa was then compared with, and resolved according to, the Taxonomic Name Resolution Service v. 4.043. Both original and resolved taxonomy is maintained in GLOWCAD. Inclusion of new published data was halted as of Dec. 31, 2020.
Dead wood C data attributes
When classifying dead wood data, we considered three primary factors associated with woody tissue decomposition and related chemical change: A) decay class (DC), B) position, and C) size (diameter and length). In the majority of publications, dead woody tissue C values were reported along a conventional 1–5 DC scale. These values were included in GLOWCAD as published, while noting the DC scale employed. In cases where DC was reported as a two-category range (e.g. DC 1–2), the higher DC was included in GLOWCAD. In cases where a multiple category DC was presented (e.g. DC 3–5), the middle DC value was used in GLOWCAD. In the few instances DC was reported along a 0–5 point scale (where DC of 0 was defined as dead and not live wood), dead wood reported with a DC of 0 was classified as DC 1. Lastly, in a subset of papers the number of years since tree death (instead of DC) was reported. In these cases, years since death were converted to DC based on published decay class transition metrics (e.g.44). When classifying position of dead wood, “standing” referred to snags and suspended woody debris, and “downed” referred to anything sampled from the forest floor. The default position was “downed” for the few publications that did not specify position.
GLOWCAD structure
The structure of GLOWCAD is simple to navigate (Fig. 1). Within GLOWCAD, all the woody tissue C data is present under the “Wood Carbon Database” spreadsheet. In this spreadsheet, a unique number (i.e., ‘unique.id’) of all woody tissue C data is specified beside the reference from which it was obtained. The value of the ‘reference.number’ corresponds to the detailed citation presented in the “References” spreadsheet, which links the ‘reference.number’ with the author(s)’ name and publication year, title, journal, volume, issue, and pages.
When inputting woody tissue C data from publications into GLOWCAD, the latitude and longitude were also recorded in the database when explicitly stated in the original publication. General climate information such as mean annual temperature (MAT) and mean annual precipitation (MAP) of the study region were recorded as an average. The study regions’ latitude and longitude were also used to further describe its climate with WorldClim (v.2) data27. However, when a range of geographic coordinates or a map was provided, climate data were not generated from these since averages MAT and MAP may be imprecise. We used MAT and MAP obtained from WorldClim (v.2) to label the study region’s dominant Whittaker biome45, and therefore categorize the region as one of Boreal forest, Subtropical desert, Temperate grassland/desert, Temperate rain forest, Temperate seasonal forest, Tropical rain forest, Tropical seasonal forest/savanna, or Woodland/shrubland. A list containing the details collected from each publication is presented (Supplementary Table 1). Bioclimatic variables and other climate data associated with each study location were retrieved from WorldClim (v.2)27 and added alongside woody tissue C data (Supplementary Table 2).
Previous versions of GLOWCAD
GLOWCAD is the fourth iteration of the woody tissue C dataset, though these earlier versions did not use the same acronym, and contained differing sets/ subsets of data based on different research questions. Three earlier versions are publicly hosted in the TRY Plant Trait Database, such that: 1) the first version contained n = 973 observations of dead wood C only, from 121 species; 2) the second version contained n = 1,145 observations of live woody tissue C only, from 415 species paired with geographic coordinates and climate data; and 3) the third version contained n = 2,432 observations of live woody tissue C only, from 636 species including all of the observations of the previous version .
GLOWCAD is a single data product which consolidates the dead and live woody tissue C observations of all prior iterations (where n = 3,405), and includes 271 new woody tissue C observations from 10 additional publications31–40,46. In sum, n = 3,676 data points in the GLOWCAD version described here. Unlike previous versions, GLOWCAD also includes information on growth habit or ‘woodiness’ (described below) and the original binomial nomenclatures as listed in their publications.
Data Records
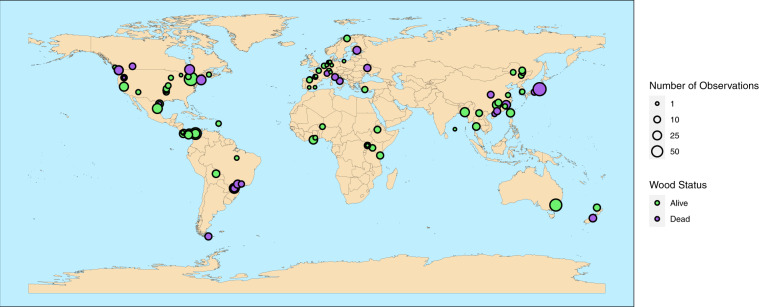
GLOWCAD is stored in .csv format at the Dryad Digital Repository (10.5061/dryad.18931zcxk). Data outputs consist of a single database of 3,676 woody tissue C observations from 130 sources17,23,24,30–40,42,44,46–159 published between 1980 and 2020 (Fig. 2), which includes C concentrations of woody tissues from 864 tree/shrub species sampled across all continents except Antarctica (Fig. 3). While data exists from papers published since 1980, the large majority (86%) of the data in GLOWCAD (n = 3,154 data points) is derived from sources published in or after 2010 (Fig. 1).
Fig. 2.
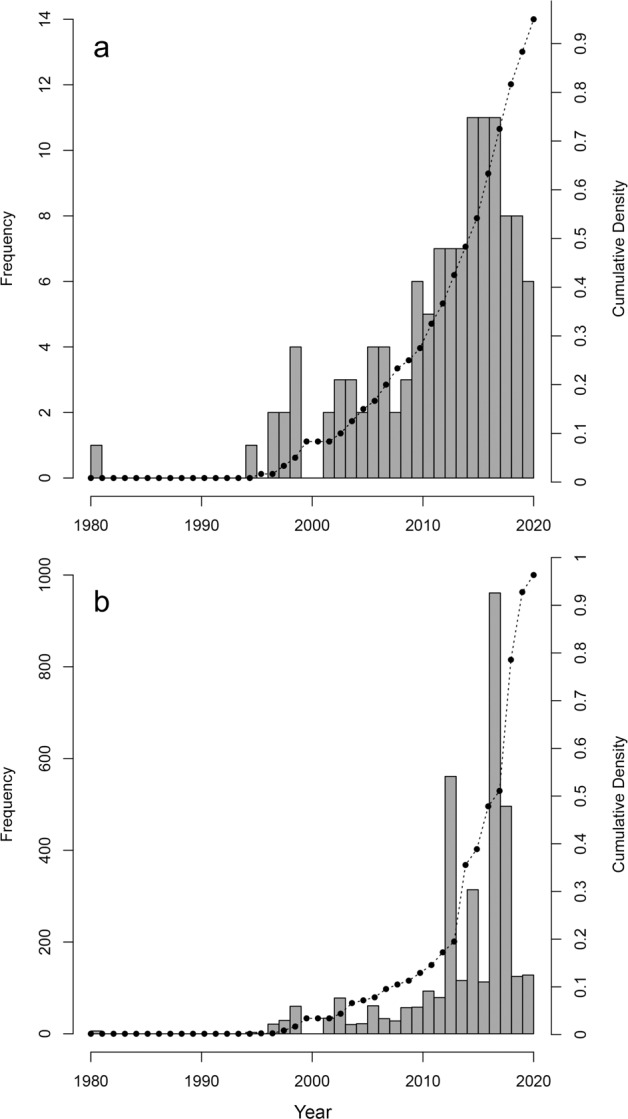
Number of peer-reviewed publications (Panel (a)) and observations (Panel (b)) included in Global Woody Tissue Carbon Concentration Database (GLOWCAD) across publication year. Gray bars indicate the number of publications or woody tissue carbon values included in GLOWCAD (corresponding to the y-axis), while black circles and dotted lines correspond to a cumulative probability density function (corresponding to the z-axis).
Fig. 3.
Woody tissue carbon concentration sampling sites for data sources included in the Global Woody Tissue Carbon Concentration Database (GLOWCAD). Data point colours correspond to tree status, where dead woody tissue is represented in green and live woody tissue is represented in purple. Point sizes are proportional to the number of woody tissue C observations recorded at a site on a continuous scale, ranging from 1–85 observations.
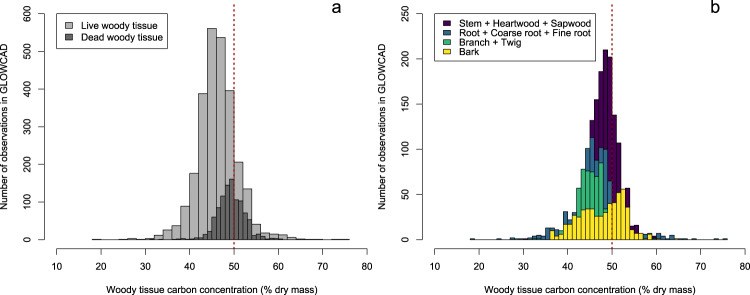
GLOWCAD includes woody tissue C values from 414 genera and 107 families, with the Pinaceae (n = 927 data points), Fabaceae (n = 383 data points), Fagaceae (n = 335 data points), Cupressaceae (n = 159 data points) and Betulaceae (n = 146 data points) being most well represented (Supplementary Tables 3–5). Across biomes, most woody tissue C data in GLOWCAD are derived from tropical forests (n = 1,513 data points), followed by temperate (n = 1,202 data points), subtropical/ Mediterranean (n = 518 data points) and boreal (n = 301 data points) forests. Across the entire database, woody tissue C ranged from 18.4–75.1% (Fig. 4).
Fig. 4.
Variation in woody tissue C concentrations (% dry mass) in GLOWCAD. Panel (a) compares the distribution of C concentrations between live woody tissues (light grey bars) and dead woody tissues (dark grey bars). Panel (b) compares the distribution of C concentrations among woody tissue types: stem, heartwood and sapwood (purple bars); root, coarse root and fine root (blue bars); branch and twig (green bars); and bark (yellow bars).
In GLOWCAD, 73% of data points were obtained from woody tissue measurements of live plants (n = 2,671 data points), while the remaining 27% (n = 1,005 data points) came from dead plant measurements. In regard to tissue types, stems (inclusive of heartwood and sapwood; n = 1,523 data points), roots (inclusive of fine-root and coarse-root; n = 986 data points) and branches (inclusive of both large and small branches/twigs; n = 619 data points) were most well represented (Table 1; Fig. 4). Additionally, woody tissue C data were retrieved from publications spanning a wide climatic range, with a MAT ranging from −5.4–29 °C (across n = 1,326 data points), and MAP ranging from 160–5,130 mm (n = 1,455 data points).
Table 1.
Sample sizes and ranges of woody tissue carbon concentration data in the Global Woody Tissue Carbon Concentration Database (GLOWCAD).
| Tissue type | Live woody tissue | Dead woody tissue | ||||
|---|---|---|---|---|---|---|
| n | Minimum | Maximum | n | Minimum | Maximum | |
| Bark | 319 | 36.0 | 65.0 | 229 | 41.0 | 59.0 |
| Branch | 337 | 28.0 | 59.2 | 85 | 41.5 | 53.8 |
| Coarse root | 345 | 28.0 | 58.3 | 0 | NA | NA |
| Fine root | 545 | 29.8 | 75.12 | 0 | NA | NA |
| Heartwood | 28 | 47.1 | 55.1 | 0 | NA | NA |
| Root | 39 | 18.4 | 51 | 57 | 44.5 | 50.0 |
| Sapwood | 33 | 47.1 | 54.1 | 0 | NA | NA |
| Stem | 828 | 30.5 | 60.68 | 634 | 29.4 | 60.2 |
| Twig | 197 | 33.0 | 54.9 | 0 | NA | NA |
The foremost drying method employed by publications incorporated into GLOWCAD was conventional oven-drying (n = 1,941 data points), while the least common was the Minimizing the Loss of Carbon (MLC) method described by Jones and O’Hara (201696; n = 9 data points). Drying temperatures ranged widely from 18–110 °C, with drying durations spanning 5–360 hours. The majority of publications made use of Elemental Analyzers (corresponding to n = 2,760 data points) when estimating woody tissue C concentrations. In sum, 34% of observations in GLOWCAD (n = 1,241 data points) were associated with exact geographic coordinates of their sampling locations (i.e, not a range of latitude and longitude), and only these observations were assigned climate information from WorldClim (v.2)27.
Technical Validation
Trait data validation
All 3,676 records included in GLOWCAD were obtained from peer-reviewed scientific journals, or indirectly, through the TRY Functional Trait Database or Global Root Traits Database. Each specific record is linked to its original reference, allowing users to verify and validate the accuracy of tissue C data and data source. All data in GLOWCAD was thoroughly screened to ensure accuracy, and appropriate methods of data acquisition. Specifically, woody tissue C values had to be measured directly, and not approximated based on secondary sources. Data that did not meet these criteria were excluded from GLOWCAD.
Taxonomic validation
Across the 40-year period during which data was collected (Fig. 1), tree species may have been misidentified or had their taxonomic information updated. To address these discrepancies and ensure that the most up-to-date taxonomic information is included in GLOWCAD, taxonomic information was directly recorded from original papers, and then verified and adjusted accordingly to reflect the appropriate name listed in the Taxonomic Name Resolution Service v. 4.033. All woody tissue C records included binomial nomenclature, and records without this degree of specificity were omitted from GLOWCAD. Phylogenetic coverage associated with the resolved taxonomy within GLOWCAD are presented in Fig. 5.
Fig. 5.
Phylogenetic coverage of species represented within the Global Woody Tissue Carbon Concentration Database (GLOWCAD). Colours mapped onto the phylogenetic tree correspond to 1) two major plant clades including gymnosperms (in blue, n = 100 species), angiosperms (in purple, n = 772 species), and 2) angiosperm ‘palm’ family, Arecaceae (in yellow, n = 8 species). Each branch represents a species in GLOWCAD (n = 864 total). Bars on the outer ring depict the sample sizes for each species (number of observations proportional to the logarithm of base 10), which are presented in full in Supplementary Tables 3–5. Phylogeny here is based on the Angiosperm Phylogeny Group megatree (R2012089.new) with branch lengths corresponding to clade ages based on fossil records165,166.
Growth habit validation
Growth habit or ‘woodiness’ was evaluated for all species included in GLOWCAD to ensure that woody tissue C data corresponded only to woody plant species, based on a functional definition of “woody”: i.e., having a persistent aboveground stem160. Therefore, all species were cross-referenced with those included in a global growth habit dataset160 and growth habits – defined here as trees, shrubs, or shrub/tree– were assigned. Species of the Arecaceae (palm) family (n = 8 species) were also included in GLOWCAD (n = 32 data points) since these 1) met the functional definition of “wood” and are 2) are important contributors to aboveground biomass C in Neotropical forests, relative to other biogeographic locations161, monocot species162 and non-conventional woody species (e.g. tree ferns)163.
Climate data validation
Bioclimatic variables were assigned to observations which were accompanied by the specific geographic coordinates (excluding ranges) of their sample location, using WorldClim (v.2). In GLOWCAD 34% of woody tissue C observations include a WorldClim-derived estimate of MAT and MAP (n = 1,241 data points). Linear regression models indicated that statistically significant positive relationships existed between (1) publication- vs. WorldClim-derived estimates of MAT (p<0.001, r2 = 0.95, model slope = 0.97 ± 0.01 (s.e.)) and (2) publication- vs. WorldClim-derived estimates of MAP (p<0.001, r2 = 0.82, model slope = 1.23 ± 0.02 (s.e.)).
Usage Notes
GLOWCAD is openly available for use in any application. It can be accessed via (1) the DRYAD Digital Repository (10.5061/dryad.18931zcxk), (2) a GitHub repository, (3) the TRY Plant Trait Database, and (4) upon request to the corresponding author. GLOWCAD is licensed under CC-BY 4.0.
Supplementary information
Acknowledgements
This research was financially supported by: 1) Discovery Grants from the Natural Science and Engineering Research Council of Canada to both A.R.M. and S.C.T.; 2) a University of Toronto Connaught New Researcher Award, and a University of Toronto Scarborough Research Impact Fund Grant to A.R.M.; 3) a University of Toronto Scarborough International Research Collaboration Fund grant to A.R.M. and G.M.D.; 4) the United States Department of Agriculture Forest Service Northern Research Station; 5) a University of Toronto Scarborough Department of Physical and Environmental Sciences graduate research bursary to both M.D. and R.K; 6) an Ontario Graduate Scholarship and a bursary from University of Toronto Scarborough’s Centre for Environmental Research in the Anthropocene (CERA) to M.D.; and 7) a University of Toronto Centre for Global Change Science Summer Intern scholarship to N.P. The findings and conclusions in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy.
Author contributions
A.R.M. conceived the study and led data compilation in preliminary versions GLOWCAD. M.D. wrote the paper and compiled and analyzed the data. N.P. and R.K. contributed to data compilation and helped write and edit the paper. Both S.C.T. and G.M.D. helped write and edit the paper, while S.C.T. conceived and contributed to earlier versions of GLOWCAD.
Code availability
All analyses used to generate figures and summary statistics were performed in R (v.4.1.2)164. No custom computer code or algorithms were used to generate the data presented in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-022-01396-1.
References
- 1.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 2.Pugh TAM, et al. Role of forest regrowth in global carbon sink dynamics. Proceedings of the National Academy of Sciences. 2019;116:4382–4387. doi: 10.1073/pnas.1810512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazdon RL, et al. Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Science Advances. 2016;2:e1501639. doi: 10.1126/sciadv.1501639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poorter L, et al. Biomass resilience of Neotropical secondary forests. Nature. 2016;530:211–214. doi: 10.1038/nature16512. [DOI] [PubMed] [Google Scholar]
- 5.Cook-Patton SC, et al. Mapping carbon accumulation potential from global natural forest regrowth. Nature. 2020;585:545–550. doi: 10.1038/s41586-020-2686-x. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SL, et al. Increasing carbon storage in intact African tropical forests. Nature. 2009;457:1003–1006. doi: 10.1038/nature07771. [DOI] [PubMed] [Google Scholar]
- 7.Hubau W, et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature. 2020;579:80–87. doi: 10.1038/s41586-020-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabuurs G-J, et al. First signs of carbon sink saturation in European forest biomass. Nature Climate Change. 2013;3:792–796. doi: 10.1038/nclimate1853. [DOI] [Google Scholar]
- 9.Köhl M, et al. Changes in forest production, biomass and carbon: results from the 2015 UN FAO Global Forest Resource Assessment. Forest Ecology and Management. 2015;352:21–34. doi: 10.1016/j.foreco.2015.05.036. [DOI] [Google Scholar]
- 10.Asner GP, et al. High-resolution forest carbon stocks and emissions in the Amazon. Proceedings of the National Academy of Sciences. 2010;107:16738–16742. doi: 10.1073/pnas.1004875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asner, G. P. Tropical forest carbon assessment: integrating satellite and airborne mapping approaches. Environmental Research Letters4, 10.1088/1748-9326/4/3/034009 (2009).
- 12.Xu L, et al. Changes in global terrestrial live biomass over the 21st century. Science Advances. 2021;7:eabe9829. doi: 10.1126/sciadv.abe9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aalde, U. et al. in IPCC Guidelines for National Greenhouse Gas Inventories Vol. 4 (eds Eggleston, S., Buendia, L., Miwa, K., Ngara, T. & Tanabe, K.) Ch. 4 (IPPC, 2006).
- 14.Brown S. Measuring carbon in forests: current status and future challenges. Environmental Pollution. 2002;116:363–372. doi: 10.1016/s0269-7491(01)00212-3. [DOI] [PubMed] [Google Scholar]
- 15.Woodall, C. W., Heath, L. S., Domke, G. M. & Nichols, M. C. Methods and equations for estimating aboveground volume, biomass, and carbon for trees in the U.S. forest inventory, 2010. (U.S. Department of Agriculture, Forest Service, Northern Research Station, 2011).
- 16.Saatchi SS, et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proceedings of the National Academy of Sciences. 2011;108:9899–9904. doi: 10.1073/pnas.1019576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamlom SH, Savidge RA. A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy. 2003;25:381–388. doi: 10.1016/S0961-9534(03)00033-3. [DOI] [Google Scholar]
- 18.Van Der Werf GR, et al. CO2 emissions from forest loss. Nature Geoscience. 2009;2:737–738. doi: 10.1038/ngeo671. [DOI] [Google Scholar]
- 19.Martin AR, Doraisami M, Thomas SC. Global patterns in wood carbon concentration across the world’s trees and forests. Nature Geoscience. 2018;11:915–920. doi: 10.1038/s41561-018-0246-x. [DOI] [Google Scholar]
- 20.Tavşanoğlu Ç, Pausas JG. A functional trait database for Mediterranean Basin plants. Scientific Data. 2018;5:180135. doi: 10.1038/sdata.2018.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chave J, et al. Towards a worldwide wood economics spectrum. Ecology Letters. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin, A. R., Domke, G. M., Doraisami, M. & Thomas, S. C. Carbon fractions in the world’s dead wood. Nature Communications12, 10.1038/s41467-021-21149-9 (2021). [DOI] [PMC free article] [PubMed]
- 23.Martin AR, Thomas SC. A Reassessment of carbon content in tropical trees. PLoS ONE. 2011;6:e23533. doi: 10.1371/journal.pone.0023533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin AR, Gezahegn S, Thomas SC. Variation in carbon and nitrogen concentration among major woody tissue types in temperate trees. Canadian Journal of Forest Research. 2015;45:744–757. doi: 10.1139/cjfr-2015-0024. [DOI] [Google Scholar]
- 25.Thomas SC, Martin AR. Carbon content of tree tissues: a synthesis. Forests. 2012;3:332–352. doi: 10.3390/f3020332. [DOI] [Google Scholar]
- 26.Doraisami M, 2022. GLOWCAD: A global database of woody tissue carbon concentrations fractions. Dryad. [DOI]
- 27.Fick SE, Hijmans RJ. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 28.Guerrero‐Ramírez NR, et al. Global root traits (GRooT) database. Global Ecology and Biogeography. 2021;30:25–37. doi: 10.1111/geb.13179. [DOI] [Google Scholar]
- 29.Kattge J, et al. TRY plant trait database – enhanced coverage and open access. Global Change Biology. 2020;26:119–188. doi: 10.1111/gcb.14904. [DOI] [PubMed] [Google Scholar]
- 30.Iversen CM, et al. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytologist. 2017;215:15–26. doi: 10.1111/nph.14486. [DOI] [PubMed] [Google Scholar]
- 31.Isaac, M. E. et al. Intraspecific trait variation and coordination: Root and Leaf Economics Spectra in coffee across environmental gradients. Frontiers in Plant Science8, 10.3389/fpls.2017.01196 (2017). [DOI] [PMC free article] [PubMed]
- 32.Liu C, et al. Variation in the functional traits of fine roots is linked to phylogenetics in the common tree species of Chinese subtropical forests. Plant and Soil. 2019;436:347–364. doi: 10.1007/s11104-019-03934-0. [DOI] [Google Scholar]
- 33.Wang R, et al. Different phylogenetic and environmental controls of first‐order root morphological and nutrient traits: Evidence of multidimensional root traits. Functional Ecology. 2018;32:29–39. doi: 10.1111/1365-2435.12983. [DOI] [Google Scholar]
- 34.Minden V, Kleyer M. Internal and external regulation of plant organ stoichiometry. Plant Biology. 2014;16:897–907. doi: 10.1111/plb.12155. [DOI] [PubMed] [Google Scholar]
- 35.Alameda D, Villar R. Linking root traits to plant physiology and growth in Fraxinus angustifolia Vahl. seedlings under soil compaction conditions. Environmental and Experimental Botany. 2012;79:49–57. doi: 10.1016/j.envexpbot.2012.01.004. [DOI] [Google Scholar]
- 36.Aubin I, et al. Traits to stay, traits to move: a review of functional traits to assess sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environmental Reviews. 2016;24:164–186. doi: 10.1139/er-2015-0072. [DOI] [Google Scholar]
- 37.Fernández-García N, et al. Intrinsic water use efficiency controls the adaptation to high salinity in a semi-arid adapted plant, henna (Lawsonia inermis L.) Journal of Plant Physiology. 2014;171:64–75. doi: 10.1016/j.jplph.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Grechi I, et al. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environmental and Experimental Botany. 2007;59:139–149. doi: 10.1016/j.envexpbot.2005.11.002. [DOI] [Google Scholar]
- 39.Ineson P, Cotrufo MF, Bol R, Harkness DD, Blum H. Quantification of soil carbon inputs under elevated CO2: C3 plants in a C4 soil. Plant and Soil. 1995;187:345–350. doi: 10.1007/bf00017099. [DOI] [Google Scholar]
- 40.Pregitzer KS, et al. Atmospheric CO2, soil nitrogen and turnover of fine roots. New Phytologist. 1995;129:579–585. doi: 10.1111/j.1469-8137.1995.tb03025.x. [DOI] [Google Scholar]
- 41.Rohatgi, A. WebPlotDigitizer: Version 4.5. https://automeris.io/WebPlotDigitizer (2021).
- 42.Harmon ME, Fasth B, Woodall CW, Sexton J. Carbon concentration of standing and downed woody detritus: effects of tree taxa, decay class, position, and tissue type. Forest Ecology and Management. 2013;291:259–267. doi: 10.1016/j.foreco.2012.11.046. [DOI] [Google Scholar]
- 43.Boyle, B. H. et al. The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinformatics14, 1-15, https://tnrs.biendata.org/ (2021). [DOI] [PMC free article] [PubMed]
- 44.Krankina ON, Harmon ME, Griazkin AV. Nutrient stores and dynamics of woody detritus in a boreal forest: modeling potential implications at the stand level. Canadian Journal of Forest Research. 1999;29:20–32. doi: 10.1139/x98-162. [DOI] [Google Scholar]
- 45.Whittaker RH. Classification of natural communities. Botanical Review. 1962;28:1–239. doi: 10.1007/BF02860872. [DOI] [Google Scholar]
- 46.Maiti R, Rodriguez HG. Wood carbon and nitrogen of 37 woody shrubs and trees in Tamaulipan thorn scrub, northeastern Mexico. Pakistan Journal of Botany. 2019;51:979–984. doi: 10.30848/PJB2019-3(25). [DOI] [Google Scholar]
- 47.Durkaya A. B. D., E. Makineci, I. Orhan Aboveground biomass and carbon storage relationship of Turkish Pines. Fresenius Environmental Bulletin. 2015;24:3573–3583. [Google Scholar]
- 48.Tesfaye MA, Bravo-Oviedo A, Bravo F, Pando V, De Aza CH. Variation in carbon concentration and wood density for five most commonly grown native tree species in central highlands of Ethiopia: The case of Chilimo dry Afromontane forest. Journal of Sustainable Forestry. 2019;38:769–790. doi: 10.1080/10549811.2019.1607754. [DOI] [Google Scholar]
- 49.Abdallah MAB, Mata-González R, Noller JS, Ochoa CG. Ecosystem carbon in relation to woody plant encroachment and control: Juniper systems in Oregon, USA. Agriculture, Ecosystems & Environment. 2020;290:106762. doi: 10.1016/j.agee.2019.106762. [DOI] [Google Scholar]
- 50.Arias D, Calvo-Alvarado J, Richter DDB, Dohrenbusch A. Productivity, aboveground biomass, nutrient uptake and carbon content in fast-growing tree plantations of native and introduced species in the Southern Region of Costa Rica. Biomass and Bioenergy. 2011;35:1779–1788. doi: 10.1016/j.biombioe.2011.01.009. [DOI] [Google Scholar]
- 51.Assefa D, Godbold DL, Belay B, Abiyu A, Rewald B. Fine Root Morphology, Biochemistry and Litter Quality Indices of Fast- and Slow-growing Woody Species in Ethiopian Highland Forest. Ecosystems. 2018;21:482–494. doi: 10.1007/s10021-017-0163-7. [DOI] [Google Scholar]
- 52.Atkin OK, Schortemeyer M, Mcfarlane N, Evans JR. The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia. 1999;120:544–554. doi: 10.1007/s004420050889. [DOI] [PubMed] [Google Scholar]
- 53.Bardulis A, Jansons A, Bardule A, Zeps M, LAzdins A. Assessment of carbon content in root biomass in Scots Pine and silver birch young stands of Latvia. Baltic Forestry. 2017;23:482–489. [Google Scholar]
- 54.Becker GS, Braun D, Gliniars R, Dalitz H. Relations between wood variables and how they relate to tree size variables of tropical African tree species. Trees. 2012;26:1101–1112. doi: 10.1007/s00468-012-0687-6. [DOI] [Google Scholar]
- 55.Bembenek M, et al. Carbon content in Juvenile and mature wood of Scots Pine (Pinus sylyestris L.) Baltic Forestry. 2015;21:279–284. [Google Scholar]
- 56.Bert D, Danjon F. Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.) Forest Ecology and Management. 2006;222:279–295. doi: 10.1016/j.foreco.2005.10.030. [DOI] [Google Scholar]
- 57.Borden KA, Anglaaere LCN, Adu-Bredu S, Isaac ME. Root biomass variation of cocoa and implications for carbon stocks in agroforestry systems. Agroforestry Systems. 2019;93:369–381. doi: 10.1007/s10457-017-0122-5. [DOI] [Google Scholar]
- 58.Borden KA, Isaac ME, Thevathasan NV, Gordon AM, Thomas SC. Estimating coarse root biomass with ground penetrating radar in a tree-based intercropping system. Agroforestry Systems. 2014;88:657–669. doi: 10.1007/s10457-014-9722-5. [DOI] [Google Scholar]
- 59.Bueno-López, S. W., García-Lucas, E. & Caraballo-Rojas, L. R. Allometric equations for total aboveground dry biomass and carbon content of Pinus occidentalis trees. Madera y Bosques25, 10.21829/myb.2019.2531868 (2019).
- 60.Bulmer RH, Schwendenmann L, Lundquist CJ. Allometric models for estimating aboveground biomass, carbon and nitrogen stocks in temperate Avicennia marina forests. Wetlands. 2016;36:841–848. doi: 10.1007/s13157-016-0793-0. [DOI] [Google Scholar]
- 61.Bütler R, Patty L, Le Bayon R-C, Guenat C, Schlaepfer R. Log decay of Picea abies in the Swiss Jura Mountains of central Europe. Forest Ecology and Management. 2007;242:791–799. doi: 10.1016/j.foreco.2007.02.017. [DOI] [Google Scholar]
- 62.Cao Y, Chen Y. Ecosystem C:N:P stoichiometry and carbon storage in plantations and a secondary forest on the Loess Plateau, China. Ecological Engineering. 2017;105:125–132. doi: 10.1016/j.ecoleng.2017.04.024. [DOI] [Google Scholar]
- 63.Castaño-Santamaría J, Bravo F. Variation in carbon concentration and basic density along stems of sessile oak (Quercus petraea (Matt.) Liebl.) and Pyrenean oak (Quercus pyrenaica Willd.) in the Cantabrian Range (NW Spain) Annals of Forest Science. 2012;69:663–672. doi: 10.1007/s13595-012-0183-6. [DOI] [Google Scholar]
- 64.Chao K-J, et al. Carbon concentration declines with decay class in tropical forest woody debris. Forest Ecology and Management. 2017;391:75–85. doi: 10.1016/j.foreco.2017.01.020. [DOI] [Google Scholar]
- 65.Chen Y, et al. Nutrient limitation of woody debris decomposition in a tropical forest: contrasting effects of N and P addition. Functional Ecology. 2016;30:295–304. doi: 10.1111/1365-2435.12471. [DOI] [Google Scholar]
- 66.Correia AC, et al. Biomass allometry and carbon factors for a Mediterranean pine (Pinus pinea L.) in Portugal. Forest Systems. 2010;19:418. doi: 10.5424/fs/2010193-9082. [DOI] [Google Scholar]
- 67.Cousins SJM, Battles JJ, Sanders JE, York RA. Decay patterns and carbon density of standing dead trees in California mixed conifer forests. Forest Ecology and Management. 2015;353:136–147. doi: 10.1016/j.foreco.2015.05.030. [DOI] [Google Scholar]
- 68.Craven D, et al. Seasonal variability of photosynthetic characteristics influences growth of eight tropical tree species at two sites with contrasting precipitation in Panama. Forest Ecology and Management. 2011;261:1643–1653. doi: 10.1016/j.foreco.2010.09.017. [DOI] [Google Scholar]
- 69.Cruz P, Bascuñan A, Velozo J, Rodriguez M. Funciones alométricas de contenido de carbono para quillay, peumo, espino y litre. Bosque (Valdivia) 2015;36:375–381. doi: 10.4067/s0717-92002015000300005. [DOI] [Google Scholar]
- 70.Currie WS, Nadelhoffer KJ. The imprint of land-use history: patterns of carbon and nitrogen in downed woody debris at the Harvard Forest. Ecosystems. 2002;5:446–460. doi: 10.1007/s10021-002-1153-x. [DOI] [Google Scholar]
- 71.Dong L, Zhang X, Li Variation in carbon concentration and allometric equations for estimating tree carbon contents of 10 broadleaf species in natural forests in northeast China. Forests. 2019;10:928. doi: 10.3390/f10100928. [DOI] [Google Scholar]
- 72.Dossa GGO, Paudel E, Cao K, Schaefer D, Harrison RD. Factors controlling bark decomposition and its role in wood decomposition in five tropical tree species. Scientific Reports. 2016;6:34153. doi: 10.1038/srep34153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durkaya B, Durkaya A, Makineci E, Ülküdür M. Estimation of above-ground biomass and sequestered carbon of Taurus Cedar (Cedrus libani L.) in Antalya, Turkey. iForest - Biogeosciences and Forestry. 2013;6:278–284. doi: 10.3832/ifor0899-006. [DOI] [Google Scholar]
- 74.Elias M, Potvin C. Assessing inter- and intra-specific variation in trunk carbon concentration for 32 neotropical tree species. Canadian Journal of Forest Research. 2003;33:1039–1045. doi: 10.1139/x03-018. [DOI] [Google Scholar]
- 75.Fang S, Xue J, Tang L. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. Journal of Environmental Management. 2007;85:672–679. doi: 10.1016/j.jenvman.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Fonseca W, Alice FE, Rey-Benayas JM. Carbon accumulation in aboveground and belowground biomass and soil of different age native forest plantations in the humid tropical lowlands of Costa Rica. New Forests. 2012;43:197–211. doi: 10.1007/s11056-011-9273-9. [DOI] [Google Scholar]
- 77.Frangi JL, Richter LL, Barrera MD, Aloggia M. Decomposition of Nothofagus fallen woody debris in forests of Tierra del Fuego, Argentina. Canadian Journal of Forest Research. 1997;27:1095–1102. doi: 10.1139/x97-060. [DOI] [Google Scholar]
- 78.Freschet GT, Cornelissen JHC, Van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology. 2010;98:362–373. doi: 10.1111/j.1365-2745.2009.01615.x. [DOI] [Google Scholar]
- 79.Fukatsu E, Fukuda Y, Takahashi M, Nakada R. Clonal variation of carbon content in wood of Larix kaempferi (Japanese larch) Journal of Wood Science. 2008;54:247–251. doi: 10.1007/s10086-007-0939-z. [DOI] [Google Scholar]
- 80.Ganamé M, Bayen P, Dimobe K, Ouédraogo I, Thiombiano A. Aboveground biomass allocation, additive biomass and carbon sequestration models for Pterocarpus erinaceus Poir. in Burkina Faso. Heliyon. 2020;6:e03805. doi: 10.1016/j.heliyon.2020.e03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganjegunte GK, Condron LM, Clinton PW, Davis MR, Mahieu N. Decomposition and nutrient release from radiata pine (Pinus radiata) coarse woody debris. Forest Ecology and Management. 2004;187:197–211. doi: 10.1016/s0378-1127(03)00332-3. [DOI] [Google Scholar]
- 82.Gao B, Taylor AR, Chen HYH, Wang J. Variation in total and volatile carbon concentration among the major tree species of the boreal forest. Forest Ecology and Management. 2016;375:191–199. doi: 10.1016/j.foreco.2016.05.041. [DOI] [Google Scholar]
- 83.Gillerot L, et al. Inter- and intraspecific variation in mangrove carbon fraction and wood specific gravity in Gazi Bay, Kenya. Ecosphere. 2018;9:e02306. doi: 10.1002/ecs2.2306. [DOI] [Google Scholar]
- 84.Gómez-Brandón M, et al. Physico-chemical and microbiological evidence of exposure effects on Picea abies – coarse woody debris at different stages of decay. Forest Ecology and Management. 2017;391:376–389. doi: 10.1016/j.foreco.2017.02.033. [DOI] [Google Scholar]
- 85.Guner ST, Comez A. Biomass equations and changes in carbon stock in afforested Black Pine (Pinus nigra Arnold. subsp. pallasiana (Lamb.) Holmboe) stands in Turkey. Fresenius Environmental Bulletin. 2017;26:2368–2379. [Google Scholar]
- 86.Guo J, Chen G, Xie J, Yang Z, Yang Y. Patterns of mass, carbon and nitrogen in coarse woody debris in five natural forests in southern China. Annals of Forest Science. 2014;71:585–594. doi: 10.1007/s13595-014-0366-4. [DOI] [Google Scholar]
- 87.Hanpattanakit P, Chidthaisong A, Sanwangsri M, Lichaikul N. Improving allometric equations to estimate biomass and carbon in secondary dry dipterocarp forest. Singapore SG. 2016;18:208–211. [Google Scholar]
- 88.Herrero De Aza C, Turrión MB, Pando V, Bravo F. Carbon in heartwood, sapwood and bark along the stem profile in three Mediterranean Pinus species. Annals of Forest Science. 2011;68:1067–1076. doi: 10.1007/s13595-011-0122-y. [DOI] [Google Scholar]
- 89.Huet S, Forgeard FO, Nys C. Above- and belowground distribution of dry matter and carbon biomass of Atlantic beech (Fagus sylvatica L.) in a time sequence. Annals of Forest Science. 2004;61:683–694. doi: 10.1051/forest:2004063. [DOI] [Google Scholar]
- 90.Jacobs DF, Selig MF, Severeid LR. Aboveground carbon biomass of plantation-grown American chestnut (Castanea dentata) in absence of blight. Forest Ecology and Management. 2009;258:288–294. doi: 10.1016/j.foreco.2009.04.014. [DOI] [Google Scholar]
- 91.Janssens IA, et al. Above- and belowground phytomass and carbon storage in a Belgian Scots pine stand. Annals of forest science. 1999;56:81–90. doi: 10.1051/forest:19990201. [DOI] [Google Scholar]
- 92.Jiménez Pérez J, Treviño Garza EJ, Yerena Yamallel JI. Concentración de carbono en especies del bosque de pino-encino en la Sierra Madre Oriental. Revista mexicana de ciencias forestales. 2013;4:50–61. doi: 10.29298/rmcf.v4i17.420. [DOI] [Google Scholar]
- 93.Jomura M, et al. Biotic and abiotic factors controlling respiration rates of above- and belowground woody debris of Fagus crenata and Quercus crispula in Japan. PLOS ONE. 2015;10:e0145113. doi: 10.1371/journal.pone.0145113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones D, O’Hara K. Variation in carbon fraction, density, and carbon density in conifer tree tissues. Forests. 2018;9:430. doi: 10.3390/f9070430. [DOI] [Google Scholar]
- 95.Jones DA, O’Hara KL. Carbon density in managed coast redwood stands: implications for forest carbon estimation. Forestry. 2012;85:99–110. doi: 10.1093/forestry/cpr063. [DOI] [Google Scholar]
- 96.Jones DA, O’Hara KL. The influence of preparation method on measured carbon fractions in tree tissues. Tree Physiol. 2016;36:1177–1189. doi: 10.1093/treephys/tpw051. [DOI] [PubMed] [Google Scholar]
- 97.Joosten R, Schulte A. Climatic Change. 2002;55:115–129. doi: 10.1023/a:1020227806137. [DOI] [Google Scholar]
- 98.Joosten R, Schumacher J, Wirth C, Schulte A. Evaluating tree carbon predictions for beech (Fagus sylvatica L.) in western Germany. Forest Ecology and Management. 2004;189:87–96. doi: 10.1016/j.foreco.2003.07.037. [DOI] [Google Scholar]
- 99.Kim C, Yoo B, Jung S, Lee K. Allometric equations to assess biomass, carbon and nitrogen content of black pine and red pine trees in southern Korea. iForest - Biogeosciences and Forestry. 2017;10:483–490. doi: 10.3832/ifor2164-010. [DOI] [Google Scholar]
- 100.Kort, J. & Turnock, R. Carbon reservoirs and biomass in Canadian prairie shelterbelts. Agroforestry Systems44, 175-186, 10.1023/a:1006226006785 (1998).
- 101.Köster K, Metslaid M, Engelhart J, Köster E. Dead wood basic density, and the concentration of carbon and nitrogen for main tree species in managed hemiboreal forests. Forest Ecology and Management. 2015;354:35–42. doi: 10.1016/j.foreco.2015.06.039. [DOI] [Google Scholar]
- 102.Kraenzel M, Castillo A, Moore T, Potvin C. Carbon storage of harvest-age teak (Tectona grandis) plantations, Panama. Forest Ecology and Management. 2003;173:213–225. doi: 10.1016/s0378-1127(02)00002-6. [DOI] [Google Scholar]
- 103.Laiho R, Laine J. Tree stand biomass and carbon content in an age sequence of drained pine mires in southern Finland. Forest Ecology and Management. 1997;93:161–169. doi: 10.1016/s0378-1127(96)03916-3. [DOI] [Google Scholar]
- 104.Laiho R, Prescott CE. The contribution of coarse woody debris to carbon, nitrogen, and phosphorus cycles in three Rocky Mountain coniferous forests. Canadian Journal of Forest Research. 1999;29:1592–1603. doi: 10.1139/x99-132. [DOI] [Google Scholar]
- 105.Lambert RL, Lang GE, Reiners WA. Loss of mass and chemical change in decaying boles of a subalpine Balsam Fir forest. Ecology. 1980;61:1460–1473. doi: 10.2307/1939054. [DOI] [Google Scholar]
- 106.Laughlin DC, Leppert JJ, Moore MM, Sieg CH. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Functional Ecology. 2010;24:493–501. doi: 10.1111/j.1365-2435.2009.01672.x. [DOI] [Google Scholar]
- 107.Li X, et al. Biomass and carbon storage in an age-sequence of Korean Pine (Pinus koraiensis) plantation forests in central Korea. Journal of Plant Biology. 2011;54:33–42. doi: 10.1007/s12374-010-9140-9. [DOI] [Google Scholar]
- 108.Lombardi F, et al. Investigating biochemical processes to assess deadwood decay of Beech and Silver Fir in Mediterranean mountain forests. Annals of Forest Science. 2013;70:101–111. doi: 10.1007/s13595-012-0230-3. [DOI] [Google Scholar]
- 109.Lutter R, Tullus A, Kanal A, Tullus T, Tullus H. The impact of former land-use type to above- and below-ground C and N pools in short-rotation hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations in hemiboreal conditions. Forest Ecology and Management. 2016;378:79–90. doi: 10.1016/j.foreco.2016.07.021. [DOI] [Google Scholar]
- 110.Mahmood H, et al. Applicability of semi-destructive method to derive allometric model for estimating aboveground biomass and carbon stock in the Hill Zone of Bangladesh. Journal of Forestry Research. 2020;31:1235–1245. doi: 10.1007/s11676-019-00881-5. [DOI] [Google Scholar]
- 111.Maiti R, Gonzalez Rodriguez H, Kumari A. Wood density of ten native trees and shrubs and its possible relation with a few wood chemical compositions. American Journal of Plant Sciences. 2016;07:1192–1197. doi: 10.4236/ajps.2016.78114. [DOI] [Google Scholar]
- 112.Mäkinen H, Hynynen J, Siitonen J, Sievänen R. Predicting The decomposition of Scots Pine, Norway Spruce, and Birch stems in Finland. Ecological Applications. 2006;16:1865–1879. doi: 10.1890/1051-0761(2006)016[1865:ptdosp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 113.Manuella S, et al. Chemical transformations in downed logs and snags of mixed boreal species during decomposition. Canadian Journal of Forest Research. 2013;43:785–798. doi: 10.1139/cjfr-2013-0086. [DOI] [Google Scholar]
- 114.Martin AR, Thomas SC. Size-dependent changes in leaf and wood chemical traits in two Caribbean rainforest trees. Tree Physiology. 2013;33:1338–1353. doi: 10.1093/treephys/tpt085. [DOI] [PubMed] [Google Scholar]
- 115.Martin AR, Thomas SC, Zhao Y. Size-dependent changes in wood chemical traits: a comparison of neotropical saplings and large trees. AoB PLANTS. 2013;5:plt039–plt039. doi: 10.1093/aobpla/plt039. [DOI] [Google Scholar]
- 116.Medlyn BE, et al. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant, Cell & Environment. 1999;22:1475–1495. doi: 10.1046/j.1365-3040.1999.00523.x. [DOI] [Google Scholar]
- 117.Moreira, A. B., Gregoire, T. G. & Do Couto, H. T. Z. Wood density and carbon concentration of coarse woody debris in native forests, Brazil. Forest Ecosystems6, 10.1186/s40663-019-0177-z (2019).
- 118.Morhart, C., Sheppard, J. P., Schuler, J. K. & Spiecker, H. Above-ground woody biomass allocation and within tree carbon and nutrient distribution of wild cherry (Prunus avium L.) – a case study. Forest Ecosystems3, 10.1186/s40663-016-0063-x (2016).
- 119.Northup BK, Zitzer SF, Archer S, Mcmurtry CR, Boutton TW. Above-ground biomass and carbon and nitrogen content of woody species in a subtropical thornscrub parkland. Journal of Arid Environments. 2005;62:23–43. doi: 10.1016/j.jaridenv.2004.09.019. [DOI] [Google Scholar]
- 120.Palviainen M, Finér L. Decomposition and nutrient release from Norway spruce coarse roots and stumps – a 40-year chronosequence study. Forest Ecology and Management. 2015;358:1–11. doi: 10.1016/j.foreco.2015.08.036. [DOI] [Google Scholar]
- 121.Peri PL, Gargaglione V, Martínez Pastur G, Lencinas MV. Carbon accumulation along a stand development sequence of Nothofagus antarctica forests across a gradient in site quality in Southern Patagonia. Forest Ecology and Management. 2010;260:229–237. doi: 10.1016/j.foreco.2010.04.027. [DOI] [Google Scholar]
- 122.Pompa-García, M. & Jurado, E. Carbon concentration in structures of Arctostaphylos pungens HBK: an alternative CO2 sink in forests. Phyton84, 385-389 (2016).
- 123.Pompa-García M, Sigala-Rodríguez J, Jurado E, Flores J. Tissue carbon concentration of 175 Mexican forest species. iForest - Biogeosciences and Forestry. 2017;10:754–758. doi: 10.3832/ifor2421-010. [DOI] [Google Scholar]
- 124.Pompa-García M, Yerena-Yamalliel JI. Concentration of carbon in Pinus cembroides Zucc: potential source of global warming mitigation. Revista Chapingo Serie Ciencias Forestales y del Ambiente. 2014;20:169–175. doi: 10.5154/r.rchscfa.2014.04.014. [DOI] [Google Scholar]
- 125.Preston CM, Trofymow JA, Flanagan LB. Decomposition, δ13C, and the “lignin paradox”. Canadian Journal of Soil Science. 2006;86:235–245. doi: 10.4141/s05-090. [DOI] [Google Scholar]
- 126.Preston CM, Trofymow JA, Nault JR. Decomposition and change in N and organic composition of small-diameter Douglas-fir woody debris over 23 years. Canadian Journal of Forest Research. 2012;42:1153–1167. doi: 10.1139/x2012-076. [DOI] [Google Scholar]
- 127.Preston CM, Trofymow JA, Niu J, Fyfe CA. PMAS-NMR spectroscopy and chemical analysis of coarse woody debris in coastal forests of Vancouver Island. Forest Ecology and Management. 1998;111:51–68. doi: 10.1016/s0378-1127(98)00307-7. [DOI] [Google Scholar]
- 128.Rana R, Langenfeld-Heyser R, Finkeldey R, Polle A. FTIR spectroscopy, chemical and histochemical characterisation of wood and lignin of five tropical timber wood species of the family of Dipterocarpaceae. Wood Science and Technology. 2010;44:225–242. doi: 10.1007/s00226-009-0281-2. [DOI] [Google Scholar]
- 129.Ray R, Majumder N, Chowdhury C, Jana TK. Wood chemistry and density: an analog for response to the change of carbon sequestration in mangroves. Carbohydrate Polymers. 2012;90:102–108. doi: 10.1016/j.carbpol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 130.Rodrigues DP, Hamacher C, Estrada GCD, Soares MLG. Variability of carbon content in mangrove species: effect of species, compartments and tidal frequency. Aquatic Botany. 2015;120:346–351. doi: 10.1016/j.aquabot.2014.10.004. [DOI] [Google Scholar]
- 131.Sakai Y, Ugawa S, Ishizuka S, Takahashi M, Takenaka C. Wood density and carbon and nitrogen concentrations in deadwood of Chamaecyparis obtusa and Cryptomeria japonica. Soil Science and Plant Nutrition. 2012;58:526–537. doi: 10.1080/00380768.2012.710526. [DOI] [Google Scholar]
- 132.Sandström F, Petersson H, Kruys N, Ståhl G. Biomass conversion factors (density and carbon concentration) by decay classes for dead wood of Pinus sylvestris, Picea abies and Betula spp. in boreal forests of Sweden. Forest Ecology and Management. 2007;243:19–27. doi: 10.1016/j.foreco.2007.01.081. [DOI] [Google Scholar]
- 133.Sanquetta MNI, Sanquetta CR, Mognon F, Corte APD, Maas GCB. Wood density and carbon content in young teak individuals from Pará, Brazil. Científica. 2016;44:608. doi: 10.15361/1984-5529.2016v44n4p608-614. [DOI] [Google Scholar]
- 134.Schwendenmann, L. & Mitchell, N. Carbon accumulation by native trees and soils in an urban park, Auckland. New Zealand Journal of Ecology38(20), 213–220 (2014).
- 135.Setälä H, Marshall VG, Trofymow JA. Influence of micro- and macro-habitat factors on collembolan communities in Douglas-fir stumps during forest succession. Applied Soil Ecology. 1995;2:227–242. doi: 10.1016/0929-1393(95)00053-9. [DOI] [Google Scholar]
- 136.Sohrabi H, Bakhtiarvand-Bakhtiari S, Ahmadi K. Above- and below-ground biomass and carbon stocks of different tree plantations in central Iran. Journal of Arid Land. 2016;8:138–145. doi: 10.1007/s40333-015-0087-z. [DOI] [Google Scholar]
- 137.Telmo C, Lousada J, Moreira N. Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresource Technology. 2010;101:3808–3815. doi: 10.1016/j.biortech.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 138.Thomas AL, et al. Carbon and nitrogen accumulation within four black walnut alley cropping sites across Missouri and Arkansas, USA. Agroforestry Systems. 2020;94:1625–1638. doi: 10.1007/s10457-019-00471-8. [DOI] [Google Scholar]
- 139.Thomas SC, Malczewski G. Wood carbon content of tree species in Eastern China: interspecific variability and the importance of the volatile fraction. Journal of Environmental Management. 2007;85:659–662. doi: 10.1016/j.jenvman.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 140.Tolunay D. Carbon concentrations of tree components, forest floor and understorey in young Pinus sylvestris stands in north-western Turkey. Scandinavian Journal of Forest Research. 2009;24:394–402. doi: 10.1080/02827580903164471. [DOI] [Google Scholar]
- 141.Tramoy R, Sebilo M, Nguyen Tu TT, Schnyder J. Carbon and nitrogen dynamics in decaying wood: paleoenvironmental implications. Environmental Chemistry. 2017;14:9. doi: 10.1071/en16049. [DOI] [Google Scholar]
- 142.Van Geffen KG, Poorter L, Sass-Klaassen U, Van Logtestijn RSP, Cornelissen JHC. The trait contribution to wood decomposition rates of 15 Neotropical tree species. Ecology. 2010;91:3686–3697. doi: 10.1890/09-2224.1. [DOI] [PubMed] [Google Scholar]
- 143.Wang G, et al. Variations in the live biomass and carbon pools of Abies georgei along an elevation gradient on the Tibetan Plateau, China. Forest Ecology and Management. 2014;329:255–263. doi: 10.1016/j.foreco.2014.06.023. [DOI] [Google Scholar]
- 144.Wang XW, Weng YH, Liu GF, Krasowski MJ, Yang CP. Variations in carbon concentration, sequestration and partitioning among Betula platyphylla provenances. Forest Ecology and Management. 2015;358:344–352. doi: 10.1016/j.foreco.2015.08.029. [DOI] [Google Scholar]
- 145.Watzlawick LF, et al. Teores de carbono em espécies da floresta ombrófila mista e efeito do grupo ecológico. Cerne. 2014;20:613–620. doi: 10.1590/01047760201420041492. [DOI] [Google Scholar]
- 146.Weber JC, et al. Variation in growth, wood density and carbon concentration in five tree and shrub species in Niger. New Forests. 2018;49:35–51. doi: 10.1007/s11056-017-9603-7. [DOI] [Google Scholar]
- 147.Weggler K, Dobbertin M, Jüngling E, Kaufmann E, Thürig E. Dead wood volume to dead wood carbon: the issue of conversion factors. European Journal of Forest Research. 2012;131:1423–1438. doi: 10.1007/s10342-012-0610-0. [DOI] [Google Scholar]
- 148.Widagdo FRA, Xie L, Dong L, Li F. Origin-based biomass allometric equations, biomass partitioning, and carbon concentration variations of planted and natural Larix gmelinii in northeast China. Global Ecology and Conservation. 2020;23:e01111. doi: 10.1016/j.gecco.2020.e01111. [DOI] [Google Scholar]
- 149.Wu, H. et al. Tree functional types simplify forest carbon stock estimates induced by carbon concentration variations among species in a subtropical area. Scientific Reports7, 10.1038/s41598-017-05306-z (2017). [DOI] [PMC free article] [PubMed]
- 150.Xing Z, et al. Carbon and biomass partitioning in balsam fir (Abies balsamea) Tree Physiology. 2005;25:1207–1217. doi: 10.1093/treephys/25.9.1207. [DOI] [PubMed] [Google Scholar]
- 151.Yang F-F, et al. Dynamics of coarse woody debris and decomposition rates in an old-growth forest in lower tropical China. Forest Ecology and Management. 2010;259:1666–1672. doi: 10.1016/j.foreco.2010.01.046. [DOI] [Google Scholar]
- 152.Yeboah D, Burton AJ, Storer AJ, Opuni-Frimpong E. Variation in wood density and carbon content of tropical plantation tree species from Ghana. New Forests. 2014;45:35–52. doi: 10.1007/s11056-013-9390-8. [DOI] [Google Scholar]
- 153.Ying, J., Weng, Y., Oswald, B. P. & Zhang, H. Variation in carbon concentrations and allocations among Larix olgensis populations growing in three field environments. Annals of Forest Science76, 10.1007/s13595-019-0877-0 (2019).
- 154.Yuan J, Cheng F, Zhu X, Li J, Zhang S. Respiration of downed logs in pine and oak forests in the Qinling Mountains, China. Soil Biology and Biochemistry. 2018;127:1–9. doi: 10.1016/j.soilbio.2018.09.012. [DOI] [Google Scholar]
- 155.Zabek LM, Prescott CE. Biomass equations and carbon content of aboveground leafless biomass of hybrid poplar in Coastal British Columbia. Forest Ecology and Management. 2006;223:291–302. doi: 10.1016/j.foreco.2005.11.009. [DOI] [Google Scholar]
- 156.Zhang, H., Jiang, Y., Song, M., He, J. & Guan, D. Improving understanding of carbon stock characteristics of Eucalyptus and Acacia trees in southern China through litter layer and woody debris. Scientific Reports10, 10.1038/s41598-020-61476-3 (2020). [DOI] [PMC free article] [PubMed]
- 157.Zhang Q, Wang C, Wang X, Quan X. Carbon concentration variability of 10 Chinese temperate tree species. Forest Ecology and Management. 2009;258:722–727. doi: 10.1016/j.foreco.2009.05.009. [DOI] [Google Scholar]
- 158.Zheng H, et al. Variation of carbon storage by different reforestation types in the hilly red soil region of southern China. Forest Ecology and Management. 2008;255:1113–1121. doi: 10.1016/j.foreco.2007.10.015. [DOI] [Google Scholar]
- 159.Zhou, L. et al. Tissue-specific carbon concentration, carbon stock, and distribution in Cunninghamia lanceolata (Lamb.) Hook plantations at various developmental stages in subtropical China. Annals of Forest Science76, 10.1007/s13595-019-0851-x (2019).
- 160.Zanne AE, et al. Three keys to the radiation of angiosperms into freezing environments. Nature. 2014;506:89–92. doi: 10.1038/nature12872. [DOI] [PubMed] [Google Scholar]
- 161.Muscarella R, et al. The global abundance of tree palms. Global Ecology and Biogeography. 2020;29:1495–1514. doi: 10.1111/geb.13123. [DOI] [Google Scholar]
- 162.Du H, et al. Mapping global bamboo forest distribution using multisource remote sensing data. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing. 2018;11:1458–1471. doi: 10.1109/JSTARS.2018.2800127. [DOI] [Google Scholar]
- 163.Iwashita DK, Litton CM, Giardina CP. Coarse woody debris carbon storage across a mean annual temperature gradient in tropical montane wet forest. Forest Ecology and Management. 2013;291:336–343. doi: 10.1016/j.foreco.2012.11.043. [DOI] [Google Scholar]
- 164.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2021).
- 165.Wikström N, Savolainen V, Chase MW. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268:2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gastauer M, Meira Neto JAA. Updated angiosperm family tree for analyzing phylogenetic diversity and community structure. Acta Botanica Brasilica. 2017;31:191–198. doi: 10.1590/0102-33062016abb0306. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Doraisami M, 2022. GLOWCAD: A global database of woody tissue carbon concentrations fractions. Dryad. [DOI]
Supplementary Materials
Data Availability Statement
All analyses used to generate figures and summary statistics were performed in R (v.4.1.2)164. No custom computer code or algorithms were used to generate the data presented in the manuscript.